Abstract
Objective:
Converging evidence indicates that brain abnormalities in autism spectrum disorders (ASDs) involve atypical network connectivity. Given the central role of social deficits in the ASD phenotype, this investigation examined functional connectivity of the amygdala – a brain structure critically involved in processing of social information – in children and adolescents with ASDs, as well as age-dependent changes and links with clinical symptoms.
Method:
Resting-state functional magnetic resonance imaging (rs-fMRI) data from 55 participants with ASDs and 50 typically developing (TD) controls, ages 7–17 years, were included. Groups were matched for age, gender, IQ, and head motion. Functional connectivity MRI (fcMRI) analysis was applied to examine intrinsic functional connectivity (iFC) of the amygdala, including cross-sectional tests of age-related changes.
Results:
Direct between-group comparisons revealed reduced functional connectivity between bilateral amygdalae and left inferior occipital cortex, accompanied by greater connectivity between right amygdala and right sensorimotor cortex in the ASD group. This atypical pattern of amygdala connectivity was associated with decreased symptom severity and better overall functioning, as specifically seen in an ASD subgroup with the most atypical amygdala iFC but least impaired social functioning. Age-related strengthening of amygdala-prefrontal connectivity, as observed in the TD group, was not detected in children with ASDs.
Conclusion:
Findings support aberrant network sculpting in ASDs, specifically atypical integration between amygdala and primary sensorimotor circuits. Paradoxical links between atypical iFC and behavioral measures suggest that abnormal amygdala functional connections may be compensatory in some individuals with ASDs.
Keywords: autism spectrum disorders (ASDs), neuroimaging, functional MRI (fMRI), functional connectivity, amygdala
Introduction
Deficits in social functioning are a core feature of autism spectrum disorders (ASDs). Although etiological and clinical heterogeneity of ASDs is widely recognized, social impairments – including diminished social responsiveness, difficulty recognizing others’ emotions and intentions, inability to form friendships or to relate to peers in a reciprocal manner – remain a defining feature of all ASDs,1 and are considered the most universal and specific characteristics of the autism spectrum.2,3 Despite having been extensively studied (as reviewed in refs.4–7), the neural circuitry supporting human social functioning remains incompletely understood, both in typical8,9 and atypical development, including in ASDs.10–12 One of the principal brain regions considered essential for social functioning is the amygdala, a relatively small structure located in the medial temporal lobe, just anterior to the hippocampus. Its role in social cognition is well established across species and with a variety of methods, from earlier studies in nonhuman primates demonstrating that its activity is linked to interpreting social signals from conspecifics,13 to more recent evidence that human adults with larger amygdala volumes or greater amygdala gray matter density have more complex social networks.14,15
As known from tract tracing studies,16,17 the amygdala has widespread connections throughout the brain, including both afferent and efferent connections with neocortex, phylogenetically older limbic cortex, and subcortical structures. Many neocortical regions connected with the amygdala also contribute to social information processing. For instance, ventromedial prefrontal cortex – directly connected with the amygdala through the uncinate fasciculus18 – is involved in social decision making and moral judgment.19,20 Visual association areas in the ventral temporal cortex – also monosynaptically connected with the amygdala21,22 – are implicated in processing social signals from others, including facial expressions. Thus, investigation of the connectivity between the amygdala and other brain regions involved in social cognition may be key to understanding the nature of social dysfunction in ASDs.
We utilized functional connectivity MRI (fcMRI) to examine intrinsic functional connectivity of the amygdala in a cohort of children and adolescents with ASDs. By quantifying the synchronization in blood oxygen level dependent (BOLD) signal fluctuations between spatially segregated brain regions (e.g., amygdala and prefrontal cortex), fcMRI allows inferences about large-scale functional networks of interacting brain regions with coordinated activity,23,24 which have been shown to subserve specialized cognitive, sensorimotor, or other mental functions and systems (cf. refs25–27). Studies utilizing fcMRI in ASDs have reported widespread abnormalities in intrinsic functional connectivity (iFC), but the precise patterns remain unclear, with often contradictory evidence ranging from reduced connectivity28–30 to partial or even exclusive overconnectivity,12,31–35 as compared to typically developing (TD) controls. The inconsistencies are largely attributed to methodological differences between iFC analytic strategies,36,37 or atypical age-related maturational effects in ASDs.38
Given extensive evidence of altered trajectories of brain development in ASDs,39,40 our study included children across a broad age range (7–17 years), permitting additional cross-sectional tests of developmental changes in amygdala connectivity during childhood and adolescence. In typical development, some cortical connections of the amygdala, including amygdala-prefrontal functional connectivity, continue to mature during adolescence,41,42 likely due to the protracted development of prefrontal cortex.43 Although these age-related effects in amygdala iFC have yet to be ascertained in ASDs, there is evidence of atypical maturation of the amygdala in children with ASDs. Namely, amygdala volume appears enlarged in toddlers with ASDs, above and beyond total brain overgrowth observed in the first years of life in autism,40,44,45 and remains atypically enlarged throughout childhood, reaching adult size as early as age 8–9 years.46 This is in contrast with typical development, during which amygdala growth accelerates later in pre-adolescence, reaching adult size only in mid- to late adolescence.47,48
The present study investigated intrinsic functional connectivity (iFC) of the amygdala, a brain region with established role in social cognition, in children and adolescents with ASDs, by utilizing resting-state fcMRI. We hypothesized that children with ASDs would exhibit altered iFC compared to matched TD controls, and that the atypical connectivity patterns would correlate with autism clinical symptoms. A further aim was to examine age-related changes in amygdala connectivity patterns cross-sectionally across childhood and adolescence (between ages 7 and 17 years).
Method
Participants.
In total, 72 children and adolescents with ASDs and 58 TD participants, ages 7–17 years, were enrolled in the study. After exclusions for excessive head motion (as defined below) during scans (16 ASD and 6 TD) or incidental finding on MRI (1 ASD and 2 TD), the final sample included 55 ASD and 50 TD participants matched at the group level on age, gender, handedness, non-verbal IQ, and head motion (Table 1). ASD diagnoses based on DSM-5 criteria1 were confirmed by the Autism Diagnostic Interview-Revised (ADI-R)49 and the Autism Diagnostic Observation Schedule, 2nd Edition (ADOS-2).50 Participants with history of autism-related medical conditions (e.g., epilepsy, Fragile-X, tuberous sclerosis) were excluded. Further exclusion criteria for TD participants were personal or family history of autism, and personal history of any other neurological, developmental or psychiatric conditions. ASD participants with comorbid ADHD, OCD, or anxiety disorders, or those reporting current psychotropic medication usage, including stimulants, antidepressants, antipsychotics, and anxiolytics, were not excluded, because of the high prevalence of such comorbid conditions51 and psychotropic medication use in ASDs52–54 (see Table 1 and Table S1, available online, for details). Overall cognitive ability was assessed by the Wechsler Abbreviated Scale of Intelligence, 2nd Edition (WASI-II).55 Core language abilities were measured with the Clinical Evaluation of Language Fundamentals, 4th Edition (CELF-4).56 In addition to the ADOS-derived indices of social behavior available only for ASD participants, social functioning was assessed in all participants using a parent questionnaire, the Social Responsiveness Scale, 2nd Edition (SRS-2).57 Hand preference was established with the Edinburgh Handedness Inventory.58 Informed assent and consent were obtained from all participants and their caregivers, respectively, in accordance with the University of California, San Diego and San Diego State University Institutional Review Boards.
Table 1.
Participant Characteristics
| ASD (n = 55) | TD (n = 50) | |||||
|---|---|---|---|---|---|---|
| M ± SD [range] | M ± SD [range] | p value | Cohen’s ds | |||
| Gender (M/F)a | 46/9 | 41/9 | 0.82 | -- | ||
| Handedness (R/L)a | 46/9 | 42/8 | 0.96 | -- | ||
| Age (years) | 13.7 ± 2.8 | [7.4–17.8] | 13.4 ± 2.7 | [8.0–17.6] | 0.50 | 0.13 |
| Verbal IQ | 102.5 ± 17.2 | [70–147] | 107.5 ± 9.0 | [87–127] | 0.07 | 0.36 |
| Non-verbal IQ | 106.3 ± 18.6 | [53–145] | 105.6 ± 13.3 | [62–137] | 0.83 | 0.04 |
| Full-scale IQ | 104.6 ± 16.9 | [66–141] | 107.2 ± 10.5 | [79–130] | 0.36 | 0.18 |
| ADOS-2 Social Affect | 10.2 ± 3.6 | [6–20] | n/a | -- | -- | |
| Repetitive Behavior | 3.1 ± 1.8 | [1–8] | n/a | -- | -- | |
| SRS-2, Total | 81.0 ± 8.9 | [58–94] | 42.1 ± 5.0 | [35–58] | <0.001 | 5.23 |
| RMSD (in-scanner head motion) | 0.063 ± 0.026 | [0.019–0.110] | 0.062 ± 0.030 | [0.017–0.135] | 0.82 | 0.03 |
Note: 26/51 participants with autism spectrum disorders (ASDs) (Rx data on 4/55 participants were missing) were reported to be taking psychoactive medications to control comorbid symptoms, as detailed in supplementary Table S1, available online. 18 participants with ASDs reported having comorbid psychiatric conditions, including attention-deficit/hyperactivity disorder (10), depression (5), and anxiety (10), with 5 / 18 participants diagnosed with more than one comorbid condition (although these numbers may underestimate true rates of comorbidity, given the greater number of participants on psychoactive medications intended to treat these conditions). Cohen’s ds is calculated as standardized mean difference between two groups (of independent observations) for the sample. ADOS-2 = Autism Diagnostic Observation Schedule, 2nd Edition; TD = typically developing; RMSD = root mean square of displacement; SRS-2, Social Responsiveness Scale, 2nd Edition.
Values denote counts and corresponding chi-square p values. Remaining comparisons reflect two-sample t-test analyses and corresponding p values.
MRI data acquisition.
Imaging data were acquired on a GE 3T MR750 scanner with an 8-channel head coil. High-resolution anatomical images were obtained using a T1-weighted whole-brain IR-SPGR sequence (TR = 8.108ms; TE = 3.172ms; flip angle = 8°; 172 slices; 1 mm3 resolution). Functional T2*-weighted images were acquired using a single-shot gradient-recalled echo-planar pulse sequence, in one resting-state scan consisting of 185 whole-brain volumes (TR = 2000ms; TE = 30ms; flip angle = 90°; FOV = 220mm; 64 × 64 matrix; 3.4mm3 isotropic resolution; 42 axial slices covering the whole brain). Throughout the 6:10 minute resting state scan, participants were instructed to lie still with their eyes open and centered on a white fixation cross displayed on black background in the center of a screen, using a rear projection display. Adherence to instructions was monitored using a miniature MRI-compatible video camera affixed inside the bore. Field maps were collected with the same spatial parameters to correct functional images for magnetic field inhomogeneities.
fMRI data preprocessing and fcMRI analyses.
Images were processed using Analysis of Functional NeuroImages (AFNI)59 and FMRIB Software Library (FSL).60 After discarding the first five volumes (10 sec of data) to reduce magnetization equilibration effects, data were corrected for slice-dependent time shifts, head motion (by realigning all volumes to the middle time point), and magnetic field inhomogeneity by applying field-maps. The resulting images were co-registered to the anatomical image using linear transformation (FSL’s FLIRT), resampled to 3.0 mm isotropic voxels, spatially normalized to the MNI atlas space, temporally band-pass filtered (.008 < f < .08 Hz) using a second-order Butterworth filter to isolate frequencies at which intrinsic network-specific BOLD correlations predominate,24,61 and spatially smoothed using a Gaussian kernel of FWHM of 6mm. Six motion parameters, mean white matter and CSF signals (extracted from participant-level masks created with FSL’s FAST automated segmentation and eroded to avoid partial-volume effects), and their first temporal derivatives (all band-pass filtered at .008 < f < .08 Hz) were regressed from the signal. Any volume with> 0.5mm shift from its preceding volume in any direction was censored from regression along with its two subsequent time points.62,63 Any time series segments with <10 consecutive uncensored volumes were also excluded. Residuals from the regression served as the BOLD time courses for iFC analyses.
Participants with < 80% of volumes remaining after censoring (15/72 participants with ASDs and 8/58 TD participants) were excluded from further analyses. The mean percentage of censored volumes in the final sample of 55 ASD and 50 TD participants did not differ between groups (MASD = 1.3%; MTD = 1.1%; p = 0.55), nor did the individual motion parameters (translation and rotation for each brain volume in the x, y, z planes; 0.43<p< 0.93) or the mean head motion (computed as the root mean square of displacement [RMSD] on all six translational and rotational axes; Table 1). Motion confounds in group comparisons were thus unlikely.
To generate iFC maps of the amygdala, Pearson correlation coefficients were computed between average BOLD time series extracted from each amygdala seed (left and right, separately) and the time courses of all voxels across the brain, for each participant. The amygdala seeds were created using probabilistic maps from the Harvard-Oxford Subcortical Structural Atlas available in FSL, including voxels that had ≥70% probability of being labeled as amygdala (left: 1269 mm3, right: 1431 mm3). Although the amygdala includes several nuclei with distinct functions, whole amygdalae were used here as seeds due to the limited spatial resolution (left and right amygdala seeds included 46 and 53 voxels in fMRI space, respectively). The resultant voxel-wise correlation coefficients were converted to normally distributed z-values, using Fisher’s r-to-z transformation, and entered into one- and two-independent-sample t-tests, with and without age as a covariate, to examine within- and between-group iFC effects. Cluster correction was used to control for multiple tests across whole brain gray matter with the recently updated 3dClustSim tool, which employs spatial smoothness estimation using spatial autocorrelation function (ACF) modeling.64 Correlational analyses were applied to examine whether iFC (average z-scores) within clusters showing significant group differences was associated with cognitive (IQ, language) and clinical (ADOS-2 and SRS-2) indices.
Results
There were no group differences on gender and handedness distribution, age, performance and full-scale IQ scores (Table 1). As expected, groups were not well matched for verbal IQ, with lower scores in participants with ASDs (t(103) = −1.84, p = 0.07).
Overall group differences in amygdala iFC, independent of age.
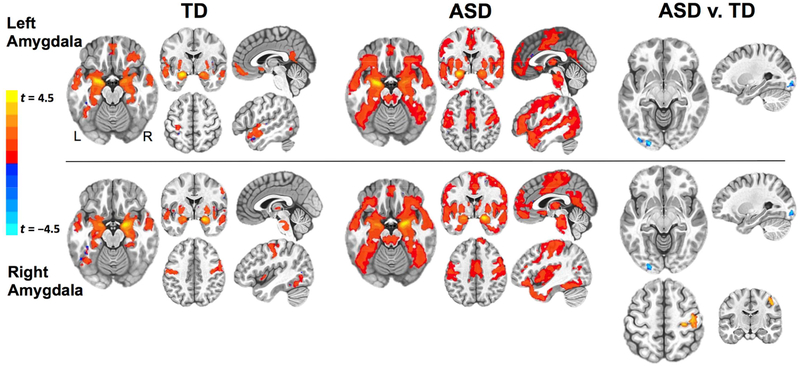
The whole-brain within-group iFC maps for left and right amygdala, thresholded at voxel-level p < 0.0001, are shown in Figure 1 (see Table S2, available online, for detailed cluster listings). Both ASD and TD groups exhibited connectivity between amygdala and thalamus, bilateral middle temporal gyri and temporal poles, posterior ventral temporal cortices, middle orbital gyri, pre- and postcentral gyri, and precuneus / posterior cingulate cortex. However, direct between-group comparisons (uncorrected voxel-level p < 0.005, cluster-corrected p < 0.05) revealed significantly reduced connectivity (ASD < TD) between both left and right amygdala seeds and left inferior occipital gyrus (IOG), and increased connectivity (ASD > TD) between right amygdala and right primary motor / somatosensory cortices (RPrG, for simplicity; see Figure 1 and Table 2 for cluster details and effect sizes).
Figure 1. Within- and Between-Group Functional Connectivity Maps for Left and Right Amygdala.
Note: Results of the within-group (p < 10−4 corrected) analyses for left and right amygdala seeds are presented separately for typically developing (TD) (left panel) and autism spectrum disorder (ASD) (middle panel) groups. Right panel presents clusters of significantly different functional connectivity (uncorrected voxel-level p < .005, cluster-corrected p < .05) in ASD v. TD group comparison. Clusters of ASD underconnectivity (ASD < TD) are indicated in blue colors, and clusters of ASD overconnectivity (ASD > TD) are indicated in red colors. L: left; R: right. Within the ASD group, the z scores in the three amygdala intrinsic functional connectivity (iFC) clusters were not significantly different between participants on psychotropic medications and those who were not (all ps > 0.6).
Table 2.
Clusters of Significant (uncorr. p < 0.005, corr. p < 0.05) Group Differences (Autism Spectrum Disorder [ASD] vs. Typically Developing [TD]) in Amygdala Intrinsic Functional Connectivity (iFC)
| % of cluster vol. | Vol. (μl) | t-score* | Cohen’s ds | MNI coordinates | ||||
|---|---|---|---|---|---|---|---|---|
| Cluster: Subregions | x | y | z | |||||
| Seed: Left amygdala | ||||||||
| L Inferior Occipital Gyrus: | L Middle Occipital Gyrus | 49.2% | 1350 | −4.08 | 0.92 | −37 | −95 | −16 |
| L Inferior Occipital Gyrus | 41.5% | |||||||
| Seed: Right amygdala | ||||||||
| R Pre-/Postcentral Gyrus: | R Precentral Gyrus | 80.4% | 2106 | 4.61 | 1.23 | 48 | −21 | 55 |
| R Postcentral Gyrus | 19.6% | |||||||
| L Inferior Occipital Gyrus: | L Middle Occipital Gyrus | 52.6% | 1269 | −3.93 | 0.76 | −22 | −98 | −9 |
| L Inferior Occipital Gyrus | 45.0% | |||||||
Note: Cohen’s ds is calculated as standardized mean difference between two groups (of independent observations) for the sample. L: left; R: right.
Positive t-values denote ASD > TD; negative t-values denote ASD < TD.
Correlations with clinical and cognitive measures.
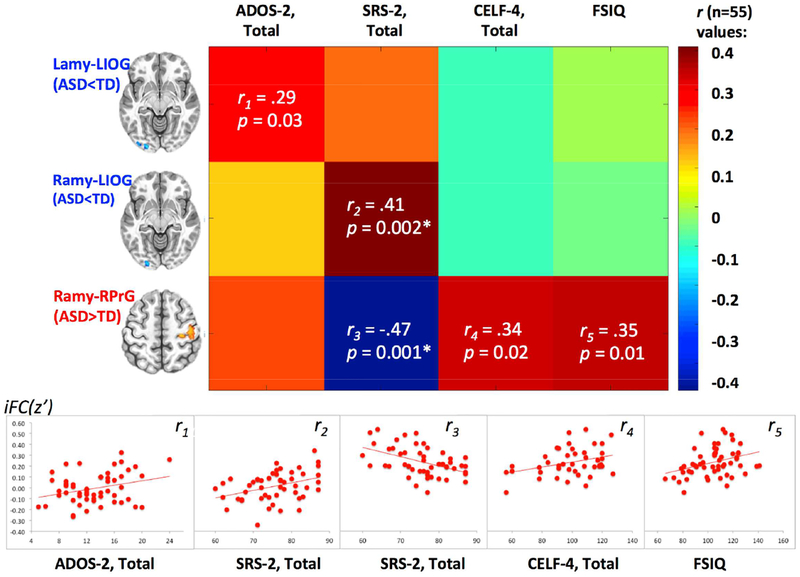
Correlational analyses were used to examine whether connectivity between the amygdala and clusters showing significant group differences (Figure 1) was associated with autism symptoms and overall level of functioning. Pearson correlations were generated between the mean z scores computed for each of the three significant between-group clusters (left amygdala – left IOG, right amygdala – left IOG, and right amygdala – right motor/somatosensory cortex) and two measures of autism symptoms (ADOS-2 and SRS-2 Total scores, with higher scores on both measures indicating greater impairment) and two cognitive measures (WASI-II Full Scale IQ and CELF-4 Core Language scores, with higher scores on both measures indicating greater level of functioning). The resulting correlations (visualized in Figure 2) revealed an unexpected pattern, indicating that for the clusters with significant underconnectivity (ASD < TD) more atypical (weaker) amygdala iFC was associated with decreased symptomatology (positive correlation between Lamy-LIOG and ADOS-2) and more normative social functioning (positive correlation between Ramy-LIOG and SRS-2). Likewise, for the overconnectivity cluster (ASD > TD), more atypical (greater) iFC with amygdala was associated with more normative social functioning (negative correlation between Ramy-RPrG and SRS-2) and better cognitive functioning (positive correlation between Ramy-RPrG and FSIQ and Core Language scores). To further understand this unexpected pattern of correlations and whether it may have been driven by subgroups within the ASD cohort, k-means clustering was performed in a post-hoc analysis.
Figure 2. Correlations Between Significant Intrinsic Functional Connectivity (iFC) Clusters (z Values) and Clinical Measures in the Autism Spectrum Disorder (ASD) group.
Note: r values represent correlations between z scores for the three clusters of significantly different (ASD v. typically developing [TD]) intrinsic functional connectivity (iFC) (clusters described in Table 2 and depicted in Figure 1, right panel) and clinical measures. Cells with r values indicate significant results (with asterisks indicating significant results at a Bonferroni adjusted p < 0.05/12 = 0.004), with corresponding scatterplots depicted below. Increasing Autism Diagnostic Observation Schedule, 2nd Edition (ADOS-2) total values indicate greater symptom count and, hence, greater impairment. Similarly, increasing Social Responsiveness Scale, 2nd Edition (SRS-2) total values indicate greater social impairment, according to parent report. In contrast, greater Clinical Evaluation of Language Fundamentals, 4th Edition (CELF-4) and full scale IQ (FSIQ) scores correspond to better language performance and overall cognitive functioning.
ASD subgroups determined by the patterns of atypical amygdala iFC.
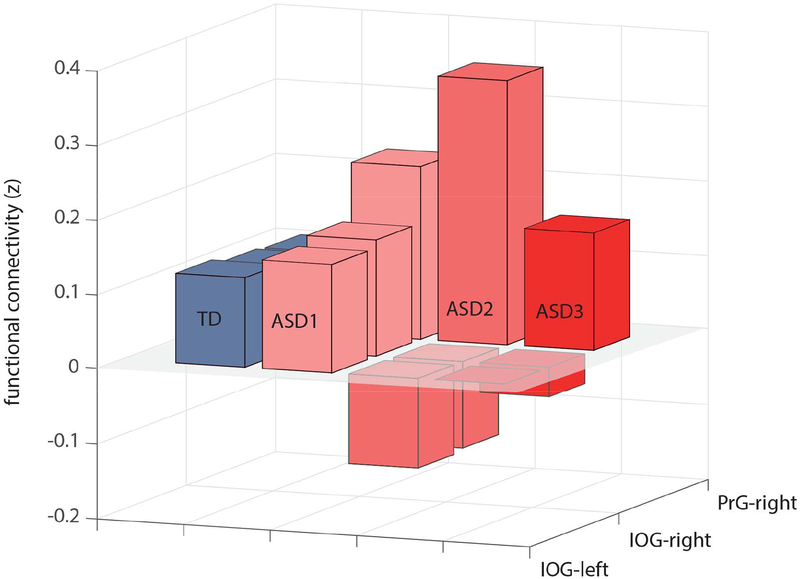
Mean z scores extracted from the three significant between-group clusters (listed in Table 2) were used to derive three subgroups of participants with ASDs by k-means clustering utilizing a squared Euclidean distance measure. The three ASD subgroups were characterized by distinct patterns of connectivity between the amygdala, IOG and primary motor/somatosensory cortices (Figure 3), but did not differ on age, in-scanner head motion, or current symptom severity based on the clinician-rated ADOS-2 (Table 3). Most notably, ASD subgroup 2 (n=15) had the lowest connectivity between both left and right amygdala and left IOG, but the highest connectivity between the right amygdala and right primary motor/somatosensory cortex, thus showing the most robust pattern of differences observed at the whole group level. ASD subgroup 2 also had significantly lower SRS-2 Total scores (reflecting mild current level of social impairment) than the other two subgroups (Table 3) and somewhat higher cognitive functioning as measured by Full Scale IQ.
Figure 3. Mean z values of three significant (Autism Spectrum Disorder [ASD] v. Typically Developing [TD]) Amygdala Intrinsic Functional Connectivity (iFC) Clusters in 3 ASD subgroups.
Note: Three ASD subgroups were determined with k-means clustering, based on the mean z scores extracted from the three significant between-group clusters (left amygdala – left inferior occipital gyrus [IOG], right amygdala – left IOG, right amygdala – right motor/somatosensory cortex [pre-/post-central gyrus, or PrG]).
Table 3.
Characteristics of Autism Spectrum Disorder (ASD) Subgroups Determined by the Amygdala Intrinsic Functional Connectivity (iFC) patterns
| ASD-1 (n = 16) | ASD-2 (n = 15) | ASD-3 (n = 24) | ||
|---|---|---|---|---|
| M ± SD [range] | M ± SD [range] | M ± SD [range] | p valuea | |
| Age (years) | 13.7 ± 2.8 [10.0–17.9] | 13.8 ± 2.7 [9.0–17.5] | 13.7 ± 2.9 [7.4–17.8] | 0.96 |
| RMSD (head motion) | 0.060 ± 0.026 [0.02–0.11] | 0.062 ± 0.024 [0.03–0.11] | 0.064 ± 0.028 [0.02–0.11] | 0.90 |
| ADOS-2, Total | 14.1 ± 5.0 [7–24] | 11.9 ± 3.5 [8–20] | 13.2 ± 3.5 [5–18] | 0.32 |
| ADOS-2, Severity | 7.6 ± 2.0 [4–10] | 6.5 ± 1.7 [4–10] | 7.5 ± 1.8 [4–10] | 0.29 |
| SRS-2, Total | 84.7 ± 6.7 [75–94] | 76.9 ± 9.8 [58–83] | 82.3 ± 8.0 [64–94] | 0.02 |
| Full-scale IQ | 105.4 ± 18.4 [81–141] | 110.5 ± 10.4 [89–124] | 98.9 ± 19.2 [66–139] | 0.11 |
| CELF −4, Core Language | 97.9 ± 26.2 [56–127] | 103.3 ± 14.7 [82–126] | 98.0 ± 13.3 [78–120] | 0.74 |
| Rx status, Rx : none | 9:7 | 7:6 (2 unknown) | 9:13 (2 unknown) | 0.56 |
Note: Rx status indicates number of participants who were on any psychotropic medications. ADOS-2 = Autism Diagnostic Observation Schedule, 2nd Edition, ADOS-2 Severity, a standardized metric for quantifying ASD symptom severity that is relevantly independent of age and verbal ability; RMSD = root mean square of displacement; SRS-2 =Social Responsiveness Scale
Values reflect one-way analysis of variance (ANOVA) and corresponding p values (except for the Rx status variable, in which case values reflect p values for χ2 comparison).
Age-related effects in amygdala iFC.
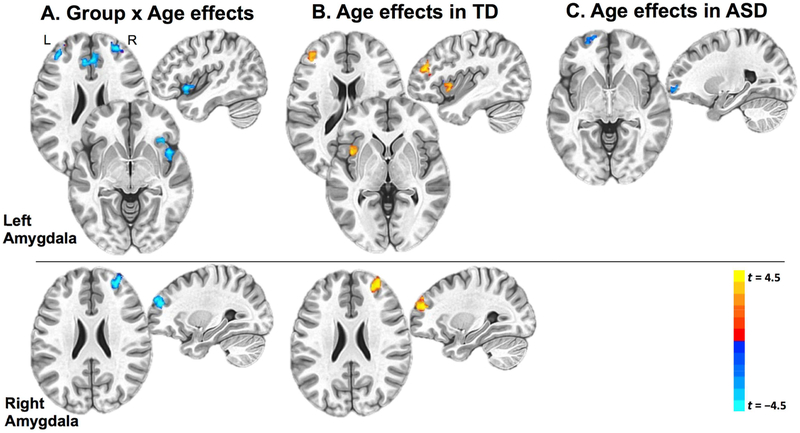
Given the broad age range of the cohort, the extent to which amygdala iFC varied across age was also examined. Including age as a covariate in AFNI 3dttest++, the interaction term was used to examine whether age moderated between-group iFC effects. A significant group × age interaction was observed in multiple prefrontal regions (voxel-level p < 0.005, cluster-corrected p < 0.05), with several clusters of amygdala iFC showing differential developmental trajectories in the ASD and TD groups (Figure 4). For the left amygdala, these regions of age-related group differences in amygdala iFC included clusters in the bilateral anterior prefrontal cortex, anterior cingulate cortex, right inferior frontal gyrus and right insula, all exhibiting more negative slopes of iFC as a function of age in the ASD (compared to the TD) group (Figure 4A). Within-group connectivity maps with age as a covariate (Figure 4B) revealed exclusively positive age-related effects in left amygdala connectivity with left insula and left inferior frontal gyrus in the TD group. In contrast, age-related effects within the ASD group (Figure 4C) showed no such strengthening of connections, with one cluster in left anterior prefrontal cortex negatively covarying with age, indicating weakening iFC with left amygdala.
Figure 4. Age-Related Effects in Amygdala Intrinsic Functional Connectivity (iFC).
Note: Results are depicted in the Montreal Neurological Institute (MNI) space, with clusters displaying negative correlations between age and amygdala iFC depicted in blue colors and clusters displaying positive correlations between age and iFC depicted in red colors. A: Age by group interaction effects. B: Clusters with significant age effect in the TD group. C: Clusters with significant age effect in the autism spectrum disorder (ASD) group. TD = typically developing.
For the right amygdala, a cluster in the right anterior prefrontal cortex exhibited age-dependent difference in iFC between the two groups (Figure 4A, bottom panel). This effect reflected age-related increase in connectivity with right prefrontal cortex among TD participants (Figure 4B, bottom panel), with no such age-dependent effects in the ASD group. In sum, the two groups exhibited differential age-dependent changes in amygdala iFC, with strengthening bilateral amygdala connections among TD but not ASD participants.
Exploratory post-hoc analyses revealed that these age-related effects in amygdala iFC varied in the three ASD subgroups, with ASD subgroup 2 exhibiting a positive trajectory similar to that observed in the TD group, contrasted by sharply negative age-related trajectory in ASD subgroup 1 (see Figure S1, available online). This difference in regression slopes was significant for left amygdala (when averaging across five age-related clusters depicted in Figure 4A), F = 4.83, p = 0.004 (effect size R2 change = 0.12), indicating that regression coefficients between age and left amygdala iFC significantly differed across four groups (TD and three ASD subgroups). Pairwise post-hoc comparisons revealed that this omnibus effect was due to the significant difference between age-related iFC trajectories in ASD subgroup 1 and the TD group (r (16) = −.58 and r (50) = .25, respectively; z (diff) = −3.72, p = 0.0004). A corresponding test for age-related effects on right amygdala iFC across four groups revealed no significant results.
Discussion
We used rs-fcMRI to examine amygdala functional connectivity in a sample of 7 – 17 year old children and adolescents with ASDs and matched TD peers. This investigation revealed three principal findings. First, direct between-group comparisons revealed that the ASD group had weaker connections between both left and right amygdalae and left inferior occipital cortex, but greater connections between right amygdala and right motor / somatosensory cortex. Second, the more atypical patterns of amygdala connectivity were unexpectedly associated with more favorable outcomes, including decreased symptomatology and better cognitive functioning. This was primarily due to a subgroup of participants with ASDs who showed most atypical amygdala iFC with those two regions, but least impaired social functioning. Third, we found that age had differential effect on amygdala iFC in TD and ASD groups, with amygdala-prefrontal and amygdala-insula connectivity increasing from childhood to adolescence in TD participants, while such age-dependent iFC increase was not present in the ASD group, and even reversed between left amygdala and left prefrontal cortex.
Amygdala connectivity in ASD is decreased in occipital, but increased in pericentral regions.
The first finding of atypical amygdala connectivity identified in participants with ASDs – increased with pericentral, but decreased with occipital regions – is consistent with recent reports describing aberrant network sculpting in ASDs.12,65,66 More specifically, amygdala-occipital underconnectivity observed in the ASD group, previously reported in older adolescents and young adults with ASDs67 (but not in preschool-age children with ASDs94) may indicate inadequate integration of visual input into the socio-affective circuits. The finding of weaker amygdala-occipital connectivity in ASDs should be interpreted in the context of the extensive axonal connections between amygdala and sensory areas reported in humans and other primates. Namely, direct connections between amygdala (especially laterobasal nuclei) and primary sensory cortices, including visual cortex and particularly inferior occipital gyrus, have been identified by axonal tracing studies in monkeys,68, 69 as well as by probabilistic tractography in humans.70 It has been proposed that these connections support associative processing and perception of salience of the sensory input by the amygdala.71, 72 Indeed, the left inferior occipital cluster showing weaker amygdala connectivity overlapped with the region of the extended face network involved in emotional face encoding,73 as well as gaze processing.74 Thus, reduced amygdala-IOG functional connectivity observed here in children and adolescents with ASDs may reflect history of disrupted coordination between amygdala and the regions involved in processing of facial expressions and cues.
Notably, in addition to the amygdala-occipital underconnectivity, the ASD group was also characterized by atypically increased iFC between amygdala and pericentral cortices (Brodmann areas 3 and 4). This pattern was particularly pronounced in a subgroup of children with ASDs who also had relatively favorable clinical and cognitive outcomes. While links between amygdala and motor/somatosensory cortices may appear unexpected, one recent diffusion tractography study75 suggested that white matter tracts directly connecting these regions may exist. It has also been shown that amygdala modulates brain circuits involved in motor control, especially in the context of fear and threat, likely allowing for rapid and adaptive changes in current behavior.76 For instance, a recent transcranial magnetic stimulation (TMS) study demonstrated that observing fearful body language and body postures – a salient sign of the presence of potential threat, shown to activate amygdala77 – suppresses intracortical facilitation of the primary motor cortex.78 This is in line with fMRI evidence of motor cortex deactivation in response to experimentally-induced state of conscious fear,79 and with a recent report of pharmacogenetic inactivation of the amygdala (in rhesus monkey) resulting in disrupted amygdala-motor coupling.93 While the exact neural pathways by which motor cortex is modulated by the parasympathetic amygdala-brainstem circuitry involved in fear (exemplified by freezing behavior in animals) remain largely unknown in humans,80 there is evidence that emotional cues can modulate primary motor cortex excitability via supplementary motor area,81 which has direct connections to both the final motor effector system (primary motor area and spinal cord) and the anterior cingulate area directly linked to the amygdala. Atypical connectivity between amygdala and pericentral cortex identified in the ASD group may thus be interpreted in light of these known links between limbic and motor systems. Overconnectivity between amygdala and pericentral cortex may indicate history of fear-related interaction between these two regions, although the directionality of the finding, which was especially pronounced in the ASD subgroup with relatively lower social symptoms and higher cognitive functioning, is not easy to interpret, as BOLD correlations do not unambiguously reflect excitatory vs. inhibitory connectivity.
Atypical amygdala connectivity in ASD is associated with reduced symptomatology.
The unexpected finding that a subgroup of children with ASDs with the most atypical patterns of amygdala connectivity was characterized by significantly lower social impairment and somewhat better cognitive functioning raises a possibility of a compensatory mechanism. Importantly, these results suggest that categorical approaches to understanding network connectivity or brain function in ASDs (i.e., via group comparisons between ASD and TD cohorts) may not be sufficient nor particularly informative. This is, of course, not surprising given the immense diversity of symptoms and level of functioning, and a number of co-morbid conditions, all of which are present heterogeneously throughout the population that makes up the autism spectrum. The findings of differential iFC patterns observed in subgroups of participants with ASDs demonstrate the value of utilizing dimensional approaches alongside categorical, group-level examinations, allowing for assessment of a full spectrum of symptom profiles (and potential etiological factors), which may lead to new insights and better understanding of the brain-behavior relationships.
Age-related changes in amygdala iFC are absent, or atypically reversed in children with ASDs.
Finally, consistent with the evidence that amygdala-prefrontal circuitry undergoes protracted development through adolescence,41,82 age-related effects were detected in the TD group, showing increasing connectivity with age between amygdala and prefrontal cortex as well as insula. This trajectory of age-related increase in amygdala connectivity was entirely absent in the ASD group, with inverse effects present in the left hemisphere, especially for some subgroups of ASD subjects. Age-related changes in both structural42 and functional83,84 connectivity between amygdala and frontal regions are thought to underlie socio-emotional development during adolescence. Ventral regions of the prefrontal cortex, including anterior cingulate cortex (which showed atypical age effects in ASD participants; Figure 4A), are presumed to provide top-down modulation of amygdala responses to aversion and threat.85–88 Age-related increases in functional connectivity observed in our TD group likely reflect facilitated communication between amygdala and prefrontal cortex associated with enhanced prefrontal regulationof amygdala activity, which has particularly salient implications for understanding self and others. Absence of this developmental effect in the ASD group may contribute to the pervasive nature of sociocommunicative impairment in ASDs. Further, given the insula’s central role in a network involved in monitoring the homeostatic, emotional, or cognitive saliency of external inputs and internal brain events (the salience network, or SN),27,89,90 the age-related increase of the amygdala connectivity with insula observed in the TD group – but absent in the ASD cohort – suggests that amygdala becomes more connected and integrated with SN during neurotypical adolescence. There is evidence that SN modulates other large-scale core networks involved in cognitive information processing, with the anterior insula playing a critical and causal role in initiating switching between major brain networks.91 Thus, the lack of developmental strengthening of the amygdala-SN connections in children with ASDs may signify a diminished (or impaired) ability to detect important, significant environmental stimuli, which may have global effects, both at the neural and behavioral levels. Notably, the atypical age-related effects observed in our ASD cohort – indicating aberrant maturation of amygdala connectivity through adolescence – likely underlie the differences between the overall amygdala iFC patterns detected here and the exclusive amygdala underconnectivity observed in younger, preschool-age children with ASDs.94
Among limitations of the present study are (a) exclusion of low-functioning children with ASDs (due to the extreme sensitivity of fcMRI to head motion92), raising the possibility that the current findings may not generalize to the lower end of the autism spectrum; (b) use of cross-sectional data in examining age-related effects; (c) lack of pubertal development measures, which would have allowed for a more precise characterization of the developmental transition from childhood into adolescence than age alone, and its effects on amygdala connectivity in ASDs; and (d) lack of reliable data on comorbid conditions (with several participants who were prescribed stimulants, mood stabilizers, anxiolytics not having a corresponding formal diagnosis of ADHD, mood or anxiety disorder), which would likely further constrain the subtyping and clustering results.
In sum, the current results demonstrate atypical functional connectivity of the amygdala in a sample of children and adolescents with ASD. Specifically, weaker connections with inferior occipital cortex and increased connections with motor / somatosensory cortex were detected. Unexpectedly, these atypical iFC patterns, which were particularly pronounced in one subgroup of children with ASDs, correlated with more favorable clinical and cognitive outcomes, raising a possibility that abnormal functional connections may be compensatory, at least in some individuals with ASDs. Finally, the increase in amygdala connectivity with prefrontal and insular cortices, accompanying transition from childhood to adolescence in typical development, was not present in the ASD participants.
Supplementary Material
Acknowledgments
The authors are grateful to Chris Fong, MA, Anna Christina Macari, MA, Sangeeta Nair, MA, Weiqi Zhao, MA, and Yangfeifei Gao, MA, of San Diego State University, for invaluable assistance with data collection. The authors’ strongest gratitude goes to the children and families who so generously dedicated their time and effort to this research.
This work was supported by grants from the National Institutes of Health (K01 MH097972 to I. Fishman and R01 MH081023 to R-A. Müller).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Drs. Fishman, Linke, Hau, Carper, and Müller report no biomedical financial interests or potential conflicts of interest.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC: American Psychiatric Press, 2013. [Google Scholar]
- 2.Tager-Flusberg H The origins of social impairments in autism spectrum disorder: studies of infants at risk. Neural Networks. 2010;23(8–9):1072–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sigman M, Dijamco A, Gratier M, Rozga A. Early detection of core deficits in autism. Mental Retardation and Developmental Disabilities Research Reviews. 2004;10(4):221–233. [DOI] [PubMed] [Google Scholar]
- 4.Adolphs R The social brain: neural basis of social knowledge. Annual Review of Psychology. 2009;60:693–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frith U, Frith C. The social brain: allowing humans to boldly go where no other species has been. Philosophical Transactions of the Royal Society B. 2010;365:165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frith CD. The social brain? Philosophical Transactions of the Royal Society B. 2007;362:671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Insel TR, Fernald RD. How the brain processes social information: Searching for the social brain. Annual Review of Neuroscience. 2004;27(1):697–722. [DOI] [PubMed] [Google Scholar]
- 8.Blakemore SJ. Development of the social brain during adolescence. The Quarterly Journal of Experimental Psychology. 2008;61(1):40–49. [DOI] [PubMed] [Google Scholar]
- 9.Grossmann T, Johnson MH. The development of the social brain in human infancy. European Journal of Neuroscience. 2007;25:909–919. [DOI] [PubMed] [Google Scholar]
- 10.Pelphrey KA, Shultz S, Hudac CM. Research review: constraining heterogeneity: the social brain and its development in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2007;2(6):631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy DP, Adolphs R. The social brain in psychiatric and neurological disorders. Trends in Cognitive Sciences. 2012;16(11):559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fishman I, Keown CL, Lincoln AJ, Pineda JA, et al. Atypical cross talk between mentalizing and mirror neuron networks in autism spectrum disorder. JAMA Psychiatry. 2014;71(7):751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brothers L The neural basis of primate social communication. Motivation and Emotion. 1990;14(2):81–91. [Google Scholar]
- 14.Bickart KC, Wright CI, Dautoff RJ. Amygdala volume and social network size in humans. Nature.2011;14(2):163–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanai R, Bahrami B, Roylance R, Rees G. Online social network size is reflected in human brain structure. Proceedings of the Royal Society B: Biological Sciences. 2012;279(1732):1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stefanacci L, Amaral DG. Topographic organization of cortical inputs to the lateral nucleus of the macaque monkey amygdala: a retrograde tracing study. Journal of Comparative Neurology. 2000;421(1): 52–79. [DOI] [PubMed] [Google Scholar]
- 17.Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. New York: Wiley-Liss, 1992; p. 1–66. [Google Scholar]
- 18.Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. European Journal of Neuroscience. 2002;16(2):291–310. [DOI] [PubMed] [Google Scholar]
- 19.Harris LT, McClure SM, van den Bos W, Cohen JD, et al. Regions of the MPFC differentially tuned to social and nonsocial affective evaluation. Cognitive, Affective, & Behavioral Neuroscience. 2007;7(4):309–316. [DOI] [PubMed] [Google Scholar]
- 20.Koenigs M, Young L, Adolphs R, Tranel D, et al. Damage to the prefrontal cortex increases utilitarian moral judgements. Nature. 2007;446(7138):908–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freese JL, Amaral DG. Synaptic organization of projections from the amygdala to visual cortical areas TE and V1 in the macaque monkey. Journal of Comparative Neurology. 2006;496(5):655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freese JL, Amaral DG. The organization of projections from the amygdala to visual cortical areas TE and V1 in the macaque monkey. Journal of Comparative Neurology. 2005;486(4):295–317. [DOI] [PubMed] [Google Scholar]
- 23.Biswal BB, Mennes M, Zuo XN, Gohel S, et al. Toward discovery science of human brain function. Proceedings of the National Academy of Sciences of the USA. 2010;107(10):4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience. 2007;8(9):700–711. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, LaViolette P, O’Keefe K, Putcha D, et al. Intrinsic connectivity between the hippocampus and posteromedial cortex predicts memory performance in cognitively intact older individuals. Neuroimage. 2010;51(2):910–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith SM, Fox PT, Miller KL, Glahn DC, et al. Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the USA. 2009;106(31):13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seeley WW, Menon V, Schatzberg AF, Keller J, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27(9):2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kana RK, Keller TA, Cherkassky VL, Minshew NJ, et al. Sentence comprehension in autism: thinking in pictures with decreased functional connectivity. Brain. 2006;129(9):2484–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127(8):1811–1821. [DOI] [PubMed] [Google Scholar]
- 30.Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, et al. Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage. 2005;24(3):810–821. [DOI] [PubMed] [Google Scholar]
- 31.Uddin LQ, Supekar K, Lynch CJ, Khouzam A, et al. Salience network-based classification and prediction of symptom severity in children with autism. JAMA Psychiatry. 2013;70(8):869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lynch CJ, Uddin LQ, Supekar K, Khouzam A, et al. Default mode network in childhood autism: posteromedial cortex heterogeneity and relationship with social deficits. Biological Psychiatry. 2013;74(3):212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Supekar K, Uddin LQ, Khouzam A, Phillips J, et al. Brain hyperconnectivity in children with autism and its links to social deficits. Cell Reports. 2013;5(3):738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shih P, Keehn B, Oram JK, Leyden KM, et al. Functional differentiation of posterior superior temporal sulcus in autism: a functional connectivity magnetic resonance imaging study. Biological Psychiatry. 2011;70(3):270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keehn B, Shih P, Brenner LA, Townsend J, et al. Functional connectivity for an “island of sparing” in autism spectrum disorder: an fMRI study of visual search. Human Brain Mapping. 2013;34(10):2524–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nair A, Keown CL, Datko M, Shih P, et al. Impact of methodological variables on functional connectivity findings in autism spectrum disorders. Human Brain Mapping. 2014;35(8):4035–4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones TB, Bandettini PA, Kenworthy L, Case LK, et al. Sources of group differences in functional connectivity: an investigation applied to autism spectrum disorder. Neuroimage. 2010;49(1):401–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uddin L, Supekar K, Menon V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Frontiers in Human Neuroscience, 2013;7:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003;290(3):337–344. [DOI] [PubMed] [Google Scholar]
- 40.Shen MD, Nordahl CW, Young GS, Wootton-Gorges SL, et al. Early brain enlargement and elevated extra-axial fluid in infants who develop autism spectrum disorder. Brain. 2013;136(9):2825–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gee DG, Humphreys KL, Flannery J, Goff B, et al. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. Journal of Neuroscience. 2013;33(10):4584–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swartz JR, Carrasco M, Wiggins JL, Thomason ME, et al. Age-related changes in the structure and function of prefrontal cortex-amygdala circuitry in children and adolescents: a multi-modal imaging approach. Neuroimage. 2014;86:212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caballero A, Granberg R, Tseng KY. Mechanisms contributing to prefrontal cortex maturation during adolescence. Neuroscience & Biobehavioral Reviews. 2016;70:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schumann CM, Barnes CC, Lord C, Courchesne E. Amygdala enlargement in toddlers with autism related to severity of social and communication impairments. Biological Psychiatry. 2009;66(10):942–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nordahl CW, Scholz R, Yang X, Buonocore MH, et al. Increased rate of amygdala growth in children aged 2 to 4 years with autism spectrum disorders: a longitudinal study. Archives of General Psychiatry. 2012;69(1):53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, et al. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. Journal of Neuroscience. 2004;24(28):6392–6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giedd JN, Vaituzis AC, Hamburger SD, Lange N, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. Journal of Comparative Neurology. 1996;366(2):223–230. [DOI] [PubMed] [Google Scholar]
- 48.Mills KL, Goddings A-L, Clasen LS, Giedd JN, et al. The developmental mismatch in structural brain maturation during adolescence. Developmental Neruoscience. 2014;36(3–4):147–160. [DOI] [PubMed] [Google Scholar]
- 49.Rutter M, LeCouteur A. Autism Diagnostic Interview, Revised (ADI-R). Torrance, CA: Western Psychological Services, 2003. [Google Scholar]
- 50.Lord C, Rutter M, DiLavore P, Risi S, et al. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2). Torrance, CA: Western Psychological Services, 2012. [Google Scholar]
- 51.Simonoff E, Pickles A, Charman T, Chandler S, et al. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47(8):921–929. [DOI] [PubMed] [Google Scholar]
- 52.Spencer D, Marshall J, Post B, Kulakodlu M, et al. Psychotropic medication use and polypharmacy in children with autism spectrum disorders. Pediatrics. 2013;132(5):833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schubart JR, Camacho F, Leslie D. Psychotropic medication trends among children and adolescents with autism spectrum disorder in the Medicaid program. Autism. 2014;18(6):631–637. [DOI] [PubMed] [Google Scholar]
- 54.Siegel M, Beaulieu A. Psychotropic medications in children with autism spectrum disorders: a systematic review and synthesis for evidence-based practice. Journal of Autism and Developmental Disorders. 2012;42(8):1592–1605. [DOI] [PubMed] [Google Scholar]
- 55.Wechsler D Wechsler Abbreviated Scale of Intelligence, 2nd Edition San Antonio, TX: Psychological Corporation, 2011. [Google Scholar]
- 56.Semel E, Wiig EH, Secord WA. Clinical Evaluation of Language Fundamentals, Fourth Edition Toronto, Canada: The Psychological Corporation, 2003. [Google Scholar]
- 57.Constantino J Social Responsiveness Scale, Second Edition (SRS-2). Torrance, CA: Western Psychological Services, 2012. [Google Scholar]
- 58.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. [DOI] [PubMed] [Google Scholar]
- 59.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–173. [DOI] [PubMed] [Google Scholar]
- 60.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(1):S208–219. [DOI] [PubMed] [Google Scholar]
- 61.Cordes D, Haughton VM, Arfanakis K, Carew JD, et al. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. American Journal of Neuroradiology. 2011;22(7):1326–1333. [PMC free article] [PubMed] [Google Scholar]
- 62.Power JD, Schlaggar BL, Petersen SE. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage. 2015;105(15):536–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Siegel JS, Power JD, Dubis JW, Vogel AC. Statistical improvements in functional magnetic resonance imaging analyses produced by censoring high motion data points. Human Brain Mapping. 2014;35(5):1981–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cox RW, Chen G, Glen DR, Reynolds RC, et al. FMRI clustering in AFNI: False-positive rates redux. Brain Connectivity. 2017;7(3):152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fishman I, Datko M, Cabrera Y, Carper R, et al. Reduced integration and differentiation of the imitation network in autism: A combined functional connectivity magnetic resonance imaging and diffusion weighted imaging study. Annals of Neurology. 2015;78(6):958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rudie JD, Shehzad Z, Hernandez LM, Colich NL, et al. Reduced functional integration and segregation of distributed neural systems underlying social and emotional information processing in autism spectrum disorders. Cerebral Cortex. 2012;22(5):1025–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rausch A, Zhang W, Haak KV, Mennes M, et al. Altered functional connectivity of the amygdaloid input nuclei in adolescents and young adults with autism spectrum disorder: a resting state fMRI study. Molecular Autism. 2016;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stefanacci L, Amaral DG. Some observations on cortical inputs to the macaque monkey amygdala: an anterograde tracing study. Journal of Comparative Neurology. 2002;451(4):301–323. [DOI] [PubMed] [Google Scholar]
- 69.Yukie M Connections between the amygdala and auditory cortical areas in the macaque monkey. Neuroscience Research. 2002;42(3):219–229. [DOI] [PubMed] [Google Scholar]
- 70.Bach DR, Behrens TE, Garrido L, Weiskopf N, et al. Deep and superficial amygdala nuclei projections revealed in vivo by probabilistic tractography. Journal of Neuroscience. 2011;31(2):618–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bzdok D, Laird AR, Zilles K, Fox PT, et al. An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Human Brain Mapping. 2013;34(12):3247–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dolan RJ. The human amygdala and orbital prefrontal cortex in behavioural regulation. Proceedings of the Royal Society B: Biological Sciences. 2007;362(1481):787–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pinabiaux C, Hertz-Pannier L, Chiron C, Rodrigo S, et al. Memory for fearful faces across development: specialization of amygdala nuclei and medial temporal lobe structures. Frontiers in Human Neuroscience. 2013;7:901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hooker CI, Paller KA, Gitelman DR, Parrish TB, et al. Brain networks for analyzing eye gaze. Brain Research. 2003;17(2):406–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grèzes J, Valabrègue R, Gholipour B, Chevallier C. A direct amygdala-motor pathway for emotional displays to influence action: A diffusion tensor imaging study. Human Brain Mapping. 2014;35(12):5974–5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sagaspe P, Schwartz S, Vuilleumier P. Fear and stop: a role for the amygdala in motor inhibition by emotional signals. Neuroimage. 2011;55(4):1825–1835. [DOI] [PubMed] [Google Scholar]
- 77.Hadjikhani N, de Gelder B. Seeing fearful body expressions activates the fusiform cortex and amygdala. Current Biology. 2003;13(24):2201–2205. [DOI] [PubMed] [Google Scholar]
- 78.Borgomaneri S, Vitale F, Gazzola V, Avenanti A. Seeing fearful body language rapidly freezes the observer’s motor cortex. Cortex. 2015;65:232–245. [DOI] [PubMed] [Google Scholar]
- 79.Butler T, Pan H, Tuescher O, Engelien A, et al. Human fear-related motor neurocircuitry. Neuroscience. 2007;150(1):1–7. [DOI] [PubMed] [Google Scholar]
- 80.Roelofs K Freeze for action: neurobiological mechanisms in animal and human freezing. Philosophical Transactions of the Royal Society B. 2017;372:1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oliveri M, Babiloni C, Filippi MM, Caltagirone C, et al. Influence of the supplementary motor area on primary motor cortex excitability during movements triggered by neutral or emotionally unpleasant visual cues. Eperimental Brain Research. 2003;149(2):214–221. [DOI] [PubMed] [Google Scholar]
- 82.Gabard-Durnam LJ, Flannery J, Goff B, Gee DG, et al. The development of human amygdala functional connectivity at rest from 4 to 23 years: a cross-sectional study. Neuroimage. 2014;95:193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perlman SB, Pelphrey KA. Developing connections for affective regulation: age-related changes in emotional brain connectivity. Journal of Experimental Child Psychology. 2011;108(3):607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Decety J, Michalska KJ, Kinzler KD. The contribution of emotion and cognition to moral sensitivity: a neurodevelopmental study. Cerebral Cortex. 2012;22(1):209–220. [DOI] [PubMed] [Google Scholar]
- 85.Ochsner KN, Ray RD, Cooper JC, Robertson ER, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23(2):483–499. [DOI] [PubMed] [Google Scholar]
- 86.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. [DOI] [PubMed] [Google Scholar]
- 87.Etkin A, Egner T, Peraza DM, Kandel ER, et al. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51(6):871–882. [DOI] [PubMed] [Google Scholar]
- 88.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. [DOI] [PubMed] [Google Scholar]
- 89.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function. 2010;214(5–6):655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Craig AD. How do you feel now? The anterior insula and human awareness. Nature Reivews Neuroscience. 2009;10(1):59–70. [DOI] [PubMed] [Google Scholar]
- 91.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the USA. 2008;105(34):12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Power J, Barnes K, Snyder A, Schlaggar B, et al. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grayson D, Bliss-Moreau E, Machado CJ, Bennett J, et al. The rhesus monkey connectome predicts disrupted functional networks resulting from pharmacogenetic inactivation of the amygdala. Neuron. 2016;91(2):453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shen MD, Li DD, Keown CL, Lee A, et al. Functional connectivity of the amygdala is disrupted in preschool-aged children with autism spectrum disorder. Journal of American Academy of Child and Adolescent Psychiatry. 2016;55:817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.