Abstract
Numerous studies of motor control have confirmed beta and gamma oscillations in the primary motor cortices during basic movements. These responses include a robust beta decrease that precedes and extends through movement onset, a transient gamma response that coincides with the movement, and a post-movement beta rebound (PMBR) response that occurs after movement offset. While the existence of these responses has been confirmed by many studies, very few studies have examined their developmental trajectory. In the current study, we utilized magnetoencephalography (MEG) to investigate age-related changes in sensorimotor cortical oscillations in a large cross-section of children and adolescents (n = 94; age range = 9–15 years-old). All participants performed a stimulus detection task with their right finger and the resulting MEG data were examined using oscillatory analysis methods and imaged using a beamformer. Consistent with adult studies, these youth participants exhibited characteristic beta (16–24 Hz) decreases prior to and during movement, as well as PMBR responses following movement offset, and a transient gamma (74–84 Hz) response during movement execution. Our primary findings were that the strength of the PMBR increased with age, while the strength of the gamma synchronization decreased with chronological age. In addition, the strength of each motor-related oscillatory response was significantly correlated with the power of spontaneous activity in the same frequency range and same voxel. This was the case for all three oscillatory responses. In conclusion, we investigated motor-related oscillatory activity in the largest cohort of children and adolescents reported to date, and our results indicated that beta and gamma cortical oscillations continue to develop as children transition into adolescents, and that these responses may not be fully matured until young to middle adulthood.
Keywords: MEG, beta, PBMR, gamma, motor cortex, hand
1. Introduction
Previous magnetoencephalographic (MEG), electroencephalographic (EEG), and electro-corticographic (ECoG) studies have identified a series of cortical oscillations that accompany hand and finger movements (Cheyne et al., 2006; Cheyne et al., 2008; Jurkiewicz et al., 2006; Pfurtscheller and Lopes da Silva, 1999; Pfurtscheller et al., 1997; Salenius et al., 1997; Schnitzler et al., 1997; Tzagarakis et al., 2010; Wilson et al., 2014; Wilson et al., 2010). For example, there is a sharp decrease in the beta band (15–30 Hz) power that begins several hundred milliseconds before the onset of movement, and this power change is sustained throughout movement duration (Cheyne et al., 2006; Engel and Fries, 2010; Gaetz et al., 2010; Wilson et al., 2014; Wilson et al., 2010). This beta event-related desynchronization (ERD) has been linked with motor planning (Cheyne et al., 2008; Wilson et al., 2010), as it occurs prior to the onset of muscular activity, occurs sooner for easier motor tasks, and is influenced by the certainty of the movement pattern to be performed (Grent-’t-Jong et al., 2014; Heinrichs-Graham and Wilson, 2015, 2016; Heinrichs-Graham et al., 2014; Jurkiewicz et al., 2006; Kaiser et al., 2001; Kurz et al., 2016; Tzagarakis et al., 2010; Tzagarakis et al., 2015; Wilson et al., 2010; Wilson et al., 2011). Once the movement has been completed, there is a sharp increase or rebound in beta band power (Cheyne et al., 2006; Gaetz et al., 2010; Heinrichs-Graham and Wilson, 2015, 2016; Heinrichs-Graham et al., 2014; Jurkiewicz et al., 2006; Wilson et al., 2014; Wilson et al., 2010), which has been termed the post-movement beta rebound (PMBR) response. This response is thought to reflect the active inhibition of cortical networks after movement termination (Neuper and Pfurtscheller, 2001; Salmelin et al., 1995; Solis-Escalante et al., 2012), afferent feedback to the sensorimotor cortices (Cassim et al., 2001; Houdayer et al., 2006), and/or the certainty of the feedforward motor actions that were executed based on the internal model (Arpin et al., 2017; Tan et al., 2016). Although these motor-related beta oscillations have been extensively studied, only a handful of investigations have evaluated their developmental trajectory. For example, one recent study showed that younger children tend to have a weaker beta ERD than older children when performing knee movements (Kurz et al., 2016), while other studies focused on index finger movements have revealed a linear increase in the amplitude of both the beta ERD and PMBR responses from childhood (4–6 years-old) to adolescence (11–13 years-old) to adulthood (Gaetz et al., 2010). However, both of these studies involved relatively small samples, with Gaetz and colleagues examining 10 participants per group in their study. Finally, it should be noted that recent studies have also suggested that the amplitude of the beta ERD may increase across the life span in adults (Heinrichs-Graham and Wilson, 2016; Rossiter et al., 2014), and that this increase may be directly tied to the level of spontaneous beta activity in the motor cortices.
In addition to the motor-related beta oscillations, numerous studies have identified an increase (or synchronization) in the high gamma-frequency range (70–90 Hz) that generally begins slightly before movement onset and is sustained for about 200 ms after movement onset (Cheyne et al., 2008; Miller et al., 2007; Pfurtscheller et al., 2003; Wilson et al., 2010). This gamma event-related synchronization (ERS) is restricted to a smaller population of neurons within the contralateral primary motor cortex, and appears to closely follow the somatosensory and motor homuncular topological organization (Cheyne et al., 2008; Dalal et al., 2008; Gaetz et al., 2010; Muthukumaraswamy, 2010; Tecchio et al., 2008). It has been proposed that the transient gamma ERS serves to initialize activation of the motor command (i.e., is a motor execution signal), as it is temporally-succinct and closely yoked to the onset of muscular activity (Cheyne & Ferrari, 2013). This interpretation is further supported by evidence suggesting that the gamma ERS is elicited during active motor tasks, but is not present when the joint is moved passively (Muthukumaraswamy, 2010). In regard to developmental effects, the gamma ERS has been identified in children as young as 3.5 years of age (Cheyne et al., 2014), however, there appears to be considerable variability in the frequency range at this age. Specifically, some children displayed this response in the upper gamma range (70–80 Hz), others had the response in a lower gamma range (30–40 Hz), while a third group exhibited responses in both frequency ranges (Cheyne et al., 2014). Such wide variations in peak frequency may be restricted to toddlers and very young children, as Gaetz et al. (2010) observed ERS responses limited to the upper gamma range in their study of children (4–6 years-old), adolescents, and adults. In fact, they found that the adolescents had stronger motor-related gamma responses relative to both the children and adults, and that there were no age-related differences in peak gamma frequency between the groups (Gaetz et al., 2010). However, data from a prior study that combined children and adolescents into a single 8–15 year-old group found that the strength of the gamma ERS during both right and left finger movements became weaker with advancing age in the contralateral supplementary motor area (SMA) (Wilson et al., 2010). Thus, while the motor-related gamma ERS appears to be a robust response seen across development and into adulthood, studies focused on the developmental trajectory have been inconsistent and at least partially contradictory. Nevertheless, the small sample sizes used in these prior investigations may be contributing to the inconsistent findings, as no study has had more than 10 participants per group.
The primary goal of the current study was to map the developmental trajectory of motor-related oscillations during the transition from childhood to adolescence. It is well appreciated that motor performance sharply improves during this developmental stage, but the accompanying neurophysiological changes are not well understood. Given the disagreements in the literature, we aimed to conduct a definitive study using MEG in a large cross-section of children and adolescents (N = 94). Our primary hypotheses were: 1) the strength of the beta ERD and PMBR responses would increase with age, 2) the strength of the gamma ERS would decrease with age, and 3) the strength of the beta ERD would correlate with the baseline beta power. Secondarily, we explored if the peak frequency and latency of these responses changed with developmental age, and tested for sex differences in the developmental trajectory of motor-related oscillations.
2. Methods and Materials
2.1. Subject Selection
Ninety-four healthy children and adolescents were recruited from the local community as part of the National Science Foundation (NSF) funded Developmental Chronnecto-Genomics (Dev-CoG) study (NSF 1539067, http://devcog.mrn.org). All participants were recruited from the University of Nebraska Medical Center (UNMC) site and were between the ages of 9 and 15 years (Mage = 11.69 years, SDage = 1.57; 46 females, 48 males). The Dev-CoG study examines changes in cognition and brain function during the transition from childhood to adolescence (i.e., puberty onset), and thus the age range is 9–15 years. Participants were generally healthy, without attention deficit disorders, mental illness or previous head trauma, and with English as their primary language. Participants were excluded according to MEG/MRI exclusionary criteria such as metal implants, dental braces or permanent retainers, or other metallic or otherwise magnetic non-removable devices. Handedness was assessed using the Edinburgh handedness inventory test (89 right-handed, 5 left-handed). Other exclusionary criteria included any medical illness affecting CNS function, any neurological or psychiatric disorder, and current substance abuse. The study protocol was approved by UNMC’s Institutional Review Board, and written informed consent was obtained from the parents and assent was acquired from all children.
2.2. Experimental Paradigm
During MEG recording, participants were seated in a nonmagnetic chair within the magnetically-shielded room with their right hand positioned on a custom-made five-finger button pad. Each button press sent a unique signal (i.e., TTL pulse/trigger code) to the MEG acquisition computer, and thus behavioral responses were temporally synced with the MEG data. For the experiment, participants initially fixated on a crosshair and this was followed by the presentation of a visual grating stimulus, auditory steady-state stimulus, or both for 0.8 s. All stimuli were supra-threshold and easily detected. Participants were instructed to respond with a button press using their right index finger when any stimulus was detected. There were a total of 300 trials (100 per condition) and these were pseudo-randomized so that no more than three trials of the same type occurred consecutively. The interstimulus interval was 2.4 to 2.6 s and consisted of a fixation cross. In the current study, we focused on the 100 visual-only trials to ensure homogeneous brain responses; data from the full experiment will be reported elsewhere.
2.3. MEG Acquisition Parameters and Coregistration with MRI
All recordings were conducted in a one-layer magnetically-shielded room with active shielding engaged. Neuromagnetic responses were sampled continuously at 1 kHz with an acquisition bandwidth of 0.1–330 Hz using an Elekta MEG system with 306 magnetic sensors (Elekta, Helsinki, Finland). Using MaxFilter (v2.2; Elekta), MEG data from each subject were individually corrected for head motion and subjected to noise reduction using the signal space separation method with a temporal extension (Taulu and Simola, 2006; Taulu et al., 2005).
Prior to starting the MEG experiment, four coils were attached to the subject’s head and localized, together with the three fiducial points and scalp surface, with a 3-D digitizer (Fastrak 3SF0002, Polhemus Navigator Sciences, Colchester, VT, USA). Once the subject was positioned for MEG recording, an electric current with a unique frequency label (e.g., 322 Hz) was fed to each of the coils. This induced a measurable magnetic field and allowed for each coil to be localized in reference to the sensors throughout the recording session. Since the coil locations were also known in head coordinates, all MEG measurements could be transformed into a common coordinate system. With this coordinate system, each participant’s MEG data were coregistered with structural T1-weighted MRI data prior to source space analyses using BESA MRI (Version 2.0). Structural T1-weighted MRI images were acquired using a Siemens Skyra 3-Tesla MRI scanner with a 32-channel head coil and a MP-RAGE sequence with the following parameters: TR = 2400 ms; TE = 1.94 ms; flip angle = 8°; FOV = 256 mm; slice thickness = 1 mm (no gap); voxel size = 1 × 1 × 1 mm. These data were aligned in parallel to the anterior and posterior commissures and transformed into standardized space. Following source analysis (i.e., beamforming), each participant’s 4.0 × 4.0 × 4.0 mm MEG functional images were transformed into standardized space using the transform that was previously applied to the structural MRI volume and spatially resampled.
2.4. MEG Time-Frequency Transformation and Statistics
Cardiac artifacts were removed from the data using signal-space projection (SSP), which was accounted for during source reconstruction (Uusitalo and Imoniemi 1997). The continuous magnetic time series was divided into epochs of 2 s duration, with the baseline being defined as −0.950 to −0.550 and 0.0 s being movement onset (i.e., button press). Epochs containing artifacts (e.g., eye blinks, muscle artifacts, etc.) were rejected based on a fixed-threshold method using individual amplitude and gradient thresholds, supplemented with visual inspection. An average of 80.78 (SD: 4.2) trials per participant were used for further analysis.
Artifact-free epochs were transformed into the time-frequency domain using complex demodulation, and the resulting spectral power estimations per sensor were averaged over trials to generate time-frequency plots of mean spectral density. These sensor-level data were normalized using the respective bin’s baseline power, which was calculated as the mean power during the −0.950 to −0.550 s time period. The specific time-frequency windows used for imaging were determined by statistical analysis of the sensor-level spectrograms across the entire array of gradiometers. To reduce the risk of false positive results while maintaining reasonable sensitivity, a two-stage procedure was followed to control for Type 1 error. In the first stage, one-sample t-tests were conducted on each data point and the output spectrogram of t-values was thresholded at p < 0.05 to define time-frequency bins containing potentially significant oscillatory deviations relative to baseline across all participants. In stage two, time-frequency bins that survived the threshold were clustered with temporally and/or spectrally neighboring bins that were also above the (p < 0.05) threshold, and a cluster value was derived by summing all of the t-values of all data points in the cluster. Nonparametric permutation testing was then used to derive a distribution of cluster-values, and the significance level of the observed clusters (from stage one) were tested directly using this distribution (Ernst, 2004; Maris and Oostenveld, 2007). For each comparison, at least 10,000 permutations were computed to build a distribution of cluster values. Based on these analyses, the time-frequency windows that corresponded to events of a priori interest (i.e., the beta ERD, PMBR, and motor-related gamma ERS) and contained significant oscillatory events across all participants were subjected to the beamforming analysis.
2.5. MEG Imaging & Statistics
Cortical networks were imaged using an extension of the linearly-constrained minimum variance vector beamformer (Gross et al., 2001; Van Veen et al., 1997), which employs spatial filters in the frequency domain to calculate source power for the entire brain volume. The single images were derived from the cross-spectral densities of all combinations of MEG gradiometers averaged over the time-frequency range of interest, and the solution of the forward problem for each location on a grid specified by input voxel space. This use of the cross-spectral densities is often referred to as the dynamic imaging of coherent sources (DICS) beamformer (Gross et al., 2001). Following convention, we computed noise-normalized, source power per voxel in each participant using active (i.e., task) and passive (i.e., baseline) periods of equal duration and bandwidth (Hillebrand et al., 2005; Van Veen et al., 1997). Such images are typically referred to as pseudo-t maps, with units (pseudo-t) that reflect noise-normalized power differences (i.e., active vs. passive) per voxel. MEG pre-processing and imaging used the Brain Electrical Source Analysis (BESA version 6.1) software.
Normalized differential source power was computed for the statistically-selected time-frequency bands (see below) over the entire brain volume per participant at 4.0 × 4.0 × 4.0 mm resolution. The resulting 3D maps of brain activity were averaged across participants to assess the neuroanatomical basis of significant oscillatory responses identified through the sensor-level analysis. We then extracted virtual sensors (i.e., voxel time series) for the peak voxel of each oscillatory response. To compute the virtual sensors, we applied the sensor weighting matrix derived through the forward computation to the preprocessed signal vector, which yielded two time series (i.e., one per orientation) for each coordinate in source space, and we used the time series with the maximal response for our analyses (Gross et al., 2001). Note that this virtual sensor extraction was done per participant, once the coordinates of interest (i.e., one per cluster) were known. Once these virtual sensors were extracted, absolute beta and gamma activity values were computed for the baseline period in each participant.
2.6. Correlation Analyses
Pearson product moments were calculated between the age in months and the respective MEG outcome variables. Pearson correlations were also computed between specific MEG variables. As an exploratory analysis, we also split the group by sex and computed Pearson correlations between age in months and the respective MEG outcome variables in each group. We then used Fisher’s z transformation to identify significant differences between these group-wise correlation coefficients. The statistical analyses were conducted with SPSS (Version 22.0; IBM Corporation, Armonk, NY).
3. Results
3.1. Demographic and Behavioral Data
Of the 94 youth who completed the task, 11 were excluded during MEG preprocessing and analysis due to excessive motion and/or other MEG artifacts. A cohort of 83 participants remained and were used in the final analyses (M = 11.67 years, SD = 1.57; see Figure S1 in the Supplementary Materials). Participants detected the stimuli at a very high rate (> 98%), with a mean reaction time of 0.461 s (SD = 0.136 s).
3.2. Sensor-level and Beamforming Analyses
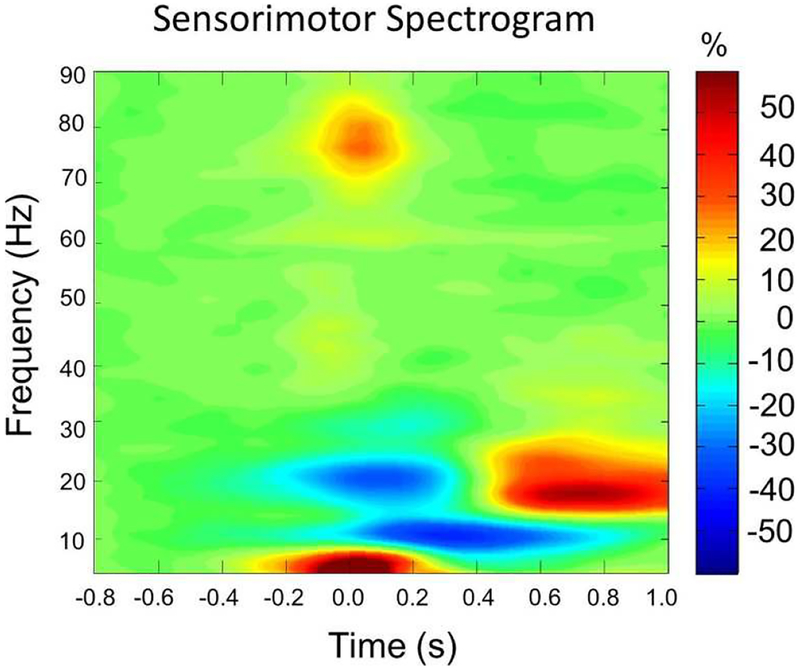
Figure 1 shows the group-averaged oscillatory responses from a MEG sensor located near the left primary motor cortex (see Figure S2 in the Supplementary Materials for a spectrogram per age group). A significant beta ERD response (16–24 Hz) was seen across a large number of sensors near the sensorimotor cortices, and this response began about −0.1 s before movement onset and persisted until about 0.3 s afterward (p < 0.0001, corrected). In addition, a classic PMBR response (18–24 Hz) emerged in largely the same MEG sensors after the movement was completed, with significant activity stretching from about 0.6 s to 0.9 s (p < 0.0001, corrected). There was also an alpha ERD that began after movement onset and extended from 0.1 to 0.7 s, but this oscillatory response was beyond our primary focus as it has commonly been linked to somatosensory activity (Hari et al., 1997). Lastly, a significant gamma ERS in the 74–84 Hz range was also detected in a smaller group of sensors near the left sensorimotor cortices from −0.05 s before movement onset until about 0.10 s afterward (p < 0.0001, corrected).
Figure 1.
Group-averaged time-frequency spectra from a gradiometer sensor located near the left sensorimotor cortices (the same sensor was selected in each participant). Time is denoted on the x-axis in seconds (s), with 0.0 s defined as movement onset. Frequency (in Hz) is shown on the y-axis. All signal power is expressed as a percent difference from baseline, with the color legend shown to the far right. Prior to and during movement, there was a strong beta event-related desynchronization (ERD) that occurred about −0.1 s before movement onset and persisted until about 0.3 s afterward. There was also a transient high-frequency gamma synchronization that began slightly before movement onset and quickly dissipated (-0.05 to 0.10 s). Finally, after movement termination, there was a post movement beta rebound (PMBR) response that extended from about 0.6 to 0.9 s. There also was an alpha ERD that began after movement onset (0.1 to 0.7 s), but this response was beyond our primary focus as it has commonly been linked to somatosensory activity. Of note, the low-frequency response centered near 0.0 s likely reflects the evoked response, as it was strongly phase-locked to movement onset. See Figure S2 in the supplementary materials for this spectrogram shown per age group.
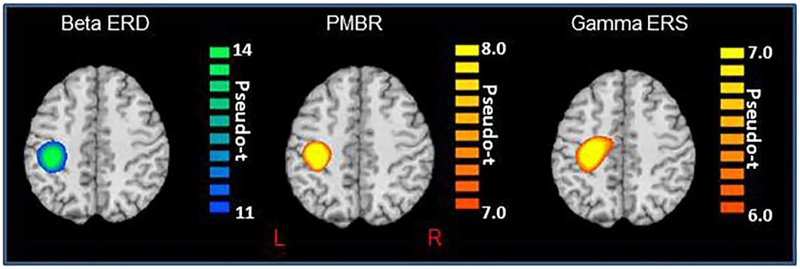
To identify the cortical origin of these responses, the significant time-frequency windows described above were independently imaged in each participant using a beamformer. Briefly, the beta ERD (16–24 Hz) was imaged from −0.1 to 0.3 s with a baseline period of −0.95 to −0.55 s, the PMBR (18–24 Hz) was imaged from 0.6 to 0.9 s with a baseline period of −0.9 to −0.6 s, and the gamma ERS (74–84 Hz) was imaged from −0.05 to 0.10 s with a baseline period of −0.7 to −0.55 s. In addition, we also imaged the alpha ERD (8–14 Hz) from 0.1 to 0.5 s with a baseline period of −0.95 to −0.55 s to inform future studies. After beamforming, the images for each oscillatory response were averaged separately across all participants to visualize their cortical origins. Consistent with prior studies, these images showed that the beta ERD, PMBR, and gamma ERS were generated by the left (contralateral) primary sensorimotor cortices (Figure 2), and the alpha ERD was generated by the contralateral left postcentral gyrus (i.e., somatosensory cortex). Next, we extracted the pseudo-t value of the peak voxel in each cluster and computed the absolute and relative (to baseline) time series corresponding to the same peak voxels for visualization of the dynamics and to quantify the baseline (i.e., spontaneous) power in each frequency window. The pseudo-t value of the peak voxel for each oscillatory response was then correlated with age in months, as well as the baseline power values to address our main hypotheses. These results are described in the following sections.
Figure 2.
Group mean beamformer images (pseudo-t; see color bar) for the beta ERD (left; 16–24 Hz, −0.1 to 0.3 s), PMBR response (middle; 18–24 Hz, 0.6 to 0.9 s), and the gamma ERS (middle; 74–84 Hz, −0.05 to 0.1 s). All images are displayed following neurological convention (Right = Right). As shown, all three motor-related oscillatory responses were centered on the left precentral gyrus near the motor-hand knob feature of this structure.
3.3. Beta ERD Response
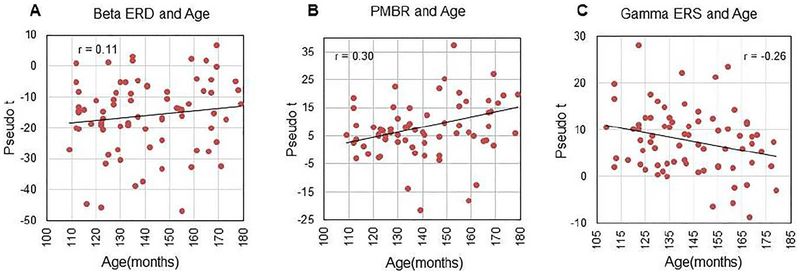
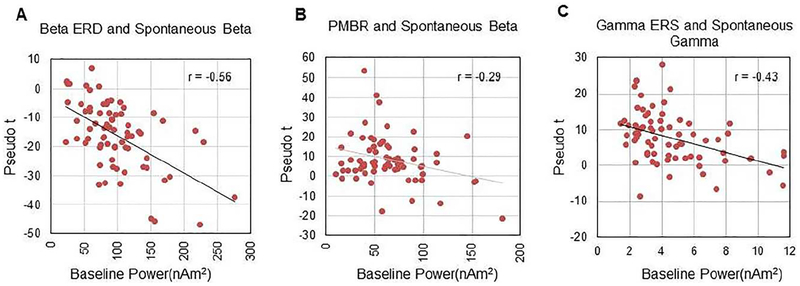
The peak amplitude (pseudo-t; Figure 3A), frequency, and latency of the beta ERD were not correlated with age. In contrast, there was a strong negative correlation between the absolute power in the baseline period and the strength of the beta ERD response (r = −0.56, p < 0.001), which indicates that the beta ERD was stronger in youth who had greater spontaneous beta power during the baseline period (Figure 4A). Finally, the strength of beta activity during the baseline did not correlate with age. As mentioned above, we also performed an exploratory analysis using Fisher’s z to evaluate whether the correlation between chronological age, beta ERD strength, and absolute beta power statistically differed by sex. These analyses indicated that sex did not significantly affect the correlation between age and these two MEG metrics.
Figure 3.
Scatter plots showing the relationship between peak amplitude of each motor-related oscillation and chronological age. In each plot, age is shown on the x-axis in months and peak voxel amplitude (pseudo-t) is shown on the y-axis. Pearson’s r-values are shown near the top of each plot. (Left) There was no relationship between age and the strength of the beta ERD response. In contrast, the PMBR became stronger with age (middle; p = 0.013), while the gamma ERS became weaker with age (right; p = 0.027).
Figure 4.
Scatter plots showing the relationship between the peak strength of each motor-related oscillation and spontaneous power in the same frequency band and brain area (i.e., voxel). In each plot, spontaneous power is shown on the x-axis (nAm2) and peak voxel amplitude (pseudo-t) is shown on the y-axis. Pearson’s r-values are shown near the top of each plot. There was a significant correlation between the beta ERD and spontaneous beta power (left; p < 0.001), which indicated that responses were stronger (more negative) in participants with higher baseline beta. Likewise, there was also significant relationship between the PMBR amplitude and spontaneous beta (middle; p = 0.015), and gamma ERS strength and spontaneous gamma power (right; p < 0.001), but in these cases oscillatory response strength was lower in those with higher baseline levels in each frequency range.
3.4. PMBR Activity
The peak amplitude of the PMBR (pseudo-t) was significantly correlated with age in months (r = 0.30, p = 0.013; Figure 3B), which indicated that the older participants tended to have a stronger PMBR. Consistent with the beta ERD findings, the peak frequency and latency of the PMBR were not correlated with age. Finally, spontaneous beta power during the baseline was significantly correlated with the strength of the PMBR (r = −0.29, p = 0.015; Figure 4B), but not with chronological age. As with the beta ERD, the relationship between chronological age, the strength of the PMBR, and spontaneous beta power did not statistically differ by sex.
3.5. Movement-Related Gamma ERS
The peak of the gamma ERS amplitude (pseudo-t) was negatively correlated with age in months (r = − 0.26, p = 0.027), indicating that the younger participants tended to have a stronger gamma ERS than their adolescent peers (Figure 3C). Like the beta responses, no significant correlation was found between gamma ERS peak frequency or latency and age, and a significant negative correlation was found between spontaneous gamma power during the baseline period and the strength of the gamma ERS (r = −0.43, p < 0.001), suggesting that the relative gamma ERS response was weaker in those with higher spontaneous gamma levels during the baseline (Figure 4C). Finally, the absolute gamma power during the baseline was not correlated with age, and neither this relationship nor that between age and peak gamma ERS strength (pseudo-t) statistically differed by sex.
4. Discussion
We examined the developmental trajectory of motor-related oscillations during the transition from childhood to adolescence using a stimulus detection task. Consistent with previous findings in children and adults, we observed a beta ERD response that preceded and extended through movement duration, a transient high-frequency gamma oscillation that coincided with movement onset, and a PMBR response that began after the movement and extended a few hundred milliseconds. All three responses were strongest in the contralateral primary motor cortices. Importantly, we found that the strength of the PMBR and the gamma ERS responses, but not the beta ERD, were linked with chronological age. Finally, extending several recent studies of baseline (i.e., spontaneous) activity in the motor cortices, we found that the strength of all three motor-related oscillations was correlated with the strength of spontaneous activity within the same frequency range and voxel. Below, we discuss the primary implications of these findings for understanding motor development during the transition to adolescence.
One of our most interesting findings was the developmental decrease observed in the strength of the gamma ERS. In other words, the strength of this response became weaker with increasing age across our 9–15 year-old sample. This finding is consistent with Gaetz et al. (2010) who found that the response was stronger in adolescents (11–13 year-olds) than in adults and very young children. Presumably, the response is weak during early childhood and gets stronger up until adolescence, then gradually decreases from adolescence into adulthood. This reduction in the gamma ERS may be related to a refinement of the neural populations that are involved in the execution of the finger motor command. Interestingly, if one focuses on the mean response amplitude for the 11–13 year-olds in our group, the values are almost identical to those observed in Gaetz et al. (2010). Thus, these findings expand the scope to include the trajectory of change in age ranges missing from the Gaetz study (i.e., 8–10 and 13–15 years), and are also consistent with Wilson et al. (2010). In contrast to the amplitude findings, we did not observe any developmental effects for the peak frequency or latency of the gamma ERS. These data are consistent with the findings from Gaetz et al. (2010), but at odds with other studies that have found the peak gamma ERS frequency to vary in children that are younger (Cheyne and Ferrari, 2013; Cheyne et al., 2014). Potentially, the frequency (and latency) of the gamma ERS may be less stable in very young children, and become more stable as children enter adolescence.
We also found that the strength of the PMBR varied with age, becoming significantly stronger during the transition from childhood to adolescence. This result is broadly consistent with what has been previously reported in the literature (Gaetz et al., 2010; Wilson et al., 2010), and provides much more detailed data on the inherent trajectory of the underlying change. The PMBR has been suggested to represent active inhibition of neuronal networks after movement termination (Neuper & Pfurtscheller, 2001; Salmelin et al, 1995; Solis-Escalante et al., 2012; Heinrichs-Graham et al., 2017). This premise is at least partially supported by the outcomes of a prior magnetic resonance spectroscopy (MRS) study that has shown that the strength of the PMBR is related to the concentration of inhibitory GABA within the sensorimotor cortices (Gaetz et al., 2011). Taken together, it is possible that the age related PMBR correlations seen in this investigation may be partially driven by increased activity in GABAergic interneurons with maturation. Recent experimental work has also suggested that a stronger PMBR is related to the certainty of the internal model of the muscular system and afferent feedback that is returned to the sensorimotor cortices (Tan et al., 2016; Arpin et al., 2017; Cassim et al., 2001; Houdayer et al., 2006; Parkes et al., 2006). It is alternatively plausible that the stronger PMBR seen in the older participants may reflect a more robust internal model of the motor actions and/or better integration of the afferent feedback returned after completing the motor action.
Our hypothesis that the strength of the beta ERD would be significantly correlated with chronological age was not supported. This finding was somewhat surprising since prior studies have found that the beta ERD during hand and finger movements becomes stronger with age in roughly the same developmental window (Gaetz et al., 2010; Wilson et al., 2010). However, this discrepancy could be due to relatively small samples in these previous studies (both had no more than 10 participants per group), or slight differences in the age range between studies. For example, the Gaetz et al. (2010) study examined three groups with the youngest being 4–6 years-old, the second being 11–13 years-old, and the third group being adults (~32 years-old). They found that the beta ERD was stronger in the 11–13 year-olds compared to the 4–6 year-olds, and that the 11–13 year-olds did not differ from the adults, although response strength was highly variable in the two older groups (Gaetz et al., 2010). This lack of a difference between the two older groups might imply that the beta ERD becomes more stable during adolescence, which would be consistent with the current findings. Of note, a recent paper focusing on the life span trajectory of the beta ERD also supports this contention, as they found no differences between adolescents and young adults (mean ~28 years-old) in beta ERD power, but that older adults significantly differed from both groups (Heinrichs-Graham et al., 2018).
Finally, across this developmental sample, we found that the strength of the gamma ERS, beta ERD, and PMBR was related to the power of spontaneous activity at the same frequency and within the same voxel during the baseline period. Similar findings have been recently reported in the motor control literature (Heinrichs-Graham and Wilson, 2016; Wilson et al., 2014). Essentially, we found that participants with the strongest spontaneous beta activity during the baseline also had the strongest beta desynchronization (i.e., beta ERD) prior to and during movement, which may suggest that beta levels in the motor cortex need to reach a specific threshold for the execution of a movement. For further development and discussion of this framework, see other recent publications (Heinrichs-Graham and Wilson, 2016; Wilson et al., 2014). Interestingly, the opposite pattern was observed for the gamma ERS and PMBR responses, as increased spontaneous gamma power during the baseline was related to weaker gamma ERS during movement execution; the same pattern was observed for the PMBR. These findings may indicate that there is an absolute maximum amplitude for beta and gamma responses in the motor cortex, and that movement-associated increases (i.e., oscillations) in power are limited to the difference between the absolute maximum and spontaneous levels during the baseline (i.e., a dynamic range). However, additional work is needed and future studies should further test this relationship and identify whether this ‘dynamic range’ for motor-related oscillations predicts behavioral performance. The basic movements performed in the current study were likely too simple to identify any such relationship.
In conclusion, we evaluated the developmental trajectory of motor-related oscillations during the transition from childhood to adolescence in a large sample of 9–15 year-olds using MEG. Our primary findings were that the strength of the motor-related gamma ERS response decreased with age, the strength of the PMBR increased with age, and that the strength of motor-related oscillatory activity was directly related to the power of spontaneous activity in the same frequency window and brain region. These relationships were not statistically different between males and females. In addition, we found no developmental effects related to the peak frequency or peak latency of the beta ERD, PMBR, or gamma ERS. To date, the current study is the largest MEG oscillatory study of motor control in this age range, and our findings extend and clarify the field’s understanding of age-related changes in motor-related oscillatory activity during the transition from childhood to adolescence. That said, an important limitation of the study is the relatively narrow age range (i.e., 9–15 years), which prevented a long range trajectory from being estimated. Future studies should evaluate the transition to young adulthood, as combining our findings with those from young adults allows specific predictions about the underlying trajectory.
Supplementary Material
Highlights.
A large cohort of 94 healthy youth completed a motor control task during MEG
MEG data were analyzed using oscillatory methods and imaged using a beamformer
The strength of movement-related gamma and post-movement beta were modulated by age
Spontaneous baseline power was correlated with the strength of beta and gamma oscillations
Motor-related neural oscillations continue to develop throughout childhood
Acknowledgements
This work was supported by grants from the National Science Foundation (NSF 1539067) and National Institutes of Health (R01HD086245, R01MH103220, R01MH116782, P20GM103472, R01EB020407, P50AA022534, and R01AA021771).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arpin DJ, Heinrichs-Graham E, Gehringer JE, Zabad R, Wilson TW, Kurz MJ, 2017. Altered sensorimotor cortical oscillations in individuals with multiple sclerosis suggests a faulty internal model. Hum Brain Mapp 38, 4009–4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. Royal Stat Soc 57, 289–300. [Google Scholar]
- Cassim F, Monaca C, Szurhaj W, Bourriez JL, Defebvre L, Derambure P, Guieu JD, 2001. Does post-movement beta synchronization reflect an idling motor cortex? Neuroreport 12, 3859–3863. [DOI] [PubMed] [Google Scholar]
- Cheyne D, Bakhtazad L, Gaetz W, 2006. Spatiotemporal mapping of cortical activity accompanying voluntary movements using an event-related beamforming approach. Hum Brain Mapp 27, 213–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyne D, Bells S, Ferrari P, Gaetz W, Bostan AC, 2008. Self-paced movements induce high-frequency gamma oscillations in primary motor cortex. Neuroimage 42, 332–342. [DOI] [PubMed] [Google Scholar]
- Cheyne D, Ferrari P, 2013. MEG studies of motor cortex gamma oscillations: evidence for a gamma “fingerprint” in the brain? Front Hum Neurosci 7, 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyne D, Jobst C, Tesan G, Crain S, Johnson B, 2014. Movement-related neuromagnetic fields in preschool age children. Hum Brain Mapp 35, 4858–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal SS, Guggisberg AG, Edwards E, Sekihara K, Findlay AM, Canolty RT, Berger MS, Knight RT, Barbaro NM, Kirsch HE, Nagarajan SS, 2008. Five-dimensional neuroimaging: localization of the time-frequency dynamics of cortical activity. Neuroimage 40, 1686–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Fries P, 2010. Beta-band oscillations--signalling the status quo? Curr Opin Neurobiol 20, 156–165. [DOI] [PubMed] [Google Scholar]
- Ernst MD, 2004. Permutation methods: A basis for exact inference. Stat Sci 19, 676–685. [Google Scholar]
- Gaetz W, Macdonald M, Cheyne D, Snead OC, 2010. Neuromagnetic imaging of movement-related cortical oscillations in children and adults: age predicts post-movement beta rebound. Neuroimage 51, 792–807. [DOI] [PubMed] [Google Scholar]
- Grent-’t-Jong T, Oostenveld R, Jensen O, Medendorp WP, Praamstra P, 2014. Competitive interactions in sensorimotor cortex: oscillations express separation between alternative movement targets. J Neurophysiol 112, 224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R, 2001. Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Natl Acad Sci U S A 98, 694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari R, Salmelin R, Mäkelä JP, Salenius S, Helle M, 1997. Magnetoencephalographic cortical rhythms. Int J Psychophysiol 26, 51–62. [DOI] [PubMed] [Google Scholar]
- Heinrichs-Graham E, McDermott TJ, Mills MS, Wiesman AI, Wang YP, Stephen JM, Calhoun VD, Wilson TW, 2018. The lifespan trajectory of neural oscillatory activity in the motor system. Dev Cogn Neurosci 30, 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Wilson TW, 2015. Coding complexity in the human motor circuit. Hum Brain Mapp 36, 5155–5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Wilson TW, 2016. Is an absolute level of cortical beta suppression required for proper movement? Magnetoencephalographic evidence from healthy aging. Neuroimage 134, 514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs-Graham E, Wilson TW, Santamaria PM, Heithoff SK, Torres-Russotto D, Hutter-Saunders JA, Estes KA, Meza JL, Mosley RL, Gendelman HE, 2014. Neuromagnetic evidence of abnormal movement-related beta desynchronization in Parkinson’s disease. Cereb Cortex 24, 2669–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand A, Singh KD, Holliday IE, Furlong PL, Barnes GR, 2005. A new approach to neuroimaging with magnetoencephalography. Hum Brain Mapp 25, 199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houdayer E, Labyt E, Cassim F, Bourriez JL, Derambure P, 2006. Relationship between event-related beta synchronization and afferent inputs: analysis of finger movement and peripheral nerve stimulations. Clin Neurophysiol 117, 628–636. [DOI] [PubMed] [Google Scholar]
- Jurkiewicz MT, Gaetz WC, Bostan AC, Cheyne D, 2006. Post-movement beta rebound is generated in motor cortex: evidence from neuromagnetic recordings. Neuroimage 32, 1281–1289. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Birbaumer N, Lutzenberger W, 2001. Event-related beta desynchronization indicates timing of response selection in a delayed-response paradigm in humans. Neurosci Lett 312, 149–152. [DOI] [PubMed] [Google Scholar]
- Kurz MJ, Proskovec AL, Gehringer JE, Becker KM, Arpin DJ, Heinrichs-Graham E, Wilson TW, 2016. Developmental Trajectory of Beta Cortical Oscillatory Activity During a Knee Motor Task. Brain Topogr 29, 824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Oostenveld R, 2007. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 164, 177–190. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Leuthardt EC, Schalk G, Rao RP, Anderson NR, Moran DW, Miller JW, Ojemann JG, 2007. Spectral changes in cortical surface potentials during motor movement. J Neurosci 27, 2424–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, 2010. Functional properties of human primary motor cortex gamma oscillations. J Neurophysiol 104, 2873–2885. [DOI] [PubMed] [Google Scholar]
- Neuper C, Pfurtscheller G, 2001. Event-related dynamics of cortical rhythms: frequency-specific features and functional correlates. Int J Psychophysiol 43, 41–58. [DOI] [PubMed] [Google Scholar]
- Parkes LM, Bastiaansen MC, Norris DG, 2006. Combining EEG and fMRI to investigate the post-movement beta rebound. Neuroimage 29, 685–696. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Graimann B, Huggins JE, Levine SP, Schuh LA, 2003. Spatiotemporal patterns of beta desynchronization and gamma synchronization in corticographic data during self-paced movement. Clin Neurophysiol 114, 1226–1236. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH, 1999. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 110, 1842–1857. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C, Andrew C, Edlinger G, 1997. Foot and hand area mu rhythms. Int J Psychophysiol 26, 121–135. [DOI] [PubMed] [Google Scholar]
- Rossiter HE, Davis EM, Clark EV, Boudrias MH, Ward NS, 2014. Beta oscillations reflect changes in motor cortex inhibition in healthy ageing. Neuroimage 91, 360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salenius S, Schnitzler A, Salmelin R, Jousmäki V, Hari R, 1997. Modulation of human cortical rolandic rhythms during natural sensorimotor tasks. Neuroimage 5, 221–228. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Hämäläinen M, Kajola M, Hari R, 1995. Functional segregation of movement-related rhythmic activity in the human brain. Neuroimage 2, 237–243. [DOI] [PubMed] [Google Scholar]
- Schnitzler A, Salenius S, Salmelin R, Jousmäki V, Hari R, 1997. Involvement of primary motor cortex in motor imagery: a neuromagnetic study. Neuroimage 6, 201–208. [DOI] [PubMed] [Google Scholar]
- Solis-Escalante T, Müller-Putz GR, Pfurtscheller G, Neuper C, 2012. Cue-induced beta rebound during withholding of overt and covert foot movement. Clin Neurophysiol 123, 1182–1190. [DOI] [PubMed] [Google Scholar]
- Tan H, Wade C, Brown P, 2016. Post-Movement Beta Activity in Sensorimotor Cortex Indexes Confidence in the Estimations from Internal Models. J Neurosci 36, 1516–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taulu S, Simola J, 2006. Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol 51, 1759–1768. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J, Kajola M, 2005. Applications of the signal space separation method (SSS). IEEE Trans Signal Process 53, 3359–3372. [Google Scholar]
- Tecchio F, Zappasodi F, Porcaro C, Barbati G, Assenza G, Salustri C, Rossini PM, 2008. High-gamma band activity of primary hand cortical areas: a sensorimotor feedback efficiency index. Neuroimage 40, 256–264. [DOI] [PubMed] [Google Scholar]
- Tzagarakis C, Ince NF, Leuthold AC, Pellizzer G, 2010. Beta-band activity during motor planning reflects response uncertainty. J Neurosci 30, 11270–11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagarakis C, West S, Pellizzer G, 2015. Brain oscillatory activity during motor preparation: effect of directional uncertainty on beta, but not alpha, frequency band. Front Neurosci 9, 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veen BD, van Drongelen W, Yuchtman M, Suzuki A, 1997. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng 44, 867–880. [DOI] [PubMed] [Google Scholar]
- Wilson TW, Heinrichs-Graham E, Becker KM, 2014. Circadian modulation of motor-related beta oscillatory responses. Neuroimage 102 Pt 2, 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Slason E, Asherin R, Kronberg E, Reite ML, Teale PD, Rojas DC, 2010. An extended motor network generates beta and gamma oscillatory perturbations during development. Brain Cogn 73, 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TW, Slason E, Asherin R, Kronberg E, Teale PD, Reite ML, Rojas DC, 2011. Abnormal gamma and beta MEG activity during finger movements in early-onset psychosis. Dev Neuropsychol 36, 596–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.