Abstract
Substantial effort has been devoted toward understanding the psychopharmacological effects of tryptamine hallucinogens, which are thought to be mediated by activation of 5-HT2A and 5-HT1A receptors. Recently, several psychoactive tryptamines based on the N,N-diallyltryptamine (DALT) scaffold have been encountered as recreational drugs. Despite the apparent widespread use of DALT derivatives in humans, little is known about their pharmacological properties. We compared the binding affinities of DALT and its 2-phenyl-, 4-acetoxy-, 4-hydroxy-, 5-methoxy-, 5-methoxy-2-methyl-, 5-fluoro-, 5-fluoro-2-methyl-, 5-bromo-, and 7-ethyl-derivatives at 45 receptor and transporter binding sites. Additionally, studies in C57BL/6J mice examined whether these substances induce the head twitch response (HTR), a 5-HT2A receptor-mediated response that is widely used as a behavioral proxy for hallucinogen effects in humans. Most of the test drugs bound to serotonin receptors, σ sites, α2-adrenoceptors, dopaminergic D3 receptors, histaminergic H1 receptors, and the serotonin transporter. DALT and several of the ring-substituted derivatives were active in the HTR assay with the following rank order of potency: 4-acetoxy-DALT > 5-fluoro-DALT > 5-methoxy-DALT > 4-hydroxy-DALT > DALT > 5-bromo-DALT. 2-Phenyl-DALT, 5-methoxy-2-methyl-DALT, 5-fluoro-2-methyl-DALT, and 7-ethyl-DALT did not induce the HTR. HTR potency was not correlated with either 5-HT1A or 5-HT2A receptor binding affinity, but a multiple regression analysis indicted that 5-HT2A and 5-HT1A receptors make positive and negative contributions, respectively, to HTR potency (R2 = 0.8729). In addition to supporting the established role of 5-HT2A receptors in the HTR, these findings are consistent with evidence that 5-HT1A activation by tryptamine hallucinogens buffers their effects on HTR.
Keywords: hallucinogen; psychedelic; mice; head twitch; 5-methoxy-N,N-diallyltryptamine; 5-MeO-DALT; 4-acetoxy-N,N-diallyltryptamine; 4-AcO-DALT; 4-hydroxy-N,N-diallyltryptamine
1. INTRODUCTION
Over the past decade there has been a renewed focus on the pharmacology and effects of serotonergic hallucinogens. This focus has been driven, in part, by accumulating evidence that serotonergic hallucinogens may have therapeutic efficacy against anxiety, depression, substance abuse, and obsessive-compulsive disorder (Bogenschutz and Ross 2017). Additionally, although hallucinogen use has remained relatively stable over the past few decades, there has been a marked increase in the availability and diversity of hallucinogens in recent years that has resulted in numerous reports of untoward effects. Some of these hallucinogens are derived from N,N- diallyltryptamine (DALT). 5-Methoxy-N,N-diallyltryptamine (5-MeO-DALT), for example, was first synthesized by Alexander T. Shulgin (A.T. Shulgin, personal communication), and was first marketed via the Internet in 2004 (Corkery et al. 2012). According to Shulgin, oral doses of 12–20 mg produce psychoactive effects with a rapid onset and a relatively brief duration of 2–4 h (Shulgin and Shulgin 2004). Subsequently, 5-MeO-DALT and other DALT derivatives have become popular recreational hallucinogen; 5-MeO-DALT has been identified in many seized samples (Nagai et al. 2007; Rasanen et al. 2014; Strano Rossi et al. 2014; Odoardi et al. 2016; Brunt et al. 2017) and DALT and 4-acetoxy-N,N-diallyltryptamine (4-AcO-DALT) have also been detected (EMCDDA 2008,2013,2015).
Despite the widespread distribution and nonmedical use of diallyltryptamines (DALTs), very little is known about their pharmacology. It was previously reported that six DALT compounds bind non-selectively to 27 different receptors including 5-HT receptors (Cozzi and Daley 2016), and 5-MeO-DALT has been shown to act as a 5-HT2A agonist (Arunotayanun et al. 2013). However, few animal behavioral assessments have been performed with these compounds, and the resulting information could provide insight into the relationship between receptor binding and the behavioral effects of these drugs. Hence, the binding of DALT and nine ring-substituted DALTs (see Fig. 1) were assessed at 45 receptor and transporter binding sites.
Figure 1.
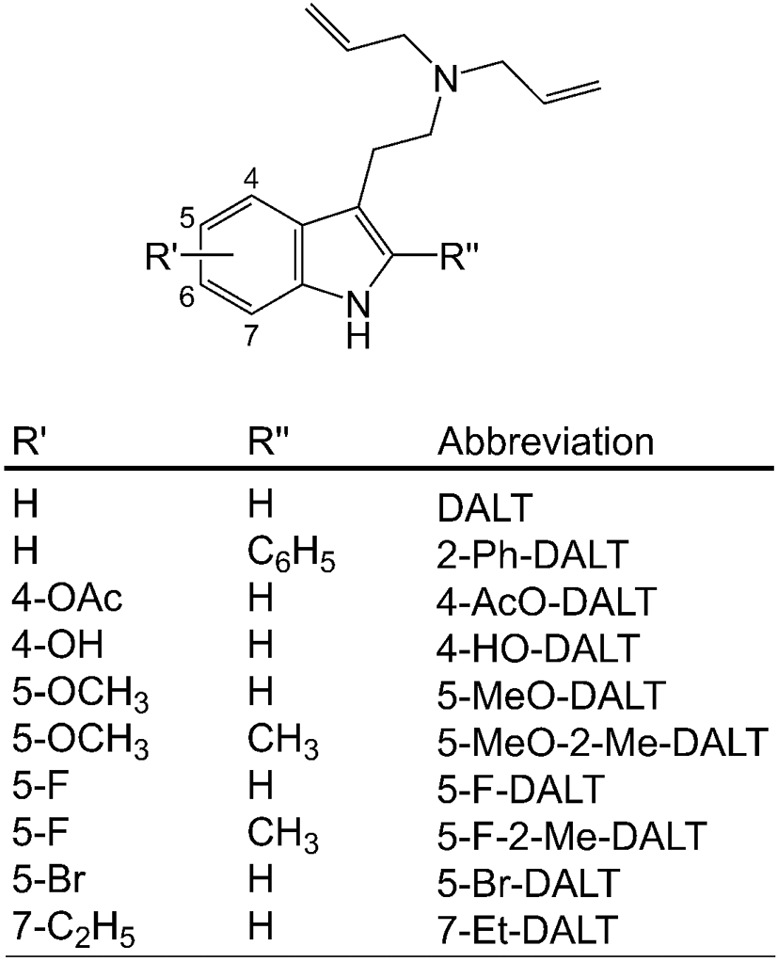
Chemical structures of N,N-diallyltryptamine (DALT) and several ring-substituted derivatives.
Serotonergic hallucinogens produce the head twitch response (HTR), a brief paroxysmal head rotation in rats and mice, via activation of the 5-HT2A receptor (Schreiber et al. 1995; Canal and Morgan 2012; Halberstadt and Geyer 2013), the same receptor responsible for the psychedelic effects of hallucinogens in humans (Quednow et al. 2012; Kometer et al. 2013; Valle et al. 2016; Kraehenmann et al. 2017; Preller et al. 2017b,a). The HTR is widely used as a behavioral proxy in rodents for human hallucinogenic effects because it is one of only a few behaviors that can reliably distinguish hallucinogenic and non-hallucinogenic 5-HT2A receptor agonists (Gonzalez-Maeso et al. 2007). We employed HTR studies with the ten DALT compounds in C57BL/6J mice to test whether these tryptamines produce LSD-like behavioral effects in vivo.
In addition to producing effects via the 5-HT2A receptor, tryptamine hallucinogens also bind to 5-HT1A receptors with moderate to high affinity and efficacy (McKenna et al. 1990; Blough et al. 2014; Rickli et al. 2016). The HTR induced by hallucinogens is attenuated by administration of 5-HT1A receptor agonists such as 8-OH-DPAT, ipsapirone, and buspirone (Darmani et al. 1990; Schreiber et al. 1995; Kleven et al. 1997), which is consistent with evidence for countervailing interactions between 5-HT1A and 5-HT2A receptors (Araneda and Andrade 1991; Ashby et al. 1994; Krebs-Thomson and Geyer 1998; Amargos-Bosch et al. 2004; Li et al. 2011). In light of this apparent cross-talk, one unanswered question is whether the ability of tryptamine hallucinogens to induce the HTR via 5-HT2A activation is modulated by their concurrent effects on 5-HT1A receptors. Pretreatment with the mixed 5-HT1A/β-adrenergic antagonist pindolol markedly augments the subjective response induced by the hallucinogen N,N-dimethyltryptamine (DMT) in human volunteers, suggesting that 5-HT1A activation by DMT may blunt its 5-HT2A-mediated effects (Strassman 1996). Based on those findings, we hypothesized that 5-HT1A activation by tryptamine hallucinogens may buffer their ability to induce the HTR in mice.
One way to gauge the involvement of 5-HT1A receptors in the behavioral response to hallucinogens is to assess the effect of combined administration with a 5-HT1A antagonist. The possibility exists, however, that 5-HT1A antagonists might alter the potency of 5-HT2A receptor-mediated responses due to interactions that are known to occur between the receptors (Krebs-Thomson and Geyer 1998; Salmi and Ahlenius 1998; Li et al. 2011). Indeed, 5-HT1A antagonists can augment the HTR induced by hallucinogen administration (Willins and Meltzer 1997), and under certain conditions can even induce head twitches through a mechanism involving indirect activation of 5-HT2A receptors (Darmani and Reeves 1996; Darmani 1998; Fox et al. 2010). As an alternative to conducting antagonist blockade studies, receptor binding studies were conducted with DALT derivatives and regression analyses were performed to determine whether potency in the HTR assay is correlated with 5-HT2A and/or 5-HT1A receptor affinities.
2. MATERIALS AND METHODS
2.1. Subjects
Male C57BL/6J mice (6–8 weeks old) obtained from Jackson Laboratories (Bar Harbor, ME, USA) were housed in a vivarium at the University of California San Diego, an AAALAC-approved animal facility that meets all Federal and State requirements for care and treatment of laboratory animals. Mice were housed up to four per cage in a climate-controlled room on a reverse-light cycle (lights on at 1900 h, off at 0700 h) and were provided with ad libitum access to food and water, except during behavioral testing. Testing was conducted between 1000 and 1800 h. All animal experiments were conducted in accordance with NIH guidelines and were approved by the UCSD animal care committee.
2.2. Drugs
The following drugs were tested: N,N-diallyltryptamine hydrochloride (DALT), 5-methoxy-N,N-diallyltryptamine hydrochloride (5-MeO-DALT), 5-fluoro-N,N-diallyltryptamine hydrochloride (5-F-DALT), 5-bromo-N,N-diallyltryptamine hydrochloride (5-Br-DALT), 4-hydroxy-N,N-diallyltryptamine fumarate (4-HO-DALT), 4-acetoxy-N,N-diallyltryptamine fumarate (4-AcO-DALT), 2-phenyl-N,N-diallyltryptamine hydrochloride (2-Ph-DALT), 5-methoxy-2-methyl-N,N-diallyltryptamine hydrochloride (5-MeO-2-Me-DALT), 5-fluoro-2-methyl-N,N-diallyltryptamine hydrochloride (5-F-2-Me-DALT), and 7-ethyl-N,N-diallyltryptamine hydrochloride (7-Et-DALT). 4-AcO-DALT fumarate and 4-HO-DALT hemifumarate were obtained from Scientific Supplies (London, UK); the other tryptamines were synthesized, fully characterized, and available from previous studies (Meyer et al. 2014; Michely et al. 2015; Dinger et al. 2016; Brandt et al. 2017a; Caspar et al. 2017; Michely et al. 2017).
2.3. Binding studies
A screening at 45 receptor and transporter binding sites was performed by the NIMH Psychoactive Drug Screening Program (NIMH PDSP). Most of these screenings were performed with cloned human receptors; exceptions are listed in Table 1. Test compounds were dissolved in DMSO and were tested at 10 μM in competition assays against radioactive probe compounds. Sites exhibiting > 50% inhibition at 10 μM were tested in secondary assays at the identified receptor or transporter using 12 concentrations of the DALT compound, measured in triplicate, to generate competition binding isotherms. Ki values were obtained from nonlinear regression of these binding isotherms from best-fit IC50 values using the Cheng-Prusoff equation (Cheng and Prusoff 1973). Ki values were converted to pKi values for data analysis. The radioligands used were as follows: [3H]8-OH-DPAT (5-HT1A), [3H]GR125743 (5-HT1B/1D), [3H]5-HT (5-HT1E), [3H]ketanserin (5-HT2A), [3H]LSD (5-HT2B/5A/6/7), [3H]mesulergine (5-HT2C), [3H]citalopram (serotonin transporter), [3H]prazocin (α1A/1B/1D), [3H]rauwolscine (α2A/2B/2C), [125I]pindolol (β1), [3H]CGP12177 (β2, β3), [3H]nisoxetine (norepinephrine transporter), [3H]SCH23390 (D1, D5), [3H]N/-methylspiperone (D2/3/4), [3H]WIN35428 (dopamine transporter), [3H]DAMGO (μ-opioid), [3H]DADLE (δ-opioid), [3H]U69593 (κ-opioid), [3H]muscimol (GABAA), [3H]funitrazepam (central benzodiazepine), [3H]PK11195 (peripheral benzodiazepine), [3H]pyrilamine (H1), [3H]tiotidine (H2), [3H]α-methylhistamine (H3), [3H]histamine (H4), [3H]QNB (M1–5), [3H](+)-pentazocine (σ1), and [3H]DTG (σ2). The experimental protocols are available from the NIMH PDSP website (Roth 2013).
Table 1.
Summary of binding data for N,N-diallyltryptamine (DALT) and ring-substituted derivatives at 33 receptors and transporters.
| Site | Speciesa | Binding Affinity (Ki, nM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DALT | 5-MeO | 5-F | 5-Br | 4-HO | 4-AcO | 2-Ph | 5-MeO-2-Me | 5-F-2-Me | 7-Et | ||
| 5-HT1A | Human | 100 | 19 | 80 | 11 | 319 | 383 | 402 | 267 | 318 | 1,013 |
| 5-HT1B | Human | > 10,000 | 735 | 1,787 | 950 | 2,494 | > 10,000 | 273 | 2,267 | 2,011 | > 10,000 |
| 5-HT1D | Human | 689 | 107 | 816 | 130 | 693 | 801 | 204 | 900 | 1,592 | 2,691 |
| 5-HT1E | Human | 378 | 500 | 474 | 512 | 238 | 467 | > 10,000 | 1,594 | 1,273 | > 10,000 |
| 5-HT2A | Human | 701 | 218 | 247 | 477 | 652 | 565 | 13 | 1,153 | 655 | 1,515 |
| 5-HT2B | Human | 61 | 59 | 16 | 53 | 2,593 | 63 | 192 | 241 | 17 | 65 |
| 5-HT2C | Human | 385 | 456 | 102 | 358 | 2,113 | 1,515 | 278 | > 10,000 | 541 | 443 |
| 5-HT5A | Human | > 10,000 | 3,312 | 4,299 | 2,389 | > 10,000 | 5,844 | 1,670 | 1,822 | 1,916 | > 10,000 |
| 5-HT6 | Human | 1,718 | 153 | 74 | 133 | 213 | 1,791 | 68 | 206 | 168 | > 10,000 |
| 5-HT7 | Human | > 10,000 | 90 | 402 | 49 | 600 | 724 | > 10,000 | > 10,000 | 493 | > 10,000 |
| SERT | Human | 150 | 499 | 36 | 127 | 5,210 | 1,089 | > 10,000 | > 10,000 | 983 | 795 |
| α1A | Human | 1,663 | > 10,000 | 1,251 | 637 | > 10,000 | > 10,000 | 75 | 1,198 | 1,570 | > 10,000 |
| α1B | Human | 1,369 | > 10,000 | > 10,000 | 2,050 | > 10,000 | > 10,000 | 904 | > 10,000 | > 10,000 | > 10,000 |
| α1D | Human | > 10,000 | > 10,000 | > 10,000 | 1,124 | > 10,000 | > 10,000 | 243 | 2,405 | > 10,000 | > 10,000 |
| α2A | Human | 124 | 215 | 119 | 83 | 1,206 | 342 | 85 | 189 | 53 | 141 |
| α2B | Human | 305 | 726 | 218 | 227 | > 10,000 | 170 | 78 | 335 | 108 | 489 |
| α2C | Human | 901 | 1,467 | 848 | 356 | > 10,000 | 748 | 159 | 888 | 184 | 682 |
| NET | Human | 1,121 | > 10,000 | 1,818 | 964 | > 10,000 | > 10,000 | 420 | > 10,000 | > 10,000 | 1,879 |
| D1 | Human | > 10,000 | > 10,000 | > 10,000 | > 10,000 | > 10,000 | > 10,000 | 2,793 | > 10,000 | > 10,000 | > 10,000 |
| D2 | Human | > 10,000 | > 10,000 | 2,463 | 4,349 | > 10,000 | > 10,000 | 388 | > 10,000 | 4,416 | > 10,000 |
| D3 | Human | 672 | > 10,000 | 120 | 240 | 1,570 | > 10,000 | 342 | 2,399 | 414 | 1,082 |
| D4 | Human | > 10,000 | > 10,000 | > 10,000 | > 10,000 | > 10,000 | > 10,000 | 1,000 | > 10,000 | > 10,000 | > 10,000 |
| D5 | Human | > 10,000 | > 10,000 | > 10,000 | > 10,000 | > 10,000 | > 10,000 | 2,003 | > 10,000 | > 10,000 | > 10,000 |
| DAT | Human | 1,406 | 3,378 | 2,150 | 2,455 | > 10,000 | > 10,000 | 746 | 2,413 | 2,208 | 1,725 |
| MOR | Human | > 10,000 | > 10,000 | > 10,000 | 1,726 | > 10,000 | > 10,000 | > 10,000 | > 10,000 | > 10,000 | 2,674 |
| DOR | Human | > 10,000 | > 10,000 | > 10,000 | > 10,000 | > 10,000 | > 10,000 | 6,789 | > 10,000 | > 10,000 | > 10,000 |
| KOR | Human | 2,477 | 1,132 | 2,184 | 898 | > 10,000 | 5,235 | 589 | 391 | 580 | 580 |
| PBR | Rat kidneyb | > 10,000 | > 10,000 | > 10,000 | > 10,000 | > 10,000 | > 10,000 | 1,929 | > 10,000 | > 10,000 | > 10,000 |
| H1 | Human | 127 | 505 | 83 | 106 | > 10,000 | 353 | 79 | 847 | 435 | 913 |
| H2 | Human | > 10,000 | > 10,000 | > 10,000 | > 10,000 | > 10,000 | > 10,000 | 367 | > 10,000 | > 10,000 | > 10,000 |
| H3 | Guinea pig | > 10,000 | 1,712 | 2,093 | 1,495 | > 10,000 | > 10,000 | > 10,000 | 1,134 | 1,397 | > 10,000 |
| σ1 | Rat brainb | 101 | 301 | 86 | 101 | 2,765 | 299 | > 10,000 | 427 | 531 | 22 |
| σ2 | Rat PC12b | 356 | 253 | 303 | 224 | > 10,000 | > 10,000 | 717 | 1,235 | 396 | 136 |
The experiments were performed using cloned receptors from the species indicated.
The experiment was performed using tissues or cells natively expressing the receptor.
Abbreviations: 2-Ph, 2-phenyl-N,N-diallyltryptamine; 4-AcO, 4-acetoxy-N,N-diallyltryptamine; 4-HO, 4-hydroxy-N,N-diallyltryptamine; 5-Br, 5-bromo-N,N-diallyltryptamine; 5-F, 5-fluoro-N,N-diallyltryptamine; 5-F-2-Me, 5-methoxy-2-fluoro-N,N-diallyltryptamine; 5-MeO, 5-methoxy-N,N-diallyltryptamine; 5-MeO-2-Me, 5-methoxy-2-methyl-N,N-diallyltryptamine; 7-Et, 7-ethyl-N,N-diallyltryptamine; DALT, N,N-diallyltryptamine; DAT, dopamine transporter; DOR, δ-opioid receptor; KOR, κ-opioid receptor; MOR, μ-opioid receptor; NET, norepinephrine transporter; PBR, peripheral benzodiazepine receptor; SERT, serotonin transporter.
2.4. Head-twitch response
The head twitch response (HTR) was assessed using a head-mounted magnet and a magnetometer detection coil (Halberstadt and Geyer 2013,2014; Nichols et al. 2015). Briefly, mice were anesthetized and a small neodymium magnet was attached to the dorsal surface of the cranium using dental cement. Following a two-week recovery period, HTR experiments were carried out in a well-lit room with at least 7-days between sessions to avoid carryover effects. Test compounds were dissolved in water containing 5% Tween 80 and administered IP at a volume of 5 or 10 mL/kg body weight immediately prior to testing. Mice (n=5–6/group) were injected with drug or vehicle and then HTR activity was recorded in a glass cylinder surrounded by a magnetometer coil for 30 minutes. Coil voltage was low-pass filtered (2–10 kHz cutoff frequency), amplified, and digitized (20 kHz sampling rate) using a Powerlab/8SP with LabChart v 7.3.2 (ADInstruments, Colorado Springs, CO, USA), then filtered off-line (40–200 Hz bandpass). Head twitches were identified manually based on the following criteria: 1) sinusoidal wavelets; 2) evidence of at least two sequential head movements (usually exhibited as bipolar peaks) with frequency ≥ 40 Hz; 3) amplitude exceeding the level of background noise; 4) duration < 0.15 s; and 5) stable coil voltage immediately preceding and succeeding each response.
2.5. Data analysis
Head twitch counts were analyzed using one-way analyses of variance (ANOVA). Post hoc pairwise comparisons between selected groups were performed using Tukey’s studentized range method. The entire 30-min recordings were examined for head twitches, but in some cases a shorter block of time was used for analysis to accommodate compounds with a brief duration-of-action (potency calculations can be confounded by extended periods of inactivity). ED50 values and 95% confidence limits were calculated using nonlinear regression. Relationships between HTR potency and binding affinities were assessed using linear regression and ordinary least-squares regression. For all analyses, significance was demonstrated by surpassing an α-level of 0.05.
3. RESULTS
3.1. Receptor binding
DALT and 9 ring-substituted derivatives were submitted to the NIMH PDSP for examination of their binding profiles at 45 neurotransmitter receptors and transporters. Ki values were determined for compounds that produced > 50% displacement of a radioactive probe compound at a concentration of 10,000 nM. The results are summarized in Table 1. The data for DALT and several of its 5-substituted derivatives (5-MeO-DALT, 5-F-DALT, and 5-Br-DALT) were reported in a previous publication (Cozzi and Daley 2016). All of the compounds were devoid of 50% displacement at M1-M5 muscarinic, β1-β3 adrenergic, H4 histaminergic, central benzodiazepine sites (labeled with [3H]flunitrazepam), and GABAA receptors.
As reported previously (Cozzi and Daley 2016), DALT binds relatively non-selectively to 5-HT1 and 5-HT2 subtypes, σ1 and σ2 sites, α2-adrenoceptors, dopaminergic D3 receptors, histaminergic H1 receptors, and the 5-HT transporter (SERT). DALT had the highest measured affinities for 5-HT2B (Ki = 61 nM), 5-HT1A (Ki = 100 nM), σ1 (Ki = 101 nM), α2A (Ki = 124 nM), H1 (Ki = 127 nM) and SERT (Ki = 150 nM). Incorporation of an oxygenated substituent at the 4-position altered the binding pattern of DALT. Compared to DALT, the 4-hydroxy and 4-acetoxy derivatives showed several-fold lower affinities for 5-HT1A, 5-HT2C, α2A-adrenergic receptors, σ1 and σ2 sites, and SERT, whereas 5-HT7 receptor affinity was increased by at least an order of magnitude. 4-Hydroxy-DALT also had low affinity for 5-HT2B receptors (Ki = 2593 nM) and moderately high affinity for 5-HT6 receptors (Ki = 213 nM).
The 2-phenyl-substituted DALT derivative (2-Ph-DALT) showed a notable binding profile. The 5-HT2A binding affinity of 2-Ph-DALT (Ki = 13 nM) was 54-fold higher than the affinity of DALT (Ki = 701 nM) and at least 10-fold higher than the affinity of any other DALT derivative. According to a previous report (Stevenson et al. 2000), 2-aryl-tryptamines such as 2-phenyl-N,N-dimethyltryptamine and 2-phenyl-N,N-diethyltryptamine act as 5-HT2A receptor antagonists and have high affinity (Ki values of 4.4 nM and 2.8 nM, respectively, vs. [3H]ketanserin). 2-Ph-DALT was the only compound tested herein that bound to D1, D4, D5, H2, δ-opioid, and peripheral benzodiazepine receptors with a Ki value < 10 μM. Compared to the other compounds, 2-Ph-DALT also had relatively high affinity for α1A and α1D adrenoceptors and D2 receptors. By contrast, 2-phenyl substitution abolished binding to σ1 sites and SERT.
The 2-methyl derivatives of 5-MeO-DALT and 5-F-DALT were also examined. Incorporation of a 2-methyl group tended to reduce the affinity of those DALT derivatives for 5-HT receptors and SERT. The affinities of 5-MeO-DALT and 5-F-DALT for 5-HT1A, 5-HT1D, 5-HT1E, 5-HT2A, and 5-HT2C receptors were consistently reduced by 2-methylation (see Table 1). Likewise, the binding of 5-MeO-DALT to SERT (Ki = 499 nM) was abolished by 2-methylation (5-MeO-2-Me-DALT: < 50% displacement at 10,000 nM), whereas the affinity of 5-F-DALT (Ki = 36 nM) was reduced almost 30-fold (5-F-2-Me-DALT; Ki = 983 nM).
Although 7-ethyl-substitution tended to reduce the binding affinity of DALT for most sites (including 5-HT1A and 5-HT2A receptors), the affinity of 7-Et-DALT for σ1 sites (Ki = 22 nM) was nearly 5-fold higher than the parent compound.
3.2. Head twitch response
DALT induced the HTR in mice with an ED50 of 3.42 mg/kg. Compared to other N,N- disubstituted tryptamines such as N,N-dipropyltryptamine and N,N-diisopropyltryptamine (Smith et al. 2014), DALT had relatively low potency. Similar to other tryptamine derivatives (Fantegrossi et al. 2008a), the response to DALT followed an inverted-U-shaped dose-response function (see Table 2).
Table 2.
Summary of head twitch response (HTR) data for N,N-diallyltryptamine (DALT) and ring-substituted derivatives.
| Drug | One-Way ANOVA | Duration (min) | N | Dose (mg/kg) | HTR Counts (mean ± SEM) | ED50 (95% CI) (mg/kg) | ED50 (95% CI) (μmol/kg) |
|---|---|---|---|---|---|---|---|
| DALT | F(5,24) = 5.71, p < 0.002 | 30 | 5 | 0 | 3.6 ± 0.9 | 3.42 (2.44–4.79) | 12.3 (8.8–17.3) |
| 5 | 0.875 | 8.2 ± 2.8 | |||||
| 5 | 1.75 | 6.8 ± 2.6 | |||||
| 5 | 3.5 | 14.2 ± 4.3 | |||||
| 5 | 7 | 21.8 ± 4.4 ** | |||||
| 5 | 14 | 20.6 ± 2.7 ** | |||||
| 5-MeO-DALT | F(5,24) = 6.63, p=0.0005 | 20 | 5 | 0 | 3.0 ± 1.5 | 2.25 (1.82–2.78) | 7.3 (5.9–9.1) |
| 5 | 1.75 | 6.6 ± 1.0 | |||||
| 5 | 3.5 | 19.8 ± 1.5 ** | |||||
| 5 | 7 | 8.8 ± 2.6 | |||||
| 5 | 14 | 8.0 ± 4.9 | |||||
| 5-F-DALT | F(5,24) = 5.12, p < 0.003 | 30 | 5 | 0 | 4.4 ± 0.6 | 1.58 (1.09–2.28) | 5.4 (3.7–7.7) |
| 5 | 0.875 | 9.8 ± 2.6 | |||||
| 5 | 1.75 | 21.0 ± 5.7 | |||||
| 5 | 3.5 | 36.0 ± 6.8 ** | |||||
| 5 | 7 | 26.8 ± 7.1 * | |||||
| 5 | 14 | 21.0 ± 4.0 | |||||
| 5-Br-DALT | F(5,24) = 5.21, p < 0.003 | 30 | 5 | 0 | 3.4 ± 0.5 | 4.80 (2.70–8.54) | 13.5 (7.6–24.0) |
| 5 | 3.5 | 5.0 ± 0.3 | |||||
| 5 | 7 | 10.8 ± 2.7 * | |||||
| 5 | 14 | 8.6 ± 2.7 | |||||
| 5 | 28 | 1.4 ± 0.4 | |||||
| 5 | 56 | 1.4 ± 1.4 | |||||
| 4-HO-DALT | F(5,24) = 12.07, p <0.0001 | 5 | 5 | 0 | 1.2 ± 0.4 | 2.60 (2.01–3.35) | 8.3 (6.4–10.6) |
| 5 | 0.875 | 4.0 ± 3.3 | |||||
| 5 | 1.75 | 9.2 ± 3.7 | |||||
| 5 | 3.5 | 28.6 ± 4.1 ** | |||||
| 5 | 7 | 31.6 ± 4.9 ** | |||||
| 5 | 14 | 24.6 ± 4.7 ** | |||||
| 4-AcO-DALT | F(5,24) = 6.87, p=0.0004 | 30 | 5 | 0 | 4.8 ± 1.0 | 1.99 (1.35–2.95) | 4.8 (3.3–7.1) |
| 5 | 0.875 | 10.4 ± 1.4 | |||||
| 5 | 1.75 | 42.0 ± 9.5 * | |||||
| 5 | 3.5 | 39.0 ± 14.1 * | |||||
| 5 | 7 | 65.0 ± 8.4 ** | |||||
| 5 | 14 | 47.8 ± 10.0 ** | |||||
| 2-Ph-DALT | F(5,24) = 2.20, NS | 30 | 5 | 0 | 3.8 ± 0.8 | ND | ND |
| 5 | 0.875 | 2.8 ± 0.5 | |||||
| 5 | 1.75 | 3.6 ± 1.3 | |||||
| 5 | 3.5 | 1.4 ± 0.5 | |||||
| 5 | 7 | 2.0 ± 0.5 | |||||
| 5 | 14 | 1.2 ± 0.5 | |||||
| 2-Me-5-MeO-DALT | F(5,24) = 1.02, NS | 30 | 5 | 0 | 3.8 ± 1.3 | ND | ND |
| 5 | 0.875 | 4.4 ± 0.2 | |||||
| 5 | 1.75 | 7.4 ± 2.2 | |||||
| 5 | 3.5 | 4.2 ± 1.0 | |||||
| 5 | 7 | 4.2 ± 0.9 | |||||
| 5 | 14 | 5.0 ± 1.4 | |||||
| 2-Me-5-F-DALT | F(5,24) = 0.19, NS | 30 | 5 | 0 | 5.4 ± 1.7 | ND | ND |
| 5 | 0.875 | 6.2 ± 1.0 | |||||
| 5 | 1.75 | 6.8 ± 0.9 | |||||
| 5 | 3.5 | 5.8 ± 2.5 | |||||
| 5 | 7 | 6.4 ± 1.6 | |||||
| 5 | 14 | 7.2 ± 0.8 | |||||
| 7-Et-DALT | F(1,10) = 0.11, NS | 30 | 6 | 0 | 10.7 ± 1.7 | ND | ND |
| 6 | 15 | 9.8 ± 1.8 | |||||
ND = not determined (the compound was not active within the dose range tested).
p < 0.05,
p < 0.01,
significant difference from the vehicle control group (Tukey’s test).
Ring-substitution on the DALT molecule resulted in active compounds, some of which were more potent than DALT (see Table 2). The 4-hydroxy and 5-methoxy derivatives induced the HTR with almost twice the potency of DALT. 4-Acetoxy- or 5-fluoro-substitution produced even greater increases in potency. By contrast, 5-bromo substitution did not significantly alter HTR potency relative to DALT. Substitution at the 2-position with either a methyl or a phenyl group (e.g., 2-Ph-DALT, 2-Me-5-MeO-DALT, 2-Me-5-F-DALT) abolished activity in the HTR assay. Similarly, 7-Et-DALT did not induce the HTR. In addition to having higher potency than DALT, the 4-hydroxy and 4-acetoxy derivatives produced a HTR with an extremely rapid onset (data not shown).
For DALT and its active derivatives, there was no correlation between HTR potency (ED50 values) and 5-HT1A receptor affinity (R2 = 0.2804; F(1,4) = 1.56, NS) or 5-HT2A receptor affinity (R2 = 0.1646; F(1,4) = 0.79, NS). A multiple regression analysis was performed to test whether HTR potency is predicted by both 5-HT1A and 5-HT2A affinity. The ordinary least-squares (OLS) regression revealed that 5-HT1A and 5-HT2A binding affinities significantly predicted HTR potency (R2 = 0.8729; F(2,3) = 10.31, p < 0.05; Figure 2). Both 5-HT2A affinity (β = 0.741, t(3) = 3.74, p < 0.04) and 5-HT1A affinity (β = −0.279, t(3) = −4.09, p < 0.03) contributed significantly to the prediction, indicating that 5-HT2A and 5-HT1A receptors make positive and negative contributions, respectively, to HTR potency. In addition to 5-HT1A and 5-HT2A receptors, several other monoaminergic sites can influence HTR expression, including 5-HT2C receptors (Fantegrossi et al. 2010), SERT (Basselin et al. 2009), and α2-adrenoceptors (Schreiber et al. 1995). To test whether these other receptors play a role in the HTR induced by DALT derivatives, additional regression analyses were performed for sites with Ki < 10,000 nM. There was no correlation between HTR potency and affinity at 5-HT2C (R2 = 0.0292; F(1,4) = 0.12, NS), SERT (R2 = 0.0661; F(1,4) = 0.28, NS), or α2A sites (R2 = 0.2197; F(1,4) = 1.12, NS). Furthermore, affinity for these sites did not significantly predict HTR potency when analyzed in combination with 5-HT2A receptor affinity using multiple regression (data not shown).
Figure 2.
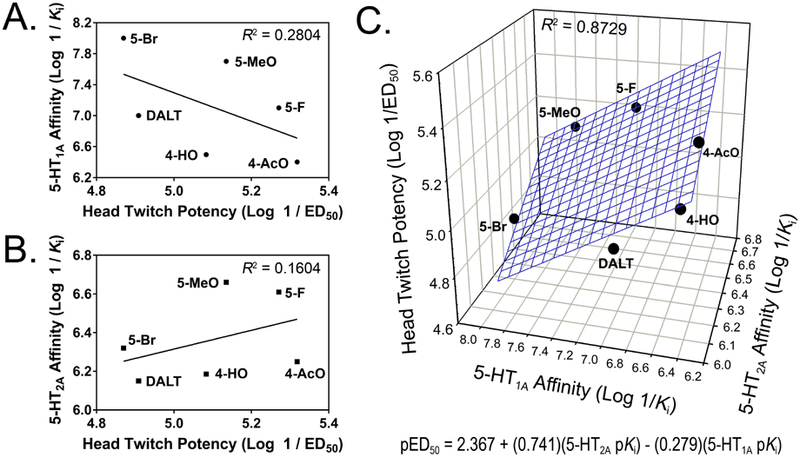
Correlation between potency in the head twitch response (HTR) assay (pED50 values) and serotonin receptor binding affinities (pKi values) for N,N-diallyltryptamine (DALT) and five ring-substituted derivatives. (A) Correlation between HTR potency and 5-HT1A receptor affinity. (B) Correlation between HTR potency and 5-HT2A receptor affinity. (C) Correlation between HTR potency and 5-HT1A and 5-HT2A receptor affinity.
4. DISCUSSION
The potency and 5-HT receptor affinities of tryptamine hallucinogens are influenced by the substituent groups present on the indole nucleus and amine nitrogen. Most compounds in this structural class contain N,N-dialkyl substituents, but tryptamines containing N,N-diallyl groups have also been synthesized (Brandt et al. 2017a). Although the structure-activity relationships and pharmacology of dialkyltryptamines such as DMT and psilocybin have been widely investigated, relatively little is known about the comparative properties of diallyltryptamines.
The present studies were conducted to investigate the pharmacology and behavioral effects of DALT and a variety of ring-substituted derivatives, some of which are used recreationally as new psychoactive substances or “research chemicals” and reportedly have hallucinogenic effects.
Consistent with the effects of other tryptamine hallucinogens (Fantegrossi et al. 2006; Fantegrossi et al. 2008b; Halberstadt et al. 2011; Carbonaro et al. 2015; Nichols et al. 2015), DALT and several of its derivatives substituted at the 4 or 5 position induced head twitches in mice. Although our studies measured 5-HT2A binding affinity and did not include a functional assessment of receptor activation, DALT, 4-HO-DALT, 4-AcO-DALT, 5-Br-DALT, 5-F-DALT and 5-MeO-DALT are likely to be 5-HT2A agonists based on their effects in the HTR assay. Importantly, 5-MeO-DALT was previously reported to act as an agonist at recombinant human 5-HT2A receptors (Arunotayanun et al. 2013). Similarly, it was recently reported (Gatch et al. 2017) that 5-MeO-DALT produces full substitution in rats trained to discriminate the hallucinogenic 5-HT2A receptor agonist 2,5-dimethoxy-4-methylamphetamine (DOM). Since the head twitch assay is routinely used to test whether 5-HT2A agonists produce LSD-like behavioral effects (Gonzalez-Maeso et al. 2007), the ability of diallyltryptamines to induce the HTR and produce DOM-like stimulus effects is thus consistent with their classification as serotonergic hallucinogens. However, few details have been published regarding the effects of these compounds in humans.
Notably, the potency of the diallyltryptamines in the HTR assay is not correlated with 5-HT2A receptor binding affinity alone but is dependent on activity at both 5-HT1A and 5-HT2A receptors. According to the multiple regression analysis, there is a positive relationship between HTR potency and 5-HT2A affinity and a negative relationship between HTR potency and 5-HT1A affinity; in other words, HTR potency increases as 5-HT2A affinity increases and decreases as 5-HT1A affinity increases. As noted earlier, the hallucinogen HTR occurs as a result of 5-HT2A activation and can be suppressed by concurrent administration of a 5-HT1A agonist (Darmani et al. 1990; Schreiber et al. 1995; Kleven et al. 1997). Based on the roles that 5-HT1A and 5-HT2A receptors are known to play in the hallucinogen HTR, the regression analysis can be interpreted as showing that 5-HT2A activation by DALT and its derivatives mediates the HTR, whereas their interaction with the 5-HT1A receptor has a countervailing influence that inhibits expression of head twitch behavior. Hence, the potency of diallyltryptamines in the HTR assay may ultimately be determined by their combined activities at 5-HT1A and 5-HT2A receptors. These findings support the hypothesis that 5-HT1A activation by tryptamine hallucinogens buffers their effects on the HTR.
Based on the ability of 5-HT1A agonists to inhibit the HTR, there has been speculation that 5-HT1A stimulation by nonselective tryptamine and lysergamide hallucinogens may reduce or inhibit the frequency of their induced head twitch behavior (Darmani et al. 1990). Our recent work has demonstrated that the LSD analog and non-selective 5-HT1A/5-HT2A agonist lysergic acid morpholide (LSM-775) does not induce the HTR in mice unless the animals are pretreated with the 5-HT1A antagonist WAY-100635 (Brandt et al. 2017b), indicating that 5-HT1A activation by LSM-775 masks its ability to induce the HTR. As far as we are aware, however, the present study is the first to show that the potency of the HTR induced by tryptamine hallucinogens may be influenced by their 5-HT1A interactions. Nevertheless, these findings remain tentative given to the small number of compounds tested; follow-up studies with a larger group of tryptamines are necessary to achieve more definitive results.
One potential confound for the regression analysis is that the binding studies were performed with cloned human 5-HT receptors whereas the behavioral experiments were performed in mice. Sequence differences between rodent and human 5-HT receptors can result in ligand binding affinity differences (Kao et al. 1992; Oksenberg et al. 1992; Parker et al. 1993; Smolyar and Osman 1993). There are reportedly species differences in the affinities of 4-hydroxytryptamines for the 5-HT2A receptor, which are potentially relevant to our studies with 4-HO-DALT and 4-AcO-DALT. Specifically, according to Gallaher et al. (1993), who studied human and rat 5-HT2A receptors labeled with [3H]ketanserin, 4-hydroxy-DMT (psilocin) has 15-fold higher affinity for the human receptor (Ki = 340 nM) than for the rat receptor (Ki = 5,100 nM), whereas its 5-hydroxy isomer bufotenine has nearly equal affinities for the human and rat receptors (Ki values of 300 nM and 520 nM, respectively). The human 5-HT2A receptor contains a serine at position 242 in helix V whereas alanine is present in the receptor in rodents, leading Gallaher et al. (1993) to speculate that psilocin may have higher affinity for the human receptor because Ser-242(5.42) can form a hydrogen-bond with the 4-hydroxyl group in psilocin. Other studies, however, failed to confirm their findings. Another group reported that both psilocin and bufotenine displace [125I]R-(–)-DOI binding to 5-HT2A receptors in rat cortex with high affinity and have nearly equivalent IC5o values (McKenna et al. 1990). Furthermore, Ser-242(5.42) in the human 5-HT2A receptor is believed to form a hydrogen-bond with the indole N1 nitrogen of tryptamines and ergolines based on mutagenesis experiments and molecular modeling (Nelson et al. 1993; Johnson et al. 1994; Almaula et al. 1996; Wacker et al. 2017), abrogating the structural basis for the species differences posited by Gallaher. Therefore, although there is no clear evidence indicating that differences between human and mouse 5-HT receptors are likely to confound our regression analysis, especially with regard to 4-substituted DALT derivatives, the potential existence of cross-species differences in 5-HT receptor pharmacology must be acknowledged as a source of potential error for the regression.
DALT and derivatives substituted at the 5-position have been shown to bind to multiple 5-HT receptors, as well as α2 adrenergic subtypes, σ1 and σ2 sites, histamine H1 receptors, and SERT (Cozzi and Daley 2016). As shown in the present investigation, substitution at other positions in the indole ring can markedly alter the binding profile of DALT. The 4-substituted derivatives displayed reduced affinity at 5-HT1A receptors compared to DALT and the 5-substituted derivatives. This is consistent with reports demonstrating that 4-hydroxy-DMT (psilocin) binds to 5-HT1A sites with 20-fold lower affinity compared to its 5-hydroxy isomer (bufotenine) or the 5-hydroxy O-methyl derivative (5-methoxy-DMT), whereas there is little difference between their 5-HT2A receptor affinities (McKenna et al. 1990; Blair et al. 2000).
Addition of a methyl group to the 2-position of 5-MeO-DALT reduced its affinity for most 5-HT binding sites, including 5-HT1A and 5-HT2A receptors, and abolished its ability to induce the HTR in mice at doses up to 14 mg/kg. These findings parallel those of Glennon et al. (2000), who found that 2-methylation or 2-ethylation of 5-methoxy-DMT reduced its affinity for 5-HT2A receptors. Similarly, although 2-methyl-5-methoxy-DMT is a hallucinogen in humans, it reportedly has significantly lower potency than 5-methoxy-DMT (Shulgin and Shulgin 1997). The 5-HT2A receptor apparently has difficulty accommodating tryptamines with a 2-alkyl substituent.
2-Ph-DALT did not induce the HTR despite having the highest 5-HT2A affinity of any compound screened (Ki = 13 nM). According to Stevenson et al. (2000), various 2-phenyl-N,N-dialkyltryptamines including the N,N-dimethyl, N,N-diethyl, and N-methyl-N-ethyl homologues bind to the 5-HT2A receptor with high (nM) affinities. However, all of these compounds blocked the stimulatory effect of 5-HT on phosphoinositide hydrolysis in CHO cells expressing the human 5-HT2A receptor. In light of the fact that other 2-phenyl-N,N-disubstituted tryptamines act as antagonists, the failure of 2-Ph-DALT to induce the HTR suggests that it may also act as a 5-HT2A antagonist.
The 7-ethyl-substituted derivative of DALT also had low affinity for 5-HT1A and 5-HT2A receptors and did not induce the HTR in mice when tested at 15 mg/kg. These findings are consistent with the behavioral effects of other 7-ethyl-substituted tryptamines. 7-Ethyl-DMT produces only partial substitution in rats trained to discriminate 5-MeO-DMT from vehicle (Glennon et al. 1980a). Rats trained to discriminate the interoceptive cue produced by 5-MeO-DMT generalize to other serotonergic hallucinogens (Glennon et al. 1980b; Young et al. 1982); hence, the absence of full substitution with 7-ethyl-DMT indicates that it does not produce hallucinogen-like stimulus effects in rodents.
The present findings also suggest that while 4- and 5-substituted DALT compounds may produce hallucinogenic effects in humans, 2- and 7-substituted DALT compounds may lack hallucinogenic effects, although further studies are necessary to test this hypothesis. While DALT, 5-MeO-DALT, and 4-AcO-DALT have already been detected by the European Early-Warning System and reported to the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA 2013, 2015), no such reports have arisen for 2- or 7-substituted DALT compounds.
To our knowledge, this analysis is the first to quantify the relative contributions of 5-HT2A and 5-HT1A receptors to the induction of HTR by a class of tryptamine hallucinogens. These findings may allow us to better predict the psychoactive potential of DALT derivatives based on their behavioral pharmacology, and suggest that similar analyses could be attempted for other classes of tryptamine hallucinogens. However, although 5-MeO-DALT produces hallucinogen-like behavioral responses in rodent behavioral paradigms including mouse HTR (the present studies) and rat drug discrimination (Gatch et al. 2017), it is not yet clear whether DALT derivatives can fully mimic the psychedelic effects produced by classical hallucinogens, allowing the possibility of subtle pharmacological differences relative to other tryptamine hallucinogens. Hence, it is not known whether the observed relationship between HTR potency and 5-HT2A and 5-HT1A binding affinities is consistent across the entire class of tryptamine hallucinogens. Nevertheless, if similar relationships do exist for other tryptamines, performing similar analyses on those classes should improve our understanding of their complex pharmacology and facilitate predictions regarding their psychoactive potencies.
HIGHLIGHTS.
A new class of recreational drugs are derived from N,N-Diallyltryptamine (DALT)
DALT derivatives are relatively nonselective for serotonin receptors
DALT derivatives induce the head twitch response (a 5-HT2A-mediated behavior) in mice
Both 5-HT2A and 5-HT1A receptors contribute to head twitch potency
5. ACKNOWLEDGEMENTS
This work was supported by an award from NIDA (R01 DA041336), as well as by the Veteran’s Administration VISN 22 Mental Illness Research, Education, and Clinical Center. Receptor binding data were generously provided by the National Institute of Mental Health’s Psychoactive Drug Screening Program (NIMH PDSP), Contract # HHSN-271–2013-00017-C). The NIMH PDSP is directed by Dr. Bryan Roth at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscol at NIMH, Bethesda, MD, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Almaula N, Ebersole BJ, Ballesteros JA, Weinstein H, Sealfon SC (1996) Contribution of a helix 5 locus to selectivity of hallucinogenic and nonhallucinogenic ligands for the human 5-hydroxytryptamine2A and 5-hydroxytryptamine2C receptors: direct and indirect effects on ligand affinity mediated by the same locus. Mol Pharmacol 50: 34–42. [PubMed] [Google Scholar]
- Amargos-Bosch M, Bortolozzi A, Puig MV, Serrats J, Adell A, Celada P, Toth M, Mengod G, Artigas F (2004) Co-expression and in vivo interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cereb Cortex 14: 281–99. [DOI] [PubMed] [Google Scholar]
- Araneda R, Andrade R (1991) 5-Hydroxytryptamine2 and 5-hydroxytryptamine 1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience 40: 399412. [DOI] [PubMed] [Google Scholar]
- Arunotayanun W, Dalley JW, Huang XP, Setola V, Treble R, Iversen L, Roth BL, Gibbons S (2013) An analysis of the synthetic tryptamines AMT and 5-MeO-DALT: Emerging ‘Novel Psychoactive Drugs’. Bioorg Med Chem Lett 23: 3411–3415. [DOI] [PubMed] [Google Scholar]
- Ashby CR Jr., Edwards E, Wang RY (1994) Electrophysiological evidence for a functional interaction between 5-HT1A and 5-HT2A receptors in the rat medial prefrontal cortex: an iontophoretic study. Synapse 17: 173–81. [DOI] [PubMed] [Google Scholar]
- Basselin M, Fox MA, Chang L, Bell JM, Greenstein D, Chen M, Murphy DL, Rapoport SI (2009) Imaging elevated brain arachidonic acid signaling in unanesthetized serotonin transporter (5-HTT)-deficient mice. Neuropsychopharmacology 34: 1695–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair JB, Kurrasch-Orbaugh D, Marona-Lewicka D, Cumbay MG, Watts VJ, Barker EL, Nichols DE (2000) Effect of ring fluorination on the pharmacology of hallucinogenic tryptamines. J Med Chem 43: 4701–10. [DOI] [PubMed] [Google Scholar]
- Blough BE, Landavazo A, Decker AM, Partilla JS, Baumann MH, Rothman RB (2014) Interaction of psychoactive tryptamines with biogenic amine transporters and serotonin receptor subtypes. Psychopharmacology (Berl) 231: 4135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenschutz MP, Ross S (2017) Therapeutic Applications of Classic Hallucinogens. Curr Top Behav Neurosci. [DOI] [PubMed] [Google Scholar]
- Brandt SD, Kavanagh PV, Dowling G, Talbot B, Westphal F, Meyer MR, Maurer HH, Halberstadt AL (2017a) Analytical characterization of N,N-diallyltryptamine (DALT) and 16 ring-substituted derivatives. Drug Test Anal 9: 115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt SD, Kavanagh PV, Twamley B, Westphal F, Elliott SP, Wallach J, Stratford A, Klein LM, McCorvy JD, Nichols DE, Halberstadt AL (2017b) Return of the lysergamides. Part IV: Analytical and pharmacological characterization of lysergic acid morpholide (LSM-775). Drug Test Anal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunt TM, Atkinson AM, Nefau T, Martinez M, Lahaie E, Malzcewski A, Pazitny M, Belackova V, Brandt SD (2017) Online test purchased new psychoactive substances in 5 different European countries: A snapshot study of chemical composition and price. Int J Drug Policy 44: 105–114. [DOI] [PubMed] [Google Scholar]
- Canal CE, Morgan D (2012) Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model. Drug Test Anal 4: 556–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonaro TM, Eshleman AJ, Forster MJ, Cheng K, Rice KC, Gatch MB (2015) The role of 5-HT2A, 5-HT 2C and mGlu2 receptors in the behavioral effects of tryptamine hallucinogens N,N-dimethyltryptamine and N,N-diisopropyltryptamine in rats and mice. Psychopharmacology (Berl) 232: 275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar AT, Gaab JB, Michely JA, Brandt SD, Meyer MR, Maurer HH (2017) Metabolism of the tryptamine-derived new psychoactive substances 5-MeO-2-Me-DALT, 5-MeO-2-Me-ALCHT, and 5-MeO-2-Me-DIPT and their detectability in urine studied by GC-MS, LC-MSn, and LC-HR-MS/MS. Drug Test Anal. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH (1973) Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22: 3099–3108. [DOI] [PubMed] [Google Scholar]
- Corkery JM, Durkin E, Elliott S, Schifano F, Ghodse AH (2012) The recreational tryptamine 5-MeO-DALT (N,N-diallyl-5-methoxytryptamine): a brief review. Prog Neuropsychopharmacol Biol Psychiatry 39: 259–62. [DOI] [PubMed] [Google Scholar]
- Cozzi NV, Daley PF (2016) Receptor binding profiles and quantitative structure-affinity relationships of some 5-substituted-N,N-diallyltryptamines. Bioorg Med Chem Lett 26: 959–64. [DOI] [PubMed] [Google Scholar]
- Darmani NA (1998) The silent and selective 5-HT1A antagonist, WAY 100635, produces via an indirect mechanism, a 5-HT2A receptor-mediated behaviour in mice during the day but not at night. Short communication. J Neural Transm (Vienna) 105: 635–43. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Pandey U, Glennon RA (1990) Do functional relationships exist between 5-HT1A and 5-HT2 receptors? Pharmacol Biochem Behav 36: 901–6. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Reeves SL (1996) The mechanism by which the selective 5-HT1A receptor antagonist S-(−)UH 301 produces head-twitches in mice. Pharmacol Biochem Behav 55: 1–10. [DOI] [PubMed] [Google Scholar]
- Dinger J, Woods C, Brandt SD, Meyer MR, Maurer HH (2016) Cytochrome P450 inhibition potential of new psychoactive substances of the tryptamine class. Toxicol Lett 241: 82–94. [DOI] [PubMed] [Google Scholar]
- EMCDDA (2008) EMCDDA-Europol 2007 Annual Report on the Implementation of Council Decision 2005/387/JHA. Publications Office of the European Union, Lisbon. [Google Scholar]
- EMCDDA (2013) New Drugs in Europe, 2012 EMCDDA-Europol 2012 Annual Report on the implementation of Council Decision 2005/387/JHA. Publications Office of the European Union, Luxembourg. doi:10.2810/99367 [Google Scholar]
- EMCDDA (2015) New Drugs in Europe, 2014 EMCDDA-Europol 2014 Annual Report on the implementation of Council Decision 2005/387/JHA. Publications Office of the European Union, Luxembourg. doi:10.2810/112317 [Google Scholar]
- Fantegrossi WE, Harrington AW, Kiessel CL, Eckler JR, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH (2006) Hallucinogen-like actions of 5-methoxy-N,N-diisopropyltryptamine in mice and rats. Pharmacol Biochem Behav 83: 122–9. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Murnane KS, Reissig CJ (2008a) The behavioral pharmacology of hallucinogens. Biochem Pharmacol 75: 17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Reissig CJ, Katz EB, Yarosh HL, Rice KC, Winter JC (2008b) Hallucinogen-like effects of N,N-dipropyltryptamine (DPT): possible mediation by serotonin 5-HT1A and 5-HT2A receptors in rodents. Pharmacol Biochem Behav 88: 358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Simoneau J, Cohen MS, Zimmerman SM, Henson CM, Rice KC, Woods JH (2010) Interaction of 5-HT2A and 5-HT2C receptors in R(−)-2,5-dimethoxy-4-iodoamphetamine-elicited head twitch behavior in mice. J Pharmacol Exp Ther 335: 728–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MA, Stein AR, French HT, Murphy DL (2010) Functional interactions between 5-HT2A and presynaptic 5-HT1A receptor-based responses in mice genetically deficient in the serotonin 5-HT transporter (SERT). Br J Pharmacol 159: 879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallaher TK, Chen K, Shih JC (1993) Higher affinity of psilocin for human than rat 5-HT2 receptor indicates binding site structure. Med Chem Res 3: 52–66. [Google Scholar]
- Gatch MB, Dolan SB, Forester MJ (2017) Locomotor and discriminative stimulus effects of four novel hallucinogens in rodents. Behav Pharmacol 28: 375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA, Lee M, Rangisetty JB, Dukat M, Roth BL, Savage JE, McBride A, Rauser L, Hufeisen S, Lee DK (2000) 2-Substituted tryptamines: agents with selectivity for 5-HT(6) serotonin receptors. J Med Chem 43: 1011–8. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Schubert E, Jacyno JM, Rosecrans JA (1980a) Studies on several 7-substituted N,N-dimethyltryptamines. J Med Chem 23: 1222–6. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, Rosecrans JA, Kallman MJ (1980b) Hallucinogenic agents as discriminative stimuli:a correlation with serotonin receptor affinities. Psychopharmacology (Berl) 68: 155–8. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA (2007) Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 53: 439–52. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL (2015) Recent advances in the neuropsychopharmacology of serotonergic hallucinogens. Behav Brain Res 277: 99–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA (2013) Characterization of the head-twitch response induced by hallucinogens in mice: detection of the behavior based on the dynamics of head movement. Psychopharmacology (Berl) 227: 727–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA (2014) Effects of the hallucinogen 2,5-dimethoxy-4-iodophenethylamine (2C-I) and superpotent N-benzyl derivatives on the head twitch response. Neuropharmacology 77: 200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Koedood L, Powell SB, Geyer MA (2011) Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. J Psychopharmacol 25: 1548–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MP, Loncharich RJ, Baez M, Nelson DL (1994) Species variations in transmembrane region V of the 5-hydroxytryptamine type 2A receptor alter the structure-activity relationship of certain ergolines and tryptamines. Mol Pharmacol 45: 277–86. [PubMed] [Google Scholar]
- Kao HT, Adham N, Olsen MA, Weinshank RL, Branchek TA, Hartig PR (1992) Site-directed mutagenesis of a single residue changes the binding properties of the serotonin 5-HT2 receptor from a human to a rat pharmacology. FEBS Lett 307: 324–8. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Assié MB, Koek W (1997) Pharmacological characterization of in vivo properties of putative mixed 5-HTiA agonist/5-HT2A/2C antagonist anxiolytics. II. Drug discrimination and behavioral observation studies in rats. J Pharmacol Exp Ther 282: 747–59. [PubMed] [Google Scholar]
- Kometer M, Schmidt A, Jancke L, Vollenweider FX (2013) Activation of serotonin 2A receptors underlies the psilocybin-induced effects on alpha oscillations, N170 visual-evoked potentials, and visual hallucinations. J Neurosci 33: 10544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraehenmann R, Pokorny D, Vollenweider L, Preller KH, Pokorny T, Seifritz E, Vollenweider FX (2017) Dreamlike effects of LSD on waking imagery in humans depend on serotonin 2A receptor activation. Psychopharmacology (Berl). [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Geyer MA (1998) Evidence for a functional interaction between 5-HT1A and 5-HT2 receptors in rats. Psychopharmacology (Berl) 140: 69–74. [DOI] [PubMed] [Google Scholar]
- Li JX, Crocker C, Koek W, Rice KC, France CP (2011) Effects of serotonin (5-HT)1A and 5-HT2A receptor agonists on schedule-controlled responding in rats: drug combination studies. Psychopharmacology (Berl) 213: 489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna DJ, Repke DB, Lo L, Peroutka SJ (1990) Differential interactions of indolealkylamines with 5-hydroxytryptamine receptor subtypes. Neuropharmacology 29: 193–8. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Caspar A, Brandt SD, Maurer HH (2014) A qualitative/quantitative approach for the detection of 37 tryptamine-derived designer drugs, 5 beta-carbolines, ibogaine, and yohimbine in human urine and plasma using standard urine screening and multi-analyte approaches. Anal Bioanal Chem 406: 225–37. [DOI] [PubMed] [Google Scholar]
- Michely JA, Brandt SD, Meyer MR, Maurer HH (2017) Biotransformation and detectability of the new psychoactive substances N,N-diallyltryptamine (DALT) derivatives 5-fluoro-DALT, 7-methyl-DALT, and 5,6-methylenedioxy-DALT in urine using GC-MS, LC-MSn, and LC-HR-MS/MS. Anal Bioanal Chem 409: 1681–1695. [DOI] [PubMed] [Google Scholar]
- Michely JA, Helfer AG, Brandt SD, Meyer MR, Maurer HH (2015) Metabolism of the new psychoactive substances N,N-diallyltryptamine (DALT) and 5-methoxy-DALT and their detectability in urine by GC-MS, LC-MSn, and LC-HR-MS-MS. Anal Bioanal Chem 407: 7831–42. [DOI] [PubMed] [Google Scholar]
- Nagai F, Nonaka R, Satoh Hisashi Kamimura K (2007) The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur J Pharmacol 559: 132–7. [DOI] [PubMed] [Google Scholar]
- Nelson DL, Lucaites VL, Audia JE, Nissen JS, Wainscott DB (1993) Species differences in the pharmacology of the 5-hydroxytryptamine2 receptor: structurally specific differentiation by ergolines and tryptamines. J Pharmacol Exp Ther 265: 1272–9. [PubMed] [Google Scholar]
- Nichols DE (2016) Psychedelics. Pharmacol Rev 68: 264–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols DE, Sassano MF, Halberstadt AL, Klein LM, Brandt SD, Elliott SP, Fiedler WJ (2015) N-Benzyl-5-methoxytryptamines as Potent Serotonin 5-HT2 Receptor Family Agonists and Comparison with a Series of Phenethylamine Analogues. ACS Chem Neurosci 6: 1165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odoardi S, Romolo FS, Strano-Rossi S (2016) A snapshot on NPS in Italy: Distribution of drugs in seized materials analysed in an Italian forensic laboratory in the period 2013–2015. Forensic Sci Int 265: 116–20. [DOI] [PubMed] [Google Scholar]
- Oksenberg D, Marsters SA, O’Dowd BF, Jin H, Havlik S, Peroutka SJ, Ashkenazi A (1992) A single amino-acid difference confers major pharmacological variation between human and rodent 5-HT1B receptors. Nature 360: 161–3. [DOI] [PubMed] [Google Scholar]
- Parker EM, Grisel DA, Iben LG, Shapiro RA (1993) A single amino acid difference accounts for the pharmacological distinctions between the rat and human 5-hydroxytryptamine1B receptors. J Neurochem 60: 380–3. [DOI] [PubMed] [Google Scholar]
- Preller KH, Herdener M, Pokorny T, Planzer A, Kraehenmann R, Stampfli P, Liechti ME, Seifritz E, Vollenweider FX (2017a) The Fabric of Meaning and Subjective Effects in LSD-Induced States Depend on Serotonin 2A Receptor Activation. Curr Biol 27: 451–457. [DOI] [PubMed] [Google Scholar]
- Preller KH, Herdener M, Pokorny T, Planzer A, Kraehenmann R, Stampfli P, Liechti ME, Seifritz E, Vollenweider FX (2017b) The Fabric of Meaning and Subjective Effects in LSD-Induced States Depend on Serotonin 2A Receptor Activation. Curr Biol. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Kometer M, Geyer MA, Vollenweider FX (2012) Psilocybin-induced deficits in automatic and controlled inhibition are attenuated by ketanserin in healthy human volunteers. Neuropsychopharmacology 37: 630–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasanen I, Kyber M, Szilvay I, Rintatalo J, Ojanpera I (2014) Straightforward single-calibrant quantification of seized designer drugs by liquid chromatography-chemiluminescence nitrogen detection. Forensic Sci Int 237: 119–25. [DOI] [PubMed] [Google Scholar]
- Rickli A, Moning OD, Hoener MC, Liechti ME (2016) Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. Eur Neuropsychopharmacol 26: 1327–37. [DOI] [PubMed] [Google Scholar]
- Roth BL (2013) National Institute of Mental Health Psychoactive Drug Screening Program (NIMH PDSP) Assay Protocol Book, Version II. Available online:https://pdspdb.unc.edu/pdspWeb/content/PDSP%20Protocols%20II%202013-03-28.pdf [Accessed: 06 May 2017]
- Salmi P, Ahlenius S (1998) Evidence for functional interactions between 5-HT1A and 5-HT2A receptors in rat thermoregulatory mechanisms. Pharmacol Toxicol 82: 122–7. [DOI] [PubMed] [Google Scholar]
- Schreiber R, Brocco M, Audinot V, Gobert A, Veiga S, Millan MJ (1995) (1-(2,5-dimethoxy-4 iodophenyl)-2-aminopropane)-induced head-twitches in the rat are mediated by 5-hydroxytryptamine (5-HT) 2A receptors: modulation by novel 5-HT2A/2C antagonists, D1 antagonists and 5-HT1A agonists. J Pharmacol Exp Ther 273: 101–12. [PubMed] [Google Scholar]
- Shulgin A, Shulgin A (1997) TIHKAL: the Continuation Transform Press, Berkeley [Google Scholar]
- Shulgin A, Shulgin A (2004) 5-MeO-DALT. Entry from a forthcoming book. Available online:https://www.erowid.org/chemicals/5meo_dalt/5meo_dalt_info1.shtml [Accessed: May 15, 2017]
- Smith DA, Bailey JM, Williams D, Fantegrossi WE (2014) Tolerance and cross-tolerance to head twitch behavior elicited by phenethylamine- and tryptamine-derived hallucinogens in mice. J Pharmacol Exp Ther 351: 485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolyar A, Osman R (1993) Role of threonine 342 in helix 7 of the 5-hydroxytryptamine type 1D receptor in ligand binding: an indirect mechanism for receptor selectivity. Mol Pharmacol 44: 882–5. [PubMed] [Google Scholar]
- Stevenson GI, Smith AL, Lewis S, Michie SG, Neduvelil JG, Patel S, Marwood R, Patel S, Castro JL (2000) 2-Aryl tryptamines: selective high-affinity antagonists for the h5-HT2A receptor. Bioorg Med Chem Lett 10: 2697–9. [DOI] [PubMed] [Google Scholar]
- Strano Rossi S, Odoardi S, Gregori A, Peluso G, Ripani L, Ortar G, Serpelloni G, Romolo FS (2014) An analytical approach to the forensic identification of different classes of new psychoactive substances (NPSs) in seized materials. Rapid Commun Mass Spectrom 28: 1904–16. [DOI] [PubMed] [Google Scholar]
- Strassman RJ (1996) Human psychopharmacology of N,N-dimethyltryptamine. Behav Brain Res 73: 1214. [DOI] [PubMed] [Google Scholar]
- Valle M, Maqueda AE, Rabella M, Rodriguez-Pujadas A, Antonijoan RM, Romero S, Alonso JF, Mananas MA, Barker S, Friedlander P, Feilding A, Riba J (2016) Inhibition of alpha oscillations through serotonin-2A receptor activation underlies the visual effects of ayahuasca in humans. Eur Neuropsychopharmacol 26: 1161–75. [DOI] [PubMed] [Google Scholar]
- Wacker D, Wang S, McCorvy JD, Betz RM, Venkatakrishnan AJ, Levit A, Lansu K, Schools ZL, Che T, Nichols DE, Shoichet BK, Dror RO, Roth BL (2017) Crystal Structure of an LSD-Bound Human Serotonin Receptor. Cell 168: 377–389 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willins DL, Meltzer HY (1997) Direct injection of 5-HT2A receptor agonists into the medial prefrontal cortex produces a head-twitch response in rats. J Pharmacol Exp Ther 282: 699–706. [PubMed] [Google Scholar]
- Young R, Rosecrans JA, Glennon RA (1982) Comparative discriminative stimulus effects of 5-methoxy-N,N-dimethyltryptamine and LSD. Life Sci 30: 2057–62. [DOI] [PubMed] [Google Scholar]


