Abstract
Purpose of Review
The objective of this review is to provide an update on the link between HIVinfection and cardiovascular disease (CVD). We will focus our review mainly on literature describing clinical CVD events and understudied topics of importance.
Recent Findings
Heart failure, peripheral artery disease, and stroke are CVD modalities deserving more attention in the context of HIV infection in the highly active antiretroviral therapy era. Incidence data on clinical CVD from HIV populations in low- and middle-income countries are limited. Multisubstance use is common in HIV, but understudied as a moderator or mediator of the association between HIV and CVD. CVD risk assessment in HIV remains challenging, but new research into novel biomarkers may provide further insights. There is also a need for inclusion of non-biologic factors in our attempts to understand, quantify, and predict CVD risk among PLWHA.
Summary
Significant attention has been paid to generating and testing hypotheses to understand the mechanisms of myocardial infarction in HIV. Similar attention is deserving for heart failure, PAD, stroke, and cardiovascular disease risk in resource-limited settings and among substance users with HIV.
Keywords: HIV, Cardiovascular disease, CVD, CVD risk, PLWHA, Review
Introduction
People living with HIV/AIDS (PLWHA) are living long enough to experience diseases of aging that are typical in the general population including cardiovascular disease. For example, multiple reports demonstrate that PLWHA have excess risk of coronary artery diseases including acute myocardial infarction. To a lesser extent, there are also reports that suggest that PLWHA also have an excess risk of ischemic stroke, heart failure, and peripheral artery disease. While the underlying mechanisms for these associations are not clear, recent research suggests that this excess risk may extend beyond traditional risk factors for atherosclerosis.
The objective of this review is to provide an update on the link between HIV infection and cardiovascular disease (CVD). We will focus our review mainly on literature describing clinical CVD events and understudied topics of importance (e.g., peripheral artery disease). We will not focus on atherosclerosis in general or the role of antiretroviral therapy in CVD but instead refer the reader to the following recent reviews, meta-analyses, and cohort studies [1–6]. Instead, we will use this opportunity to address CVD among PLWHA in the following under-represented research areas: low- and middle-income countries and substance use. Lastly, we will also discuss the state of CVD risk assessment in HIV and novel biomarkers that may inform our understanding of CVD risk mechanisms in HIV. See Appendix 1 for search methods.
Clinical CVD Events
Heart Failure
In the pre-HAART (highly active antiretroviral therapy) era, HIV-associated cardiomyopathy was predominantly driven by infection of the HIV virus or associated opportunistic pathogens infecting heart muscle and often resulted in myocarditis [7]. With access to HAART, the proportion of heart failure secondary to infective myocarditis is diminishing. However, more recent studies report that HIV infection remains a risk factor for heart failure even in the HAART era. Since there are settings with limited access to HAART (e.g., LMICs), the heart failure story in HIV is split into at least two parts—a concept recently reviewed by Lumsden and Bloomfield [8].
Several recent studies have examined the prevalence and incidence of and risk factors and prognosis for heart failure. In the Veterans Aging Cohort Study (VACS), we reported excess rates and risk of heart failure among PLWHA compared to uninfected people in age decades from < 40 up to < 70 years [9•]. Recent cross-sectional data suggest a 66% greater prevalence of heart failure diagnoses among PLWHA compared to uninfected people using data from a hospital database aggregator (Explorys) [10]. Other cross-sectional studies have shown over 2-fold increased odds of adjudicated heart failure among those with peak HIV viremia ≥ 100,000 copies/mL and nadir CD4 < 200 cells/mm3 [11]. Traditional risk factors for heart failure identified in the general population also increase risk among PLWHA, e.g., older age, black race, overweight, hypertension, smoking, and prior myocardial infarction. Additional risk factors that are particularly relevant among PLWHA include HIV viral replication, immunosuppression, and some antiretroviral therapy regimens, in addition to liver fibrosis and depression [12, 13]. Pulmonary artery hypertension related to HIV infection is prevalent (more so than idiopathic pulmonary artery hypertension) and may be another important driver of heart failure risk among PLWHA [14, 15].
Part of the changing epidemiology of heart failure in the HAART era includes a shift from a mainly dilated cardiomyopathy phenotype which may be thought of as reduced ejection fraction heart failure (HFrEF) to include both HFrEF and heart failure with preserved ejection fraction (HFpEF). Data regarding incidence of heart failure with preserved versus reduced ejection fraction (HFpEF vs. HFrEF) are sparse. We reported 21% increased risk of HFpEF and 37% increased risk of HFrEF in PLWHA compared to uninfected people [9•]. In the general population, HFpEF has mortality rates comparable with HFrEF, can be difficult to diagnose, and when diagnosed, can be difficult to treat [16–18]. This may lead to delayed care seeking among PLWHA who are already burdened by multimorbidity and polypharmacy [19, 20]. Additionally, it remains unclear how disparities in cardiovascular disease care contribute to excess heart failure risk, e.g., PLWHA are less likely to receive appropriate invasive management after myocardial infarction, a heart failure risk factor, compared to people without HIV [21, 22]. These disparities could be further exacerbated given recent reports of unequal access to advanced heart failure care like heart transplantation [23].
Future research in HIV and heart failure should focus on early detection of asymptomatic heart failure, appropriate intervention and risk reduction, and ways to mitigate health disparities in heart failure care among PLWHA.
Peripheral Artery Disease
As the spectrum of cardiovascular diseases continue to change in the HAART era, peripheral artery disease surfaces as an important understudied comorbidity. Lower extremity PAD follows coronary artery disease and stroke as the third leading cause of atherosclerotic cardiovascular disease in the general population [24]. PAD is common with aging and occurs when there is partial or complete blockage of one or more peripheral arteries most often in the lower extremities [25]. This leads to insufficient perfusion of peripheral arteries which is often asymptomatic but may lead to pain and increasing disability [26]. Many of the same conditions and behaviors that co-occur with PAD are drivers of atherosclerosis and occur with higher prevalence in HIV-infected populations, e.g., cigarette smoking, dyslipidemia, diabetes, hypertension, and chronic kidney disease [25, 26]. Given this potential convergence of risk factors and the aging of the HIV population, we highlight recent work characterizing PAD in the setting of HIV.
Most of these studies reflect cross-sectional designs and use the ankle brachial index (ABI) to determine the existence of PAD. ABI uses the ratio of systolic blood pressure between the ankle and arm to detect atherosclerosis in the leg (ABI below 0.9) [26]. The prevalence of PAD among HIV-infected people ranged from 2 to 27% [27–32]. This wide range was reflective of different definitions of PAD employed, e.g., altered ABI defined as ABI < 0.9 or ABI > 1.3 [33, 34], or requiring post exercise ABI < 0.9 [28, 35]. None of these have been validated in HIV, and there is controversy about interpreting ABI > 1.3 [36]. Variables associated with greater PAD prevalence in some but not all studies included male gender, dyslipidemia, older age, smoking, and CD4 count < 200 copies/mL [28, 33, 35, 37]. Only one of these studies showed HIV to be associated with lower ABI in adjusted models [38]. The VACS cohort has provided some of the limited recent longitudinal data in HIV excluding those with prevalent cardiovascular disease at baseline, and following participants till an incident PAD event defined using ICD-9 and CPT administrative codes [39]. Comparing demographically and behaviorally similar PLWHA and uninfected Veterans, we reported 30% increased risk of PAD associated with HIV status (presented at International Aids Society Conference, 2017; Abstract #4098). The relative risk of PAD was higher among PLWHA who were immunocompromised or had active viral replication.
Future PAD studies in the setting of HIV would benefit from longitudinal designs to estimate how quickly the PAD problem is growing and mechanistic studies to understand the role treated HIV infection plays in PAD prognosis.
Ischemic Stroke
There is a much better representation of longitudinal cohort studies for stroke than for PAD. Several of these have described an approximately 30% increased risk of ischemic stroke among HIV infected compared to uninfected people. A recent systematic review and meta-analysis on risk of vascular disease by HIV status reported similar unadjusted incidence rates of ischemic stroke by HIV status (1.08 [0.98–1.17] and 1.05 [0.98–1.12] events per 1000 person years for people with HIV compared to those without HIV) [40•]. Incidence rates in the USA were higher than those in Europe for both populations. No incidence rate estimates were available for low-income countries in this study. In adjusted models, stroke risk was consistently elevated in PLWHA compared to uninfected people (pooled risk ratio 1.27 [1.15, 1.39]). Several recent individual studies confirm this risk estimate including a population based study in Taiwan [41, 42, 43•].
Importantly, there is a small number of studies suggesting a reduction in the excess risk of ischemic stroke associated with HIV among PLWHA with preserved immune function and suppressed viremia [42, 43•, 44, 45]. Others have studied stroke etiology by HIV status and reported stroke secondary to large artery atherosclerosis and stroke of undetermined etiology to be predominant stroke subtypes among PLWHA [46, 47].
While traditional stroke risk factors (e.g., hypertension) certainly play a role in driving excess risk [41, 42, 43•], recent mechanistic studies in stroke among PLWHA provide interesting HIV-specific hypotheses. One study with potential mechanistic implications involves effects of HIV treatment on the blood brain barrier and subsequent effects on stroke severity [48•]. Earlier work has focused on gastrointestinal barrier dysfunction in HIV and its potential role in driving systemic inflammation and thus potentially, atherosclerosis [49, 50]. Whether similar mechanisms are at play with the blood brain barrier and cerebrovascular disease could be a fascinating area of research, which can leverage our understanding in the more accessible gut/periphery to make and investigate inferences about HIV in the nervous system.
Myocardial Infarction
Much of the recent work on myocardial infarctions in HIV is focused on improving our understanding of why PLWHA have higher risk for myocardial infarctions and what happens after the first myocardial infarction event. Three recent studies provide important context for progress in HIV/myocardial infarction research. A study within the Kaiser Permanente health system reported decreasing rate ratios (by HIV status) for myocardial infarction from 1996 till 2011. This suggests that the excess risk of myocardial infarction associated with HIV infection diminished over time, likely a reflection of improved HIV care and growing literature during that time regarding the association of HIV status with cardiovascular disease risk [51•]. We reported in VACS that despite a greater relative risk of myocardial infarction associated with HIV infection, PLWHA experience myocardial infarctions at approximately the same age as people without HIV [52•]. Given that myocardial infarction is a disease of aging, this study suggests that HIV is associated with accentuated aging (comorbidity risk is higher at all ages) rather than accelerated aging (comorbidities occur at younger ages). Data from the Center for AIDS Research Network of Integrated Clinical Systems (CNICS) cohort highlight the need to consider type of myocardial infarction. Type 1 (due to atherosclerotic plaque rapture) and type 2 (not due to atherosclerotic plaque rupture) myocardial infarction are prevalent among PLWHA, but their etiologies, risk factors, and therefore prevention and treatment strategies differ [53•]. Nonetheless, these data, as well as data from the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), confirm the elevated risk of type 1 myocardial infarction associated with HIV infection [54].
A series of studies focused on parsing out HIV-specific effects from traditional and other cardiovascular disease risk factor effects. Data from the Danish HIV Cohort Study assessed myocardial infarction risk by HIV status separately among never smokers and ever smokers, finding no significant association among never smokers but 78% increased risk associated with HIV infection among ever smokers [55]. They translated this finding into population attributable fractions suggesting that if all current smokers stopped smoking, 42% (95% CI 21–57%) and 21% (12–28%) of all myocardial infarctions could potentially be avoided among HIV infected and uninfected people, respectively. This contrasts with earlier analyses limited to never smokers that reported excess myocardial infarction risk persisted among PLWHA compared to uninfected people [56]. In the VACS, we grouped HIV infected and uninfected people by cardiovascular disease risk factor control and assessed myocardial infarction risk [57•]. Though very few people had optimal cardiac health, we still observed a 2-fold increased risk of myocardial infarction among those with HIV compared to uninfected people. However, absolute rates of myocardial infarction were much lower among those with optimal cardiovascular health compared to those with at least one major risk factor, suggesting that control of traditional cardiovascular disease risk factors is critical to reducing absolute risk in HIV. The finding of low prevalence of optimal cardiac health was consistent with recent data from a Mediterranean cohort [58].
There has been recent interest in recurrence of and morbidity and mortality after myocardial infarction. Data from the Data Collection on Adverse Drug Events (D:A:D) study indicates that the proportion of PLWHA dying from a CVD cause after their initial myocardial infarction decreased from 73% in 1999–2002 to 41% in 2011–2014 [59]. They conclude that short-term survival improvements following a myocardial infarction are primarily due to better management of CVD risk factors in PLWHA who have an incident myocardial infarction. US hospital discharge data indicate that PLWHA had higher mortality after admission for myocardial infarction or stroke only among those with a prior AIDS diagnosis [44]. The inference here being that maintaining immune function (e.g., with new test and treat strategies) may improve cardiovascular outcomes in HIV populations. This was consistent with other work pooling data from the USA, Europe, and South Africa [60]. In contrast, a study linking myocardial infarction registry data with data from the Swiss HIV Cohort found that well-controlled HIV infection was still associated with increased risk of death 1 year after incident myocardial infarction adjusting for age, sex, year of myocardial infarction, smoking, hypertension, and diabetes (HR 4.42 [95% CI 1.73–11.27]) [61]. This study did not find an increased risk of recurrent myocardial infarctions or hospitalization rates among HIV-infected people though others have reported disparities in guideline-recommended cardiovascular care among PLWHA compared to uninfected people [62].
Mechanistic studies comparing vascular health by HIV status among people with similar levels of coronary artery disease provide insight as to how HIV may be driving excess risk of clinical events and raises important questions. In men with acute coronary syndromes (i.e., myocardial infarction or high-risk chest pain), there was no difference in the total number of diseased coronary vessels by HIV status; however, HIV infection was associated with lower Gensini scores indicating lower disease severity [63]. Gensini scoring provides a sense of disease severity by taking into account both the amount of stenosis (blockage) in vessels and the location of stenosis in the coronary artery tree (e.g., stenosis in the left main artery indicates higher severity than stenosis in less critical distal vessels). From these findings, the authors conclude, as have earlier studies, that plaque vulnerability (i.e., likelihood that the clot causing vessel narrowing breaks off and completely blocks the vessel) may be driving more of the risk of clinical events than quantity of plaque. Among patients who had similar coronary artery disease burden and were undergoing percutaneous coronary intervention, PLWHAwere more likely to have myocardial scarring after myocardial infarction compared to uninfected patients [64•]. This raises important questions about whether myocardial cell death is more severe during a myocardial infarction in a PLWHA compared to an uninfected person and whether this explains excess risk of heart failure and sudden cardiac death [65].
Myocardial infarction has received a lot of attention in the context of cardiovascular comorbidities in HIV. While the exact mechanisms driving excess myocardial infarction risk among PLWHA are being elucidated, there is sufficient evidence for intervening on traditional CVD risk factors while suppressing HIV viremia and maintaining CD4 T levels with combination antiretroviral therapy. An important next step is implementation of science studies to figure out optimal strategies to successfully execute such interventions among PLWHA and their care providers.
CVD and HIV in Special Populations
Low and Middle-Income Countries
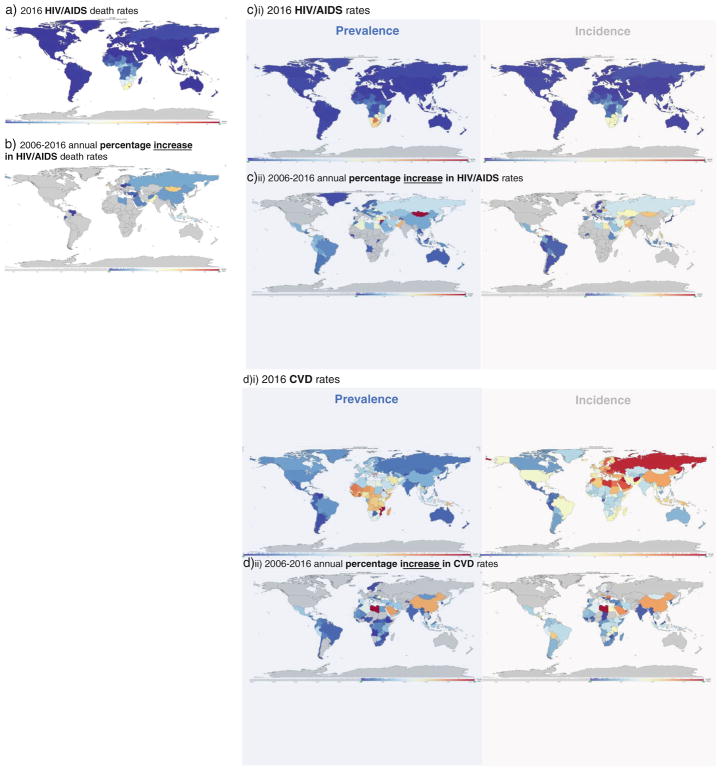
In this section, we focus on recent knowledge gained in understanding the cardiovascular disease story among HIV populations in low- and middle-income countries. Many of these regions (e.g., sub-Saharan Africa) host the majority of HIV prevalence (Fig. 1), have a growing burden of cardiovascular disease (Fig. 1), and lack the infrastructure to manage non-AIDS comorbidities. Thus, there is rationale for leveraging knowledge and, where feasible, resources from the HIV chronic care context in these settings, extending them to reduce cardiovascular disease risk in HIV, and subsequently extending these efforts to non-HIV populations.
Fig. 1.
a 2016 HIV/AIDS death rates. b 2006–2016 annual percentage increase in HIV/AIDS death rates, c)i) 2016 prevalence and incidence rates of HIV/AIDS; c)ii) 2006–2016 annual percentage increase in prevalence and incidence rates of HIV/AIDS d)i) 2016 prevalence and incidence rates of CVD; d)ii) 2006–2016 annual percentage increase in prevalence and incidence rates of CVD. Institute for Health Metrics and Evaluation (IHME). GBD Compare Data Visualization. Seattle, WA: IHME, University of Washington, 2016. Available from https://vizhub.healthdata.org/gbd-compare/. (Accessed December 4, 2016)
As described previously, cardiovascular disease in the absence of HAART is primarily driven by infective etiologies and present as pericardial effusion, infective myocarditis, and subsequent systolic heart failure. In the presence of HAART, PLWHA live long enough to establish atherosclerotic lesions, develop coronary artery disease and acute coronary syndromes (e.g., myocardial infarction), and suffer from subsequent heart failure.
Most of the recent studies we identified focus on prevalence of cardiovascular disease risk factors with particular attention paid to hypertension. Prevalence tends to be high of risk factors like dyslipidemia, hypertension, obesity, tobacco use, and malnutrition [66•, 67–80]. In contrast, knowledge about and awareness of these risk factors and their relationship with cardiovascular disease tends to be low, which impedes linkage to cardiovascular care where available [66•, 74]. Several studies describe cardiometabolic disturbances associated with certain antiretroviral therapies in low- and middle-income countries [81, 82]. However, given patients’ perceptions of their most pressing health needs (stress [53%], AIDS [17%], and depression [14%], heart disease [1%]) [66•], and that access to HAART is not universal in these settings, careful consideration is required when prioritizing and implementing CVD risk reduction strategies in these settings.
The second-largest area of focus was subclinical atherosclerosis. Feinstein et al. confirmed that ideal cardiac health, infrequent in their Ugandan HIV cohort, was associated with lower carotid intima-media thickness [83]. Siedner et al. reported twice the prevalence of arterial stiffness defined as ABI > 1.2 in Ugandans with HIV compared to uninfected Ugandans [84]. Ssinabulya et al. characterized carotid intima media thickness (cIMT), an ultrasound-based measure of early atherosclerotic changes in arterial walls, among 186 Ugandans with HIV [85]. Using a cIMT cutoff of ≥ 0.78 mm to define the presence of subclinical atherosclerosis, they found that 18% of their sample met this criterion. Median cIMT was not reported. Schoffelen et al. characterized cIMT among 866 South Africans with HIV [86]. Median for mean CIMT for women was 0.579 mm [95% CI 0.517–0.663] and for men was 0.609 [0.547–0.745]. Based on the cross-sectional age/cIMT distribution in this study, the authors estimated that this South African sample would reach a threshold cIMT value of 0.78 mm at least 10 years earlier than would have been expected in a reference group of healthy Dutch people. Both of these cIMT studies confirmed that traditional cardiovascular disease risk factors (age, obesity, total cholesterol) were associated with higher cIMT among African PLWHA.
We did not find any recent longitudinal studies reporting incidence of clinical cardiovascular disease events in low- and middle-income countries. At least two recently described cohort studies are set to start enrollment and may provide some of this epidemiological incidence data—the Ndlovu Cohort Study [87••] and the EndoAfrica Study [88••] in South Africa. The REPRIEVE clinical trial (Evaluating the Use of Pitavastatin to Reduce the Risk of Cardiovascular Disease in HIV-Infected Adults) has sites in southern Africa, Latin America, the Caribbean, India, and Thailand. This trial may have the ability to describe cardiovascular disease event incidence in HIV populations across multiple income settings as has been done with prevalence data from the START (Strategic Timing of AntiRetroviral Treatment) trial [89]. Other potential approaches involve estimation of incidence at the population level from cross-sectional prevalence data. This approach has been used in Africa to estimate HIV incidence and is being investigated to estimate cardiovascular disease incidence in high-income countries [90]. Assuming growing availability of the necessary inputs for this kind of modeling (longitudinal prevalence data, demographic change data, and mortality data), it may be possible to apply such methods to estimate cardiovascular disease incidence at the population level in low- and middle-income countries.
Case control studies thus provide best estimates of association of HIV and clinical events in these settings. A study from Malawi found distinct ischemic stroke etiology and features by HIV status [47]. Compared to HIV-uninfected people with stroke, PLWHA who had a stroke had higher prevalence of large artery disease and basal ganglia ischemia. Another study from Malawi among 222 adults with acute stroke and 504 non-stroke controls identified HIV as the predominant risk factor for stroke among participants ≤ 45 years [91]. A small pilot study comparing ≥ 35-year-old women with isolated right heart failure to similarly aged controls reported an adjusted odds ratio for HIV status of 40 [95% CI 3.7–441], i.e., women with heart failure had a 40-fold greater odds of having HIV compared to women without heart failure—note: wide confidence intervals reflect small sample size of 31 cases and 65 controls [92•]. A cross-sectional echocardiographic study of cardiac structure and function among children (mean 8 years) reported increased prevalence of left ventricular systolic dysfunction among those with HIV compared to those without HIV [93]. Left ventricular systolic dysfunction was defined as a fractional shortening ≤ 28% or ejection fraction < 40% with otherwise normal left ventricular dimensions. A separate study of Ugandan children estimated prevalence of left ventricular systolic dysfunction at 7% with a further 7% displaying electrocardiogram abnormalities [94]. A systematic review and meta-analysis that attempted to estimate incidence and prevalence of pulmonary hypertension among African adults with HIV did not find any incidence data but estimated pulmonary hypertension prevalence at 14% [95% CI 6–23%] [95].
Low- and middle-income countries bear majority of the burden of HIV prevalence and a large and growing burden of cardiovascular disease and cardiovascular disease risk factors (Fig. 1). Better estimates of cardiovascular disease incidence are needed to appropriately allocate resources for the growing double burden of HIVand CVD in low- and middle-income countries (Fig. 1).
Substance Users
The only substance of abuse included in cardiovascular disease risk prediction scores is tobacco, and recent studies have confirmed smoking as a risk factor for atherosclerosis in HIV [96]. However, multisubstance use is common among PLWHA [97], and these substances may have direct effects on cardiovascular disease risk or modify the effect of HIV status on cardiovascular disease risk.
The association between heavy alcohol use and cardiovascular events has been studied extensively in the general population but less so among PLWHA. A meta-analysis between 1999 and 2014 found only 13 eligible studies and estimated that heavy alcohol use was associated with 37% [95% CI 1.02–1.84] increased risk of cardiovascular disease compared to non-heavy use [98]. Recent longitudinal data from the Women’s Interagency HIV Study (WIHS) and the Multicenter AIDS Cohort Study (MACS) did not find an association of heavy alcohol use and incident atherosclerosis [99]. A prior cross-sectional study from MACS found an association between heavy alcohol use and the presence of coronary artery stenosis with no significant association for low/moderate drinking among PLWHA [96].
Other studies have focused on illicit substances. MACS reported 20% prevalence of heavy marijuana use (daily/weekly use) and linked heavy marijuana use to incident cardiovascular disease events independently of cigarette smoking [100]. In the general population, cocaine has been linked to acute coronary events. Among predominantly African American PLWHA, current or past cocaine use was associated with higher prevalence of carotid plaque but not carotid plaque progression, cIMT, or change in cIMT over 3 years [101]. The Johns Hopkins HIV Clinical Cohort investigated incident HIV-associated non-AIDS comorbidities including cardiovascular disease by history of injection drug use. They did not detect a significant difference by injection drug use status on the risk of stroke or myocardial infarction accounting for competing risk of death. However, no information was provided regarding the specific substances being injected [102].
Given the current opioid overdose epidemic, future studies should assess the impact of opioid misuse on cardiovascular disease risk in the context of HIV infection.
CVD Risk Assessment and Novel CVD Risk Factors in HIV
Risk Assessment
Since earlier studies suggested that the existing cardiovascular disease risk scores may inaccurately estimate the risk of clinical events in HIV population, more recent publications have attempted to confirm and extend this work [103, 104, 105•, 106–112]. There remains conflicting data about how well Framingham and other non-HIV-specific algorithms predict cardiovascular disease risk with some studies showing good discrimination or calibration [104, 106] and others, less so [105•, 106, 107, 109, 110]. The D:A:D cardiovascular disease risk score was developed using data from PLWHA and has recently been updated to facilitate use in everyday clinical practice [113•]. Given the role of lipids in estimating cardiovascular disease risk, studies of HIV co-morbidities that further perturb lipid levels and independently contribute to cardiovascular disease risk (e.g., hepatitis C) are relevant in the discussion of cardiovascular disease risk prediction in HIV [111].
Novel CVD Risk Biomarkers in HIV
Recent studies have looked beyond traditional cardiovascular disease risk factors in an attempt to explain the excess cardiovascular disease risk observed in HIV. These novel biomarkers span immunology, the intestinal microbiome [114, 115], red blood cells [116], and en-dothelial function [117]. The approaches being used are diverse and include imaging [118, 119], proteomics [120•], genomics [121, 122], metagenomics and metabolomics [123], and assessment of soluble and cellular proteins [124–130].
One of the central hypotheses driving these approaches is that HIV infection and its treatment, even with successful viral suppression, increases intestinal permeability and bacterial translocation, increases systemic inflammation, alters coagulation pathways, drives abnormal vascular function, and promotes plaque formation and subsequent destabilization all contributing to increased risk for ischemic and non-ischemic cardiovascular disease. Additional hypotheses focus on morbidity that co-occurs with HIV ranging from viral hepatitis to depression, which can modify the risk of cardiovascular disease among PLWHA.
Of equal relevance as these biologically focused mechanisms are societal and environmental factors that often do not make it into cardiovascular disease prediction models. These include issues ranging from polypharmacy associated with managing multiple chronic diseases (e.g., HIV and heart failure) [131], healthcare disparities associated with HIV status that impact cardiovascular disease prevention and care [21–23], economic disparities that impact exposure to environmental pollution [92•], and clinical cardiovascular guidelines that may not account for HIV status when designating high-risk populations that could benefit from strategies beyond usual care.
Conclusions
In a prior review on HIV and cardiovascular disease, we described a conceptual model linking biological and environmental factors to increased cardiovascular disease risk in PLWHA [132]. With this review, we highlight heart failure, peripheral artery disease, and stroke as modalities deserving more attention as the epidemiology of cardiovascular disease evolves in the HAART era. We also provide an update on myocardial infarction in HIV, suggesting implementation science work on risk reduction as a relevant next step. We identify lack of incidence data as a gap in HIV/cardiovascular disease work from low- and middle-income countries and propose research into substance use including opioids and cardiovascular disease risk in HIV. Cardiovascular disease risk assessment in HIV remains challenging, but new research into novel biomarkers may provide further insights. There is also a need for inclusion of non-biologic factors in our attempts to understand, quantify, and predict cardiovascular disease risk among PLWHA.
Appendix
Methods
We searched PubMed for relevant papers in English published between January 01, 2014 and October 31, 2017. For peripheral artery disease, we relaxed the time requirement given the dearth of available studies.
Footnotes
Compliance with Ethical Standards
Conflict of Interest Both authors received NIH grants.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
This article is part of the Topical Collection on Complications of Antiretroviral Therapy
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Stein JH, Currier JS, Hsue PY. Arterial disease in patients with human immunodeficiency virus infection: what has imaging taught us? JACC Cardiovasc Imaging. 2014;7(5):515–25. doi: 10.1016/j.jcmg.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun D, Wu Y, Yuan Y, Wang Y, Liu W, Yang J. Is the atheroscle-rotic process accentuated under conditions of HIV infection, anti-retroviral therapy, and protease inhibitor exposure? Meta-analysis of the markers of arterial structure and function. Atherosclerosis. 2015;242(1):109–16. doi: 10.1016/j.atherosclerosis.2015.06.059. [DOI] [PubMed] [Google Scholar]
- 3.Kearns A, Gordon J, Burdo TH, Qin X. HIV-1-associated atherosclerosis: Unraveling the Missing Link. J Am Coll Cardiol. 2017;69(25):3084–98. doi: 10.1016/j.jacc.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Ascenzo F, Cerrato E, Calcagno A, Grossomarra W, Ballocca F, Omede P, et al. High prevalence at computed coronary tomography of non-calcified plaques in asymptomatic HIV patients treated with HAART: a meta-analysis. Atherosclerosis. 2015;240(1):197–204. doi: 10.1016/j.atherosclerosis.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Dorjee K, Baxi SM, Reingold AL, Hubbard A. Risk of cardiovascular events from current, recent, and cumulative exposure to abacavir among persons living with HIV who were receiving an-tiretroviral therapy in the United States: a cohort study. BMC Infect Dis. 2017;17(1):708. doi: 10.1186/s12879-017-2808-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabin CA, Reiss P, Ryom L, Phillips AN, Weber R, Law M, et al. Is there continued evidence for an association between abacavir usage and myocardial infarction risk in individuals with HIV? A cohort collaboration. BMC Med. 2016;14:61. doi: 10.1186/s12916-016-0588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pugliese A, Isnardi D, Saini A, Scarabelli T, Raddino R, Torre D. Impact of highly active antiretroviral therapy in HIV-positive patients with cardiac involvement. J Inf Secur. 2000;40(3):282–4. doi: 10.1053/jinf.2000.0672. [DOI] [PubMed] [Google Scholar]
- 8.Lumsden RH, Bloomfield GS. The causes of HIV-associated car-diomyopathy: a tale of two worlds. Biomed Res Int. 2016;2016:8196560. doi: 10.1155/2016/8196560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9•.Freiberg MS, Chang CH, Skanderson M, Patterson OV, DuVall SL, Brandt CA, et al. Association between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the veterans aging cohort study. JAMA Cardiol. 2017;2(5):536–46. doi: 10.1001/jamacardio.2017.0264. Documents independent association of HIV infection with incident heart failure (including type heart failure) in the combination ART era using a behaviorally and demographically similar comparator group of uninfected people. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Kindi SG, ElAmm C, Ginwalla M, Mehanna E, Zacharias M, Benatti R, et al. Heart failure in patients with human immunodeficiency virus infection: epidemiology and management disparities. Int J Cardiol. 2016;218:43–6. doi: 10.1016/j.ijcard.2016.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steverson AB, Pawlowski AE, Schneider D, Nannapaneni P, Sanders JM, Achenbach CJ, et al. Clinical characteristics of HIV-infected patients with adjudicated heart failure. Eur J Prev Cardiol. 2017;24(16):1746–58. doi: 10.1177/2047487317732432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.So-Armah KA, Lim JK, Lo Re V, Tate JP, Chung-Chou HC, Butt AA, et al. FIB-4 stage of liver fibrosis predicts incident heart failure among HIV infected and uninfected patients. Hepatology. 2017;66:1286–95. doi: 10.1002/hep.29285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White JR, Chang CC, So-Armah KA, Stewart JC, Gupta SK, Butt AA, et al. Depression and human immunodeficiency virus infection are risk factors for incident heart failure among veterans: veterans aging cohort study. Circulation. 2015;132(17):1630–8. doi: 10.1161/CIRCULATIONAHA.114.014443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwarze-Zander C, Pabst S, Hammerstingl C, Ohlig J, Wasmuth JC, Boesecke C, et al. Pulmonary hypertension in HIV infection: a prospective echocardiographic study. HIV Med. 2015;16(9):578–82. doi: 10.1111/hiv.12261. [DOI] [PubMed] [Google Scholar]
- 15.Brittain EL, Duncan MS, Chang J, Patterson OV, DuVall SL, Brandt CA, et al. Increased echocardiographic pulmonary pressure in HIV-infected and uninfected individuals in the veterans aging cohort study. Am J Respir Crit Care Med. 2017 doi: 10.1164/rccm.201708-1555OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 17.Lourenco AP, Leite-Moreira AF, Balligand JL, Bauersachs J, Dawson D, de Boer RA, et al. An integrative translational approach to study heart failure with preserved ejection fraction: a position paper from the Working Group on Myocardial Function of the European Society of Cardiology. Eur J Heart Fail. 2017 doi: 10.1002/ejhf.1059. [DOI] [PubMed] [Google Scholar]
- 18.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383–92. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 19.Edelman EJ, Gordon KS, Glover J, McNicholl IR, Fiellin DA, Justice AC. The next therapeutic challenge in HIV: polypharmacy. Drugs Aging. 2013;30(8):613–28. doi: 10.1007/s40266-013-0093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.High KP, Brennan-Ing M, Clifford DB, Cohen MH, Currier J, Deeks SG, et al. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr. 2012;60(Suppl 1):S1–18. doi: 10.1097/QAI.0b013e31825a3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smilowitz NR, Gupta N, Guo Y, Coppola JT, Bangalore S. Influence of human immunodeficiency virus seropositive status on the in-hospital management and outcomes of patients presenting with acute myocardial infarction. J Invasive Cardiol. 2016;28(10):403–9. [PubMed] [Google Scholar]
- 22.Singh V, Mendirichaga R, Savani GT, Rodriguez AP, Dabas N, Munagala A, et al. Coronary revascularization for acute myocardial infarction in the HIV population. J Interv Cardiol. 2017;30(5):405–14. doi: 10.1111/joic.12433. [DOI] [PubMed] [Google Scholar]
- 23.Uriel N, Nahumi N, Colombo PC, Yuzefpolskaya M, Restaino SW, Han J, et al. Advanced heart failure in patients infected with human immunodeficiency virus: is there equal access to care? J Heart Lung Transplant. 2014;33(9):924–30. doi: 10.1016/j.healun.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 24.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382(9901):1329–40. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 25.McDermott MM. Lower extremity manifestations of peripheral artery disease: the pathophysiologic and functional implications of leg ischemia. Circ Res. 2015;116(9):1540–50. doi: 10.1161/CIRCRESAHA.114.303517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116(9):1509–26. doi: 10.1161/CIRCRESAHA.116.303849. [DOI] [PubMed] [Google Scholar]
- 27.Knudsen A, Malmberg CA, Kjaer A, Lebech AM. Low prevalence of peripheral arterial disease in a cross-sectional study of Danish HIV-infected patients. Infect Dis (Lond) 2015;47(11):776–82. doi: 10.3109/23744235.2015.1061204. [DOI] [PubMed] [Google Scholar]
- 28.Qaqa AY, DeBari VA, Isbitan A, Mohammad N, Sison R, Slim J, et al. The role of postexercise measurements in the diagnosis of peripheral arterial disease in HIV-infected patients. Angiology. 2011;62(1):10–4. doi: 10.1177/0003319710385339. [DOI] [PubMed] [Google Scholar]
- 29.Sharma A, Holman S, Pitts R, Minkoff HL, Dehovitz JA, Lazar J. Peripheral arterial disease in HIV-infected and uninfected women. HIV Med. 2007;8(8):555–60. doi: 10.1111/j.1468-1293.2007.00509.x. [DOI] [PubMed] [Google Scholar]
- 30.Qaqa AY, Debari VA, El-Kersh K, Sison R, Isbitan A, Mohammad N, et al. Epidemiologic aspects of abnormal ankle brachial index in the HIV infected population. Int Angiol. 2012;31(3):227–33. [PubMed] [Google Scholar]
- 31.Johns K, Saeedi R, Mancini GB, Bondy G. Ankle brachial index screening for occult vascular disease is not useful in HIV-positive patients. AIDS Res Hum Retrovir. 2010;26(9):955–9. doi: 10.1089/aid.2009.0275. [DOI] [PubMed] [Google Scholar]
- 32.Bernal E, Masia M, Padilla S, Hernandez I, Gutierrez F. Low prevalence of peripheral arterial disease in HIV-infected patients with multiple cardiovascular risk factors. J Acquir Immune Defic Syndr. 2008;47(1):126–7. doi: 10.1097/QAI.0b013e318157b0b3. [DOI] [PubMed] [Google Scholar]
- 33.Olalla J, Salas D, Del Arco A, De la Torre J, Prada J, Machin-Hamalainen S, et al. Ankle-branch index and HIV: the role of antiretrovirals. HIV Med. 2009;10(1):1–5. doi: 10.1111/j.1468-1293.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- 34.Kwiatkowska W, Knysz B, Arczynska K, Drelichowska J, Czarnecki M, Gasiorowski J, et al. Peripheral arterial disease and ankle-brachial index abnormalites in young and middle-aged HIV-positive patients in lower Silesia, Poland. PLoS One. 2014;9(12):e113857. doi: 10.1371/journal.pone.0113857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Periard D, Cavassini M, Taffe P, Chevalley M, Senn L, Chapuis-Taillard C, et al. High prevalence of peripheral arterial disease in HIV-infected persons. Clin Infect Dis. 2008;46(5):761–7. doi: 10.1086/527564. [DOI] [PubMed] [Google Scholar]
- 36.Gutierrez F, Bernal E, Masia M. Considerations on ankle-brachial index interpretation in HIV-1 infected patients. HIV Med. 2009;10(6):395. doi: 10.1111/j.1468-1293.2009.00732.x. author reply 395–396. [DOI] [PubMed] [Google Scholar]
- 37.Palacios R, Alonso I, Hidalgo A, Aguilar I, Sanchez MA, Valdivielso P, et al. Peripheral arterial disease in HIV patients older than 50 years of age. AIDS Res Hum Retrovir. 2008;24(8):1043–6. doi: 10.1089/aid.2008.0001. [DOI] [PubMed] [Google Scholar]
- 38.Ye Y, Zeng Y, Li X, Zhang S, Fang Q, Luo L, et al. HIV infection: an independent risk factor of peripheral arterial disease. J Acquir Immune Defic Syndr. 2010;53(2):276–8. doi: 10.1097/QAI.0b013e3181ba1c31. [DOI] [PubMed] [Google Scholar]
- 39.Bali V, Yermilov I, Coutts K, Legorreta AP. Novel screening metric for the identification of at-risk peripheral artery disease patients using administrative claims data. Vasc Med. 2016;21(1):33–40. doi: 10.1177/1358863X15616687. [DOI] [PubMed] [Google Scholar]
- 40•.Gutierrez J, Albuquerque ALA, Falzon L. HIV infection as vascular risk: a systematic review of the literature and meta-analysis. PLoS One. 2017;12(5):e0176686. doi: 10.1371/journal.pone.0176686. Provides recent global estimates of rates/risk for multiple CVD etiologies from larger longitudinal studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yen YF, Chen M, Jen I, Lan YC, Chuang PH, Liu YL, et al. Association of HIV and opportunistic infections with incident stroke: a nationwide population-based cohort study in Taiwan. J Acquir Immune Defic Syndr. 2017;74(2):117–25. doi: 10.1097/QAI.0000000000001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sico JJ, Chang CC, So-Armah K, Justice AC, Hylek E, Skanderson M, et al. HIV status and the risk of ischemic stroke among men. Neurology. 2015;84(19):1933–40. doi: 10.1212/WNL.0000000000001560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•.Marcus JL, Leyden WA, Chao CR, Chow FC, Horberg MA, Hurley LB, et al. HIV infection and incidence of ischemic stroke. AIDS. 2014;28(13):1911–9. doi: 10.1097/QAD.0000000000000352. Provides important data on secular trends in stroke incidence rates showing differences differ by HIV status and age group. [DOI] [PubMed] [Google Scholar]
- 44.Okeke NL, Hicks CB, McKellar MS, Fowler VG, Jr, Federspiel JJ. History of AIDS in HIV-infected patients is associated with higher in-hospital mortality following admission for acute myocardial infarction and stroke. J Infect Dis. 2016;213(12):1955–61. doi: 10.1093/infdis/jiw082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chow FC, Bacchetti P, Kim AS, Price RW, Hsue PY. Effect of CD4+ cell count and viral suppression on risk of ischemic stroke in HIV infection. AIDS. 2014;28(17):2573–7. doi: 10.1097/QAD.0000000000000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chow FC, Price RW, Hsue PY, Kim AS. Greater risk of stroke of undetermined etiology in a contemporary HIV-infected cohort compared with uninfected individuals. J Stroke Cerebrovasc Dis: Off J Natl Stroke Assoc. 2017;26(5):1154–60. doi: 10.1016/j.jstrokecerebrovasdis.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benjamin LA, Allain TJ, Mzinganjira H, Connor MD, Smith C, Lucas S, et al. The role of human immunodeficiency virus-associated vasculopathy in the etiology of stroke. J Infect Dis. 2017;216(5):545–53. doi: 10.1093/infdis/jix340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48•.Bertrand L, Dygert L, Toborek M. Antiretroviral treatment with Efavirenz disrupts the blood-brain barrier integrity and increases stroke severity. Sci Rep. 2016;6:39738. doi: 10.1038/srep39738. Pre-clinical study that can and should complement existing exiting work on effects of HIV infection on gastrointestinal barrier function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 50.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 51•.Klein DB, Leyden WA, Xu L, Chao CR, Horberg MA, Towner WJ, et al. Declining relative risk for myocardial infarction among HIV-positive compared with HIV-negative individuals with access to care. Clin Infect Dis. 2015;60(8):1278–80. doi: 10.1093/cid/civ014. Provides important data on secular trends in myocardial infarction incidence rates showing differences differ by HIV status. [DOI] [PubMed] [Google Scholar]
- 52•.Althoff KN, McGinnis KA, Wyatt CM, Freiberg MS, Gilbert C, Oursler KK, et al. Comparison of risk and age at diagnosis of myocardial infarction, end-stage renal disease, and non-AIDS-defining cancer in HIV-infected versus uninfected adults. Clin Infect Dis. 2015;60(4):627–38. doi: 10.1093/cid/ciu869. Provides data to test a frequently repeated theory that HIV accelerates aging and thus diseases common with aging like myocardial infarction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53•.Crane HM, Paramsothy P, Drozd DR, Nance RM, Delaney JA, Heckbert SR, et al. Types of myocardial infarction among human immunodeficiency virus-infected individuals in the United States. JAMA Cardiol. 2017;2(3):260–7. doi: 10.1001/jamacardio.2016.5139. Provides increased specificity for describing the association between HIV status and myo-cardial infarction by differentiating types of myocardial infaraction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Drozd DR, Kitahata MM, Althoff KN, Zhang J, Gange SJ, Napravnik S, et al. Increased risk of myocardial infarction in HIV-infected individuals in North America compared with the general population. J Acquir Immune Defic Syndr. 2017;75(5):568–76. doi: 10.1097/QAI.0000000000001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rasmussen LD, Helleberg M, May MT, Afzal S, Kronborg G, Larsen CS, et al. Myocardial infarction among Danish HIV-infected individuals: population-attributable fractions associated with smoking. Clin Infect Dis. 2015;60(9):1415–23. doi: 10.1093/cid/civ013. [DOI] [PubMed] [Google Scholar]
- 56.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8):614–22. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57•.Paisible AL, Chang CC, So-Armah KA, Butt AA, Leaf DA, Budoff M, et al. HIV infection, cardiovascular disease risk factor profile, and risk for acute myocardial infarction. J Acquir Immune Defic Syndr. 2015;68(2):209–16. doi: 10.1097/QAI.0000000000000419. Provides data enabling comparison of contribution of HIV status versus traditional cardiovascular disease risk factors to absolute cardiovascular disease risk while also demonstrating that even with control of cardiovascular disease risk factors, relative risk of myocardial infarction remains elevated in PLWHA compared to un-infected people. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Echeverria P, Domingo P, Llibre JM, Gutierrez M, Mateo G, Puig J, et al. Prevalence of ischemic heart disease and management of coronary risk in daily clinical practice: results from a Mediterranean cohort of HIV-infected patients. Biomed Res Int. 2014;2014:823058. doi: 10.1155/2014/823058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hatleberg CI, Ryom L, El-Sadr W, Smith C, Weber R, Reiss P, et al. Improvements over time in short-term mortality following myocardial infarction in HIV-positive individuals. AIDS. 2016;30(10):1583–96. doi: 10.1097/QAD.0000000000001076. [DOI] [PubMed] [Google Scholar]
- 60.D’Ascenzo F, Cerrato E, Appleton D, Moretti C, Calcagno A, Abouzaki N, et al. Prognostic indicators for recurrent thrombotic events in HIV-infected patients with acute coronary syndromes: use of registry data from 12 sites in Europe, South Africa and the United States. Thromb Res. 2014;134(3):558–64. doi: 10.1016/j.thromres.2014.05.037. [DOI] [PubMed] [Google Scholar]
- 61.Carballo D, Delhumeau C, Carballo S, Bahler C, Radovanovic D, Hirschel B, et al. Increased mortality after a first myocardial infarction in human immunodeficiency virus-infected patients; a nested cohort study. AIDS Res Ther. 2015;12:4. doi: 10.1186/s12981-015-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ladapo JA, Richards AK, DeWitt CM, Harawa NT, Shoptaw S, Cunningham WE, et al. Disparities in the quality of cardiovascular care between HIV-infected versus HIV-uninfected adults in the United States: a cross-sectional study. J Am Heart Assoc. 2017;6(11):e007107. doi: 10.1161/JAHA.117.007107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O’Dwyer EJ, Bhamra-Ariza P, Rao S, Emmanuel S, Carr A, Holloway CJ. Lower coronary plaque burden in patients with HIV presenting with acute coronary syndrome. Open Heart. 2016;3(2):e000511. doi: 10.1136/openhrt-2016-000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64•.Feinstein MJ, Mitter SS, Yadlapati A, Achenbach CJ, Palella FJ, Jr, Gonzalez PE, et al. HIV-related myocardial vulnerability to infarction and coronary artery disease. J Am Coll Cardiol. 2016;68(18):2026–7. doi: 10.1016/j.jacc.2016.07.771. Though a small pilot study, provides interesting mechanistic insight into why prognosis after a myocardial infarction may be worse among PLWHA compared to uninfected people. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tseng ZH, Secemsky EA, Dowdy D, Vittinghoff E, Moyers B, Wong JK, et al. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol. 2012;59(21):1891–6. doi: 10.1016/j.jacc.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66•.Temu TM, Kirui N, Wanjalla C, Ndungu AM, Kamano JH, Inui TS, et al. Cardiovascular health knowledge and preventive practices in people living with HIV in Kenya. BMC Infect Dis. 2015;15:421. doi: 10.1186/s12879-015-1157-8. Provides data indicating cardiovascular disease risk knowledge and prioritization among PLWHA is low in Kenya, an example of a setting with high HIV prevalence, high cardiovascular disease risk factor prevalence, and constrained resources to tackle a double burden of HIV and CVD simultaneously. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rodriguez-Arboli E, Mwamelo K, Kalinjuma AV, Furrer H, Hatz C, Tanner M, et al. Incidence and risk factors for hypertension among HIV patients in rural Tanzania—a prospective cohort study. PLoS One. 2017;12(3):e0172089. doi: 10.1371/journal.pone.0172089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Naidu S, Ponnampalvanar S, Kamaruzzaman SB, Kamarulzaman A. Prevalence of metabolic syndrome among people living with HIV in developing countries: a systematic review. AIDS Patient Care STDs. 2017;31(1):1–13. doi: 10.1089/apc.2016.0140. [DOI] [PubMed] [Google Scholar]
- 69.Divala OH, Amberbir A, Ismail Z, Beyene T, Garone D, Pfaff C, et al. The burden of hypertension, diabetes mellitus, and cardiovascular risk factors among adult Malawians in HIV care: consequences for integrated services. BMC Public Health. 2016;16(1):1243. doi: 10.1186/s12889-016-3916-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Angkurawaranon C, Nitsch D, Larke N, Rehman AM, Smeeth L, Addo J. Ecological study of HIV infection and hypertension in sub-Saharan Africa: is there a double burden of disease? PLoS One. 2016;11(11):e0166375. doi: 10.1371/journal.pone.0166375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kwarisiima D, Balzer L, Heller D, Kotwani P, Chamie G, Clark T, et al. Population-based assessment of hypertension epidemiology and risk factors among HIV-positive and general populations in rural Uganda. PLoS One. 2016;11(5):e0156309. doi: 10.1371/journal.pone.0156309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kingery JR, Alfred Y, Smart LR, Nash E, Todd J, Naguib MR, et al. Short-term and long-term cardiovascular risk, metabolic syndrome and HIV in Tanzania. Heart. 2016;102(15):1200–5. doi: 10.1136/heartjnl-2015-309026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dimala CA, Atashili J, Mbuagbaw JC, Wilfred A, Monekosso GL. Prevalence of hypertension in HIV/AIDS patients on highly active antiretroviral therapy (HAART) compared with HAART-naive patients at the Limbe regional hospital, Cameroon. PLoS One. 2016;11(2):e0148100. doi: 10.1371/journal.pone.0148100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Naanyu V, Vedanthan R, Kamano JH, Rotich JK, Lagat KK, Kiptoo P, et al. Barriers influencing linkage to hypertension care in Kenya: qualitative analysis from the LARK hypertension study. J Gen Intern Med. 2016;31(3):304–14. doi: 10.1007/s11606-015-3566-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mashinya F, Alberts M, Van Geertruyden JP, Colebunders R. Assessment of cardiovascular risk factors in people with HIV infection treated with ART in rural South Africa: a cross sectional study. AIDS Res Ther. 2015;12:42. doi: 10.1186/s12981-015-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Muyanja D, Muzoora C, Muyingo A, Muyindike W, Siedner MJ. High prevalence of metabolic syndrome and cardiovascular disease risk among people with HIV on stable ART in southwestern Uganda. AIDS Patient Care STDs. 2016;30(1):4–10. doi: 10.1089/apc.2015.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eholie SP, Lacombe K, Krain A, Diallo Z, Ouiminga M, Campa P, et al. Metabolic disorders and cardiovascular risk in treatment-naive HIV-infected patients of sub-saharan origin starting antiretrovirals: impact of westernized lifestyle. AIDS Res Hum Retrovir. 2015;31(4):384–92. doi: 10.1089/AID.2014.0164. [DOI] [PubMed] [Google Scholar]
- 78.Sander LD, Newell K, Ssebbowa P, Serwadda D, Quinn TC, Gray RH, et al. Hypertension, cardiovascular risk factors and antihyper-tensive medication utilisation among HIV-infected individuals in Rakai, Uganda. Tropical Med Int Health. 2015;20(3):391–6. doi: 10.1111/tmi.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Osegbe ID, Soriyan OO, Ogbenna AA, Okpara HC, Azinge EC. Risk factors and assessment for cardiovascular disease among HIV-positive patients attending a Nigerian tertiary hospital. Pan Afr Med J. 2016;23:206. doi: 10.11604/pamj.2016.23.206.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Menanga AP, Ngomseu CK, Jingi AM, Mfangam BM, Noubiap JJ, Gweth MN, et al. Patterns of cardiovascular disease in a group of HIV-infected adults in Yaounde, Cameroon. Cardiovasc Diagn Ther. 2015;5(6):420–7. doi: 10.3978/j.issn.2223-3652.2015.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Botha S, Fourie CM, van Rooyen JM, Kruger A, Schutte AE. Cardiometabolic changes in treated versus never treated HIV-infected black South Africans: the PURE study. Heart Lung Circ. 2014;23(2):119–26. doi: 10.1016/j.hlc.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 82.Shaffer D, Hughes MD, Sawe F, Bao Y, Moses A, Hogg E, et al. Cardiovascular disease risk factors in HIV-infected women after initiation of lopinavir/ritonavir- and nevirapine-based antiretroviral therapy in sub-Saharan Africa: A5208 (OCTANE) J Acquir Immune Defic Syndr. 2014;66(2):155–63. doi: 10.1097/QAI.0000000000000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feinstein MJ, Kim JH, Bibangambah P, Sentongo R, Martin JN, Tsai AC, et al. Ideal cardiovascular health and carotid atherosclerosis in a mixed cohort of HIV-infected and uninfected Ugandans. AIDS Res Hum Retrovir. 2017;33(1):49–56. doi: 10.1089/aid.2016.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Siedner MJ, Kim JH, Nakku RS, Hemphill L, Triant VA, Haberer JE, et al. HIV infection and arterial stiffness among older-adults taking antiretroviral therapy in rural Uganda. AIDS. 2016;30(4):667–70. doi: 10.1097/QAD.0000000000000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ssinabulya I, Kayima J, Longenecker C, Luwedde M, Semitala F, Kambugu A, et al. Subclinical atherosclerosis among HIV-infected adults attending HIV/AIDS care at two large ambulatory HIV clinics in Uganda. PLoS One. 2014;9(2):e89537. doi: 10.1371/journal.pone.0089537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schoffelen AF, de Groot E, Tempelman HA, Visseren FL, Hoepelman AI, Barth RE. Carotid intima media thickness in mainly female HIV-infected subjects in rural South Africa: association with cardiovascular but not HIV-related factors. Clin Infect Dis. 2015;61(10):1606–14. doi: 10.1093/cid/civ586. [DOI] [PubMed] [Google Scholar]
- 87••.Vos A, Tempelman H, Deville W, Barth R, Wensing A, Kretzschmar M, et al. HIV and risk of cardiovascular disease in sub-Saharan Africa: rationale and design of the Ndlovu Cohort Study. Eur J Prev Cardiol. 2017;24(10):1043–50. doi: 10.1177/2047487317702039. Will provide much needed prospective, longitudinal cardiovascular disease incidence data in regions of the world with some of the highest HIV burden. [DOI] [PubMed] [Google Scholar]
- 88••.Strijdom H, De Boever P, Walzl G, Essop MF, Nawrot TS, Webster I, et al. Cardiovascular risk and endothelial function in people living with HIV/AIDS: design of the multi-site, longitudinal EndoAfrica study in the Western Cape Province of South Africa. BMC Infect Dis. 2017;17(1):41. doi: 10.1186/s12879-016-2158-y. Will provide much needed prospective, longitudinal cardiovascular disease incidence data in regions of the world with some of the highest HIV burden. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Soliman EZ, Sharma S, Arasteh K, Wohl D, Achhra A, Tambussi G, et al. Baseline cardiovascular risk in the INSIGHT Strategic Timing of AntiRetroviral Treatment (START) trial. HIV Med. 2015;16(Suppl 1):46–54. doi: 10.1111/hiv.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hu XF, Young K, Chan HM. Estimating cardiovascular disease incidence from prevalence: a spreadsheet based model. BMC Med Res Methodol. 2017;17(1):9. doi: 10.1186/s12874-016-0288-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Benjamin LA, Corbett EL, Connor MD, Mzinganjira H, Kampondeni S, Choko A, et al. HIV, antiretroviral treatment, hypertension, and stroke in Malawian adults: a case-control study. Neurology. 2016;86(4):324–33. doi: 10.1212/WNL.0000000000002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92•.Lagat DK, DeLong AK, Wellenius GA, Carter EJ, Bloomfield GS, Velazquez EJ, et al. Factors associated with isolated right heart failure in women: a pilot study from western Kenya. Glob Heart. 2014;9(2):249–54. doi: 10.1016/j.gheart.2014.04.003. Identifies and highlights important cardiovascular disease risk factors that do not typically get factored into conceptual models of HIV-related cardiovascular disease risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Arodiwe I, Ikefuna A, Obidike E, Arodiwe E, Anisuba B, Ibeziako N, et al. Left ventricular systolic function in Nigerian children infected with HIV/AIDS: a cross-sectional study. Cardiovasc J Afr. 2016;27(1):25–9. doi: 10.5830/CVJA-2015-066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Namuyonga J, Lubega S, Musiime V, Lwabi P, Lubega I. Cardiac dysfunction among Ugandan HIV-infected children on antiretro-viral therapy. Pediatr Infect Dis J. 2016;35(3):e85–8. doi: 10.1097/INF.0000000000000997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bigna JJ, Nansseu JR, Um LN, Noumegni SR, Sime PS, Aminde LN, et al. Prevalence and incidence of pulmonary hypertension among HIV-infected people in Africa: a systematic review and meta-analysis. BMJ Open. 2016;6(8):e011921. doi: 10.1136/bmjopen-2016-011921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kelly SG, Plankey M, Post WS, Li X, Stall R, Jacobson LP, et al. Associations between tobacco, alcohol, and drug use with coronary artery plaque among HIV-infected and uninfected men in the multicenter AIDS cohort study. PLoS One. 2016;11(1):e0147822. doi: 10.1371/journal.pone.0147822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Green TC, Kershaw T, Lin H, Heimer R, Goulet JL, Kraemer KL, et al. Patterns of drug use and abuse among aging adults with and without HIV: a latent class analysis of a US veteran cohort. Drug Alcohol Depend. 2010;110(3):208–20. doi: 10.1016/j.drugalcdep.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kelso NE, Sheps DS, Cook RL. The association between alcohol use and cardiovascular disease among people living with HIV: a systematic review. Am J Drug Alcohol Abuse. 2015;41(6):479–88. doi: 10.3109/00952990.2015.1058812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kelso-Chichetto NE, Plankey M, Sheps DS, Abraham AG, Chen X, Shoptaw S, et al. The impact of long-term moderate and heavy alcohol consumption on incident atherosclerosis among persons living with HIV. Drug Alcohol Depend. 2017;181:235–41. doi: 10.1016/j.drugalcdep.2017.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lorenz DR, Dutta A, Mukerji SS, Holman A, Uno H, Gabuzda D. Marijuana use impacts midlife cardiovascular events in HIV-infected men. Clin Infect Dis. 2017;65(4):626–35. doi: 10.1093/cid/cix391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lucas GM, Atta MG, Fine DM, McFall AM, Estrella MM, Zook K, et al. HIV, cocaine use, and hepatitis C virus: a triad of nontra-ditional risk factors for subclinical cardiovascular disease. Arterioscler Thromb Vasc Biol. 2016;36(10):2100–7. doi: 10.1161/ATVBAHA.116.307985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lesko CR, Moore RD, Tong W, Lau B. Association of injection drug use with incidence of HIV-associated non-AIDS-related morbidity by age, 1995–2014. AIDS. 2016;30(9):1447–55. doi: 10.1097/QAD.0000000000001087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pinto Neto L, Dias FR, Bressan FF, Santos CRO. Comparison of the ACC/AHA and Framingham algorithms to assess cardiovascular risk in HIV-infected patients. Braz J Infect Dis. 2017;21(6):577–80. doi: 10.1016/j.bjid.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.De Socio GV, Pucci G, Baldelli F, Schillaci G. Observed versus predicted cardiovascular events and all-cause death in HIV infection: a longitudinal cohort study. BMC Infect Dis. 2017;17(1):414. doi: 10.1186/s12879-017-2510-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105•.Feinstein MJ, Nance RM, Drozd DR, Ning H, Delaney JA, Heckbert SR, et al. Assessing and refining myocardial infarction risk estimation among patients with human immunodeficiency virus: a study by the centers for AIDS research network of integrated clinical systems. JAMA Cardiol. 2017;2(2):155–62. doi: 10.1001/jamacardio.2016.4494. Results of this study raises the question of whether we should be looking beyond HIV viremia, CD4 cell count, antiretroviral therapy and traditional cardiovascular disease risk factors in tailoring cardiovascular disease risk prediction for PLWHA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thompson-Paul AM, Lichtenstein KA, Armon C, Palella FJ, Jr, Skarbinski J, Chmiel JS, et al. Cardiovascular disease risk prediction in the HIVoutpatient study. Clin Infect Dis. 2016;63(11):1508–16. doi: 10.1093/cid/ciw615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Raggi P, De Francesco D, Manicardi M, Zona S, Bellasi A, Stentarelli C, et al. Prediction of hard cardiovascular events in HIV patients. J Antimicrob Chemother. 2016;71(12):3515–8. doi: 10.1093/jac/dkw346. [DOI] [PubMed] [Google Scholar]
- 108.Salinas JL, Rentsch C, Marconi VC, Tate J, Budoff M, Butt AA, et al. Baseline, time-updated, and cumulative HIV care metrics for predicting acute myocardial infarction and all-cause mortality. Clin Infect Dis. 2016;63(11):1423–30. doi: 10.1093/cid/ciw564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Herrera S, Guelar A, Sorli L, Vila J, Molas E, Grau M, et al. The Framingham function overestimates the risk of ischemic heart disease in HIV-infected patients from Barcelona. HIV Clin Trials. 2016;17(4):131–9. doi: 10.1080/15284336.2016.1177266. [DOI] [PubMed] [Google Scholar]
- 110.Krikke M, Hoogeveen RC, Hoepelman AI, Visseren FL, Arends JE. Cardiovascular risk prediction in HIV-infected patients: comparing the Framingham, atherosclerotic cardiovascular disease risk score (ASCVD), Systematic Coronary Risk Evaluation for the Netherlands (SCORE-NL) and Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) risk prediction models. HIV Med. 2016;17(4):289–97. doi: 10.1111/hiv.12300. [DOI] [PubMed] [Google Scholar]
- 111.Chew KW, Bhattacharya D, McGinnis KA, Horwich TB, Tseng CH, Currier JS, et al. Short communication: coronary heart disease risk by Framingham risk score in hepatitis C and HIV/hepatitis C-coinfected persons. AIDS Res Hum Retrovir. 2015;31(7):718–22. doi: 10.1089/aid.2014.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Markowicz S, Delforge M, Necsoi C, De Wit S. Cardiovascular risk evaluation of HIV-positive patients in a case-control study: comparison of the D:A:D and Framingham equations. J Int AIDS Soc. 2014;17(4 Suppl 3):19515. doi: 10.7448/IAS.17.4.19515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113•.Friis-Moller N, Ryom L, Smith C, Weber R, Reiss P, Dabis F, et al. An updated prediction model of the global risk of cardiovascular disease in HIV-positive persons: the Data-collection on Adverse Effects of Anti-HIV Drugs (D:A:D) study. Eur J Prev Cardiol. 2016;23(2):214–23. doi: 10.1177/2047487315579291. Provides an update on a cardiovascular disease risk prediction model built using data from PLWHA. [DOI] [PubMed] [Google Scholar]
- 114.Haissman JM, Knudsen A, Hoel H, Kjaer A, Kristoffersen US, Berge RK, et al. Microbiota-dependent marker TMAO is elevated in silent ischemia but is not associated with first-time myocardial infarction in HIV infection. J Acquir Immune Defic Syndr. 2016;71(2):130–6. doi: 10.1097/QAI.0000000000000843. [DOI] [PubMed] [Google Scholar]
- 115.Srinivasa S, Fitch KV, Lo J, Kadar H, Knight R, Wong K, et al. Plaque burden in HIV-infected patients is associated with serum intestinal microbiota-generated trimethylamine. AIDS. 2015;29(4):443–52. doi: 10.1097/QAD.0000000000000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Al-Kindi SG, Kim CH, Morris SR, Freeman ML, Funderburg NT, Rodriguez B, et al. Brief report: elevated red cell distribution width identifies elevated cardiovascular disease risk in patients with HIV infection. J Acquir Immune Defic Syndr. 2017;74(3):298–302. doi: 10.1097/QAI.0000000000001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Haissman JM, Haugaard AK, Knudsen A, Kristoffersen US, Seljeflot I, Pedersen KK, et al. Marker of endothelial dysfunction asymmetric dimethylarginine is elevated in HIV infection but not associated with subclinical atherosclerosis. J Acquir Immune Defic Syndr. 2016;73(5):507–13. doi: 10.1097/QAI.0000000000001148. [DOI] [PubMed] [Google Scholar]
- 118.Zanni MV, Toribio M, Wilks MQ, Lu MT, Burdo TH, Walker J, et al. Application of a novel CD206+ macrophage-specific arterial imaging strategy in HIV-infected individuals. J Infect Dis. 2017;215(8):1264–9. doi: 10.1093/infdis/jix095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Knudsen A, Hag AM, Loft A, von Benzon E, Keller SH, Moller HJ, et al. HIV infection and arterial inflammation assessed by (18)F-fluorodeoxyglucose (FDG) positron emission tomography (PET): a prospective cross-sectional study. J Nucl Cardiol. 2015;22(2):372–80. doi: 10.1007/s12350-014-0032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120•.Rasheed S, Hashim R, Yan JS. Possible biomarkers for the early detection of HIV-associated heart diseases: a proteomics and bioinformatics prediction. Comput Struct Biotechnol J. 2015;13:145–52. doi: 10.1016/j.csbj.2015.02.001. Provides an example of how proteomics and bioinformatics can be used to provide new insights into how HIV contributes to cardiovascular disease risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yong YK, Shankar EM, Westhorpe CL, Maisa A, Spelman T, Kamarulzaman A, et al. Genetic polymorphisms in the CD14 gene are associated with monocyte activation and carotid intima-media thickness in HIV-infected patients on antiretroviral therapy. Medicine (Baltimore) 2016;95(31):e4477. doi: 10.1097/MD.0000000000004477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shendre A, Irvin MR, Aouizerat BE, Wiener HW, Vazquez AI, Anastos K, et al. RYR3 gene variants in subclinical atherosclerosis among HIV-infected women in the Women’s Interagency HIV Study (WIHS) Atherosclerosis. 2014;233(2):666–72. doi: 10.1016/j.atherosclerosis.2014.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.El-Far M, Tremblay CL. Gut microbial diversity in HIV infection post combined antiretroviral therapy: a key target for prevention of cardiovascular disease. Curr Opin HIV AIDS. 2017 doi: 10.1097/COH.0000000000000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dirajlal-Fargo S, Sattar A, Kulkarni M, Funderburg N, McComsey GA. Soluble TWEAK may predict carotid atherosclerosis in treated HIV infection. HIV Clin Trials. 2017;18(4):156–63. doi: 10.1080/15284336.2017.1366001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vos AG, Idris NS, Barth RE, Klipstein-Grobusch K, Grobbee DE. Pro-inflammatory markers in relation to cardiovascular disease in HIV infection. A systematic review. PLoS One. 2016;11(1):e0147484. doi: 10.1371/journal.pone.0147484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vos AG, Hulzebosch A, Grobbee DE, Barth RE, Klipstein-Grobusch K. Association between immune markers and surrogate markers of cardiovascular disease in HIV positive patients: a systematic review. PLoS One. 2017;12(1):e0169986. doi: 10.1371/journal.pone.0169986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Secemsky EA, Scherzer R, Nitta E, Wu AH, Lange DC, Deeks SG, et al. Novel biomarkers of cardiac stress, cardiovascular dysfunction, and outcomes in HIV-infected individuals. JACC Heart Fail. 2015;3(8):591–9. doi: 10.1016/j.jchf.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rasmussen LJ, Knudsen A, Katzenstein TL, Gerstoft J, Obel N, Jorgensen NR, et al. Soluble urokinase plasminogen activator receptor (suPAR) is a novel, independent predictive marker of myo-cardial infarction in HIV-1-infected patients: a nested case-control study. HIV Med. 2016;17(5):350–7. doi: 10.1111/hiv.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bellasi A, Raggi P, Rossi R, Rochira V, Stentarelli C, Zona S, et al. Intact parathyroid hormone levels are associated with increased carotid intima media thickness in HIV infected patients. Atherosclerosis. 2014;237(2):618–22. doi: 10.1016/j.atherosclerosis.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 130.Knudsen A, Katzenstein TL, Benfield T, Jorgensen NR, Kronborg G, Gerstoft J, et al. Plasma plasminogen activator inhibitor-1 predicts myocardial infarction in HIV-1-infected individuals. AIDS. 2014;28(8):1171–9. doi: 10.1097/QAD.0000000000000247. [DOI] [PubMed] [Google Scholar]
- 131.Smith JM, Flexner C. The challenge of polypharmacy in an aging population and implications for future antiretroviral therapy development. AIDS. 2017;31(Suppl 2):S173–84. doi: 10.1097/QAD.0000000000001401. [DOI] [PubMed] [Google Scholar]
- 132.So-Armah K, Freiberg MS. Cardiovascular disease risk in an aging HIV population: not just a question of biology. Curr Opin HIV AIDS. 2014;9(4):346–54. doi: 10.1097/COH.0000000000000065. [DOI] [PMC free article] [PubMed] [Google Scholar]