Abstract
Theory of mind (ToM) encompasses a range of abilities that show different developmental time courses. However, relatively little work has examined the neural correlates of ToM during early childhood. In this study, we investigated the neural correlates of ToM in typically developing children aged 4–8 years using resting-state functional magnetic resonance imaging. We calculated whole brain functional connectivity with the right temporo-parietal junction (RTPJ), a core region involved in ToM, and examined its relation to children’s early, basic, and advanced components of ToM competence assessed by a parent-report measure. Total ToM and both basic and advanced ToM components, but not early, consistently showed a positive correlation with connectivity between RTPJ and posterior cingulate cortex/precuneus; advanced ToM was also correlated with RTPJ to left TPJ connectivity. However, early and advanced ToM components showed negative correlation with the right inferior/superior parietal lobe, suggesting that RTPJ network differentiation is also related to ToM abilities. We confirmed and extended these results using a Bayesian modeling approach demonstrating significant relations between multiple nodes of the mentalizing network and ToM abilities, with no evidence for differences in relations between ToM components. Our data provide new insights into the neural correlates of multiple aspects of ToM in early childhood and may have implications for both typical and atypical development of ToM.
Keywords: theory of mind, resting-state fMRI, functional connectivity, right temporo-parietal junction, young children
Introduction
The ability to understand other people’s minds is crucial in everyday life and plays a key role in successful interactions with others. Theory of mind (ToM) is a multifaceted construct, which encompasses a variety of components, such as, inferring emotions and intentions, mental representations, reasoning about beliefs, and making pragmatic inferences, to name a few (for a review, see Schaafsma et al., 2015). These aspects of ToM may show a consistent developmental progression (Peterson et al., 2005; Wellman and Liu, 2004). For example, children at 1–2 years of age are already able to engage in joint attention and implicitly represent others mental states, but more complex representations such as explicitly reporting on other’s false beliefs are seen after 4 years of age (Gweon and Saxe, 2013). Similarly, children accurately report on other’s desires before beliefs and on diverse beliefs before false beliefs (Wellman & Liu, 2004). Further, ToM continues to develop into later childhood as children advance in their ability to make pragmatic inferences, social judgments, and recursively represent belief states (Bosacki and Astington, 1999; Miller, 2012).
A consistent pattern of brain regions, termed the “ mentalizing network” are engaged in a variety of tasks relevant to ToM reasoning including the bilateral temporo-parietal junction (LTPJ and RTPJ), posterior cingulate cortex/precuneus (PCC), and medial prefrontal cortex (mPFC). Many of these regions overlap with regions associated with mental state attribution, self- and other-related processing, and socio-affective processing (for reviews, see Carrington and Bailey, 2009; Frith and Frith, 2003; Gweon and Saxe, 2013; Molenberghs et al., 2016; Schilbach et al., 2012; Schurz et al., 2014; Van Overwalle, 2009) and are part of the default mode network (DMN) (Amft et al., 2015; Schilbach et al., 2012; Spreng et al., 2009). The DMN was originally identified as regions demonstrating deactivation during task processing and thus referred to as a “task-negative” network (Raichle et al., 2001; Fox et al., 2005; Shulman et al., 1997; Sridharan et al., 2008), but research has shown that DMN regions are also engaged during social tasks (for a review, see Mars et al., 2012). Although these regions are engaged across various types of social tasks, there is specificity within nodes of this network (Molenberghs et al., 2016). For example, some studies have found that the more lateral nodes are associated with knowledge of others’ mental states whereas the midline regions are more associated with affective or motivational components of ToM (e.g., Koster-Hale et al., 2017; Sebastian et al., 2012). Within lateral regions, the RTPJ has been shown to be selectively engaged for the mental representation of others’ beliefs, intentions, and desires (Aichhorn et al., 2009; Perner et al., 2006; Saxe and Kanwisher, 2003; Saxe and Wexler, 2005; Saxe and Powell, 2006; Sommer et al., 2007). The mPFC and PCC, on the other hand, may play a more general role in ToM-relevant processes such as processing traits of one’s self and others and affective processing (Moran et al., 2006; Saxe et al., 2006; for reviews, see Amft et al., 2015; Amodio and Frith, 2006; Schilbach et al., 2012). Further, within mPFC there is a distinction between a more ventral region (ventral mPFC, vmPFC) associated with affect and self-related processing and a more dorsal region (dorsal mPFC, dmPFC) associated with both emotion and social cognition (Schilbach et al., 2012). Taken together, evidence primarily from adults suggests that distinct brain regions support the processing of different aspects of ToM, with the RTPJ playing a key role in representational ToM. These distinct regions may also play different roles in the emergence of those components of ToM.
To date, most of the ToM-related neuroimaging studies have been conducted with adults, in which brain activation in response to specific experimental tasks is examined, and little research has investigated the neural correlates of ToM in developing children (see Bowman et al., 2018; Gweon et al., 2012; Kobayashi et al., 2007; Richardson et al., 2018; Sabbagh et al., 2009; Saxe et al., 2009). Kobayashi et al. (2007) compared 8- to 12-year-old children to adults and reported age-related changes in the bilateral TPJ for ToM understanding in both verbal and non-verbal false belief tasks, showing the engagement of the TPJ in ToM throughout childhood. In children aged 6–11, greater activation was seen in the RTPJ (as well as LTPJ and PCC) for thinking about people’s thoughts than for physical and social facts about people, suggesting selectivity of these regions for mental state reasoning (Saxe et al., 2009). Moreover, this selectivity to mental compared to social facts within RTPJ increased with age (from 5 to 11 years) and was related to behavioral performance on ToM (Saxe et al., 2009; Gweon et al., 2012). Further, a recent fMRI study demonstrated that brain regions associated with physical pain and mental states are already functionally segregated by 3 years of age and this functional segregation increases with age and ToM ability (Richardson et al., 2018). In addition, a previous electroencephalogram (EEG) study with 4-year-old children has linked the RTPJ and mPFC to explicit, representational ToM (Sabbagh et al., 2009). A follow-up study with a subsample of children from Sabbagh et al. (2009) demonstrated that EEG alpha coherence within dmPFC and ToM abilities at 4 years of age predicted dmPFC specialization for ToM at 7 to 8 years (Bowman et al., 2018).
Taken together previous studies in children suggest continuity of the ToM network, and specifically dMPFC and RTPJ, in mental state reasoning from early childhood through preadolescence; however, some limitations are worthy of note. First, many previous studies relied on tasks to assess specific aspects of ToM (e.g., belief representation) (but see Richardson et al., 2018). However, ToM is a multifaceted construct (for a review, see Schaafsma et al., 2015) that comprises multiple different components (e.g., emotion perception and processing, face/gaze processing, joint attention, self-reference, mental states inferences), which may show different developmental time courses (Hutchins et al., 2012). Thus, focusing on specific tasks doesn’t allow for a comprehensive understanding of the brain correlates of ToM nor the emergence of various facets of ToM within the typically developing brain. Second, the few studies that have used neuroimaging to investigate the neural correlates of ToM in early childhood mostly rely on EEG (e.g., Sabbagh et al., 2009), which lacks the spatial resolution of fMRI. The one study that has used fMRI during early childhood focused on correlations within and between the networks as a whole and their relationships with ToM development while children viewed a movie (Richardson et al., 2018). Nevertheless, it remains unknown how connectivity between specific brain regions within the ToM network is associated with the development of ToM, particularly early, basic, and advanced aspects of ToM.
To close this gap, in the present study, we utilized the resting-state functional magnetic resonance imaging (rs-fMRI) technique, which allows for the study of intrinsic brain networks devoid of any explicit tasks in very young children (e.g., Brauer et al., 2016; Riggins et al., 2016; Xiao et al., 2016; Vanderwal et al., 2015). We explored how connectivity between nodes of the ToM network at rest relates to different components of ToM in typically developing young children aged 4–8 years, a key period in ToM development (Hogrefe et al., 1986; Wellman et al., 2001; Wimmer and Perner, 1983; for a review, see Gweon and Saxe, 2013). Moreover, we used a parent-report ToM measure (i.e., Theory of Mind Inventory, ToMI) (Hutchins et al., 2012) which offers an evaluation of multiple developmental aspects of ToM in young children. Specifically, factor 3, referred to as early ToM, is the ToM competence that emerges in typical development during infancy and toddlerhood and reflects reading affect and sharing attention of others; factor 2, referred to as basic ToM, is relevant to metarepresentation and developmentally related understanding, emerging around age 4 years; and factor 1, referred to as advanced ToM, has more complex social functions, including complex recursion (e.g., second-order belief) and advanced metalinguistic understanding, emerging during 6 and 8 years. These distinct ToM components with different developmental milestones likely relate to different brain activity patterns. However, little is known about the brain correlates of these early, basic, and advanced components of ToM in the developing brain.
We selected the RTPJ as a region of interest for functional connectivity analysis given its role in representational aspects of ToM, which are developing throughout early childhood (Gweon et al., 2012; Saxe et al., 2009). Notably, RTPJ comprises regions surrounding temporal and parietal lobes and has been shown to be involved in multiple cognitive functions, such as social cognition, language, attention, to name a few (for a review, see Carter and Huettel, 2013). In order to locate the RTPJ associated with social cognition, ToM in particular, we used the seed coordinates provided by previous meta-analyses (Amft et al., 2015; Schilbach et al., 2012).
Consistent with previous work, we hypothesized that overall ToM as well as basic and advanced components of ToM would develop significantly from ages 4–8 years but not the early component of ToM since it emerges at a younger age and might be well-established by the age of 4 years. Second, we would expect that connections between RTPJ and ToM-relevant regions change with age as a function of gradual development in ToM performance by performing an exploratory whole-brain correlation analysis. Third, we tested the following two hypotheses by examining the relations between the RTPJ connectivity and the ToM components (including the overall ToM). 1) There are distinct but partially overlapping systems contributing to ToM behaviors, so we would expect to see different connectivity patterns associated with different developmental components of ToM. 2) There is a common underlying neural substrate across diverse types of ToM abilities during development despite the different developmental time courses of those components. For that, we expected no differences between the relation between RTPJ connectivity and the three ToM components.
Materials and Methods
Participants
We recruited a total of 200 children aged 4–8 years (100 males; mean age 6.29 ± 1.49 years, range 4 to 8.94 years) from local families to participate in a large study on cognitive and brain development. All children completed a battery of behavioral measures, EEG, and an MRI session, but only data from the ToMI measurement, digit span working memory assessment, intelligence test, and MRI scans are included in this report. We excluded 76 children for the following reasons: 25 children did not have rs-fMRI scan; 3 children fell into sleep during rs-fMRI scan; 42 children did not have or did not complete the ToMI measurement; 1 child did not have IQ data and 1 child didn’t have verbal IQ subtest data; 12 children had head motion beyond the criteria (see head motion section below for detailed descriptions). In the final sample, we included 124 children (54 males; mean age 6.61 ± 1.41 years, range 4 to 8.93 years) who contributed both usable rs-fMRI data and behavioral measurements; Fig. 1A depicts the age distribution of children in the study. Prior to participation, all children’s parents gave written assent; in addition, children aged 4–6 years gave verbal assent and children aged 7–8 years gave written assent for participation. All children were fluent English speakers with no history of neurological, medical, or psychological disorders. The study was approved by the University of Maryland Institutional Review Board.
Fig. 1.

Age distribution of participants in the present dataset (A) and the correlation between age and head motion (mean FD). The scatter plot shows the correlation between age and head motion which is not significant (r(122) = 0.008, p = 0.93). The red line indicates the relationship between age and head motion.
Behavioral Measures
Theory of mind inventory
ToMI is an evaluation of caregiver’s perception of children’s ToM competence (Hutchins et al., 2012). Although reported by caregivers, ToMI is highly correlated with child performance on ToM tasks (Hutchins et al., 2012). And, as a parent-report measure, ToMI can assess explicit ToM abilities while avoiding task effects related to different language and cognitive skills while still being appropriate for children of this age range. This measure was decomposed into 3 main factors (i.e., factors 1–3), corresponding to advanced, basic, and early components based on previous work (Hutchins et al., 2012). The mean scores of all 42 items were considered as total performance, and we calculated average subscale scores, which were considered as the performance for the corresponding component. Out of a total of 42 items in the measure used here, 5, 17, and 14 items were used for early, basic, and advanced components, respectively (https://www.theoryofmindinventory.com/).
IQ assessment
Children’s IQ was assessed by two subtests of the Wechsler Intelligence assessment, i.e., visuo-spatial and verbal IQ. Children aged 4–5 years performed Wechsler Preschool and Primary Scale of Intelligence (WPPSI) and children aged 7–8 years carried out Wechsler Intelligence Scale for Children (WISC). Children aged 6 years performed either WPPSI or WISC. We used the scaled scores on both subtests and used the average to index children’s IQ performance. This assessment was included as a measure of general cognitive ability to be used as a covariate in the brain-behavior correlation analysis.
Working memory assessment
Children’s working memory was assessed via digit span that is similar to the digit span test in NEPSY-II (Korkman et al., 2007). Children were asked to recall a series of numbers that an experimenter read to them. Before the test, a practice was done with two numbers per list. A total of four sets of numbers were comprised each level and the child was required to pass at least two of the four sets to move to a higher level, which would increase in number by one. The percent correct on the task out of all 24 possible number sets was recorded for each child as their working memory performance. This measure was included since working memory has been shown to be associated with ToM ability in developing children (Arslan et al., 2017; Mutter et al., 2006; Davis and Pratt, 1995), and so it was taken into account as a covariate in the brain-behavior correlation analysis.
Data acquisition
MRI data were collected with a 12-channel coil on a Siemens 3.0-T scanner (MAGNETOM Trio Tim System, Siemens Medical Solutions, Erlangen, Germany). Prior to data acquisition, children completed training in a mock scanner to help them become acclimated to the scanner environment and understand instructions. During the resting-state scan, children were instructed to lie as still as possible with eyes open while watching Inscapes, a movie paradigm designed for collecting “resting-state” fMRI data to reduce pote ntial head motion (Vanderwal et al., 2015). A total of 210 whole-brain rs-fMRI data were collected using a T2*-weighted gradient-echo echo-planner imaging sequence (TR 2 s, TE 25 ms, slice thickness 3.5 mm, voxel size 3.0 mm × 3.0 mm × 3.5 mm, voxel matrix 64 × 64, flip angle 70°, field of view 192 mm, 36 slices), duration of 7 minutes and 6 seconds. The following high-resolution structural images were acquired with a T1-weighted magnetization prepared rapid gradient echo sequence: TR 1.9s; TE 2.32 ms; slice thickness 0.9 mm with no gap; voxel size 0.9×0.9×0.9 mm; matrix 256×256 mm; flip angle 9°; field of volume 230 * 230 mm, duration of 4 minutes and 26 seconds.
Preprocessing
In the analyses, all 210 collected rs-fMRI images were included as the first 4 volumes were discarded before data collection due to the instability of the initial MRI signal and the adaptation of the subjects to the circumstances. The preprocessing included the following steps. First, slice timing, head motion correction, realignment with anatomical image, new segment, and regression of nuisance covariates were performed using DPABI 1.3 (a toolbox for Data Processing & Analysis for Brain Imaging, version 1.3) (Yan et al., 2016). Considering the brain size and tissues differences in young children, we first obtained 6 tissue maps, i.e., white matter (WM), grey matter (GM), cerebral spinal fluid (CSF), plus 3 background classes, based on the current dataset by using the Template-O-Matic toolbox (Wilke et al., 2008), and then segmented the structural images into WM, GM, and CSF using the New Segment procedure in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). The segmented individual WM and CSF tissues were used for the subsequent regression. Nuisance covariates regression parameters included: 1) Friston 24-motion parameters (6 head motion parameters, 6 head motions one time point before, and the 12 corresponding squared items) (Friston et al., 1996), 2) the first 5 principal components extracted from subject-specific WM and CSF tissues employing a component based noise correction method (CompCor) (Behzadi et al., 2007), and 3) a binary file representing head motion scrubbing results (see Head motion section below for details). The CompCor procedure was comprised of detrending, variance (i.e., WM and CSF) normalization, and principle component analysis according to Behzadi et al. (2007). To achieve a better registration and normalization, we used the Advanced Normalization Tools ( ANTs) (Avants et al., 2011), which has been proven to be reliable and flexible to create customized T1-template. ANTs created a group-specific template based on the segmented brain tissues, and then all functional images were normalized to this template. Finally, we performed spatial smoothing with a 5 mm full-width-at-half-maximum Gaussian kernel and temporal bandpass filtering (0.01–0.1 Hz) in AFNI (Cox, 1996).
Head motion
A well-known concern is that small volume-to-volume head movements could potentially influence resting-state functional connectivity (Power et al., 2014; Power et al., 2012; Power et al., 2015; Satterthwaite et al., 2012; Van Dijk et al., 2012). In the preprocessing, we calculated the framewise displacement (FD) following Power et al. (2012) to quantify the head motion of each volume. In order to minimize the head motion effect, we applied the following two procedures: 1) any volumes with FD greater than 0.7 mm as well as 1 back and 1 forward volumes were identified as “bad” volumes and then e ach “bad” volume was treated as a separate regressor in the regression models (Satterthwaite et al., 2013); 2) any children with remaining volumes less than 80% of the total volumes or with mean FD greater than 0.5 mm were excluded. We scrubbed the volumes with bad motion by regressing them out rather than removing them. This approach, on the one hand, can result in an equivalent effect as performing regression only within the “good” data (Power et al., 2013), and on the other hand, it can avoid removal of time points. Notably, given the young age range of the current sample, we used a relatively lenient threshold of 0.7 mm for bad volumes scrubbing (Riggins et al., 2016), which was a tradeoff between stringent data quality and reasonable data quantity. Under the aforementioned criteria, 12 children were excluded. In the final sample, head motion (i.e., mean FD) did not show significant correlations with either age (see Fig. 1B) or ToMI scores (see Table 1). In addition, we included mean FD as a nuisance covariate in the group analyses to further control the effect of head motion.
Table 1.
Summary of geographic information and behavioral measures as well as their correlations with ToMI scores
| mean (SD) |
correlations | ||||
|---|---|---|---|---|---|
| total ToM | early ToM | basic ToM | advanced ToM | ||
| age (years) | 6.61 (1.41) |
r = .47*** | r = .16 | r = .50*** | r = .47*** |
| gender | 54 boys 70 girls |
r = .14 | r = .12 | r = .13 | r = .12 |
| IQ | 12.77 (2.18) |
r = .11 | r = −.04 | r = .11 | r = .13 |
| FD (mm) | 0.21 (0.11) |
r = −.06 | r = −.05 | r = −.04 | r = −.09 |
| working memory |
0.66 (0.16) |
r = .37*** | r = .17 | r = .40*** | r = .36*** |
| total ToM | 16.86 (2.09) |
||||
| early ToM | 18.38 (1.88) |
r = .77*** | |||
| basic ToM | 16.65 (2.15) |
r = .97*** | r = .72*** | ||
| advanced ToM | 16.6 (2.3) |
r = .96*** | r = .65*** | r = .89*** | |
Note.*** p < .001, sample size n = 124.
Functional connectivity analysis
Resting state functional connectivity (RSFC) was performed by using functions in DPABI version 1.3 (Yan et al., 2016). We selected the RTPJ (MNI coordinates: 50, −60, 18) as a seed due to its core involvement in mentalizing and metarepresentation (Amft et al., 2015). Specifically, the mean time series of the RTPJ were computed across subjects within a 6-mm-radius sphere centered around the RTPJ coordinates, and then the connectivity between the time series of the seed region and those of the whole brain was calculated to generate the individual RSFC map (r-map). Subsequently, we used Fisher’s r-to-z transformation to convert r-maps into z-maps to obtain normally distributed values of the connectivity maps.
Age-related changes in RTPJ connectivity
We performed group analyses using general linear models with AFNI’s 3dttest++ program. First, we examined the age-related changes in RTPJ connectivity using the regression model,
where mean FD was included as a nuisance covariate to dissociate potential effects of head motion.
Brain-behavior correlation analysis
In order to evaluate the neural basis of ToM components, we conducted the following regression model on the whole-brain RSFC maps with the total ToMI as well as each ToM components, separately:
In separate models, total, early, basic, and advanced ToM scores were included as regressors of interest; age, gender, working memory, IQ, and mean FD were included as nuisance covariates. Both age and working memory were included in the model due to the correlations with ToMI performance (see Table 1). In addition, gender, IQ, and mean FD were also included to control for the potential effects from these covariates. Because some covariates were correlated with each other (e.g., total ToM and three components, age, and working memory), we performed Belsley collinearity diagnostics (Belsley et al., 1980) to confirm that the regressors included in the regression models were not multicollinear. Importantly, we did not investigate age by behavior interactions because high collinearity was identified in these models.
To determine whether connectivity associated with ToM differed depending on the specific ToM component investigated, we compared the correlation maps of RTPJ connectivity with different ToM components by subtracting one correlation map from the other correlation map and thresholding as described below.
All the resulting maps were transformed into Z maps and corrected for family-wise error (FWE) rate through Monte Carlo simulations using 3dClustSim program in AFNI (Cox, 1996) at a voxel wise p = 0.005 combined with a minimal cluster size of 66 voxels (cluster wise p < 0.05, FWE corrected). This spatial cluster correction took into account spatial autocorrelation by using the ‘–acf’ option in 3dClustSim (Cox et al., 2017).
Bayesian multilevel modeling
As aforementioned, we used the conventional whole-brain linear regression analysis to investigate the correlation between RTPJ and behavior, i.e., total ToM and three ToM components. This whole-brain approach induces a multiple comparisons issue due to separate inferences at each voxel. Recent studies suggested to set voxel-wise threshold at 0.001 or below or use nonparametric methods (e.g., permutation tests) (Eklund et al., 2016; Woo et al., 2014). However, these strategies might unnecessarily lose detection power due to over-conservatively controlling for false positive rates. Thus, Chen et al. (2018) proposed a novel approach, Bayesian multilevel (BML) modeling, to serve as an alternative, confirmatory or supplementary method. BML is implemented with one model for an ensemble of regions of interest (ROIs), which can be defined from previous studies, anatomical or functional atlas, or an independent dataset, and it solves the multiple testing issue through partial pooling among the ROIs. As demonstrated in Chen et al. (2018), BML can gain detection sensitivity at specific regions, compared to the conventional approaches.
Given the advantages of BML (Chen et al., 2018), we employed this approach to confirm the results from the conventional whole brain analysis and also to check whether or not those results were compromised by the correction for multiple comparisons. Specifically, we selected 21 regions of interest (ROIs) from two meta-analyses (Amft et al., 2015; Schurz et al., 2014) as shown in Table 2, which were not only relevant to ToM measure in the present study but also provided broader regions associated with social cognition, affective processing, and motivational processes associated with the development of ToM abilities. The Schurz et al. (2014) ROIs were selected from a meta-analysis specific to theory of mind tasks in adults whereas the Amft et al. (2015) ROIs included nodes of an extended socio-affective network identified through resting-state functional connectivity and meta-analytic connectivity modeling. A sphere with a radius of 6 mm was created for each ROI and then the mean z-score of each sphere was extracted from the z-maps for each subject. To make sure that these spheres were spatially separate from each other, we calculated the distance between centers of spheres and excluded one ROI (i.e., right middle temporal gyrus in Mind in the eyes studies) from Schurz et al. (2014) because it partially overlapped with the right superior temporal gyrus in False belief vs. photo studies. For more extensive details on the approach see Chen et al. (2018).
Table 2.
Regions of interest included in the Bayesian multilevel modeling test
| Nr. | source | Region | MNI Coordinates |
||
|---|---|---|---|---|---|
| x | y | z | |||
| 1 |
Table 2 (False belief vs. photo) (Schurz et al., 2014) |
R PCC | 8 | −59 | 35 |
| 2 | R TPJp | 56 | −56 | 25 | |
| 3 | R insula | 49 | −8 | −11 | |
| 4 | L IPL | −55 | −65 | 27 | |
| 5 | L SFG | −7 | 58 | 21 | |
| 6 |
Table 2 (Mind in the eyes) (Schurz et al., 2014) |
R IFG (BA45) | 47 | 22 | 6 |
| 7 | R IFG (BA9) | 60 | 25 | 19 | |
| 8 | L MTG | −51 | −62 | 5 | |
| 9 | L CG | −5 | 8 | 42 | |
| 10 | L IFG | −46 | 24 | 7 | |
| 11 | Amft et al. (2015) | ACC | 0 | 38 | 10 |
| 12 | SGC | −2 | 32 | −8 | |
| 13 | PCC | −2 | −52 | 26 | |
| 14 | dmPFC | −2 | 52 | 14 | |
| 15 | L TPJ | −46 | −66 | 18 | |
| 16 | L vBG | −6 | 10 | −8 | |
| 17 | R vBG | 6 | 10 | −8 | |
| 18 | L aMTS/aMTG | −54 | −10 | −20 | |
| 19 | R Amy/Hippo | 24 | −8 | −22 | |
| 20 | L Amy/Hippo | −24 | −10 | −20 | |
| 21 | vmPFC | −2 | 50 | −10 | |
Note. L, left; R, right. Abbreviations were following those used in previous studies (Amft et al., 2015; Schurz et al., 2014). PCC, posterior cingulate cortex/precuneus; TPJp, posterior temporo-parietal junction; IPL, inferior parietal lobe; SFG, superior frontal gyrus; IFG, inferior frontal gyrus; aMTS/aMTG, anterior middle temporal sulcus/gyrus; CG, cingulate gyrus; ACC, anterior cingulate cortex; SGC, subgenual cingulate cortex; dmPFC, dorsomedial prefrontal cortex; vBG, ventral basal ganglia; Amy/Hippo, amygdala/hippocampus; vmPFC, ventromedial prefrontal cortex.
Validation analysis with a control region
We used a control region to examine the specificity of these findings to RTPJ by selecting the anterior cingulate cortex (ACC) (MNI coordinates:0, 38, 10) from the same meta-analysis (Amft et al., 2015). The ACC is a region of relevance to social cognitive processes, such as emotion, reward, and motivation, but not part of the canonical mentalizing network as confirmed in a Neurosynth meta-analysis (http://neurosynth.org; Yarkoni et al., 2011). We tested the brain-behavior correlation using the same models as those used for RTPJ connectivity. In addition, the same ROIs (except for ACC, which was seed region here) were entered into BML model to confirm the whole brain analysis. The analysis procedures were the same as outlined above.
Results
Behavioral results
As predicted, the scores for early ToM were significantly higher than the other two more advanced components (early vs. basic: t(123) = 12.62, p < .001; early vs. advanced: t(123) = 11.09, p < .001), whereas basic and advanced components did not differ (t(123) = .48, p = .63). Further, we tested for differences across ages by using a one-way between-group analysis of variance with ages binned by year (4, 5, 6, 7, 8) and observed a significant effect of age for total ToM, basic, and advanced ToM (total ToM: F(4, 119) = 9.44, p < .001; basic ToM: F(4, 119) = 11.39, p < .001; advanced ToM: F(4, 119) = 8.73, p < .001) while the early ToM did not show significant age-related differences in this period (F(4, 119) = 1.79, p = .14) (see Fig. 2). The results were similar when using age as a continuous regressor, which showed significant correlations with total ToM and two more advanced components (see Table 1). In addition, children’s working memory was also significantly correlated with their performance in total ToM and the two more advanced components (see Table 1).
Fig. 2.
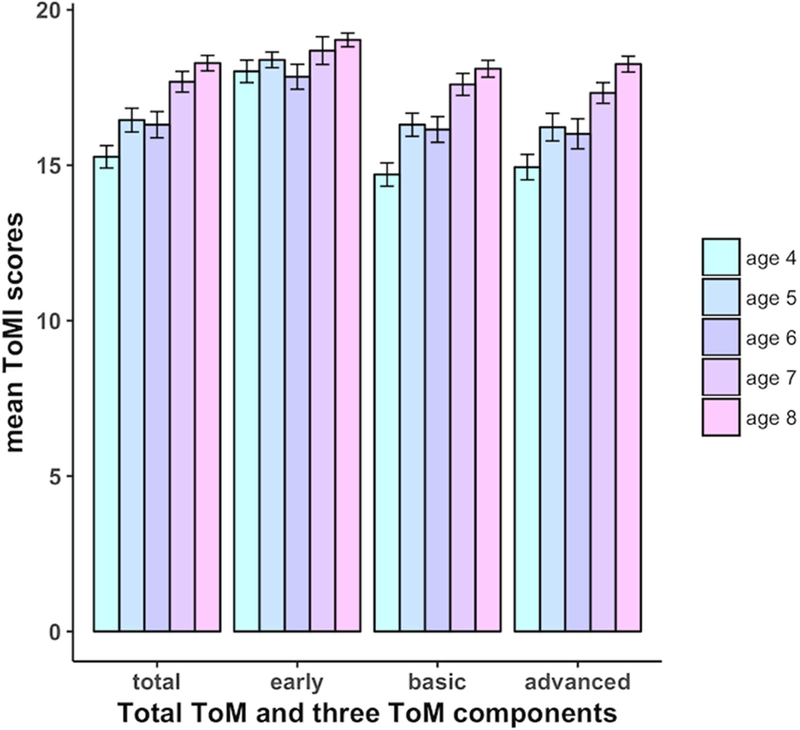
Illustration of mean scores for total ToM and three ToM components from ages 4 to 8 years. Except for early component, Total ToM, as well as early and basic components showed significant age-related improvement. Error bars represent standard error of the mean.
Age-related changes in RTPJ connectivity
We first evaluated the age effects on RTPJ functional connectivity. As shown in Supplementary Fig. S1A, only one region survived after multiple comparisons correction (bilateral caudate; peak MNI coordinates: −8, 14, 10; 144 voxels). Nevertheless, with a lenient threshold (voxel wise p = 0.05, , combined with minimal cluster size of 70 voxels), we additionally observed regions including bilateral PCC, LTPJ, mPFC, right lingual gyrus, and right middle occipital gyrus (see Supplementary Fig. S1B).
Brain-behavior correlation analysis
In a second step, we explored age-independent correlation patterns of total ToM and different ToM components separately. As shown in Fig. 3 and Table 3, the RTPJ connectivity was positively correlated with total ToM as well as basic and advanced ToM components in the bilateral PCC and negatively correlated with total ToM as well as early and advanced ToM components in the right inferior/superior parietal lobe (IPL/SPL). The negative correlation between RTPJ connectivity and early ToM also included right inferior temporal gyrus (ITG) extending to fusiform gyrus. Additionally, in the late developing component only, significant connectivity between RTPJ and LTPJ was related to ToM ability. However, whole-brain comparison of the connectivity between each of the three ToM components did not reveal any significant differences. This high similarity among components can be seen more clearly as shown in the uncorrected correlation maps (voxel-wise p = 0.0214, , combined with minimal cluster size of 70 voxels) (see Supplementary Fig. S2). Given that the ToMI has several items that load onto more than one component, we ran a post-hoc analysis using only items that loaded onto a single factor (see Hutchins et al., 2012). Even though the components show significant differences behaviorally, there were still no significant differences in the relations between RTPJ connectivity and ToM components.
Fig. 3.

Correlations between RTPJ functional connectivity and performance in total ToM and three ToM components. The red-yellow color indicates positive correlations and the blue color indicates negative correlations. The black circle denotes the seed region, i.e., RTPJ. All maps are thresholded at the cluster level through Monte Carlo simulations (cluster wise p < 0.05, FEW corrected) and visualized with BrainNet Viewer (Xia et al., 2013, http://www.nitrc.org/projects/bnv/). L, left; R, right; RTPJ, right temporo-parietal junction; FC, functional connectivity.
Table 3.
Clusters showing significant relations in RTPJ FC– ToM correlation analysis
| FC – behavior correlation |
region | BA | Peak MNI coordinates |
Cluster size (3*3*3 mm3 voxels) |
Peak Z | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| RTPJ FC – total ToM |
L/R PCC | 7, 31 | −8 | −64 | 47 | 280 | 4.08 |
| R IPL/SPL | 40 | 35 | −50 | 55 | 178 | −4.34 | |
| RTPJ FC – early ToM |
R IPL/SPL | 40 | 32 | −64 | 49 | 336 | −4.69 |
| R ITG, fusiform gyrus | 37 | 50 | −47 | −17 | 116 | −4.0 | |
| RTPJ FC – basic ToM |
L/R PCC | 7, 31 | 5 | −70 | 40 | 183 | 3.86 |
| RTPJ FC – advanced ToM |
L/R PCC | 7, 31 | −8 | −44 | 43 | 480 | 4.37 |
| R IPL/SPL | 40 | 35 | −50 | 55 | 99 | −4.25 | |
| L TPJ | 39 | −59 | −62 | 13 | 66 | 3.59 | |
Note. FC, functional connectivity. BA, Brodmann area; L, left; R, right. RTPJ, right temporo-parietal junction; PCC, posterior cingulate cortex/precuneus; IPL/SPL, inferior/superior parietal lobe; ITG, inferior temporal gyrus. Positive/negative Z values indicate positive/negative correlations. All correlational regions are significant at cluster-wise p < 0.05 (FWE corrected).
Bayesian multilevel modeling results
We used a novel BML approach to examine the relations between ToM and RTPJ connectivity, including differences in relations between components, using a non-binary approach while avoiding potentially overly-stringent correction for false positive rates. The BML was used to identify regions showing strong evidence of correlation (i.e., within the positive 95% quantile interval under BML corresponding to a two-tailed p-value of 0.05 under conventional statistical testing) or moderate evidence of correlation (i.e., within the positive 90% quantile interval under BML corresponding to a one-tailed p-value of 0.05 under conventional statistical testing) with the total ToM and each of the three ToM components. Regions showing strong evidence across all components and the ToM total measure included bilateral PCC, left IPL, LTPJ, right posterior TPJ, left anterior middle temporal sulcus and gyrus (aMTS/aMTG), vmPFC, and dmPFC (see Fig. 4 and Supplementary Table S1). Notably, the positive correlation patterns shown in BML were similar to the uncorrected results from the whole brain analysis (see Supplementary Fig. S2). Further, we tested whether relations between RTPJ connectivity and behavior differed across the different components, and there was no strong evidence to indicate differences between components in the current data, i.e., for all ROIs the range of values within the 10–90% quantile interval contain 0.
Fig. 4.

Regions in red indicate strong evidence of correlation within positive 95% quantile interval under BML (corresponding to a one-tailed p-value of 0.05 under conventional statistical testing); regions in green indicate moderate evidence of correlation within the positive 90% quantile interval under BML (corresponding to a one-tailed p-value of 0.05 under conventional statistical testing). L, left; R, right; FC, functional connectivity; RTPJ, right temporo-parietal junction; PCC, posterior cingulate cortex/precuneus; TPJp, posterior temporo-parietal junction; IPL, inferior parietal lobe; aMTS/aMTG, anterior middle temporal sulcus/gyrus; dmPFC, dorsomedial prefrontal cortex; vmPFC, ventromedial prefrontal cortex.
Results of the validation analysis
When seeded in the control region, i.e., ACC, we neither saw any significant results for brain-behavior relation in the whole-brain analysis nor found any regions showing strong or moderate evidence of correlation with the BML model.
Discussion
In this study we explored how intrinsic functional connectivity is related to ToM abilities overall (total ToM), as well as early, basic, and advanced developmental components of ToM in typically developing children. As predicted, the total ToM ability as well as the basic and advanced components showed significant age-related differences from ages 4 to 8 years, whereas the early component didn’t show significant change during this age period. In the whole brain correlation analysis, both basic and advanced components showed correlations with the connectivity between RTPJ and bilateral PCC, a key region in the mentalizing network; both early and advanced components demonstrated negative correlations between RTPJ and right IPL/SPL. Further, although only advanced ToM showed a significant correlation with LTPJ, there were no significant differences in the relations between RTPJ connectivity and ToM ability between the different components. We confirmed these results by a Bayesian modeling approach (i.e., BML), and observed that connectivity between RTPJ and key regions of the mentalizing network including LTPJ, PCC, and mPFC were related to different components of ToM and that there was no strong evidence of differences between three ToM components. Taken together, these findings demonstrate a common neural basis across diverse types of ToM in typically developing young children from the perspective of resting-state functional connectivity.
Developments in different components of ToM in children between 4 and 8 years
Behaviorally, children’s performance in the earlier-developing ToM competence, related to fundamental skills of social cognition such as affect recognition and sharing attention, was high by the age of 4 years and did not show significant improvement from ages 4 to 8 years. By contrast, children’s performance in basic and advanced ToM, which relies more heavily on meta-representational abilities, increased steadily with age. Notably, basic and advanced ToM components were not significantly different from each other, in contrast to the previous behavioral study (Hutchins et al., 2012). Basic ToM is suggested to emerge at an earlier age than advanced ToM that includes items requiring more complex social cognitive ability such as complex recursion and advanced pragmatic abilities. However, the extent to which these factors are fully dissociable requires further testing given that some items are complex and loaded onto other factors more weakly. Further, both basic and advanced components of ToM also rely on similar underlying cognitive abilities (Hutchins et al., 2012), and these similarities might account for the lack of age-related differences between these two components in the current study. Finally, because these data are cross-sectional, age-related differences might be confounded with individual differences which could belie true developmental effects. Future studies should examine these brain-behavior relations using a longitudinal design.
PCC connectivity with RTPJ is positively related to ToM abilities
Bilateral PCC connectivity with RTPJ was the only connection to show consistent significant positive correlations with total ToM and two components (i.e., basic and advanced ToM) in the whole brain analysis. The PCC is a versatile region, which is involved in multiple cognitive functions (for a review, see Leech and Sharp, 2014). A number of adult studies have demonstrated that along with TPJ, PCC is activated during the processing of mental compared to non-mental state information (Gallagher et al., 2000; Saxe and Kanwisher, 2003; Saxe and Powell, 2006; Saxe and Wexler, 2005; Young et al., 2010). However, Saxe et al. (2006) found the PCC was activated by both ToM and self-related processing, suggesting that PCC is not selectively involved in belief representation and instead is also involved in other aspects of social-cognitive processing. Furthermore, despite that the PCC is frequently reported in ToM studies, it doesn’t respond selectively to information about mental states compared to social information about people (Gweon et al., 2012; Saxe et al., 2009), suggesting a more general role in social processing. In addition, Sebastian et al. (2012) reported stronger PCC activity during tasks requiring affective ToM than during tasks requiring cognitive ToM. Collectively, these findings show that PCC is not specific to the representational aspects of ToM (i.e., representing another’s thoughts, beliefs, and intentions as representational); rather, it plays a general role in social and affective aspects of ToM. The correlation between RTPJ – PCC connectivity and ToM competence suggests the coactivation between RTPJ and PCC is a significant contributor to ToM representation and reasoning in early childhood.
In addition to the PCC, the BML approach identified consistent involvement of multiple key nodes of the mentalizing network, including bilateral TPJ, mPFC (i.e., vmPFC and dmPFC), and left aMTS/aMTG. Bilateral TPJ is consistently engaged during ToM-related tasks and mental representation in particular in studies of adults (e.g., Gallagher et al., 2000; Perner et al., 2006; Saxe et al., 2006; Saxe and Kanwisher, 2003; Saxe and Powell, 2006). In children, the functional profile of the TPJ changes during middle and late childhood (Gweon et al., 2012; Kobayashi et al., 2007; Saxe et al., 2009) such that it shows a more selective response with age. In line with those findings, we observed moderate evidence in connectivity between RTPJ and LTPJ for early ToM and strong evidence for basic and advanced ToM. Further, at the whole-brain level, only advanced ToM showed a significant relation between RTPJ to LTPJ connectivity and ToM ability. However, importantly, there was no evidence of significant differences across components in these brain-behavior relations.
The dmPFC, on the other hand, demonstrated no significant relations with any ToM component in the whole-brain analysis and only moderate support in the BML. This inconsistent support for the dmPFC was surprising given that it is identified as a key node for mentalizing and social interaction (Gallagher et al., 2002; McCabe et al., 2001; for reviews, see Frith and Frith, 2006; Gallagher and Frith, 2003) and across multiple diverse ToM tasks (Schurz et al., 2014), and developmentally (Sabbagh et al., 2009; Bowman et al., 2018; Grossmann, 2013; Grossmann and Johnson, 2010). Of note, however, the non-significant correlation with RTPJ – dmPFC connectivity (or moderate support in BML) shown in the current data does not necessarily suggest dmPFC activation is not important to ToM. Rather, it might imply the covariation of these two brain regions (i.e., RTPJ and dmPFC) does not contribute to individual differences in the development of ToM in early and middle childhood. Nevertheless, future studies using both resting state and task fMRI could disentangle relations between RTPJ – dmPFC connectivity and dmPFC activation during ToM development.
A similar positive neural correlate across diverse aspects of ToM abilities
ToM is a complex, multifaceted construct and recent empirical and theoretical work has argued against a monolithic treatment of this ability and its associated neural correlates (e.g., Schaafsma et al., 2015; Schurz et al., 2014), but little research has addressed this question developmentally. Indeed, we predicted that each of these developmentally-diverse components would be associated with distinct patterns of connectivity with the RTPJ. Contrary to our predictions, in the current data, we found little evidence of differences in correlation patterns of RTPJ connectivity and the different ToM components within both the whole-brain analysis and the BML approach, suggesting a common rather than diverse neural substrate underlying the development of different ToM abilities in early childhood. These abilities spanned shared attention and affect to more complex belief representation and pragmatic abilities. One possibility for these commonalities in RTPJ connectivity relations with diverse behaviors is that the behavioral constructs still tapped multiple common basic cognitive and social processes (for a review, see Schaafsma et al., 2015).
Specificity of the ToM network to ToM development
Notably, however, these relations were specific to nodes associated with ToM and social cognition and not those regions of the extended socio-affective network associated with affective or motivational processing (except vmPFC), suggesting those distinct “basic processes” may still rely on components within the social-cognitive or mentalizing network rather than more general contributions of emotion or motivational brain regions, even for the earliest developing component involving shared affect. Further, this specificity within the mentalizing network to ToM abilities is supported by the validation results with the ACC. There were no relations between ACC connectivity and ToM behaviors, nor any evidence of correlation from the BML model, showing a functional dissociation between regions of mentalizing network and regions associated with more general affective or emotional processing in support of developing ToM abilities – a finding consistent with Richardson et al. (2018). Nevertheless, these results should be interpreted with an important caveat that the regions showing evidence of correlation in the BML model were selected from previous studies rather than localized with a ToM task in the current study.
Functional specialization of RTPJ connectivity is correlated with better ToM performance
Although positive relations with RTPJ connectivity were seen within the mentalizing network, a consistent pattern of negative correlations were found with the connectivity between RTPJ and neighboring regions, i.e., right IPL/SPL, was related to both early and advanced ToM components, as was a negative relation with the connectivity between RTPJ and right ITG extending to fusiform gyrus in early ToM. The RTPJ is a multifunctional region involved in a variety of cognitive functions, including language, memory, attention, and social processing (Carter and Huettel, 2013; Igelström et al., 2016). Anatomically, the RTPJ and surrounding regions are a convergence zone for multiple large-scale brain networks, including the default mode network, fronto-parietal network, and dorsal and ventral attention networks (Mars et al., 2012; Yeo et al., 2011). Thus, we speculate these negative correlations might indicate an increasing segregation from neighboring regions of RTPJ that are not involved in ToM and suggest this enhanced functional specialization within the mentalizing network is associated with better ToM abilities. This decrease in local connectivity is consistent with developmental theory and evidence suggesting that network organization progresses from more local to long-distance patterns of connectivity and that these changes are related to both age and experience (Johnson, 2011). Accordantly, the decreasing involvement of ToM unrelated regions (i.e., right IPL/SPL and right ITG extending to fusiform gyrus) might demonstrate a trend of diffuse to focal connectivity within the mentalizing network for the components of ToM, which is related to better ToM abilities.
Complementary results from the BML model
In addition to the whole-brain analysis, we adopted a complimentary ROI-based Bayesian approach, i.e., BML, to confirm the results from the whole brain analysis. Some studies (e.g., Amrhein et al., 2017; Chen et al., 2017; Cohen, 1994) have argued that the current practice of controlling for false positive rate under the traditional null hypothesis significance testing might be problematic because the null hypothesis is not pragmatically meaningful. BML, instead, handles multiple comparisons by conservatively shrinking the original effect toward the center among the regions, and statistical inferences are constructed through the posterior distributions (Chen et al., 2017). The BML analysis with our data revealed evidence of connectivity between RTPJ and a wide range of regions associated with ToM reasoning, including bilateral TPJ, mPFC, PCC, and left aMTS/aMTG, for total ToM and three components, while only the PCC and LTPJ survived rigorous correction under the suggested criterion in the whole brain analysis. Furthermore, unlike the null hypothesis significance testing approach, BML enables us to demonstrate that the current data did not provide strong evidence for differences between ToM components, offering more solid support for a common neural basis for distinct developmental components of ToM. Taken together, these results suggest that BML can serve as at least a complementary approach with enhanced spatial specificity and detection sensitivity of the data over conventional approaches.
Limitations of the current study
In the current study, we used a parent-report measure, which comprises multifaceted aspects of ToM tagged by diverse items and enables us to probe comprehensive ToM ability in children rather than a single, more narrow aspect of ToM. Given that children’s ToM competence is reported by their caretaker, it would not be constrained by their cognitive and verbal skills which may confound performance on specific tasks. Further, this measure records the continuum of children’s ToM understanding instead of their dichotomous performance and thus can be used to examine individual differences. However, limitations of this measure are also worth noting. First, some items are complex and load onto more than one factor to different extents. These shared items could be contributing to similarity in the relations between connectivity patterns across components. However, in a post-hoc analysis we tested whether connectivity differed between components when all shared items were removed and found no difference in the relations with RTPJ connectivity between these components. Second, parents may have a bias for children’s social understandings and might underestimate or overestimate the reasoning skills of their children, although it has been shown the parent-report scores are highly correlated with child performance on ToM tasks (Hutchins et al., 2012). Nonetheless, future studies should incorporate both parent-report and children assessments to gain a more complete picture of children’s ToM.
In addition, some potential limitations of resting-state functional connectivity should be taken into account when interpreting the current findings. Although research has shown high correlations between resting-state connectivity and task activations (e.g., Smith et al., 2009), there may be discrepancies between functional connectivity during rest and during task (Di et al., 2013). Thus, some components of ToM could be reflected in differential connectivity patterns during social tasks but not at rest. For example, co-activations between regions such as dmPFC and RTPJ could change with age or tasks, but these relations may not be identifiable at rest. Therefore, the interpretation of the current results should take into account the use of resting-state fMRI. While comparison of task and resting-state functional connectivity within the same participants is an important question for future research, we do note that our findings of specificity of functional connectivity within the mentalizing network to ToM behavior are broadly consistent with a study utilizing task-related functional connectivity in this age range (Richardson et al., 2018).
Conclusion
To conclude, our study presents a link between a comprehensive ToM evaluation investigating early- and late-emerging components of ToM and functional connectivity within the developing brain. The large cohort of developing children in the current study allows for investigating the neural correlates of diverse types of ToM abilities as well as their development in early childhood. Our data demonstrate a common neural correlation pattern across different components of ToM from the perspective of functional connectivity by using both whole-brain analysis and BML. Specifically, the whole brain analysis revealed the relations of the three ToM components to the mentalizing network, i.e., connectivity between RTPJ and PCC for both basic and advanced components and connectivity between RTPJ and LTPJ for advanced ToM component, whereas BML provided evidence of connectivity between RTPJ and more regions associated with ToM, such as connectivity between RTPJ and bilateral TPJ, mPFC, PCC, and left aMTS/aMTG, for total ToM and three components of ToM. Though these ToM abilities emerge at different ages, no strong evidence was found regarding the differences among their correlation patterns. Further, significant positive correlations between RTPJ connectivity and ToM abilities were only found within social-cognitive regions whereas negative correlations were seen outside the social-cognitive network, i.e., right IPL/SPL and ITG. These negative correlations within neighboring regions to the TPJ suggest enhanced functional segregation of the mentalizing network from anatomically proximal but functionally unrelated networks is associated with better ToM abilities. These novel findings from young children offer new insights into underpinnings of multiple aspects of ToM in the developing brain and thus may have implications for both typical and atypical ToM development in childhood.
Supplementary Material
Acknowledgements
This research was supported by NIMH R01HD079518 awarded to T.R. We thank the Neurocognitive Development Lab for data collection, and the Maryland Neuroimaging Center and staff for project assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aichhorn M, Perner J, Weiss B, Kronbichler M, Staffen W, Ladurner G, 2009. Temporoparietal junction activity in theory-of-mind tasks: falseness, beliefs, or attention. J. Cogn. Neurosci 21, 1179–1192. [DOI] [PubMed] [Google Scholar]
- Amft M, Bzdok D, Laird AR, Fox PT, Schilbach L, Eickhoff SB, 2015. Definition and characterization of an extended social-affective default network. Brain Struct. Funct 220, 1031–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio DM, Frith CD, 2006. Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci 7, 268–277. [DOI] [PubMed] [Google Scholar]
- Amrhein V, Korner-Nievergelt F, Roth T, 2017. The earth is flat (p > 0.05): significance thresholds and the crisis of unreplicable research. PeerJ Preprints 5, e2921v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan B, Hohenberger A, Verbrugge R, 2017. Syntactic Recursion Facilitates and Working Memory Predicts Recursive Theory of Mind. PLoS One 12, e0169510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC, 2011. A Reproducible Evaluation of ANTs Similarity Metric Performance in Brain Image Registration. Neuroimage 54, 2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT, 2007. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsley DA, Kuh E, Welsh RE, 1980. Regression Diagnostics: Identifying Influential Data and Sources of Collinearity New York: Wiley. [Google Scholar]
- Bosacki S, Astington JW, 1999. Theory of Mind in Preadolescence: Relations Between Social Understanding and Social Competence. Soc. Dev 8, 237–255. [Google Scholar]
- Bowman LC, Dodell-Feder D, Saxe R, Sabbagh MA, 2017. Stability in the Neural System A Stable Neural System Supporting for Children’s Theory of Mind Development: Longitudinal links between task-independent EEG and task-dependent fMRI Under review [DOI] [PMC free article] [PubMed]
- Brauer J, Xiao Y, Poulain T, Friederici AD, Schirmer A, 2016. Frequency of Maternal Touch Predicts Resting Activity and Connectivity of the Developing Social Brain. Cereb. Cortex 26, 3544–3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington SJ, Bailey AJ, 2009. Are there theory of mind regions in the brain? A review of the neuroimaging literature. Hum. Brain Mapp 30, 2313–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RM, Huettel SA, 2013. A nexus model of the temporal–parietal junction. Trends Cogn. Sci 17, 328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Xiao Y, Taylora P, Riggins T, Geng F, Redcay E, Cox RW, 2018. Handling Multiplicity in Neuroimaging through Bayesian Lenses with Hierarchical Modeling. bioRxiv [DOI] [PMC free article] [PubMed]
- Cohen J, 1994. The earth is round (p < .05). Am. Psychol 49, 997–1003. [Google Scholar]
- Cox RW, 1996. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Comput. Biomed. Res 29, 162–173. [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA, 2017. fMRI clustering and false-positive rates. Proc. Natl. Acad. Sci. U. S. A 114, E3370–E3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis HL, Pratt C, 1995. The development of children’s theory of mind: The working memory explanation. Aust. J. Psychol 47, 25–31. [Google Scholar]
- Di X, Gohel S, Kim EH, Biswal BB, 2013. Task vs. rest—different network configurations between the coactivation and the resting-state brain networks. Front. Hum. Neurosci 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H, 2016. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. U. S. A 113, 7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME, 2005. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A 102, 9673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R, 1996. Movement-related effects in fMRI time-series. Magn. Reson. Med 35, 346–355. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U, 2006. The Neural Basis of Mentalizing. Neuron 50, 531–534. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD, 2003. Development and neurophysiology of mentalizing. Philos. Trans. R. Soc. B Biol. Sci 358, 459–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD, 2003. Functional imaging of ‘theory of mind.’ Trends Cogn. Sci 7, 77–83. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Happé F, Brunswick N, Fletcher PC, Frith U, Frith CD, 2000. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’in verbal and nonverbal tasks. Neuropsychologia 38, 11–21. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Jack AI, Roepstorff A, Frith CD, 2002. Imaging the Intentional Stance in a Competitive Game. Neuroimage 16, 814–821. [DOI] [PubMed] [Google Scholar]
- Grossmann T, 2013. The role of medial prefrontal cortex in early social cognition. Front. Hum. Neurosci 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann T, Johnson MH, 2010. Selective prefrontal cortex responses to joint attention in early infancy. Biol. Lett 6, 540–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gweon H, Dodell-Feder D, Bedny M, Saxe R, 2012. Theory of Mind Performance in Children Correlates With Functional Specialization of a Brain Region for Thinking About Thoughts. Child Dev 83, 1853–1868. [DOI] [PubMed] [Google Scholar]
- Gweon H, Saxe R, 2013. Developmental Cognitive Neuroscience of Theory of Mind, In: Rubenstein J and Rakic P (Eds.), Neural circuit development and function in the brain (pp. 367–377). San Diego, CA: Academic Press. [Google Scholar]
- Hogrefe G-J, Wimmer H, Perner J, 1986. Ignorance versus False Belief: A Developmental Lag in Attribution of Epistemic States. Child Dev 57, 567–582. [Google Scholar]
- Hutchins TL, Prelock PA, Bonazinga L, 2012. Psychometric Evaluation of the Theory of Mind Inventory (ToMI): A Study of Typically Developing Children and Children with Autism Spectrum Disorder. J. Autism Dev. Disord 42, 327–341. [DOI] [PubMed] [Google Scholar]
- Igelström KM, Webb TW, Kelly YT, Graziano MSA, 2016. Topographical Organization of Attentional, Social, and Memory Processes in the Human Temporoparietal Cortex. eNeuro 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH, 2011. Interactive specialization: a domain-general framework for human functional brain development? Dev. Cogn. Neurosci 1, 7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi C, Glover GH, Temple E, 2007. Children’s and adults’ neural bases of verbal and nonverbal ‘Theory of Mind.’ Neuropsychologia 45, 1522–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp S, 2007. NEPSY-II: A developmental neuropsychological assessment San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Koster-Hale J, Richardson H, Velez N, Asaba M, Young L, Saxe R, 2017. Mentalizing regions represent distributed, continuous, and abstract dimensions of others’ beliefs. Neuroimage 161, 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Sharp DJ, 2014. The role of the posterior cingulate cortex in cognition and disease. Brain 137, 12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mars RB, Sallet J, Schüffelgen U, Jbabdi S, Toni I, Rushworth MFS, 2012. Connectivity-Based Subdivisions of the Human Right “Temporoparietal Junction Area”: Evidence for Different Areas Participating in Different Cortical Networks. Cereb. Cortex 22, 1894–1903. [DOI] [PubMed] [Google Scholar]
- McCabe K, Houser D, Ryan L, Smith V, Trouard T, 2001. A functional imaging study of cooperation in two-person reciprocal exchange. Proc. Natl. Acad. Sci. U. S. A 98, 11832–11835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SA, 2012. Theory of Mind: Beyond the Preschool Years New York, NY: Psychology Press. [Google Scholar]
- Molenberghs P, Johnson H, Henry JD, Mattingley JB, 2016. Understanding the minds of others: A neuroimaging meta-analysis. Neurosci. Biobehav. Rev 65, 276–291. [DOI] [PubMed] [Google Scholar]
- Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM, 2006. Neuroanatomical evidence for distinct cognitive and affective components of self. J. Cogn. Neurosci 18, 1586–1594. [DOI] [PubMed] [Google Scholar]
- Mutter B, Alcorn MB, Welsh M, 2006. Theory of Mind and Executive Function: Working-Memory Capacity and Inhibitory Control as Predictors of False-Belief Task Performance. Percept. Mot. Skills 102, 819–835. [DOI] [PubMed] [Google Scholar]
- Perner J, Aichhorn M, Kronbichler M, Staffen W, Ladurner G, 2006. Thinking of mental and other representations: The roles of left and right temporo-parietal junction. Soc. Neurosci 1, 245–258. [DOI] [PubMed] [Google Scholar]
- Peterson CC, Wellman HM, Liu D, 2005. Steps in Theory-of-Mind Development for Children With Deafness or Autism. Child Dev 76, 502–517. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE, 2013. Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to Carp. Neuroimage 76, 439–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE, 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE, 2014. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage 84, 320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Petersen SE, 2015. Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage 105, 536–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL, 2001. A default mode of brain function. Proc. Natl. Acad. Sci. U. S. A 98, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson H, Lisandrelli G, Riobueno-Naylor A, Saxe R, 2018. Development of the social brain from age three to twelve years. Nat. Commun 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggins T, Geng F, Blankenship SL, Redcay E, 2016. Hippocampal functional connectivity and episodic memory in early childhood. Dev. Cogn. Neurosci 19, 58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh MA, Bowman LC, Evraire LE, Ito J, 2009. Neurodevelopmental correlates of theory of mind in preschool children. Child Dev 80, 1147–1162. [DOI] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K, Loughead J, Calkins ME, Eickhoff SB, Hakonarson H, Gur RC, Gur RE, 2013. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage 64, 240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, Gur RC, Gur RE, 2012. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage 60, 623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N, 2003. People thinking about thinking people: The role of the temporo-parietal junction in theory of mind? Neuroimage 19, 1835–1842. [DOI] [PubMed] [Google Scholar]
- Saxe R, Moran JM, Scholz J, Gabrieli J, 2006. Overlapping and non-overlapping brain regions for theory of mind and self reflection in individual subjects. Soc. Cogn. Affect. Neurosci 1, 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, Powell LJ, 2006. It’s the thought that counts: specific brain regions for one component of theory of mind. Psychol. Sci 17, 692–699. [DOI] [PubMed] [Google Scholar]
- Saxe R, Wexler A, 2005. Making sense of another mind: The role of the right temporo-parietal junction. Neuropsychologia 43, 1391–1399. [DOI] [PubMed] [Google Scholar]
- Saxe RR, Whitfield-Gabrieli S, Scholz J, Pelphrey KA, 2009. Brain Regions for Perceiving and Reasoning About Other People in School-Aged Children. Child Dev 80, 1197–209. [DOI] [PubMed] [Google Scholar]
- Schaafsma SM, Pfaff DW, Spunt RP, Adolphs R, 2015. Deconstructing and reconstructing theory of mind. Trends Cogn. Sci 19, 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L, Bzdok D, Timmermans B, Fox PT, Laird AR, Vogeley K, Eickhoff SB, 2012. Introspective Minds: Using ALE Meta-Analyses to Study Commonalities in the Neural Correlates of Emotional Processing, Social & Unconstrained Cognition. PLoS One 7, e30920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurz M, Radua J, Aichhorn M, Richlan F, Perner J, 2014. Fractionating theory of mind: A meta-analysis of functional brain imaging studies. Neurosci. Biobehav. Rev 42, 9–34. [DOI] [PubMed] [Google Scholar]
- Sebastian CL, Fontaine NMG, Bird G, Blakemore S-J, De Brito SA, McCrory EJP, Viding E, 2012. Neural processing associated with cognitive and affective Theory of Mind in adolescents and adults. Soc. Cogn. Affect. Neurosci 7, 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE, 1997. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J. Cogn. Neurosci 9, 648–663. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, others, 2009. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci. U. S. A 106, 13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer M, Döhnel K, Sodian B, Meinhardt J, Thoermer C, Hajak G, 2007. Neural correlates of true and false belief reasoning. Neuroimage 35, 1378–1384. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS, 2009. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J. Cogn. Neurosci 21, 489–510. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V, 2008. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. U. S. A 105, 12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL, 2012. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage 59, 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F, 2009. Social cognition and the brain: A meta-analysis. Hum. Brain Mapp 30, 829–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwal T, Kelly C, Eilbott J, Mayes LC, Castellanos FX, 2015. Inscapes : A movie paradigm to improve compliance in functional magnetic resonance imaging. Neuroimage 122, 222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman HM, Cross D, Watson J, 2001. Meta-analysis of theory-of-mind development: the truth about false belief. Child Dev 72, 655–684. [DOI] [PubMed] [Google Scholar]
- Wellman HM, Liu D, 2004. Scaling of Theory-of-Mind Tasks. Child Dev 75, 523–541. [DOI] [PubMed] [Google Scholar]
- Wilke M, Holland SK, Altaye M, Gaser C, 2008. Template-O-Matic: A toolbox for creating customized pediatric templates. Neuroimage 41, 903–913. [DOI] [PubMed] [Google Scholar]
- Wimmer H, Perner J, 1983. Beliefs about beliefs: Representation and constraining function of wrong beliefs in young children’s understanding of deception. Cognition 13, 103–128. [DOI] [PubMed] [Google Scholar]
- Woo C-W, Krishnan A, Wager TD, 2014. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage 91, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M, Wang J, He Y, 2013. BrainNet Viewer: a network visualization tool for human brain connectomics. PloS One 8, e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Friederici AD, Margulies DS, Brauer J, 2016. Longitudinal changes in resting-state fMRI from age 5 to age 6 years covary with language development. Neuroimage 128, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C-G, Wang X-D, Zuo X-N, Zang Y-F, 2016. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics 1–13. [DOI] [PubMed]
- Yarkoni T, Poldrack RA, Nichols TE, Essen DCV, Wager TD, 2011. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods 8, 665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, Fischl B, Liu H, Buckner RL, 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol 106, 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L, Dodell-Feder D, Saxe R, 2010. What gets the attention of the temporo-parietal junction? An fMRI investigation of attention and theory of mind. Neuropsychologia 48, 2658–2664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


