Abstract
Esophageal squamous cell carcinoma (ESCC) is a deadly disease that requires extensive research. Here, we review the current understanding of the functions of the NRF2 signaling pathway in the esophagus. Genomic data suggest that gene mutations and several other mechanisms result in NRF2 hyperactivation in human ESCC. As a consequence, NRF2high ESCC is more resistant to chemoradiotherapy and has poorer survival than NRF2low ESCC. Mechanistically, we believe NRF2, functioning as a transcription factor, causes an esophageal phenotype through regulation of gene transcriptional. We discuss metabolism, mitochondria, proteasomes, and several other signaling pathways as downstream players that may contribute to esophageal phenotype due to NRF2 hyperactivation. Finally, strategies are proposed to target the NRF2 signaling pathway for future therapy of NRF2high ESCC.
Keywords: esophageal squamous cell carcinoma, targeted therapy, NRF2, KEAP1
Graphical abstract
Genomic data suggest that gene mutations and several other mechanisms result in NRF2 hyperactivation in human esophageal squamous cell carcinoma (ESCC). As a consequence, NRF2high ESCC is more resistant to chemoradiotherapy and has poorer survival than NRF2low ESCC. We discuss metabolism, mitochondria, proteasomes, and other signaling pathways as downstream players that may contribute to phenotypes due to NRF2 hyperactivation
As a major cellular defense mechanism, the nuclear factor erythroid-derived 2–like 2 (NRF2 or NFE2l2) signaling pathway is known to regulate the expression of enzymes involved in detoxification and the antioxidative stress response. NRF2 forms heterodimers with small Maf proteins and binds to the antioxidant response elements of target genes when cells are exposed to oxidative stress or xenobiotics. Kelch-like ECH-associated protein 1 (KEAP1) inhibits the function of NRF2 by retaining NRF2 in the cytoplasm under normal physiological conditions and allowing nuclear translocation of NRF2 under stress conditions.1 Although the cancer-preventive function of the NRF2 signaling pathway has been well documented, recent studies have revealed that NRF2 activity is a double-edged sword, as it can be carcinogenic when hyperactive. NRF2 was found to prevent initiation but accelerate the progression of lung carcinogenesis in vivo.2,3 Many studies have repeatedly shown that NRF2 helps cancer cells survive chemoradiation-induced oxidative stress and accelerates drug metabolism4–7 thus contributing to chemoradioresistance.8 NRF2 overexpression is associated with poor prognosis in cancer.9 Genetic targeting of the NRF2 signaling pathway impaired tumorigenesis in the lung, pancreas, and colon.10–12
NRF2 in the esophagus
The role of the NRF2 signaling pathway in the esophagus was first revealed in a mouse study by Yamamoto’s group in 2003. Genetic activation of NRF2 in Keap1−/− mice resulted in esophageal hyperproliferation and hyperkeratosis. These mice died from poor nutrition due to esophageal blockage, and Nrf2−/−–Keap1−/− mice completely rescued the esophageal phenotype.13 The esophageal phenotype of Keap1−/− mice was further attributable to constitutive activation of NRF2 with the assistance of small Maf proteins.14,15 Tissue-specific deletion of esophageal NRF2 in Keap1−/− mice (K5Cre Nrf2fl/fl Keap1−/−) allowed survival until adulthood. However, these mice developed polyuria with low osmolality and bilateral hydronephrosis due to defects in water reabsorption as a result of reduced expression of aquaporin 2 in the kidney.16 Consistent with these findings using genetic models, chemical activation of NRF2 by tert-butylated hydroxyanisole or its metabolite tert-butylhydroquinone caused hyperkeratosis and squamous cell carcinoma in rodent forestomach.17–19 It should be noted that hyperkeratosis is a precursor lesion of carcinogen-induced esophageal squamous cell carcinoma (ESCC) in rodents.20–22 In humans, esophageal hyperkeratosis has also been reported as a result of gastroesophageal reflux, vitamin A deficiency, or tylosis A.22 On the other hand, Nrf2−/− mice were more susceptible to 4-nitroquinoline-1-oxide–induced tongue and esophageal carcinogenesis than wild-type mice, whereas KEAP1 knockdown mice were resistant.23
When we compared differential gene expression between the normal esophagus and Barrett’s esophagus,24 NRF2 was found to be one of the transcriptional factors enriched in normal esophageal squamous epithelium. We then studied how the NRF2 signaling pathway regulates morphogenesis of the esophageal epithelium in mice by comparing gene expression profiles in wild-type, Nrf2−/−, Keap1−/− and Nrf2−/− Keap1−/− esophagi using gene microarrays. We found that the NRF2 signaling pathway had a baseline activity at the early stage and was further activated later during the development of mouse esophageal squamous epithelium. Keap1−/− esophagus had an increased expression of keratinization genes, PI3K/Akt pathway genes, and PPARβ/δ.22 Since the keratinized layer is the major protective layer against physical stress and chemical injuries,25 and terminally differentiated keratinocytes express proteins that can provide protection by quenching reactive oxygen species,26 we hypothesized that NRF2 may be involved in esophageal epithelial barrier function and may therefore play a protective role during gastroesophageal reflux. Indeed, NRF2 deficiency reduced transepithelial electrical resistance and increased intercellular space in the esophageal epithelium through downregulation of claudin 4 (CLDN4). Chromatin immunoprecipitation (ChIP) analysis clearly showed binding of NRF2 to the predicted sites in the promoter region of mouse Cldn4. Meanwhile, NRF2 target genes and gene sets associated with oxidoreductase activity, mitochondrial biogenesis, and energy production were downregulated in Nrf2−/− esophagus. Consistent with these observations, ATP biogenesis and CoxIV (a mitochondrial marker) were also downregulated.27 These data suggested that energy-dependent tight junction integrity was subject to NRF2 regulation. Activating NRF2 may potentially strengthen esophageal epithelial barrier function as a therapeutic approach for gastroesophageal reflux disease.28–30
Que’s group further demonstrated that basal progenitor cell–specific expression of constitutively active bone morphogenetic protein (BMP) promoted squamous differentiation in mouse esophagus. The action of BMP was mediated through increased intracellular oxidative stress and an NRF2-mediated antioxidative response. This mechanism is further involved in the development of eosinophilic esophagitis, in which reduced squamous differentiation is associated with high levels of follistatin (a BMP inhibitor) and disrupted BMP/NRF2 pathways.31
Gene mutations and NRF2 hyperactivation in human ESCC
With the recent technological advances in next-generation sequencing, human ESCC samples from North and South America, China, Japan, Vietnam, and Malawi have been sequenced.32–46 ESCC shares similar genomic profiles with head and neck SCC and lung SCC, but not esophageal adenocarcinoma, suggesting common etiological factors.47–49 In fact, NRF2 and KEAP1 have been classified among 291 high-confidence cancer-driver genes acting on 3205 tumors from 12 different cancer types.50
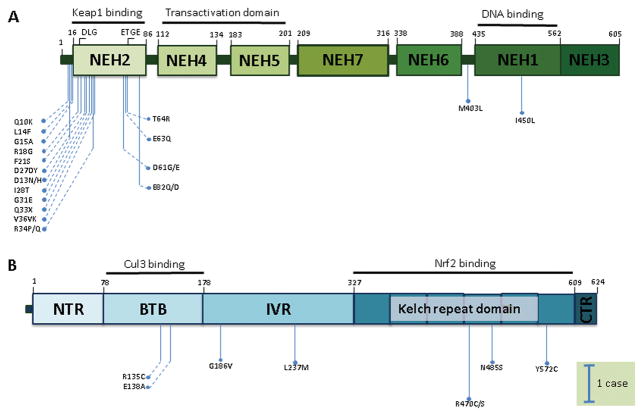
Among many gene mutations, NRF2 mutations were commonly seen, with a frequency over 5%, even up to ~ 20% in certain reports. Mutations in other genes of the NRF2 signaling pathway (KEAP1 and CUL3) were relatively less common. NRF2 mutations were mostly located in the DLG and ETGE motifs (KEAP1-binding domain) and the DNA-binding domain, while KEAP1 mutations tended to be scattered across the whole gene (Fig. 1). NRF2 mutations and KEAP1 mutations were mutually exclusive in human lung cancer cell lines.51 In human ESCC tissue samples, such mutual exclusivity was also suggested.33,52
Figure 1.
Point mutations in NRF2 and KEAP1 in human ESCC based on original data from four recent next-generation sequencing studies from China.32–34,40
On the basis of the genomics data, ESCC can be clustered into three subtypes, with subtype 1 (56%, 50/90) characterized by genomic alterations in the NRF2 signaling pathway. This subtype had a higher frequency of SOX2 and/or p63 amplification and potential involvement of the Hippo pathway, similar to head and neck SCC and lung SCC.44 Asian patients tended to be clustered in subtype 1, whereas Eastern European and South American patients clustered in subtype 2, and North American patients in subtype 3.44 ESCC in African American patients also tended to involve the NRF2 signaling pathway.53 Similar to ESCC, a molecular subtype with NRF2 activation has also been reported in head and neck SCC based on microarray data.54,55
NRF2 mutations have not been reported in esophageal squamous hyperplasia and non-tumorous dysplasia. However, they were present in low-grade dysplasia and high-grade dysplasia associated with ESCC.36–38 Phylogenetic analysis showed that NRF2 mutation as a driver mutation tended to be located on the branches of the tumor phylogenetic tree, while mutations of tumor suppressor genes (e.g., p53) tended to be located on the trunk, suggesting that NRF2 mutation may be a relatively late event during the development of ESCC.37,46
Many point mutations in NRF2 have been shown to activate NRF2 as a result of altered interaction between NRF2 and KEAP156–58 and increased nuclear localization of NRF2.59 Certain KEAP1 mutations, when heterozygous, had a dominant-negative effect on the wild-type KEAP1 and thus gave rise to NRF2 activation.60 Genomic mutations of the NRF2 signaling pathway correlated with the transcriptional activity of the NRF2 signaling pathway in ESCC.45 We also found that human ESCC can be clustered into NRF2high and NRF2low cases according to gene microarray data of several esophagus-specific NRF2 target genes.
Other than mutations, at least five additional mechanisms are known to activate NRF2 in cancer: hypomethylation of KEAP1, accumulation of disruptor proteins, increased production of NRF2, electrophoretic attack of KEAP1 by oncometabolites, and downregulation of NRF2-targeting microRNAs (miRNAs).61,62 This explains why there is a much higher percentage of ESCC with NRF2 hyperactivation than with point mutations.
Consequences of NRF2 hyperactivation in ESCC
Significant correlations were found between positive NRF2 expression and unfavorable response to chemoradiotherapy in ESCC patients. NRF2 overexpression was significantly correlated with lymph node metastasis, postoperative recurrence, and overall survival.36,63,64 Multivariate analysis showed that NRF2 expression status was an independent prognostic factor.64 Even the molecular signatures due to NRF2 mutations were significantly predictive and prognostic for clinical response. Mutant NRF2 conferred increased cell proliferation, attachment-independent survival, and resistance to 5-fluorouracil and γ-irradiation.36 Blockage of NRF2 suppresses the migration and invasion of ESCC cells in a hypoxic microenvironment.63 These data support the notion that NRF2 hyperactivation plays an important role in ESCC and can be targeted to improve the therapeutic efficacy of conventional therapy.
How does NRF2 hyperactivation contribute to ESCC?
NRF2, working as a transcription factor, regulates gene transcription.65 Previous ChIP-Seq experiments have shown that NRF2 potentially up- or downregulates transcription of hundreds of genes.66–69 We hypothesized that NRF2 hyperactivation caused esophageal hyperproliferation and hyperkeratosis through gene transcriptional regulation in esophageal squamous epithelial cells. Due to the nature of cell and tissue specificity in transcription factor binding,70–72 the ChIP-Seq experiment will need to be repeated with esophageal samples. Several pathways are known to be regulated by NRF2.
Metabolism
In addition to their involvement with the metabolic phenotype of NRF2 hyperactivation in the esophagus, mitochondria also regulate oxidative stress, cell signaling, and cell death during carcinogenesis.73,74 Our previous study showed that the number of mitochondria was decreased in NRF2−/− esophagus compared with wild-type esophagus. Mitochondrion-related gene sets were downregulated in NRF2−/− esophagus. Reduction of mitochondria was confirmed by downregulation of a mitochondrial marker protein (Cox IV). These data were consistent with other studies showing that NRF2 regulates mitochondrial biogenesis and cellular bioenergetics.75–77 It is also known that KEAP1 and NRF2 are tethered to mitochondria through PGAM5 and p62.78,79 NRF2 regulates productions of reactive oxygen species and thus protects against mitochondrial decay.80,81 Therefore, mitochondria may be potentially critical for the phenotypes attributable to NRF2 hyperactivation.82,83
Proteasome
Proteasomal subunits are known to be regulated by the NRF2 signaling pathway.84 Proteasome inhibitors may be effective for NRF2high ESCC.85 It has been reported that NRF2 contributes to colon carcinogenesis through its regulation of proteasomes.86 Inhibition of NRF2 by the alkaloid trigonelline rendered pancreatic cancer cells more susceptible to apoptosis through decreased proteasomal gene expression and proteasome activity.87
Notch signaling
Recent literature suggests a cross talk between the NRF2 and Notch signaling pathways.88,89 We also showed that expression of both NICD1 and HES1 in oral squamous epithelial cells were regulated by NRF2,90 consistent with previous findings in hematopoietic stem cells,91 airway basal stem cells,92 and mouse embryonic fibroblasts.93 Although the Notch signaling pathway is believed to be anti-carcinogenic through its regulation of squamous epithelial cell differentiation,94,95 several recent studies indicated that upregulation of the Notch signaling pathway may contribute to the malignant phenotype in these cells as well.96–98 It remains to be determined whether NRF2-activated Notch signaling pathway plays a dual role in ESCC.
PI3K/Akt signaling
The epidermal growth factor (EGF) signaling pathway has long been associated with human ESCC. With eight ligands and four receptors, this pathway has several downstream signaling paths, one of which is the PI3K/Akt pathway.99–101 Recent next-generation sequencing studies have confirmed PIK3CA mutations as drivers in ESCC.32–34 Phospho-Akt levels were increased in the KEAP1−/− esophagus.22 In the literature, transgenic overexpression of EGF ligand or receptor (AREG, ERBB2) and a constitutively active Akt or transgenic knockout of PTEN (an inhibitor of the PI3K/Akt pathway) caused esophageal hyperkeratosis in mice.102–105 It appears that NRF2 and PI3K/Akt regulate each other in a reciprocal manner. Loss of PTEN increased NRF2 activity.106 Since PI3K/Akt mutations and activation are commonly seen in human ESCC,33,52 it would be interesting to further understand how these two signaling pathways interact with each other. More importantly, targeting both signaling pathways may have synergistic effects on ESCC.
The NRF2 signaling pathway as a therapeutic target in ESCC
We believe that the esophagus is a unique organ site for studies on NRF2 hyperactivation owing to the strong esophageal phenotype in the KEAP1−/− esophagus.13 Targeting the NRF2 signaling pathway in the esophagus will not only help us develop a better therapy for NRF2high ESCC but also potentially contribute to therapy of NRF2high cancers of other organ sites (e.g., head and neck, lung). However, tissue of origin and environment are critical factors implicated in carcinogenesis driven by genetic alterations. It will be essential to focus on the esophagus to develop therapeutic strategies for NRF2high ESCC. Additionally, in vivo studies will likely be more reliable than in vitro cell culture studies, as seen in recent cancer metabolism studies.107,108
Several strategies have been proposed to target the NRF2 signaling pathway for cancer therapy: transcriptional downregulation of NRF2; increased degradation of NRF2 mRNA or decreased translation; enhancement of NRF2 degradation through upregulation/activation of KEAP1–CUL3, β-TrCP-SCF, or HRD1; blocking the dimerization of NRF2 with small Maf proteins; and blocking the NRF2–sMaf DNA-binding domain.109 In addition, NRF2 downstream pathways may also be targeted if they can be shown to be functionally critical for NRF2high ESCC.110 For example, a recent study used chemical proteomics to map druggable proteins that are selectively expressed in NRF2high lung cancer. NR0B1 was identified as a downstream druggable target, and small molecules were found to disrupt NR0B1 protein complexes and thus inhibit NRF2-dependent lung cancer.111 Several small molecule NRF2 inhibitors––halofuginone,112 brusatol,113 AEM1,114 and ML385115––have been identified by high-throughput screening. We are also in the process of screening NRF2 inhibitors from chemical libraries for NRF2high ESCC. Yet, targeting a transcription factor can be a challenge. Most known NRF2 inhibitors may actually target mechanisms other than NRF2 itself. In addition to small molecule inhibitors, miRNA can be an alternative approach. A reporter-coupled miRNA library screen identified four miRNAs (miR-507, -634, -450a, and -129-5p) that negatively regulate the NRF2 signaling pathway. Administration of miR-507 alone or in combination with cisplatin inhibited tumor growth in vivo.62
It should be noted that the location of NRF2 mutations on the branches of tumor phylogenetic trees suggests that targeting NRF2 may not be as effective as targeting the trunks (e.g., p53). Targeting branches may even lead to growth acceleration of non-mutated subpopulations.46 In fact, Clemons proposed targeting the glutathione biosynthesis pathway (or NRF2 signaling pathway) in p53-mutanted cancers, considering that more than 80% of ESCCs harbor mutations in the p53 gene.116
Conclusions
NRF2 hyperactivation is one of the commonly seen molecular alterations in human ESCC. Multiple studies have clearly shown a poor prognosis in cases with hyperactive NRF2. Therefore, it is critical to understand the molecular mechanisms of ESCC associated with hyperactive NRF2 and develop targeted therapy directed at NRF2 signaling. We believe NRF2 as a transcription factor causes an esophageal phenotype through gene transcriptional regulation. Several strategies have been proposed to target the NRF2 signaling pathway for future therapy of NRF2high ESCC.
Acknowledgments
The authors are supported by a Faculty Development Fund from Peking University Third Hospital, NIH/NCI U54 CA156735, and NIH/NIMHD U54 MD012392. The authors have not received research funding from other funding agencies or the industry for the research work discussed in this manuscript.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the KEAP1-NRF2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 2.Satoh H, Moriguchi T, Takai J, et al. NRF2 prevents initiation but accelerates progression through the Kras signaling pathway during lung carcinogenesis. Cancer Res. 2013;73:4158–4168. doi: 10.1158/0008-5472.CAN-12-4499. [DOI] [PubMed] [Google Scholar]
- 3.Satoh H, Moriguchi T, Saigusa D, et al. NRF2 Intensifies Host Defense Systems to Prevent Lung Carcinogenesis, but After Tumor Initiation Accelerates Malignant Cell Growth. Cancer Res. 2016;76:3088–3096. doi: 10.1158/0008-5472.CAN-15-1584. [DOI] [PubMed] [Google Scholar]
- 4.Mine N, Yamamoto S, Kufe DW, et al. Activation of NRF2 Pathways Correlates with Resistance of NSCLC Cell Lines to CBP501 In Vitro. Mol Cancer Ther. 2014;13:2215–2225. doi: 10.1158/1535-7163.MCT-13-0808. [DOI] [PubMed] [Google Scholar]
- 5.Jaramillo MC, Zhang DD. The emerging role of the NRF2-KEAP1 signaling pathway in cancer. Genes Dev. 2013;27:2179–2191. doi: 10.1101/gad.225680.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang DD. The NRF2-KEAP1-ARE signaling pathway: The regulation and dual function of NRF2 in cancer. Antioxid Redox Signal. 2010;13:1623–1626. doi: 10.1089/ars.2010.3301. [DOI] [PubMed] [Google Scholar]
- 7.Wang XJ, Sun Z, Villeneuve NF, et al. NRF2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of NRF2. Carcinogenesis. 2008;29:1235–1243. doi: 10.1093/carcin/bgn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamadori T, Ishii Y, Homma S, et al. Molecular mechanisms for the regulation of NRF2-mediated cell proliferation in non-small-cell lung cancers. Oncogene. 2012;31:4768–4777. doi: 10.1038/onc.2011.628. [DOI] [PubMed] [Google Scholar]
- 9.Sporn MB, Liby KT. NRF2 and cancer: the good, the bad and the importance of context. Nat Rev Cancer. 2012;12:564–571. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeNicola GM, Karreth FA, Humpton TJ, et al. Oncogene-induced NRF2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauer AK, Cho HY, Miller-Degraff L, et al. Targeted deletion of NRF2 reduces urethane-induced lung tumor development in mice. PLoS One. 2011;6:e26590. doi: 10.1371/journal.pone.0026590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim TH, Hur EG, Kang SJ, et al. NRF2 blockade suppresses colon tumor angiogenesis by inhibiting hypoxia-induced activation of HIF-1alpha. Cancer Res. 2011;71:2260–2275. doi: 10.1158/0008-5472.CAN-10-3007. [DOI] [PubMed] [Google Scholar]
- 13.Wakabayashi N, Itoh K, Wakabayashi J, et al. KEAP1-null mutation leads to postnatal lethality due to constitutive NRF2 activation. Nat Genet. 2003;35:238–245. doi: 10.1038/ng1248. [DOI] [PubMed] [Google Scholar]
- 14.Taguchi K, Maher JM, Suzuki T, et al. Genetic analysis of cytoprotective functions supported by graded expression of KEAP1. Mol Cell Biol. 2010;30:3016–3026. doi: 10.1128/MCB.01591-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motohashi H, Katsuoka F, Engel JD, et al. Small Maf proteins serve as transcriptional cofactors for keratinocyte differentiation in the KEAP1-NRF2 regulatory pathway. Proc Natl Acad Sci U S A. 2004;101:6379–6384. doi: 10.1073/pnas.0305902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki T, Seki S, Hiramoto K, et al. Hyperactivation of NRF2 in early tubular development induces nephrogenic diabetes insipidus. Nature communications. 2017;8:14577. doi: 10.1038/ncomms14577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kroes R, Wester PW. Forestomach carcinogens: possible mechanisms of action. Food Chem Toxicol. 1986;24:1083–1089. doi: 10.1016/0278-6915(86)90292-9. [DOI] [PubMed] [Google Scholar]
- 18.Clayson DB, Iverson F, Nera EA, et al. The significance of induced forestomach tumors. Annu Rev Pharmacol Toxicol. 1990;30:441–463. doi: 10.1146/annurev.pa.30.040190.002301. [DOI] [PubMed] [Google Scholar]
- 19.Gharavi N, Haggarty S, El-Kadi AO. Chemoprotective and carcinogenic effects of tert-butylhydroquinone and its metabolites. Curr Drug Metab. 2007;8:1–7. doi: 10.2174/138920007779315035. [DOI] [PubMed] [Google Scholar]
- 20.Baden T, Yamamichi K, Michiura T, et al. Sequential endoscopic findings and histological changes of N-nitrosomethylbenzylamine-induced esophageal carcinogenesis in rats. Oncol Rep. 2006;16:965–970. [PubMed] [Google Scholar]
- 21.Hsu NY, Yeh KT, Chiang IP, et al. Cortactin overexpression in the esophageal squamous cell carcinoma and its involvement in the carcinogenesis. Dis Esophagus. 2008;21:402–408. doi: 10.1111/j.1442-2050.2007.00775.x. [DOI] [PubMed] [Google Scholar]
- 22.Chen H, Li J, Li H, et al. Transcript profiling identifies dynamic gene expression patterns and an important role for NRF2/KEAP1 pathway in the developing mouse esophagus. PLoS One. 2012;7:e36504. doi: 10.1371/journal.pone.0036504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohkoshi A, Suzuki T, Ono M, et al. Roles of KEAP1-NRF2 system in upper aerodigestive tract carcinogenesis. Cancer Prev Res (Phila) 2013;6:149–159. doi: 10.1158/1940-6207.CAPR-12-0401-T. [DOI] [PubMed] [Google Scholar]
- 24.Wang J, Qin R, Ma Y, et al. Differential gene expression in normal esophagus and Barrett’s esophagus. Journal of gastroenterology. 2009;44:897–911. doi: 10.1007/s00535-009-0082-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simpson CL, Patel DM, Green KJ. Deconstructing the skin: cytoarchitectural determinants of epidermal morphogenesis. Nat Rev Mol Cell Biol. 2011;12:565–580. doi: 10.1038/nrm3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vermeij WP, Alia A, Backendorf C. ROS quenching potential of the epidermal cornified cell envelope. J Invest Dermatol. 2011;131:1435–1441. doi: 10.1038/jid.2010.433. [DOI] [PubMed] [Google Scholar]
- 27.Chen H, Hu Y, Fang Y, et al. NRF2 deficiency impairs the barrier function of mouse oesophageal epithelium. Gut. 2014;63:711–719. doi: 10.1136/gutjnl-2012-303731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen H, Fang Y, Li W, et al. NFkB and NRF2 in esophageal epithelial barrier function. Tissue barriers. 2013;1:e27463. doi: 10.4161/tisb.27463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woodland P, Sifrim D. Oesophageal mucosal barrier: a key factor in the pathophysiology of non-erosive reflux disease (NERD) and a potential target for treatment. Gut. 2014;63:705–706. doi: 10.1136/gutjnl-2013-305101. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Chen XL, Shaker A, et al. Contribution of immunomodulators to gastroesophageal reflux disease and its complications: stromal cells, interleukin 4, and adiponectin. Annals of the New York Academy of Sciences. 2016;1380:183–194. doi: 10.1111/nyas.13157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang M, Ku WY, Zhou Z, et al. BMP-driven NRF2 activation in esophageal basal cell differentiation and eosinophilic esophagitis. J Clin Invest. 2015;125:1557–1568. doi: 10.1172/JCI78850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao YB, Chen ZL, Li JG, et al. Genetic landscape of esophageal squamous cell carcinoma. Nat Genet. 2014;46:1097–1102. doi: 10.1038/ng.3076. [DOI] [PubMed] [Google Scholar]
- 33.Song Y, Li L, Ou Y, et al. Identification of genomic alterations in oesophageal squamous cell cancer. Nature. 2014;509:91–95. doi: 10.1038/nature13176. [DOI] [PubMed] [Google Scholar]
- 34.Lin DC, Hao JJ, Nagata Y, et al. Genomic and molecular characterization of esophageal squamous cell carcinoma. Nat Genet. 2014;46:467–473. doi: 10.1038/ng.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agrawal N, Jiao Y, Bettegowda C, et al. Comparative genomic analysis of esophageal adenocarcinoma and squamous cell carcinoma. Cancer Discov. 2012;2:899–905. doi: 10.1158/2159-8290.CD-12-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shibata T, Kokubu A, Saito S, et al. NRF2 mutation confers malignant potential and resistance to chemoradiation therapy in advanced esophageal squamous cancer. Neoplasia. 2011;13:864–873. doi: 10.1593/neo.11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen XX, Zhong Q, Liu Y, et al. Genomic comparison of esophageal squamous cell carcinoma and its precursor lesions by multi-region whole-exome sequencing. Nature communications. 2017;8:524. doi: 10.1038/s41467-017-00650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X, Zhang M, Ying S, et al. Genetic Alterations in Esophageal Tissues From Squamous Dysplasia to Carcinoma. Gastroenterology. 2017;153:166–177. doi: 10.1053/j.gastro.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 39.Qin HD, Liao XY, Chen YB, et al. Genomic Characterization of Esophageal Squamous Cell Carcinoma Reveals Critical Genes Underlying Tumorigenesis and Poor Prognosis. Am J Hum Genet. 2016;98:709–727. doi: 10.1016/j.ajhg.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang L, Zhou Y, Cheng C, et al. Genomic analyses reveal mutational signatures and frequently altered genes in esophageal squamous cell carcinoma. Am J Hum Genet. 2015;96:597–611. doi: 10.1016/j.ajhg.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu W, Snell JM, Jeck WR, et al. Subtyping sub-Saharan esophageal squamous cell carcinoma by comprehensive molecular analysis. JCI Insight. 2016;1:e88755. doi: 10.1172/jci.insight.88755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng C, Zhou Y, Li H, et al. Whole-Genome Sequencing Reveals Diverse Models of Structural Variations in Esophageal Squamous Cell Carcinoma. Am J Hum Genet. 2016;98:256–274. doi: 10.1016/j.ajhg.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu N, Kadota M, Liu H, et al. Genomic Landscape of Somatic Alterations in Esophageal Squamous Cell Carcinoma and Gastric Cancer. Cancer Res. 2016;76:1714–1723. doi: 10.1158/0008-5472.CAN-15-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cancer Genome Atlas Research Network. Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541:169–175. doi: 10.1038/nature20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sawada G, Niida A, Uchi R, et al. Genomic Landscape of Esophageal Squamous Cell Carcinoma in a Japanese Population. Gastroenterology. 2016;150:1171–1182. doi: 10.1053/j.gastro.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 46.Hao JJ, Lin DC, Dinh HQ, et al. Spatial intratumoral heterogeneity and temporal clonal evolution in esophageal squamous cell carcinoma. Nat Genet. 2016;48:1500–1507. doi: 10.1038/ng.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hammerman PS, Hayes DN, Wilkerson MD, et al. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agrawal N, Frederick MJ, Pickering CR, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333:1154–1157. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stransky N, Egloff AM, Tward AD, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tamborero D, Gonzalez-Perez A, Perez-Llamas C, et al. Comprehensive identification of mutational cancer driver genes across 12 tumor types. Sci Rep. 2013;3:2650. doi: 10.1038/srep02650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim JW, Botvinnik OB, Abudayyeh O, et al. Characterizing genomic alterations in cancer by complementary functional associations. Nat Biotechnol. 2016;34:539–546. doi: 10.1038/nbt.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Du P, Huang P, Huang X, et al. Comprehensive genomic analysis of Oesophageal Squamous Cell Carcinoma reveals clinical relevance. Sci Rep. 2017;7:15324. doi: 10.1038/s41598-017-14909-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Erkizan HV, Johnson K, Ghimbovschi S, et al. African-American esophageal squamous cell carcinoma expression profile reveals dysregulation of stress response and detox networks. BMC Cancer. 2017;17:426. doi: 10.1186/s12885-017-3423-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walter V, Yin X, Wilkerson MD, et al. Molecular subtypes in head and neck cancer exhibit distinct patterns of chromosomal gain and loss of canonical cancer genes. PLoS One. 2013;8:e56823. doi: 10.1371/journal.pone.0056823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chung CH, Parker JS, Karaca G, et al. Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell. 2004;5:489–500. doi: 10.1016/s1535-6108(04)00112-6. [DOI] [PubMed] [Google Scholar]
- 56.Hast BE, Cloer EW, Goldfarb D, et al. Cancer-derived mutations in KEAP1 impair NRF2 degradation but not ubiquitination. Cancer Res. 2014;74:808–817. doi: 10.1158/0008-5472.CAN-13-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shibata T, Ohta T, Tong KI, et al. Cancer related mutations in NRF2 impair its recognition by KEAP1-Cul3 E3 ligase and promote malignancy. Proc Natl Acad Sci U S A. 2008;105:13568–13573. doi: 10.1073/pnas.0806268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ooi A, Dykema K, Ansari A, et al. CUL3 and NRF2 mutations confer an NRF2 activation phenotype in a sporadic form of papillary renal cell carcinoma. Cancer Res. 2013;73:2044–2051. doi: 10.1158/0008-5472.CAN-12-3227. [DOI] [PubMed] [Google Scholar]
- 59.Kim YR, Oh JE, Kim MS, et al. Oncogenic NRF2 mutations in squamous cell carcinomas of oesophagus and skin. J Pathol. 2010;220:446–451. doi: 10.1002/path.2653. [DOI] [PubMed] [Google Scholar]
- 60.Suzuki T, Maher J, Yamamoto M. Select heterozygous KEAP1 mutations have a dominant-negative effect on wild-type KEAP1 in vivo. Cancer Res. 2011;71:1700–1709. doi: 10.1158/0008-5472.CAN-10-2939. [DOI] [PubMed] [Google Scholar]
- 61.Taguchi K, Yamamoto M. The KEAP1-NRF2 System in Cancer. Frontiers in oncology. 2017;7:85. doi: 10.3389/fonc.2017.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamamoto S, Inoue J, Kawano T, et al. The impact of miRNA-based molecular diagnostics and treatment of NRF2-stabilized tumors. Mol Cancer Res. 2014;12:58–68. doi: 10.1158/1541-7786.MCR-13-0246-T. [DOI] [PubMed] [Google Scholar]
- 63.Shen H, Yang Y, Xia S, et al. Blockage of NRF2 suppresses the migration and invasion of esophageal squamous cell carcinoma cells in hypoxic microenvironment. Dis Esophagus. 2014;27:685–692. doi: 10.1111/dote.12124. [DOI] [PubMed] [Google Scholar]
- 64.Kawasaki Y, Okumura H, Uchikado Y, et al. NRF2 is useful for predicting the effect of chemoradiation therapy on esophageal squamous cell carcinoma. Ann Surg Oncol. 2014;21:2347–2352. doi: 10.1245/s10434-014-3600-2. [DOI] [PubMed] [Google Scholar]
- 65.Tonelli C, Chio IIC, Tuveson DA. Transcriptional Regulation by NRF2. Antioxid Redox Signal. 2017 doi: 10.1089/ars.2017.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chorley BN, Campbell MR, Wang X, et al. Identification of novel NRF2-regulated genes by ChIP-Seq: influence on retinoid X receptor alpha. Nucleic Acids Res. 2012;40:7416–7429. doi: 10.1093/nar/gks409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Malhotra D, Portales-Casamar E, Singh A, et al. Global mapping of binding sites for NRF2 identifies novel targets in cell survival response through ChIP-Seq profiling and network analysis. Nucleic Acids Res. 2010;38:5718–5734. doi: 10.1093/nar/gkq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kobayashi EH, Suzuki T, Funayama R, et al. NRF2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nature communications. 2016;7:11624. doi: 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hirotsu Y, Katsuoka F, Funayama R, et al. NRF2-MafG heterodimers contribute globally to antioxidant and metabolic networks. Nucleic Acids Res. 2012;40:10228–10239. doi: 10.1093/nar/gks827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Handstad T, Rye M, Mocnik R, et al. Cell-type specificity of ChIP-predicted transcription factor binding sites. BMC genomics. 2012;13:372. doi: 10.1186/1471-2164-13-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gertz J, Savic D, Varley KE, et al. Distinct properties of cell-type-specific and shared transcription factor binding sites. Molecular cell. 2013;52:25–36. doi: 10.1016/j.molcel.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee BK, Bhinge AA, Battenhouse A, et al. Cell-type specific and combinatorial usage of diverse transcription factors revealed by genome-wide binding studies in multiple human cells. Genome research. 2012;22:9–24. doi: 10.1101/gr.127597.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vyas S, Zaganjor E, Haigis MC. Mitochondria and Cancer. Cell. 2016;166:555–566. doi: 10.1016/j.cell.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zong WX, Rabinowitz JD, White E. Mitochondria and Cancer. Molecular cell. 2016;61:667–676. doi: 10.1016/j.molcel.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Piantadosi CA, Carraway MS, Babiker A, et al. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via NRF2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res. 2008;103:1232–1240. doi: 10.1161/01.RES.0000338597.71702.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holmstrom KM, Baird L, Zhang Y, et al. NRF2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol Open. 2013;2:761–770. doi: 10.1242/bio.20134853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ludtmann MH, Angelova PR, Zhang Y, et al. NRF2 affects the efficiency of mitochondrial fatty acid oxidation. Biochem J. 2014;457:415–424. doi: 10.1042/BJ20130863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lo SC, Hannink M. PGAM5 tethers a ternary complex containing KEAP1 and NRF2 to mitochondria. Exp Cell Res. 2008;314:1789–1803. doi: 10.1016/j.yexcr.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kwon J, Han E, Bui CB, et al. Assurance of mitochondrial integrity and mammalian longevity by the p62-KEAP1-NRF2-Nqo1 cascade. EMBO Rep. 2012;13:150–156. doi: 10.1038/embor.2011.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Strom J, Xu B, Tian X, et al. NRF2 protects mitochondrial decay by oxidative stress. FASEB J. 2016;30:66–80. doi: 10.1096/fj.14-268904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kovac S, Angelova PR, Holmstrom KM, et al. NRF2 regulates ROS production by mitochondria and NADPH oxidase. Biochim Biophys Acta. 2015;1850:794–801. doi: 10.1016/j.bbagen.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dinkova-Kostova AT, Abramov AY. The emerging role of NRF2 in mitochondrial function. Free radical biology & medicine. 2015;88:179–188. doi: 10.1016/j.freeradbiomed.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Holmstrom KM, Kostov RV, Dinkova-Kostova AT. The multifaceted role of NRF2 in mitochondrial function. Current opinion in toxicology. 2016;1:80–91. doi: 10.1016/j.cotox.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kwak MK, Wakabayashi N, Greenlaw JL, et al. Antioxidants enhance mammalian proteasome expression through the KEAP1-NRF2 signaling pathway. Mol Cell Biol. 2003;23:8786–8794. doi: 10.1128/MCB.23.23.8786-8794.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chapple SJ, Siow RC, Mann GE. Crosstalk between NRF2 and the proteasome: therapeutic potential of NRF2 inducers in vascular disease and aging. The international journal of biochemistry & cell biology. 2012;44:1315–1320. doi: 10.1016/j.biocel.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 86.Arlt A, Bauer I, Schafmayer C, et al. Increased proteasome subunit protein expression and proteasome activity in colon cancer relate to an enhanced activation of nuclear factor E2-related factor 2 (NRF2) Oncogene. 2009;28:3983–3996. doi: 10.1038/onc.2009.264. [DOI] [PubMed] [Google Scholar]
- 87.Arlt A, Sebens S, Krebs S, et al. Inhibition of the NRF2 transcription factor by the alkaloid trigonelline renders pancreatic cancer cells more susceptible to apoptosis through decreased proteasomal gene expression and proteasome activity. Oncogene. 2013;32:4825–4835. doi: 10.1038/onc.2012.493. [DOI] [PubMed] [Google Scholar]
- 88.Sparaneo A, Fabrizio FP, Muscarella LA. NRF2 and Notch Signaling in Lung Cancer: Near the Crossroad. Oxid Med Cell Longev. 2016;2016:7316492. doi: 10.1155/2016/7316492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wakabayashi N, Chartoumpekis DV, Kensler TW. Crosstalk between NRF2 and Notch signaling. Free radical biology & medicine. 2015;88:158–167. doi: 10.1016/j.freeradbiomed.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fan H, Paiboonrungruan C, Zhang X, et al. NRF2 regulates cellular behaviors and Notch signaling in oral squamous cell carcinoma cells. Biochemical and biophysical research communications. 2017;493:833–839. doi: 10.1016/j.bbrc.2017.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim JH, Thimmulappa RK, Kumar V, et al. NRF2-mediated Notch pathway activation enhances hematopoietic reconstitution following myelosuppressive radiation. J Clin Invest. 2014;124:730–741. doi: 10.1172/JCI70812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paul MK, Bisht B, Darmawan DO, et al. Dynamic changes in intracellular ROS levels regulate airway basal stem cell homeostasis through NRF2-dependent Notch signaling. Cell Stem Cell. 2014;15:199–214. doi: 10.1016/j.stem.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wakabayashi N, Shin S, Slocum SL, et al. Regulation of notch1 signaling by NRF2: implications for tissue regeneration. Sci Signal. 2010;3:ra52. doi: 10.1126/scisignal.2000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Duan L, Yao J, Wu X, et al. Growth suppression induced by Notch1 activation involves Wnt-beta-catenin down-regulation in human tongue carcinoma cells. Biology of the cell. 2006;98:479–490. doi: 10.1042/BC20060020. [DOI] [PubMed] [Google Scholar]
- 95.Sakamoto K, Fujii T, Kawachi H, et al. Reduction of NOTCH1 expression pertains to maturation abnormalities of keratinocytes in squamous neoplasms. Laboratory investigation; a journal of technical methods and pathology. 2012;92:688–702. doi: 10.1038/labinvest.2012.9. [DOI] [PubMed] [Google Scholar]
- 96.Lee SH, Do SI, Lee HJ, et al. Notch1 signaling contributes to stemness in head and neck squamous cell carcinoma. Laboratory investigation; a journal of technical methods and pathology. 2016;96:508–516. doi: 10.1038/labinvest.2015.163. [DOI] [PubMed] [Google Scholar]
- 97.Sun W, Gaykalova DA, Ochs MF, et al. Activation of the NOTCH pathway in head and neck cancer. Cancer Res. 2014;74:1091–1104. doi: 10.1158/0008-5472.CAN-13-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhong R, Bao R, Faber PW, et al. Notch1 Activation or Loss Promotes HPV-Induced Oral Tumorigenesis. Cancer Res. 2015;75:3958–3969. doi: 10.1158/0008-5472.CAN-15-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schneider MR, Wolf E. The epidermal growth factor receptor ligands at a glance. J Cell Physiol. 2009;218:460–466. doi: 10.1002/jcp.21635. [DOI] [PubMed] [Google Scholar]
- 100.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13:140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nyati MK, Morgan MA, Feng FY, et al. Integration of EGFR inhibitors with radiochemotherapy. Nat Rev Cancer. 2006;6:876–885. doi: 10.1038/nrc1953. [DOI] [PubMed] [Google Scholar]
- 102.Cook PW, Piepkorn M, Clegg CH, et al. Transgenic expression of the human amphiregulin gene induces a psoriasis-like phenotype. J Clin Invest. 1997;100:2286–2294. doi: 10.1172/JCI119766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xie W, Chow LT, Paterson AJ, et al. Conditional expression of the ErbB2 oncogene elicits reversible hyperplasia in stratified epithelia and up-regulation of TGFalpha expression in transgenic mice. Oncogene. 1999;18:3593–3607. doi: 10.1038/sj.onc.1202673. [DOI] [PubMed] [Google Scholar]
- 104.Murayama K, Kimura T, Tarutani M, et al. Akt activation induces epidermal hyperplasia and proliferation of epidermal progenitors. Oncogene. 2007;26:4882–4888. doi: 10.1038/sj.onc.1210274. [DOI] [PubMed] [Google Scholar]
- 105.Suzuki A, Itami S, Ohishi M, et al. Keratinocyte-specific Pten deficiency results in epidermal hyperplasia, accelerated hair follicle morphogenesis and tumor formation. Cancer Res. 2003;63:674–681. [PubMed] [Google Scholar]
- 106.Rojo AI, Rada P, Mendiola M, et al. The PTEN/NRF2 Axis Promotes Human Carcinogenesis. Antioxid Redox Signal. 2014;21(2):498–2514. doi: 10.1089/ars.2014.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mayers JR, Vander Heiden MG. Nature and Nurture: What Determines Tumor Metabolic Phenotypes? Cancer Res. 2017;77:3131–3134. doi: 10.1158/0008-5472.CAN-17-0165. [DOI] [PubMed] [Google Scholar]
- 108.Luengo A, Gui DY, Vander Heiden MG. Targeting Metabolism for Cancer Therapy. Cell chemical biology. 2017;24:1161–1180. doi: 10.1016/j.chembiol.2017.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.de la Vega MR, Dodson M, Chapman E, et al. NRF2-targeted therapeutics: New targets and modes of NRF2 regulation. Current opinion in toxicology. 2016;1:62–70. doi: 10.1016/j.cotox.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shibata T, Saito S, Kokubu A, et al. Global downstream pathway analysis reveals a dependence of oncogenic NF-E2-related factor 2 mutation on the mTOR growth signaling pathway. Cancer Res. 2010;70:9095–9105. doi: 10.1158/0008-5472.CAN-10-0384. [DOI] [PubMed] [Google Scholar]
- 111.Bar-Peled L, Kemper EK, Suciu RM, et al. Chemical Proteomics Identifies Druggable Vulnerabilities in a Genetically Defined Cancer. Cell. 2017;171:696–709 e623. doi: 10.1016/j.cell.2017.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tsuchida K, Tsujita T, Hayashi M, et al. Halofuginone enhances the chemo-sensitivity of cancer cells by suppressing NRF2 accumulation. Free radical biology & medicine. 2017;103:236–247. doi: 10.1016/j.freeradbiomed.2016.12.041. [DOI] [PubMed] [Google Scholar]
- 113.Ren D, Villeneuve NF, Jiang T, et al. Brusatol enhances the efficacy of chemotherapy by inhibiting the NRF2-mediated defense mechanism. Proc Natl Acad Sci U S A. 2011;108:1433–1438. doi: 10.1073/pnas.1014275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bollong MJ, Yun H, Sherwood L, et al. A Small Molecule Inhibits Deregulated NRF2 Transcriptional Activity in Cancer. ACS chemical biology. 2015;10:2193–2198. doi: 10.1021/acschembio.5b00448. [DOI] [PubMed] [Google Scholar]
- 115.Singh A, Venkannagari S, Oh KH, et al. Small Molecule Inhibitor of NRF2 Selectively Intervenes Therapeutic Resistance in KEAP1-Deficient NSCLC Tumors. ACS chemical biology. 2016;11:3214–3225. doi: 10.1021/acschembio.6b00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu DS, Duong CP, Haupt S, et al. Inhibiting the system xC-/glutathione axis selectively targets cancers with mutant-p53 accumulation. Nature communications. 2017;8:14844. doi: 10.1038/ncomms14844. [DOI] [PMC free article] [PubMed] [Google Scholar]