Abstract
Introduction
Porphyria cutanea tarda (PCT), the most common human porphyria, due to hepatic deficiency of uroporphyrinogen decarboxylase (UROD) activity, which is acquired in the presence of multiple susceptibility factors. PCT presents clinically with cutaneous blistering photosensitivity and is readily treatable with either repeated phlebotomy or 4-aminoquinoline antimalarial. We performed a systematic review and meta-analysis to compare the effectiveness of these quite different treatment approaches, especially on relapse rates after achieving remission.
Methods
Published studies that included follow up for at least one year after treatment of PCT were included. The primary study outcome was PCT relapse. Pooled data are reported as relapse rate per person year of follow up with 95% confidence intervals (CI).
Results
Of 375 articles identified as pertaining to PCT treatment, 12 were eligible for analysis, and of these 5 used high dose 4-aminoquinoline regimens (2 combined with phlebotomy and 3 without phlebotomy), 5 used low dose 4-aminoquinoline regimens and 3 used phlebotomy. Relapse rates during the year after treatment were similar for the high and low dose 4-aminoquinoline groups (35–36%) and lower in the phlebotomy group (20%). Pooled relapse rates (RR) with their 95% confidence intervals (CI) were 8.6 (3.9–13.3) per 100 person-years in the high dose 4-aminoquinoline group, 17.1 (8.9–25.3) per 100 person-years in the low dose 4-aminoquinoline group, and 5.1 (0.5–10.6) per 100 person-years in the phlebotomy group. Subgroup and sensitivity analyses showed similar results.
Conclusion
Clinical or biochemical relapse rates ranged 5–17 per 100 person years after remission of PCT. Relapses were somewhat more frequent after remission with 4-aminoquinoline regimens as compared to remission after phlebotomy. Prospective studies are needed to better define how often relapses occur with these treatments after documenting both clinical and biochemical remission of PCT.
Keywords: Porphyria cutanea tarda, phlebotomy, hydroxychloroquine, 4-aminoquinolines
Introduction
Porphyria cutanea tarda (PCT) is the most common human porphyria, and is due to an acquired deficiency of hepatic uroporphyrinogen decarboxylase (UROD) activity, resulting in accumulation of the uroporphyrinogen and other highly carboxylated porphyrinogens in the liver, which then enter the plasma mostly as oxidized porphyrins and are excreted in urine.(1) Elevated levels of these porphyrins in plasma cause chronic blistering cutaneous photosensitivity affecting sun exposed areas. The deficient activity of hepatic UROD results from inhibition by a uroporphomethene,(2) which is generated from uroporphyrinogen by an oxidative process in the presence of iron and susceptibility factors that may include alcohol, smoking, hepatitis C virus (HCV) infection, estrogen use, hemochromatosis gene (HFE) mutations, and human immunodeficiency virus (HIV) infection. Approximately 20% of patients are heterozygous for UROD mutation, which reduces UROD to 50% of normal in all tissues from birth, and this represents an additional susceptibility factor. PCT develops only after hepatic UROD activity is further reduced by inhibition to less than ~20% of normal.(3; 4; 5; 6; 7)
PCT is effectively treatable by either repeated phlebotomy, which decreases hepatic iron and interrupts generation of the UROD inhibitor, or 4-aminoquinoline antimalarial (chloroquine or hydroxychloroquine), which mobilize porphyrins that have accumulated in lysosomes and other organelles in hepatocytes.(1; 8; 9; 10; 11) Efficacy of both therapies is high and comparable in terms of rates of remission.(9; 12) Also, we previously reported that times to remission with hydroxychloroquine (HCQ) 100 mg twice weekly or phlebotomy were comparable.(13) But relapse of PCT after successful treatment occurs in some patients after each treatment and comparative data is lacking. In this systematic review and meta-analysis we examined the frequency of PCT relapse after reported clinical remission after phlebotomy or either hydroxychloroquine or chloroquine.
Methods
Search strategy
We followed the MOOSE (Meta-analysis of Observational Studies in Epidemiology) guidelines.(14) A literature search was performed using the search engines Pubmed, Embase, Scopus, CINAHL, and Cochrane database. The initial medical subject headings (MeSH) search terms were: “porphyria cutanea tarda”, “phlebotomy”, “4-aminoquinoline”, “chloroquine” and “hydroxychloroquine”. All databases were searched through December 2017. References cited in these identified articles were also searched to identify additional studies, which may have been missed on the initial search.
Protocol and PICO (Population Intervention Comparison Outcome) format
Population: Patients with PCT achieving remission; Intervention and Comparison: Phlebotomy, high-dose 4-aminoquinolines, or low-dose 4-aminoquinolines; Outcome: Relapse of PCT defined as biochemical (elevated porphyrins in plasma and/or urine) or clinical (appearance of skin lesions of PCT).
Study selection and data extraction
Eligible studies fulfilled the following criteria: (1) study population of PCT patients was treated with either phlebotomy or 4-aminoquinolines, (2) data on relapse of PCT after achieving remission was reported, and (3) relapse was defined as biochemical (elevation of plasma and/or urine porphyrins) or clinical (appearance of skin lesions), and (4) median follow up period was at least one year. Studies with other medical interventions, review articles, interim reports, case reports, and pooled analyses were excluded. Titles, abstracts, and full manuscripts were reviewed independently by three authors (HS, HS, and AKS) for selection of studies for analysis. Selected studies were reviewed independently by the two investigators (HS and HS) for data extraction on patient demographics, treatment regimens and duration, and PCT remission and relapse. Any discrepancies were resolved after joint reviews of the published data and manuscripts by all authors.
End Points and Outcomes
The primary outcome was the pooled relapse rate during follow-up in patients who had achieved remission.
Quality Assessment
The quality of included studies was assessed independently by the first two investigators using the MINORS statement (Methodological Index for Nonrandomized Studies), which is a risk-of-bias assessment tool for nonrandomized studies.(15) Items assessed included selection of cases or cohorts and controls, comparability and information on exposure and outcome. This index provides a maximum possible score of 16 for non-comparative studies, as in this analysis, and 24 for comparative studies. The MINORS checklist was preferred over the Newcastle-Ottawa Quality Assessment Scale(16) because we included studies without a control group and the MINORS checklist allows a quality evaluation in studies with or without a control group. Any discrepancies in the assessments by the two investigators were addressed by discussion among the investigators after further review of the manuscripts.
Statistical analysis
As the follow up period after achieving remission was variable across studies, median follow up was weighted for sample size in each study to calculate the person years of follow up in each study with the assumption that each patient in the study was followed for the median follow up time. Relapse rate for individual studies was calculated by dividing the number of relapses by follow-up person years, with corresponding 95% confidence interval (CI). A random effects model was used for analyzing pooled data for all the analyses.(17) Heterogeneity was measured using I2 statistics for inter-study variance with higher I2 values and lower P values suggesting higher heterogeneity.(18) Publication bias was assessed using the Egger regression and the Begg-Mazumdar rank correlation tests.(19; 20; 21) The Egger test is a regression method checking for association between effect sizes and standard error and uses actual effect size for each study.(21) The Begg-Mazumdar rest is a rank correlation test examining the potential association between effect estimates (taken as a rank and not exact effect size) and sampling variance (or standard error).(20) Funnel plots on each analysis were also developed (Supplementary file).
For analyses for publication bias, the analyses were repeated either by performing sensitivity analysis or using the Duval and Tweedie Trim and Fill method, which is a nonparametric (rank-based) data augmentation technique.(22) This method can be used to estimate the number of studies missing from a meta-analysis, which suppresses the most extreme results on one side of the funnel plot. Then it amplifies the observed data so that the funnel plot is more symmetric and re-computes the summary estimate based on the comprehensive data.(23) All statistical analyses were performed using the Comprehensive Meta-analysis program (Biostat, Englewood, NJ).
Results
Baseline Characteristics
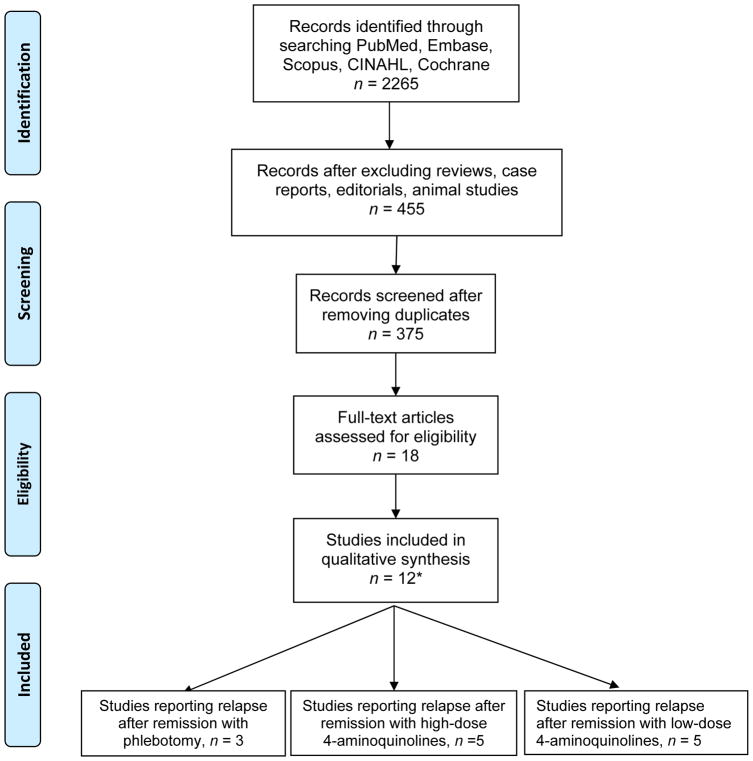
The initial search identified 2,265 citations, of which 12 studies were included in the final analysis (Figure 1). Twelve studies that evaluated treatment of 525 PCT patients by phlebotomy, 4-aminoquinolines (using a low dose regimen, defined as twice weekly use of chloroquine 125 mg or hydroxychloroquine 100 mg, a high dose regimen defined as using higher doses, or both phlebotomy and a 4-aminoquinoline were included in the analysis.(11; 24; 25; 26; 27; 28; 29; 30; 31; 32; 33; 34) Studies were stratified into three categories based on the treatment regimen; the first group was treated with high dose 4-aminoquinoline regimens, (11; 24; 25; 26; 27) the second one with low dose regimens (28; 29; 30; 31; 32) and the third underwent repeated phlebotomies.(32; 33; 34) Data about specific dosages, phlebotomy schedules and duration of therapy are provided in (Table 1). Three studies combined high dose 4-aminoquinoline antimalarial treatment and phlebotomy.(24; 26; 27) Also, 4-aminoquinoline regimens varied in terms of dosages, schedule and duration of treatment, and median follow up. Person-year of follow up was highest in the high dose group followed by low dose and then phlebotomy groups.
Figure 1.
PRISMA flow chart on selection of studies for the analysis
Table 1.
Baseline characteristics of studies included in the meta-analysis
| Study (year) | N | Male (%) | Mean Age |
Therapy | Regimen | Therapy Duration |
End-point | Median FU (years) |
Relapse definition |
Relapse | Person-years | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High Dose 4-aminoquinolines | ||||||||||||
| No phlebotomy | Malkinson (1980)(11) | 6 | 42(66.7) | 50.9 | HCQ | varying dose that increased up to 400 mg daily | 5–13 months | UP 15–281 (ULN<26) mcg/d | 2.4 | Biochemical and/or Clinical | 4 (67%) | 14.4 |
| Tsega (1987)(25) | 46 | 44(95.7) | 46 | Chloroquine | 500 mg/d | 10 days | - | 2.7 | Biochemical and Clinical | 6 (13%) | 124.2 | |
| Phlebotomy | Wennersten (1982)(24) | 21 | 14(66.7) | 55.3 | Chloroquine | 250 mg/d* | 7 days | Drug course | 2.5 | Clinical or Biochemical | 9 (43%) | 52.5 |
| Petersen (1992)(26) | 65 | 42 (64.6) | 43 | HCQ | 250 mg three times daily** | 3 days | Drug course | 2.8 | Clinical and/or Biochemical | 23 (35%) | 182 | |
| Rossmann-Ringdahl (2007)(27) | 57 | 36(63.2) | 59.7 | Chloroquine | 250 mg/d*** | 7 days | Drug course | 11 | Biochemical | 27 (47%) | 627 | |
| Total | 195 | 69 (35%) | 1000.1 | |||||||||
| Low Dose 4-aminoquinolines | ||||||||||||
| Kordac (1977)(28) | 112 | - | - | Chloroquine | 125 mg biw | 8–18 months | UP<100 mcg/d | 4 | - | 38 (34%) | 448 | |
| Ashton (1984)(29) | 7 | 7(100) | 57 | Chloroquine | 125 mg biw | 14.9 months | UP<~100 nmol/d | 2.4 | Biochemical | 4 (57%) | 16.8 | |
| Valls (1994)(30) | 53 | 51 (96.2) | 47.4 | Chloroquine | 125 mg biw x 2wks. then 250 mg biw | 1–12 months (median 8 months) | UP<100 mcg/L | 3 | - | 22 (49%) | 159 | |
| Wollina (2009)(31) | 55 | 41 (65.1) | - | Chloroquine | 125 or 250 mg biw | 12 months | Normal UP | 1 | Biochemical and Clinical | 16 (29%) | 63 | |
| Singal(2015)(32) | 15 | - | - | HCQ | 100 mg twice weekly | - | Plasma porphyrin concentration < 0.9 mcg/dl with cessation of skin lesions | 1 | Biochemical | 8 (53%) | 15 | |
| Total | 242 | 88 (36.3%) | 701.8 | |||||||||
| Phlebotomy | ||||||||||||
| Epstein (1968)(33) | 20 | 14 (70) | Phlebotomy | 0.5 L every ~2 weeks | 2.5–8.5 months | Reduced hemoglobin, serum iron or iron saturation | 3 | Biochemical | 2 (10%) | 60 | ||
| Lundvall (1982)(34) | 44 | - | Phlebotomy | 0.4–0.5 L weekly | 1–7 months | Hemoglobin constantly 10–11 mg/dL | 6 | Biochemical | 9 (20%) | 264 | ||
| Singal (2015)(32) | 24 | - | Phlebotomy | - | - | Plasma porphyrin concentration < 0.9 mcg/dl with cessation of skin lesions | 1 | Biochemical | 7(29%) | 24 | ||
| Total | 88 | 18 (20.5%) | 348 | |||||||||
1–2 phlebotomies 300 mL each at 3–7-day interval,
Phlebotomy 500 mL 1–3 weeks before in 32 patients with very high UP or cirrhosis,
2–3 phlebotomies of average 750 mL of blood,
-; missing data in the original studies, biw; bi-weekly, CI; confidence internal, HCQ; hydroxychloroquine, N; total number of cases, MINORS; Methodological index for non-randomized studies, ULN; upper limit of normal, UP; uroporphyrin, wk.; week,
Study Quality Assessment
Based on the MINORS statement, all studies had a score of 9 or higher. The clear majority of the studies fell short in detected bias and prospective sample size calculation. All were fully published manuscripts except one(32). Two studies had a score of nine(26; 34), four with a score of ten(25; 28; 32), five with a score of eleven(11; 24; 27; 29; 31), and one for score of twelve(33) and another for a score of thirteen(30) (Table 2).
Table 2.
MINORS quality assessment tool of non-randomized studies.(15) Each criterion carries a maximum of two points
| Study | A clearly stated aim | Inclusion of consecutive patients | Prospective collection of data | Endpoints appropriate to the aim of the study | Unbiased assessment of the study endpoint | Follow-up period appropriate to the aim of the study | Loss to follow up less than 5% | Prospective calculation of the study size | Total |
|---|---|---|---|---|---|---|---|---|---|
| Malkinson(11) | 2 | 2 | 2 | 2 | 0 | 1 | 2 | 0 | 11 |
| Wennersten(24) | 2 | 2 | 2 | 2 | 0 | 1 | 2 | 0 | 11 |
| Tsega(25) | 2 | 1 | 2 | 2 | 0 | 2 | 1 | 0 | 10 |
| Petersen (26) | 2 | 0 | 2 | 2 | 0 | 1 | 2 | 0 | 9 |
| Rossmann (27) | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 0 | 11 |
| Kordac (28) | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 0 | 10 |
| Ashton(29) | 2 | 2 | 2 | 2 | 0 | 1 | 2 | 0 | 11 |
| Valls (30) | 2 | 1 | 2 | 2 | 0 | 2 | 2 | 2 | 13 |
| Wollina (31) | 2 | 2 | 2 | 2 | 0 | 1 | 2 | 0 | 11 |
| Singal(32) | 2 | 0 | 2 | 2 | 0 | 2 | 2 | 0 | 10 |
| Epstein (33) | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 |
| Lundvall (34) | 2 | 0 | 2 | 2 | 0 | 2 | 1 | 0 | 9 |
Outcomes
High dose 4-aminoquinoline regimens
As shown in Table 1, relapse was reported in 69 (35.4%, range 13–67%) of the 195 patients in 5 studies treated with high dose regimens of hydroxychloroquine or chloroquine. The median follow-up in these studies ranged from 2.4 to 11 years, with a total follow up of 1000.1 person-years. The pooled relapse rate (RR) with 95% confidence interval was 8.6 (3.9–13.3) per 100 person-years (Figure 2). The data were heterogeneous (I2=75%, P<0.01), without evidence of publication bias as assessed by the Begg-Mazumdar test (P=0.46) and the Egger test (P=0.06). Notably, the regimens in this group of 5 studies were quite variable, and subgroup analysis was carried out based on whether or not a preceding phlebotomy schedule was used before administering high dose 4-aminoquinolines. The pooled relapse rate (95% CI) from two studies using high dose 4-aminoquinolines without preceding phlebotomy was 12.2 (−0.09–33.2) per 100 person-years, with heterogeneous data (I2=62%, P=0.1). The pooled relapse rate (95% CI) from three studies using high dose 4-aminoquinolines with preceding phlebotomy was 10.2 (2.5–17.9) per 100 person-years with heterogeneous data (I2=85%, P<0.01). Further analyses were not done due to low number of studies.
Figure 2.
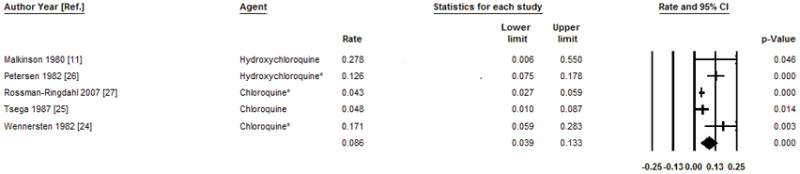
Pooled annual relapse rate and 95% confidence intervals per 100 person year after treatment of PCT with high dose 4-aminoquinolines. The bottom line shows the pooled annual relapse rate using the random effects model.
Low dose 4-aminoquinoline treatment
In this group of 5 studies, which included 242 patients treated with low dose 4-aminoquinoline regimens, the median follow-up ranged from 1 to 4 years, with a total of 701.8 person-years of follow up. Relapse was reported in 88 patients (36.3%). The pooled relapse rate (RR) with 95% confidence interval was 17.5 (8.9–25.3) per 100 person-years (Figure 3). The data were heterogeneous (I2=75%, P=0.003), with evidence of publication bias as assessed Egger’s test (P=0.014) but not by the Begg-Mazumdar test (P=0.46). Sensitivity analysis after excluding the study reported as an abstract, (31) showed similar effect size 14.5 (7.5–21.5) per 100 person-years, with heterogeneous data (I2=70, P=0.018) without publication bias (Egger’s test P=0.37 and Begg-Mazumdar test P=0.99).
Figure 3.
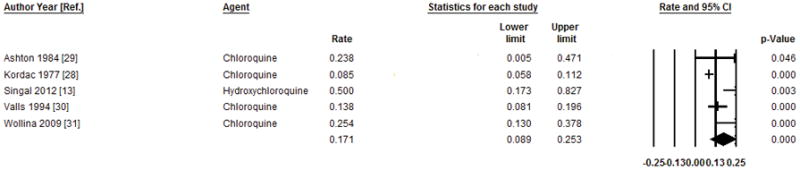
Pooled annual relapse rate and 95% confidence intervals per 100 person year after treatment of PCT with low dose 4-aminoquinolines. The bottom line shows the pooled annual relapse rate using the random effects model.
Treatment by phlebotomy
Phlebotomy treatment was reported in 3 studies comprised of 88 patients; 18 patients relapsed (20.5%) during median follow-up periods ranging from 1 to 6 years with a total of 348 person-years of follow up. Relapse rates among the 3 studies ranged from 10% to 29%. The pooled relapse rate (RR) with 95% confidence interval was 6.5 (1.2–11.7) per 100 person-years (Figure 4). The data were heterogeneous (I2=66%, P=0.03), without evidence of publication bias as assessed by Begg-Mazumdar test (P=0.09) and Egger’s test (P=0.09). Sensitivity analysis after excluding the data from the study reported only in abstract form(32), showed a similar effect size with a pooled relapse rate of 4.2 (1.1–7.2) per 100 person-years with homogenous data (I2=32%, P=0.23).
Figure 4.

Pooled annual relapse rate and 95% confidence intervals per 100 person year after treatment of PCT with phlebotomy regimen. The bottom line shows the pooled annual relapse rate using the random effects model.
Discussion
Phlebotomy is considered first line therapy for PCT at most centers, especially when the disease is associated with substantial iron overload.(7; 8; 33; 35; 36; 37; 38; 39) However, phlebotomy is expensive, time-consuming, inconvenient, and uncomfortable, and may cause side effects such as syncope or anemia. Therefore, compliance with a scheduled regimen of repeated phlebotomy is often challenging.(13) Most available evidence suggests similar rates of remission, with either repeated phlebotomy or a 4-aminoquinoline regimen. Also, we found in a previous prospective study that times to remission were comparable in patients treated with low dose hydroxychloroquine or phlebotomy.(13) However, there is less comparative information on relapse rates, which is an important issue in choosing treatment, but requires long-term follow up after treatment is completed and remission is documented. In this meta-analysis, we examine published data on relapse rates with these two quite different approaches to treatment of PCT. To our knowledge, this is the first meta-analysis that formally compares relapse rates after these treatments.
We found that PCT relapse rates are similar for high and low dose 4-aminoquinoline regimens (35.4% vs. 36.3%), but were somewhat lower with phlebotomy (20.5%). Likewise, the pooled relapse rate (RR) of PCT per 100 person-years is lowest after achieving remission with phlebotomy compared to when the remission was achieved with low or high dose 4-aminoquinolines.
It should be noted that, with few exceptions, the reports examined in this study included patients who underwent only one of these treatments, and therefore did not directly compare relapse rates with the alternative treatment. As a result, relapse rates in each study might in part reflect clinical or other reasons for choosing one type of treatment over the other. Also, the choice of treatment among the studies included in this analysis may have been affected by the presence of certain susceptibility factors or the degree of iron overload. Whether susceptibility factors, such as alcohol or estrogen use, smoking, HCV or HIV infection, or their removal during treatment, can influence treatment response is uncertain. But it seems likely that relapse rate is especially likely to be influenced by removal, treatment or the continued presence of such factors, and this deserves attention in future prospective studies. Relapse rate is also likely to depend upon the endpoint chosen for discontinuation of treatment. For phlebotomy this has been standardized by achievement of a ferritin level near the lower limit of normal. For 4-aminoquinolines, treatment is usually discontinued after normalization of porphyrin levels. The endpoints for both treatments and criteria for diagnosis of remission of PCT were not uniform within and across all studies we examined, and the number of studies was too small to ascertain whether such differences might influence rates of relapse.
An effect of chloroquine to increase urinary porphyrin levels and photosensitivity in patients with PCT was first reported by Davis et al in 1957,(40) and the first therapeutic uses of chloroquine in PCT were also reported in 1957.(41; 42) “High-dose” regimens of chloroquine and hydroxychloroquine correspond to standard doses used for other treatment indications, and in PCT cause a transient toxic hepatitis, with fever, nausea, vomiting, abdominal pain, headache, and myalgia,(43; 44; 45; 46) accompanied by liver enzyme elevations and further marked elevation of plasma and urine porphyrins.(24; 26; 43; 44; 45; 46; 47) The transient increases in porphyrins to levels exceeding those before treatment is due to rapid mobilization of porphyrins from lysosomes and other intracellular organelles. The highest liver enzyme elevations were encountered in phlebotomized women during high dose hydroxychloroquine treatment, with peak liver enzyme elevations on days 3–4 of treatment and normalization of enzymes by day 30.(26) Patients were usually hospitalized for such high dose treatment regimens,(26) and development of transient ascites and hemolysis were described.(46) High dose regimens have fallen out of favor, and low dose regimens are more attractive due to a much lower side effects profile.(26; 29) As an alternative to phlebotomy, we prefer a regimen of hydroxychloroquine of 100 mg twice weekly until plasma and urine porphyrins are normalized as this dose has been reported to achieve remission as rapidly as repeated phlebotomy.(13) Although, both low dose hydroxychloroquine and phlebotomy regimen were safe in this study, side effects even at this low dose regimen may occasionally occur, and would likely be greater with somewhat higher doses.
The current meta-analysis, like others, is limited by the possibility of publication bias and subsequent overestimation of the true effect size due to negative study identification failure.(48) We combined searches from PubMed/Medline, Embase and Cochrane with manual searches in order to minimize this possibility. Although we used procedures in agreement with current guidelines, we may have overlooked studies that were not accessible.(48) Another limitation is the inclusion of case-control studies which are prone to biases in terms of case selection and reporting and to inherent confounding factors. However, no randomized controlled studies are available and are unlikely to be reported comparing relapse after these treatment regimens, because patients or physicians frequently chose one or the other treatment regimen based on patient characteristics and/or preference. Also, two of the included thirteen studies in the meta-analysis had a short one-year median follow-up (31; 32) and PCT may recur after more than one year. Finally, all initial analyses were heterogeneous for which subgroup analysis addressed this issue except for the high dose group.
Our findings of relapse rates of about 5–17 per 100 person years after remission of PCT justify at least annual follow up visit for clinical assessment for skin lesions, biochemical profile for porphyrins, and to check patient compliance on control of susceptibility factors. Further, the heterogeneous data as observed from these analyses clearly identifies clinical unmet need for developing homogeneous prospective long-term follow up data to examine relapse rates of PCT after remission by phlebotomy or low dose hydroxychloroquine. Properly designed studies overcoming the limitations identified in this analysis would form the basis for developing strategies and maintenance therapies to prevent such relapses. Prospective studies of well characterized patients with PCT to examine relapse rates after documenting remission with phlebotomy or low dose hydroxychloroquine are currently being conducted by the Porphyrias Consortium.
Supplementary Material
Acknowledgments
We acknowledge the expert assistance of Carolyn Holmes at the University of Alabama in conducting the literature searches for this study.
Supported in part by grants from the Institute for Translational Sciences at the University of Texas Medical Branch, supported in part by a Clinical and Translational Science Award (UL1TR000071) from the National Center for Advancing Translational Sciences, National Institutes of Health, and the American Porphyria Foundation.
List of Abbreviations
- PCT
Porphyria Cutanea Tarda
- UROD
Uroporphyrinogen Decarboxylase
References
- 1.Elder G. Porpheria cutanea tarda and related disorders (chapter 88) In: Kadish KM, Smith K, Guilard R, editors. Porphyrin Handbook, Part II. Vol. 2003. San Diego: Academic Press; 2003. pp. 67–92. [Google Scholar]
- 2.Phillips JD, Bergonia HA, Reilly CA, et al. A porphomethene inhibitor of uroporphyrinogen decarboxylase causes porphyria cutanea tarda. Proc Natl Acad Sci U S A. 2007;104:5079–5084. doi: 10.1073/pnas.0700547104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lundvall O, Weinfeld A, Lundin P. Iron storage in porphyria cutanea tarda. Acta medica Scandinavica. 1970;1–2:37–53. doi: 10.1111/j.0954-6820.1970.tb08003.x. [DOI] [PubMed] [Google Scholar]
- 4.Bygum A, Brandrup F, Christiansen L, et al. Porphyria cutanea tarda. Ugeskr Laeger. 2000;162:2020–2024. [PubMed] [Google Scholar]
- 5.Bonkovsky HL, Lambrecht RW, Shan Y. Iron as a co-morbid factor in nonhemochromatotic liver disease. Alcohol. 2003;30:137–144. doi: 10.1016/s0741-8329(03)00127-7. [DOI] [PubMed] [Google Scholar]
- 6.Gorman N, Zaharia A, Trask HS, et al. Effect of an oral iron chelator or iron-deficient diets on uroporphyria in a murine model of porphyria cutanea tarda. Hepatology. 2007;46:1927–1834. doi: 10.1002/hep.21903. [DOI] [PubMed] [Google Scholar]
- 7.Dereure O, Jumez N, Bessis D, et al. Measurement of liver iron content by magnetic resonance imaging in 20 patients with overt porphyria cutanea tarda before phlebotomy therapy: a prospective study. Acta Derm Venereol. 2008;88:341–345. doi: 10.2340/00015555-0472. [DOI] [PubMed] [Google Scholar]
- 8.Rocchi E, Gibertini P, Cassanelli M, et al. Serum ferritin in the assessment of liver iron overload and iron removal therapy in porphyria cutanea tarda. The Journal of laboratory and clinical medicine. 1986;107:36–42. [PubMed] [Google Scholar]
- 9.Malina L, Chlumsky J. A comparative study of the results of phlebotomy therapy and low-dose chloroquine treatment in porphyria cutanea tarda. Acta Derm Venereol. 1981;61:346–350. [PubMed] [Google Scholar]
- 10.Goerz G, Bolsen K, Merk H. Influence of chloroquine on the porphyrin metabolism. Archives of dermatological research. 1985;277:114–117. doi: 10.1007/BF00414107. [DOI] [PubMed] [Google Scholar]
- 11.Malkinson FD, Levitt L. Hydroxychloroquine treatment of porphyria cutanea tarda. Arch Dermatol. 1980;116:1147–1150. [PubMed] [Google Scholar]
- 12.Cainelli T, Di Padova C, Marchesi L, et al. Hydroxychloroquine versus phlebotomy in the treatment of porphyria cutanea tarda. Br J Dermatol. 1983;108:593–600. doi: 10.1111/j.1365-2133.1983.tb01062.x. [DOI] [PubMed] [Google Scholar]
- 13.Singal AK, Kormos-Hallberg C, Lee C, et al. Low-dose hydroxychloroquine is as effective as phlebotomy in treatment of patients with porphyria cutanea tarda. Clin Gastroenterol Hepatol. 2012;10:1402–1409. doi: 10.1016/j.cgh.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 15.Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 16.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [accessed 03/24/2014 2014];2014 [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Bmj. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.JJD, DGA, MJB . Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger DSGM, Altman DG, editors. Systematic Reviews in Health Care: Meta- Analysis in Context. 2. London: BMJ Books; 2005. pp. 285–312. [Google Scholar]
- 20.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 21.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 23. [accessed June 20 2016];R: Trim and Fill Analysis for ‘rma.uni’ Objects. 2016 http://finzi.psych.upenn.edu/library/metafor/html/trimfill.html.
- 24.Wennersten G, Ros AM. Chloroquine in treatment of porphyria cutanea tarda. Long-term efficacy of combined phlebotomy and high-dose chloroquine therapy. Acta dermato-venereologica Supplementum. 1982;100:119–123. [PubMed] [Google Scholar]
- 25.Tsega E. Long-term effect of high-dose, short-course chloroquine therapy on porphyria cutanea tarda. Q J Med. 1987;65:953–957. [PubMed] [Google Scholar]
- 26.Petersen CS, Thomsen K. High-dose hydroxychloroquine treatment of porphyria cutanea tarda. J Am Acad Dermatol. 1992;26:614–619. doi: 10.1016/0190-9622(92)70090-3. [DOI] [PubMed] [Google Scholar]
- 27.Rossmann-Ringdahl I, Olsson R. Porphyria cutanea tarda: effects and risk factors for hepatotoxicity from high-dose chloroquine treatment. Acta Derm Venereol. 2007;87:401–405. doi: 10.2340/00015555-0260. [DOI] [PubMed] [Google Scholar]
- 28.Kordac V, Papezova R, Semradova M. Chloroquine in the treatment of porphyria cutanea tarda. N Engl J Med. 1977;296:949. [PubMed] [Google Scholar]
- 29.Ashton RE, Hawk JL, Magnus IA. Low-dose oral chloroquine in the treatment of porphyria cutanea tarda. The British journal of dermatology. 1984;111:609–613. doi: 10.1111/j.1365-2133.1984.tb06632.x. [DOI] [PubMed] [Google Scholar]
- 30.Valls V, Ena J, Enriquez-De-Salamanca R. Low-dose oral chloroquine in patients with porphyria cutanea tarda and low-moderate iron overload. J Dermatol Sci. 1994;7:169–175. doi: 10.1016/0923-1811(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 31.Wollina U, Kostler E, Koch A, et al. Does chloroquine therapy of porphyria cutanea tarda influence liver pathology? Int J Dermatol. 2009;48:1250–1253. doi: 10.1111/j.1365-4632.2009.04205.x. [DOI] [PubMed] [Google Scholar]
- 32.Singal AK, Gou E, Rizwan M, et al. Relapse of Porphyria Cutanea Tarda After Achieving Remission With Phlebotomy or Low Dose Hydroxychloroquine. Hepatology. 2015;62:1231A–1232A. [Google Scholar]
- 33.Epstein JH, Redeker AG. Porphyria cutanea tarda. A study of the effect of phlebotomy. N Engl J Med. 1968;279:1301–1304. doi: 10.1056/NEJM196812122792402. [DOI] [PubMed] [Google Scholar]
- 34.Lundvall O. Phlebotomy treatment of porphyria cutanea tarda. Acta dermato-venereologica Supplementum. 1982;100:107–118. [PubMed] [Google Scholar]
- 35.Ratnaike S, Blake D, Campbell D, et al. Plasma ferritin levels as a guide to the treatment of porphyria cutanea tarda by venesection. The Australasian journal of dermatology. 1988;29:3–8. doi: 10.1111/j.1440-0960.1988.tb01216.x. [DOI] [PubMed] [Google Scholar]
- 36.Ippen H. Treatment of porphyria cutanea tarda by phlebotomy. Semin Hematol. 1977;14:253–259. [PubMed] [Google Scholar]
- 37.Ramsay CA, Magnus IA, Turnbull A, et al. The treatment of porphyria cutanea tarda by venesection. Q J Med. 1974;43:1–24. [PubMed] [Google Scholar]
- 38.Lundvall O. The effect of phlebotomy therapy in porphyria cutanea tarda. Its relation to the phlebotomy-induced reduction of iron stores. Acta medica Scandinavica. 1971;189:33–49. doi: 10.1111/j.0954-6820.1971.tb04337.x. [DOI] [PubMed] [Google Scholar]
- 39.Epstein JH, Redeker AG. Porphyria cutanea tarda symptomatica (PCT-S). A study of the effect of phlebotomy therapy. Archives of dermatology. 1965;92:286–289. discussion 289–290. [PubMed] [Google Scholar]
- 40.Davis MJ, Ploeg DE. Acute porphyria and coproporphyrinuria following chloroquine therapy; a report of two cases. AMA archives of dermatology. 1957;75:769–800. [PubMed] [Google Scholar]
- 41.London ID. Porphyria cutanea tarda; report of case successfully treated with chloroquine. AMA archives of dermatology. 1957;75:801–803. doi: 10.1001/archderm.1957.01550180015004. [DOI] [PubMed] [Google Scholar]
- 42.Colomb D. Three cases of delayed cutaneous porphyria in adults and two cases of photodermatitis with good reaction to nivaquine therapy. Bull Soc Fr Dermatol Syphiligr. 1957;39:420–421. [PubMed] [Google Scholar]
- 43.Felsher BF, Redeker AG. Effect of chloroquine on hepatic uroporphyrin metabolism in patients with porphyria cutanea tarda. Medicine (Baltimore) 1966;45:575–583. doi: 10.1097/00005792-196645060-00024. [DOI] [PubMed] [Google Scholar]
- 44.Sweeney GD, Saunders SJ, Dowdle EB, et al. EFFECTS OF CHLOROQUINE ON PATIENTS WITH CUTANEOUS PORPHYRIA OF THE “SYMPTOMATIC” TYPE. Br Med J. 1965;1:1281–1285. doi: 10.1136/bmj.1.5445.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kowertz MJ. The therapeutic effect of chloroquine. Hepatic recovery in porphyria cutanea tarda. Jama. 1973;223:515–519. [PubMed] [Google Scholar]
- 46.Vogler WR, Galambos JT, Olansky S. Biochemical effects of chloroquine therapy in porphyria cutanea tarda. Am J Med. 1970;49:316–321. doi: 10.1016/s0002-9343(70)80022-5. [DOI] [PubMed] [Google Scholar]
- 47.Swanbeck G, Wennersten G. Treatment of porphyria cutanea tarda with chloroquine and phlebotomy. Br J Dermatol. 1977;97:77–81. doi: 10.1111/j.1365-2133.1977.tb15431.x. [DOI] [PubMed] [Google Scholar]
- 48.Thornton A, Lee P. Publication bias in meta-analysis: its causes and consequences. J Clin Epidemiol. 2000;53:207–216. doi: 10.1016/s0895-4356(99)00161-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.