Abstract
Background
We hypothesized that maternal nutrient restriction (NR) would increase activity and behavioral indicators of anxiety (self-directed behaviors, SDB) in captive baboons (Papio sp.), and result in more protective maternal styles.
Methods
Our study included 19 adult female baboons. Seven females ate ad libitum (control group) and eight females ate 30% less (NR group) and were observed through pregnancy and lactation.
Results
Control females engage in higher rates of SDB than NR females overall (p ≤ 0.018) and during the prenatal period (p ≤ 0.001) and engage in more aggressive behavior (p ≤ 0.033). Control females retrieved infants more than NR females during weeks 5-8 postpartum (p ≤ 0.019).
Conclusions
Lower SDB rates among prenatal NR females reduce energy expenditure and increase available resources for fetal development when nutritionally restricted. Higher infant retrieval rates by controls may indicate more infant independence rather than maternal style differences.
Keywords: developmental programming, nutrition, mother-infant interactions
Introduction
Poor nutrition during pregnancy has significant negative effects on fetal growth, offspring health, and subsequent cognitive, behavioral, and emotional function in humans and several animal species [1–8]. While low birth weight is one outcome observable at birth, poor maternal nutrition has also been found to increase the risk for adult-onset conditions, including hypertension, cardiovascular disease, obesity, and diabetes [1]. In addition to physiological changes, prenatal undernutrition has been shown to alter cognitive performance of the offspring in a variety of species. For example, in sheep, Erhard et al. [9] found that ewes fed 50% less than control ewes during the first two-thirds of gestation had offspring with increased emotional reactivity and male offspring showed reduced cognitive flexibility, having difficulty transferring learning between tasks. Both male and female sheep also displayed sex-specific changes in side preferences during a T-maze test with males shifting from a right-bias to neutral and females shifting to a left-bias. In baboons, juvenile offspring of nutrient restricted mothers demonstrated less motivation and impaired working memory and male offspring of these same mothers showed impaired learning and increased impulsivity [8]. Similar deficits in cognitive flexibility and increased emotional reactivity have been found in other animal models [10]. Interestingly, cognitive inflexibility has been found in both prenatal and grand-maternal malnutrition in rats [11], suggesting developmental malnutrition may lead to permanent and heritable changes in cognition.
The field of developmental programming suggests that these enduring outcomes are the result of specific insults during critical periods in the development of the organism [3, 5, 12–15]. The mechanisms by which these behavioral changes occur remain elusive. A number of nonexclusive biological mechanisms have been proposed, including changes in organ structure, changes in organ cell number, clonal selection, metabolic differentiation (including changes to hormone and enzyme activity and epigenetic gene regulation), and the polyploidization of the main cells of the liver [2, 14, 15]. The role of hormones as the programming mechanism, particularly those involved with regulation of the hypothalamic-pituitary-adrenal (HPA) axis, has received considerable attention from researchers examining nutrient restriction (NR) in rodents and sheep [16–19].
Our research on the long-term effects of moderate global maternal nutrient restriction during pregnancy and lactation in socially-housed baboons (Papio sp.) looks at the effects of nutrient restriction across a variety of physiological processes [8, 20–34]. The results from these studies have confirmed that offspring born to mothers restricted to 70% of normal nutrient intake weigh approximately 12% less than offspring born to unrestricted mothers and said offspring have thus been described as intrauterine growth restricted (IUGR) [35]. The IUGR individuals included in our studies demonstrate a number of physiological, cognitive, and behavioral differences when compared to individuals who developed under nutritional control conditions. For example, IUGR resulted in upregulation of the HPA axis [26], increased fetal cortisol [34], impaired ventricular function [33], insulin resistance [21], premature brain aging among females [32], reduced cognitive flexibility [8], and behavioral changes including low arousal, poor attention, and a difficulty modulating activity [36]. Furthermore, IUGR offspring displayed increased aggression at 3-5 years of age, particularly among males [23]. In that study, Huber and colleagues speculated that one unexplored explanation for the increased aggression may have been social, with aggression being a behavior learned from their NR mothers, as maternal behavior in nonhuman primates has been shown to have considerable effects on adult offspring behavior [37, 38]. However, little is currently known about how changes in maternal behavior and performance contribute to long term behavioral/cognitive outcomes in offspring of nutrient restricted mothers [17, 39]. Therefore, in the current study, we explore maternal behavior and performance during NR to better understand the variety of mechanisms that may be influencing these negative outcomes.
A number of studies have demonstrated that maternal behavior, especially that pertaining to anxiety and mother-infant interactions, can have long-lasting effects on offspring behavior during adulthood among nonhuman primates [40–44] and other animals [9, 17, 45, 46]. In particular, maternal style has been determined to play a central role in the development of individual temperament [47–50], including patterns of reactions to stress [40, 41, 51, 52] and future maternal behavior [42].
In this study we investigated self-directed behavior (SDB), an often used indicator of anxiety in nonhuman primates [41, 53–55], and social interactions in NR mothers both before and after parturition, as well as a number of aspects of mother-infant interactions during infancy. Research on NR has generated (albeit mostly anecdotal) reports of adverse effects on sociability and temperament in nonhuman primates [56], increases in activity levels and stereotypic behaviors in nonhuman primates [57], and increased levels of glucocorticoids in rodents [18, 19, 58]. Our team has now demonstrated that NR in baboons can result in increased fetal cortisol levels and an up-regulation of the HPA axis in fetal baboons [26, 59]. We therefore hypothesized that NR females would show increased activity levels, reduced sociability, increased rates of aggression, and increased rates of SDB compared to control females. Also, because the presence of an infant following birth increases overall rates of sociality [60] and SDB rates have been shown to increase after birth [41], we expected that rates of aggression and SDB would be greatest in NR mothers during the postpartum period.
If NR results in an increase in stress, maternal style also may be affected. Female baboons of low rank are known to be more protective of their infants [42, 61–64] and it has been suggested that this is the result of increased stress due to limited social support networks [41]. Furthermore, baboon females typically experience an increase in cortisol during the last two weeks of gestation that has been correlated with increased mother-infant affiliative interactions after parturition [65] and mothers who are more attentive to infant distress signals during the first eight weeks of life [66]. It thus follows that anxiety due to nutritional stress may also lead to an increase in protectiveness by baboon mothers. We therefore hypothesized that, relative to controls, NR mothers will exhibit more time in contact with infants, less time out of arm’s reach, and higher rates of infant restraint.
Materials and Methods
Humane Care Guidelines
The study animals were housed in two intact social groups at The Texas Biomedical Research Institute (TBRI), formerly the Southwest Foundation for Biomedical Research. Each group consisted of 1 vasectomized male and up to 16 females per cage (3.5m high with a floor area of 37m2); social grouping and feeding procedures have been described in detail elsewhere [67]. Briefly, once daily all animals were transferred to individual feeding cages for a 2-hour period where they were fed a controlled diet of Purina Monkey Diet 5038 biscuits. Control animals (N = 8) were fed ad libitum, while NR females (N = 11) were fed 70% of feed consumed by controls on a weight-adjusted basis throughout the study period [68]. All procedures were approved by the TBRI Institutional Animal Care and Use Committee and conducted in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care.
Maternal Behavior
Beginning 8 weeks prior to the expected date of parturition the behavior of each pregnant socially-housed female was recorded with a handheld video for three 20-minute sample periods per week whenever possible. Video collection continued until the infant reached 12 weeks of age (416 observations, 139 hours total). Care was taken to ensure that observation samples were equally distributed between morning and afternoon hours. All videotaped observations were subsequently coded by the first author (LEOL) using The Observer Video-Pro® (Noldus Information Technology Inc., Leesburg, VA).
Activity level was measured by the percent time per observation period the subject spent engaged in walking, running, climbing, hanging, or fighting states; due to the infrequency or short duration of many of these behaviors, all active behaviors were also combined into a general “active" category. All other states were categorized as “inactive” behaviors, including sitting, laying down, and sleeping. Social behaviors were defined following Ramirez [60] and include the percent time engaged in social grooming (either as the recipient or groomer), the rate per minute observed of affiliative events (e.g., approach, attempt touch, embrace, enlist groom, groom other, huddle, inspect genitals, muzzle/muzzle, present, touch, receive affiliative behavior from other), and the rate per minute observed of aggressive behaviors (e.g., bite, display, fight, hit, lunge, open mouth, pull, pull hair, round mouth, stare, yawn, receive aggressive behavior from other). Rates of receiving aggression from others were also analyzed separately. Following Bardi, French, Ramirez and Brent [65], SDB include scratch self, muzzle wipe, brow wipe, and mantle shake. Stereotypical self-directed behaviors (e.g., pull/eat own hair, head toss, clasping own body, suck self, other abnormal behavior) were also included in the ethogram but were rarely observed (n = 8) and therefore excluded from the analyses. All behavioral categories were analyzed as behavioral category aggregates as well as individual behaviors.
Mother-Infant Interactions
Maternal behavioral was coded based on categories developed by Ramirez [60] and included two main dimensions: interaction behaviors and proximity. Mother-infant interaction behaviors included break contact, dangle, detach infant, drag infant, keep from nursing, make contact, restrain infant, retrieve infant, and rough handling. Rejection was categorized as breaking contact, detaching infant, and keeping infant from nursing. Protective behaviors included make contact, restrain infant, and retrieve infant. Mother-infant proximity was categorized as ventral contact, dorsal contact, inappropriate contact, other contact, within arm’s reach, or greater than arm’s reach. Different forms of contact (ventral, dorsal, inappropriate, and other) were then aggregated and proximity was analyzed as in contact, within arm’s reach, or greater than arm’s reach. Proximity and several mother-infant interaction behaviors (including dangle, drag infant, restrain infant, retrieve infant, and rough handling) were recorded as states and calculated as percent time per observation, while all other behaviors were recorded as events and calculated as rate per minute observed. Infants were expected to transition from dependence to independence during the postpartum observation period. Consequently, the postpartum period was divided into three four-week sub-periods (weeks 1-4, 5-8, and 9-12).
Dominance Coding and Infant Sex
We examined the effects of dominance on both female behavior and mother-infant interactions with a dominance hierarchy established for each cage prior to the onset of detailed behavioral observations following methods described by Bramblett [69]. We also included the sex of the infant when analyzing rates of protective behaviors. However, because neither dominance nor sex of the infant resulted in any significant effects, these analyses are not presented.
Statistical Analysis
Statistical analyses were performed using SPSS® statistical software to identify statistically significant differences due to time, treatment group (control vs. NR), and their interaction. Effect sizes were also calculated using eta. Event frequency rates per minute were calculated for all event behavior categories. Durations of active and inactive behaviors as well as social grooming were calculated as percentages of total time. Prenatal, postpartum, and overall means for each behavior were calculated for all subjects. Repeated measures ANOVAs were then used to simultaneously test for the effects of treatment group, time (prenatal vs. post-partum or age of infant), and the interaction between treatment group and time. The significance level for all tests was set at a two-tailed alpha level of p ≤ 0.05 (indicated in figures and captions as *).
Results
Female Behavior
Activity level
Activity level (the percent time per observation the subject spent engaged in walking, running, climbing, hanging, or fighting states) did not differ between the prenatal and post-partum periods (p > 0.05) (Table 1). Activity level did not differ between control females and NR females (p > 0.05). The interaction between treatment and maternal condition did not affect activity level (p > 0.05).
Table 1.
Female Behavior Results
| Categorical comparison (value ± SE)
|
||
|---|---|---|
| Prenatal | Post-partum | |
| Effect of parturition | ||
| Active | 4.81% ± 0.66% | 5.01% ± 0.54% |
| Affiliative | 0.510/min ± 0.04 | 1.113/min ± 0.10 |
| Aggressive | 0.030/min ± 0.01 | 0.066/min ± 0.01 |
| Rec aggression | 0.009/min ± 0.00 | 0.024/min ± 0.00 |
| SDB | 1.067/min ± 0.07 | 0.631/min ± 0.06 |
| Scratch self | 0.606/min ± 0.05 | 0.336/min ± 0.05 |
| Categorical comparison (value ± SE)
|
||
|---|---|---|
| Control | NR | |
| Effect of treatment | ||
| Activity | 4.6% ± 0.8% | 5.2% ± 0.8% |
| Affiliative | 0.916/min ± 0.08 | 0.706/min ± 0.08 |
| Aggression | 0.064/min ± 0.01 | 0.032/min ± 0.01 |
| Rec aggression | 0.023/min ± 0.00 | 0.012/min ± 0.00 |
| SDB | 0.987/min ± 0.07 | 0.711/min ± 0.07 |
| Scratch self | 0.569/min ± 0.06 | 0.372/min ± 0.06 |
| Categorical comparison (value ± SE)
|
||
|---|---|---|
| Control | NR | |
| Interaction of parturition and treatment | ||
| Activity | ||
| prenatal | 4.2% ± 1.0% | 5.3% ± 0.1% |
| post-partum | 5.0% ± 0.1% | 5.0% ± 0.1% |
| Affiliative | ||
| prenatal | 0.563/min ± 0.05 | 0.457/min ± 0.05 |
| post-partum | 1.269/min ± 0.14 | 0.956/min ± 0.13 |
| Aggression | ||
| prenatal | 0.044/min ± 0.02 | 0.017/min ± 0.01 |
| post-partum | 0.084/min ± 0.01 | 0.047/min ± 0.01 |
| Rec aggression | ||
| prenatal | 0.013/min ± 0.00 | 0.006/min ± 0.00 |
| post-partum | 0.032/min ± 0.01 | 0.018/min ± 0.01 |
| SDB | ||
| prenatal | 1.277/min ± 0.10 | 0.857/min ± 0.09 |
| post-partum | 0.696/min ± 0.09 | 0.566/min ± 0.09 |
| Scratch self | ||
| prenatal | 0.761/min ± 0.07 | 0.450/min ± 0.07 |
| post-partum | 0.377/min ± 0.08 | 0.295/min ± 0.07 |
Affiliative behavior
Affiliative behavior was significantly higher after the birth of an infant (F(1,13) = 41.417, p ≤ 0.001, eta = 0.87) (Figure 1A). Rates of affiliative behaviors did not differ between control females and NR females (p > 0.05). The interaction between treatment and maternal condition did not affect rates of affiliative behaviors (p > 0.05).
Figure 1. Rates per minute of female behaviors.

1A Rates of affiliative behaviors; 1B Rates of aggressive behaviors; and 1C Rates of self-directed behaviors. Data are means, with one standard error of the mean (SEM) represented by vertical bars. Control N = 7, NR N = 8. *There was a significant effect of parturition on prenatal and post-partum means (p ≤ 0.05; repeated measures ANOVA). ** There was a significant effect of treatment group between control and NR females (p ≤ 0.05; repeated measures ANOVA). † While the interaction between parturition and treatment group only approached significance (p ≤ 0.1; repeated measures ANOVA), during the prenatal period control females exhibited higher rates of SDB than NR females (p ≤ 0.05; one-way ANOVA).
Aggression
Aggression was significantly higher after the birth of an infant (F(1,13) = 6.934, p = 0.021, eta = 0.59) (Figure 1B). There was a significant simple main effect of treatment group on rates of aggressive behavior (F(1,13) = 5.705, p = 0.033, eta = 0.55) with control females exhibiting higher rates than NR females. The interaction between treatment group and maternal condition did not affect rates of aggressive behavior (p > 0.05).
Rates of receiving aggression were higher after the birth of an infant (F(1,13) = 11.318, p = 0.005, eta = 0.68). Rates of receiving aggression between control and NR females approached significance (F(1,13) = 3.285, p = 0.093, eta = 0.202) with control females receiving aggression more frequently compared to NR females. The interaction between treatment group and maternal condition did not affect rates of receiving aggression (p > 0.05).
Self-directed behaviors (SDB)
Self-directed behaviors (SDB) were significantly lower after the birth of an infant (F(1,13) = 30.779, p ≤ 0.001, eta = 0.84) (Figure 1C). There was a significant simple main effect of treatment group on SDB rates (F(1,13) = 7.359, p = 0.018, eta = 0.60) with control females exhibiting higher rates than NR females. The interaction between treatment group and maternal condition on rates of SDB only approached significance (F (1,13) = 3.385, p = .089, eta = 0.45). Post-hoc analysis revealed a significant difference in SDB rates between control and NR females during the prenatal period (F(1,16) = 16.428, p = 0.001) with control females exhibiting higher rates than NR females.
Within SDB behaviors, there was also a significant decrease in rates of self-scratching (F(1,13) = 17.435, p = 0.001, eta = 0.76). There was a significant simple main effect of treatment group on scratch self (F(1,13) = 6.036, p = 0.029, eta = 0.56) with control females exhibiting higher rates than NR females. The interaction between treatment group and maternal condition only approached significance for scratch self (F(1,13) = 3.158, p = 0.099, eta = 0.44). Post-hoc analysis revealed a significant difference between control and NR females during the prenatal period (F(1,16) = 16.428, p = 0.001) with control females exhibiting higher rates than NR females (Figure 2).
Figure 2. Rates per minute of individual SDB during the prenatal period.
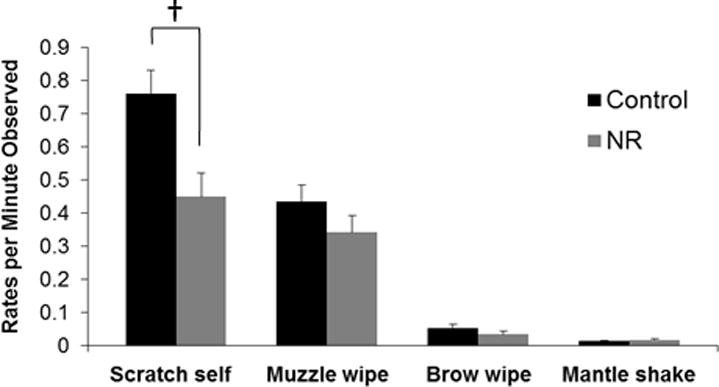
Data are means, with one SEM represented by vertical bars. † While the interaction between parturition and treatment group only approached significance (p ≤ 0.1; repeated measures ANOVA), during the prenatal period NR mean value was significantly lower from that of the control group for rates of scratch self (p ≤ 0.05; one-way ANOVA).
Mother-infant interactions
Protective behavior
There was no effect of infant age for protective behaviors in aggregate (make contact, restrain infant, and retrieve infant), make contact, or restrain infant (p > 0.05). However, rates of females retrieving infants approached significance (F(2,18) = 2.908, p = 0.080, eta = 0.49) (Table 2). There were no significant differences in protective behaviors between control and NR mothers for aggregated protective behavior, make contact, restrain infant, or retrieve infant (p > 0.05). The interaction between NR and infant age did not affect protective behavior in aggregate, make contact, or restrain infant (p > 0.05). However, our results indicate a trend in the interaction between NR and infant age for rates of retrieving infants (F(2,18) = 2.863, p = 0.083, eta = 0.49). Post-hoc analysis indicated that control and NR females differed significantly from each other during weeks 5-8 (one-way between subjects ANOVA: F(1,13) = 7.200, p = 0.019) (Figure 3).
Table 2.
Mother-Infant Interaction Results
| Categorical comparison (value ± SE)
|
|||
|---|---|---|---|
| Weeks 1-4 | Weeks 5-8 | Weeks 9-12 | |
| Effect of infant age | |||
| Protective behavior | 0.169/min ± 0.06 | 0.497/min ± 0.12 | 0.448/min ± 0.27 |
| Make Contact | 0.089/min ± 0.03 | 0.270/min ± 0.07 | 0.241/min ± 0.13 |
| Restrain Infant | 0.059/min ± 0.03 | 0.169/min ± 0.05 | 0.177/min ± 0.12 |
| Retrieve Infant | 0.021/min ± 0.01 | 0.059/min ± 0.01 | 0.030/min ± 0.02 |
| Time in Contact | 90.3% ± 2.3% | 69.5% ± 5.4% | 57.3% ± 6.1% |
| Within AR | 5.6% ± 1.9% | 16.1% ± 3.6% | 20.2% ± 6.3% |
| Greater than AR | 4.0% ± 1.4% | 14.3% ± 4.5% | 22.3% ± 4.2% |
| Categorical comparison (value ± SE)
|
||
|---|---|---|
| Control | NR | |
| Effect of treatment | ||
| Protective behavior | 0.458/min ± 0.22 | 0.285/min ± 0.17 |
| Make Contact | 0.261/min ± 0.12 | 0.139/min ± 0.09 |
| Restrain Infant | 0.148/min ± 0.10 | 0.122/min ± 0.07 |
| Retrieve Infant | 0.049/min ± 0.02 | 0.024/min ± 0.01 |
| Time in Contact | 67.8% ± 6.0% | 76.9% ± 4.5% |
| Within AR | 14.2% ± 5.8% | 13.8% ± 4.4% |
| Greater than AR | 17.9% ± 4.5% | 9.2% ± 3.4% |
| Categorical comparison (value ± SE)
|
||
|---|---|---|
| Control | NR | |
| Interaction of treatment and infant age | ||
| Protective behavior | ||
| weeks 1-4 | 0.213/min ± 0.09 | 0.125/min ± 0.07 |
| weeks 5-8 | 0.695/min ± 0.19 | 0.300/min ± 0.15 |
| weeks 9-12 | 0.465/min ± 0.43 | 0.431/min ± 0.32 |
| Make Contact | ||
| weeks 1-4 | 0.124/min ± 0.05 | 0.054/min ± 0.04 |
| weeks 5-8 | 0.381/min ± 0.11 | 0.158/min ± 0.09 |
| weeks 9-12 | 0.277/min ± 0.21 | 0.205/min ± 0.16 |
| Restrain Infant | ||
| weeks 1-4 | 0.061/min ± 0.04 | 0.057/min ± 0.03 |
| weeks 5-8 | 0.220/min ± 0.08 | 0.117/min ± 0.06 |
| weeks 9-12 | 0.162/min ± 0.19 | 0.191/min ± 0.14 |
| Retrieve Infant | ||
| weeks 1-4 | 0.028/min ± 0.01 | 0.013/min ± 0.01 |
| weeks 5-8 | 0.094/min ± 0.02 | 0.025/min ± 0.02 |
| weeks 9-12 | 0.026/min ± 0.03 | 0.035/min ± 0.02 |
| Time in Contact | ||
| weeks 1-4 | 84.8% ± 3.7% | 95.9% ± 2.8% |
| weeks 5-8 | 61.0% ± 8.6% | 78.0% ± 6.5% |
| weeks 9-12 | 57.7% ± 9.8% | 56.9% ± 7.4% |
| Within AR | ||
| weeks 1-4 | 7.5% ± 3.1% | 3.7% ± 2.3% |
| weeks 5-8 | 17.9% ± 5.8% | 14.2% ± 4.4% |
| weeks 9-12 | 17.1% ± 10.1% | 23.3% ± 7.6% |
| Greater than AR | ||
| weeks 1-4 | 7.7% ± 2.2% | 0.4% ± 1.7% |
| weeks 5-8 | 21.0% ± 7.2% | 7.6% ± 5.4% |
| weeks 9-12 | 24.9% ± 6.7% | 19.7% ± 5.1% |
Figure 3. Rates of retrieve infant.

Data are means, with one SEM represented by vertical bars. † While the interaction between infant age and treatment group only approached significance (p ≤ 0.1; repeated measures ANOVA), NR mean value was significantly lower from that of the control group for weeks 5-8 (p ≤ 0.05; one-way ANOVA; control N = 4, NR N = 7).
Proximity
Time spent in contact decreased significantly as infants increased in age (F(2,18) = 18.700, p ≤ 0.001, eta = 0.82) (Figure 4A). Time spent within arm’s reach increased (F(2,18) = 6.633, p = 0.007, eta = 0.65), and time spent at greater than arm’s reach increased significantly (F(2,18) = 10.454, p = 0.001, eta = 0.73) (Figure 4B). There were no significant differences between control and NR mothers for time spent in contact, time spent within arm’s reach, or time spent at greater than arm’s reach (p > 0.05). The interaction between NR and infant age did not affect time spent in contact, within arm’s reach, or at greater than arm’s reach (p > 0.05).
Figure 4. Rates of mother-infant interaction behaviors.
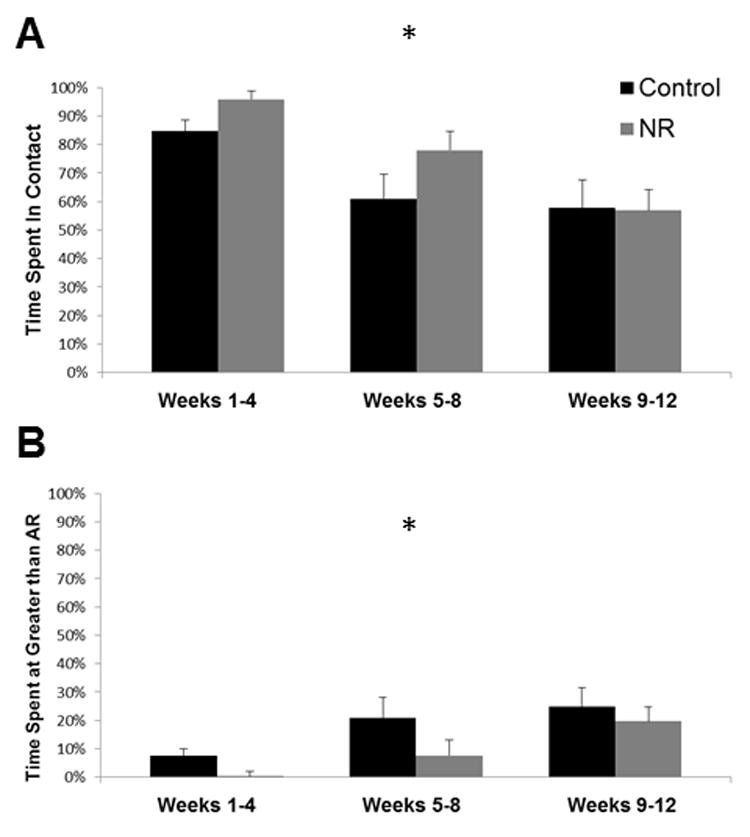
4A Proportion of time mothers spent in contact with infants, 4B Proportion of time infants spent at distances greater than arms reach from their mothers. Data are means, with one SEM represented by vertical bars. Control N = 4, NR N = 7. *There was a significant effect of age of infant on time spent in contact, time spent within arm’s reach (not shown), and time spent at greater than arm’s reach (p ≤ 0.05; repeated measures ANOVA). Offspring of control females spend more time at greater distance during all periods, though in no case did this difference approach significance (p ≥ 0.05; repeated measures ANOVA).
Discussion
Parturition and female behavior
As expected, parturition affected female behavior with rates of affiliative and aggressive behaviors increasing after the birth of the infant. Our results are consistent with previous studies in regards to the effects of parturition on social behavior. Specifically, females with infants received more social attention, increasing affiliative and aggressive interactions, including receiving aggression from other individuals. SDB rates decreased after parturition, contrary to the findings of a previous study with this species at this facility [41]. However, Brendt et al. [41] suggest that the difference in rates between the two periods in their study may be the result of a suppression of SDB in the prenatal period rather than an increase during the postpartum period. Females in our study were fed in individual enclosures, eliminating feeding competition and likely decreasing the amount of psychosocial stress factors that might affect SDB rates.
Nutritional regime and female behavior overall
Overall, there were no observed differences in activity level due to treatment group, contrary to our prediction that NR females would show higher activity levels than control females. However, it is important to note that activity levels among all study subjects were consistently very low with activity rates averaging only 5% of the time observed and ranging from 2-10% of the time. While previous studies of NR in Old World primates have shown increased levels of activity as a result of NR [57], these were studies that were using socially housed but also socially fed primate groups. Vitousek and colleagues [39] have suggested that the increased activity levels reported by Weed and colleagues [57] are due to intense activity prior to feeding times and in fact mask overall lower activity rates throughout the rest of the day.
While rates of affiliative behaviors rose significantly following the birth of the infant as expected, no differences were detected between treatment groups. Rates of aggression were higher in control females, contrary to our prediction that NR females would engage in higher rates of aggressive behaviors. Nevertheless, the very low levels of both affiliative and aggressive behaviors observed may be the result of the very low activity levels in general. Aggressive behaviors and affiliative behaviors in the context of reconciliation are frequently associated with feeding competition, particularly in free-ranging animals [71, 72]; our study protocol eliminated this motivation for social interaction. Notably, the lack of differences in aggressive behaviors between control and NR females are not consistent with Huber et al.’s suggestion that that increased aggression in IUGR offspring might be the result of socially learned behaviors acquired early in life.
Contrary to expectations, NR females had significantly lower SDB rates than controls. If pregnancy is a period of relative anxiety, then this result may indicate that the typical behavioral response to prenatal psychosocial stress is suppressed in NR females. Indeed, our results for control females are similar to other studies with the same species (Papio sp.) conducted at TBRI, where females exhibited prenatal self-scratching rates at 0.817/minute [41], suggesting that control females engage in SDB at rates typical for this study population. It is also possible that the lower rates of SDB among NR females are a strategic optimization of energy use. It has been suggested that displacement behaviors such as SDB provide a behavioral coping strategy through which individuals can lessen the physiological response to anxiety [73]. Yet such behaviors require energetic output. Individuals who are not energetically taxed may benefit from engaging in SDB to minimize the glucocorticoid output while energetically stressed individuals may forego such behaviors to minimize energy use, increasing glucocorticoid output but maximizing energy available for offspring development. Despite research demonstrating an increase in fetal cortisol due to nutrient restriction, our research team did not find significant differences in maternal cortisol among this study group [34], suggesting that SDB was not an effective means of mitigating glucocorticoid output. Therefore, it is more likely that SDB are reduced in NR females during pregnancy due to decreased available energy.
As mentioned above, the differences in activity rates in the current study were not statistically significant; however, in previous work by our team using the same model of NR and the same species, activity levels derived from accelerometers attached to the animals’ collars revealed significant differences in activity levels even in the absence of clear differences in activity budgets [31]. Those results suggest that accelerometers may record inconspicuous, but metabolically important, movements. SDB may fit this definition. In other words, eliminating subtle activities may yield adaptively significant energetic savings that protect fetal growth during NR [31].
Nutrition restriction and maternal behavior
The second aim of this project was to determine if NR led to significant differences in mother-infant interactions and maternal style. While we found no significant differences in protective behaviors across the entire study period, we did find a decrease in the percentage of time mothers spent in contact with infants, as expected. In the wild, two months after birth marks an important stage in infant development, with infants traveling independently and engaging in more social interactions at this age [74, 75]. Furthermore, our data show clear trends with NR mothers spending more time in contact with infants and infants of NR females closer to their mothers.
In the absence of other clear differences in female behavior post-partum, we attribute the noticeable trends in contact and proximity to differences in activity rates of the infants born to NR females – presumably lower due to energetic deficits – rather than to differences in maternal style. Therefore, we reason that well-fed infants are more interested in leaving their mothers while those who are nutritionally taxed remain in contact with their mothers [76–78]. A second possibility is that the trends in contact and proximity between control and NR subjects is due to differences in offspring temperament, as has been documented in IUGR offspring of both rodents and sheep [9, 10] and in nonhuman primate studies investigating the effects of differences in available milk energy and cortisol in mother’s milk [78–80]. Our team also found less motivation in IUGR offspring during adolescence [8], which may also lead to more time spent in close proximity to mothers during infancy.
In conclusion, NR resulted in lower rates of aggressive behaviors and lower rates of SDB, particularly during the prenatal period. However, because rates of activity were lower than expected, confirming our findings would require further research with an expanded sample size. Nutrient restriction clearly influenced rates of SDB. It is possible that moderate global NR prompts an adaptive suppression of SDB in pregnant female baboons as a means of mitigating the insult of NR on fetal development.
Furthermore, our findings suggest that maternal-infant interactions are influenced by NR in regards to contact between the mother and infant, but any future studies should consider the role of the infant in determining mother-infant behavior. One limitation of our own study was the use of handheld video to conduct behavioral data collection. As the mother was the study subject, infants often left the field of view for long periods. One remedy for this during future projects would be to focus on the infant during behavioral data collection. By doing so, one could analyze the infant’s role in maintaining proximity. Once moving independently from the mother, infants are active independent agents and should thus be considered as equally contributing to mother-infant interactions, rather than viewed as passive entities whose behaviors are shaped solely by the constraints of maternal style.
Acknowledgments
This research was supported by NICHD-021350 and HD 21350. The project described was supported by Award Number R21HD057480 from the Eunice Kennedy Shriver National Institute of Child Health &Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health. This research was also supported by a grant from San Antonio Life Sciences Institute.
We would like to thank Michael Bauman, Jesse Rodriguez, Katie Smith, Heath Neville, William Ramirez, Meghan Egan, and Stephanie Ramirez for assistance on this project.
Supported by: NICHD-021350 and HD 21350
Footnotes
DR. LYDIA E. O. LIGHT (Orcid ID : 0000-0001-9791-3487)
DR. THAD QUINCY BARTLETT (Orcid ID : 0000-0001-5994-9454)
DR. HILLARY FRIES HUBER (Orcid ID : 0000-0001-9734-427X)
References
- 1.Armitage JA, Khan IY, Taylor PD, Nathanielsz PW, Poston L. Developmental programming of the metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in mammals? Journal of Physiology. 2004;561:355–377. doi: 10.1113/jphysiol.2004.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiological Reviews. 2005:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 3.McMullen S, Mostyn A. Animal models for the study of the developmental origins of health and disease. Proceedings of the Nutrition Society. 2009;68:306–320. doi: 10.1017/S0029665109001396. [DOI] [PubMed] [Google Scholar]
- 4.Ojeda NB, Grigore D, Alexander BT. Developmental Programming of Hypertension. Hypertension. 2008;52:44–50. doi: 10.1161/HYPERTENSIONAHA.107.092890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Symonds ME, Stephenson T, Gardner DS, Budge H. Long-term effects of nutritional programming of the embryo and fetus: mechanisms and critical windows. Reproduction, Fertility and Development. 2006;19:53–63. doi: 10.1071/rd06130. [DOI] [PubMed] [Google Scholar]
- 6.Baker PN, Wheeler SJ, Sanders TA, Thomas JE, Hutchinson CJ, Clarke K, Berry JL, Jones RL, Seed PT, Poston L. A prospective study of micronutrient status in adolescent pregnancy. The American Journal of Clinical Nutrition. 2009;89:1114–1124. doi: 10.3945/ajcn.2008.27097. [DOI] [PubMed] [Google Scholar]
- 7.Morgane PJ, Mokler DJ, Galler JR. Effects of prenatal protein malnutrition on the hippocampal formation. Neuroscience & Biobehavioral Reviews. 2002;26:471–483. doi: 10.1016/s0149-7634(02)00012-x. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez JS, Bartlett TQ, Keenan KE, Nathanielsz PW, Nijland MJ. Sex-Dependent Cognitive Performance in Baboon Offspring Following Maternal Caloric Restriction in Pregnancy and Lactation. Reproductive Sciences. 2012;19:493–504. doi: 10.1177/1933719111424439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erhard HW, Boissy A, Rae MT, Rhind SM. Effects of prenatal undernutrition on emotional reactivity and cognitive flexibility in adult sheep. Behavioural Brain Research. 2004;151:25–35. doi: 10.1016/j.bbr.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Strupp BJ, Levitsky DA. Enduring cognitive effects of early malnutrition: a theoretical reappraisal. Journal of Nutrition. 1995;125:2221S–2232S. doi: 10.1093/jn/125.suppl_8.2221S. [DOI] [PubMed] [Google Scholar]
- 11.Bresler D, Ellison G, Zamenhov S. Learning deficits in rats with malnourished grandmothers. Developmental Psychobiology. 1975;8:315–323. doi: 10.1002/dev.420080405. [DOI] [PubMed] [Google Scholar]
- 12.Langley-Evans SC. Intrauterine programming of hypertension by glucocorticoids. Life Sciences. 1997;60:1213–1221. doi: 10.1016/s0024-3205(96)00611-x. [DOI] [PubMed] [Google Scholar]
- 13.Langley-Evans SC. Nutritional programming of disease: unravelling the mechanism. Journal of Anatomy. 2009;215:36–51. doi: 10.1111/j.1469-7580.2008.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucas A. The Childhood Environment and Adult Disease. Vol. 156. Wiley; 1991. Programming by Early Nutrition in Man; pp. 38–55. (CIBA Foundation Symposium). [PubMed] [Google Scholar]
- 15.Waterland RA, Garza C. Potential mechanisms of metabolic imprinting that lead to chronic disease. The American Journal of Clinical Nutrition. 1999;69:179–197. doi: 10.1093/ajcn/69.2.179. [DOI] [PubMed] [Google Scholar]
- 16.Bispham J, Gopalakrishnan GS, Dandrea J, Wilson V, Budge H, Keisler DH, Broughton Pipkin F, Stephenson T, Symonds ME. Maternal Endocrine Adaptation throughout Pregnancy to Nutritional Manipulation: Consequences for Maternal Plasma Leptin and Cortisol and the Programming of Fetal Adipose Tissue Development. Endocrinology. 2003;144:3575–3585. doi: 10.1210/en.2003-0320. [DOI] [PubMed] [Google Scholar]
- 17.Dwyer CM, Lawrence AB, Bishop SC, Lewis M. Ewe–lamb bonding behaviours at birth are affected by maternal undernutrition in pregnancy. British Journal of Nutrition. 2003;89:123–136. doi: 10.1079/BJN2002743. [DOI] [PubMed] [Google Scholar]
- 18.Han E-S, Evans TR, Shu JH, Lee S, Nelson JF. Food restriction enhances endogenous and corticotropin-induced plasma elevations of free but not total corticosterone throughout life in rats. The Journals of Gerontology. 2001;56A:B391. doi: 10.1093/gerona/56.9.b391. [DOI] [PubMed] [Google Scholar]
- 19.Leakey JEA, Chen SHU, Manjgaladze M, Turturro A, Duffy PH, Pipkin JL, Hart RW. Role of Glucocorticoids and “Caloric Stress” in Modulating the Effects of Caloric Restriction in Rodents. Annals of the New York Academy of Sciences. 1994;719:171–194. doi: 10.1111/j.1749-6632.1994.tb56828.x. [DOI] [PubMed] [Google Scholar]
- 20.Antonow-Schlorke I, Schwab M, Cox LA, Li C, Stuchlik K, Witte OW, Nathanielsz PW, McDonald TJ. Vulnerability of the fetal primate brain to moderate reduction in maternal global nutrient availability. Proceedings of the National Academy of Sciences. 2011;108:3011–3016. doi: 10.1073/pnas.1009838108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi J, Li C, McDonald TJ, Comuzzie A, Mattern V, Nathanielsz PW. Emergence of insulin resistance in juvenile baboon offspring of mothers exposed to moderate maternal nutrient reduction. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2011;301:R757–R762. doi: 10.1152/ajpregu.00051.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox L, Nijland M, Gilbert J, Schlabritz-Loutsevitch N, Hubbard G, McDonald T, Shade R, Nathanielsz P. Effect of 30 per cent maternal nutrient restriction from 0.16 to 0.5 gestation on fetal baboon kidney gene expression. The Journal of Physiology. 2006;572:67–85. doi: 10.1113/jphysiol.2006.106872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huber HF, Ford SM, Bartlett TQ, Nathanielsz PW. Increased aggressive and affiliative display behavior in intrauterine growth restricted baboons. Journal of Medical Primatology. 2015;44:143–157. doi: 10.1111/jmp.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li C, Levitz M, Hubbard G, Jenkins S, Han V, Ferry R, McDonald T, Nathanielsz P, Schlabritz-Loutsevitch N. The IGF axis in baboon pregnancy: placental and systemic responses to feeding 70% global ad libitum diet. Placenta. 2007;28:1200–1210. doi: 10.1016/j.placenta.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C, McDonald TJ, Wu G, Nijland MJ, Nathanielsz PW. Intrauterine growth restriction alters term fetal baboon hypothalamic appetitive peptide balance. Journal of Endocrinology. 2013;217:275–282. doi: 10.1530/JOE-13-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li C, Ramahi E, Nijland MJ, Choi J, Myers DA, Nathanielsz PW, McDonald TJ. Up-regulation of the fetal baboon hypothalamo-pituitary-adrenal axis in intrauterine growth restriction: coincidence with hypothalamic glucocorticoid receptor insensitivity and leptin receptor down-regulation. Endocrinology. 2013;154:2365–2373. doi: 10.1210/en.2012-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li C, Schlabritz-Loutsevitch NE, Hubbard GB, Han V, Nygard K, Cox LA, McDonald TJ, Nathanielsz PW. Effects of maternal global nutrient restriction on fetal baboon hepatic insulin-like growth factor system genes and gene products. Endocrinology. 2009;150:4634–4642. doi: 10.1210/en.2008-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDonald TJ, Wu G, Nijland MJ, Jenkins SL, Nathanielsz PW, Jansson T. Effect of 30% nutrient restriction in the first half of gestation on maternal and fetal baboon serum amino acid concentrations. The British Journal of Nutrition. 2013;109:1382. doi: 10.1017/S0007114512003261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nijland MJ, Mitsuya K, Li C, Ford S, McDonald TJ, Nathanielsz PW, Cox LA. Epigenetic modification of fetal baboon hepatic phosphoenolpyruvate carboxykinase following exposure to moderately reduced nutrient availability. The Journal of Physiology. 2010;588:1349–1359. doi: 10.1113/jphysiol.2009.184168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nijland MJ, Schlabritz-Loutsevitch NE, Hubbard GB, Nathanielsz PW, Cox LA. Non-human primate fetal kidney transcriptome analysis indicates mammalian target of rapamycin (mTOR) is a central nutrient-responsive pathway. Journal of Physiology. 2007;579:643–656. doi: 10.1113/jphysiol.2006.122101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlabritz-Loutsevitch NE, Dudley CJ, Gomez JJ, Nevill CH, Smith BK, Jenkins SL, McDonald TJ, Bartlett TQ, Nathanielsz PW, Nijland MJ. Metabolic adjustments to moderate maternal nutrient restriction. British Journal of Nutrition. 2007;98:276–284. doi: 10.1017/S0007114507700727. [DOI] [PubMed] [Google Scholar]
- 32.Franke K, Clarke GD, Dahnke R, Gaser C, Kuo AH, Li C, Schwab M, Nathanielsz PW. Premature brain aging in baboons resulting from moderate fetal undernutrition. Frontiers in Aging Neuroscience. 2017;9 doi: 10.3389/fnagi.2017.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuo AH, Li C, Huber HF, Schwab M, Nathanielsz PW, Clarke GD. Maternal nutrient restriction during pregnancy and lactation leads to impaired right ventricular function in young adult baboons. The Journal of Physiology. 2017;595 doi: 10.1113/JP273928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li C, Jenkins S, Mattern V, Comuzzie AG, Cox LA, Huber HF, Nathanielsz PW. Effect of moderate, 30 percent global maternal nutrient reduction on fetal and postnatal baboon phenotype. Journal of Medical Primatology. 2017 doi: 10.1111/jmp.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie L, Antonow-Schlorke I, Schwab M, McDonald T, Nathanielsz P, Li C. The frontal cortex IGF system is down regulated in the term, intrauterine growth restricted fetal baboon. Growth Hormone & Igf Research. 2013;23:187–192. doi: 10.1016/j.ghir.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keenan K, Bartlett TQ, Nijland M, Rodriguez JS, Nathanielsz PW, Zürcher NR. Poor nutrition during pregnancy and lactation negatively affects neurodevelopment of the offspring: evidence from a translational primate model. The American Journal of Clinical Nutrition. 2013;98:396–402. doi: 10.3945/ajcn.112.040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bardi M, Huffman MA. Maternal behavior and maternal stress are associated with infant behavioral development in macaques. Developmental Psychobiology. 2006;48:1–9. doi: 10.1002/dev.20111. [DOI] [PubMed] [Google Scholar]
- 38.Fairbanks LA. What Is a Good Mother? Adaptive Variation in Maternal Behavior of Primates. Current Directions in Psychological Science (Wiley-Blackwell) 1993;2:179–183. [Google Scholar]
- 39.Vitousek KM, Manke FP, Gray JA, Vitousek MN. Caloric restriction for longevity: II—The systematic neglect of behavioural and psychological outcomes in animal research. European Eating Disorders Review. 2004;12:338–360. [Google Scholar]
- 40.Bardi M, Bode AE, Ramirez SM, Brent LY. Maternal care and development of stress responses in baboons. American Journal of Primatology. 2005;66:263–278. doi: 10.1002/ajp.20143. [DOI] [PubMed] [Google Scholar]
- 41.Brent L, Koban T, Ramirez S. Abnormal, abusive, and stress-related behaviors in baboon mothers. Biological Psychiatry. 2002;52:1047–1056. doi: 10.1016/s0006-3223(02)01540-8. [DOI] [PubMed] [Google Scholar]
- 42.Maestripieri D. Maternal anxiety in rhesus macaques (Macaca mulatta). II. Emotional bases of individual differences in mothering style. Ethology. 1993;95:32–42. [Google Scholar]
- 43.Maestripieri D, Lindell SG, Higley JD. Intergenerational transmission of maternal behavior in rhesus macaques and its underlying mechanisms. Developmental Psychobiology. 2007;49:165–171. doi: 10.1002/dev.20200. [DOI] [PubMed] [Google Scholar]
- 44.Parker KJ, Maestripieri D. Identifying key features of early stressful experiences that produce stress vulnerability and resilience in primates. Neuroscience & Biobehavioral Reviews. 2011;35:1466–1483. doi: 10.1016/j.neubiorev.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sarkar P, Bergman K, O’Connor TG, Glover V. Maternal Antenatal Anxiety and Amniotic Fluid Cortisol and Testosterone: Possible Implications for Foetal Programming. Journal of Neuroendocrinology. 2008;20:489–496. doi: 10.1111/j.1365-2826.2008.01659.x. [DOI] [PubMed] [Google Scholar]
- 46.Mogi K, Nagasawa M, Kikusui T. Developmental consequences and biological significance of mother–infant bonding. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35:1232–1241. doi: 10.1016/j.pnpbp.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 47.Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, Stevens VJ, Vollmer WM, Lin P-H, Svetkey LP. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA: Journal of the American Medical Association. 2003 doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- 48.Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiology & Behavior. 2003;79:359–371. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 49.Szyf M, Weaver I, Meaney M. Maternal care, the epigenome and phenotypic differences in behavior. Reproductive Toxicology. 2007;24:9–19. doi: 10.1016/j.reprotox.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 50.Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 51.Fish EW, Shahrokh D, Bagot R, Caldji C, Bredy T, Szyf M, Meaney MJ. Epigenetic programming of stress responses through variations in maternal care. Annals of the New York Academy of Sciences. 2004;1036:167–180. doi: 10.1196/annals.1330.011. [DOI] [PubMed] [Google Scholar]
- 52.Meaney MJ, Szyf M, Seckl JR. Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends in Molecular Medicine. 2007;13:269–277. doi: 10.1016/j.molmed.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 53.Maestripieri D, Schino G, Aureli F, Troisi A. A modest proposal: displacement activities as an indicator of emotions in primates. Animal Behaviour. 1992;44:967–979. [Google Scholar]
- 54.Easley SP, Coelho AM, Jr, Taylor LL. Scratching, dominance, tension, and displacement in male baboons. American Journal of Primatology. 1987;13:397–411. doi: 10.1002/ajp.1350130405. [DOI] [PubMed] [Google Scholar]
- 55.Maestripieri D. Maternal anxiety in rhesus macaques (Macaca mulatta). I. Measurement of anxiety and identification of anxiety-eliciting situations. Ethology. 1993;95:19–31. [Google Scholar]
- 56.Loy J. Behavioral responses of free-ranging rhesus monkeys to food shortage. American Journal of Physical Anthropology. 1970;33:263–272. doi: 10.1002/ajpa.1330330212. [DOI] [PubMed] [Google Scholar]
- 57.Weed JL, Lane MA, Roth GS, Speer DL, Ingram DK. Activity measures in rhesus monkeys on long-term calorie restriction. Physiology & Behavior. 1997;62:97–103. doi: 10.1016/s0031-9384(97)00147-9. [DOI] [PubMed] [Google Scholar]
- 58.Sabatino F, Masoro EJ, McMahan CA, Kuhn RW. Assessment of the Role of the Glucocorticoid System in Aging. Journal of Gerontology. 1991;46:B171. doi: 10.1093/geronj/46.5.b171. [DOI] [PubMed] [Google Scholar]
- 59.Guo C, Li C, Myatt L, Nathanielsz PW, Sun K. Sexually dimorphic effects of maternal nutrient reduction on expression of genes regulating cortisol metabolism in fetal baboon adipose and liver tissues. Diabetes. 2013;62:1175–1185. doi: 10.2337/db12-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramirez SM. Department of Anthropology. Vol. 146. The University of Texas at San Antonio; 2004. The evolutionary function of allomothering among female baboons (Papio hamadryas anubis sp.) [Google Scholar]
- 61.Altmann J. Baboon Mothers and Infants. Cambridge: Harvard University Press; 1980. [Google Scholar]
- 62.Berman C, Rasmussen K, Suomi S. Reproductive consequences of maternal care patterns during estrus among free-ranging rhesus monkeys. Behavioral Ecology and Sociobiology. 1993;32:391–399. [Google Scholar]
- 63.Silk JB. Social mechanisms of population regulation in a captive group of bonnet macaques (Macaca radiata) American Journal of Primatology. 1988;14:111–124. doi: 10.1002/ajp.1350140202. [DOI] [PubMed] [Google Scholar]
- 64.Fairbanks LA. Parenting. 2003 [Google Scholar]
- 65.Bardi M, French JA, Ramirez SM, Brent L. The role of the endocrine system in baboon maternal behavior. Biological Psychiatry. 2004;55(7):724–732. doi: 10.1016/j.biopsych.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 66.Nguyen N, Gesquiere LR, Wango EO, Alberts SC, Altmann J. Late pregnancy glucocorticoid levels predict responsiveness in wild baboon mothers (Papio cynocephalus) Animal Behaviour. 2008;75:1747–1756. [Google Scholar]
- 67.Schlabritz-Loutsevitch NE, Howell K, Rice K, Glover EJ, Nevill CH, Jenkins SL, Bill Cummins L, Frost PA, McDonald TJ, Nathanielsz PW. Development of a system for individual feeding of baboons maintained in an outdoor group social environment. Journal of Medical Primatology. 2004;33:117–126. doi: 10.1111/j.1600-0684.2004.00067.x. [DOI] [PubMed] [Google Scholar]
- 68.Cox LA, Nijland MJ, Gilbert JS, Schlabritz-Loutsevitch NE, Hubbard GB, McDonald TJ, Shade RE, Nathanielsz PW. Effect of 30 per cent maternal nutrient restriction from 0.16 to 0.5 gestation on fetal baboon kidney gene expression. Journal of Physiology. 2006;572:67–85. doi: 10.1113/jphysiol.2006.106872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bramblett CA. Dominance tabulation: Giving form to concepts. Behavioral and Brain Science. 1981;4:435–436. [Google Scholar]
- 70.Mundry R, Fischer J. Use of statistical programs for nonparametric tests of small samples often leads to incorrect P values: examples from Animal Behaviour. Animal Behaviour. 1998;56:256–259. doi: 10.1006/anbe.1998.0756. [DOI] [PubMed] [Google Scholar]
- 71.Paterson JD. Ecologically differentiated patterns of aggressive and sexual behavior in two troops of Ugandan baboons, Papio anubis. American Journal of Physical Anthropology. 1973;38:641–647. doi: 10.1002/ajpa.1330380281. [DOI] [PubMed] [Google Scholar]
- 72.Sapolsky R. Social cultures among nonhuman primates. Current Anthropology. 2006;47:641–656. [Google Scholar]
- 73.Higham JP, Maclarnon A, Heistermann M, Ross C, Semple S. Rates of self-directed behavior and faecal glucocorticoid levels are not correlated in female wild olive baboons (Papio hamadryas anubis) Stress. 2009;12:526–532. doi: 10.3109/10253890902756565. [DOI] [PubMed] [Google Scholar]
- 74.Altmann J, Altmann SA, Hausfater G, McCuskey SA. Life history of yellow baboons: physical development, reproductive parameters, and infant mortality. Primates. 1977;18:315–330. [Google Scholar]
- 75.Altmann J, Samuels A. Costs of maternal care: infant-carrying in baboons. Behavioral Ecology and Sociobiology. 1992;29:391–398. [Google Scholar]
- 76.Hinde K, Power ML, Oftedal OT. Rhesus macaque milk: magnitude, sources, and consequences of individual variation over lactation. American Journal of Physical Anthropology. 2009;138:148–157. doi: 10.1002/ajpa.20911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hinde K, Milligan LA. Primate milk: proximate mechanisms and ultimate perspectives. Evolutionary Anthropology: Issues, News, and Reviews. 2011;20:9–23. doi: 10.1002/evan.20289. [DOI] [PubMed] [Google Scholar]
- 78.Fairbanks LA, Hinde K. Behavioral response of mothers and infants to variation in maternal condition: adaptation, compensation, and resilience. Building Babies Springer. 2013:281–302. [Google Scholar]
- 79.Sullivan EC, Hinde K, Mendoza SP, Capitanio JP. Cortisol concentrations in the milk of rhesus monkey mothers are associated with confident temperament in sons, but not daughters. Developmental Psychobiology. 2011;53:96–104. doi: 10.1002/dev.20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hinde K, Capitanio JP. Lactational programming? Mother’s milk energy predicts infant behavior and temperament in rhesus macaques (Macaca mulatta) American Journal of Primatology. 2010;72:522–529. doi: 10.1002/ajp.20806. [DOI] [PMC free article] [PubMed] [Google Scholar]


