Abstract
We examined two potentially interacting, connected pathways by which parental supportiveness during early adolescence (ages 11–13) may come to be associated with later African American young adult smoking. The first pathway is between parental supportiveness and young adult stress (age 19), with stress, in turn, predicting increased smoking at age 20. The second pathway is between supportive parenting and TNF-methylation (i.e., TNFm), a pro-inflammatory epitype, with low levels indicating greater inflammatory potential and forecasting increased risk for smoking in response to young adult stress. In a sample of 382 African American youth residing in rural Georgia, followed from early adolescence (age 10–11) to young adulthood (age 20), supportive parenting indirectly predicted smoking via associations with young adult stress (IE = −.071, 95%(−.132, −.010). In addition, supportive parenting was associated with TNFm measured at age 20 (r = .177, p = .001). Further, lower TNFm was associated with a significantly steeper slope (b = .583, p = .003) of increased smoking in response to young adult stress compared to those with higher TNFm. (b = .155, p = .291), indicating an indirect, amplifying role for supportive parenting via TNFm. Results suggest that supportive parenting in early adolescence may play a role in understanding the emergence of smoking in young adulthood.
Keywords: epigenetic, methylation, African American, parenting, CpG, TNF, SES
Smoking is the leading preventable cause of morbidity and mortality in the U.S., with complications of smoking causing nearly half a million US deaths per year as well as higher population rates of preventable, serious illness (Mokdad et al., 2004; CDC, 2011; 2014). Unfortunately, many youths currently between the ages of 12 to 20 will initiate cigarette smoking, comprising a large portion of the 1.5 million individuals initiating daily smoking each year. As a consequence, it is expected that 5.6 million children who are alive today will experience premature death attributable to cigarette smoking (CDC, 2014). Cigarette smoking has also become increasingly tied to SES, making it a primary driver of SES related health disparities (e.g., Fagan et al., 2007), with those most economically disadvantaged at greatest risk for smoking related illness. At the same time, in the US, people of African descent suffer worse outcomes for smoking-related illnesses (CDC, 2010; Haiman, et al., 2006), even after adjusting for covariates such as socioeconomic status [SES] and healthcare access, suggesting that the need for attention to smoking prevention is particularly acute among African American youth for whom it could have especially pronounced health benefits.
Family Influences and Stress.
Family influences have emerged as a key element in models identifying potential points of intervention for early prevention of smoking (e.g., Ennett, Bauman, Pemberton, et al., 2001). Parenting is a significant predictor of all forms of substance use across early to late adolescence (Ryzin Fosco, & Dishion, 2012; Piko & Balazs, 2012), the time during which the vast majority of adult smokers initiated smoking (Brynin, 1999). Increases in smoking across this age range also predict development of substance abuse disorders, poor psychosocial functioning, and poorer mental health outcomes (e.g. Conger, Ge, Elder, & Lorenz, 1994; Simmons, Burgeson, Carlton-Ford, & Blyth, 1987; Windle, M., & Windle, R. C., 2009; 2012). Accordingly, there are many reasons for a continuing focus on ways that families influence smoking outcomes during adolescence.
Another well-studied predictor of cigarette smoking among young adults is the experience of stress (Cerbone and Larison, 2000; Sinha, 2001; Wills, 1990). Level of stress experienced by young adult African Americans as they transition into adult roles is predictive of their smoking (Aseltine & Gore, 2005; Brody, Chen, Kogan, Smith, & Brown, 2010; Paschall, Flewelling, & Faulkner, 2000). This is not surprising given the well-documented effect of stress on increased desire to smoke (Buchmann, et al., 2010; Colamussi, Bovbjerg, & Erblich, 2007; Erblich, Boyarsky, Spring, Niaura, & Bovbjerg, 2003; Niaura et al., 2002;). Given the effect of young adult stress on smoking, one potential pathway connecting supportive parenting to smoking may be from early adolescent parenting to later experience of stress in young adulthood, and then ultimately to later cigarette smoking.
Inflammation and Epigenetics.
The emergence of a literature on potential biological effects of parenting suggests an additional potential pathway from supportive parenting to smoking. Recent work has identified ways in which experiences in childhood or early adolescence may “get under the skin,” becoming biologically embedded risk factors that manifest later in adulthood (Brody, Chen, Kogan, 2010; De Bellis, 2002; Gordon, 2002; Sinha, 2008). Predictive-adaptive response (PAR) models (Cole, et al., 2011; Gluckman, Hanson & Spencer, 2005; Rickard & Lummaa, 2007), in particular, suggest that social adversity and perceived threat in childhood or early adolescence should lead to greater pro-inflammatory propensities. From an evolutionary perspective this shift is thought to be adaptive because it prepares the individual for potentially elevated risk for tissue damage across the life span, with effects on pro-inflammatory potential that persist into young adulthood. The presence of chronic stressors, including poverty, neighborhood crime, and discrimination, may increase the importance of supportive parenting as a protective factor.
Building on this broad foundation, an integrative model recently put forward by Nusslock & Miller (2015), posits that biologically embedded changes developed by youth growing up in difficult circumstances may be linked to later behavioral outcomes, such as smoking, in part, because they are linked to broader neuro-hormonal-immune system network changes that serve to amplify links between inflammatory propensities on the one hand and the neural circuitry of anxiety and reward on the other. On this view, if biologically embedded vulnerabilities are triggered by young adult stressors, they may amplify resulting behavioral effects. For example, such vulnerabilities might amplify increases in cigarette use in young adulthood in response to stress.
Parenting and Biological Embedding
Because supportive parenting may counter the impact of life stress on behavioral outcomes (Luthar, 2006), and parental emotional support buffers youths’ physiological stress reactions, supportive parenting may be particularly important for minority youth growing up in challenging environments. In keeping with this view, supportive parenting may ameliorate hormonal, metabolic, inflammatory, and cardiovascular risk following childhood and early adolescent adversity (Brody, Yu, Beach, Kogan, Windle, & Philibert, 2014; Chen, Miller, Kobor, & Cole, 2011), potentially protecting against the development of pro-inflammatory epigenetic changes (Beach, Lei, Brody, Dogan, Philibert, 2015), accelerated weathering (Geronimus, Hicken, Keene, & Bound, 2006), and development of vulnerabilities to poorer health in young adulthood (Beach, Lei, Barton, et al., 2016). In contrast, parenting that is harsh or abusive may amplify inflammatory profiles later in life (Dube et al., 2009; Miller & Chen, 2010). Together, these finding suggest that supportive parenting during early adolescence may protect against development of a pro-inflammatory epitype, providing another indirect pathway of influence from supportive parenting to later smoking by youth, in this case, by reducing the impact of later stress.
Why Examine Epigentic Markers of Vulnerability?
The way in which protective parenting during early adolescence can be turned into biological changes with health consequences for young adulthood is likely complex (cf. Hertzman, 1999). However, one likely mechanism mediating such effects is epigenetic programming of immune cells (Miller, Chen, & Parker, 2011). Methylation of specific CpG sites (i.e., regions of DNA in which cytosine occurs next to guanine separated by only one phosphate bond), can influence access to key regulatory elements controlling the rate of gene transcription, and so influence downstream effects. Because methylation associated with the first exon is particularly predictive of gene expression (e.g., Brenet et al., 2011; Plume, Beach, Brody, & Philibert, 2012), characterizing individual differences in methylation of inflammation-related genes in the region of the first exon may be particularly informative, and provides a useful starting point for examination of potential epigenetic mediators. Quantifying the expectation of larger effects when methylation occurs in the first exon, Brenet, et al., 2011 found that DNA methylation of the first exon was more tightly linked to transcriptional silencing than was methylation elsewhere in the genome (e.g., compared to effects for methylation of introns, internal exons, and last exons, and even methylation of the promoter region), with hypomethylation of the first exon producing a large effect on gene expression (LOR = −2.8). Follow-up work to examine the impact of experimentally manipulated demethylation confirmed these conclusions (Brenet, et al., 2011). In terms of Cohen’s effect sizes, an LOR of −2.8 can be characterized as a large effect size (Chen, Cohen, & Chen, 2010), suggesting that a focus on methylation of the first exon is a good starting point for characterization of epigenetic effects.
Why focus on TNFm as a pro-inflammatory epitype?
Supportive parenting has been shown to be associated with epigenetic effects on cell-signaling processes generally (Beach, Lei, Brody, Kim, Barton, Dogan, Philibert, 2016), and also has been shown to predict greater methylation of the first exon of TNF (TNFm) (Beach, Lei, Brody, Dogan, Philibert, 2015). TNF is the gene that encodes Tumor necrosis factor (TNF), often referred to as TNFα or TNF alpha. We assessed TNFm as a window on a pro-inflammatory epitype because TNF is a key regulator of the inflammatory response (Bradley, 2007; Dhama K, et al., 2013). It stimulates, for example, production of inflammatory cytokines (e.g., IL-1β and IL6) that are prominent in many types of pathology and it activates pro-inflammatory transcription factors such as NF-kB (Bradley , 2007). Further, vascular endothelial cells demonstrate several pro-inflammatory changes in response to TNF and it has been linked to autoimmune and inflammatory conditions (Bradley , 2007; Dhama K, et al., 2013). Indeed, treatment of many inflammatory disorders involves medication designed to block the action of TNF (Bradley , 2007; Dhama K, et al., 2013), which is seen as central to inflammatory control efforts and a specific target of medical intervention. Accordingly, we focused on methylation of the first exon of TNF to identify individuals whose relatively greater methylation in this location would indicate down-regulation of TNF production and decreased production of TNF relative to those with lower methylation. In humans TNF is commonly produced by activated macrophages, i.e. monocytes that have migrated from blood to tissues and differentiated. Accordingly, as a central element of the innate inflammatory response, epigenetic change via shifts in the level of methylation of TNF (i.e., TNFm) is a mechanism that has strong theoretical links to existing theory, and that we can access using a readily available peripheral tissue to characterize individual differences.
Better understanding of the way family processes during early adolescence contribute to, or protect against, inflammation is particularly relevant for African Americans who tend to show higher levels of inflammatory markers (Chyu & Upchurch, 2011; Geronimus, Hicken, Keene, & Bound, 2006; Paalani, Lee, Haddad, , & Tonstad, 2011) than do whites. As a result, better understanding family processes, like supportive parenting, that potentially protect against a pro-inflammatory epitype, i.e., low TNFm, may be particularly useful in identifying risk and protective factors relevant to African American health and health behavior in young adulthood.
Heuristic model of parenting, inflammation, young adult stress and smoking.
The foregoing considerations suggest that supportive parenting in early adolescence may be important for understanding initiation and escalation of smoking among African American youth growing up in the rural southeast, a context that is challenging in many respects. In particular, African American youth residing in the Southern coastal plain are often exposed to economic disadvantage and socio-economic status (SES) related risks, setting the stage for a pro-inflammatory shift in transcriptional responses (Cole, 2010, 2012; 2014; Kiecolt-Glaser et al. 2003, Miller et al. 2008, Ranjit et al. 2007a)). In this context, supportive parenting that conveys a sense of safety and reduced stress could be powerful in protecting against the pro-inflammatory processes that might otherwise be engendered (cf. Gruenewald et al., 2009; Loucks, et al., 2010).
Among youth who are more stressed and/or develop a pro-inflammatory epitype, we hypothesized increased smoking for several reasons. As noted above, for smokers, there is a well-documented effect of stress on increased desire to smoke. This is due, in part, to the increased reward value of smoking in the context of stressful experiences (Childs & DeWitt, 2010). Second, as suggested by Nusslock and Miller (2015), for youth raised in more difficult circumstances, pro-inflammatory processes may lead as well to long-lasting changes in reward processing (cf. Gianaros, et al., 2011; Miller, Maltic, Raison, 2009; Maier, Watkins, 1988),), resulting in blunted reward sensitivity and greater nicotine craving among smokers (Peechatka, Whitton, Farmer, Pizzagalli, & Janes, 2015). This suggests that a pro-inflammatory epitype could contribute to more rapid escalation of smoking in the context of heightened stress because nicotine temporarily normalizes blunted reward responsiveness (Janes, Pedrelli, Whitton, et al., 2015), enhancing smoking’s attractiveness for those with a pro-inflammatory response pattern.
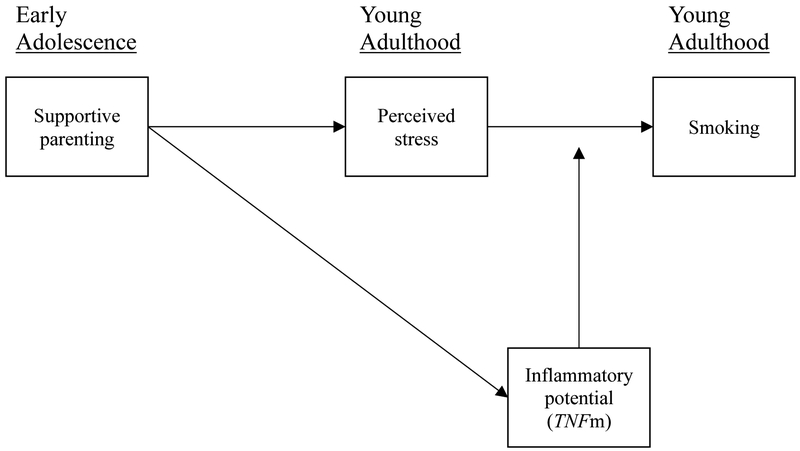
These expectations are represented in Figure 1. It can be seen that parenting during early adolescence is predicted to be associated with both TNFm and with young adult stress. Young adult stress is assumed to be an important driver of increased smoking across the young adult years, leading to an indirect effect of early adolescent parenting with young adult smoking, but the effects of young adult stress on smoking are predicted to be greater among those with a pro-inflammatory profile for TNFm.
Figure 1.
Theoretical model showing effects of early adolescent parenting on TNF methylation, young adult stress, and smoking in young adulthood in response to stress.
Preliminary and Supplemental Analyses.
In addition to the direct tests of the model described below, as a preliminary step we first described the pattern of change in smoking from early adolescence to early adulthood for the current sample of African American youth (see Figure 2), and this pattern informed our decision to control smoking through age 14. Among our control variables are factors capturing cell-type variation, our derivation of these control variables is more extensively described in the supplemental material (Table S1). We also provide supplemental material examining the individual residues that comprise TNFm and show the high consistency of their individual ranges and mean values (Table S2). Because our key outcome, smoking reports at age 20, is skewed and over dispersed, we used negative binomial regressions to better represent the dependent variable when examining factors influencing young adult smoking. Supporting this decision, we provide supplemental material reporting comparisons of residuals for the negative binomial with alternative statistical models appropriate for count data. Specifically, we compare residuals for negative binomial distributions with Poisson and zero-inflated analytic models (Table S3). To examine the robustness of effects to changes in our measurement of young adult stress, we provide supplemental analyses using an alternative, broader, characterization of young adult stress comprised of the average of standardized stress measures for ages 17, 18, and 19. This analysis shows an equivalent pattern of results to those resulting from a focus on stress at age 19 only (See Supplemental Table S4). Stress measures were significantly, but only moderately correlated across waves and measures (average r = .196). To examine robustness with regard to concurrent use of alcohol and marijuana, we also conducted a supplemental analysis including concurrent alcohol and marijuana use as control variables, and found that including them did not change the observed pattern (See Table S5). Finally, we also provide supplemental material showing the association of TNFm with methylation genomewide using gominer to describe broader patterns of methylation associated with the index (Table S6).
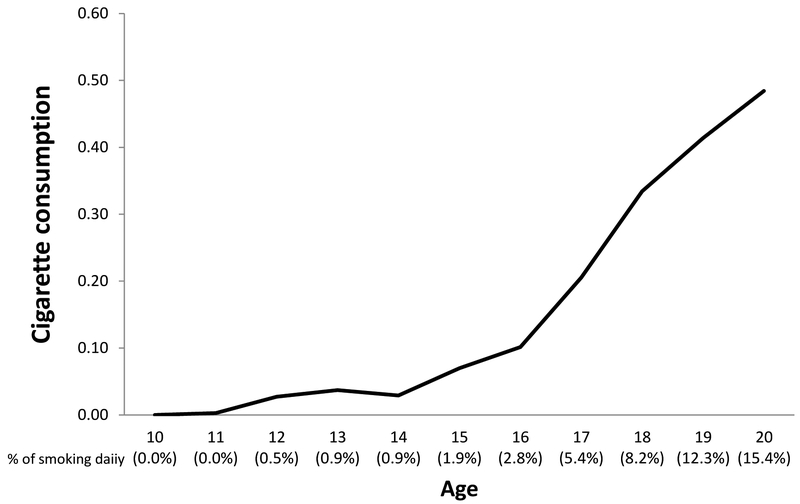
Figure 2.
African American youth smoking from ages 10 to 20. The curve shows little increase in average consumption or daily smoking prior to age 14, but substantial escalation thereafter
Hypotheses to be tested
The primary hypotheses derived from model displayed in Figure 1 are that supportive parenting may influence young adult smoking in two ways. First, it may influence young adult smoking by influencing young adult stress, and second it may influence smoking by influencing TNFm. In turn, we hypothesize that higher TNFm, resulting from more supportive parenting, will reduce the effect of young adult stress on smoking at age 20. We examine whether lower TNFm is associated with increases in smoking in response to stress beyond effects attributable to other circulating inflammatory markers (i.e. C-reactive protein (CRP)), a marker of current systemic inflammation known to be associated with BMI), as well as individual differences in cell-type composition that could be responsive to initiation of smoking. To address these issues we examine the following specific hypotheses:
- Supportive parenting during early adolescence (Ages 11–13), indexed by reports from both adolescents and their primary caregiver, will be associated with less perceived stress and greater TNFm in young adulthood.
- This association will be reflected in significant associations between early adolescent supportive parenting and both young adult stress (negative association) and TNFm (positive association).
- In addition, both associations will be robust to controls for sex, inflammatory cytokine level (CRP), and cell type variation.
- Young adult stress will predict smoking at age 20, controlling for early smoking (prior to age 14).
- The effect of stress on smoking at age 20 will be robust to controls for sex, early smoking, inflammatory cytokine levels (CRP), and cell type variation.
- There will be a significant indirect association of supportive parenting with smoking through young adult stress.
- Level of TNFm will be consequential with regard to later onset of smoking in response to stress.
- Lower TNFm will be associated with a greater impact of stress on cigarette smoking at age 20, controlling for gender, CRP, level of SES risk across early adolescence (ages 11–13), variation in blood cell type, and early smoking.
- The effect of young adult stress on smoking at age 20 will be significant for those with low TNFm and significantly greater for those lower in TNFm than for those higher in TNFm.
Method
Participants
At the first assessment, 667 families were selected randomly from lists of fifth-grade students, residing in nine rural counties in Georgia, using names that schools provided (see Brody et al., 2004, for a full description). From the sample of 561 available at the age 18 data collection (a retention rate of 84%), 500 emerging adults were selected randomly to continue participating in biological assessments going forward. Costs associated with proposed biological assessments necessitated the drawing of the subsample. From this subsample, 398 (79.8% of the original sample) provided blood samples for genome-wide methylation analyses around age 20 (see Brody et al., 2014 for additional details). Of these, 16 did not provide data at age 19, leaving an effective sample of N = 382 for the current analyses. Comparisons with participants who did not provide blood samples or complete all study measures did not reveal any significant differences on any variables at baseline.
Youth lived in small towns and communities with poverty and unemployment rates among the highest in the U.S. (Dalaker, 2001), and all youth self-described as African American. Based on feedback from local communities and focus groups of rural African American community members (Brody et al., 2004), community liaisons were used to aid in the recruitment and retention of participants. At the first assessment, primary caregivers in the sample worked full time, on average, for an average of 39.9 hours per week, but 42.3% lived below federal poverty standards, with a majority living below 150% of the poverty threshold. Median monthly family income was $1,644 at age 19 and $1,840 at the age of 20. In this and other regards they are representative of the Georgia counties in which they reside (Boatright & Bachtel, 2003).
Youth mean age was 10.66 years at the first wave of assessment and 20.46 years, on average, at the time of the blood draw used for epigenetic analyses, and 20.43 years, on average when reporting on wave 9 cigarette smoking. Of the young adults whose outcomes are the focus of the investigation, 45.4% are male and 54.6% are female. Approximately one-quarter (24%) had less than a 12th grade education.
The current sample has been the focus of prior research described in Beach et al. (2016), Beach et al. (2014), and Beach et al. (2015).
Procedure
A standardized assessment protocol lasting two hours, on average, collected in participants’ residences, was used at each wave of data collection. Self-report questionnaires were administered to youth in an interview format. Each interview was conducted privately, with no other family members present or able to overhear the conversation. Youth reported on their primary caregiver’s supportive parenting, their own smoking behavior, and also provided blood for epigenetic assessments. Primary caregivers reported on their parenting and family SES. To further enhance rapport with participants, African American students and community members served as home visitors to collect data at all visits.
Primary caregivers consented to their own and the youths’ participation in the study, and the youths under 18 assented to their own participation and then consented when they participated as adults. All procedures were approved by the University of Georgia Institutional Review Board.
Measures
Parenting.
Supportive parenting was assessed using target youth and parent reports when targets were 10.7, 12.4 and 13.2, on average. The short form of the Interaction Behavior Questionnaire (IBQ; Prinz, Foster, Kent, & O’Leary, 1979) was used to assess both youth and parent report. The short form includes the items with the highest phi coefficients and the highest item-total correlations among the 75 original items. It is correlated .96 with the full length scale. The 15 true-false items comprising the scale ask about listening, understanding, enjoying, and getting along. Cronbach’s alpha was over .70 for each reporter and at each wave (i.e., youth IBQ: .76 at age 10.7 (first wave), .79 at age 12.4 (second wave), and .82 at age 13.2 (third wave). Parent IBQ had Cronbach’s alpha of .84 at wave 1, .86 at wave 2, and .88 at wave 3. Parenting total scores were standardized and summed across youth and parent report and across ages 11 to 13 to form an overall index of supportive parenting during early adolescence (i.e. ages 11–13). Primary caregiver and target reports were correlated significantly at each wave, r = .232, r = .188, and r = .245, at waves 1, 2, and 3 respectively.
SES-Risk.
Caregiver reports collected when youth were 10.7 to 13.2 (waves 1 to 3) were used to create our measure of Socio-economic risk. Early adolescent cumulative SES risk was assessed across six indicators. Each indicator was scored dichotomously (0 if absent, 1 if present). Cumulative SES risk was defined as the average number of risk factors across the three assessments, yielding an index with a theoretical range of 0 to 6 (M = 2.33, SD = 1.35). The six risk indicators were (a) family poverty, defined as being below the poverty level, taking into account both family income and number of family members; (b) primary caregiver non-completion of high school or an equivalent; (c) primary caregiver unemployment; (d) single-parent family structure; (e) family receipt of Temporary Assistance for Needy Families; and (f) income rated by the primary caregiver as not adequate to meet all needs.
Perceived Stress.
Participating young adults responded to ten items from the Perceived Stress Scale (PSS; Cohen & Mermelstein, 1983) when targets were 19.1 years old, on average. The response format ranged from 1 (never) to 5 (very often). An example item is, “How often in the past 30 days have you been upset because of something happening unexpectedly.” Cronbach’s alpha was .81, with higher scores indicating greater perceived stress. As noted above, a parallel analysis is provided in the supplemental material showing that a combination of stress measures collected across ages 17.1 and 18.5 (waves 6 and 7) combined with the measure of perceived stress used at age 19.1 (wave 8) yields the same pattern of results (see supplemental table S4).
Cigarette consumption.
At each wave of data collection, subjects were asked “In the past month, how much did you smoke cigarettes?” Response options included: 0 - None at all; 1 - Less than 1 cigarette a day; 2 – 1 to 5 cigarettes a day; 3 - About a half a pack a day; 4 - About a pack a day; 5 - About 1 and a half packs a day; 6 - About 2 packs a day. This allowed us to chart changes across adolescence and early adulthood as well as to identify those smoking daily. Changes in smoking responses as a function of age (not wave of assessment) is provided in figure 2. Percent of the sample reporting “daily smoking” is also provided for each age. As can be seen, the graph is relatively flat from 11to 14 and then rises thereafter continuously until 15.4% of the sample is smoking daily at the final wave of assessment, age 20. Examination of self-reported smoking at age 20.4 (Wave 9) indicated that reports of smoking are over dispersed (mean = .484; variance = 1.185; Skewness = 2.680; Kurtosis = 7.848). Accordingly, we used a negative binominal regression to predict age 20 smoking (alternative models compared for residuals are shown in supplemental table S3, indicating that they provide inferior fit).
C-Reactive Protein (CRP).
Certified phlebotomists went to each participant’s home to draw blood when participants were 20.46 on average. At time of the blood draw, one tube of blood was drawn into a serum separator tube by the certified phlebotomist and this tube was frozen and delivered to the Psychiatric Genetics Lab at the University of Iowa for assaying. Serum levels of CRP were determined using a Duo Set Kit (DY1707; R&D Systems, Minneapolis, MN, USA) according to the manufacturer’ s directions. A normal concentration of CRP in healthy human serum is usually lower than 10 mg/L. No participants had CRP levels outside the normal range. Because CRP is characterized by a skewed distribution (skewness = 1.90, kurtosis = 2.94), we applied a log transformation to normalize the readings, resulting in substantial improvement of the distribution (skewness = 0.91, kurtosis = -0.31 after the transformation).
Methylation.
Certified phlebotomists also drew whole blood (30 ml) from each participant and shipped it to a lab in Iowa the same day for preparation. At the lab the blood tubes were inspected to ensure anticoagulation and aliquots of blood were diluted, mononuclear cell pellets were separated from the diluted blood specimen by density-gradient centrifugation, and the mononuclear cell layer was removed from the tube using a transfer pipette, resuspended, and frozen at –80 degree C until use. Genomic DNA was was prepared using a QiaAmp (Qiagen, Germany) according to manufacturer’s directions. A typical DNA yield for each mononuclear cell pellet was between 10 and 15 µg.
The Illumina (San Diego, CA) HumanMethylation450 Beadchip was used to assess genome-wide DNA methylation. Participants were randomly assigned to 12 sample “slides/chips” with groups of 8 slides being bisulfite converted in a single batch, resulting in five “batches/plates.” A replicated sample of DNA was included in each plate to aid in assessment of batch variation and to ensure correct handling of specimens. The replicated sample was examined for average correlation of beta values between plates, resulting in average correlations greater than 0.99. Prior to normalization, methylation data were filtered based on these criteria: 1) samples containing 1% of CpG sites with detection p-value >0.05 were removed, 2) sites were removed if a beadcount of < 3 was present in 5% of samples and 3) sites with a detection p-value of >0.05 in 1% of samples were removed. More than 99.76% of the 485,577 probes yielded statistically reliable data.
Quantile Normalization of Methylation data.
Quantile normalization methods with separate normalization of Type I and Type II assays in the Illumina array produce marked improvement in detection of relationships by correcting distributional problems inherent in the manufacturers default method for calculating β (i.e., β = M/(M + U + 100; where M and U are methylated and unmethylated signal intensities, respectively) (Pidsley et al., 2013). Accordingly, in the current investigation all loci across all plates were quantile normalized concurrently, separating methylated and unmethylated intensities, and using the wateRmelon (2013) R package (Team, 2012) to institute the “dasen” function recommended by Pidsley et al. (2013). This method equalizes the backgrounds of Type I and Type II probes prior to normalization and conducts between-array normalization of Type I and Type II probes separately.
Identifying and Correcting for Chip and Batch effects.
As demonstrated by Sun et al. (2011), quantile normalization typically reduces, but may not eliminate, batch and chip effects. Accordingly, after cleaning and quantile normalizing the data, all samples were examined for batch and chip effects. The distribution of quantile normalized average β values for all samples in each chip and batch were contrasted with all others using a box and density plot to indicate both the mean and confidence intervals around the mean in each case. The results of this examination indicated that both batch and chip effects were eliminated through quantile normalization. Absence of plate effects was confirmed via direct examination of the sample replicated across plates.
Assessing and Controlling Proportion of Cell types in Mixed Cell Populations.
Mononuclear cell pellets of the sort used in the current investigation are comprised of several different cell types (e.g., primarily T-helper and cytotoxic cells, monocytes, B cells, and natural killer (NK) cells, (Reinius, et al., 2012). Accordingly, we controlled for individual differences in cell types by using a regression calibration approach similar to that developed by Houseman and colleagues (2012), except we used Illumina HumanMethylation 450K BeadChip data to identify the 100 sites best differentiating to the five cell types of interest. A locus determined to be on the X chromosome was dropped from subsequent analyses. Then, we performed a principal components analysis (PCA) to identify principle components characterizing dimensions of individual variability in cell-type in the current sample. Regressions linking each factor with proportion of cell types can be found in supplementary material (See Supplemental Table S1 for details).
TNF methylation.
An index of pro-inflammatory tendencies was created by examining degree of methylation of the first exon of TNF (TNFm). Eight CpG sites were identified as being associated with the first exon of TNF based on the manufacturer’s documentation. Greater methylation of the first exon of TNF in cells capable of expressing TNF-α should result in less expression of this gene product and, all other things being equal, lower pro-inflammatory response. The inter-correlation of the eight CpG values on exon one was examined (rs ranging from .736 to .942; all ps < .00001). A factor analysis of the eight CpGs identified a single factor with all loadings above .85. Accordingly, to index overall methylation of the first exon of TNF (TNFm), βs for CpGs on the first exon were standardized prior to creating an average score with a Cronbach alpha of .98. TNFm averaged .265 (range .14 - .43) with all loci showing similar distributions (See Supplemental Table S2 for details).
Results
H1: Effect of Supportive parenting on Stress and TNFm.
As can be seen in Table 1, primary study variables were correlated at a zero-order level. Supportive parenting was significantly (and negatively) associated with SES-risk in early adolescence. Only SES risk and later smoking at age 20 were significantly associated with very early onset smoking and only supportive parenting in early adolescence (ages 11–13) and sex were significantly associated with (age 19) reports of young adult stress. Only supportive parenting was significantly associated with TNFm. All zero-order effects of interest were small to medium in size.
Table 1.
Correlation Matrix along with Means and Standard Deviations for Primary Study Variables (N = 382).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Supportive parenting (ages 11-13) | —— | |||||||||
| 2. Perceived stress (age 19) | −.202** | —— | ||||||||
| 3. Cigarette consumption (ages 11-14) | −.109* | .029 | —— | |||||||
| 4. Cigarette consumption (age 20) | −.100† | .093† | .122* | —— | ||||||
| 5. TNFm | .177** | −.071 | −.054 | −.045 | —— | |||||
| 6. Log of CRP | −.004 | .055 | −.096† | −.007 | −.064 | —— | ||||
| 7. Sex (1 = males) | .081 | −.177** | .081 | .289** | .064 | −.282** | —— | |||
| 8. SES-risk (ages 11-13) | −.196** | .033 | .144** | .095† | −.063 | .039 | −.014 | —— | ||
| Mean | −.021 | 27.374 | .026 | .484 | .007 | −1.131 | .456 | 6.869 | ||
| SD | .688 | 5.967 | .161 | 1.088 | .947 | 2.464 | .499 | 3.981 | ||
p ≤ .05.
p ≤ .01.
p ≤ .10, two-tailed. Factors 1-4 are the four principle components reflecting cell-type variation in the current data.
To further explicate the association of early adolescent parenting (ages 11–13) with TNF methylation and young adult stress, thereby examining the first stage of the theoretical mode (Figure 1), we examined these associations introducing multivariate controls including sex, SES risk, log (CRP), and cell type. As can be seen in table 2, the association of early adolescent supportive parenting (ages 11–13) with TNFm and young adult stress were robust to the introduction of these controls, supporting the first step of the theoretical model presented in Figure 1.
Table 2.
Regression models indicating that supportive parenting is a predictor of TNFm (age 20) and young adult perceived stress (age 19). (N = 382).
|
TNFm |
Stress (age 19) |
|||
|---|---|---|---|---|
| b | β | b | β | |
| Supportive parenting (ages 11-13) | .055* (.026) |
.058 | −1.107** (.289) |
−.185 |
| Sex (1= males) | .022 (.061) |
.012 | −1.904** (.677) |
−.159 |
| SES-risk (ages 11-13) | .041 (.029) |
.043 | −.053 (.294) |
−.009 |
| Factor 1 cell-type | .569** (.031) |
.601 | −.171 (.305) |
−.029 |
| Factr 2 cell-type | .489** (.032) |
.517 | −.048 (.328) |
−.008 |
| Factor 3 cell-type | −.104** (.028) |
−.110 | .218 (.337) |
.036 |
| Factor 4 cell-type | −.139** (.036) |
−.147 | −.007 (.290) |
−.001 |
| Log of CRP | −.034 (.025) |
−.036 | .031 (.291) |
.005 |
| Constant | −.003 (.036) |
28.242** (.424) |
||
| R2 | .677 | .069 | ||
Notes: Unstandardized (b) and standardized coefficients (β) shown with robust standard errors in parentheses; supportive parenting (ages 11-13), SES-risk (ages 11-13), factors cell-type, and CRP are standardized by z-transformation (mean = 0 and SD = 1).
p ≤ .10,
p ≤ .05,
p ≤ .01 (two-tailed tests).
H2: Stress effects on smoking and mediation of supportive parenting.
We next examined the association of young adult stress (age 19) with smoking in young adulthood (age 20). We controlled for the effect of very early smoking 11–14 (i.e. waves 1 to 4), as well as sex, SES risk, log (CRP), and cell type. As can be seen in Table 3, model 1, the association of young adult stress (age 19) with smoking (age 20) remained significant after including controls. As predicted, there was evidence of a significant indirect pathway from early parenting to change in young adult smoking through associations with young adult perceived stress (IE = −.071, 95% CI = (−.132, −.010). Examination of the column labeled IRR (incidence rate ratio) indicates that a standard deviation increase in perceived stress at wave 8 was associated with an increase in the expected response regarding cigarette consumption at wave 9 by 47.30%, holding all other variables, including smoking prior to age 14, constant. This relatively large effect is put in context by the even larger effect of sex (> 6), indicating that smoking was reported much more frequently by males.
Table 3.
Negative binomial regression models depicting the joint effect of perceived stress and TNFm on cigarette consumption at age 20 (N = 382).
| Cigarette consumption (age 20) |
||||
|---|---|---|---|---|
| Model 1 |
Model 2 |
|||
| b | IRR | b | IRR | |
| Perceived stress (age 19) | .387** (.137) |
1.473 | .369** (.137) |
1.447 |
| TNFm | −.331 (.221) |
.718 | ||
| Perceived stress (age 19) × TNFm |
−.214* (.103) |
.807 | ||
| Supportive parenting (ages 11-13) | −.076 (.113) |
.926 | −.087 (.109) |
.917 |
| Sex (1= males) | 1.928** (.258) |
6.877 | 1.925** (.255) |
6.854 |
| SES-risk (ages 11-13) | .193 (.129) |
1.213 | .180 (.118) |
1.197 |
| Cigarette consumption (ages 11-14) | .141 (.175) |
1.152 | .157 (.190) |
1.171 |
| Factor 1 cell-type | .173 (.111) |
1.189 | .393* (.167) |
1.481 |
| Factor 2 cell-type | −.078 (.110) |
.925 | .131 (.164) |
1.140 |
| Factor 3 cell-type | .089 (.093) |
1.093 | .029 (.093) |
1.030 |
| Factor 4 cell-type | .061 (.097) |
1.063 | .020 (.107) |
1.021 |
| Log of CRP | .192† (.106) |
1.211 | .168 (.107) |
1.182 |
| Constant | −2.006** (.227) |
−2.049** (.222) |
||
| −2LL | 614.638 | 609.992 | ||
| ∆ Chi-square (df = 1) | 4.646* | |||
Notes: Unstandardized (b) shown with robust standard errors in parentheses; IRR = incident rate ratio; supportive parenting (ages 11-13), SES-risk (ages 11-13), cigarette consumption (ages 11-14), factors cell-type, and CRP are standardized by z-transformation (mean = 0 and SD = 1).Using KHB methods (Breen, Karlson, & Holm, 2013), the test of the indirect effect of supportive parenting (ages 11-13) on cigarette consumption (age 20) through perceived stress (age 19) is significant [indirect effect = −.071, 95%(−.132, −.010)].
p ≤ .10,
p ≤ .05,
p ≤ .01 (two-tailed tests).
There was no evidence of multicollinearity in the regression. Diagnostic VIF scores for all variables in the regression were below 10, ranging from 1.056 to 3.097, indicating no evidence of multicollinearity among the study variables.
H3: Amplification of stress by TNFm.
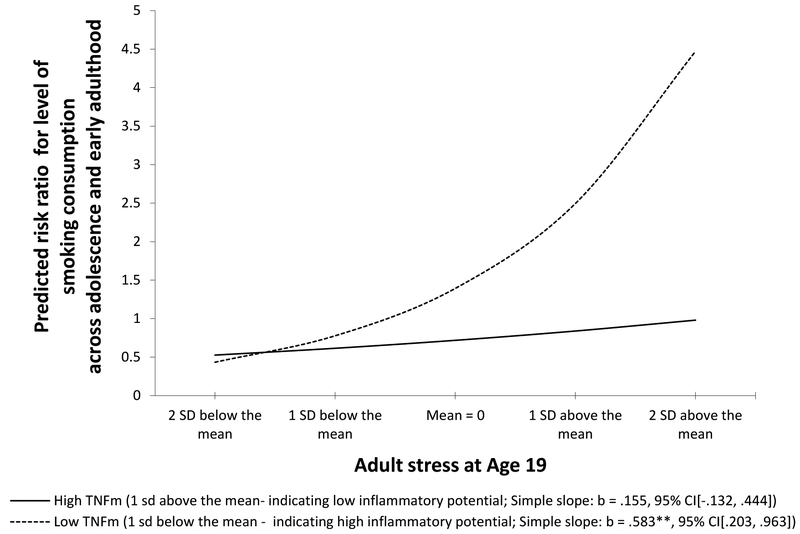
In model 2 of table 3 we examined the potential role of TNFm as a moderator of stress effects on increases in smoking by adding TNFm and the interaction term created by the product of TNFm with young adult stress to the regression. As can be seen in model 2, the effect of stress remained significant, and there was no significant main effect of TNFm, but the interaction term reflecting the joint impact of TNFm and young adult stress was significant, indicating significant moderation. To explicate the significant interaction effect we graphed slopes for high (1 sd above the mean) and low (1 sd below the mean) TNFm. As can be seen in figure 3, greater TNF-related inflammatory potential (i.e., low TNFm) was associated with significantly increased impact of young adult stress (age 19) on cigarette use at age 20. For low TNFm (high inflammatory potential), the association was significant (b = .583, p = .003) whereas for high TNFm (low inflammatory potential), the association was not significant (b = .155, p = .291). A standard deviation increase in perceived stress at wave 8 for respondents with low TNFm was associated with a 79.14% increase in reported cigarette consumption at wave 9, holding all other variables constant. Conversely among those with high TNFm, a standard deviation increase in stress was associated with a non-significant (16.77%) increase in smoking. Accordingly, the indirect effect of supportive parenting during early adolescence on change in young adult smoking was significant only among youth with lower TNFm (IE = −.039; 95% CI (−.084, −.008). To assess whether smoking at wave 8 (age 19) or wave 9 (age 20) might predict TNFm or CRP, potentially suggesting a role for smoking in predicting inflammatory potential, rather than the reverse, we examined a series of simple correlations. As can be seen in table 4, there is no evidence of a significant association of smoking at age 19 or 20 with either TNFm or log(CRP). Only the association of BMI with CRP was significant, as would be expected. Finally, to examine robustness with regard to concurrent use of alcohol and marijuana, we also conducted a supplemental analysis including concurrent alcohol and marijuana use as additional control variables in the negative binomial regression models examining the joint effect of perceived stress and TNFm on cigarette consumption, and found that including them did not change the observed pattern (See Table S5).
Figure 3.
Explication of interaction between TNFm and stress in the prediction of change in smoking using a negative binomial regression model, controlling for sex, SES-risk (ages 11–13), cell-type, and early cigarette consumption (ages 11–14). The lines represent the regression lines for different levels of TNFm (low: 1 SD below the mean; high: 1 SD above the mean). Simple slopes and confidence intervals provided in parentheses.
Table 4.
Smoking is not predictive of either inflammatory potential (TNFm) or circulating inflammatory markers (CRP) at age 20.
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| 1. Log of CRP (age 20) | —— | ||||
| 2. BMI (age 20) | .519** | —— | |||
| 3. TNFm (age20) | −.064 | −.088† | —— | ||
| 4. Cigarette consumption (age 19) | −.017 | −.031 | −.067 | —— | |
| 5. Cigarette consumption (age 20) | −.007 | −.099† | −.045 | .572** | —— |
| Mean | −1.131 | 28.527 | .007 | .414 | .48 |
| SD | 2.464 | 8.268 | .947 | 1.010 | 1.088 |
p ≤ .05.
p ≤ .01.
p ≤ .10.
Discussion
Results supported two pathways by which supportive parenting in early adolescence may have an impact on smoking in young adulthood. As indicted in the theoretical model presented in Figure 1, there was an indirect effect from early adolescent supportive parenting (ages 11 – 13) to young adult smoking (age 20) via associations with young adult stress (age 19). In addition, supportive parenting during early adolescence was associated with TNFm, indicating that supportive parenting provides protection that gets “under the skin,” or prevents other stressors from doing so, and works against pro-inflammatory propensities. As predicted, those with the less protective epitype experienced an amplified effect of young adult stress on smoking by age 20. As a consequence, among African American youth growing up in economically disadvantaged circumstances, even after controlling for sex, circulating inflammatory cytokines, early SES related risk, and variation due to individual differences in cell-types comprising the blood samples, more supportive parenting in early adolescence was associated with decreased smoking in young adulthood.
The results are consistent with theorizing by Nusslock & Miller (2015) and others (Cole et al., 2011; Gluckman, et al., 2005) that experiences in childhood or early adolescence may contribute to biological vulnerabilities, particularly inflammation-related vulnerabilities, that can be maintained into adulthood and can be consequential for young adult health behavior. The current results indicate that supportive parenting may be protective against a pro-inflammatory epitype that amplifies the effect of later young adult stress, highlighting supportive parenting as a potential early target of intervention to prevent rapid escalation in smoking behavior among rural African American youth as they enter young adulthood. Although stress effects on smoking are clearly evident in the current results, they suggest that the impact of stress encountered in the transition into adulthood may be influenced by earlier, modifiable family factors that can confer some protection. In the current sample the impact of stress was moderated by level of TNFm. There was no significant increase in smoking in response to young adult stress among those with higher TNFm, and this protection was associated with reports of greater parental supportiveness provided by both youth and their primary caregiver in early adolescence. Indeed, by looking at the association of stress with smoking across levels of parenting support and TNFm simultaneously, it can be seen that parenting support had no effect for those experiencing low stress, but had a substantial effect for those experiencing higher stress, and the impact of high stress on smoking was particularly noticeable among those who also had low methylation of TNF (See Supplemental Figure S1).
It has been shown previously that longer-term patterns of smoking trigger an inflammatory response that is maintained over time (See Shaykhiev et al., 2009; Willemse, Postma, Timens, & ten Hacken, 2004). In particular, among adolescents with heavier past-month smoking, an association between smoking status and elevated CRP has been observed (O’Loughlin, et al., 2008). Consequently, it was important to see if TNFm could be construed as a result of current smoking or if other inflammatory markers such as CRP might better capture observed associations. We examined smoking at age 19 and 20 and found that it was not associated with either TNFm or CRP in the current sample, suggesting that our observations occurred sufficiently early in the development of smoking patterns to avoid an effect of smoking on indicators of systemic inflammation.
There are several potential implications of the current results for the development of preventive interventions designed to reduce health problems among young adult African Americans. In addition to interventions to reduce young adult stress, it appears that interventions focused on increasing supportive parenting in early adolescence, before the onset of normative experimentation with smoking, may have the potential to modify epigenetic vulnerability and reduce vulnerability for later smoking in response to stress. Theoretically we might also expect an epitype associated with greater inflammatory potential to be associated with health outcomes at older ages. Accordingly, intervention to increase supportive parenting in early adolescence would appear to have substantial beneficial potential on later adult health. However, because the epigenetic vulnerability we examined appears to be correlated with parenting at ages 11 – 13, the earliest assessment for the current sample, it is not possible to discern with certainty at what age a focus on enhancing supportive parenting would have its maximum effect on TNFm. That is, it is possible that the association between epitype and parenting was established at an earlier age than those we assessed, suggesting that investigation of samples at earlier ages is warranted. Indeed, some theorists would suggest that parenting potentially influences the development of inflammation related patterns of differential methylation beginning much earlier in childhood (Cf. Miller & Chen, 2010; Miller et al., 2011). Accordingly, further research is needed to examine whether epigenetic vulnerabilities can be modified later in adolescence or in young adulthood.
Because youth can also exert influence on parenting behavior (e.g., Kiff, et al., 2011), it is possible that, in addition to the non-specific influence of environmental stressors, some of the observed impact of early adolescent supportive parenting on young adult outcomes may reflect early adolescent temperament and other behavioral characteristics that were not directly measured in the current study. Likewise, the current research depends on a single wave of assessment of methylation, reducing confidence in causal conclusions. It is to be hoped that future research, incorporating multiple assessments sufficiently powered to examine change in both differential methylation and change in parenting relationships, will further clarify the time course of differential methylation, the direction of effects, and perhaps identify the age at which maximum effects of suppportive parenting on differential methylation are obtained.
Importantly, there are several indications that the TNFm pro-inflammatory epitype was not merely a response to cigarette smoking. First, it is a precursor variable rather than an inflammatory agent itself, making it less probable that it would vary as part of an inflammatory reaction to smoking. Second, there are significant correlations between TNFm and theoretically plausible precursors, i.e. early adolescent parenting but no correlations with smoking at ages 19 or 20, i.e., the ages that should have shown an effect if smoking was a driver of TNFm. Third, as was shown in Figure 2, increases in cigarette smoking began after age 14, later than the age for which parenting variables are predictive of TNFm. Accordingly, it seems unlikely that observed effects are attributable to an effect of smoking on TNFm. Nonetheless, direct measurement of TNFm at multiple ages, including assessment prior to the onset of smoking would help solidify conclusions about causal direction.
Contrary to expectations, we did not find an effect of SES risk on TNFm. Although SES-risk was associated significantly with suppportive parenting and early smoking, it was not associated with TNFm. This may be due in part to selection of a sample with relatively low variability in SES, with all participants experiencing a challenging economic context. In addition, results suggest a need to examine other pro-inflammatory changes that may be predicted by SES-risk to see how they may differ from or interact with TNFm. Alternatively, it may be useful to examine broader networks of pro-inflammatory changes to better capture the impact of a range of childhood adversities and examine the role of supportive parenting in relation to each of them. To better characterize significantly enriched pathways using controls for multiple comparisons, we did an exporatory genomewide examination of CpG sites, also on first exons, that were significantly associated with TNFm using GoMiner™. We used “all gene ontology” as the root category setting, and used the 105 genes reflecting the 128 CpG sites associated at a genomewide significance level (i.e., p< 10e-7) as the “changed” gene set (Zeeberg et al., 2003). The top 10 pathways are reported in supplemental table S6. TNFm was robustly asssociated with gene pathways linked to immune functioning and cell type activation and signalling among others
Limitations of the current investigation notwithstanding, the current research provides an initial step in explicating the complex and important ways in which early adversity and early family environment may become biologically embedded and set the stage for, or protect against, later health problems. As illustrated in the current investigation, biologically embedded consequences of early experience, reflected in differential methylation, may be consequential for later behavioral response to stress, helping explain the increased vulnerability of some youth to rapid escalation in smoking in young adulthood. In addition, the influence of early supportive parenting on the development of latent vulnerability to smoking in young adulthood has particular relevance for prevention programs because parenting practices are potentially modifiable (Brody, et al., 2012; (Brody, Yu, Beach, Kogan, Windle, & Philibert, 2014; Chen, Miller, Kobor, & Cole, 2011), and they appear to provide promising potential points of intervention upon which smoking prevention programs can expand. As a consequence, the current research contributes to much needed translational research efforts that identify new potential points of preventive intervention for smoking.
Supplementary Material
Acknowledgments
This research was supported by Award 5R01HD030588–16A1 from the National Institute of Child Health and Human Development and Award 1P30DA027827 from the National Institute on Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Child Health and Human Development, the National Institute on Drug Abuse, or the National Institutes of Health.
Abbreviations:
- CpG
regions of DNA in which a cytosine nucleotide occurs next to a guanine nucleotide separated by only one phosphate
- TNF
Tumor necrosis factor
- TNF
the gene encoding TNF-alpha.
- TNFm
TNF methylation.
- SES
socioeconomic status
- DMR
Differentially methylated Region
Contributor Information
Steven R. H. Beach, Center for Family Research, University of Georgia
Man Kit Lei, Center for Family Research, University of Georgia.
Gene H. Brody, Center for Family Research, University of Georgia
Gregory E. Miller, Institute for Policy Research and Department of Psychology, Northwestern University
Edith Chen, Institute for Policy Research and Department of Psychology, Northwestern University.
Jelani Mandara, Human Development and Social Policy, Northwestern University.
Robert A. Philibert, Department of Psychiatry, University of Iowa
References
- Aseltine RH & Gore S (2005). Work, postsecondary education, and psychosocial functioning following the transition from high school. Journal of Adolescent Research 20, 615–639. [Google Scholar]
- Beach SRH, Brody GH, Lei MK, Kim S, Cui J, & Philibert RA (2014). Is serotonin transporter genotype associated with epigenetic susceptibility or vulnerability? Examination of the impact of socioeconomic status risk on African American youth. Development and Psychopathology, 26(2), 289–304. Doi: 10.1017/S0954579413000990 NIHMSID:604984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach SRH, Lei MK, Brody GH, Kim S, Barton AW, Dogan MV, Philibert RA (2016). Parenting, SES-risk, and later Young Adult Health: Exploration of opposing indirect effects via DNA methylation. Child Development, 87(1), 111–121. doi: 10.1111/cdev.12486.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach SRH, Lei MK, Brody GH, Dogan MV, Philibert RA (2015). Higher Levels of Protective Parenting are Associated with Better Young Adult Health: Exploration of Mediation through Epigenetic Influences on Pro-Inflammatory Processes. Frontiers in Psychology, 6, 676. doi: 10.3389/fpsyg.2015.00676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatright SR, Bachtel DC (2003). The Georgia County Guide, 19th Edn. Athens, GA: University of Georgia Cooperative Extension Service. [Google Scholar]
- Bradley JR (2007). TNF-mediated inflammatory disease. Journal of Pathology, 214, 149–160. [DOI] [PubMed] [Google Scholar]
- Breen R, Karlson KB, & Holm A (2013). Total, direct, and indirect effects in logit and probit models. Sociological Methods & Research, 42(2), 164–191. doi: 10.1177/0049124113494572.. [DOI] [Google Scholar]
- Brenet F, Moh M, Funk P, Feierstein E, Viale AJ, Socci ND, Scandura JM (2011). DNA Methylation of the First Exon Is Tightly Linked to Transcriptional Silencing. PLoS One. 6(1): e14524. Published online 2011 Jan 18. doi: 10.1371/journal.pone.0014524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Chen Y. f., Kogan SM, Yu T, Molgaard VK, DiClemente RJ, & Wingood GM (2012). Family-centered program to prevent substance use, conduct problems, and depressive symptoms in Black adolescents. Pediatrics, 129(1), 108–115. doi: 10.1542/peds.2011-0623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Chen Y & Kogan SM (2010). A Cascade Model Connecting Life Stress to Risk Behavior Among Rural African American Emerging Adults. Development and Psychopathology 22, 667–678. doi: 10.1017/S0954579410000350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Chen YF, Yu T, Beach SRH, Kogan SM, Simons RL, Windle M, Philibert RA (2012). Life stress, the dopamine receptor gene, and emerging adult drug use trajectories: A longitudinal, multilevel, mediated moderation analysis. Development and Psychopathology 24, 941–951, doi: 10.1017/S0954579412000466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Murry VM, Gerrard M, Gibbons FX, Molgaard V, McNair LD, Neubaum-Carlan E (2004). The Strong African American Families program: Translating research into prevention programming. Child Development, 75, 900–917. doi: 10.1111/j.1467-8624.2004.00713.x.. [DOI] [PubMed] [Google Scholar]
- Brody GH, Yu T, Beach SRH, Kogan SM, Windle M, & Philibert RA (2014). Harsh parenting and adolescent health: A longitudinal analysis with genetic moderation. Health Psychology, 33, 401–409. doi: 10.1037/a0032686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Chen Y. f., Kogan SM, Smith K & Brown AC (2010). Resilience Effects of a Family-Based Preventive Intervention on African American Emerging Adults. Journal of Marriage and Family 72, 1426–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann AF, Laucht M, Schmid B, Wiedemann K, Mann K, Zimmermann US. (2010). Cigarette craving increases after a psychosocial stress test and is related to cortisol stress response but not to dependence scores in daily smokers. Journal of Psychopharmacology. 24(2):247–55. doi: 10.1177/0269881108095716.doi:10.1177/0269881108095716. [DOI] [PubMed] [Google Scholar]
- Brynin M (1999). Smoking behaviour: Predisposition or adaptation? Journal of Adolescence, 22, 635–646. [DOI] [PubMed] [Google Scholar]
- CDC (2010).. Racial disparities in smoking-attributable mortality and years of potential life lost---Missouri, 2003–2007. MMWR. Morbidity and mortality weekly report 59, 1518. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2011). Excessive Alcohol Use. Retrieved April 14, 2015 from http://www.cdc.gov/chronicdisease/resources/publications/aag/pdf/2011/alcohol_aag_web_508.pdf
- Centers for Disease Control and Prevention (2014). The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention; [accessed 2015 Apr 7 from http://www.cdc.gov/tobacco/data_statistics/sgr/50th-anniversary/index.htm]. [Google Scholar]
- Cerbone FG, Larison CL. (2000). A bibliographic essay: The relationship between stress and substance use. Substance Use & Misuse, 35, 757–786 [DOI] [PubMed] [Google Scholar]
- Chen H, Cohen P, Chen S (2010). How Big is a Big Odds Ratio? Interpreting the Magnitudes of Odds Ratios in Epidemiological Studies. Communications in Statistics: Simulation and Computation, 39 (4), 860–864. [Google Scholar]
- Chen E, Miller GE, Kobor MS, & Cole SW (2011). Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Molecular Psychiatry, 16, 729–737. doi: 10.1038/mp.2010.53.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs E, & de Witt H (2010). Effects of acute psychosocial stress on cigarette craving and smoking. Nicotine and Tobacco Research, 12(4), 449–453. doi: 10.1093/ntr/ntp214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chyu L, & Upchurch DM (2011). Racial and Ethnic Patterns of Allostatic Load among Adult Women in the United States: Findings from the National Health and Nutrition Examination Survey 1999–2004. Journal of Womens Health, 20(4), 575–583. doi: 10.1089/jwh.2010.2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24, 385–396. [PubMed] [Google Scholar]
- Colamussi L, Bovbjerg DH, Erblich J (2007). Stress- and cue-induced cigarette craving: Effects of a family history of smoking. Drug and Alcohol Dependence, 88, 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW (2010). Elevating the perspective on human stress genomics. Psychoneuroendocrinology 35, 955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW (2014). Human social genomics. PLOS Genetics 10(8) e1004601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Conti G, Arevalo JM, Ruggiero AM, Heckman JJ, Suomi SJ (2012). Transcriptional modulation of the developing immune system by early life social adversity. Proceedings of the National Academy of Sciences USA, 109 (50), 20578–20583. doi: 10.1073/pnas.1218253109.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JMG, Cacioppo JT (2011). Transcript origin analysis identifies antigen presenting cells as primary targets of socially regulated gene expression in leukocytes. Proceedings of the National Academy of Sciences USA, 108, 3080–3085. doi: 10.1073/pnas.1014218108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger RD, Ge X, Elder GH, & Lorenz FO (1994). Economic stress, coercive family process, and developmental problems of adolescents. Child Development, 65(2), 541–561. [PubMed] [Google Scholar]
- Dalaker J (2001, September). Poverty in the United States, 2000 (U. S. Census Bureau Current Population Reports Series P60–214). Washington, DC: U. S. Government Printing Office. [Google Scholar]
- De Bellis MD (2002). Developmental traumatology: a contributory mechanism for alcohol and substance use disorders. Psychoneuroendocrinology 27, 155–170. [DOI] [PubMed] [Google Scholar]
- Dhama K, Latheef SK, Samad HA, Chakraborty S, Tiwari R, Kumar A, Raha A. (2013). Tumor necrosis factor as mediator of inflammatory diseases and its therapeutic targeting: A review. Journal of Medical Sciences, 13(4), 226–235. DOI: 10.3923/jms.2013.226.235 [DOI] [Google Scholar]
- Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, & Croft JB (2009). Cumulative childhood stress and autoimmune diseases in adults. Psychosomatic Medicine, 71, 243–250. doi: 10.1097/PSY.0b013e3181907888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erblich J, Boyarsky Y, Spring B, Niaura R, Bovbjerg DH (2003). A family history of smoking predicts heightened levels of stress-induced cigarette craving. Addiction, 98, 657–664. [DOI] [PubMed] [Google Scholar]
- Ennett ST, Bauman KE, Pemberton M, Foshee VA, Chuang Y-C, King T, et al. (2001). Mediation in a family-directed program for prevention of adolescent tobacco and alcohol use. Preventive Medicine, 33, 333–346. [DOI] [PubMed] [Google Scholar]
- Fagan P, Moolchanm ET Lawrence D, Fernander A, Ponder PK (2007). Identifying health disparities across the tobacco continuum. Addiction, 102(supplement), 5–29. [DOI] [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, & Bound J (2006). “Weathering” and Age Patterns of Allostatic Load Scores among Blacks and Whites in the United States. American Journal of Public Health, 96(5), 826–833. doi: 10.2105/ajph.2004.060749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Manuck SB, Sheu LK, Kuan DC, Votruba-Drzal E, Craig AE, et al. (2011). Parental education predicts corticostriatal functionality in adulthood. CerebralCortex, 21, 896–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, & Spencer HG (2005). Predictive adaptive responses and human evolution. Trends in Ecology & Evolution, 20(10), 527–533. doi: 10.1016/j.tree.2005.08.001 [DOI] [PubMed] [Google Scholar]
- Gordon HW (2002). Early environmental stress and biological vulnerability to drug abuse. Psychoneuroendocrinology 27, 115–126. doi: 10.1016/S0306-4530(01)00039-7. [DOI] [PubMed] [Google Scholar]
- Gruenewald TL, Cohen S, Matthews KA, Tracy R, & Seeman TE (2009). Association of socioeconomic status with inflammation markers in black and white men and women in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Social Science & Medicine, 69(3), 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiman CA, Stram DO, Wilkens LR, Pike MC, Kolonel LN, Henderson BE & Le Marchand L (2006).. Ethnic and racial differences in the smoking-related risk of lung cancer. New England Journal of Medicine, 354, 333–342. [DOI] [PubMed] [Google Scholar]
- Hertzman C (1999). The biological embedding of early experience and its effects on health in adulthood. Annals of the New York Academy of Sciences, 896, 85–95. [DOI] [PubMed] [Google Scholar]
- Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, Wiencke JK, Kelsey KT (2012). DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics, 13, 86–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Pedrell P, Whitton AE, Pechtel P, Douglas S, Martinson MA, Huz I, Fava M, Pizzagalli DA, Evins AE (2015). Reward Responsiveness Varies by Smoking Status in Women with a History of Major Depressive Disorder. Neuropsychopharmacology, 40, 1940–1946. doi: 10.1038/npp.2015.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R (2003). Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proceedings of the. National Academy of Sciences, 100, 9090–95. doi: 10.1073/pnas.1531903100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiff CJ, Lengua LJ, Zalewski M (2011). Nature and nurturing: parenting in the context of child temperament. Clinical Child and Family Psychology Review, 14(3), 251–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loucks EB Pilote L, Lynch JW, Richard H, Almeida N, Benjamin EJ, Murabito JM (2010). Life course socioeconomic position is associated with inflammatory markers: The Framingham Offspring Study. Social Science & Medicine, 71(1), 187–195. doi: 10.1016/j.socscimed.2010.03.012.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthar SS (2006). Resilience in development: A synthesis of research across five decades In Cicchetti Dante and Cohen DJ (Eds.), Developmental Psychopathology: Risk, Disorder, and Adaptation. New York: Wiley; pp. 740–795. [Google Scholar]
- Maier SF, Watkins LR (1998). Cytokines for psychologiests: Implications of bidirectional immune-to-brain communication for understanding behavior, mood,and cognition. Psychological review, 105, 83–107. [DOI] [PubMed] [Google Scholar]
- Miller GE & Chen E (2010). Harsh family climate in early life presages the emergence of pro-inflammatory phenotype in adolescence. Psychological Science, 21, 848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Sze J, Marin TJ, Doll RM, Ma R, & Cole SW 2008. A functional genomic fingerprint of chronic stress in humans: decreased glucocorticoid and increased NF-κB signaling. Biological Psychiatry, Biological Psychiatry, 64, 266–272. doi: 10.1016/j.biopsych.2008.03.017.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Parker KJ (2011). Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychological Bulletin, 137, 959–997. doi: 10.1037/a0024768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, & Cole SW (2012). Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biological Psychiatry, 72(1), 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maltic V, Raison CL (2009). Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biological Psychiatry, 65, 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL (2004). Actual causes of death in the United States, 2000. Journal of the American Medical Association, 291(10), 1238–1245. [DOI] [PubMed] [Google Scholar]
- Niaura R, Shadel WG, Britt DM, Abrams DB (2002). Response to social stress, urge to smoke, and smoking cessation. Addictive Behaviors, 27, 241–250. [DOI] [PubMed] [Google Scholar]
- Nusslock R, & Miller G (2015). Early-life adversity and physical and emotional health across the lifespan: A neuro-immune network hypothesis. Biological Psychiatry, S0006–3223(15)00466–7. doi: 10.1016/j.biopsych.2015.05.017.. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Loughlin J, Lambert M, Karp I, McGrath J, Gray-Donald K, Barnett TA, Delvin EE, Levy E, Paradis G (2008). Association between cigarette smoking and C-reactive protein in a representative, population-based sample of adolescents. Nicotine and Tobacco Research, 10(3), 525–532. doi: 10.1080/14622200801901997.. [DOI] [PubMed] [Google Scholar]
- Paalani M, Lee JW, Haddad E, & Tonstad S (2011). Determinants of Inflammatory Markers in a Bi-Ethnic Population. Ethnicity & Disease, 21(2), 142–149. [PMC free article] [PubMed] [Google Scholar]
- Paschall MJ, Flewelling RL & Faulkner DL (2000). Alcohol misuse in young adulthood: Effects of race, educational attainment, and social context. Substance Use & Misuse, 35, 1485–1506. [DOI] [PubMed] [Google Scholar]
- Peechatka AL, Whitton AE, Farmer SL, Pizzagalli DA, Janes AC (2015). Cigarette craving is associated with blunted reward processing in nicotine-dependent smokers. Drug and Alcohol Dependence,155,:202–207. doi: 10.1016/j.drugalcdep.2015.07.015.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidsley R, Wong CCY, Volta M, Lunnon K, Mill J, Schalkwyk LC (2013). A data-driven approach to preprocessing Illumina 450K methylation array data. BMC Genomics, 14, 293–302. doi: 10.1186/1471-2164-14-293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piko B,F & Balazs MA (2012). Authoritative parenting style and adolescent smoking and drinking Addictive Behaviors, 37 (3), 353–356. doi.org/10.1016/j.addbeh.2011.11.022 [DOI] [PubMed] [Google Scholar]
- Plume J, Beach SRH, Brody GH, & Philibert RA (2012). A Cross Platform genome wide comparison of the relationship of promoter DNA methylation to gene expression. Frontiers in Epigenomics, 3, 1–8. Epub Feb 12. doi: 10.3389/fgene.2012.00012 PMID [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz RJ, Foster SL, Kent RN, & O’Leary KD (1979). Multivariate assessment of conflict in distressed and nondistressed mother–adolescent dyads. Journal of Applied Behavior Analysis, 12, 691–700. doi: 10.1901/jaba.1979.12-691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinius LE, Acevedo N, Joerink M, Pershagen G, Dahlén SE, Greco D, Söderhäll C, Scheynius A, Kere J (2012). Differential DNA methylation in purified human blood cells: implications for cell lineage and studies on disease susceptibility. PLoS One, 7, e41361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjit N, Diez-Roux AV, Shea S, Cushman M, Ni H, Seeman T (2007). Socioeconomic position race/ethnicity and inflammation in the multi-ethnic study of atherosclerosis. Circulation, 116, 2383–2390. [DOI] [PubMed] [Google Scholar]
- Rickard IJ, & Lummaa V (2007). The predictive adaptive response and metabolic syndrome: challenges for the hypothesis. Trends in Endocrinology and Metabolism, 18(3), 94–99. doi: 10.1016/j.tem.2007.02.004 [DOI] [PubMed] [Google Scholar]
- Ryzin MJ, Fosco GM, Dishion TJ (2012). Family and peer predictors of substance use from early adolescence to early adulthood: An 11-year prospective analysis. Addictive Behaviors, 37 (12), 1314–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaykhiev R, Krause A, Salit J, Strulovici-Barel Y, Harvey BG, O’Connor TP, Crystal RG (2009). Smoking-dependent reprogramming of alveolar macrophage polarization: implication for pathogenesis of chronic obstructive pulmonary disease. Journal of Immunology, 183, 2867–2883. doi: 10.4049/jimmunol.0900473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons RG, Burgeson R, Carlton-Ford S, Blyth DA (1987). The impact of cumulative change in early adolescence. Child Development, 58(5), 1220–1234. [DOI] [PubMed] [Google Scholar]
- Sinha R (2008). Chronic Stress, Drug Use, and Vulnerability to Addiction. Annals of the New York Academy of Sciences, 1141, 105–130, doi: 10.1196/annals.1441.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R (2001). How does stress increase risk of drug abuse and relapse? Psychopharmacology. 158, 343–359. [DOI] [PubMed] [Google Scholar]
- Sun Z, Chai HS, Wu Y, White WM, Donkena KV, Klein CJ, Garovic VD, Therneau TM, Kocher JP (2011). Batch effect correction for genome-wide methylation data with Illumina Infinium platform. BMC Medical Genomics, 16(4):84. doi: 10.1186/1755-8794-4-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- wateRmelon: Illumina 450 methylation array normalization and metrics [computer program]. Version R package 1.5.1.2013.
- Willemse BWM, Postma DS, Timens W & ten Hacken NHT (2004). The impact of smoking cessation on respiratory symptoms, lung function, airway hyperresponsiveness and inflammation. European Respiratory Journal, 23, 464–476. [DOI] [PubMed] [Google Scholar]
- Wills TA (1990). Stress and Coping Factors in the Epidemiology of Substance Use. in Research advances in alcohol and drug problems, Vol. 10 Annis HM Cappell HD Glaser FB Goodstadt MS Kozlowski LT (Eds.), pp. 215–250. New York: Springer; 10.1007/978-1-4899-1669-3 [DOI] [Google Scholar]
- Windle M, & Windle RC (2009). Adolescent alcohol use In DiClemente RJ Santelli JS Crosby RA (Eds.) , Adolescent health: Understanding and preventing risk behaviors (pp. 165–178). San Francisco, CA, US: Jossey-Bass. [Google Scholar]
- Windle M, & Windle RC (2012). Early onset problem behaviors and alcohol, tobacco, and other substance use disorders in young adulthood. Drug and alcohol dependence, 121(1), 152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeberg B, Feng W, Wang G, Wang MD, Fojo AT, Sunshine M, Weinstein JN (2003). GoMiner: A resource for biological interpretation of genomic and proteomic data. Genome Biology 4, 1–8. Retrived from: http://genomebiology.com/2003/4/4/R28 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.