Abstract
To date, the major focus of diagnostic modalities and interventions to treat coronary artery disease has been the large epicardial vessels. Despite substantial data showing that microcirculatory dysfunction is a strong predictor of future adverse cardiovascular events, very little research has gone into developing techniques for in vivo diagnosis and therapeutic interventions to improve microcirculatory function. In this review, we will discuss the pathophysiology of coronary arteriolar dysfunction, define its prognostic implications, evaluate the diagnostic modalities available, and provide speculation on current and potential therapeutic opportunities.
Introduction
Current approaches to diagnose and treat atherosclerosis-related disorders arise from decades of research into the associated pathological changes that occur in large conduit vessels, such as lipid-laden, neointimal plaque formation in coronary arteries (33). Coronary angiography is the gold-standard technique to evaluate coronary stenosis severity. Medical treatment seeks to reduce lipid deposition and counteract thrombus formation in large coronary vessels, and interventional approaches, like coronary stenting, target sites of plaque-induced obstruction. However, a number of early studies identified commonalities between arterial and arteriolar pathophysiology in atherosclerotic development, such as increased endothelial adhesion molecule expression and lipid deposition in both large and small vessels (24, 33, 55), despite absence of plaque formation in arterioles. Descriptions of myocardial ischemia and chest pain in patients with normal angiograms (52, 61) and awareness of abnormalities in arteriolar vasomotor responses in atherosclerotic animals (21, 92, 105) also provided early indications of pathological changes in otherwise plaque-free arterioles. Still, the major historical emphasis on large vessels is not surprising. Several factors have contributed to this trend, including a relative dearth of clinical tools to directly evaluate in vivo microvascular function in humans (37), and lack of overt plaque formation in microscopically visible arterioles vs. arteries where macroscopic plaque formation has been noted for over a century (33, 55). This has resulted in a virtual absence of FDA-approved therapies developed to specifically address microvascular dysfunction (69).
Challenging the status quo and rapidly reshaping our understanding of coronary atherosclerosis and coronary artery disease (CAD) are emerging reports that microvascular dysfunction may occur before large-vessel dysfunction (88) and may be the predominant driver of major adverse cardiac events in the presence or absence of diseased coronary arteries (3, 15, 38, 103, 104). Therefore, although pathology in this segment of the circulation is more difficult to detect clinically, it may carry greater prognostic significance than the degree of obstruction in larger coronary arteries. This review summarizes key findings related to coronary arteriolar dysfunction that define its critical role in cardiovascular disease and suggest that arterioles should be prioritized in the ongoing development of novel diagnostic and therapeutic strategies to combat CAD.
Overview of Coronary Arteriolar Function
Coronary arterioles have an endothelial and well-innervated smooth muscle cell layer and are typically defined as resistance microvesssels with an internal luminal diameter of ~80–100 µm (22, 46). During resting conditions, most of the heart’s vascular resistance is generated by coronary arterioles that maintain a relatively high threshold level of myogenic tone (22, 68), resulting in a major drop in pressure across the arterioles (46). Accordingly, vasodilator reserve is typically between three and five times basal flow, providing oxygen for potentially large increases in metabolic demand associated with conditions like exercise (46, 67). Various factors [metabolites, extravascular compression, pressure, flow (flow-mediated dilation, FMD), and neurohumoral factors] influence vascular tone, and the signaling pathways responsible for the control of arteriolar tone in humans have been reviewed elsewhere (12, 37).
Benefitting from the fractal branching pattern of the vascular tree (8), the microcirculation (arterioles, capillaries, and venules) maximizes its spatial distribution and adjacency to each parenchymal cardiomyocyte, while minimizing its volume. As a result, the local arteriolar environment is poised to profoundly influence cardiac physiology on a cellular and tissue level, and any changes in surrounding cell types can likewise impact arteriolar function. For example, cross talk between arterioles and cardiomyocytes is essential for metabolic dilation (89, 90). It is also believed that factors released from endothelial or smooth muscle cells can modulate, for example, collagen metabolism in cardiac fibroblasts (34).
Emerging Evidence Implicates Coronary Arteriolar Dysfunction in Disease
Techniques to Assess Arteriolar Function
Several technological approaches provide insight into coronary vascular dynamics. In vivo methods to detect coronary blood flow include the TIMI frame count, which requires intracoronary dye injection but has been well-validated as a predictor of outcomes post-infarction (4). Intracoronary Doppler velocimetry can be used in the cath laboratory to assess coronary flow velocity reserve as an indicator of microvascular dilator capacity (102). Coronary flow reserve can also be determined non-invasively by echo Doppler using the instantaneous wave-free ratio (23). Contrast echocardiography can also be used to assess vasodilator reserve in the heart (93). Positron emission tomography (PET) supplies quantitative information about myocardial perfusion non-invasively and is considered the “gold standard” for measuring myocardial perfusion (26). PET can measure endocardial vs. epicardial flow distribution, which is critical for evaluating the extent of myocardial ischemia since altered transmural distribution can augment pathophysiological changes arising from arteriolar dysfunction. Magnetic resonance (MR) angiography can also assess, with contrast, myocardial perfusion (29). A new non-invasive approach based on T1 cardiac MR removes the need for contrast or radiation and corresponds well with invasive measurements of epicardial and microvascular dysfunction (62).
Direct evaluation of the local arteriolar environment is not clinically feasible in vivo, so clinical studies using the above techniques currently categorize arteriolar dysfunction as an impairment in regional or global blood flow. However, coronary conduit arteries can impair blood flow independent of arteriolar dysfunction. To distinguish between abnormal flow associated with a flow-limiting epicardial stenosis and abnormal flow arising from microvascular dysfunction, two different measurement techniques are applied. A widespread classification scheme uses fractional flow reserve (FFR), an indicator of obstructive epicardial CAD, and coronary flow velocity reserve (CFR), an indicator of coronary microvascular dilator reserve. FFR is captured during maximal hyperemia—typically after infusion of adenosine—and measures the change in pressure across a stenosis using a Doppler guidewire (42). CFR compares the level of blood flow at rest and during peak flow, and assesses microvascular function when FFR is normal (71). Although these studies can be used to interrogate the effect of plaques on blood flow and categorize different clinical populations, they only describe measurements of resting and peak blood flow (a relatively crude and limited assessment of arteriolar function), and fail to address nuances of arteriolar dysfunction such as endothelium-specific changes in mediators of vasodilation or separation of endothelial and smooth muscle contributions to dilator compromise. Furthermore, most approaches only serve to confirm or suggest associations between arteriolar dysfunction and disease pathology.
To address this limitation of in vivo approaches, ex vivo videomicroscopy allows researchers to explore mechanisms of arteriolar dysfunction in greater detail. This technique involves isolating arterioles from harvested tissue and cannulating these vessels in a warmed organ bath. Once cannulated, the isolated arterioles are pressurized, and changes in internal diameter are recorded on a video monitor attached to an inverted microscope. A wide range of experimental conditions are possible with this technique, such as endothelial denudation, pretreatment of arterioles with compounds that affect mRNA and protein levels (e.g., viruses and pharmacological agents), use of fluorescently labeled compounds to assess changes in redox state or to track intracellular trafficking of molecules, evaluation of flow and agonist-induced dilator mechanisms, and more. This technique has generated substantial data regarding the specifics of arteriolar physiology, described in the next section, and removes the potential confounding influence of neurohumoral, extravascular, myogenic, and many paracrine influences that modulate coronary arteriolar resistance (68). Although more mechanistic than in vivo measurements of CFR, this approach does not yield insight into all nuances of arteriolar dysfunction, such as the possible interaction between arterioles and cardiac fibroblasts, neurohumoral modulation, or the role for blood constituents on vascular function.
Clinical Outcomes and Effect of Arteriolar Dysfunction on Coronary Physiology
Studies examining the relationship between outcomes and microvascular vs. large-vessel dysfunction (CFR vs. FFR, respectively) have risen sharply in recent years. Four distinct patient populations have been identified: 1) patients with normal epicardial and microvascular function (normal FFR and CFR), 2) patients with epicardial dysfunction but no microvascular dysfunction (normal FFR and abnormal CFR), 3) those with epicardial and microvascular dysfunction (abnormal FFR and CFR), and 4) patients with sole microvascular dysfunction (normal CFR and abnormal FFR) (2, 60, 103). Stratifying patient responses in this way has identified a strikingly high rate of cardiac events in those with microvascular dysfunction (populations 3 and 4). That is, the rate of adverse cardiac events seems to follow CFR more than FFR (70, 81, 101, 103) and is independent of sex (74). These recent reports have increasingly shifted attention from obstructive epicardial CAD to the influence of microvascular dysfunction within the context of CAD.
Why might arteriolar dysfunction more than large-vessel disease strongly influence CAD prognosis (103)? It is important to note that, although the location of pathology and the specific mechanisms involved may differ, many of the underlying physiological concepts that explain the detrimental effect of large-vessel disease recur in a discussion of the consequences of arteriolar dysfunction. These concepts relate to impaired blood flow and resulting ischemia, loss of nitric oxide with endothelial dysfunction, and widespread inflammation due to immune cell activation (17, 36, 84).
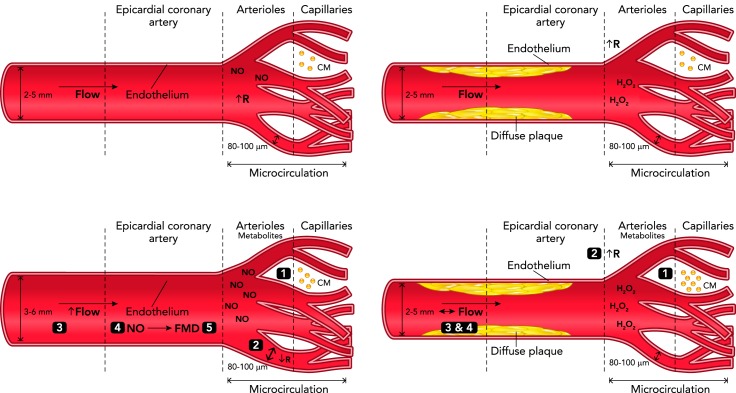
Since arterioles rather than arteries are the gatekeepers of myocardial perfusion, and the heart needs to precisely modify its flow on a rapid beat-to-beat time frame based on changing metabolic needs, dysregulation of cardiac blood flow arising from arteriolar dysfunction has grave consequences. The critical role of arterioles in the control of blood flow to tissues and their tight integration into surrounding tissue structures suggest that arteriolar dysfunction can profoundly influence development of tissue ischemia. In regions of the circulation subserved by conduit arteries with obstructive disease, the responsibility for maintaining blood flow depends on compensatory downstream arteriolar dilation through autoregulatory changes in overall vascular resistance. If arteriolar dysfunction is present, CFR declines, microvascular resistance is elevated, and this robust compensatory dilation cannot be achieved (28, 103). As a result, the local tissue is more susceptible to the development of ischemia (FIGURE 1). Even in the absence of epicardial stenoses (normal angiogram) and any cardiac or systemic diseases characterized by microvascular dysfunction, coronary arteriolar dysfunction may occur and lead to clinically detectable tissue ischemia on EKG (1). These findings emphasize that, although the affected area of the coronary circulation may differ, the result is similar.
FIGURE 1.
Arteriolar dysfunction can produce ischemia in the absence of a flow-limiting stenosis
Top left: at rest, arterioles display a high level of resting tone, which determines the basal blood flow through the upstream arteries. Bottom left: during times of increased oxygen demand, CM release vasoactive metabolites (1), which leads to a drop in arteriolar resistance (2). The decreased arteriolar resistance elicits increased flow in upstream arteries (3), causing increased NO release and oxygen delivery to downstream capillaries (4), as well as FMD in the artery (5), further increasing flow. Top right: in the presence of a diffuse but non-flow-limiting stenosis, arterial resistance and coronary flow are not affected. Bottom right: when arteriolar dysfunction is present, CM release vasoactive metabolites (1) but arterioles do not respond with appropriate vasodilation (2), and arteriolar resistance remains high. In the absence of a drop in arteriolar resistance, the necessary increase in flow is attenuated (3), FMD does not occur (4), and ischemia results from the mismatch between metabolic demand and flow. R, resistance; FMD, flow-mediated dilation; NO, nitric oxide; CM, cardiomyocytes.
Aside from impairments in overall blood flow, the local environment is likely altered with disease onset, and arteriolar-released factors may contribute to this change. For example, it has been postulated that a transition from endothelial release of nitric oxide to hydrogen peroxide (H2O2) observed in human coronary arterioles affected by CAD may be an early pathogenic step in the progression of CAD (37, 64). Since hydrogen peroxide can readily move across cell membranes, this may define a novel pathway by which arteriolar dysfunction—defined here as a transition in the release of endothelial-derived factors rather than compromised blood flow—could exert a persistent proinflammatory influence on cardiac tissue and precipitate adverse outcomes, independent of the extent of upstream plaque formation. Although yet untested, in the presence of arterial plaques, release of hydrogen peroxide could compound existing pathology through activation of inflammation and worsen outcomes, for example, by increasing susceptibility to plaque rupture. Until in vivo approaches allow for evaluation of microvascular parameters beyond blood flow, these provocative pathways lack strong clinical validation. Such an investigation should be essential to demonstrate whether arteriolar dysfunction drives development of epicardial stenosis over time.
Mechanisms of Arteriolar Dysfunction in CAD
The presence of arteriolar dysfunction, its impact on blood flow and tissue-arteriole cross talk, and its prognostic importance in patients with cardiac disease is increasingly apparent. Looking toward the future, to develop effective arteriolar-targeted therapies for CAD, we must understand what mechanisms and pathways contribute to arteriolar dysfunction. Based on previous reports, largely performed using the videomicroscopy preparation, key candidates can be grouped into several major categories: endothelial-derived factors [nitric oxide (NO), epoxyeicosatrienoic acids (EETs), mitochondrial hydrogen peroxide (H2O2)], ion channels, metabolic factors, and myogenic mechanisms.
Therapies to enhance NO delivery, including direct NO donors and inhibitors of NO breakdown, have been studied (65). Some of these drug classes may have advantageous effects on arterioles—although they have largely been tested within the context of large-vessel dysfunction, necessitating additional arteriole-specific studies—but there are unique aspects of the microcirculation that suggest that refined strategies are needed. Loss of NO as a mediator of FMD does indeed occur in arterioles with the onset of CAD (64), and this appears to be specific to CAD rather than age-related arteriolar pathology (14). However, direct NO donors, in doses sub-threshold for dilation, may paradoxically compromise rather than improve arteriolar dilation in the presence of CAD by inhibiting flow-induced mitochondrial production of H2O2 (14).
EETs are endogenous lipids generated by metabolism of arachidonic acid via cytochrome P450 epoxygenases. EETs represent an important but relatively understudied vasodilator pathway in coronary arterioles (57, 59). EET-mediated signaling is masked in arterioles from patients with CAD but can emerge as a vasodilator when H2O2 is inhibited (58). The pleiotropic protective effects of EETs on the coronary circulation are known (57). Stimulating EET release or inhibiting its catabolism to inactive dihydroxy metabolites (DHETE) with soluble epoxide hydrolase inhibitors could enhance both arteriolar dilation and non-vasomotor pathways to promote arteriolar health, although some concerns for off-target effect in the pulmonary circulation exist (43, 56).
Mitochondrial H2O2 is known to be elevated in response to shear in arterioles from patients with CAD (11, 49). Although mitochondrial H2O2 appears to preserve dilation when NO levels fall (64), it can promote inflammation in the vessel wall itself and in surrounding structures (37). Since EETs can maintain dilation when H2O2 is inhibited, targeting mitochondrial H2O2 should limit its damaging effects on arterioles and surrounding tissues without compromising arteriolar dilation. Recent studies have also expanded this research area and identified factors that affect mitochondrial H2O2 release in arterioles by either reducing it (including the enzyme telomerase reverse transcriptase and the transcriptional coactivator PGC-1alpha) or increasing its generation (e.g., sphingolipid ceramide) (11, 32, 37, 49). In addition to mitochondrial release of H2O2, several other mitochondrial pathways have been identified that contribute to the regulation of vascular tone, such as depolarization (51), damage associated molecular patterns (48), coupling factor 6 (77, 78), and humanin (7).
Similar to the EET-H2O2 interaction described above, there are many reports demonstrating the resilience of arteriolar dilation; that is, arterioles demonstrate a remarkable capacity to maintain dilation when these individual dilator pathways are compromised. For example, in mice lacking eNOS, skeletal muscle arteriolar dilation is preserved by compensatory release of an endothelial hyperpolarizing factor (females) or cyclooxygenase-derived prostaglandins (males) (40, 97). In human arterioles isolated from patients without cardiovascular disease, NO produces dilation at rest, but its bioavailability is compromised by abrupt increases in intraluminal pressure. However, dilation is maintained by H2O2 and thus serves as a compensatory vasodilator (9). Given the critical role of arterioles in maintaining perfusion to downstream tissue beds, possessing these backup vasodilator pathways may help to maintain perfusion in the presence of acute vascular stress. Conversely, arteriolar dysfunction likely occurs when this vasodilatory reserve is exhausted. Clarifying the cellular mechanisms controlling these compensatory pathways may expose new therapeutic targets to restore vasodilatory plasticity in diseased arterioles.
In many studies, these individual factors are often described as exclusive and independent vasodilators (e.g., fully NO- or H2O2-dependent dilation). Studies have also characterized a provocative and potentially unique aspect of the microcirculation: the ability to simultaneously recruit multiple vasodilator agents (e.g., NO and H2O2) (37, 40, 58). Notably, this phenomenon appears to be unique to arterioles (94, 99). Perhaps more importantly, recent studies even suggest that optimal arteriolar function exists when multiple concurrent vasodilators are present, particularly as a protective mechanism against acute vascular stress (47, 49, 50, 86, 99). In contrast, arterioles relying on a single vasodilator agent—either NO or H2O2—display blunted dilation after exposure to an abrupt rise in intraluminal pressure (9, 49), a pathogenic stimulus with great relevance for acute atherosclerosis-related cardiac events (95). This dysfunction does not occur in arterioles that rely on multiple vasodilator agents (47, 49). This evidence underscores the complexity of arteriolar dilation and supports a “poly-pill” (i.e., multiple pharmacological agents in a single formulation) rather than single-target approach to guide drug development. That is, instead of restoring only NO or EET bioavailability in the presence of arteriolar dysfunction, encouraging release of multiple vasodilator factors may be preferable. The feasibility and benefit of introducing >1 vasodilator in arterioles affected by CAD was recently validated (49).
Aside from endothelial-derived changes, shifts in smooth muscle vasomotor properties are also observed in arterioles from subjects with CAD. Ion channels are a prime example. In healthy human coronary arterioles, arteriolar dilation relies on both voltage-gated and large-conductance calcium-activated potassium channels (75). In contrast, in arterioles affected by CAD, dilation depends on endothelial transient receptor potential vanilloid type 4 (TRPV4) channel activation (16). Regarding ion channels localized to smooth muscle cells, the activation of large-conductance potassium channels (106) rather than voltage-gated channels (75) contributes more to arteriolar dilation in the context of CAD.
Most in vitro studies examining mechanisms of arteriolar dysfunction in CAD have largely been performed during flow- or agonist-induced dilation. However, given that both myogenic and metabolic responses also have a prominent governing effect on arteriolar tone (19), more effort should be directed at exploring the possible dysregulation of these key physiological processes. Both myogenic and metabolic vasomotor responsiveness may be altered in CAD, which could adversely influence arteriolar regulation of blood flow. There is some early support for disrupted NO-dependent metabolic dilation in the coronary microcirculation of patients with atherosclerosis or its risk factors due to loss of NO bioavailability (85). It is possible to evaluate both myogenic responses and metabolic dilation using videomicroscopy (72, 89, 100), and studies exploring how these processes are corrupted in CAD may yield greater understanding of underlying pathophysiology and identify new therapeutic directions. Similarly, the interaction between myogenic and flow-induced dilation can be studied in vitro (54) and may be altered by disease.
Although these mechanisms emphasize the highly specialized and distinct physiology of coronary arterioles relative to that of coronary arteries, it is critical to mention that these mechanisms are often conserved across arteriolar beds. For example, a rise in mitochondrial H2O2 with the onset of CAD occurs in peripheral adipose, pericardial adipose, and atrial arterioles obtained from human subjects (32, 49). Although not described specifically in relation to CAD, these changes have also been observed in renal arterioles with clear effects on arteriolar function (41). Therefore, given the growing number of diseases that have been linked to arteriolar dysfunction (37), the relevance of widespread investment in arteriole-related research extends beyond CAD.
Other Disease States in Which Microvascular Dysfunction Occurs
Since arterioles are predominant regulators of vascular tone and resistance across most vascular beds, it is perhaps least surprising that arteriolar dysfunction has been identified within the context of systemic hypertension (30). Here too the definition of arteriolar dysfunction is largely restricted to its physiological role in regulating vascular tone and predominantly categorized as impaired dilation or enhanced constriction, but can also include rarefaction, inflammation, thrombogenicity, loss of barrier function, and vascular remodeling (30). Blunted myogenic- and agonist-induced responses in various vascular beds (mesenteric, cerebral, renal) have been reported with hypertension (10, 13, 44).
Diabetes is a classic example of microvascular dysfunction and seems to incorporate the broadest definition of arteriolar dysfunction, encompassing compromised vascular wall integrity with increased permeability, impaired dilation, oxidative stress, remodeling, and luminal obstruction (31, 76), as well as impaired angiogenesis (98). Arteriolar consequences manifest as diabetic retinopathy, peripheral vascular disease, cognitive decline, and renal dysfunction. Impaired dilation in coronary arterioles has also been noted, similar to that observed in CAD (5, 6, 53, 83). In both conditions, coronary arteriolar dysfunction has been linked to disturbances in ion channel function (63, 73). The striking overlap between coronary arteriolar dysfunction in CAD and diabetes helps to explain why diabetes accelerates progression of CAD. Even in the absence of CAD, diabetic microvascular dysfunction may give rise to diabetic cardiomyopathy (45).
Several cardiac-specific disorders are characterized by arteriolar dysfunction. The classic example is syndrome X, which refers to clinically detectable ischemia on EKG but normal coronary angiograms (1). Syndrome X is more likely to appear in postmenopausal females. A rapidly expanding area of interest is the involvement of arterioles in heart failure with preserved ejection fraction (HFpEF) (27, 80, 96), possibly as an early and inciting event (18). Although systolic function is normal in these patients, morbidity and mortality parallel that of the more traditional form of heart failure with reduced ejection fraction. Preliminary evidence classifies this arteriolar dysfunction as an impairment in the dilatory response to bradykinin (25). Both arteriolar dysfunction (blunted endothelium-dependent dilation and reduced hyperemic coronary flow responses, reflective primarily of arteriolar smooth muscle dysfunction) and impaired diastolic filling occur in an aged rat model free from hypertension and atherosclerosis. These changes are reversed by exercise training (39).
It is possible that increased awareness of arteriolar dysfunction in other human diseases besides CAD will catalyze interest in arteriolar dysfunction across disciplines and may eventually urge clinicians to more aggressively target arterioles in patients, particularly those with multiple diseases characterized by arteriolar dysfunction (e.g., common scenario of single patient carrying a diagnosis of both CAD and HTN).
Therapeutic Considerations
There is thus great importance in emphasizing development of novel approaches to combat arteriolar dysfunction. With respect to possible pharmacological approaches, existing therapies developed and validated for various cardiovascular disorders (statins, aspirin, ACE-inhibitors, and beta-blockers) may serve as effective treatments for microvascular dysfunction. However, the results summarized in recent reviews (69, 91) are disappointing; most of the listed drugs failed to improve symptoms (angina) or CFR, and some even worsened these parameters. These studies primarily evaluated effects on symptoms or microvascular resistance rather than more objective clinical outcomes (69). This lack of efficacy of existing drugs is unfortunate but could be explained by the previously mentioned pathological heterogeneity between conduit and arteriolar phenotypes in patients with CAD. Even in different areas of the same arterial tree, arterial and microvascular-derived ECs are characterized by profoundly different genetic (and presumably proteomic) profiles (20). Like ECs, smooth muscle cells represent a heterogenous population throughout the vascular system, with diverse embryological origins (66). Given these differences, traditional cardiovascular pharmacological agents may not be targeting responsible pathways at the level of the arterioles.
Moving forward, it will be important to continue to search for possible treatment options for arteriolar dysfunction with a refined approach informed by the unique nature of arteriolar physiology. Some possibilities based on available drugs include PDE-3 inhibitors (increase cAMP; e.g., cilostazol), ranolazine (blocks late inward sodium currents in cardiomyocytes and reduces stiffness, thus alleviating extravascular compressive forces on the microcirculation), PDE-5 inhibitors (indirectly restore NO), or mitochondrial reactive oxygen species inhibitors [noted to play a key role in microvascular pathologies, especially in diabetes (79)]. For a more comprehensive discussion of existing and emerging agents to treat microvascular dysfunction, the reader is referred to Ref. 35. One available drug that more selectively affects arteriolar dilation is adenosine. However, adenosine was developed to assess coronary function during maximal hyperemia, not treat arteriolar dysfunction; in fact, adenosine may reduce rather than increase coronary perfusion through a coronary steal phenomenon. Although adenosine has arteriolar effects, flooding the system with supraphysiological doses of a direct vasodilator like adenosine—especially a vasodilator that is not implicated in the development of cardiovascular disease—is not a tailored therapeutic approach.
Adenosine serves as a case-in-point of the glaring issue facing the available therapeutic toolbox to treat arteriolar dysfunction. Most of the indicated drugs were not developed with restoration of arteriolar function as a primary goal. As a result, the beneficial effects of these drugs on microvascular parameters are serendipitous rather than intentional. One potential approach is to identify arteriole-specific mechanisms occurring during development of CAD. For example, restoring physiological release of a dilator or dilator pathway that is compromised during onset of cardiovascular disease may be one such mechanistic, evidence-based approach. Several of these pathways were noted above, like the transition in ion channel-based signaling in the presence of CAD. Although some arteriolar changes are observed across vascular beds (e.g., transition to H2O2-dependent dilation), other studies have also demonstrated some organ-specific (patho)physiological mechanisms that may allow for organ-specific drug targeting.
Likewise, there is also the potential to develop arteriole-specific agents that are trafficked to the (coronary) arterioles in the same way that novel chemotherapeutic agents are harnessing the specificity of nanoparticle-based carriers (82) or the tumor cell membrane’s electric properties (87) to deliver treatment directly to the cancer cells. For example, targeting cell surface markers expressed primarily on damaged or diseased microvascular endothelium or developing therapeutics based on the unique genetic profile of arteriolar endothelial and smooth muscle cells may enable this directed therapeutic approach.
Aside from suggesting how we may develop novel tools to address specific mechanisms of arteriolar dysfunction precisely at the arteriolar level, it is also important to note the strong additional clinical appeal of targeting the arterial system. Relative to other organ systems, the arteriolar system is most ideally suited for therapeutics since any agent delivered via IV first interacts with the vasculature. It is the only organ that has the potential to be targeted by systemic approaches without effects on other tissue beds. Together, this appreciation of arteriolar dysfunction in various diseases, specific underlying (patho)physiological mechanisms, the unique profile and make-up of arterioles, and the ability to obtain direct access to the arteriolar system frames the fight against arteriolar dysfunction as a pressing, personalized, and clinically feasible endeavor. Given the arteriole-specific mechanisms noted here and above, there are already several candidates to exploit in future drug development.
Summary and Future Directions
Clinical studies have described the clear contribution of coronary microvascular dysfunction to coronary artery disease. Future work should continue to unravel underlying mechanisms. Arteriole-specific therapeutic approaches are lacking and represent a dire clinical void. Development of these therapies should allow clinicians and researchers to evaluate the clinical benefit of improving arteriolar function in humans.
Acknowledgments
This work was supported by the National Institutes of Health Grants R01-HL-135901-01 (to D. D. Gutterman) and T32-GM-080202 (to MCW Medical Scientist Training Program), an endowment from Northwestern Mutual Foundation, and the American Heart Association Predoctoral Fellowship Grant 16PRE29130003 (to A. O. Kadlec).
No conflicts of interest, financial or otherwise, are declared by the author(s).
H.A., D.D.G., and A.O.K. drafted manuscript; H.A., D.D.G., and A.O.K. edited and revised manuscript; H.A., D.D.G., and A.O.K. approved final version of manuscript.
References
- 1.Agrawal S, Mehta PK, Bairey Merz CN. Cardiac syndrome X: update. Heart Fail Clin 12: 141–156, 2016. doi: 10.1016/j.hfc.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Ahn SG, Suh J, Hung OY, Lee HS, Bouchi YH, Zeng W, Gandhi R, Eshtehardi P, Gogas BD, Samady H. Discordance between fractional flow reserve and coronary flow reserve: insights from intracoronary imaging and physiological assessment. JACC Cardiovasc Interv 10: 999–1007, 2017. doi: 10.1016/j.jcin.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Albertal M, Voskuil M, Piek JJ, de Bruyne B, Van Langenhove G, Kay PI, Costa MA, Boersma E, Beijsterveldt T, Sousa JE, Belardi JA, Serruys PW; Doppler Endpoints Balloon Angioplasty Trial Europe (DEBATE) II Study Group . Coronary flow velocity reserve after percutaneous interventions is predictive of periprocedural outcome. Circulation 105: 1573–1578, 2002. doi: 10.1161/01.CIR.0000012514.15806.DD. [DOI] [PubMed] [Google Scholar]
- 4.Amin ST, Morrow DA, Braunwald E, Sloan S, Contant C, Murphy S, Antman EM. Dynamic TIMI risk score for STEMI. J Am Heart Assoc 2: e003269, 2013. doi: 10.1161/JAHA.112.003269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ammar RF Jr, Gutterman DD, Brooks LA, Dellsperger KC. Free radicals mediate endothelial dysfunction of coronary arterioles in diabetes. Cardiovasc Res 47: 595–601, 2000. doi: 10.1016/S0008-6363(00)00094-8. [DOI] [PubMed] [Google Scholar]
- 6.Ammar RF Jr, Gutterman DD, Brooks LA, Dellsperger KC. Impaired dilation of coronary arterioles during increases in myocardial O(2) consumption with hyperglycemia. Am J Physiol Endocrinol Metab 279: E868–E874, 2000. doi: 10.1152/ajpendo.2000.279.4.E868. [DOI] [PubMed] [Google Scholar]
- 7.Bachar AR, Scheffer L, Schroeder AS, Nakamura HK, Cobb LJ, Oh YK, Lerman LO, Pagano RE, Cohen P, Lerman A. Humanin is expressed in human vascular walls and has a cytoprotective effect against oxidized LDL-induced oxidative stress. Cardiovasc Res 88: 360–366, 2010. doi: 10.1093/cvr/cvq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bassingthwaighte JB, King RB, Roger SA. Fractal nature of regional myocardial blood flow heterogeneity. Circ Res 65: 578–590, 1989. doi: 10.1161/01.RES.65.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beyer AM, Durand MJ, Hockenberry J, Gamblin TC, Phillips SA, Gutterman DD. An acute rise in intraluminal pressure shifts the mediator of flow-mediated dilation from nitric oxide to hydrogen peroxide in human arterioles. Am J Physiol Heart Circ Physiol 307: H1587–H1593, 2014. doi: 10.1152/ajpheart.00557.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beyer AM, Fredrich K, Lombard JH. AT1 receptors prevent salt-induced vascular dysfunction in isolated middle cerebral arteries of 2 kidney-1 clip hypertensive rats. Am J Hypertens 26: 1398–1404, 2013. doi: 10.1093/ajh/hpt129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beyer AM, Freed JK, Durand MJ, Riedel M, Ait-Aissa K, Green P, Hockenberry JC, Morgan RG, Donato AJ, Peleg R, Gasparri M, Rokkas CK, Santos JH, Priel E, Gutterman DD. Critical role for telomerase in the mechanism of flow-mediated dilation in the human microcirculation. Circ Res 118: 856–866, 2016. doi: 10.1161/CIRCRESAHA.115.307918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beyer AM, Gutterman DD. Regulation of the human coronary microcirculation. J Mol Cell Cardiol 52: 814–821, 2012. doi: 10.1016/j.yjmcc.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beyer AM, Raffai G, Weinberg BD, Fredrich K, Rodgers MS, Geurts AM, Jacob HJ, Dwinell MR, Lombard JH. Amelioration of salt-induced vascular dysfunction in mesenteric arteries of Dahl salt-sensitive rats by missense mutation of extracellular superoxide dismutase. Am J Physiol Heart Circ Physiol 306: H339–H347, 2014. doi: 10.1152/ajpheart.00619.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beyer AM, Zinkevich N, Miller B, Liu Y, Wittenburg AL, Mitchell M, Galdieri R, Sorokin A, Gutterman DD. Transition in the mechanism of flow-mediated dilation with aging and development of coronary artery disease. Basic Res Cardiol 112: 5, 2017. doi: 10.1007/s00395-016-0594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Britten MB, Zeiher AM, Schächinger V. Microvascular dysfunction in angiographically normal or mildly diseased coronary arteries predicts adverse cardiovascular long-term outcome. Coron Artery Dis 15: 259–264, 2004. doi: 10.1097/01.mca.0000134590.99841.81. [DOI] [PubMed] [Google Scholar]
- 16.Bubolz AH, Mendoza SA, Zheng X, Zinkevich NS, Li R, Gutterman DD, Zhang DX. Activation of endothelial TRPV4 channels mediates flow-induced dilation in human coronary arterioles: role of Ca2+ entry and mitochondrial ROS signaling. Am J Physiol Heart Circ Physiol 302: H634–H642, 2012. doi: 10.1152/ajpheart.00717.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buffon A, Biasucci LM, Liuzzo G, D’Onofrio G, Crea F, Maseri A. Widespread coronary inflammation in unstable angina. N Engl J Med 347: 5–12, 2002. doi: 10.1056/NEJMoa012295. [DOI] [PubMed] [Google Scholar]
- 18.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ Jr, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D, Calkins H, Kuck KH, Cappato R, Chen S-A, Prystowsky EN, Kuck KH, Natale A, Haines DE, Marchlinski FE, Calkins H, Davies DW, Lindsay BD, Damiano R, Packer DL, Brugada J, Camm AJ, Crijns HJG, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haissaguerre M, Hindricks G, Iesaka Y, Jackman WM, Jais P, Jalife J, Kalman J, Keane D, Kim Y-H, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Mansour M, Marchlinski F, McCarthy P, Mont JL, Morady F, Nademanee K, Nakagawa H, Nattel S, Pappone C, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao H-M, Wilber D, Ad N, Cummings J, Gillinov AM, Heidbuchel H, January C, Lip G, Markowitz S, Nair M, Ovsyshcher IE, Pak H-N, Tsuchiya T, Shah D, Siong TW, Vardas PE. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 14: 528–606, 2012. doi: 10.1093/europace/eus027. [DOI] [PubMed] [Google Scholar]
- 19.Carlson BE, Arciero JC, Secomb TW. Theoretical model of blood flow autoregulation: roles of myogenic, shear-dependent, and metabolic responses. Am J Physiol Heart Circ Physiol 295: H1572–H1579, 2008. doi: 10.1152/ajpheart.00262.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chi JT, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, van de Rijn M, Botstein D, Brown PO. Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci USA 100: 10623–10628, 2003. doi: 10.1073/pnas.1434429100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chilian WM, Dellsperger KC, Layne SM, Eastham CL, Armstrong MA, Marcus ML, Heistad DD. Effects of atherosclerosis on the coronary microcirculation. Am J Physiol Heart Circ Physiol 258: H529–H539, 1990. [DOI] [PubMed] [Google Scholar]
- 22.Chilian WM, Eastham CL, Marcus ML. Microvascular distribution of coronary vascular resistance in beating left ventricle. Am J Physiol Heart Circ Physiol 251: H779–H788, 1986. [DOI] [PubMed] [Google Scholar]
- 23.Cook CM, Jeremias A, Petraco R, Sen S, Nijjer S, Shun-Shin MJ, Ahmad Y, de Waard G, van de Hoef T, Echavarria-Pinto M, van Lavieren M, Al Lamee R, Kikuta Y, Shiono Y, Buch A, Meuwissen M, Danad I, Knaapen P, Maehara A, Koo BK, Mintz GS, Escaned J, Stone GW, Francis DP, Mayet J, Piek JJ, van Royen N, Davies JE. Fractional flow reserve/instantaneous wave-free ratio discordance in angiographically intermediate coronary stenoses: an analysis using doppler-derived coronary flow measurements. JACC Cardiovasc Interv 10: 2514–2524, 2017. doi: 10.1016/j.jcin.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cybulsky MI, Gimbrone MA Jr. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science 251: 788–791, 1991. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 25.Davila AC, Dou H, Patel V, Fulton D, Weintraub N, Bagi Z. Impaired conducted coronary arteriole dilation in patients with HFpEF. FASEB J 31: 833.834–833.834, 2017. [Google Scholar]
- 26.Driessen RS, Raijmakers PG, Stuijfzand WJ, Knaapen P. Myocardial perfusion imaging with PET. Int J Cardiovasc Imaging 33: 1021–1031, 2017. doi: 10.1007/s10554-017-1084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dryer K, Gajjar M, Narang N, Lee M, Paul J, Shah AP, Nathan S, Butler J, Davidson CJ, Fearon WF, Shah SJ, Blair JEA. Coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Am J Physiol Heart Circ Physiol 314: H1033–H1042, 2018. doi: 10.1152/ajpheart.00680.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Echavarria-Pinto M, Escaned J, Macías E, Medina M, Gonzalo N, Petraco R, Sen S, Jimenez-Quevedo P, Hernandez R, Mila R, Ibañez B, Nuñez-Gil IJ, Fernández C, Alfonso F, Bañuelos C, García E, Davies J, Fernández-Ortiz A, Macaya C. Disturbed coronary hemodynamics in vessels with intermediate stenoses evaluated with fractional flow reserve: a combined analysis of epicardial and microcirculatory involvement in ischemic heart disease. Circulation 128: 2557–2566, 2013. doi: 10.1161/CIRCULATIONAHA.112.001345. [DOI] [PubMed] [Google Scholar]
- 29.Feher A, Sinusas AJ. Quantitative assessment of coronary microvascular function: dynamic single-photon emission computed tomography, positron emission tomography, ultrasound, computed tomography, and magnetic resonance imaging. Circ Cardiovasc Imaging 10: e006427, 2017. doi: 10.1161/CIRCIMAGING.117.006427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feihl F, Liaudet L, Waeber B, Levy BI. Hypertension: a disease of the microcirculation? Hypertension 48: 1012–1017, 2006. doi: 10.1161/01.HYP.0000249510.20326.72. [DOI] [PubMed] [Google Scholar]
- 31.Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes 26: 77–82, 2008. doi: 10.2337/diaclin.26.2.77. [DOI] [Google Scholar]
- 32.Freed JK, Beyer AM, LoGiudice JA, Hockenberry JC, Gutterman DD. Ceramide changes the mediator of flow-induced vasodilation from nitric oxide to hydrogen peroxide in the human microcirculation. Circ Res 115: 525–532, 2014. doi: 10.1161/CIRCRESAHA.115.303881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gimbrone MA Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res 118: 620–636, 2016. doi: 10.1161/CIRCRESAHA.115.306301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guarda E, Myers PR, Brilla CG, Tyagi SC, Weber KT. Endothelial cell induced modulation of cardiac fibroblast collagen metabolism. Cardiovasc Res 27: 1004–1008, 1993. doi: 10.1093/cvr/27.6.1004. [DOI] [PubMed] [Google Scholar]
- 35.Guarini G, Huqi A, Morrone D, Capozza P, Todiere G, Marzilli M. Pharmacological approaches to coronary microvascular dysfunction. Pharmacol Ther 144: 283–302, 2014. doi: 10.1016/j.pharmthera.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Gutiérrez E, Flammer AJ, Lerman LO, Elízaga J, Lerman A, Fernández-Avilés F. Endothelial dysfunction over the course of coronary artery disease. Eur Heart J 34: 3175–3181, 2013. doi: 10.1093/eurheartj/eht351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gutterman DD, Chabowski DS, Kadlec AO, Durand MJ, Freed JK, Ait-Aissa K, Beyer AM. The human microcirculation: regulation of flow and beyond. Circ Res 118: 157–172, 2016. doi: 10.1161/CIRCRESAHA.115.305364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation 106: 653–658, 2002. doi: 10.1161/01.CIR.0000025404.78001.D8. [DOI] [PubMed] [Google Scholar]
- 39.Hotta K, Chen B, Behnke BJ, Ghosh P, Stabley JN, Bramy JA, Sepulveda JL, Delp MD, Muller-Delp JM. Exercise training reverses age-induced diastolic dysfunction and restores coronary microvascular function. J Physiol 595: 3703–3719, 2017. doi: 10.1113/JP274172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang A, Sun D, Carroll MA, Jiang H, Smith CJ, Connetta JA, Falck JR, Shesely EG, Koller A, Kaley G. EDHF mediates flow-induced dilation in skeletal muscle arterioles of female eNOS-KO mice. Am J Physiol Heart Circ Physiol 280: H2462–H2469, 2001. doi: 10.1152/ajpheart.2001.280.6.H2462. [DOI] [PubMed] [Google Scholar]
- 41.Huang Q, Wang Q, Zhang S, Jiang S, Zhao L, Yu L, Hultström M, Patzak A, Li L, Wilcox CS, Lai EY. Increased hydrogen peroxide impairs angiotensin II contractions of afferent arterioles in mice after renal ischaemia-reperfusion injury. Acta Physiol (Oxf) 218: 136–145, 2016. doi: 10.1111/apha.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ihdayhid AR, Yong A, Harper R, Rankin J, Wong C, Brown AJ, Leung M, Ko B. A practical guide for fractional flow reserve guided revascularisation. Heart Lung Circ 27: 406–419, 2018. doi: 10.1016/j.hlc.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 43.Imig JD. Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiol Rev 92: 101–130, 2012. doi: 10.1152/physrev.00021.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ito S, Juncos LA, Carretero OA. Pressure-induced constriction of the afferent arteriole of spontaneously hypertensive rats. Hypertension 19, Suppl: II164–II167, 1992. doi: 10.1161/01.HYP.19.2_Suppl.II164. [DOI] [PubMed] [Google Scholar]
- 45.Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res 122: 624–638, 2018. doi: 10.1161/CIRCRESAHA.117.311586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson PC. Overview of the microcirculation. Compr Physiol 1: xi–xxiv, 2011. doi: 10.1002/cphy.cp0204fm02 [DOI] [Google Scholar]
- 47.Kadlec AO, Barnes C, Durand MJ, Gutterman DD. Microvascular adaptations to exercise: protective effect of PGC-1 alpha. Am J Hypertens 31: 240–246, 2018. doi: 10.1093/ajh/hpx162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kadlec AO, Beyer AM, Ait-Aissa K, Gutterman DD. Mitochondrial signaling in the vascular endothelium: beyond reactive oxygen species. Basic Res Cardiol 111: 26, 2016. doi: 10.1007/s00395-016-0546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kadlec AO, Chabowski DS, Ait-Aissa K, Hockenberry JC, Otterson MF, Durand MJ, Freed JK, Beyer AM, Gutterman DD. PGC-1α (peroxisome proliferator-activated receptor γ coactivator 1-α) overexpression in coronary artery disease recruits NO and hydrogen peroxide during flow-mediated dilation and protects against increased intraluminal pressure. Hypertension 70: 166–173, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kadlec AO, Gutterman DD. The yin and yang of endothelium-derived vasodilator factors. Am J Physiol Heart Circ Physiol 314: H892–H894, 2018. doi: 10.1152/ajpheart.00019.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katakam PV, Wappler EA, Katz PS, Rutkai I, Institoris A, Domoki F, Gáspár T, Grovenburg SM, Snipes JA, Busija DW. Depolarization of mitochondria in endothelial cells promotes cerebral artery vasodilation by activation of nitric oxide synthase. Arterioscler Thromb Vasc Biol 33: 752–759, 2013. doi: 10.1161/ATVBAHA.112.300560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kemp HG., Jr Left ventricular function in patients with the anginal syndrome and normal coronary arteriograms. Am J Cardiol 32: 375–376, 1973. doi: 10.1016/S0002-9149(73)80150-X. [DOI] [PubMed] [Google Scholar]
- 53.Kibel A, Selthofer-Relatic K, Drenjancevic I, Bacun T, Bosnjak I, Kibel D, Gros M. Coronary microvascular dysfunction in diabetes mellitus. J Int Med Res 45: 1901–1929, 2017. doi: 10.1177/0300060516675504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuo L, Chilian WM, Davis MJ. Interaction of pressure- and flow-induced responses in porcine coronary resistance vessels. Am J Physiol 261: H1706–H1715, 1991. [DOI] [PubMed] [Google Scholar]
- 55.Kuo L, Davis MJ, Cannon MS, Chilian WM. Pathophysiological consequences of atherosclerosis extend into the coronary microcirculation. Restoration of endothelium-dependent responses by L-arginine. Circ Res 70: 465–476, 1992. doi: 10.1161/01.RES.70.3.465. [DOI] [PubMed] [Google Scholar]
- 56.Larsen BT, Campbell WB, Gutterman DD. Beyond vasodilatation: non-vasomotor roles of epoxyeicosatrienoic acids in the cardiovascular system. Trends Pharmacol Sci 28: 32–38, 2007. doi: 10.1016/j.tips.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 57.Larsen BT, Gutterman DD, Hatoum OA. Emerging role of epoxyeicosatrienoic acids in coronary vascular function. Eur J Clin Invest 36: 293–300, 2006. doi: 10.1111/j.1365-2362.2006.01634.x. [DOI] [PubMed] [Google Scholar]
- 58.Larsen BT, Gutterman DD, Sato A, Toyama K, Campbell WB, Zeldin DC, Manthati VL, Falck JR, Miura H. Hydrogen peroxide inhibits cytochrome p450 epoxygenases: interaction between two endothelium-derived hyperpolarizing factors. Circ Res 102: 59–67, 2008. doi: 10.1161/CIRCRESAHA.107.159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Larsen BT, Miura H, Hatoum OA, Campbell WB, Hammock BD, Zeldin DC, Falck JR, Gutterman DD. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BK(Ca) channels: implications for soluble epoxide hydrolase inhibition. Am J Physiol Heart Circ Physiol 290: H491–H499, 2006. doi: 10.1152/ajpheart.00927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee JM, Jung JH, Hwang D, Park J, Fan Y, Na SH, Doh JH, Nam CW, Shin ES, Koo BK. Coronary Flow Reserve and Microcirculatory Resistance in Patients With Intermediate Coronary Stenosis. J Am Coll Cardiol 67: 1158–1169, 2016. doi: 10.1016/j.jacc.2015.12.053. [DOI] [PubMed] [Google Scholar]
- 61.Likoff W, Segal BL, Kasparian H. Paradox of normal selective coronary arteriograms in patients considered to have unmistakable coronary heart disease. N Engl J Med 276: 1063–1066, 1967. doi: 10.1056/NEJM196705112761904. [DOI] [PubMed] [Google Scholar]
- 62.Liu A, Wijesurendra RS, Liu JM, Greiser A, Jerosch-Herold M, Forfar JC, Channon KM, Piechnik SK, Neubauer S, Kharbanda RK, Ferreira VM. Gadolinium-free cardiac MR stress T1-mapping to distinguish epicardial from microvascular coronary disease. J Am Coll Cardiol 71: 957–968, 2018. doi: 10.1016/j.jacc.2017.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Liu Y, Xie A, Singh AK, Ehsan A, Choudhary G, Dudley S, Sellke FW, Feng J. Inactivation of endothelial small/intermediate conductance of calcium-activated potassium channels contributes to coronary arteriolar dysfunction in diabetic patients. J Am Heart Assoc 4: e002062, 2015. doi: 10.1161/JAHA.115.002062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res 93: 573–580, 2003. doi: 10.1161/01.RES.0000091261.19387.AE. [DOI] [PubMed] [Google Scholar]
- 65.Lundberg JO, Gladwin MT, Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat Rev Drug Discov 14: 623–641, 2015. doi: 10.1038/nrd4623. [DOI] [PubMed] [Google Scholar]
- 66.Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol 27: 1248–1258, 2007. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- 67.Marcus ML. The Coronary Circulation in Health and Disease. New York: McGraw-Hill, 1983, p. xi. [Google Scholar]
- 68.Marcus ML, Chilian WM, Kanatsuka H, Dellsperger KC, Eastham CL, Lamping KG. Understanding the coronary circulation through studies at the microvascular level. Circulation 82: 1–7, 1990. doi: 10.1161/01.CIR.82.1.1. [DOI] [PubMed] [Google Scholar]
- 69.Marinescu MA, Löffler AI, Ouellette M, Smith L, Kramer CM, Bourque JM. Coronary microvascular dysfunction, microvascular angina, and treatment strategies. JACC Cardiovasc Imaging 8: 210–220, 2015. doi: 10.1016/j.jcmg.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation 135: 1075–1092, 2017. doi: 10.1161/CIRCULATIONAHA.116.024534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meuwissen M, Chamuleau SA, Siebes M, Schotborgh CE, Koch KT, de Winter RJ, Bax M, de Jong A, Spaan JA, Piek JJ. Role of variability in microvascular resistance on fractional flow reserve and coronary blood flow velocity reserve in intermediate coronary lesions. Circulation 103: 184–187, 2001. doi: 10.1161/01.CIR.103.2.184. [DOI] [PubMed] [Google Scholar]
- 72.Miller FJ Jr, Dellsperger KC, Gutterman DD. Myogenic constriction of human coronary arterioles. Am J Physiol 273: H257–H264, 1997. [DOI] [PubMed] [Google Scholar]
- 73.Miura H, Wachtel RE, Loberiza FR Jr, Saito T, Miura M, Nicolosi AC, Gutterman DD. Diabetes mellitus impairs vasodilation to hypoxia in human coronary arterioles: reduced activity of ATP-sensitive potassium channels. Circ Res 92: 151–158, 2003. doi: 10.1161/01.RES.0000052671.53256.49. [DOI] [PubMed] [Google Scholar]
- 74.Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, Dorbala S, Blankstein R, Rimoldi O, Camici PG, Di Carli MF. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation 129: 2518–2527, 2014. doi: 10.1161/CIRCULATIONAHA.113.008507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nishijima Y, Cao S, Chabowski DS, Korishettar A, Ge A, Zheng X, Sparapani R, Gutterman DD, Zhang DX. Contribution of KV1.5 channel to hydrogen peroxide-induced human arteriolar dilation and its modulation by coronary artery disease. Circ Res 120: 658–669, 2017. doi: 10.1161/CIRCRESAHA.116.309491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Orasanu G, Plutzky J. The pathologic continuum of diabetic vascular disease. J Am Coll Cardiol 53, Suppl: S35–S42, 2009. doi: 10.1016/j.jacc.2008.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Osanai T, Okada S, Sirato K, Nakano T, Saitoh M, Magota K, Okumura K. Mitochondrial coupling factor 6 is present on the surface of human vascular endothelial cells and is released by shear stress. Circulation 104: 3132–3136, 2001. doi: 10.1161/hc5001.100832. [DOI] [PubMed] [Google Scholar]
- 78.Osanai T, Tanaka M, Kamada T, Nakano T, Takahashi K, Okada S, Sirato K, Magota K, Kodama S, Okumura K. Mitochondrial coupling factor 6 as a potent endogenous vasoconstrictor. J Clin Invest 108: 1023–1030, 2001. doi: 10.1172/JCI11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pangare M, Makino A. Mitochondrial function in vascular endothelial cell in diabetes. J Smooth Muscle Res 48: 1–26, 2012. doi: 10.1540/jsmr.48.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62: 263–271, 2013. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 81.Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G, Bairey Merz CN. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol 55: 2825–2832, 2010. doi: 10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pérez-Herrero E, Fernández-Medarde A. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm 93: 52–79, 2015. doi: 10.1016/j.ejpb.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 83.Picchi A, Capobianco S, Qiu T, Focardi M, Zou X, Cao JM, Zhang C. Coronary microvascular dysfunction in diabetes mellitus: A review. World J Cardiol 2: 377–390, 2010. doi: 10.4330/wjc.v2.i11.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pries AR, Reglin B. Coronary microcirculatory pathophysiology: can we afford it to remain a black box? Eur Heart J 38: 478–488, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Quyyumi AA, Dakak N, Andrews NP, Gilligan DM, Panza JA, Cannon RO III. Contribution of nitric oxide to metabolic coronary vasodilation in the human heart. Circulation 92: 320–326, 1995. doi: 10.1161/01.CIR.92.3.320. [DOI] [PubMed] [Google Scholar]
- 86.Robinson AT, Franklin NC, Norkeviciute E, Bian JT, Babana JC, Szczurek MR, Phillips SA. Improved arterial flow-mediated dilation after exertion involves hydrogen peroxide in overweight and obese adults following aerobic exercise training. J Hypertens 34: 1309–1316, 2016. doi: 10.1097/HJH.0000000000000946. [DOI] [PubMed] [Google Scholar]
- 87.Rodzinski A, Guduru R, Liang P, Hadjikhani A, Stewart T, Stimphil E, Runowicz C, Cote R, Altman N, Datar R, Khizroev S. Targeted and controlled anticancer drug delivery and release with magnetoelectric nanoparticles. Sci Rep 6: 20867, 2016. doi: 10.1038/srep20867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rubinshtein R, Yang EH, Rihal CS, Prasad A, Lennon RJ, Best PJ, Lerman LO, Lerman A. Coronary microcirculatory vasodilator function in relation to risk factors among patients without obstructive coronary disease and low to intermediate Framingham score. Eur Heart J 31: 936–942, 2010. doi: 10.1093/eurheartj/ehp459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saitoh S, Kiyooka T, Rocic P, Rogers PA, Zhang C, Swafford A, Dick GM, Viswanathan C, Park Y, Chilian WM. Redox-dependent coronary metabolic dilation. Am J Physiol Heart Circ Physiol 293: H3720–H3725, 2007. doi: 10.1152/ajpheart.00436.2007. [DOI] [PubMed] [Google Scholar]
- 90.Saitoh S, Zhang C, Tune JD, Potter B, Kiyooka T, Rogers PA, Knudson JD, Dick GM, Swafford A, Chilian WM. Hydrogen peroxide: a feed-forward dilator that couples myocardial metabolism to coronary blood flow. Arterioscler Thromb Vasc Biol 26: 2614–2621, 2006. doi: 10.1161/01.ATV.0000249408.55796.da. [DOI] [PubMed] [Google Scholar]
- 91.Samim A, Nugent L, Mehta PK, Shufelt C, Bairey Merz CN. Treatment of angina and microvascular coronary dysfunction. Curr Treat Options Cardiovasc Med 12: 355–364, 2010. doi: 10.1007/s11936-010-0083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sellke FW, Armstrong ML, Harrison DG. Endothelium-dependent vascular relaxation is abnormal in the coronary microcirculation of atherosclerotic primates. Circulation 81: 1586–1593, 1990. doi: 10.1161/01.CIR.81.5.1586. [DOI] [PubMed] [Google Scholar]
- 93.Seol SH, Lindner JR. A primer on the methods and applications for contrast echocardiography in clinical imaging. J Cardiovasc Ultrasound 22: 101–110, 2014. doi: 10.4250/jcu.2014.22.3.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, Takayanagi T, Nagao T, Egashira K, Fujishima M, Takeshita A. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol 28: 703–711, 1996. doi: 10.1097/00005344-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 95.Smyth A, O’Donnell M, Lamelas P, Teo K, Rangarajan S, Yusuf S, Investigators I; INTERHEART Investigators . Physical activity and anger or emotional upset as triggers of acute myocardial infarction: The INTERHEART Study. Circulation 134: 1059–1067, 2016. doi: 10.1161/CIRCULATIONAHA.116.023142. [DOI] [PubMed] [Google Scholar]
- 96.Sucato V, Evola S, Novo G, Sansone A, Quagliana A, Andolina G, Assennato P, Novo S. Angiographic evaluation of coronary microvascular dysfunction in patients with heart failure and preserved ejection fraction. Microcirculation 22: 528–533, 2015. doi: 10.1111/micc.12223. [DOI] [PubMed] [Google Scholar]
- 97.Sun D, Liu H, Yan C, Jacobson A, Ojaimi C, Huang A, Kaley G. COX-2 contributes to the maintenance of flow-induced dilation in arterioles of eNOS-knockout mice. Am J Physiol Heart Circ Physiol 291: H1429–H1435, 2006. doi: 10.1152/ajpheart.01130.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tahergorabi Z, Khazaei M. Imbalance of angiogenesis in diabetic complications: the mechanisms. Int J Prev Med 3: 827–838, 2012. doi: 10.4103/2008-7802.104853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tanaka S, Shiroto T, Godo S, Saito H, Ikumi Y, Ito A, Kajitani S, Sato S, Shimokawa H. Important role of endothelium-dependent hyperpolarization in the pulmonary microcirculation in male mice: implications for hypoxia-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol 314: H940–H953, 2018. doi: 10.1152/ajpheart.00487.2017. [DOI] [PubMed] [Google Scholar]
- 100.Tanikawa T, Kanatsuka H, Koshida R, Tanaka M, Sugimura A, Kumagai T, Miura M, Komaru T, Shirato K. Role of pertussis toxin-sensitive G protein in metabolic vasodilation of coronary microcirculation. Am J Physiol Heart Circ Physiol 279: H1819–H1829, 2000. doi: 10.1152/ajpheart.2000.279.4.H1819. [DOI] [PubMed] [Google Scholar]
- 101.Taqueti VR, Hachamovitch R, Murthy VL, Naya M, Foster CR, Hainer J, Dorbala S, Blankstein R, Di Carli MF. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation 131: 19–27, 2015. doi: 10.1161/CIRCULATIONAHA.114.011939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Treasure CB, Vita JA, Cox DA, Fish RD, Gordon JB, Mudge GH, Colucci WS, Sutton MG, Selwyn AP, Alexander RW. Endothelium-dependent dilation of the coronary microvasculature is impaired in dilated cardiomyopathy. Circulation 81: 772–779, 1990. doi: 10.1161/01.CIR.81.3.772. [DOI] [PubMed] [Google Scholar]
- 103.van de Hoef TP, van Lavieren MA, Damman P, Delewi R, Piek MA, Chamuleau SA, Voskuil M, Henriques JP, Koch KT, de Winter RJ, Spaan JA, Siebes M, Tijssen JG, Meuwissen M, Piek JJ. Physiological basis and long-term clinical outcome of discordance between fractional flow reserve and coronary flow velocity reserve in coronary stenoses of intermediate severity. Circ Cardiovasc Interv 7: 301–311, 2014. doi: 10.1161/CIRCINTERVENTIONS.113.001049. [DOI] [PubMed] [Google Scholar]
- 104.van Kranenburg M, Magro M, Thiele H, de Waha S, Eitel I, Cochet A, Cottin Y, Atar D, Buser P, Wu E, Lee D, Bodi V, Klug G, Metzler B, Delewi R, Bernhardt P, Rottbauer W, Boersma E, Zijlstra F, van Geuns RJ. Prognostic value of microvascular obstruction and infarct size, as measured by CMR in STEMI patients. JACC Cardiovasc Imaging 7: 930–939, 2014. doi: 10.1016/j.jcmg.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 105.Yamamoto H, Bossaller C, Cartwright J Jr, Henry PD. Videomicroscopic demonstration of defective cholinergic arteriolar vasodilation in atherosclerotic rabbit. J Clin Invest 81: 1752–1758, 1988. doi: 10.1172/JCI113516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang DX, Borbouse L, Gebremedhin D, Mendoza SA, Zinkevich NS, Li R, Gutterman DD. H2O2-induced dilation in human coronary arterioles: role of protein kinase G dimerization and large-conductance Ca2+-activated K+ channel activation. Circ Res 110: 471–480, 2012. doi: 10.1161/CIRCRESAHA.111.258871. [DOI] [PMC free article] [PubMed] [Google Scholar]