Abstract
Hypoxia signaling in the vasculature controls vascular permeability, inflammation, vascular growth, and repair of vascular injury. In this review, we summarize recent insights in this burgeoning field and highlight the importance of studying the heterogeneity of hypoxia responses among individual patients, distinct vascular beds, and even individual vascular cells.
Introduction
Vascular homeostasis during adult life is the result of balancing vascular injury and damage with repair and regeneration while also integrating environmental cues to optimize vascular function and blood vessel growth, thus ensuring adequate supply of nutrients and oxygen to tissues. Vascular regeneration may occur either through the de novo formation of blood vessels via endothelial progenitor cells (vasculogenesis), by the expansion of the existing vascular network (angiogenesis), or by the combination of both (19). Vascular homeostasis refers to the maintenance of vascular function over time and thus also includes the adaptation to persistent environmental signals such as chronic hypoxia. It is noteworthy that the vasculature also has the ability to rapidly respond to acute signals to restore homeostatic function, such as what is observed during acute hypoxia. Recent studies have identified several adaptive vascular responses to chronic hypoxia, including changes in vascular permeability, arterial vs. venous specification of the developing or growing vasculature, and the regulation of inflammatory processes. These recent findings not only provide important insights into basic mechanisms by which environmental oxygen levels regulate vascular function but also suggest potential novel therapeutic approaches that target hypoxia-regulated pathways in the vasculature to minimize vascular injury or enhance vascular repair and regeneration. With the emergence of precision medicine and precision science—popular catchphrases that signify the growing specificity as well as greater appreciation of biological complexity and heterogeneity—it is becoming apparent that the heterogeneity of cellular responses to hypoxia may be a critical factor in determining how tissues respond to low oxygen levels. This heterogeneity has been observed at an inter-individual level in which distinct patients may exhibit genetic differences in the key signaling pathways mediating hypoxia responses. In addition to inter-individual heterogeneity, hypoxic responses of the vasculature also differ widely between distinct vascular cell types and tissue beds, and even between individual cells in one vascular bed (FIGURE 1). Finally, a word of caution is also required since systemic manipulation of hypoxic signaling pathways might have untoward side effects due to the central role of hypoxic signaling in homeostasis. Understanding the complexity and heterogeneity of tissue responses to hypoxia is a prerequisite for designing targeted therapies that minimize unintended collateral effects.
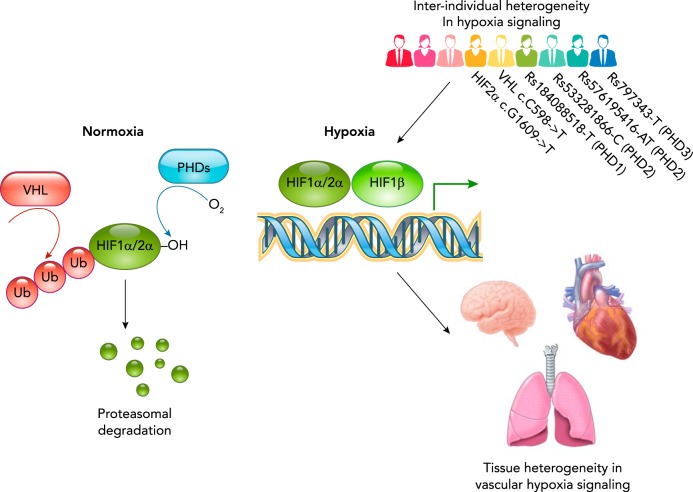
FIGURE 1.
Heterogeneity of hypoxia signaling
Under normal oxygen levels, HIF-1α and HIF-2α are hydroxylated on conserved prolyl residues by a set of oxygen-dependent prolyl hydroxylases (PHD1–3). This results in the recognition of HIF-1α and HIF-2α by the von Hippel-Lindau protein, leading to ubiquitination and subsequent proteasomal degradation. In response to lower oxygen levels, PHD enzymes will be inhibited, allowing for the accumulation of HIF-1α and HIF-2α. They subsequently form a complex with constitutively expressed HIF-1β and induce the expression of target genes. These genes are involved both in systemic responses such as increased angiogenesis and in changes of cellular energy metabolism. There is considerable heterogeneity in the hypoxic response between individuals, between different vascular beds, and even between individual endothelial cells. Understanding this complexity and heterogeneity is a prerequisite for designing targeted therapies that minimize unintended collateral effects.
Hypoxia Signaling Pathways
Cells respond to low oxygen levels in several ways, and probably the best-studied system to sense hypoxia consists of the hypoxia-inducible factors (HIFs), which are transcription factors consisting of the HIF-1α or HIF-2α subunits that dimerize with the HIF-1β subunit to initiate the transcription of hypoxia-responsive genes. Oxygen-dependent prolyl hydroxylases (PHDs) hydroxylate HIF-1α and HIF-2α when oxygen is present at high levels, leading to ubiquitination by the von Hippel-Lindau (VHL) protein and subsequent proteasomal degradation. Low oxygen levels inhibit PHDs, allowing for the accumulation of HIF-1α and HIF-2α (FIGURE 1), and the expression of specific genes involved in blood vessel growth, anaerobic metabolism, and enhanced delivery of oxygen to tissues (13, 52, 66). In addition, factor inhibiting HIF (FIH) is an asparagine hydroxylase that negatively regulates the transcriptional activity of HIF and can also serve as a regulatory mechanism for modulating hypoxia responses (84).
It is noteworthy that some animals such as sponges and comb jellies maintain their gene expression levels despite exposure to extremely low oxygen levels, probably because they lack a VHL ortholog and their HIF orthologs lack hydroxylation domains (49, 64). Therefore, HIF-mediated oxygen-sensing pathways were not present when single-celled ancestors started to evolve into the first animals ~800 million years ago and appear to have evolved since then.
In addition to HIF-mediated shifts in gene expression, cells can also respond more rapidly to changes in oxygen levels by alternate mechanisms. For example, hypoxic pulmonary vasoconstriction is mediated by the mitochondria (17) and complex III of the electron transport chain has been identified as an important oxygen sensor (76), indicating that hypoxia can also be sensed independently of the PHD/HIF pathway and can lead to rapid hypoxic responses that do not require de novo gene expression. Similarly, hypoxia can modulate reactive oxygen species (ROS) in vascular cells (24, 77) and thereby modulate vascular barrier integrity (51, 80) and the angiogenic response (46). Therefore, vascular homeostasis depends on both HIF-dependent and -independent mechanisms, which can act in concert to regulate vascular function and adaptation to environmental oxygen levels.
Role of Hypoxia Signaling on Blood Vessel Permeability and Stability
During acute lung injury (ALI)/acute respiratory distress syndrome (ARDS), the endothelial barrier in the lung is disrupted, which in turn leads to edema formation and the infiltration of inflammatory cells. Lung edema impairs the gas-exchange function of the lung, thus resulting in local and systemic hypoxia. The high levels of oxygen consumption by inflammatory neutrophils can also induce local tissue hypoxia even in the absence of environmental hypoxia, as has been reported for mucosal inflammation (8). It is thus likely that the combination of edema-impaired gas exchange and inflammation-induced oxygen consumption results in profound hypoxia of the lung vasculature during ALI/ARDS. This prompted researchers to study whether hypoxic signaling in the vascular endothelium may regulate endothelial barrier permeability as a possible adaptive mechanism to contain tissue injury during ALI/ARDS. Mice with HIF-2α-deficient ECs develop normally but exhibit increased vessel permeability and focal lymphocyte infiltration in the lungs and adipose tissue, whereas vascular permeability in the liver and kidney were not altered (67). Endothelial cells lacking HIF-2α showed reduced adhesion in hypoxic conditions and expressed lower levels of the NOTCH ligand Dll4 (67). Reduced Dll4 levels can increase vessel branching, but the resulting vessels have a smaller diameter, are often not perfused, and fail to recruit pericytes (65). Similar findings were made in tumors where Dll4 inhibition also leads to increased vascularization but poor perfusion of the blood vessels (53). Therefore, Dll4 downregulation appears to destabilize blood vessels and can ultimately lead to increased tissue hypoxia.
At a molecular level, vascular endothelial cadherin (VE-cadherin) homodimer formation leads to the formation of endothelial adherens junctions (AJs), which are critical regulators of endothelial barrier integrity. Vascular endothelial protein tyrosine phosphatase (VE-PTP) is an important stabilizer of AJs, which prevents VE-cadherin phosphorylation and internalization (21). It was recently shown that endothelial-specific deletion of HIF-2α increased lung vascular permeability in ALI/ARDS and that, conversely, activation of HIF-2α by inhibiting PHD2 suppresses lung vascular leakiness in ALI/ARDS, thus indicating an important adaptive barrier-stabilizing role of hypoxic signaling in the lung vasculature (21). In contrast, HIF activation has been found to lead to a disruption of the blood-brain barrier in response to inhaled anesthetics such as isoflurane by downregulating the tight-junction protein occludin as well as collagen type IV in brain blood vessels (9). In line with these findings, deletion of HIF-1α in endothelial cells can prevent blood-brain barrier leakiness after transient middle cerebral artery occlusion of diabetic mice (86). These findings demonstrate that each vascular bed may respond to hypoxia differently and that HIF-1α and HIF-2α can act in a distinct manner in each vascular bed.
Arterial vs. Venous Specialization of Endothelial Cells
Early embryonic development occurs in a relatively hypoxic environment, which stimulates the formation of endothelial cells (37, 59). For example, a reduced oxygen environment (5% O2) during the first 6 days of human-induced pluripotent stem cell differentiation into endothelial cells leads to increased numbers of endothelial cells that are more functional in forming a vascular network (37).
Recent work has shown that oxygen-derived free radicals stimulate the differentiation of pluripotent stem cells by inducing the degradation of the master pluripotency regulator OCT4 (45). Pluripotent stem cells maintain a reduced intracellular environment, probably as a protection against reactive oxygen species. Metabolic shifts that enhance the cellular oxidation state significantly enhance the loss of pluripotency and facilitate vascular differentiation of pluripotent embryonic stem cells (45).
Hypoxia also guides endothelial cells toward an arterial cell fate, as evidenced by the upregulation of arterial markers, likely indicating an adaptive response to enhance arterial blood flow and oxygenation (72). Subsequent work in mouse embryonic stem cells has shown that increased formation of endothelial cells due to hypoxia is the result of a HIF-1α-dependent downregulation of the pluripotency markers Oct4 and Nanog, and upregulation of VEGF (41). The initial phase of endothelial differentiation is of critical importance, and genetic deletion of HIF-1α prevented the upregulation of the Etv2 transcription factor, which regulates endothelial differentiation (72).
During embryonic development, the arterial or venous identity of endothelial cells is established very early, even before blood is circulated through the vasculature, as evidenced by the expression of Ephrin B2 in arteries and its receptor EphB4 in veins (74). One of the earliest signals that differentiates between both fates is the expression of Sonic Hedgehog (Shh), which is required for arterial differentiation (39). Downstream of Shh, VEGF-mediated induction of Notch signaling was shown to be required for the formation of arterial endothelial cells (39), and loss of Notch signaling leads to ectopic expression of venous markers in the dorsal aorta of zebrafish (38). Continuous hypoxia induced the differentiation of mouse embryonic stem cells into arterial endothelial cells in hypoxia, an effect that depended on HIF-1α-mediated upregulation of Notch1 (72).
In a mouse brain endothelial cell line, hypoxia induced the arterial-specific Notch ligand Dll4, resulting in the upregulation of several Notch target genes. These target genes in turn suppress the venous regulator Coup-TFII (16). Therefore, hypoxia-induced Notch activation can lead to a switch between a venous and arterial phenotype, but it remains to be seen whether hypoxic enhancement of vascular barrier function (21) is also in part mediated by a phenotype switch of endothelial cells.
Role of Hypoxia Signaling in Inflammation
The inflammatory environment is often characterized by low oxygen levels due to a high metabolic demand of the inflammatory cells (8), and, in more severe cases, thrombosis or vascular damage can further impair oxygen delivery to the inflammatory environment. Interestingly, several observations point to an induction of HIF in inflammatory cells even in a normoxic environment. Stimulation of macrophages with both Gram-positive and Gram-negative bacteria leads to a robust induction of HIF-1α (58), which depends on the activation of NF-κB (62). Furthermore, macrophages lacking the NF-κB activator IκB kinase beta (IKKβ) demonstrate blunted HIF-1α accumulation in response to hypoxia (62). Several cytokines, including TNF-α (88) and IL-1β (32), similarly upregulate HIF-1α, and it is noteworthy that this response is to a large extent dependent on increased HIF-1α mRNA transcription and not due to a suppression of PHD-mediated HIF-1α degradation (32, 88). Conversely, macrophages lacking HIF-1α have impaired bacterial killing, and mice lacking HIF-1α in myeloid cells were not able to contain a skin infection of group A Streptococcus (58). Pharmacological activation of HIF-1α with AKB-4924 in contrast protected mice from Staphylococcus aureus infection (55). These findings indicate that signaling via HIF-1α is critical for intact immune function.
Much of the work on the role of hypoxic signaling in inflammation has focused on leukocytes, and because leukocytes are required to migrate through the endothelial layer to reach inflamed tissues, targeted modulation of hypoxic signaling in endothelial cells could emerge as a potential novel approach to regulating acute or chronic inflammation. Interestingly, inflammatory activation and tissue hypoxia may also create an environmental niche that not only recruits additional leukocytes but also recruits endothelial progenitor cells, which may participate in blood vessel repair and growth. Hypoxia-mediated upregulation of intercellular adhesion molecule-1 (ICAM-1) on endothelial cells, for example, allows endothelial progenitor cells to home to ischemic areas (83), which is dependent on their expression of β2 integrins (10).
Radiation Sensitivity of Endothelial Cells
Radiation therapy dosage in cancer patients is limited by the risk for normal tissue injury. For example, intestinal injury is often seen after irradiation of patients with abdominal and pelvic tumors. Several studies have shown that irradiation-induced endothelial apoptosis and capillary loss adversely affects the repair capacity of the epithelium (75). Moreover, endothelial deletion of HIF-1α protects against injury induced by localized intestinal irradiation by improving vascular integrity, which reduced both intestinal hypoxia and macrophage infiltration (71). Reduction of HIF-1α signaling led to a less inflammatory profile of endothelial cells, with lower levels of P-selectin, IL-1β, and VCAM1 expression after irradiation (71). Deletion of HIF-1α in gut epithelial cells, on the other hand, had no impact on disease severity.
When all three PHD enzymes were removed in gut epithelial cells, mice showed a remarkably improved survival after abdominal irradiation, an effect that was almost entirely dependent on the upregulation of HIF-2α (70). HIF signaling was further shown to improve epithelial integrity of the gastrointestinal tract and prevent hypernatremia and hyperglycemia. This study also showed that treating mice with DMOG to activate HIF signaling did not enhance xenograft growth of colorectal cancer cells or inhibit the tumoricidal effect of irradiation (70). These studies underscore the complexity of modulating HIF signaling in vivo: on the one hand, enhanced HIF-2α signaling might improve epithelial integrity, but, on the other hand, reducing HIF-1α signaling would improve endothelial function. Therefore, it might be necessary to develop therapeutic approaches that can be targeted to specific cell types and specifically modulate HIF-1α or HIF-2α.
Microvascular Repair After Organ Transplantation
Upon organ transplantation, the blood vessels of the transplanted organ are exposed to the host immune system, and alloimmune rejection negatively impacts graft survival. Microvascular dropout is an important factor in chronic rejection for transplantation of several solid organs, and HIF activation can mitigate this response. For example, after lung transplant, local ischemia and loss of microvasculature is linked to small airway fibrosis or bronchiolitis obliterans, a major complication that determines long-term survival of transplanted lungs (23). By using an orthotopic tracheal transplantation model in mice, researchers found that donor endothelial cells increased HIF-1α levels and grafts with adenoviral-mediated enhanced HIF-1α levels had better microvascular perfusion and less fibrotic remodeling, whereas the opposite was true if HIF-1α was deleted (30).
Mechanistically, HIF-1α increased the expression of proangiogenic factors and enhanced the recruitment of recipient Tie2+ angiogenic cells to the allograft to replace donor endothelial cells (30). In addition, VHL-haplodeficient pulmonary endothelial cells are more angiogenic and resistant to serum withdrawal-induced cell death (29), whereas VHL-haplodeficient mice were better able to contain a pulmonary infection of Aspergillus fumigatus, possibly because of the improved microvascular integrity (29). Topical application of the HIF activator desferrioxamine (DFO) on airway transplants at the time of transplantation promoted microvascular repair, suggesting that HIF activation might be a clinically relevant therapy (31). Similarly, treating a donor rat with a PHD inhibitor (FG-4497) improves the long-term survival of allogeneic kidney transplants (7).
Genetic Instability in Response to Hypoxia
Hypoxia has been studied extensively as a driver of genetic instability of tumors (43), and several genes involved in DNA damage repair were found to be regulated by hypoxia. However, similar observations about hypoxia-mediated downregulated DNA repair enzymes have been made in non-cancer cells, suggesting that endothelial cells exposed to hypoxia might be more prone to accumulate mutations.
Normal human fibroblasts maintained in hypoxia have an impaired ability to repair DNA double-strand breaks in response to irradiation, and this results in increased chromosomal instability (36). Hypoxia was subsequently shown to downregulate several proteins involved in homology directed repair, including Rad51, BRCA1, and BRCA2 (47).
In a mouse dermal fibroblast cell line, exposure to 48 h of hypoxia or desferrioxamine led to a substantial downregulation in MLH1 and PMS2 proteins involved in DNA mismatch repair (48). This resulted in a twofold increase in mutation frequency and increased replication slippage at DNA repeat regions. Hypoxic downregulation of the MLH1 protein depended on histone deacetylation and could largely be prevented by the histone deacetylase inhibitor TSA (48). A similar HIF-1α-dependent inhibition of mismatch repair enzymes was also found in colon cancer cells (35).
Endothelial cells from renal cell carcinomas were shown to exhibit a high proportion of aneuploidy (2), and further work from the same group showed that, when tumor-associated endothelial cells from high-metastatic and low-metastatic melanoma xenografts were compared, endothelial cells isolated from high-metastatic tumors proliferated faster and had a more invasive phenotype (54). Interestingly, freshly isolated endothelial cells from high-metastatic tumors also had a strikingly higher aneuploidy rate and even double-minute chromosomes (54). Intravital imaging of oxygen levels using pimonidazole further showed that blood vessels in high-metastatic tumors were exposed to hypoxia, suggesting that the tumor oxygen environment can impact the genetic stability of tumor endothelial cells (54), and these genetic changes in endothelial cells might in turn stimulate metastasis (44).
Certain types of tumor cells appear to have the ability to differentiate into endothelial-like cells as well. For example, endothelial cells present in B-cell lymphomas contained similar chromosomal translocations as the lymphoma cells, suggesting that tumor endothelial cells may also be derived from malignant cells (69). The precise origin and phenotype of the tumor endothelium can vary among tumors, and some studies suggest that tumors can form their own microvascular channels that do not require the recruitment of preexisting tissue endothelial cells, a process that is referred to as vasculogenic mimicry. Vasculogenic mimicry has been observed in several types of tumors (18) and correlates with a poor survival (15). During vasculogenic mimicry, tumor cells are even able to acquire typical endothelial markers such as VE-cadherin, but it is not clear that they have necessarily fully transdifferentiated into mature endothelial cells and likely represent a hybrid cell type (15).
It should also be noted that a clonal expansion of endothelial cells has been observed in plexiform lesions of patients with pulmonary artertial hypertension (PAH) (40), and, since hypoxia is a widely used model to study PAH, it would be interesting to see whether hypoxia-induced genomic instability might be involved in the formation of hyperproliferative endothelial cells in patients with PAH.
Genetics of Hypoxia Responses and Implications for Personalized Medicine
Modulating HIF signaling might be an attractive target, yet it is important to appreciate that there is considerable heterogeneity in the individual response to hypoxia, and this needs to be taken into account when customizing care to patients (FIGURE 1). Several mutations in the HIF signaling pathway have a clear phenotype, including the mutation in VHL that leads to Chuvash disease (3, 4). In addition, recent genome-wide association studies (GWAS) have identified several genetic variants in the vicinity of genes involved in the cellular and physiological response to hypoxia. Further work is necessary to clarify the importance of these SNPs and how the presence of these SNPs affects hypoxia signaling in individuals.
HIF-1α and HIF-2α
Mutations in HIF-2α are associated with erythrocytosis and cancer development. For example, a heterozygous G1609->T mutation in exon 12 of HIF-2α was identified in a family with erythrocytosis, and the resulting amino acid change led to a weaker binding of PHD2 to Pro531 and reduced degradation of HIF-2α (57). Similar gain-of-function mutations in HIF-2α have also been found in two patients with multiple paragangliomas (catecholamine-producing tumors from the chromaffin cells of the extra-adrenal paraganglia) with or without somatostatinoma (somatostatin-producing tumors derived from neural crest cells) (89). Both patients were found to have mutations in exon 12, leading to a disruption of prolyl hydroxylation of HIF-2α and increased half-life of HIF-2α (89).
More subtle variants within or in the proximity of HIF-2α have been identified by using GWAS. For example, Tibetans have adapted to living at high altitude by having lower hemoglobin levels preventing blood hyperviscosity. Several studies have implicated HIF-2α in this adaptation (56, 78, 82). In the biggest of these studies, a GWAS on >1,000 native Tibetans identified several variants in HIF-2α that were much less frequent in people with a Han Chinese or Japanese ancestry (56). Variants in the promoter region of HIF-2α have similarly been associated with high-altitude adaptation (79).
A multi-ancestry GWAS study of >150,000 individuals also identified that a SNP upstream of HIF-2α is associated with birth weight (26), whereas an intronic SNP in HIF-2α is associated with atrial P-wave duration in a GWAS study comprising 44,000 individuals (14). Craniofacial microsomia is a congenital condition for which the pathogenesis is unknown; however, a case/control GWAS identified a SNP upstream of HIF-2α (85).
The association of this large number of seemingly unrelated conditions with variants in the vicinity or intronic regions of HIF-2α might be surprising but probably reflects variants that influence tissue-specific enhancers. It also points at how modulating HIF-2α can affect multiple tissues and the potential variability in how individuals will respond.
Overall, few GWAS studies have identified SNPs in the vicinity of HIF-1α, perhaps pointing to the importance of tightly controlling HIF-1α expression levels. In one exception, a GWAS in a subpopulation of the Framingham heart study found that a SNP in an intronic region of HIF-1α is correlated with CD40 ligand serum levels (6). Serum CD40 ligand levels are biomarkers for increased cardiovascular risk (81), although changes in HIF-1α levels themselves have not been associated with increased cardiovascular risk.
PHDs and VHL
An intronic SNP in the PHD1 gene is associated with smoking behavior (number of cigarettes/day) (20), and a SNP upstream of PHD1 is associated with the development of chronic obstructive pulmonary disease (COPD) (12). However, it should be pointed out that the major nicotine-inactivating enzyme Cyp2A6 lies just 30 kb downstream of PHD1 and that these SNPs might simply be in linkage disequilibrium. Further research needs to be done to determine the exact role of PHD1 in COPD.
A GWAS looking at hematological traits in >170,000 individuals of European ancestry found a strong association between a 5′ UTR variant of the PHD2 gene and hematocrit, hemoglobin levels, and red blood cell count. A separate intronic SNP was similarly associated with hematocrit levels and red blood cell counts (5).
No SNPs in the vicinity of VHL have been identified using GWAS studies; however, both loss-of-function and hypomorphic mutations in VHL lead to disease. For example, VHL mutations lead to the development of a number of tumors, including highly vascular hemangioblastomas and clear-cell renal carcinomas (33). In addition, the hypomorphic VHL R200W allele leads to the development of Chuvash polycythemia and pulmonary hypertension (3, 4), probably because of the activation of HIF-2α (25). Also, neutrophils from patients with heterozygous mutations in VHL are apoptosis resistant and have an increased phagocytic activity (73), and a full loss of VHL results in an almost complete resistance to viral infection of renal clear-cell carcinoma and glioblastoma cell lines (27).
Novel Approaches to Study Hypoxia in the Vasculature
Local oxygen levels can be measured indirectly by detecting the incorporation of pimonidazole in cells exposed to a Po2 of <10 Torr (22). This approach presents an all or nothing measurement at a single time point, and novel tools have been developed recently to circumvent these limitations (50). For example, the phosphorescent PtP-C343 probe has a phosphorescence decay time that depends on local oxygen levels, and this can be imaged in vivo, for example, inside the bone marrow, using two-photon microscopy (68). This technique allows oxygen concentration measurements along an individual blood vessel in a live animal.
The development of large-scale transcriptomic analyses using single-cell RNA-Seq is revolutionizing our understanding of vascular heterogeneity at a single-cell level (34, 87). For example, endothelial cells from different organs could readily be distinguished based on their G-protein-coupled receptor (GPCR) expression levels, but even within one organ there were significant differences between the expression levels in individual endothelial cells (34). Similarly, endothelial cells from different organs have clearly distinguishable gene regulatory networks (28). This heterogeneity is likely also present for the response of endothelial cells to hypoxia, but it is an open question whether some endothelial cells are primed to respond to hypoxia, whereas others might be relatively insensitive.
Importantly, endothelial cells in vivo are exposed to spatial hypoxia gradients and not to uniform oxygen levels. This spatial complexity of the oxygen environment can be mimicked in vitro by culturing cells in a microfluidic device consisting of an oxygen-permeable polydimethylsiloxane membrane perfused with gas of different oxygen concentrations (60, 61). By combining different channels and gas mixtures, linear, exponential, and more complex gradients can be generated, and these gradients can be modified over time (1). The use of hydrogels even allows the generation of 3D hypoxia gradients, where the thickness of the hydrogel can be manipulated to establish different gradients, and this system can be studied for up to 2 wk (42). In addition to regulating local oxygen concentrations using gas mixtures, it is also possible to use chemical reactions to locally produce or consume oxygen (11). Even more complex 3D structures can be generated by seeding cells together with collagen into a porous cellulose scaffold. This scaffold can then be rolled up to generate a 3D structure. Unrolling the sheet allows for the isolation of cells from specific locations within the 3D structure (63). These approaches enable the generation of ex vivo oxygen gradients, which permits the dissection of how spatial oxygen gradients may differentially impact hypoxia responses.
Translational Perspective
Enhancing HIF-1α or HIF-2α signaling has a clear beneficial effect in the settings of organ transplant, irradiation, or pathological increases in vascular permeability. Desferrioxamine, which activates both HIF-1α and HIF-2α, is already in clinical use as an iron chelator, and therefore safety has already been established. Nevertheless, it should be clear from the aforementioned studies that such a broad activation of both HIF-1α and HIF-2α throughout the body might have unintended consequences, such as activating inflammation, which limit the therapeutic efficacy. The endothelium can easily be reached through the circulation, and we expect that novel drug delivery methods will be developed that allow specific activation of HIF-1α and HIF-2α signaling in the endothelium. Moreover, because of the divergence between HIF-1α and HIF-2α signaling pathways in endothelial cells, it should be a high priority to develop drugs that specifically inhibit or activate either isoform.
Understanding the cellular and tissue-specific heterogeneity of hypoxia responses on a molecular level in the vasculature should be critical for future therapeutic approaches that take advantage of the adaptive and reparative pathways activated by hypoxia while containing hypoxia-induced tissue injury.
Acknowledgments
This work was supported in part by an American Heart Scientist Development Grant 15SDG23250002 (G.M.) and National Institutes of Health Grants P01-HL-60678 (J.R.), R01-HL-118068 (J.R.), R01-HL-90152 (J.R.), and R21-CA-223915 (J.R.).
No conflicts of interest, financial or otherwise, are declared by the author(s).
G.M. and J.R. drafted manuscript; G.M. and J.R. edited and revised manuscript; G.M. and J.R. approved final version of manuscript.
References
- 1.Adler M, Polinkovsky M, Gutierrez E, Groisman A. Generation of oxygen gradients with arbitrary shapes in a microfluidic device. Lab Chip 10: 388–391, 2010. doi: 10.1039/B920401F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akino T, Hida K, Hida Y, Tsuchiya K, Freedman D, Muraki C, Ohga N, Matsuda K, Akiyama K, Harabayashi T, Shinohara N, Nonomura K, Klagsbrun M, Shindoh M. Cytogenetic abnormalities of tumor-associated endothelial cells in human malignant tumors. Am J Pathol 175: 2657–2667, 2009. doi: 10.2353/ajpath.2009.090202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ang SO, Chen H, Gordeuk VR, Sergueeva AI, Polyakova LA, Miasnikova GY, Kralovics R, Stockton DW, Prchal JT. Endemic polycythemia in Russia: mutation in the VHL gene. Blood Cells Mol Dis 28: 57–62, 2002. doi: 10.1006/bcmd.2002.0488. [DOI] [PubMed] [Google Scholar]
- 4.Ang SO, Chen H, Hirota K, Gordeuk VR, Jelinek J, Guan Y, Liu E, Sergueeva AI, Miasnikova GY, Mole D, Maxwell PH, Stockton DW, Semenza GL, Prchal JT. Disruption of oxygen homeostasis underlies congenital Chuvash polycythemia. Nat Genet 32: 614–621, 2002. doi: 10.1038/ng1019. [DOI] [PubMed] [Google Scholar]
- 5.Astle WJ, Elding H, Jiang T, Allen D, Ruklisa D, Mann AL, Mead D, Bouman H, Riveros-Mckay F, Kostadima MA, Lambourne JJ, Sivapalaratnam S, Downes K, Kundu K, Bomba L, Berentsen K, Bradley JR, Daugherty LC, Delaneau O, Freson K, Garner SF, Grassi L, Guerrero J, Haimel M, Janssen-Megens EM, Kaan A, Kamat M, Kim B, Mandoli A, Marchini J, Martens JHA, Meacham S, Megy K, O’Connell J, Petersen R, Sharifi N, Sheard SM, Staley JR, Tuna S, van der Ent M, Walter K, Wang SY, Wheeler E, Wilder SP, Iotchkova V, Moore C, Sambrook J, Stunnenberg HG, Di Angelantonio E, Kaptoge S, Kuijpers TW, Carrillo-de-Santa-Pau E, Juan D, Rico D, Valencia A, Chen L, Ge B, Vasquez L, Kwan T, Garrido-Martín D, Watt S, Yang Y, Guigo R, Beck S, Paul DS, Pastinen T, Bujold D, Bourque G, Frontini M, Danesh J, Roberts DJ, Ouwehand WH, Butterworth AS, Soranzo N. The allelic landscape of human blood cell trait variation and links to common complex disease. Cell 167: 1415–1429.e19, 2016. doi: 10.1016/j.cell.2016.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benjamin EJ, Dupuis J, Larson MG, Lunetta KL, Booth SL, Govindaraju DR, Kathiresan S, Keaney JF Jr, Keyes MJ, Lin JP, Meigs JB, Robins SJ, Rong J, Schnabel R, Vita JA, Wang TJ, Wilson PW, Wolf PA, Vasan RS. Genome-wide association with select biomarker traits in the Framingham Heart Study. BMC Med Genet 8, Suppl 1: S11, 2007. doi: 10.1186/1471-2350-8-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernhardt WM, Gottmann U, Doyon F, Buchholz B, Campean V, Schödel J, Reisenbuechler A, Klaus S, Arend M, Flippin L, Willam C, Wiesener MS, Yard B, Warnecke C, Eckardt KU. Donor treatment with a PHD-inhibitor activating HIFs prevents graft injury and prolongs survival in an allogenic kidney transplant model. Proc Natl Acad Sci USA 106: 21276–21281, 2009. doi: 10.1073/pnas.0903978106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell EL, Bruyninckx WJ, Kelly CJ, Glover LE, McNamee EN, Bowers BE, Bayless AJ, Scully M, Saeedi BJ, Golden-Mason L, Ehrentraut SF, Curtis VF, Burgess A, Garvey JF, Sorensen A, Nemenoff R, Jedlicka P, Taylor CT, Kominsky DJ, Colgan SP. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity 40: 66–77, 2014. doi: 10.1016/j.immuni.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao Y, Li Z, Li H, Ni C, Li L, Yang N, Shi C, Zhong Y, Cui D, Guo X. Hypoxia-inducible factor-1α is involved in isoflurane-induced blood-brain barrier disruption in aged rats model of POCD. Behav Brain Res 339: 39–46, 2018. doi: 10.1016/j.bbr.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Chavakis E, Aicher A, Heeschen C, Sasaki K, Kaiser R, El Makhfi N, Urbich C, Peters T, Scharffetter-Kochanek K, Zeiher AM, Chavakis T, Dimmeler S. Role of beta2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J Exp Med 201: 63–72, 2005. doi: 10.1084/jem.20041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen YA, King AD, Shih HC, Peng CC, Wu CY, Liao WH, Tung YC. Generation of oxygen gradients in microfluidic devices for cell culture using spatially confined chemical reactions. Lab Chip 11: 3626–3633, 2011. doi: 10.1039/c1lc20325h. [DOI] [PubMed] [Google Scholar]
- 12.Cho MH, Castaldi PJ, Wan ES, Siedlinski M, Hersh CP, Demeo DL, Himes BE, Sylvia JS, Klanderman BJ, Ziniti JP, Lange C, Litonjua AA, Sparrow D, Regan EA, Make BJ, Hokanson JE, Murray T, Hetmanski JB, Pillai SG, Kong X, Anderson WH, Tal-Singer R, Lomas DA, Coxson HO, Edwards LD, MacNee W, Vestbo J, Yates JC, Agusti A, Calverley PM, Celli B, Crim C, Rennard S, Wouters E, Bakke P, Gulsvik A, Crapo JD, Beaty TH, Silverman EK, Investigators I, Investigators E, Investigators CO; ICGN Investigators; ECLIPSE Investigators; COPDGene Investigators . A genome-wide association study of COPD identifies a susceptibility locus on chromosome 19q13. Hum Mol Genet 21: 947–957, 2012. doi: 10.1093/hmg/ddr524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choudhry H, Harris AL. Advances in hypoxia-inducible factor biology. Cell Metab 27: 281–298, 2018. doi: 10.1016/j.cmet.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Christophersen IE, Magnani JW, Yin X, Barnard J, Weng LC, Arking DE, Niemeijer MN, Lubitz SA, Avery CL, Duan Q, Felix SB, Bis JC, Kerr KF, Isaacs A, Müller-Nurasyid M, Müller C, North KE, Reiner AP, Tinker LF, Kors JA, Teumer A, Petersmann A, Sinner MF, Buzkova P, Smith JD, Van Wagoner DR, Völker U, Waldenberger M, Peters A, Meitinger T, Limacher MC, Wilhelmsen KC, Psaty BM, Hofman A, Uitterlinden A, Krijthe BP, Zhang ZM, Schnabel RB, Kääb S, van Duijn C, Rotter JI, Sotoodehnia N, Dörr M, Li Y, Chung MK, Soliman EZ, Alonso A, Whitsel EA, Stricker BH, Benjamin EJ, Heckbert SR, Ellinor PT. Fifteen genetic loci associated with the electrocardiographic P wave. Circ Cardiovasc Genet 10: e001667, 2017. doi: 10.1161/CIRCGENETICS.116.001667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delgado-Bellido D, Serrano-Saenz S, Fernández-Cortés M, Oliver FJ. Vasculogenic mimicry signaling revisited: focus on non-vascular VE-cadherin. Mol Cancer 16: 65, 2017. doi: 10.1186/s12943-017-0631-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diez H, Fischer A, Winkler A, Hu CJ, Hatzopoulos AK, Breier G, Gessler M. Hypoxia-mediated activation of Dll4-Notch-Hey2 signaling in endothelial progenitor cells and adoption of arterial cell fate. Exp Cell Res 313: 1–9, 2007. doi: 10.1016/j.yexcr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Dunham-Snary KJ, Wu D, Sykes EA, Thakrar A, Parlow LRG, Mewburn JD, Parlow JL, Archer SL. Hypoxic pulmonary vasoconstriction: from molecular mechanisms to medicine. Chest 151: 181–192, 2017. doi: 10.1016/j.chest.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan YL, Zheng M, Tang YL, Liang XH. A new perspective of vasculogenic mimicry: EMT and cancer stem cells (Review). Oncol Lett 6: 1174–1180, 2013. doi: 10.3892/ol.2013.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer C, Schneider M, Carmeliet P. Principles and therapeutic implications of angiogenesis, vasculogenesis and arteriogenesis. Handb Exp Pharmacol 176: 157–212, 2006. doi: 10.1007/3-540-36028-X_6. [DOI] [PubMed] [Google Scholar]
- 20.Furberg H, Ostroff J, Lerman C, Sullivan PF. The public health utility of genome-wide association study results for smoking behavior. Genome Med 2: 26, 2010. doi: 10.1186/gm147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong H, Rehman J, Tang H, Wary K, Mittal M, Chaturvedi P, Zhao YY, Komarova YA, Vogel SM, Malik AB. HIF2α signaling inhibits adherens junctional disruption in acute lung injury. J Clin Invest 125: 652–664, 2015. doi: 10.1172/JCI77701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gross MW, Karbach U, Groebe K, Franko AJ, Mueller-Klieser W. Calibration of misonidazole labeling by simultaneous measurement of oxygen tension and labeling density in multicellular spheroids. Int J Cancer 61: 567–573, 1995. doi: 10.1002/ijc.2910610422. [DOI] [PubMed] [Google Scholar]
- 23.Grossman EJ, Shilling RA. Bronchiolitis obliterans in lung transplantation: the good, the bad, and the future. Transl Res 153: 153–165, 2009. doi: 10.1016/j.trsl.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Hamanaka RB, Chandel NS. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci 35: 505–513, 2010. doi: 10.1016/j.tibs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hickey MM, Richardson T, Wang T, Mosqueira M, Arguiri E, Yu H, Yu QC, Solomides CC, Morrisey EE, Khurana TS, Christofidou-Solomidou M, Simon MC. The von Hippel-Lindau Chuvash mutation promotes pulmonary hypertension and fibrosis in mice. J Clin Invest 120: 827–839, 2010. doi: 10.1172/JCI36362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horikoshi M, Beaumont RN, Day FR, Warrington NM, Kooijman MN, Fernandez-Tajes J, Feenstra B, van Zuydam NR, Gaulton KJ, Grarup N, Bradfield JP, Strachan DP, Li-Gao R, Ahluwalia TS, Kreiner E, Rueedi R, Lyytikäinen LP, Cousminer DL, Wu Y, Thiering E, Wang CA, Have CT, Hottenga JJ, Vilor-Tejedor N, Joshi PK, Boh ETH, Ntalla I, Pitkänen N, Mahajan A, van Leeuwen EM, Joro R, Lagou V, Nodzenski M, Diver LA, Zondervan KT, Bustamante M, Marques-Vidal P, Mercader JM, Bennett AJ, Rahmioglu N, Nyholt DR, Ma RCW, Tam CHT, Tam WH, Ganesh SK, van Rooij FJ, Jones SE, Loh PR, Ruth KS, Tuke MA, Tyrrell J, Wood AR, Yaghootkar H, Scholtens DM, Paternoster L, Prokopenko I, Kovacs P, Atalay M, Willems SM, Panoutsopoulou K, Wang X, Carstensen L, Geller F, Schraut KE, Murcia M, van Beijsterveldt CE, Willemsen G, Appel EVR, Fonvig CE, Trier C, Tiesler CM, Standl M, Kutalik Z, Bonas-Guarch S, Hougaard DM, Sánchez F, Torrents D, Waage J, Hollegaard MV, de Haan HG, Rosendaal FR, Medina-Gomez C, Ring SM, Hemani G, McMahon G, Robertson NR, Groves CJ, Langenberg C, Luan J, Scott RA, Zhao JH, Mentch FD, MacKenzie SM, Reynolds RM, Lowe WL Jr, Tönjes A, Stumvoll M, Lindi V, Lakka TA, van Duijn CM, Kiess W, Körner A, Sørensen TI, Niinikoski H, Pahkala K, Raitakari OT, Zeggini E, Dedoussis GV, Teo YY, Saw SM, Melbye M, Campbell H, Wilson JF, Vrijheid M, de Geus EJ, Boomsma DI, Kadarmideen HN, Holm JC, Hansen T, Sebert S, Hattersley AT, Beilin LJ, Newnham JP, Pennell CE, Heinrich J, Adair LS, Borja JB, Mohlke KL, Eriksson JG, Widén EE, Kähönen M, Viikari JS, Lehtimäki T, Vollenweider P, Bønnelykke K, Bisgaard H, Mook-Kanamori DO, Hofman A, Rivadeneira F, Uitterlinden AG, Pisinger C, Pedersen O, Power C, Hyppönen E, Wareham NJ, Hakonarson H, Davies E, Walker BR, Jaddoe VW, Jarvelin MR, Grant SF, Vaag AA, Lawlor DA, Frayling TM, Davey Smith G, Morris AP, Ong KK, Felix JF, Timpson NJ, Perry JR, Evans DM, McCarthy MI, Freathy RM, Freathy RM; CHARGE Consortium Hematology Working Group . Genome-wide associations for birth weight and correlations with adult disease. Nature 538: 248–252, 2016. doi: 10.1038/nature19806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang II, Watson IR, Der SD, Ohh M. Loss of VHL confers hypoxia-inducible factor (HIF)-dependent resistance to vesicular stomatitis virus: role of HIF in antiviral response. J Virol 80: 10712–10723, 2006. doi: 10.1128/JVI.01014-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jambusaria A, Klomp J, Hong Z, Rafii S, Dai Y, Malik AB, Rehman J. A computational approach to identify cellular heterogeneity and tissue-specific gene regulatory networks. BMC Bioinformatics 19: 217, 2018. doi: 10.1186/s12859-018-2190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang X, Hsu JL, Tian W, Yuan K, Olcholski M, Perez VJ, Semenza GL, Nicolls MR. Tie2-dependent VHL knockdown promotes airway microvascular regeneration and attenuates invasive growth of Aspergillus fumigatus. J Mol Med (Berl) 91: 1081–1093, 2013. doi: 10.1007/s00109-013-1063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang X, Khan MA, Tian W, Beilke J, Natarajan R, Kosek J, Yoder MC, Semenza GL, Nicolls MR. Adenovirus-mediated HIF-1α gene transfer promotes repair of mouse airway allograft microvasculature and attenuates chronic rejection. J Clin Invest 121: 2336–2349, 2011. doi: 10.1172/JCI46192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang X, Malkovskiy AV, Tian W, Sung YK, Sun W, Hsu JL, Manickam S, Wagh D, Joubert LM, Semenza GL, Rajadas J, Nicolls MR. Promotion of airway anastomotic microvascular regeneration and alleviation of airway ischemia by deferoxamine nanoparticles. Biomaterials 35: 803–813, 2014. doi: 10.1016/j.biomaterials.2013.09.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J 17: 2115–2117, 2003. doi: 10.1096/fj.03-0329fje. [DOI] [PubMed] [Google Scholar]
- 33.Kaelin WG., Jr Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer 2: 673–682, 2002. doi: 10.1038/nrc885. [DOI] [PubMed] [Google Scholar]
- 34.Kaur H, Carvalho J, Looso M, Singh P, Chennupati R, Preussner J, Günther S, Albarrán-Juárez J, Tischner D, Classen S, Offermanns S, Wettschureck N. Single-cell profiling reveals heterogeneity and functional patterning of GPCR expression in the vascular system. Nat Commun 8: 15700, 2017. doi: 10.1038/ncomms15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koshiji M, To KK, Hammer S, Kumamoto K, Harris AL, Modrich P, Huang LE. HIF-1alpha induces genetic instability by transcriptionally downregulating MutSalpha expression. Mol Cell 17: 793–803, 2005. doi: 10.1016/j.molcel.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 36.Kumareswaran R, Ludkovski O, Meng A, Sykes J, Pintilie M, Bristow RG. Chronic hypoxia compromises repair of DNA double-strand breaks to drive genetic instability. J Cell Sci 125: 189–199, 2012. doi: 10.1242/jcs.092262. [DOI] [PubMed] [Google Scholar]
- 37.Kusuma S, Peijnenburg E, Patel P, Gerecht S. Low oxygen tension enhances endothelial fate of human pluripotent stem cells. Arterioscler Thromb Vasc Biol 34: 913–920, 2014. doi: 10.1161/ATVBAHA.114.303274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development 128: 3675–3683, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Lawson ND, Vogel AM, Weinstein BM. sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell 3: 127–136, 2002. doi: 10.1016/S1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- 40.Lee SD, Shroyer KR, Markham NE, Cool CD, Voelkel NF, Tuder RM. Monoclonal endothelial cell proliferation is present in primary but not secondary pulmonary hypertension. J Clin Invest 101: 927–934, 1998. doi: 10.1172/JCI1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee SW, Jeong HK, Lee JY, Yang J, Lee EJ, Kim SY, Youn SW, Lee J, Kim WJ, Kim KW, Lim JM, Park JW, Park YB, Kim HS. Hypoxic priming of mESCs accelerates vascular-lineage differentiation through HIF1-mediated inverse regulation of Oct4 and VEGF. EMBO Mol Med 4: 924–938, 2012. doi: 10.1002/emmm.201101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis DM, Blatchley MR, Park KM, Gerecht S. O2-controllable hydrogels for studying cellular responses to hypoxic gradients in three dimensions in vitro and in vivo. Nat Protoc 12: 1620–1638, 2017. doi: 10.1038/nprot.2017.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luoto KR, Kumareswaran R, Bristow RG. Tumor hypoxia as a driving force in genetic instability. Genome Integr 4: 5, 2013. doi: 10.1186/2041-9414-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maishi N, Ohba Y, Akiyama K, Ohga N, Hamada J, Nagao-Kitamoto H, Alam MT, Yamamoto K, Kawamoto T, Inoue N, Taketomi A, Shindoh M, Hida Y, Hida K. Tumour endothelial cells in high metastatic tumours promote metastasis via epigenetic dysregulation of biglycan. Sci Rep 6: 28039, 2016. doi: 10.1038/srep28039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marsboom G, Zhang GF, Pohl-Avila N, Zhang Y, Yuan Y, Kang H, Hao B, Brunengraber H, Malik AB, Rehman J. Glutamine Metabolism Regulates the Pluripotency Transcription Factor OCT4. Cell Reports 16: 323–332, 2016. doi: 10.1016/j.celrep.2016.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maulik N, Das DK. Redox signaling in vascular angiogenesis. Free Radic Biol Med 33: 1047–1060, 2002. doi: 10.1016/S0891-5849(02)01005-5. [DOI] [PubMed] [Google Scholar]
- 47.Meng AX, Jalali F, Cuddihy A, Chan N, Bindra RS, Glazer PM, Bristow RG. Hypoxia down-regulates DNA double strand break repair gene expression in prostate cancer cells. Radiother Oncol 76: 168–176, 2005. doi: 10.1016/j.radonc.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 48.Mihaylova VT, Bindra RS, Yuan J, Campisi D, Narayanan L, Jensen R, Giordano F, Johnson RS, Rockwell S, Glazer PM. Decreased expression of the DNA mismatch repair gene Mlh1 under hypoxic stress in mammalian cells. Mol Cell Biol 23: 3265–3273, 2003. doi: 10.1128/MCB.23.9.3265-3273.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mills DB, Francis WR, Vargas S, Larsen M, Elemans CP, Canfield DE, Wörheide G. The last common ancestor of animals lacked the HIF pathway and respired in low-oxygen environments. eLife 7: e31176, 2018. doi: 10.7554/eLife.31176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mirabello V, Cortezon-Tamarit F, Pascu SI. Oxygen Sensing, Hypoxia Tracing and in Vivo Imaging with Functional Metalloprobes for the Early Detection of Non-communicable Diseases. Front Chem 6: 27, 2018. doi: 10.3389/fchem.2018.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monaghan-Benson E, Burridge K. The regulation of vascular endothelial growth factor-induced microvascular permeability requires Rac and reactive oxygen species. J Biol Chem 284: 25602–25611, 2009. doi: 10.1074/jbc.M109.009894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakazawa MS, Keith B, Simon MC. Oxygen availability and metabolic adaptations. Nat Rev Cancer 16: 663–673, 2016. doi: 10.1038/nrc.2016.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, Lin HC, Yancopoulos GD, Thurston G. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature 444: 1032–1037, 2006. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 54.Ohga N, Ishikawa S, Maishi N, Akiyama K, Hida Y, Kawamoto T, Sadamoto Y, Osawa T, Yamamoto K, Kondoh M, Ohmura H, Shinohara N, Nonomura K, Shindoh M, Hida K. Heterogeneity of tumor endothelial cells: comparison between tumor endothelial cells isolated from high- and low-metastatic tumors. Am J Pathol 180: 1294–1307, 2012. doi: 10.1016/j.ajpath.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 55.Okumura CY, Hollands A, Tran DN, Olson J, Dahesh S, von Köckritz-Blickwede M, Thienphrapa W, Corle C, Jeung SN, Kotsakis A, Shalwitz RA, Johnson RS, Nizet V. A new pharmacological agent (AKB-4924) stabilizes hypoxia inducible factor-1 (HIF-1) and increases skin innate defenses against bacterial infection. J Mol Med (Berl) 90: 1079–1089, 2012. doi: 10.1007/s00109-012-0882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peng Y, Yang Z, Zhang H, Cui C, Qi X, Luo X, Tao X, Wu T, Ouzhuluobu, Basang, Ciwangsangbu, Danzengduojie, Chen H, Shi H, Su B. Genetic variations in Tibetan populations and high-altitude adaptation at the Himalayas. Mol Biol Evol 28: 1075–1081, 2011. doi: 10.1093/molbev/msq290. [DOI] [PubMed] [Google Scholar]
- 57.Percy MJ, Furlow PW, Lucas GS, Li X, Lappin TR, McMullin MF, Lee FS. A gain-of-function mutation in the HIF2A gene in familial erythrocytosis. N Engl J Med 358: 162–168, 2008. doi: 10.1056/NEJMoa073123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peyssonnaux C, Datta V, Cramer T, Doedens A, Theodorakis EA, Gallo RL, Hurtado-Ziola N, Nizet V, Johnson RS. HIF-1alpha expression regulates the bactericidal capacity of phagocytes. J Clin Invest 115: 1806–1815, 2005. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prado-Lopez S, Conesa A, Armiñán A, Martínez-Losa M, Escobedo-Lucea C, Gandia C, Tarazona S, Melguizo D, Blesa D, Montaner D, Sanz-González S, Sepúlveda P, Götz S, O’Connor JE, Moreno R, Dopazo J, Burks DJ, Stojkovic M. Hypoxia promotes efficient differentiation of human embryonic stem cells to functional endothelium. Stem Cells 28: 407–418, 2010. [DOI] [PubMed] [Google Scholar]
- 60.Rexius-Hall ML, Mauleon G, Malik AB, Rehman J, Eddington DT. Microfluidic platform generates oxygen landscapes for localized hypoxic activation. Lab Chip 14: 4688–4695, 2014. doi: 10.1039/C4LC01168F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rexius-Hall ML, Rehman J, Eddington DT. A microfluidic oxygen gradient demonstrates differential activation of the hypoxia-regulated transcription factors HIF-1α and HIF-2α. Integr Biol 9: 742–750, 2017. doi: 10.1039/C7IB00099E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 453: 807–811, 2008. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodenhizer D, Gaude E, Cojocari D, Mahadevan R, Frezza C, Wouters BG, McGuigan AP. A three-dimensional engineered tumour for spatial snapshot analysis of cell metabolism and phenotype in hypoxic gradients. Nat Mater 15: 227–234, 2016. doi: 10.1038/nmat4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rytkönen KT. Evolution: Oxygen and early animals. eLife 7: e34756, 2018. doi: 10.7554/eLife.34756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scehnet JS, Jiang W, Kumar SR, Krasnoperov V, Trindade A, Benedito R, Djokovic D, Borges C, Ley EJ, Duarte A, Gill PS. Inhibition of Dll4-mediated signaling induces proliferation of immature vessels and results in poor tissue perfusion. Blood 109: 4753–4760, 2007. doi: 10.1182/blood-2006-12-063933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Semenza GL. Vascular responses to hypoxia and ischemia. Arterioscler Thromb Vasc Biol 30: 648–652, 2010. doi: 10.1161/ATVBAHA.108.181644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Skuli N, Liu L, Runge A, Wang T, Yuan L, Patel S, Iruela-Arispe L, Simon MC, Keith B. Endothelial deletion of hypoxia-inducible factor-2alpha (HIF-2alpha) alters vascular function and tumor angiogenesis. Blood 114: 469–477, 2009. doi: 10.1182/blood-2008-12-193581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, Zaher W, Mortensen LJ, Alt C, Turcotte R, Yusuf R, Côté D, Vinogradov SA, Scadden DT, Lin CP. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 508: 269–273, 2014. doi: 10.1038/nature13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Streubel B, Chott A, Huber D, Exner M, Jäger U, Wagner O, Schwarzinger I. Lymphoma-specific genetic aberrations in microvascular endothelial cells in B-cell lymphomas. N Engl J Med 351: 250–259, 2004. doi: 10.1056/NEJMoa033153. [DOI] [PubMed] [Google Scholar]
- 70.Taniguchi CM, Miao YR, Diep AN, Wu C, Rankin EB, Atwood TF, Xing L, Giaccia AJ. PHD inhibition mitigates and protects against radiation-induced gastrointestinal toxicity via HIF2. Sci Transl Med 6: 236ra64, 2014. doi: 10.1126/scitranslmed.3008523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Toullec A, Buard V, Rannou E, Tarlet G, Guipaud O, Robine S, Iruela-Arispe ML, François A, Milliat F. HIF-1 α deletion in the endothelium, but not in the epithelium, protects from radiation-induced enteritis. Cell Mol Gastroenterol Hepatol 5: 15–30, 2017. doi: 10.1016/j.jcmgh.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsang KM, Hyun JS, Cheng KT, Vargas M, Mehta D, Ushio-Fukai M, Zou L, Pajcini KV, Rehman J, Malik AB. Embryonic stem cell differentiation to functional arterial endothelial cells through sequential activation of ETV2 and NOTCH1 signaling by HIF1α. Stem Cell Reports 9: 796–806, 2017. doi: 10.1016/j.stemcr.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walmsley SR, Cowburn AS, Clatworthy MR, Morrell NW, Roper EC, Singleton V, Maxwell P, Whyte MK, Chilvers ER. Neutrophils from patients with heterozygous germline mutations in the von Hippel Lindau protein (pVHL) display delayed apoptosis and enhanced bacterial phagocytosis. Blood 108: 3176–3178, 2006. doi: 10.1182/blood-2006-04-018796. [DOI] [PubMed] [Google Scholar]
- 74.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 93: 741–753, 1998. doi: 10.1016/S0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 75.Wang J, Boerma M, Fu Q, Hauer-Jensen M. Significance of endothelial dysfunction in the pathogenesis of early and delayed radiation enteropathy. World J Gastroenterol 13: 3047–3055, 2007. doi: 10.3748/wjg.v13.i22.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Waypa GB, Marks JD, Guzy RD, Mungai PT, Schriewer JM, Dokic D, Ball MK, Schumacker PT. Superoxide generated at mitochondrial complex III triggers acute responses to hypoxia in the pulmonary circulation. Am J Respir Crit Care Med 187: 424–432, 2013. doi: 10.1164/rccm.201207-1294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weir EK, Archer SL. The role of redox changes in oxygen sensing. Respir Physiol Neurobiol 174: 182–191, 2010. doi: 10.1016/j.resp.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu S, Li S, Yang Y, Tan J, Lou H, Jin W, Yang L, Pan X, Wang J, Shen Y, Wu B, Wang H, Jin L. A genome-wide search for signals of high-altitude adaptation in Tibetans. Mol Biol Evol 28: 1003–1011, 2011. doi: 10.1093/molbev/msq277. [DOI] [PubMed] [Google Scholar]
- 79.Xu XH, Huang XW, Qun L, Li YN, Wang Y, Liu C, Ma Y, Liu QM, Sun K, Qian F, Jin L, Wang J. Two functional loci in the promoter of EPAS1 gene involved in high-altitude adaptation of Tibetans. Sci Rep 4: 7465, 2014. doi: 10.1038/srep07465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamaoka-Tojo M, Tojo T, Kim HW, Hilenski L, Patrushev NA, Zhang L, Fukai T, Ushio-Fukai M. IQGAP1 mediates VE-cadherin-based cell-cell contacts and VEGF signaling at adherence junctions linked to angiogenesis. Arterioscler Thromb Vasc Biol 26: 1991–1997, 2006. doi: 10.1161/01.ATV.0000231524.14873.e7. [DOI] [PubMed] [Google Scholar]
- 81.Yan JC, Zhu J, Gao L, Wu ZG, Kong XT, Zong RQ, Zhan LZ. The effect of elevated serum soluble CD40 ligand on the prognostic value in patients with acute coronary syndromes. Clin Chim Acta 343: 155–159, 2004. doi: 10.1016/j.cccn.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 82.Yi X, Liang Y, Huerta-Sanchez E, Jin X, Cuo ZX, Pool JE, Xu X, Jiang H, Vinckenbosch N, Korneliussen TS, Zheng H, Liu T, He W, Li K, Luo R, Nie X, Wu H, Zhao M, Cao H, Zou J, Shan Y, Li S, Yang Q, Asan, Ni P, Tian G, Xu J, Liu X, Jiang T, Wu R, Zhou G, Tang M, Qin J, Wang T, Feng S, Li G, Huasang, Luosang J, Wang W, Chen F, Wang Y, Zheng X, Li Z, Bianba Z, Yang G, Wang X, Tang S, Gao G, Chen Y, Luo Z, Gusang L, Cao Z, Zhang Q, Ouyang W, Ren X, Liang H, Zheng H, Huang Y, Li J, Bolund L, Kristiansen K, Li Y, Zhang Y, Zhang X, Li R, Li S, Yang H, Nielsen R, Wang J, Wang J. Sequencing of 50 human exomes reveals adaptation to high altitude. Science 329: 75–78, 2010. doi: 10.1126/science.1190371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yoon CH, Hur J, Oh IY, Park KW, Kim TY, Shin JH, Kim JH, Lee CS, Chung JK, Park YB, Kim HS. Intercellular adhesion molecule-1 is upregulated in ischemic muscle, which mediates trafficking of endothelial progenitor cells. Arterioscler Thromb Vasc Biol 26: 1066–1072, 2006. doi: 10.1161/01.ATV.0000215001.92941.6c. [DOI] [PubMed] [Google Scholar]
- 84.Zhang N, Fu Z, Linke S, Chicher J, Gorman JJ, Visk D, Haddad GG, Poellinger L, Peet DJ, Powell F, Johnson RS. The asparaginyl hydroxylase factor inhibiting HIF-1alpha is an essential regulator of metabolism. Cell Metab 11: 364–378, 2010. doi: 10.1016/j.cmet.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang YB, Hu J, Zhang J, Zhou X, Li X, Gu C, Liu T, Xie Y, Liu J, Gu M, Wang P, Wu T, Qian J, Wang Y, Dong X, Yu J, Zhang Q. Genome-wide association study identifies multiple susceptibility loci for craniofacial microsomia. Nat Commun 7: 10605, 2016. doi: 10.1038/ncomms10605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Z, Yan J, Shi H. Role of hypoxia inducible factor 1 in hyperglycemia-exacerbated blood-brain barrier disruption in ischemic stroke. Neurobiol Dis 95: 82–92, 2016. doi: 10.1016/j.nbd.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao Q, Eichten A, Parveen A, Adler C, Huang Y, Wang W, Ding Y, Adler A, Nevins T, Ni M, Wei Y, Thurston G. Single-cell transcriptome analyses reveal endothelial cell heterogeneity in tumors and changes following anti-angiogenic treatment. Cancer Res 78: 2370–2382, 2018. doi: 10.1158/0008-5472.CAN-17-2728. [DOI] [PubMed] [Google Scholar]
- 88.Zhou J, Schmid T, Brüne B. Tumor necrosis factor-alpha causes accumulation of a ubiquitinated form of hypoxia inducible factor-1alpha through a nuclear factor-kappaB-dependent pathway. Mol Biol Cell 14: 2216–2225, 2003. doi: 10.1091/mbc.e02-09-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhuang Z, Yang C, Lorenzo F, Merino M, Fojo T, Kebebew E, Popovic V, Stratakis CA, Prchal JT, Pacak K. Somatic HIF2A gain-of-function mutations in paraganglioma with polycythemia. N Engl J Med 367: 922–930, 2012. doi: 10.1056/NEJMoa1205119. [DOI] [PMC free article] [PubMed] [Google Scholar]