Abstract
Breathing’s remarkable ability to adapt to changes in metabolic, environmental, and behavioral demands stems from a complex integration of its rhythm-generating network within the wider nervous system. Yet, this integration complicates identification of its specific rhythmogenic elements. Based on principles learned from smaller rhythmic networks of invertebrates, we define criteria that identify rhythmogenic elements of the mammalian breathing network and discuss how they interact to produce robust, dynamic breathing.
Introduction
Breathing is vital. Its pivotal role in regulating blood gases positions this rhythmic behavior at the core of physiology and pathophysiology. Much has been learned about the neural networks that generate the breathing rhythm, and several comprehensive reviews discuss our current understanding (1, 7, 22, 32, 51, 103, 104, 113, 119). Reviewing the mechanisms underlying respiratory rhythmogenesis is timely, since many new, and often unexpected, insights have been gained due to the advent of transgenic and optogenetic approaches. But it has been a long journey from the early discovery that the isolated brain stem is sufficient to generate a basic rhythm (2) to our current understanding of how the underlying networks are integrated to generate breathing in the intact organism. Along this journey, there have been many controversies, confusions, and discussions about where and how the respiratory rhythm originates (31, 32, 93, 106, 115, 119, 131). Indeed, several key questions continue to be debated and seem to remain unresolved despite a wealth of new insights. For example, we continue to debate whether the respiratory rhythm is based on synaptic inhibition, recurrent excitation, or pacemaker properties (1, 54, 75), a topic already discussed in 1960 (125). Defining the role of the pons in respiratory rhythmogenesis is another issue that has been discussed since the early 1900s (26, 28, 71, 72). The role of inhibitory mechanisms as an inspiratory off-switch was first hypothesized by Euler (155) and is still considered as a possible mechanism for rhythmogenesis (27, 33, 100, 168). Many of these questions remain unresolved because the criteria used for rhythm generation are vaguely defined or misunderstood. Thus the purpose of this review is to systematically address and define the key processes that govern the generation of breathing in the context of our current understanding.
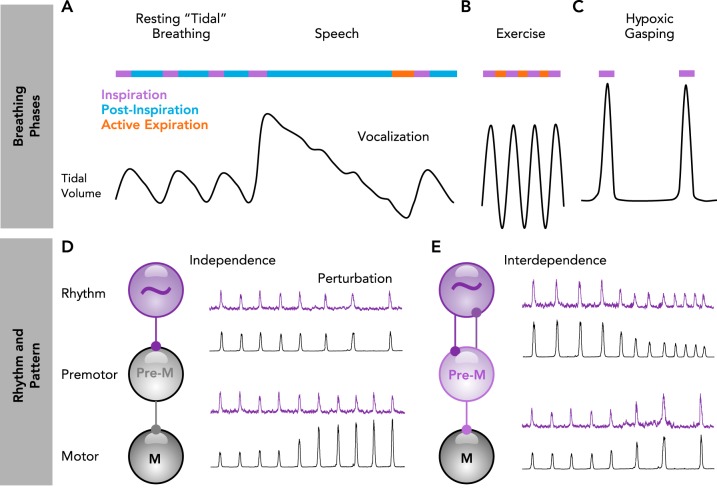
On the surface, breathing appears to be a relatively simple rhythmic behavior that, like locomotion, controls skeletal muscles. Although breathing can be voluntary, conscious awareness is not required for it to continuously adapt to changing metabolic, environmental, and behavioral demands. Breathing’s remarkable ability to adjust in a cycle-to-cycle and well-coordinated manner may be best exemplified for human speech or singing (63) (FIGURE 1A). During the inspiratory phase before vocalization, the amount of inhaled air is accurately adjusted to the length of the subsequent sentence. Inspiration must also be precisely timed between sentences to produce seemingly uninterrupted speech or singing. Vocalization occurs following inspiration during a breathing phase referred to as postinspiration (28, 137), when expiratory airflow is precisely regulated by the diaphragm, upper airway muscles, and vocal cords to produce sounds. Speech becomes more difficult under conditions of high metabolic demand, such as exercise (120), when breathing must become more rapid (79), and thoracic and abdominal muscles are recruited to forcefully expel air from the lungs, a breathing phase called active expiration (FIGURE 1B). The three breathing phases, inspiration, postinspiration, and active expiration, can be reconfigured and recombined to produce a breathing rhythm that is surprisingly dynamic and adaptable (103). For example, during severe hypoxia, breathing switches to gasping, a one-phase inspiratory rhythm (156) (FIGURE 1C).
FIGURE 1.
Breathing phases arise from a combination of rhythm- and pattern-generating mechanisms
A–C: schematic of breathing phase coordination and reconfiguration during rest, speech, exercise, and severe hypoxia. A: during rest, breathing alternates between inspiration (purple) and postinspiration (blue). Prior to speaking, the depth of inspiration (tidal volume) is adjusted to the anticipated length of the vocalization. Vocalization occurs during an extended postinspiratory phase. B: during heavy exercise, breathing generally alternates rapidly between inspiration and active expiration (red) to match increased metabolic demands. C: during severe hypoxia, breathing reconfigures to produce a slow one-phase inspiratory rhythm. D–E: processes of rhythm and pattern generation can be independent or interdependent. D: when independent, perturbations of rhythm (purple) do not alter pattern (black), and perturbations of pattern do not alter rhythm. E: when interdependent, perturbations of rhythm also alter pattern, and perturbations of pattern also alter rhythm.
Breathing’s complex control is often differentiated into two principle processes: rhythm vs. pattern generation (FIGURE 1, D AND E). In general, rhythm-generating mechanisms control breathing frequency and are involved in reconfiguring breathing into a one-, two-, or three-phase rhythm (103). In contrast, regulation of tidal volume and the coordinated and differential activation of the many upper airway and respiratory pump muscles is considered “pattern generation” (33). The notion of distinct rhythm- and pattern-generating mechanisms is supported by a variety of neuronal and modulatory properties that differentiate rhythm vs. pattern (34, 141) (FIGURE 1D). These two principle processes are differentially controlled by rhythmogenic microcircuits, premotor, and motoneuron pools (117). However, rhythm and pattern generation are not always separate and distinct. Processes of rhythm and pattern generation can be interdependent such that manipulations that influence the breathing rhythm also alter its pattern or vice versa (9) (FIGURE 1E). Indeed, neuronal networks that generate the rhythm within the CNS are generally referred to as “central pattern generators,” which is consistent with the notion that processes of rhythm and pattern generation are intermingled. This review will focus primarily on rhythm generation, since it aims to define the criteria required to be considered “rhythmogenic.” As we discuss the neuronal origins of mammalian breathing, we consider fundamental principles learned in smaller, well-characterized rhythm-generating networks of invertebrates.
Defining a Rhythm-Generating Network, and the Concept of a Central Pattern Generator (CPG)
Graham Brown first proposed the concept that rhythmic behaviors arise from specialized networks in the CNS, referred to as central pattern generators (CPGs) (37, 135, 161). A CPG must fulfill one criterion: the putative CPG needs to generate a behaviorally relevant rhythmic activity even when physically isolated from all other central and peripheral inputs. However, after Graham Brown’s proposal, it took several years before convincing evidence for a CPG was demonstrated. Adrian and Buytendijk discovered that the isolated, completely deafferented brain stem of the goldfish continued to generate a respiratory-related rhythm (2); but these experiments did not specifically identify the region/network within the brain stem responsible for rhythmogenesis. It took another 60 years before it was discovered that isolated slices from the medulla of neonatal rodents contained a small network, the so-called pre-Bötzinger complex (preBötC), that was sufficient to generate a breathing-related rhythm (134).
Although the persistence of rhythmic activity in isolation may seem like a straight-forward criterion for a CPG, demonstrating its behavioral relevance can be more difficult. One complication is that the rhythmic output of an isolated CPG may depend on the specific experimental context. In the preBötC, not only one but three distinct types of rhythms can be generated. The rhythm that underlies the inspiratory component of normal eupneic activity and sigh activity are concurrently generated under control conditions, whereas gasping activity is generated during hypoxia (66). These in vitro rhythms are often cautiously referred to as “fictive” activities since there is no actual drive to the muscles, and they may only be a representation of the actual behavior in vivo. Furthermore, in the intact system, a CPG will be under the influence of many neuromodulatory and synaptic inputs that may significantly alter the characteristics of its rhythmic output. Thus demonstrating the behavioral relevance of a putative CPG often requires interrogating the functional consequences of manipulating it in the context of the whole animal. Indeed, the discovery of the preBötC led to numerous follow-up studies and 25 years of intense research aimed at better defining this “in vitro” rhythm and its role in vivo (39, 78, 112, 140, 160). As a result, it is now generally accepted that the preBötC is a microcircuit that is central to the generation of mammalian breathing.
Defining the Neuronal Elements of a Rhythm-Generating Network
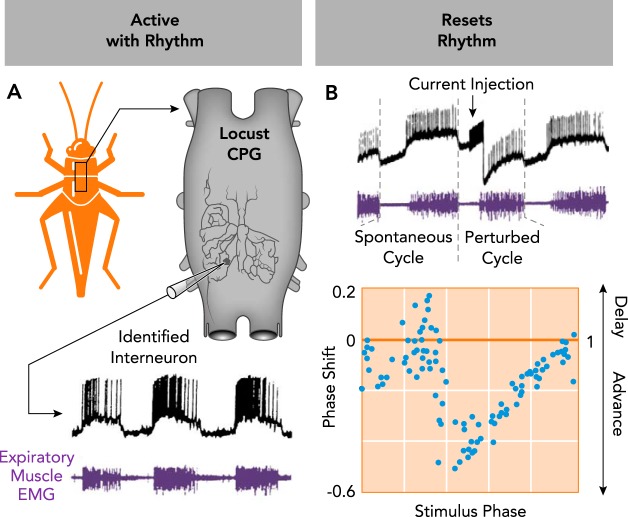
Today, there is overwhelming evidence that CPGs exist for most, if not all, rhythmic motor behaviors in invertebrates and vertebrates. Yet, identifying the CPG for a rhythmic behavior is only a first step toward understanding how a neural rhythm is generated. Next is to identify the specific element(s) within the CPG that make it rhythmic. As first established in invertebrate model systems, two criteria must be met to define a neuron as an important element of a rhythm-generating network: 1) a neuron or group of neurons must be active in phase with the rhythm and 2) the neuron(s) need to reset the rhythm in a characteristic phase-dependent manner in response to a brief stimulus. Neurons that fulfill these two characteristics typically also modulate the frequency of the rhythm during a sustained stimulus and entrain the rhythm when stimulated repetitively. These criteria are illustrated for an anatomically identified neuron in the respiratory network of the locust, which is rhythmically active in phase with expiration (FIGURE 2A, criterion 1) and resets the rhythm when stimulated with a current pulse (FIGURE 2B, criterion 2). The resetting characteristics of these stimulations are visualized in a phase-shift curve that reveals a neuron’s ability to advance or delay the rhythm depending on the stimulus phase, providing critical insights into a neuron’s role in rhythmogenesis (102, 108).
FIGURE 2.
Characteristics of an identified rhythmogenic neuron in the respiratory central pattern generator of the locust
A: the neuron (subesophogeal ganglion interneuron 378) is active in phase with the rhythm (criterion 1), as indicated by intracellular recording of spiking activity (black) concurrent with expiratory muscle EMG activity (purple). B: stimulation of the neuron resets the respiratory rhythm (criterion 2). Direct current stimulation of subesophogeal ganglion interneuron 378 shortens the perturbed respiratory cycle relative to a spontaneous cycle. Resetting characteristics for the neuron are visualized in a phase-shift plot, demonstrating stimulus phase-dependent resetting of the rhythm. Data pooled from experiments in three different animals. A and B are adapted from Ref. 102 with permission from the Journal of Neurophysiology.
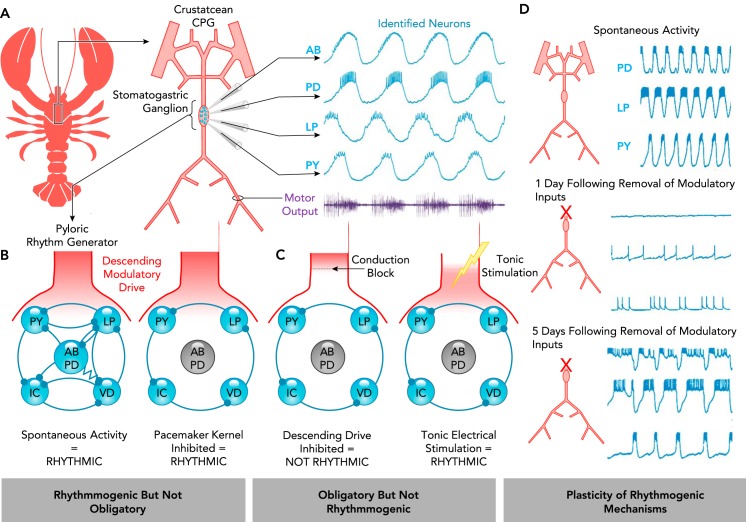
Whether a given neuron, neuron group, or microcircuit is obligatory for rhythmicity is often also used as a criterion to identify rhythmogenic mechanisms. However, this can be a source of considerable confusion. Common pitfalls associated with this strategy are illustrated using a small invertebrate network, in which all neuronal elements of the CPG are well defined (FIGURE 3A) (76). The network diagram shown in FIGURE 3B illustrates the connectivity of the network that generates the pyloric rhythm within the stomatogastric ganglion (STG) of crustacean. Within this rhythmogenic network, two types of electrically coupled neurons, the AB and PD neurons, form the pacemaker kernel of this CPG (30, 44, 48). These neurons possess intrinsic bursting properties (83, 123) and are rhythmically active in phase with the pyloric rhythm, and stimulating these neurons resets the rhythm (48). Yet, when lesioning AB, PD, or other core neurons within this network, additional rhythmogenic mechanisms allow the pyloric rhythm to continue (FIGURE 3B) (48, 129). Thus these neurons are not “obligatory” for rhythmogenesis, even though they are interconnected with all other rhythmogenic neurons and are important elements of the normal operation of this rhythm-generating network. These experiments have important general implications: although lesioning a neuron or neuron group can provide insight into its functional role (29, 30, 129), the persistence of a rhythm, i.e., the finding that a neuron or neuron group is not obligatory for rhythmicity, does not negate its important role in rhythmogenesis.
FIGURE 3.
The rhythmogenic role of an element may not be determined solely based on whether it is obligatory
A: schematic of the crustacean stomatogastric nervous system and intracellular recordings of identified stomatogastric ganglion neurons of the pyloric rhythm generator (AB, anterior burster; PD, pyloric dilator; LP, lateral pyloric; PY, pyloric). The electrically coupled AB and PD neurons have autonomous bursting properties and comprise the “pacemaker kernel.” These neurons are active in phase with rhythmic motor output (purple) and can also reset the rhythm. Adapted from Ref. 77, with permission from the Annual Review of Physiology. B–C: schematic of the pyloric rhythm-generating circuit. All synapses are inhibitory; resistor indicates electrical connections. B: in the presence of descending neuromodulatory drive, silencing the AB/PD pacemaker kernel does not abolish rhythmogenesis. C: subsequent removal of neuromodulatory drive eliminates the rhythm; however, it can be restored with artificial tonic stimulation (129). D: the extent to which a given mechanism is obligatory for rhythmogenesis may be altered by plasticity. Spontaneous rhythmicity of the pyloric rhythm generator is initially lost following removal of neuromodulatory inputs. However, rhythmicity returns as the rhythm-generating properties of the network adapt. Adapted from Ref. 143, with permission from the Journal of Neurophysiology.
Conversely, there are many reasons why a neuron or neuron group may be obligatory for rhythmicity, but not rhythmogenic. For example, removing descending drive to the STG stops rhythmic activity produced by the pyloric network (FIGURE 3C). However, rhythmicity can be reestablished by tonic, non-rhythmic electrical stimulation of the descending input. Therefore, it is a tonic neuromodulatory influence rather than a rhythmic drive that makes this descending input obligatory (129). Indeed, the synaptic and intrinsic properties of the pyloric neurons are tuned such that their rhythmogenic properties are dependent on neuromodulation (143). Although rhythmicity ceases upon acute removal of neuromodulatory input, after several days, rhythmicity returns spontaneously in a neuromodulator-independent mode as mechanisms of homeostatic plasticity reestablish a balance of ionic and synaptic conductances (65, 73, 143) (FIGURE 3D). Indeed, it seems that rhythmogenic networks are more plastic and adaptable if their membrane properties are tuned to be neuromodulator-dependent. These considerations illustrate why being “obligatory” or “not obligatory” for rhythmogenesis cannot be unambiguously used to either identify or negate a neuron’s role as a rhythmogenic element. Instead, for a neuron or neuron group to be considered rhythmogenic, it must be active in phase with the network rhythm and be able to reset the rhythm when stimulated.
Defining Rhythmogenic Elements in the Mammalian Respiratory Network
Similar to the crustacean model network discussed above, there are numerous neuromodulatory inputs to the rhythmogenic networks underlying mammalian breathing (25, 77). Based on the principles learned in invertebrates, removal of modulatory inputs should be expected to produce drastic changes in the breathing rhythm. Indeed, early lesion experiments that sectioned the brain stem at the mid-pontine level together with the transection of the vagus nerves caused “apneusis,” prolonged inspiratory activity (71, 72, 98). These findings led to the concept of a “pneumotactic center” within the pons and contributed to the idea that the pons has an important role as an “inspiratory off-switch mechanism.” However, such lesion experiments can only provide limited insights into the rhythmogenic role of the pons. Many early investigators were fully aware of these limitations. In von Euler’s publication (155) on the potential existence of a pontine “off-switch” mechanism, he carefully stated, “it must be emphasized, however, that so far there is only correlational evidence for the proposed functions of any of these neurons.” Yet, these concepts remain influential to this day, despite convincing studies demonstrating that the medulla can generate rhythmic breathing in the absence of the pons and sensory feedback (14, 46, 125). Although there is no doubt that the pons, including the Kölliker-Fuse region, plays an important role in the integration of sensory inputs and the modulation of the respiratory rhythm (26), it has yet to be convincingly demonstrated that the pons is a respiratory CPG, i.e., can be rhythmogenic when isolated. Moreover, evidence that selectively stimulating identified rhythmically active pontine neurons can reset the respiratory rhythm is, to the best of our knowledge, still missing. Thus we conclude that the pons provides important neuromodulatory inputs to the rhythm-generating neurons of the medulla, but whether the pons, or a region within the pons, plays a role in generating the breathing rhythm remains to be determined.
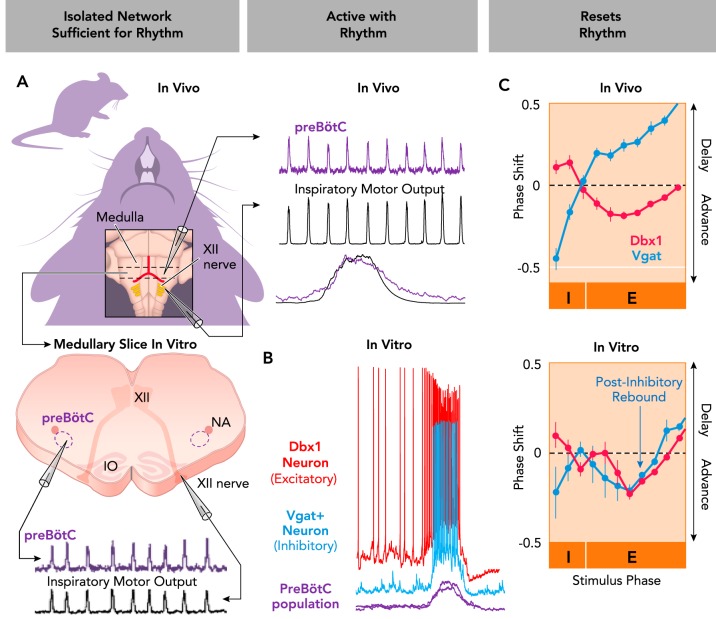
The mammalian breathing CPG, as identified within the medulla (FIGURE 4A), lacks the anatomical specificity of smaller invertebrate rhythm-generating networks that allow repeated identification of individual neurons with consistent functional roles (see FIGURES 2 AND 3). However, the criteria used to define an element of a rhythm-generating network may still be applied to specific subtypes of neurons in the mammalian medulla. Two landmark studies identified a specific class of excitatory, glutamatergic neurons within the preBötC (13, 38). These neurons are derived from precursors that express the transcription factor Dbx1. Many Dbx1-lineage neurons are rhythmically active in phase with inspiration (3, 45, 97) (FIGURE 4B), and optogenetic stimulation of these neurons resets the rhythm produced by the preBötC in isolated medullary slices in vitro, as well as the breathing rhythm produced by intact mice in vivo (9, 19, 149, 150) (FIGURE 4C). Thus these neurons fulfill the criteria indicative of elements of a rhythm-generating network. In addition to these principle criteria for rhythmogenesis, Dbx1 neurons also seem to be obligatory, because silencing these neurons in medullary slices (157, 158) or in adult mice (150) stops or significantly disrupts the breathing rhythm. Thus there is little doubt that Dbx1 preBötC neurons are core elements of the respiratory rhythm-generating network. It must be emphasized, however, that Dbx1 neurons are not a functionally homogenous population. Some Dbx1 neurons may play a more significant role in pattern vs. rhythm generation (or vice versa) (19, 117, 158), and some may have other non-respiratory functions such as modulation of arousal (163). Further identification and characterization of the specific subset of Dbx1 neurons that contribute to rhythmogenesis should be an important avenue of continued research.
FIGURE 4.
Rhythmogenic roles of excitatory and inhibitory neurons in the mammalian breathing CPG
A: the pre-Bötzinger Complex (preBötC) continues to produce a rhythm (purple) that is in phase with inspiratory hypoglossal (XII) motor output (black) when isolated in medullary slices from neonatal mice. The preBötC is also active in phase with inspiratory motor activity in intact adult mice. B: excitatory (red) and inhibitory (blue) preBötC neurons are concurrently active in phase with rhythmic inspiratory activity (purple). Intracellular current-clamp recordings of genetically identified Dbx1+ (excitatory) and Vgat+ (inhibitory) neurons active with integrated extracellular preBötC population bursts generated in vitro. C: optogenetic stimulation of excitatory (Dbx1+) or inhibitory (Vgat+) preBötC neurons resets the breathing rhythm in vivo and in vitro. Stimulations can advance or delay the next inspiratory cycle depending on stimulus phase (I, inspiratory phase; E, expiratory phase). Note that postinhibitory rebound advances the next cycle during relatively slow rhythms in vitro but not in vivo. Figure adapted from Ref. 9, with permission from Nature Communications.
The preBötC also contains many types of neurons that are not derived from Dbx1 precursors. Among preBötC neurons that are functionally active in phase with inspiration, an estimated 37% are inhibitory (9). These neurons, characterized by the vesicular GABA transporter (Vgat), are activated concurrently with Dbx1 neurons (FIGURE 4B), and optogenetic stimulations of these neurons elicit a stimulus phase-dependent reset of the breathing rhythm both in vitro and in vivo (FIGURE 4C) (9, 130). Thus inhibitory preBötC neurons, like Dbx1 neurons, fulfill the principle criteria that define a neuron as being an element of a rhythm-generating network.
Excitatory and inhibitory neurons are both important for controlling rhythmicity in the preBötC, and their interactions have been explored in modeling studies (43). However, these neuronal populations have different functional roles with distinct resetting characteristics (FIGURE 4C). Interestingly, during the inspiratory phase, concurrent with preBötC population bursts, stimulation of excitatory Dbx1 neurons delays the onset of the subsequent breath. In contrast, the equivalent stimulation of inhibitory Vgat neurons advances the next breath (9). During the expiratory phase, between preBötC population bursts, stimulation of Dbx1 neurons can advance the subsequent breath. However, as will be discussed in detail below, this effect is limited by refractory properties of Dbx1 neurons. Conversely, stimulation of inhibitory neurons during the expiratory phase has distinct effects depending on the experimental preparation: in vivo, the next breath is delayed (9, 130), and during the much slower frequencies generated in vitro, post-inhibitory rebound advances the next breath (9, 17). These experiments serve as an important general reminder that, unless specific neurons are selectively stimulated during their active phase, it is difficult to determine whether and how they contribute to rhythmogenesis. Furthermore, since it has been repeatedly demonstrated that blockade of synaptic inhibition may alter, but does not stop, the preBötC rhythm (54, 166), these experiments further illustrate how a given element may play an important role in rhythmogenesis without being obligatory.
The Relationship Between Synchronization and the Duration of a Rhythmic Cycle
Mechanisms of Refractoriness
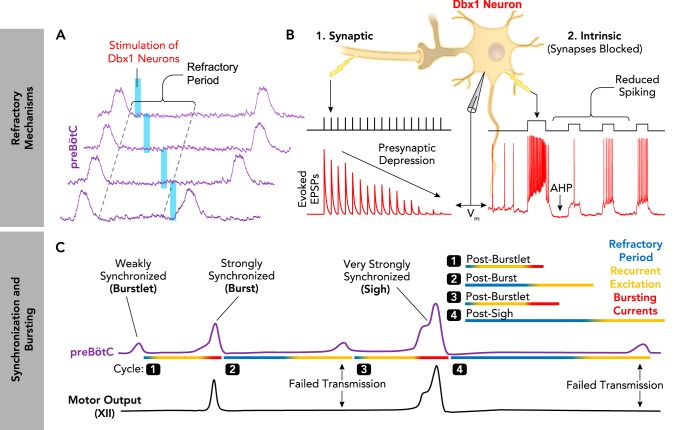
Like other rhythmic systems (64, 74, 126, 138, 162, 167), the preBötC has an inherent refractoriness (4). During rhythmogenesis, recurrently connected excitatory Dbx1 neurons (and non-Dbx1 neurons) periodically synchronize and desynchronize within the preBötC. Following a synchronized population “burst,” during the early expiratory phase, stimulation of Dbx1 neurons typically fails to evoke another burst, whereas stimulation during late expiration almost always evokes a burst (FIGURE 5A) (9, 62). This refractory period following synchronized preBötC activity is determined by at least two mechanisms (FIGURE 5B). 1) High firing rates during each inspiratory burst deplete presynaptic vesicles, limiting synaptic transmission between excitatory neurons until the vesicle pool is restored (40). This has been observed in vitro as a progressive reduction in evoked EPSP amplitude recoded in Dbx1 neurons during repeated electrical stimulation of excitatory presynaptic inputs (62). 2) The depolarizing “drive potential” during each inspiratory burst activates intrinsic membrane properties that cause a transient hyperpolarization and reduced excitability of excitatory neurons until resting membrane potential is restored. In Dbx1 neurons following blockade of synaptic transmission, a depolarizing current step is followed by afterhyperpolarization and reduced spiking evoked during smaller current pulses (9). Identifying the specific ionic conductance(s) underlying this intrinsic membrane property should be an important next step in unravelling the mechanisms of refractoriness in the preBötC.
FIGURE 5.
The relationship between synchronization, bursting, and refractory mechanisms in the preBötC
A: the preBötC has an inherent refractoriness. The probability of evoking preBötC population bursts by optogenetic stimulation of Dbx1 neurons is transiently reduced following spontaneous preBötC bursts, i.e., during the refractory period (9) B: synaptic and intrinsic properties of excitatory Dbx1+ neurons contribute to the refractory period (1). Schematic demonstrating presynaptic depression of excitatory synaptic transmission between preBötC neurons. The amplitude of evoked excitatory postsynaptic potentials (EPSPs; red) recorded in Dbx1 neurons is progressively reduced during repeated electrical stimulations (black) of presynaptic inputs (62). 2: intrinsic properties transiently reduce the excitability of Dbx1 neurons following preBötC bursts. Intracellular current-clamp recording of a synaptically isolated Dbx1 neuron demonstrating membrane afterhyperpolarization (AHP) and transiently reduced spiking following an initial, larger depolarizing burst (9). C: schematic representing the different amounts of synchronization during distinct patterns of preBötC activity (purple) and associated hypoglossal (XII) motor output (black). During burstlets, synchronization is weak, bursting currents are only activated in some neurons, and the refractory period is minimal. During normal “eupneic” bursts, synchronization is strong and bursting currents are activated in many neurons, resulting in a period of relative refractoriness. During periodic sigh bursts, synchronization is very strong and bursting currents are strongly activated, leading to large depolarizing drive potentials in preBötC neurons and an exaggerated refractory period. Note that strong synchronization facilitated by activation of bursting currents promotes successful transmission of preBötC activity to XII motor output.
The extent to which refractory mechanisms influence rhythmogenesis is determined by the amount of synchronized activity during each preBötC population burst. This is illustrated for different patterns of preBötC activity in FIGURE 5C. During normal “eupneic” bursts (106, 147), recurrent synaptic excitation and intrinsic bursting currents (discussed in detail below) strongly synchronize preBötC neurons, leading to a refractory period. As neurons recover from the refractory period, recurrent excitation can begin to initiate another cycle. During much weaker synchronizations, referred to as “burstlets” (56), low spiking rates and small drive potentials in excitatory neurons are expected to elicit a relatively short refractory period. The opposite is expected during very strongly synchronized bi-phasic sigh bursts generated by the preBötC (147), when long refractory periods likely result in a “post-sigh apnea” (9). These considerations illustrate how rhythm generation (i.e., cycle period) and pattern generation (i.e., burst amplitude) can be interdependent processes (see FIGURE 1E).
Intrinsic Bursting
An important mechanism that promotes synchronization is the activity of intrinsic bursting properties, sometimes also referred to as “pacemaker properties.” First described in small invertebrate neuronal networks (see, e.g., Refs. 5, 122), intrinsic bursting is a fundamental feature of most, if not all, neuronal networks (8, 21, 69, 70, 114, 142, 146, 148). Although, these intrinsic bursting properties allow neurons to generate bursts of action potentials in the absence of synaptic input, this is rarely the case in a functional network where neurons are bombarded by excitatory and inhibitory synaptic inputs. In this context, bursting neurons play a critical role as nonlinear amplifiers of synaptic drive (107). In the preBötC, it is thought that intrinsic bursting properties mediate the transition of weakly synchronized burstlets into normal inspiratory bursts (111) (FIGURE 5C). The transition to the burst also depends on the interplay between concurrent excitation and inhibition, which will vary from cycle to cycle for each individual neuron. If inhibition is predominant or excitability is low, the bursting threshold may not be reached in many neurons, which can lead to a burstlet at the population level (56, 57, 110, 111). We refer to this balance between excitation, inhibition, and intrinsic bursting as the rhythmogenic triangle (FIGURE 6): the onset and synchronization of a rhythmic cycle, as well as the resulting refractory period, will depend on the close interdependence between these three principle rhythmogenic mechanisms (103).
FIGURE 6.
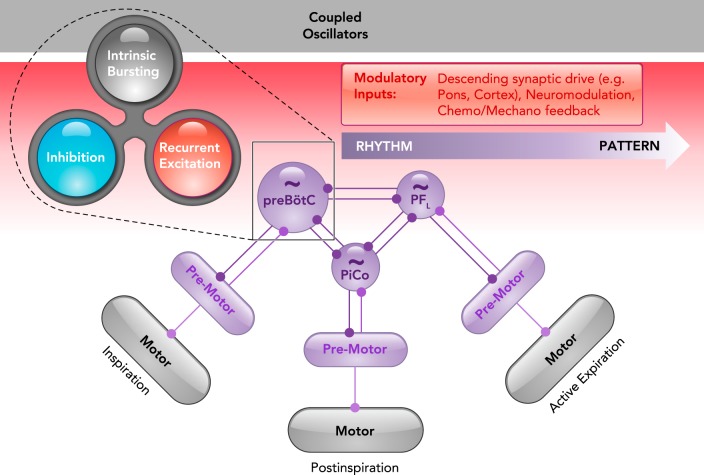
A contemporary view on the origins of mammalian breathing
The triple oscillator hypothesis (6) proposes that each breathing phase is generated by a distinct microcircuit in the medulla: the preBötzinger complex (preBötC) generates inspiration, the postinspiratory complex (PiCo) generates postinspiration, and the lateral parafacial region (pFL) generates active expiration. Each oscillator is coupled by excitatory and inhibitory connections, although inhibition typically dominates to coordinate the timing of each breathing phase. As shown in the preBötC, rhythmicity within each microcircuit is controlled by a balance between recurrent synaptic excitation, inhibition, and intrinsic bursting properties, i.e., the rhythmogenic triangle (103). A gradient of rhythm (purple) and pattern (gray) generating properties can be independent or interdependent depending on the connectivity between specific rhythm, premotor, and motor elements. The activity of rhythm- and pattern-generating elements can be differentially tuned by various modulatory inputs (red) to endow breathing with exquisite metabolic-, state-, and behavior-dependent control.
Intrinsic bursting can be mediated by a variety of voltage- or calcium-dependent mechanisms. In the preBötC, the persistent sodium current (INaP) is an important voltage-dependent bursting mechanism, whereas the calcium-activated nonselective cation current (ICAN) is largely voltage-independent, but dependent on the intracellular calcium concentration (10, 18, 45, 94). Neuromodulators (e.g., norepinephrine, serotonin, and substance P) can differentially modulate INaP- and ICAN-dependent bursting properties, allowing the network to amplify synaptic inputs in a voltage- and calcium-dependent manner (94–96, 151–153). Whether these bursting properties can be homeostatically adjusted, similar to bursting in the pyloric rhythm generator of the crustacean (see FIGURE 3C), remains unknown. Nevertheless, the ability to differentially modulate bursting characteristics among subsets of preBötC neurons is likely one mechanism that contributes to the impressive flexibility of the breathing rhythm.
Integrated Local Inhibition
Synaptic inhibition plays a critical role in regulating synchronization (43). Bursting properties of preBötC neurons are suppressed by inhibition (148), and in the absence of synaptic inhibition, Dbx1 neurons become hyperactive and synchronize more efficiently. However, this results in long refractory periods and, in vivo, such slow breathing frequencies are not physiologically sustainable. Conversely, increasing the activity of inhibitory neurons during inspiration weakens synchronization, which dramatically accelerates breathing (9). Indeed, modulation of refractory mechanisms can explain the phase-dependent resetting characteristics of inhibitory neurons (see FIGURE 4C). Activation during inspiration weakens synchronization of the inspiratory network and reduces the refractory period, whereas activation of inhibitory neurons during expiration slows breathing, presumably by disrupting the propagation of excitatory activity through the network. Thus, by regulating the synchronization of excitatory neurons, the activation of bursting properties, and the subsequent refractory period, inhibitory neurons exert a powerful influence on breathing frequency. However, there must be a precise balance of excitation and inhibition in the preBötC, as indicated by computational models (43): too much inhibition integrated within the network desynchronizes and irregularizes the rhythm until it eventually falls apart. Future modeling studies should be useful to better understand how the balance between excitation and inhibition controls refractory mechanisms, cycle duration, and the stability of the preBötC rhythm.
Network Connectivity
As mentioned previously, the preBötC lacks the same level of anatomical specificity available in smaller invertebrate rhythm-generating networks (see FIGURES 2 AND 3). As a result, we are far from having established a detailed connectome of the rhythmogenic network that gives rise to mammalian breathing. Moreover, it is unknown to what extent network connectivity is unique among individuals and whether connectivity may be altered throughout life by synaptic plasticity. However, a functionally important property of the preBötC seems to be sparsity. Combined modeling and experimental studies suggest that sparse connectivity contributes to the high degree of stochasticity and onset variability that is observed in the activity of individual preBötC neurons in medullary slices (15, 16), but also among neurons recorded in vivo (68, 87, 127). These computational models predict that networks with sparse connectivity can have weakly synchronized cycles (i.e., burstlets), and that close to 300 excitatory neurons need to synchronize to maintain network activity with regular burst frequency and amplitude (15, 16). These modeling studies also predict that connectivity within the preBötC is tuned near the limit of network stability, which may endow the network with a great deal of flexibility, but in some cases may make it vulnerable to pathology. For example, the preBötC rhythm becomes irregular, and weak synchronizations become more common in neonatal mice chronically exposed to intermittent episodes of hypoxia (35, 36). These weak burstlets generated in the preBötC fail to fully activate hypoglossal (XII) motoneurons, leading to breathing cycles without XII motor output (110) (FIGURE 5C). This finding has important clinical implications in the context of obstructive sleep apnea, where patients experience chronic intermittent hypoxia and have reduced inspiratory drive to muscles of the upper airway (innervated by the XII nerve), making it prone to collapse (105).
Integrated Rhythmogenesis
Overall, existing data suggest that respiratory rhythmogenesis is not the deterministic result of a single homogenous mechanism. However, history has shown that semantics can cause widespread confusion, and invertebrate networks can once again serve as an important reminder. In the STG, a “pacemaker kernel,” consisting of the AB-PD bursting neurons, is important for rhythmogenesis. This may imply that bursting properties of these two cells are the primary determinants of rhythmogenesis. Yet, many characteristics of bursting neurons and their temporal activation depend on the dynamic integration of multiple neuromodulatory and synaptic mechanisms as well as the interaction with other rhythmogenic neurons of the STG network (see FIGURE 3A). Thus the desire to identify and attribute rhythmogenesis to one essential rhythmogenic mechanism may not reflect how a network actually operates. A popular hypothesis for respiratory rhythmogenesis, the so called “group pacemaker hypothesis,” posits that periodic inspiratory bursts originate from intrinsic currents that are ordinarily latent until synaptically evoked in the context of network function (116). This hypothesis describes the integration between intrinsic and synaptic mechanisms, but by emphasizing the synaptic mechanisms that are necessary to activate the “ordinarily latent, intrinsic” properties, it infers that excitatory synaptic transmission is the primary rhythmogenic mechanism (23, 24, 121). However, we suggest that assigning a primary rhythmogenic mechanism can be misleading. Instead, the emphasis should be on the dynamic integration between synaptic excitation, inhibition, and intrinsic bursting, which assembles the respiratory rhythm in a cycle-to-cycle manner. Any given cycle may occur with or without bursting properties (103) and also with or without synaptic inhibition (e.g., in gasping). But it is the dynamic interplay between heterogeneous mechanisms that imbues rhythmogenesis with the ability to support breathing during the many physiological and pathophysiological conditions that may occur throughout life.
Coupled Oscillators and the Multiphase Breathing Rhythm
Thus far we have focused on mechanisms that govern the inspiratory rhythm generated by the preBötC. However, despite being a core rhythmogenic microcircuit, the preBötC is just one component in a wider network; and mammalian breathing does not only consist of inspiration but also postinspiration and active expiration (118, 119). Coordination of the three breathing phases requires a different role for synaptic inhibition. Instead of controlling synchronization as discussed above, synaptic inhibition needs to control the temporal sequence of each breathing phase (119). However, consistent with the compartmental model (124, 131–133), computational network models predict that inhibition cannot generate multiple phases within the preBötC alone (43). Specifically, to generate neurons that are inhibited during inspiration and active during expiration, the fraction of inhibitory neurons within the network needs to be increased. Yet, increasing inhibition desynchronizes network activity, and only a small number of neurons (maximally ~15%) can be forced to fire during the expiratory phase before rhythmicity falls apart (43). Indeed, experimental data indicate that only a small fraction of preBötC neurons are active out of phase with inspiration (15, 16, 131). Thus the computational model makes an important prediction: to produce a multiphase rhythm, multiple independently rhythmic networks must be coupled by synaptic inhibition. Such networks have been identified in the medulla as the postinspiratory complex (PiCo), active during postinspiration (6, 7), and the lateral parafacial nucleus (pFL), a subdivision of the retrotrapezoid nucleus/parafacial respiratory group (RTN/pFRG) region that is recruited during active expiration (49, 50).
The PiCo, located rostral to the preBötC, continues to produce a rhythm when isolated in a medullary slice, contains neurons that are rhythmically active in phase with postinspiration, and stimulation of these neurons resets the rhythm in vitro and in vivo (6). Thus the PiCo fulfills all criteria that define a rhythmogenic network or CPG. PiCo neurons (or at least a subset of them) are characterized by a glutamatergic, cholinergic transmitter phenotype. Cholinergic signaling seems to have primarily modulatory functions, whereas, like the preBötC, glutamatergic transmission is required for synchronization of the network. GABAergic inhibition establishes the phase relationship between the PiCo and the preBötC; however, to what extent inhibition also regulates synchronization and refractory mechanisms within the PiCo is still unknown. Further characterization of the rhythmogenic elements within PiCo and their role in the generation of postinspiration and potentially other behaviors, such as vocalization, will be critical to gain further insights into the functional implications of this medullary microcircuit.
The RTN/pFRG is located rostral to the preBötC and ventral to the PiCo (49, 50, 52). This region has been subdivided into rhythmic lateral (pFL) and non-rhythmic ventral (pFV) areas (49). However, the degree to which these areas are distinct is a matter of debate. The pFL is typically silent but becomes rhythmic when disinhibited (92). In adult rodents, rhythmicity in the pFL depends on (presumably tonic) excitation from the preBötC (50). However, in younger rodents, the pFRG region can generate a rhythm without rhythmic preBötC activity (52, 139), even when physically isolated from the preBötC (91). In vivo, neurons in the pFL fire during active expiration, and stimulation of the pFL evokes expiratory activity and resets the breathing rhythm (92), presumably through inhibitory interactions with the preBötC (49). Neurons that give rise to rhythmicity within the pFL may (90) or may not (92) include neurons that express Phox2b. However, like Dbx1 neurons in the preBotC, neurons within the pFL and parafacial region in general are heterogeneous, and unraveling exactly which neuronal subtypes are responsible for rhythmogenesis, chemosensation, and blood pressure regulation is an important research avenue (41, 42, 90, 91).
As illustrated in the invertebrate STG network (102), CPGs are often tuned to neuromodulation to allow precise control of rhythmic behavior. In this context, it is interesting that the three coupled oscillators for breathing are differentially tuned with regard to their sensitivity to neuromodulators. For example, the PiCo is strongly stimulated by norepinephrine and inhibited by somatostatin and the µ-opioid agonist DAMGO (6). In contrast, the pFL is strongly activated by acetylcholine (12) but is insensitive to opioids (52, 80). The enhanced sensitivity of the PiCo and pFL to neuromodulation may contribute to their higher threshold for rhythmicity, which is likely an important property allowing these networks to be rhythmic only during the appropriate metabolic, environmental, or behavioral context. The preBötC is also regulated by a host of neuromodulators (25). However, the preBötC has a relatively low threshold for rhythmicity and less dependence on neuromodulatory input, reflecting its role as the “master clock” for breathing-related behaviors (86) and the paramount importance of the inspiratory phase in mammalian breathing.
Conclusions and Open Questions
A contemporary view on the origins of mammalian breathing is conceptualized in FIGURE 6. The organization of the breathing network is consistent with the idea that rhythmic activity emerges from the interactions between three coupled oscillators, in which each phase, inspiration, postinspiration, and active expiration, is generated within the medulla by its own dedicated microcircuit, referred to as the “triple oscillator hypothesis” (7). The dynamic balance between recurrent synaptic excitation, inhibition, and intrinsic bursting properties, i.e., the “rhythmogenic triangle” (103), controls synchronization within each microcircuit, although this concept has yet to be tested in the PiCo and pFL. Mutually inhibitory interactions between the microcircuits seem to control the temporal sequence of the rhythm generated by the network. However, interactions between the PiCo and pFL have not been described. Rhythmogenic microcircuits project to premotor and motor pools, and the connectivity of a given element will determine its role in rhythm generation, pattern generation, or both. The complex integration of the network with the rest of the central and peripheral nervous system allows exquisite modulation of all rhythm- and pattern-generating elements. This complex organization may have evolved in mammals to match their increased metabolic and behavioral demands on breathing (104).
An important next step in understanding the breathing rhythm will be to examine in further detail how the three rhythmogenic microcircuits interact. Indeed, the triple oscillator hypothesis and the concept of the rhythmogenic triangle raise many unresolved questions: How is the so-called Bötzinger complex (124, 131, 132) integrated with the three rhythmogenic microcircuits? Are there separate sets of inhibitory neurons dedicated to controlling synchronization within the microcircuits and temporal control between them? How dynamic are these interactions? Do burstlets, bursts, and sighs generated by the preBötC differentially activate or inhibit the other rhythmogenic microcircuits? A sigh associated with arousal may be differentially connected to the expiratory networks than a sigh of relief. Do hypoxic and hypercapnic conditions differentially alter and reconfigure these microcircuits? How do the roles of rhythmogenic elements within and the interactions between each microcircuit change during the variety of non-ventilatory behaviors, such as coughing and swallowing, that are known to involve reconfiguration of the network (11, 67, 99, 128)? As discussed here for the preBötC, it will be important to unravel to what extent the neurons active during non-ventilatory behaviors can be identified as belonging to multiple CPGs. Specifically, do neurons that reset breathing also reset other rhythmic behaviors like swallowing and coughing? The discovery of more functionally specific molecular and genetic markers should allow exploration of these possibilities. Along the way, principles learned from invertebrate networks can continue to provide guidance in unraveling the reconfiguration and control of different, yet partially overlapping, rhythmic behaviors (102, 108, 109, 159).
Many additional open questions relate to the supramedullary control of breathing and the functional integration of the breathing rhythm with higher brain regions. It is well known that breathing is under cognitive and emotional control, likely involving descending control from regions such as the parabrachial nucleus, the hypothalamus, the periaqueductal gray, various limbic regions, and the prefrontal and anterior cingulate cortex (20, 26, 28, 47, 58, 84, 89, 136, 165). All of these regions are likely connected with the rhythmogenic microcircuits in the medulla to regulate breathing; and this detailed connectivity is beginning to be worked out (164). There are also important ascending influences of the breathing rhythm that may contribute to the function of supramedullary regions. Activity in the prefrontal cortex, the locus coeruleus, hippocampus, and olfactory bulb can all be modulated by the breathing rhythm (55, 59, 61, 81, 85, 88). Indeed, breathing has been described as a global timing mechanism in the brain (144, 145). Thus, in addition to its critical role in regulating blood gasses, breathing likely also plays an important role in regulating higher brain functions and emotions. Yet, how the rhythmogenic microcircuits in the medulla are connected with supramedullary regions and the functional implications of this ascending integration are only beginning to be revealed (163). Perhaps one day we will understand the neuronal mechanisms of why we sigh when we are sad, in love, or relieved (101); why we sometimes feel “inspired”; and why panic attacks and fear can be controlled with breathing (60, 82, 154). Although it has taken almost a century to unravel the basic medullary circuitry underlying generation of the mammalian breathing rhythm, we are in an exciting time when powerful new experimental approaches promise rapid progress toward understanding these important unanswered questions.
Acknowledgments
The article was supported by National Heart, Lung, and Blood Institute Grants RO1 HL-126523 and PO1 HL-090554.
No conflicts of interest, financial or otherwise, are declared by the author(s).
J.-M.R. and N.A.B. drafted manuscript; J.-M.R. and N.A.B. edited and revised manuscript; J.-M.R. and N.A.B. approved final version of manuscript; N.A.B. prepared figures.
References
- 1.Abdala AP, Paton JF, Smith JC. Defining inhibitory neurone function in respiratory circuits: opportunities with optogenetics? J Physiol 593: 3033–3046, 2015. doi: 10.1113/jphysiol.2014.280610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adrian ED, Buytendijk FJ. Potential changes in the isolated brain stem of the goldfish. J Physiol 71: 121–135, 1931. doi: 10.1113/jphysiol.1931.sp002720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akins VT, Weragalaarachchi K, Picardo MCD, Revill AL, Del Negro CA. Morphology of Dbx1 respiratory neurons in the preBötzinger complex and reticular formation of neonatal mice. Sci Data 4: 170097, 2017. doi: 10.1038/sdata.2017.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alsahafi Z, Dickson CT, Pagliardini S. Optogenetic excitation of preBötzinger complex neurons potently drives inspiratory activity in vivo. J Physiol 593: 3673–3692, 2015. doi: 10.1113/JP270471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alving BO. Spontaneous activity in isolated somata of Aplysia pacemaker naurons. J Gen Physiol 51: 29–45, 1968. doi: 10.1085/jgp.51.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson TM, Garcia AJ III, Baertsch NA, Pollak J, Bloom JC, Wei AD, Rai KG, Ramirez JM. A novel excitatory network for the control of breathing. Nature 536: 76–80, 2016. doi: 10.1038/nature18944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson TM, Ramirez JM. Respiratory rhythm generation: triple oscillator hypothesis. F1000 Res 6: 139, 2017. doi: 10.12688/f1000research.10193.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arshavsky YI. Cellular and network properties in the functioning of the nervous system: from central pattern generators to cognition. Brain Res Brain Res Rev 41: 229–267, 2003. doi: 10.1016/S0165-0173(02)00249-7. [DOI] [PubMed] [Google Scholar]
- 9.Baertsch NA, Baertsch HC, Ramirez JM. The interdependence of excitation and inhibition for the control of dynamic breathing rhythms. Nat Commun 9: 843, 2018. doi: 10.1038/s41467-018-03223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ben-Mabrouk F, Tryba AK. Substance P modulation of TRPC3/7 channels improves respiratory rhythm regularity and ICAN-dependent pacemaker activity. Eur J Neurosci 31: 1219–1232, 2010. doi: 10.1111/j.1460-9568.2010.07156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolser DC, Gestreau C, Morris KF, Davenport PW, Pitts TE. Central neural circuits for coordination of swallowing, breathing, and coughing: predictions from computational modeling and simulation. Otolaryngol Clin North Am 46: 957–964, 2013. doi: 10.1016/j.otc.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boutin RC, Alsahafi Z, Pagliardini S. Cholinergic modulation of the parafacial respiratory group. J Physiol 595: 1377–1392, 2017. doi: 10.1113/JP273012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouvier J, Thoby-Brisson M, Renier N, Dubreuil V, Ericson J, Champagnat J, Pierani A, Chédotal A, Fortin G. Hindbrain interneurons and axon guidance signaling critical for breathing. Nat Neurosci 13: 1066–1074, 2010. doi: 10.1038/nn.2622. [DOI] [PubMed] [Google Scholar]
- 14.Breckenridge CG, Hoff HE. Pontine and medullary regulation of respiration in the cat. Am J Physiol 160: 385–394, 1950. [DOI] [PubMed] [Google Scholar]
- 15.Carroll MS, Ramirez JM. Cycle-by-cycle assembly of respiratory network activity is dynamic and stochastic. J Neurophysiol 109: 296–305, 2013. doi: 10.1152/jn.00830.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carroll MS, Viemari JC, Ramirez JM. Patterns of inspiratory phase-dependent activity in the in vitro respiratory network. J Neurophysiol 109: 285–295, 2013. doi: 10.1152/jn.00619.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cregg JM, Chu KA, Dick TE, Landmesser LT, Silver J. Phasic inhibition as a mechanism for generation of rapid respiratory rhythms. Proc Natl Acad Sci USA 114: 12815–12820, 2017. doi: 10.1073/pnas.1711536114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crowder EA, Saha MS, Pace RW, Zhang H, Prestwich GD, Del Negro CA. Phosphatidylinositol 4,5-bisphosphate regulates inspiratory burst activity in the neonatal mouse preBötzinger complex. J Physiol 582: 1047–1058, 2007. doi: 10.1113/jphysiol.2007.134577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui Y, Kam K, Sherman D, Janczewski WA, Zheng Y, Feldman JL. Defining preBötzinger complex rhythm- and pattern-generating neural microcircuits in vivo. Neuron 91: 602–614, 2016. doi: 10.1016/j.neuron.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damasceno RS, Takakura AC, Moreira TS. Regulation of the chemosensory control of breathing by Kölliker-Fuse neurons. Am J Physiol Regul Integr Comp Physiol 307: R57–R67, 2014. doi: 10.1152/ajpregu.00024.2014. [DOI] [PubMed] [Google Scholar]
- 21.Dekin MS, Richerson GB, Getting PA. Thyrotropin-releasing hormone induces rhythmic bursting in neurons of the nucleus tractus solitarius. Science 229: 67–69, 1985. doi: 10.1126/science.3925552. [DOI] [PubMed] [Google Scholar]
- 22.Del Negro CA, Funk GD, Feldman JL. Breathing matters. Nat Rev Neurosci 19: 351–367, 2018. doi: 10.1038/s41583-018-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Del Negro CA, Hayes JAA. A ‘group pacemaker’ mechanism for respiratory rhythm generation. J Physiol 586: 2245–2246, 2008. doi: 10.1113/jphysiol.2008.153627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Del Negro CA, Hayes JA, Pace RW, Brush BR, Teruyama R, Feldman JL. Synaptically activated burst-generating conductances may underlie a group-pacemaker mechanism for respiratory rhythm generation in mammals. Prog Brain Res 187: 111–136, 2010. doi: 10.1016/B978-0-444-53613-6.00008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doi A, Ramirez JM. Neuromodulation and the orchestration of the respiratory rhythm. Respir Physiol Neurobiol 164: 96–104, 2008. doi: 10.1016/j.resp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dutschmann M, Dick TE. Pontine mechanisms of respiratory control. Compr Physiol 2: 2443–2469, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dutschmann M, Herbert H. The Kölliker-Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. Eur J Neurosci 24: 1071–1084, 2006. doi: 10.1111/j.1460-9568.2006.04981.x. [DOI] [PubMed] [Google Scholar]
- 28.Dutschmann M, Jones SE, Subramanian HH, Stanic D, Bautista TG. The physiological significance of postinspiration in respiratory control. Prog Brain Res 212: 113–130, 2014. doi: 10.1016/B978-0-444-63488-7.00007-0. [DOI] [PubMed] [Google Scholar]
- 29.Eisen JS, Marder E. A mechanism for production of phase shifts in a pattern generator. J Neurophysiol 51: 1375–1393, 1984. doi: 10.1152/jn.1984.51.6.1375. [DOI] [PubMed] [Google Scholar]
- 30.Eisen JS, Marder E. Mechanisms underlying pattern generation in lobster stomatogastric ganglion as determined by selective inactivation of identified neurons. III. Synaptic connections of electrically coupled pyloric neurons. J Neurophysiol 48: 1392–1415, 1982. doi: 10.1152/jn.1982.48.6.1392. [DOI] [PubMed] [Google Scholar]
- 31.Feldman JL, Del Negro CA, Gray PA. Understanding the rhythm of breathing: so near, yet so far. Annu Rev Physiol 75: 423–452, 2013. doi: 10.1146/annurev-physiol-040510-130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feldman JL, Kam K. Facing the challenge of mammalian neural microcircuits: taking a few breaths may help. J Physiol 593: 3–23, 2015. doi: 10.1113/jphysiol.2014.277632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fogarty MJ, Mantilla CB, Sieck GC. Breathing: motor control of diaphragm muscle. Physiology (Bethesda) 33: 113–126, 2018. doi: 10.1152/physiol.00002.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuller DD, Mitchell GS. Respiratory neuroplasticity: overview, significance and future directions. Exp Neurol 287: 144–152, 2017. doi: 10.1016/j.expneurol.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 35.Garcia AJ III, Dashevskiy T, Khuu MA, Ramirez JM. Chronic intermittent hypoxia differentially impacts different states of inspiratory activity at the level of the preBötzinger complex. Front Physiol 8: 571, 2017. doi: 10.3389/fphys.2017.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia AJ III, Zanella S, Dashevskiy T, Khan SA, Khuu MA, Prabhakar NR, Ramirez JM. Chronic intermittent hypoxia alters local respiratory circuit function at the level of the preBötzinger complex. Front Neurosci 10: 4, 2016. doi: 10.3389/fnins.2016.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graham Brown T. The intrinsic factors in the act of progression in mammals. Proc R Soc Lond B 84: 308–319, 1911. doi: 10.1098/rspb.1911.0077. [DOI] [Google Scholar]
- 38.Gray PA, Hayes JA, Ling GY, Llona I, Tupal S, Picardo MC, Ross SE, Hirata T, Corbin JG, Eugenín J, Del Negro CA. Developmental origin of preBötzinger complex respiratory neurons. J Neurosci 30: 14883–14895, 2010. doi: 10.1523/JNEUROSCI.4031-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBötzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci 4: 927–930, 2001. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guerrier C, Hayes JA, Fortin G, Holcman D. Robust network oscillations during mammalian respiratory rhythm generation driven by synaptic dynamics. Proc Natl Acad Sci USA 112: 9728–9733, 2015. doi: 10.1073/pnas.1421997112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guyenet PG, Bayliss DA. Neural control of breathing and CO2 homeostasis. Neuron 87: 946–961, 2015. doi: 10.1016/j.neuron.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guyenet PG, Stornetta RL, Abbott SB, Depuy SD, Kanbar R. The retrotrapezoid nucleus and breathing. Adv Exp Med Biol 758: 115–122, 2012. doi: 10.1007/978-94-007-4584-1_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harris KD, Dashevskiy T, Mendoza J, Garcia AJ III, Ramirez JM, Shea-Brown E. Different roles for inhibition in the rhythm-generating respiratory network. J Neurophysiol 118: 2070–2088, 2017. doi: 10.1152/jn.00174.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hartline DK, Maynard DM. Motor patterns in the stomatogastric ganglion of the lobster Panulirus argus. J Exp Biol 62: 405–420, 1975. [DOI] [PubMed] [Google Scholar]
- 45.Hayes JA, Kottick A, Picardo MCD, Halleran AD, Smith RD, Smith GD, Saha MS, Del Negro CA. Transcriptome of neonatal preBötzinger complex neurones in Dbx1 reporter mice. Sci Rep 7: 8669, 2017. doi: 10.1038/s41598-017-09418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoff HE, Breckenridge CG. The medullary origin of respiratory periodicity in the dog. Am J Physiol 158: 157–172, 1949. [DOI] [PubMed] [Google Scholar]
- 47.Holstege G. The periaqueductal gray controls brainstem emotional motor systems including respiration. Prog Brain Res 209: 379–405, 2014. doi: 10.1016/B978-0-444-63274-6.00020-5. [DOI] [PubMed] [Google Scholar]
- 48.Hooper RM, Tikidji-Hamburyan RA, Canavier CC, Prinz AA. Feedback control of variability in the cycle period of a central pattern generator. J Neurophysiol 114: 2741–2752, 2015. doi: 10.1152/jn.00365.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huckstepp RT, Cardoza KP, Henderson LE, Feldman JL. Role of parafacial nuclei in control of breathing in adult rats. J Neurosci 35: 1052–1067, 2015. doi: 10.1523/JNEUROSCI.2953-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huckstepp RT, Henderson LE, Cardoza KP, Feldman JL. Interactions between respiratory oscillators in adult rats. eLife 5: e14203, 2016. doi: 10.7554/eLife.14203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ikeda K, Kawakami K, Onimaru H, Okada Y, Yokota S, Koshiya N, Oku Y, Iizuka M, Koizumi H. The respiratory control mechanisms in the brainstem and spinal cord: integrative views of the neuroanatomy and neurophysiology. J Physiol Sci 67: 45–62, 2017. doi: 10.1007/s12576-016-0475-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J Physiol 570: 407–420, 2006. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Janczewski WA, Tashima A, Hsu P, Cui Y, Feldman JL. Role of inhibition in respiratory pattern generation. J Neurosci 33: 5454–5465, 2013. doi: 10.1523/JNEUROSCI.1595-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jessberger J, Zhong W, Brankačk J, Draguhn A. Olfactory bulb field potentials and respiration in sleep-wake states of mice. Neural Plast 2016: 4570831, 2016. doi: 10.1155/2016/4570831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kam K, Worrell JW, Janczewski WA, Cui Y, Feldman JL. Distinct inspiratory rhythm and pattern generating mechanisms in the preBötzinger complex. J Neurosci 33: 9235–9245, 2013. doi: 10.1523/JNEUROSCI.4143-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kam K, Worrell JW, Ventalon C, Emiliani V, Feldman JL. Emergence of population bursts from simultaneous activation of small subsets of preBötzinger complex inspiratory neurons. J Neurosci 33: 3332–3338, 2013. doi: 10.1523/JNEUROSCI.4574-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaur S, Wang JL, Ferrari L, Thankachan S, Kroeger D, Venner A, Lazarus M, Wellman A, Arrigoni E, Fuller PM, Saper CB. A genetically defined circuit for arousal from sleep during hypercapnia. Neuron 96: 1153–1167.e5, 2017. doi: 10.1016/j.neuron.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kay LM. Circuit oscillations in odor perception and memory. Prog Brain Res 208: 223–251, 2014. doi: 10.1016/B978-0-444-63350-7.00009-7. [DOI] [PubMed] [Google Scholar]
- 60.Kinkead R, Tenorio L, Drolet G, Bretzner F, Gargaglioni L. Respiratory manifestations of panic disorder in animals and humans: a unique opportunity to understand how supramedullary structures regulate breathing. Respir Physiol Neurobiol 204: 3–13, 2014. doi: 10.1016/j.resp.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 61.Kőszeghy Á, Lasztóczi B, Forro T, Klausberger T. Spike-timing of orbitofrontal neurons is synchronized with breathing. Front Cell Neurosci 12: 105, 2018. doi: 10.3389/fncel.2018.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kottick A, Del Negro CA. Synaptic depression influences inspiratory-expiratory phase transition in Dbx1 interneurons of the preBötzinger complex in neonatal mice. J Neurosci 35: 11606–11611, 2015. doi: 10.1523/JNEUROSCI.0351-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leanderson R, Sundberg J, von Euler C. Role of diaphragmatic activity during singing: a study of transdiaphragmatic pressures. J Appl Physiol (1985) 62: 259–270, 1987. doi: 10.1152/jappl.1987.62.1.259. [DOI] [PubMed] [Google Scholar]
- 64.Leng G, Hashimoto H, Tsuji C, Sabatier N, Ludwig M. Discharge patterning in rat olfactory bulb mitral cells in vivo. Physiol Rep 2: e12021, 2014. doi: 10.14814/phy2.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lett KM, Garcia VJ, Temporal S, Bucher D, Schulz DJ. Removal of endogenous neuromodulators in a small motor network enhances responsiveness to neuromodulation. J Neurophysiol 118: 1749–1761, 2017. doi: 10.1152/jn.00383.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lieske SP, Thoby-Brisson M, Telgkamp P, Ramirez JM. Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps Nat Neurosci 3: 600–607, 2000. doi: 10.1038/75776. [DOI] [PubMed] [Google Scholar]
- 67.Lindsey BG, Morris KF, Shannon R, Gerstein GL. Repeated patterns of distributed synchrony in neuronal assemblies. J Neurophysiol 78: 1714–1719, 1997. doi: 10.1152/jn.1997.78.3.1714. [DOI] [PubMed] [Google Scholar]
- 68.Lindsey BG, Rybak IA, Smith JC. Computational models and emergent properties of respiratory neural networks. Compr Physiol 2: 1619–1670, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Llinás R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol 305: 197–213, 1980. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Llinás RR. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science 242: 1654–1664, 1988. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- 71.Lumsden T. Observations on the respiratory centres. J Physiol 57: 354–367, 1923. doi: 10.1113/jphysiol.1923.sp002073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lumsden T. Observations on the respiratory centres in the cat. J Physiol 57: 153–160, 1923. doi: 10.1113/jphysiol.1923.sp002052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luther JA, Robie AA, Yarotsky J, Reina C, Marder E, Golowasch J. Episodic bouts of activity accompany recovery of rhythmic output by a neuromodulator- and activity-deprived adult neural network. J Neurophysiol 90: 2720–2730, 2003. doi: 10.1152/jn.00370.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Machhada A, Ang R, Ackland GL, Ninkina N, Buchman VL, Lythgoe MF, Trapp S, Tinker A, Marina N, Gourine AV. Control of ventricular excitability by neurons of the dorsal motor nucleus of the vagus nerve. Heart Rhythm 12: 2285–2293, 2015. doi: 10.1016/j.hrthm.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marchenko V, Koizumi H, Mosher B, Koshiya N, Tariq MF, Bezdudnaya TG, Zhang R, Molkov YI, Rybak IA, Smith JC. Perturbations of respiratory rhythm and pattern by disrupting synaptic inhibition within pre-Botzinger and Botzinger complexes. eNeuro 3: ENEURO.0011-16.2016, 2016. doi: 10.1523/ENEURO.0011-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marder E. Roles for electrical coupling in neural circuits as revealed by selective neuronal deletions. J Exp Biol 112: 147–167, 1984. [DOI] [PubMed] [Google Scholar]
- 77.Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol 69: 291–316, 2007. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- 78.McKay LC, Feldman JL. Unilateral ablation of pre-Botzinger complex disrupts breathing during sleep but not wakefulness. Am J Respir Crit Care Med 178: 89–95, 2008. doi: 10.1164/rccm.200712-1901OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meckel Y, Rotstein A, Inbar O. The effects of speech production on physiologic responses during submaximal exercise. Med Sci Sports Exerc 34: 1337–1343, 2002. doi: 10.1097/00005768-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 80.Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron 37: 821–826, 2003. doi: 10.1016/S0896-6273(03)00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Melnychuk MC, Dockree PM, O’Connell RG, Murphy PR, Balsters JH, Robertson IH. Coupling of respiration and attention via the locus coeruleus: effects of meditation and pranayama. Psychophysiology e13091, 2018. doi: 10.1111/psyp.13091. [DOI] [PubMed] [Google Scholar]
- 82.Meuret AE, Ritz T, Wilhelm FH, Roth WT, Rosenfield D. Hypoventilation therapy alleviates panic by repeated induction of dyspnea. Biol Psychiatry Cogn Neurosci Neuroimaging 3: 539–545, 2018. doi: 10.1016/j.bpsc.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miller JP, Selverston AI. Mechanisms underlying pattern generation in lobster stomatogastric ganglion as determined by selective inactivation of identified neurons. II. Oscillatory properties of pyloric neurons. J Neurophysiol 48: 1378–1391, 1982. doi: 10.1152/jn.1982.48.6.1378. [DOI] [PubMed] [Google Scholar]
- 84.Miller JR, Zuperku EJ, Stuth EAE, Banerjee A, Hopp FA, Stucke AG. A Subregion of the parabrachial nucleus partially mediates respiratory rate depression from intravenous remifentanil in young and adult rabbits. Anesthesiology 127: 502–514, 2017. doi: 10.1097/ALN.0000000000001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moberly AH, Schreck M, Bhattarai JP, Zweifel LS, Luo W, Ma M. Olfactory inputs modulate respiration-related rhythmic activity in the prefrontal cortex and freezing behavior. Nat Commun 9: 1528, 2018. doi: 10.1038/s41467-018-03988-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moore JD, Deschênes M, Furuta T, Huber D, Smear MC, Demers M, Kleinfeld D. Hierarchy of orofacial rhythms revealed through whisking and breathing. Nature 497: 205–210, 2013. doi: 10.1038/nature12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morris KF, Nuding SC, Segers LS, Iceman KE, O’Connor R, Dean JB, Ott MM, Alencar PA, Shuman D, Horton KK, Taylor-Clark TE, Bolser DC, Lindsey BG. Carotid chemoreceptors tune breathing via multipath routing: reticular chain and loop operations supported by parallel spike train correlations. J Neurophysiol 119: 700–722, 2018. doi: 10.1152/jn.00630.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nguyen Chi V, Müller C, Wolfenstetter T, Yanovsky Y, Draguhn A, Tort AB, Brankačk J. Hippocampal respiration-driven rhythm distinct from theta oscillations in awake mice. J Neurosci 36: 162–177, 2016. doi: 10.1523/JNEUROSCI.2848-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nobis WP, Schuele S, Templer JW, Zhou G, Lane G, Rosenow JM, Zelano C. Amygdala-stimulation-induced apnea is attention and nasal-breathing dependent. Ann Neurol 83: 460–471, 2018. doi: 10.1002/ana.25178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Onimaru H, Ikeda K, Kawakami K. Phox2b, RTN/pFRG neurons and respiratory rhythmogenesis. Respir Physiol Neurobiol 168: 13–18, 2009. doi: 10.1016/j.resp.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 91.Onimaru H, Kumagawa Y, Homma I. Respiration-related rhythmic activity in the rostral medulla of newborn rats. J Neurophysiol 96: 55–61, 2006. doi: 10.1152/jn.01175.2005. [DOI] [PubMed] [Google Scholar]
- 92.Pagliardini S, Janczewski WA, Tan W, Dickson CT, Deisseroth K, Feldman JL. Active expiration induced by excitation of ventral medulla in adult anesthetized rats. J Neurosci 31: 2895–2905, 2011. doi: 10.1523/JNEUROSCI.5338-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Paton JF, St-John WM. Counterpoint: Medullary pacemaker neurons are essential for gasping, but not eupnea, in mammals. J Appl Physiol (1985) 103: 718–720, 2007. doi: 10.1152/japplphysiol.00003.2007a. [DOI] [PubMed] [Google Scholar]
- 94.Peña F, Parkis MA, Tryba AK, Ramirez JM. Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron 43: 105–117, 2004. doi: 10.1016/j.neuron.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 95.Peña F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci 22: 11055–11064, 2002. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peña F, Ramirez JM. Substance P-mediated modulation of pacemaker properties in the mammalian respiratory network. J Neurosci 24: 7549–7556, 2004. doi: 10.1523/JNEUROSCI.1871-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Picardo MC, Weragalaarachchi KT, Akins VT, Del Negro CA. Physiological and morphological properties of Dbx1-derived respiratory neurons in the pre-Botzinger complex of neonatal mice. J Physiol 591: 2687–2703, 2013. doi: 10.1113/jphysiol.2012.250118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pitts RF. Organization of the respiratory center. Physiol Rev 26: 609–630, 1946. doi: 10.1152/physrev.1946.26.4.609. [DOI] [PubMed] [Google Scholar]
- 99.Pitts T. Airway protective mechanisms. Lung 192: 27–31, 2014. doi: 10.1007/s00408-013-9540-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Poon CS, Song G. Bidirectional plasticity of pontine pneumotaxic postinspiratory drive: implication for a pontomedullary respiratory central pattern generator. Prog Brain Res 209: 235–254, 2014. doi: 10.1016/B978-0-444-63274-6.00012-6. [DOI] [PubMed] [Google Scholar]
- 101.Ramirez JM. The integrative role of the sigh in psychology, physiology, pathology, and neurobiology. Prog Brain Res 209: 91–129, 2014. doi: 10.1016/B978-0-444-63274-6.00006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ramirez JM. Reconfiguration of the respiratory network at the onset of locust flight. J Neurophysiol 80: 3137–3147, 1998. doi: 10.1152/jn.1998.80.6.3137. [DOI] [PubMed] [Google Scholar]
- 103.Ramirez JM, Baertsch NA. The dynamic basis of respiratory rhythm generation: one breath at a time. Annu Rev Neurosci. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ramirez JM, Dashevskiy T, Marlin IA, Baertsch N. Microcircuits in respiratory rhythm generation: commonalities with other rhythm generating networks and evolutionary perspectives. Curr Opin Neurobiol 41: 53–61, 2016. doi: 10.1016/j.conb.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ramirez JM, Garcia AJ III, Anderson TM, Koschnitzky JE, Peng YJ, Kumar GK, Prabhakar NR. Central and peripheral factors contributing to obstructive sleep apneas. Respir Physiol Neurobiol 189: 344–353, 2013. doi: 10.1016/j.resp.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ramirez JM, Lieske SP. Commentary on the definition of eupnea and gasping. Respir Physiol Neurobiol 139: 113–119, 2003. doi: 10.1016/S1569-9048(03)00195-2. [DOI] [PubMed] [Google Scholar]
- 107.Ramirez JM, Pearson KG. Alteration of bursting properties in interneurons during locust flight. J Neurophysiol 70: 2148–2160, 1993. doi: 10.1152/jn.1993.70.5.2148. [DOI] [PubMed] [Google Scholar]
- 108.Ramirez JM, Pearson KG. Distribution of intersegmental interneurones that can reset the respiratory rhythm of the locust. J Exp Biol 141: 151–176, 1989. [Google Scholar]
- 109.Ramirez JM, Pearson KG. Generation of motor patterns for walking and flight in motoneurons supplying bifunctional muscles in the locust. J Neurobiol 19: 257–282, 1988. doi: 10.1002/neu.480190307. [DOI] [PubMed] [Google Scholar]
- 110.Ramirez JM, Quellmalz UJ, Richter DW. Postnatal changes in the mammalian respiratory network as revealed by the transverse brainstem slice of mice. J Physiol 491: 799–812, 1996. doi: 10.1113/jphysiol.1996.sp021258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ramirez JM, Richter DW. The neuronal mechanisms of respiratory rhythm generation. Curr Opin Neurobiol 6: 817–825, 1996. doi: 10.1016/S0959-4388(96)80033-X. [DOI] [PubMed] [Google Scholar]
- 112.Ramirez JM, Schwarzacher SW, Pierrefiche O, Olivera BM, Richter DW. Selective lesioning of the cat pre-Bötzinger complex in vivo eliminates breathing but not gasping. J Physiol 507: 895–907, 1998. doi: 10.1111/j.1469-7793.1998.895bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ramirez JM, Severs LJ, Ramirez SC, Agosto-Marlin IM. Advances in cellular and integrative control of oxygen homeostasis within the central nervous system. J Physiol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ramirez JM, Tryba AK, Peña F. Pacemaker neurons and neuronal networks: an integrative view. Curr Opin Neurobiol 14: 665–674, 2004. doi: 10.1016/j.conb.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 115.Ramirez JM, Zuperku EJ, Alheid GF, Lieske SP, Ptak K, McCrimmon DR. Respiratory rhythm generation: converging concepts from in vitro and in vivo approaches? Respir Physiol Neurobiol 131: 43–56, 2002. doi: 10.1016/S1569-9048(02)00036-8. [DOI] [PubMed] [Google Scholar]
- 116.Rekling JC, Feldman JL. PreBötzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu Rev Physiol 60: 385–405, 1998. doi: 10.1146/annurev.physiol.60.1.385. [DOI] [PubMed] [Google Scholar]
- 117.Revill AL, Vann NC, Akins VT, Kottick A, Gray PA, Del Negro CA, Funk GD. Dbx1 precursor cells are a source of inspiratory XII premotoneurons. eLife 4: e12301, 2015. doi: 10.7554/eLife.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Richter DW. Generation and maintenance of the respiratory rhythm. J Exp Biol 100: 93–107, 1982. [DOI] [PubMed] [Google Scholar]
- 119.Richter DW, Smith JC. Respiratory rhythm generation in vivo. Physiology (Bethesda) 29: 58–71, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rotstein A, Meckel Y, Inbar O. Perceived speech difficulty during exercise and its relation to exercise intensity and physiological responses. Eur J Appl Physiol 92: 431–436, 2004. doi: 10.1007/s00421-004-1160-z. [DOI] [PubMed] [Google Scholar]
- 121.Rubin JE, Hayes JA, Mendenhall JL, Del Negro CA. Calcium-activated nonspecific cation current and synaptic depression promote network-dependent burst oscillations. Proc Natl Acad Sci USA 106: 2939–2944, 2009. doi: 10.1073/pnas.0808776106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Russell DF, Hartline DK. Bursting neural networks: a reexamination. Science 200: 453–456, 1978. doi: 10.1126/science.644309. [DOI] [PubMed] [Google Scholar]
- 123.Russell DF, Hartline DK. Slow active potentials and bursting motor patterns in pyloric network of the lobster, Panulirus interruptus. J Neurophysiol 48: 914–937, 1982. doi: 10.1152/jn.1982.48.4.914. [DOI] [PubMed] [Google Scholar]
- 124.Rybak IA, Abdala AP, Markin SN, Paton JF, Smith JC. Spatial organization and state-dependent mechanisms for respiratory rhythm and pattern generation. Prog Brain Res 165: 201–220, 2007. doi: 10.1016/S0079-6123(06)65013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Salmoiraghi GC, Burns BD. Notes on mechanism of rhythmic respiration. J Neurophysiol 23: 14–26, 1960. doi: 10.1152/jn.1960.23.1.14. [DOI] [PubMed] [Google Scholar]
- 126.Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci 3: 1027–1034, 2000. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- 127.Segers LS, Nuding SC, Ott MM, Dean JB, Bolser DC, O’Connor R, Morris KF, Lindsey BG. Peripheral chemoreceptors tune inspiratory drive via tonic expiratory neuron hubs in the medullary ventral respiratory column network. J Neurophysiol 113: 352–368, 2015. doi: 10.1152/jn.00542.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Segers LS, Nuding SC, Vovk A, Pitts T, Baekey DM, O’Connor R, Morris KF, Lindsey BG, Shannon R, Bolser DC. Discharge identity of medullary inspiratory neurons is altered during repetitive fictive cough. Front Physiol 3: 223, 2012. doi: 10.3389/fphys.2012.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Selverston AI, Miller JP. Mechanisms underlying pattern generation in lobster stomatogastric ganglion as determined by selective inactivation of identified neurons. I. Pyloric system. J Neurophysiol 44: 1102–1121, 1980. doi: 10.1152/jn.1980.44.6.1102. [DOI] [PubMed] [Google Scholar]
- 130.Sherman D, Worrell JW, Cui Y, Feldman JL. Optogenetic perturbation of preBötzinger complex inhibitory neurons modulates respiratory pattern. Nat Neurosci 18: 408–414, 2015. doi: 10.1038/nn.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Smith JC, Abdala AP, Borgmann A, Rybak IA, Paton JF. Brainstem respiratory networks: building blocks and microcircuits. Trends Neurosci 36: 152–162, 2013. doi: 10.1016/j.tins.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol 98: 3370–3387, 2007. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Smith JC, Abdala AP, Rybak IA, Paton JF. Structural and functional architecture of respiratory networks in the mammalian brainstem. Philos Trans R Soc Lond B Biol Sci 364: 2577–2587, 2009. doi: 10.1098/rstb.2009.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254: 726–729, 1991. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stuart DG, Hultborn H. Thomas Graham Brown (1882–1965), Anders Lundberg (1920-), and the neural control of stepping. Brain Res Brain Res Rev 59: 74–95, 2008. doi: 10.1016/j.brainresrev.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 136.Subramanian HH, Holstege G. The midbrain periaqueductal gray changes the eupneic respiratory rhythm into a breathing pattern necessary for survival of the individual and of the species. Prog Brain Res 212: 351–384, 2014. doi: 10.1016/B978-0-444-63488-7.00017-3. [DOI] [PubMed] [Google Scholar]
- 137.Sugiyama Y, Shiba K, Mukudai S, Umezaki T, Hisa Y. Activity of respiratory neurons in the rostral medulla during vocalization, swallowing, and coughing in guinea pigs. Neurosci Res 80: 17–31, 2014. doi: 10.1016/j.neures.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 138.Swiercz W, Cios K, Hellier J, Yee A, Staley K. Effects of synaptic depression and recovery on synchronous network activity. J Clin Neurophysiol 24: 165–174, 2007. doi: 10.1097/WNP.0b013e318033756f. [DOI] [PubMed] [Google Scholar]
- 139.Takeda S, Eriksson LI, Yamamoto Y, Joensen H, Onimaru H, Lindahl SG. Opioid action on respiratory neuron activity of the isolated respiratory network in newborn rats. Anesthesiology 95: 740–749, 2001. doi: 10.1097/00000542-200109000-00029. [DOI] [PubMed] [Google Scholar]
- 140.Tan W, Janczewski WA, Yang P, Shao XM, Callaway EM, Feldman JL. Silencing preBötzinger complex somatostatin-expressing neurons induces persistent apnea in awake rat. Nat Neurosci 11: 538–540, 2008. doi: 10.1038/nn.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Telgkamp P, Ramirez JM. Differential responses of respiratory nuclei to anoxia in rhythmic brain stem slices of mice. J Neurophysiol 82: 2163–2170, 1999. doi: 10.1152/jn.1999.82.5.2163. [DOI] [PubMed] [Google Scholar]
- 142.Thoby-Brisson M, Ramirez JM. Identification of two types of inspiratory pacemaker neurons in the isolated respiratory neural network of mice. J Neurophysiol 86: 104–112, 2001. doi: 10.1152/jn.2001.86.1.104. [DOI] [PubMed] [Google Scholar]
- 143.Thoby-Brisson M, Simmers J. Long-term neuromodulatory regulation of a motor pattern-generating network: maintenance of synaptic efficacy and oscillatory properties. J Neurophysiol 88: 2942–2953, 2002. doi: 10.1152/jn.00482.2001. [DOI] [PubMed] [Google Scholar]
- 144.Tort ABL, Brankačk J, Draguhn A. Respiration-entrained brain rhythms are global but often overlooked. Trends Neurosci 41: 186–197, 2018. doi: 10.1016/j.tins.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 145.Tort ABL, Ponsel S, Jessberger J, Yanovsky Y, Brankačk J, Draguhn A. Parallel detection of theta and respiration-coupled oscillations throughout the mouse brain. Sci Rep 8: 6432, 2018. doi: 10.1038/s41598-018-24629-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Traub RD, Llinás R. Hippocampal pyramidal cells: significance of dendritic ionic conductances for neuronal function and epileptogenesis. J Neurophysiol 42: 476–496, 1979. doi: 10.1152/jn.1979.42.2.476. [DOI] [PubMed] [Google Scholar]
- 147.Tryba AK, Peña F, Lieske SP, Viemari JC, Thoby-Brisson M, Ramirez JM. Differential modulation of neural network and pacemaker activity underlying eupnea and sigh-breathing activities. J Neurophysiol 99: 2114–2125, 2008. doi: 10.1152/jn.01192.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tryba AK, Peña F, Ramirez JM. Stabilization of bursting in respiratory pacemaker neurons. J Neurosci 23: 3538–3546, 2003. doi: 10.1523/JNEUROSCI.23-08-03538.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vann NC, Pham FD, Dorst KE, Del Negro CA. Dbx1 pre-Botzinger complex interneurons comprise the core inspiratory oscillator for breathing in unanesthetized adult mice. eNeuro 5: ENEURO.0130-18.2018, 2018. doi: 10.1523/ENEURO.0130-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vann NC, Pham FD, Hayes JA, Kottick A, Del Negro CA. Transient suppression of Dbx1 PreBotzinger interneurons disrupts breathing in adult mice. PLoS One 11: e0162418, 2016. doi: 10.1371/journal.pone.0162418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Viemari JC, Garcia AJ III, Doi A, Elsen G, Ramirez JM. β-Noradrenergic receptor activation specifically modulates the generation of sighs in vivo and in vitro. Front Neural Circuits 7: 179, 2013. doi: 10.3389/fncir.2013.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Viemari JC, Garcia AJ III, Doi A, Ramirez JM. Activation of alpha-2 noradrenergic receptors is critical for the generation of fictive eupnea and fictive gasping inspiratory activities in mammals in vitro. Eur J Neurosci 33: 2228–2237, 2011. doi: 10.1111/j.1460-9568.2011.07706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Viemari JC, Ramirez JM. Norepinephrine differentially modulates different types of respiratory pacemaker and nonpacemaker neurons. J Neurophysiol 95: 2070–2082, 2006. doi: 10.1152/jn.01308.2005. [DOI] [PubMed] [Google Scholar]
- 154.Vlemincx E, Meulders M, Luminet O. A sigh of relief or a sigh of expected relief: Sigh rate in response to dyspnea relief. Psychophysiology 55: e12979, 2018. doi: 10.1111/psyp.12979. [DOI] [PubMed] [Google Scholar]
- 155.von Euler C. On the central pattern generator for the basic breathing rhythmicity. J Appl Physiol Respir Environ Exerc Physiol 55: 1647–1659, 1983. [DOI] [PubMed] [Google Scholar]
- 156.Wang W, Fung ML, Darnall RA, St John WM. Characterizations and comparisons of eupnoea and gasping in neonatal rats. J Physiol 490: 277–292, 1996. doi: 10.1113/jphysiol.1996.sp021143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang X, Hayes JA, Picardo MC, Del Negro CA. Automated cell-specific laser detection and ablation of neural circuits in neonatal brain tissue. J Physiol 591: 2393–2401, 2013. doi: 10.1113/jphysiol.2012.247338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang X, Hayes JA, Revill AL, Song H, Kottick A, Vann NC, LaMar MD, Picardo MC, Akins VT, Funk GD, Del Negro CA. Laser ablation of Dbx1 neurons in the pre-Bötzinger complex stops inspiratory rhythm and impairs output in neonatal mice. eLife 3: e03427, 2014. doi: 10.7554/eLife.03427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Weimann JM, Meyrand P, Marder E. Neurons that form multiple pattern generators: identification and multiple activity patterns of gastric/pyloric neurons in the crab stomatogastric system. J Neurophysiol 65: 111–122, 1991. doi: 10.1152/jn.1991.65.1.111. [DOI] [PubMed] [Google Scholar]
- 160.Wenninger JM, Pan LG, Klum L, Leekley T, Bastastic J, Hodges MR, Feroah TR, Davis S, Forster HV. Large lesions in the pre-Bötzinger complex area eliminate eupneic respiratory rhythm in awake goats. J Appl Physiol (1985) 97: 1629–1636, 2004. doi: 10.1152/japplphysiol.00953.2003. [DOI] [PubMed] [Google Scholar]
- 161.Whelan PJ. Control of locomotion in the decerebrate cat. Prog Neurobiol 49: 481–515, 1996. doi: 10.1016/0301-0082(96)00028-7. [DOI] [PubMed] [Google Scholar]
- 162.Wilson DM. Relative refractoriness and patterned discharge of locust flight motor neurons. J Exp Biol 41: 191–205, 1964. [DOI] [PubMed] [Google Scholar]
- 163.Yackle K, Schwarz LA, Kam K, Sorokin JM, Huguenard JR, Feldman JL, Luo L, Krasnow MA. Breathing control center neurons that promote arousal in mice. Science 355: 1411–1415, 2017. doi: 10.1126/science.aai7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yang CF, Feldman JL. Efferent projections of excitatory and inhibitory preBötzinger Complex neurons. J Comp Neurol 526: 1389–1402, 2018. doi: 10.1002/cne.24415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yokota S, Oka T, Asano H, Yasui Y. Orexinergic fibers are in contact with Kölliker-Fuse nucleus neurons projecting to the respiration-related nuclei in the medulla oblongata and spinal cord of the rat. Brain Res 1648, Pt A: 512–523, 2016. doi: 10.1016/j.brainres.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 166.Zanella S, Doi A, Garcia AJ III, Elsen F, Kirsch S, Wei AD, Ramirez JM. When norepinephrine becomes a driver of breathing irregularities: how intermittent hypoxia fundamentally alters the modulatory response of the respiratory network. J Neurosci 34: 36–50, 2014. doi: 10.1523/JNEUROSCI.3644-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zheng J, Lee S, Zhou ZJ. A transient network of intrinsically bursting starburst cells underlies the generation of retinal waves. Nat Neurosci 9: 363–371, 2006. doi: 10.1038/nn1644. [DOI] [PubMed] [Google Scholar]
- 168.Zuperku EJ, Stucke AG, Hopp FA, Stuth EA. Characteristics of breathing rate control mediated by a subregion within the pontine parabrachial complex. J Neurophysiol 117: 1030–1042, 2017. doi: 10.1152/jn.00591.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]