Abstract
5-Methyltetrahydrofolate (5-MTHF) is important for nitric oxide (NO)-mediated cutaneous microvascular vasodilation. Ultraviolet B (UVB) radiation may deplete 5-MTHF, either directly or via production of reactive oxygen species (ROS), decreasing NO-mediated vasodilation. We hypothesized that 1) acute UVB exposure would attenuate NO-dependent cutaneous vasodilation, 2) local perfusion of 5-MTHF or ascorbate (ASC) (antioxidant) would augment NO-dependent vasodilation after UVB, and 3) darker skin pigmentation would be UVB-protective. Intradermal microdialysis fibers (n = 3) placed in each forearm of 22 healthy young adults (23 ± 1 yr; 8M/14F) locally delivered lactated Ringer’s (control), 5 mM 5-MTHF, or 10 mM ASC. One arm was UVB-exposed (300 mJ/cm2), and the other served as a nonexposed control (CON). Following UVB exposure, a standardized local heating (42°C) protocol induced cutaneous vasodilation. After attaining a plateau blood flow, 15 mM NG-nitro-l-arginine methyl ester (nitric oxide synthase inhibiter) was infused at all sites to quantify the NO contribution. Red cell flux was measured at each site by laser-Doppler flowmetry (LDF), and cutaneous vascular conductance (CVC = LDF/mean arterial pressure) was expressed as a percentage of maximum (28 mM sodium nitroprusside + 43°C). UVB attenuated NO-mediated vasodilation compared with CON (23.1 ± 3.6 vs. 33.9 ± 3.4%; P = 0.001). Delivery of 5-MTHF or ASC improved NO-mediated vasodilation versus lactated Ringer’s in the UVB-exposed arm (MTHF: 30.1 ± 4.8% vs. 23.1 ± 3.8%; P = 0.03; ASC: 30.9 ± 4.3% vs. 23.1 ± 3.8%; P = 0.02). Neither treatment affected the response in the nonexposed arm (P ≥ 0.09). Skin pigmentation (melanin index) was not predictive of the UVB response (P ≥ 0.34). These data suggest that acute UVB exposure attenuates NO-mediated vasodilation via direct and/or ROS-induced reductions in 5-MTHF, independent of skin pigmentation.
NEW & NOTEWORTHY Endothelial-derived nitric oxide (NO) contributes to normal healthy function of the human cutaneous microvasculature. Bioavailability of 5-methyltetrahydrofolate (5-MTHF) is important for the production of NO. Ultraviolet (UV) radiation exposure, specifically UVB, may deplete cutaneous 5-MTHF, thereby reducing NO-mediated microvascular function. Our findings suggest that acute UVB exposure attenuates NO-mediated vasodilation of the cutaneous microvasculature via degradation of 5-MTHF. These findings advance our understanding of the potential negative health impacts of acute UV exposure.
Keywords: folate, microvascular function, skin blood flow, UV radiation
INTRODUCTION
Endothelium-derived nitric oxide (NO) is an important component of the vasodilatory responses of the cutaneous microvasculature (3, 23) and is associated with a healthy vascular phenotype. 5-methyltetrahydrofolate (5-MTHF), the bioactive metabolite of folate, contributes to the synthesis and vascular bioavailability of endothelial NO (6, 36) by stabilizing endothelial NO synthase (NOS) via (1) increasing bioavailable tetrahydrobiopterin (BH4), an essential cofactor in the coupling of the NOS dimer, and (2) direct scavenging of reactive oxygen species (ROS) (6, 36). Under normal conditions, thermally induced increases in skin blood flow are primarily mediated by NO, which in turn depends on adequate 5-MTHF bioavailability.
In vitro studies have suggested that 5-MTHF is sensitive to photodegradation by ultraviolet (UV) radiation (25, 28, 37), but it remains unclear how cutaneous vascular 5-MTHF is affected by UV in vivo. Importantly, 5-MTHF appears to be sensitive to UVB and UVC but not UVA radiation (25, 37). UVC radiation is unable to penetrate the earth’s atmosphere, and UVB may be unable to penetrate the dermis to reach the dermal circulation (37). Alternatively, 5-MTHF may be indirectly degraded in vivo by UV exposure-induced increases in ROS. Indeed, 5-MTHF is rapidly degraded by UV in the presence, but not in the absence, of endogenous photosensitizers (25, 38). Such naturally occurring photosensitizers in the skin or blood produce ROS upon exposure to solar radiation (7). It is unknown, however, whether direct and/or indirect photodegradation of 5-MTHF occurs with exposure to UVB in vivo.
Differences in skin pigmentation are suggested to result from evolutionary adaptations to geographical variations in UV exposure (13) designed to enhance reproductive viability. Protection of 5-MTHF is of evolutionary importance because of its role in the prevention of macrocytic anemia in pregnant women and the development of neural tube defects in utero (13, 29). Thus, early human populations that inhabited equatorial Africa and were subjected to high levels of UV exposure are thought to have developed a darkened pigment to protect from potentially negative effects of UV, including sunburn, skin cancer, and photolysis of bioavailable folate (13, 14, 30). As humans began to move to geographical regions distant from the equator, depigmentation of the skin is reasoned to have occurred to allow for adequate vitamin D biosynthesis under conditions of reduced UV exposure. Lightening of skin pigment, however, may increase susceptibility to UV-induced degradation of bioavailable 5-MTHF.
To date, no studies have determined how cutaneous vascular 5-MTHF is affected by acute UV exposure in vivo, and it is unknown whether UV-induced degradation of 5-MTHF affects NO-mediated vasodilation of the human cutaneous microvasculature. Furthermore, whether a darkened skin pigment “protects” the cutaneous microvasculature from the negative effects of acute UV is unknown. Therefore, the purpose of the current study was to elucidate the impact of UV, specifically in the UVB region, on NO-mediated vasodilation in the human cutaneous microvasculature and to examine the role of direct 5-MTHF photodegradation and ROS in these responses. Furthermore, we sought to examine whether there is an association between UVB effects on NO-dependent vasodilation of the skin microvasculature and skin pigmentation. We hypothesized that NO-mediated cutaneous vasodilation would be attenuated after acute UVB exposure but that this effect would be smaller in darkly vs. lightly pigmented individuals. We further hypothesized that local infusion of either 5-MTHF or ascorbate (ASC) (a nonspecific antioxidant), would augment NO-mediated vasodilation after UVB exposure, suggesting that one or both mechanisms are involved.
METHODS
Subjects.
Experimental protocols were approved by the Institutional Review Board at The Pennsylvania State University. Men and women aged 18–30 who were normally active, healthy, nonsmokers, free from cardiovascular disease, and not taking any prescription medications with primary or secondary vascular effects were tested. Additional measures were taken to recruit subjects across a wide range of skin pigmentation. All subjects underwent an initial screening that included physical examination, lipid profile, and blood chemistry (Quest Diagnostics, Pittsburgh, PA). Subjects with rash, skin disease, disorders of pigmentation, known skin allergies, allergies to folic acid, or kidney disease were excluded. Written and verbal consent were obtained voluntarily from all subjects before participation, according to the Declaration of Helsinki.
Assessment of skin pigmentation.
Skin pigmentation was measured by reflectance spectrophotometry (DermaSpectrometer; Cortex Technology, Hadsund, Denmark) to determine the melanin index (M-index) of the skin on the subject’s inner aspect of the upper arm. The M-index is routinely measured in this region because of its relatively low sun exposure and ease of access (30). Additional M-index measurements were taken at the forehead and both forearms. Lower and higher M-indices are related to lighter and darker skin pigments, respectively.
Instrumentation.
All protocols were performed in a thermoneutral laboratory with the subject in a semisupine position and both arms supported at heart level. Three intradermal microdialysis fibers (10 mm, 55-kDa cut-off membrane; CMA, Holliston, MA) were placed into the dermal layer of the ventral aspect of each forearm (6 sites total) for the local delivery of pharmacological agents (3, 19). Pharmacological agents were dissolved in lactated Ringer’s solution just before use, sterilized using syringe microfilters (Acrodisc; Pall, Ann Arbor, MI), and wrapped in foil to prevent degradation because of light exposure. In each forearm, microdialysis sites were randomly assigned for local delivery of either 5 mM 5-MTHF (USP, Rockville, MD) (34), 10 mM ASC (Sigma, St. Louis, MO) (33), or lactated Ringer’s solution alone (control site). All solutions were perfused through their respective microdialysis fibers at a rate of 2 µl/min (Bee Hive controller and Baby Bee microinfusion pumps; Bioanalytical Systems, West Lafayette, IN) (3).
Local red blood cell flux was measured directly over each microdialysis site throughout the protocol using an integrated laser-Doppler flowmetry probe placed in a local heating unit (moorLab, Temperature Monitor, SHO2; Moor Instruments, Axminster, UK). Mean arterial pressure was calculated for each phase of the protocol using blood pressure taken from an automated blood pressure monitor (CardioCap; GE Healthcare, Milwaukee, WI).
Experimental protocol.
After placement of microdialysis fibers and following a 60-min period for resolution of hyperemia associated with fiber placement, one arm was randomly exposed to 300 mJ/cm2 (75 s) UVB (SolRx 500; SolArc Systems, Minesing, Canada), whereas the other served as a nonexposed control. We then collected baseline data and initiated a local heating protocol (local temperature increased to 42°C at a rate of 0.5°C/5 s) as described previously (3, 23). This protocol elicits an initial peak that is mediated by sensory nerves, followed by a plateau (16, 17). After ~40 min of local heating and establishment of a stable local heating plateau, each microdialysis site was perfused with 15 mM l-NG-nitroarginine methyl ester (l-NAME) (Calbiochem, San Diego, CA) at a rate of 4 µl/min to fully inhibit NOS (1, 35). Following a stable l-NAME plateau, 28 mM sodium nitroprusside (USP) were perfused through each site, and local temperature was increased to 43°C to elicit maximal cutaneous vascular conductance (CVCmax) (15, 23).
Data acquisition and analysis.
Laser-Doppler flowmetry data were collected using Windaq (Windaq; DATAQ Instruments) at a frequency of 40 Hz and stored on a personal computer for subsequent analysis. CVC was calculated as red blood cell flux divided by mean arterial pressure and expressed as a percentage of CVCmax for each phase of the local heating protocol (22, 23). The percent of cutaneous vasodilation mediated by NO was calculated from the difference between the local heating and L-NAME plateaus.
Two-way repeated measures ANOVA was used to detect differences between pharmacological treatment sites in UVB-exposed and nonexposed arms at each phase of the local heating protocol (arm × MD site × phase) (SAS v9.4, SAS Institute Inc.). NO-dependent vasodilation was regressed against the M-index to ascertain whether skin pigmentation was predictive of the response to UVB. Bonferroni post hoc corrections were performed to account for multiple comparisons in the ANOVA, when necessary, and significance was set a priori and accepted at α = 0.05. All values are presented as mean ± standard error.
RESULTS
Subject characteristics are presented in Table 1. All baseline variables and health indicators were normal for this age group, and a broad range of continuous and heterogeneous M-index values were included. Forearm M-indices are expressed as the average of both forearms.
Table 1.
Subject characteristics
| Mean ± SE | Range | |
|---|---|---|
| n (M/F) | 22 (8/14) | |
| Age, yr | 23 ± 1 | 18–28 |
| BMI, kg/m2 | 24 ± 1 | 19–31 |
| Systolic BP, mmHg | 112 ± 2 | 94–120 |
| Diastolic BP, mmHg | 70 ± 2 | 56–88 |
| Heart rate, beats/min | 65 ± 3 | 44–80 |
| M-index, a.u. | ||
| Inner arm | 46 ± 4 | 30–79 |
| Forehead | 52 ± 4 | 32–98 |
| Forearm | 45 ± 4 | 31–83 |
| Blood biochemistry | ||
| HbA1c, % | 5.0 ± 0.1 | 4.2–5.6 |
| Total cholesterol, mg/dl | 154 ± 8 | 83–209 |
| HDL, mg/dl | 60 ± 4 | 37–79 |
| LDL, mg/dl | 79 ± 6 | 21–118 |
n = no. of participants. a.u., arbitrary units; BMI, body mass index; BP, blood pressure; M-index, a skin-reflectance measure of melanization; HbA1c, hemoglobin A1C; HDL, high density lipoprotein; LDL, low density lipoprotein.
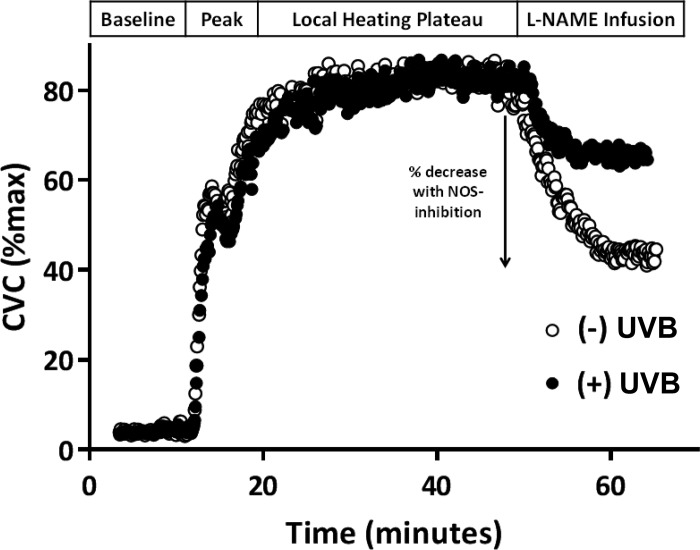
Figure 1 illustrates representative tracings of %CVCmax in response to the standardized local heating protocol for one subject, showing the control site in both the UVB-exposed and nonexposed arm. The percent decrease in %CVCmax with NOS inhibition by local administration of L-NAME is indicated.
Fig. 1.
Representative tracings from one subject demonstrating the blood flow response [percent maximal cutaneous vascular conductance (%CVCmax)] during the local heating protocol in the control (lactated Ringer’s) sites of the ultraviolet B (UVB)-exposed (closed circles) and nonexposed (open circles) arms. The arrow indicates the percentage decrease with nitric oxide synthase (NOS) inhibition [l-NG-nitroarginine methyl ester (l-NAME infusion)].
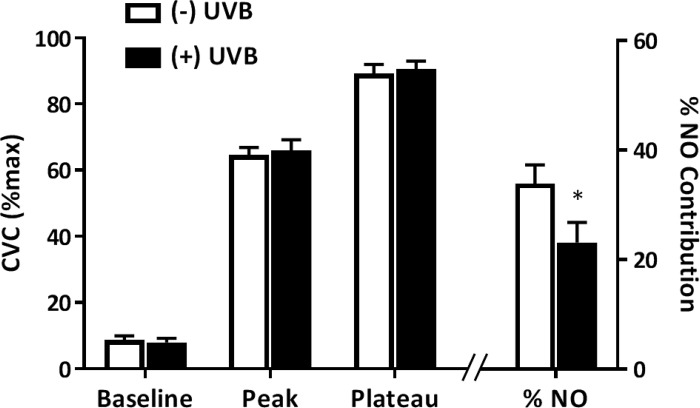
Figure 2 presents %CVCmax for the control site at each phase of the local heating protocol in UVB-exposed and nonexposed arms (left axis). There were no differences in %CVCmax due to acute UVB exposure during baseline, at the initial peak, or during the local heating plateau. As illustrated by the right-most bars and right axis, %NO-mediated vasodilation was attenuated in UVB-exposed, compared with nonexposed, arms (23.1 ± 3.8% vs. 33.9 ± 3.4%; P = 0.001). Importantly, as shown in Table 2, there were no between-arm or intersite differences in the maximal heating values.
Fig. 2.
Percent maximal cutaneous vascular conductance (%CVCmax) at each phase of the local heating protocol as well as the percent contribution of nitric oxide (NO) to the local heating plateau in the lactated Ringer’s sites in ultraviolet B (UVB)-exposed (+UVB; closed bars) and nonexposed (−UVB; open bars) arms. *P < 0.05 compared with nonexposed arm; n = 22.
Table 2.
Maximal CVC values
| Ringer’s | Ascorbate | 5-MTHF | |
|---|---|---|---|
| Control | 2.29 ± 0.25 | 2.16 ± 0.19 | 2.34 ± 0.18 |
| UVB | 2.34 ± 0.20 | 1.89 ± 0.17 | 2.20 ± 0.14 |
All values are expressed as mean ± SE in flux/mmHg. 5-MTHF, 5-methyltetrahydrofolate; CVC, cutaneous vascular conductance; UVB, ultraviolet B.
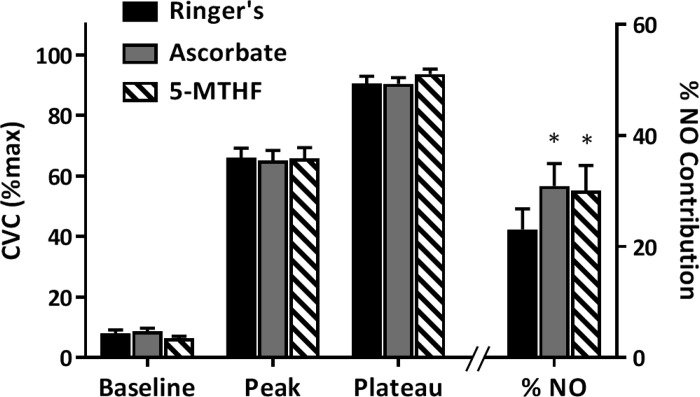
Figure 3 depicts CVC responses to the local heating protocol in Ringer’s-, ASC-, and 5-MTHF-treated sites in the UVB-exposed arm only. There were no differences between sites during baseline, initial peak, or the local heating plateau phases (left axis). The %NO-mediated vasodilation (right axis) was augmented by local administration of both 5-MTHF (30.1 ± 4.8% vs. 23.1 ± 3.8%; P = 0.03) and ASC (30.1 ± 4.3% vs. 23.1 ± 3.8%; P = 0.02) compared with the control site.
Fig. 3.
Percent maximal cutaneous vascular conductance (%CVCmax) responses at each phase of the local heating protocol as well as the percent contribution of nitric oxide (NO) to the local heating plateau in lactated Ringer’s-, ascorbate-, and 5-methyltetrahydrofolate (5-MTHF)-treated sites in the ultraviolet B exposed arm. *P < 0.05 compared with Ringer’s; n = 22.
Neither of the localized treatments (5-MTHF or ASC) had an effect on cutaneous vascular responses to local heating in the non-UVB exposed arm (P ≥ 0.09). In the UVB-exposed arm, both 5-MTHF and ASC restored the %NO-mediated vasodilation such that there were no differences between the control site in the nonexposed arm and the treated (5-MTHF: 33.9 ± 3.4% vs. 30.1 ± 4.8%, P = 0.27 and ASC: 33.9 ± 3.4% vs. 30.9 ± 4.3%, P = 0.32) sites in the UVB-exposed arm.
The magnitude of change in %NO-mediated vasodilation after UVB exposure was not associated with the M-index at the inner arm (R2 = 0.04, P = 0.42), forehead (R2 = 0.02, P = 0.59), or forearms (R2 = 0.05, P = 0.34) across the range tested. Furthermore, there was no relation between the responses to 5-MTHF or ASC and skin pigmentation at the inner arm (5-MTHF: R2 = 0.02, P = 0.53 and ASC: R2 = 0.03, P = 0.48), forehead (5-MTHF: R2 = 0.02, P = 0.53 and ASC: R2 = 0.004, P = 0.78), or forearms (5-MTHF: R2 = 0.005, P = 0.76 and ASC: R2 = 0.008, P = 0.71).
DISCUSSION
The primary finding of this study was that a brief bout of acute UVB exposure attenuated NO-mediated vasodilation in the human cutaneous microvasculature. Additionally, local delivery of either 5-MTHF or ASC augmented the NO-mediated contribution to vasodilation after UVB exposure such that NO-mediated vasodilation in the 5-MTHF and ASC sites of the UVB-exposed arm were similar to that of the control site in the nonexposed arm. Taken together, these findings suggest that reductions in the NO-mediated contribution to vasodilation after UVB exposure are mediated, at least in part, by degradation of 5-MTHF, either directly or via local UVB-induced increases in ROS. Lastly, no relation was found between skin pigmentation and the magnitude of change in %NO-mediated vasodilation consequent to UVB exposure, suggesting that darker skin pigment may not protect against the deleterious effects of acute UVB exposure on cutaneous microvascular function.
In healthy young subjects, ~40%–60% of the vasodilatory response to local heating in the cutaneous microcirculation is mediated by NO under control conditions (3, 11, 34). 5-MTHF plays an indirect but essential role in the coupling of NOS by increasing the production of tetrahydrobiopterin (BH4) from its inactive form, dihydrobiopterin (BH2). 5-MTHF also directly scavenges ROS, which likewise increases NO bioavailability by stabilizing the NOS dimer (36). Thus, full expression of NO-mediated vasodilation is reliant upon adequate bioavailable 5-MTHF. Our results suggested that NO-dependent cutaneous vasodilation is attenuated by acute UVB-exposure because of the degradation of 5-MTHF, either by direct photodegradation, via local increases in ROS, or both.
Despite significant reductions in the NO-mediated component of the vasodilatory response following UVB-exposure, which were similar in magnitude to attenuations observed in aging and various diseased states (10, 24, 32), there was no difference in the magnitude of the local heating plateau between UVB-exposed and nonexposed arms. This is likely explained by redundant dilator pathways, such as endothelium-derived hyperpolarizing factors (4), which may compensate for the acute reduction in NO bioavailability in young, healthy subjects (3). This is similar to our previous finding in healthy middle-aged adults in which the NO-dependent dilation response is attenuated, but the magnitude of the overall dilator response to local heating is preserved by a compensatory increase in other dilator mechanisms (3). The activity of such redundant pathways, however, is diminished in aging adults (24) and those with various pathologies, such as hypertension and hypercholesterolemia (10, 32). As such, attenuated production and reductions in NO-dependent dilation after UVB exposure may present an avenue for future risk of cutaneous microvascular dysfunction in otherwise healthy adults.
Although UVB (wavelength = 280 to 315 nm) directly degrades 5-MTHF in vitro, the dermis is relatively impenetrable by UVB (37) and has therefore been suggested to be unlikely to directly affect the cutaneous circulation. Conversely, UVA (315 to 400 nm) can penetrate the dermis, but 5-MTHF does not appear to be sensitive to UVA (25, 37). Our findings demonstrate that reductions in NO-mediated vasodilation of the cutaneous microvasculature after UVB exposure were improved by local administration of 5-MTHF, suggesting that the reductions were a result of UVB-induced reductions in 5-MTHF bioavailability. Taken together, while it cannot be ruled out based on the present data, it is unlikely that 5-MTHF is directly degraded by UVB in vivo. Alternatively, UVB may be capable of indirectly degrading 5-MTHF in vivo by increasing local production of ROS. Local delivery of ASC augmented NO-mediated vasodilation after UVB exposure, indicating that local increases in ROS contribute to the reduction in NO-mediated dilation with UVB. Consequently, UVB-induced degradation of 5-MTHF may be mediated by local increases in ROS.
Standard erythema dose (SED) is a standardized measure of the UV dose required to elicit a reddening response of the skin (16). UV exposures in the range of 2–6 SED per hour have been documented in triathletes and cyclists during competition (26, 27). Likewise, hikers and tennis players have been demonstrated to experience 8.1 and 7.5 SED with exposure times of 6.4 and 4.0 h, respectively (31). UVB exposure in the current study was equivalent to ~1.8 SED. As such, the magnitude of UV exposure experienced by individuals participating in outdoor activities can far exceed that of the present study.
The evolution of dark, eumelanin-rich skin is believed to have occurred early in the evolution of the genus Homo in equatorial Africa, at the same time as the reduction of somatic body hair, to protect from the deleterious effects of UV radiation, including photodegradation of 5-MTHF (12–14, 30). As humans dispersed to areas with greater seasonal variations in sunlight and lower intensities of UV radiation, depigmentation is thought to have occurred to facilitate adequate UVB-induced vitamin D3 synthesis. Indeed, strong correlations have been demonstrated between skin pigmentation and the absolute latitude at which a population lives (13) and between skin pigmentation and the rate of cutaneous vitamin D3 synthesis (5, 9). We, therefore, hypothesized that individuals with constitutively darker skin would be protected from UVB-induced degradation of 5-MTHF and resultant reductions in NO-dependent vasodilation of the cutaneous microvasculature. Our data indicate that NO-dependent vasodilation was attenuated in the cutaneous microvasculature after acute UVB exposure but that this effect was independent of skin color. Furthermore, local 5-MTHF or ASC treatments had similar effects regardless of the level of skin pigmentation.
It may be that darkly and lightly pigmented individuals are equally susceptible to the acute effects of UV radiation but that those with darker skin are better able to adapt to the effects of UV radiation and are thereby better protected from repeated exposures. Indeed, immediate pigment darkening after UV radiation exposure protects 5-MTHF from sensitization to UV radiation by natural photosensitizers, such as riboflavin and uroporphyrin (25). Future studies may be warranted to observe the role of immediate pigment darkening in protecting from UV radiation-induced reductions in 5-MTHF and NO-mediated vasodilation in the cutaneous microvasculature. The effects of UVA on 5-MTHF status and NO-mediated vasodilation also warrant study. UVA penetrates the dermis of the skin more deeply than UVB, its passage is more strongly dependent on melanin concentration, and it may have a greater effect on dermal capillaries than UVB (2, 8). UVA is also present at higher levels throughout most of the year at most latitudes (14). It will also be worth examining the effects of multiple UV radiation exposures over the course of days or weeks, a situation that would more closely simulate the conditions of chronic outdoor sun exposure experienced by humans during most of evolution.
Blunted cutaneous vasodilation in response to local heating, as well as an attenuated NO contribution to the response, has been demonstrated in healthy young African American compared with Euro-American subjects (11, 20). The contrary findings in the present study are likely explained by heritage. Subjects were included in the previous studies only if both parents were of African American or Euro-American descent. In contrast, our study included subjects independent of specific heritage, and comparisons were made based solely upon skin pigmentation. It is, therefore, possible that differences in vascular function between African American and Euro-American subjects demonstrated previously may be explained by genetic factors or mediated by a chemical intermediary and not directly by skin pigment.
Limitations.
Data collection for this study occurred in the months from August through April. Thus, there was likely substantial variation in the amount of daily UV radiation from the sun that subjects were exposed to, depending upon the time of year during which they participated. To limit this variability, we tested the skin of the ventral forearm, which is exposed to less UV radiation compared with the dorsal aspect of the arm during daily activities. We found that there was no variation (P = 0.87) in the response to acute UV radiation exposure as a function of the time of year at which data were collected.
UV radiation exposure in the present study was limited to wavelengths in the UVB region. We chose to utilize only UVB because 5-MTHF may be degraded by UVB either directly or indirectly via production of ROS (25, 37), although the effects of these two mechanisms in vivo are unclear. Conversely, UVA is unable to directly affect 5-MTHF. Therefore, the current study sought to elucidate the specific effects of UVB exposure on 5-MTHF bioavailability in vivo. Our findings suggest that 5-MTHF is degraded by UVB in vivo, at least in part because of UVB-induced production of ROS. Further research is needed to examine how broad-spectrum UV radiation exposure may affect bioavailable 5-MTHF and NO-mediated cutaneous vasodilation.
UVB exposure was limited to the ventral aspect of the forearm, allowing us to observe local effects of UVB on NO-dependent vasodilation and, indirectly, bioavailability of 5-MTHF. Although our results demonstrated reductions in the NO component of cutaneous vasodilation via 5-MTHF-related mechanisms, we are unable to speculate as to how systemic vascular function and bioavailability of 5-MTHF would be affected by whole-body UV radiation exposure. Recent data have demonstrated a negative relation between blood folate concentration and UV radiation exposure accumulated over 42 days, suggesting that repeated UV radiation exposures may result in diminished systemic 5-MTHF bioavailability (21). However, it remains unclear how accumulated UV radiation exposure may affect cutaneous 5-MTHF bioavailability and microvascular function. Future investigation is warranted to further elucidate the role of UV radiation on 5-MTHF and cutaneous microvascular function.
Perspectives.
The results of the current study suggest that NO-mediated vasodilation is attenuated after acute exposure to UVB. Additionally, the observed reduction in NO-dependent vasodilation is mediated by reductions in bioavailable 5-MTHF, most likely through ROS production. Vasodilation of the cutaneous microvasculature is an important facet of the physiological response to local (23) and whole-body heating (17), and proper function of this response is reliant upon adequate bioavailability of NO (17, 18). Although we have demonstrated that UVB exposure acutely diminishes this response to local heat, it is unknown how chronic UV radiation exposure may affect long-term cutaneous microvascular function and/or thermoregulatory increases in skin blood flow. The data from the present study exemplify the importance of improving our understanding of the effects of UV radiation on cutaneous microvascular function, as well as how to mitigate these effects.
In summary, NO-mediated vasodilation in the cutaneous microvasculature was attenuated by acute UVB exposure. This attenuation was due to reductions in bioavailable 5-MTHF and/or UVB-induced increases in ROS. The effects of UVB on NO-mediated vasodilation were independent of skin pigmentation, suggesting that a dark constitutive skin color does not play a protective role in these outcome measures after acute exposure to UVB. Further investigation is warranted to better understand the role of UV radiation on vascular function, as well as the impact of various interventions on this response.
GRANTS
This research was supported by the Pennsylvania State University Center for Human Evolution and Diversity Research Endowment Grant (to W. L. Kenney) and NIH T-32 Grant no. 5T-32-AG-049676–03 (to S. T. Wolf).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.E.S., N.G.J., and W.L.K. conceived and designed research; S.T.W. and A.E.S. performed experiments; S.T.W. analyzed data; S.T.W., A.E.S., and W.L.K. interpreted results of experiments; S.T.W. prepared figures; S.T.W. drafted manuscript; S.T.W., A.E.S., N.G.J., and W.L.K. edited and revised manuscript; S.T.W., A.E.S., N.G.J., and W.L.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful for the subjects’ participation and the assistance of Jane Pierzga and Susan Slimak.
REFERENCES
- 1.Alexander LM, Kutz JL, Kenney WL. Tetrahydrobiopterin increases NO-dependent vasodilation in hypercholesterolemic human skin through eNOS-coupling mechanisms. Am J Physiol Regul Integr Comp Physiol 304: R164–R169, 2013. doi: 10.1152/ajpregu.00448.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson RR, Parrish JA. The optics of human skin. J Invest Dermatol 77: 13–19, 1981. doi: 10.1111/1523-1747.ep12479191. [DOI] [PubMed] [Google Scholar]
- 3.Bruning RS, Santhanam L, Stanhewicz AE, Smith CJ, Berkowitz DE, Kenney WL, Holowatz LA. Endothelial nitric oxide synthase mediates cutaneous vasodilation during local heating and is attenuated in middle-aged human skin. J Appl Physiol (1985) 112: 2019–2026, 2012. doi: 10.1152/japplphysiol.01354.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunt VE, Minson CT. KCa channels and epoxyeicosatrienoic acids: major contributors to thermal hyperaemia in human skin. J Physiol 590: 3523–3534, 2012. doi: 10.1113/jphysiol.2012.236398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen TC, Chimeh F, Lu Z, Mathieu J, Person KS, Zhang A, Kohn N, Martinello S, Berkowitz R, Holick MF. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys 460: 213–217, 2007. doi: 10.1016/j.abb.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crabtree MJ, Channon KM. Synthesis and recycling of tetrahydrobiopterin in endothelial function and vascular disease. Nitric Oxide 25: 81–88, 2011. doi: 10.1016/j.niox.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eichler M, Lavi R, Shainberg A, Lubart R. Flavins are source of visible-light-induced free radical formation in cells. Lasers Surg Med 37: 314–319, 2005. doi: 10.1002/lsm.20239. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmann K, Kaspar K, Altmeyer P, Gambichler T. UV transmission measurements of small skin specimens with special quartz cuvettes. Dermatology 201: 307–311, 2000. doi: 10.1159/000051543. [DOI] [PubMed] [Google Scholar]
- 9.Holick MF, MacLaughlin JA, Doppelt SH. Regulation of cutaneous previtamin D3 photosynthesis in man: skin pigment is not an essential regulator. Science 211: 590–593, 1981. doi: 10.1126/science.6256855. [DOI] [PubMed] [Google Scholar]
- 10.Holowatz LA, Kenney WL. Acute localized administration of tetrahydrobiopterin and chronic systemic atorvastatin treatment restore cutaneous microvascular function in hypercholesterolaemic humans. J Physiol 589: 4787–4797, 2011. doi: 10.1113/jphysiol.2011.212100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurr C, Patik JC, Kim K, Christmas KM, Brothers RM. Tempol augments the blunted cutaneous microvascular thermal reactivity in healthy young African Americans. Exp Physiol 103: 343–349, 2018. doi: 10.1113/EP086776. [DOI] [PubMed] [Google Scholar]
- 12.Jablonski NG. The evolution of human skin and skin color. Annu Rev Anthropol 33: 585–623, 2004. doi: 10.1146/annurev.anthro.33.070203.143955. [DOI] [Google Scholar]
- 13.Jablonski NG, Chaplin G. The evolution of human skin coloration. J Hum Evol 39: 57–106, 2000. doi: 10.1006/jhev.2000.0403. [DOI] [PubMed] [Google Scholar]
- 14.Jablonski NG, Chaplin G. Colloquium paper: human skin pigmentation as an adaptation to UV radiation. Proc Natl Acad Sci USA 107, Suppl 2: 8962–8968, 2010. doi: 10.1073/pnas.0914628107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson JM, O’Leary DS, Taylor WF, Kosiba W. Effect of local warming on forearm reactive hyperaemia. Clin Physiol 6: 337–346, 1986. doi: 10.1111/j.1475-097X.1986.tb00239.x. [DOI] [PubMed] [Google Scholar]
- 16.Karppinen T, Ala-Houhala M, Ylianttila L, Kautiainen H, Viljakainen H, Reunala T, Snellman E. Narrowband ultraviolet B exposures maintain vitamin D levels during winter: a randomized controlled trial. Acta Derm Venereol 96: 490–493, 2016. doi: 10.2340/00015555-2269. [DOI] [PubMed] [Google Scholar]
- 17.Kellogg DL Jr, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol (1985) 85: 824–829, 1998. doi: 10.1152/jappl.1998.85.3.824. [DOI] [PubMed] [Google Scholar]
- 18.Kellogg DL Jr, Liu Y, Kosiba IF, O’Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol (1985) 86: 1185–1190, 1999. doi: 10.1152/jappl.1999.86.4.1185. [DOI] [PubMed] [Google Scholar]
- 19.Kenney WL. Edward F. Adolph distinguished lecture: skin-deep insights into vascular aging. J Appl Physiol (1985) 123: 1024–1038, 2017. doi: 10.1152/japplphysiol.00589.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim K, Hurr C, Patik JC, Matthew Brothers R. Attenuated cutaneous microvascular function in healthy young African Americans: role of intradermal l-arginine supplementation. Microvasc Res 118: 1–6, 2018. doi: 10.1016/j.mvr.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Lucock M, Beckett E, Martin C, Jones P, Furst J, Yates Z, Jablonski NG, Chaplin G, Veysey M. UV-associated decline in systemic folate: implications for human nutrigenetics, health, and evolutionary processes. Am J Hum Biol 29: e22929, 2017. doi: 10.1002/ajhb.22929. [DOI] [PubMed] [Google Scholar]
- 22.Minson CT. Thermal provocation to evaluate microvascular reactivity in human skin. J Appl Physiol (1985) 109: 1239–1246, 2010. doi: 10.1152/japplphysiol.00414.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol (1985) 91: 1619–1626, 2001. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 24.Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol (1985) 93: 1644–1649, 2002. doi: 10.1152/japplphysiol.00229.2002. [DOI] [PubMed] [Google Scholar]
- 25.Moan J, Nielsen KP, Juzeniene A. Immediate pigment darkening: its evolutionary roles may include protection against folate photosensitization. FASEB J 26: 971–975, 2012. doi: 10.1096/fj.11-195859. [DOI] [PubMed] [Google Scholar]
- 26.Moehrle M. Ultraviolet exposure in the Ironman triathlon. Med Sci Sports Exerc 33: 1385–1386, 2001. doi: 10.1097/00005768-200108000-00021. [DOI] [PubMed] [Google Scholar]
- 27.Moehrle M, Heinrich L, Schmid A, Garbe C. Extreme UV exposure of professional cyclists. Dermatology 201: 44–45, 2000. doi: 10.1159/000018428. [DOI] [PubMed] [Google Scholar]
- 28.Off MK, Steindal AE, Porojnicu AC, Juzeniene A, Vorobey A, Johnsson A, Moan J. Ultraviolet photodegradation of folic acid. J Photochem Photobiol B 80: 47–55, 2005. doi: 10.1016/j.jphotobiol.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Olney RS, Mulinare J. Trends in neural tube defect prevalence, folic acid fortification, and vitamin supplement use. Semin Perinatol 26: 277–285, 2002. doi: 10.1053/sper.2002.34773. [DOI] [PubMed] [Google Scholar]
- 30.Parra EJ, Kittles RA, Shriver MD. Implications of correlations between skin color and genetic ancestry for biomedical research. Nat Genet 36, Suppl: S54–S60, 2004. doi: 10.1038/ng1440. [DOI] [PubMed] [Google Scholar]
- 31.Serrano MA, Cañada J, Moreno JC, Gurrea G. Personal UV exposure for different outdoor sports. Photochem Photobiol Sci 13: 671–679, 2014. doi: 10.1039/C3PP50348H. [DOI] [PubMed] [Google Scholar]
- 32.Smith CJ, Santhanam L, Bruning RS, Stanhewicz A, Berkowitz DE, Holowatz LA. Upregulation of inducible nitric oxide synthase contributes to attenuated cutaneous vasodilation in essential hypertensive humans. Hypertension 58: 935–942, 2011. doi: 10.1161/HYPERTENSIONAHA.111.178129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stanhewicz AE, Alba BK, Kenney WL, Alexander LM. Dairy cheese consumption ameliorates single-meal sodium-induced cutaneous microvascular dysfunction by reducing ascorbate-sensitive oxidants in healthy older adults. Br J Nutr 116: 658–665, 2016. doi: 10.1017/S0007114516002579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stanhewicz AE, Alexander LM, Kenney WL. Folic acid supplementation improves microvascular function in older adults through nitric oxide-dependent mechanisms. Clin Sci (Lond) 129: 159–167, 2015. doi: 10.1042/CS20140821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stanhewicz AE, Greaney JL, Kenney WL, Alexander LM. Sex- and limb-specific differences in the nitric oxide-dependent cutaneous vasodilation in response to local heating. Am J Physiol Regul Integr Comp Physiol 307: R914–R919, 2014. doi: 10.1152/ajpregu.00269.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stanhewicz AE, Kenney WL. Role of folic acid in nitric oxide bioavailability and vascular endothelial function. Nutr Rev 75: 61–70, 2017. doi: 10.1093/nutrit/nuw053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steindal AH, Juzeniene A, Johnsson A, Moan J. Photodegradation of 5-methyltetrahydrofolate: biophysical aspects. Photochem Photobiol 82: 1651–1655, 2006. doi: 10.1111/j.1751-1097.2006.tb09826.x. [DOI] [PubMed] [Google Scholar]
- 38.Tam TT, Juzeniene A, Steindal AH, Iani V, Moan J. Photodegradation of 5-methyltetrahydrofolate in the presence of uroporphyrin. J Photochem Photobiol B 94: 201–204, 2009. doi: 10.1016/j.jphotobiol.2008.12.003. [DOI] [PubMed] [Google Scholar]