Key Clinical Message
In the treatment of immunotherapy with immune checkpoint inhibitors, we often experience immune‐related adverse event which manifest most frequently as a skin disorder, and very rarely as a renal disorder. In our manuscript, we report the case of a 71‐year‐old man with nivolumab‐induced severe acute kidney injury (AKI) in which the time from treatment initiation to the onset of AKI was the longest among the previously reported cases (377 days). Prolonged follow‐up is therefore warranted to detect late‐onset AKI.
Keywords: acute kidney injury, immune checkpoint inhibitors, immune‐related adverse event, nivolumab
1. INTRODUCTION
Immune checkpoint inhibitors (ICIs) have become the mainstream treatment of lung cancer, and the anti‐programmed cell death‐1 (PD‐1) antibody, nivolumab, has been widely used as an ICI. ICIs have a unique adverse event known as immune‐related adverse event (irAE) which manifest most frequently as a skin disorder, and very rarely as a renal disorder.1 Recently, it has been known that the histopathology of acute kidney injury (AKI) associated with irAE show acute interstitial nephritis (AIN) in most cases.2 It is speculated that the mechanism of ICI‐induced AIN differs from other medications‐induced AIN. Moreover, it might cause late‐onset AKI, which is characteristic of ICI‐induced AIN.
Herein, we present a case of nivolumab‐induced severe AKI in which the time from treatment initiation to the onset of AKI was the longest among the reported cases. This case report contributes to the understanding of the complex mechanism underlying the effect of ICIs on systemic organs and the safety of rechallenging with this immunotherapy.
2. CASE PRESENTATION
A 71‐year‐old man was admitted to our hospital for investigation of an elevated tumor marker of carbohydrate antigen 19‐9 level. His past medical history included hypertension and chronic kidney disease (with baseline serum creatinine level of 1.4 mg/dL), and he was a past smoker (70 pack‐years). Computed tomography (CT) showed a pulmonary nodule in the left lower lobe and a liver nodule. These lesions showed fluorodeoxyglucose avidity on positron emission tomography‐CT. Biopsy of the liver nodule revealed adenocarcinoma with no driver mutation, and the histology of the remaining liver showed no abnormality. His past medical history and radiological findings were consistent with primary lung cancer with hepatic metastasis. The clinical stage was cT1aN3M1b stage IV. In December 2014, carboplatin (CBDCA) + pemetrexed (PEM) + bevacizumab (BV) were administered as first‐line chemotherapy. After four cycles, partial response was achieved and PEM +BV was continued as maintenance therapy. After four cycles, the tumor marker level was elevated again, and the regimen was changed to docetaxel (DTX) + BV as 2nd line chemotherapy. Maintenance BV monotherapy was started after four cycles of 2nd line therapy. During BV maintenance therapy, the patient developed pneumothorax twice, and was managed conservatively. After five cycles of maintenance therapy, radiological evaluation showed progressive disease and nivolumab was selected as 3rd line chemotherapy in July 2016. After 13 cycles, the patient was diagnosed with asymptomatic hypothyroidism (serum thyroid stimulating hormone level was 87.5 μU/mL and serum free tetraiodothyronine level was 0.45 ng/mL), which was thought to be caused by nivolumab as an immune‐related adverse event (irAE), and 50 μg of levothyroxine was administered. The level of serum creatinine was stable during the 26 treatment cycles and the best clinical response achieved was partial response. In July 2017, the patient was admitted to our hospital with anorexia and the level of serum creatinine was found to be elevated, at 4.61 mg/dL. This AKI was thought to be caused by nivolumab, as an irAE. Immunotherapy was stopped, and corticosteroid therapy was initiated, and was continued for 3 months. Two weeks after corticosteroid withdrawal, the level of serum creatinine was elevated to 10.64 mg/dL. Corticosteroid therapy was restarted, and serum creatinine level decreased to 2.2 mg/dL. In January 2018, nivolumab was rechallenged after the recovery of kidney function. After two cycles, serum creatinine level was elevated again to 8.05 mg/dL and urine test indicated pyuria and proteinuria (Urine microscopy test showed 18 white blood cells/high power field (HPF) and three red blood cells/HPF, and urine protein‐creatinine ratio was 1.66 g/g Cre). The high level of urinary α1‐microglobulin excretion was found (144 mg/L), which indicated renal tubular disorder. The patient complained of vomiting and general malaise due to uremia, which required hemodialysis for relief. Renal biopsy which was performed after it, showed acute tubulointerstitial nephritis with infiltrating neutrophils and lymphocytes (Figure 1A,B), which corresponded with urinary test findings. The serum creatinine level decreased to 2.82 mg/dL with corticosteroid therapy. The clinical course is shown in Figure 2. The management plan was shifted to best supportive care without chemotherapy.
Figure 1.
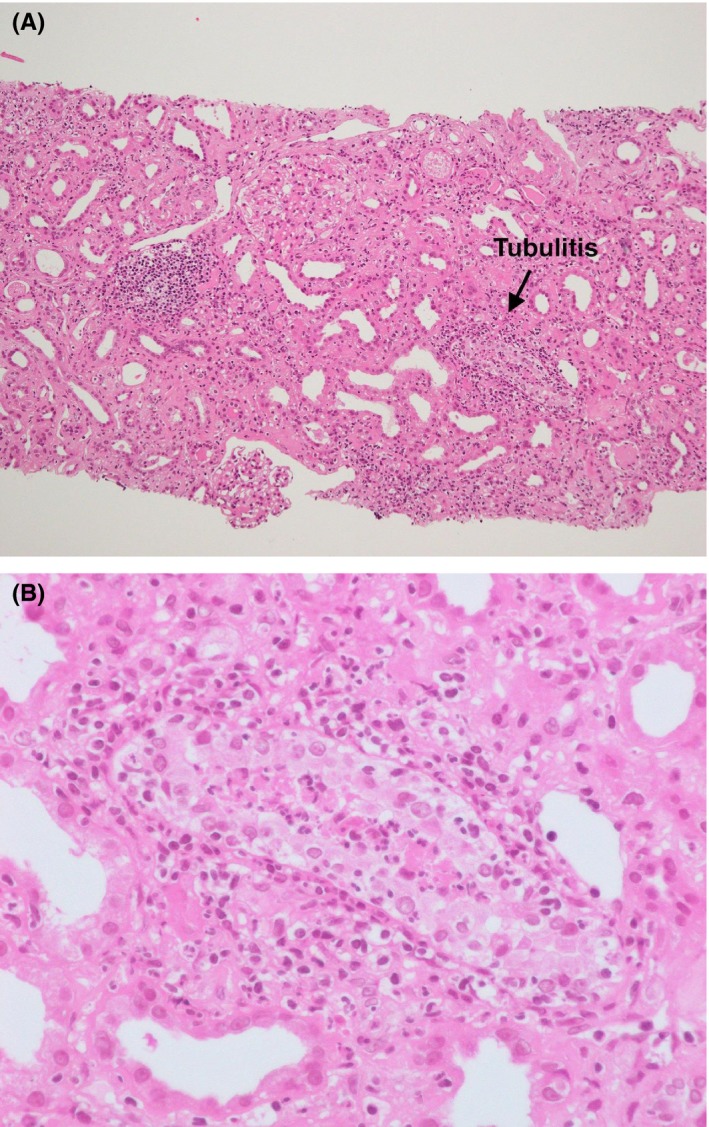
Pathology of acute interstitial nephritis. A, Hematoxylin and eosin staining. The glomeruli are mostly intact. On the other hand, tubulitis is shown and lymphocytes and plasma cells are invading the fibrosed tubulointerstitium (×100). B, The portion of tubulitis is enlarged (arrow, A). Neutrophils are infiltrating the tubules and tubular epithelial cells, most of which show degenerative changes
Figure 2.
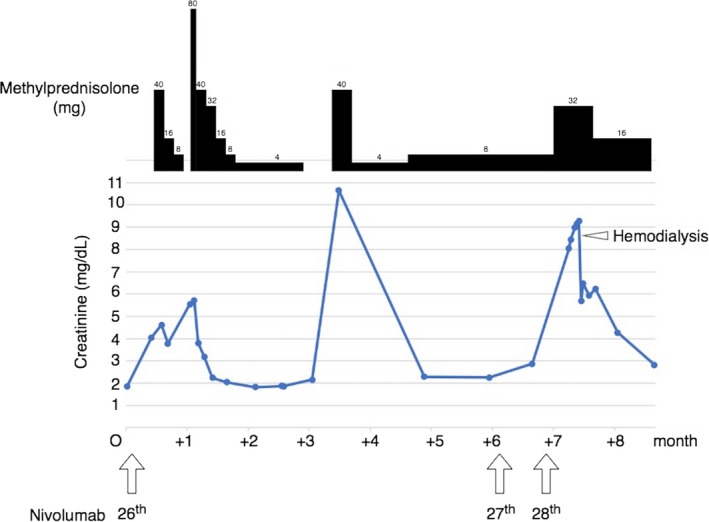
Clinical course of the patient after the onset of acute kidney injury. The serum creatinine was elevated after the 26th cycle of nivolumab, although it was stable until the 25th cycle, and corticosteroid therapy resulted in an improved renal function. After two cycles of rechallenging with nivolumab, severe acute kidney injury occurred and hemodialysis was required
3. DISCUSSION
Immune‐related adverse events, which are induced by ICIs, can affect various organ systems. A meta‐analysis reported that in patients treated with PD‐1 inhibitors the overall incidence of irAEs was 26.82% (any grade) and 6.10% (severe grade).1 The organ most frequently affected with irAEs is the skin, followed by the gastrointestinal tract, endocrine system, liver, lung, and kidney.1 The incidence of nephritis secondary to irAEs was very low in comparison with other organs; Cortazar FB et al reported that the overall incidence of AKI was 2.2% among a total of 3695 patients treated with ICI monotherapy.2 In this review, all the patients who underwent kidney biopsy after ICI‐induced AKI showed histological features of AIN which resembled those of drug‐induced AIN.
Drug hypersensitivity reactions which occur as adverse events are immediate, mediated by immunoglobulin‐E (type I reaction) or delayed, mediated by T cells (type IV reaction).3 T‐cell‐mediated reaction has a major role in drug‐induced AINs. Antigen‐presenting cells (APCs), such as dendritic cells, present the core structure of the drug to T cells, leading to an inflammatory response. On the other hand, down‐regulation of major histocompatibility complex class II, which is expressed on the surface of APCs, or activation of suppressor T cells, prevent drug hypersensitivity.
PD‐1 ligation inhibits T‐cell receptor signaling in both CD 8+ and CD 4+ T cells, causing immune suppression.4 The inhibition of PD‐1/PD‐L1 signaling with anti‐PD‐1 antibody up‐regulates T‐cell‐mediated inflammation, which may result in AIN.5
By definition, drug hypersensitivity reaction occurs 48‐72 hours after exposure to the causative medication.3 However, kidney injury is not often detected until an increase in serum creatinine is manifested, which might take 7‐10 days after exposure.3 The time from initiation of ICI to AKI was much longer than that of other medications; Cortazar FB et al reported that the median time was 91 (range, 21‐245) days.2 In our case, the duration from initiation of nivolumab to onset of AKI was 377 days, which was longer than any other cases in that paper. The plausible reason for delayed onset of AKI is that ICI, itself, is not likely to be the antigen responsible for triggering the AKI, in contrast to other medications. ICI may reprogram the immune system, leading to loss of tolerance against endogenous kidney antigens.2 Shirali et al reported six lung cancer patients with biopsy‐proven AIN during treatment with nivolumab or pembrolizumab.4 In this report, all the six cases received proton‐pump inhibitors (PPIs) and/or nonsteroidal inflammatory drugs which were well associated with the development of AIN. In four cases, the medications were started long before PD‐1 inhibitor therapy. One case was managed with PPI discontinuation only, without administration of corticosteroid, and nivolumab was continued for 8 months after discontinuation of PPI, indicating that PPI was the key drug causing AIN in this case. In a case report, lansoprazole‐induced AIN after administration of nivolumab and drug‐induced lymphocyte stimulation test for lansoprazole was proven to be positive.6 In our case, however, no culprit medication associated with AIN was administered, indicating that an endogenous antigen attacked the kidney.
ICI rechallenging was undertaken following improvement of AKI in three cases and AKI recurrence developed in one case.2, 4 In that case, serum creatinine level was elevated to 1.7 mg/dL from a baseline level of 1.2 mg/dL, while our patient showed more severe AKI requiring hemodialysis.
Though the incidence of AKI induced by ICIs is less than that of other organ dysfunction, patients may develop severe AKI requiring hemodialysis. ICIs‐induced AKI is characterized by late onset after administration, and in our case, it was the longest duration among the reported cases. It is speculated that ICIs reprogram the immune system, leading to the loss of tolerance to endogenous and exogenous antigens, which is different from the mechanism underlying AKI induced by other medications. Therefore, rechallenging ICIs after recovery of the renal function should be tried. Nevertheless, prolonged follow‐up is needed to detect the late recurrence of severe AKI as reported in our case.
In conclusion, we have to consider ICIs‐induced AKI even after long‐term treatment with them. However, rechallenging of them can be selected according to each situation because the pathophysiology of AKI caused by ICIs is different from one caused by other medications.
AUTHOR CONTRIBUTION
UN, ST, KF, and MK: cared and followed up the patient. KM: provided pathological analysis of patient. TM: supported clinical management of patient care. UN, MK, and KF: wrote and revised the manuscript. ST, KM, and TM: supervised the manuscript.
Uchida N, Tsuji S, Fujita K, Koizumi M, Moriyoshi K, Mio T. Nivolumab‐induced severe acute kidney injury with a long latent phase in a patient with non‐small‐cell lung cancer: A case report. Clin Case Rep. 2018;6:2185–2188. 10.1002/ccr3.1848
REFERENCES
- 1. Wang PF, Chen Y, Song SY et al. Immune‐related adverse events associated with anti‐PD‐1/PD‐L1 treatment for malignancies: a meta‐analysis. Front Pharmacol. 2017;18(8):730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cortazar FB, Marrone KA, Troxell ML et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 2016;90(3):638‐647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Raghavan R, Shawar S. Mechanisms of drug‐induced interstitial nephritis. 1. Adv Chronic Kidney Dis. 2017;24(2):64‐71. [DOI] [PubMed] [Google Scholar]
- 4. Shirali AC, Perazella MA, Gettinger S. Association of acute interstitial nephritis with programmed cell death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis. 2016;68(2):287‐291. [DOI] [PubMed] [Google Scholar]
- 5. Wanchoo R, Karam S, Uppal NN et al. Adverse renal effects of immune checkpoint inhibitors: a narrative review. Am J Nephrol. 2017;45(2):160‐169. [DOI] [PubMed] [Google Scholar]
- 6. Koda R, Watanabe H, Tsuchida M et al. Immune checkpoint inhibitor (nivolumab)‐associated kidney injury and the importance of recognizing concomitant medications known to cause acute tubulointerstitial nephritis: a case report. BMC Nephrol. 2018;19(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]


