Key Clinical Message
Splinting and mechanical disruption of the mitral valve apparatus is an important limitation of an endocardial left ventricular (LV) pacing lead. Further, long‐term data are required before this approach is more widely adopted.
Keywords: cardiac resynchronization therapy, endocardial LV, mitral regurgitation, mitral valve
1. CASE
Stimulation of the left ventricular endocardium to deliver cardiac resynchronization therapy is an option for patients in whom conventional epicardial pacing is not possible. One potential limitation of pacing the endocardium via the trans‐atrial septal approach is that the pacing lead may disrupt the integrity of the mitral valve.
A 65‐year‐old man with a history of myocardial infarction, pulmonary embolism and Factor V Leiden mutation underwent a dual‐chamber pacemaker insertion for sick sinus syndrome. He had ongoing, symptomatic atrial tachyarrhythmias despite six ablation procedures, with evidence of significantly impaired left ventricular (LV) systolic function. Medications included warfarin, bisoprolol 10 mg od and ramipril 10 mg od. He was also prescribed mexiletine 200 mg bd for a high burden of frequent ventricular ectopics.
He underwent upgrade to a biventricular defibrillator (CRTD) (ongoing breathlessness, poor LV systolic function and 80% right ventricular, RV pacing) and subsequent catheter ablation of the atrioventricular node. At the time of implant, echocardiography demonstrated mild mitral regurgitation. A LV lead (Attain Performa, Medtronic, Minneapolis, USA) was implanted via the coronary sinus into a posterolateral vein, where it gave acceptable pacing parameters (thresholds of <2.5 V without phrenic nerve stimulation in LV2‐LV3, LV2‐LV4, LV3‐RV coil). However, the lead required deactivation within a month due to insurmountable phrenic nerve stimulation (despite testing in all available vectors with higher pulse widths and lower outputs). In addition, the atrial lead had displaced. Other than the large posterolateral vein, there were no other viable venous epicardial tributaries for LV lead placement.
Given the lack of alternative favorable, epicardial venous options for LV stimulation, he was referred for a trans‐septal LV endocardial lead to deliver cardiac resynchronization therapy (CRT), in addition to a right atrial (RA) lead revision. An LV endocardial lead (Medtronic 6254, 85 cm) was successfully implanted via a trans‐atrial septal approach (Figure 1), using a previously described technique1 and the epicardial LV lead was removed. Despite excellent pacing parameters during follow‐up and an initial clinical improvement, his breathlessness was worse by 6 months. Transthoracic echocardiography demonstrated severe mitral regurgitation; trans‐esophageal echocardiography (TEE) confirmed that this was likely to be due to a combination of splinting of the mitral valve apparatus (posterior leaflet, P2 scallop) by the LV lead, in addition to ischemic tethering (Figure 2, Video S1).
Figure 1.

Postprocedural posteroanterior (PA) X‐ray. LV endocardial lead placed into the basal posterolateral wall. RA lead, RV pace‐sense and separate, defibrillator lead in conventional positions
Figure 2.
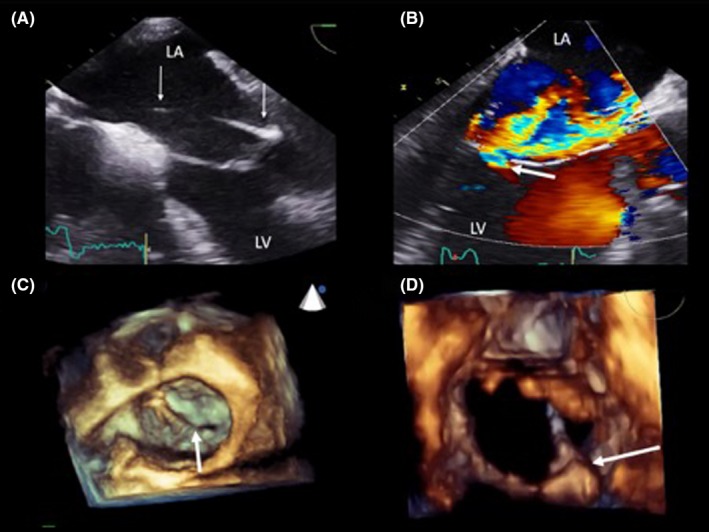
Trans‐esophageal echocardiogram (TEE). The LV lead is crossing the LA toward the posterior mitral valve leaflet at 0 degree (panel A). There is significant valvular regurgitation depicted by the white arrow at 120 degrees (panel B). Three‐dimensional reconstruction shows an en‐face view of the mitral valve with the white arrow demonstrating the endocardial LV lead preventing complete apposition of mitral valve leaflets at the P2/A2 scallops (panel C). The lead penetrates the mitral valve orifice at P2 (panel D)
The case was discussed at a multidisciplinary cardiac devices meeting in the presence of electrophysiologists, cardiac surgeons, general cardiologists and cardiac imaging specialists. The LV endocardial lead was considered to be contributing significantly to the severe mitral regurgitation. The options of a surgical lead removal with or without mitral valve repair/replacement or percutaneous lead extraction were considered. At that stage, the LV lead had been implanted for 10 months and it was concluded that a percutaneous approach would be less invasive and carry less procedural risk than cardiothoracic surgery. The patient was in full agreement with this decision and consented to percutaneous removal.
The procedure was performed under general anesthesia, with intraprocedural TEE. The generator and leads were mobilized. A firm standard stylet was advanced to the tip of the LV endocardial lead and the active fixation helix retracted. With gentle traction alone, the lead detached from the LV endocardium and was pulled through the interatrial septum (Video S2), leaving a residual atrial septal defect (Figure 3). There was an improvement of mitral leaflet apposition but persistence of mitral regurgitation due to ischemic tethering of P2/3 (Figure 3). The body of a 7F femoral sheath was passed over the LV lead in order to maintain venous access without additional venepuncture. A Boston Scientific CS Hook and 130‐degree subselector facilitated coronary venography. The main (and only) posterolateral vein had several sub‐branches. A quadripolar LV lead (Attain Performa, Medtronic, Minneapolis, USA) was placed in the same posterolateral cardiac vein; however, the final lead position was different here to that of the original CRT implant (Figure4 ). On this occasion, the capture threshold in this position was 1.8 V at 1 ms without evidence of phrenic nerve stimulation at high output. These parameters were accepted and the lead attached to the existing generator and placed in a prepectoral pocket. The wound was closed in routine fashion. The patient made a good recovery from the general anesthetic, was without any neurological insult upon discharge, and reported an improved exercise tolerance and less breathlessness at 6 months postprocedure.
Figure 3.

Trans‐esophageal echocardiography (TEE). The LV lead is traversing the interatrial septum inferiorly en route to the LA, prior to extraction at 90 degrees (panel A). A residual left to right shunt demonstrated using color Doppler after removal of the lead at 90 degrees (panel B). There is residual mitral regurgitation but significantly less than prior to removal (panel C). Three‐dimensional en‐face reconstruction of the mitral valve apparatus showing a residual deficit at the P2 scallop (panel D)
Figure 4.

PA X‐Ray with original CRTD in situ and displaced right atrial lead (panel A). Selective coronary sinus venogram showing a large posterolateral vein with several tributaries and a middle cardiac vein along the inferior surface of the heart (panel B). PA projection X‐Ray of the final, new LV lead position (panel C). The cardiac silhouette is significantly larger; splaying of the carina suggests enlarged atria as a consequence of the severe mitral regurgitation compared with panel A, one year earlier. The quadripolar LV lead was reimplanted into a different distal sub‐branch of the posterolateral vein compared with the original implant.
2. DISCUSSION
Left ventricular endocardial leads are an alternative way of providing LV stimulation and thus delivering biventricular pacing in those in whom the epicardial venous route has failed. There is evidence from both animal and clinical studies that, compared to epicardial stimulation, LV endocardial stimulation by activating the ventricular myocardium in a more physiological fashion may deliver superior electrical and hemodynamic properties and be less likely to cause phrenic nerve stimulation.2, 3, 4, 5 For a select group of patients who have failed conventional CRT delivered via an epicardial LV lead, this approach may be of value. A growing body of clinical data now exists on the feasibility of delivering an LV lead via the trans‐atrial septal route to the LV endocardium. The Alternate Site Cardiac Resynchronization (ALSYNC) study demonstrated successful LV endocardial lead implantation in 89% (120/138) patients.6 Safety concerns include systemic thromboembolism, disruption of the mitral valve apparatus, and the feasibility and safety of lead extraction; however, there is limited published data. A recent systematic review by Gamble et al7 examined 23 studies with 384 patients who underwent LV endocardial pacing for CRT delivery. Procedural success was over 95%, with a stroke risk of 2.5 events per 100 patient years and mortality rate 4.5 per 100 patient years. In ALSYNC, the largest body of data on LV endocardial pacing for CRT, 5 patients had a system infection requiring extraction; all cases were reported to be successful without complication but further detail was not given.6
In this patient, the LV lead had been in situ for 10 months prior to extraction. Given it was splinting the mitral valve apparatus, there was concern about potential damage to the valve upon removal in addition to arterial embolization. The risk of embolic stroke during interventions within the arterial system is well described within the transcatheter aortic valve implantation (TAVI) cohort of patients; there is a high prevalence of subclinical infarcts on neurological imaging and clinical cerebrovascular accidents.8 There are, however, no published reports of transcutaneous extraction of endocardial LV leads, and therefore, the prevalence of such complications is unknown.
Importantly, stimulation of the LV endocardium is also possible without interference with the mitral valve apparatus. Betts et al have described delivering energy via a radiofrequency needle from within a steerable sheath placed against the mid‐septum of the right ventricle, creating a transventricular passage to the LV endocardium. As there is no lead in the left atrium (LA), it may be that the risk of embolic stroke is lower; no strokes were reported in 10 patients over an 8‐month follow‐up period, but the longer term outcomes are needed.9 In addition, the LV lead in this technique does not traverse the mitral valve apparatus, and therefore, mechanical disruption and splinting of the valve leaflets as occurred in this case may be avoided using this approach.
Finally, the clinical feasibility of wireless intracardiac LV endocardial stimulation for the delivery of biventricular pacing has been described among patients who have failed to improve with a conventional CRT approach. Unlike the single cardiac pacing units currently used for RV pacing,10 the WiSE‐CRT pacing system (EBR Systems, Inc., Sunnyvale, California, USA) comprises a subcutaneous ultrasound pulse generator and battery in addition to a separate transcatheter‐delivered 9‐mm electrode. This can deliver LV endocardial stimulation within 3 ms of sensing a RV pacing spike from a preexisting pacemaker, enabling LV endocardial stimulation but without the presence of a pacing lead in the arterial system of the left heart. Among the 54 patients implanted across two small studies, the mean clinical response to CRT was 74% (which is notable given this group are self selected non responders to conventional CRT).11, 12 Longer term efficacy and safety data are awaited, but results are certainly promising and this too may represent a tangible approach for delivering CRT via LV endocardial stimulation, without the risk of disruption to the mitral valve.
3. CONCLUSION
Splinting and mechanical disruption of the mitral valve apparatus is an important longer‐term consideration in patients receiving an endocardial LV lead. Percutaneous removal of an endocardial LV lead is feasible and safe. A multidisciplinary team approach is important to appreciate and plan for the risks specific to the case. Finally, TEE guidance is invaluable to provide real‐time visualization of the mitral valve apparatus before, during, and after instrumentation.
CONFLICT OF INTEREST
None declared.
AUTHORSHIP
JMB: prepared and wrote the manuscript. SB: carried out the TOE during the procedure and prepared the images/videos for publication. KR, SS, and MD: cared for the patient and contributed to writing and revising the manuscript.
CONSENT
Verbal informed consent to publish this manuscript was obtained from the patient.
Supporting information
Behar JM, Ranjan K, Bhattacharyya S, Sporton S, Dhinoja M. Splinting and mechanical disruption of the mitral valve apparatus by an endocardial left ventricular lead while delivering cardiac resynchronization therapy. Clin Case Rep. 2018;6:2081–2085. 10.1002/ccr3.1758
REFERENCES
- 1. Domenichini G, Diab I, Campbell NG, et al. A highly effective technique for transseptal endocardial left ventricular lead placement for delivery of cardiac resynchronization therapy. Heart Rhythm. 2015;12:943‐949. [DOI] [PubMed] [Google Scholar]
- 2. Derval N, Steendijk P, Gula LJ, et al. Optimizing hemodynamics in heart failure patients by systematic screening of left ventricular pacing sites: the lateral left ventricular wall and the coronary sinus are rarely the best sites. J Am Coll Cardiol. 2010;55:566‐575. [DOI] [PubMed] [Google Scholar]
- 3. Spragg DD, Dong J, Fetics BJ, et al. Optimal left ventricular endocardial pacing sites for cardiac resynchronization therapy in patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2010;56:774‐781. [DOI] [PubMed] [Google Scholar]
- 4. Behar JM, Jackson T, Hyde E, et al. Optimized left ventricular endocardial stimulation is superior to optimized epicardial stimulation in ischemic patients with poor response to cardiac resynchronization therapy. JACC Clin Electrophysiol. 2016;2(7):799‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shetty AK, Sohal M, Chen Z, et al. A comparison of left ventricular endocardial, multisite, and multipolar epicardial cardiac resynchronization: an acute haemodynamic and electroanatomical study. Europace. 2014;16:873‐879. [DOI] [PubMed] [Google Scholar]
- 6. Morgan JM, Biffi M, Gellér L, et al. ALternate Site Cardiac ResYNChronization (ALSYNC): a prospective and multicentre study of left ventricular endocardial pacing for cardiac resynchronization therapy. Eur Heart J. 2016;37(27):2118‐2127. [DOI] [PubMed] [Google Scholar]
- 7. Gamble JHP, Herring N, Ginks M, Rajappan K, Bashir Y, Betts TR. OUP accepted manuscript. Europace. 2016;9:1798‐1804. [Google Scholar]
- 8. Kapadia S, Agarwal S, Miller DC, et al. Insights into timing, risk factors, and outcomes of stroke and transient ischemic attack after transcatheter aortic valve replacement in the PARTNER trial (placement of aortic transcatheter valves). Circ Cardiovasc Interv. 2016;9:e002981. [DOI] [PubMed] [Google Scholar]
- 9. Betts TR, Gamble JHP, Khiani R, Bashir Y, Rajappan K. Development of a technique for left ventricular endocardial pacing via puncture of the interventricular septum. Circ Arrhythm Electrophysiol. 2014;7:17‐22. [DOI] [PubMed] [Google Scholar]
- 10. Miller MA, Neuzil P, Dukkipati SR, Reddy VY. Leadless cardiac pacemakers. J Am Coll Cardiol. 2015;66:1179‐1189. [DOI] [PubMed] [Google Scholar]
- 11. Auricchio A, Delnoy P‐PP‐P, Butter C, et al. Feasibility, safety, and short‐term outcome of leadless ultrasound‐based endocardial left ventricular resynchronization in heart failure patients: results of the Wireless Stimulation Endocardially for CRT (WiSE‐CRT) study. Europace. 2014;16:681‐688. [DOI] [PubMed] [Google Scholar]
- 12. Reddy VY, Miller MA, Neuzil P, et al. Cardiac resynchronization therapy with wireless left ventricular endocardial pacing: the SELECT‐LV study. J Am Coll Cardiol. 2017;69:2119‐2129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


