Abstract
An increased prevalence of malignant lymphoma and of gastric cancer has been observed in large cohorts of patients with common variable immunodeficiency (CVID), the most frequently symptomatic primary immunodeficiency. Surveillance strategies for cancers in CVID should be defined based on epidemiological data. Risks and mortality for cancers among 455 Italian patients with CVID were compared to cancer incidence data from the Italian Cancer Registry database. CVID patients showed an increased cancer incidence for all sites combined (Obs = 133, SIR = 2.4; 95%CI = 1.7–3.5), due to an excess of non-Hodgkin lymphoma (Obs = 33, SIR = 14.3; 95%CI = 8.4–22.6) and of gastric cancer (Obs = 25; SIR = 6.4; 95%CI = 3.2–12.5). CVID patients with gastric cancer and lymphoma had a worse survival in comparison to cancer-free CVID (HR: 4.8, 95%CI: 4.2–44.4 and HR: 4.2, 95%CI: 2.8–44.4). Similar to what observed in other series, CVID-associated lymphomas were more likely to be of B cell origin and often occurred at extra-nodal sites. We collected the largest case-series of gastric cancers in CVID subjects. In contrast to other reports, gastric cancer was the leading cause of death in CVID. Standardized mortality ratio indicated a 10.1-fold excess mortality among CVID patients with gastric cancer. CVID developed gastric cancer 15 years earlier than the normative population, but they had a similar overall survival. Only CVID diagnosed at early stage gastric cancer survived >24 months. Stomach histology from upper endoscopy performed before cancer onset showed areas of atrophic gastritis, intestinal metaplasia or dysplasia. CVID patients might progress rapidly to an advanced cancer stage as shown by patients developing a III-IV stage gastric cancer within 1 year from an endoscopy without signs of dysplasia. Based on high rate of mortality due to gastric cancer in Italian CVID patients, we hereby suggest a strategy aimed at early diagnosis, based on regular upper endoscopy and on Helicobacter pylori infection treatment, recommending an implementation of national guidelines.
Keywords: common variable immunodeficiency: cancer, gastric cancer, lymphoma, IgA, upper endoscopy, risk, guidelines
Introduction
Inherited conditions affecting immune system function are classified as primary immune deficiencies (PID) (1). As the PID life expectancy increased because of improvements in the surveillance, prevention, and treatment, the occurrence of cancer increased (2, 3). In PID, hematological and non-hematological malignancies occur mainly in the fourth to the seventh decades of life while rare case of malignancies are commonly observed in the pediatric population (4). Among PID, an increased prevalence of cancer is recognized in patients affected by common variable immunodeficiency (CVID), the most common symptomatic primary antibody defect. In CVID, the antibody deficiency might derive from decreased diversity of the naive pool, decreased hyper mutation in memory repertoires, an unusual clonal expansion of un-mutated B cells, and from a number of defects in innate and adaptive immune mechanisms (5). Other than sino-pulmonary infections, CVID patients suffer from associated clinical conditions, including autoimmune and inflammatory diseases and neoplasia, mainly lymphoma and gastric cancer (6, 7). Ten years ago, our group described a higher prevalence of lymphoma and gastric carcinomas in the Italian cohort of CVID in comparison to the normative population (8). Five years later, we confirmed this high prevalence rate of lymphoma and gastric carcinomas in a four decades study showing that 21% of adult CVID patients developed cancers. We also observed that deaths from cancer occurred in 10.2%, a percentage double than that reported in a study from a CVID cohort in New York over the same length of time (2, 9). We suggested that the discrepancy in cancer survival, between the two cohorts, might have been due to the high prevalence of deaths for malignancies other than lymphoma in the Italian CVID cohort, and to deaths for gastric cancer. The percentage of patients who died for lymphoma, indeed, was similar in the two studies.
Herein, we analyzed data on the prevalence of hematological and non-hematological malignancies, on cancer risk, on mortality and on survival rate in a cohort of 455 Italian adult CVID patients compared to normative population. Detailed data on CVID patients diagnosed with gastric cancer, histopathology of gastric lesions, cancer outcome and possible associated risk-factors were reported. Based on the high rate of mortality for gastric cancer in Italian CVID patients, we highlight the need of a strategy for an earlier diagnosis and we suggest a new schedule for gastric endoscopy in CVID patients.
Methods
Study design
Data on adult CVID patients (>18 years old), regularly followed in three University-based PID referral centers located in Central Italy (Rome), Southern Italy (Naples), and Northern Italy (Padua-Treviso) were prospectively collected from 01/01/2001 to 31/12/2017 and retrospectively collected from 01/01/1993 to 31/12/2000. To be considered for analysis, subjects needed to fulfill the 2016 ESID revised criteria (http://esid.org/Working-Parties/Registry/Diagnosis-criteria). A set of variables was recorded for each patient including: gender, date of birth, date of CVID diagnosis, data on cancer diagnosis and histology, date of last follow up visit, vital status information, date and cause of death, CVID-associated diseases (infections, cancer, autoimmunity, unexplained persistent proliferation, and unexplained persistent enteropathy) and Helicobacter pylori (H. pylori) status. We excluded from the analysis patients whose data on date cancer occurrence and its outcome and on date of cancer diagnosis, death and last follow-up were lacking. The follow-up period before the occurrence of cancer was calculated since the year of immunodeficiency onset. All subjects were followed until date of death or date of the end of the study (31 December 2017). For the subset of patients who developed cancer, medical records were traced to verify cancer diagnosis, treatments received, clinical complications, and outcome.
AIRTUM estimated cancer incidence
The Associazione Italiana Registro Tumori (AIRTUM) (www.registri-tumori.it) is a coordinated system of population-based cancer registries that collects cancer incidence and survival data from 20 geographic areas throughout Italy, covering 70% of the Italian population (without age restriction). Detailed information is available at http://www.registri-tumori.it/. We used AIRTUM published data to estimate the expected incidence of cancer. Among skin malignancies, melanoma was the only one cancer with data on incidence and mortality reported in the AIRTUM database. For this reason, we did not collected data for not-malignant skin cancer.
Statistical analysis
Demographics of the CVID database were summarized with descriptive statistics. Sociodemographic and clinical variables were compared between the patients who developed cancer and cancer-free patients. Statistical analysis was performed using frequency distributions. The X2 test was used for categorical variables and the t-test was used for continuous variables. The observed numbers of cancer cases among CVID were compared with the expected numbers calculated based on AIRTUM data on incidence rates of cancer in 5-year interval to yield the standardized incidence ratio (SIR). “All cancer” and site-specific cancer SIRs were calculated for the entire cohort, and separately for men and women. For mortality analysis, the time since diagnosis was determined using the age at the time of CVID diagnosis or the age at birth. The endpoint used was the time of last known follow-up or the date of death. Probabilities of survival after the diagnosis of CVID and after the diagnosis of cancer were estimated from Kaplan Meier life Table. Mortality rates (crude death rates, CDRs) of the general population were used to calculate the standardized mortality ratio (SMRs). The CDR was obtained from AIRTUM. SMRs were calculated using the formula, SMR = Observed (Obs) deaths/expected (Exp) deaths. We calculated SMRs as incident cases divided by the contributed person-years. However, general population incidence and mortality data for Italy before 2003 were not available, so only cancer and death occurred after 2003 were included in the analysis. Statistical Package for Social Sciences version 15 (SPSS Inc., 233 South Wacker Drive, 11th Floor, Chicago) was used for the analysis. Confidence Intervals (CI95%) were calculated by R-3.4.4 version.
Results
Patients
As of 31 December 2017, 501 subjects with a CVID diagnosis were included in the dataset. We excluded from the analysis 46 subjects who did not satisfy ESID criteria and patients whose date of death, date of cancer occurrence and outcome, and date of last follow-up could not be accurately determined. Data on 455 CIVD patients were included in the analysis. The characteristics of CVID patients enrolled in the study are summarized in Table 1. The mean age at last follow-up was 51.1 ± 15.0 years, with a 1:1 female: male ratio. Patients were followed-up for a cumulative period of 5,169 person-years with a mean time of follow-up of 11.5 ± 8.9 years. H. pylori status was available in 325/455 patients. H. pylori infection by histology was found in 40 patients (12%).
Table 1.
Characteristics of 455 CVID patients enrolled in the study.
| Characteristics | All patients | Cancer | Cancer-free |
|---|---|---|---|
| AGE INTERVAL—n. (SD) | |||
| 18-35 years | 75 (16.5) | 7 (6.0) | 68 (20.0)*** |
| 36–50 years | 142 (31.2) | 29 (25.0) | 113 (33.3) |
| 51–65 years | 150 (32.9) | 45(38.8) | 105 (31.0) |
| 66–80 years | 82 (18.0) | 33 (28.5) | 49 (14.5)** |
| >80 years | 6 (1.3) | 2 (1.7) | 4 (1.2) |
| Sex (female)—n. (%) | 235 (51.6) | 58 (50.0) | 162 (47.8) |
| Age at CVID diagnosis—mean (SD) | 40.1 (15.4) | 45.8 (13.2) | 38.8 (15.7)*** |
| SERUM IMMUNOGLOBULIN AT DIAGNOSIS (mg/dL)—MEAN (SD) | |||
| IgG | 250.3 (172.3) | 256.2 (168.6) | 248.7 (173.6) |
| IgA | 21.6 (34.2) | 19.9 (30.8) | 22.1 (35.1) |
| IgM | 25.2 (49.8) | 37.0 (89.8) | 21.9 (30.4)* |
| Bronchiectasis | 118 (31) | 26 (31) | 92 (30) |
| Autoimmunity | 130 (28) | 27 (28) | 103 (29) |
| Lymphoproliferative | 113 (31) | 28 (33) | 85 (29) |
| Enteropathy | 52 (14) | 11 (14) | 41 (14) |
| Time of follow-up person-year—mean (SD) | 11.5 (8.9) | 11.8 (8.4) | 11.4 (9.2) |
| Patients with cancer—n. (%) | 116 (25.5) | – | – |
| Patients with more than one cancer—n. (%) | 18 (4.0) | – | 18(15.5) |
| Patients alive at the last follow up—n. (%) | 377 (82.9) | 51(44.0) | 27 (8.0) |
p < 0.01,
p < 0.001, and
p < 0.0001 (cancer vs. cancer-free CVID patients).
SD, standard deviation; CVID, common variable immunodeficiency; Ig, immunoglobulin. Immunoglobulin normal range (adults): IgG 700–1600 mg/dL; IgA 68–400 mg/dL; IgM 40–259 mg/dL.
Cancer prevalence and risk in CVID patients
During the study time, 132 separate cancers were diagnosed in 116 patients (25.5%). Eighteen patients (4%) developed more than one cancer. The age at CVID onset was higher for patients who developed cancer in comparison to those who did not (45.9 ± 13.2 vs. 38.1 ± 15.7 yrs, p < 0.0001, Table 1). The mean age at the first cancer diagnosis was 52.5 ± 13.8 (range: 26–85 yrs). Sixty-seven cancers were diagnosed in women and 65 cancers in men. Malignancies diagnosed were: lymphoma (38; 29%), gastrointestinal cancers (35; 26%), genitourinary cancers (14; 8%), breast cancers (10; 7%), uterine cancers (7; 5.3%), thyroid cancers (6; 4%), lung cancers (4; 3%), liver cancers (4; 3%), prostatic cancer (3; 2%), and pancreatic cancer (3; 2%). The overall and sex-related prevalence of single cancer was summarized in Table 2.
Table 2.
Prevalence of cancer diagnosis in 455 Italian CVID patients.
| Cancer diagnosis—n. % |
All patients n. 455 |
Female n. 235 |
Male n. 220 |
|||
|---|---|---|---|---|---|---|
| n. | % | n. | % | n. | % | |
| Non-Hodgkin lymphoma | 33 | 7.3 | 15 | 6.4 | 18 | 8.2 |
| Gastric cancer | 25 | 5.5 | 9 | 3.8 | 16 | 7.1 |
| Colorectal cancer | 10 | 2.2 | 4 | 1.7 | 6 | 2.7 |
| Breast cancer | 10 | 2.2 | 10 | 4.3 | - | - |
| Thyroid cancer | 6 | 1.3 | 3 | 1.3 | 3 | 1.3 |
| Hodgkin lymphoma | 5 | 1.1 | 2 | 0.9 | 3 | 1.3 |
| Large Granular Lymphocytic Leukemia | 5 | 1.1 | 3 | 1.3 | 2 | 0.9 |
| Lung cancer | 4 | 0.9 | 2 | 0.9 | 2 | 0.9 |
| Liver cancer | 4 | 0.9 | 2 | 0.9 | 2 | 0.9 |
| Uterine cancer, body | 4 | 0.9 | 4 | 1.7 | – | – |
| Uterine cancer, cervical | 3 | 0.7 | 3 | 1.3 | – | – |
| Prostatic cancer | 3 | 0.7 | – | – | 3 | 1.3 |
| Pancreatic cancer | 3 | 0.7 | 2 | 0.9 | 1 | 0.4 |
| Other blood cancer (CML, polycythemia vera) | 3 | 0.7 | 1 | 0.4 | 2 | 0.9 |
| Kaposi sarcoma | 1 | 0.2 | 0 | – | 1 | 0.4 |
| Others#x0002A; | 13 | 3.5 | 7 | 3.0 | 6 | 2.7 |
Others: Bladder cancer, meningioma, melanoma, neuro-endocrine carcinoma, ocular carcinoma, kidney carcinoma, adrenal carcinoma.
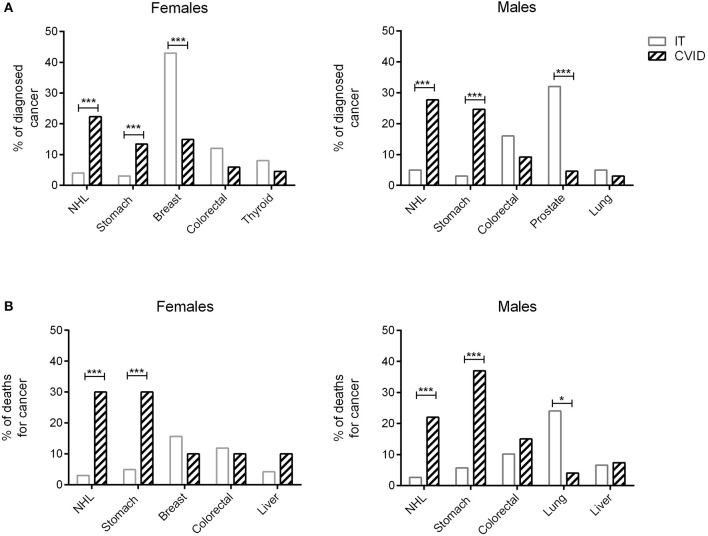
Figures 1A,B showed the percentage of the top five diagnosed cancers and the top five fatal cancers seen in CVID in comparison to the normative Italian population. The most common malignancy diagnosed in the AIRTUM database was breast cancer (women only), prostate cancer (men only), lung, and colorectal cancers. The incidence of these cancers was not increased in CVID patients in comparison to the AIRTUM database (Table 3). Ten female CVID patients were diagnosed with breast cancer (Exp: 10.0; SIR: 1.0; 95%CI: 0.7–1.2). Three male CVID patients were diagnosed with prostate cancer (Exp: 7.1; SIR: 0.4, 95%CI: 0.1–1.0). There was no increase in the rates of lung cancer (Obs: 4, Exp: 28.0, SIR 0.1; 95%CI: 0.2–0.7) and colon cancer (Obs: 10; Exp: 8.2; SIR 1.2; 95%CI: 0.0–1.9) among CVID patients vs. normative population (Table 3). In contrast to Italian normative population, the most commonly diagnosed malignancies in female and male subjects with CVID were non-Hodgkin lymphoma (NHL) and gastric cancer (Figure 1A). The risk for NHL and Hodgkin's lymphoma (HD) was increased by 14.3- and 12.5-fold, respectively, based on 33 and 5 cases observed. The risk for gastric cancer was increased 6.4-fold based on 25 cases observed (Table 3).
Figure 1.
Cancers diagnosis and death for cancer in CVID and in the normative population. Data related to the proportion of the five most frequently diagnosed cancers in male and female CVID patients (dashed bars) are shown in comparison to the normative population (IT, white bars) (A). Proportion of deaths for cancer in male and female CVID patients (dashed bars) are shown in comparison to the normative population (IT, white bars) (B). In CVID, NHL and gastric cancer were the most commonly diagnosed cancers in both sexes, whereas breast cancer and prostate cancer were the most frequently recorded malignancies in Italian normative population. Gastric cancer was the first cause of death for cancer in CVID females and males, followed by NHL; breast and lung cancers were the most common cause of death for cancer in normative population. Data of normative population referred to 2017 AIRTUM report. NHL, non-Hodgkin lymphoma. *p < 0.01; ***p < 0.0001.
Table 3.
Observed (Obs) and Expected (Exp) numbers and Standardized Incidence Ratio (SIR) of cancer among 455 Italian patients with CVID.
| Obs | Exp | SIR | 95%CI | |
|---|---|---|---|---|
| CANCER | ||||
| All malignant neoplasms | 133 | 55.1 | 2.4 | 1.7–3.5 |
| Non-Hodgkin lymphoma | 33 | 2.3 | 14.3 | 8.4–22.6 |
| Gastric cancer | 25 | 3.9 | 6.4 | 3.2–12.5 |
| Colorectal cancer | 10 | 8.2 | 1.2 | 0.0–1.9 |
| Breast cancer | 10 | 10 | 1 | 0.7–1.2 |
| Thyroid cancer | 5 | 1.7 | 2.9 | 0.0–6.4 |
| Hodgkin Disease | 5 | 0.4 | 12.5 | 3.4–22.4 |
| Lung cancer | 4 | 28 | 0.1 | 0.2–0.7 |
| Liver cancer | 4 | 2.1 | 1.9 | 0.3–5.6 |
| Uterine cancer, body | 4 | 1.2 | 3.3 | 0.1–6.5 |
| Uterine cancer, cervical | 3 | 1.2 | 2.5 | 0.1–4.8 |
| Prostatic cancer | 3 | 7.1 | 0.4 | 0.1–1.0 |
| Pancreatic cancer | 3 | 1.9 | 1.6 | 0.3–3.9 |
Survival and mortality
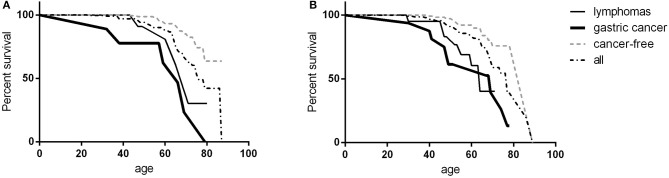
Three-hundred and seventy-four (82.4%) patients were alive at the end of the study-time. During the study time, we observed 78 deaths in the patient population. Malignancies were the first cause of death, accounting for 60.3% of deaths. Gastric cancer was the leading cause of death (20.5%). Infections accounted for 19.2% of deaths, 80% due to lower tract respiratory infections. Causes of mortality in patients are detailed in Table 4. The CVID overall survival (OS) was 85.1% (SE 2.3%) at 60 years and 61.8% (SE 4.2%) at 70 years. No differences were observed between males and females. Cancer-free CVID had a better survival in comparison to those with gastric cancer (Log-Rank p < 0.0001, HR: 4.8, 95%CI: 4.2–44.4, Figure 2A) and lymphomas (Log-Rank p = 0.001, HR: 4.2, 95%CI: 2.8–44.4, Figure 2B).
Table 4.
Cause of death in CVID patients.
| Causes of death | n. | % |
|---|---|---|
| Cancer | 47 | 60.3 |
| Gastric cancer | 16 | 20.5 |
| Non-Hodgkin Lymphoma | 14 | 17.9 |
| Colorectal cancer | 6 | 7.7 |
| Liver cancer | 4 | 5.1 |
| Pancreatic cancer | 2 | 2.6 |
| Breast cancer | 2 | 2.6 |
| Hodgkin Disease | 1 | 1.3 |
| Lung cancer | 1 | 1.3 |
| Uterine cancer | 1 | 1.3 |
| Infections | 15 | 19.2 |
| LRTI (respiratory failure) | 12 | 10.3 |
| Other infections (sepsis, CMV) | 3 | 10.3 |
| Cardiovascular disease | 5 | 6.4 |
| Autoimmune manifestations: AHA, AIH | 4 | 5.1 |
| Others* | 7 | 9.0 |
| Total | 78 | – |
LRTI, lower respiratory tract infections; AHA, autoimmune hemolytic anemia; AIH, autoimmune hepatitis.
Parkinson disease, cirrhosis, accident, suicide.
Figure 2.
CVID survival. Survival in female (A) and male (B) CVID participants: data were shown as overall survival (black dashed line), in CVID patients with gastric cancer (black bold line), in patients with lymphoma (black line) and in cancer-free CVID patients (gray dashed line). No survival differences were observed between females and males; CVID subjects with gastric cancer or lymphoma had a worse survival in comparison to cancer-free CVID population.
Cancer excess of mortality was expressed as SMRs (Table 5). The most fatal cancers in the AIRTUM database were lung, colorectal and breast cancer. We found no significant increase in the mortality for colorectal cancer (Obs: 6; Exp: 2.1; SMR: 2.8, 95%CI: 0.1–6.3) and a significant lower mortality for lung and breast cancers in CVID patients (lung cancer: Obs: 1; Exp: 6.6; SMR: 0.1; 95%CI: 0.3–0.5; breast cancer: Obs; 2; Exp: 7.0; SMR: 0.3; 95%CI: 0.0–0.5, Table 5). Moreover, in CVID we found an excess of mortality for NHL (Obs: 14; Exp: 0.8; SMR: 16.5; 95%CI: 8.8–31.4) and gastric cancer (Obs: 16; Exp: 2.0; SMR: 10.1; 95%CI: 3.8–16.3) (Table 5).
Table 5.
Standardized mortality ratios (SMRs) for cancers causing death in CVID.
| Cancers | Obs | Exp | SMR | 95%CI |
|---|---|---|---|---|
| All malignant neoplasm | 47 | 44.5 | 1.0 | 0.5–1.6 |
| Gastric cancer | 16 | 2.0 | 10.1 | 3.8–16.3 |
| Non-Hodgkin lymphoma | 14 | 0.8 | 16.5 | 8.8–31.4 |
| Colorectal cancer | 6 | 2.1 | 2.8 | 0.1–6.3 |
| Liver cancer | 4 | 1.8 | 2.9 | 0.1–5.9 |
| Pancreatic cancer | 2 | 1.7 | 1.2 | 0.6–3.2 |
| Breast cancer | 2 | 7.0 | 0.3 | 0.0–0.5 |
| Hodgkin Disease | 1 | 0.1 | 10.0 | 0.0–45.2 |
| Lung cancer | 1 | 6.6 | 0.1 | 0.3–0.5 |
| Uterine cancer | 1 | 0.3 | 2.8 | 0.0–8.3 |
Gastric cancer
Gastric cancer was the second most frequent cancer diagnosed in CVID accounting for 18.9% of cancer diagnosed, and the first cause of death. Of the 25 cases of gastric cancer, 16 (64%) occurred in men. The age at cancer diagnosis was 51.8 ± 13.7 years (range: 30–75), 15 years younger than that reported in the Italian population (10). The diagnosis of cancer occurred within 10 years from the CVID diagnosis in two thirds of these patients. CVID subjects with gastric cancer had a similar age at immunodeficiency onset than the entire CVID cohort (40.0 ± 15.0 vs.39.9 ± 15.4, p = 0.975). Undetectable IgA (<7 mg/dL) and IgM (<6 mg/dL) serum levels at time of CVID diagnosis were more likely in patients with gastric cancer in comparison to those without that complication (IgA: OR 27.5, 95%CI: 1.5–475.9, p = 0.027; IgM: OR 7.4, 95% CI: 1.5–36.1, p = 0.013). Seven out of 25 patients had an additional malignancy: three patients were diagnosed with lymphoma, two with colorectal cancer, one with gallbladder cancer, and one with meningioma. In addition, two patients had a multifocal gastric adenocarcinoma treated by two-stage gastrectomy (Table 6). One patient (n. 1) had a positive family history for gastric cancer: three relatives, including his IgA-deficient brother, developed the malignancy. In these two brothers, mutations of CDH1 gene were not found. Fifteen patients with gastric cancer died during the study time. Overall, the average SMR indicated a 10.1-fold excess mortality among CVID patients with gastric cancer. The 10-year survival probability of the entire cohort of patients with gastric cancer was 25%. Clinical staging was available for 16/25 patients. Patients classified as stage I had a better survival in comparison to those with stage III-IV (HR: 0.01, 95%CI: 0.0–0.1, p < 0.0001, Figure 3). H. pylori status and histology of gastric endoscopic biopsies collected before the diagnosis of cancer was available for 7 patients (Table 7): areas of dysplasia were identified in two subjects whereas areas of atrophic gastritis and/or intestinal metaplasia were found in all patients. In the two patients with dysplasia, the following endoscopy revealed a stage I malignancy, 6 months apart (patient n. 23). Patient n. 25 agreed to undergo a further gastroscopy only 15 months apart, which allowed diagnosis of a stage IV gastric cancer. Interesting to note, patient n. 20 and n. 22 developed a high-grade gastric cancer <14 months after the preceding endoscopy whose histology did not show any signs of dysplasia. At least one H. pylori detection was significantly related to gastric cancer (43 vs. 13%, OR: 5.3, 95%CI: 1.1–24.8, p = 0.042).
Table 6.
Age at PID and at cancer diagnosis, survival, outcome, histology, cancer stage, and cancer treatment in 25 CVID patients with gastric cancer.
| ID | Sex |
Age at PID diagnosis (years) |
Age at cancer diagnosis (years) |
Survival after cancer diagnosis (months) |
Outcome | Histology | Stage | Treatment | Additional cancer | Enteropathy (before cancer diagnosis) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 39 | 40 | 408 | Alive | Early gastric cancer, pT1N0 G1 |
stage I | Gastrectomy (total) | Kaposi sarcoma, colorectal carcinoma | No |
| 2 | F | 25 | 31 | 12 | Deceased (cancer) | Gastric adenocarcinoma, NOS | NA | Chemotherapy, NOS | No | Yes |
| 3 | F | 45 | 45 | 252 | Alive | Gastric adenocarcinoma, NOS | NA | Gastrectomy, NOS | Meningioma | No |
| 4 | F | 67 | 69 | 120 | Deceased (cancer) | Early gastric cancer pT1N0 G2, |
stage I | Gastrectomy (total) | Biliary tract carcinoma |
NA |
| 5 | M | 40 | 49 | 7 | Deceased (cancer) | Gastric adenocarcinoma, NOS | NA | Gastrectomy (total) | No | Yes |
| 6 | M | 27 | 51 | 204 | Alive | Gastric adenocarcinoma, intestinal type pT1N0 G2 |
stage I | Gastrectomy (total) | NHL (duodenal) lymphoma |
No |
| 7 | M | 58 | 74 | 36 | Deceased (cancer) | Gastric adenocarcinoma, intestinal type, PT1, N0, M0 |
stage IA | Gastrectomy (subtotal) | No | Yes |
| 8 | F | 35 | 45 | 144 | Alive | Gastric adenocarcinoma, NOS pT1bN1 G1 | stage IB | Gastrectomy (subtotal) | No | No |
| 9 | M | 67 | 67 | 132 | Alive | Gastric adenocarcinoma, NOS | Gastrectomy (subtotal) | Colorectal carcinoma |
Yes | |
| 10 | F | 32 | 38 | 132 | Alive | Early gastric cancer, pT1N0 G2 | stage I | Gastrectomy (subtotal) | HD | Yes |
| 11 | M | 64 | 67 | 30 | Deceased (cancer) | Gastric adenocarcinoma, intestinal type, PT1, N0, Mx, G3 |
stage I | Gastrectomy (subtotal), Chemotherapy (lederfolin, xeloda, 5-fluorouracil) | No | Yes |
| 12 | M | 69 | 75 | 24 | Deceased (cancer) | Gastric adenocarcinoma, NOS | NA | Supportive | No | Yes |
| 13 | F | 35 | 68 | 12 | Deceased (cancer) | Gastric Adenocarcinoma, G3 | NA | Supportive | NHL | Yes |
| 14 | F | 27 | 38 | 9 | Deceased (cancer) | Gastric adenocarcinoma, NOS | NA | Supportive | No | NA |
| 15 | M | 30 | 47 | 12 | Deceased (cancer) | Gastric adenocarcinoma, NOS | stage IIIB | Chemotherapy, NOS | No | Yes |
| 16 | M | 40 | 40 | 84 | Alive | Multifocal Gastric Adenocarcinoma pT2bN0 G3 CMV+ |
stage IB | Gastrectomy (total, two-step) | No | No |
| 17 | M | 59 | 68 | 12 | Deceased (cancer) | Gastric adenocarcinoma, NOS | NA | Supportive | No | Yes |
| 18 | M | 36 | 48 | 11 | Deceased (respiratory failure) | Gastric adenocarcinoma, NOS | NA | Chemotherapy, NOS | No | NA |
| 19 | M | 18 | 40 | 15 | Deceased (cancer) | Gastric adenocarcinoma, intestinal type pT3N3bMx G3 |
stage IIIC | Gastrectomy (total), Chemotherapy (platinum/5-fluorouracil) | No | Yes |
| 20 | F | 43 | 64 | 24 | Deceased (cachexia, meningitis) | Gastric adenocarcinoma, intestinal type pT3N2 G3 | stage IIIA | Gastrectomy (subtotal), + Capecitabine | No | No |
| 21 | M | 22 | 30 | 8 | Deceased (cancer) | Gastric adenocarcinoma, NOS | stage IV | Supportive | No | No |
| 22 | M | 29 | 40 | 7 | Deceased (cancer) | Gastric adenocarcinoma, NOS | stage IV | Chemotherapy, NOS | No | Yes |
| 23 | M | 49 | 51 | 15 | Alive | Multifocal gastric adenocarcinoma, intestinal type, pT1pN0 G3 |
stage I | Gastrectomy (total, two-step) | No | No |
| 24 | M | 47 | 50 | 14 | Alive | Early gastric cancer, intestinal type, pT1bNx G3 |
stage I | Gastrectomy | No | No |
| 25 | F | 46 | 59 | 6 | Deceased (cancer) | Gastric adenocarcinoma G3 |
stage IV | Chemotherapy (epirubicine, platinum, 5-fluorouracil) | No | Yes |
NOS, not otherwise specified; NA, not available M, Males, F, Females; NHL, Non-Hodgkin lymphoma; HD, Hodgkin disease.
Figure 3.
Gastric cancer survival by sex and staging. Survival in the cancer free CVID subjects (black bold line) and in CVID females (gray dashed line) and males (gray line) with gastric cancer was shown in (A). Survival in patients scored as stage I (gray line) and in patients scored as stage III-IV (dashed line) was shown in (B). No difference was observed between CVID females and males with gastric cancer; patients scored as stage I had a better survival in comparison to patients scored as stage III-IV.
Table 7.
H. pylori status and histology of gastric biopsies from the endoscopy preceding the examination leading to gastric cancer diagnosis in seven CVID patients.
| ID | H. pylori (pos/neg) | Histology of biopsy taken at the endoscopy preceding the one with gastric cancer diagnosis | Interval between endoscopies (months) | Stage at cancer diagnosis | Outcome |
|---|---|---|---|---|---|
| 9 | pos | Active chronic gastritis, intestinal metaplasia | 36 | NA | Alive |
| 12 | neg | Atrophic gastritis | 35 | NA | Death |
| 13 | pos | Atrophic gastritis | 12 | NA | Death |
| 20 | neg | Active chronic gastritis (moderate) with incomplete intestinal metaplasia | 14 | Stage IIIA | Death |
| 22 | pos | Active chronic gastritis (moderate), intestinal metaplasia | 14 | Stage IV | Death |
| 23 | neg | Atrophic gastritis, high grade dysplasia | 6 | Stage I | Alive |
| 25 | neg | Intestinal metaplasia, high-grade dysplasia | 15 | Stage IV | Death |
NA, not available.
Lymphoma
Lymphoma was the most frequent cancer diagnosed in CVID and the second cause of death for cancer (Tables 2, 4). The age at lymphoma diagnosis was 32.8 ± 4.6 years for HD and 52.4 ± 13.1 years for NHL. The age at CVID diagnosis was higher in patients with lymphoma in comparison to those without lymphoma (45.7 ± 12.4 vs. 39.6 ± 15.6, p = 0.008). Three patients first presented with lymphoma prior to CVID diagnosis, raising the question if hypogammaglobulinemia might be secondary to the lymphoproliferative disease (Table 8). However, the longtime state of antibody defect after the diagnosis and treatment of lymphoma might suggest this possibility. As widely described (2–9, 11), also in our cohort, CVID-associated lymphoma was more likely to be of B cell origin (88.4%) with a predominance of NHL (81.8%). T-cell lymphomas (peripheral T-cell lymphoma, angio-immunoblastic T-cell lymphoma and anaplastic T-cell lymphoma) and one primitive effusion cavity (PEL) lymphoma were also observed (Table 8). Similar to what observed in other series of CVID patients about 30% were extra-nodal lymphomas. Patients with lymphoma were more likely to have lymphopenia (lymphocytes < 1,000 cell/mm3) (OR: 3.0, 95%CI: 1.1–8.3, p = 0.030) and polyclonal lymphocytic infiltration phenotype (OR: 2.7, 95%CI: 1.2–6.3, p = 0.016) before cancer diagnosis.
Table 8.
Characteristics of CVID patients diagnosed with lymphoma.
| ID | Sex |
Age at cancer onset, years |
Age at CVID diagnosis, years | Survival after cancer, months | Outcome (cause) | Histology and stage | Treatment | Additional cancer |
|---|---|---|---|---|---|---|---|---|
| 6 | M | 50 | 37 | 216 | Alive | Diffuse large B cell lymphoma (small bowel) | Chemotherapy NOS | Gastric cancer |
| 10 | F | 32 | 42 | 336 | Alive | HD | CHOP, ABV | Gastric cancer |
| 13 | F | 67 | 35 | 12 | Deceased (gastric cancer) |
Diffuse large B cell lymphoma | NA | Gastric cancer |
| 26 | F | 47 | 47 | 8 | Deceased (lymphoma) | NHL not further classified | NA | No |
| 27 | M | 38 | 37 | 120 | Alive | Diffuse large B cell lymphoma of small bowel, stage IVE | R-CHOP | No |
| 28 | F | 50 | 44 | 13 | Deceased (lymphoma) |
T-cell lymphoma (peripheral T cell lymphoma) | CHOP, autologous HSCT, Brentuximab | No |
| 29 | F | 64 | 58 | 12 | Deceased (lymphoma) |
T-cell lymphoma (angioimmunoblastic T cell lymphoma) | Prednisone | No |
| 30 | M | 40 | 47 | 144 | Deceased (lymphoma) |
NHL not further classified | NA | No |
| 31 | M | 48 | 41 | 132 | Alive | NHL not further classified | NA | No |
| 32 | F | 74 | 47 | 24 | Alive | Diffuse large B cell lymphoma (large bowel) | R-CHOP, RTX | No |
| 33 | M | 53 | 47 | 9 | Alive | Diffuse large B cell lymphoma (T-cells rich) | R-CHOP | No |
| 34 | M | 29 | 30 | 14 | Alive | Diffuse large B cell lymphoma | R-CHOP | No |
| 35 | M | 58 | 55 | 36 | Alive | NHL not further classified | Chemotherapy NOS | No |
| 36 | M | 59 | 35 | 12 | Deceased (lymphoma) |
Cutaneous diffuse large B cell lymphoma leg-type | R-CHOP, radiotherapy | No |
| 37 | F | 67 | 43 | 84 | Alive | Marginal Zone Lymphoma (Splenic) | Splenectomy | Lung cancer |
| 38 | M | 54 | 47 | 84 | Alive | NHL not further classified | NA | No |
| 39 | F | 29 | 28 | 24 | Alive | Lymphoplasmacytic lymphoma | Chemotherapy NOS | No |
| 40 | M | 47 | 45 | 6 | Deceased (lymphoma) |
NHL not further classified | NA | No |
| 41 | F | 65 | 62 | 8 | Deceased (lymphoma) | Diffuse large B cell lymphoma | NA | No |
| 42 | F | 62 | 56 | 12 | Alive | Marginal Zone Lymphoma (nodal and extra nodal) | RTX, bendamustine | No |
| 43 | M | 60 | 60 | 36 | Alive | Marginal Zone Lymphoma (nodal and extra nodal) | R-CHOP | No |
| 44 | M | 47 | 47 | 72 | Alive | Anaplastic T cell Lymphoma ALK- stage IVB (skin) | CHOEP, FEAM and autologous HSCT | No |
| 45 | F | 67 | 64 | 11 | Deceased (lymphoma) | NHL not further classified | NA | No |
| 46 | F | 61 | 70 | 168 | Deceased (cardiovascular) |
Diffuse large B cell lymphoma (small bowel) | Ileocecal resection + R-CHOP | No |
| 47 | F | 41 | 38 | 24 | Alive | Marginal Zone Lymphoma | NA | No |
| 48 | M | 52 | 56 | 120 | Alive | NHL, not further classified, stage IV | R-FN, R-CHOP | No |
| 49 | M | 70 | 59 | 12 | Alive | Marginal Zone Lymphoma (nodal, indolent behavior) | Rituximab + bendamustine | Prostatic cancer |
| 50 | M | 41 | 40 | 15 | Alive | Kaposi sarcoma/Primitive effusion lymphoma, HHV8+/EBV+ | CDE | No |
| 51 | F | 74 | 62 | 72 | Alive | MALT Lymphoma (gastric) | NA | No |
| 52 | M | 59 | 59 | 60 | Deceased (lymphoma) | Diffuse large B cell lymphoma (lung), stage IVB | R-CHOP, R-COMP | No |
| 53 | F | 45 | 54 | 216 | Deceased (lymphoma) | NHL not further classified (low grade) | R-CHOP + Etoposide | Uterine cancer, body |
| 54 | M | 55 | 55 | 10 | Deceased (lymphoma) | NHL not further classified | NA | No |
| 55 | M | 36 | 21 | 108 | Alive | HD, classic type, stage IIIsB | ABVD | No |
| 56 | M | 30 | 28 | 12 | Deceased (lymphoma) |
HD, classical type, lymphocyte-depleted, stage IV | Chemotherapy NOS | Thyroid cancer |
| 57 | F | 28 | 38 | 12 | Deceased (T-cell lymphoma) |
HD, mixed-cellularity type | Radiotherapy | Thyroid cancer, angio-immunoblastic T cell lymphoma) |
| 58 | M | 29 | 18 | 8 | Alive | HD, sclero-nodular type | ABVD, radiotherapy | No |
| 59 | M | 39 | 38 | 96 | Deceased (lymphoma) |
HD, classical type, stage IVA | VEBEP, HSCT | No |
ABV, Adriamycin, Hydroxydaunorubicin, Bleomycin, Vinblastine; ABVD, Adriamycin, Hydroxydaunorubicin, Bleomycin, Vinblastine, Dacarbazine; CHOEP, Cyclophosphamide, Doxorubicin, Etoposide, Vincristine, Prednisone; CHOP, Cyclophosphamide, Hydroxydaunorubicin, Vincristine, Prednisone; CDE, Cyclophosphamide, Doxorubicin, Etoposide; COPP, Cyclophosphamide, Vincristine, Procarbazine, Prednisone; FEAM, Fotemustine, Etoposide, Cytarabine, Melphalan; R-CHOP, Rituximab, Cyclophosphamide, Hydroxydaunorubicin, Vincristine, Prednisone; FN-R, Rituximab, Fludarabine, Mitoxantrone; HD, Hodgkin disease, NHL, Non-Hodgkin lymphoma, HSCT, Hematopoietic stem cell transplantation; R-COMP, Rituximab, Cyclophosphamide, Vincristine, Myocet, Prednisone; RTX, Rituximab; VEBEP, Etoposide, Epirubucin, Bleomycin, Cyclophosphamide, Prednisolone; NOS, not otherwise specified; NA, not available.
Discussion
This longitudinal study on a large cohort of CVID patients over a cumulative period of 5,169 person-years showed that one fourth of patients developed a malignancy. Cancer represents the first cause of death in our patient's population. The most commonly diagnosed malignancies in CVID were NHL and the first cause of death was gastric cancer. The excess of mortality for lymphoma and gastric carcinoma in CVID was increased by more than 10-fold in comparison to normative population. Several studies reported a high frequency of malignancies in CVID patients (2, 3, 6–9, 11–14) with a prevalence ranging from 1.5 to 20.7%. However, only few studies provided SIR, allowing the comparison of data on CVID to data on normative population. These surveys showed an excess of incidence ranging from 4- to 30-fold for NHL and from 3- to 47-fold for gastric cancer (3, 6, 7, 15). Nevertheless, the prevalence other malignancies was not increased, confirming that patients with antibody deficiencies have a narrow range of cancers (6).
Lymphoma is considered as one of the more severe complications of CVID. The prevalence registered in our cohort was similar to that found across the different countries examined (2–9, 11, 12). The histological types reported in the different series of CVID-associated lymphoma were also similar to our findings, with a predominance of non-Hodgkin B cell lymphomas, possibly occurring at extra nodal sites. We confirmed the observation by Chapel et al. showing that CVID patients with polyclonal lymphadenopathy phenotype have an increased risk of lymphoid malignancy that generally occurs late in the disease course (16). In addition, we found that CVID patients with lymphopenia had a 3-fold increased risk to develop lymphomas. In CVID patients, diagnosis of lymphoma may be particularly challenging. Immune-histochemical analysis, studies on clonality and molecular studies might be helpful to distinguish reactive from neoplastic lymphoproliferative diseases, even if CVID patients with clonal B cell expansion, who survive without developing an overt lymphoma, have been described (12, 17). Treatment of CVID-associated lymphoma was usually like the treatment of lymphoma in other settings and usually it included rituximab.
Herein, to the best of our knowledge, we collected the largest case-series of gastric cancers in CVID subjects ever described, showing a high prevalence and an excess of mortality for gastric cancer. However, the SIR for gastric cancer was similar to that found across other studies providing this kind of figure (3, 6, 7). This difference might be related to the observation that gastric cancer prevalence may vary significantly within and between countries (18). In comparison to the normative population, CVID patients were on the average 15-years younger at the time of cancer onset. As reported for non-CVID subjects (19), CVID patients with early-stages gastric cancer had a better prognosis in comparison to those with more advanced stage, who died within 2 years since cancer diagnosis. According to our data, chronic atrophic gastritis and extensive intestinal metaplasia are invariably associated with gastric cancer in CVID. Similarly, De Petris et al. (20) showed that these adenocarcinomas were diagnosed at a young age and were of intestinal type. They were also associated with increased numbers of intra-tumoral lymphocytes, paucity of plasma cells and nodular lymphoid hyperplasia, all features suggestive of chronic inflammation of the gastric mucosa.
These observations gave us the chance to suggest the implementation of current screening strategy, aimed to an early diagnosis. The appropriate timing of upper endoscopy in CVID is a matter of debate. In the general population, Rugge et al. suggested performing upper endoscopy every 2 years in subjects with gastritis scored as stage III–IV (10). In CVID, Dhalla et al. (21) suggested to perform upper endoscopy in patients with risk factors for gastric cancer (H. pylori positivity, low serum vitamin B12 and iron concentrations) with an interval between the subsequent endoscopic assessment based on histological findings: every 1–3 years in CVID patients with metaplasia, every 3 years in patients with atrophic gastritis, and every 6–12 months in those with dysplasia. However, this interval may not be suitable for CVID, who rapidly develop advanced-stage cancer with poor prognosis. In fact, we showed that some CVID developed a high-grade gastric cancer already 12–14 months after an endoscopy showing no histologic signs of dysplasia. This rapid cancer development in CVID was unexpected since no epithelial gastric cancer was identified in patients without signs of dysplasia on a cohort of 1,615 Italian non-CVID followed over a 1–5 years period (10).
H. pylori eradication represents the main strategy to reduce the lifetime risk of gastric cancer since H. pylori is widely recognized as the leading cause of gastric cancer (22). In CVID, serological tests are not useful to identify H. pylori positive patients and only direct diagnostic methods for H. pylori detection should be considered (23). However, follow-up strategies targeted at gastric cancer secondary prevention cannot rely only on H. pylori identification, since the eradication of H. pylori might not abolish the risk for neoplastic progression (24–26). This is supported by our observation in two CVID patients who developed gastric cancer 1–2 years after a H. pylori-negative gastric biopsy.
On the basis of our data, we recommend the implementation of national guidelines based on regular upper endoscopy and on treatment of H. pylori infection. We propose to always perform upper endoscopy at the time of CVID diagnosis; to repeat endoscopy every 24 months in patients with normal histology; every 12 months in patients with atrophic gastritis or intestinal metaplasia, and every 6 months in patients with dysplastic lesions. Diagnosis of H. pylori infection should be actively ruled out at diagnosis and during the course of CVID disease. The prevalence and the risk of gastric cancer detected in Italian CVID patients might be related to the epidemiology of H. pylori infection in our country. Thus, further studies should be undertaken in other countries before they adopt our suggested measures of disease management. However, a careful endoscopic monitoring of gastric cancer should be advisable also in countries with low H. pylori prevalence, since the rate of antibiotic-resistant strains is increasing worldwide (22).
Our study has some limitations. First of all, we included in the analysis also retrospective data with possible survival bias. Second, we did not include in the analysis the genetic diagnoses of the cohort. Since it has been shown the risk of gastric cancer was not increased among relatives of CVID patients (7), however, it is possible that cancer morbidity might be related to the immunodeficiency per se rather than to family habits or environmental factors, including H. pylori sharing. Finally, preliminary data suggested spontaneous gastric cancer in models of NFkappaB1 deficiency (27) and recent papers suggested that significant proportion of CVID patients may harbor haploinsufficient NFKB1 mutations (28). Additional studies on alterations of gastric mucosal immunity and microbiota and on genetic alterations are needed to better understand the gastric carcinogenesis in CVID patients.
Ethics statement
This study was carried out in accordance with the Good Clinical Practice guidelines, the International Conference on Harmonization guidelines, and the most recent version of the Declaration of Helsinki. The protocol was approved by Ethics Committee of Sapienza University of Rome and Azienda Policlinico Umberto I: Protocollo di osservazionale retrospettivo-prospettico sui soggetti affetti da Immunodeficienza Comune Variabile arruolati nei centri AIEOP/IPINET. Rif. CE:4063 on 04/14/2016.
Author contributions
FP, GS, CA, and IQ: conceived and designed the study; FP, AP, FC, ES, LC, FR, and CM data collection; FP, CM, GS, CA, MV, ST, and IQ: data analysis and interpretation; FP, CM, GS, CA, ST, and IQ: manuscript preparation.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank our patients and their families and the Jeffrey Modell Foundation for the continuous support to our Centre. We thank Dr. Raffaella Neri, Dr. Saitto, and Dr. Paci for the manuscript revision.
Footnotes
Funding. This work was funded by Progetto Ateneo 2016, 2017.
References
- 1.Ochs HD, Petroni D. From clinical observations and molecular dissection to novel therapeutic strategies for primary immunodeficiency disorders. Am J Med Genet. (2018) 176:784–803. 10.1002/ajmg.a.38480 [DOI] [PubMed] [Google Scholar]
- 2.Resnick ES, Moshier EL, Godbold JH, Cunningham-Rundles C. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood (2012) 119:1650–7. 10.1182/blood-2011-09-377945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayor PC, Eng KH, Singel KL, Abrams SI, Odunsi K, Moysich KB, et al. Cancer in primary immunodeficiency diseases: cancer incidence in the United States Immune Deficiency Network Registry. J Allergy Clin Immunol. (2018) 141:1028–35. 10.1016/j.jaci.2017.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mortaz E, Tabarsi P, Mansouri D, Khosravi A, Garssen J, Velayati A, et al. Cancers related to immunodeficiencies: update and perspectives. Front Immunol. (2016) 7:365. 10.3389/fimmu.2016.00365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonilla FA, Barlan I, Chapel H, Costa-Carvalho BT, Cunningham-Rundles C, de la Morena MT, et al. International consensus document (ICON): common variable immunodeficiency disorders. J Allergy Clin Immunol Pract. (2016) 4:38–59. 10.1016/j.jaip.2015.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vajdic CM, Mao L, van Leeuwen MT, Kirkpatrick P, Grulich AE, Riminton S. Are antibody deficiency disorders associated with a narrower range of cancers than other forms of immunodeficiency? Blood (2010) 116:1228–34. 10.1182/blood-2010-03-272351 [DOI] [PubMed] [Google Scholar]
- 7.Mellemkjaer L, Hammarstrom L, Andersen V, Yuen J, Heilmann C, Barington T, et al. Cancer risk among patients with IgA deficiency or common variable immunodeficiency and their relatives: a combined Danish and Swedish study. Clin Exp Immunol. (2002) 130:495–500. 10.1046/j.1365-2249.2002.02004.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinti I, Soresina A, Spadaro G, Martino S, Donnanno S, Agostini C, et al. Long-term follow-up and outcome of a large cohort of patients with common variable immunodeficiency. J Clin Immunol. (2007) 27:308–16. 10.1007/s10875-007-9075-1 [DOI] [PubMed] [Google Scholar]
- 9.Quinti I, Agostini C, Tabolli S, Brunetti G, Cinetto F, Pecoraro A, et al. Malignancies are the major cause of death in patients with adult onset common variable immunodeficiency. Blood (2012) 120:1953–4. 10.1182/blood-2012-05-431064 [DOI] [PubMed] [Google Scholar]
- 10.Rugge M, Meggio A, Pravadelli C, Barbareschi M, Fassan M, Gentilini M, et al. Gastritis staging in the endoscopic follow-up for the secondary prevention of gastric cancer: a 5-year prospective study of 1755 patients. Gut (2018) gutjnl-2017-314600. 10.1136/gutjnl-2017-314600 [DOI] [PubMed] [Google Scholar]
- 11.Filipovich AH, Mathur A, Kamat D, Kersey JH, Shapiro RS. Lymphoproliferative disorders and other tumors complicating immunodeficiencies. Immunodeficiency (1994) 5:91–112. 10.1016/j.leukres.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 12.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. (1999) 92:34–48. 10.1006/clim.1999.4725 [DOI] [PubMed] [Google Scholar]
- 13.Gompels MM, Hodges E, Lock RJ, Angus B, White H, Larkin A, et al. Lymphoproliferative disease in antibody deficiency: a multi-centre study. Clin Exp Immunol. (2003). 134:314–20. 10.1046/j.1365-2249.2003.02253.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rezaei N, Hedayat M, Aghamohammadi A, Nichols KE. Primary immunodeficiency diseases associated with increased susceptibility to viral infections and malignancies. J Allergy Clin Immunol. (2011) 127:1329–41. 10.1016/j.jaci.2011.02.047 [DOI] [PubMed] [Google Scholar]
- 15.Kinlen LJ, Webster AD, Bird AG, Haile R, Peto J, Soothill JF, et al. Prospective study of cancer in patients with hypogammaglobulinaemia. Lancet (1985) 1:263–6. [DOI] [PubMed] [Google Scholar]
- 16.Chapel H, Lucas M, Lee M, Bjorkander J, Webster D, Grimbacher B, et al. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood (2008) 112:277–86. 10.1182/blood-2007-11-124545 [DOI] [PubMed] [Google Scholar]
- 17.Chua I, Quinti I, Grimbacher B. Lymphoma in common variable immunodeficiency: interplay between immune dysregulation, infection and genetics. Curr Opin Hematol. (2008) 15:368–74. 10.1097/MOH.0b013e328302c7b6 [DOI] [PubMed] [Google Scholar]
- 18.Hunt RH, Xiao SD, Megraud F, Leon-Barua R, Bazzoli F, van der Merwe S, et al. Helicobacter pylori in developing countries. World Gastroenterology Organisation Global Guideline. J Gastrointestin Liver Dis. (2011) 20:299–304. 10.1097/MCG.0b013e31820fb8f6 [DOI] [PubMed] [Google Scholar]
- 19.Lin JX, Lin JP, Li P, Xie JW, Wang JB, Lu J, et al. Which staging system better predicts 10-year survival for gastric cancer? A study using an international multicenter database. Eur J Surg Oncol. (2018) 44:1205–11. 10.1016/j.ejso.2018.05.014 [DOI] [PubMed] [Google Scholar]
- 20.De Petris G, Dhungel BM, Chen L, Chang YH. Gastric adenocarcinoma in common variable immunodeficiency: features of cancer and associated gastritis may be characteristic of the condition. Int J Surg Pathol. (2014) 22:600–6. 10.1177/1066896914532540 [DOI] [PubMed] [Google Scholar]
- 21.Dhalla F, da Silva SP, Lucas M, Travis S, Chapel H. Review of gastric cancer risk factors in patients with common variable immunodeficiency disorders, resulting in a proposal for a surveillance programme. Clin Exp Immunol. (2001) 165:1–7. 10.1111/j.1365-2249.2011.04384.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shichijo S, Hirata Y. Characteristics and predictors of gastric cancer after Helicobacter pylori eradication. World J Gastroenterol. (2018) 24:2163–72. 10.3748/wjg.v24.i20.2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rugge M. Gastric cancer risk in patients with helicobacter pylori infection and following its eradication. Gastroenterol Clin North Am. (2015) 44:609–24. 10.1016/j.gtc.2015.05.009 [DOI] [PubMed] [Google Scholar]
- 24.Choi JH, Yang YJ, Bang CS, Lee JJ, Baik GH. Current status of the third-line helicobacter pylori eradication. Gastroenterol Res Pract. (2018) 2018:6523653. 10.1155/2018/6523653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Vries AC, Kuipers EJ, Rauws EA. Helicobacter pylori eradication and gastric cancer: when is the horse out of the barn? Am J Gastroenterol. (2009) 104:1342–5. 10.1038/ajg.2008.15 [DOI] [PubMed] [Google Scholar]
- 26.Kuipers EJ. When is endoscopic follow-up appropriate after helicobacter pylori eradication therapy? Gastroenterol Clin North Am. (2015) 44:597–608. 10.1016/j.gtc.2015.05.006 [DOI] [PubMed] [Google Scholar]
- 27.O'Reilly LA, Putoczki TL, Mielke LA, Low JT, Lin A, Preaudet A, et al. Loss of NF-κB1 causes gastric cancer with aberrant inflammation and expression of immune checkpoint regulators in a STAT-1-dependent manner. Immunity (2018) 48:570–83.e8. 10.1016/j.immuni.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 28.Fliegauf M, Bryant VL, Frede N, Slade C, Woon ST, Lehnert K, et al. Haploinsufficiency of the NF-κB1 subunit p50 in common variable immunodeficiency. Am J Hum Genet. (2015) 97:389–403. 10.1016/j.ajhg.2015.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]