Key Clinical Message
The link between Myobacterium avium‐intracellulare and lung cancer remains underemphasized in literature. Our objective is to increase awareness of Myobacterium avium‐intracellulare and coexisting lung cancer and to emphasize the need in establishing prevalence and specific testing guidelines in this patient population.
Keywords: diagnosis, incidence, lung cancer, mycobacterium avium‐intracellulare, non‐tuberculous mycobacterium
1. INTRODUCTION
While a correlation between pulmonary Mycobacterium avium‐intracellulare and development of lung cancer has been reported, specific guidelines regarding timing of screening and testing modalities for malignancy in patients with Mycobacterium avium‐intracellulare are not established. Mycobacterium avium‐intracellulare can be ubiquitous in its clinical course and misdiagnosis can greatly affect patient mortality.
Pulmonary Mycobacterium avium‐intracellulare (MAI) has been increasingly recognized as a common infection in patients with chronic obstructive pulmonary disease (COPD). Non‐tuberculous mycobacterium (NTM) pulmonary disease has been found in 2.0%‐5.8% of patients with lung cancer.1, 2Given the varied presentation of MAI, it is often difficult to diagnose patients with other developing underlying lung processes, specifically malignancy when MAI is present. This presents providers with a conundrum as patients with COPD are at high risk for malignancy. While the association between MAI and lung cancer has been previously reported in case studies, the link remains underemphasized in guidelines.1, 3 Most recently, a case report of co‐existing Mycobacterium avium and lung cancer within the same cavitary mass was reported; the coexistence was not recognized until definitive surgical removal of the mass.2 Established guidelines would hasten these diagnoses. Here we report a case of a 62‐year‐old female who was treated for MAI for a prolonged duration, eventually being found to have a concomitant diagnosis of lung cancer, discovered at a late stage. The objective of this case is report is to increase the awareness of the development of MAI and co‐existing lung cancer, necessitating the need for establishing prevalence and specific testing guidelines in this patient population.
2. CASE REPORT
A 62‐year‐old female with multiple medical problems to include mild COPD presented with the complaint of a persistent cough despite 2 years of therapy for presumed coccidioidomycosis. Two years prior when she first developed the cough she presented to a hospital in Arizona and was diagnosed with “pneumonia”. Patient's chest x‐ray was atypical at the time and a follow‐up CT scan revealed the presence of a cavitary mass. Patient underwent multiple lung biopsies at the outside hospital to rule out malignancy. No evidence of cancer was found, although necrotizing granulomatous changes were noted on the pathology report without further evidence of tuberculosis. Given the high prevalence of coccidioidomycosis in Arizona, despite initially negative sputum cultures for fungi, she was placed on oral fluconazole. While on the fluconazole treatment, she continued to have a mild cough and fatigue but denied feeling acutely ill. Serial CT imaging during this timeframe demonstrated bronchiectasis and worsening cavitary and nodular changes in her lungs.
Records review noted a positive MAI culture from a previous bronchiolar lavage (BAL) 2 years prior that had not been previously treated. Repeat sputum samples during the current evaluation demonstrated continued heavy growth of MAI. She was placed on standard antibiotic therapy of clarithromycin, ethambutol, and rifampin. In addition, patient was offered aminoglycoside therapy due to her cavitary disease, but declined due to side effects. Initially, her symptoms mildly improved with evidence of decreased cough and a resolution of her fevers; however, 4 months later her AFB cultures remained strongly positive for MAI (4+) with continued growth on cultures despite adherence to the treatment regimen. A repeat CT chest was obtained at the time to help assess the clinical status of the patient's ongoing MAI infection, which demonstrated evidence of a new cavitary lesion within the left upper lobe measuring 1.5 × 4.4 cm, as well as an airspace consolidation within the right upper lobe measuring approximately 1.9 × 3.6 cm, and increasing mediastinal lymphadenopathy (Figure 1).
Figure 1.
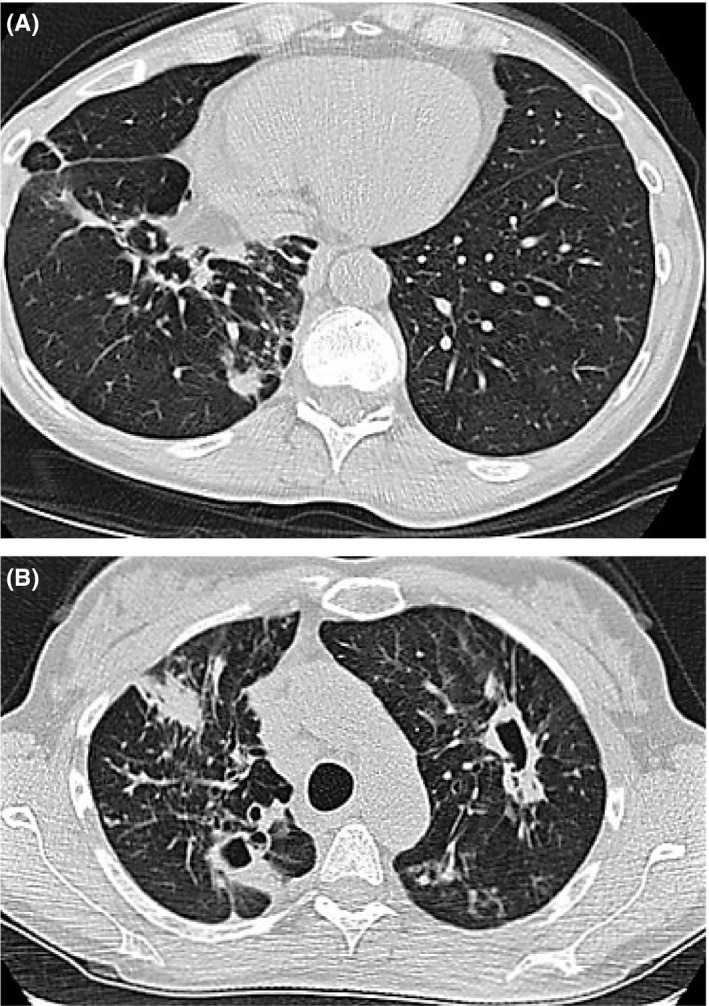
A, Airspace consolidation within the right upper lobe measuring approximately 1.9 × 3.6 cm and increasing mediastinal lymphadenopathy. B, Progression with evidence of new cavitary lesion within the left upper lobe (lower scan) measuring 1.5 × 4.4 cm
At that point, no repeat biopsies were obtained as the patient clinically felt well and the new lesions were thought to be due to the presumptive slow progression of the patient's known pulmonary MAI disease. At this time the patient's macrolide was changed from clarithromycin to azithromycin to allow higher blood levels when interacting with the other antibiotics and her ethambutol dose was increased. Other thoughts included adding intermittent amikacin for 2‐3 months and, as well as oral clofazimine. Traditionally, an anti‐leprosy drug, clofazimine, has been noted as a successful 2nd line agent for MAI in case reports, however would take time to obtain via special approval from FDA and this was not obtained prior to further progression of her symptoms. Her sensitivities at this time revealed susceptibilities to rifampin + ethambutol combo as well clarithromycin, clofazimine, however was resistant to amikacin.
Over the next 3 months, 2 repeat CTs were performed for hemoptysis and a brief episode of pleuritic chest pain–neither of which revealed any changes. Approximately 6 months later while awaiting the arrival and approval of the additional MAI medications, she began to complain of bone pain and low back pain radiating to her posterior thigh. She was initially thought to have discogenic sciatica, but her symptoms progressed to include worsening pain as well as numbness along the lateral aspect of the entire left leg and foot, which resulted in hospitalization. She received an expedited MRI which revealed a large vertebral mass extending from L4 to S1 with compression of the lumbosacral nerve roots (Figure 2). At this point, with previous known new infiltrate and having not yet started the additional MAI treatment, disseminated MAI was a consideration, but metastatic cancer was also high in the differential. Patient underwent a tissue biopsy of the vertebral mass with a preliminary result revealing undifferentiated carcinoma. Subsequently, a CT pulmonary angiography was obtained due to the patient's new complaint of shortness of breath and symptoms of SVC syndrome. The CTPA revealed a new large heterogeneous, necrotic appearing mediastinal mass, in addition to a metastatic appearing T4 lesion as well as an elevated right diaphragm likely due to impingement of her phrenic nerve by the mass. Ultimately, patient was diagnosed with metastatic neuroendocrine lung cancer from the biopsy of her vertebral mass (Figure 3). A couple weeks after her diagnosis, she elected palliative radiotherapy for treatment of the mediastinal mass and treatment of SCV syndrome due to its severity, and no further biopsy for typing of her cancer.
Figure 2.
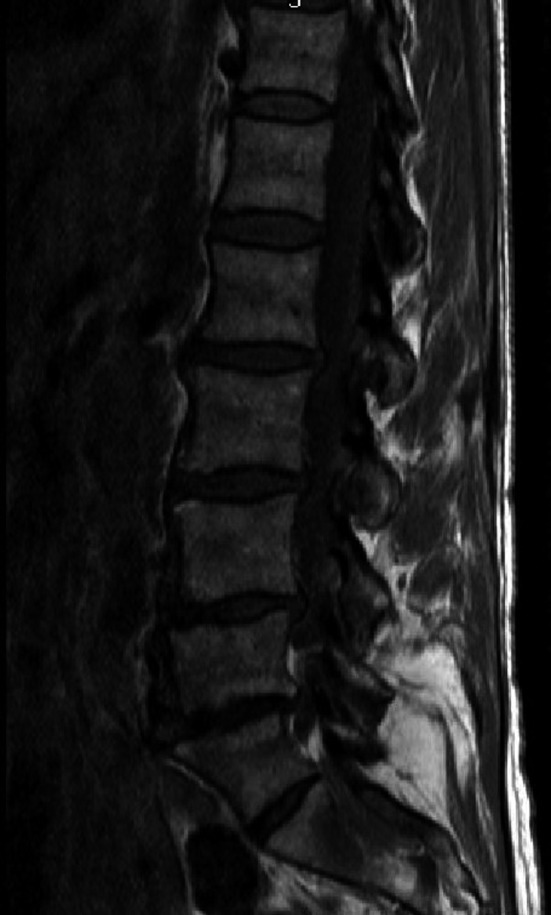
MRI lumbar spine exhibiting vertebral mass extending from L4 to S1 with compression of the lumbosacral nerve roots
Figure 3.
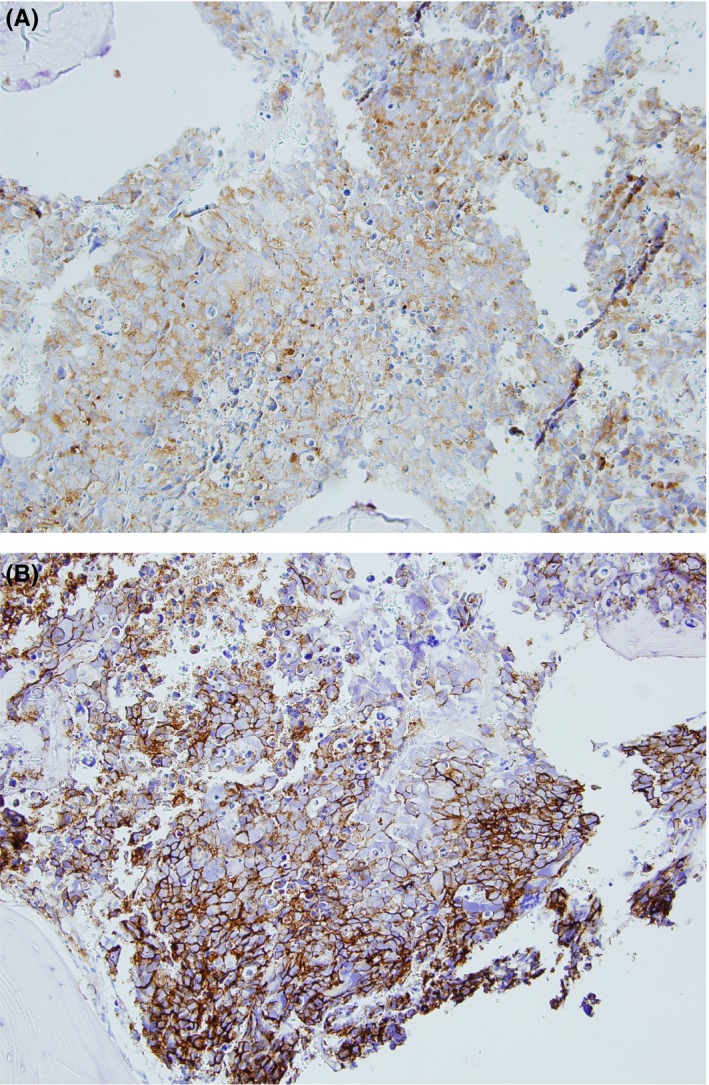
A, Lung pathology specimen‐core needle biopsy of pedicle L4 vertebrae showing neoplastic cells. B, Core needle biopsy from pedicle L4 vertebrae with neoplastic cells with immunoreactivity to Ber‐EP4 and CD56 stain
3. DISCUSSION
A retrospective analysis in 2012 of patients with pulmonary nontuberculous mycobacterial disease (NTM), 8.6% of men and 6.3% of women also had lung cancer.1 A particular association between squamous cell carcinoma, MAC and women was also noted with approximately 40% of women with MAC also developing squamous cell cancer as opposed to 28% of women with lung cancer alone.1 Of note, more nonsmokers were noted in the MAC/lung cancer group than the lung cancer group alone. Some literature postulates that the persistence of mycobacterial organisms leads to stimulation of a proinflammatory response creating chronic inflammation which nurtures developing malignancies. Other theories claim that growth of mycobacterial organisms is in fact a result of the chronic immunosuppression caused by the malignancy.4 It is difficult to ascertain which process develops first. This case represents a case of pulmonary MAI in which a concomitant diagnosis of lung cancer was potentially not detected at an earlier stage.
Microbiologic procedures such as bronchial washings and sputum culture are essential in diagnosing NTM disease. However, they must be interpreted with caution in patients with a mass‐like consolidation as NTM species are saprophytes and may colonize airways rather than infect them, therefore increasing the rate of false positive results. A false positive culture result for sputum or bronchial washing fluid does not exclude the possibility of concomitant lung cancer. This is important to recognize as a retrospective analysis in 2016 showed that NTM can manifest as a solitary pulmonary nodule, solitary mass, or mass‐like consolidation. The study analyzed radiologic features overlapping those of primary lung cancer and found the incidence of NTM pulmonary disease mimicking lung cancer clinically and radiologically to be 3.6% (14 of 388 patients). The NTM lesions typically showed poor contrast enhancement and frequent internal calcification on CT.3 Literature also suggests that both NTM and lung cancer have 18F‐FDG and 11C‐choline focal uptake has been described in these nodules; however, the degree of uptake was usually less than that seen in cancer.5 The study found that percutaneous needle biopsy was useful for microbiologic diagnosis of NTM disease manifesting as an SPN, mass, or mass‐like consolidation, which may argue that each new lesion arising in a patient with established NTM may require testing.
A requisite component of high value care includes ordering appropriate tests and ensuring that guidelines are followed. However, one should argue that in patients at high risk for malignancy, such as patients with chronic inflammatory states or COPD, repeat biopsies for new lesions may be needed. The current American Thoracic Society goals for MAI state that the goals of MAI therapy are to obtain: “symptomatic, radiographic, and microbiologic improvement,” which, except for the criteria for microbiological improvement are not well defined. The guidelines specify that although radiographic improvement is expected and desirable; radiographic assessment can be difficult both because of concomitant lung disease as well as the limited potential for improvement of MAI‐related abnormalities. They also state that patients should show clinical improvement within a 6‐month timeframe, which they acknowledge is often complicated to assess and may occur at an even later time frame in patients with concomitant COPD,6 such as our patient. Failure of treatment is most definitively diagnosed microbiologically when sputum samples do not covert to negative within 12 months on a macrolide‐containing regimen. However, as in this case, months of watchful waiting may not be practical if an underlying malignancy is being considered.
Searching the medical literature for additional surrogate markers of disease activity might help a provider clinically follow the possibility of multiple such serious chronic diseases at once. For example, an article published in 2015 suggested that changes in a baseline score derived from the semiquantitative measure of MAI growth over a 3‐month period was highly predictive of long‐term sputum conversion and treatment success in pulmonary MAI.7 Regarding pneumonias and other opacities seen on chest imaging of patients at risk for lung cancer, recommendations have suggested follow‐up radiographs or CT scans in 6‐12 weeks post pneumonia treatment to ensure resolution. In retrospect, the use of the semiquantitative MAC growth score in conjunction with sooner follow‐up CT imaging, might have prompted the provider and patient to consider an additional lung biopsy.
4. CONCLUSION
A clear association has been established between MAI and malignancy; however, little guidance regarding further testing such as additional biopsies in the setting of new lesions is offered in medical literature. It is critical for this guidance to be present especially for earlier detection of lung cancer which can greatly impact a patient's morbidity and mortality.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTION
NG: was the primary author. CP: was the secondary author, editor, and corresponding author. MS, and CS: involved in the editor and expert opinion.
DISCLAIMER
The views expressed are those of the authors and do not reflect the official policy of the Department of the Army, the Department of Defense or the U.S. Government.
Garg N, Punch C, Stein M, Schofield C. When occam's razor can fail– active mycobacteria infection and lung cancer: A case of neuroendocrine lung cancer diagnosed in the setting of refractory mycobacterium avium‐intracellulare. Clin Case Rep. 2018;6:2156–2159. 10.1002/ccr3.1813
References
- 1. Lande L, Peterson D, Gogoi R, et al. Association between pulmonary mycobacterium avium complex infection and lung cancer. J Thorac Oncol. 2012;7(9):1345‐1351. [DOI] [PubMed] [Google Scholar]
- 2. Taira N, Kawasaki H, Takahara S, Chibana K, Atsumi E, Kawabata T. The presence of coexisting lung cancer and non‐tuberculous mycobacterium in a solitary mass. Am J Case Rep. 2018;19:748‐751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hong S, Kim T, Lee J, Park J. Nontuberculous mycobacterial pulmonary disease mimicking lung cancer. Clinicoradiologic features and diagnostic implications. Open Respir Med J. 2016;10:20‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tamura A, Hebisawa A, Kusaka K, et al. Relationship between lung cancer and complex isolated using bronchoscopy. Open Respir Med J. 2016;10(1):20‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martinez S, McAdams P, Batchu C. The many faces of pulmonary nontuberculous mycobacterial infection. Am J Roentgenol. 2007;189:177‐186. [DOI] [PubMed] [Google Scholar]
- 6. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. American Journal of Respiratory and Critical Care Medicine. Vol 175, No 4 [Internet]. Atsjournals.org. 2016. http://www.atsjournals.org/doi/full/10.1164/rccm.200604-571ST#.V5FA0Hn2ZMs. Accessed December 21, 2016. [DOI] [PubMed] [Google Scholar]
- 7. Griffith D, Adjemian J, Brown‐Elliott B, et al. Semiquantitative culture analysis during therapy for mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2015;192(6):754‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]


