Abstract
We have recently demonstrated that whole egg ingestion induces a greater muscle protein synthetic (MPS) response when compared with isonitrogenous egg white ingestion after resistance exercise in young men. Our aim was to determine whether whole egg or egg white ingestion differentially influenced colocalization of key regulators of mechanistic target of rapamycin complex 1 (mTORC1) as means to explain our previously observed divergent postexercise MPS response. In crossover trials, 10 healthy resistance-trained men (21 ± 1 yr; 88 ± 3 kg; body fat: 16 ± 1%; means ± SE) completed lower body resistance exercise before ingesting whole eggs (18 g protein, 17 g fat) or egg whites (18 g protein, 0 g fat). Muscle biopsies were obtained before exercise and at 120 and 300 min after egg ingestion to assess, by immunofluorescence, protein colocalization of key anabolic signaling molecules. After resistance exercise, tuberous sclerosis 2-Ras homolog enriched in brain (Rheb) colocalization decreased (P < 0.01) at 120 and 300 min after whole egg and egg white ingestion with concomitant increases (P < 0.01) in mTOR-Rheb colocalization. After resistance exercise, mTOR-lysosome-associated membrane protein 2 (LAMP2) colocalization significantly increased at 120 and 300 min only after whole egg ingestion (P < 0.01), and mTOR-LAMP2 colocalization correlated with rates of MPS at rest and after exercise (r = 0.40, P < 0.05). We demonstrated that the greater postexercise MPS response with whole egg ingestion is related in part to an enhanced recruitment of mTORC1-Rheb complexes to the lysosome during recovery. These data suggest nonprotein dietary factors influence the postexercise regulation of mRNA translation in human skeletal muscle.
Keywords: anabolic signaling, immunofluorescence, muscle protein synthesis, Rheb, TSC2
INTRODUCTION
The mechanistic target of rapamycin complex 1 (mTORC1) signaling cascade is a major regulator of nutrient and contraction-induced increases in human muscle protein synthesis (7, 8). While mTORC1 activity is fundamentally regulated by changes in protein phosphorylation, traditional Western blot readouts of pathway activation can be dissociated from acute changes in muscle protein synthesis (14) and protein kinase activity (19). However, it is becoming increasingly apparent that protein-protein interactions and intracellular translocation represent important loci of control for mTORC1 pathway regulation (2) that ultimately mediate downstream kinase activity (15, 25). Consistent with in vitro events of mTORC1 regulation (16, 34), we recently demonstrated in human muscle that the negative regulator tuberous sclerosis 2 (TSC2) dissociates from mTOR and that there is a concomitant increase in mTOR association with its positive regulator Ras homolog enriched in brain (Rheb) after endurance (1) and resistance exercise (25). These mTOR regulatory events potentially contribute to increased mTOR activity and postexercise muscle protein synthesis. Further, intracellular redistribution of mTOR towards the cell periphery—a potential regulatory “hub” in human muscle—occurs in response to feeding at rest and/or after exercise (1, 25). It is unclear whether this cell periphery-translocation, combined with persistent mTORC1-lysosome association (25), is unique to human skeletal muscle since in vitro (23) and rodent work (17) suggests that the lysosome is the key regulatory site of mTORC1 activity.
The postexercise consumption of dietary amino acids increases skeletal muscle protein synthesis and mTOR pathway activation in human muscle (8, 21). Research in humans utilizing isolated protein sources (i.e., whey, casein, soy) (27, 30) and/or crystalline amino acids (22) dissolved in liquid-based beverages have demonstrated robust increases in mTOR activity and postexercise muscle protein synthesis, which is generally related to the essential amino acid content of the ingested protein source (28). However, postprandial dietary amino acid availability may not be the only variable regulating postexercise muscle protein synthesis after the consumption of protein-dense whole foods (3, 10), suggesting a potential role for nonprotein nutritive factors in the acute regulation of mTORC1 and human muscle protein synthesis. In support, we recently demonstrated that ingestion of whole eggs stimulated a greater myofibrillar protein synthetic response when compared with isonitrogenous egg whites after resistance exercise in young men (29). However, the greater increases in myofibrillar protein synthesis we observed with the whole egg versus egg white ingestion did not appear to be related to differences in postprandial dietary leucine availability in circulation (or the amino acid composition of the ingested food) or muscle anabolic protein signaling phosphorylation (29), all of which are important variables influencing postexercise muscle protein synthesis (7, 8, 30). Therefore, the purpose of the present study was to determine whether the greater whole egg-induced myofibrillar protein synthesis response we previously observed postexercise was mediated by differences in mTOR colocalization.
MATERIALS AND METHODS
Participants and ethical approval.
Ten healthy resistance-trained young (age: 21 ± 1 yr, weight: 88 ± 3 kg, lean body mass: 72 ± 2 kg; 10-repetition maximum (RM) leg press 242 ± 32 kg, 10-RM leg extensions 112 ± 10 kg; means ± SE) men were recruited for this study. The participants were part of a larger investigation conducted in our laboratories (29). All participants were deemed healthy on the basis of responses to a routine medical screening questionnaire. Participants provided written informed consent to a protocol that was approved by the University of Toronto Research Ethics Board and the University of Illinois at Urbana-Champaign Institutional Review Board and that conformed to the standards set by the most recent revision of the Declaration of Helsinki.
Experimental design.
The experimental design used for this study has been described previously (29). The present study was registered at clinicaltrials.gov as NCT03117127. Briefly, participants reported to the laboratory at 0700 h after an overnight fast and having refrained from vigorous physical activity for 3 days. After baseline muscle biopsies were taken (t = −30 min), participants performed lower body resistance exercise consisting of 4 sets of 10 repetitions at 80% of 10-RM for leg press and leg extensions. Immediately after exercise (t = 0 min), three whole eggs or an isonitrogenous quantity of egg whites (~6 egg whites) were consumed in a randomized, counter-balance fashion. The whole eggs or egg whites were scrambled in a skillet until solid with no visible liquid remaining the morning of the experiment. The macronutrient composition and energy content for the whole eggs was 18 g protein, 17 g fat, and 226 kcals, and 18 g protein, 0 g fat, and 73 kcals for the egg whites. Muscle biopsies were obtained from the vastus lateralis, as previously described (29), at t = 120 and 300 min of postexercise recovery from the contralateral leg and were subsequently used for anabolic-related colocalization analysis. Muscle biopsy samples (~25 mg) were freed from any visible blood, adipose, and connective tissue and were immediately mounted in Optimal Cutting Temperature Compound (Tissue-Tek, VWR) and frozen in isopentane cooled by liquid nitrogen before storage at −80°C for subsequent immunofluorescence analysis.
Plasma analysis.
Plasma samples were analyzed for cholecystokinin (CCK) concentrations by an enzyme immunoassay kit according to the manufacturer’s instructions (RayBiotech, Norcross, GA). Cholesterol samples were measured using an enzymatic-based kit according to the manufacturer’s instructions (Pointe Scientific, Canton, MI).
Immunofluorescence.
Serial cross sections (7 μm) were stained for mTOR colocalization and digital images were captured using an EVOS FL Auto Cell imaging microscope (Thermo Fisher) at ×40 0.75 numerical aperture (NA) magnification as previously described (25). All image capturing parameters were kept constant between images, including exposure time, gain, image frame, and light intensity. For colocalization analysis, at least six images of randomly selected fields of view were captured per section with each image including approximately six fibers on average. For each subject, muscle samples were taken from three time points/trial (six samples total), and average values were calculated from randomly selected fields of two serial sections for each time point. All image processing and quantitation was carried out in ImagePro Premier (version 9.2, (Media Cybernetics) and Pearson’s correlation coefficient was used to quantify correlations between mTOR/TSC2 with Rheb, and mTOR with lysosome-associated membrane protein 2 (LAMP2) to be consistent with previous human in vivo research (1, 15, 25). To assess the nonspecificity/autofluorescence of our antibodies, primary antibody was omitted from a single cross section on each slide for each participant. Fluorescence intensity of these secondary‐only sections was subsequently utilized as a set point for determining positive staining/thresholds on all other sections on the slide. This allowed us to determine the background noise and variability on every slide for each participant whereby the pixels whose intensities fell below the range of non- specificity were omitted from analysis.
Antibodies.
The mouse monoclonal anti-mTOR (no. 05-1592) antibody was purchased from Millipore (Toronto, ON, Canada) and mouse monoclonal anti-TSC2 (no. AM1919b-EV) was purchased from FroggaBio (Toronto, ON, Canada). The corresponding conjugated secondary antibody to this was goat anti-mouse IgGγ1 Alexa 594 (no. ab 150116; Abcam). Antibodies targeting rabbit polyclonal-LAMP2 (no. ABGAP1824DEV) were purchased from Biolynx (Toronto, ON, Canada) and Rheb (no. ab92313; Abcam) was purchased from Abcam (Toronto, ON, Canada). The corresponding antibody to this was goat anti-rabbit IgG(H&L) Alexa 488 secondary antibody (no. ab150113; Abcam). Finally, wheat germ agglutinin (WGA-350, no. W11263, FisherScientific) was used to identify the cell periphery of muscle fibers. To assess whether the mTOR and Rheb signals are primarily localized internal to or adjacent with the WGA, sections from one participant were viewed at ×60 1.30 NA oil immersion objective on a Leica DMI8 confocal microscope (Supplemental Figure S1; Supplemental Material for this article is available online at the Journal website).
Statistical analysis.
Colocalization and plasma analysis data were analyzed by two-way repeated measures ANOVA (time × condition) with Tukey post hoc analysis when P < 0.05. For colocalization analysis we analyzed the raw Pearson's r values using linear correlation and present the data as fold-change to better reflect the physiological changes. Time series and area under the curve (AUC) data were analyzed using two-way repeated-measures ANOVA and Student’s t-test, respectively. Using average data from 120- and 300-min time points, Pearson's r was calculated for mTOR-LAMP2 correlation with myofibrillar protein synthesis, phospho-ribosomal protein S6 kinase 1 (p-S6K1), and phospho 4E-binding protein 1 (4E-BP1) correlation with myofibrillar protein synthesis, and p-S6K1 correlation with mTOR-LAMP2, using myofibrillar protein synthesis and p-S6K1/p-4E-BP1 data from our previous publication (29). An average of the 120- and 300-min postprandial mTOR-related signal (either via immunofluorescence or Western blotting) was taken given that the myofibrillar protein synthetic response represents and average kinetic rate over the entire 5-h period. In contrast, a single fasted mTOR-signal was taken to reflect the basal, fasted myofibrillar protein synthetic response. GraphPad for Windows (version 6.00, GraphPad Software, San Diego, CA) was used for all statistical analyses. All data are expressed as means ± SE.
RESULTS
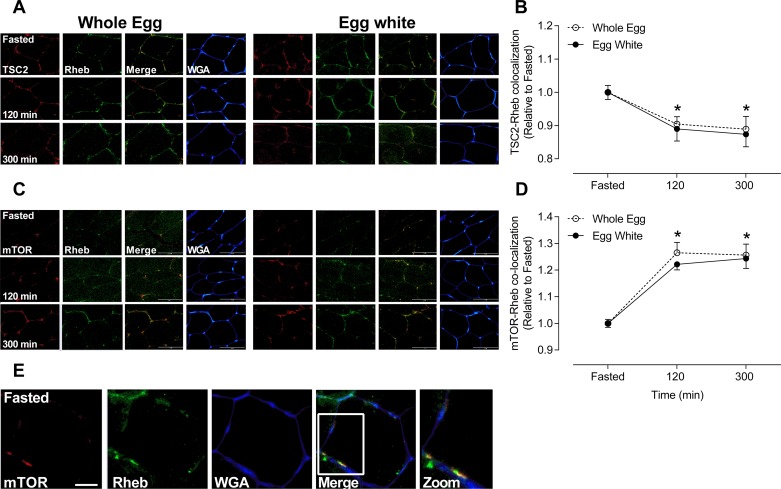
Similar decreases in TSC2 colocalization with Rheb with whole eggs vs. egg whites after resistance exercise.
After resistance exercise, TSC2 colocalization with Rheb decreased by ~11 and ~12% at 120 and 300 min, respectively (P < 0.01), with no difference between whole eggs or egg whites (P = 0.97) (Fig. 1, A and B).
Fig. 1.
Tuberous sclerosis 2 (TSC2) and mechanistic target of rapamycin (mTOR) colocalization with Ras homolog enriched in brain (Rheb) in response to whole eggs or egg whites following resistance exercise. Immunofluorescence quantification of mTOR/TSC2 (red) and Rheb (green) colocalization, displayed as a composite image (merge) and WGA (blue) (n = 10/condition). Yellow/orange regions represent TSC2 and Rheb (A) mTOR and Rheb colocalization (C). Each panel represents one subject from whole eggs and egg whites across the experimental time course. Group data are quantified and reported as TSC2 and Rheb colocalization (B) and mTOR and Rheb colocalization (D). Open circles represent whole eggs and closed circles represent egg whites. All data are presented relative to Fasted. Scale bar represents 100-μm area. Data are presented as means ± SE and were analyzed using two-way repeated-measures ANOVA. *P < 0.01, different from Fasted in same condition. Immunofluorescence image was obtained on a Leica DMI8 confocal microscope of mTOR (red) and Rheb (green) interaction, displayed as a composite image (merge) and WGA (blue) (E). Sections were viewed at ×60 1.30 numerical aperture oil immersion objective to visualize the signal being primarily intracellular and/or within the fiber junctions. Scale bar represents 20-μm area (E).
Similar increases in mTOR colocalization with Rheb with whole eggs vs egg whites after resistance exercise.
After resistance exercise, mTOR colocalization with Rheb increased by ~20 and ~20% at 120 and 300 min, respectively (P < 0.01), with no difference between whole eggs or egg whites (P = 0.52) (Fig. 1, C and D).
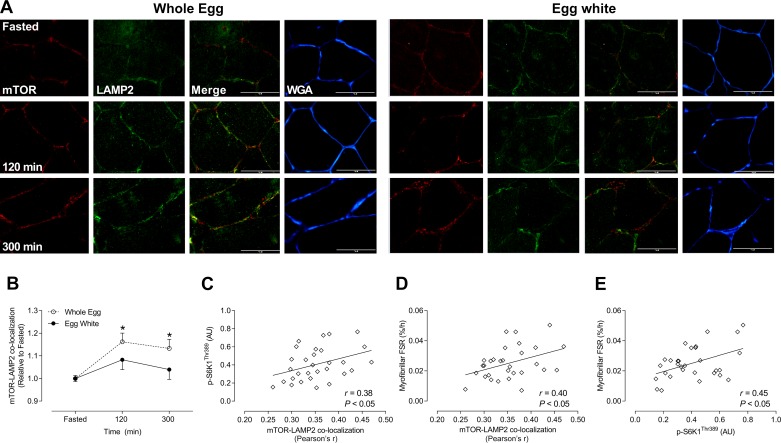
Whole eggs but not egg whites induce mTOR colocalization with LAMP2 following resistance exercise.
After resistance exercise, mTOR colocalization with LAMP2 significantly increased by ~14 and ~12% at 120 and 300 min, respectively, following whole egg ingestion (P < 0.01). There was no change in mTOR colocalization with LAMP2 (P = 0.12) in response to egg white ingestion (Fig. 2B).
Fig. 2.
Mechanistic target of rapamycin (mTOR) colocalization with lysosome-associated membrane protein 2 (LAMP2) in response to whole eggs or egg whites after resistance exercise and mTOR-LAMP and phosphorylation of S6 kinase 1 (p-S6K1) against myofibrillar protein synthesis in response to whole eggs and egg whites. Immunofluorescence quantification of mTOR (red) and LAMP2 (green) colocalization, displayed as a composite image (merge) and wheat germ agglutinin (WGA; blue; n = 10/condition). Yellow/orange regions represent mTOR and LAMP2 colocalization (A). Each panel represents one subject from whole eggs and egg whites across the experimental time course. Group data are quantified and reported; open circles represent whole eggs and closed circles represent egg whites (B). Correlation analysis of mTOR-LAMP2 and myofibrillar fractional synthetic rate (FSR; C), p-S6K1 with myofibrillar FSR (D), and p-S6K1 with mTOR-LAMP2 (E). All data are presented relative to Fasted. Scale bar represents 100-μm area. Data are presented as means ± SE and were analyzed using two-way repeated-measures ANOVA. *P < 0.01, different from Fasted in whole eggs. AU, arbitrary units. [Myofibrillar FSR data from Ref. 29.]
Greater mTOR-LAMP2 colocalization and phosphorylation of S6K1 is positively associated with myofibrillar protein synthesis.
p-S6K1 was positively associated with mTOR-LAMP2 colocalization (P < 0.05; r = 0.38) (Fig. 2B) but not with p-4E-BP1 or TSC2-Rheb (both P ≥ 0.40; data not shown). mTOR-LAMP2 colocalization (P < 0.05; r = 0.40) and p-S6K1 (P < 0.05; r = 0.45) were positively correlated with myofibrillar protein synthesis (Fig. 2, D and E). There were no significant correlations between p-4E-BP1/TSC2-Rheb with fasted or postprandial myofibrillar protein synthesis as well as p-4E-BP1 with mTOR-LAMP2 (all P ≥ 0.082; data not shown).
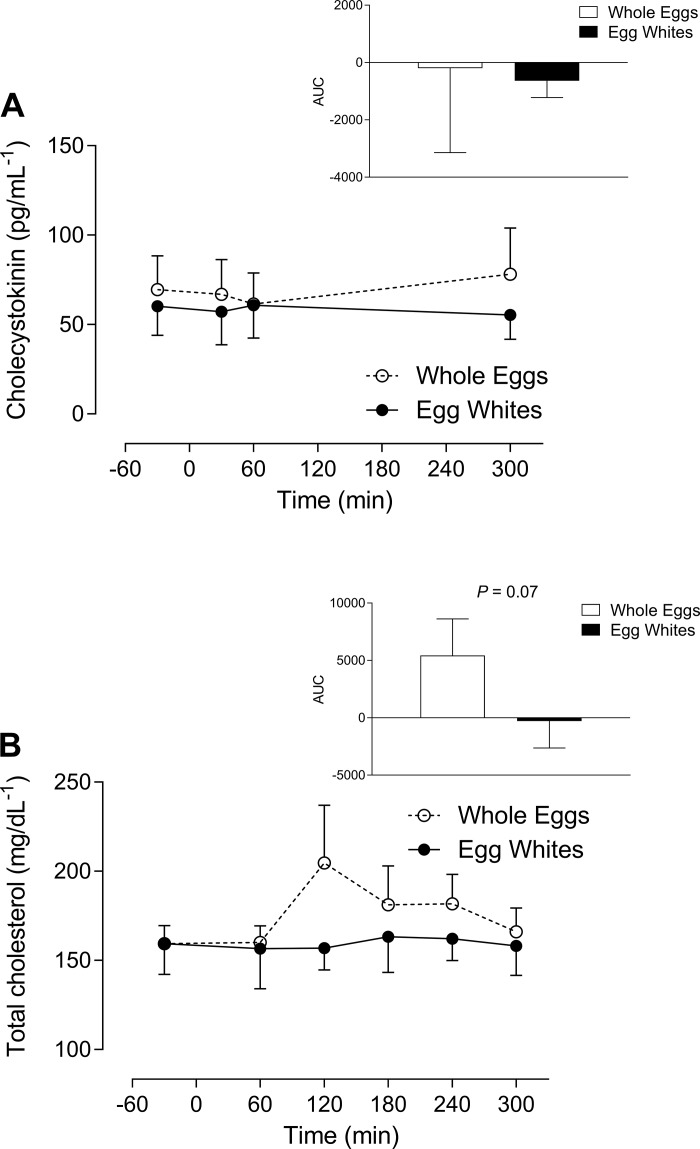
Increase in cholesterol in response to whole egg but not egg white ingestion.
There was no effect of combined nutrition and exercise on plasma CCK (Fig. 3A). Plasma cholesterol peaked at 120 min in the whole egg and remained in the egg whites, and there was a greater trend with the AUC in response to the whole egg (P = 0.07) (Fig. 3B).
Fig. 3.
Plasma cholecystokinin (CCK) and cholesterol availability in response to whole eggs or whites following resistance exercise. Total CCK (pg/ml) (A) and cholesterol (mg/dl) (B) were analyzed in response to whole eggs and egg whites (n = 10/condition); open circles represent whole eggs and closed circles represent egg whites. Data are presented as means ± SE and were analyzed using two-way repeated-measures ANOVA. AUC, area under the curve.
DISCUSSION
The mTORC1 pathway is generally considered to be the main regulator of anabolic stimuli in skeletal muscle (7, 8). In response to amino acids, Rag GTPases recruit mTORC1 to the lysosomal surface where it can interact with Rheb (24). Consistent with previous observations of a dissociation of TSC2 from the lysosome in response to nutrient- (12) and contraction-derived (17) stimuli, we found that TSC2-Rheb colocalization decreased below fasted levels at 120 and 300 min of recovery with a reciprocal increase in mTOR-Rheb colocalization. These events are consistent with previous findings from our lab suggesting that the TSC2-Rheb dissociation, mirrored by an increase in mTOR-Rheb colocalization, may be important regulatory steps in the postprandial stimulation of myofibrillar protein synthesis at rest and after endurance exercise (1). Thus, the association of mTOR-Rheb would be consistent with molecular events that would support enhanced mRNA translation in response muscle contraction (13). Nevertheless, the similarity in these subcellular events between the whole eggs and egg whites conditions suggest that they did not underpin our previously observed differences in postexercise myofibrillar protein synthetic response (29).
Studies in vitro and in rodents suggest that mTORC1 recruitment to the lysosome is important for its kinase activity (17, 23). We observed that whole egg, but not egg white, ingestion induced mTOR-LAMP2 (lysosomal marker) colocalization at 120 and 300 min of postexercise recovery. Importantly, the degree of mTOR-LAMP2 colocalization was associated with previously determined S6K1 phosphorylation, a readout of mTORC1 activity, and myofibrillar protein synthesis (29), suggesting that our the greater anabolic effect of whole eggs after resistance exercise may be mediated, in part, by a greater lysosomal targeting of mTORC1. These results in human muscle are in agreement with studies in vitro and in rodents demonstrating that mTORC1 translocates towards the lysosome, the site of its activation (2, 17, 23), in response to amino acid administration (23) and/or contractile activity (17). Our finding of marginal changes in mTOR-LAMP2 colocalization after egg whites is in agreement with previous human data (25), which reported that mTOR-LAMP2 (lysosomal marker) colocalization does not increase in response to combined mixed meal ingestion (i.e., 20 g protein as whey and casein proteins, 44 g carbohydrate, 1 g fat) and resistance exercise (25). Additionally, in a previous study from our lab (1), we did not observe an increase in mTOR-LAMP2 colocalization during recovery from endurance exercise despite consumption of whole eggs and carbohydrates (18 g protein, 60 g carbohydrate, 17 g fat) (1). Thus, the greater mTOR-LAMP2 colocalization concomitant with enhanced rates of myofibrillar protein synthesis with whole eggs suggest that mTORC1 association with the lysosome may be a requisite event to further support elevated rates of mRNA translation in human skeletal muscle during postexercise recovery.
An egg yolk is enriched in lipids, phospholipids, and cholesterol (5), which may have played a role, either independently or synergistically, in mediating the greater mTORC1-lysosomal recruitment. For example, it has recently been demonstrated that lysosomal cholesterol activates mTORC1 through SLC38A9-mediated activation of Rag GTPases and mTORC1 recruitment to the lysosome (4). We therefore quantified postprandial plasma cholesterol availability and observed a slight increase in with whole egg ingestion at 120 min, which is cotemporal with mTOR-LAMP2 colocalization. Thus, the greater net exposure of cholesterol (AUC) for muscle receptor-mediated endocytosis may have played a role in the greater mTORC1 recruitment with whole eggs. However, glycerophospholipid phosphatidic acid (PA) is also important for contraction-induced mTOR activation (18), which ostensibly would include mTORC1 recruitment to the lysosome (17). The egg yolk is enriched in oleic acid and phosphatidylcholine which can be converted to PA (20, 31). Furthermore, the egg yolk is enriched with diacylglycerol (32) whereby PA is rapidly metabolized to diacylglycerol intracellularly (33). Given that exercise stimulates PA production and that exogenous PA or precursors (such as oleic acid and phosphatidylcholine) enhance mTOR activity (18, 20, 33), we cannot discount the possibility that the phospholipid content of the whole eggs may have contributed to the greater mTORC1 activity via PA-mediated mechanism.
The macronutrient composition and energy content for the whole eggs was 18 g protein, 17 g fat, and 226 kcals, and 18 g protein, 0 g fat, and 73 kcals for the egg whites. The energy content of a meal is unlikely to direct the differential MPS response between whole eggs and egg whites (26). However, CCK is a gut-derived secondary messenger that is released rapidly in response to nutrients (including fat) (11) and stimulates growth via an mTOR-dependent mechanism in pancreatic tissue (6). Despite the potential anabolic effect of CCK, we observed no effects for time or condition in our study, suggesting that the relatively low energy content (226 and 73 kcals in whole eggs and egg whites, respectively) was insufficient to elevate CCK and thus mediate our greater mTORC1 pathway activation and MPS.
Image capture in the present study was determined using light microscopy and therefore may not be sufficient to identify molecular and/or protein-protein interactions (9). To clearly identify physical apposition of molecules, higher-resolution techniques such as fluorescence resonance energy transfer and/or electron microscopy would be preferable and represent future opportunities to study the in vivo molecular regulation of human muscle protein synthesis. Pearson's correlation coefficient was used in the present study as this is generally robust in group comparison designs with data that are visually aligned with linear correlations (9). However, we acknowledge that alternative analyses (e.g., Maunders correlation coefficient) are possible in colocalization studies if data are nonlinear and/or if background staining is nonuniform (9). Thus, although we interpret the greater mTOR-LAMP2 colocalization with whole eggs as being related to an enhanced lysosomal targeting of mTOR, future research is warranted with alternative image capture methods and/or analysis parameters to confirm whether our data indeed represent increased protein-protein interaction.
In conclusion, we demonstrate that whole egg ingestion, but not egg whites, induces mTOR colocalization to the lysosome during recovery from resistance exercise. This molecular event may provide insight as to how postexercise MPS is stimulated to a greater extent after whole eggs versus isonitrogenous amounts of egg whites consumption in healthy young males. Additional research is required to identify the dietary component and the synergistic mechanism(s) that drives mTOR towards the lysosome after combined resistance exercise and whole egg ingestion.
GRANTS
S. Abou Sawan was supported by the Ontario Graduate Scholarship. Funding for the study was provided by grants from the Faculty of Kinesiology and Physical Education at the University of Toronto and Natural Sciences and Engineering Research Council of Canada (D. R. Moore) and the University of Illinois Center on Health, Aging, and Disability (N. A. Burd).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.v.V., N.A.B., and D.R.M. conceived and designed research; S.A.S., D.W.W., J.W.B., S.A.P., and N.A.B. performed experiments; S.A.S., S.v.V., D.W.W. analyzed data; S.A.S., S.v.V., D.W.W., S.A.P., N.A.B., and D.R.M. interpreted results of experiments; S.A.S., D.W.W. prepared figures; S.A.S., D.W.W. drafted manuscript; S.A.S., S.v.V., D.W.W., J.W.B., S.A.P., N.A.B., and D.R.M. edited and revised manuscript; S.A.S., S.v.V., D.W.W., J.W.B., S.A.P., N.A.B., and D.R.M. approved final version of manuscript.
Supplemental Data
REFERENCES
- 1.Abou Sawan S, van Vliet S, Parel JT, Beals JW, Mazzulla M, West DWD, Philp A, Li Z, Paluska SA, Burd NA, Moore DR. Translocation and protein complex co-localization of mTOR is associated with postprandial myofibrillar protein synthesis at rest and after endurance exercise. Physiol Rep 6: e13628, 2018. doi: 10.14814/phy2.13628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Betz C, Hall MN. Where is mTOR and what is it doing there? J Cell Biol 203: 563–574, 2013. doi: 10.1083/jcb.201306041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burd NA, Gorissen SH, van Vliet S, Snijders T, van Loon LJ. Differences in postprandial protein handling after beef compared with milk ingestion during postexercise recovery: a randomized controlled trial. Am J Clin Nutr 102: 828–836, 2015. doi: 10.3945/ajcn.114.103184. [DOI] [PubMed] [Google Scholar]
- 4.Castellano BM, Thelen AM, Moldavski O, Feltes M, van der Welle RE, Mydock-McGrane L, Jiang X, van Eijkeren RJ, Davis OB, Louie SM, Perera RM, Covey DF, Nomura DK, Ory DS, Zoncu R. Lysosomal cholesterol activates mTORC1 via an SLC38A9-Niemann-Pick C1 signaling complex. Science 355: 1306–1311, 2017. doi: 10.1126/science.aag1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cotterill OJ, Marion WW, Naber EC. A nutrient re-evaluation of shell eggs. Poult Sci 56: 1927–1934, 1977. doi: 10.3382/ps.0561927. [DOI] [PubMed] [Google Scholar]
- 6.Crozier SJ, Sans MD, Guo L, D’Alecy LG, Williams JA. Activation of the mTOR signalling pathway is required for pancreatic growth in protease-inhibitor-fed mice. J Physiol 573: 775–786, 2006. doi: 10.1113/jphysiol.2006.106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickinson JM, Fry CS, Drummond MJ, Gundermann DM, Walker DK, Glynn EL, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr 141: 856–862, 2011. doi: 10.3945/jn.111.139485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol 587: 1535–1546, 2009. doi: 10.1113/jphysiol.2008.163816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn KW, Kamocka MM, McDonald JH. A practical guide to evaluating colocalization in biological microscopy. Am J Physiol Cell Physiol 300: C723–C742, 2011. doi: 10.1152/ajpcell.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elliot TA, Cree MG, Sanford AP, Wolfe RR, Tipton KD. Milk ingestion stimulates net muscle protein synthesis following resistance exercise. Med Sci Sports Exerc 38: 667–674, 2006. doi: 10.1249/01.mss.0000210190.64458.25. [DOI] [PubMed] [Google Scholar]
- 11.Feinle C, O’Donovan D, Doran S, Andrews JM, Wishart J, Chapman I, Horowitz M. Effects of fat digestion on appetite, APD motility, and gut hormones in response to duodenal fat infusion in humans. Am J Physiol Gastrointest Liver Physiol 284: G798–G807, 2003. doi: 10.1152/ajpgi.00512.2002. [DOI] [PubMed] [Google Scholar]
- 12.Garami A, Zwartkruis FJ, Nobukuni T, Joaquin M, Roccio M, Stocker H, Kozma SC, Hafen E, Bos JL, Thomas G. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell 11: 1457–1466, 2003. doi: 10.1016/S1097-2765(03)00220-X. [DOI] [PubMed] [Google Scholar]
- 13.Goodman CA, Frey JW, Mabrey DM, Jacobs BL, Lincoln HC, You JS, Hornberger TA. The role of skeletal muscle mTOR in the regulation of mechanical load-induced growth. J Physiol 589: 5485–5501, 2011. doi: 10.1113/jphysiol.2011.218255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, Wackerhage H, Smith K, Atherton P, Selby A, Rennie MJ. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab 295: E595–E604, 2008. doi: 10.1152/ajpendo.90411.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodson N, McGlory C, Oikawa SY, Jeromson S, Song Z, Rüegg MA, Hamilton DL, Phillips SM, Philp A. Differential localization and anabolic responsiveness of mTOR complexes in human skeletal muscle in response to feeding and exercise. Am J Physiol Cell Physiol 313: C604–C611, 2017. doi: 10.1152/ajpcell.00176.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev 17: 1829–1834, 2003. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobs BL, You JS, Frey JW, Goodman CA, Gundermann DM, Hornberger TA. Eccentric contractions increase the phosphorylation of tuberous sclerosis complex-2 (TSC2) and alter the targeting of TSC2 and the mechanistic target of rapamycin to the lysosome. J Physiol 591: 4611–4620, 2013. doi: 10.1113/jphysiol.2013.256339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joy JM, Gundermann DM, Lowery RP, Jager R, McCleary SA, Purpura M, Roberts MD, Wilson SM, Hornberger TA, Wilson JM. Phosphatidic acid enhances mTOR signaling and resistance exercise induced hypertrophy. Nutr Metab (Lond) 11: 29, 2014. doi: 10.1186/1743-7075-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGlory C, White A, Treins C, Drust B, Close GL, Maclaren DP, Campbell IT, Philp A, Schenk S, Morton JP, Hamilton DL. Application of the [γ-32P] ATP kinase assay to study anabolic signaling in human skeletal muscle. J Appl Physiol (1985) 116: 504–513, 2014. doi: 10.1152/japplphysiol.01072.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menon D, Salloum D, Bernfeld E, Gorodetsky E, Akselrod A, Frias MA, Sudderth J, Chen PH, DeBerardinis R, Foster DA. Lipid sensing by mTOR complexes via de novo synthesis of phosphatidic acid. J Biol Chem 292: 6303–6311, 2017. doi: 10.1074/jbc.M116.772988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore DR, Atherton PJ, Rennie MJ, Tarnopolsky MA, Phillips SM. Resistance exercise enhances mTOR and MAPK signalling in human muscle over that seen at rest after bolus protein ingestion. Acta Physiol (Oxf) 201: 365–372, 2011. doi: 10.1111/j.1748-1716.2010.02187.x. [DOI] [PubMed] [Google Scholar]
- 22.Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr 89: 161–168, 2009. doi: 10.3945/ajcn.2008.26401. [DOI] [PubMed] [Google Scholar]
- 23.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141: 290–303, 2010. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320: 1496–1501, 2008. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song Z, Moore DR, Hodson N, Ward C, Dent JR, O'Leary MF, Shaw AM, Hamilton DL, Sarkar S, Gangloff YG, Hornberger TA, Spriet LL, Heigenhauser GJ, Philp A. Resistance exercise initiates mechanistic target of rapamycin (mTOR) translocation and protein complex co-localisation in human skeletal muscle. Sci Rep 7: 5028, 2017. doi: 10.1038/s41598-017-05483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staples AW, Burd NA, West DW, Currie KD, Atherton PJ, Moore DR, Rennie MJ, Macdonald MJ, Baker SK, Phillips SM. Carbohydrate does not augment exercise-induced protein accretion versus protein alone. Med Sci Sports Exerc 43: 1154–1161, 2011. doi: 10.1249/MSS.0b013e31820751cb. [DOI] [PubMed] [Google Scholar]
- 27.Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol (1985) 107: 987–992, 2009. doi: 10.1152/japplphysiol.00076.2009. [DOI] [PubMed] [Google Scholar]
- 28.Tipton KD, Gurkin BE, Matin S, Wolfe RR. Nonessential amino acids are not necessary to stimulate net muscle protein synthesis in healthy volunteers. J Nutr Biochem 10: 89–95, 1999. doi: 10.1016/S0955-2863(98)00087-4. [DOI] [PubMed] [Google Scholar]
- 29.van Vliet S, Shy EL, Abou Sawan S, Beals JW, West DW, Skinner SK, Ulanov AV, Li Z, Paluska SA, Parsons CM, Moore DR, Burd NA. Consumption of whole eggs promotes greater stimulation of postexercise muscle protein synthesis than consumption of isonitrogenous amounts of egg whites in young men. Am J Clin Nutr 106: 1401–1412, 2017. doi: 10.3945/ajcn.117.159855. [DOI] [PubMed] [Google Scholar]
- 30.West DW, Burd NA, Coffey VG, Baker SK, Burke LM, Hawley JA, Moore DR, Stellingwerff T, Phillips SM. Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise. Am J Clin Nutr 94: 795–803, 2011. doi: 10.3945/ajcn.111.013722. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto I, Konto A, Handa T. Regulation of phospholipase D activity by neutral lipids in egg-yolk phosphatidylcholine small unilamellar vesicles and by calcium ion in aqueous medium. Biochim Biophys Acta 1233: 21–26, 1995. doi: 10.1016/0005-2736(94)00220-J. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto I, Mazumi T, Handa T, Miyajima K. Effects of 1,2-diacylglycerol and cholesterol on the hydrolysis activity of phospholipase D in egg-yolk phosphatidylcholine bilayers. Biochim Biophys Acta 1145: 293–297, 1993. doi: 10.1016/0005-2736(93)90302-G. [DOI] [PubMed] [Google Scholar]
- 33.You JS, Lincoln HC, Kim CR, Frey JW, Goodman CA, Zhong XP, Hornberger TA. The role of diacylglycerol kinase ζ and phosphatidic acid in the mechanical activation of mammalian target of rapamycin (mTOR) signaling and skeletal muscle hypertrophy. J Biol Chem 289: 1551–1563, 2014. doi: 10.1074/jbc.M113.531392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol 5: 578–581, 2003. doi: 10.1038/ncb999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.