Abstract
Osteoblasts secrete collagen and isolate bone matrix from extracellular space. In the matrix, alkaline phosphatase generates phosphate that combines with calcium to form mineral, liberating 8 H+ per 10 Ca+2 deposited. However, pH-dependent hydroxyapatite deposition on bone collagen had not been shown. We studied the dependency of hydroxyapatite deposition on type I collagen on pH and phosphate by surface plasmon resonance in 0–5 mM phosphate at pH 6.8–7.4. Mineral deposition saturated at <1 mM Ca2+ but was sensitive to phosphate. Mineral deposition was reversible, consistent with amorphous precipitation; stable deposition requiring EDTA removal appeared with time. At pH 6.8, little hydroxyapatite deposited on collagen; mineral accumulation increased 10-fold at pH 7.4. Previously, we showed high expression Na+/H+ exchanger (NHE) and ClC transporters in osteoblasts. We hypothesized that, in combination, these move protons across osteoblasts to the general extracellular space. We made osteoblast membrane vesicles by nitrogen cavitation and used acridine orange quenching to characterize proton transport. We found H+ transport dependent on gradients of chloride or sodium, consistent with apical osteoblast ClC family Cl−,H+ antiporters and basolateral osteoblast NHE family Na+/H+ exchangers. Little, if any, active H+ transport, supported by ATP, occurred. Major transporters include cariporide-sensitive NHE1 in basolateral membranes and ClC3 and ClC5 in apical osteoblast membranes. The mineralization inhibitor levamisole reduced bone formation and expression of alkaline phosphatase, NHE1, and ClC5. We conclude that mineral deposition in bone collagen is pH-dependent, in keeping with H+ removal by Cl−,H+ antiporters and Na+/H+-exchangers. Periodic orientation hydroxyapatite is organized on type I collagen-coiled coils.
Keywords: chloride-hydrogen antiport, hydroxyapatite deposition, sodium-hydrogen exchange, vacuolar H+-ATPase
INTRODUCTION
The mineralization of bone occurs within an organized bone matrix compartment enclosed and produced by osteoblasts (3). After forming a tight epithelium over the bone surface, osteoblasts secrete type I collagen from their apical surface, forming the structured collagen fibrils that are mineralized to form the composite bone matrix. The osteoblast layer contains tight junctions that include claudin and cell-to-cell communications, including by CX43 gap junctions. In the bone matrix space, phosphate is generated by phosphate transport and phosphatases, whereas the available calcium is sufficient, as the usual calcium channels and exchangers are present (3). Together these yield amorphous calcium phosphate crystals to form on the collagen template, but these must be matured further into a stable hydroxyapatite-collagen composite (31). The process of hydroxyapatite formation generates protons in large quantities. Since bone forms in a closed space, osteoblasts must remove these protons to the extracellular space as indicated by the equation:
| (1) |
Thus, the overall process of transport for dense mineral accumulation can be expressed as an equation, indicating production of acid with precipitation of alkaline apatites of calcium and phosphates.
Our previous studies established that osteoblasts express ClC3 and ClC5 Cl−,H+ antiporters in their apical surfaces (17), and NHE1 and NHE6 type 1 Na+/H+ exchangers in their basolateral surfaces (22). However, direct H+ translocation by ClC and NHE transporters across osteoblast membranes had not been demonstrated. Furthermore, matrix mineralization includes intermediate steps and is coordinated with accessory proteins and collagen in a maturation process (13, 30). Two of the major accessory proteins, osteopontin and osteocalcin, have been genetically excised in mice and in each case, the pups are born with normal skeletons, and adult mineralization is not affected to any major extent (10, 10a). Thus, we suggest that maturation of amorphous calcium phosphates at nucleation sites of the collagen fibrils (32) into stable hydroxyapatite crystals based largely on mineral transport and pH. Much of this has been studied in detail in vitro and where the maturation requires a mildly alkaline pH (22, 25). However, despite numerous studies, the minimum requirements for mineral deposition and maturation of bone matrix remain unclear.
Here, to assess the pH dependency of calcium phosphate-collagen association in the earliest steps of collagen mineralization, we generated a pure type I tropocollagen layer on carboxymethyl dextran-modified surface plasmon resonance chips activated by reaction with N-hydroxysuccinimide and N-ethyl-N′-(dimethylaminopropyl)-carbodiimide HCl. Using this preparation, we studied the association of calcium-phosphate complexes onto this surface as a function of pH and phosphate concentration in the absence of cells, matrix vesicles, or accessory proteins. Furthermore, we isolated and reconstituted surface membranes from human osteoblasts differentiated in culture and used these vesicles to study directly the membrane H+ transporters they contain. This included testing for the presence of ATP-dependent active H+ transporters and whether chloride or sodium gradient-dependent H+ transport occurs in isolated membrane vesicles. We demonstrate the occurrence of these transporters in the basolateral and apical surfaces of mouse bone. Furthermore, we used the mineralization inhibitor levamisole in mouse bone to show that, in addition to reducing alkaline phosphatase activity and mineral formation, the transporters NHE1 and ClC5 are inhibited.
METHODS
Surface plasmon resonance.
To approximate the mineralization of collagen in situ, surface plasmon resonance experiments were done using triple helical, mature type I collagen (kind gift of Professor Robert Mecham, Washington University, St. Louis, MO) immobilized on a carboxymethyl dextran hydrogen sensor chip by linkage with EDC/NHS coupling of 700 RU to the sensor surface (16), studied in a Reichert SR7000DC (Buffalo, NY) microfluidic surface plasmon resonance instrument. This method is widely used to study ligand binding to immobilized protein and has been shown to immobilize collagen without destroying its biological function (29). Hydroxyapatite has a high refractive index (1.62) and is detectible at concentrations where its individual components, Ca2+, PO43−, and HPO42− (1.35–1.52), are not interfering.
In situ labeling for light and electron microscope microscopy.
Primary antibodies for the vacuolar H+-ATPase (V-ATPase) were rabbit anti-whole bovine kidney H+-ATPase, used at 1:200, visualized using anti-rabbit peroxidase anti-peroxidase (4) for labeling avian tissue or for labeling mouse tissue antibody to the 31 kD E subunit of the V-ATPase raised in chickens at 1:500 (from GenWay), visualized with goat anti-chicken rhodamine isothiocyanate (1:100; Jackson Immunologicals) (21a). Alkaline phosphatase was visualized as alkaline phosphatase activity by hydrolysis of α-naphthol AS-phosphate in the coupling reagent fast blue RR (Sigma-Aldrich, St Louis, MO) or by electron microscopy using monoclonal anti-alkaline phosphatase SP8 (Thermo Fisher) visualized with anti-mouse 20 nm gold. Fluorescent labeling of nuclei used the Bisbenzimide dye Hoechst 33342 dye with ultraviolet excitation and blue emission. Antibodies for visualization of NHE1, ClC3, and ClC5 were goat anti-NHE1 (anti-COOH-terminal 20-mer peptide; sc-16097) from Santa Cruz Biotechnology (Santa Cruz, CA), goat polyclonal anti-ClC5 D-17, recognizing a ClC5-specific internal epitope (Santa Cruz), or rabbit polyclonal anti-ClC3 raised to amino acids 80–125 of human ClC3 (Bioss, Woburn, MA). They were applied at 4 mg/ml for 1 h at room temperature, followed by donkey anti-goat labeled with cyanine 2 (green fluorescence) or cyanine 3 (red fluorescence) (Jackson Laboratories, West Grove, PA) for 1 h at room temperature. Fluorescent-labeled proteins were photographed using 1.3 NA 40× or 100× oil objectives on a Nikon TE2000 microscope. Images were acquired using a 14 bit 2,048 × 2,048-pixel cooled charge-coupled detector array (Diagnostic Instruments, Sterling Heights, MI). The mineral binding green fluor calcein was added three days before mice were killed and was visualized directly as in previous studies (21). The green channel indicates signal from excitation 450–490 nm, a 510-nm dichroic mirror, and a 520-nm barrier; the red signal represents excitation 536–556 nm, a 580-nm dichroic mirror, and a 590-nm barrier.
Quantitative PCR.
Messenger RNA was extracted from cells by oligo dT binding, followed by reverse transcription as described (21). Real time PCR was run using as an internal standard glycerol-3-phosphate dehydrogenase expression; DNA primer sequences are previously published (17, 21).
Mesenchymal stem cells, in vitro bone differentiation, and the effects of levamisole.
Media were from Thermo-Fischer (Waltham, MA) or as stated. Normal human bone marrow-derived mesenchymal stem cells (MSCs) and normal human osteoblasts, a cell fraction with MSC multi-lineage potential but improved osteoblast differentiation, were from Lonza (Allendale, NJ). Mouse MSCs were isolated and differentiated as described (21) using ClC3−/− animals (17). Cells were grown in Dulbecco's modified essential medium with 1 g/l glucose, 10% fetal bovine serum, penicillin, streptomycin, and amphotericin-B. For osteoblast differentiation, cells were grown to confluence, and then media were supplemented with 10 mM glycerol-2-phosphate and 30 µg/ml of ascorbic acid. Differentiation media were replaced every 3 days. To study the effects of the inhibitor levamisole (14) on bone formation and bone transporters, we used this compound at 50 µg/ml in mouse osteoblasts differentiated from MSCs isolated from CLCN3 knockout animals, which mineralize well and express ClC5 at high levels (17).
Membrane vesicles.
Membrane vesicles from osteoblasts were prepared as described (15), with minor modifications. Cell fragmentation was by nitrogen cavitation in 250 mM sucrose, 1 mM EGTA, 1 mM dithiothreitol, and 10 mM Tris, pH 7.0 (lysis buffer). Disruption of 4 × l07 cells in 20 ml of lysis buffer, contained in a 50-ml centrifuge tube placed in the cavitation bomb, was initiated by pressurization to 35 atmospheres of N2 (500 psi) for 45 min at 4°C. Decompression occurred over ~1 s, through a 3.5-mm diameter orifice. After explosive decompression, nuclei and large cell fragments were removed by centrifugation at 1,000 × g for 5 min, mitochondria by centrifugation at 4,700 g for 10 min, and the membrane fraction was recovered by centrifugation at 48,000 g for 40 min in 4 aliquots to produce four membrane pellets. These pellets were frozen at −80°C; each aliquot contained ~1 mg of protein in a small amount (~10 µl) of residual lysis buffer and could be stored several weeks with recovery of activity on reconstitution. These membranes are largely free of intracellular vesicles and have been shown to contain <5% of the β-glucuronidase or N-acetylhexosaminidase of initial cell lysates (5). Osteoclast vesicle ATP-dependent acidification is shown as a positive control, as reported (33).
Acridine orange uptake to monitor acid transport.
Membranes from l07 osteoblasts were thawed rapidly and diluted to 300 µl in 120 mM KCl, 20 mM NaCl, 10 mM HEPES, pH 7.0 (intracellular buffer), or other reconstitution buffer described in specific experiments. The membrane suspension was thoroughly mixed and incubated for a minimum of 30 min at 4°C to allow vesicles to form (5). Proton uptake was determined using the fluorescent weak base, acridine orange (AO, N,N,N′,N′-tetramethylacridine-3,6-diamine) at 2 µM or as specified. Vesicles were diluted into cuvettes containing 2.4 ml of the same buffer with stirring and held until fluorescence was stable, 30–40 min, after which transport was initiated (Fig. 3) using MgCl2 and ATP or other energy source as stated. Dozens of membrane preparations were used; data are from over 100 acridine uptake assays. In individual preparations, vesicle inside volume varied and could not be quantified. However, in each preparation, repeat measurements were consistent to pipetting accuracy (±10%), in the absence of changes in conditions. Ion gradient-dependent H+ exchange was studied by the addition of 30–100 µl of vesicles in buffers as specified to acridine orange in 2.4 ml of 120 mM KCl and 20 mM NaCl with 10 mM HEPES, pH 7.0, with stirring, at 22°C, or isotonic sucrose as specified to prevent Na exchange-dependent effects (Fig. 4, D and E). Quenching of acridine orange was quantified by fluorescence with excitation at 450 nm, measuring emission at 548 nm. The decrease in E648 was used to follow vesicle acidification, since concentration of the dye in acidified vesicles quenches its fluorescence (4, 9, 15). Acridine quenching was reversed with the nonfluorescent weak base ammonium chloride, 1 mM or as specified, or with the H-K ionophore nigericin, 10 µM, to demonstrate the specificity of quenching for H+ transport. To allow comparison of quenching under varying conditions, baseline fluorescence emission was normalized for conditions compared in individual figures. To prevent interference from strong Na/H exchange activity in osteoblast membranes (21), effects on intravesicular anion exchange were studied with extracellular buffer replaced with 250 mM sucrose, 10 mM HEPES (prepared with HEPES base and HCl), pH 7.0 (or other pH indicated).
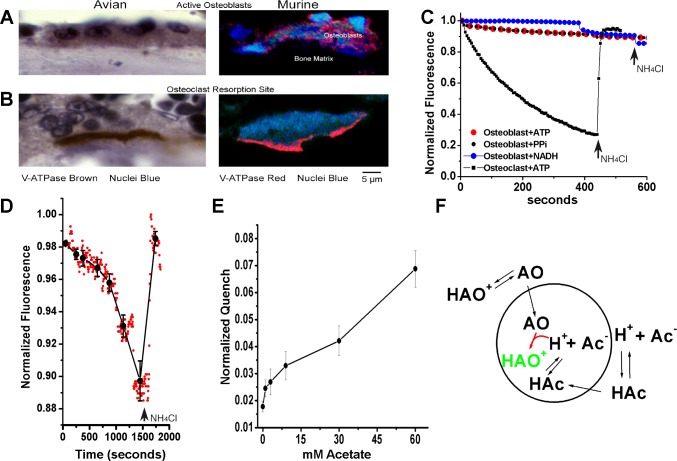
Fig. 3.
Energy-dependent H+ translocation was not detected in osteoblast vesicles. A and B: vacuolar H+-ATPase (V-ATPase) in osteoblasts and osteoclasts. The vacuolar ATPase is highly expressed in osteoblasts but is not localized to the basolateral surface (upper left) in contrast to osteoclasts (bottom left). Antibodies used were whole bovine V-ATPase (peroxidase, brown stain) for the avian preparations and chicken antibody to the E-subunit (rhodamine, red stain) for murine preparations. C: ATP-dependent acid transport in osteoclast, but not osteoblast, plasma membrane vesicles. In osteoblast plasma membrane vesicles reconstituted in 120 mM KCl, there is no detectable ATP-dependent acid transport in contrast with osteoclast membrane vesicles shown as a positive control. Two separate osteoblast membrane preparations were used. As further controls, NADH and pyrophosphate dependent acid transport were assayed and also were negative. Osteoblast membranes resuspended in 140 mM NaCl and 1 µM acridine orange were held until fluorescence reached steady-state, typically 40 min, before the addition of energy sources. While there was minor drift in osteoblast curves, in no case was fluorescence recovered on addition of the nonfluorescent weak base, 1 mM NH4Cl, replacing acridine in vesicles (arrows). Representative traces are shown; two separate osteoblast preparations were used for duplicate experiments. D: acridine orange quenching induced by acetate, demonstrating that vesicles capable of retaining acid are present. Osteoblast membrane vesicles as in C. Quenching, with reversal by the nonfluorescent weak base ammonium chloride, 1 mM, confirms the pH gradient imparted by the acetic acid, weak acid, and the response of acridine orange quenching. E: quenching of acridine orange as a function of acetate concentration, using data from D. Mean ± SD for 10–20 determinations at each acetate concentration. For demonstration of acetate effects on cellular pH with and without Na+/H+ exchange activity, see Liu et al. (21). F: model for acetate effect on acridine quenching. A vesicle is indicated by the circular figure. Ac, acetate; AO, acridine orange; HAc, acetic acid; HAO, protonated acridine orange. Quenched AO is charged and cannot diffuse out of the vesicle and is highlighted in green.
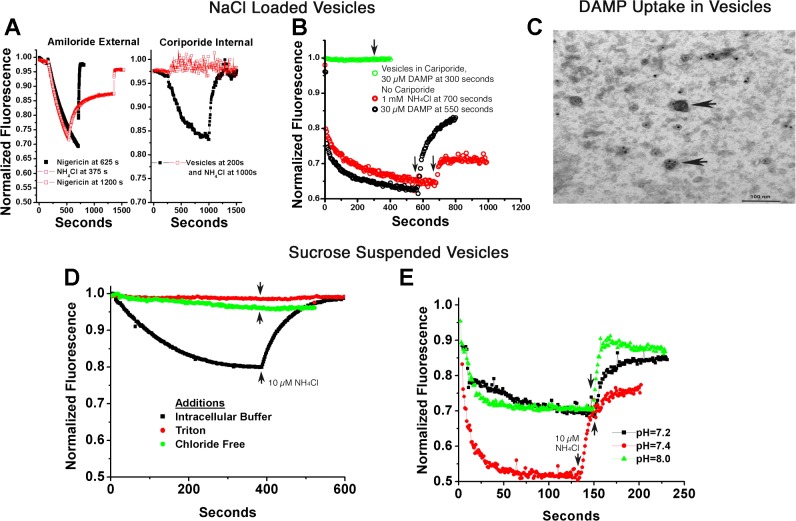
Fig. 4.
Ion gradient-coupled acid transport in human membrane vesicles. A–C: vesicles were reconstituted in 140 mM NaCl and then suspended in 120 mM KCl plus 2 µM acridine orange to study Na+ gradient acidification. A, left: minimal effect of Na/H exchange inhibitors on the outside of inside-out vesicles. Extensive acid uptake occurred (acridine quenching), reversed slowly with 1 mM NH4Cl or rapidly with the H-K ionophore nigericin, 10 µM. The black curve had 100 µM amiloride added with slightly slower quenching, but no claim is made of the relevancy of this small effect. This concentration of amiloride blocked Na/H exchange in recordings of whole cell pH, as reported in Ref. 21. Note that the time course in both curves is much slower for ATP-dependent acid transport in osteoclast membranes (Fig. 3C, black trace). Right: strong inhibition by Na/H exchange inhibitors on the inside of inside-out vesicles. In contrast, reconstitution of the vesicles in 1 mM cariporide, that is, with the inhibitor inside, stops vesicle acidification and acridine orange quenching. The strong dependence of the acidification on the Na+ gradient inside to outside and the requirement for the inhibitor on the same side as the Na+ confirms that acidifying vesicles were reconstituted with the extracellular face of the cell on the inside of the vesicles. This is consistent with Na+/H+ exchanger (NHE) inhibitors being on the same side as the sodium substrate. B: interaction of the weak base DAMP (N-(3-[(2,4-dinitrophenyl)-amino]propyl)-N-(3-aminopropyl)methylamine), with acidified vesicles. Both ammonium chloride (10 mM) and DAMP (30 µM) reduced vesicle acidification, as shown by acridine orange quenching. However, when acidification was blocked by 1 mM intravesicular cariporide, the addition of DAMP had no effect on acridine fluorescence, indicating and the DAMP weak base had no detectible interaction with non-acid vesicles. C: DAMP labeling of acid vesicles. Samples of acid vesicles were treated with DAMP, fixed, and then DAMP was antibody-localized, as described in methods, so that DAMP in vesicles could be visualized by electron microscopy (2, 26). In brief, acid vesicles were reacted with 30 µM DAMP as in B and fixed in glutaraldehyde 1%. Vesicles were recovered by centrifugation and rinsed with goat anti-dinitrophenol, and then horseradish peroxidase anti-goat antibody to make a dense precipitate. The preparation was postlabeled with 20 nM gold antialkaline phosphatase. Vesicles with and without alkaline phosphatase labeling are acidified as shown by election-dense material in random vesicles (arrows); ATP was not added. D: chloride gradient-dependent acid transport. Vesicles reconstituted in intracellular buffer (120 mM KCl) and assayed in isotonic sucrose acidify, and acid uptake is reversed with ammonium chloride (bottom trace). Detergent disruption (top trace) abolishes quenching. A representative trace is also shown with replacement of KCl by potassium gluconate (second trace from top), which eliminated all but trace quenching, consistent with a Cl−-dependent process (see discussion). E: transport at varying extravesicular pH is similar to pH 8. Vesicles reconstituted in intracellular KCl buffer and assayed in isotonic sucrose with 10 mM HEPES adjusted to pH 7.2, 7.4, and 8 acidified similarly, indicating that the process works at slightly alkaline pH. This is important relative to mineral deposition as shown in Fig. 1).
Acid vesicle labeling with antibodies and weak base antibody pairs.
For electron microscopy of vesicle preparations for alkaline phosphatase, membrane vesicles from osteoblast preparations were lysed for 7 min in 0.1% Triton and then blocked in normal goat serum 1:20, followed by incubation with goat anti-alkaline phosphatase and anti-goat 20 nM gold sequentially. Vesicles were then washed and postfixed in 2.5% glutaraldehyde for 5 min and with uranyl acetate for 2 min before drying on methyl cellulose-coated grids overnight and electron microscopy. A separate set of membranes reconstituted in intracellular buffer and allowed to acidify (Fig. 4B) was treated with 30 µM of the weak base, N-(3-[(2,4-dinitrophenyl)amino]propyl)-N-(3-aminopropyl) methylamine (DAMP), as described by Anderson et al. (1), after our earlier work (5). After 30 min, the preparations were fixed and permeabilized in 1% glutaraldehyde, recovered by centrifugation, and washed, followed by goat anti-dinitrophenol and then horseradish peroxidase-coupled anti-goat antibody to make a dense precipitate labeling the DAMP, indicating acid vesicles (1, 2, 26). The preparation was postlabeled with 20 nM gold anti-alkaline phosphatase, as described above. Controls omitted the weak base. Vesicles were postfixed and applied to methyl cellulose-coated grids, as described above.
RESULTS
Mineral deposition on pure type I collagen driven solely by pH and phosphate.
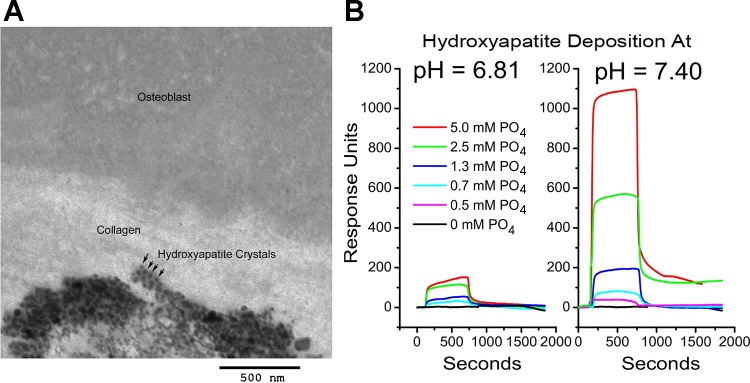
Transmission electron microscopy of our human osteoblasts differentiated and depositing mineral in culture clearly display periodic hydroxyapatite deposition (Fig. 1A). There is an extensive literature supporting pH dependent periodic mineral deposition but suggesting that nucleation might require additional components. We tested the hypothesis that pH and phosphate are sufficient using surface plasmon resonance. Purified type I collagen (kind gift of Professor Robert Mecham, Washington University, St. Louis, MO) was deposited on carboxymethyl dextran-modified gold activated by reaction with N-hydroxysuccinimide and N-ethyl-N′-(dimethylaminopropyl)-carbodiimide HCl, followed by capture of collagen triple helix in PBS at pH 7 (16). This chip was used for analysis of mineral deposition as a function of time, phosphate concentration, and pH (Fig. 1B) in 1 mM CaCl2 with EDTA washes as controls to remove mineral and reduce resonance to baseline. At time zero in each trace, responses are overlain for injections of buffer, 1 mM CaCl2, and phosphate from 0 to 5 mM in increments. At 1 mM CaCl2 and pH 7.4, dramatic phosphate dependence of hydroxyapatite aggregate deposition occurs. This is reduced ~90% by dropping pH to 6.8. At the permissive pH, phosphate injections of 1.3 to 5 mM generated stable hydroxyapatite deposits that were removed by subsequent EDTA washes. These results suggest multistep models of mineralization where initial deposition depends only collagen, pH, and phosphate.
Fig. 1.
pH-dependent mineral deposition on type I collagen studied by surface plasmon resonance. A: transmission electron micrograph of a partially mineralized bone nodule in vitro showing collagen and periodic hydroxyapatite deposition. Arrows demonstrate periodic hydroxyapatite. B: surface plasmon resonance showing phosphate and pH dependency of hydroxyapatite deposition in an artificial type I collagen layer. At time zero, responses are overlain for identical injection protocols. The baseline curves are identical for buffer (140 mM NaCl, 10 mM HEPES, pH = 7.4 or 6.81), 1 mM CaCl2, or 5 mM phosphate (alone with buffer). At ~700 s, the injection ended, and the surface was washed with buffer. Later, the surface was washed with 10 mM EDTA to remove any stabilized calcium salts and return the response to baseline. At 1 mM CaCl2, a dramatic phosphate dependency of large hydroxyapatite aggregate deposition occurs. It is reduced by dropping pH to 6.8. At the permissive pH, 5 and 2.5 mM phosphate injections generate stable hydroxyapatite deposition. These results indicate a strong pH dependence and multistep model for collagen mineralization.
Osteoblast membrane vesicles.
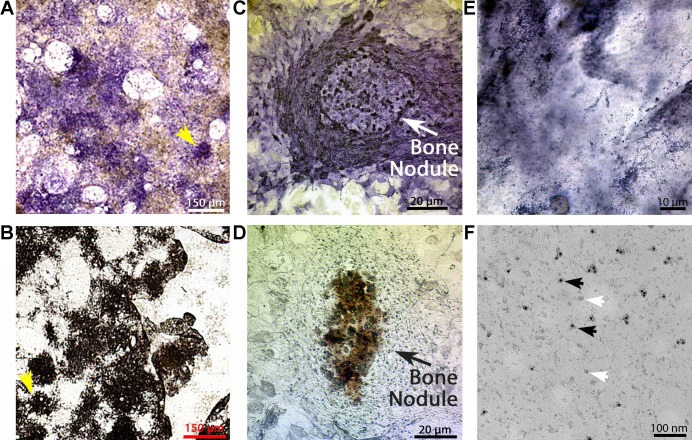
Osteoblasts were differentiated in culture as described in methods and previously (17). This made it possible to isolate plasma membrane fragments by nitrogen cavitation and differential centrifugation (4, 5). These preparations generated membrane pellets that remained active when stored at −80°C for a few weeks, and 4–6 of the following vesicle assays could be reconstituted from each tube. Therefore, the data shown is representative, and each experiment was replicated from more than one membrane preparation. By testing H+ transport with acridine orange quenching after different times in culture, we observed significant activity only in reconstituted vesicles from cell cultures, which had been in osteoblast differentiation medium for 3–4 wk. These active cultures displayed mineralizing nodules that were identified by eye or lower power microscopic examination (3). This is confirmed in Fig. 2, A and B, after representative cultures were stained. The culture cell morphology typical of osteoblasts surrounding the mineral nodules is shown in higher power views in Fig. 2, C and D. Active osteoblast membranes classically display bone alkaline phosphatase (32a) as an ectoenzyme that produces phosphate for mineralization. After nitrogen cavitation and centrifugation, the membrane preparations were positive for human bone alkaline phosphatase, although the membrane fragments themselves were too small to be seen by light microscopy (Fig. 2E). Electron microscopy of the preparation on methyl cellulose-coated grids resolved the individual membrane fragments (Fig. 2F), which averaged ~50 nm in size and about half of which (black arrows) were labeled with anti-alkaline phosphatase-20 nm gold, where 50% is in keeping with expression of alkaline phosphatase as a membrane enzyme (ectoenzyme) on the apical but not the basolateral surfaces of osteoblasts.
Fig. 2.
Membrane vesicles from mineralizing osteoblasts. A and B: mineralization and alkaline phosphatase in whole osteoblast cultures (). Mesenchymal stem cells were placed into culture as described in methods. After 3–4 wk, the cultures developed characteristic nodules of alkaline phosphatase and mineral-containing cells that can be seen directly in low power scans of cultures (1-mm fields). C and D: photomicrographs of representative bone nodules in osteoblast cultures. Low-power photomicrographs of nodules similar to the regions identified with arrows in A and B. Nodules of cells with strong alkaline phosphatase activity (C) and mineralization (D) are present. Only cultures with mineralizing osteoblasts had significant transport activity in isolated membranes (3, 21). E: oil immersion photomicrograph of osteoblast membranes labeled for alkaline phosphatase activity. Plasma membrane fragments were prepared by nitrogen cavitation in an isotonic HEPES-buffered sucrose solution (5). After alkaline phosphatase activity labeling, viewing at ×100 oil immersion revealed that the membrane fragments are alkaline phosphatase labeled, although the individual membranes are too small to resolve by light microscopy. F: electron microscopic analysis of osteoblast membrane fragments with alkaline phosphatase labeling. Examination by electron microscopy on methyl cellulose grids after alkaline phosphatase 20 nM gold labeling. About half of the membranes are labeled, consistent with alkaline phosphatase expression in apical but not basolateral membranes. Black arrows indicate labeled membrane fragments; white arrows indicate unlabeled membrane fragments.
Energy-dependent H+ translocation in osteoblast membrane vesicles was not detected.
In whole genome cRNA screening of mineralizing osteoblasts, we found that the V-ATPase was expressed. Thus, a simple hypothesis was that osteoblasts might export protons by basolateral H+ pump activity. However, while osteoclasts express the V-type ATPase at their bone attachment, as demonstrated in avian or murine osteoclasts (Fig. 3A, bottom), no polarized expression was seen in osteoblasts. Rather, a classical intracellular vacuolar pattern typical of secretory cells was observed (Fig. 3A, top). Whether this intracellular V-type ATPase functions in bone formation is unknown.
With reconstitution into vesicles, it is possible for transport to generate a pH gradient across the vesicle membrane because of primary active transport or secondary active transport in the presence of imposed ion gradients. Reconstitution into high potassium and chloride vesicles as previously described (4, 5) was suitable for studying primary active transport as in osteoclast membranes. For osteoclast vesicles, the addition of 2.5 mM ATP and MgCl2 produced a strong quenching gradient [Fig. 3C, lower trace, reversed rapidly with 1 mM NH4Cl (arrows at right)] (22a). In contrast, results with osteoblast membranes (Fig. 3C, upper traces) reveal no substrate-based primary active transport acidification in preparations of osteoblasts that were mineralizing in culture but were active in secondary active transport acidification (Fig. 4). We also assessed for NADH or pyrophosphate dependent transport, with negative results. While there were small baseline shifts in osteoblast membrane preparations, the addition of 1 mM NH4Cl did not recover fluorescence, indicating no specific acridine orange uptake.
It was possible that lack of acidification might reflect lack of membrane vesicle formation in osteoblasts. To test this, we examined the effect of neutral acetate on acridine orange uptake. This showed that vesicles capable of retaining acid are present; as a control, 1 mM NH4Cl reversed the process (Fig. 3D). This indicated that vesicles competent to retain acid were present. The normalized acridine orange quenching as a function of acetate concentration is shown in Fig. 3E, including mean ± SD for 10–20 determinations at each acetate concentration. Acetate effects on cellular pH with and without Na+/H+ exchange activity were previously shown by monitoring intracellular pH as a function of acetate addition with and without sodium present in the supernatant, as shown by Liu et al. (21). For clarity, a model of acetate effect on acridine quenching of vesicles is shown (Fig. 3F). Acridine (AO) is trapped in acid vesicles by protonation (HAO+ green). Acetic acid diffuses into the vesicles and is dissociated, providing acid for acridine protonation. Protonated acridine orange is quenched and is detected as reduced fluorescence. Note that the intravesicular volume is a few femtoliters in 2.4 ml of extravesicular acridine orange solution; the concentration of acridine orange in vesicles, while it cannot be calculated specifically, is large relative to the extravesicular volume.
Osteoblast membrane vesicles show secondary, ion gradient-dependent, active acid transport.
By reconstituting frozen membrane fragments, we define the intracellular environment when the vesicles form. Following that, we can dilute the vesicles into appropriate ionic composition to study the secondary active pathways available for proton transport. In vesicles reconstituted in 140 mM NaCl and then suspended in 120 mM KCl plus 2 µM acridine orange, we studied Na+ gradient-dependent acidification. Extensive acid uptake occurred (acridine quenching) that was reversed slowly with 1 mM NH4Cl or rapidly with the H-K ionophore nigericin, 10 µM (Fig. 4A). The time course of acidification was much slower than for ATP-dependent acid transport in osteoclast membranes (Fig. 2C). The black curve in Fig. 4A had 100 mM amiloride added to the extravesicular solution. There was slightly slower quenching, although no claims are made as to whether this might be significant. However, 100 mM amiloride blocked Na/H exchange of cultured osteoblasts completely (21). On the other hand, there was strong inhibition by an intravesicular Na/H exchange inhibitor. When vesicles were reconstituted in in 1 mM cariporide, this completely halted vesicle acidification, as shown by acridine orange quenching relative to a control using the same membrane preparation but without cariporide (Fig. 4A, right). This observation is consistent with NHE inhibitors and sodium encountering the transport protein from the same side.
The weak base, DAMP, has been used to track acid vesicles (1, 5). However, it is possible that DAMP also binds cholesterol and might not be specific in given contexts (18). To determine whether nonspecific DAMP binding might be relevant to osteoblast membranes, we studied DAMP effects on non-acid and acidifying osteoblast membrane vesicles. Ammonium chloride, 10 mM, or DAMP, 30 µM, eliminated vesicle acidification (reversed acridine orange quenching) (Fig. 4B). When acid uptake was blocked by 1 mM intravesicular cariporide, addition of DAMP had no effect on acridine fluorescence. This indicates that in osteoblast membranes, DAMP does not detectably interact with non-acid vesicles. Acid vesicles were treated with DAMP and reacted with antibodies so that vesicle acidification could be visualized by electron microscopy (Fig. 4C) (2, 26). Postlabeling with 20 nM gold anti-alkaline phosphatase showed membrane alkaline phosphatase, indicating cell surface membranes. The sequence of labeling was a reaction of acid vesicles with 30 µM DAMP, fixation in 1% glutaraldehyde, recovery by centrifugation, goat anti-dinitrophenol, and then horseradish peroxidase anti-goat antibody treatment, with appropriate rinse steps, to make a dense precipitate. Only complete vesicles were labeled with DAMP; nonfusing membrane fragments were not labeled.
In addition to Na/H exchange for removal of acid from osteoblasts, cells must take up acid; previous work indicated that Cl−,H+ antiporters mediate this process, at least in part (17), but direct Cl−,H+ antiport activity in osteoblast membranes had not been demonstrated. We used vesicles reconstituted in intracellular buffer (120 mM KCl) and assayed acid uptake in isotonic sucrose to eliminate any contribution from Na/H exchange. These vesicles acidify, and acid uptake was reversed with ammonium chloride (Fig. 4D). Detergent disruption abolished transport, controlling for possible artifacts, and replacement of KCl with potassium gluconate eliminated acidification, demonstrating chloride dependency and consistent with Cl−,H+ antiport activity.
For the osteoblast acid transport process to mediate mineral deposition (Fig. 1), the process must work at slightly basic pH. We tested this using vesicles reconstituted in intracellular KCl buffer and assayed in isotonic sucrose with 10 mM HEPES at pH 7.2, 7.4, and 8. While there were small differences in quantitative activity, for which no claims of significance are made, the process is clearly active over this range of pH values (Fig. 4E).
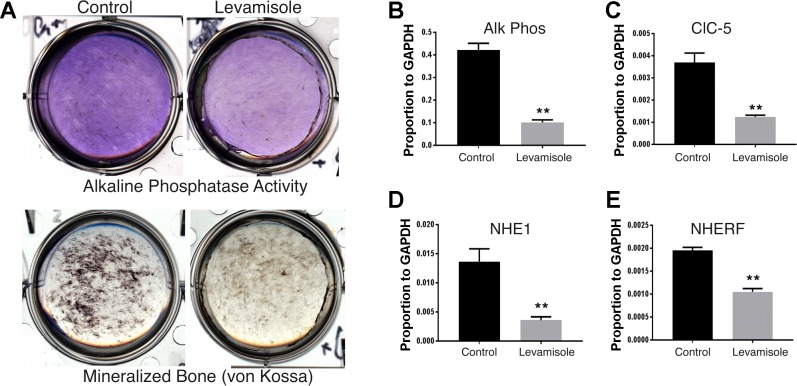
Parallel effects of the inhibitor levamisole on mineralization, alkaline phosphatase, and acid transporter expression.
Levamisole is a pleotropic inhibitor that, among other activities, is documented to inhibit alkaline phosphatase with strong inhibition of bone formation in mice (14). We compared the effects of levamisole on mineralization, alkaline phosphatase activity, and expression of mRNAs for alkaline phosphatase and key transporters related to acid extrusion. We used osteoblasts differentiated from CLCN3−/− mouse mesenchymal stem cells, which express increased ClC5 and mineralize consistently in vitro (17). Cultures were maintained in bone-supporting medium for 2 wk with or without 50 µg/ml of levamisole (250 µM). Levamisole clearly reduced mineral deposition and reduced alkaline phosphatase activity at 3 wk by 50% (Fig. 5A). To measure the effects on expression of key acid transporters, we used quantitative PCR. This showed parallel decreases at 3 wk in alkaline phosphatase, ClC5, the Cl−,H+ antiport-5, NHE1, the Na+/H+ exchanger-1, and NHERF1, the Na+/H+-exchanger regulatory factor 1 (Fig. 5, B–E). Results are shown relative to glyceraldehyde-3-phosphatase dehydrogenase mRNA; all are statistically different from untreated controls at P < 0.01.
Fig. 5.
Effect of the inhibitor levamisole on bone formation. This study used CLCN3−/− mouse mesenchymal stem cells, which mineralize well in vitro and express increased ClC5 (17) (Fig. 6). A: effect of 50 µg/ml levamisole on alkaline phosphatase activity and formation of mineralized bone. Cultures were maintained in bone-supporting medium for 2 wk, with or without levamisole (50 µg/ml) added. Both mineralization (von Kossa) and alkaline phosphatase activity were suppressed by levamisole. B–E: parallel effects of levamisole-inhibiting expression of mRNAs for alkaline phosphatase, Cl−/H+ antiport-5 (ClC5), Na+/H+ exchanger 1 (NHE1), and Na+/H+ exchanger regulatory factor 1 (NHERF1). In each case, results are relative to glyceraldehyde-3-phosphatase dehydrogenase mRNA and are shown as n = 3; mean ± SD. **P < 0.01, signficant differences.
Osteoblast epithelial structure in vivo and a model of osteoblast epithelial acid transport.
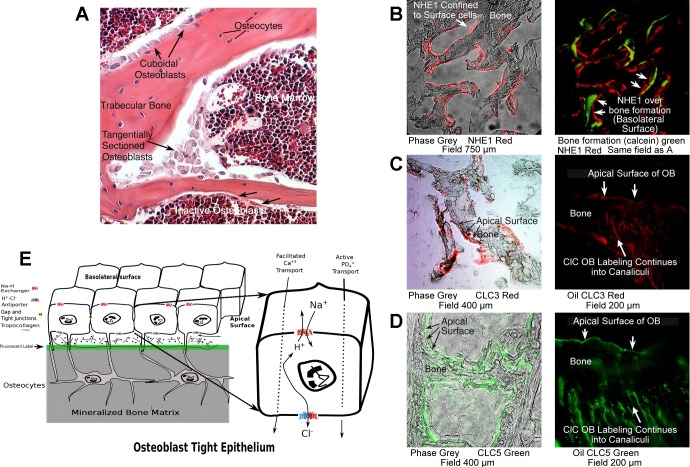
Bone in vivo is bounded by an epithelial layer of osteoblasts (3); in our view, this informs significantly the mechanism of osteoblast bone formation. Here, we show that the morphology of the osteoblast layer in a 300-µm wide portion of murine bone was rapidly fixed in 2% glutaraldehyde. This was decalcified, paraffin-embedded, and 6-µm sections were stained with hematoxylin-eosin for viewing (Fig. 6A). Gaps between osteoblasts and bone matrix are sectioning artifacts; other methods of embedding show direct osteoblast-bone integration (3). As shown in Fig. 6A, from a rapidly fixed section of mouse vertebral bone, two classes of osteoblasts are clearly indicated. The plump cuboidal osteoblasts surround active bone mineralizing regions; flat epithelial osteoblasts are found in nonmineralizing areas. A key point is that in all cases, there is a cellular separation of bone matrix and bone marrow, the general extracellular space. This is essential for pH control during mineralization and, thereby, for supporting mineralization. Further, to illustrate this point, in Fig. 6B, a section of mouse bone labeled with soluble calcein during life, accumulating in new bone, is cut and labeled for NHE1, which is confined to the basolateral (marrow adjacent) surface of osteoblasts. A fluorescent photograph of the same field shows accumulation of calcein during mineralization under groups of adjacent cells. We observe a constant relation between new bone formation and the NHE1 being at opposite poles of the active osteoblast (Fig. 6B). Fluorescent weak bases, including calcein and tetracycline, have been known to label bone during formation since the 1950s [reviewed in (3)]. In contrast, the Cl−,H+ antiporters ClC-3 and ClC-5 are present on the apical surfaces of osteoblasts (that is, the bone matrix surface) but expression extends into the canalicular system of osteocytes, the layers of osteoblasts that have been incorporated in bone (Fig. 6, C and D).
Fig. 6.
Osteoblast epithelial structure in vivo, labeling of key osteoblast transport proteins in mice, and a model for osteoblast epithelial acid transport. A: bone is bounded by an epithelial layer of osteoblasts. Shown is a 300-µm wide section of murine bone rapidly fixed in glutaraldehyde, decalcified, and a 6-µm section stained with hematoxylin-eosin. White gaps between osteoblasts and bone matrix are artifacts of sectioning. What is clearly shown is that osteoblasts are between the bone marrow or extracellular space and the bone matrix. B. images of a region of mouse bone at ×20 magnification in phase (gray, left) and Na+/H+ exchanger (NHE) 1 antibody labeling (red fluorescence, left and right). The NHE1 is confined to surface cells. In the right, bone formation is labeled with calcein, as well as the NHE1, which is identical to the red label in A. Calcein is taken up during mineralization and labels a cohort of osteoblasts acting in concert. C. low-power (left, phase gray) and ×100 oil immersion images of normal mouse bone with labeling of Cl−/H+ antiport- (ClC)3 in green. At high power (right), the labeling of the apical surface of osteoblasts and continuity of label into the canalicular system (which connects osteocytes and osteoblasts) is seen. D. labeling, similar to C, of ClC5 in ClC3−/− animals. ClC5 is present in wild-type animals, but its expression increases to compensate for ClC3 in the ClC3 knockout (17). This animal has consistent and strong in vitro bone mineral formation, making it particularly useful for the study of metabolic effects (Fig. 5). E: model of proton transport in osteoblasts. Key transport proteins are shown with main membrane positions of expression. The epithelial nature of osteoblasts in a bone-forming unit is shown; the cells are connected by gap and tight junctions (3). When active, small hydroxyapatite binding fluors, such as calcein, accumulate in the mineral rapidly (green line; cf. sections in B). After accumulation of matrix, lines of osteoblasts buried in matrix are called osteocytes; see A. These remain alive and are connected to the surface cells by gap junctions (3) (not illustrated). Osteoblasts produce bone matrix and mineral-producing proteins, including type I collagen and alkaline phosphatase, as well as sodium hydrogen exchangers and Cl−,H+ antiports in a coordinated fashion (Fig. 5). The sodium hydrogen exchangers and Cl−,H+ antiports are key proteins for transcytosis (see detailed figure, right) of protons to allow mineral deposition at a slightly alkaline pH (Fig. 1), despite production of significant acid by mineral precipitation (Eq. 1, introduction).
To summarize the findings in Figs. 1–4 and localization of key transport proteins, a diagram is provided (Fig. 6E). This illustrates the epithelial nature of osteoblasts in a bone forming unit. Cells are connected by gap junctions and tight junctions (3); overall, the unit functionally is called an osteon. Osteoblasts produce bone matrix and mineral-producing proteins, including type I collagen and alkaline phosphatase, as well as sodium hydrogen exchangers and Cl−,H+ antiporters in a coordinated fashion, (Fig. 5). When bone formation is active, small hydroxyapatite binding fluorophores accumulate in the mineral rapidly (green line). The Na+/H+ exchangers and Cl−,H+ antiporters are key proteins for transcytosis (see detailed subfigure in Fig. 6E).
DISCUSSION
We addressed an important and controversial point, the adequacy of chemical gradients and pH for bone mineral deposition. This involves complex issues. The overall process of transport for dense mineral accumulation can be put simply (Eq. 1 in the introduction). However, the process includes intermediate steps and might be coordinated with accessory proteins and collagen crosslinking in a maturation process (13, 29). Maturation of calcified matrix might require days.
Nonetheless, the first step is deposition of calcium phosphates at nucleation sites on collagen (or possibly accessory proteins) to form stable hydroxyapatite crystals. As others have observed, the microcrystalization during mineralization obeys a periodic pattern (Fig. 1A) consistent with the pH-dependent features occurring periodically in type 1 collagen. While we did not study maturation, we determined that it is sufficient to have 1.3–5 mM phosphate held at pH 7.4 in 1 mM CaCl2 to nucleate hydroxyapatite on pure collagen (Fig. 1B). Physical chemistry provides a useful insight, specifically, repeating charge alignments on collagen have suggested a nucleation lattice (7, 8, 32). An interesting aspect of mineralization is that it only occurs experimentally when the pH is held at mildly alkaline levels (22). Our work (Fig. 4B) is completely consistent with these models and data, and indicates that, at least, the initial stages of mineral deposition are supported by the presence of adequate phosphate and calcium at mildly alkaline pH and do not require other support for nucleation.
We further address the functional location of transporters important in bone mineralization. In earlier work, we established that the basolateral membranes of active bone forming osteoblasts express a high-capacity sodium-dependent H+ transport that is associated with the expression of NHE1 and NHE6 proteins (21). The protons produced in mineral deposition, as shown in Eq. 1, make their way into osteoblasts lining the active osteon and are exported using the sodium gradient between the cytoplasm and the extracellular space. Subsequently, we demonstrated that active mouse osteoblasts differentiated in culture had bone mineralization severely curtailed by the genetic removal of chloride-proton antiporters ClC3 and ClC5, normally found to be abundant in the apical membranes of mineralizing osteoblasts (17). We summarized these findings and the resulting hypothesis that the two transporters work in tandem removing protons from the mineralizing osteon (3). In the present work, we support this model with functional studies on the isolated and reconstituted osteoblast and osteoclast surface membranes. Nitrogen cavitation can be tuned so that curvature of the membranes being disrupted is 1−10 microns and intracellular vesicles with curvature <200 nm remaining intact. After removal of the intracellular organelles by centrifugation and sedimenting the isolated the membrane fragments at ~50,000 g, the pellets are stored at −80°C until reconstituted into vesicles.
After thawing, the membrane fragments will reconstitute into vesicles during ~45 min on ice. The reconstitution medium defines the intravesicular composition, and by dilution, an outer composition is defined. In this way, primary and secondary active transport can be studied. In each case, proton gradients generated by H+-ATPase, sodium hydrogen exchange, and Cl−,H+ antiport activity were followed by the fluorescent quenching of acridine added to the external buffer (5, 23, 27). Furthermore, activity of reagents that might be membrane side-dependent, such as for NHE inhibitors (Fig. 4A), can specifically be studied by reconstituting membranes with or without inhibitors inside.
We used nitrogen cavitation previously in the study of the surface membrane of the osteoclast, and in combination with cultured cell studies, we were able to describe pH homeostasis for the massive proton transport that occurs in bone metabolism by that cell (5, 22a, 28). In the osteoblast cultures, we stained for whole bovine V-ATPase, or for its E subunit, in mouse preparations. We incidentally discovered that the TCIRG subunit of the V-ATPase, discovered in osteoclasts (19), important in the polarized expression of the V-ATPase in osteoclasts and required to avoid osteopetrosis (11), is expressed, albeit at a relatively low level, in osteoblasts (data not shown). In animals, its absence does not produce a clear osteoblast phenotype. Using the osteoblast membrane preparation, we specifically explored and verified that H+-ATPase is not captured in the vesicle membranes isolated from osteoblasts (Fig. 3C), although membrane vesicles quite capable of retaining acid are produced (Fig. 3, D and E). In fact, we could detect no primary active transport in osteoblast membranes prepared in this manner. We conclude that the V-ATPase detected immunologically in the osteoblast is used in their secretory function and are intracellular membranes that are excluded by our method of preparation. As an additional control, we studied osteoblast vesicles in symmetrical solutions based on 140 mM NaCl, which, as expected, showed no acridine quenching. Here, we added acetate in increasing amounts, passively to acidify the membranes, as expected (Fig. 3, D and E); this is similar to acetate addition in whole cells during studies of NHE expression by measuring intracellular pH with and without sodium in the extracellular fluid (21). As expected, vesicle acid uptake was reversed by the addition of excess ammonium chloride as a control for specificity (Fig. 3D).
As for secondary ion gradient-dependent active transport (Fig. 4), we performed several studies. Using acridine orange quenching; we observed vesicle acidification in two distinct cases. One was when Na+inside > Na+outside (Fig. 4, A and B) and the other when Cl−inside > Cl−outside (Fig. 4, D and E). Vesicles reconstituted in 140 mM NaCl and then diluted into 120 mM KCl and 20 mM NaCl should have acidification that is, to a large extent, due to NHE1 and -6. Using active osteoblasts in culture, we previously showed that this activity was sensitive to amiloride (21); however, in the reconstituted vesicles, the amiloride effect on the cytoplasmic side is small, and no claims of significance are made (Fig. 4A, left graph). This might, therefore, be due to the side-dependent effects of NHE inhibitors. When we repeated this study reconstituting vesicles reconstituted with 1 mM cariporide inside, transport was completely inhibited (Fig. 4A, right graph), confirming the role of NHE transport in Na-dependent acidification (Fig. 4C). Included in this work, we show that acidifying, but not non-acidifying membranes take up the weak base DAMP, reducing the acidification, showing that nonspecific binding of DAMP to membranes is not quantitatively important in this case (Fig. 4B), as has been reported in some intracellular membranes (18).
These studies, overall, demonstrate acid transport by acridine orange quenching, consistent with Na+/H+ and Cl−,H+ antiport activity. Furthermore, to establish the role of chloride transport in the acid uptake by osteoblasts, vesicles reconstituted from membranes of active bone forming cells were diluted into isotonic sucrose before being studied for acridine quenching (Fig. 4, D and E). This eliminates possible interference due to the very strong Na/H exchange activity. When the vesicles had been reconstituted into 120 mM KCl with no added sodium, a significant acidification occurred. The rate and extent of the quenching was less than observed for the vesicles containing both chloride and sodium, but it was consistent with the conclusion that chloride was a central ion in the acidification (Fig. 4D). To test this, we replaced the KCl with 120 mM K gluconate, which generated essentially no acidification. Likewise, and unsurprisingly, disrupting the vesicles with triton eliminated acidification. Previously, we demonstrated that the Cl−,H+ antiporters ClC3 and ClC5 are required for production of mineralizing nodules in cultured osteoclasts (17). Our hypothesis is that mineralization operates most efficiently at the slightly alkaline pH (7.4–7.6) that has been reported for the osteon matrix (6). We observe that amorphous apatite deposition on collagen is dramatically increased at pH 7.4 (Fig. 1B) and that the chloride-dependent acidification of reconstituted vesicles is similar at neutral and slightly alkaline pH (Fig. 4E).
An additional, interesting series of controls was made using the mineralization inhibitor levamisole (Fig. 5). As expected from previous work, both mineralization and alkaline phosphatase activity were suppressed (14). Completely in accord with the presence of a coordinated complex of acid-transporting and phosphate-producing genes, messenger RNAs for alkaline phosphatase, NHE1, ClC5, and the regulatory protein NHERF1 (21) were suppressed 50%–80%. However, despite this very interesting correlation of effects, whether these reflect a common mechanism for coordinated expression will require further study. In Fig. 6, we demonstrate basolateral expression of NHE1 in mouse bone in vivo with apical and canalicular expression of ClCs 3 and 5, in keeping with the transport studies above.
Study of hydroxyapatite deposition in layers of pure type I collagen by surface plasmon resonance indicates that pH- and phosphate-dependent mineral deposition in collagen is of major importance, although accessory processes, including other bone proteins, might affect the efficiency and maturation of the matrix. Our findings support a model of removal of H+ to drive bone hydroxyapatite deposition in bone by concatenated Cl−,H+ antiport and NHE exchangers, which is summarized in the cartoon shown in Fig. 6G.
GRANTS
This work was supported in part by the Department of Veterans Affairs Grant I01BX002490 and by NIH Grants AR-065407 and HL-125076.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.C.B., Q.C.L., L.L., D.B.S., D.J.N., and P.H.S. conceived and designed research; H.C.B., Q.C.L., L.L., J.H.B., D.B.S., and P.H.S. performed experiments; H.C.B., Q.C.L., L.L., and P.H.S. analyzed data; H.C.B., Q.C.L., L.L., D.B.S., D.J.N., and P.H.S. interpreted results of experiments; H.C.B., Q.C.L., I.L.T., L.L., D.B.S., and P.H.S. prepared figures; H.C.B. and P.H.S. drafted manuscript; H.C.B., I.L.T., D.J.N., and P.H.S. edited and revised manuscript; H.C.B. and P.H.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Professor Tom Kleyman, University of Pittsburgh, for the labeling of murine V-ATPase in bone and Professor Robert Mecham, Washington University, St. Louis, for purified type I collagen monomers.
Present address of Q. C. Larrouture: Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, Botnar Research Centre, Windmill Rd., Oxford, OX3 7LD, UK.
REFERENCES
- 1.Anderson RG, Falck JR, Goldstein JL, Brown MS. Visualization of acidic organelles in intact cells by electron microscopy. Proc Natl Acad Sci USA 81: 4838–4842, 1984. doi: 10.1073/pnas.81.15.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson RG, Orci L. A view of acidic intracellular compartments. J Cell Biol 106: 539–543, 1988. doi: 10.1083/jcb.106.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blair HC, Larrouture QC, Li Y, Lin H, Beer-Stoltz D, Liu L, Tuan RS, Robinson LJ, Schlesinger PH, Nelson DJ. Osteoblast differentiation and bone matrix formation in vivo and in vitro. Tissue Eng Part B Rev 23: 268–280, 2017. doi: 10.1089/ten.teb.2016.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blair HC, Teitelbaum SL, Ghiselli R, Gluck S. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science 245: 855–857, 1989. doi: 10.1126/science.2528207. [DOI] [PubMed] [Google Scholar]
- 5.Blair HC, Teitelbaum SL, Tan HL, Koziol CM, Schlesinger PH. Passive chloride permeability charge coupled to H(+)-ATPase of avian osteoclast ruffled membrane. Am J Physiol 260: C1315–C1324, 1991. doi: 10.1152/ajpcell.1991.260.6.C1315. [DOI] [PubMed] [Google Scholar]
- 6.Chakkalakal DA, Mashoof AA, Novak J, Strates BS, McGuire MH. Mineralization and pH relationships in healing skeletal defects grafted with demineralized bone matrix. J Biomed Mater Res 28: 1439–1443, 1994. doi: 10.1002/jbm.820281209. [DOI] [PubMed] [Google Scholar]
- 7.Chapman JA. The staining pattern of collagen fibrils. I. An analysis of electron micrographs. Connect Tissue Res 2: 137–150, 1974. doi: 10.3109/03008207409152099. [DOI] [PubMed] [Google Scholar]
- 8.Chapman JA, Hardcastle RA. The staining pattern of collagen fibrils. II. A comparison with patterns computer-generated from the amino acid sequence. Connect Tissue Res 2: 151–159, 1974. doi: 10.3109/03008207409152100. [DOI] [PubMed] [Google Scholar]
- 9.Clerc S, Barenholz Y. A quantitative model for using acridine orange as a transmembrane pH gradient probe. Anal Biochem 259: 104–111, 1998. doi: 10.1006/abio.1998.2639. [DOI] [PubMed] [Google Scholar]
- 10.Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, Bradley A, Karsenty G. Increased bone formation in osteocalcin-deficient mice. Nature 382: 448–452, 1996. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- 10a.Franzén A, Hultenby K, Reinholt FP, Onnerfjord P, Heinegård D. Altered osteoclast development and function in osteopontin deficient mice. J Orthop Res 26: 721–728, 2008. doi: 10.1002/jor.20544. [DOI] [PubMed] [Google Scholar]
- 11.Frattini A, Orchard PJ, Sobacchi C, Giliani S, Abinun M, Mattsson JP, Keeling DJ, Andersson AK, Wallbrandt P, Zecca L, Notarangelo LD, Vezzoni P, Villa A. Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat Genet 25: 343–346, 2000. doi: 10.1038/77131. [DOI] [PubMed] [Google Scholar]
- 13.Fujisawa R, Tamura M. Acidic bone matrix proteins and their roles in calcification. Front Biosci (Landmark Ed) 17: 1891–1903, 2012. doi: 10.2741/4026. [DOI] [PubMed] [Google Scholar]
- 14.Garba MT, Marie PJ. Alkaline phosphatase inhibition by levamisole prevents 1,25-dihydroxyvitamin D3-stimulated bone mineralization in the mouse. Calcif Tissue Int 38: 296–302, 1986. doi: 10.1007/BF02556610. [DOI] [PubMed] [Google Scholar]
- 15.Gluck S, Al-Awqati Q. An electrogenic proton-translocating adenosine triphosphatase from bovine kidney medulla. J Clin Invest 73: 1704–1710, 1984. doi: 10.1172/JCI111378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnsson B, Löfås S, Lindquist G. Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Anal Biochem 198: 268–277, 1991. doi: 10.1016/0003-2697(91)90424-R. [DOI] [PubMed] [Google Scholar]
- 17.Larrouture QC, Nelson DJ, Robinson LJ, Liu L, Tourkova I, Schlesinger PH, Blair HC. Chloride-hydrogen antiporters ClC-3 and ClC-5 drive osteoblast mineralization and regulate fine-structure bone patterning in vitro. Physiol Rep 3: e12607, 2015. doi: 10.14814/phy2.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li W, Wang R, Zhang S, Li X. DAMP, an acidotropic pH indicator, can be used as a tool to visualize non-esterified cholesterol in cells. Acta Biochim Biophys Sin (Shanghai) 47: 73–79, 2015. doi: 10.1093/abbs/gmu123. [DOI] [PubMed] [Google Scholar]
- 19.Li YP, Chen W, Stashenko P. Molecular cloning and characterization of a putative novel human osteoclast-specific 116-kDa vacuolar proton pump subunit. Biochem Biophys Res Commun 218: 813–821, 1996. doi: 10.1006/bbrc.1996.0145. [DOI] [PubMed] [Google Scholar]
- 21.Liu L, Schlesinger PH, Slack NM, Friedman PA, Blair HC. High capacity Na+/H+ exchange activity in mineralizing osteoblasts. J Cell Physiol 226: 1702–1712, 2011. doi: 10.1002/jcp.22501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a.Liu W, Pastor-Soler NM, Schreck C, Zavilowitz B, Kleyman TR, Satlin LM. Luminal flow modulates H+-ATPase activity in the cortical collecting duct (CCD). Am J Physiol Renal Physiol 302: F205–F215, 2012. doi: 10.1152/ajprenal.00179.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marelli B, Ghezzi CE, Zhang YL, Rouiller I, Barralet JE, Nazhat SN. Fibril formation pH controls intrafibrillar collagen biomineralization in vitro and in vivo. Biomaterials 37: 252–259, 2015. doi: 10.1016/j.biomaterials.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 22a.Moriyama Y, Takano T, Ohkuma S. Acridine orange as a fluorescent probe for lysosomal proton pump. J Biochem 92: 1333–1336, 1982. doi: 10.1093/oxfordjournals.jbchem.a134053. [DOI] [PubMed] [Google Scholar]
- 23.Neves JS, Perez SA, Spencer LA, Melo RC, Weller PF. Subcellular fractionation of human eosinophils: isolation of functional specific granules on isoosmotic density gradients. J Immunol Methods 344: 64–72, 2009. doi: 10.1016/j.jim.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niu LN, Jee SE, Jiao K, Tonggu L, Li M, Wang L, Yang YD, Bian JH, Breschi L, Jang SS, Chen JH, Pashley DH, Tay FR. Collagen intrafibrillar mineralization as a result of the balance between osmotic equilibrium and electroneutrality. Nat Mater 16: 370–378, 2017. doi: 10.1038/nmat4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orci L, Ravazzola M, Amherdt M, Madsen O, Perrelet A, Vassalli JD, Anderson RG. Conversion of proinsulin to insulin occurs coordinately with acidification of maturing secretory vesicles. J Cell Biol 103: 2273–2281, 1986. doi: 10.1083/jcb.103.6.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park E, Campbell EB, MacKinnon R. Structure of a CLC chloride ion channel by cryo-electron microscopy. Nature 541: 500–505, 2017. doi: 10.1038/nature20812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin A, Cheng TS, Pavlos NJ, Lin Z, Dai KR, Zheng MH. V-ATPases in osteoclasts: structure, function and potential inhibitors of bone resorption. Int J Biochem Cell Biol 44: 1422–1435, 2012. doi: 10.1016/j.biocel.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Ramachandran A, Ravindran S, Huang CC, George A. TGF β receptor II interacting protein-1, an intracellular protein has an extracellular role as a modulator of matrix mineralization. Sci Rep 6: 37885, 2016. doi: 10.1038/srep37885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reznikov N, Shahar R, Weiner S. Bone hierarchical structure in three dimensions. Acta Biomater 10: 3815–3826, 2014. doi: 10.1016/j.actbio.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 31.Sakae T, Nakada H, LeGeros JP. Historical review of biological apatite crystallography. J Hard Tissue Biol 24: 111–122, 2015. doi: 10.2485/jhtb.24.111. [DOI] [Google Scholar]
- 32.Silver FH, Landis WJ. Deposition of apatite in mineralizing vertebrate extracellular matrices: A model of possible nucleation sites on type I collagen. Connect Tissue Res 52: 242–254, 2011. doi: 10.3109/03008207.2010.551567. [DOI] [PubMed] [Google Scholar]
- 32a.Whyte MP. Hypophosphatasia and how alkaline phosphatase promotes mineralization (2nd ed.) In: Genetics of Bone Biology and Skeletal Disease, edited by Thakker RV, Whyte MP, Eisman JA, Igarashi T. Cambridge, MA: Academic, 2018, p. 481–505. doi: 10.1016/B978-0-12-804182-6.00028-9 [DOI] [Google Scholar]
- 33.Williams JP, McDonald JM, McKenna MA, Jordan SE, Radding W, Blair HC. Differential effects of tamoxifen-like compounds on osteoclastic bone degradation, H(+)-ATPase activity, calmodulin-dependent cyclic nucleotide phosphodiesterase activity, and calmodulin binding. J Cell Biochem 66: 358–369, 1997. doi:. [DOI] [PubMed] [Google Scholar]