Abstract
Background:
Exposure to multiple types of air pollution may contribute to and exacerbate allergic diseases including asthma and wheezing. However, few studies have examined chronic air pollution exposure and allergic disease outcomes among an adult population. Associations between potential estimates of annual average fine particulate matter (PM2.5), traffic related air pollution, and industrial source air emissions and three allergic disease outcomes (asthma, allergies and wheezing) were examined in a state-wide general population of adults.
Methods:
The study includes a representative sample of 3381 adult Wisconsin residents who participated in the 2008–2013 Survey of the Health of Wisconsin (SHOW) program. Participant data were geographically linked to The United States Environmental Protection Agency (USEPA) Baysian space-time downscaler air pollution model for PM2.5, the United States Census roadway, and USEPA's Toxic Release Inventory data. Self-report and lung function (FEV1) estimates were used to define prevalence of asthma, allergies and wheezing symptoms.
Results:
Annual mean exposure to fine particulate matter (PM2.5) was between 6.59 and 15.14 μg/m3. An increase of 5 μg/m3 in the annual mean PM2.5 resulted in a 3.58 (2.36, 5.43) increase in the adjusted odds (95% CI) of having asthma. Exposure to vehicle traffic increased the odds of both current allergies [OR (95% CI)=1.35 (1.07, 1.35)] and current asthma [OR (95% CI)=1.51 (1.14, 2.00)]. Living within 300 m of an Interstate roadway was associated with a 3-fold increase in the odds of asthma. Those living within 800 m of an industrial site were 47% more likely to have asthma. No significant associations were seen with wheezing.
Conclusions:
Within this population exposed to overall annual average levels of estimated low level chronic exposure to fine particulate matter (PM2.5) at or near 12 μg/m3, the USEPA standard for air quality, significant association between both modeled PM2.5 exposure and proximity to roadways with asthma and allergies but not wheezing were found. Industrial source emissions were not associated with any allergic disease outcomes.
Keywords: Air pollution, Fine particulate matter, Baysian space-time downscaler model, Allergic disease, Asthma
1. Introduction
Over the past several decades there has been growing evidence air pollution is a risk factor for allergic diseases, including asthma, and there is growing concern outdoor air pollutants may contribute to the prevalence of allergic diseases (D’Amato, 2011). Allergic disease outcomes are more commonly studied among children, however, the prevalence of allergic disease among adults is also on the rise (Zhang et al., 2013). While the association between exposure to fine particulate matter and allergic disease is well supported throughout the literature (Kim and Bernstein, 2009) few of these studies have examined associations in general population-based studies of adults. Numerous epidemiological studies have demonstrated short-term exposure to elevated concentrations of ambient air pollutants, including mixtures of fine particulate matter (PM2.5), can exacerbate pre-existing asthma and trigger wheezing (Greenbaum, 2010; Jerrett and Shankardass, 2008; McConnell et al., 2010; Nishimura and Galanter, 2013; O’Connor et al., 2008; Weinmayr and Romeo, 2010). However inconsistent results have been found between ambient and regional air pollution and allergies (Bowatte et al., 2014; Fuertes et al., 2013; Leung et al., 2012; Weir et al., 2013; Zhang et al., 2011). Furthermore, adverse effects of long-term chronic exposure to regional air pollution and allergic disease outcomes are less clear (Anderson et al., 2013; Kunzli et al., 2009; Modig and Torén, 2009). Additional investigation into the association between chronic exposure to air pollutants and allergic disease is needed.
Epidemiologic studies have identified exposure to traffic emissions as potentially a main driver of air pollution contributing to allergic disease health outcomes (Greenbaum, 2010). However, most studies focus on short-term exposure in children or adolescents (Batterman et al., 2014; Khreis et al., 2016; Urman et al., 2013). Less is known about the prevalence of allergic disease among adults exposed to traffic emissions over an extended period of time. Additionally, very little research has looked at allergic disease outcomes and exposure to air pollution from other stationary sources such as industries (Mirabelli and Wing, 2006; Patel et al., 2011).
The objective of this study was to assess the association of potential chronic exposure to fine particulate matter (PM2.5), traffic related air pollution, and industrial related air pollution with the prevalence of three different allergic disease outcomes: allergies, asthma and wheezing in an adult population. Given the paucity of data on chronic exposure to air pollution and adult allergic disease prevalence, we examined associations between potential exposure to air pollution and allergic disease using not only regional ambient PM2.5 which comes from all sources (including traffic and industries), but also estimates of traffic related and industrial related air pollution separately. Traffic related and industrial related air pollution may be greater triggers or sources of allergic disease, especially among a population exposed to regional fine particulates near or below the current United States Environmental Protection Agency (USEPA) National Ambient Air Quality Standard (NAAQS) for annual three-year mean fine-particulate matter (PM2.) estimates of 12.0 μg/m3 (U.S. EPA, 2012). Three different measures including annual average PM2.5 at home address, the distance from residential address to nearest major roadway as a measure of traffic related air pollution, and the distance from residential address to nearest industrial site as a measure of industrial air pollution were used to estimate potential exposures. This study adds to the existing evidence by improving understanding of the relative associations between different potential measures of air pollution exposure and allergic disease outcomes in a randomly selected population-based sample of adults.
2. Methods
2.1. Study sample
Data came from the 2008–2013 Survey of the Health of Wisconsin (SHOW). SHOW is an ongoing health examination survey of adults ages 21–74 (n=3381). The SHOW probabilistic sampling design, procedures, and data collection are described in detail elsewhere (Nieto and Peppard, 2010). In brief, a two-stage cluster sampling strategy is used to randomly select household addresses. At the time of household based recruitment all age-eligible adults are invited to participate. After consent, information is collected through in-person interviews, self-administered questionnaires, and physical exams, including objective measures of height, weight, blood pressure and spirometry-based estimates of lung function. The SHOW protocol and informed consent documents are approved by the Health Sciences Institutional Review Board of the University of Wisconsin-Madison.
For this study subjects with missing data for any one of the three outcomes of interest were excluded from descriptive and unadjusted analyses. Subjects with a history of chronic bronchitis or emphysema (n=222) were also excluded from analysis when the outcome of interest is wheezing, resulting in final sample sizes of 3343 for current allergy, 3375 for current asthma, and 3150 for wheezing.
2.2. Exposure assessment
2.2.1. Geocoding
Each SHOW participant address at the time of consent into the SHOW program was geocoded using CENTRUS software (Pitney Bowes Inc., Stamford, CT). The geocoded addresses were used to link participants to three different types of air pollution data including ambient fine particulate matter (PM2.5), traffic related air pollution, and industrial related air pollution using ArcGIS v10.2 software (ESRI, Redlands, CA).
2.2.2. Fine Particulate Matter (PM 25)
The USEPA Fused Air Quality Surface Downscaler model (FAQSD) (U.S. EPA, 2016a) was used to derive annual average PM2.5 estimates for each SHOW participant. Data were downloaded for years 2007–2012 from the USEPA Remote Sensing Information Gateway (RSIG) data files (U.S. EPA, 2016a). FAQSD is a Bayesian space-time model that fuses together 24-h average monitoring data from the National Air Monitoring Stations and State and Local Air Monitoring Stations (NAMS/SLAMS) with 12 km gridded output from the Models-3/Community Multiscale Air Quality (CMAQ) v4.6 model (U.S. EPA, 2016b). The CMAQ model integrates USEPA's National Emissions Inventory data, daily continuous emissions monitoring data for major nitrogen oxide (NOx) point sources, and meterological data (U.S. EPA, 2016b). The final FASQD model provides 24-h PM2.5 predictions at the 2010 US Census Tract centroid locations. Oridinary kriging was applied to the irregularly spaced FAQSD data point estimates to create a continuous raster image (pixel size=1 mi sq) for the entire state of Wisconsin. Kriging was chosen in order to reduce estimate bias by giving greater weight to values which are spatially closer. The stable variogram was selected as the best fit model based on mean standardized error (MSE) and root mean square standardized error (RMSSE).
Kriged daily and annual PM2.5 estimates were then linked to SHOW participant data using the Spatial Analyst Tool in ArcGIS. We estimated potential chronic PM2.5 exposures using a 1 year-lagged model. For example, 2008 participants were linked to 2007 air pollution data, and 2009 participants were linked to 2008 air pollution data and so forth. Annual average PM2.5 was examined as a continuous variable (for every 5 μg/m3 increase), and also by quartile (comparing those exposed to an annual average PM2.5 in the second, third, and forth quartile to those in the lowest quartile). Additionally, we examined the number of days that exceed a 24-h mean of 30 μg/m3 and 35 μg/m3. The current NAAQS for 24 –hr. mean PM2.5 is 35 μg/m3 (U.S. EPA, 2012). Since our study population had few participants with more than 1 or 2 days 24-h mean PM2.5 concentrations above 35 μg/m3, we examined a threshold of 30 μg/m3 in addition to 35 μg/m3.
2.2.3. Traffic Related Air Pollution (TRAP)
Proximty to the nearest major roadway was used as a proxy measure for potential exposure to traffic related air pollution. Data came from the United States Census 2010, and the MAF/TIGER Feature Class Code (MTFCC) and Road Type Code (RTTYP) were used to identify roadway segments as Primary, Secondary, Primary – Interstate, and Primary – non-Interstate roadways (U.S. Census Bureau, 2010). Previous research has shown the majority of normalized pollutant concentrations diminish to background levels 100–300 m from the edge of major roadways (Karner et al., 2010; Zhou and Levy, 2007). Therefore, subjects were dichotomized into categories of potential high vs. low exposure to TRAP using a cutpoint of 300 m from each of the nearest road types. Distances were calculated using the proximity “Near” tool in ArcGIS.
2.2.4. Proximity to industrial facilities
Similar to TRAP estimates, the USEPA's Toxic Release Inventory site database (U.S. EPA, 2008) was used to examine potential industrial source air pollution exposures. Industries that are required to report fugitive or stack air emissions annually to the USEPA were downloaded for the year 2008 from the EPA website (U.S. EPA, 2008); this year of data had the most comprehensive list of industries. Only industries which had been reporting for at least 1 year prior and continued to be reporting through 2013 were included.
A dichotomous variable was created that classified participants as living less than or equal to 400 m from the nearest industrial site (high air pollution) or living greater than 400 m from the nearest industrial site (low air pollution) and was spatially joined to SHOW participants using the proximity “Near” tool in ArcGIS. Since a relatively small percentage of the study sample lived within 400 m of an industrial site, we additionally ran analysis using an 800 m (about ½ mile) cut point. An 800 m buffer from an industrial has been cited in the literature when analyzing total respiratory health effects from nearby industrial site exposures (Maantay, 2007).
2.3. Health, outcome measures
Both self-report and objective exam based measures were used to define current allergy, current asthma, and wheezing. Forced expiratory volume in one second (FEV1) was measured using an electronic peak flow meter (Jaeger AM, Yorba Linda, CA), a validated instrument (Richter et al., 1998), which was given during the exam center portion of the SHOW study. Trained technicians gave study participants explicit directions on how to breath into the spirometry device. The highest FEV1 measure was used among a minimum of three valid measures. Spirometry measurements were valid if two FEV1 readings were within 10% of the highest value measured. Participants who reported having one or more of the following items were excluded from spirometry testing: heart attack, major surgical procedure, hernia repair, eye surgery, pregnancy or stroke in the last 6 months; chest infection in last 6 weeks; unstable angina episode in last 24 h; current ear infection; ever having had a sudden, unexpected collapsed lung or an aortic aneurysm or bulging wall of the aorta, coughed up blood with unknown cause, or been diagnosed with tuberculosis. Predicted FEV1 was calculated using sex, race, age, and height as defined by the NHANES general U.S. population (Hankinson et al., 1999).
Current allergies is defined as having reported “yes” to the survey question “Do you still have allergies or hay fever?” This is a follow-up question asked only of subjects who reported “yes” to the question “Has a doctor or other health professional ever told you that you had allergies or hay fever?” Current asthma is defined as being previously diagnosed with asthma or having forced expiratory volume in one second (FEV1) less than 80% of the predicted value. Previous diagnosis of asthma comes from the survey question “Do you still have asthma?” which is a follow-up question asked only of subjects who reported “yes” to the question “Has a doctor or other health professional ever told you that you had asthma?” FEV1 < 80% predicted is used as a measure of airway inflammation and obstruction because at FEV1 < 80% predicted is used for diagnosing persistent-moderate to persistent-severe asthma as defined by The National Heart, Lung, and Blood Institute asthma guidelines for diagnosing and managing asthma (NIH, 2016). Prevalence of wheezing is defined as having reported “yes” to the survey question “In the past 12 months have you had wheezing or whistling in your chest?”
2.4. Covariates and confounding
Self-reported demographic data including age, gender, education and income as well as housing data were gathered using Computer Assisted Personal Interviews (CAPI). Age was left as a continuous variable in all statistical models but divided by ten so that a one-unit increase was equivalent to a ten year age increase. In descriptive Table 1, age is categorized in order to better display the characteristics of the study population. Participants were categorized as owning a pet if they owned at least one of the following: dog, cat, bird, hamster, mouse, rate, guinea pig, gerbil, or ferret. Participants were categorized as allowing smoking in the home if they reported smoking was allowed everywhere in the home, in certain rooms in the home, or allowed for special guests in the home. Participants reported whether they smelled mildew or mold inside their home in the past 12 months. Participants also reported yes or no to whether any pesticides were used inside their home to kill or control insects or other pests in the past 12 months.
Table 1.
Characteristics of the study population by three different air pollution measures.
| Descriptive characteristics |
Total study samplea (n=3381) |
Highest quartile of annual average PM2.5 (n=842) |
800 m or less from Industrial site (n=470) |
400 m or less from primary or secondary road (n=1076) |
|||
|---|---|---|---|---|---|---|---|
| N | % | p- trend |
% | p- trend |
% | p- trend |
|
| Gender | 0.90 | 0.57 | 0.80 | ||||
| Male | 1480 | 24.8 | 13.5 | 38.6 | |||
| Female | 1901 | 25.0 | 14.2 | 39.0 | |||
| Age (in years) | 0.04 | < 0.0001 |
0.004 | ||||
| 21–39 | 1104 | 26.8 | 17.8 | 42.2 | |||
| 40–59 | 1503 | 25.1 | 13.3 | 38.6 | |||
| 60–72 | 774 | 21.7 | 9.4 | 34.6 | |||
| Education | < 0.0001 |
0.01 | < 0.0001 |
||||
| H.S./GED or less | 992 | 21.5 | 16.8 | 45.1 | |||
| Some college | 1309 | 23.5 | 14.8 | 41.3 | |||
| Bachelors or | 1073 | 29.5 | 12.1 | 33.4 | |||
| higher | |||||||
| Income | 0.02 | < 0.0001 |
< 0.0012 |
||||
| < $25,000 | 764 | 21.1 | 19.2 | 43.3 | |||
| $25,000 - $49,000 | 800 | 24.1 | 12.7 | 41.4 | |||
| $50,000 - $99,000 | 1102 | 27.5 | 11.5 | 36.8 | |||
| > $99,000 | 579 | 25.9 | 9.5 | 27.6 | |||
|
Smoking Status |
0.24 | < 0.0001 |
0.0017 | ||||
| Current | 555 | 21.1 | 22.0 | 44.7 | |||
| Former | 825 | 23.2 | 10.5 | 35.4 | |||
| Never | 1535 | 24.6 | 11.9 | 37.7 | |||
|
Smoking in Home |
0.74 | < 0.0001 |
< 0.0001 |
||||
| Yes | 541 | 23.7 | 21.0 | 45.6 | |||
| No | 2534 | 23.0 | 12.0 | 36.8 | |||
| BMI | 0.002 | 0.23 | 0.25 | ||||
| < 25 | 1264 | 28.2 | 13.4 | 39.5 | |||
| 25–30 | 936 | 23.6 | 12.8 | 36.6 | |||
| > 30 | 1181 | 22.4 | 15.2 | 40.0 | |||
|
Physical Activity |
0.40 | < 0.0001 |
0.0002 | ||||
| < 600 Met Min/ week |
902 | 25.9 | 18.3 | 44.0 | |||
| > =600 Met min/week | 2479 | 24.5 | 12.3 | 37.0 | |||
| Pet Owner | 0.02 | 0.61 | 0.08 | ||||
| Yes | 1906 | 23.3 | 13.7 | 37.2 | |||
| No | 1475 | 26.9 | 14.4 | 40.5 | |||
| Mold in home | 0.16 | 0.19 | 0.003 | ||||
| Yes | 503 | 25.0 | 15.7 | 44.4 | |||
| No | 2652 | 22.2 | 13.5 | 37.5 | |||
|
Indoor chemical use |
0.04 | 0.16 | 0.10 | ||||
| Yes | 818 | 20.7 | 15.1 | 40.9 | |||
| No | 2369 | 24.1 | 13.2 | 37.7 | |||
| Location | < 0.0001 |
< 0.0001 |
< 0.0001 |
||||
| Urban | 2139 | 32.7 | 20.1 | 43.0 | |||
| Rural | 1241 | 11.5 | 3.1 | 31.8 | |||
|
Residence Duration |
0.24 | < 0.0001 |
0.003 | ||||
| < =5 years | 1234 | 24.5 | 17.6 | 41.3 | |||
| > 5 years | 1305 | 25.3 | 10.0 | 36.2 | |||
| Allergies | 0.44 | 0.42 | 0.34 | ||||
| Yes | 1080 | 25.7 | 14.5 | 40.0 | |||
| No | 2301 | 24.5 | 13.5 | 38.3 | |||
| Asthma | 0.32 | 0.0015 | 0.001 | ||||
| Yes | 502 | 26.7 | 19.8 | 47.5 | |||
| No | 2879 | 24.6 | 13.2 | 37.9 | |||
| Wheezing | 0.15 | < 0.0001 |
0.0002 | ||||
| Yes | 657 | 22.7 | 19.8 | 45.2 | |||
| No | 2712 | 25.4 | 12.5 | 37.4 | |||
p-trend: statistical significance by Chi-square test.
Abbreviations: GRE - General Education Development test; BMI - body mass index.
Total study sample size varies due to missing data on outcomes and confounders. See Methods sections for details.
Residential addresses, and length of time at residence, were also verified during the in-home interview. Location type was derived from the 2010 Census Urban and Rural Classification. Urbanized Areas (50,000 or more people) and Urban Clusters (2500 to 50,000 people) were classified as “urban.” All populations, territories, and housing not included within an Urban area or Urban cluster were classified as “rural.” Due to Wisconsin being a relatively homogeneous population with few non-whites, and a high correlation between race and exposure and low sample of racial diversity across all three stratum of air pollution exposure in our sample, we did not control for race in regression models.
2.5. Statistical analysis
All statistical analyses were run using SAS v9.3 (SAS Institute, Cary, NC) and all adjusted analyses included sampling weights to account for sampling design, response rates and spatial clustering.
Logistic regression analysis was used to examine the association between chronic air pollution and the prevalence of allergic disease outcomes. Two different metrics were used for measuring associations between potential PM2.5 exposures and allergic diseases. Adjusted odds ratios were estimated comparing the odds of having an allergic disease versus not for every five unit increase in PM2.5. Similarly, odds of having an allergic disease versus not were compared across quartiles of potential PM2.5 exposure. Potential confounders were selected a priori from the literature. All confounders were examined in univariate analyses with each outcome and with all dichotomous exposures to assess confounding. Covariates that were associated with both the exposure and the outcome at p < 0.25 level using the likelihood ratio chi-square test were selected as confounders for subsequent multivariate models. Confounders that did not change the main effect estimate by more than 10% were excluded from the multivariate models. Statistically significant results were reported as having an adjusted Odds Ratio with p-value < 0.05.
Stratified analyses were run to check for effect modification by urbanicity since different chemical composition of outdoor air pollution may exist in urban vs. rural areas. Stratified analyses were also run by smoking status, household smoking policy, pets, mold, and indoor chemical use. If differences were seen in stratified analyses, an interaction term was added to the model to test its statisitical significance at the p < 0.05 level. These interactions were run to test the theory that those who are exposed to more allergens may be more or less susceptible to the effect outdoor air pollution has on allergic disease (Krämer et al., 2009; Künzli et al., 2009).
We also conducted additional sensitivity analyses examining associations only among individuals reporting length of residence greater than five years in current home. The hypothesis is that air pollution measures linked to SHOW participants will be a more accurate measure of chronic exposure among those who have lived at their residence longer.
3. Results
3.1. Demographic characteristics
Table 1 displays the sociodemographic characteristics and allergic outcomes that are in the “high exposure” category for each of the three air pollution exposures used in analyses. The study population was exposed to annual average PM2.5 estimates ranging from 6.59 to 15.14 μg/m3. High exposure was defined as annual average PM2.5 in the highest quartile 10.86–15.12 μg/m3 PM2.5), living within 300 m from a primary or secondary roadway, and living within 800 m of an industrial site. The percentage of participants living within the highest quartile of annual average PM2.5, 300 m from roadways, and 400 m of an industrial site all decreased as age group, education, and income level increased. Non-smokers and those having a no-smoking policy in the home also had a smaller percentage of subjects exposed to higher levels of air pollution. Among those living in urban areas, 10–40% more adults were exposed to higher levels of air pollution. Also, adults living in their homes less than five years were more likely to be in the high exposure of air pollution, when compared to adults living in their homes more than five years. A higher proportion of adults with current asthma and wheezing were also more likely to live within 300 m of the nearest primary and secondary roadway or 400 m of the nearest industrial facility. No differences in exposure were seen among those with and without current allergies.
3.2. Fine Particulate Matter (PM2 5)
Table 2 shows the adjusted odds ratios for having allergic disease outcomes, compared to not having allergic disease outcomes, for every 5 μg/m3 increase in annual average PM2.5 and by quartile of annual average PM2.5 exposure. The adjusted odds of having allergies was 1.38 (95% CI=1.03, 1.76) times greater in the second quartile and 1.33 (95% = 1.00, 1.76) times greater in the third quartile when compared to individuals in the lowest quartile. Surprising, the adjusted odds were non-significant when comparing the odds of allergies between the fourth and first quartile [OR (95% CI)=1.18 (0.89, 1.58)] and for every 5 μg/m3 increase in annual average PM2.5 [OR (95% CI)=1.06 (0.74, 1.53)]. In contrast, the largest effects were seen with asthma, where an increase of 5 μg/m3 in the annual mean PM2.5 resulted in an adjusted OR (95% CI) of 3.58 (2.36, 5.43) for being asthmatic versus non-asthmatic (Table 2). When comparing quartiles of annual average PM2.5, the odds of being asthmatic vs. non-asthmatic was 3.23 (95% CI 2.11, 4.95) times more likely among participants living in the fourth quartile of potential exposure compared to the first quartile. However, no statistically significant associations were found among the lower quartiles and asthma. PM2.5 was not found to be associated with wheezing.
Table 2.
Associations between Annual Average PM2.5 for 2008 and Allergic Disease Outcomes.
| Outcome | OR for every 5 μg/m3 increase in annual average PM2.5 |
ORa (95% CI) comparing quartiles of annual average PM2.5 (in μg/m3) |
||||
|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) | Adjusteda OR (95% CI) | Q1 (6.59–9.31) | Q2 (9.32–10.20) | Q3 (10.21–10.85) | Q4 (10.86–15.12) | |
| Allergy | 1.29 (1.00, 1.67) | 1.06 (0.74, 1.53) | Ref. | 1.38 (1.03, 1.76) | 1.33 (1.00, 1.76) | 1.18 (0.89, 1.58) |
| Asthma | 3.49 (2.39, 5.09) | 3.58 (2.36, 5.43) | Ref. | 1.57 (0.96, 2.57) | 1.44 (0.91, 2.28) | 3.23 (2.11, 4.95) |
| Wheezing | 0.96 (0.69, 1.35) | 0.97 (0.58, 1.63) | Ref. | 0.96 (0.65, 1.43) | 1.09 (0.73, 1.63) | 0.96 (0.65, 1.44) |
Abbreviations: OR - Odds ratio, CI - Confidence interval.
Adjusted for gender, age, BMI, smoking status, education, income.
Table 3 presents the odds of allergic disease outcomes by quartile of the number of annual days of PM2.5 concentration exceedances greater that 30 μg/m3. Similar to results examining annual average exposures, every additional day that exceeds 30 μg/m3 PM2.5 resulted in an 1.05 (95% CI=1.02, 1.08) increased odds for asthma, but no significant associations were seen with allergy and wheezing (Table 3). When the number of 24-h days that exceed PM2.5 concentrations of 30 μg/m3 was analyzed by quartiles, those who experienced 7–21 days above 30 μg/m3 were 1.67 times (95% CI=1.14, 2.46) more likely to have asthma than those who experienced no days above 30 μg/m3 (Table 3). As one would expect, similar results were found when the number of days exceeding 35 μg/m3 were analyzed by quartile (Table A.1). Those in the fourth and third quartiles were more likely to have asthma when compared with those in the lowest quartile [OR (95% CI)=1.68 (1.20, 2.35) and 2.12 (1.38, 3.26) respectively]. No associations were seen with the number of days exceeding 30 or 35 μg/m3 PM2.5 and allergies or wheezing.
Table 3.
Associations between the number of 24-h days that exceed PM2.5 concentrations of 30 μg/m3 and allergic disease outcomes.
| Outcome | OR for every additional day that exceeds 30 μg/m3 PM2.5 |
ORa (95% CI) comparing quartiles of annual days exceeding 30 μg/m3 PM2.5 |
||||
|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) | Adjusteda OR (95% CI) | Q1 (0 days) | Q2 (1–3 days) | Q3 (4–6 days) | Q4 (7–21 days) | |
| Allergy | 1.02 (1.00, 1.04) | 0.99 (0.96, 1.02) | Ref. | 1.05 (0.81, 1.38) | 1.09 (0.82, 1.46) | 0.92 (0.70, 1.21) |
| Asthma | 1.06 (1.03, 1.09) | 1.05 (1.02, 1.08) | Ref. | 1.11 (0.73, 1.71) | 1.31 (0.82, 2.09) | 1.67 (1.14, 2.46) |
| Wheezing | 1.00 (0.98, 1.03) | 1.00 (0.95, 1.05) | Ref. | 1.20 (0.74, 1.61) | 1.09 (0.72, 1.65) | 1.03 (0.67, 1.58) |
Abbreviations: OR - odds ratio, CI - confidence interval.
Adjusted for gender, age, BMI, smoking status, education, income.
3.3. Proximity to roadways
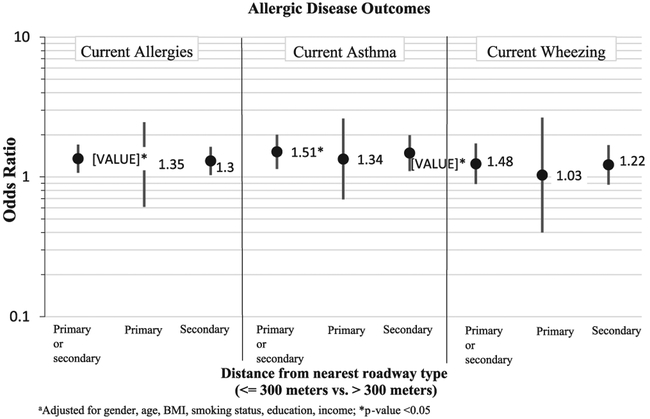
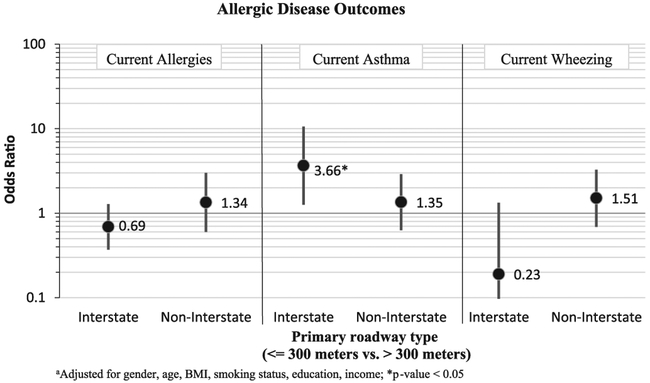
Potential exposure to traffic related air pollution (TRAP) appeared to increase odds of both current allergies [OR (95% CI)=1.35 (1.07, 1.35)] and current asthma [OR (95% CI)=1.51 (1.14, 2.00)] (see Fig. 1). Effects were seen with wheezing, but were not statistically significant. When proximity to primary and secondary roadways were analyzed as separate exposures, similar effects were seen for current allergy, although there was a decrease in precision. The decreased precision is partially due to having fewer exposed adults when examining proximity to primary and secondary roadways separately. Results from analyses where primary roadways were split into two different primary road types: primary – Interstate, and primary – non-Interstate is shown in Fig. 2. The effect between the distance to the nearest roadway and asthma was strengthened when primary-Interstate roadway was examined [OR (95% CI)=3.66 (1.26, 10.6)] and when primary-non-Interstate was examined [OR (95% CI)=1.35 (0.63, 2.90)]. For both allergy and wheezing, effects were strengthened for proximity to non-interstate, yet inverse associations were seen for proximity to Interstate (see Fig. 2). However, none of the associations with allergy and wheezing were statistically significant.
Fig. 1.
Adjusteda odds ratios (95% CI) of having allergies, asthma, or wheezing comparing those living < =300 m from nearest roadway type vs. those living further away.
Fig. 2.
Adjusteda odds ratios (95% CI) of having allergies, asthma, or wheezing comparing those living < =300 m from nearest Primary roadway type vs. those living further away.
3.4. Proximity to industrial sites
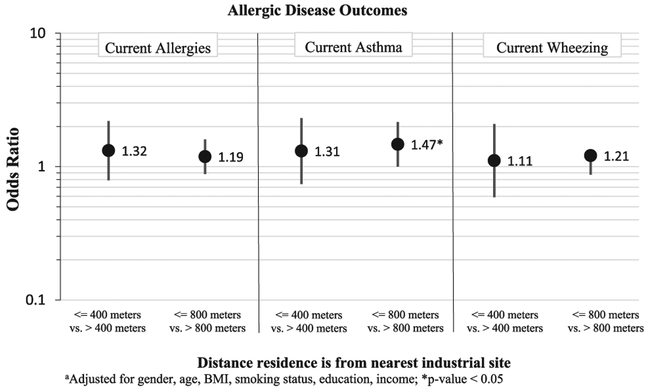
Potential exposure to air pollution from the nearest industrial site (both the 400 m and 800 m distance models) also was correlated with allergic disease outcomes, but only associations with asthma were statistically significant (Fig. 3). The adjusted ORs for participants living within 400 m of an industrial site were greater for allergy [OR (95% CI) =1.32 (0.79, 2.20)] than for participants living within 800 m of an industrial site [OR (95% CI)=1.19 (0.88, 1.60)] for allergy. However the same trend was not seen for asthma. The adjusted ORs for participants living within 400 m of an industrial site were smaller for asthma [OR (95% CI)=1.31 (0.74, 2.31)] than for participants living within 800 m of an industrial site [OR (95% CI)=1.47 (1.00, 2.16)]. A weaker association was found with wheezing among those living within 400 m of an industrial site [OR (95% CI)=1.11 (0.59, 2.09)], however the effect among those living within 800 m of an industrial site with wheezing [OR (95% CI) =1.21 (0.87, 1.68)] was similar to the effects seen with asthma.
Fig. 3.
Adjusteda odds ratios (95% CI) of having allergies, asthma, or wheezing comparing those living near an industrial site vs. those living further away.
When the sample was restricted to include only those who have lived in their home for five or more years, effect sizes did not change but the precision of the effects deceased (results not shown), likely due to decreased sample size. Stratified analyses showed different effects by smoking status in the proximity to roadways and industrial site models, but when added as an interaction term the effect was not statistically significant. None of the other tested variables showed effect modification.
4. Discussion
This study contributes important findings in furthering our understanding of the allergic disease health effects of chronic exposure to air pollution using three different measures of potential air pollution exposure among an adult population. In areas with low background ambient air pollution exposure as measured by annual average PM2.5 and total number of days of exceedance, we saw a large association with ambient air pollution and asthma prevalence, a smaller association with allergies, and no association with wheezing. The study setting was unique in that the annual estimated PM2.5 exposure levels ranged from 6.59 to 15.14 μg/m3, with the highest quartile of exposure 10.86–15.12 μg/m3) spanning the current NAAQS for annual mean PM2.5 of 12.0 μg/m3 (U.S. EPA, 2012). Additionally, associations were seen for proxy measures of traffic-related and industrial-related pollutants with asthma and allergies, but not for wheezing. While not all adjusted analyses were statistically significant at the p < .05 level, effect estimates showed trends reflective of those seen in other epidemiologic studies with higher levels of air pollution (Bowatte et al., 2016; Guarnieri and Balmes, 2014; Kim et al., 2013).
4.1. PM2.5
Our study resulted in a more than 3-fold increase in odds of asthma for every 5 μg/m3 increase in annual average PM2.5, and a 3-fold increase in odds of asthma when the highest quartile of annual average PM2.5 was compared with the lowest quartile (Table 2). These effects were substantially higher than those seen from similar studies examining ambient air pollution measures of fine particulate matter and asthma outcomes. Weber et al. (2016) used the USEPA's hierarchical baysian model (HBM) of fine particulate matter and linked daily measures preceding hospital and emergency room visits related to asthma and found a 10-unit change in PM2.5 resulted in odds ratios of inpatient and emergency room visits slightly over 1.00 for those older than 14 years of age. Nachman and Paker (2012) assessed fine particulate matter and respiratory outcomes in adults using two national cross-sectional datasets and annual average PM2.5 data derived from USEPA AirData and found an adjusted odds ratio for current asthma of 0.97 (95% CI=0.87, 1.07). Other studies that assessed PM2.5 exposure and asthma outcomes have found similarly small assocations with effect sizes lower than those seen in this study (Anderson et al., 2013).
A few things could explain why our effect sizes with PM2.5 and asthma are larger than those seen in other studies. First, the fused PM2.5 data used was newly modeled and uploaded for public use by the USEPA in 2016. Studies have yet to publish population-level asthma prevalence using the FASQD PM2.5 estimates, which replaced the USEPA's previous hierarchical bayesian model (HBM) estimates. The range of variability and types of pollutants captured in the fused data may be different enough to capture different effects with respiratory health. More importantly, the larger effects could be due to differences in definition of asthma. Many studies have used either hospital reported data or self-reported data of asthma. Few studies have used a combination of clinical and self-reported asthma. Studies which rely soley on self-reported doctor diagnosed asthma or hospital data may be underrepresenting the true number of asthmatics as not everyone with asthma symptoms seeks diagnosis from a physician or experiences severe enough of symptoms to seek emergency care or hospitalization. Rice et al. (2015) found every 2 μg/m3 increase in annual average PM2.5 (data from satellite measurements of aerosol optical depth) was associated with a 13.5 ml lower FEV1 of adults in the Framingham Heart Study. Their population range of annual average exposure to PM2.5 was 7.3–21.7 μg/m3, a slightly higher range of exposure than seen in our study. However, these findings are consistent with this study and suggest annual average PM2.5 may significantly affect lung function, obstruct airways, and lead to asthmatic symptoms.
Surprisingly, we found smaller associations between allergies when comparing the highest quartile with the lowest compared to the second and third quartiles. However, the highest quartile of annual average PM2.5 when compared with the lowest quartile, did produce the greatest effect on asthma compared to the second and third quartiles. One of our most surprising findings was the associations found when the number of annual days experienced where PM2.5 concentrations were above 30 and 35 μg/m3 was analyzed by quartile with asthma. Just having 7–21 days out of the year above 30 μg/m3 PM2.5 resulted in a 67% increase in the prevalence of asthma when compared with those who experienced no annual days above 30 μg/m3. Similar results were seen with a higher cutpoint, where experiencing just 4–12 days above 35 μg/m3 PM2.5 resulted in a 68% increase in the prevalence of asthma.
4.2. Proximity to roadways- TRAP
Adults living within 300 m of a primary or secondary roadway had a 51% increase in the odds of having current asthma and 35% increase in the odds of having current allergy. When road types were analyzed separately, associations were stronger for secondary roadways and not primary roadways. Adults living within 300 m of a secondary roadway had a 48% increase in the odds of current asthma, but not for primary roadways. The stronger association seen with secondary roadways rather than primary roadways may be due to differences in traffic volume, the vehicle distribution and driving cycle differences across the primary and secondary roads.
We also wanted to examine further how different roadway types, indicative of different types of traffic, are associated with allergic outcomes. Our most interesting finding was when primary roadways were examined separately as primary “interstate only” and “non-interstate.” The largest association was found among those living within 300 m of an interstate, where the odds of asthma more than tripled. This was not a surprising finding, as those living near the interstate would likely experience heavier flows of traffic. Surprisingly, allergies and wheezing showed much stronger associations with living near non-interstate primary roads than with living near the interstate, but associations were not statistically significant. Porebski et al. (2014) found similar results among children and adolescents, comparing those living < 200 m from major roadway versus those living further than 500 m from the nearest major roadway, statistically significant odds ratios ranged from 2.0 to 5.7 for self-reported asthma and asthma symptoms. Bowatte et al. (2016) also found a slightly increased odds ratio of 1.10 (0.97–1.24) among an adult population when comparing those living within 200 m of a major road with those living further away. However, this study did not separate out different types of roadways, but rather a major roadway included “freeways, highways, arterial roads, and subarterial roads.” Furthermore, the outcome of asthma relied soley on self-report. Both factors may have contributed to odds ratio biased towards the null. Despite the differences in human health effects from different road types, our results further strengthen findings from prior studies that found traffic-related air pollution to be associated with, and likely a contributor of, asthmatic and allergic disease health outcomes (Codispoti and LeMasters, 2014; Delfino et al., 2014; Fuertes et al., 2013; Greenbaum, 2010; Jerrett and Shankardass, 2008; McConnell et al., 2010).
4.3. Proximity to industrial sites
We found the prevalence of asthma and wheezing to be greater among adults living within 800 m of an industrial site. The only statistically significant results were found with asthma, where we found a 47% increase in the odds of asthma among those living less than 800 m from an industrial site compared with those living further away. Our findings were nearly identical to Patel et al. (2011) who found the odds ratio for every 33.3% increase in residential area located within 800 m of a industrial site to be 1.43 (95% CI: 1.03, 1.97) for self-reported asthma among children in the Columbia Center for Children's Environmental Health birth cohort. Furthermore, Maantay (2007) found those age 16 and older living in the Bronx, New York, who reside within 800 m of an industrial site were up to 60% more likely to be hospitalized for asthma than those living further than 800 m from an industrial site.
Surprisingly, the odds of asthma and wheezing was roughly 10% lower among those living within 400 m of an industrial site. This is likely due to having a small sample of adults living within 400 m of an industrial site, or the fact some of the important emissions from industrial facilities are emitted via an elevated stack which prevents emissions from reaching ground level until downwind dispersion occurs further from the facility. Our ability to detect an association may have simply been an issue of power. We did not examine the distribution of stack height from the industrial sources nor their average dispersion characteristics and future work would be needed to test this hypothesis.
When examining associations between prevalence of wheezing with potential industrial facilities and TRAP exposures, insignificant results could be due to issues of collinearity or unmeasured confounding. For example, individuals of lower socioeconomic status may be more susceptible to wheezing for reasons other than proximity to industrial sites and would also explain why associations were more frequently seen in the unadjusted, but not the adjusted models. Future studies should identify vulnerable sub-populations when conducting research investigating the associations between localized air pollution and allergic disease outcomes.
When analyses were run with only participants who have lived in their home for five or more years, associations were no longer statistically significant. This was likely due to a decrease in sample size and limited power. Finding non-significance when smoking status, household smoking policy, pets, mold, and indoor chemical use were tested as interaction terms was not entirely surprising as only a few studies have reported finding any of these characteristics to be interactions when assessing air pollution and allergic disease outcomes.
4.4. Limitations
Despite the novelty of findings in exploring associations in a unique population setting there are several limitations in this study. First, the cross sectional nature of this study and the self-reported outcomes limit any estimates of causal associations. While we incorporated objective measure of lung function into the definition of asthma, an individual's FEV1 values can vary and one time point of lung function may not be reflective of an individual's lung function. Furthermore, participants are not asked to use their bronchodilater inhalers before completing the FEV1 test, so for some asthmatics, the FEV1 measure is not post bronchodilation.
Second, the nature of the exposure assessment may have resulted in some misclassification bias which might explain some of the null findings. Despite efforts to use the most refined downscaled model and use of kriging, the FAQSD air pollution estimates still may not be refined enough to capture air pollution estimates that are far from monitors. It has been shown that the extent of the spatial impacts of traffic related air pollutants is related to factors including the type of roadway, traffic volume and intensity, meteorology and background concentrations (Zhou and Levy, 2007; Zhu et al., 2004, 2006), which were not captured in this study. Furthermore, underreporting of fugitive and stack emissions has been shown among the Toxic Release Inventory database (De Marchi Scott, 2006; Grant and Jones, 2003; Koehler and Spengler, 2007), and this study did not capture varying quantities of emissions, nor the variation in chemical speciation. Associations seen with distance to industry may have been stronger if analyses had been stratified by type of and amount of industry emissions released; something to consider for future studies. Given the overall associations with proximity industrial facilities and allergic diseases was weak or null, we did not think looking at associations by different emission sources would yield any statistically significant results in our sample. Rather, the surrogate measures in this study are an indicator of exposure that may reflect the magnitude of the intra-urban spatial variability due to traffic-related and industrial-related pollutants.
An additional concern in this study is the effect of residential mobility on chronic exposure to air pollution in our study population. Our findings suggest that individuals who move more often tend to live in areas with higher levels of air pollution. This is likely due to sociodemographic reasons, which poses a challenge for this type of research. It was important to run analyses on only those participants residing in their current home for five years or longer in order to minimize any misclassification bias that may have occurred when assigning air pollution measures to participants of residential durations of five years or less. However, our analyses from residents of more than five years resulted in similar associations as seen with the entire study sample.
Given the nature of the study design, a number of confounding variables included in the analysis such as smoking status and smoking policy in the home, were self-reported with known issues of reporting bias. However, it is likely that these factors are uniformly mis-classified across the entire study population and thus should not have significantly biased our results. Finally, our ability to detect some associations may also have been simply an issue of power. The population size may have been too small for a study using estimates of air pollution exposure.
5. Conclusions
This study expands on existing literature by examining associations between potential exposure to traffic, industries, and PM2.5 with allergic diseases in a general population sample of adults exposed to annual mean fine particulate matter at or below the current USEPA standards. The current USEPA standard for annual average PM2.5 is 12 μg/m3, the upper quartile range of exposure in the population was between 10.86 and 15.12 μg/m3. We found significant associations between three different measures of potential air pollution exposure with allergic disease outcomes. The strongest effects were seen with annual average exposures to regional PM2.5 and proximity to roadways less than 300 m with increased odds of asthma. These findings suggest traffic may be a primary driver behind the association between PM2.5 and asthma in our study. Studies examining the health effects seen among populations exposed to regional fine particulate matter near or below the current national USEPA standard for annual average PM2.5 are important to the development and design of air pollution standards directed at reducing the impact of air pollution on public health. Future studies of this kind would benefit from in-person observations and repeated measures of lung function in a longitudinal analyses.
Acknowledgements
The authors thank the staff and graduate students at the Survey of the Health of Wisconsin (SHOW) for assistance in data processing and analysis. We would also like to thank the Wisconsin Partnership Program for continued funding of this work. Finally, we would like to thank the SHOW participants for their time and contribution to this work. Funding for SHOW comes from the Wisconsin Division of Public Health, the Wisconsin Partnership Program (PERC) Award (223 PRJ 25DJ), the National Institutes of Health's Clinical and Translational Science Award (5UL 1RR025011), and the National Heart Lunch and Blood Institute (1 RC2 HL101468) and a core grant to the Center for Demography and Ecology at the University of Wisconsin-Madison (P2C HD047873).
Sources of financial support
This work was supported by the National Institutes of Health's Clinical and Translational Science Award [5UL 1RR025011]; the National Heart Lung and Blood Institute [1 RC2 HL101468]; and the University of Wisconsin's (UW) Wisconsin Partnership Program PERC Award [233PRJ25DJ] and a core grant to the Center for Demography and Ecology at the University of Wisconsin-Madison (P2C HD047873).
Abbreviations:
- USEPA
United States Environmental Protection Agency
- OR
Odds Ratio
- CI
Confidence Interval
Appendix
Table A.1.
ORa (95% CI) comparing quartiles of annual days exceeding 35 μg/m3 PM2.5.
| Outcome | Q1 (0 days) |
Q2 (1 day) | Q3 (2–3 days) |
Q4 (4–12 days) |
|---|---|---|---|---|
| Allergy | Ref. | 1.21 (0.91, 1.61) |
1.22 (0.93, 1.60) |
0.91 (0.72, 1.16) |
| Asthma | Ref. | 1.11 (0.72, 1.70) |
2.12 (1.38, 3.26) |
1.68 (1.20, 2.35) |
| Wheezing | Ref. | 1.18 (0.79, 1.75) |
1.13 (0.72, 1.78) |
1.19 (0.82, 1.71) |
Abbreviations: OR - Odds ratio, CI - Confidence interval.
Adjusted for gender, age, BMI, smoking status, education, income.
Footnotes
Conflict of Interest
The authors report no conflicts of interest.
References
- Anderson H, Favarato G, Atkinson R, 2013. Long-term exposure to outdoor air pollution and the prevalence of asthma: meta-analysis of multi-community prevalence studies. Air Qual. Atmos. Health [Google Scholar]
- Batterman S, Ganguly R, Isakov V, Burke J, Arunachalam S, Snyder M, Robins T, Lewis T, 2014. Dispersion modeling of traffic-related air pollutant exposures and health effects among children with asthma in detroit, Michigan. Transp. Res. Rec 2452, 105–112. 10.3141/2452-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowatte G, Lodge C, Lowe A, Erbas B, 2014. The influence of childhood traffic-related air pollution exposure on asthma, allergy and sensitization: a systematic review and a meta-analysis of birth cohort studies. Allergy. [DOI] [PubMed] [Google Scholar]
- Bowatte G, Lodge CJ, Knibbs LD, Lowe AJ, Erbas B, Dennekamp M, Marks GB, Giles G, Morrison S, Thompson B, Thomas PS, Hui J, Perret JL, Abramson MJ, Walters H, Matheson MC, Dharmage SC, 2016. Traffic-related air pollution exposure is associated with allergic sensitization, asthma, and poor lung function in middle age. J. Allergy Clin. Immunol, 1–9. 10.1016/j.jaci.2016.05.008. [DOI] [PubMed] [Google Scholar]
- Codispoti C, LeMasters G, 2014. Traffic pollution is associated with early childhood aeroallergen sensitization. Ann. Allergy Asthma Immunol 114, 126–133. 10.1016/j.anai.2014.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amato G, 2011. Effects of climatic changes and urban air pollution on the rising trends of respiratory allergy and asthma. Multidiscip. Respir. Med 6, 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marchi Scott HJT, 2006. Assessing the accuracy of self-reported data: an Evaluaton of the Toxics release Inventory. J. Risk Uncertain 32, 57–76. [Google Scholar]
- Delfino R, Wu J, Tjoa T, 2014. Asthma morbidity and ambient air pollution: effect modification by residential traffic-related air pollution. Epidemiology 25, 48–57, (doi:1097/EDE.0000000000000016). [DOI] [PubMed] [Google Scholar]
- Fuertes E, Standl M, Cyrys J, Berdel D, 2013. A longitudinal analysis of associations between traffic-related air pollution with asthma, allergies and sensitization in the GINIplus and LISAplus birth cohorts. Peer J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant D, Jones AW, 2003. Are subsidiaries more prone to pollute? New evidence from the EPA's toxics release inventory. Soc. Sci. Q 84, 162–173. 10.1111/1540-6237.t01-1-8401010. [DOI] [Google Scholar]
- Greenbaum D, 2010. Panel on the Health Effects of Traffic-Related Air Pollution Traffic-related air pollution: a critical review of the literature on emissions, exposure, and health effects. Boston, MA. [Google Scholar]
- Guarnieri M, Balmes J, 2014. Outdoor air pollution and asthma. Lancet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankinson JL, Odencrantz JR, Fedan KB, 1999. Spirometric reference values from a sample of the general U.S. Population. Am. J. Respir. Crit. Care Med 159, 179–187. 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- Jerrett M, Shankardass K, 2008. Traffic-related air pollution and asthma onset in children: a prospective cohort study with individual exposure measurement. Environ. Health Perspect 116, 1433–1488. 10.1289/ehp.10968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karner AA, Eisinger DS, Niemeier DA, 2010. Near-roadway air quality: synthesizing the findings from real-world data. Environ. Sci. Technol 44, 5334–5344. 10.1021/es100008x. [DOI] [PubMed] [Google Scholar]
- Khreis H, Kelly C, Tate J, Parslow R, Lucas K, Nieuwenhuijsen M, 2016. Exposure to traffic-related air pollution and risk of development of childhood asthma: a systematic review and meta-analysis. Environ. Int, 34–40. 10.1016/j.envint.2016.11.012. [DOI] [PubMed] [Google Scholar]
- Kim KH, Jahan SA, Kabir E, 2013. A review on human health perspective of air pollution with respect to allergies and asthma. Environ. Int, 41–52. [DOI] [PubMed] [Google Scholar]
- Kim H, Bernstein JA, 2009. Air pollution and allergic disease. Curr. Allergy Asthma Rep 9, 128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler DA, Spengler JD, 2007. The toxic release inventory: fact or fiction? A case study of the primary aluminum industry. J. Environ. Manag 85, 296–307. 10.1016/j.jenvman.2006.09.025. [DOI] [PubMed] [Google Scholar]
- Krämer U, Sugiri D, Ranft U, Krutmann J, von Berg A, Berdel D, Behrendt H, Kuhlbusch T, Hochadel M, Wichmann H, Heinrich J, 2009. Eczema, respiratory allergies, and traffic-related air pollution in birth cohorts from small-town areas. J. Dermatol. Sci 56, 99–105. http://dx.doi.Org/10.1016/j.jdermsci.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Künzli N, Bridevaux P, Liu S, 2009. Traffic-related air pollution correlates with adult-onset asthma among never-smokers. Thorax. [DOI] [PubMed] [Google Scholar]
- Leung TF, Ko FWS, Wong GWK, 2012. Roles of pollution in the prevalence and exacerbations of allergic diseases in Asia. J. Allergy Clin. Immunol 129, 42–47. 10.1016/j.jaci.2011.11.031. [DOI] [PubMed] [Google Scholar]
- Maantay J, 2007. Asthma and air pollution in the Bronx: methodological and data considerations in using GIS for environmental justice and health research. Health Place 13, 32–56. 10.1016/j.healthplace.2005.09.009. [DOI] [PubMed] [Google Scholar]
- McConnell R, Islam T, Shankardass K, Jerrett M, Lurmann F, Gilliland F, Gauderman J, Avol E, Künzli N, Yao L, Peters J, Berhane K, 2010. Childhood incident asthma and traffic-related air pollution at home and school. Environ. Health Perspect 118, 1021–1026. 10.1289/ehp.0901232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabelli MC, Wing S, 2006. Proximity to pulp and paper mills and wheezing symptoms among adolescents in North Carolina. Environ. Res 102, 96–100. 10.1016/j.envres.2005.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modig L, Torén K, 2009. Vehicle exhaust outside the home and onset of asthma among adults. Eur. Respir. J 33, 1261–1267. 10.1183/09031936.00101108. [DOI] [PubMed] [Google Scholar]
- Nachman KE, Parker JD, 2012. Exposures to fine particulate air pollution and respiratory outcomes in adults using two national datasets: a cross-sectional study. Environ. Health 11, 25 10.1186/1476-069X-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto F, Peppard P, 2010. The survey of the health of Wisconsin (show), a novel infrastructure for population health research: rationale and methods. BMC Public Heal. Heal 10, 1 10.1186/1471-2458-10-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH, 2016. National Heart, Lung, and Blood Institute. Asthma Care Quick Reference: Diagnosing and Managing Asthma [WWW Document]. ⟨https://www.nhlbi.nih.gov/health-pro/guidelines/current/asthma-guidelines/quick-reference-html⟩ (accessed 01.04.17).
- Nishimura K, Galanter J, 2013. Early-life air pollution and asthma risk in minority children. The GALA II and SAGE II studies. Am. J. Respir. Crit. Care Med 188, 309–318. 10.1164/rccm.201302-0264OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor G, Neas L, Vaughn B, 2008. Acute respiratory health effects of air pollution on children with asthma in US inner cities. J. Allergy Clin. Immunol 121, 1133–1139. 10.1016/j.jaci.2008.02.02. [DOI] [PubMed] [Google Scholar]
- Patel MM, Quinn JW, Jung KH, Hoepner L, Diaz D, Perzanowski M, Rundle A, Kinney PL, Perera FP, Miller RL, 2011. Traffic density and stationary sources of air pollution associated with wheeze, asthma, and immunoglobulin E from birth to age 5 years among New York City children. Environ. Res 111, 1222–1229. 10.1016/j.envres.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porebski G, Wozniak M, Czarnobilska E, 2014. Residential proximity to major roadways is associated with increased prevalence of allergic respiratory symptoms in children. Ann. Agric. Environ. Med 21, 21. [DOI] [PubMed] [Google Scholar]
- Rice MB, Ljungman PL, Wilker EH, Dorans KS, Gold DR, Schwartz J, Koutrakis P, Washko GR, O’Connor GT, Mittleman MA, 2015. Long-term exposure to traffic emissions and fine particulate matter and lung function decline in the Framingham Heart Study. Am. J. Respir. Crit. Care Med 191, 656–664. 10.1164/rccm.201410-1875OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K, Kanniess F, Mark B, Jorres RA, Magnussen H, 1998. Assessment of accuracy and applicability of a new electronic peak flow meter and asthma monitor. Eur. Respir. J 2, 457–462. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau, 2010. TIGER/Line Shapefiles and TIGER/line Files [WWW Document]. ⟨ftp://ftp2.census.gov/geo/pvs/tiger2010st/55_Wisconsin/55/⟩ (accessed 02.08.15)
- U.S. EPA, 2016a. RSIG-Related Downloadable Data Files [WWW Document]. ⟨https://www.epa.gov/hesc/rsig-related-downloadable-data-file⟩ (accessed 9.15.16).
- U.S. EPA, 2016b. RSIG-Related Downloadable Data Files. Technical Information about Fused Air Quality Surface Using Downscaling Tool: Metadata Description [WWW Document]. ⟨https://www.epa.gov/sites/production/files/2016-07/documents/data_fusion_meta_file_july_2016.pdf⟩ (accessed 09.15.16).
- U.S. EPA, 2012. National Ambient Air Quality Standards (NAAQS) | Criteria Air Pollutants | US EPA [WWW Document]. US Environ. Prot. Agency ⟨https://www.epa.gov/criteria-air-pollutants/naaqs-table⟩ (accessed 12.8.16).
- U.S. EPA, 2008. Toxic Release Inventory (TRI) Program. TRI Basic Data Files: Calendar Years 1987–2014 [WWW Document]. ⟨https://www.epa.gov/toxics-release-inventory-tri-program/tri-basic-data-files-calendar-years-1987-2014⟩ (accessed 02.05.15).
- Urman R, McConnell R, Islam T, Avol EL, Lurmann FW, Vora H, Linn WS, Rappaport EB, Gilliland FD, Gauderman WJ, 2013. Associations of children's lung function with ambient air pollution: joint effects of regional and near-roadway pollutants. Thorax, 1–8. 10.1136/thoraxjnl-2012-203159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber SA, Insaf TZ, Hall ES, Talbot TO, Huff AK, 2016. Assessing the impact of fine particulate matter (PM2.5) on respiratory-cardiovascular chronic diseases in the New York City Metropolitan area using Hierarchical Bayesian Model estimates. Environ. Res 151, 399–409. 10.1016/j.envres.2016.07.012. [DOI] [PubMed] [Google Scholar]
- Weinmayr G, Romeo E, 2010. Short-term effects of PM10 and NO2 on respiratory health among children with asthma or asthma-like symptoms: a systematic review and meta-analysis. Environ. Heal. Perspect. Heal. Persectives 118, 449–457. 10.1289/ehp.0900844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir C, Yeatts K, Sarnat J, Vizuete W, 2013. Nitrogen dioxide and allergic sensitization in the 2005–2006 National Health and Nutrition Examination Survey. Respir. Med 107, 1763–1772. 10.1016/j.rmed.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang W, Lv J, Krafft T, Xu J, 2011. Time-series studies on air pollution and daily outpatient visits for allergic rhinitis in Beijing. China Sci. Total Environ 409, 2486–2492. 10.1016/j.scitotenv.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Zhang X, Morrison-Carpenter T, Holt JB, Callahan DB, 2013. Trends in adult current asthma prevalence and contributing risk factors in the United States by state: 2000–2009. BMC Public Health 13, 1156 10.1186/1471-2458-13-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Levy J, 2007. Factors influencing the spatial extent of mobile source air pollution impacts: a meta-analysis. BMC Public Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Hinds WC, Shen S, Sioutas C, 2004. Seasonal trends of concentration and size distribution of ultrafine particles near major highways in Los Angeles. Aerosol Sci. Technol 38, 5–13. 10.1080/02786820390229156. [DOI] [Google Scholar]
- Zhu Y, Kuhn T, Mayo P, Hinds WC, 2006. Comparison of daytime and nighttime concentration profiles and size distributions of ultrafine particles near a major highway. Environ. Sci. Technol 40, 2531–2536. 10.1021/es0516514. [DOI] [PubMed] [Google Scholar]