Abstract
Lysophosphatidic acid (LPA) is a bioactive lipid molecule, which regulates a broad range of pathophysiological processes. Recent studies have demonstrated that LPA modulates electrolyte flux in the intestine, and its potential as an antidiarrheal agent has been suggested. Of six LPA receptors, LPA5 is highly expressed in the intestine. Recent studies by our group have demonstrated activation of Na+/H+ exchanger 3 (NHE3) by LPA5. However, much of what has been elucidated was achieved using colonic cell lines that were transfected to express LPA5. In the current study, we engineered a mouse that lacks LPA5 in intestinal epithelial cells, Lpar5ΔIEC, and investigated the role of LPA5 in NHE3 regulation and fluid absorption in vivo. The intestine of Lpar5ΔIEC mice appeared morphologically normal, and the stool frequency and fecal water content were unchanged compared with wild-type mice. Basal rates of NHE3 activity and fluid absorption and total NHE3 expression were not changed in Lpar5ΔIEC mice. However, LPA did not activate NHE3 activity or fluid absorption in Lpar5ΔIEC mice, providing direct evidence for the regulatory role of LPA5. NHE3 activation involves trafficking of NHE3 from the terminal web to microvilli, and this mobilization of NHE3 by LPA was abolished in Lpar5ΔIEC mice. Dysregulation of NHE3 was specific to LPA, and insulin and cholera toxin were able to stimulate and inhibit NHE3, respectively, in both wild-type and Lpar5ΔIEC mice. The current study for the first time demonstrates the necessity of LPA5 in LPA-mediated stimulation of NHE3 in vivo.
NEW & NOTEWORTHY This study is the first to assess the role of LPA5 in NHE3 regulation and fluid absorption in vivo using a mouse that lacks LPA5 in intestinal epithelial cells, Lpar5ΔIEC. Basal rates of NHE3 activity and fluid absorption, and total NHE3 expression were not changed in Lpar5ΔIEC mice. However, LPA did not activate NHE3 activity or fluid absorption in Lpar5ΔIEC mice, providing direct evidence for the regulatory role of LPA5.
Keywords: LPA, mouse, NHE3
INTRODUCTION
Na+/H+ Exchanger 3 (NHE3, SLC9A3) is predominantly expressed on the apical membrane of the intestine and proximal tubule of the kidney, where it mediates the electroneutral exchange of luminal sodium for intracellular hydrogen (9). NHE3 is often functionally linked to an anion exchanger, downregulated in adenoma (DRA, SLC26a3) or putative anion transporter-1 (PAT-1, SLC26A6), where it can also facilitate the absorption of NaCl and NaHCO3 across the plasma membrane. As such, NHE3 has a major role in controlling electrolyte and fluid balance and is a frequent target of inhibition in many diarrheal diseases (32, 38). Mutations in NHE3 cause congenital sodium diarrhea, and inhibition of NHE3 has been reported in both enterotoxigenic and inflammatory diarrhea (1, 11, 14, 31). In addition, reduced NHE3 activity can predispose humans and animals to inflammatory bowel disease (13, 18).
Acute regulation of NHE3 is mediated by changes of protein expression at the brush border, and this process is tightly regulated by a range of factors, including neurohormones, glucocorticoids, and second messenger transmitters. Lysophosphatidic acid (LPA) is a naturally occurring phospholipid that mediates multiple biological effects through six G protein-coupled receptors, LPA1–LPA6 (37). LPA is present in biological fluids, including plasma, saliva, follicular fluid, and malignant effusion (37). In addition, increasing evidence demonstrates the presence of LPA in a variety of foods (27, 33). LPA evokes a diverse range of signaling processes that modulate cell migration, proliferation, survival, and inflammatory responses (3, 12). Recent studies have demonstrated that LPA modulates electrolyte transport in the intestinal tract. LPA activates NHE3 to stimulate Na+ and fluid absorption as well as preventing fluid loss by inhibition of the cystic fibrosis transmembrane conductance regulator (CFTR) (22, 25, 34). We have shown previously that LPA activates NHE3 in Caco-2bbe cells and mouse ileum (25). However, it is important to note that our current understanding of how LPA regulates NHE3 activity and the potential role of LPA5 in NHE3 regulation are primarily achieved using cell lines. While in vitro methods have been indispensable for determining cellular signaling pathways of LPA-induced NHE3 regulation, cell lines used in these studies rely on overexpression of LPA5 (2, 25, 29, 34). In a range of clinical disorders, including hypertension, acute kidney injury, and diarrheal diseases, where dysregulation of NHE3 is implicated, in vitro findings of NHE3 regulation have at times failed to be recapitulated when studied in animals or in a clinical setting (7). This underscores the importance of defining the LPA-induced stimulation of NHE3 by LPA5 in a whole-organism model.
In this study, we provide in vivo evidence that LPA-induced activation of NHE3 and fluid absorption in the intestine is mediated by LPA5. The current study utilized a mouse strain that lacks LPA5 in the intestinal epithelial cells (IECs). Our findings demonstrated that LPA did not regulate NHE3 or intestinal fluid in the absence of LPA5.
EXPERIMENTAL PROCEDURES
Chemicals and materials.
Preparation of LPA purchased from Avanti Polar Lipids (Alabaster, AL) was performed as previously described (25). Briefly, stock concentrations of 0.3 mM LPA (18:1) was prepared in PBS solution containing 0.1% BSA (wt/vol), pH 7.2. LPA was used at the final concentration of 1 µM. An equal volume of PBS containing 0.1% BSA was used as a control. Rabbit anti-NHE3 antibody was obtained from Alpha Diagnostics (San Antonio, TX). Cholera toxin (CTX) was purchased from Sigma (St. Louis, MO), and stock solutions of 1 mg/ml were prepared in sterile PBS. All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO) or EMD Millipore (Billerica, MA) unless otherwise specified.
Lpar5 gene targeting.
A 16.8-kb ApaI/ApaI genomic subclone containing exon 2 of Lpar5 from a C57BL/6J BAC clone was used as source DNA. The targeting construct was generated by inserting three PCR fragments, 8.7, 1,7, and 6.2 kb, into pFlexible (Fig. 1A). The targeting vector was linearized and electroporated into embryonic stem (ES, Sv129/R1) cells. Following selection by neomycin, ES cell clones were screened for homologous recombination by Southern blot analysis. The Frt-flanked phosphoglyceride kinase-neomycin selection cassette was removed by adenoviral Cre recombinase (16). Neosensitive-targeted ES cells were microinjected into blastocysts of C57BL/6J mice to generate chimeric mice with one targeted allele, Lpa5f/+ mice. Lpa5f/+ mice were bred to C57BL/6J mice, generating Lpa5f/f mice. To delete LPA5 in IECs, Lpa5f/+ mice were then crossed with villin-Cre mice, which harbor the villin promoter-driven Cre recombinase (6). Genotypes were determined by PCR using the following primers: Villin-Cre forward, CAA GCC TGG CTC GAC GGC C and reverse, CGC GAA CAT CTT CAG GTT CT; loxP forward, CCA GGC AGA GAG AGG AAG TG and reverse, TGG CCT CAG AAG ATT TGC TC. Experiments with animals were carried out under approval by the Institutional Animal Care and Use Committee of Emory University and in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals.
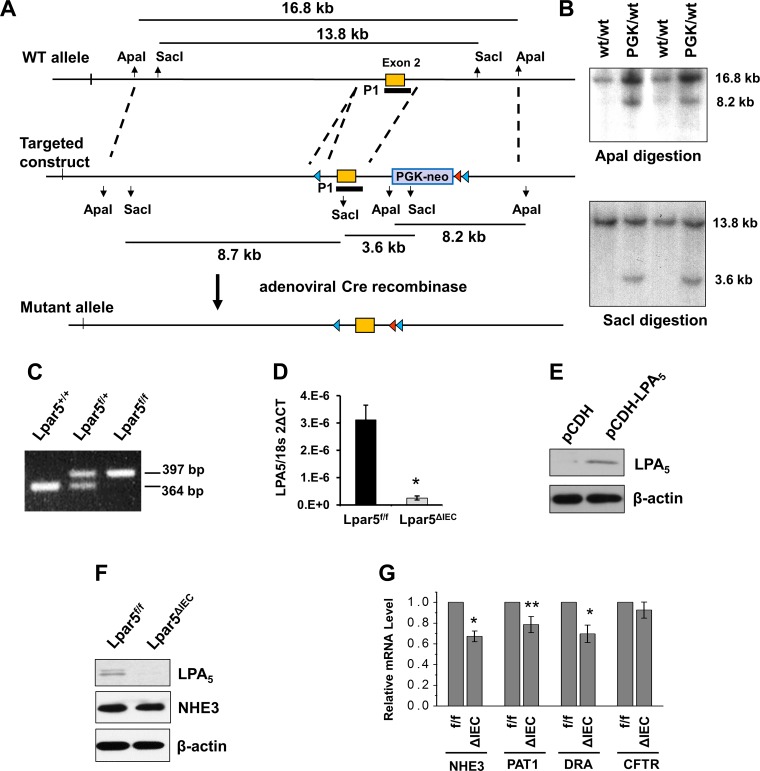
Fig. 1.
Generation of the floxed lysophosphatidic acid receptor 5 (Lpar5) allele in mice. A: design of cloning vectors and targeting strategies. Part of the Lpar5 gene containing exon 2 is shown at top (WT allele). LoxP and Frt are marked by blue triangle and red triangle, respectively. The targeting construct was generated by inserting 3 Lpar5 fragments into pFlexible. The Frt-flanked phosphoglyceride kinase-neomycin (Pgk-neo) selection cassette was removed using adenoviral Cre recombinase. B: Southern blots of embryonic stem (ES) cell genomic DNAs digested with ApaI or SacI using probe P1 are shown. Homologous recombination produced a 8.2-kb (top) and a 3.6-kb (bottom) band. C: PCR genotyping showing the wild-type (WT; 364 bp) and altered Lpar5 (397 bp) products. D: quantitative (q)RT-PCR for Lpar5 mRNA in intestinal epithelial cells (IECs) indicate knockdown of Lpar5 in Lpar5ΔIEC mice. *P < 0.01; n = 3. E: Western blot of Caco-2 cells transfected with the lentiviral pCDH-carrying LPA5 is shown. F: Western blot analysis showing absence of LPA5 and unaltered Na+/H+ exchanger 3 (NHE3) expression in Lpar5ΔIEC mouse intestine. G: qRT-PCR comparing mRNA expression of NHE3, putative anion transporter 1 (PAT1), downregulated in adenoma (DRA), and cystic fibrosis transmembrane conductance regulator (CFTR) in jejunum of Lpar5f/f (f/f) and Lpar5ΔIEC (ΔIEC) mice. *P < 0.01, **P < 0.05 vs. Lpar5f/f; n = 6.
Animal treatment.
For insulin treatments, mice were given 5 U/kg insulin (Sigma) or an equivalent volume of 0.9% saline vehicle by intraperitoneal injection 30 min before euthanasia (10). For CTX treatments, mice were orally administered 200 µg of CTX with or without 20 µM LPA in 200 µl total volume and then left to recover for 60 min before euthanasia.
Production of anti-LPA5 antiserum.
The cDNA encoding the carboxyl terminus (aa 297–372) of human LPA5 was subcloned into the bacterial hexahistidine (His6) fusion vector pET-16b (Novagen, Gibbstown, NJ) to generate the LPA5 (297–372)-His6 recombinant protein. The LPA5 recombinant protein was then purified using nickel-nitrilotriacetic acid-agarose resin (Qiagen) and used for immunization of New Zealand White rabbits by Covance Research Products (Denver, PA) to produce anti-LPA5 serum.
Quantitative RT-PCR and Western blot analyses.
Ileal mucosa was scraped and total RNA was prepared using an RNAeasy kit (Qiagen). One microgram of total RNA was used for cDNA synthesis using the First Strand cDNA Synthesis kit (Invitrogen, Carlsbad, CA) according to the manufacture’s instructions. Quantitative PCR was performed using iQ SYBR Green Supermix (Bio-Rad) on the Eppendorf Mastercycler Realplex. The primer sequences are listed in Table 1. To determine the expression of LPA5 and NHE3 protein, ileal mucosa scrapes were isolated and homogenized in lysis buffer (Cell Signaling Technology) containing protease inhibitor cocktails and 1 mM phenylmethylsulfonyl fluoride (PMSF). Thirty microgram of total protein lysate was separated on SDS-PAGE gel, and the expression of LPA5 and NHE3 was determined with rabbit anti-LPA5 and anti-NHE3 antibodies (35).
Table 1.
Primers used for RT-PCR
| Primer Sequence | |
|---|---|
| LPA1 | |
| Forward | ACA CCA GCC TGA CAG CTT CT |
| Reverse | CTG TAG AGG GGT GCC ATG TT |
| LPA2 | |
| Forward | TCA CTG GTC AAT GCA GTG GT |
| Reverse | AAG GGT GGA GTC CAT CAG TG |
| LPA3 | |
| Forward | AGG GCT CCC ATG AAG CTA AT |
| Reverse | TTC ATG ACG GAG TTG AGC AG |
| LPA4 | |
| Forward | TGC ATC AGT GTG GAT CGT TT |
| Reverse | GAA GCC TTC AAA GCA AGT GG |
| LPA5 | |
| Forward | GCT CCA GTG CCC TGA CTA TC |
| Reverse | GGG AAG TGA CAG GGT GAA GA |
| LPA6 | |
| Forward | ACT TTG CAA CAC GGA ATT GG |
| Reverse | TCA CTG TGA ACC ACA CAG CA |
| NHE3 | |
| Forward | ACA GAA GCG GAG GAA TAA CA |
| Reverse | TAT CAA TTC CTG CCC CAG AG |
| PAT1 | |
| Forward | TCC ATA GCC TCA TCC TGG AC |
| Reverse | CAG AGG CAA AGA CAT GCT GC |
| DRA | |
| Forward | AAT GCT GAT GCA GTT TGC TG |
| Reverse | TGC TCC TTC CAA CAT TAG CC |
| CFTR | |
| Forward | AGA GCA GTT TCC TGG ACA GC |
| Reverse | CCA GCG AAG GCT TGT TTT AG |
| 18S rRNA | |
| Forward | GTA ACC CGT TGA ACC CCA TT |
| Reverse | CCA TCC AAT CGG TAG TAG CG |
LPA1–6, lysophosphatidic acid receptors 1–6; NH3, Na+/H+ exchanger 3; PAT1, putative anion transporter 1; DRA, downregulated in adenoma; CFTR, cystic fibrosis transmembrane conductance regulator.
Na+-dependent intracellular pH recovery.
Isolation of villi from mouse ileum was performed as previously described (8). In brief, mice were anesthetized by inhalation of isoflurane and euthanized by cervical dislocation, and immediately a 1-cm section of proximal ileum was taken and placed on a cooled stage, cut longitudinally and washed with Na+ buffer in mM: 130 NaCl, 20 HEPES, 5 KCl, 1 tetramethylammonium (TMA)-PO4, 2 CaCl2, and 1 MgSO4). Villi were extracted from ileum sections using microdissection scissors and transferred onto coverslips using a 200-µl pipette. Samples were then covered in a permeable, 3-μm polycarbonate film (GE Water and Process Technologies) and incubated for 10 min with Na+ buffer containing 10 µM pH-sensitive dye, 2′,7′-bis(2-carboxyethyl)-5,6 carboxyfluorescein (BCECF, Sigma-Aldrich; excitation at 495 nm and 440 nm, emission at 530 nm), as described previously (8). Following the dye-loading period, coverslips were mounted on a perfusion chamber on an inverted microscope (TE200 inverted microscope; Nikon, Melville, NY) and superfused with NH4+ buffer (in mM: 40 NH4Cl, 90 NaCl, 20 HEPES, 5 KCl, 1 TMA-PO4 2 CaCl2, 1 MgSO4, and 18 glucose) for 5 min at 37°C. Individual regions of interest at the tip of the villi were outlined and monitored during the course of the measurement. Villi were then exposed for 1.5 min with TMA+ buffer (in mM: 130 TMA-Cl, 20 HEPES, 5 KCl, 1 TMA-PO4, 2 CaCl2, 1 MgSO4. And 18 glucose). Villi were then superfused with Na+ buffer to drive Na+-dependent pH recovery. Calibration of the florescent signal was performed using the K+/H+ ionophore nigericin, as previously described (17). Photometric data were acquired using the Metafluor software (Molecular Devices, San Jose, CA). The rate of Na+-dependent pH recovery was calculated by determining slopes along the early stage of intracellular pH recovery by a least squares analysis over a minimum of 5 s. Comparisons of Na+/H+ exchange were made between measurements on the same day.
Intestinal water flux measurement.
Intestinal water flux was measured as previously reported, with minor modifications (25). Briefly, mice anesthetized with pentobarbital sodium (Nembutal) were placed on a 37°C heating platform, and a small incision was made in the abdomen. A 5-cm length of ileum ~10 cm distal to the cecum was cannulated at either end with 0.76-mm polyethylene tubing. The abdominal cavity was covered using a moist Kimwipe for the duration of the experiment. The cannulated section was flushed for 10 min at a rate of 750 µl/min for 10 min using a peristaltic pump with buffer containing (in mM) 118 NaHCO3, 4.7 KCl, 2.52 CaCl2, 1.18 MgSO4, 25 NaHCO3, and 1.18 KH2PO4, pH 7.4, warmed to 37°C. The section was then perfused with perfusion buffer containing (in mM) 118.4 NaCl, 4.7 KCl, 2.52 CaCl2, 1.18 MgSO4, 1.18 KH2PO4, and 25 Na-gluconate for 2 h. When required, either 1 µM LPA or 0.1% BSA vehicle for control was added to the perfusion buffer. Intestinal water flux was recorded by calculating the change in weight of perfusion buffer at the end of the 2-h perfusion period. Mice were monitored in real time, with changes in weight of the perfusion buffer recorded every 20 min to record a fluid absorption trace over the perfusion period.
Confocal immunofluorescence microscopy.
Ileum sections were either treated ex vivo for 10 min with 1 µM LPA or 1% BSA vehicle for control, or alternatively, samples were taken from tissue treated for 2 h following intestinal water flux measurement. Tissue sections were fixed in 4% paraformaldehyde solution for 45 min, followed by immersion in 30% sucrose PBS solution overnight. Sections were then placed in Tissue-Tek OCT solution (Sakura Finetek, Torrance, CA) and stored at −80°C until required for analysis. Five-micrometer sections were placed onto Superfrost Plus (ThermoFisher, Waltham, MA) slides, and, after removal of OCT, tissues were blocked for 30 min in PBS solution containing 0.5% Triton X and 5% goat serum. Fluorescence staining procedures were described previously (8). NHE3 was stained with rabbit anti-NHE3, followed by anti-rabbit Alexa fluor 488. F-actin was stained using Alexa fluor 568 phalloidin, and nuclei staining was performed using Hoescht (Invitrogen). The specimens were then mounted with ProLong Gold Anti-Fade Reagent (Invitrogen) and observed under a Leica TCS SP8 confocal laser microscope (Leica Mycrosystems, Buffalo Glove, IL).
Semiquantification of NHE3 trafficking to microvilli region.
For quantification of NHE3 trafficking in response to LPA treatment from the terminal web to the microvilli region, images were analyzed using ImageJ (National Institutes of Health), where a 6-µm line was drawn perpendicular to the membrane, and fluorescent pixel density of both actin and NHE3 along the line was recorded. The peak of actin florescent intensity at the midway point of the line drawn (3 µm) was considered a marker dividing terminal web and microvilli. The terminal web region was found to be located between the regions of 2.7 and 3.2 µm and the microvilli region between 3.3 and 3.9 µm in all analyses. For each animal, seven to nine individual regions were captured, and seven or more florescent curves were analyzed per image.
Statistical analysis.
Statistical significance was assessed by paired Student’s t-test. When more than two groups were analyzed, a two-way ANOVA was performed followed by Bonferroni’s test for multiple comparisons (Graphpad Prism, La Jolla, CA). Results are presented as means ± SE. A P value of < 0.05 was considered significant.
RESULTS
Characteristics of Lpar5ΔIEC mice.
LPA5 is highly expressed in the intestinal tract, and our previous studies have demonstrated activation of NHE3 by LPA via LPA5 in vitro (19, 25, 34) However, Caco-2bbe cells used as a model for polarized IECs lack endogenous LPA5 expression; hence, the previous studies were based on overexpression of LPA5 in these cells. For functional studies of LPA5 in vivo, we developed a mouse strain in which exon 2 of Lpar5 was flanked by loxP sites so that it could be deleted upon spatiotemporally controlled expression of Cre recombinase. The strategy to generate the floxed Lpar5 allele in mice is illustrated in Fig. 1A. An example of Southern blot analysis comparing targeted and wild-type (WT) alleles in ES cells is shown in Fig. 1B. Targeted allele had 8.2 and 3.6 kb compared with 16.8 and 13.8 for WT. Genotypes of mice generated were determined by PCR (Fig. 1C). Mice heterozygous for floxed Lpar5, Lpar5f/+, were crossed with villin-Cre mice to generate a strain that lacked LPA5 in IECs, Lpar5f/f;villin-Cre, which were termed Lpar5ΔIEC (6). The deletion of LPA5 mRNA in IECs of Lpar5ΔIEC mice was determined by real-time PCR (Fig. 1D). We generated a rabbit polyclonal antiserum against human LPA5, which cross-reacted with exogenously expressed LPA5 in Caco-2 cells (Fig. 1E). Western blotting of intestinal mucosa for LPA5 using the anti-LPA5 serum demonstrated the absence of LPA5 in IECs (Fig. 1F). LPA5 deletion did not have a significant effect on the cellular level of NHE3 protein in IECs. On the other hand, we found that the mRNA expression of NHE3 was decreased in Lpar5ΔIEC mice compared with WT mice. Similarly, mRNA levels of PAT1 and DRA, but not CFTR, were decreased in Lpar5ΔIEC IECs. We did not determine protein expressions of PAT1, DRA, or CFTR.
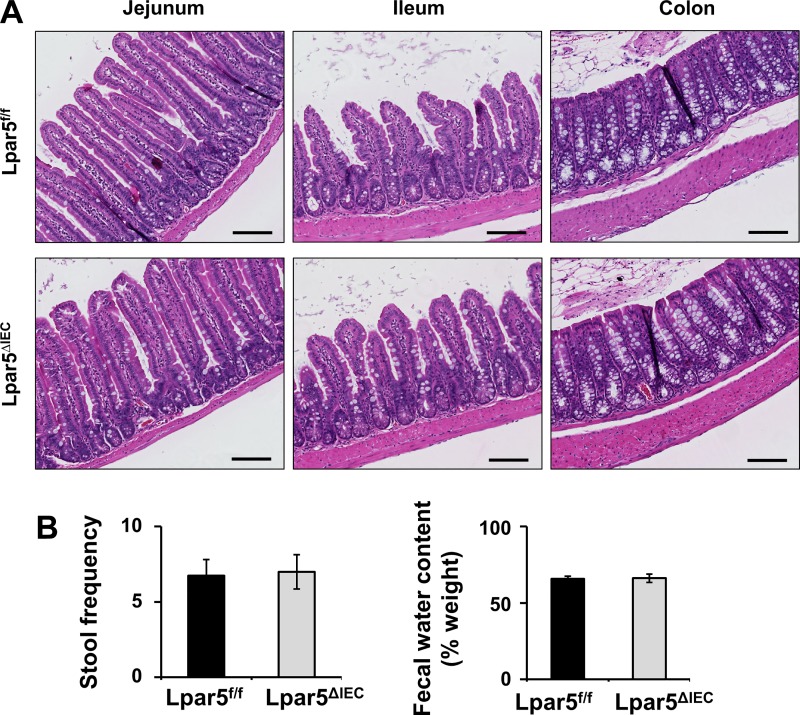
The phenotype of Lpar5ΔIEC mice was not different from their Lpar5f/f control littermates. No gross morphological differences in the intestine were observed, and the appearance of villi and crypt was unchanged (Fig. 2A). A gross assessment of the intestinal function by stool frequency and fecal water content, which was used as a measure of diarrhea, further corroborated that there was no gross effect on the basal functions of intestine by IEC-specific deletion of LPA5 (Fig. 2B).
Fig. 2.
Deletion of lysophosphatidic acid receptor 5 (LPA5) in intestinal epithelial cells (IECs) does not result in gross changes in mouse intestine. Lpar5ΔIEC mice lacking intestinal expression of LPA5 did not show any gross morphological or physiological gastrological defects compared with LPA5f/f controls. A: representative images of H&E-stained intestinal sections of LPA5f/f (top) and Lpar5ΔIEC mice (bottom) are shown. Scale bars, 100 µm. B: stool frequency over a 45-min period was determined. Fecal water content was estimated determining the weights of fresh stool and air-dried stool; n = 8.
Regulation of NHE3 activity by LPA in LPA5 ΔIEC mice.
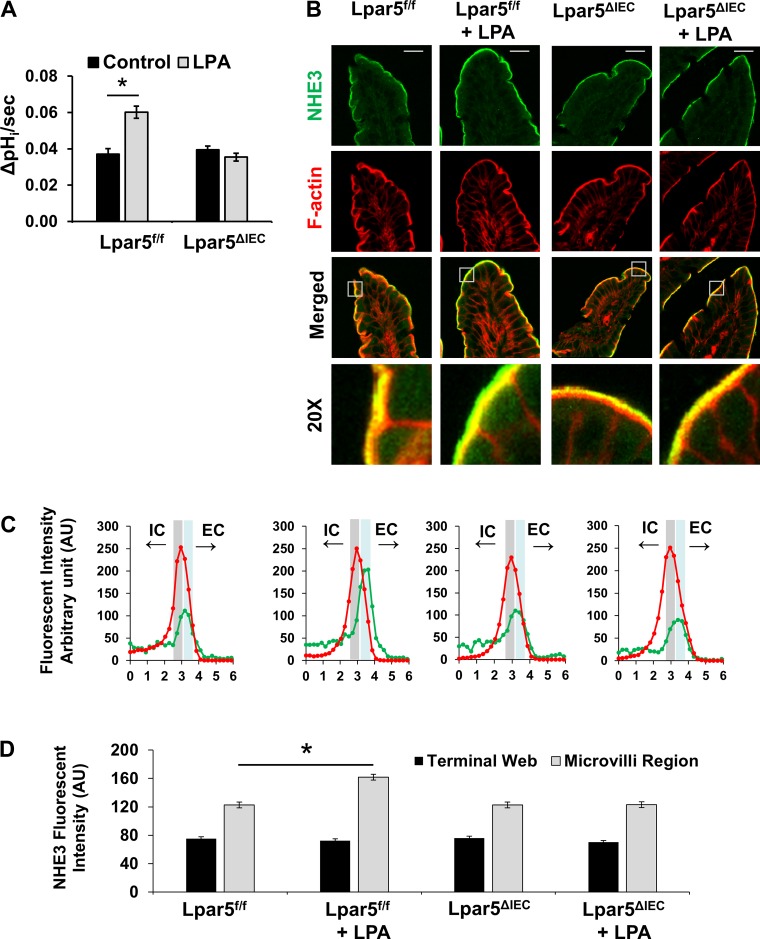
To determine the role of LPA5 on NHE3 regulation, we initially determined NHE3 activity in isolated ileal villi of Lpar5f/f and Lpar5ΔIEC mice. Basal NHE3 activity was unchanged by LPA5 deletion, and NHE3 activities were comparable between control and Lpar5ΔIEC mice. As we demonstrated in vitro (25), a significant increase in NHE3 activity was observed in Lpar5f/f villi when treated with 1 µM LPA for 10 min compared with control treatment (0.037 ± 0.003 vs. 0.060 ± 0.003 ΔpH/s, P < 0.05; Fig. 3A). However, in Lpar5ΔIEC enterocytes, LPA was unable to stimulate NHE3 activity (0.040 ± 0.002 vs. 0.035 ± 0.002 ΔpH/s), demonstrating that LPA5 is necessary for LPA-mediated stimulation of NHE3 activity.
Fig. 3.
Lysophosphatidic acid receptor 5 (LPA5)-mediated activation of Na+/H+ exchanger 3 (NHE3) activity is abolished in Lpar5ΔIEC mice. A: NHE3 transport activity was determined in ileal villi isolated from LPA5f/f and Lpar5ΔIEC mice in the presence of NHE1 and NHE2 inhibitor Hoe-694 (40 μM). LPA was added to the villi 10 min before the start of activity measurement. NHE3 activity is represented as the rate of Na+-dependent intracellular pH change, ΔpHi/s, at pHi 6.5; n = 7. *P < 0.01. B: representative confocal immunofluorescence images showing NHE3 (green) and F-actin (red) in mouse ileum. Scale bar in top, 20 µm. Bottom: ×20 magnification of insets in “merged” images. C: representative graphs of fluorescent intensity of NHE3 (green) and F-actin (red) along a 6-µm line drawn perpendicular to the membrane are shown. The terminal web (gray area) and microvilli regions (blue area) are indicated on each graph. IC, intracellular. EC, extracellular. D: average NHE3 fluorescence intensity in microvilli and terminal web determined above are presented as means ± SE. For each condition, 4 mice, 7 sections/mouse, and 7 measurements/section were made. *P < 0.01.
Stimulation of NHE3 activity often involves trafficking of NHE3 molecules to the brush border membrane (BBM). Our previous studies have shown that LPA increased BBM NHE3 in Caco-2bbe cells that were transfected to express exogenous LPA5 (34). To investigate whether NHE3 BBM expression is altered in mice in response to LPA, mouse ileal sections were treated ex vivo with 1 µM LPA for 10 min. Sections were then fixed and stained for NHE3 (green) and F-actin (red) (Fig. 3B). Immunofluorescence confocal microscopy analysis showed that NHE3 was predominantly expressed at the terminal web of the microvilli region where immunofluorescence signaling of NHE3 and F-actin overlapped. Actin was used as a relative marker to standardize fluorescence intensity across the terminal web and microvilli regions, with the peak of actin expression designated at the midway point across a 6-µm line analyzed for each fluorescent curve. Representative curves shown in Fig. 3C depict typical expression of NHE3 and actin across the terminal web (highlighted in gray) and microvilli region (highlighted in blue). LPA treatment significantly shifted NHE3 expression toward the tip of the microvilli (Fig. 3, C and D). This shift in NHE3 fluorescence signaling relative to actin by LPA is consistent with our previous study (25). Lpar5ΔIEC mice exhibited similar distribution of NHE3 relative to actin as WT mice. However, no significant change of NHE3 at the microvilli region was observed when exposed to LPA, indicating that LPA-mediated NHE3 mobility was impaired in Lpar5ΔIEC mice.
LPA-mediated water absorption and NHE3 trafficking are abolished in Lpar5ΔIEC mice.
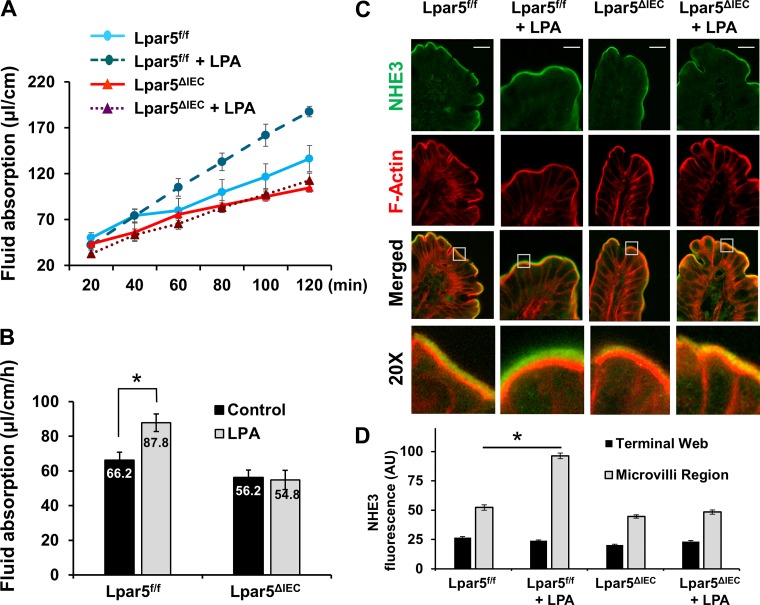
We previously demonstrated that LPA stimulates NHE3-dependent fluid absorption in the intestine in vivo and that this process requires the presence of the scaffolding protein Na+/H+ exchanger regulator factor 2 (NHERF2) (25). Although we have shown NHERF2-dependent regulation of LPA5, NHERF2 interacts with LPA5 as well as LPA2 and it is necessary for LPA2-dependent inhibition of CFTR, suggesting that NHERF2 deletion could have a compounding effect on NHE3 and CFTR (22, 25, 36). Therefore, we compared fluid absorption in control Lpar5f/f and Lpar5ΔIEC mice by using an in vivo perfusion model where fluid is perfused from one end of a short intestinal segment to the other end in a circulating manner (5, 25). In this model, despite the externalization of the intestine, the vasculature and innervation of the intestinal Musca are intact, making it an in vivo analysis. Figure 4A shows representative traces of fluid absorption under different conditions where fluid was absorbed at a constant rate for at least 2 h in all cases. Consistent with our previous studies (25), LPA significantly stimulated fluid absorption in control mouse ileum (87.8 ± 6.5 µl·cm−1·h−1 with LPA compared with basal conditions (66.2 ± 4.6 µl·cm−1·h−1 basal, P < 0.01) (Fig. 4B). The basal rate of water absorption in Lpar5ΔIEC ileum was similar to that of control mouse ileum, consistent with the finding above that basal NHE3 basal activities were similar in these mice. Importantly, there was no significant change in fluid absorption in Lpar5ΔIEC mouse ileum in the presence of LPA (51.6 ± 6.8 with LPA vs. 56.2 ± 4.4 µl·cm−1·h−1 control, P = 0.86).
Fig. 4.
Stimulation of intestinal water absorption by lysophosphatidic acid (LPA) requires LPA receptor 5 (LPA5). A: fluid absorption in mouse intestine was determined by in vivo perfusion system. Representative traces of fluid absorption in the absence or presence of 2 μM LPA in perfusion buffer are shown. B: rates of fluid absorption in the presence or absence of LPA in Lpar5f/f and Lpar5ΔIEC mouse intestine are shown. **P < 0.05, n ≥ 5. C: ileum sections used in water flux experiments were stained for Na+/H+ exchanger 3 (NHE3; green) and F-actin (red), and representative confocal immunofluorescence images of control and LPA perfused sections are shown. Scale bar in top, 20 µm. Bottom: ×20 magnification of insets in “merged” images. D: quantification of NHE3 fluorescence pixel intensity in the microvilli and terminal web. For each condition, 4 mice, 7 sections/mouse, and 5 measurements/section were made. *P < 0.01.
Immunofluorescence microscopic analysis of the ileal segments used in the fluid absorption showed that LPA resulted in a significant movement of NHE3 (green) toward the tip of microvilli in control Lpa5f/f mice (Fig. 4C: 52.3 ± 2.2 AU for control vs. 96.3 ± 2.3 AU for LPA, P < 0.01). On the other hand, the 2-h-long perfusion with LPA had no significant effect on NHE3 distribution in Lpar5ΔIEC mouse ilium (Fig. 4, C and D: 44.6 ± 1.4 AU for control vs. 48.4 ± 1.9 AU for LPA). These findings are congruent with the acute ex vivo LPA treatments, indicating that both acute and chronic treatment with LPA lead to NHE3 movement to BBM that is dependent on the presence of LPA5.
Deletion of LPA5 in IECs does not compromise other regulatory effects on NHE3.
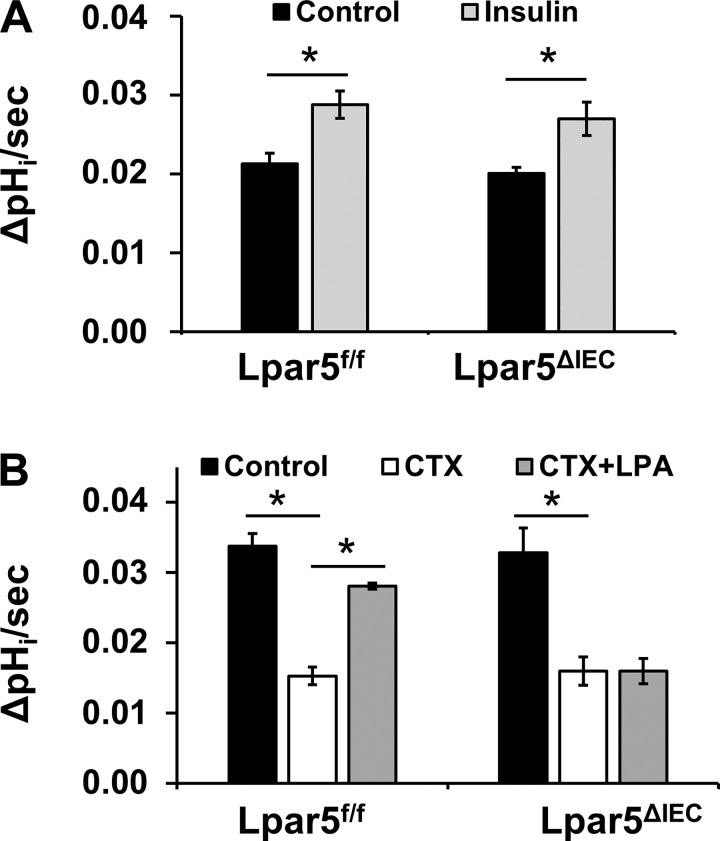
To ascertain that the loss of LPA5 does not abolish the overall regulatory capacity of NHE3, we compared the effect of insulin on NHE3. Mice were treated with insulin intraperitoneally, and NHE3 activity was determined in isolated ileal villi. In both Lpa5f/f and Lpa5ΔIEC IECs, insulin significantly stimulated NHE3 activity to the same extent, demonstrating that insulin-mediated regulation of NHE3 was not altered by the loss of LPA5 (Fig. 5A).
Fig. 5.
Deletion of lysophosphatidic acid receptor 5 (LPA5) does not affect Na+/H+ exchanger 3 (NHE3) regulation by insulin or cholera toxin (CTX). NHE3 activity was determined in freshly isolated intestinal villi of mice treated with insulin (A) or CTX (B) with or without LPA. Hoe-694 (40 μM) was used to block NHE1 and NHE2. NHE3 activity is represented as the rate of Na+-dependent intracellular pH change, ΔpHi/s; n ≥ 5. *P < 0.01.
We next investigated whether the inhibitory regulation of NHE3 by CTX was preserved in Lpa5ΔIEC enterocytes. To this end, mice were orally administered CTX, and intestinal villi were collected 60 min later. NHE3 activity in CTX-treated mice was significantly lowered regardless of the LPA5 presence (Fig. 5B), demonstrating that LPA5 deletion did not affect CTX-dependent inhibition of NHE3. Because our previous studies had shown that LPA was able to reverse water loss induced by CTX (25), we determined whether coadministration of LPA with CTX could restore NHE3 activity in mouse IECs. In Lpar5f/f mice, LPA almost completely reversed CTX-induced inhibition of NHE3. In contrast, LPA had no effect on NHE3 activity in CTX-treated Lpa5f/f mice. These results demonstrated that deletion of LPA5 in IECs specifically ablated LPA-dependent stimulation of NHE3.
DISCUSSION
In this study, we investigated the role of LPA5 in vivo by determining how intestinal loss of LPA5 affects the regulation of NHE3 activity and fluid absorption. We found that IEC-specific deletion of LPA5 abolished activation of NHE3 activity and intestinal fluid absorption, providing direct evidence for LPA5-dependent regulation of NHE3 in vivo. The effect of LPA on NHE3 has been demonstrated in human colonic Caco-2bbe cells and opossum renal proximal tubule cells (2, 34). Though the use of cell culture has been invaluable in extending our understanding of NHE3 regulation, it is important to note that our current knowledge of NHE3 regulation by LPA has been achieved exclusively in cell lines, which required overexpression of LPA5 (2, 34). In contrast, high levels of LPA5 were reported in the intestinal tract (4, 19). Given the disparities between the expression levels of LPA5 in cultured epithelial lines vs. intestinal tract of animals, it is important to determine whether the effect of LPA5 in NHE3 stimulation can be recapitulated in whole organisms. The current study is the first that demonstrates that the presence of LPA5 is essential for the stimulation of NHE3 activity and NHE3-dependent water absorption by LPA in vivo.
LPA5 and LPA2 are unique among the LPA receptors in that they have the carboxyl-terminal motif that interacts with the PDZ domains. This interaction permits specific effects through a number of cellular proteins, such as NHERF2, MAGI-3 (membrane-associated guanylate kinase, WW and PDZ domain-containing protein-3), Rho guanine nucleotide-exchange factor (PDZ-RhoGEF), and leukemia-associated Rho-GEF (21, 26, 36, 39). Specifically, LPA5 interacts with NHERF2, which is necessary for the stimulation of NHE3 via transactivation of the epidermal growth factor receptor (EGFR) (25, 34). We have shown that LPA was unable to activate NHE3 and intestinal fluid absorption in mice lacking NHERF2, but LPA-mediated intestinal fluid absorption was intact in Lpar2−/− mice, excluding LPA2 in NHE3 regulation despite its interaction with NHERF2 (25). However, NHERF2 is also involved in inhibition of CFTR by LPA such that the regulation of CFTR by LPA was lost in Nherf2−/− mice (22, 30). Therefore, studies thus far have indirectly implicated the role of LPA5 in NHE3 regulation in vivo, and direct evidence supporting its role has been lacking.
To overcome these limitations, we engineered a mouse strain lacking LPA5 in IECs. Lpar5ΔIEC mice were viable and fertile. The gross appearance of the intestinal tract of Lpar5ΔIEC mice was indistinguishable from that of WT mice. The normal morphological appearance of Lpar5ΔIEC mice was anticipated based on the previous study that global LPA5 knockout did not displace any gross phenotypic defect although these mice were reported to have a defective neuropathic pain perception (23). The characterization of Lpar5ΔIEC mice in the current study was limited to the features that are directly related to NHE3 regulation. However, we reported recently that, despite the normal intestinal morphology, Lpar1−/− mice have defective epithelial repair and epithelial barrier dysfunction, which predispose them to colitis (20, 24). Hence, additional studies of Lpar5ΔIEC mice are warranted to fully decode any defect outside the regulation of NHE3.
It was shown that Nherf2−/− mice have a reduced villus-to-crypt ratio in the ileum with decreased NHE3 activity, although the expression level of NHE3 in Nherf2−/− mice is similar to that of WT mice (28). In Lpar5ΔIEC mice, NHE3 protein expression at the terminal web and villus tip of enterocytes was not altered by LPA5 deletion. Furthermore, the baseline levels of NHE3 activity and fluid absorption in the ileum were not found to be significantly altered in Lpar5ΔIEC mice compared with WT mice. These findings indicated that LPA5 is not necessary for the maintenance of basal NHE3 expression or activity. It was shown that activation of LPA5 elevates cAMP levels, which is involved in neuropathic development (15, 19). Whether cAMP level is altered in the absence of LPA5 is not known, but it was reported that the pain-related marker expression was not affected in Lpar5−/− mice despite the protection of Lpar5−/− mice from neuropathic pain (23). Therefore, LPA5 does not appear to play a critical role in the maintenance of basal physiological conditions.
Our data showed that LPA5 was necessary for NHE3 regulation, so that LPA was unable to activate NHE3 activity or intestinal fluid absorption in Lpar5ΔIEC mice. We did not investigate the mechanism of NHE3 regulation by LPA, since our previous studies using Caco-2bbe cells had laid out the signaling pathway between LPA5 and NHE3 (29, 34). As we showed previously (25), the movement of NHE3 from the terminal web toward the tip of microvilli was stimulated by both ex vivo and in vivo application of LPA. This apical trafficking of NHE3 was not observed in Lpar5ΔIEC mice, confirming our previous studies in Caco-2bbe cells (34). Despite the defective regulation by LPA, NHE3 activation by insulin and inhibition by CTX were intact in Lpar5ΔIEC mice. NHE3 regulation by LPA involves transactivation of EGFR. Downstream of EGFR, proline-rich tyrosine kinase-2-phosphoinositide-dependent kinase-1 (PDK1) pathway and the mitogen-activated protein kinase/extracelllular signal-regulated kinase (MAPK/ERK) kinase (MEK)-ERK pathway are independently activated. PDK1 and ERK act on p90 ribosomal S6 kinase-2, which phosphorylates NHE3, inducing NHE3 mobility toward the BBM (29, 34). In contrast to LPA-mediated signaling, insulin activates NHE3 via a phosphoinositide 3-kinase-protein kinase C-protein kinase D pathway (10). The LPA- and insulin-induced regulation of NHE3 also differs in the involvement of NHERF proteins. As discussed above, LPA5 interacts with NHERF2 but not with NHERF1. On the contrary, NHERF1 is needed for the activation of NHE3 by insulin (10). In agreement with these differences, deletion of LPA5 did not affect the regulation of NHE3 by insulin. Similarly, the inhibition of NHE3 by CTX was similar between WT and Lpar5ΔIEC mice. These data demonstrated that Lpar5ΔIEC mice retained the ability to up- and downregulate NHE3 activity via other signaling pathways, indicating that these mice are a robust model that can be used to investigate LPA regulation of NHE3 without altering other regulatory arms.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-061418 and DK-107719 and a Veterans Affairs Merit Award I01BX002540 (to C. C. Yun) and American Heart Association Grant 13SDG1623001 (to P. He). Confocal microscopic analyses were supported in part by the Integrated Cellular Imaging Shared Resources of Winship Cancer Institute of Emory University and National Institutes of Health/National Cancer Institute under award P30 CA-138292. Funding for Lpar5f/f mouse generation was provided by Emory University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.A.J., P.H., and C.C.Y. conceived and designed research; K.A.J. and P.H. performed experiments; K.A.J., P.H., and C.C.Y. analyzed data; K.A.J., P.H., and C.C.Y. interpreted results of experiments; K.A.J., P.H., and C.C.Y. prepared figures; K.A.J. and C.C.Y. drafted manuscript; K.A.J., P.H., and C.C.Y. edited and revised manuscript; K.A.J., P.H., and C.C.Y. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Mouse Transgenic Core at Emory University and the former director Dr. W. David Martin for the generation of Lpa5 knockout mice.
REFERENCES
- 1.Barmeyer C, Harren M, Schmitz H, Heinzel-Pleines U, Mankertz J, Seidler U, Horak I, Wiedenmann B, Fromm M, Schulzke JD. Mechanisms of diarrhea in the interleukin-2-deficient mouse model of colonic inflammation. Am J Physiol Gastrointest Liver Physiol 286: G244–G252, 2004. doi: 10.1152/ajpgi.00141.2003. [DOI] [PubMed] [Google Scholar]
- 2.Cha B, Chen T, Sarker R, Yang J, Raben D, Tse CM, Kovbasnjuk O, Donowitz M. Lysophosphatidic acid stimulation of NHE3 exocytosis in polarized epithelial cells occurs with release from NHERF2 via ERK-PLC-PKCδ signaling. Am J Physiol Cell Physiol 307: C55–C65, 2014. doi: 10.1152/ajpcell.00045.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, Lin ME, Teo ST, Park KE, Mosley AN, Chun J. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol 50: 157–186, 2010. doi: 10.1146/annurev.pharmtox.010909.105753. [DOI] [PubMed] [Google Scholar]
- 4.Choi S, Lee M, Shiu AL, Yo SJ, Halldén G, Aponte GW. GPR93 activation by protein hydrolysate induces CCK transcription and secretion in STC-1 cells. Am J Physiol Gastrointest Liver Physiol 292: G1366–G1375, 2007. doi: 10.1152/ajpgi.00516.2006. [DOI] [PubMed] [Google Scholar]
- 5.Clayburgh DR, Barrett TA, Tang Y, Meddings JB, Van Eldik LJ, Watterson DM, Clarke LL, Mrsny RJ, Turner JR. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J Clin Invest 115: 2702–2715, 2005. doi: 10.1172/JCI24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.el Marjou F, Janssen KP, Chang BH, Li M, Hindie V, Chan L, Louvard D, Chambon P, Metzger D, Robine S. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 39: 186–193, 2004. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- 7.Girardi AC, Di Sole F. Deciphering the mechanisms of the Na+/H+ exchanger-3 regulation in organ dysfunction. Am J Physiol Cell Physiol 302: C1569–C1587, 2012. doi: 10.1152/ajpcell.00017.2012. [DOI] [PubMed] [Google Scholar]
- 8.He P, Lee SJ, Lin S, Seidler U, Lang F, Fejes-Toth G, Naray-Fejes-Toth A, Yun CC. Serum- and glucocorticoid-induced kinase 3 in recycling endosomes mediates acute activation of Na+/H+ exchanger NHE3 by glucocorticoids. Mol Biol Cell 22: 3812–3825, 2011. doi: 10.1091/mbc.e11-04-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He P, Yun CC. Mechanisms of the regulation of the intestinal Na+/H+ exchanger NHE3. J Biomed Biotechnol 2010: 238080, 2010. doi: 10.1155/2010/238080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He P, Zhao L, Zhu L, Weinman EJ, De Giorgio R, Koval M, Srinivasan S, Yun CC. Restoration of Na+/H+ exchanger NHE3-containing macrocomplexes ameliorates diabetes-associated fluid loss. J Clin Invest 125: 3519–3531, 2015. doi: 10.1172/JCI79552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodges K, Gill R. Infectious diarrhea: cellular and molecular mechanisms. Gut Microbes 1: 4–21, 2010. doi: 10.4161/gmic.1.1.11036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houben AJ, Moolenaar WH. Autotaxin and LPA receptor signaling in cancer. Cancer Metastasis Rev 30: 557–565, 2011. doi: 10.1007/s10555-011-9319-7. [DOI] [PubMed] [Google Scholar]
- 13.Janecke AR, Heinz-Erian P, Müller T. Reduced NHE3 activity results in congenital diarrhea and can predispose to inflammatory bowel disease. Am J Physiol Regul Integr Comp Physiol 312: R311, 2017. doi: 10.1152/ajpregu.00545.2016. [DOI] [PubMed] [Google Scholar]
- 14.Janecke AR, Heinz-Erian P, Yin J, Petersen BS, Franke A, Lechner S, Fuchs I, Melancon S, Uhlig HH, Travis S, Marinier E, Perisic V, Ristic N, Gerner P, Booth IW, Wedenoja S, Baumgartner N, Vodopiutz J, Frechette-Duval MC, De Lafollie J, Persad R, Warner N, Tse CM, Sud K, Zachos NC, Sarker R, Zhu X, Muise AM, Zimmer KP, Witt H, Zoller H, Donowitz M, Müller T. Reduced sodium/proton exchanger NHE3 activity causes congenital sodium diarrhea. Hum Mol Genet 24: 6614–6623, 2015. doi: 10.1093/hmg/ddv367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jongsma M, Matas-Rico E, Rzadkowski A, Jalink K, Moolenaar WH. LPA is a chemorepellent for B16 melanoma cells: action through the cAMP-elevating LPA5 receptor. PLoS One 6: e29260, 2011. doi: 10.1371/journal.pone.0029260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaartinen V, Nagy A. Removal of the floxed neo gene from a conditional knockout allele by the adenoviral Cre recombinase in vivo. Genesis 31: 126–129, 2001. doi: 10.1002/gene.10015. [DOI] [PubMed] [Google Scholar]
- 17.Lamprecht G, Weinman EJ, Yun CH. The role of NHERF and E3KARP in the cAMP-mediated inhibition of NHE3. J Biol Chem 273: 29972–29978, 1998. doi: 10.1074/jbc.273.45.29972. [DOI] [PubMed] [Google Scholar]
- 18.Larmonier CB, Laubitz D, Thurston RD, Bucknam AL, Hill FM, Midura-Kiela M, Ramalingam R, Kiela PR, Ghishan FK. NHE3 modulates the severity of colitis in IL-10-deficient mice. Am J Physiol Gastrointest Liver Physiol 300: G998–G1009, 2011. doi: 10.1152/ajpgi.00073.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J Biol Chem 281: 23589–23597, 2006. doi: 10.1074/jbc.M603670200. [DOI] [PubMed] [Google Scholar]
- 20.Lee SJ, Leoni G, Neumann PA, Chun J, Nusrat A, Yun CC. Distinct phospholipase C-β isozymes mediate lysophosphatidic acid receptor 1 effects on intestinal epithelial homeostasis and wound closure. Mol Cell Biol 33: 2016–2028, 2013. doi: 10.1128/MCB.00038-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SJ, Ritter SL, Zhang H, Shim H, Hall RA, Yun CC. MAGI-3 competes with NHERF-2 to negatively regulate LPA2 receptor signaling in colon cancer cells. Gastroenterology 140: 924–934, 2011. doi: 10.1053/j.gastro.2010.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C, Dandridge KS, Di A, Marrs KL, Harris EL, Roy K, Jackson JS, Makarova NV, Fujiwara Y, Farrar PL, Nelson DJ, Tigyi GJ, Naren AP. Lysophosphatidic acid inhibits cholera toxin-induced secretory diarrhea through CFTR-dependent protein interactions. J Exp Med 202: 975–986, 2005. doi: 10.1084/jem.20050421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin ME, Rivera RR, Chun J. Targeted deletion of LPA5 identifies novel roles for lysophosphatidic acid signaling in development of neuropathic pain. J Biol Chem 287: 17608–17617, 2012. doi: 10.1074/jbc.M111.330183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin S, Han Y, Jenkin K, Lee SJ, Sasaki M, Klapproth JM, He P, Yun CC. Lysophosphatidic acid receptor 1 is important for intestinal epithelial barrier function and susceptibility to colitis. Am J Pathol 188: 353–366, 2018. doi: 10.1016/j.ajpath.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin S, Yeruva S, He P, Singh AK, Zhang H, Chen M, Lamprecht G, de Jonge HR, Tse M, Donowitz M, Hogema BM, Chun J, Seidler U, Yun CC. Lysophosphatidic acid stimulates the intestinal brush border Na(+)/H(+) exchanger 3 and fluid absorption via LPA(5) and NHERF2. Gastroenterology 138: 649–658, 2010. doi: 10.1053/j.gastro.2009.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mikelis CM, Palmby TR, Simaan M, Li W, Szabo R, Lyons R, Martin D, Yagi H, Fukuhara S, Chikumi H, Galisteo R, Mukouyama Y-S, Bugge TH, Gutkind JS. PDZ-RhoGEF and LARG are essential for embryonic development and provide a link between thrombin and LPA receptors and Rho activation. J Biol Chem 288: 12232–12243, 2013. doi: 10.1074/jbc.M112.428599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morishige J, Touchika K, Tanaka T, Satouchi K, Fukuzawa K, Tokumura A. Production of bioactive lysophosphatidic acid by lysophospholipase D in hen egg white. Biochim Biophys Acta 1771: 491–499, 2007. doi: 10.1016/j.bbalip.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Murtazina R, Kovbasnjuk O, Chen TE, Zachos NC, Chen Y, Kocinsky HS, Hogema BM, Seidler U, de Jonge HR, Donowitz M. NHERF2 is necessary for basal activity, second messenger inhibition, and LPA stimulation of NHE3 in mouse distal ileum. Am J Physiol Cell Physiol 301: C126–C136, 2011. doi: 10.1152/ajpcell.00311.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.No YR, He P, Yoo BK, Yun CC. Regulation of NHE3 by lysophosphatidic acid is mediated by phosphorylation of NHE3 by RSK2. Am J Physiol Cell Physiol 309: C14–C21, 2015. doi: 10.1152/ajpcell.00067.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh AK, Riederer B, Krabbenhöft A, Rausch B, Bonhagen J, Lehmann U, de Jonge HR, Donowitz M, Yun C, Weinman EJ, Kocher O, Hogema BM, Seidler U. Differential roles of NHERF1, NHERF2, and PDZK1 in regulating CFTR-mediated intestinal anion secretion in mice. J Clin Invest 119: 540–550, 2009. doi: 10.1172/JCI35541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan S, Alex P, Dassopoulos T, Zachos NC, Iacobuzio-Donahue C, Donowitz M, Brant SR, Cuffari C, Harris ML, Datta LW, Conklin L, Chen Y, Li X. Downregulation of sodium transporters and NHERF proteins in IBD patients and mouse colitis models: Potential contributors to IBD-associated diarrhea. Inflamm Bowel Dis 15: 261–274, 2009. doi: 10.1002/ibd.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Surawicz CM. Mechanisms of diarrhea. Curr Gastroenterol Rep 12: 236–241, 2010. doi: 10.1007/s11894-010-0113-4. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka T, Kassai A, Ohmoto M, Morito K, Kashiwada Y, Takaishi Y, Urikura M, Morishige J, Satouchi K, Tokumura A. Quantification of phosphatidic acid in foodstuffs using a thin-layer-chromatography-imaging technique. J Agric Food Chem 60: 4156–4161, 2012. doi: 10.1021/jf300147y. [DOI] [PubMed] [Google Scholar]
- 34.Yoo BK, He P, Lee SJ, Yun CC. Lysophosphatidic acid 5 receptor induces activation of Na+/H+ exchanger 3 via apical epidermal growth factor receptor in intestinal epithelial cells. Am J Physiol Cell Physiol 301: C1008–C1016, 2011. doi: 10.1152/ajpcell.00231.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoo BK, Yanda MK, No YR, Yun CC. Human intestinal epithelial cell line SK-CO15 is a new model system to study Na+/H+ exchanger 3. Am J Physiol Gastrointest Liver Physiol 303: G180–G188, 2012. doi: 10.1152/ajpgi.00069.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yun CC, Sun H, Wang D, Rusovici R, Castleberry A, Hall RA, Shim H. LPA2 receptor mediates mitogenic signals in human colon cancer cells. Am J Physiol Cell Physiol 289: C2–C11, 2005. doi: 10.1152/ajpcell.00610.2004. [DOI] [PubMed] [Google Scholar]
- 37.Yung YC, Stoddard NC, Chun J. LPA receptor signaling: pharmacology, physiology, and pathophysiology. J Lipid Res 55: 1192–1214, 2014. doi: 10.1194/jlr.R046458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zachos NC, Tse M, Donowitz M. Molecular physiology of intestinal Na+/H+ exchange. Annu Rev Physiol 67: 411–443, 2005. doi: 10.1146/annurev.physiol.67.031103.153004. [DOI] [PubMed] [Google Scholar]
- 39.Zhang H, Wang D, Sun H, Hall RA, Yun CC. MAGI-3 regulates LPA-induced activation of Erk and RhoA. Cell Signal 19: 261–268, 2007. doi: 10.1016/j.cellsig.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]