Abstract
Zrt/Irt-like protein 8 (ZIP8) (encoded by Slc39a8) is a multifunctional membrane transporter that influxes essential metal cations Zn2+, Mn2+, Fe2+, and nonmetal inorganic selenite (HSeO3−). Physiological roles of ZIP8 in different cell types and tissues remain to be elucidated. We aimed to investigate ZIP8 functions in liver. Two mouse models were used in this study: 1) 13- to 21-mo-old Slc39a8(+/neo) hypomorphs having diminished ZIP8 levels and 2) a liver-specific ZIP8 acute knockdown mouse (Ad-shZip8). Histology, immunohistochemistry, and Western blotting were used to investigate ZIP8-deficiency effects on hepatic injury, inflammatory changes, and oxidative stress. Selenium (Se) and zinc (Zn) were quantified in tissues by inductively coupled plasma-mass spectrophotometry. We found that ZIP8 is required to maintain normal liver function; moderate or acute decreases in ZIP8 activity resulted in hepatic pathology. Spontaneous liver neoplastic nodules appeared in ~50% of Slc39a8(+/neo) between 13 and 21 mo of age, exhibiting features of inflammation, fibrosis, and liver injury. In Ad-shZip8 mice, significant hepatomegaly was observed; histology showed ZIP8 deficiency was associated with hepatocyte injury, inflammation, and proliferation. Significant decreases in Se, but not Zn, were found in Ad-shZip8 liver. Consistent with this Se deficit, liver expression of selenoproteins glutathione peroxidases 1 and 2 was downregulated, along with decreases in antioxidant superoxide dismutases 1 and 2, consistent with increased oxidative stress. Thus, ZIP8 plays an important role in maintaining normal hepatic function, likely through regulating Se homeostasis and redox balance. Hepatic ZIP8 deficiency is associated with liver pathology, including oxidative stress, inflammation, proliferation, and hepatocellular injury.
NEW & NOTEWORTHY Zrt/Irt-like protein 8 (ZIP8) is a multifunctional membrane transporter that facilitates biometal and mineral uptake. The role of ZIP8 in liver physiology has not been previously investigated. Liu et al. discovered unique ZIP8 functions, i.e., regulation of hepatic selenium content and association of ZIP8 deficiency in mouse liver with liver defects.
Keywords: ICP-MS, oxidative stress, selenium, ZIP8
INTRODUCTION
Membrane transporters play essential roles in regulating access to nutrients and other endogenous small molecules. Zrt/Irt-like protein 8 (ZIP8) is a multifunctional membrane transporter protein that facilitates uptake of several essential and nonessential divalent metals, including Zn2+, Mn2+, and Fe2+ (15, 18, 34, 57). More recently, ZIP8 was found to be a major transporter of inorganic selenite (HSeO3−); transport of selenite requires both Zn2+ and bicarbonate as cosubstrates (41). ZIP8 substrates Zn2+, Mn2+, Fe2+, and selenite are critically required in maintaining normal cellular function. For example, Zn2+ is an essential micronutrient that serves as a cofactor for many enzymes and participates in numerous signaling and transcription factors (8). Selenium (Se) is essential for synthesis of selenoproteins, which are critical in maintaining cellular redox balance (48). Tissue homeostasis of these biometals and minerals is tightly controlled by membrane transporters, including both importers and exporters.
ZIP8 belongs to the SLC39A8 gene subfamily, which encodes 14 ZIP members, ZIP1 through ZIP14 (25). Dysregulation of these influx transporters has been implicated in several human chronic diseases, including certain cancers (3). For example, ZIP4 is found to be overexpressed in human pancreatic cancer tissues compared with surrounding normal tissue, suggesting ZIP4 promotes human pancreatic cancer pathogenesis (32). Downregulation of ZIP14 is found in hepatocellular cancer (HCC) tissue compared with normal liver; this is consistent with markedly lower levels of zinc (Zn) in malignant liver tissue, indicating ZIP14 dysregulation is likely associated with decreased Zn in HCCs (14).
The highly conserved ZIP8 is expressed in embryonic stem cells and is known to be indispensable from early embryogenesis onward (55). Deficiency (≥80% in all tissues examined) in ZIP8 expression in Slc39a8(neo/neo) homozygous mice leads to fetal death in utero as early as gestational day (GD) 16.5 (15). In adult mice, ZIP8 is most abundantly expressed in lung followed by testis, kidney, and liver (24, 56). Genome-wide association studies and clinical studies have shown that several variants of SLC39A8 that encode ZIP8 with decreased function are correlated with clinical pathology and traits, such as high-density lipoprotein-cholesterol level (58), body mass index (37), blood pressure (22), cerebellar atrophy (6, 44), and inflammation-related diseases, including metabolic syndrome (13, 29) and Crohn’s disease (31).
ZIP8 also participates in Zn2+-regulated NF-κB, which plays a critical role in immune responses, particularly in inflammation. Inflammatory stimuli, such as TNF, IL1β, and lipopolysaccharide, all induce ZIP8 expression in cell culture and mammalian cell types, such as monocytes, macrophages, lung (5, 35), chondrocytes, and cartilage (28). ZIP8 serves as a negative regulator in the canonical NF-kB pathway through recruiting Zn2+ and inhibiting IκB kinase (35). These findings show that ZIP8 serves as an immune modulator for balancing pro-versus anti-inflammatory responses in mammals.
Many factors contribute to the pathogenesis of liver disease, which result from persistent cellular injury (39), dysregulated inflammation (16, 47), and oxidative stress (40). Se and Zn play pivotal roles in these processes, and disruption of Se and Zn homeostasis is commonly seen in a range of liver disorders in both animal models and clinical studies.
Low Se levels in liver and in the general circulation are found in patients with chronic alcoholic fatty liver disease (AFLD) (53) and in some patients with cirrhosis (7); epidemiological evidence shows a high prevalence of primary liver cancer in populations of marginally low Se status, such as European (20). Se deficiency or genetic variation of selenoprotein has been shown to impair endogenous antioxidative defense systems and negatively regulate the immune process, leading to chronic inflammation (12, 30, 60). Zn decreases have been found to affect liver disease at multiple levels, including modulation of signaling cascades (51), induction of oxidative stress (27), impairment of proliferation and survival (26), and inflammation (46). Hypozincemia is observed in many liver disorders, including AFLD (26, 27), viral hepatitis (23), cirrhosis (17), and virtually all HCC tumors (11). Some studies have also shown a synergistic deficiency of Se and Zn to be prevalent in viral hepatic disease and cirrhosis patients (33, 42). Dietary supplementation of Se or Zn, or both, has demonstrated improvement in liver function in animal models and patients with liver disease (27, 43); Se and Zn supplementation has therefore been proposed as a preventive measure against liver cancer (11, 20).
In our present study, spontaneous liver pathology was found in Slc39a8(+/neo) having diminished ZIP8 levels; observed defects included fatty liver, inflammation, fibrosis, and neoplastic changes consistent with HCC. Sustained inflammation is likely a direct result of ZIP8 deficiency, which contributes ultimately to hepatocellular tumorigenesis in these mice.
Moreover, in mice having liver-specific ZIP8 knockdown for 7 days by adenovirus-delivered shRNA that targets Slc39a8, we observed substantial hepatomegaly, inflammation, and proliferation. Inductively coupled plasma-mass spectrometry (ICP-MS) quantitation revealed intracellular Se to be significantly lower in liver, and the selenoproteins glutathione peroxidase (GPX)1 and GPX2 and the antioxidants SOD1 and SOD2 were decreased, all consistent with Se deficiency-associated increases in oxidative stress. Together, these results suggest that ZIP8 is required to maintain normal liver Se homeostasis, which in turn protects against oxidative stress, inflammation, and liver injury. We propose that ZIP8-mediated Se transport in hepatocytes plays a pivotal role in the prevention of liver pathology.
METHODS
Animals.
Slc39a8(+/neo) mice were maintained on a mixed C57BL/6J and 129S6/SvEvTac genetic background, originally constructed in the Nebert laboratory, University of Cincinnati (55). In brief, the Slc39a8(neo) allele was developed by insertion of Frt-flanked neomycin-resistance (neo) mini-cassette into intron 3 and loxP sites in introns 3 and 6 of the Slc39a8 gene. Retention of the neo insert inadvertently resulted in Slc39a8(neo/neo) homozygotes having diminished Slc39a8 mRNA and ZIP8 protein expression by ≥80%. Slc39a8(neo/neo) mice die between GD 16.5 and postnatal day 1. Slc39a8(+/+) wild-type (WT) and Slc39a8(+/neo) heterozygotes, aged 13–21 mo, were used in all studies herein, without bias of males or females.
With regard to the shRNA knockdown mouse, male Slc39a8(+/+) mice from the same litter were used at age 8 to 10 wk; they were injected with either Ad-shZip8 or Ad-control at a dose of 1 × 1011 viral particles per mouse by a single tail-vein injection. Animals were euthanized 7 days following adenovirus injection, and liver weight and body weight were recorded. Tissue samples were snap-frozen in liquid nitrogen for further analysis. All mice were housed in a pathogen-free facility with a 12-h:12-h light-dark cycle and free access to water and a regular chow diet. All animal studies were approved by the Oakland University Institute of Animal Care and Use Committee.
Generation of recombinant adenovirus-expressing shRNA.
To make recombinant adenovirus-expressing shRNA against mouse Slc39a8, a specific RNAi sequence designed by The RNAi Consortium was selected, 5′-CAACGCGGGAAGGCATTTAAT-3′, which specifically targets the 3′-untranslated region of mouse Slc39a8, following analysis using National Center for Biotechnology Information BLASTN on the mouse genomic and transcript database (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Recombinant adenovirus (Ad5)-containing shRNA was constructed, amplified, and purified (Welgen); the resultant Ad-shZip8 mouse was compared in all experiments with mice injected with recombinant adenovirus-containing scrambled shRNA as the control (Ad-control mouse). The control shRNA sequence was GCGCGCGATTGTAGGATTCA.
AML12 cells and primary hepatocyte cultures.
The alpha mouse liver 12 (AML12) (ATCC CRL-2254) cell line was established from hepatocytes of a CD1 mouse transgenic for human TGFα; cells were maintained in DMEM/F12 medium with 10% FBS and supplemented with insulin-transferrin-Se (ITS-G; Thermo Fisher Scientific) and dexamethasone. Primary hepatocytes from 8-wk-old Slc39a8(+/+) males were isolated by a two-step collagenase perfusion procedure as described in Ref. 49. Hepatocytes were cultured in William’s E medium with 2% FBS, glutamine, and dexamethasone on 35-mm collagen-coated dishes at 80% confluency. Testing the efficiency of Ad-shZip8 to knock down ZIP8 mRNA was first performed in AML12 cells and mouse primary hepatocytes with multiplicity of infection 10. Recombinant Ad-shZip8 or Ad-control was diluted in culture medium and incubated with AML12 and primary hepatocytes for 12 h, and then harvested after an additional 12 h for determination of RNA expression.
RNA isolation and real-time quantitative PCR.
Total RNA was isolated from mouse tissues and cells using Qiagen RNeasy mini prep kit, following the manufacturer’s instructions. The cDNAs were synthesized by the M-MuMLV-transcriptase system (New England Biolabs). The quantitative (q)PCR for individual gene expression was carried out with Luna Universal qPCR Master Mix (New England Biolabs) and gene-specific primers; cycling conditions included 95°C for 1 min, followed by amplification for 40 cycles at 95°C for 15 s, and then 60°C for 30 s in the CFX96 Real-Time PCR system (Bio-Rad). Relative expression of mRNA levels was normalized to that of the housekeeping control gene Gapdh, using the ΔΔCq method. Gene-specific primer sequences are provided in Table 1.
Table 1.
Primers for quantitative RT-PCR
| Forward Primer | Reverse Primer | |
|---|---|---|
| Acta2 | GTACCACCATGTACCCAGGC | GCTGGAAGGTAGACAGCGAA |
| Afp | AAGTGGATCACACCCGCTTC | TGTTCACTTCCTCCTCGGTG |
| Gapdh | CCAATGTGTCCGTCGTGGATCT | GTTGAAGTCGCAGGAGACAACC |
| Il1β | TGCCACCTTTTGACAGTGATG | ATGTGCTGCTGCGAGATTTG |
| Il6 | ACAAAGCCAGAGTCCTTCAGAG | AGAGCATTGGAAATTGGGGTAGG |
| Tgfb1 | AGCTGCGCTTGCAGAGATTA | AGCCCTGTATTCCGTCTCCT |
| Timp | TGGCATCTGGCATCCTCTTG | GGTCTCGTTGATTTCTGGGGA |
| Tnf | CCACCACGCTCTTCTGTCTAC | AGGGTCTGGGCCATAGAACT |
| Slc39a8 | GGGACTAGCTTTCGGCATT | GCATGTCGTTCATCTCTGGA |
Histology and immunohistochemistry.
Liver tissues were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned at 5 μm. Hematoxylin-eosin (H&E) and picrosirius red staining was performed according to standard procedures. For immunohistochemistry (IHC), paraffin-fixed liver sections were routinely deparaffinized and rehydrated. Antigen retrieval was performed, using either citrate buffer (10 mM, pH 6.0) or EDTA buffer (1 mM, pH 8.0) in a water bath (95°C). Endogenous peroxidase activity was blocked in PBS with 3% H2O2 for 30 min. Prior to staining, nonspecific antibody binding was blocked by incubating the sections with PBS plus 0.025% Triton X-100, 1% BSA, and 10% serum compatible with the secondary antibody. Primary antibodies were applied in PBS with 1% BSA at 4°C overnight. Secondary antibodies were applied for 1 h at room temperature in PBS with 1% BSA. ImmPACT DAB Substrate (Vector) was used for final chromogen formation. Sections were counterstained with hematoxylin, dehydrated, and mounted in a mounting buffer. The following primary antibodies were used: anti-8-hydroxy-2′-deoxyguanosine (8-OHdG) (sc-393871), anti-γH2AX (sc-517348), anti-α-fetoprotein (AFP) (sc-130302), anti-F4/80 (sc-52664), anti-myeloperoxidase (MPO) (sc-390109), and anti-proliferating cell nuclear antigen (PCNA) (sc-56) from Santa Cruz Biotechnology, anti-Ki67 (bs-23102R; Bioss Antibodies), and anti-cleaved caspase 3 (No. 9664; Cell Signaling Technology). Matching secondary antibodies were used for detecting primary antibodies, goat-anti-rabbit IgG (H+L) horseradish peroxidase (HRP)-conjugate (SA00001-2; ProteinTech Group) and m-IgGk-BP-HRP (sc-516102; Santa Cruz Biotechnology).
Immunoblot analysis.
Liver lysate supernatant was prepared by homogenizing ~20 mg liver in radioimmunoprecipitation assay buffer supplemented with protease inhibitors (Roche). Lysate was centrifuged at 4°C at 14,000 g for 20 min. The protein concentrations were measured using the Pierce bicinchoninic acid protein assay kit (Thermo Fisher Scientific). Protein (40 μg) was separated using SDS-PAGE and transferred to PVDF membranes for detection of targeting proteins. The following primary antibodies were used: anti-Gpx1/2 (sc-133160), anti-cyclinD1 (sc-450), anti-SOD2 (sc-137254), and anti-PCNA (sc-56) from Santa Cruz Biotechnology, anti-SOD1 (GTX100554, Genetex), and anti-tubulin (BioLegend). Matching secondary antibodies included goat anti-rabbit IgG (H+L) HRP-conjugate (SA00001-2; ProteinTech Group) and m-IgGk-BP-HRP (sc-516102; Santa Cruz Biotechnology); these were used to amplify the signal from the primary antibodies, and ECL reagent (Advansta) was used to visualize the signal.
Quantification of metals.
The elements Zn and Se were measured in liver, heart, kidney, and spleen by ICP-MS (ELAN DRC-e; PerkinElmer). Ad-shZip8 mice and Ad-control mice were euthanized, and the tissues were isolated and weighed. Tissue samples were processed in 100 μl 70% HNO3 at 70°C until completely digested. Following centrifugation, the supernatant was diluted in deionized water to a final volume of 2 ml and subjected to quantitation of the intracellular metals. Content (μg/l) was measured using a calibration curve of standards prepared at four different concentrations of each element; absolute metal content (ng/mg wet tissue) was determined.
Statistical analysis.
Univariate comparison of two group means was performed, using the unpaired Student’s t-test. Mean values and standard errors were calculated by Sigma Plot 10.0. Data are expressed as mean s± SE. P < 0.05 was used to confirm significance. Group sizes were n = 3–7, as labeled in each experiment.
RESULTS
Chronic ZIP8 deficiency in Slc39a8(+/neo) mice is associated with liver pathology.
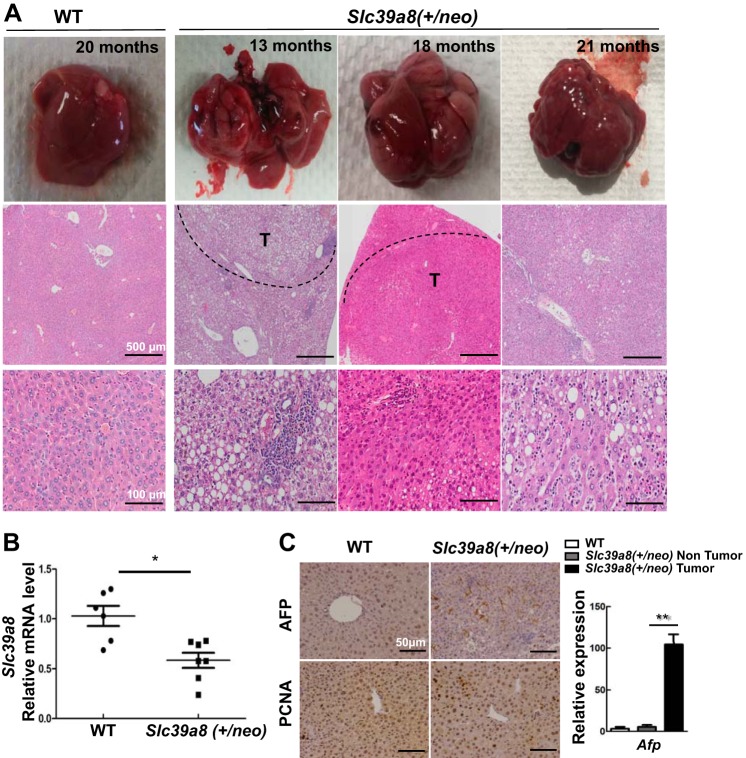
During the first 12 mo in our mouse colony, Slc39a8(+/neo) did not show any visible morphological or behavioral abnormalities. We postulated that Slc39a8(+/neo), having some degree of ZIP8 deficiency, might lead to metal transport problems being cumulative over time; therefore, we investigated possible long-term effects at ages 13–21 mo. Indeed, we found about half Slc39a8(+/neo) with liver pathology; 10 of 22 mice developed tumors, as shown in Fig. 1A. Grossly, each tumor formed discrete nodules in liver. H&E sections of tumors showed well-demarcated masses. The neoplastic cells were arranged in trabecular growth patterns without normal liver architecture and showed severe cytological atypia with some fatty changes and ballooning changes. Diminished ZIP8 expression was observed in Slc39a8(+/neo) liver (Fig. 1B).
Fig. 1.
Liver pathology showing significant nodular tumors in Slc39a8(+/neo) mice. A: wild-type (WT) mouse (far left column) was 20 mo of age; Slc39a8(+/neo) mice (three right columns) were 13, 18 and 21 mo of age, respectively. Macroscopic livers (top row) and microscopic hematoxylin-eosin (H&E) staining (bottom two rows) is shown. Nodular tumors are visualized in parts or in the entire liver of Slc39a8(+/neo) mice. Out of 22 hypomorph mice, 10 were identified with similar nodular tumors. Scale bars: 500 μm (middle row); 100 μm (bottom row). B: hepatic Slc39a8 mRNA expression in WT (n = 6) and Slc39a8(+/neo) mice ages 13–21 mo (n = 7). C: typical immunohistochemistry (IHC) staining for α-fetoprotein (AFP) and proliferating cell nuclear antigen (PCNA) in WT and Slc39a8(+/neo) mice (20 mo of age) (top). Bottom: quantitative PCR analysis of Afp in WT and in nontumor portion and tumor of Slc39a8(+/neo) (n = 3 for each of the three histograms). All data represent means ± SE, with 1.0 designated for WT mean expression. *P ≤ 0.05 by Student’s t-test. T, tumor.
Histopathological features of increased AFP and PCNA strongly suggest the possibility of hepatocellular adenoma and carcinoma (Fig. 1C). IHC staining and qPCR for AFP revealed highly significantly increased AFP in neoplastic regions but not in normal hepatocellular regions; tumor cells exhibited increases in PCNA. The histopathology supports a diagnosis of hepatocellular neoplasm. In the tumorous parts, diffuse inflammation infiltration was also present (Fig. 1A).
Looking at age-matched Slc39a8(+/+) WT mice, we found that only 1 of 14 animals had developed a spontaneous hepatic tumor. In other tissues of the WT and all Slc39a8(+/neo) mice, no visible gross pathology was noted.
Hepatocellular injury, inflammation, and fibrosis in Slc39a8(+/neo) mice.
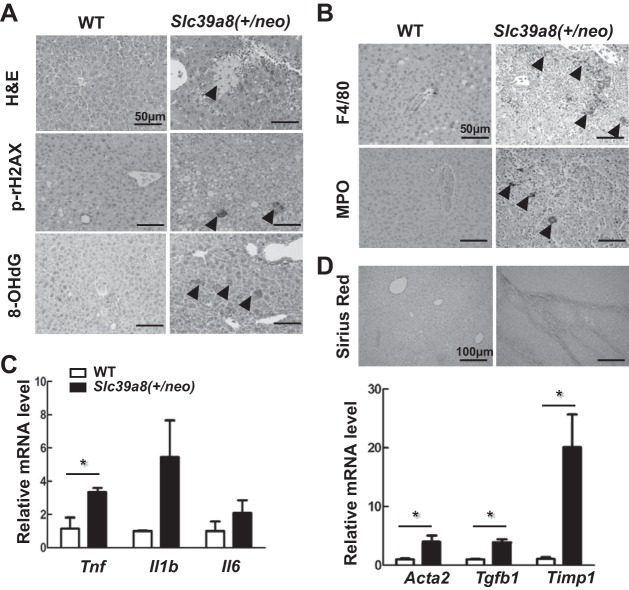
In Slc39a8(+/neo) mice of 13 to 21 mo of age, severe multifocal necrosis was observed in tumor regions (Fig. 2A); however, we found no evidence of apoptosis, as determined by IHC staining of cleaved caspase 3 (data not illustrated). IHC staining for phosphorylation of histone H2AX (γH2AX) was positive in Slc39a8(+/neo) hepatocytes, suggesting increased DNA damage (Fig. 2A). Along with elevated DNA damage, the mice also showed strong positive staining of 8-OHdG, the biomarker for oxidative stress (Fig. 2A). These results suggested that cell injury and increased oxidative stress both contribute to the tumor development we observed in the aged heterozygotes mice.
Fig. 2.
Slc39a8(+/neo) mouse liver showing necrosis, proliferation, fibrosis, and inflammation. A: Exemplary hematoxylin-eosin (H&E) staining for cell necrosis (top), immunohistochemistry (IHC) staining for phosphorylation of histone H2AX (γH2AX) (middle), and 8-hydroxy-2′-deoxyguanosine (8-OHdG) (bottom) in wild-type (WT) vs. Slc39a8(+/neo) liver. Arrowheads denote focal necrosis in Slc39a8(+/neo) hepatocytes. B: representative IHC staining for F4/80 and myeloperoxidase (MPO) in WT vs. Slc39a8(+/neo) liver. C: relative expression levels of the proinflammatory cytokines Tnf, Il1β, and Il6 mRNA in WT (n = 3) vs. Slc39a8(+/neo) (n = 5) liver samples. D: exemplary picrosirius red (PSR) staining for fibrosis in WT vs. Slc39a8(+/neo) liver (top). Relative mRNA levels of Acta2, Tgfb1, and Timp1 (markers of liver fibrosis) in WT (n = 3) vs. Slc39a8(+/neo) (n = 5) liver samples (bottom). Arrowheads indicate IHC-positive staining. Scale bars: H&E and IHC, 50 μm; PSR, 100 μm. Bar graphs represent means ± SE, with 1.0 designated for WT mean expression. *P ≤ 0.05 and **P ≤ 0.01 by Student’s t-test.
Liver injury triggers an inflammatory response, and H&E staining showed prominent immune cell infiltration (Fig. 2B). IHC staining also showed positive staining for F4/80, a macrophage biomarker, and MPO, a granulocyte biomarker (Fig. 2B), consistent with the histological appearance of macrophage and granulocytic immune cells in Slc39a8(+/neo) mice in Fig. 1A. We also found that mRNA levels of the proinflammatory cytokine Tnf, but not Il1b or Il6 (Fig. 2C), are elevated in the tumor nodules. Picrosirius red staining (Fig. 2D) indicating mild fibrotic changes, mostly around the hepatic vein, were identified. Increased expression of Acta2, Tgfb1, and Timp1 mRNA, indicative of fibrosis, were significantly increased in Slc39a8(+/neo) but not WT liver (Fig. 2D).
Collectively, these results demonstrate that chronic low-grade ZIP8 deficiency for 13- to 21-mo-old Slc39a8(+/neo) mice with a regular laboratory chow diet results in substantial liver pathology, including disruption of normal hepatocellular architecture, necrosis, inflammation, and fibrosis. The ultimate result is the typical appearance of HCC-like nodules.
Liver-specific acute ZIP8 knockdown leads to distinctive liver pathology.
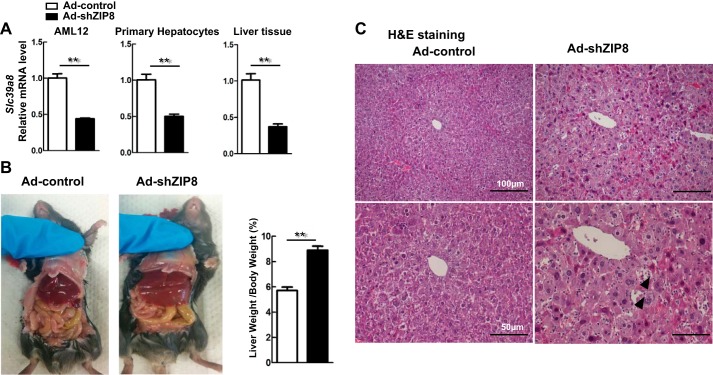
Chronic low-grade ZIP8 deficiency in Slc39a8(+/neo) mice between 13 and 21 mo of age resulted in pathological liver changes, including HCC, and no other tissues appeared macroscopically abnormal. Although unlikely, it is possible that chronic, moderate ZIP8 deficiency causes these liver changes by means of some extrahepatic process. To rule this out, we studied the Ad-shZip8 mouse livers with an acute ZIP8 deficiency for 7 days. Adenovirus reportedly accumulates predominantly in liver tissue, affecting several liver cell types, including hepatocytes, Kupfer cells, and macrophages (50). We tested Ad-shZip8 knockdown efficiency in the mouse-hepatocyte AML12-established cell line and in mouse primary isolated hepatocytes. We found decreases in ZIP8 levels of ~60% following Ad-shZip8 infection in AML12 cells and in primary hepatocyte cultures (Fig. 3A).
Fig. 3.
Liver pathology found in the Ad-shZip8 mouse. A: relative Slc39a8 mRNA expression levels in alpha mouse liver 12 (AML12) cultures, primary hepatocyte cultures, and in liver of intact mice, comparing Ad-shZip8 (n = 8) with Ad-control (n = 6). B: gross anatomical views of Ad-shZip8 vs. Ad-control mice, showing hepatomegaly in the Ad-shZip8 mouse 7 days after adenovirus injection. The ratio of liver-weight-to-body-weight demonstrates this hepatomegaly quantitatively (n = 6 samples for Ad-controls; n = 5 samples for Ad-shZip8). C: comparison of H&E staining in Ad-shZip8 or Ad-control liver. Arrowheads show lymphocytic infiltration. Bar graphs represent means ± SE, with 1.0 designated for mean control expression. *P ≤ 0.05 by Student’s t-test.
Ad-shZip8 mice continued to feed normally, body weight remained unaffected, and their behavior was normal for 1 wk before euthanizing for experiments. Decreases in ZIP8 levels of ~60% were also seen in these intact mice (Fig. 3A). Interestingly, significant hepatomegaly was observed; the ratio of liver-weight-to-body-weight of these animals was ~1.6-fold greater than that of controls (Fig. 3B). Histopathology diagnosis of severe hepatocyte abnormalities in H&E sections includes hepatocytic ballooning changes and severe diffuse inflammatory cell infiltration. Atypical hepatocytes with enlarged hyperchromatic nuclei were also identified (Fig. 3C). These results demonstrated that liver injury, cell death, and inflammation are immediate events following acute ZIP8 downregulation.
Ad-shZip8 knockdown shows increased oxidative stress and cell proliferation.
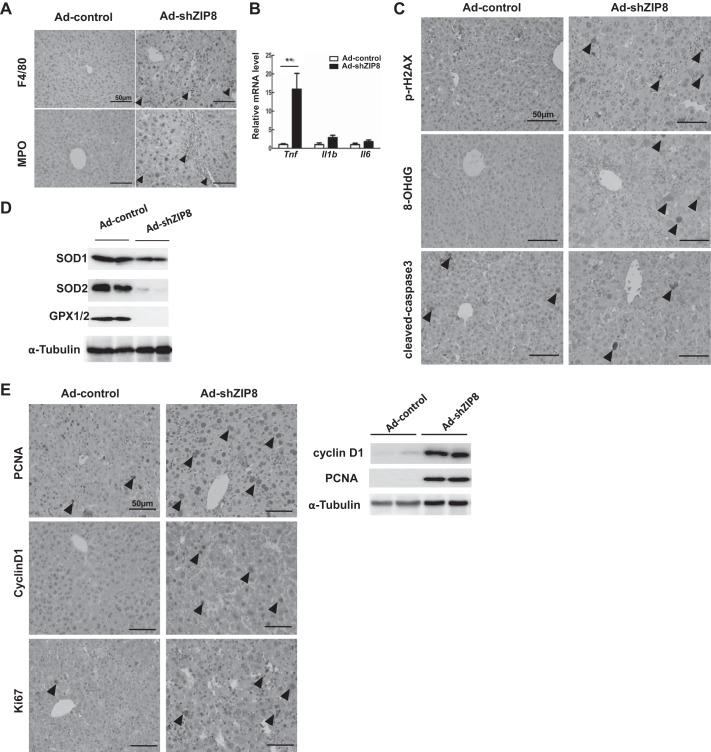
Massive lobular immune-cell infiltration was observed in Ad-shZip8 liver; IHC-staining with F4/80 and MPO showed elevated accumulation of macrophages and granulocytes in these mice (Fig. 4A). Hepatic mRNA expression of inflammatory genes, including Tnf but not Il1β or Il6, was significantly increased (Fig. 4B), which implies that ZIP8 downregulation may exacerbate TNF-associated liver injury in response to adenovirus administration.
Fig. 4.
Hepatic inflammation, DNA damage, oxidative stress, and proliferation in Ad-shZip8 vs. Ad-control mice. A: representative immunohistochemistry (IHC) staining for F4/80 and myeloperoxidase (MPO) in Ad-shZip8 vs. Ad-control liver. B: relative mean expression of the proinflammatory cytokines Tnf, Il1β, and Il6 mRNA in Ad-shZip8 (n = 4 samples) vs. Ad-control (n = 4 samples) liver. C: relative amounts of IHC staining for γH2AX, 8-hydroxy-2′-deoxyguanosine (8-OHdG), and cleaved caspase 3 protein in Ad-shZip8 vs. Ad-control liver. D: expression of SOD1, SOD2, glutathione peroxidase (GPX)1 and GPX2 in Ad-shZip8 vs. Ad-control liver, as determined by Western blot and normalized with α-tubulin. E: IHC staining for proliferating cell nuclear antigen (PCNA), Cyclin D1, and cleaved Ki67 proteins in Ad-shZip8 vs. Ad-control liver (left). Expression of Cyclin D1 and PCNA in Ad-shZip8 and Ad-control liver, as detected by Western blot and normalized with α-tubulin. Arrowheads represent IHC-positive staining. Scale bar: 50 μm. Bar graphs represent means ± SE, with mean expression in controls set to 1.0. *P ≤ 0.05 by Student’s t-test.
Observed inflammation was accompanied by ballooning of hepatocytes, which is a sign of multiple organelle injury and cell death. Cell death, DNA injury, and oxidative stress were examined by using well-established biomarkers (Fig. 4, C and D). We noted an increased number of γH2AX-positive nuclei and 8-OHdG-positive hepatocytes (Fig. 4C), indicating elevated DNA damage and oxidative stress (38, 54). However, no differences in cleaved caspase 3 were seen (Fig. 4C), indicating that apoptosis is not related to ZIP8 knockdown; lack of apoptosis was noted earlier in aged Slc39a8(+/neo) liver. Antioxidant enzymes SOD1, SOD2, GPX1, and GPX2 were all significantly decreased in Ad-shZip8 mice (Fig. 4D), consistent with increased oxidative stress (Fig. 4C). GPX1 and GPX2 are both selenoproteins in which expression is modulated by Se homeostasis.
Because compensatory hepatocyte proliferation is frequently accompanied by cell death, we examined tissue-regenerative responses. Increased levels of PCNA- and cyclin D1-positive hepatocytes, indicative of proliferation, were both significantly upregulated in Ad-shZip8 liver, as shown by IHC and Western blotting (Fig. 4E). These data are consistent with tissue regeneration and proliferation associated with acute ZIP8 deficiency. In addition to hepatocytes, immune cells also undergo proliferation, as shown by positive staining by biomarker Ki67 in nuclei (Fig. 4E).
Collectively, our results demonstrate that normal ZIP8 expression is critically important for liver homeostasis. Decreases in ZIP8 for 7 days enhanced oxidative stress, leading to hepatocellular injury, which subsequently induces massive inflammation as well as compensatory proliferation.
Se and Zn content in Ad-shZip8 tissues.
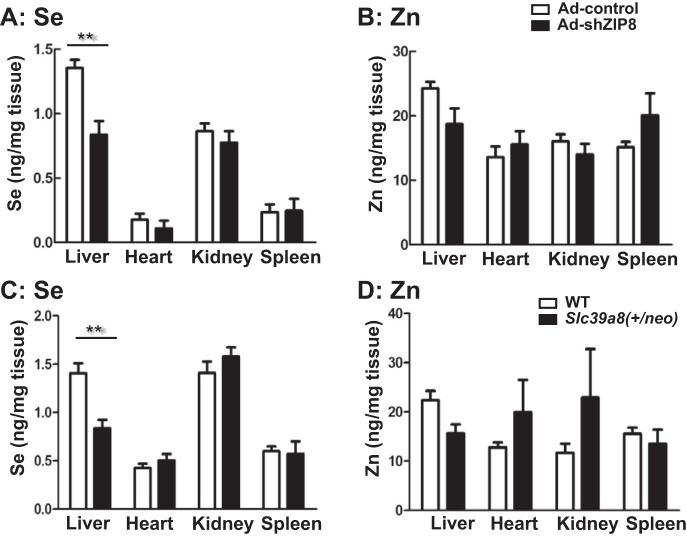
We hypothesized that ZIP8 substrates might be involved in ZIP8-associated liver pathology. We therefore quantified Se and Zn in the liver, heart, kidney, and spleen of the two mouse models: Slc39a8(+/neo) versus WT and Ad-shZip8 versus Ad-control mice (Fig. 5). Interestingly, the most statistically significant difference seen (~38%) was Se content in the liver of Slc39a8(+/neo) and Ad-shZip8 ZIP8 knockdown mice (Fig. 5, A and C). Total Zn content showed no differences between ZIP8 knockdown and control (Fig. 5, B and D).
Fig. 5.
Intracellular selenium (Se) and zinc (Zn) content in four tissues of Ad-shZip8 vs. Ad-control mice and Slc39a8(+/neo) vs. WT mice. Inductively coupled plasma-mass spectrometry (ICP-MS) quantification (ng/mg wet wt tissue) was carried out for total Se (A) and total Zn (B) in the liver, heart, kidney, and spleen of Ad-shZip8 and Ad-control mice. The total Se (C) and total Zn (D) were measured in Slc39a8(+/neo) and WT mice ages 13–21 mo. Bar graphs represent means ± SE, with n = 6 samples. **P ≤ 0.01 by Student’s t-test.
Heart, kidney, and spleen Se and Zn content revealed no significant differences between Ad-shZip8 mice and controls; these results are consistent with the liver-specific properties of adenovirus infection. Our data are consistent with the likely importance of the nutritional mineral Se being associated with ZIP8 deficiency, specifically in liver.
DISCUSSION
In the present study of two mouse models, Slc39a8(+/neo) ages 13–21 mo and Ad-Zip8 knockdown mice, we found that ZIP8 plays a critical role in maintaining normal liver physiology. Mild decreases in whole-body ZIP8 expression were found to be associated with a high incidence of liver nodules; significant elevation of the HCC biomarkers AFP and PCNA is strong evidence of hepatocarcinogenesis. Chronic inflammation is known to precede development of such tumors, and evidence of inflammatory changes was shown.
The ZIP8 decreases seen in Slc39a8(+/neo) liver at ages 13–21 mo were not substantial, which makes it difficult to explain any direct cause-and-effect relationship between ZIP8 deficiency and liver pathology. In fetal and postnatal day 1 Slc39a8(+/neo) liver, a ZIP8 decrease by ~50% was demonstrated (15). It is possible such a deficiency can be slowly compensated for over time by other mechanisms, allowing Se and Zn to return to near-normal WT levels. Ideally, it would have been best to take liver biopsies of Slc39a8(+/neo) versus WT liver as a function of time between 1 wk and 12 mo of age; had this been possible, perhaps we would have been able to see that ZIP8 deficiency in the neonate is slowly compensated for by other redundant divalent-cation uptake transporters. Yet, while this purported compensation is taking place during the first 13 mo of life, the liver tissue has been sufficiently affected by some degree of ZIP8 deficiency such that pathological changes (loss of tissue organization, necrosis, inflammation, fibrosis, and tumorigenesis) have begun irreversibly to occur.
This speculation is not without precedent. Comparing Slc39a8(neo/neo) with Slc39a8(+/+) and performing RNA sequencing analysis on GD 13.5 yolk sac and placenta, as well as GD16.5 fetal liver, kidney, lung, heart, and cerebellum, 27 genes in the solute-carrier (Slc) transporter superfamily (besides Slc39a8) were found to be differentially expressed in one or another tissue; 21 of the 52 known Slc gene families were represented, and none of the 14 genes in the Slc39 family (other than Slc39a8) were differentially expressed (Chen et al., unpublished observations).
On the other hand, during acute ZIP8 downregulation over a 7-day period in Ad-shZip8 mice, we observed hepatomegaly, inflammation, and increased oxidative stress. Hepatomegaly is frequently observed with infection, drug overdose, metabolic disorders, vascular congestion, and biliary obstruction (59). We propose that acute downregulation of ZIP8 stimulates inflammatory changes and aggravates the oxidative stress induced by Se decrease, which leads to hepatomegaly in only 7 days time. Hepatomegaly might also be associated with some other ZIP8 functions, such as alterations in energy metabolism, another observation noted during RNA sequencing analysis (Chen et al., unpublished observations). Ablation of the Slc39a14 gene, evolutionarily the most closely related to Slc39a8, has also been found to be correlated with hepatic glucose metabolism impairment in liver (2).
Intriguingly, in both the Slc39a8(+/neo) and Ad-shZip8 models, inflammation was observed as an outcome of deficient ZIP8 expression. These data are consistent with ZIP8 being highly responsive to the inflammatory stimulus lipopolysaccharide and the proinflammatory cytokines IL1β and TNF (5, 28, 35). ZIP8 upregulation during inflammation can be advantageous or disadvantageous, depending on the tissue and cell type. For example, persistent IL1β-induced elevation of ZIP8 expression in chondrocytes increases matrix metalloproteinase and aggrecanase expression, eventually leading to cartilage degeneration and development of osteoarthritis (28). On the other hand, ZIP8 upregulation in response to pulmonary inflammatory stress protects the lung epithelium against cytotoxic effects of TNF by recruiting intracellular Zn2+ to maintain normal mitochondrial function (5). In addition, ZIP8 was shown to be a negative regulator of the proinflammatory response; Zn deficiency induces excessive inflammation during polymicrobial sepsis (35). In Slc39a8(neo/neo) fetal liver having >80% ZIP8 deficiency, increased CD11b+ cells and inflammatory changes were observed (15). All these results indicate that ZIP8 participates in beneficial and detrimental responses, depending on the tissue and cell type.
The ZIP8 substrates Zn and Se are both involved in anti-inflammatory and antioxidant processes (19, 45). Inflammation-induced upregulation of ZIP8 can accelerate intracellular uptake of Zn and Se to combat inflammatory injury. In our Ad-shZip8 mice, extensive inflammation and oxidative stress were observed, which were not seen in Ad-control mice (Fig. 4). This result is consistent with ZIP8 deficiency being unable to protect hepatocytes against inflammatory stimuli perhaps exacerbated by adenovirus exposure.
The role of hepatic ZIP8 was recently investigated by comparing a liver-specific ZIP8-conditional knockout mouse (ZIP8-LSKO) with a global ZIP8-knockout mouse (ZIP8-iKO) (34). In both lines, ZIP8 liver expression was significantly reduced (98 and 75% decreases in ZIP8-iKO and ZIP8-LSKO, respectively). Surprisingly, the ZIP8 knockout in hepatocytes did not result in any severe pathology; these mice exhibited a significant decrease in manganese (Mn) levels and defects in the activity of Mn-dependent enzymes and protein glycosylation (34).
Why might this ZIP8-LSKO and our Ad-shZip8 mouse show such differences? Although it is possible that some unknown ZIP8 off-target exists in Ad-shZip8 mice to explain the difference, we did not find any evidence of a potential off-target using BLASTN when designing the Ad-shZip8 construct; furthermore, Slc39a8 mRNA was shown to be downregulated in our experiments (Fig. 3A). There are three other possible explanations for the discrepancy between the ZIP8-LSKO and Ad-shZip8 data. 1) differences in the mouse genetic background, 2) differences in liver cell types affected by the Slc39a8 downregulation methodology used, and 3) differences in effects of secondary stress. A liver-specific conditional knockout targets only hepatocytes, whereas adenovirus-delivered shRNA-mediated knockdowns are able to affect hepatocytes, Kupfer cells, and macrophages (50, 52). Malfunction of Kupfer cells and macrophages can alter overall liver function, leading to acute inflammation. While a conditional knockout is achieved solely by genetic modification, adenovirus-delivered shRNA-mediated knockdowns introduce adenovirus, which can act autonomously as an intracellular stress. Since ZIP8 is critically required for normal immune function, any additional viral exposure may aggravate ZIP8 deficiency-induced liver inflammation and injury.
How might ZIP8 functionality be involved in liver pathology? ZIP8 has previously been shown to facilitate uptake of Zn2+, Mn2+, Fe2+, and (HSeO3)−, any or all of which transport processes might be pivotal in regulation of their intracellular levels. For example, Zn2+ content is decreased in Slc39a8(neo/neo) fetal tissues, including liver and heart (15). In liver-specific ZIP8 knockout mouse liver, Zn2+ levels are marginally decreased, whereas Mn levels are significantly decreased (34). In both of these studies, Se levels were not investigated. In the present study, comparing Ad-shZip8 with Ad-controls, we found no significant differences in Zn content (Fig. 5). As mentioned above, return to Zn2+ homeostasis might be achieved by redundancy of other transporters, e.g., ZIP14 (1, 36) and SLC30A10 (ZnT10) (21).
The data presented herein show that in Ad-shZip8 liver, Se content was decreased by ~38%. (Fig. 5). Lowered intracellular levels of Se are expected to contribute to increased oxidative stress, which was observed. Se deficiency was also consistent with significant decreases in GPX1 and GPX2 (Fig. 4D), which are selenoenzymes that act as important antioxidants in liver. GPX1 is an antioxidant selenoprotein and a cellular marker for Se status (10). This suggests an association between ZIP8 deficiency and lowered Se tissue retention, which subsequently results in increased oxidative stress in hepatocytes. Because Se is well known to maintain cell redox balance, Se deficiency is frequently linked to a variety of liver disorders, including chronic AFLD, hepatitis, and hepatocarcinogenesis (7, 20, 33, 53). ZIP8 has been shown to be a major selenite transporter (41), and liver is the principal tissue for Se accumulation. Adenovirus can act as a secondary stress to aggravate Se deficiency in ZIP8 knockdown; under conditions of Se deficiency, exposure to various viruses is believed to introduce secondary stress, for example, to trigger cardiomyopathy pathologies (4). In the present study, SOD1 and especially SOD2 were decreased in Ad-shZip8 liver (Fig. 4D); mitochondrial SOD2 is an Mn-dependent enzyme. This finding might reflect Mn deficiency because of the ZIP8 knockdown, which is consistent with the finding that disrupted ZIP8 function causes increased oxidative stress through reducing the activity of SOD2 (9). The lower SOD enzyme concentrations, along with decreased GPX1 and GPX2, are consistent with increased oxidative stress in Ad-shZip8 liver.
In summary (Fig. 6), the studies herein are consistent with ZIP8 serving as important biometal transporter that helps maintain normal liver physiology. Furthermore, Se, more so than Zn or Mn, appears to be the most likely critical ZIP8 substrate responsible for this correlation.
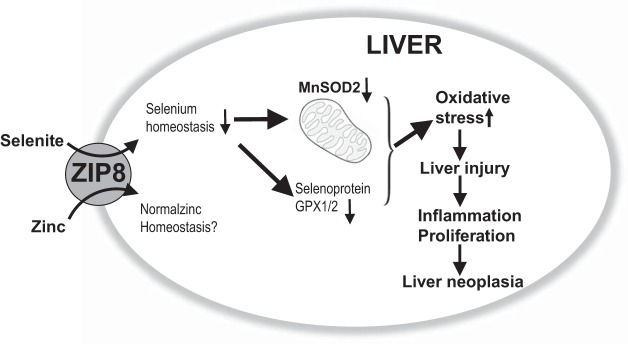
Fig. 6.
Schematic diagram, summarizing what was found in the present study described herein. Zrt/Irt-like Protein 8 (ZIP8) deficiency in Slc39a8(+/neo) mice between 13 and 21 mo of age results in increased oxidative stress, liver injury, inflammation, proliferation, and hepatocellular cancer (HCC) formation. ZIP8 deficiency in Ad-shZip8 mice exhibiting an acute knockdown of Zip8 mRNA results in striking hepatomegaly, decreased SOD2, increased oxidative stress and inflammation within 1 wk after adenovirus injection, and significantly lowered selenium (Se) in hepatocytes whereas zinc (Zn) content appears not to be decreased. GPX, glutathione peroxidase.
GRANTS
This work was supported by the Oakland University Research Excellence Fund and National Institute of Environmental Health Sciences Grant ES-022800 (to Z. Liu) and National Natural Science Foundation of China Grants 81522009/H0317 and 81770588/H0315 (to H. Wang). This work was also supported by Pilot Project Funding from the Herbert Wertheim College of Medicine, Florida International University (to M. Yoshinaga).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.L., H.W., and Z.L. conceived and designed research; L.L., X.G., and M.Y. performed experiments; L.L., Y.C., J.S., D.W.N., H.W., and Z.L. analyzed data; L.L., X.G., B.L.C., and Z.L. interpreted results of experiments; L.L. and Z.L. prepared figures; L.L., J.S., D.W.N., and Z.L. drafted manuscript; L.L., Y.C., M.Y., D.W.N., H.W., and Z.L. edited and revised manuscript; L.L., X.G., Y.C., B.L.C., M.Y., J.S., D.W.N., H.W., and Z.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Joseph R. McDermott (Chinese Academy of Sciences) for critiques and comments. We acknowledge Dr. Liangyou Rui (University of Michigan) for valuable input. We also thank Dr. Sang Rhee (Oakland University) for collegial discussion of this project and Mei Chen (Oakland University) for technical support in animal work.
REFERENCES
- 1.Aydemir TB, Kim M-H, Kim J, Colon-Perez LM, Banan G, Mareci TH, Febo M, Cousins RJ. Metal transporter Zip14 (Slc39a14) deletion in mice increases manganese deposition and produces neurotoxic signatures and diminished motor activity. J Neurosci 37: 5996–6006, 2017. doi: 10.1523/JNEUROSCI.0285-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aydemir TB, Troche C, Kim MH, Cousins RJ. Hepatic ZIP14-mediated zinc transport contributes to endosomal insulin receptor trafficking and glucose metabolism. J Biol Chem 291: 23939–23951, 2016. doi: 10.1074/jbc.M116.748632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bafaro E, Liu Y, Xu Y, Dempski RE. The emerging role of zinc transporters in cellular homeostasis and cancer. Signal Trans Target Ther 2: 17029, 2017. doi: 10.1038/sigtrans.2017.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck MA, Levander OA, Handy J. Selenium deficiency and viral infection. J Nutr 133, Suppl 1: 1463S–1467S, 2003. doi: 10.1093/jn/133.5.1463S. [DOI] [PubMed] [Google Scholar]
- 5.Besecker B, Bao S, Bohacova B, Papp A, Sadee W, Knoell DL. The human zinc transporter SLC39A8 (Zip8) is critical in zinc-mediated cytoprotection in lung epithelia. Am J Physiol Lung Cell Mol Physiol 294: L1127–L1136, 2008. doi: 10.1152/ajplung.00057.2008. [DOI] [PubMed] [Google Scholar]
- 6.Boycott KM, Beaulieu CL, Kernohan KD, Gebril OH, Mhanni A, Chudley AE, Redl D, Qin W, Hampson S, Küry S, Tetreault M, Puffenberger EG, Scott JN, Bezieau S, Reis A, Uebe S, Schumacher J, Hegele RA, McLeod DR, Gálvez-Peralta M, Majewski J, Ramaekers VT, Nebert DW, Innes AM, Parboosingh JS, Abou Jamra R; Care4Rare Canada Consortium . Autosomal-recessive intellectual disability with cerebellar atrophy syndrome caused by mutation of the manganese and zinc transporter gene SLC39A8. Am J Hum Genet 97: 886–893, 2015. doi: 10.1016/j.ajhg.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burk RF, Early DS, Hill KE, Palmer IS, Boeglin ME. Plasma selenium in patients with cirrhosis. Hepatology 27: 794–798, 1998. doi: 10.1002/hep.510270322. [DOI] [PubMed] [Google Scholar]
- 8.Chasapis CT, Loutsidou AC, Spiliopoulou CA, Stefanidou ME. Zinc and human health: an update. Arch Toxicol 86: 521–534, 2012. doi: 10.1007/s00204-011-0775-1. [DOI] [PubMed] [Google Scholar]
- 9.Choi EK, Nguyen TT, Gupta N, Iwase S, Seo YA. Functional analysis of SLC39A8 mutations and their implications for manganese deficiency and mitochondrial disorders. Sci Rep 8: 3163, 2018. doi: 10.1038/s41598-018-21464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Combs GF., Jr Biomarkers of selenium status. Nutrients 7: 2209–2236, 2015. doi: 10.3390/nu7042209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costello LC, Franklin RB. The status of zinc in the development of hepatocellular cancer: an important, but neglected, clinically established relationship. Cancer Biol Ther 15: 353–360, 2014. doi: 10.4161/cbt.27633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curran JE, Jowett JB, Elliott KS, Gao Y, Gluschenko K, Wang J, Abel Azim DM, Cai G, Mahaney MC, Comuzzie AG, Dyer TD, Walder KR, Zimmet P, MacCluer JW, Collier GR, Kissebah AH, Blangero J. Genetic variation in selenoprotein S influences inflammatory response. Nat Genet 37: 1234–1241, 2005. doi: 10.1038/ng1655. [DOI] [PubMed] [Google Scholar]
- 13.Fall T, Ingelsson E. Genome-wide association studies of obesity and metabolic syndrome. Mol Cell Endocrinol 382: 740–757, 2014. doi: 10.1016/j.mce.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Franklin RB, Levy BA, Zou J, Hanna N, Desouki MM, Bagasra O, Johnson LA, Costello LC. ZIP14 zinc transporter downregulation and zinc depletion in the development and progression of hepatocellular cancer. J Gastrointest Cancer 43: 249–257, 2012. doi: 10.1007/s12029-011-9269-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gálvez-Peralta M, He L, Jorge-Nebert LF, Wang B, Miller ML, Eppert BL, Afton S, Nebert DW. ZIP8 zinc transporter: indispensable role for both multiple-organ organogenesis and hematopoiesis in utero. PLoS One 7: e36055, 2012. doi: 10.1371/journal.pone.0036055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao B, Jeong WI, Tian Z. Liver: An organ with predominant innate immunity. Hepatology 47: 729–736, 2008. doi: 10.1002/hep.22034. [DOI] [PubMed] [Google Scholar]
- 17.Grüngreiff K, Reinhold D, Wedemeyer H. The role of zinc in liver cirrhosis. Ann Hepatol 15: 7–16, 2016. [DOI] [PubMed] [Google Scholar]
- 18.He L, Girijashanker K, Dalton TP, Reed J, Li H, Soleimani M, Nebert DW. ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: characterization of transporter properties. Mol Pharmacol 70: 171–180, 2006. doi: 10.1124/mol.106.024521. [DOI] [PubMed] [Google Scholar]
- 19.Huang Z, Rose AH, Hoffmann PR. The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 16: 705–743, 2012. doi: 10.1089/ars.2011.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes DJ, Duarte-Salles T, Hybsier S, Trichopoulou A, Stepien M, Aleksandrova K, Overvad K, Tjønneland A, Olsen A, Affret A, Fagherazzi G, Boutron-Ruault MC, Katzke V, Kaaks R, Boeing H, Bamia C, Lagiou P, Peppa E, Palli D, Krogh V, Panico S, Tumino R, Sacerdote C, Bueno-de-Mesquita HB, Peeters PH, Engeset D, Weiderpass E, Lasheras C, Agudo A, Sánchez MJ, Navarro C, Ardanaz E, Dorronsoro M, Hemmingsson O, Wareham NJ, Khaw KT, Bradbury KE, Cross AJ, Gunter M, Riboli E, Romieu I, Schomburg L, Jenab M. Prediagnostic selenium status and hepatobiliary cancer risk in the European Prospective Investigation into Cancer and Nutrition cohort. Am J Clin Nutr 104: 406–414, 2016. doi: 10.3945/ajcn.116.131672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutchens S, Liu C, Jursa T, Shawlot W, Chaffee BK, Yin W, Gore AC, Aschner M, Smith DR, Mukhopadhyay S. Deficiency in the manganese efflux transporter SLC30A10 induces severe hypothyroidism in mice. J Biol Chem 292: 9760–9773, 2017. doi: 10.1074/jbc.M117.783605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. International Consortium for Blood Pressure Genome-Wide Association Studies; Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, Smith AV, Tobin MD, Verwoert GC, Hwang SJ, Pihur V, Vollenweider P, O’Reilly PF, . et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478: 103–109, 2011. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwata K, Enomoto H, Nishiguchi S, Aizawa N, Sakai Y, Iwata Y, Tanaka H, Ikeda N, Takashima T, Saito M, Imanishi H, Iijima H, Tsuda Y, Higuchi K. Serum zinc value in patients with hepatitis virus-related chronic liver disease: association with the histological degree of liver fibrosis and with the severity of varices in compensated cirrhosis. J Clin Biochem Nutr 55: 147–152, 2014. doi: 10.3164/jcbn.14-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenkitkasemwong S, Wang CY, Mackenzie B, Knutson MD. Physiologic implications of metal-ion transport by ZIP14 and ZIP8. Biometals 25: 643–655, 2012. doi: 10.1007/s10534-012-9526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeong J, Eide DJ. The SLC39 family of zinc transporters. Mol Aspects Med 34: 612–619, 2013. doi: 10.1016/j.mam.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang X, Song Z, McClain CJ, Kang YJ, Zhou Z. Zinc supplementation enhances hepatic regeneration by preserving hepatocyte nuclear factor-4α in mice subjected to long-term ethanol administration. Am J Pathol 172: 916–925, 2008. doi: 10.2353/ajpath.2008.070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang X, Zhong W, Liu J, Song Z, McClain CJ, Kang YJ, Zhou Z. Zinc supplementation reverses alcohol-induced steatosis in mice through reactivating hepatocyte nuclear factor-4α and peroxisome proliferator-activated receptor-α. Hepatology 50: 1241–1250, 2009. doi: 10.1002/hep.23090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JH, Jeon J, Shin M, Won Y, Lee M, Kwak JS, Lee G, Rhee J, Ryu JH, Chun CH, Chun JS. Regulation of the catabolic cascade in osteoarthritis by the zinc-ZIP8-MTF1 axis. Cell 156: 730–743, 2014. doi: 10.1016/j.cell.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Kraja AT, Chasman DI, North KE, Reiner AP, Yanek LR, Kilpeläinen TO, Smith JA, Dehghan A, Dupuis J, Johnson AD, . et al. Pleiotropic genes for metabolic syndrome and inflammation. Mol Genet Metab 112: 317–338, 2014. doi: 10.1016/j.ymgme.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leighton D, Goua M, Dolan E, Burgess K, Bermano G. Can selenium supplementation modify oxidative stress in-vitro? A role for selenium supplementation in the prevention of cardiovascular disease. J Inflamm 12, Suppl 1: 7, 2015. doi: 10.1186/1476-9255-12-S1-P7. [DOI] [Google Scholar]
- 31.Li D, Achkar JP, Haritunians T, Jacobs JP, Hui KY, D’Amato M, Brand S, Radford-Smith G, Halfvarson J, Niess JH, Kugathasan S, Büning C, Schumm LP, Klei L, Ananthakrishnan A, Aumais G, Baidoo L, Dubinsky M, Fiocchi C, Glas J, Milgrom R, Proctor DD, Regueiro M, Simms LA, Stempak JM, Targan SR, Törkvist L, Sharma Y, Devlin B, Borneman J, Hakonarson H, Xavier RJ, Daly M, Brant SR, Rioux JD, Silverberg MS, Cho JH, Braun J, McGovern DP, Duerr RH. A pleiotropic missense variant in SLC39A8 is associated with Crohn’s disease and human gut microbiome composition. Gastroenterology 151: 724–732, 2016. doi: 10.1053/j.gastro.2016.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li M, Zhang Y, Liu Z, Bharadwaj U, Wang H, Wang X, Zhang S, Liuzzi JP, Chang S-M, Cousins RJ, Fisher WE, Brunicardi FC, Logsdon CD, Chen C, Yao Q. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc Natl Acad Sci USA 104: 18636–18641, 2007. doi: 10.1073/pnas.0709307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin CC, Huang JF, Tsai LY, Huang YL. Selenium, iron, copper, and zinc levels and copper-to-zinc ratios in serum of patients at different stages of viral hepatic diseases. Biol Trace Elem Res 109: 15–24, 2006. doi: 10.1385/BTER:109:1:015. [DOI] [PubMed] [Google Scholar]
- 34.Lin W, Vann DR, Doulias P-T, Wang T, Landesberg G, Li X, Ricciotti E, Scalia R, He M, Hand NJ, Rader DJ. Hepatic metal ion transporter ZIP8 regulates manganese homeostasis and manganese-dependent enzyme activity. J Clin Invest 127: 2407–2417, 2017. doi: 10.1172/JCI90896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu M-J, Bao S, Gálvez-Peralta M, Pyle CJ, Rudawsky AC, Pavlovicz RE, Killilea DW, Li C, Nebert DW, Wewers MD, Knoell DL. ZIP8 regulates host defense through zinc-mediated inhibition of NF-κB. Cell Reports 3: 386–400, 2013. doi: 10.1016/j.celrep.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liuzzi JP, Lichten LA, Rivera S, Blanchard RK, Aydemir TB, Knutson MD, Ganz T, Cousins RJ. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci USA 102: 6843–6848, 2005. doi: 10.1073/pnas.0502257102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, Croteau-Chonka DC, Esko T, Fall T, . et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 518: 197–206, 2015. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mah LJ, El-Osta A, Karagiannis TC. gammaH2AX: a sensitive molecular marker of DNA damage and repair. Leukemia 24: 679–686, 2010. doi: 10.1038/leu.2010.6. [DOI] [PubMed] [Google Scholar]
- 39.Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology 134: 1641–1654, 2008. doi: 10.1053/j.gastro.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mantena SK, King AL, Andringa KK, Eccleston HB, Bailey SM. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol- and obesity-induced fatty liver diseases. Free Radic Biol Med 44: 1259–1272, 2008. doi: 10.1016/j.freeradbiomed.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDermott JR, Geng X, Jiang L, Gálvez-Peralta M, Chen F, Nebert DW, Liu Z. Zinc- and bicarbonate-dependent ZIP8 transporter mediates selenite uptake. Oncotarget 7: 35327–35340, 2016. doi: 10.18632/oncotarget.9205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Milman N, Laursen J, Pødenphant J, Asnaes S. Trace elements in normal and cirrhotic human liver tissue. I. Iron, copper, zinc, selenium, manganese, titanium and lead measured by X-ray fluorescence spectrometry. Liver 6: 111–117, 1986. doi: 10.1111/j.1600-0676.1986.tb00276.x. [DOI] [PubMed] [Google Scholar]
- 43.Mousavi SN, Faghihi A, Motaghinejad M, Shiasi M, Imanparast F, Amiri HL, Shidfar F. Zinc and selenium co-supplementation reduces some lipid peroxidation and angiogenesis markers in a rat model of NAFLD-fed high fat diet. Biol Trace Elem Res 181: 288–295, 2018. doi: 10.1007/s12011-017-1059-2. [DOI] [PubMed] [Google Scholar]
- 44.Park JH, Hogrebe M, Grüneberg M, DuChesne I, von der Heiden AL, Reunert J, Schlingmann KP, Boycott KM, Beaulieu CL, Mhanni AA, Innes AM, Hörtnagel K, Biskup S, Gleixner EM, Kurlemann G, Fiedler B, Omran H, Rutsch F, Wada Y, Tsiakas K, Santer R, Nebert DW, Rust S, Marquardt T. SLC39A8 deficiency: A disorder of manganese transport and glycosylation. Am J Hum Genet 97: 894–903, 2015. doi: 10.1016/j.ajhg.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prasad AS. Zinc is an antioxidant and anti-inflammatory agent: its role in human health. Front Nutr 1: 14, 2014. doi: 10.3389/fnut.2014.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Read SA, O’Connor KS, Suppiah V, Ahlenstiel CL, Obeid S, Cook KM, Cunningham A, Douglas MW, Hogg PJ, Booth D, George J, Ahlenstiel G. Zinc is a potent and specific inhibitor of IFN-λ3 signalling. Nat Commun 8: 15245, 2017. doi: 10.1038/ncomms15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson MW, Harmon C, O’Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol 13: 267–276, 2016. doi: 10.1038/cmi.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roman M, Jitaru P, Barbante C. Selenium biochemistry and its role for human health. Metallomics 6: 25–54, 2014. doi: 10.1039/C3MT00185G. [DOI] [PubMed] [Google Scholar]
- 49.Severgnini M, Sherman J, Sehgal A, Jayaprakash NK, Aubin J, Wang G, Zhang L, Peng CG, Yucius K, Butler J, Fitzgerald K. A rapid two-step method for isolation of functional primary mouse hepatocytes: cell characterization and asialoglycoprotein receptor based assay development. Cytotechnology 64: 187–195, 2012. doi: 10.1007/s10616-011-9407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shayakhmetov DM, Li ZY, Ni S, Lieber A. Analysis of adenovirus sequestration in the liver, transduction of hepatic cells, and innate toxicity after injection of fiber-modified vectors. J Virol 78: 5368–5381, 2004. doi: 10.1128/JVI.78.10.5368-5381.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun Q, Zhong W, Zhang W, Li Q, Sun X, Tan X, Sun X, Dong D, Zhou Z. Zinc deficiency mediates alcohol-induced apoptotic cell death in the liver of rats through activating ER and mitochondrial cell death pathways. Am J Physiol Gastrointest Liver Physiol 308: G757–G766, 2015. doi: 10.1152/ajpgi.00442.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tao N, Gao GP, Parr M, Johnston J, Baradet T, Wilson JM, Barsoum J, Fawell SE. Sequestration of adenoviral vector by Kupffer cells leads to a nonlinear dose response of transduction in liver. Mol Ther 3: 28–35, 2001. doi: 10.1006/mthe.2000.0227. [DOI] [PubMed] [Google Scholar]
- 53.Thuluvath PJ, Triger DR. Selenium in chronic liver disease. J Hepatol 14: 176–182, 1992. doi: 10.1016/0168-8278(92)90155-I. [DOI] [PubMed] [Google Scholar]
- 54.Valavanidis A, Vlachogianni T, Fiotakis C. 8-Hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Science Health C Environ Carcinog Ecotoxicol Rev 27: 120–139, 2009. doi: 10.1080/10590500902885684. [DOI] [PubMed] [Google Scholar]
- 55.Wang B, He L, Dong H, Dalton TP, Nebert DW. Generation of a Slc39a8 hypomorph mouse: markedly decreased ZIP8 Zn2+/(HCO3−)2 transporter expression. Biochem Biophys Res Commun 410: 289–294, 2011. doi: 10.1016/j.bbrc.2011.05.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang B, Schneider SN, Dragin N, Girijashanker K, Dalton TP, He L, Miller ML, Stringer KF, Soleimani M, Richardson DD, Nebert DW. Enhanced cadmium-induced testicular necrosis and renal proximal tubule damage caused by gene-dose increase in a Slc39a8-transgenic mouse line. Am J Physiol Cell Physiol 292: C1523–C1535, 2007. doi: 10.1152/ajpcell.00409.2006. [DOI] [PubMed] [Google Scholar]
- 57.Wang CY, Jenkitkasemwong S, Duarte S, Sparkman BK, Shawki A, Mackenzie B, Knutson MD. ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading. J Biol Chem 287: 34032–34043, 2012. doi: 10.1074/jbc.M112.367284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, Ganna A, Chen J, Buchkovich ML, Mora S, Beckmann JS, Bragg-Gresham JL, . et al. Discovery and refinement of loci associated with lipid levels. Nat Genet 45: 1274–1283, 2013. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolf AD, Lavine JE. Hepatomegaly in neonates and children. Pediatr Rev 21: 303–310, 2000. doi: 10.1542/pir.21-9-303. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Z, Gao X, Cao Y, Jiang H, Wang T, Song X, Guo M, Zhang N. Selenium deficiency facilitates inflammation through the regulation of TLR4 and TLR4-related signaling pathways in the mice uterus. Inflammation 38: 1347–1356, 2015. doi: 10.1007/s10753-014-0106-9. [DOI] [PubMed] [Google Scholar]