Abstract
Bile acid transporters, including the ileal apical sodium-dependent bile acid transporter (ASBT) and the hepatic sodium-taurocholate cotransporting polypeptide (NTCP), are crucial for the enterohepatic circulation of bile acids. Our objective was to develop a method for measuring bile acid transporter activity in real time to precisely evaluate rapid changes in their function. We designed a reporter system relying on a novel probe: cholic acid attached to luciferin via a disulfide-containing, self-immolating linker (CA-SS-Luc). Incubation of human embryonic kidney-293 cells coexpressing luciferase and ASBT with different concentrations of CA-SS-Luc (0.01–1 μM) resulted in bioluminescence with an intensity that was concentration- and time-dependent. The bioluminescence measured during incubation with 1 μM CA-SS-Luc was dependent on the levels of ASBT or NTCP expressed in the cells. Coincubation of CA-SS-Luc with natural bile acids enhanced the bioluminescence in a concentration-dependent manner with kinetic parameters for ASBT similar to those previously reported using conventional methods. These findings suggest that this method faithfully assesses ASBT function. Further, incubation with tyrosine phosphatase inhibitor III (PTPIII) led to significantly increased bioluminescence in cells expressing ASBT, consistent with previous studies showing an increase in ASBT function by PTPIII. We then investigated CA-SS-Luc in isolated mouse intestinal epithelial cells. Ileal enterocytes displayed significantly higher luminescence compared with jejunal enterocytes, indicating a transport process mediated by ileal ASBT. In conclusion, we have developed a novel method to monitor the activity of bile acid transporters in real time that has potential applications both for in vitro and in vivo studies.
NEW & NOTEWORTHY This article reports the development of a real-time method for measuring the uptake of bile acids using a bioluminescent bile acid-based probe. This method has been validated for measuring uptake via the apical sodium-dependent bile acid transporter and the sodium-taurocholate cotransporting polypeptide in cell culture and ex vivo intestinal models.
Keywords: bile acids, bile acid transport, intestinal epithelial cell
INTRODUCTION
Bile acids (BAs) are synthesized in the liver from cholesterol and secreted into the small intestine, where they solubilize lipids and fat-soluble vitamins to facilitate their absorption. To prevent excessive loss of bile acids in the stool, the apical sodium-dependent bile acid transporter (ASBT; SLC10A2) actively reabsorbs ∼95% of bile acids in the distal ileum. These bile acids are returned to the liver via the portal circulation and actively transported into the hepatocyte via the Na+-taurocholate cotransporting polypeptide (NTCP; SLC10A1). Reabsorbed bile acids are subsequently reexcreted into the bile, establishing the enterohepatic circulation of bile acids. Thus, ASBT and NTCP are critical for maintaining the enterohepatic circulation and ensuring bile acid homeostasis (2).
Changes in ASBT or NTCP function lead to disturbances in bile acid homeostasis and are implicated in the pathophysiology of several intestinal and hepatic diseases. For instance, inactivating mutations in ASBT are the cause of primary bile acid diarrhea, which is characterized by excessive loss of bile acids in the stool, lipid malabsorption, and steatorrhea that requires bile acid supplementation (24, 35). Furthermore, decreased expression of ASBT was associated with the development of colonic BA accumulation and subsequent colitis-associated cancer in mice (9). Studies have also shown that enhanced ASBT function is associated with several diseases. Indeed, an increase in ASBT has been implicated in the pathophysiology of diabetes mellitus (3, 11), necrotizing enterocolitis (17), and progressive familial intrahepatic cholestasis (10). Moreover, ASBT inhibitors were shown to improve glucose tolerance in models of diabetes mellitus (11), attenuate disease severity of necrotizing enterocolitis (NEC) (18), and reduce liver damage in cases of nonalcoholic steatohepatitis (NASH) (27). In the same respect, dysregulation of NTCP has been implicated in several liver diseases, including cholestasis (2), and inhibition of NTCP may be of interest in the treatment of cholestatic diseases (30). In light of their essential roles in the normal physiology of bile acids, there has been an immense interest in investigating the cellular mechanisms involved in the regulation of ASBT and NTCP function and expression.
Because bile acid secretion in response to food intake causes rapid changes in local bile acid concentrations throughout the enterohepatic system, the function of bile acid transport proteins must acutely adapt to ensure efficient enterohepatic circulation in response to this rapidly changing milieu (2, 12). Indeed, recent evidence has demonstrated that the activities of ASBT and NTCP are rapidly regulated by signaling pathways and changes in plasma membrane microdomains. For instance, ASBT activity is subject to rapid posttranslational modulation by protein tyrosine phosphatase (4). Furthermore, both ASBT and NTCP functions were shown to be modulated by protein kinase C signaling pathways (28, 31) and by changes in plasma membrane lipid rafts (5, 22). Therefore, it is essential to capture the rapid changes in ASBT and NTCP function to fully understand their acute regulation by cellular signaling pathways.
The conventional methodology to assess the transport function of ASBT and other bile acid transporters (BATs) relies mainly on measuring the uptake of radiolabeled bile acids. Using this method to study the effect of a given treatment requires cells to first be treated and then incubated with radiolabeled bile acids, washed to remove excess bile acids, lysed, and counted by scintillation. Because this process allows for measurement of the transport function only at the end of a treatment, rapid changes in BAT function may not be fully captured by this technique. For in vivo use, the delay between treatment and radioactive uptake also makes these studies impractical for measuring rapid posttranslational regulation of ASBT or NTCP in vivo. Thus, development of a real-time method for assessment of BAT function in living systems is necessary to sufficiently study dynamic changes in activity.
Although several attempts have been made to monitor bile acids in real time (16, 33), none have been designed to assess bile acid transporter function directly. Recently, bioluminescence-based methods have been used to measure the real-time function of fatty acid transporters (19, 26). Therefore, our objective was to develop a bioluminescence-based method for measuring bile acid transport activity in real time to precisely evaluate the rapid changes in their function. In this study, we describe a method that relies on a novel probe: cholic acid attached to luciferin via a disulfide-containing, self-immolating linker (CA-SS-Luc). The probe is designed such that upon entry into cells expressing luciferase, the linker is cleaved to release free luciferin, generating light that can be measured by a sensitive in vivo imaging system (IVIS). This quantitative approach allows for sensitive measurement of ASBT and NTCP function in vitro, and further development will confirm its in vivo utility.
MATERIALS AND METHODS
Cell culture and materials.
The human embryonic kidney fibroblast cell line (HEK-293) was obtained from ATCC and grown in plastic flasks at 37°C in an atmosphere consisting of 5% CO2-95% room air. The cells were cultured in Dulbecco’s modified Eagle’s medium containing 4 mM l-glutamine, 4.5 g/l glucose, 1 mM sodium pyruvate, and 1.5 g/l sodium bicarbonate supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 mg/ml gentamicin (Invitrogen). HEK-293 cells stably transfected with human ASBT-V5 (2BT) were cultured in the same media containing 7 μg/ml puromycin. All chemicals were obtained from Sigma-Aldrich unless otherwise specified.
Plasmid construction.
Human ASBT cDNA was previously generated in our laboratory (5), and human NTCP cDNA was purchased from Origene. These cDNAs were amplified by PCR using the primers 5′-GATTACGCGGCCGCACCATGGCCAATGATCCGAACAGC-3′ and 5′-GATTACCTCGAGCTTTTCGTCAGGTTGAAATC-3′ for ASBT amplification and 5′-GATTACGCGGCCGCACCATGGCCGAGGCCCACAACG-3′ and 5′-GATTACCTCGAGGGCTGTGCAAGGGGAG-3′ for NTCP amplification. PCR products were digested using NotI and XhoI restriction enzymes (New England Biolabs) and ligated into the vector pSF-CMV-Puro-COOH-TEV-V5 (Oxford Genetics) using T4 DNA Ligase (New England Biolabs).
Measurement of CA-SS-Luc bioluminescence.
Transfections were performed using Lipofectamine 2000 (Invitrogen), following the manufacturer’s instructions. 2BT cells, which stably express ASBT-V5, were transfected with 1 μg/well pmirGLO (Promega) for the expression of luciferase and plated on 24-well TC-treated black plastic plates with glass bottoms (Perkin-Elmer) at a density of 2 × 105 cells/well. Wild-type HEK-293 cells were cotransfected with 500 ng/well pmirGLO and 1) 750 ng/well ASBT-V5 or NTCP-V5, 2) 250 ng/well ASBT-V5 or NTCP-V5 and 500 ng/well serotonin transporter (SERT-V5), or 3) 750 ng/well SERT-V5. Media was changed the next day, and experiments were performed on the 2nd day following transfection. Cells were washed twice at 25°C with uptake buffer containing (in mM) 110 NaCl (with sodium) or choline chloride (without sodium), 4 KCl, 1 MgSO4, 1 CaCl2, 45 mannitol, 5 glucose, and 10 HEPES (pH 7.4). Cells were then incubated with the same buffer containing CA-SS-Luc (1 μM, unless otherwise specified) and immediately placed into a Xenogen IVIS Spectrum in vivo imaging system (Caliper Life Sciences). Bioluminescence production was measured using the IVIS camera set to obtain images with 1-min exposure times unless otherwise specified. The luminescence produced from each well was quantified using Living Image software (Perkin-Elmer). Images were either taken directly from this software or further processed using ImageJ [National Institutes of Health (NIH)].
Western blot analysis.
Cells were lysed in cell lysis buffer (Cell Signaling Technology) supplemented with protease inhibitor cocktail (Roche) and 1% SDS. Protein samples were prepared in Laemmli buffer (Bio-Rad) containing 2.5% 2-mercaptoethanol, subjected to SDS-PAGE on 10% polyacrylamide gel, transferred to nitrocellulose membranes, and probed with anti-V5-HRP antibodies (Invitrogen) or anti-ASBT antibodies (a generous gift from Dr. Paul Dawson, Emory University).
Treatment with PTPIII.
2BT cells were transfected with pmirGLO and plated as described previously. On the day of the experiment, cells were treated with protein tyrosine phosphatase inhibitor III (PTPIII; 500 μM; Millipore) or DMSO for 60 min before imaging. Bioluminescence was measured as described earlier.
Isolation of primary intestinal epithelial cells.
Transgenic mice expressing luciferase [LucTg;FVB-Tg(CAG-luc,-GFP)L2G85Chco/J0] were obtained from The Jackson Laboratory and housed in the animal facility at the Jesse Brown Veterans Affairs (VA) Medical Center according to an approved Institutional Animal Care and Use Committee protocol. At age 10–16 wk, male and female mice were euthanized, and epithelial cells from the jejunum and ileum were isolated as previously described (3). Briefly, the small intestine was removed and flushed, and the proximal (jejunum) and distal (ileum) 5 cm were cut into small pieces and washed with ice-cold PBS. The intestines from five animals were pooled for each experiment. Intestinal pieces were then washed in Ringer’s solution without calcium or magnesium and supplemented with 2% BSA and 2% glucose. Tissues were then incubated with shaking at 37°C in Ringer’s solution supplemented with 0.5 mM DTT and 1.5 mM EDTA for 15 min. Cells were collected with centrifugation at 500 g for 5 min, resuspended in uptake buffer containing 1 μM CA-SS-Luc, and imaged by IVIS with 5-min camera exposure.
Statistical analysis.
Results were expressed as means ± SE of three to four experiments performed on multiple occasions. Student’s t-test or two-way ANOVA was used for statistical analysis. P ≤ 0.05 was considered statistically significant.
RESULTS
To develop a method for the assessment of bile acid transporter activity in real time, we have designed a reporter system relying on a novel bioluminescent probe: CA-SS-Luc. Upon entry into the cells, a two-step process initiated by intracellular glutathione results in the release of free luciferin, the oxidation of which will be catalyzed by luciferase expressed in the cells to generate light, as shown in Fig. 1.
Fig. 1.
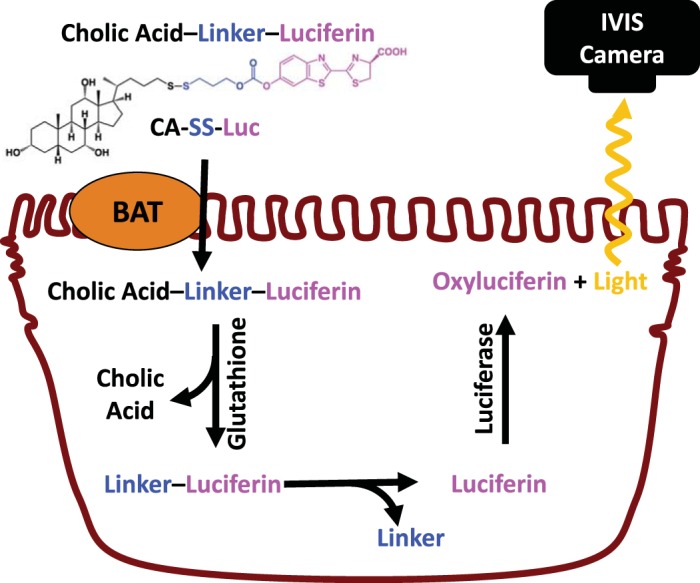
Schematic of luminescence production by CA-SS-Luc. CA-SS-Luc enters cells via a bile acid transporter (BAT). Intracellular glutathione cleaves the disulfide linker, which self-immolates, yielding free luciferin. Luciferase oxidizes luciferin, producing light that is measured by an in vivo imaging system (IVIS).
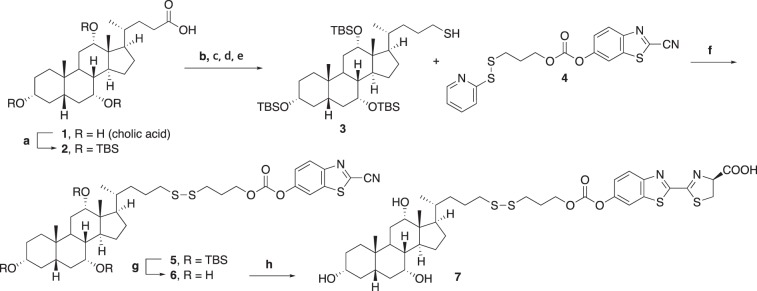
Synthesis of CA-SS-Luc.
The abbreviated synthetic route for the disulfide-based probe CA-SS-Luc compound 7 is shown in Fig. 2. Full details regarding the synthesis are provided in the supplemental information (Supplemental Material for this article can be found on the AJP-Gastrointestinal and Liver Physiology website). The synthesis began with protection of three hydroxyl groups of cholic acid compound 1 with tert-butyldimethylsilyl (TBS) groups to generate compound compound 2. Compound 2 was subjected to reduction of the carboxylic acid moiety, iodination, substitution with KSCN, and reduction of the thiocyanide moiety to generate the thiol compound 3. Sulfide exchange of compound 4 with compound 3 proceeded smoothly to generate compound 5. Removal of TBS groups followed by treatment of compound 6 with d-cysteine provided CA-SS-Luc compound 7.
Fig. 2.
Synthesis of CA-SS-Luc. tert-Butyldimethylsilylchloride (TBSCl), imidazole, dimethylformamide (DMF), reflux 10 h (a); LiAlH4, Et2O, 0°C to rt, 3 h (b); I2, PPh3, pyridine, CH2Cl2, 0°C to rt, 1 h (c); KSCN, acetone, rt, overnight (d); LiAlH4, Et2O, 0°C to rt, 5 h (e); Et3N, DMF, rt, 2 h (f); p-Toluenesulfonic acid (p-TsOH), MeOH, rt, overnight (g); d-cysteine, K2CO3, DCM, MeOH, H2O, rt, 5 min (h).
Transporter dependence of CA-SS-Luc uptake.
We examined the hypothesis that the putative CA-SS-Luc probe will be transported into cells specifically via a bile acid transporter, producing bioluminescence that is proportional to the amount of probe entering the cells over a given period of time. We first tested the hypothesis in HEK-293 cells stably transfected with the ileal ASBT fused to a COOH-terminal V5 tag (2BT cells) and transiently transfected with luciferase. Forty-eight hours posttransfection with luciferase, these cells were incubated with different concentrations of CA-SS-Luc probe and then continuously imaged by IVIS for 45 min. As shown in Fig. 3, the detected bioluminescence was found to be proportional to the concentration of CA-SS-Luc. Furthermore, the luminescent signal was time dependent and reached equilibrium after ∼20 min of incubation, indicating that the signal likely represents a transport activity. Because a robust signal intensity was generated by 1 μM of CA-SS-Luc, all subsequent experiments were performed using this concentration of probe.
Fig. 3.
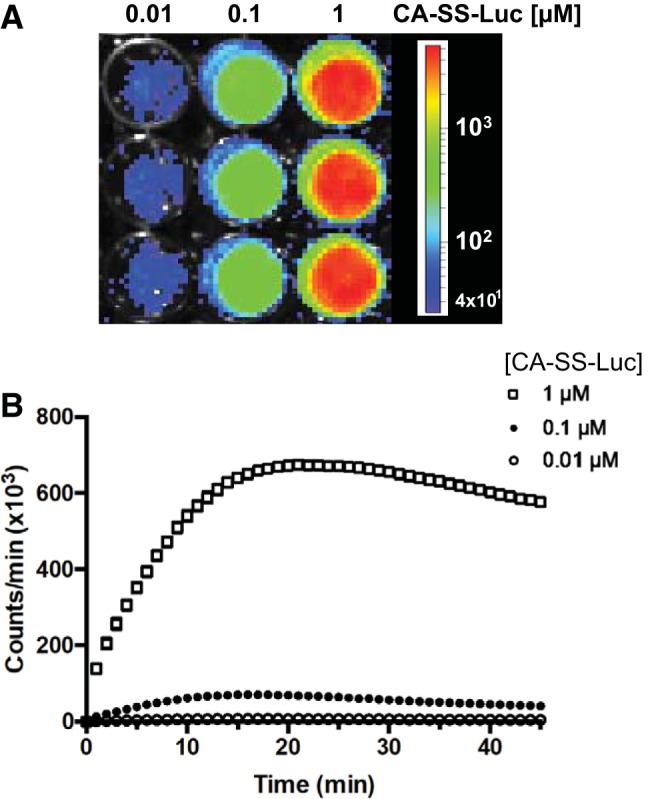
The bioluminescence generated by CA-SS-Luc in 2BT cells is concentration and time dependent. 2BT cells treated with increasing concentrations of CA-SS-Luc were imaged using an in vivo imaging system (IVIS). A: a representative IVIS image following 20-min incubation. B: time course of luminescence production by 0.01 (○), 0.1 (●), and 1 μM (□) of CA-SS-Luc; n = 3. Error bars representing SE are displayed but are too small to be seen for most points. Two-way ANOVA: time (P < 0.0001) and dose (P < 0.0001).
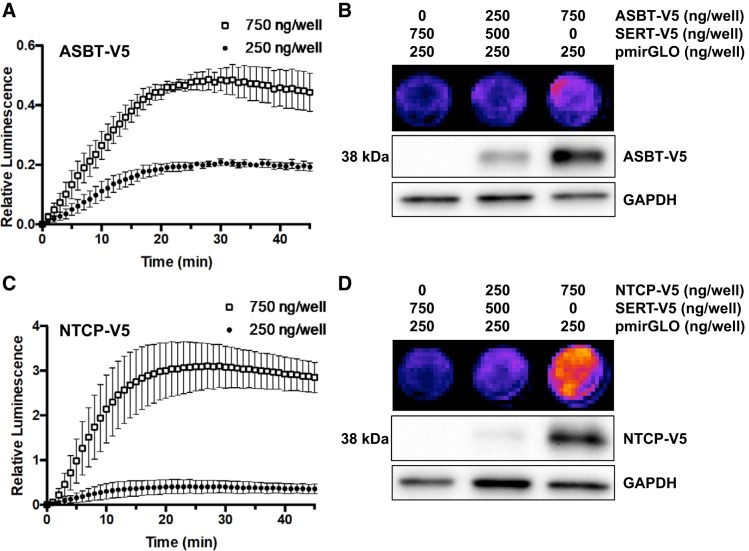
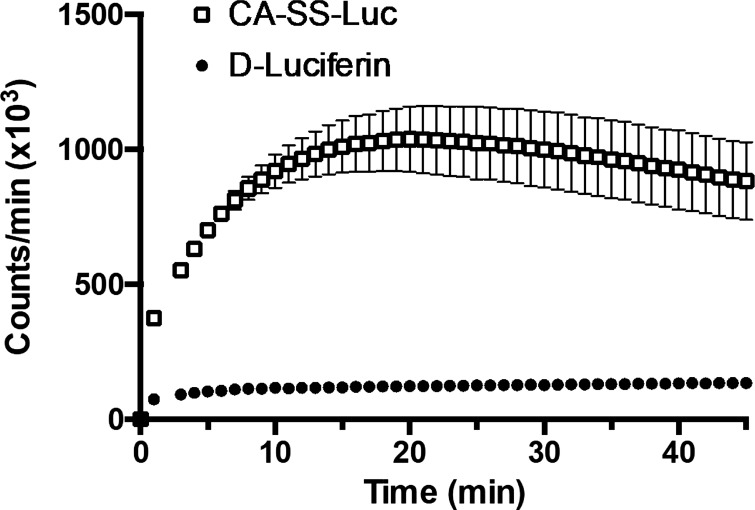
We next examined the dependence of the luminescence produced in the cells on cellular expression of ASBT. HEK-293 cells were transiently cotransfected with luciferase plasmid DNA (pmirGLO) and either 1) 750 ng/well of the serotonin transporter SERT-V5, 2) 250 ng/well ASBT-V5 and 500 ng/well SERT-V5, or 3) 750 ng/well ASBT-V5. Cells were imaged as above 48 h posttransfection. As shown in Fig. 4A, when compared with cells transfected with 750 ng/well SERT-V5, luminescence was dependent on the amount of transfected ASBT-V5. Cells transfected with 250 ng/well ASBT-V5 displayed greater luminescence than SERT-transfected cells, and this luminescence was further increased when 750 ng/well of ASBT-V5 was transfected. This corresponded to the cellular expression of ASBT-V5, as determined by Western blotting (Fig. 4B). Similar results were obtained when HEK-293 cells were transiently cotransfected with luciferase and the hepatic bile acid transporter NTCP. The bioluminescence was also significantly increased by higher levels of NTCP-V5 fusion protein in HEK-293 cells, and this luminescence was even greater than that seen in cells transfected with ASBT-V5 (Fig. 4, C and D). It is important to note that the serotonin transporter (SERT), which does not transport BAs, was used as a control plasmid in cells transfected with <750 ng/well of BAT. Thus, luminescence from SERT-transfected cells was minimal. To demonstrate that luminescence is the result of active uptake rather than extracellular cleavage of CA-SS-Luc followed by passive diffusion of d-luciferin, the luminescence of CA-SS-Luc was compared with that of d-luciferin. At a concentration of 1 μM, CA-SS-Luc produced several-fold greater luminescence than d-luciferin for the duration of the experiment (Fig. 5). These results suggest that extracellular hydrolysis of CA-SS-Luc is not responsible for the produced bioluminescence.
Fig. 4.
Bioluminescence production is dependent on cellular levels of bile acid transporters (BAT). A and C: time course of bile acid transporter-dependent bioluminescence production in human embryonic kidney (HEK)-293 transiently transfected with luciferase and 250 (●) or 750 ng/well (□) of apical sodium-dependent bile acid transporter (ASBT)-V5 (A) or sodium-taurocholate cotransporting polypeptide (NTCP)-V5 (C) relative to cells transfected with luciferase and SERT-V5 (750 ng/well). B and D: bioluminescence production (top) and a bile acid transporter (BAT) expression (middle) by increasing amounts of transfected ASBT (B) or NTCP (D); n = 3. Two-way ANOVA: time (P < 0.0001) and plasmid dose (P < 0.0001).
Fig. 5.
Incubation with CA-SS-Luc produces greater bioluminescence than d-luciferin. Luminescence production by 1 μM d-luciferin (●) and 1 μM CA-SS-Luc (□) in 2BT cells; n = 4. Two-way ANOVA: time (P < 0.0001) and CA-SS-Luc (P < 0.0001).
Measuring real-time changes in ASBT function.
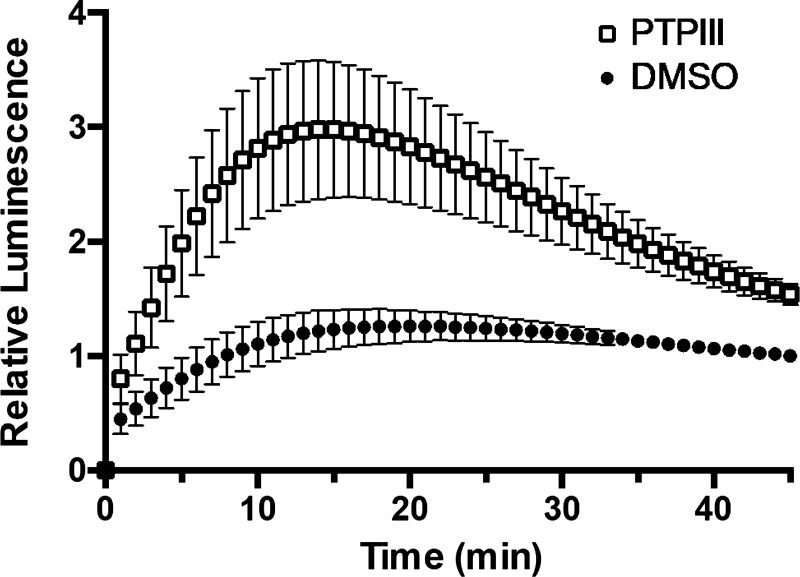
We next examined whether this assay could detect rapid changes in ASBT activity. 2BT cells stably transfected with ASBT-V5 were transiently transfected with luciferase as above. Forty-eight hours posttransfection, cells were treated with vehicle or 500 μM tyrosine phosphatase inhibitor III (PTPIII; Millipore) for 1 h, which has previously been shown to enhance ASBT activity (4). The treatment was removed, and cells were imaged by IVIS as above. As shown in Fig. 6, bioluminescence from PTPIII-treated cells increased more rapidly compared with untreated cells in the first 10 min of imaging and reached a maximum approximately threefold increase in luminescence before rapidly decreasing to near control-treated luminescence.
Fig. 6.
Tyrosine phosphatase inhibitor (PTPIII) enhances CA-SS-Luc bioluminescence production. CA-SS-Luc luminescence in 2BT cells with 1-h pretreatment with 500 μM PTPIII (□) or DMSO (●); n = 6. Two-way ANOVA: time (P < 0.0001) and PTPIII (P < 0.0001).
Induction of CA-SS-Luc bioluminescence by coincubation with natural bile acids.
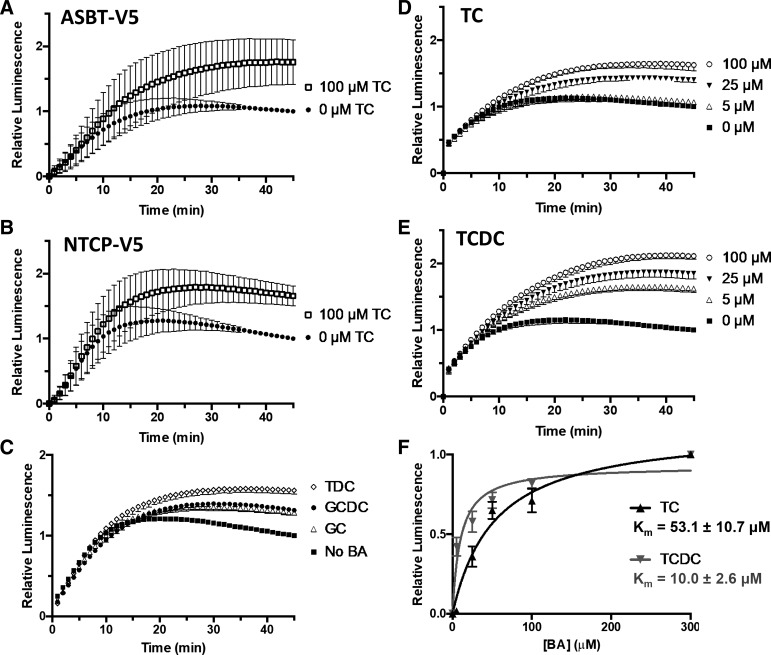
We then examined whether coincubation of CA-SS-Luc with excess of natural bile acids could compete with CA-SS-Luc for entry into the cell. Interestingly, addition of 100 μM taurocholate (TC) to the uptake solution enhanced the luminescence ∼1.5-fold in HEK-293 cells transiently transfected with either ASBT-V5 (Fig. 7A) or NTCP-V5 (Fig. 7B) but not in cells transfected with SERT, which does not transport BAs (data not shown). Similar results were seen with the bile acids taurodeoxycholate (TDC), glycocholate (GC), and glycochenodeoxycholate (GCDC), demonstrating that natural bile acids induce CA-SS-Luc bioluminescence in a bile acid transporter-dependent manner (Fig. 7C).
Fig. 7.
Natural bile acids enhance CA-SS-Luc bioluminescence. A and B: relative bioluminescence production by CA-SS-Luc cotreated with 100 μM taurocholate (TC; □) or 0 μM TC (●) in human embryonic kidney (HEK) cells transiently expressing apical sodium-dependent bile acid transporter (ASBT; A) or sodium-taurocholate cotransporting polypeptide (NTCP; B); n = 3. Two-way ANOVA: time (P < 0.0001) and TC (P < 0.0001). C: induction of CA-SS-Luc luminescence by 0 μM bile acid (BA; ■) or 50 μM of either glycocholate (GC; △), glycochenodeoxycholate (GCDC; ●), or taurodeoxycholate (TDC; ◇). D and E: induction of CA-SS-Luc luminescence by 0 (■), 5 (△), 25 (▲), and 100 μM (○) TC (D) and TCDC (E) in 2BT cells; n = 4. Two-way ANOVA: time (P < 0.0001) and dose (P < 0.0001). F: luminescence induction relative to 300 μM BA by different concentrations of TC (black) or TCDC (gray). Curve fit to the Michaelis-Menten equation and apparent Km calculation by GraphPad Prism 6.0; n = 4, P < 0.05.
We further investigated BA-dependent luminescence induction in the presence of different BA concentrations. As seen in Fig. 7D, concentrations of TC as low as 5 μM resulted in enhanced luminescence and were further increased with TC concentrations ≤100 μM in stable 2BT cells transiently expressing luciferase. Interestingly, addition of taurochenodeoxycholate (TCDC) enhanced CA-SS-Luc luminescence to a greater extent and at lower concentrations than TC (Fig. 7E). We then plotted the bile acid-dependent increase in luminescence at 15 min against the concentration of bile acid (5, 25, 50 100, and 300 μM) and fit to the Michaelis-Menten equation (Fig. 7F). After fitting, the apparent Km of TC was calculated as 53.1 ± 10.7 μM, and the apparent Km of TCDC was calculated as 10.0 ± 2.6 μM.
CA-SS-Luc uptake in isolated intestinal epithelial cells.
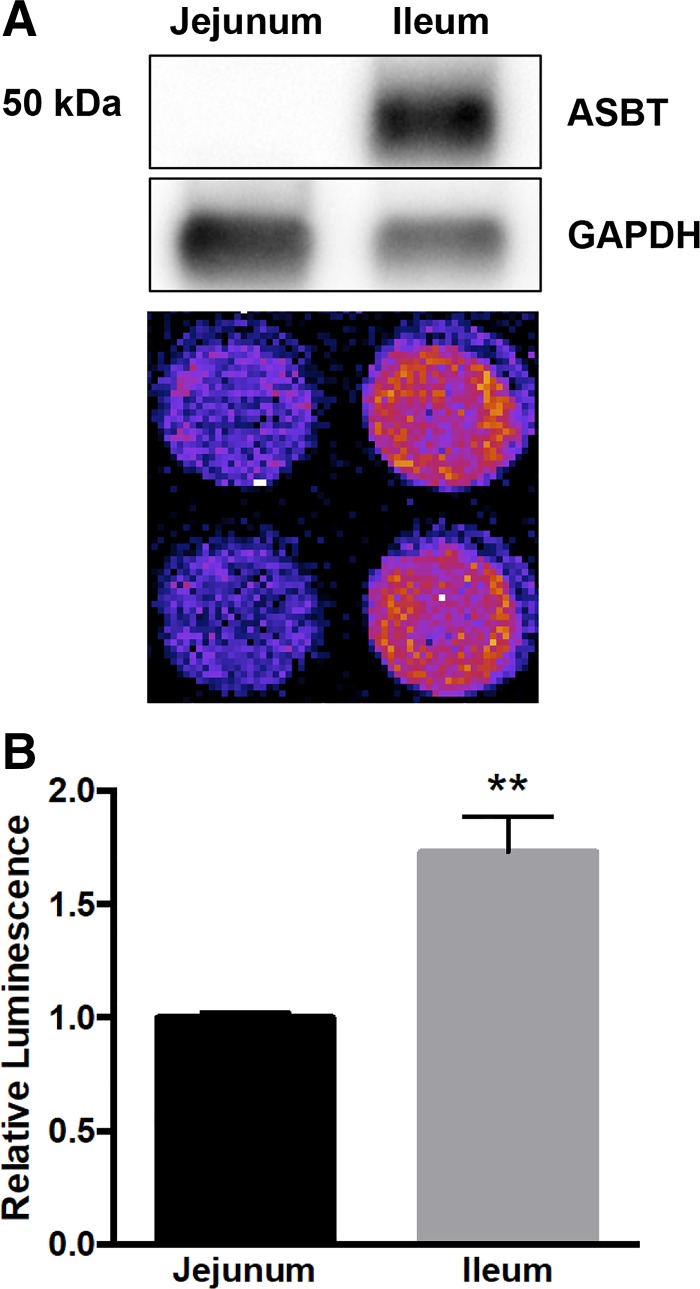
Because our in vitro studies indicated that luminescence generated by CA-SS-Luc likely represents bile acid transport activity, we hypothesized that CA-SS-Luc could be used to measure ASBT activity in native ileal enterocytes. Intestinal epithelial cells (IECs) were isolated from the jejunum and ileum of transgenic mice expressing luciferase (LucTg). Viability of these IECs was ∼70% following the isolation, as determined by trypan blue staining. Isolated IECs were immediately incubated with 1 μM CA-SS-Luc in uptake buffer and imaged by IVIS with a single 5-min exposure time. The bioluminescence generated by ileal IECs was significantly higher than that generated by an equal number of jejunal IECs, which corresponds to ASBT protein expression as determined by Western blot analysis (Fig. 8). These data demonstrate that CA-SS-Luc luminescence from IECs is enhanced in ileal cells, consistent with the localization and mechanism of ASBT.
Fig. 8.
CA-SS-Luc uptake is enhanced in ileal intestinal epithelial cells. A: apical sodium-dependent bile acid transporter (ASBT) protein expression in jejunal and ileal intestinal epithelial cells (IECs) from transgenic mice expressing luciferase (LucTg; representative Western blot from n = 3 mice) corresponds to CA-SS-Luc bioluminescence production. B: CA-SS-Luc luminescence relative to jejunal IECs; n = 4. **P < 0.01.
DISCUSSION
We have developed and validated a novel, bioluminescence-based method for assessing the cellular uptake of bile acids and real-time monitoring of the transport activity of ileal ASBT and hepatic NTCP. Recent work in the field has focused on developing methods for real-time imaging of bile acids. Specifically, taurocholic acid has been conjugated to fluorophores and 19F-labeled lysine to generate probes to trace bile acid translocation within the enterohepatic system (16, 34). Although these labeled probes could be used as tracers, they are not suitable to directly measure the function of BATs in real time.
The traditional methods for directly assessing BAT activity, which rely on radiolabeled probe uptake, are unable to sufficiently assess rapid functional changes in these transporters. Recently, bioluminescence-based methods have become increasingly useful tools for measuring real-time transporter activity and other cellular processes (19, 25, 26). In our study, we designed a novel method that relies on a small molecule probe, designated as CA-SS-Luc, which is composed of a cholic acid moiety conjugated to firefly luciferin via a cleavable disulfide linker. The use of a bile acid-based probe is an attractive method for measuring BAT function because these transporters have a fairly broad substrate specificity. ASBT and NTCP have been shown to transport bile acids conjugated to synthetic molecules, a property that has also led to interest in using BATs as prodrug targets (29). We designed CA-SS-Luc to adhere to known structural requirements for ASBT substrates because ASBT has a narrower substrate specificity compared with NTCP (6). In this regard, all known ASBT substrates possess a bile acid ring structure. Studies have shown that many high-affinity substrates for ASBT contain the bile acid ring structure conjugated to other molecules at the terminus of the C-24 sidechain (6). Also, a negatively charged C-24 side chain improves translocation of substrates via ASBT (7). The structure of CA-SS-Luc is consistent with all of these parameters, making it a likely substrate for BATs. It will be interesting to determine in future studies whether probes with luciferin attached to other sites of the bile acid, such as the 3-hydroxy moiety, have different affinity and transport properties compared with CA-SS-Luc. The previously described self-immolating linker was designed such that, upon CA-SS-Luc entry into the cell, glutathione cleaves the disulfide bond, releasing free luciferin and leading to luminescence in cells expressing firefly luciferase (20). Notably, because CA-SS-Luc itself is not a substrate for luciferase, light is only generated after cellular uptake and free luciferin release. This allows for real-time measurement of uptake with minimal extracellular background, which provides an advantage over fluorescence- or MRI-based methods, whose extracellular signal prevents a direct measurement of cellular uptake (16, 34).
In this study, we demonstrate that CA-SS-Luc is useful for the measurement of BAT function in a cell culture model. In HEK-293 cells expressing luciferase and either ASBT or NTCP, CA-SS-Luc bioluminescence is both concentration and time dependent. It is important to note that incubation with 15 μM CA-SS-Luc produced significantly higher bioluminescence that saturated the signal collected by IVIS (data not shown), indicating that at the CA-SS-Luc concentrations used in this study, neither intracellular glutathione nor luciferase expression was limiting. The bioluminescence signal, which represents a velocity of the transport activity, increases linearly for ∼20 min and then reaches a plateau representing an equilibrium state consistent with a transporter-mediated process for CA-SS-Luc cellular entry. Importantly, bioluminescence is dependent on the levels of BAT expression but not expression of other transport proteins that are not involved in bile acid transport, such as SERT. This provides strong evidence that the bioluminescence signal generated by CA-SS-Luc is induced specifically by BATs. It was also interesting that the relative luminescence in cells transfected with NTCP-V5 was considerably higher than the luminescence in cells transfected with an equal amount of ASBT-V5 plasmid DNA. This may simply be the result of different expression levels of these transporters at the plasma membrane of transfected cells, different levels of expression from plasmid DNA, or different transfection efficiency. Future studies will test the utility of this system to measure bile acid transport via other BATs, such as the hepatic bile salt export pump (BSEP) and organic anion transporting polypeptide (OATP), as well as the ileal organic solute transporter-α/β. Notably, this method was also able to measure rapid changes in ASBT activity. We have shown previously that ASBT-dependent [3H]TC uptake was significantly increased by 30-min incubation with the phosphatase inhibitor PTPIII (4). Here, we show that CA-SS-Luc bioluminescence rapidly increased in the presence of PTPIII, thus providing strong evidence for the ability of CA-SS-Luc to assess rapid changes in ASBT function in real time. This observation will be further tested using other compounds known to rapidly modulate BAT function (28, 36).
Interestingly, coincubation with natural bile acids to compete with CA-SS-Luc for cellular uptake instead caused a significant increase in the luminescence signal. This induction was sodium dependent and occurred only in cells expressing BATs (data not shown). Additionally, coincubation with TCDC produced greater induction than with TC. Because ASBT was previously shown to have more affinity for TCDC as compared with TC, we considered that the observed enhanced luminescence is directly related to the transport activity of ASBT. When the induction of CA-SS-Luc luminescence by increasing concentrations (5–300 μM) of TC or TCDC was fit to the Michaelis-Menten equation, we calculated the apparent Km of TC as 53.1 ± 10.7 μM and the apparent Km of TCDC as 10.0 ± 2.6 μM. Interestingly, these values are consistent with those previously measured by radioactive probe uptake (1, 13, 15, 21, 32). It should be noted that the induction of the bioluminescence by coincubation with higher levels of natural bile acids is unexpected, as previous data from our laboratory has demonstrated that the uptake of radiolabeled tracer [3H]TC is decreased in the presence of higher concentrations of cold TC. The TC-dependent induction of bioluminescence in our system is not the result of increased ASBT expression, as the induction occurs within minutes and expression of ASBT is driven by a constitutive CMV promoter from transfected plasmid DNA. It is possible that the presence of extracellular BAs increases luminescence either by disrupting membrane integrity and allowing passive diffusion or by promoting extracellular cleavage of CA-SS-Luc, leading to diffusion of free luciferin. However, both of these possibilities are unlikely, because 1) the BA-dependent increase in luminescence only occurred in cells expressing ASBT or NTCP and not cells expressing other transporters and 2) our results showed that incubating the cells with d-luciferin alone produced minimal signal compared with CA-SS-Luc. Another possibility is that intracellular BAs increase luminescence, potentially through enhancing the intracellular cleavage of CA-SS-Luc. This also is quite unlikely, as intracellular bile acid accumulation tends to produce oxidative stress (23) and could reduce intracellular glutathione, which would instead be expected to decrease the cleavage of CA-SS-Luc and thus decrease luminescence. A likely explanation for this increased luminescence is that CA-SS-Luc and natural bile acids bind to distinct sites on ASBT and NTCP and hence, are not competitors. Rather, the binding and transport of natural bile acids appears to enhance the transport of CA-SS-Luc. Delineating the molecular basis for this speculation will be the subject of future studies. While investigating the different binding sites for CA-SS-Luc on ASBT and NTCP, it is important to note that both ASBT and NTCP form functional dimers (8, 14). It should also be mentioned that ASBT has recently been shown to exhibit a second functional mode, acting as a receptor for large compounds behaving as ligands and leading to their rapid internalization. Therefore, it is possible that the copresence of CA-SS-Luc and natural bile acids stimulates a functional transport mechanism distinct from the transport of individual bile acids. Nevertheless, we provide strong evidence here that this novel method in the presence of natural bile acids faithfully captures BAT function.
It is of particular importance that our method can be used to assess ASBT activity in native intestinal epithelial cells, a more complex and physiologically relevant model. Our present data demonstrate that in primary IECs isolated from LucTg mice, CA-SS-Luc bioluminescence is significantly higher in cells isolated from the ileum, the intestinal segment where ASBT is expressed. This indicates that in native mouse intestinal cells, CA-SS-Luc uptake is dependent on ASBT expression. It is notable that in vivo measurement of BAT function using this method will be relatively straightforward, as it will require only the CA-SS-Luc compound and commercially available LucTg mice. This provides an advantage over the recently described fluorescence resonance energy transfer (FRET)-based biosensor that measures intracellular bile acid concentrations (33). This FRET-based method allows for efficient, real-time measurement of bile acid concentration at subcellular resolution in vitro. Although the FRET-based method provides an attractive approach to assess the intracellular concentrations in a single cell, the application of such an approach to animal models may be challenging. On the other hand, our novel luciferase-based method may offer an in vivo approach to evaluate the function of intestinal and hepatic bile acid transporters in living animals as well as from primary cells isolated from LucTg mice. However, it is important to note that this method will not allow for the measurement of BAT activity in human patients, as it does require expression of luciferase in intestinal epithelial cells. Thus, its utility is limited to in vitro and in vivo preclinical models. Ongoing studies in our laboratory are focusing on developing novel methods to measure bile acid transport and distribution in humans.
In conclusion, we have developed and validated a method for assessing BAT activity in real time using a novel bioluminescent probe. This method will be applied to developing assays to identify compounds that modulate BAT activity as well as pursuing the in vivo use of CA-SS-Luc. Future in vivo studies will not only allow for real-time measurement of BAT activity in the intestine and liver but may also identify novel bile acid transport processes in other organ systems.
GRANTS
These studies were supported by a Veterans Affairs (VA) Research Career Scientist Award and Merit Award No. BX000152 (W. A. Alrefai). The studies are also partially supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-109709 (W. A. Alrefai), DK-81858 (P. K. Dudeja), and DK-98170 (R. K. Gill) and VA Merit Awards BX002011 (P. K. Dudeja) and BX002867 (S. Saksena). P. K. Dudeja is also supported by a VA Senior Research Career Scientist Award.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.L.T., R.K.G., D.L., and W.A.A. conceived and designed research; A.L.T. and H.L. performed experiments; A.L.T., H.L., D.L., and W.A.A. analyzed data; A.L.T., H.L., R.K.G., P.K.D., S.S., D.L., and W.A.A. interpreted results of experiments; A.L.T. and H.L. prepared figures; A.L.T., H.L., and W.A.A. drafted manuscript; A.L.T., H.L., R.K.G., P.K.D., S.S., D.L., and W.A.A. edited and revised manuscript; A.L.T., H.L., R.K.G., P.K.D., S.S., D.L., and W.A.A. approved final version of manuscript.
Supplemental Data
Chemical Syntheses and NMR Data - .docx (1.33 MB)
REFERENCES
- 1.Alpini G, Glaser SS, Rodgers R, Phinizy JL, Robertson WE, Lasater J, Caligiuri A, Tretjak Z, LeSage GD. Functional expression of the apical Na+-dependent bile acid transporter in large but not small rat cholangiocytes. Gastroenterology 113: 1734–1740, 1997. doi: 10.1053/gast.1997.v113.pm9352879. [DOI] [PubMed] [Google Scholar]
- 2.Alrefai WA, Gill RK. Bile acid transporters: structure, function, regulation and pathophysiological implications. Pharm Res 24: 1803–1823, 2007. doi: 10.1007/s11095-007-9289-1. [DOI] [PubMed] [Google Scholar]
- 3.Annaba F, Ma K, Kumar P, Dudeja AK, Kineman RD, Shneider BL, Saksena S, Gill RK, Alrefai WA. Ileal apical Na+-dependent bile acid transporter ASBT is upregulated in rats with diabetes mellitus induced by low doses of streptozotocin. Am J Physiol Gastrointest Liver Physiol 299: G898–G906, 2010. doi: 10.1152/ajpgi.00139.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Annaba F, Sarwar Z, Gill RK, Ghosh A, Saksena S, Borthakur A, Hecht GA, Dudeja PK, Alrefai WA. Enteropathogenic Escherichia coli inhibits ileal sodium-dependent bile acid transporter ASBT. Am J Physiol Gastrointest Liver Physiol 302: G1216–G1222, 2012. doi: 10.1152/ajpgi.00017.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Annaba F, Sarwar Z, Kumar P, Saksena S, Turner JR, Dudeja PK, Gill RK, Alrefai WA. Modulation of ileal bile acid transporter (ASBT) activity by depletion of plasma membrane cholesterol: association with lipid rafts. Am J Physiol Gastrointest Liver Physiol 294: G489–G497, 2008. doi: 10.1152/ajpgi.00237.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anwer MS, Stieger B. Sodium-dependent bile salt transporters of the SLC10A transporter family: more than solute transporters. Pflugers Arch 466: 77–89, 2014. doi: 10.1007/s00424-013-1367-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balakrishnan A, Wring SA, Coop A, Polli JE. Influence of charge and steric bulk in the C-24 region on the interaction of bile acids with human apical sodium-dependent bile acid transporter. Mol Pharm 3: 282–292, 2006. doi: 10.1021/mp0600135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bijsmans IT, Bouwmeester RA, Geyer J, Faber KN, van de Graaf SF. Homo- and hetero-dimeric architecture of the human liver Na+-dependent taurocholate co-transporting protein. Biochem J 441: 1007–1016, 2012. doi: 10.1042/BJ20111234. [DOI] [PubMed] [Google Scholar]
- 9.Cao L, Che Y, Meng T, Deng S, Zhang J, Zhao M, Xu W, Wang D, Pu Z, Wang G, Hao H. Repression of intestinal transporters and FXR-FGF15 signaling explains bile acids dysregulation in experimental colitis-associated colon cancer. Oncotarget 8: 63665–63679, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen F, Ananthanarayanan M, Emre S, Neimark E, Bull LN, Knisely AS, Strautnieks SS, Thompson RJ, Magid MS, Gordon R, Balasubramanian N, Suchy FJ, Shneider BL. Progressive familial intrahepatic cholestasis, type 1, is associated with decreased farnesoid X receptor activity. Gastroenterology 126: 756–764, 2004. doi: 10.1053/j.gastro.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Yao X, Young A, McNulty J, Anderson D, Liu Y, Nystrom C, Croom D, Ross S, Collins J, Rajpal D, Hamlet K, Smith C, Gedulin B. Inhibition of apical sodium-dependent bile acid transporter as a novel treatment for diabetes. Am J Physiol Endocrinol Metab 302: E68–E76, 2012. doi: 10.1152/ajpendo.00323.2011. [DOI] [PubMed] [Google Scholar]
- 12.Chiang JY. Bile acid metabolism and signaling. Compr Physiol 3: 1191–1212, 2013. doi: 10.1002/cphy.c120023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chignard N, Mergey M, Veissière D, Parc R, Capeau J, Poupon R, Paul A, Housset C. Bile acid transport and regulating functions in the human biliary epithelium. Hepatology 33: 496–503, 2001. doi: 10.1053/jhep.2001.22345. [DOI] [PubMed] [Google Scholar]
- 14.Chothe PP, Czuba LC, Moore RH, Swaan PW. Human bile acid transporter ASBT (SLC10A2) forms functional non-covalent homodimers and higher order oligomers. Biochim Biophys Acta 1860: 645–653, 2018. doi: 10.1016/j.bbamem.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Craddock AL, Love MW, Daniel RW, Kirby LC, Walters HC, Wong MH, Dawson PA. Expression and transport properties of the human ileal and renal sodium-dependent bile acid transporter. Am J Physiol Gastrintest Liver Physiol 274: G157–G169, 1998. doi: 10.1152/ajpgi.1998.274.1.G157. [DOI] [PubMed] [Google Scholar]
- 16.De Bruyn T, Sempels W, Snoeys J, Holmstock N, Chatterjee S, Stieger B, Augustijns P, Hofkens J, Mizuno H, Annaert P. Confocal imaging with a fluorescent bile acid analogue closely mimicking hepatic taurocholate disposition. J Pharm Sci 103: 1872–1881, 2014. doi: 10.1002/jps.23933. [DOI] [PubMed] [Google Scholar]
- 17.Halpern MD, Holubec H, Saunders TA, Dvorak K, Clark JA, Doelle SM, Ballatori N, Dvorak B. Bile acids induce ileal damage during experimental necrotizing enterocolitis. Gastroenterology 130: 359–372, 2006. doi: 10.1053/j.gastro.2005.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halpern MD, Weitkamp JH, Mount Patrick SK, Dobrenen HJ, Khailova L, Correa H, Dvorak B. Apical sodium-dependent bile acid transporter upregulation is associated with necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 299: G623–G631, 2010. doi: 10.1152/ajpgi.00242.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henkin AH, Cohen AS, Dubikovskaya EA, Park HM, Nikitin GF, Auzias MG, Kazantzis M, Bertozzi CR, Stahl A. Real-time noninvasive imaging of fatty acid uptake in vivo. ACS Chem Biol 7: 1884–1891, 2012. doi: 10.1021/cb300194b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones LR, Goun EA, Shinde R, Rothbard JB, Contag CH, Wender PA. Releasable luciferin-transporter conjugates: tools for the real-time analysis of cellular uptake and release. J Am Chem Soc 128: 6526–6527, 2006. doi: 10.1021/ja0586283. [DOI] [PubMed] [Google Scholar]
- 21.Kramer W, Stengelin S, Baringhaus KH, Enhsen A, Heuer H, Becker W, Corsiero D, Girbig F, Noll R, Weyland C. Substrate specificity of the ileal and the hepatic Na(+)/bile acid cotransporters of the rabbit. I. Transport studies with membrane vesicles and cell lines expressing the cloned transporters. J Lipid Res 40: 1604–1617, 1999. [PubMed] [Google Scholar]
- 22.Molina H, Azocar L, Ananthanarayanan M, Arrese M, Miquel JF. Localization of the Sodium-Taurocholate cotransporting polypeptide in membrane rafts and modulation of its activity by cholesterol in vitro. Biochim Biophys Acta 1778: 1283–1291, 2008. doi: 10.1016/j.bbamem.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 23.Nomoto M, Miyata M, Yin S, Kurata Y, Shimada M, Yoshinari K, Gonzalez FJ, Suzuki K, Shibasaki S, Kurosawa T, Yamazoe Y. Bile acid-induced elevated oxidative stress in the absence of farnesoid X receptor. Biol Pharm Bull 32: 172–178, 2009. doi: 10.1248/bpb.32.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oelkers P, Kirby LC, Heubi JE, Dawson PA. Primary bile acid malabsorption caused by mutations in the ileal sodium-dependent bile acid transporter gene (SLC10A2). J Clin Invest 99: 1880–1887, 1997. doi: 10.1172/JCI119355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paley MA, Prescher JA. Bioluminescence: a versatile technique for imaging cellular and molecular features. MedChemComm 5: 255–267, 2014. doi: 10.1039/C3MD00288H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park HM, Russo KA, Karateev G, Park M, Dubikovskaya E, Kriegsfeld LJ, Stahl A. A System for In Vivo Imaging of Hepatic Free Fatty Acid Uptake. Gastroenterology 152: 78–81.e2, 2017. doi: 10.1053/j.gastro.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao A, Kosters A, Mells JE, Zhang W, Setchell KDR, Amanso AM, Wynn GM, Xu T, Keller BT, Yin H, Banton S, Jones DP, Wu H, Dawson PA, Karpen SJ. Inhibition of ileal bile acid uptake protects against nonalcoholic fatty liver disease in high-fat diet-fed mice. Sci Transl Med 8: 357ra122–357ra122, 2016. doi: 10.1126/scitranslmed.aaf4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarwar Z, Annaba F, Dwivedi A, Saksena S, Gill RK, Alrefai WA. Modulation of ileal apical Na+-dependent bile acid transporter ASBT by protein kinase C. Am J Physiol Gastrointest Liver Physiol 297: G532–G538, 2009. doi: 10.1152/ajpgi.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sievänen E. Exploitation of bile acid transport systems in prodrug design. Molecules 12: 1859–1889, 2007. doi: 10.3390/12081859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slijepcevic D, van de Graaf SFJ. Bile Acid Uptake Transporters as Targets for Therapy. Dig Dis 35: 251–258, 2017. doi: 10.1159/000450983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stross C, Helmer A, Weissenberger K, Görg B, Keitel V, Häussinger D, Kubitz R. Protein kinase C induces endocytosis of the sodium taurocholate cotransporting polypeptide. Am J Physiol Gastrointest Liver Physiol 299: G320–G328, 2010. doi: 10.1152/ajpgi.00180.2010. [DOI] [PubMed] [Google Scholar]
- 32.Sun AQ, Balasubramaniyan N, Chen H, Shahid M, Suchy FJ. Identification of functionally relevant residues of the rat ileal apical sodium-dependent bile acid cotransporter. J Biol Chem 281: 16410–16418, 2006. doi: 10.1074/jbc.M600034200. [DOI] [PubMed] [Google Scholar]
- 33.van der Velden LM, Golynskiy MV, Bijsmans IT, van Mil SW, Klomp LW, Merkx M, van de Graaf SF. Monitoring bile acid transport in single living cells using a genetically encoded Förster resonance energy transfer sensor. Hepatology 57: 740–752, 2013. doi: 10.1002/hep.26012. [DOI] [PubMed] [Google Scholar]
- 34.Vivian D, Cheng K, Khurana S, Xu S, Kriel EH, Dawson PA, Raufman JP, Polli JE. In vivo performance of a novel fluorinated magnetic resonance imaging agent for functional analysis of bile acid transport. Mol Pharm 11: 1575–1582, 2014. doi: 10.1021/mp400740c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walters JR, Pattni SS. Managing bile acid diarrhoea. Therap Adv Gastroenterol 3: 349–357, 2010. doi: 10.1177/1756283X10377126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Y, Aquino CJ, Cowan DJ, Anderson DL, Ambroso JL, Bishop MJ, Boros EE, Chen L, Cunningham A, Dobbins RL, Feldman PL, Harston LT, Kaldor IW, Klein R, Liang X, McIntyre MS, Merrill CL, Patterson KM, Prescott JS, Ray JS, Roller SG, Yao X, Young A, Yuen J, Collins JL. Discovery of a highly potent, nonabsorbable apical sodium-dependent bile acid transporter inhibitor (GSK2330672) for treatment of type 2 diabetes. J Med Chem 56: 5094–5114, 2013. doi: 10.1021/jm400459m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Chemical Syntheses and NMR Data - .docx (1.33 MB)