Abstract
The reflexive activities of the gastrointestinal tract are regulated, in part, by precise interactions between neurons and glia in the enteric nervous system (ENS). Intraganglionic enteric glia are a unique type of peripheral glia that surround enteric neurons and regulate neuronal function, activity, and survival. Enteric glia express numerous neurotransmitter receptors that allow them to sense neuronal activity, but it is not clear if enteric glia monitor acetylcholine (ACh), the primary excitatory neurotransmitter in the ENS. Here, we tested the hypothesis that enteric glia detect ACh and that glial activation by ACh contributes to the physiological regulation of gut functions. Our results show that myenteric enteric glia express both the M3 and M5 subtypes of muscarinic receptors (MRs) and that muscarine drives intracellular calcium (Ca2+) signaling predominantly through M3R activation. To elucidate the functional effects of activation of glial M3Rs, we used GFAP::hM3Dq mice that express a modified human M3R (hM3Dq) exclusively on glial fibrillary acidic protein (GFAP) positive glia to directly activate glial hM3Dqs using clozapine-N-oxide. Using spatiotemporal mapping analysis, we found that the activation of glial hM3Dq receptors enhances motility reflexes ex vivo. Continuous stimulation of hM3Dq receptors in vivo, drove changes in gastrointestinal motility without affecting neuronal survival in the ENS and glial muscarinic receptor activation did not alter neuron survival in vitro. Our results provide the first evidence that GFAP intraganglionic enteric glia express functional muscarinic receptors and suggest that the activation of glial muscarinic receptors contributes to the physiological regulation of functions.
NEW & NOTEWORTHY Enteric glia are emerging as novel regulators of enteric reflex circuits, but little is still known regarding the effects of specific transmitter pathways on glia and the resulting consequences on enteric reflexes. Here, we provide the first evidence that enteric glia monitor acetylcholine in the enteric nervous system and that glial activation by acetylcholine is a physiological mechanism that contributes to the functional regulation of intestinal reflexes.
Keywords: cholinergic signaling, enteric glia, muscarine receptors
INTRODUCTION
The enteric nervous system (ENS) is the principal regulator of gastrointestinal functions and neural reflex pathways within the ENS coordinate fluid exchange across the mucosa, local blood flow, and patterns of motility (19). Disruptions to the synaptic architecture of the ENS in conditions such as enteric neuropathies contribute to a wide range of functional bowel disorders (16). These conditions often exhibit a dysregulated balance of excitatory and inhibitory neurotransmission in enteric reflexes. The major inhibitory transmitters within the ENS are nitric oxide, adenosine triphosphate (ATP), and vasoactive intestinal peptide, whereas the major excitatory pathways within the ENS are cholinergic and tachykinergic, with acetylcholine (ACh) and tachykinins producing excitatory potentials in postsynaptic effector cells (35).
Enteric reflexes are modulated by signaling between enteric neurons and enteric glia (8, 23). Intraganglionic enteric glia are a distinct type of peripheral glia cell that surround enteric neurons and interact with neural circuits in the ENS (23). Enteric glia detect neuronal activity (6, 7, 8, 22, 26, 31) through the expression of receptors for multiple neurotransmitters (6, 35) and glial activation subsequently modulates the activity of neural circuits (6–8, 22, 26, 27, 31, 32). Glial activation has a major influence over enteric circuits involved in the regulation of motility (32) and secretion (24), and the direct activation of enteric glia is sufficient to drive intestinal reflexes (8, 24, 32). In addition, glial activation by danger cues is responsible for driving neurodegeneration during intestinal inflammation (9). Therefore, understanding the circumstances under which glial activation is triggered and the downstream consequences of various glial signal transduction pathways is important to understand the functional regulation of the intestine.
Enteric glial activity is recruited by multiple neurotransmitters and modulators in the ENS. To date, enteric glia are known to express receptors for adenosine diphosphate (P2Y1 receptors; 9, 33), ATP and uridine triphosphate (both through P2Y4 receptors; 26), adenosine (A2B receptors; 12), norepinephrine (α2a adrenergic receptors; 38), glutamate (metabotropic glutamate receptor 5, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, N-methyl-d-aspartate; 39, 53), serotonin (5-HT2; 6), bioactive lipids (SP1R, lysophosphatidic acid receptor 1; 47, 48), endothelin (likely endothelin B receptors; 56), protease-activate receptors (1 and 2; 20), and bradykinin (β2 receptors) (36). Thus, enteric glia can respond to multiple neurotransmitters and modulators within the ENS, but whether glia encode unique responses to individual transmitter pathways is still unknown. Importantly, whether enteric glia respond to ACh, the principal excitatory neurotransmitter in the ENS, and what the functional consequences of glia action by ACh might be are still unanswered questions. Cholinergic receptor expression is well established in other types of neuroglia such as perisynaptic schwann cells (PSCs) in the neuromuscular junction (21), cortical astrocytes (40, 50, 51), and cochlear astrocytes (43). The similarities between enteric glia and these related populations of neuroglia suggest that enteric glia may also have the potential to sense cholinergic transmission.
Here, we addressed this issue by testing the hypothesis that enteric glia detect ACh and that glial activation by ACh contributes to the physiological regulation of gut functions. Our results show that enteric glia express the M3 and M5 subtypes of muscarinic receptors (MRs) and that muscarine drives glial calcium (Ca2+) responses that are primarily driven by M3Rs and are independent of neuronal activation. We assessed the functional consequences of glial M3R activation using GFAP::hM3Dq+/− mice that express a modified human M3R (hM3Dq) exclusively on glial fibrillary acidic protein (GFAP)-positive glia and found that the selective activation of glial M3Rs with clozapine-N-oxide (CNO) enhances distension-induced motility reflexes. Chronic stimulation of glial M3Rs in vivo altered intestinal motility but did not cause overt damage or affect neuron survival in the ENS. Overall, our results provide the first evidence, to our knowledge, that enteric glia express functional MRs and suggest that the activation of glial MRs contributes to the functional regulation of intestinal reflexes.
MATERIALS AND METHODS
Declaration of animal use approval.
All work involving animals was conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and was approved by the Institutional Animal Care and Use Committee at Michigan State University.
Animals.
Male and female C57BL/6 mice between 8 and 10 wk of age were used for immunohistochemistry and for the in situ model of neuroinflammation (Charles River, Hollister, CA). All mice were maintained on a 12-h:12-h light-dark cycle in a temperature-controlled environment with access to food and water ad libitum. Transgenic mice expressing the genetically-encoded Ca2+ indicator GCaMP5g in enteric glia (Sox10CreERT2::Polr2atm1(CAG-GCaMP5g,-tdTomato)Tvrd mice) were bred in house and were generated as previously described (34) by crossing Sox10CreERT2 mice (a gift from Dr. Vassilis Pachnis, Francis Crick Institute, London, England) with Polr2atm1(CAG-GCaMP5g,-tdTomato)Tvrd mice [PC::G5-tdT (Jackson, Bar Harbor, ME; stock no. 024477; RRID: IMSR_JAX:024477)]. Transgenic mice expressing the designer receptor hM3Dq under the transcriptional control of the GFAP promotor [GFAP::hM3Dq+/− (RRID: MMRRC_042286-UNC)] were a gift from Dr. Ken McCarthy (University of North Carolina at Chapel Hill) and were bred in house as heterozygous as previously described (32). All double transgenic mice were maintained as hemizygous for Cre (Sox10CreERT2+/−) and homozygous for the floxed allele (Polr2atm1(CAG-GCaMP5g,-tdTomato)Tvrd). CreERT2 activity was induced in Sox10CreERT2::Polr2atm1(CAG-GCaMP5g,-tdTomato)Tvrd mice by feeding animals with chow containing tamoxifen citrate (400 mg/kg) for 2 wk followed by 1 wk of normal chow before use. Genotyping was performed by the Research Technology Support Facility Genomics Core at Michigan State University and by Transnetyx (Cordova, TN).
Whole-mount immunohistochemistry.
Longitudinal muscle myenteric plexus (LMMP) whole mount preparations were microdissected from mouse colonic tissue preserved in Zamboni’s fixative. Processing of LMMPs via immunohistochemistry was conducted as previously described (10) with the primary and secondary antibodies listed in Tables 1 and 2, respectively. Briefly, LMMP preparations were washed 3 times for 10 min in 0.1% Triton X-100 in PBS followed by a 45-min incubation in blocking solution containing 4% normal goat serum, 0.4% Triton X-100, and 1% bovine serum in PBS. Preparations were incubated with primary antibodies overnight at room temperature and with secondary antibodies for 2 h at room temperature before mounting. Antibody specificity was confirmed by preadsorption with the corresponding control peptides. Fluorescent labeling was visualized by confocal imaging through the Plan-Apochromat 60X oil immersion objective (1.42 numerical aperture) of an inverted Olympus Fluoview FV-1000 microscope (Olympus, Center Valley, PA).
Table 1.
Primary antibodies used
| Antibody | Source | Dilution | Cat. No. | Resource ID No. |
|---|---|---|---|---|
| Chicken anti-GFAP | Abcam, Cambridge, MA | 1:1,000 | AB-4674 | AB_304558 |
| Biotinylated mouse anti-human HuC/D | Invitrogen, Carlsbad, CA | 1:200 | A21272 | AB_2535822 |
| Rabbit anti-M1 muscarinic receptor | Alomone, Jerusalem, Israel | 1:200 | AMR-010 | AB_2340994 |
| Rabbit anti-M2 muscarinic receptor | Alomone | 1:200 | AMR-002 | AB_2039995 |
| Rabbit anti-M3 muscarinic receptor | Alomone | 1:200 | AMR-006 | AB_2039997 |
| Rabbit anti-M4 muscarinic receptor | Alomone | 1:200 | AMR-004 | AB_11219338 |
| Rabbit anti-M5 muscarinic receptor | Alomone | 1:200 | AMR-005 | AB_10658757 |
GFAP, glial fibrillary acidic protein.
Table 2.
Secondary antibodies used
| Antibody | Source | Dilution | Cat. No. | Resource ID No. |
|---|---|---|---|---|
| Alexa Fluor 488 goat anti-chicken | Invitrogen, Carlsbad, CA | 1:400 | A-11039 | AB_2534096 |
| Alexa Fluor 594 donkey anti-rabbit | Jackson ImmunoResearch, West Grove, PA | 1:400 | 711-585-152 | AB_2340621 |
| Dylight 405-conjugated streptavidin | Jackson ImmunoResearch | 1:400 | 016-470-084 | AB_2337248 |
| Alexa Fluor 594-conjugated streptavidin | Jackson ImmunoResearch | 1:400 | 016-580-084 | AB_2337250 |
Ca2+ imaging.
LMMP whole-mount preparations from Sox10CreERT2::Polr2atm1(CAG-GCaMP5g,-tdTomato)Tvrd mice were microdissected from mouse distal colons and incubated for 15 min at room temperature in an enzyme mixture consisting of 150 U/ml Collagenase type II and 1 U/ml Dispase (Life Technologies). Images were acquired at 1 Hz through the ×40 water immersion objective (LUMPlan N, 0.8 numerical aperture) of an upright Olympus BX51WI fixed-stage microscope (Olympus) using MetaMorph software (Molecular Devices, Sunnyvale, CA) and a Neo sCMOS camera (Andor, South Windsor, CT). We continually perfused whole-mounts at 2–3 ml/min with buffer using a gravity flow perfusion system. Agonists were dissolved in buffer and bath applied for 30 s. Antagonists were dissolved in buffer and applied for either 20 min or 3 min before imaging.
In situ model of neuroinflammation.
Enteric neuron death was driven as previously described (9). LMMP preparations were incubated with the P2X7R agonist 2′-(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate triethylammonium salt (BzATP; 300 µM) or (+)-(2S,4R,5S)-tetrahydro-4-hydroxy-N,N,N,5-tetramethyl-2-furanmethanammonium chloride [(+)-muscarine; 20 µM] in the presence or absence of the nonselective muscarinic ACh antagonist (−)-scopolamine hydrochloride (10 µM) for 2 h in 95% air-5% CO2 at 37°C. LMMP preparations were then rinsed with fresh buffer, incubated for an additional 2 h in buffer only, and fixed overnight in Zamboni’s fixative.
Motility reflex recordings using spatiotemporal maps.
Mouse colons from GFAP::hM3Dq+/− and wild-type (WT) mice were removed and placed in a bath containing DMEM/F-12 maintained at 37°C. Luminal contents were flushed, and the colon was cannulated at both ends (oral and anal) and secured using silk suture. Intraluminal pressure was hydrostatically maintained using DMEM/F-12 at room temperature. After a 30-min acclimation period, baseline and pharmacologically-induced contractile patterns were recorded using a 2-megapixel digital camera (Olympus DP21, Olympus, Tokyo, Japan) placed above the organ bath at a rate of 25 frames/s. The recordings were subsequently processed offline to generate and analyze spatiotemporal maps.
CNO administration in drinking water.
GFAP::hM3Dq+/− mice and WT littermates were administered 0.13 mg/ml of clozapine-N-oxide (CNO) dissolved in 0.1% dimethyl sulfoxide and 3.5% sucrose in drinking water. Mice were monitored closely, and weight was recorded daily. Mice were euthanized after 4 days of treatment with CNO. Macroscopic damage was recorded with an established scoring system (49).
Colonic migrating motor complex recordings.
Mouse colons were removed and placed in a bath containing DMEM/F-12 maintained at 37°C. Luminal contents were flushed, and a stainless-steel rod was inserted into the lumen. Tissue was secured at both ends with surgical silk and force transducers (Grass Instruments, West Warwick, RI) were placed 2 cm apart and attached by metal hooks. The initial tension was adjusted to 0.5 g, and the development of spontaneous colonic migrating motor complexes (CMMCs) was monitored over an acclimatization period of 20 min using LabChart 8 (ADInstruments, Colorado Springs, CO). The last 6-min interval of this acclimation period was used as baseline. Drugs were added after the acclimation period, and CMMCs were recorded for an additional 10-min interval.
Endogenous pellet production.
Mice were individually housed, and fecal pellet output was measured on 3 consecutive days after 2 days of acclimation. Pellets were collected for 1 h beginning at 9:00 AM (Zeitgeber +3). The wet weight of fecal matter was measured immediately, and the dry weight was obtained the next day after dehydration. Data from 3 days were averaged.
Colon bead assay.
Mice were lightly anesthetized with isoflurane, and a 2-mm plastic bead was inserted 3 cm into the colon. Distal colonic transit time was assessed by recording the time to expulsion of the bead. Data from 2 days were averaged.
Solutions.
Live tissue was maintained in DMEM/F-12 nutrient mixture (Life Technologies) during collection and microdissection. Ca2+ imaging experiments were performed in modified Krebs buffer containing (in mM): 121 NaCl, 5.9 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 10 HEPES, 21.2 NaHCO3, 1 pyruvic acid, and 8 glucose (pH adjusted to 7.4 with NaOH).
Chemicals and reagents.
Unless otherwise stated, all chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO). Tetrodotoxin, Fugu sp. [tetrodotoxin (TTX)] was purchased from Millipore Sigma (Burlington, MA). (αR)-α-cyclopentyl-α-hydroxy-N-[1-(4-methyl-3-pentenyl)-4-piperidinyl]benzeneacetamide fumarate (J104129-fumarate) was purchased from Tocris (Minneapolis, MN). 5-[(3-Acetylphenoxy)methyl]-N-methyl-N-[(1S)-1-pyridin-2-ylethyl]-1,2-oxazole-3-carboxamide [ML381 (VU0488130) Fumarate] was purchased from Aobious (Glousester, MA). 8-Chloro-11-(4-methyl-4-oxido-1-piperazinyl)-5H-dibenzo[b,e][1,4]diazepine (CNO) was obtained from the National Institute on Drug Abuse Supply Program.
Statistical analysis.
For Ca2+ imaging, traces represent the average change in fluorescence over time for all glial cells within a single ganglion. Neuron packing density was determined by counting the number of HuC/D-immunoreactive neurons per ganglionic area in 10 ganglia per LMMP preparation using the cell counter plug-in tool on ImageJ Software, version 1.48 (NIH). Changes in intestinal diameter and speed of propagation were assessed using an edge detection routine that measures the diameter of each position along the length of the colon. All results are presented as means ± SE, and statistically significant differences were determined using an analysis of variance (ANOVA) or t-test as appropriate with P < 0.05 considered statistically significant (GraphPad Prism; GraphPad Software, San Diego, CA).
RESULTS
Enteric glia express M3R and M5R.
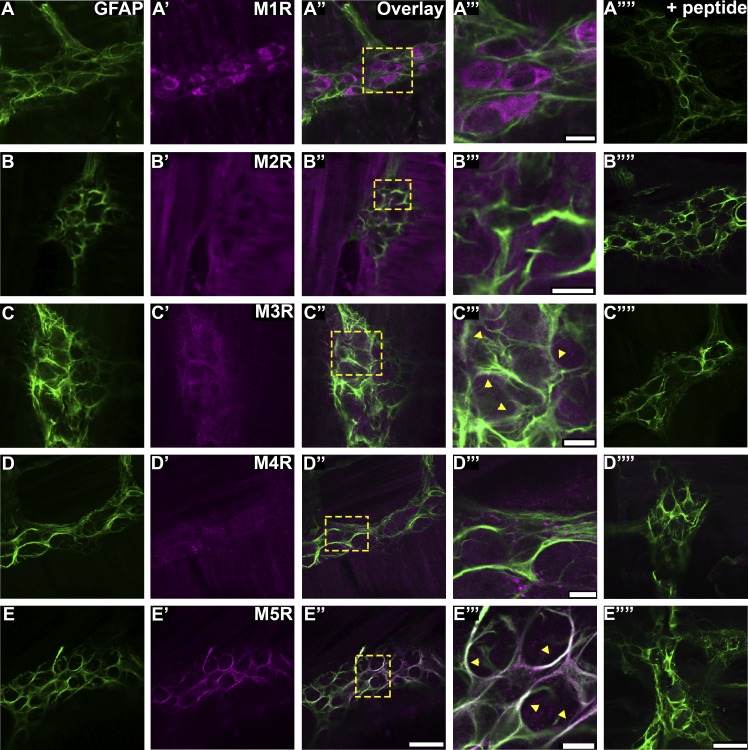
Acetylcholine is the predominant neurotransmitter in the ENS, and MRs are involved in multiple intestinal reflexes (14, 18, 28). To test whether enteric glia have the potential to respond to acetylcholine, we colabeled with antibodies against GFAP and MR subtypes (Fig. 1). Our results show that enteric glia primarily express M3R (Fig. 1, C–C′′′) and M5R (Fig. 1, E–E′′′). Preadsorption controls for the different MR subtypes (Fig. 1, A′′′′–E′′′′) confirmed labeling. These results demonstrate that enteric glia express muscarinic receptors and have the potential to respond to ACh.
Fig. 1.
Enteric glia in the mouse myenteric plexus express M3R and M5R. Images (confocal single optical planes, 1 µm) show dual-label immunohistochemistry for GFAP (green, A–E) and either M1R (magenta, A′), M2R (magenta, B′), M3R (magenta, C′), M4R (magenta, D′), or M5R (magenta, E′) in the distal colon myenteric plexus of mice. Overlays of each combination are shown in A′′– E′′. Areas demarcated by the dashed boxes in A′–E′′ are enlarged in panels A′′′– E′′′. *A′′′– E′′′: areas of colocalization. Overlays of each combination with preabsorption controls are shown in A′′′′– E′′′′. The scale bar in E′′ represents 50 µm and applies to A′– E′′. All scale bars in the enlarged images represent 10 µm. The scale bar in E′′′′ represents 50 µm and applies to A′′′′– E′′′′. Images are representative of labeling performed on tissue from a minimum of three mice. GFAP, glial fibrillary acidic protein; M1R, muscarinic type 1 receptor; M2R, muscarinic type 2 receptor; M3R, muscarinic type 3 receptor; M4R, muscarinic type 4 receptor; M5R, muscarinic type 5 receptor.
Muscarine drives robust glial Ca2+ responses independent of neuronal depolarization.
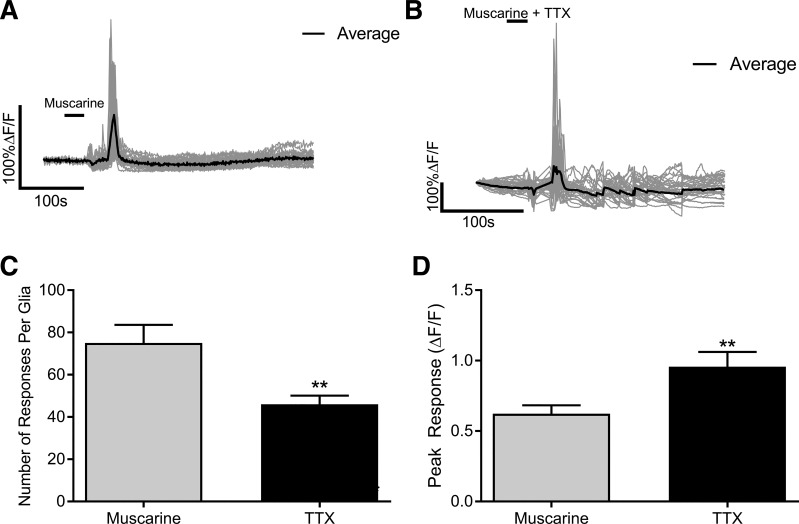
M3 and M5Rs couple to Gq signal transduction pathways that lead to increases in intracellular Ca2+ (11). Therefore, we used Ca2+ imaging to determine whether glial MRs are functional. In these experiments, we used Sox10CreERT2::Polr2atm1(CAG-GCaMP5g,-tdTomato)Tvrd mice to study glial Ca2+ responses selectively (34) and to avoid the confounding effects of other nearby cells. We first stimulated myenteric plexus preparations with the prototypical muscarinic agonist muscarine (20 µM; Fig. 2A). A 30-s application of muscarine evoked numerous individual glial Ca2+ responses (75 on average) with an average magnitude of 0.62 ± 0.06 change in fluorescence (Fig. 2, C and D). We next used TTX (1 µM) to test whether glial responses to muscarine require reciprocal interactions with neurons (Fig. 2B). Although TTX did not abolish glial-Ca2+ responses to muscarine, it did significantly reduce the number of responses per glia by 46% (P = 0.0026; Fig. 2C) and significantly increased the glial peak response by 43% (P = 0.0059; Fig. 2D). Together with our immunohistochemistry data above, these data show that glia are capable of directly responding to muscarinic agonists. However, the bath application of muscarine also evokes intercellular neuron-glia communication that contributes to cholinergic transmission in the ENS.
Fig. 2.
Muscarine drives Ca2+ responses in enteric glia that are independent of neuronal activation. Representative Ca2+ responses evoked by muscarine in enteric glia in the absence (A) or presence of tetrodotoxin (TTX; B). Gray traces show the responses of individual glial cells and the averaged response of all glia within the ganglion is shown in the black trace. Quantification of the effects of TTX in the number (C) and peak response (D) of glial Ca2+ responses induced by muscarine (n = 26–34 glia from 3 to 4 mice; Student’s t-test; **P < 0.01). ΔF/F, change in fluorescence over time.
Glial Ca2+ responses evoked by muscarine are primarily mediated through M3Rs.
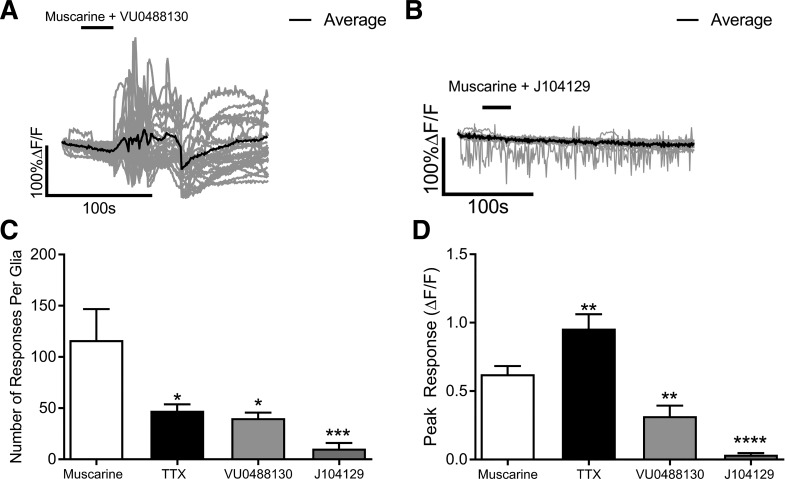
We next conducted experiments with M3 and M5 selective drugs to test the relative contribution of each receptor subtype to muscarine-driven glial Ca2+ responses. In these experiments, we again used Sox10CreERT2::Polr2atm1(CAG-GCaMP5g,-tdTomato)Tvrd mice to study glial Ca2+ responses selectively. We began by testing the contribution of M5Rs to glial Ca2+ responses by using the selective M5R antagonist VU0488130 (Fig. 3). Muscarine still reliably evoked glial Ca2+ responses in the presence of 10 µM VU0488130 (Fig. 3A), but the number of responses per enteric glia was significantly reduced by 65% (P = 0.0108; Fig. 3C), and glial peak responses were significantly reduced by 64% compared with muscarine alone (P = 0.0045; Fig. 3D). To determine the contribution of M3Rs to muscarine-induced glial Ca2+ responses, we used the potent and selective M3R antagonist J104129 (100 nM). Treatment with J104129 alone was sufficient to abolish glial Ca2+ responses (Fig. 3B), significantly reducing the number of individual glial responses (P = 0.0009; Fig. 3C) and the peak response (P = 0.0001; Fig. 3D). Together, these data suggest that glial Ca2+ responses to muscarine are primarily mediated through M3Rs, but M5Rs also contribute to the response.
Fig. 3.
Glial Ca2+ responses driven by muscarine are primarily mediated through M3Rs. Representative glial Ca2+ responses evoked by muscarine in the presence of the M5R selective antagonist VU0488130 (A) or the M3R selective antagonist J104129 (B). Gray traces show the responses of individual glial cells and the averaged response of all glia within the ganglion is shown in the black trace. Quantification of the effects of VU0488130 or J104129 on the number (D) and peak response (E) of glial Ca2+ responses induced by muscarine. (n = 21–39 glia from 4 to 5 mice; one-way ANOVA with multiple comparisons; *P < 0.05; **P < 0.005; ***P = 0.0009; ****P < 0.0001). ΔF/F, change in fluorescence over time; J104129, (αR)-α-cyclopentyl-α-hydroxy-N-[1-(4-methyl-3-pentenyl)-4-piperidinyl]benzeneacetamide fumarate; M3R, muscarinic type 3 receptor; M5R, muscarinic type 5 receptor; TTX, tetrodotoxin; VU0488130, 5-[(3-acetylphenoxy)methyl]-N-methyl-N-[(1S)-1-pyridin-2-ylethyl]-1,2-oxazole-3-carboxamide.
Selective activation of glial M3Rs in GFAP::hM3Dq mice modulates distension evoked motility reflexes.
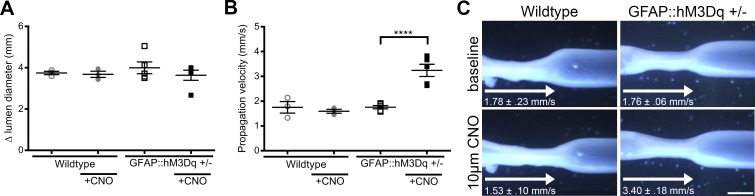
The observation that M3R activation is the major component of glial calcium responses suggest that M3R activation on enteric glia plays a role in the functional regulation of ENS reflexes. To test this notion, we used a chemogenetic model that expresses a modified hM3Dq receptor under the transcriptional control of the GFAP promoter (GFAP::hM3Dq; 32). This is an ideal model to study the effects of glial M3 receptor activation specifically because the hM3Dq is an M3R that can be selectively activated by the agonist CNO. This allows a clear assessment of the downstream effects subsequent to the stimulation of glial M3R signaling. In these experiments, we found no significant difference in lumen diameter between GFAP::hM3Dq and WT mice in both baseline conditions (GFAP::hM3Dq, 3.99 ± 0.28 mm; WT, 3.74 ± 0.08) and in the presence of 10 µM CNO (GFAP::hM3Dq, 3.63 ± 0.25 mm; WT, 3.69 ± 0.23; Fig. 4A). Propagation velocity in the presence of 10 µM CNO was significantly increased in the GFAP::hM3Dq mice [3.40 ± 0.18 mm/s as compared with WT (1.527 ± 0.10 mm/s) Fig. 4B]. However, there were no significant differences in propagation velocity at baseline conditions (GFAP::hM3Dq, 1.76 ± 0.06 mm/s; WT, 1.78 ± 0.23 mm/s). These data suggest that glial M3R activation has the ability to potentiate functional reflexes in the ENS.
Fig. 4.
Selective activation of glial hM3Dq receptors with clozapine-n-oxide (CNO) modulates distention evoked motility reflexes. A: quantification of proximal colon lumen diameter from wildtype and GFAP::hM3Dq+/− mice in baseline conditions and in the presence of 10 µM CNO. (n = 3–5 mice; one-way ANOVA with multiple comparisons). B: propagation velocity from wildtype and GFAP::hM3Dq+/− mice in baseline conditions and in the presence of 10 µM CNO. (n = 3–5 mice; one-way ANOVA with multiple comparisons; ****P < 0.0001). C: representative images of distention evoked motility reflexes in the proximal colon of wild-type and GFAP::hM3Dq +/− mice at baseline or in the presence of 10 µM CNO. Scale bar represents 250 µm and applies to all images. Δ, change in; GFAP, glial fibrillary acidic protein; hM3Dq, modified human M3R; M3R, muscarinic type 3 receptor.
Cholinergic activation of enteric glia does not modify purine-driven neurodegeneration.
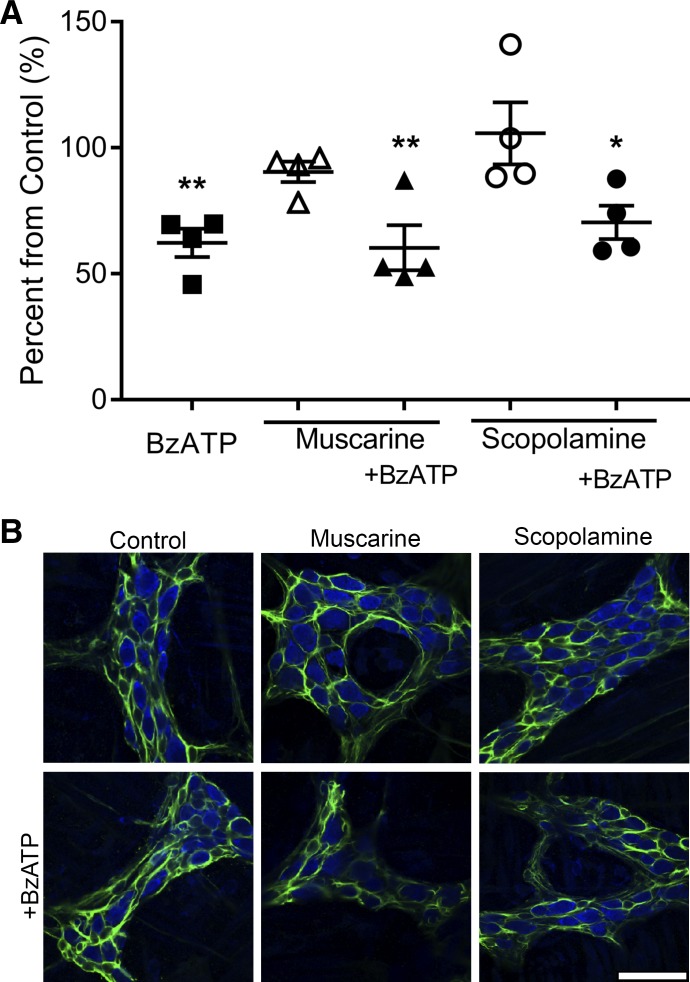
Purinergic signaling has a pivotal role in the modulation of inflammation in the colon (46). We and others have shown that ATP levels are significantly elevated during colitis (3, 9) and that this increase in ATP levels drives enteric neuron death through neuronal P2X7 receptors (P2X7Rs; 25) and decreases cholinergic signaling in the myenteric plexus (52). In addition, cholinergic transmission affects immune cell proliferation, cytokine production, T-helper differentiation, and antigen presentation providing anti-inflammatory benefits (29, 41, 45). This suggests that cholinergic anti-inflammatory pathways could compete and protect against purinergic proinflammatory pathways. To determine if ACh signaling through MRs modulates P2X7R-mediated neuron death in inflammation, we used an in situ model of neuroinflammation in whole-mount preparations of myenteric plexus to study potential interactions between the purinergic and cholinergic activation of glia (Fig. 5). Similar to our previous data, 300 µM BzATP, a P2X7R agonist, was sufficient to drive a significant 36% reduction in myenteric neuron-packing density (P = 0.0082; 9). Muscarine (20 µM) alone was not sufficient to drive neuron loss in situ and neurodegeneration in the presence of 20 µM muscarine and 300 µM BzATP was comparable (40% reduction, P = 0.0054) to neurodegeneration in the presence of BzATP alone. This suggests that glial MR activation does not potentiate or protect against P2X7R-mediated neurodegeneration.
Fig. 5.
Muscarine does not drive neurodegeneration in situ. Quantification (A) and representative images (B) of mean packing density of HuC/D-immunoreactive neurons in the myenteric ganglia after in situ activation of P2X7 receptors with BzATP; activation of muscarinic receptors with muscarine in the presence or absence of BzATP; or the nonselective muscarinic receptor antagonist scopolamine in the presence or absence of BzATP. (n = 4 mice; one-way ANOVA with multiple comparisons; *P < 0.05; **P < 0.01). Enteric neurons are labeled with HuC/D (blue) and enteric glia are labeled with GFAP (green) in all panels. Scale bar represents 20 µm and applies to all images. BzATP, 2′(3′)-O-(4-benzoylbenzoyl)adenosine 5′-triphosphate triethylammonium salt; GFAP, glial fibrillary acidic protein.
Increases in ATP during inflammation contribute to decreases in ACh levels (52). Therefore, we tested if decreasing ACh signaling through MRs potentiates P2X7R-mediated neuron death. To this end, we used 10 µM scopolamine, a nonspecific muscarinic receptor antagonist, to prevent muscarine receptor activation. Scopolamine (10 µM) alone did not drive neurodegeneration and treatment of 10 µM scopolamine in conjunction with 300 µM BzATP did not potentiate or prevent neuronal loss (30% neuron loss, P = 0.042). Together, these data suggest muscarinic receptors are not involved in pathophysiological signaling pathways in the ENS.
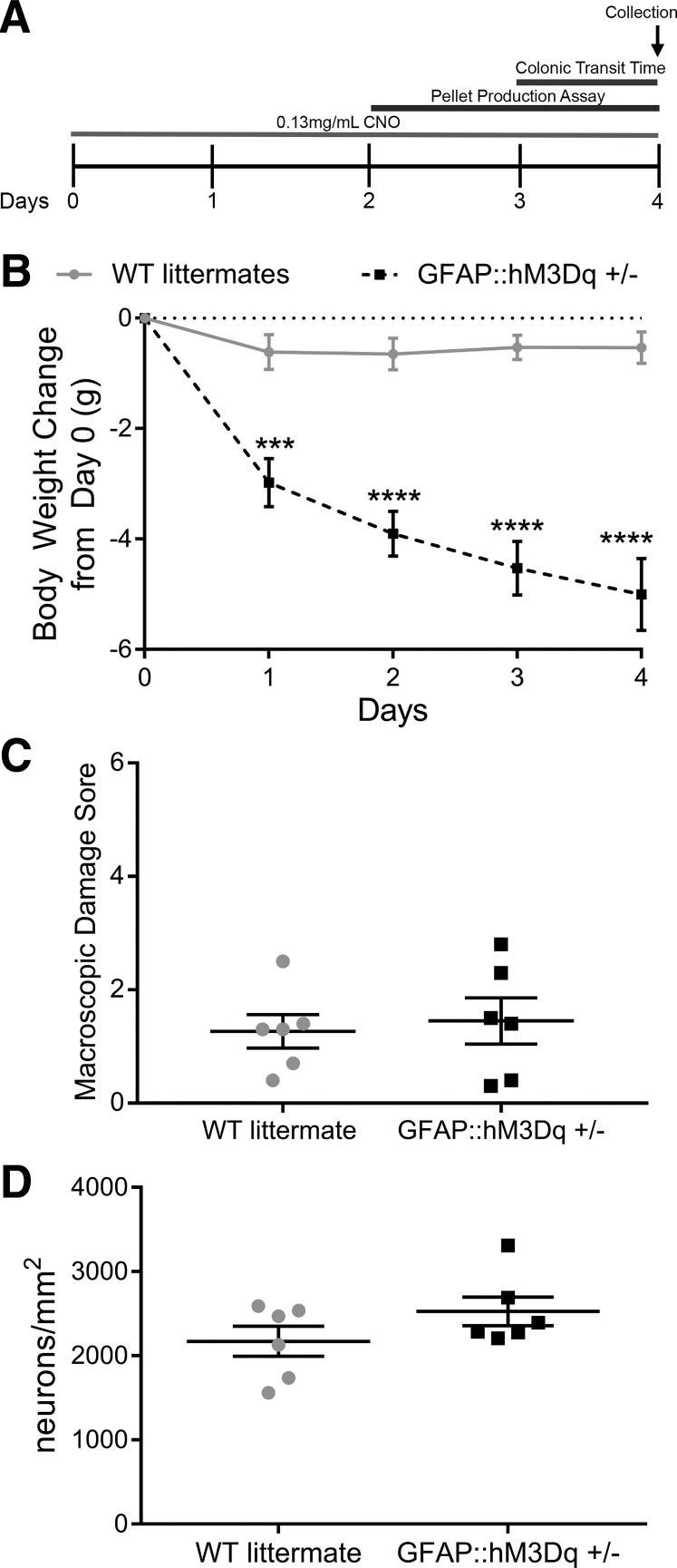
Chronic stimulation of glial M3Rs in vivo drives weight loss but does not induce overt inflammation or affect neuron survival.
To assess potential pathophysiological contributions of glial muscarinic receptor activation pathways, we chronically activated glial M3R pathways over 4 days by administering CNO (0.13 mg/ml) to the drinking water of GFAP::hM3Dq+/− mice and WT littermates (Fig. 6A). In agreement with earlier reports (55), CNO treatment alone drove significant body weight loss in GFAP::hM3Dq+/− mice compared with WT littermates (P < 0.0001; Fig. 6B). This effect is likely caused by increased heart rate in this model (2, 55). However, the chronic stimulation of glial M3 receptors did not increase macroscopic damage to the distal colon (P = 0.7231; Fig. 6C) or drive neurodegeneration (P = 0.1823; Fig. 6D). These in vivo data are in agreement with our in vitro data and show that cholinergic signaling in enteric glia mediates functional but not pathophysiological pathways.
Fig. 6.
Continuous stimulation of glial hM3Dq receptors with clozapine-n-oxide (CNO) in vivo decreases mouse body weight without decreasing neuronal survival. Schematic representation of the timeline of assays performed on WT littermate and GFAP::hM3Dq mice (A). Weight loss pattern at 4 days after start of treatment with 0.13 mg/ml CNO in water (n = 6 mice; two-way ANOVA with multiple comparisons; ***P = 0.002; ****P < 0.0001; B). In vivo stimulation of GFAP::hM3Dq with CNO does not increase macroscopic damage (C) or decrease neuronal survival (D; n = 6 mice; Student’s t-test). GFAP, glial fibrillary acidic protein; hM3Dq, modified human M3R; M3R, muscarinic type 3 receptor; WT, wild-type.
Chronic activation of glial cholinergic pathways alters gastrointestinal motility.
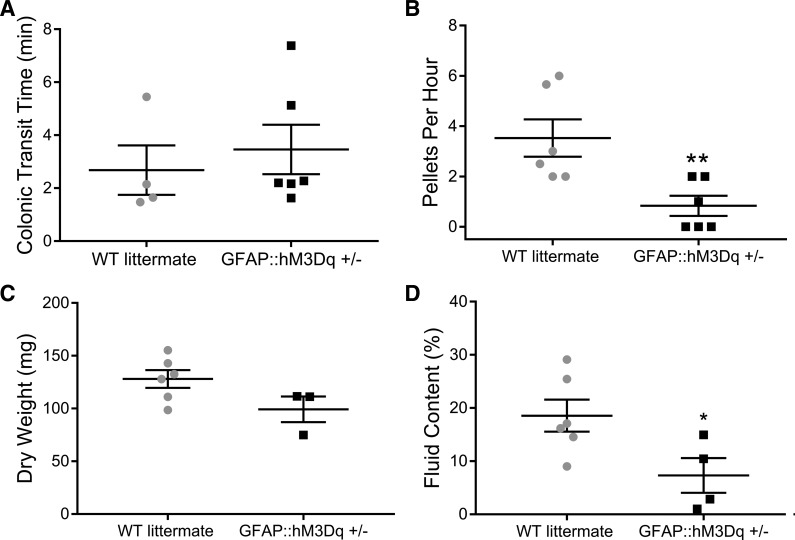
We next assessed how the chronic stimulation of glial M3Rs affects gastrointestinal motility in vivo (Fig. 7). The chronic treatment with CNO did not significantly alter colonic transit time as measured by the colon bead assay in GFAP::hM3Dq+/− mice (Fig. 7A), but fecal pellet production was significantly reduced by 60% compared with WT littermates (P = 0.0098; Fig. 7B). Pellet dry weight was not significantly different between groups (Fig. 7C), but chronic glial M3R stimulation did significantly reduce pellet fluid content in GFAP::hM3Dq+/− mice by 87% compared with WT littermates (P = 0.04; Fig. 7D).
Fig. 7.
Continuous activation of glial hM3Dq receptors in vivo decreases pellet production and pellet fluid content without increasing colonic transit time. Effects of 0.13 mg/ml CNO in drinking water on colonic transit time (A); endogenous pellet production (B); pellet dry weight (C) and pellet water content (D) from GFAP::hM3Dq mice and WT littermates. (n = 3–6 mice; Student’s t-test; *P < 0.05; **P < 0.02). CNO, clozapine-N-oxide; GFAP, glial fibrillary acidic protein; hM3Dq, modified human M3R; M3R, muscarinic type 3 receptor; WT, wild-type.
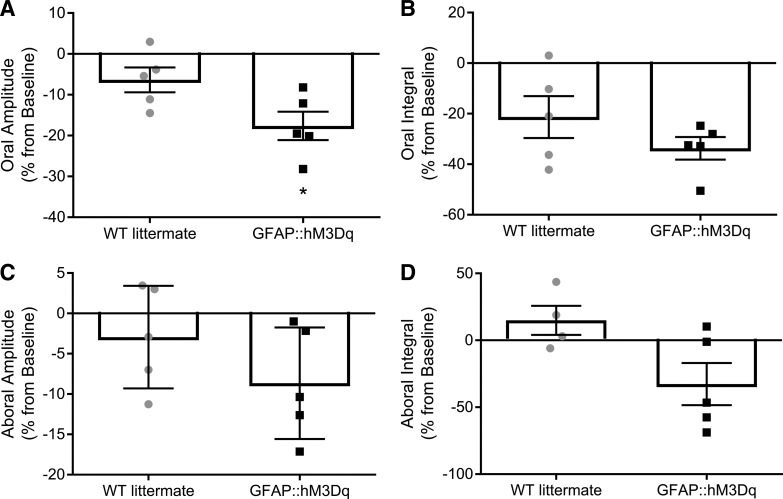
We also tested how chronic glial M3R activation affects the ongoing pattern of CMMCs in the isolated colon. Similar to our published data, we observed spontaneous, regular CMMCs in both GFAP::hM3Dq+/− mice and WT littermates (32). However, oral CMMC contraction amplitude was reduced by 93% in colons from mice with chronic glial M3R stimulation as compared with WT littermate controls (P = 0.0402; Fig. 8A). Although chronic CNO treatment trended to decrease other contractile aspects of CMMCs, we did not observe any other significant changes (Fig. 8). This is in agreement with other studies demonstrating that muscarinic receptors are involved in the excitatory, but not inhibitory, component of CMMCs (28). Overall, our data suggest that the cholinergic activation of enteric glial cells is primarily a physiological receptor pathway.
Fig. 8.
Continuous activation of glial hM3Dq receptors in vivo decreases oral contraction amplitude of colonic migrating motor complexes (CMMCs). Effects of 0.13 mg/ml CNO in drinking water on CMMC characteristics including oral contraction amplitude (A) and integral (B); and aboral contraction amplitude (C) and integral (D). All data are expressed as percentage change from baseline after acclimation period. (n = 4–6 mice; Student’s t-test; *P < 0.05). CNO, clozapine-N-oxide; GFAP, glial fibrillary acidic protein; hM3Dq, modified human M3R; M3R, muscarinic type 3 receptor; WT, wild-type.
DISCUSSION
The ENS regulates gastrointestinal reflexes by precise interactions between enteric neurons and glia (19). Intraganglionic enteric glia are a distinct type of peripheral glia that surround enteric neurons and play important roles in regulating neuronal function, activity, and survival (23). Enteric glial activity is recruited by multiple neurotransmitters and modulators. However, it is still not clear if enteric glia can sense and respond to ACh, the principal excitatory neurotransmitter in the ENS. Here, we addressed this issue by testing the hypothesis that enteric glia detect ACh and that glial activation by ACh contributes to physiological regulation of gut functions. Our results show that enteric glia express two subtypes of muscarinic receptors and the stimulation of these receptors induces Ca2+ responses independent of neuronal depolarization, primarily through M3Rs. Importantly, the direct activation of glial hM3Dq receptors enhanced distension-evoked motility reflexes and the continuous stimulation of glial hM3Dq receptors in vivo drove changes in pellet production and colonic contractions without affecting neuronal survival in the ENS. Together, our data provide the first evidence, to our knowledge, for functional MRs on enteric glia and suggest that activation of glial MRs contributes to cholinergic pathways in the ENS.
MR signaling is well established in other glial cells, including PSCs, astrocytes, oligodendrocytes, and Müller cells (14, 15, 21, 43, 51, 53, 54). PSCs express MRs that when activated can protect against GFAP upregulation associated with glial reactivity (21). Cortical and cochlear astrocytes express multiple MR subtypes and muscarine stimulation drives intracellular Ca2+ responses (42, 43, 51). Similarly, Müller cells, a type of retinal glial cell, express functional M1Rs in culture (54). The myelinating glial cells of the central nervous system, oligodendrocytes, also express functional MRs that upon activation enhance oligodendrocyte proliferation (15).
The similarities between enteric glia and these other populations of neuroglia provide strong rationale for our original hypothesis that enteric glia express functional muscarinic receptors. In support, we found that enteric glia predominantly express M3Rs and M5Rs. Both M3Rs and M5Rs signal through Gq transduction cascades that involve phosphoinositol and the release of Ca2+ from intracellular stores (11). Therefore, we used Ca2+ imaging to understand the mechanisms of MR activation on enteric glia. We found that muscarine drives Ca2+ responses in enteric glia. Interestingly, TTX treatment significantly reduced the number of responses per glia to muscarine, suggesting there is a neuronal component to muscarine-induced glial Ca2+ signaling. Our data using MR subtype selective drugs demonstrate that muscarine-mediated glial Ca2+ responses are primarily driven through M3Rs because antagonizing M3Rs completely abolished glial responses. However, antagonizing M5Rs also significantly reduced glial Ca2+ responses. These data suggest that glial M5Rs may also play a role in cholinergic signaling but to a lesser extent.
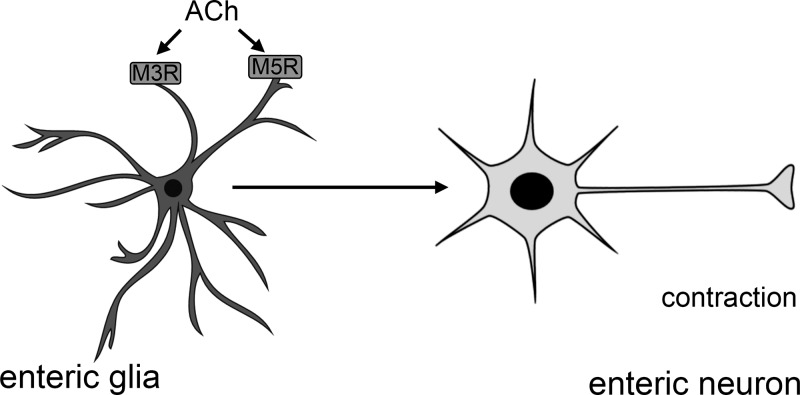
ACh is the principal excitatory neurotransmitter in the ENS and the stimulation of MRs is involved in neurotransmission mediating intestinal muscle reflexes and ion secretion within the gut (13, 17). Importantly, enteric glia modulate enteric neuronal networks involved in gastrointestinal motility and the stimulation of glial Ca2+ responses has strong, excitatory effects on motor circuits (32). Additionally, enteric glial activity contributes to the regulation of electrogenic ion transport in the intestine through effects on enteric neuronal circuits (24). Our current data suggest that the cholinergic activation of glia contributes to the regulation of these gastrointestinal reflexes (Fig. 9). Enteric glia could function similar to astrocytes in tripartite synapses to detect ACh and respond with release of gliotransmitters to modulate synaptic strength and neuronal excitability (1, 44). In support, stimulation of MRs on cortical astrocytes elicits gliotransmitter release to enhance synaptic plasticity in the somatosensory cortex (50). This suggests that glial M3R activation may function to enhance synaptic strength and potentiate excitatory reflexes through gliotransmitter release. However, more work is needed to decipher the exact mechanisms and gliotransmitters involved.
Fig. 9.
Schematic model of muscarinic receptor activation on enteric glia. Enteric glia express M3Rs and M5Rs that could detect acetylcholine (ACh) and respond to modulate synaptic strength and neuronal excitability, potentially through the release of gliotransmitters. M3R, muscarinic type 3 receptor; M5R, muscarinic type 5 receptor.
Enteric glia are actively involved in pathophysiological pathways during inflammation in the ENS. Purines are an important mediator that drives pathophysiological glial mechanisms including reactive gliosis (9) and neurodegeneration (25). Increased purine levels during colitis (9, 52) also contribute to decreases in ACh levels (52). This suggests that changes in the cholinergic activation of glia could contribute to pathophysiological pathways in the ENS. Our data show that MRs on enteric glia primarily mediate physiological and not pathophysiological pathways in the ENS. The activation of MRs in an in situ model of neuroinflammation did not drive neuron death and did not potentiate P2X7R-mediated neuron loss. Similarly, antagonizing MRs had no effect on P2X7R-mediated neuron death. This is important because MRs are important mediators of enteric neurotransmission (13, 17, 28). In addition, both MRs and the purinergic P2Y1 receptor signal through Gq transduction cascades that involve phosphoinositol and the release of Ca2+ from intracellular stores (4, 11). Activation of glial P2Y1 drives enteric neuron death (9); in contrast, MR activation on enteric glia did not drive enteric neuron death in this study, suggesting enteric glia have intracellular signaling specificity. However, more work is needed to elucidate the mechanisms that drive this intracellular signaling specificity. Similarly, chronic stimulation of glial M3Rs did not drive inflammation or neuron death. Together, these results strongly suggest that enteric glial MRs are primarily involved in physiological signaling in the ENS.
MRs, particularly M3Rs, are functionally significant in mediating ACh-induced smooth muscle contraction and motility reflexes (30). However, the majority of studies on M3Rs in the intestine have focused on isolated smooth muscle cell preparations. Here we show that M3Rs are also expressed by enteric glia. This observation suggests that some of the functional effects of M3Rs on gastrointestinal motility could be mediated through MRs on enteric glia. In this study, we used a chemogenetic model that expresses a modified human M3 (hM3Dq) receptor under the transcriptional control of a GFAP promotor (GFAP::hM3Dq+/−) (32) to specifically assess the role of glial M3Rs and avoid the potential confounding effects of MRs expressed by other cell types. These modified M3 receptors are designer receptors that are activated by the hM3Dq agonist CNO and allowed us to selectively stimulate M3R pathways in glial cells. Importantly, hM3Dq receptors stimulate the same phosphoinositol cascade as endogenous M3Rs and provide an effective model to study downstream mechanisms (2). Using this model, we demonstrate that direct activation of M3R pathways on enteric glia enhances distension-evoked motility reflexes. These data support the conclusion that glial M3R signaling contributes to the regulation of gastrointestinal motility.
Enteric glia play an active role in the regulation of enteric circuits that exert control over motility and manipulating enteric glia affects gastrointestinal functions (8, 32–34, 37). The chronic stimulation of glia M3Rs in vivo did not drive neurodegeneration, overt intestinal inflammation, or other major changes to gut health. This is important, because it shows that the effects of glial activation on neural circuits are receptor dependent and not merely an effect of general glial activation. For example, purinergic pathways may have a more prominent role driving glial responses involved in neuroinflammation whereas cholinergic pathways may be more prominent in modulating physiological reflexes. However, the chronic stimulation of glial M3Rs did drive some alterations to motility and to body weight. Changes in body weight are likely caused by the activation of other populations of peripheral and central glia in this in vivo model that modulate metabolism, cardiovascular function, locomotor activity, salivation, and body temperature (2, 55). Similarly, high metabolism driven by the chronic CNO treatment in our study also likely explains the decrease in fecal pellet fluid content and endogenous pellet production observed. Although we did not find changes in propulsive motility using a colon bead assay, we did observe a significant decrease in CMMC oral contractile amplitude. The maintenance of CMMCs requires functional connectivity of neuronal and glial networks (32). Therefore, disrupting enteric glia in our chronic stimulation model had a major effect on CMMCs. In contrast, it is likely that redundant mechanisms are in place to ensure propulsive motility is maintained and as such we did not observe significant changes in our chronic stimulation model. Importantly, the decrease in CMMC oral contractile amplitude suggests that local changes to enteric glia, such as the desensitization of enteric glial M3Rs and/or a decrease in the neurotransmitter pool, could contribute to the effects on motility.
Together, our results provide the first evidence, to our knowledge, of functional muscarinic receptors on enteric glia. Importantly, our results show that activation of glial hM3Dq receptors has effects on gut physiology through modulation of excitatory reflexes involved in gastrointestinal motility. Thus, enteric glia could detect ACh release and enhance enteric neuron excitability possibly through the release of glial mediators. This is particularly important when considering gastrointestinal disorders such as slow-transit constipation where glial network activity is disrupted (5). In these disorders, modifying M3R activity on enteric glia could provide a novel therapeutic target to restore gastrointestinal motility.
GRANTS
This project was supported by grants to B. D. Gulbransen from the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK-103723) and the Crohn’s and Colitis Foundation of America (Senior Research Award 327058).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.M.D., D.E.F., and B.D.G. conceived and designed research; N.M.D., D.E.F., G.R.-L., and L.G. performed experiments; N.M.D., D.E.F., G.R.-L., L.G., and B.D.G. analyzed data; N.M.D., D.E.F., G.R.-L., L.G., and B.D.G. interpreted results of experiments; N.M.D., D.E.F., and G.R.-L. prepared figures; N.M.D. drafted manuscript; N.M.D., D.E.F., and B.D.G. edited and revised manuscript; N.M.D., D.E.F., G.R.-L., L.G., and B.D.G. approved final version of manuscript.
REFERENCES
- 1.Acton D, Miles GB. Gliotransmission and adenosinergic modulation: insights from mammalian spinal motor networks. J Neurophysiol 118: 3311–3327, 2017. doi: 10.1152/jn.00230.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agulhon C, Boyt KM, Xiaoqiao X, Friocourt F, Roth BL, McCarthy KD. Modulation of the autonomic nervous system and behaviour by acute glial cell Gq protein- coupled receptor activation in vivo. J Physiol 591: 5599–5609, 2013. doi: 10.1113/jphysiol.2013.261289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonioli L, Giron MC, Colucci R, Pellegrini C, Sacco D, Caputi V, Orso G, Tuccori M, Scarpignato C, Blandizzi C, Fornai M. Involvement of the P2X7 purinergic receptor in colonic motor dysfunction associated with bowel inflammation in rats. PLoS One 9: e116253, 2014. doi: 10.1371/journal.pone.0116253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barańska J, Czajkowski R, Pomorski P. P2Y1 receptors- properties and functional activities. Adv Exp Med Biol 1051: 71–89, 2017. doi: 10.1007/5584_2017_57. [DOI] [PubMed] [Google Scholar]
- 5.Bassotti G, Villanacci V, Maurer CA, Fisogni S, Di Fabio F, Cadei M, Morelli A, Panagiotis T, Cathomas G, Salerni B. The role of glial cells and apoptosis of enteric neurons in the neuropathology of intractable slow transit constipation. Gut 55: 41–46, 2006. doi: 10.1136/gut.2005.073197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boesmans W, Cirillo C, Van den Abbeel V, Van den Haute C, Depoortere I, Tack J, Vanden Berghe P. Neurotransmitters involved in fast excitatory neurotransmission directly activate enteric glial cells. Neurogastroenterol Motil 25: e151–e160, 2013. doi: 10.1111/nmo.12065. [DOI] [PubMed] [Google Scholar]
- 7.Boesmans W, Martens MA, Weltens N, Hao MM, Tack J, Cirillo C, Vanden Berghe P. Imaging neuron-glia interactions in the enteric nervous system. Front Cell Neurosci 7: 183, 2013. doi: 10.3389/fncel.2013.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broadhead MJ, Bayguinov PO, Okamoto T, Heredia DJ, Smith TK. Ca2+ transients in myenteric glial cells during the colonic migrating motor complex in the isolated murine large intestine. J Physiol 590: 335–350, 2012. doi: 10.1113/jphysiol.2011.219519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown IAM, McClain JL, Watson RE, Patel BA, Gulbransen BD. Enteric glia mediate neuron death in colitis through purinergic pathways that require connexin-43 and nitric oxide. Cell Mol Gastroenterol Hepatol 2: 77–91, 2016. doi: 10.1016/j.jcmgh.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bubenheimer RK, Fried DE, McClain JL, Gulbransen BD. Sirtuin-3 is expressed by enteric neurons but it does not play a major role in their regulation of oxidative stress. Front Cell Neurosci 10: 73, 2016. doi: 10.3389/fncel.2016.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev 50: 279–290, 1998. [PubMed] [Google Scholar]
- 12.Christofi FL, Zhang H, Yu JG, Guzman J, Xue J, Kim M, Wang YZ, Cooke HJ. Differential gene expression of adenosine A1, A2a, A2b, and A3 receptors in the human enteric nervous system. J Comp Neurol 439: 46–64, 2001. doi: 10.1002/cne.1334. [DOI] [PubMed] [Google Scholar]
- 13.Cooke HJ. Neurotransmitters in neuronal reflexes regulating intestinal secretion. Ann N Y Acad Sci 915: 77–80, 2000. doi: 10.1111/j.1749-6632.2000.tb05225.x. [DOI] [PubMed] [Google Scholar]
- 14.Costa M, Brookes SJ, Steele PA, Gibbins I, Burcher E, Kandiah CJ. Neurochemical classification of myenteric neurons in the guinea-pig ileum. J Neurosci 75: 949–967, 1996. doi: 10.1016/0306-4522(96)00275-8. [DOI] [PubMed] [Google Scholar]
- 15.De Angelis F, Bernardo A, Magnaghi V, Minghetti L, Tata AM. Muscarinic receptor subtypes as potential targets to modulate oligodendrocyte progenitor survival, proliferation, and differentiation. Dev Neurobiol 72: 713–728, 2012. doi: 10.1002/dneu.20976. [DOI] [PubMed] [Google Scholar]
- 16.De Giorgio R, Bianco F, Latorre R, Caico G, Clavenzani P, Bonora E. Eneric neuropahies: yesterday, today and tomorrow. Adv Exp Med Biol 891: 123–133, 2016. doi: 10.1007/978-3-319-27592-5_12. [DOI] [PubMed] [Google Scholar]
- 17.Eglen RM. Muscarinic receptors and gastrointestinal tract smooth muscle function. Life Sci 68: 2573–2578, 2001. doi: 10.1016/S0024-3205(01)01054-2. [DOI] [PubMed] [Google Scholar]
- 18.Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol 817: 39–71, 2014. doi: 10.1007/978-1-4939-0897-4_3. [DOI] [PubMed] [Google Scholar]
- 19.Furness JB. The enteric nervous system: normal functions and enteric neuropathies. Neurogastroenterol Motil 20, Suppl 1: 32–38, 2008. doi: 10.1111/j.1365-2982.2008.01094.x. [DOI] [PubMed] [Google Scholar]
- 20.Garrido R, Segura B, Zhang W, Mulholland M. Presence of functionally active protease activated receptors 1 and 2 in myenteric glia. J Neurochem 83: 556–564, 2002. doi: 10.1046/j.1471-4159.2002.01119.x. [DOI] [PubMed] [Google Scholar]
- 21.Georgiou J, Robitaille R, Charlton MP. Muscarinic control of cytoskeleton in perisynaptic glia. J Neurosci 19: 3836–3846, 1999. doi: 10.1523/JNEUROSCI.19-10-03836.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomes P, Chevalier J, Bosemans W, Roosen L, van den Abbeel V, Neunlist M, Tack J, Vanden Berghe P. ATP-dependent paracrine communication between enteric neurons and glia in a primary cell culture derived from embryonic mice. Neurogastroenterol Motil 21: 870–e62, 2009. doi: 10.1111/j.1365-2982.2009.01302.x. [DOI] [PubMed] [Google Scholar]
- 23.Grubišić V, Gulbransen BD. Enteric glia: the most alimentary of glia. J Physiol 595: 557–570, 2017. doi: 10.1113/JP271021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grubišić V, Gulbransen BD. Enteric glial activity regulates secretomotor function in the mouse colon but does not acutely affect gut permeability. J Physiol 595: 3409–3424, 2017. doi: 10.1113/JP273492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gulbransen BD, Bashashati M, Hirota SA, Gui X, Roberts JA, MacDonald JA, Muruve DA, McKay DM, Beck PL, Mawe GM, Thompson RJ, Sharkey KA. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med 18: 600–604, 2012. doi: 10.1038/nm.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gulbransen BD, Sharkey KA. Purinergic neuron-to-glia signaling in the enteric nervous system. Gastroenterology 136: 1349–13.58, 2009. doi: 10.1053/j.gastro.2008.12.058. [DOI] [PubMed] [Google Scholar]
- 27.Gulbransen BD. Enteric Glia. San Rafael, CA: Morgan & Claypool, 2014, p. 1–70. [Google Scholar]
- 28.Harrington AM, Hutson JM, Southwell BR. Cholinergic neurotransmission and muscarinic receptors in the enteric nervous system. Prog Histochem Cytochem 44: 173–202, 2010. doi: 10.1016/j.proghi.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Jeremias IC, Victorino VJ, Barberio HV, Kubo SA, Prado CM, Lima TM, Soriano FG. The role of acetylcholine in the inflammatory response in animals surviving sepsis induced by cecal ligation and puncture. Mol Neurobiol 53: 6635–6643, 2016. doi: 10.1007/s12035-015-9538-y. [DOI] [PubMed] [Google Scholar]
- 30.Kerr PM, Hillier K, Wallis RM, Garland CJ. Characterization of muscarinic receptors mediating contractions of circular and longitudinal muscle of human isolated colon. Br J Pharmacol 115: 1518–1524, 1995. doi: 10.1111/j.1476-5381.1995.tb16645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimball BC, Mulholland MW. Enteric glia exhibit P2U receptors that increase cytosolic calcium by a phospholipase C-dependent mechanism. J Neurochem 66: 604–612, 1996. doi: 10.1046/j.1471-4159.1996.66020604.x. [DOI] [PubMed] [Google Scholar]
- 32.McClain J, Fried DE, Gulbransen BD. Agonist-evoked Ca2+ signaling in enteric glia drives neural programs that regulate intestinal motility in mice. Cell Mol Gastroenterol Hepatol 1: 631–645, 2015. doi: 10.1016/j.jcmgh.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McClain JL, Grubisic V, Fried D, Gomez-Suarez RA, Leinninger GM, Sevigny J, Parpura V, Gulbransen BD. Ca2+ responses in enteric glia are mediated by connexin-43 hemichannels and modulate colonic transit in mice. Gastroenterology 146: 497–507, 2014. doi: 10.1053/j.gastro.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McClain JL, Gulbransen BD. The acute inhibition of enteric glial metabolism with fluoroacetate alters calcium signaling, hemichannel function and the expression of key proteins. J Neurophysiol 117: 365–375, 2017. doi: 10.1152/jn.00507.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McConalogue K, Furness JB. Gastrointestinal neurotransmitters. Baillieres Clin Endocrinol Metab 8: 51–76, 1994. doi: 10.1016/S0950-351X(05)80226-5. [DOI] [PubMed] [Google Scholar]
- 36.Murakami M, Ohta T, Otsuguro KI, Ito S. Involvement of prostaglandin E(2) derived from enteric glial cells in the action of bradykinin in cultured rat myenteric neurons. Neuroscience 145: 642–653, 2007. doi: 10.1016/j.neuroscience.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 37.Nasser Y, Fernandez E, Keenan CM, Ho W, Oland LD, Tibbles LA, Schemann M, MacNaughton WK, Rühl A, Sharkey KA. Role of enteric glia in intestinal physiology: effects of the gliotoxin fluorocitrate on motor and secretory function. Am J Physiol Gastrointest Liver Physiol 291: G912–G927, 2006. doi: 10.1152/ajpgi.00067.2006. [DOI] [PubMed] [Google Scholar]
- 38.Nasser Y, Ho W, Sharkey KA. Distribution of adrenergic receptors in the enteric nervous system of the guinea pig, mouse, and rat. J Comp Neurol 495: 529–553, 2006. doi: 10.1002/cne.20898. [DOI] [PubMed] [Google Scholar]
- 39.Nasser Y, Keenan CM, Ma AC, McCafferty DM, Sharkey KA. Expression of a functional metabotropic glutamate receptor 5 on enteric glia is altered in states of inflammation. Glia 55: 859–872, 2007. doi: 10.1002/glia.20507. [DOI] [PubMed] [Google Scholar]
- 40.Navarrete M, Perea G, Fernandez de Sevilla D, Gómez-Gonzalo M, Núñez A, Martín ED, Araque A. Astrocytes mediate in vivo cholinergic-induced synaptic plasticity. PLoS Biol 10: e1001259, 2012. doi: 10.1371/journal.pbio.1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nizri E, Brenner T. Modulation of inflammatory pathways by the immune cholinergic system. Amino Acids 45: 73–85, 2013. doi: 10.1007/s00726-011-1192-8. [DOI] [PubMed] [Google Scholar]
- 42.Oda S, Tsuneoka Y, Yoshida S, Adachi-Akahana S, Ito M, Kuroda M, Funato H. Immunolocalization of muscarinic M1 receptor in the rat medial prefrontal cortex. J Comp Neurol 526: 1329–1350, 2018. doi: 10.1002/cne.24409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pap P, Kőszeghy Á, Szűcs G, Rusznák Z. Cytoplasmic Ca2+ concentration changes evoked by cholinergic stimulation in primary astrocyte cultures prepared from the rat cochlear nucleus. Hear Res 255: 73–83, 2009. doi: 10.1016/j.heares.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 44.Papouin T, Dunphy J, Tolman M, Foley JC, Haydon PG. Astrocytic control of synaptic function. Philos Trans R Soc Lond B Biol Sci 372: 20160154, 2017. doi: 10.1098/rstb.2016.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol Med 9: 125–134, 2013. [PMC free article] [PubMed] [Google Scholar]
- 46.Roberts JA, Lukewich MK, Sharkey KA, Furness JB, Mawe GM, Lomax AE. The roles of purinergic signaling during gastrointestinal inflammation. Curr Opin Pharmacol 12: 659–666, 2012. doi: 10.1016/j.coph.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Segura BJ, Zhang W, Cowles RA, Xiao L, Lin TR, Logsdon C, Mulholland MW. Lysophosphatidic acid stimulates calcium transients in enteric glia. Neuroscience 123: 687–693, 2004. doi: 10.1016/j.neuroscience.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Segura BJ, Zhang W, Xiao L, Turner D, Cowles RA, Logsdon C, Mulholland MW. Sphingosine-1-phosphate mediates calcium signaling in guinea pig enteroglial cells. J Surg Res 116: 42–54, 2004. doi: 10.1016/S0022-4804(03)00281-6. [DOI] [PubMed] [Google Scholar]
- 49.Storr MA, Keenan CM, Zhang H, Patel KD, Makriyannis A, Sharkey KA. Activation of the cannabinoid 2 receptor (CB2) protects against experimental colitis. Inflamm Bowel Dis 15: 1678–1685, 2009. doi: 10.1002/ibd.20960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takata N, Mishima T, Hisatsune C, Nagai T, Ebisui E, Mikoshiba K, Hirase H. Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. J Neurosci 31: 18155–18165, 2011. doi: 10.1523/JNEUROSCI.5289-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Der Zee EA, De Jong GI, Strosberg AD, Luiten PG. Muscarinic acetylcholine receptor-expression in astrocytes in the cortex of young and aged rats. Glia 8: 42–50, 1993. doi: 10.1002/glia.440080106. [DOI] [PubMed] [Google Scholar]
- 52.Vieira C, Ferreirinha F, Magalhaes-Cardoso MT, Silva I, Marques P, Correia-de-Sa P. Post-inflammatory ileitis induces non-neuronal purinergic signaling adjustments of cholinergic neurotransmission in the myenteric plexus. Front Pharmacol 8: 811, 2017. doi: 10.3389/fphar.2017.00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Von Boyen GB, Steinkamp M, Adler G, Kirsch J. Glutamate receptor subunit expression in primary enteric glia cultures. J Recept Signal Transduct Res 26: 329–336, 2006. doi: 10.1080/10799890600778821. [DOI] [PubMed] [Google Scholar]
- 54.Wakakura M, Utsunomiya-Kawasaki I, Ishikawa S. Rapid increase in cytosolic calcium ion concentration mediated by acetylcholine receptors in cultured retinal neurons and Müller cells. Graefes Arch Clin Exp Ophthalmol 236: 934–939, 1998. doi: 10.1007/s004170050183. [DOI] [PubMed] [Google Scholar]
- 55.Xie AX, Lee JJ, McCarthy KD. Ganglionic GFAP+ glial Gq-GPCR signaling enhances heart functions in vivo. JCI Insight. 2: e90565, 2017. doi: 10.1172/jci.insight.90565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang W, Sarosi G, Barnhart D, Yule DI, Mulholland MW. Endothelin-activated calcium signaling in enteric glia derived from neonatal guinea pig. Am J Physiol Gastrointest Liver Physiol 272: G1175–G1185, 1997. doi: 10.1152/ajpgi.1997.272.5.G1175. [DOI] [PubMed] [Google Scholar]