Abstract
Cyclic GMP (cGMP) is an important intracellular regulator of endochondral bone growth and skeletal remodeling. Tadalafil, an inhibitor of the phosphodiesterase (PDE) type 5 (PDE5) that specifically hydrolyzes cGMP, is increasingly used to treat children with pulmonary arterial hypertension (PAH), but the effect of tadalafil on bone growth and strength has not been previously investigated. In this study, we first analyzed the expression of transcripts encoding PDEs in primary cultures of chondrocytes from newborn rat epiphyses. We detected robust expression of PDE5 as the major phosphodiesterase hydrolyzing cGMP. Time-course experiments showed that C-type natriuretic peptide increased intracellular levels of cGMP in primary chondrocytes with a peak at 2 min, and in the presence of tadalafil the peak level of intracellular cGMP was 37% greater (P < 0.01) and the decline was significantly attenuated. Next, we treated 1-mo-old Sprague Dawley rats with vehicle or tadalafil for 3 wk. Although 10 mg·kg−1·day−1 tadalafil led to a significant 52% (P < 0.01) increase in tissue levels of cGMP and a 9% reduction (P < 0.01) in bodyweight gain, it did not alter long bone length, cortical or trabecular bone properties, and histological features. In conclusion, our results indicate that PDE5 is highly expressed in growth plate chondrocytes, and short-term tadalafil treatment of growing rats at doses comparable to those used in children with PAH has neither obvious beneficial effect on long bone growth nor any observable adverse effect on growth plate structure and trabecular and cortical bone structure.
Keywords: bone, endochondral ossification, growth plate, PDE5 inhibitor, young rat
INTRODUCTION
In mammals, development and growth of skeletal long bones occur through a process of endochondral ossification in which the skeletal cartilage anlagen are replaced by mineralized bone tissue. Postnatally, endochondral ossification at the growth plate is essential for longitudinal bone growth (36). During this process, chondrocytes proliferate and undergo hypertrophy and apoptosis. A cartilage template is synthesized by the dividing chondrocytes, which is later invaded by blood vessels that bring osteoclasts that resorb the matrix. In turn, osteoblasts, the bone-forming cells, are recruited and deposit bone matrix on remnants of the cartilage model. With maturation of the skeleton and closure of epiphyseal growth plates, longitudinal growth of bone ceases. Nevertheless, skeletal remodeling persists throughout life as part of a continuous process of bone replacement and repair that involves a coordinated interplay between osteoclastic bone resorption and osteoblastic bone formation.
Multiple intracellular signals are necessary to regulate cartilage development and bone remodeling, and recently much interest has been focused on the role of intracellular cyclic GMP (cGMP), generated by both soluble and particulate forms of guanylate cyclase that are stimulated by nitric oxide and C-type natriuretic peptide (CNP), respectively (43, 48). Like cyclic AMP (cAMP), cGMP is an intracellular second messenger that mediates the action of various hormones and chemicals on a large variety of cells. In bone, proliferation of osteoblasts can be increased by mechanical stimulation via a mechanism that is cGMP-dependent and associated with nitric oxide-induced stimulation of soluble guanylate cyclase (sGC; 40). Interestingly, at least part of the anabolic effect of estrogen on osteoblasts appears to be mediated via this same pathway (24, 25, 29). For example, cinaciguat, a small molecule agonist of sGC, can reverse the effects of estrogen deficiency on bone, producing improved trabecular bone microarchitecture in ovariectomized animals with effect sizes similar to those obtained with estrogen (25). Similarly, osteoblasts and chondrocytes express the transmembrane receptor protein NPR2, a particulate guanylate cyclase that is activated upon binding of its primary ligand CNP (34, 39, 44). In chondrocytes, CNP-induced cGMP antagonizes FGFR3 signaling and thereby regulates the rate of chondrocyte hypertrophy (37). Genetic mutations that increase NPR2-dependent cGMP elevations in chondrocytes, either through increased serum CNP levels (8) or gain-of-function missense mutations of NPR2 (17, 30, 31), cause skeletal overgrowth in humans and mice (23). In addition, in mice the loss of Npr3, a decoy receptor for CNP and therefore an inhibitor for CNP action or overexpression of brain natriuretic peptide, another ligand for Npr2 in mice, also result in skeletal overgrowth (23, 45). By contrast, in humans inactivating mutations in one or both NPR2 alleles lead to proportionate decreases in synthesis of cGMP in patients with short stature or acromesomelic dysplasia (type Maroteaux dwarfism), respectively (4, 6, 13, 35, 49, 51, 52). Moreover, deletion of either the Cnp or Npr2 gene in mice results in severe dwarfism caused by reduced postnatal endochondral ossification (10, 49).
These examples provide compelling evidence for a concentration-dependent effect of cGMP on long bone growth and bone remodeling and suggest that pharmacological manipulation of chondrocyte cGMP levels might have important effects on skeletal development and statural growth. In children, agents that inhibit phosphodiesterase (PDE) type 5 (PDE5), which selectively hydrolyzes cGMP, are commonly used to treat pulmonary arterial hypertension (PAH), a disease associated with impaired growth, particularly in younger children (38). Approximately 6% to 18% of extremely low-birth weight infants (birth weight less than 1,000 g) may develop signs of PAH (3), with a median age of diagnosis of 112 days of life (7). Incidence data from the Netherlands have revealed an annual incidence and point prevalence of 0.7 and 4.4 for idiopathic PAH and 2.2 and 15.6 for PAH related to congenital heart disease cases per million children (50). Initial oral therapy usually consists of pulmonary vasodilators, including endothelin receptor antagonists or more commonly a PDE5 inhibitor (32) such as tadalafil (47) or sildenafil (5), which can increase levels of cGMP in vascular cells. There is greater enthusiasm for oral use of tadalafil (0.5–1 mg·kg−1·day−1) in children based on studies showing increased clinical efficacy and safety and improved compliance in children with PAH compared with sildenafil (42, 47). However, because of the importance of cGMP in skeletal development, a possible effect of tadalafil on bone growth in these young patients remains a theoretical concern. In this study, we first analyzed Pde5 expression profile in epiphyseal chondrocytes from newborn rats and then performed a comprehensive analysis of skeletal development in young rats receiving tadalafil to identify potential effect(s) of this PDE5 inhibitor on bone growth.
METHODS
Animals.
Male Sprague Dawley rats, purchased from Charles River, were grouped randomly and treated with the following reagents via oral gavage daily starting from 1 mo of age: vehicle (0.5% methyl cellulose; cat. no. M-638, Sigma-Aldrich, St. Louis, MO, 2.5 mg·kg−1·day−1 tadalafil, and 10 mg·kg−1·day−1 tadalafil (Lilly USA LLC, Indianapolis, IN) (n = 10 rats/group). Animals were weighed each week and euthanized 3 wk later for harvest of the long bone and brain. Additional 1-mo-old rats were treated with 10 mg/kg tadalafil and euthanized right before the treatment or at 3, 6, and 24 h after treatment (n = 3/time point) for harvest of the brain. In accordance with the standards for animal housing, rats were group-housed at 23°C–25°C with a 12-h light/dark cycle and allowed free access to water and standard laboratory pellets. All animal work performed in this report was approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania. The study design and organization respected the Animal Research: Reporting of In Vivo Experiments guidelines.
Primary chondrocyte culture and real-time RT-PCR.
Primary rat chondrocytes from newborn pups were harvested from the knee epiphyseal region and cultured as described previously (55). Cells were plated at a density of 4 × 104/cm2 in 6-well plates in growth medium (DMEM containing 15% FBS, 100 μg/ml streptomycin, and 100 U/ml penicillin). RNA was harvested when cells reached 90% confluence for qRT-PCR of PDEs using primers listed in Table 1.
Table 1.
Sequences of rat primers used for RT-PCR
| Gene | 5′ Primer | 3′ Primer |
|---|---|---|
| β-actin | CGCGAGTACAACCTTGC | CGCAGCGATATCGTCATCCA |
| Pde1a | CCACTTTGTGATCGGAAGTC | TTCTGCTGAATGATGTCCACC |
| Pde1b | CAGGGTGACAAGGAGGCAGAG | GACATCTGGTTGGTGATGCC |
| Pde1c | TCTCAAAGGATGACTGGAGG | GCTTCTCTGTCACCCTGTC |
| Pde2a | CCTCCTGTGACCTCTCTGACC | TGAACTTGTGGGACACCTTGG |
| Pde3a | TCACAGGGCCTTAACTTACAC | GGAGCAAGAATTGGTTTGTCC |
| Pde3b | CCTCAGGCAGTTTTATACAATG | TGCTTCTTCATCTCCCTGCTC |
| Pde4a | GTGGAGAAGTCTCAGGTGGG | TGGAACTTGTCAGGCAGGG |
| Pde4b | TAGAAGATAACAGGAACTGG | GCAATGTCTATGTCAGTCTC |
| Pde4c | ACGTGGCGTACCACAACAGC | TACCGCGAGGTGATGGTTCTC |
| Pde4d | GGATAATGGAGGAGTTCTTCC | CGATTGTCCTCCAAAGTGTCC |
| Pde5a | CCCTGGCCTATTCAACAACGG | GTGGGTCAGGGCCTCATACAG |
| Pde6a | CCGAGATTGTCTTCCCTTTG | GCTCATCCTCTTCTGTGTTG |
| Pde6b | TTCTCTGGTCAGCCAATAAG | GAGTACCGGTCACAGTTTAG |
| Pde6c | CCAGTTTCTTGGATGGTCTC | GGCTTTGGTGAGGTTCATAA |
| Pde7a | TGGACAAGCCAAGTGTATGCTG | TTTAAGTAACAGTGCATGGCC |
| Pde7b | AAAGCCCAGTGGAAGAGC | CGAAGGGAGGTGGTAAATG |
| Pde8a | CAACAAGCCTCTGAAAGC | TCGGTCTGGGAGAAATAC |
| Pde8b | ACCACAACTCCACCCATG | AGAGGCTTGTTGATGCTG |
| Pde9a | TGGGTGGACTGTTTACTGG | CGGTCTTCATTGTCTTTCG |
| Pde10a | TGCTTGGTGGCGTTTGTTAG | TTCTCTGATGCCTGGGATGTAC |
| Pde11a | TTTAGCGGTGATTGTGGG | TCTCGAAGTACAGCGTGAGG |
Measurement of cGMP.
The rat cerebrum was dissected out and homogenized. Primary chondrocytes were cultured to near confluence and then treated with 100 nM recombinant CNP (cat. no. N-8768, Sigma-Aldrich) in the absence or presence of 50 µM tadalafil (cat. no. SML-1877, Sigma-Aldrich) and 250 µM IBMX (cat. no. I-5879, Sigma-Aldrich). The cGMP concentrations in brain or cell culture were quantified by an ELISA immunoassay kit (cat. no. ADI-900-164, Enzo Life Sciences, Farmingdale, NY) following the manufacturer’s instructions.
Microcomputed tomography analysis.
Rats were anesthetized by isoflurane on day 0, day 7, day 14, and day 21 for measuring the long bone growth. To do so, their right tibiae were secured by a customized holder to ensure minimal motion effect before they were scanned by an in vivo microcomputed tomography (microCT) system (VivaCT 40, Scanco Medical AG). A scout view of the entire tibia was performed to measure the length of the tibia from the upper extremity to the lower extremity.
After euthanasia, the right tibiae were harvested for trabecular bone analysis using VivaCT 40. The proximal end of the tibia corresponding to a 0- to 4.4-mm region below the lowest point of growth plate was scanned at 10.5-µm isotropic voxel size. All images were smoothened by a Gaussian filter (sigma = 1.2, support = 2.0) and then thresholded corresponding to 579 mg HA/cm3. The images of the secondary spongiosa regions located at 1.5–3.0 mm below the lowest point of the growth plate were contoured for trabecular bone analysis. Trabecular volumetric bone mineral density (BMD), bone volume fraction, trabecular thickness, trabecular separation, trabecular number, and structure model index were calculated by three-dimensional standard microstructural analysis (9).
For cortical bone analysis, the right femurs were harvested and scanned via scout view to determine the midline (the central region with the same distance to both ends of femurs). The midshaft region (from 0.5 mm above to 0.5 mm below the midline) was scanned at 10.5-µm isotropic voxel size. All images were smoothened by a Gaussian filter (sigma = 1.2, support = 2.0) and then thresholded corresponding to 643.1 mg HA/cm3. Cortical thickness, cortical tissue mineral density, polar moment of inertia, cortical area, periosteal perimeter, and endosteal perimeter were measured using the microCT scanner software (Scanco Medical).
Histomorphometry.
The right tibiae were fixed in 4% PFA overnight, washed under tap water, and then decalcified in 0.5 M EDTA (pH 7.4) for 3 wk before embedding in paraffin. Six-micrometer-thick sections were cut for H&E staining. The left tibiae were processed for embedding in methylmethacrylate. Six-micrometer-thick longitudinal sections were cut with a Polycut-S motorized microtome (Reichert) and stained with Goldner’s trichrome solutions. Images corresponding to the secondary spongiosa region were acquired via a Nikon Eclipse 90i and quantified using Bioquant Osteo Software (Bioquant Image Analysis). Osteoblast number per bone surface and osteoclast number per bone surface were calculated as described earlier (11). The thicknesses of the proliferative zone and hypertrophic zone of the growth plate were expressed as the average of 12 measurements that were taken at sites that were evenly distributed across the entire growth plate in the paraffin sections as described previously (55). The proliferative zone is defined as the area that contains flattened cells in a longitudinal and columnar arrangement. The hypertrophic zone is defined as the area at which the cell size starts to increase to the area where cartilage becomes bone.
Statistical analysis.
Data are expressed as means ± standard error (SE) and analyzed by unpaired Student’s t-test or with a one-way or two-way ANOVA with Bonferroni’s post hoc test for multiple comparisons using Prism 5 software (GraphPad Software). Values of P < 0.05 were considered significant.
RESULTS
Tadalafil enhances the cGMP response to CNP in primary chondrocytes.
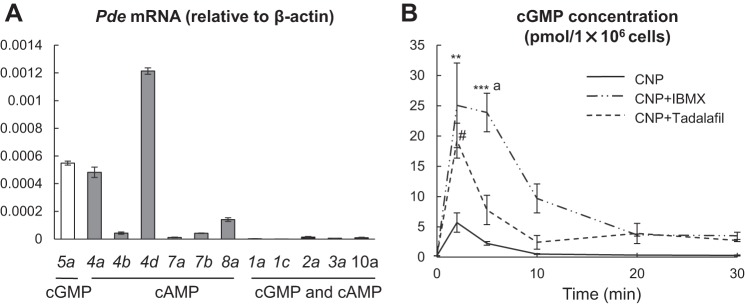
Previous studies have demonstrated the expression of PDE5 in human articular cartilage, a chondrogenic cell line (12), and an osteoblastic cell line (2). However, the expression profile of all PDE isoforms and their activities in epiphyseal chondrocytes, particularly PDE5, were still largely unknown. To determine which PDEs play a primary role in targeting cGMP signaling pathway in chondrocytes, we first measured mRNA levels of all PDEs, including those that specifically hydrolyze cAMP (Pde4, Pde7, and Pde8), those that specifically hydrolyze cGMP (Pde5, Pde6, and Pde9), and those that hydrolyze both (Pde1, Pde2, Pde3, Pde10, and Pde11), in primary chondrocytes derived from the epiphyseal region of rat long bones. Among those PDEs, we detected the expression of Pde1a, 1c, 2a, 3a, 4a, 4b, 4d, 5a, 7a, 7b, 8a, and 10a (Fig. 1A). Therefore, Pde5 is the only cGMP-specific PDE expressed in primary chondrocytes. Although chondrocytes also express PDEs that hydrolyze both cGMP and cAMP, such as 1a, 1c, 2a, 3a, and 10a, their expression levels were 100 times lower compared with that of Pde5.
Fig. 1.
Tadalafil enhances the cGMP response to CNP in primary chondrocytes. A: qRT-PCR analysis of Pde isoforms in primary chondrocytes. n = 3 rats. B: time-courses of CNP-stimulated intracellular cGMP levels in primary chondrocytes with or without pretreatment of IBMX (250 µM) or tadalafil (50 µM). Cells were collected from indicated time points after CNP (100 nM) addition and analyzed by ELISA to measure intracellular cGMP amounts (n = 3). #P < 0.05, CNP + tadalafil vs. CNP; **P < 0.01, CNP + IBMX vs. CNP; ***P < 0.001, CNP + IBMX vs. CNP; aP < 0.05, CNP + IBMX vs. CNP + tadalafil by two-way ANOVA with Bonferroni’s post hoc test for multiple comparisons. cGMP, cyclic GMP; CNP, C-type natriuretic peptide; qRT-PCR, quantitative RT-PCR.
Next, we investigated whether PDE5 regulates cGMP levels in chondrocytes after CNP stimulation. Time course experiments revealed that CNP rapidly and transiently increases the intracellular cGMP level in primary chondrocytes with a peak at 2 min and a rapid fall in cGMP thereafter (Fig. 1B). Combined treatment of cells with CNP plus either tadalafil, which specifically inhibits PDE5, or IBMX, which inhibits all PDEs, not only significantly elevated the peak level of intracellular cGMP but also attenuated the subsequent decline of cGMP compared with CNP alone. Overall, IBMX showed a stronger effect of elevating cGMP level at all time points than tadalafil. These results suggest that PDE5-dependent hydrolysis of cGMP plays an important role in cGMP homeostasis in chondrocytes but other PDEs may also be involved in cGMP hydrolysis.
In vivo treatment of tadalafil increases tissue cGMP level in rats.
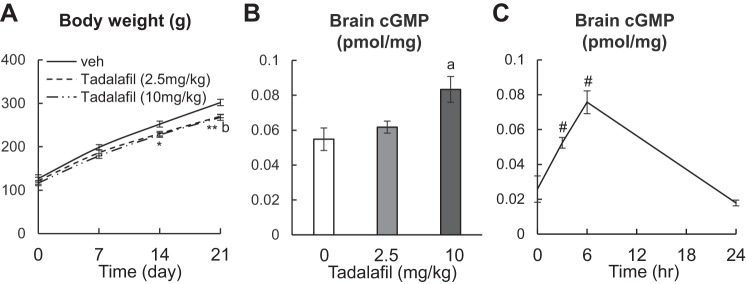
To study whether tadalafil affects skeletal development in vivo, we treated 1-mo-old male rats with vehicle, 2.5 mg/kg or 10 mg/kg tadalafil daily by oral gavage for 3 wk. Those dosages attempt to mimic clinical treatment of children with PAH at 0.5–1 mg·kg−1·day−1. Both doses significantly decreased the gain of body weight during this period (Fig. 2A). At the end of treatment, we validated the efficacy of tadalafil by measuring the tissue level of cGMP. ELISA analysis revealed that although 2.5 mg·kg−1·day−1 of tadalafil treatment does not alter cGMP level, 10 mg·kg−1·day−1 of tadalafil treatment causes a significant 52% increase in brain cGMP compared with vehicle only (Fig. 2B). Furthermore, rats given a single injection of 10 mg tadalafil showed that tissue cGMP levels were 2.0-fold greater at 3 h than at baseline, with a peak increase of 2.9-fold around 6 h. These data are consistent with the role of tadalafil as a potent inhibitor of PDE5 activity. Taken together, our results demonstrate that our tadalafil treatment regimens are effective in increasing tissue levels of cGMP level in young rats and they produce a known pharmacological effect on body weight.
Fig. 2.
Tadalafil treatment increases the tissue cGMP level in young rats. A: rats were weighed weekly during the 3-wk tadalafil treatment period. n = 10 rats/group. B: ELISA analysis of cGMP amount in brain tissue of rats treated with vehicle, 2.5 mg/kg or 10 mg/kg tadalafil daily for 3 wk. n = 5 rats/group. C: ELISA analysis of cGMP amount in brain tissue of rats at 0, 3, 6, and 24 h after receiving 1 dose of 10 mg/kg tadalafil. n = 3 rats/time point. *P < 0.05, 2.5 mg/kg tadalafil vs. vehicle; **P < 0.01, 2.5 mg/kg tadalafil vs. vehicle; aP < 0.05, 10 mg/kg tadalafil vs. vehicle; bP < 0.01, 10 mg/kg tadalafil vs. vehicle; #P < 0.05 vs. 0 h by two-way (A) or one-way ANOVA (B and C) with Bonferroni’s post hoc test for multiple comparisons. cGMP, cyclic GMP; veh, vehicle.
Tadalafil treatment does not affect longitudinal bone growth.
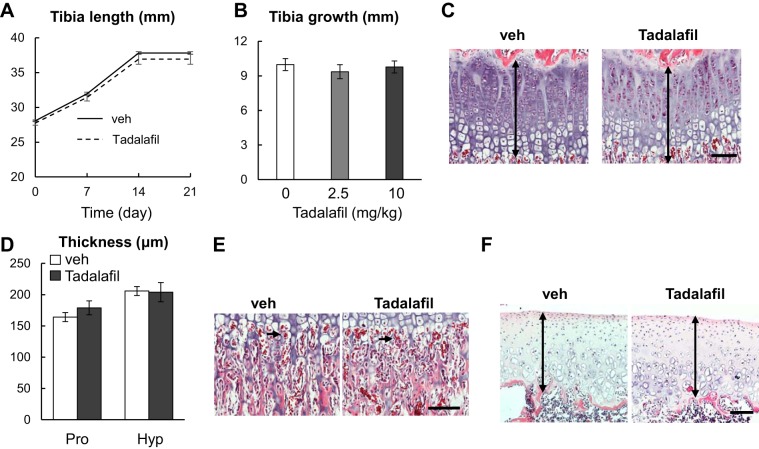
To examine the possible effect of tadalafil on the endochondral bone formation, we first measured tibial growth in rats during the treatment period by weekly in vivo microCT scans. As shown in Fig. 3, A and B, the longitudinal elongation of tibiae was not affected by tadalafil when given at either 2.5 or 10 mg/kg per day. Three weeks later, rats were euthanized, and their tibiae were harvested for histology assessment. Surprisingly, the structure of the growth plate was very similar in vehicle and tadalafil groups (Fig. 3C). Quantification did not detect any change in the thicknesses of the proliferative zone and hypertrophic zone of the growth plate (Fig. 3D). The vascular invasion at the chondral-osseous junction remained the same after 3 wk of tadalafil treatment (Fig. 3E). Moreover, we did not observe any abnormality in the knee articular cartilage (Fig. 3F). Therefore, our data suggested that tadalafil treatment in vivo does not influence chondrocyte proliferation, hypertrophy, and cartilage remodeling into bone during endochondral ossification.
Fig. 3.
Three weeks of tadalafil treatment does not affect longitudinal skeletal growth. A: tibia length of vehicle-treated and 10 mg·kg−1·day−1 tadalafil-treated rats was measured weekly by in vivo microcomputed tomography. n = 10 rats/group. B: length of tibial in rats after 3 wk of vehicle, 2 mg·kg−1·day−1, and 10 mg·kg−1·day−1 tadalafil treatments. n = 10 rats/group. C: representative H&E staining of growth plate from vehicle group and 10 mg·kg−1·day−1 tadalafil group. Double-arrowed lines point to the growth plate. D: thickness of the proliferative zone (Pro) and hypertrophic zone (Hyp) of the growth plate was quantified. n = 5 rats/group. E: H&E staining of chondral-ossification junction of vehicle-treated and 10 mg·kg−1·day−1 tadalafil-treated rats. Arrows point to erythrocytes within blood vessels that invade growth plate cartilage. F: H&E staining of tibial articular cartilage of vehicle-treated and 10 mg·kg−1·day−1 tadalafil-treated rats. Double arrowed lines point to the articular cartilage. Scale bar: 100 µm. Results were analyzed by two-way (A, D) or one-way (B) ANOVA with Bonferroni’s post hoc test for multiple comparisons. veh, vehicle.
Tadalafil treatment does not alter trabecular bone structure or bone cell metabolism.
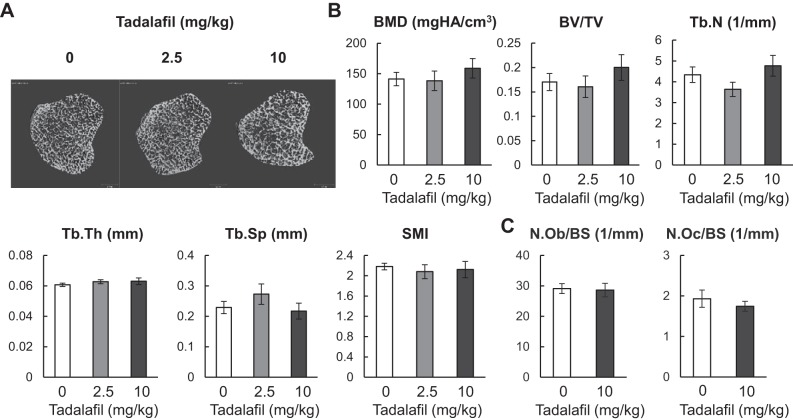
Growth plate cartilage is eventually replaced by bone to form trabecular bone in the primary and secondary spongiosa. MicroCT analysis of the proximal tibial metaphysis indicated that all trabecular bone structural parameters (BMD, bone volume fraction, trabecular number, trabecular thickness, trabecular separation, and structure model index) are the same among vehicle, 2.5 mg·kg−1·day−1, and 10 mg·kg−1·day−1 tadalafil treatment groups (Fig. 4, A and B). Furthermore, bone histomorphometric analyses indicated that there are no significant changes in osteoblast and osteoclast numbers in the trabecular bone (Fig. 4C), suggesting that tadalafil treatment does not alter either bone formation or bone resorption in vivo.
Fig. 4.
Three weeks of tadalafil treatment does not change bone metabolism in the trabecular bone. A: representative 3-D reconstructed microcomputed tomography (microCT) images of tibial trabecular bone in rats treated with vehicle, 2.5 mg·kg−1·day−1 or 10 mg·kg−1·day−1 tadalafil. B: microCT measurement of trabecular bone structural parameters. n = 10 rats/group. C: osteoblast numbers (N.Ob) and osteoclast numbers (N.Oc) per bone surface (BS) were quantified by histomorphometric analysis. n = 5 rats/group. Results were analyzed by one-way ANOVA with Bonferroni’s post hoc test for multiple comparisons (B) or by unpaired t-test (C). 3-D, three-dimensional; BMD, bone mineral density; BV/TV, bone volume fraction; SMI, structure model index; Tb.N, trabecular number; Tb.Sp, trabecular separation; Tb.Th, trabecular thickness.
Tadalafil treatment did not alter cortical bone structure.
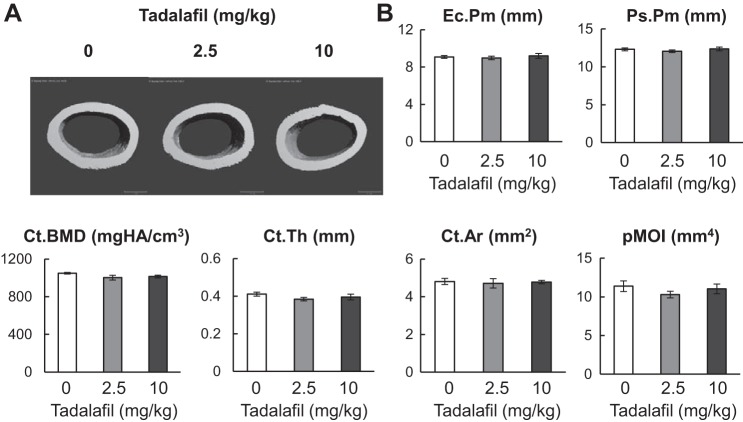
Cortical bone is derived from perichondrium surrounding the epiphyseal cartilage during endochondral ossification. To characterize the effects of tadalafil treatment on cortical bone, we scanned femoral midshaft region at the end of treatment. Similar to trabecular bone, we did not observe any significant changes in cortical bone structural parameters, such as endosteal perimeter, periosteal perimeter, cortical BMD, cortical thickness, cortical area, and polar moment of inertia after 3 wk of tadalafil treatment (Fig. 5, A and B).
Fig. 5.
Cortical bone structure is not altered by 3 wk of tadalafil treatment. A: representative 3-D reconstructed microcomputed tomography (microCT) images of cortical bone in femoral midshaft region of rats treated with vehicle, 2.5 mg·kg−1·day−1 or 10 mg·kg−1·day−1 tadalafil. B: microCT measurement of cortical bone structural parameters. n = 10 rats/group. Results were analyzed by one-way ANOVA with Bonferroni’s post hoc test for multiple comparisons. Ct.Ar, corticol area; Ct.BMD, cortical tissue bone mineral density; Ct.Th; cortical thickness; Ec.Pm, endosteal perimeter; pMOI, polar moment of inertia; Ps.Pm, periosteal perimeter.
DISCUSSION
Recent guidelines from the American Heart Association (AHA) and American Thoracic Society (ATS) recommend the use of PDE5 inhibitors, including tadalafil, for the treatment of pediatric PAH (1). Although no adverse (or beneficial) effects of tadalafil on patient skeletons have been reported to date, the low prevalence of pediatric PAH, and the untoward consequences of PAH itself on normal growth, make it difficult to assess a potential clinical effect of these agents on skeletal development and statural growth. This uncertainty prompted the current study in which we used adolescent rats to investigate whether tadalafil treatment might be associated with changes in growth plate development and long bone structure. To our knowledge, our cell culture studies are the first comprehensive analysis of PDE isoforms expressed in chondrocytes and our rat experiments are the first animal study to validate the clinical observation of tadalafil effects on the skeleton.
Although tadalafil enhanced CNP-induced accumulation of cGMP in chondrocytes in vitro, which might predict altered endochondral bone growth (17, 30, 40, 41) or remodeling (21) in vivo, we found no evidence for a skeletal effect of tadalafil in young rats using doses (2.5 and 10 mg·kg−1·day−1) that are comparable to or slightly in excess of the AHA/ATS-suggested dose of 0.5–1 mg·kg−1·day−1 for pediatric patients (33). By contrast, our tadalafil treatments were effective in blunting weight gain in growing rats, an effect that has been ascribed to the ability of increased cGMP to enhance energy expenditure by inducing differentiation of brown adipocytes and “browning” of primary white adipocytes (14, 18, 19, 53), thus providing evidence that our dose regimen was capable of producing a pharmacological effect. We envision several possible explanations to account for the inability of therapeutic doses of tadalafil to affect skeletal development. First, the dose of tadalafil that we administered may have been too low to achieve effective concentrations of the drug in growth plate chondrocytes because of the avascular nature of cartilage. In our experiments, we observed a dosage-dependent effect in terms of upregulation of concentration of cGMP in brain tissue, and as described above, achieved a recognized physiological end point of weight reduction. Although it is possible that higher doses of tadalafil might affect the skeleton, these doses would be in excess of currently prescribed amounts of tadalafil and therefore, of no clinical consequence. Second, it is possible that other PDEs, such as PDE1, 2, 3, and 10, which are expressed at much lower levels than PDE5 in primary chondrocytes, might exert significant effects in vivo and compensate for the tadalafil-induced loss of PDE5 activity in chondrocytes, thereby preventing an increase in intracellular cGMP. A previous study has showed that in ATDC5 chondrogenic cells (Mouse 129 teratocarcinoma AT805 derived), PDE1, a calcium-calmodulin stimulated PDE, plays an important role in cGMP hydrolysis (12). Consistent with this notion, we found that CNP-induced accumulation and maintenance of cGMP in primary cultures of chondrocytes is greater in the presence of IBMX, a generalized inhibitor of PDEs, than in the presence of tadalafil, which is a PDE5-specific inhibitor. Third, tadalafil may have increased cGMP levels in chondrocytes but not to an extent that would permit an alteration in cellular behavior or growth. Gain-of-function mutations that activate NPR2 and lead to skeletal overgrowth are associated with intracellular levels of cGMP that are 20-fold greater than normal (41), which are far greater than 4- to 5-fold increases we observed with either IBMX or tadalafil treatment in primary chondrocytes. Indeed, a separate study using riociguat, an allosteric stimulator of the sGC, to treat newborn rat pups generated a similar conclusion that drugs effective in treating PAH in newborn rat pups by elevating the cGMP level do not affect skeletal development in vivo (11a).
The CNP/Npr/cGMP signaling pathway regulates not only cartilage homeostasis but also bone metabolism. A recent study showed that overexpressing CNP in mice results in bone loss in intact femurs and accelerated fracture healing because of increased bone turnover (27). A series of studies at earlier time points demonstrated that in culture, CNP stimulates osteoblast proliferation, differentiation, and mineralization via elevating the cGMP level (16, 22, 28, 46, 54). However, another in vivo study found that tadalafil treatment in 2-mo-old mice for 2 mo reduces trabecular bone mass by suppressing Wnt-induced osteoblastogenesis (15). This study used extremely high doses of tadalafil (45 and 75 mg·kg−1·day−1), which greatly exceed the equivalent human doses, and which may have caused toxic effects. Nevertheless, no obvious growth plate phenotype was reported in this study. The role of CNP/Npr/cGMP signaling pathway in osteoclastogenesis is also ambiguous. Although an early report suggested that CNP increases osteoclast bone resorption in a mixture of bone marrow cells (20), another study identified an opposite and inhibitory action of CNP on osteoclast formation from bone marrow macrophages (26). Based on these prior studies, it is likely that our inability to demonstrate any changes in our microCT analyses of tibial trabecular bone and femoral cortical bone and static histomorphometry of tibiae in young rats treated with tadalafil for 3 wk represents a failure of tadalafil to induce a robust increase in intracellular cGMP in bone cells as high as CNP does to cause any alteration in either bone formation or resorption activity.
Our studies have several limitations. First, the rat model used in this study is at the adolescent stage. Pediatric PAH affects children as young as the new born. The skeletal responses to tadalafil might be age-dependent. Second, we only studied male rats, but pediatric PAH affects both women and men. Last, we exposed rats to tadalafil for only a short period of time. However, as the goal of our study was to determine the potential effects of tadalafil on children, we limited exposure of rats to tadalafil to only 3 wk, which corresponds to roughly 2 yr in humans. Although it might require a longer treatment period to identify tadalafil-related skeletal changes, we believe that such effects if detected should be minor and more related to adults instead of children.
In conclusion, our results provide a comprehensive gene expression profile of PDEs in rat cartilage chondrocytes. We also demonstrated that treatment with tadalafil for 3 wk at doses of up to 10 mg·kg−1·day−1, which are much higher than doses used in children with PAH, has no observable effect on long bone growth, including growth plate structure and trabecular and cortical bone structure, in growing rats. Therefore, our studies indicate that despite the important roles of cGMP in skeletal regulation at commonly used dosages, tadalafil is unlikely to affect the skeleton of growing children.
GRANTS
This study was supported by NIH grants NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases grant nos. R01-AR-066098 and R01-DK-095803 (to L. Qin) and the Penn Center for Musculoskeletal Disorders Histology Core (grant no. P30-AR06919).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.W., H.J., M.A.L., and L.Q. conceived and designed research; L.W., and H.J. performed experiments; L.W, H.J., R.J.T., and L.Q. analyzed data; L.W., H.J., M.A.L., and L.Q. interpreted results of experiments; L.W., and L.Q. prepared figures; L.Q. drafted manuscript; M.A.L. edited and revised manuscript; M.A.L. and L.Q. approved final version of manuscript.
REFERENCES
- 1.Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, Hanna BD, Rosenzweig EB, Raj JU, Cornfield D, Stenmark KR, Steinhorn R, Thébaud B, Fineman JR, Kuehne T, Feinstein JA, Friedberg MK, Earing M, Barst RJ, Keller RL, Kinsella JP, Mullen M, Deterding R, Kulik T, Mallory G, Humpl T, Wessel DL; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; and the American Thoracic Society . Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society. Circulation 132: 2037–2099, 2015. [Erratum in Circulation 133: e368, 2016]. doi: 10.1161/CIR.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 2.Ahlström M, Lamberg-Allardt C. Inactivation of atrial natriuretic factor-stimulated cyclic guanosine 3′,5′-monophosphate (cGMP) in UMR-106 osteoblast-like cells. Biochem Pharmacol 59: 1133–1139, 2000. doi: 10.1016/S0006-2952(00)00236-7. [DOI] [PubMed] [Google Scholar]
- 3.Ali Z, Schmidt P, Dodd J, Jeppesen DL. Predictors of bronchopulmonary dysplasia and pulmonary hypertension in newborn children. Dan Med J 60: A4688, 2013. [PubMed] [Google Scholar]
- 4.Amano N, Mukai T, Ito Y, Narumi S, Tanaka T, Yokoya S, Ogata T, Hasegawa T. Identification and functional characterization of two novel NPR2 mutations in Japanese patients with short stature. J Clin Endocrinol Metab 99: E713–E718, 2014. doi: 10.1210/jc.2013-3525. [DOI] [PubMed] [Google Scholar]
- 5.Barst RJ, Beghetti M, Pulido T, Layton G, Konourina I, Zhang M, Ivy DD; STARTS-2 Investigators . STARTS-2: long-term survival with oral sildenafil monotherapy in treatment-naive pediatric pulmonary arterial hypertension. Circulation 129: 1914–1923, 2014. doi: 10.1161/CIRCULATIONAHA.113.005698. [DOI] [PubMed] [Google Scholar]
- 6.Bartels CF, Bükülmez H, Padayatti P, Rhee DK, van Ravenswaaij-Arts C, Pauli RM, Mundlos S, Chitayat D, Shih LY, Al-Gazali LI, Kant S, Cole T, Morton J, Cormier-Daire V, Faivre L, Lees M, Kirk J, Mortier GR, Leroy J, Zabel B, Kim CA, Crow Y, Braverman NE, van den Akker F, Warman ML. Mutations in the transmembrane natriuretic peptide receptor NPR-B impair skeletal growth and cause acromesomelic dysplasia, type Maroteaux. Am J Hum Genet 75: 27–34, 2004. doi: 10.1086/422013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhat R, Salas AA, Foster C, Carlo WA, Ambalavanan N. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics 129: e682–e689, 2012. doi: 10.1542/peds.2011-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bocciardi R, Giorda R, Buttgereit J, Gimelli S, Divizia MT, Beri S, Garofalo S, Tavella S, Lerone M, Zuffardi O, Bader M, Ravazzolo R, Gimelli G. Overexpression of the C-type natriuretic peptide (CNP) is associated with overgrowth and bone anomalies in an individual with balanced t(2;7) translocation. Hum Mutat 28: 724–731, 2007. doi: 10.1002/humu.20511. [DOI] [PubMed] [Google Scholar]
- 9.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25: 1468–1486, 2010. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 10.Chusho H, Tamura N, Ogawa Y, Yasoda A, Suda M, Miyazawa T, Nakamura K, Nakao K, Kurihara T, Komatsu Y, Itoh H, Tanaka K, Saito Y, Katsuki M, Nakao K. Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc Natl Acad Sci USA 98: 4016–4021, 2001. doi: 10.1073/pnas.071389098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 28: 2–17, 2013. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11a.Donda K, Zambrano R, Moon Y, Percival J, Vaidya R, Dapaah-Siakwan F, Luo S, Duncan MR, Bao Y, Wang L, Qin L, Benny M, Young K, Wu S. Riociguat prevents hyperoxia-induced lung injury and pulmonary hypertension in neonatal rats without effects on long bone growth. PLoS One 13: e0199927, 2018. doi: 10.1371/journal.pone.0199927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujishige K, Kotera J, Yanaka N, Akatsuka H, Omori K. Alteration of cGMP metabolism during chondrogenic differentiation of chondroprogenitor-like EC cells, ATDC5. Biochim Biophys Acta 1452: 219–227, 1999. doi: 10.1016/S0167-4889(99)00141-X. [DOI] [PubMed] [Google Scholar]
- 13.Geister KA, Brinkmeier ML, Hsieh M, Faust SM, Karolyi IJ, Perosky JE, Kozloff KM, Conti M, Camper SA. A novel loss-of-function mutation in Npr2 clarifies primary role in female reproduction and reveals a potential therapy for acromesomelic dysplasia, Maroteaux type. Hum Mol Genet 22: 345–357, 2013. doi: 10.1093/hmg/dds432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glöde A, Naumann J, Gnad T, Cannone V, Kilic A, Burnett JC Jr, Pfeifer A. Divergent effects of a designer natriuretic peptide CD-NP in the regulation of adipose tissue and metabolism. Mol Metab 6: 276–287, 2017. doi: 10.1016/j.molmet.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong Y, Xu CY, Wang JR, Hu XH, Hong D, Ji X, Shi W, Chen HX, Wang HB, Wu XM. Inhibition of phosphodiesterase 5 reduces bone mass by suppression of canonical Wnt signaling. Cell Death Dis 5: e1544, 2014. doi: 10.1038/cddis.2014.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagiwara H, Inoue A, Yamaguchi A, Yokose S, Furuya M, Tanaka S, Hirose S. cGMP produced in response to ANP and CNP regulates proliferation and differentiation of osteoblastic cells. Am J Physiol Cell Physiol 270: C1311–C1318, 1996. doi: 10.1152/ajpcell.1996.270.5.C1311. [DOI] [PubMed] [Google Scholar]
- 17.Hannema SE, van Duyvenvoorde HA, Premsler T, Yang RB, Mueller TD, Gassner B, Oberwinkler H, Roelfsema F, Santen GW, Prickett T, Kant SG, Verkerk AJ, Uitterlinden AG, Espiner E, Ruivenkamp CA, Oostdijk W, Pereira AM, Losekoot M, Kuhn M, Wit JM. An activating mutation in the kinase homology domain of the natriuretic peptide receptor-2 causes extremely tall stature without skeletal deformities. J Clin Endocrinol Metab 98: E1988–E1998, 2013. doi: 10.1210/jc.2013-2358. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann LS, Etzrodt J, Willkomm L, Sanyal A, Scheja L, Fischer AW, Stasch JP, Bloch W, Friebe A, Heeren J, Pfeifer A. Stimulation of soluble guanylyl cyclase protects against obesity by recruiting brown adipose tissue. Nat Commun 6: 7235, 2015. doi: 10.1038/ncomms8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffmann LS, Larson CJ, Pfeifer A. cGMP and Brown Adipose Tissue. Handb Exp Pharmacol 233: 283–299, 2016. doi: 10.1007/164_2015_3. [DOI] [PubMed] [Google Scholar]
- 20.Holliday LS, Dean AD, Greenwald JE, Gluck SL. C-type natriuretic peptide increases bone resorption in 1,25-dihydroxyvitamin D3-stimulated mouse bone marrow cultures. J Biol Chem 270: 18983–18989, 1995. doi: 10.1074/jbc.270.32.18983. [DOI] [PubMed] [Google Scholar]
- 21.Homer BL, Morton D, Bagi CM, Warneke JA, Andresen CJ, Whiteley LO, Morris DL, Tones MA. Oral administration of soluble guanylate cyclase agonists to rats results in osteoclastic bone resorption and remodeling with new bone formation in the appendicular and axial skeleton. Toxicol Pathol 43: 411–423, 2015. doi: 10.1177/0192623314546559. [DOI] [PubMed] [Google Scholar]
- 22.Inoue A, Hayakawa T, Otsuka E, Kamiya A, Suzuki Y, Hirose S, Hagiwara H. Correlation between induction of expression of biglycan and mineralization by C-type natriuretic peptide in osteoblastic cells. J Biochem 125: 103–108, 1999. doi: 10.1093/oxfordjournals.jbchem.a022245. [DOI] [PubMed] [Google Scholar]
- 23.Jaubert J, Jaubert F, Martin N, Washburn LL, Lee BK, Eicher EM, Guénet JL. Three new allelic mouse mutations that cause skeletal overgrowth involve the natriuretic peptide receptor C gene (Npr3). Proc Natl Acad Sci USA 96: 10278–10283, 1999. doi: 10.1073/pnas.96.18.10278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joshua J, Kalyanaraman H, Marathe N, Pilz RB. Nitric oxide as a mediator of estrogen effects in osteocytes. Vitam Horm 96: 247–263, 2014. doi: 10.1016/B978-0-12-800254-4.00010-6. [DOI] [PubMed] [Google Scholar]
- 25.Joshua J, Schwaerzer GK, Kalyanaraman H, Cory E, Sah RL, Li M, Vaida F, Boss GR, Pilz RB. Soluble guanylate cyclase as a novel treatment target for osteoporosis. Endocrinology 155: 4720–4730, 2014. doi: 10.1210/en.2014-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koide N, Kondo Y, Odkhuu E, Ulziisaikhan J, Ukaji T, Yokochi T, Umezawa K. Inhibition of receptor activator of nuclear factor-κB ligand- or lipopolysaccharide-induced osteoclast formation by conophylline through downregulation of CREB. Immunol Lett 161: 31–37, 2014. doi: 10.1016/j.imlet.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Kondo E, Yasoda A, Fujii T, Nakao K, Yamashita Y, Ueda-Sakane Y, Kanamoto N, Miura M, Arai H, Mukoyama M, Inagaki N, Nakao K. Increased bone turnover and possible accelerated fracture healing in a murine model with an increased circulating C-Type natriuretic peptide. Endocrinology 156: 2518–2529, 2015. doi: 10.1210/en.2014-1801. [DOI] [PubMed] [Google Scholar]
- 28.Lenz A, Bennett M, Skelton WP IV, Vesely DL. Vessel dilator and C-type natriuretic peptide enhance the proliferation of human osteoblasts. Pediatr Res 68: 405–408, 2010. [DOI] [PubMed] [Google Scholar]
- 29.Marathe N, Rangaswami H, Zhuang S, Boss GR, Pilz RB. Pro-survival effects of 17β-estradiol on osteocytes are mediated by nitric oxide/cGMP via differential actions of cGMP-dependent protein kinases I and II. J Biol Chem 287: 978–988, 2012. doi: 10.1074/jbc.M111.294959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miura K, Kim OH, Lee HR, Namba N, Michigami T, Yoo WJ, Choi IH, Ozono K, Cho TJ. Overgrowth syndrome associated with a gain-of-function mutation of the natriuretic peptide receptor 2 (NPR2) gene. Am J Med Genet A 164A: 156–163, 2014. doi: 10.1002/ajmg.a.36218. [DOI] [PubMed] [Google Scholar]
- 31.Miura K, Namba N, Fujiwara M, Ohata Y, Ishida H, Kitaoka T, Kubota T, Hirai H, Higuchi C, Tsumaki N, Yoshikawa H, Sakai N, Michigami T, Ozono K. An overgrowth disorder associated with excessive production of cGMP due to a gain-of-function mutation of the natriuretic peptide receptor 2 gene. PLoS One 7: e42180, 2012. doi: 10.41371/journal.pone.0042180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montani D, Chaumais MC, Savale L, Natali D, Price LC, Jaïs X, Humbert M, Simonneau G, Sitbon O. Phosphodiesterase type 5 inhibitors in pulmonary arterial hypertension. Adv Ther 26: 813–825, 2009. doi: 10.1007/s12325-009-0064-z. [DOI] [PubMed] [Google Scholar]
- 33.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm 7: 27–31, 2016. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nashida T, Matsumoto H, Imai A, Kameda A, Shimomura H. Effect of cyclic GMP produced by natriuretic peptides on osteoblast-like MC3T3-E1 cells. Biochem Mol Biol Int 40: 1243–1251, 1996. [DOI] [PubMed] [Google Scholar]
- 35.Olney RC, Bükülmez H, Bartels CF, Prickett TC, Espiner EA, Potter LR, Warman ML. Heterozygous mutations in natriuretic peptide receptor-B (NPR2) are associated with short stature. J Clin Endocrinol Metab 91: 1229–1232, 2006. doi: 10.1210/jc.2005-1949. [DOI] [PubMed] [Google Scholar]
- 36.Olsen BR, Reginato AM, Wang W. Bone development. Annu Rev Cell Dev Biol 16: 191–220, 2000. doi: 10.1146/annurev.cellbio.16.1.191. [DOI] [PubMed] [Google Scholar]
- 37.Ornitz DM, Marie PJ. Fibroblast growth factor signaling in skeletal development and disease. Genes Dev 29: 1463–1486, 2015. doi: 10.1101/gad.266551.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ploegstra MJ, Ivy DD, Wheeler JG, Brand M, Beghetti M, Rosenzweig EB, Humpl T, Iriart X, Rouzic EM, Bonnet D, Berger RM. Growth in children with pulmonary arterial hypertension: a longitudinal retrospective multiregistry study. Lancet Respir Med 4: 281–290, 2016. doi: 10.1016/S2213-2600(16)00069-2. [DOI] [PubMed] [Google Scholar]
- 39.Potter LR. Guanylyl cyclase structure, function and regulation. Cell Signal 23: 1921–1926, 2011. doi: 10.1016/j.cellsig.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rangaswami H, Marathe N, Zhuang S, Chen Y, Yeh JC, Frangos JA, Boss GR, Pilz RB. Type II cGMP-dependent protein kinase mediates osteoblast mechanotransduction. J Biol Chem 284: 14796–14808, 2009. doi: 10.1074/jbc.M806486200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson JW, Dickey DM, Miura K, Michigami T, Ozono K, Potter LR. A human skeletal overgrowth mutation increases maximal velocity and blocks desensitization of guanylyl cyclase-B. Bone 56: 375–382, 2013. doi: 10.1016/j.bone.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sabri MR, Beheshtian E. Comparison of the therapeutic and side effects of tadalafil and sildenafil in children and adolescents with pulmonary arterial hypertension. Pediatr Cardiol 35: 699–704, 2014. doi: 10.1007/s00246-013-0840-z. [DOI] [PubMed] [Google Scholar]
- 43.Saura M, Tarin C, Zaragoza C. Recent insights into the implication of nitric oxide in osteoblast differentiation and proliferation during bone development. Sci World J 10: 624–632, 2010. doi: 10.1100/tsw.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suda M, Komatsu Y, Tanaka K, Yasoda A, Sakuma Y, Tamura N, Ogawa Y, Nakao K. C-Type natriuretic peptide/guanylate cyclase B system in rat osteogenic ROB-C26 cells and its down-regulation by dexamethazone. Calcif Tissue Int 65: 472–478, 1999. doi: 10.1007/s002239900735. [DOI] [PubMed] [Google Scholar]
- 45.Suda M, Ogawa Y, Tanaka K, Tamura N, Yasoda A, Takigawa T, Uehira M, Nishimoto H, Itoh H, Saito Y, Shiota K, Nakao K. Skeletal overgrowth in transgenic mice that overexpress brain natriuretic peptide. Proc Natl Acad Sci USA 95: 2337–2342, 1998. doi: 10.1073/pnas.95.5.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suda M, Tanaka K, Fukushima M, Natsui K, Yasoda A, Komatsu Y, Ogawa Y, Itoh H, Nakao K. C-type natriuretic peptide as an autocrine/paracrine regulator of osteoblast. Evidence for possible presence of bone natriuretic peptide system. Biochem Biophys Res Commun 223: 1–6, 1996. doi: 10.1006/bbrc.1996.0836. [DOI] [PubMed] [Google Scholar]
- 47.Takatsuki S, Calderbank M, Ivy DD. Initial experience with tadalafil in pediatric pulmonary arterial hypertension. Pediatr Cardiol 33: 683–688, 2012. doi: 10.1007/s00246-012-0180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teixeira CC, Agoston H, Beier F. Nitric oxide, C-type natriuretic peptide and cGMP as regulators of endochondral ossification. Dev Biol 319: 171–178, 2008. doi: 10.1016/j.ydbio.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsuji T, Kunieda T. A loss-of-function mutation in natriuretic peptide receptor 2 (Npr2) gene is responsible for disproportionate dwarfism in cn/cn mouse. J Biol Chem 280: 14288–14292, 2005. doi: 10.1074/jbc.C500024200. [DOI] [PubMed] [Google Scholar]
- 50.van Loon RL, Roofthooft MT, Hillege HL, ten Harkel AD, van Osch-Gevers M, Delhaas T, Kapusta L, Strengers JL, Rammeloo L, Clur SA, Mulder BJ, Berger RM. Pediatric pulmonary hypertension in the Netherlands: epidemiology and characterization during the period 1991 to 2005. Circulation 124: 1755–1764, 2011. doi: 10.1161/CIRCULATIONAHA.110.969584. [DOI] [PubMed] [Google Scholar]
- 51.Wang SR, Jacobsen CM, Carmichael H, Edmund AB, Robinson JW, Olney RC, Miller TC, Moon JE, Mericq V, Potter LR, Warman ML, Hirschhorn JN, Dauber A. Heterozygous mutations in natriuretic peptide receptor-B (NPR2) gene as a cause of short stature. Hum Mutat 36: 474–481, 2015. doi: 10.1002/humu.22773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang W, Song MH, Miura K, Fujiwara M, Nawa N, Ohata Y, Kitaoka T, Kubota T, Namba N, Jin DK, Kim OH, Ozono K, Cho TJ. Acromesomelic dysplasia, type maroteaux caused by novel loss-of-function mutations of the NPR2 gene: Three case reports. Am J Med Genet A 170A: 426–434, 2016. doi: 10.1002/ajmg.a.37463. [DOI] [PubMed] [Google Scholar]
- 53.Wu W, Shi F, Liu D, Ceddia RP, Gaffin R, Wei W, Fang H, Lewandowski ED, Collins S. Enhancing natriuretic peptide signaling in adipose tissue, but not in muscle, protects against diet-induced obesity and insulin resistance. Sci Signal 10: eaam6870, 2017. doi: 10.1126/scisignal.aam6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yeh LC, Zavala MC, Lee JC. C-type natriuretic peptide enhances osteogenic protein-1-induced osteoblastic cell differentiation via Smad5 phosphorylation. J Cell Biochem 97: 494–500, 2006. doi: 10.1002/jcb.20657. [DOI] [PubMed] [Google Scholar]
- 55.Zhang X, Siclari VA, Lan S, Zhu J, Koyama E, Dupuis HL, Enomoto-Iwamoto M, Beier F, Qin L. The critical role of the epidermal growth factor receptor in endochondral ossification. J Bone Miner Res 26: 2622–2633, 2011. doi: 10.1002/jbmr.502. [DOI] [PMC free article] [PubMed] [Google Scholar]