Abstract
Mitochondrial dysfunction in adipose tissue is involved in the pathophysiology of obesity-induced systemic metabolic complications, such as type 2 diabetes, insulin resistance, and dyslipidemia. However, the mechanisms responsible for obesity-induced adipose tissue mitochondrial dysfunction are not clear. The aim of present study was to test the hypothesis that nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase sirtuin-3 (SIRT3) in adipocytes plays a critical role in adipose tissue mitochondrial biology and obesity. We first measured adipose tissue SIRT3 expression in obese and lean mice. Next, adipocyte-specific mitochondrial Sirt3 knockout (AMiSKO) mice were generated and metabolically characterized. We evaluated glucose and lipid metabolism in adult mice fed either a regular-chow diet or high-fat diet (HFD) and in aged mice. We also determined the effects of Sirt3 deletion on adipose tissue metabolism and mitochondrial biology. Supporting our hypothesis, obese mice had decreased SIRT3 gene and protein expression in adipose tissue. However, despite successful knockout of SIRT3, AMiSKO mice had normal glucose and lipid metabolism and did not change metabolic responses to HFD-feeding and aging. In addition, loss of SIRT3 had no major impact on putative SIRT3 targets, key metabolic pathways, and mitochondrial function in white and brown adipose tissue. Collectively, these findings suggest that adipocyte SIRT3 is dispensable for maintaining normal adipose tissue mitochondrial function and whole body metabolism. Contrary to our hypothesis, loss of SIRT3 function in adipocytes is unlikely to contribute to the pathophysiology of obesity-induced metabolic complications.
Keywords: adipocyte, adipose tissue, glucose metabolism, mitochondria, NAD+, obesity, SIRT3
INTRODUCTION
Obesity is an important risk factor for developing systemic metabolic abnormalities, such as type 2 diabetes, insulin resistance, β-cell dysfunction, nonalcoholic fatty liver disease (NAFLD), and dyslipidemia (8, 21). The complex mechanisms responsible for obesity-induced metabolic complications are not entirely clear but could involve mitochondrial dysfunction in adipose tissue. Interestingly, it has been reported that obesity is closely associated with impaired adipose tissue mitochondrial biogenesis and function in both rodents and humans (2, 16, 32, 41, 52). In adipocytes, mitochondria play a critical role in regulating key metabolic pathways, such as production and secretion of adipokines, lipid storage and mobilization, oxidative stress defense, and adipogenesis (7, 26). Emerging evidence has suggested that adipocyte-specific modulation of key mitochondrial regulators, such as peroxisome proliferator-activated receptor-γ coactivator 1α (PGC1α) (22), MitoNEET (25), mitochondrial transcription factor A (TFAM) (49), and mammalian target of rapamycin (mTOR) signaling (35), has a tremendous impact not only on adipose tissue mitochondrial biology but also on whole body metabolism. Collectively, these findings have shed light on the potential role of adipose tissue mitochondrial dysfunction as a key mediator of obesity-induced metabolic complications. However, the molecular mechanism underlying obesity-induced adipose tissue mitochondrial dysfunction is not still clear.
Sirtuin-3 (SIRT3) is a nicotinamide adenine dinucleotide (NAD+)-dependent protein deacetylase localized in mitochondria. SIRT3 deacetylates numerous mitochondrial enzymes and regulates key metabolic functions, such as fatty acid β-oxidation, electron transport, glycolysis, and oxidative stress defense, in a wide variety of cell types (1, 11, 13, 15, 24, 31, 33, 47). Indeed, data obtained from previous studies have revealed close relationships between impaired SIRT3 function and obesity-induced metabolic complications. For example, in liver, high-fat diet (HFD) feeding decreases SIRT3 expression and activity, and loss of SIRT3 function reduces fatty acid β-oxidation and mitochondrial respiratory capacity, contributing to the development of NAFLD (14, 20, 27). In skeletal muscle, SIRT3 expression is decreased by HFD-induced obesity and diabetes, whereas it is increased by caloric restriction (17, 34). Sirt3 deletion increases mitochondrial reactive oxygen species production and impairs substrate metabolism and insulin signaling in skeletal muscle (17, 18, 28, 34, 48). Finally, Sirt3-deficient mice have accelerated obesity-associated metabolic complications, such as glucose intolerance, insulin resistance, NAFLD, and dyslipidemia (14). Although these findings clearly demonstrate the importance of SIRT3 in whole body metabolism and obesity, the tissue-specific metabolic function of SIRT3 in adipose tissue is poorly understood.
Accordingly, the purpose of the present study was to investigate the functional role of adipocyte SIRT3 in adipose tissue metabolism and obesity. We hypothesized that SIRT3 plays a pivotal role in regulating adipose tissue metabolic function and mitochondrial biology and, thus, that loss of SIRT3 function in adipocytes contributes to the development of obesity-induced systemic metabolic complications. To test these hypotheses, we first measured adipose tissue SIRT3 expression in obese and lean mice. Next, adipocyte-specific mitochondrial Sirt3 knockout (AMiSKO) mice were generated by using Cre/loxP technology. We carefully evaluated whole body glucose and lipid metabolism in adult AMiSKO and control mice under regular chow diet (RCD) and HFD conditions, and in aged mice. In addition, we determined the effects of Sirt3 deletion on key cellular metabolic pathways and mitochondrial biology in white and brown adipose tissue.
MATERIALS AND METHODS
Animal experimentation.
AMiSKO mice were generated by using adiponectin (Adipoq)-Cre transgenic mice (9) and floxed-Sirt3 (flox/flox) mice; flox/flox mice were generated and provided by Dr. Eric Verdin at the University of California, San Francisco (UCSF). flox/flox mice and Adipoq-Cre transgenic mice were backcrossed at least 14 times to C57BL/6J mice before generation of AMiSKO mice. Mice were housed in a barrier facility with 12:12-h light-dark cycles and were maintained on a RCD (LabDiet 5053; LabDiet, St. Louis, MO) ad libitum. For the HFD study, mice were placed on a HFD (45% kcal from fat, Research Diets D12451; Research Diets, New Brunswick, NJ) starting from 3 to 7 wk of age. Diet-induced obese C57BL/6J male mice (no. 380050) and leptin receptor deficient C57/BLKS/J db/db male mice (no. 000697) were purchased from the Jackson Laboratory (Bar Harbor, ME) and used to evaluate SIRT3 expression in adipose tissue. Tissue samples were collected, immediately frozen in liquid nitrogen, and stored at −80°C until analyses. For the histological assessments, tissue samples were fixed in formalin and embedded in paraffin. All animal studies were approved by the Washington University Institutional Animal Care and Use Committee.
Glucose and energy metabolism.
Body composition was determined using a NMR instrument (EchoMRI, Echo Medical Systems, Waco, TX). After mice were fasted for ~15 h, 50% dextrose solution (2 g/kg-body wt) was injected intraperitoneally for intraperitoneal glucose tolerance tests (IPGTTs) at Zeitgeber time 3 (ZT3). Tail blood was taken at 0, 15, 30, 60, and 120 min, and glucose levels were determined using the Accu-Chek II glucometer (Roche Diagnostics, Indianapolis, IN), as we previously described (46). For insulin tolerance tests (ITTs), mice were injected with human insulin (0.75 U/kg body wt; Lilly, Indianapolis, IN) at ZT7 after they had been fasted for ~4 h. Blood glucose levels were measured at 0, 15, 30, 45, and 60 min after insulin injection. Blood samples were also collected for plasma insulin measurements with the Erenna immunoassay system (Singulex, Alameda, CA) at the Washington University Core Laboratory for Clinical Studies. Whole body oxygen consumption (V̇o2), CO2 production (V̇co2), and energy expenditure (EE) were determined using a Phenomaster system (TSE Systems, Bad Homburg, Germany). For the cold exposure experiments, mice were placed in a cold room at 4°C and body temperature was monitored using a rectal probe.
Plasma and tissue lipids.
Plasma concentrations of triglyceride (TG), total cholesterol (TC), free fatty acids (FFA), and hepatic TG contents were determined at the Washington University Animal Model Research Core as previously described (46). Hepatic TG contents were normalized to tissue weight.
Adipocyte and SVF isolation.
Visceral adipose tissue (VAT) samples were obtained from age-matched AMiSKO and flox/flox mice and incubated in Krebs-Ringer buffer containing 1 mg/ml collagenase type I (LS004196; Worthington Biochemical, Lakewood, NJ) at 37°C for 45 min. After collagenase digestion, white adipocytes and stromal vascular fraction (SVF) cells were separated by centrifuge and subjected to real-time polymerase chain reaction (PCR) and Western blot.
RNA isolation and real-time PCR.
Total RNA was isolated by using RNeasy Mini Kit (no. 74104; Qiagen, Chatsworth, CA) or TRIzol Reagent (no. 15596018; Invitrogen, Carlsbad, CA). Gene expression was determined by using an ABI 7500 real-time PCR system (Invitrogen) and SYBR Green Master Mix (Invitrogen) as we previously described (46). The expression of each gene was normalized with glyceraldehyde-3-phosphate dehydrogenase (Gapdh) in VAT and with ribosomal protein (36b4) in brown adipose tissue (BAT). Primer details are listed in Table 1.
Table 1.
Sequence of primers for real-time PCR
| Primer Sequences (5′-3′) | |||
|---|---|---|---|
| Gene | Accession Number | Forward | Reverse |
| Sirt3 | NM_001177804 | ATCCCGGACTTCAGATCCCC | CAACATGAAAAAGGGCTTGGG |
| Sirt4 | NM_001167691 | GATTGACTTTCAGGCCGACAA | GCGGCACAAATAACCCCGA |
| Sirt5 | NM_178848 | CTCCGGGCCGATTCATTTCC | GCGTTCGCAAAACACTTCCG |
| Pgc1α | NM_008904 | TATGGAGTGACATAGAGTGTGCT | CCACTTCAATCCACCCAGAAAG |
| Adipoq | NM_009605 | TGTTCCTCTTAATCCTGCCCA | CCAACCTGCACAAGTTCCCTT |
| Lep | NM_008493 | GAGACCCCTGTGTCGGTTC | CTGCGTGTGTGAAATGTCATTG |
| Tnfα | NM_013693 | CCCTCACACTCAGATCATCTTCT | GCTACGACGTGGGCTACAG |
| Il6 | NM_031168 | TAGTCCTTCCTACCCCAATTTCC | TTGGTCCTTAGCCACTCCTTC |
| Mcp1 | NM_011333 | TTAAAAACCTGGATCGGAACCAA | GCATTAGCTTCAGATTTACGGGT |
| Glut4 | NM_009204 | CTGTCGCTGGTTTCTCCAA | CTGCTCTAAAAGGGAAGGTGTC |
| Atgl | NM_001163689 | GGATGGCGGCATTTCAGACA | CAAAGGGTTGGGTTGGTTCAG |
| Hsl | NM_001039507 | CCAGCCTGAGGGCTTACTG | CTCCATTGACTGTGACATCTCG |
| Lcad | NM_007381 | TCTTTTCCTCGGAGCATGACA | GACCTCTCTACTCACTTCTCCAG |
| MnSOD | NM_013671 | CAGACCTGCCTTACGACTATGG | CTCGGTGGCGTTGAGATTGTT |
| Catalase | NM_009804 | AGCGACCAGATGAAGCAGTG | TCCGCTCTCTGTCAAAGTGTG |
| Foxo3a | NM_019740 | CTGGGGGAACCTGTCCTATG | TCATTCTGAACGCGCATGAAG |
| Idh2 | NM_173011 | GGAGAAGCCGGTAGTGGAGAT | GGTCTGGTCACGGTTTGGAA |
| Gpx1 | NM_008160 | AGTCCACCGTGTATGCCTTCT | GAGACGCGACATTCTCAATGA |
| Ucp1 | NM_009463 | AGGCTTCCAGTACCATTAGGT | CTGAGTGAGGCAAAGCTGATTT |
| Pparα | NM_011144 | AGAGCCCCATCTGTCCTCTC | ACTGGTAGTCTGCAAAACCAAA |
| Dio2 | NM_010050 | AATTATGCCTCGGAGAAGACCG | GGCAGTTGCCTAGTGAAAGGT |
| Cidea | NM_007702 | TGACATTCATGGGATTGCAGAC | GGCCAGTTGTGATGACTAAGAC |
| mtCO1 | YP_003024028 | GGATTTGTTCACTGATTCCCATTA | GCATCTGGGTAGTCTGAGTAGCG |
| Gapdh | NM_001289726 | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
| 36b4 | NM_007475 | GCAGACAACGTGGGCTCCAAGCAGAT | GGTCCTCCTTGGTGAACACGAAGCCC |
Western blot.
Mitochondria were prepared from VAT adipocytes and BAT using sucrose step density gradient. Frozen tissue and isolated mitochondria were homogenized in lysis buffer containing protease and phosphatase inhibitor cocktail, and proteins were extracted as described previously (46). The following primary antibodies were used: rabbit monoclonal anti-SIRT3 antibody (no. 5490, Cell Signaling Technology), mouse monoclonal anti-α-tubulin (no. T5168; Sigma, St. Louis, MO), rabbit polyclonal anti-acetylated-lysine antibody (no. 9441, Cell Signaling Technology), rabbit monoclonal anti-cytochrome c oxidase (COX) IV antibody (no. 4850, Cell Signaling Technology), and total OXPHOS antibody cocktail (no. ab110413; Abcam, Cambridge, UK). Secondary antibodies were horseradish peroxidase (HRP)-conjugated anti-rabbit antibody (no. 7074, Cell Signaling Technology) and HRP-conjugated anti-mouse antibody (no. sc-2005; Santa Cruz Biotechnology, Santa Cruz, CA). Blots were developed by using the ECL Select Western Blotting Detection Reagent (no. RPN2235; GE Healthcare Life Sciences, Piscataway, NJ). Western blot densitometry was quantitated using ImageJ software (NIH ImageJ 1.47; https://imagej.nih.gov/ij).
Quantification of mitochondrial DNA content.
DNA was isolated by using QIAamp DNA Mini Kit (no. 51034, Qiagen). Mitochondrial DNA (mtDNA) contents were determined by quantitating expression of a mitochondria-encoded gene, 16S ribosomal RNA (rRNA) (forward: 5′-CCGCAAGGGAAAGATGAAAGAC-3′; reverse: 5′-TCGTTTGGTTTCGGGGTTTC-3′) and a nuclear-encoded gene, hexokinase 2 (forward: 5′-GCCAGCCTCTCCTGATTTTAGTGT-3′; reverse: 5′-GGGAACACAAAAGACCTCTTCTGG-3′), as described previously (37). The expression of 16S rRNA was normalized to the expression of hexokinase 2.
Adipocyte sizing.
Adipocyte size and number were measured as described previously (6). Briefly, VAT samples obtained from AMiSKO and flox/flox mice were fixed in a solution containing collidine HCl (0.2 mol/l) and osmium tetraoxide (31 mg/ml) and dissociated in a solution containing urea (8 mol/l) and NaCl (154 mmol/l). Samples were then analyzed on a Multisizer-3 (Beckman Coulter, Fullerton, CA), using a 400-μm aperture (dynamic linear range 12–320 m). The adipocyte diameter and size distribution were determined by using the Multisizer 3.
Glutathione and glutathione disulfide.
Frozen tissue samples were homogenized in 50% methanol-water. After centrifuge, the supernatant was mixed with chloroform, and the aqueous phase was lyophilized and stored until analysis. The samples were reconstituted with ammonium formate (5 mmol/l) and loaded into an Agilent 1290 high-performance liquid chromatography (HPLC) system (Agilent Technologies, Waldbronn, Germany) equipped with an Atlantis T3 column (Waters, Milford, MA). Glutathione (GSH) and glutathione disulfide (GSSG) were analyzed with an Agilent triple quadrupole mass spectrometer in the positive electrospray ionization (ESI) mode and quantitated by multiple reaction monitoring (MRM) mode. The following MRM transitions were monitored: m/z 308.09/162.02 (GSH) and 613.16/355.07 (GSSG). The amounts of GSH and GSSG were quantitated using the Agilent MassHunter software. GSH and GSSG concentrations were normalized to tissue weight.
NAD+ and NADH.
NAD+ and NADH concentrations were determined using a HPLC system as previously described (38, 46, 53). Briefly, NAD+ and NADH were extracted from frozen tissues by using perchloric acid and potassium hydroxide, respectively. The extracts were loaded to a HPLC system (Prominence; Shimadzu Scientific Instruments, Columbia, MD) with a Supelco LC-18-T column (no. 58970-U, Sigma). VAT NADH was quantified with a commercially available kit (no. MAK037, Sigma). NAD+ and NADH concentrations were normalized to tissue weight.
Plasma adipokine concentrations.
Blood samples were collected under fed conditions to determine plasma concentrations of adiponectin (no. MRP300) and leptin (no. MOB00; R&D Systems, Minneapolis, MN).
Adipose tissue respiration.
Adipose tissue oxygen consumption rate (OCR) was measured ex vivo by using the Seahorse XF24 extracellular flux analyzer (Seahorse Bioscience, North Billerica, MA) as described previously (3). Briefly, VAT was obtained from age-matched male AMiSKO and flox/flox mice and immediately plated in an XF24-well microplate. After OCR was measured under basal conditions, VAT was treated with 30 μmol/l oligomycin, 20 μmol/l carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP), and 20 μmol/l rotenone-antimycin to evaluate the uncoupled respiration, maximal respiration, and nonmitochondrial respiration, respectively. OCR was normalized to dry tissue weight.
Statistical analyses.
Data are presented as means ± SE. Differences in continuous variables (e.g., body weight, blood glucose levels during IPGTTs and ITTs) between groups were evaluated using repeated-measures ANOVA. Differences in other parameters were assessed using Student's unpaired t-test. A P value < 0.05 was considered statistically significant.
RESULTS
Obesity decreases SIRT3 expression in adipose tissue.
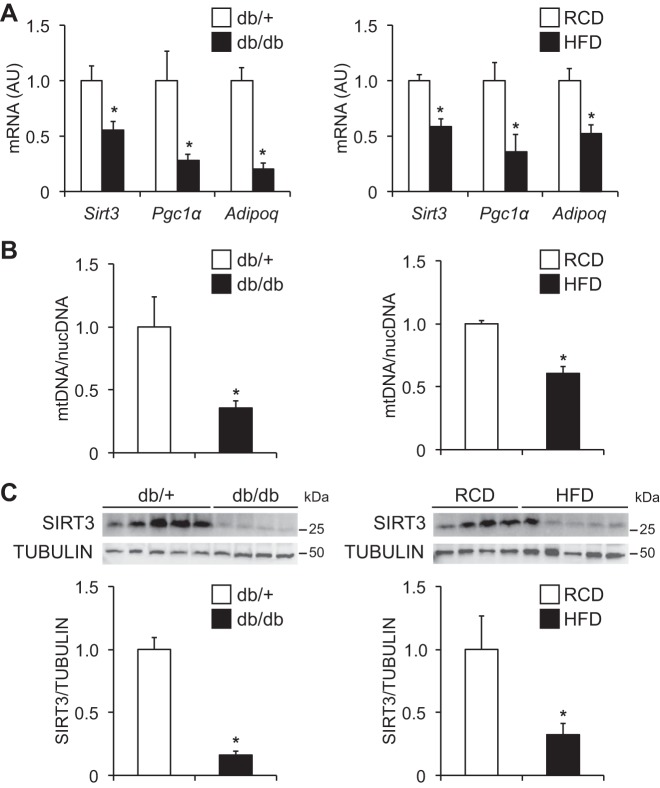
Adipose tissue gene expression of Pgc1α, a master regulator of mitochondrial function, and adiponectin, a key insulin-sensitizing adipokine (Fig. 1A), and mtDNA contents (Fig. 1B) were significantly decreased in genetically obese (db/db) and HFD-induced obese mice, compared with their lean counterparts. Interestingly, obese mice markedly reduced SIRT3 gene and protein expression in adipose tissue (Fig. 1, A and C). These findings prompted us to hypothesize that decreased SIRT3 expression is involved in obesity-induced adipose tissue mitochondrial dysfunction and metabolic complications.
Fig. 1.
Adipose tissue sirtuin 3 (SIRT3) expression in obese mice. A: gene expression of Sirt3, peroxisome proliferator-activated receptor-γ coactivator 1α(Pgc1α) and adiponectin (Adipoq), was determined in visceral adipose tissue (VAT) obtained from genetically obese (db/db) and high-fat diet (HFD)-induced obese mice and their lean controls (db/+ mice and regular-chow diet [RCD]-fed mice, respectively; n = 5 per group). B: VAT mitochondrial DNA (mtDNA) contents were normalized to nuclear DNA (nucDNA) contents (n = 3–5 per group). C: Western blot analysis of SIRT3 in VAT (n = 4–5 per group). Densitometric analysis of SIRT3 protein normalized to tubulin is shown. *Value significantly different from control value (Student’s t-test, P < 0.05). Values are means ± SE.
Generation of AMiSKO mice.
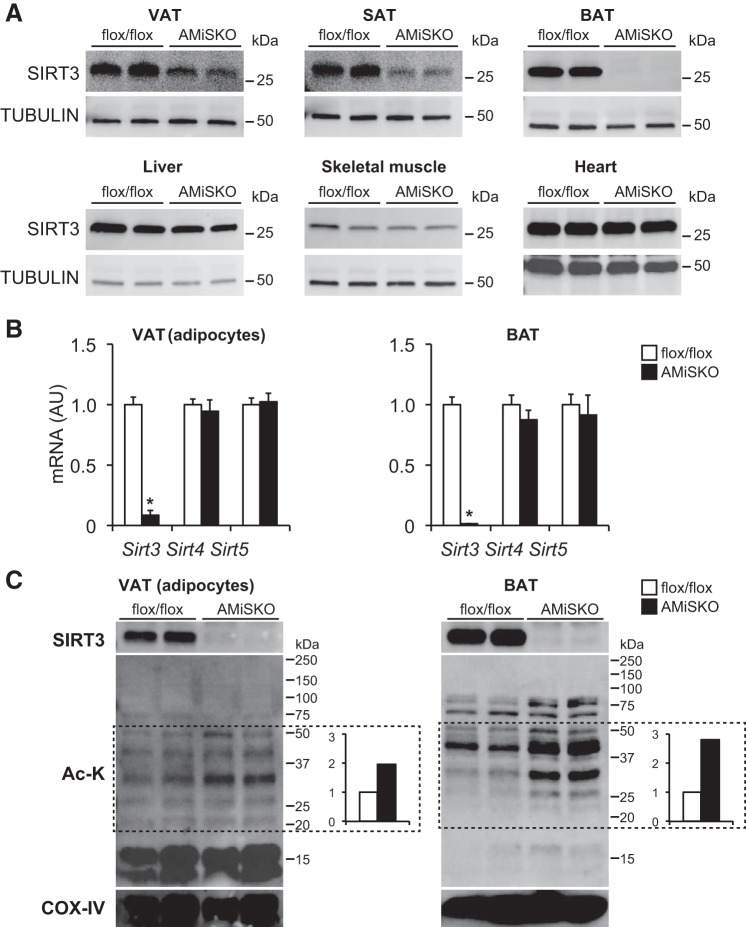
To test this hypothesis, we generated AMiSKO mice by using Adipoq-Cre transgenic mice (9) and Sirt3-floxed (flox/flox) mice. AMiSKO mice were born in the expected Mendelian ratios and appeared grossly normal. In AMiSKO mice, SIRT3 protein expression was markedly reduced in VAT, subcutaneous adipose tissue (SAT), and BAT but not in other metabolic organs (Fig. 2A). Other mitochondrial sirtuins, Sirt4 and Sirt5, were unaffected in VAT adipocytes and BAT (Fig. 2B). None of the mitochondrial sirtuins were changed in stromal vascular fraction (SVF) cells of VAT (data not shown). SIRT3 protein expression was completely abolished in VAT adipocytes and in BAT mitochondria obtained from AMiSKO mice (Fig. 2C). In both VAT adipocytes and BAT, AMiSKO mice had increased mitochondrial lysine acetylation of multiple protein bands, particularly between 20 and 50 kDa. Mitochondrial protein hyperacetylation was more evident in BAT than in VAT adipocytes. These findings are consistent with data obtained from the germ line Sirt3 knockout mice (5, 28, 29) and suggest a decrease in adipose tissue SIRT3 deacetylase activity in AMiSKO mice.
Fig. 2.
Generation of adipocyte-specific mitochondrial Sirt3 knockout (AMiSKO) mice. AMiSKO mice were generated by using Adipoq-Cre transgenic mice and floxed-Sirt3 (flox/flox) mice. A: Western blot analysis of SIRT3 in VAT, subcutaneous adipose tissue (SAT), brown adipose tissue (BAT), liver, skeletal muscle, and heart. B: gene expression of mitochondrial sirtuins (Sirt3, Sirt4, Sirt5) in VAT adipocytes and BAT (n = 3–5 per group). C: Western blot analysis of SIRT3, acetylated lysine (Ac-K), and cytochrome c oxidase (COX) IV. Mitochondrial proteins isolated from VAT adipocytes and BAT were separated on Any-kD gels (Bio-Rad). Densitometric analysis of acetylated mitochondrial protein bands (between 20 and 50 kDa) is shown. *Value significantly different from control value (Student’s t-test, P < 0.05). Values are means ± SE.
Adipocyte-specific Sirt3 deletion does not affect whole body glucose and lipid metabolism.
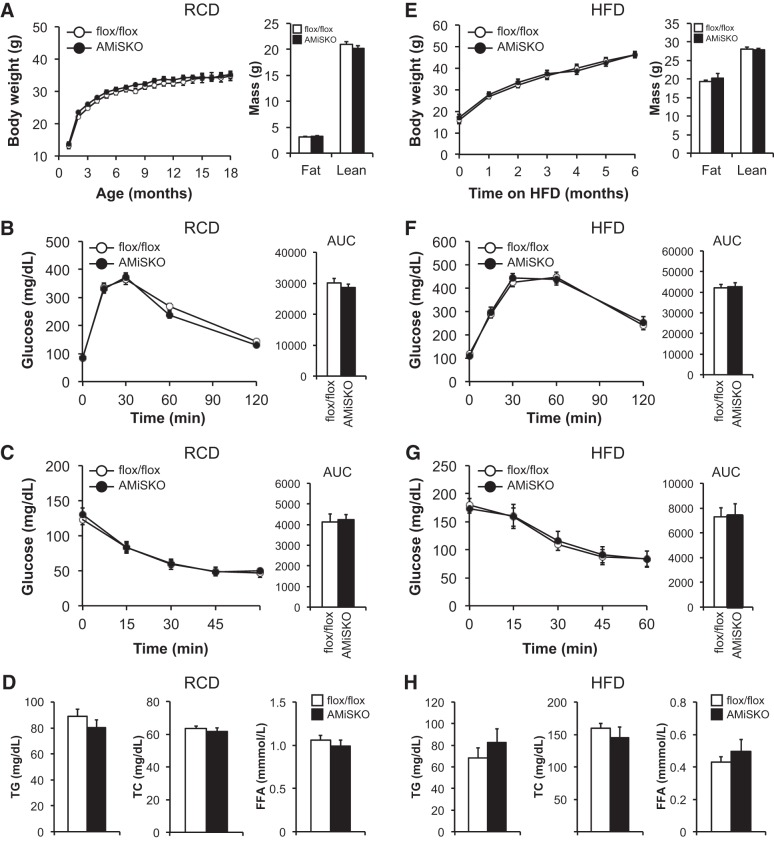
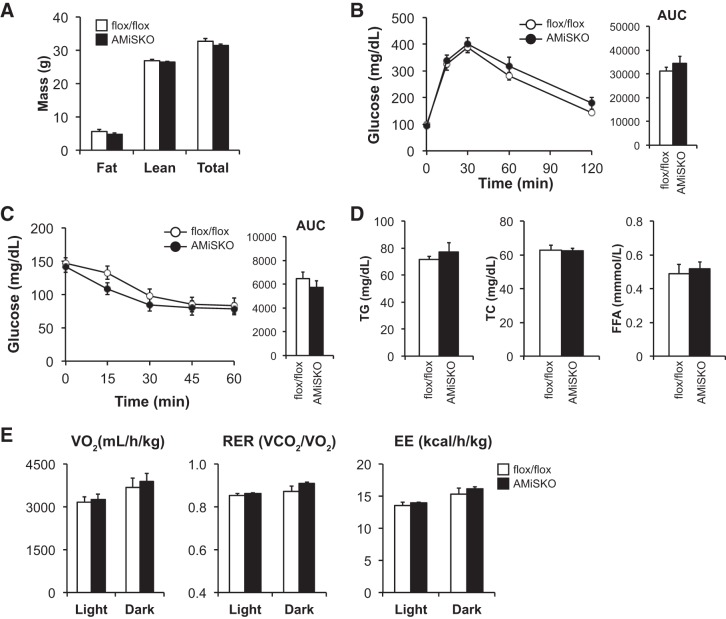
We first metabolically characterized male AMiSKO and flox/flox mice under RCD conditions. Adipocyte-specific Sirt3 deletion did not influence body weight gain over a period of 18 mo, fat mass, or lean mass (Fig. 3A). AMiSKO mice had normal glucose tolerance and insulin sensitivity (Fig. 3, B and C), and fasting plasma insulin concentrations (9- to 12-mo-old AMiSKO mice = 213 ± 28; flox/flox mice = 152 ± 10 pg/ml). In addition, plasma lipid profile (Fig. 3D) and hepatic TG contents (3-mo-old AMiSKO mice = 9.0 ± 1.0; flox/flox mice = 8.2 ± 0.8 μg/mg tissue wt) were unaffected in AMiSKO mice, compared with flox/flox mice. We next tested the possibility that AMiSKO mice were more susceptible to HFD-induced obesity. However, unlike the germ line Sirt3 knockout mice (14), AMiSKO mice did not worsen HFD-induced metabolic abnormalities, compared with flox/flox mice (Fig. 3, E–H). Similarly, adipocyte-specific Sirt3 deletion had no effect on glucose and lipid metabolism in female mice fed RCD or HFD (data not shown).
Fig. 3.
Metabolic phenotype of AMiSKO mice under RCD and HFD conditions. Characterization of male AMiSKO and flox/flox mice fed either a RCD (A–D) or HFD (E–H). For HFD study, mice were placed on a HFD starting from 3 to 7 wk of age. Body weight and body composition were measured in 3- to 6-mo-old RCD-fed mice (A) and in mice after 6 mo of HFD feeding (E) (n = 5–9 per group). Blood glucose concentrations during the intraperitoneal glucose tolerance tests (IPGTTs) in 9- to 12-mo-old RCD-fed mice (B) and in mice after 4 mo of HFD feeding (F) (n = 9–10 per group). Area under the curve (AUC) for glucose is shown next to each curve. Blood glucose concentrations during insulin tolerance tests (ITTs) in 9- to 12-mo-old RCD-fed mice (C) and in mice after 4 mo of HFD feeding (G) (n = 9–10 per group). AUC for glucose is shown next to each curve. Plasma concentrations of triglyceride (TG), total cholesterol (TC), and free fatty acids (FFA) were determined in 9- to 12-mo-old RCD- fed mice (D) and in mice after 6 mo of HFD feeding (H) (n = 5–10 per group). There was a significant time effect (P < 0.05) but no group × time interaction in body weight, IPGTT, and ITT data (ANOVA). Values are means ± SE.
Loss of SIRT3 has no major impact on adipose tissue metabolism and mitochondrial biology.
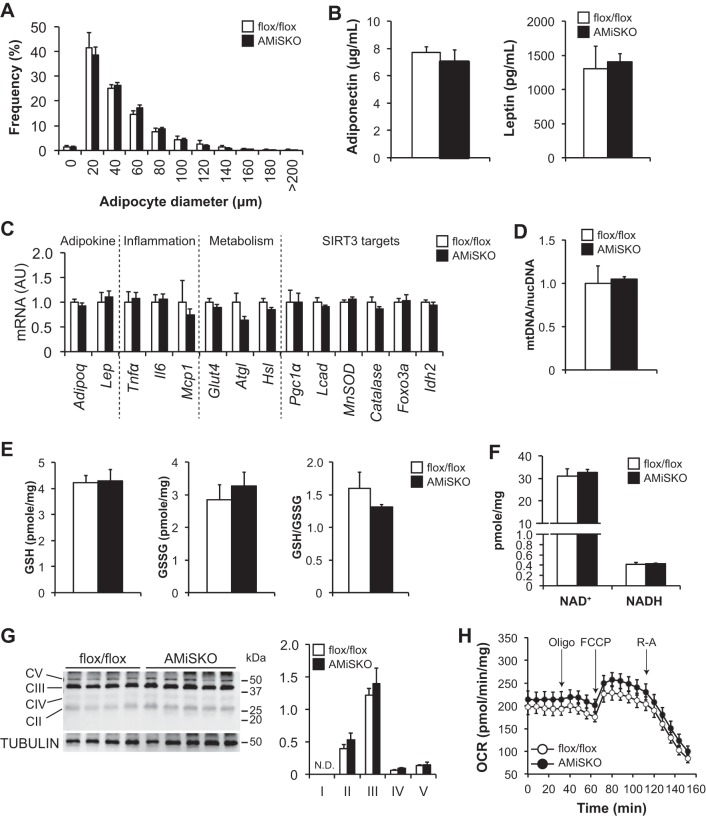
We next evaluated putative SIRT3 targets, key cellular metabolic pathways, and mitochondrial biology in VAT of AMiSKO and flox/flox mice. AMiSKO mice did not change adipocyte size (Fig. 4A), histology (data not shown), or plasma concentrations and gene expression of the key adipokines adiponectin and leptin compared with flox/flox mice (Fig. 4, B and C). Loss of SIRT3 had no effect on gene expression of inflammatory markers [tumor necrosis factor α (Tnfα), interleukin-6 (Il6), monocyte chemoattractant protein-1 (Mcp1)], key metabolic regulators [glucose transporter 4 (Glut4), adipose triglyceride lipase (Atgl), hormone-sensitive lipase (Hsl)], and proteins involved in putative SIRT3 target metabolic pathways, such as mitochondrial biogenesis and fatty acid β-oxidation [Pgc1α, long-chain acyl-CoA dehydrogenase (Lcad)], and oxidative stress defense (manganese superoxide dismutase (MnSOD), catalase, forkhead box O3 (Foxo3a), isocitrate dehydrogenase 2 (Idh2)] (Fig. 4C). Consistently, AMiSKO mice did not change mtDNA contents (Fig. 4D), SIRT3-regulated redox metabolites, namely GSH and GSSG (12, 36, 45) (Fig. 4E), or NAD+ and NADH (Fig. 4F). Finally, loss of SIRT3 had no impact on protein contents of the subunits of the electron transport chain (ETC) complex (Fig. 4G), basal OCR (3- to 5-mo-old male AMiSKO mice = 214 ± 17; flox/flox mice = 196 ± 17 pmol·min−1·mg tissue wt−1), and OCR responses to metabolic inhibitors (Fig. 4H). Taken together, these results suggest a minimal role of SIRT3 in regulating adipose tissue metabolism and mitochondrial function.
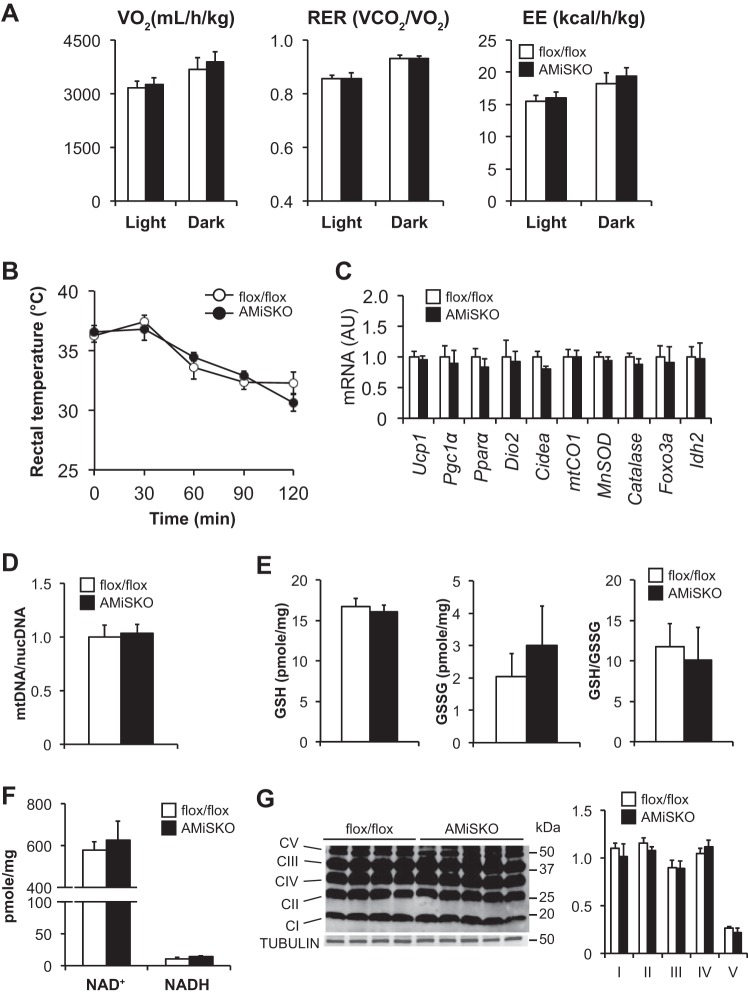
Fig. 4.
Adipose tissue metabolism and mitochondrial biology in AMiSKO mice. SIRT3 targets and mitochondrial metabolic pathways in VAT of AMiSKO and flox/flox mice. Adipocyte size (n = 4 per group; A), plasma adiponectin and leptin concentrations (n = 5 per group; B), gene expression of SIRT3 targets and proteins involved in adipose tissue metabolism, inflammation, mitochondrial function, and oxidative stress defense (n = 5 per group; C), mtDNA contents (n = 5 per group; D), GSH and GSSG concentrations and their ratios (n = 3–5 per group; E), and NAD+ and NADH concentrations (n = 4 per group; F) in VAT obtained from 3- to 5-mo-old male mice. G: Western blot analysis of subunits of electron transport chain (ETC) complex (C) in VAT obtained from 3- to 4-mo-old female mice. Densitometric analysis of each protein normalized to tubulin is shown. H: ex vivo respiratory function was evaluated in VAT obtained from 3- to 5-mo-old male mice by using the Seahorse system (n = 6–9 per group). Oxygen consumption rate (OCR) was measured during the basal conditions and in responses to oligomycin (Oligo), carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP), and rotenone-antimycin (R-A). Values are means ± SE.
Loss of SIRT3 does not affect BT function or energy homeostasis.
Compared with flox/flox mice, AMiSKO mice did not change whole body V̇o2, respiratory exchange ratio (RER), EE (Fig. 5A), or cold tolerance (Fig. 5B). Loss of SIRT3 had no effect on BAT histology (data not shown), gene expression of key SIRT3 downstream targets in brown adipocytes [Pgc1α and uncoupling protein-1 (Ucp1)] (44) and other proteins involved in regulating mitochondrial function and thermogenesis [Pparα, iodothyronine deiodinase 2 (Dio2), cell death-inducing DFFA-like effector a (Cidea), cytochrome c oxidase I (mtCO1)] and oxidative stress defense (MnSOD, Catalase, Foxo3a, Idh2) (Fig. 5C), mtDNA contents (Fig. 5D), GSH and GSSG (Fig. 5E), NAD+ and NADH (Fig. 5F), and protein contents of the subunits of the ETC complex (Fig. 5G). The absence of overt BAT phenotypes in AMiSKO mice is consistent with that in the germ line Sirt3 knockout mice (29).
Fig. 5.
Brown adipose tissue (BAT) function and energy metabolism in AMiSKO mice. BAT function and energy metabolism were evaluated in AMiSKO and flox/flox mice. A: whole body oxygen consumption (V̇o2), respiratory exchange rate (RER), and energy expenditure (EE) were determined in 4- to 5-mo-old male mice (n = 4 per group). B: rectal body temperature was monitored during cold exposure in 4- to 5-mo-old male mice (n = 5 per group). There was a significant time effect (P < 0.05) but no group × time interaction (ANOVA). Gene expression of SIRT3 targets and proteins involved in regulating thermogenesis, mitochondrial function, and oxidative stress defense (n = 5 per group; C), mtDNA contents (n = 4 per group; D), GSH and GSSG concentrations and their ratios (n = 3–5 per group; E), and NAD+ and NADH concentrations (n = 4 per group; F), in BAT obtained from 3- to 5-mo-old male mice. G: Western blot analysis of subunits of ETC complex (C) in BAT obtained from 3- to 4-mo-old female mice. Densitometric analysis of each protein normalized to tubulin is shown. Values are means ± SE.
Adipocyte-specific Sirt3 deletion does not change whole body metabolism in aged mice.
Previous studies have shown that SIRT3 is critically involved in various age-associated pathophysiologies and diseases (11, 13, 15, 31, 47). However, aged male AMiSKO mice did not change body weight, body composition, glucose tolerance, insulin sensitivity, plasma lipid profile, or whole body energy metabolism compared with age-matched flox/flox mice (Fig. 6).
Fig. 6.
Metabolic phenotype of aged AMiSKO mice. Characterization of aged male AMiSKO and flox/flox mice. A: body composition in 20- to 23-mo-old mice (n = 5–7 per group). Blood glucose concentrations during the IPGTTs (B) and ITTs (C) were determined in 18- to 21-mo-old mice (n = 8–9 per group). AUC for glucose is shown next to each curve. D: plasma lipid profile was determined in 18- to 21-mo-old mice (n = 7–9 per group). E: whole body V̇o2, RER, and EE were determined in 21- to 23-mo-old mice (n = 4 per group). There was a significant time effect (P < 0.05) but no group × time interaction in IPGTTs and ITTs data (ANOVA). Values are means ± SE.
DISCUSSION
In the present study, we investigated the functional role of adipocyte SIRT3 in adipose tissue metabolism and obesity. We found that obese mice had a marked reduction in adipose tissue SIRT3 gene and protein expression compared with their lean counterparts. However, contrary to our hypothesis, adipocyte-specific Sirt3 deletion had no significant impact on putative SIRT3 targets, key metabolic pathways and mitochondrial biology in WAT and BAT, whole body glucose and lipid metabolism, energy homeostasis, and metabolic responses to HFD-feeding and aging, two major risk factors of obesity. Collectively, these findings suggest that adipocyte SIRT3 is dispensable for maintaining normal adipose tissue mitochondrial function and whole body metabolism.
The results of the present study demonstrate reduced SIRT3 expression in adipose tissue of obese mice, which is consistent with data obtained from studies conducted with human adipose tissue biopsies (19, 39). Interestingly, it has been also reported that obesity is associated with decreased adipose tissue expression of a key NAD+ biosynthetic enzyme, nicotinamide phosphoribosyltransferase (NAMPT), and other NAD+-dependent sirtuins such as SIRT1 and SIRT6 in both rodents and humans (4, 19, 23, 39, 51, 54). These findings indicate the importance and translational potential of adipose tissue NAD+-sirtuins axes in the pathophysiology of obesity. Indeed, we recently reported that adipocyte-specific Nampt deletion causes obesity-associated systemic metabolic abnormalities, including adipose tissue dysfunction and multiorgan insulin resistance (46, 51). Similarly, recent data obtained from studies conducted in adipocyte-specific knockout mouse models have demonstrated that nuclear sirtuins, SIRT1 (4, 30) and SIRT6 (23, 50), are involved in obesity and its metabolic complications. In contrast, our current results suggest that mitochondrial SIRT3 in adipocytes does not play an important role in adipose tissue metabolism or obesity. However, we cannot exclude the possibility that functional redundancy of SIRT3 and other sirtuins exists in adipocytes. Interestingly, data obtained from recent studies using proteomic approaches have identified a significant overlap of SIRT3-regulated and SIRT5-regulated lysine residues in numerous liver mitochondrial enzymes (1, 24, 40). Moreover, SIRT1 and SIRT3 also have common molecular targets, such as PGC1α and FOXO3, in various cell types (11, 13, 33). Therefore, it will be of great importance to examine double-knockout mice lacking Sirt3 and other sirtuin(s) and dissect the fascinating interplay between nuclear and mitochondrial sirtuins in adipose tissue mitochondrial biology.
In a recent study conducted by Johan Auwerx’s group, liver- or muscle-specific Sirt3 knockout mice did not change cellular mitochondrial function or whole body metabolism (10). Similarly, we found that adipocyte-specific Sirt3 knockout mice have normal adipose tissue mitochondrial function and whole body metabolism. However, in contrast to these findings, germ line Sirt3 knockout mice had enhanced susceptibility to HFD-induced obesity and displayed systemic metabolic complications such as insulin resistance, glucose intolerance, NAFLD, and dyslipidemia (14). Mechanisms explaining these phenotypic differences between germ line and conditional knockout mice remain unclear but could involve Sirt3 deficiency in a different organ or tissue. For example, it has been recently reported that SIRT3 has a protective role against mitochondrial oxidative and metabolic stress in neurons (5, 43). Given the importance of brain neurons in systemic metabolic regulation (42) loss of neuronal SIRT3 expression could impair metabolic function in peripheral organs, such as liver and skeletal muscle. It is also possible that loss of SIRT3 function in multiple organs is required to cause systemic metabolic abnormalities observed in the germ line Sirt3 knockout mice. In addition, we cannot exclude the possibilities that AMiSKO mice display metabolic phenotypes over a longer HFD feeding period and that aged AMiSKO mice change their susceptibility to HFD-induced obesity. Additional studies are needed to further determine tissue-specific function of SIRT3 in whole body metabolism and obesity.
In conclusion, the results of the present study conducted in adipocyte-specific Sirt3 knockout mice demonstrate a minimal role of SIRT3 in adipose tissue mitochondrial biology and whole body metabolism. Loss of adipocyte Sirt3 has no major impact on metabolic responses to HFD feeding and aging. Hence, although we found that adipose tissue SIRT3 expression was markedly decreased in obese mice, impaired SIRT3 function in adipocytes is unlikely to play a causative role in the pathogenesis of obesity-induced adipose tissue mitochondrial dysfunction and systemic metabolic complications. Future studies are warranted to understand the complex molecular mechanisms that link NAD+-sirtuins axes and mitochondrial biology in adipocytes.
GRANTS
This study was supported by NIH Grants DK-104995 (J.Y.), EY-019287 (R. S. Apte), EY-02687 (Vision Core Grant), DK-56341 (Nutrition and Obesity Research Center), DK-37948 and DK-20579 (Diabetes Research Center), and DK-52574 (Digestive Diseases Research Core Center), T32 GM-07200, UL1 TR-002345, and TL1 TR-002344 (J. B. Lin), and Jeffrey Fort Innovation Fund; Starr Foundation (R. S. Apte), the Carl Marshall Reeves and Mildred Almen Reeves Foundation (R. S. Apte), the Bill and Emily Kuzma Family Gift for retinal research (R. S. Apte), a Physician-Scientist Award and a Nelson Trust Award from Research to Prevent Blindness (R.S.A.), the Thome Foundation (R. S. Apte), and an unrestricted grant from Research to Prevent Blindness, Inc., to the Washington University School of Medicine Department of Ophthalmology.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.C.P., M.P.F., Y.S., E.V., R.S.A., and J.Y. conceived and designed research; L.C.P., M.P.F., T.P., S.Y., J.B.L., Y.S., and J.Y. performed experiments; L.C.P., M.P.F., T.P., Y.S., and J.Y. analyzed data; L.C.P., M.P.F., T.P., Y.S., and J.Y. interpreted results of experiments; J.Y. prepared figures; L.C.P. and J.Y. drafted manuscript; L.C.P., M.P.F., T.P., S.Y., J.B.L., Y.S., E.V., R.S.A., and J.Y. edited and revised manuscript; L.C.P., M.P.F., T.P., S.Y., J.B.L., Y.S., E.V., R.S.A., and J.Y. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Dmitri Samovski (Dept. of Medicine, Washington University School of Medicine), Takeshi Egawa (Dept. of Pathology and Immunology, Washington University School of Medicine) and Sangeeta Adak (Washington University Diabetes Research Center) for technical assistance.
REFERENCES
- 1.Anderson KA, Hirschey MD. Mitochondrial protein acetylation regulates metabolism. Essays Biochem 52: 23–35, 2012. doi: 10.1042/bse0520023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogacka I, Xie H, Bray GA, Smith SR. Pioglitazone induces mitochondrial biogenesis in human subcutaneous adipose tissue in vivo. Diabetes 54: 1392–1399, 2005. doi: 10.2337/diabetes.54.5.1392. [DOI] [PubMed] [Google Scholar]
- 3.Bugge A, Dib L, Collins S. Measuring respiratory activity of adipocytes and adipose tissues in real time. Methods Enzymol 538: 233–247, 2014. doi: 10.1016/B978-0-12-800280-3.00013-X. [DOI] [PubMed] [Google Scholar]
- 4.Chalkiadaki A, Guarente L. High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab 16: 180–188, 2012. doi: 10.1016/j.cmet.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng A, Yang Y, Zhou Y, Maharana C, Lu D, Peng W, Liu Y, Wan R, Marosi K, Misiak M, Bohr VA, Mattson MP. Mitochondrial SIRT3 mediates adaptive responses of neurons to exercise and metabolic and excitatory challenges. Cell Metab 23: 128–142, 2016. doi: 10.1016/j.cmet.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craft CS, Pietka TA, Schappe T, Coleman T, Combs MD, Klein S, Abumrad NA, Mecham RP. The extracellular matrix protein MAGP1 supports thermogenesis and protects against obesity and diabetes through regulation of TGF-β. Diabetes 63: 1920–1932, 2014. doi: 10.2337/db13-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Pauw A, Tejerina S, Raes M, Keijer J, Arnould T. Mitochondrial (dys)function in adipocyte (de)differentiation and systemic metabolic alterations. Am J Pathol 175: 927–939, 2009. doi: 10.2353/ajpath.2009.081155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature 444: 881–887, 2006. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 9.Eguchi J, Wang X, Yu S, Kershaw EE, Chiu PC, Dushay J, Estall JL, Klein U, Maratos-Flier E, Rosen ED. Transcriptional control of adipose lipid handling by IRF4. Cell Metab 13: 249–259, 2011. doi: 10.1016/j.cmet.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez-Marcos PJ, Jeninga EH, Canto C, Harach T, de Boer VC, Andreux P, Moullan N, Pirinen E, Yamamoto H, Houten SM, Schoonjans K, Auwerx J. Muscle or liver-specific Sirt3 deficiency induces hyperacetylation of mitochondrial proteins without affecting global metabolic homeostasis. Sci Rep 2: 425, 2012. doi: 10.1038/srep00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giblin W, Skinner ME, Lombard DB. Sirtuins: guardians of mammalian healthspan. Trends Genet 30: 271–286, 2014. doi: 10.1016/j.tig.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han C, Someya S. Maintaining good hearing: calorie restriction, Sirt3, and glutathione. Exp Gerontol 48: 1091–1095, 2013. doi: 10.1016/j.exger.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He W, Newman JC, Wang MZ, Ho L, Verdin E. Mitochondrial sirtuins: regulators of protein acylation and metabolism. Trends Endocrinol Metab 23: 467–476, 2012. doi: 10.1016/j.tem.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Hirschey MD, Shimazu T, Jing E, Grueter CA, Collins AM, Aouizerat B, Stančáková A, Goetzman E, Lam MM, Schwer B, Stevens RD, Muehlbauer MJ, Kakar S, Bass NM, Kuusisto J, Laakso M, Alt FW, Newgard CB, Farese RV Jr, Kahn CR, Verdin E. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol Cell 44: 177–190, 2011. doi: 10.1016/j.molcel.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imai S, Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol 24: 464–471, 2014. doi: 10.1016/j.tcb.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jahansouz C, Serrot FJ, Frohnert BI, Foncea RE, Dorman RB, Slusarek B, Leslie DB, Bernlohr DA, Ikramuddin S. Roux-en-Y gastric bypass acutely decreases protein carbonylation and increases expression of mitochondrial biogenesis genes in subcutaneous adipose tissue. Obes Surg 25: 2376–2385, 2015. doi: 10.1007/s11695-015-1708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jing E, Emanuelli B, Hirschey MD, Boucher J, Lee KY, Lombard D, Verdin EM, Kahn CR. Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc Natl Acad Sci USA 108: 14608–14613, 2011. doi: 10.1073/pnas.1111308108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jing E, O’Neill BT, Rardin MJ, Kleinridders A, Ilkeyeva OR, Ussar S, Bain JR, Lee KY, Verdin EM, Newgard CB, Gibson BW, Kahn CR. Sirt3 regulates metabolic flexibility of skeletal muscle through reversible enzymatic deacetylation. Diabetes 62: 3404–3417, 2013. doi: 10.2337/db12-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jukarainen S, Heinonen S, Rämö JT, Rinnankoski-Tuikka R, Rappou E, Tummers M, Muniandy M, Hakkarainen A, Lundbom J, Lundbom N, Kaprio J, Rissanen A, Pirinen E, Pietiläinen KH. Obesity is associated with low NAD(+)/SIRT pathway expression in adipose tissue of BMI-discordant monozygotic twins. J Clin Endocrinol Metab 101: 275–283, 2016. doi: 10.1210/jc.2015-3095. [DOI] [PubMed] [Google Scholar]
- 20.Kendrick AA, Choudhury M, Rahman SM, McCurdy CE, Friederich M, Van Hove JL, Watson PA, Birdsey N, Bao J, Gius D, Sack MN, Jing E, Kahn CR, Friedman JE, Jonscher KR. Fatty liver is associated with reduced SIRT3 activity and mitochondrial protein hyperacetylation. Biochem J 433: 505–514, 2011. doi: 10.1042/BJ20100791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein S, Wadden T, Sugerman HJ. AGA technical review on obesity. Gastroenterology 123: 882–932, 2002. doi: 10.1053/gast.2002.35514. [DOI] [PubMed] [Google Scholar]
- 22.Kleiner S, Mepani RJ, Laznik D, Ye L, Jurczak MJ, Jornayvaz FR, Estall JL, Chatterjee Bhowmick D, Shulman GI, Spiegelman BM. Development of insulin resistance in mice lacking PGC-1α in adipose tissues. Proc Natl Acad Sci USA 109: 9635–9640, 2012. doi: 10.1073/pnas.1207287109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuang J, Zhang Y, Liu Q, Shen J, Pu S, Cheng S, Chen L, Li H, Wu T, Li R, Li Y, Zou M, Zhang Z, Jiang W, Xu G, Qu A, Xie W, He J. Fat-specific Sirt6 ablation sensitizes mice to high-fat diet-induced obesity and insulin resistance by inhibiting lipolysis. Diabetes 66: 1159–1171, 2017. doi: 10.2337/db16-1225. [DOI] [PubMed] [Google Scholar]
- 24.Kumar S, Lombard DB. Mitochondrial sirtuins and their relationships with metabolic disease and cancer. Antioxid Redox Signal 22: 1060–1077, 2015. doi: 10.1089/ars.2014.6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kusminski CM, Holland WL, Sun K, Park J, Spurgin SB, Lin Y, Askew GR, Simcox JA, McClain DA, Li C, Scherer PE. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat Med 18: 1539–1549, 2012. doi: 10.1038/nm.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kusminski CM, Scherer PE. Mitochondrial dysfunction in white adipose tissue. Trends Endocrinol Metab 23: 435–443, 2012. doi: 10.1016/j.tem.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwon S, Seok S, Yau P, Li X, Kemper B, Kemper JK. Obesity and aging diminish sirtuin 1 (SIRT1)-mediated deacetylation of SIRT3, leading to hyperacetylation and decreased activity and stability of SIRT3. J Biol Chem 292: 17312–17323, 2017. doi: 10.1074/jbc.M117.778720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lantier L, Williams AS, Williams IM, Yang KK, Bracy DP, Goelzer M, James FD, Gius D, Wasserman DH. SIRT3 is crucial for maintaining skeletal muscle insulin action and protects against severe insulin resistance in high-fat-fed mice. Diabetes 64: 3081–3092, 2015. doi: 10.2337/db14-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, Chen Y, Hirschey MD, Bronson RT, Haigis M, Guarente LP, Farese RV Jr, Weissman S, Verdin E, Schwer B. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol 27: 8807–8814, 2007. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayoral R, Osborn O, McNelis J, Johnson AM, Oh DY, Izquierdo CL, Chung H, Li P, Traves PG, Bandyopadhyay G, Pessentheiner AR, Ofrecio JM, Cook JR, Qiang L, Accili D, Olefsky JM. Adipocyte SIRT1 knockout promotes PPARγ activity, adipogenesis and insulin sensitivity in chronic-HFD and obesity. Mol Metab 4: 378–391, 2015. doi: 10.1016/j.molmet.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonnell E, Peterson BS, Bomze HM, Hirschey MD. SIRT3 regulates progression and development of diseases of aging. Trends Endocrinol Metab 26: 486–492, 2015. doi: 10.1016/j.tem.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mustelin L, Pietiläinen KH, Rissanen A, Sovijärvi AR, Piirilä P, Naukkarinen J, Peltonen L, Kaprio J, Yki-Järvinen H. Acquired obesity and poor physical fitness impair expression of genes of mitochondrial oxidative phosphorylation in monozygotic twins discordant for obesity. Am J Physiol Endocrinol Metab 295: E148–E154, 2008. doi: 10.1152/ajpendo.00580.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nogueiras R, Habegger KM, Chaudhary N, Finan B, Banks AS, Dietrich MO, Horvath TL, Sinclair DA, Pfluger PT, Tschöp MH. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev 92: 1479–1514, 2012. doi: 10.1152/physrev.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward JL III, Goodyear LJ, Tong Q. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging (Albany NY) 1: 771–783, 2009. doi: 10.18632/aging.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polak P, Cybulski N, Feige JN, Auwerx J, Rüegg MA, Hall MN. Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab 8: 399–410, 2008. doi: 10.1016/j.cmet.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab 12: 662–667, 2010. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 37.Quiros PM, Goyal A, Jha P, Auwerx J. Analysis of mtDNA/nDNA ratio in mice. Curr Protoc Mouse Biol 7: 47–54, 2017. doi: 10.1002/cpmo.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rajagopal R, Zhang S, Wei X, Doggett T, Adak S, Enright J, Shah V, Ling G, Chen S, Yoshino J, Hsu FF, Semenkovich CF. Retinal de novo lipogenesis coordinates neurotrophic signaling to maintain vision. JCI Insight 3: e97076, 2018. doi: 10.1172/jci.insight.97076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rappou E, Jukarainen S, Rinnankoski-Tuikka R, Kaye S, Heinonen S, Hakkarainen A, Lundbom J, Lundbom N, Saunavaara V, Rissanen A, Virtanen KA, Pirinen E, Pietiläinen KH. Weight loss is associated with increased NAD(+)/SIRT1 expression but reduced PARP activity in white adipose tissue. J Clin Endocrinol Metab 101: 1263–1273, 2016. doi: 10.1210/jc.2015-3054. [DOI] [PubMed] [Google Scholar]
- 40.Rardin MJ, He W, Nishida Y, Newman JC, Carrico C, Danielson SR, Guo A, Gut P, Sahu AK, Li B, Uppala R, Fitch M, Riiff T, Zhu L, Zhou J, Mulhern D, Stevens RD, Ilkayeva OR, Newgard CB, Jacobson MP, Hellerstein M, Goetzman ES, Gibson BW, Verdin E. SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab 18: 920–933, 2013. doi: 10.1016/j.cmet.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rong JX, Qiu Y, Hansen MK, Zhu L, Zhang V, Xie M, Okamoto Y, Mattie MD, Higashiyama H, Asano S, Strum JC, Ryan TE. Adipose mitochondrial biogenesis is suppressed in db/db and high-fat diet-fed mice and improved by rosiglitazone. Diabetes 56: 1751–1760, 2007. doi: 10.2337/db06-1135. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz MW, Porte D Jr. Diabetes, obesity, and the brain. Science 307: 375–379, 2005. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- 43.Shi H, Deng HX, Gius D, Schumacker PT, Surmeier DJ, Ma YC. Sirt3 protects dopaminergic neurons from mitochondrial oxidative stress. Hum Mol Genet 26: 1915–1926, 2017. doi: 10.1093/hmg/ddx100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi T, Wang F, Stieren E, Tong Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem 280: 13560–13567, 2005. doi: 10.1074/jbc.M414670200. [DOI] [PubMed] [Google Scholar]
- 45.Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 143: 802–812, 2010. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stromsdorfer KL, Yamaguchi S, Yoon MJ, Moseley AC, Franczyk MP, Kelly SC, Qi N, Imai S, Yoshino J. NAMPT-mediated NAD(+) biosynthesis in adipocytes regulates adipose tissue function and multi-organ insulin sensitivity in mice. Cell Reports 16: 1851–1860, 2016. doi: 10.1016/j.celrep.2016.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van de Ven RAH, Santos D, Haigis MC. Mitochondrial sirtuins and molecular mechanisms of aging. Trends Mol Med 23: 320–331, 2017. doi: 10.1016/j.molmed.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vassilopoulos A, Pennington JD, Andresson T, Rees DM, Bosley AD, Fearnley IM, Ham A, Flynn CR, Hill S, Rose KL, Kim HS, Deng CX, Walker JE, Gius D. SIRT3 deacetylates ATP synthase F1 complex proteins in response to nutrient- and exercise-induced stress. Antioxid Redox Signal 21: 551–564, 2014. doi: 10.1089/ars.2013.5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vernochet C, Damilano F, Mourier A, Bezy O, Mori MA, Smyth G, Rosenzweig A, Larsson NG, Kahn CR. Adipose tissue mitochondrial dysfunction triggers a lipodystrophic syndrome with insulin resistance, hepatosteatosis, and cardiovascular complications. FASEB J 28: 4408–4419, 2014. doi: 10.1096/fj.14-253971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiong X, Zhang C, Zhang Y, Fan R, Qian X, Dong XC. Fabp4-Cre-mediated Sirt6 deletion impairs adipose tissue function and metabolic homeostasis in mice. J Endocrinol 233: 307–314, 2017. doi: 10.1530/JOE-17-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamaguchi S, Yoshino J. Adipose tissue NAD+ biology in obesity and insulin resistance: From mechanism to therapy. BioEssays 39: 1600227, 2017. doi: 10.1002/bies.201600227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yin X, Lanza IR, Swain JM, Sarr MG, Nair KS, Jensen MD. Adipocyte mitochondrial function is reduced in human obesity independent of fat cell size. J Clin Endocrinol Metab 99: E209–E216, 2014. doi: 10.1210/jc.2013-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshino J, Imai S. Accurate measurement of nicotinamide adenine dinucleotide (NAD+) with high-performance liquid chromatography. Methods Mol Biol 1077: 203–215, 2013. doi: 10.1007/978-1-62703-637-5_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab 14: 528–536, 2011. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]