Abstract
ErbB4, a member of the EGF receptor family, plays a variety of roles in physiological and pathological states. Genetic studies have indicated a link between ErbB4 and type 2 diabetes and obesity, but its role in metabolic syndrome (MetS) has not been reported. In the current study we found that mice with ErbB4 deletion developed MetS after 24 wk on a medium-fat diet (MFD), as indicated by development of obesity, dyslipidemia, hepatic steatosis, hyperglycemia, hyperinsulinemia, and insulin resistance, compared with wild-type mice. ErbB4 deletion mice also exhibited increased amounts of subcutaneous and visceral fat, with increased serum leptin levels, compared with wild-type mice, whereas levels of adiponectin were not significantly different. Histologically, severe inflammation, indicated by F4/80 immunostaining and M1 macrophage polarization, was detected in inguinal and epididymal white adipose tissue in ErbB4 deletion mice. ErbB4 expression decreased during 3T3-L1 preadipocyte differentiation. Administration of neuroregulin 4, a specific ligand for ErbB4, to 3T3-L1 adipocytes had no effect on adipogenesis and lipolysis but significantly inhibited lipogenesis, promoted browning, induced GLUT4 redistribution to the cell membrane, and increased glucose uptake. Neuroregulin 4 also significantly increased glucose uptake in adipocytes isolated from wild-type mice, while these effects were significantly decreased in adipocytes isolated from ErbB4 deletion mice. In conclusion, our results indicate that ErbB4 may play an important role in glucose homeostasis and lipogenesis. ErbB4 deficiency-related obesity and adipose tissue inflammation may contribute to the development of MetS.
Keywords: ErbB4, inflammation, insulin resistance, lipogenesis, neuregulin 4
INTRODUCTION
Metabolic syndrome (MetS) is characterized by a constellation of metabolic disorders, including obesity, insulin resistance, dyslipidemia, hypertension, and hyperglycemia. Obesity and obesity-associated insulin resistance are thought to play key roles in the pathogenesis of MetS (9). With the rising obesity rates, the prevalence of MetS is rapidly increasing and has become a major healthcare challenge worldwide due to its close association with, and increased risk for, numerous pathological conditions, including type 2 diabetes, cardiovascular disease, stroke, several types of cancer, and all-cause mortality (7, 8, 11). Obesity and MetS have significant genetic components. Therefore, genome-wide association studies have been conducted in the search for the relevant gene variants (13, 24). ErbB4 was found to be one of the genes with linkage to obesity and diabetes, illustrating a potential contribution of the ErbB4 signaling pathway to the pathogenesis of MetS (1, 13, 16, 23).
ErbB4, a type I transmembrane receptor tyrosine kinase, belongs to the EGFR superfamily, which consists of four receptors, ErbB1 (EGFR), ErbB2 (Neu), ErbB3, and ErbB4 (21), and can be activated through the binding of their ligands, followed by receptor dimerization, auto- and transphosphorylation of COOH-terminal tyrosine residues, and initiation of downstream signaling pathways (5). Seven known ligands are able to bind and activate ErbB4; they can be grouped into three categories: 1) heparin-binding EGF (HB-EGF), epiregulin, and betacellulin, which also binds to EGFR, 2) neuroregulin (NRG) 1 and NRG2, which also bind to ErbB3, and 3) NRG3 and NRG4, ligands for ErbB4 only. All these ligands have been reported to play important roles in glucose transport, lipogenesis, or browning of the adipocytes, critical steps in glucose homeostasis and energy expenditure. Multiple studies have reported that NRG1 mediates glucose transport and improves glucose tolerance (14, 26). Although NRG1 can bind to both ErbB3 and ErbB4, activation of ErbB4 was proven to be critical for the increased glucose uptake, as ErbB4-blocking antibodies impaired NRG1-induced glucose uptake (3). NRG4, a ligand that specifically binds to ErbB4, has been reported to promote “browning” of white fat, fuel oxidation, prevention of high-fat diet-induced obesity, and improvement of insulin sensitivity (6, 15, 29). These findings suggest that ErbB4 may play an important role in metabolic regulation. In the current study we found that mice with ErbB4 deletion developed MetS when fed a medium-fat diet (MFD).
RESEARCH DESIGN AND METHODS
Animals and animal care.
Mice were housed at the Vanderbilt Medical Center veterinary facility. Animal care and all experimental protocols complied with the regulations of, and were approved by, Vanderbilt University’s Institutional Animal Care and Usage Committee. Heart-rescued ErbB4 deletion (ErbB4−/−ht+) mice are described elsewhere (30). They were originally on C57BL/6J and /FVB mixed background and were backcrossed for >10 generations to C57BL/6J before they were used for the study. Genotyping was performed using PCR and primers as previously reported (30). Age-matched male mice with ErbB4−/−ht+ were used in the experimental group, and mice with ht+ only were used as wild-type. For regular chow diet feeding, mice were fed the 5001 laboratory rodent diet (LabDiet), which contains 4.5 g% (13 kcal%) fat. For MFD diet feeding, mice were fed the PicoLab 5LJ5 diet (LabDiet), which contains 11 g% (25 kcal%) fat, for 24 wk starting at 4 wk of age. In our preliminary studies we found no significant differences in body weight between mice with and mice without ErbB4 deletion when fed the regular chow diet (data not shown). Therefore, in the current study, all mice were fed the MFD. Body weight was monitored bimonthly, and food intake was measured biweekly. At the end of the MFD feeding, mice were euthanized, blood samples were collected for analysis of metabolic parameters, and organs were collected, weighed, and processed for histological analysis.
Intraperitoneal glucose tolerance test.
At 28 wk of age, ErbB4−/−ht+ (n = 9) and wild-type (n = 10) mice were fasted for 16 h. Fasting (baseline) blood glucose in a drop of tail vein blood was measured with an Accu-Check Aviva glucometer and glucose test strips (Roche Diagnostics). Animals were injected with filter-sterilized glucose (2 mg/g body wt ip) in 0.9% NaCl, and blood glucose was measured 15, 30, 60, 90, and 120 min after glucose injection. Plasma used for insulin analysis was collected from the tail vein at 0 (baseline), 15, and 30 min after the glucose loads. Statistical analyses for glucose handling were performed using the differences from baseline of the area under the curve by GraphPad Prism 5. Insulin content was analyzed in duplicates using radioimmunoassay by the Vanderbilt University Hormone Assay and Analytical Services Core. Insulin resistance was assessed by homeostatic model assessment (HOMA-IR), which was calculated, as previously described (17), using the following formula: HOMA-IR = [glucose (mg/dl)] × [insulin (mU/l)]/405.
Intraperitoneal insulin tolerance test.
The intraperitoneal insulin tolerance test (ipITT) was performed on random-fed mice at around 10 AM. The mice were injected intraperitoneally with insulin (human regular, U-100; catalog no. NDC 0169-1833-11, Novolin) at 0.75 U/kg in 0.9% NaCl. Blood glucose levels were measured before and 15, 30, 45, 60, and 120 min after the insulin injection.
Plasma chemistry and metabolic parameters.
At the end of experiments, blood levels of leptin and adiponectin were measured with a Luminex 100 system by the Vanderbilt University Hormone Assay and Analytical Services Core. Plasma alanine aminotransferase levels were measured by the Vanderbilt University Pathology Core. Lipid profiles, including total cholesterol, triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and free fatty acids were measured by the Vanderbilt University Metabolic Core.
Histology and immunochemistry studies.
Upon euthanasia, liver and adipose tissue (AT), including interscapular brown AT (BAT), inguinal white AT (iWAT), and epididymal white AT (eWAT), were fixed in 4% paraformaldehyde and embedded in paraffin or Tissue-Tek OCT medium. Tissue was stained with hematoxylin-eosin for the morphological studies. Liver samples from cryosections were stained with oil red O (ORO). Immunofluorescence staining for macrophages using F4/80 antibody (Bio-Rad, Hercules, CA) was carried out as previously described (31).
ORO staining and lipid content measurement.
ORO staining was used to evaluate the lipid content in the liver and 3T3-L1 cells. Frozen sections (8 µm) of liver or 3T3-L1 adipocytes in 12-well plates were fixed with 4% paraformaldehyde for 30 min and then gently rinsed with PBS followed by 60% isopropanol for 5 min and ORO working solution for 30 min. Hematoxylin was used to counterstain the nucleus. The images were examined and photographed using a Zeiss microscope. To extract ORO dye, cells were washed with 60% isopropanol, and 500 µl of 100% isopropanol were added for each well. Two aliquots of 200 μl were transferred to 96-well plates, and the optical density reading of ORO was measured at 520 nm using an Omega multimode microplate reader (BMG-Labtech, Cary, NC).
Adipocyte isolation.
Adipocytes were isolated from eWAT of 12-wk-old wild-type or ErbB4−/−ht+ mice (4–5 mice in each group) that were fed the MFD for 8 wk using methods previously described (4) with a few modifications. Briefly, eWAT was dissected, minced, and digested with 1 mg/ml collagenase type IV (Invitrogen, Thermo Fisher Scientific, Waltham, MA) in Krebs-Ringer HEPES buffer (KRHB) containing 1.5% fatty acid-free bovine serum albumin (catalog no. A8806, Sigma) at 37°C for 60 min with gentle shaking every 5 min. Digested eWAT was filtered through a 300-µm pluriStrainer (product no. 43-50300-03, PluriSelect) to remove large debris and passed through a 40-µm cell strainer (catalog no. 352340, BD Falcon) to remove stromal vascular fractions and red blood cells. The mature adipocytes on the top of the strainer were carefully transferred to a 1.5-ml tube using a wide-bore pipette tip, washed three times with 0.5 ml of KRHB containing 1.5% fatty acid-free bovine serum albumin, resuspended in the same buffer, and used for glucose uptake assay.
Cell culture.
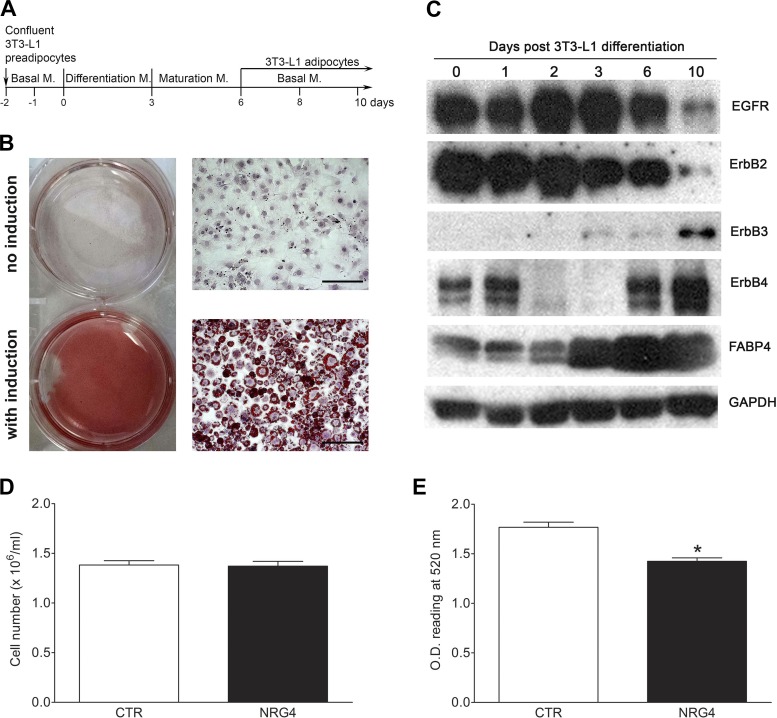
Murine 3T3-L1 preadipocytes were obtained from American Type Culture Collection (Bethesda, MD) and cultured in high-glucose (4.5 g/l) DMEM (Corning) supplemented with 10% fetal bovine serum, streptomycin (100 µg/ml), and penicillin (100 U/ml), designated “basal medium.” Two-days-postconfluent 3T3-L1 preadipocytes were induced to differentiate for 3 days in MDI differentiation medium (basal medium + 1 μM dexamethasone, 0.5 mM IBMX, and 10 μg/ml insulin), which was replaced with mature medium containing basal medium plus 10 μg/ml insulin, and the cells were cultured for another 2 days and then maintained in the basal medium and utilized for the experiments as adipocytes, which were verified using ORO staining (see Fig. 5, A and B). For GLUT4 localization studies, 3T3-L1 adipocytes were serum-starved for 4 h and then treated with 10 µg/ml insulin or 20 ng/ml NRG4 (Reprokine, Valley Cottage, NY) for 15 min.
Fig. 5.
EGFR family member expression during 3T3-L1 differentiation and neuroregulin 4 (NRG4) effects on lipogenesis. A: 3T3-L1 differentiation protocol and time line. M, medium. B: oil red O staining of 3T3-L1 preadipocytes (no induction) and adipocytes (with induction) on differentiation day 10. C: representative Western blots of EGFR family member expression levels during 3T3-L1 preadipocyte differentiation. FABP4, marker for adipocyte maturation (fatty acid-binding protein 4). GAPDH was used as loading control. D: NRG4 treatment did not affect cell proliferation. E: NRG4 treatment significantly reduced lipid content in 3T3-L1 adipocytes. CTR, control; OD, optical density. Values are means ± SE. n = 5. *P < 0.05.
Gene expression analyses.
Total RNA from adipose tissue or 3T3-L1 adipocytes was extracted using the TRIzol reagent (Invitrogen) and purified using an RNeasy minikit (Qiagen). For real-time PCR analysis, an equal amount of RNA was reverse-transcribed into cDNA using SuperScript III reverse transcriptase (Thermo Fisher Scientific) followed by real-time-PCRs using IQ SYBR green supermix (Bio-Rad). All assays were run on a 7900HT Fast real-time PCR system (Applied Biosystems) at Vanderbilt Technologies for Advanced Genomics (VANTAGE). The relative abundance of mRNA was normalized to GAPDH and expressed as ΔΔCt, as previously described (32). The primers used for quantitative RT-PCR are listed in Table 1.
Table 1.
Primers used for gene expression assay
| Gene Symbol | Gene Description | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|---|
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | TGGAGAAACCTGCCAAGTATGA | GAAGAGTGGGAGTTGCTGTTGA |
| iNOS | Inducible nitric oxide synthase | TGGTGGTGACAAGCACATTT | AAGGCCAAACACAGCATACC |
| Agr1 | Arginase 1 | AAAGCTGGTCTGCTGGAAAA | AGACCGTGGGTTCTTCAC |
| TNF-α | Tumor necrosis factor-α | GAACTGGCAGAAGAGGCACT | GGTCTGGGCCATAGAACTGA |
| IL-6 | Interleukin 6 | CCGGAGAGGAGACTTCACAG | TCCAGTTTGGTAGCATCCATC |
| IL-1β | Interleukin 1β | GGGCCTCAAAGGAAAGAATC | CTCTGCTTGTGAGGTGCTGA |
| CCL2 | C-C motif chemokine ligand 1 | AGCACCAGCCAACTCTCACT | CGTTAACTGCATCTGGCTGA |
| CXCL1 | Chemokine (C-X-C motif) ligand 1 | CTTGACCCTGAAGCTCCCTT | AGGTGCCATCAGAGCAGTCT |
| IL-10 | Interleukin 10 | CCAAGCCTTATCGGAAATGA | TTTTCACAGGGGAGAAATCG |
| IL-4 | Interleukin 4 | CCTCACAGCAACGAAGAACA | TTCAAGCATGGAGTTTTCCC |
| PAT2 | Proton/amino acid transporter 2 | GTGCCAAGAAGCTGCAGAG | TGTTGCCTTTGACCAGATGA |
| CD137 | Cluster of differentiation 37 | CGTGCAGAACTCCTGTGATAAC | GTCCACCTATGCTGGAGAAGG |
| UCP1 | Uncoupling protein 1 | CTTTGCCTCACTCAGGATTGG | ACTGCCACACCTCCAGTCATT |
| PRDM16 | PR domain 16 | CAGCACGGTGAAGCCATTC | GCGTGCATCCGCTTGTG |
For hepatic lipogenic gene expression, cDNA was synthesized from equal amounts of liver RNA extracted from wild-type or ErbB4 deletion mice. Real-time PCR was performed using TaqMan master mix and RNA primers for mouse sterol regulatory element-binding transcription factor 1 (Srebf1; Mm00550338), fatty acid synthase (Fasn; Mm00662319), glucokinase (Gck; Mm00439129), and mouse ribosomal protein S18 (RPS18; Mm02601777), as described above, and values were normalized to RPS18.
Lipolysis assay.
For lipolysis assays, 3T3-L1 preadipocytes were plated in 24-well plates and differentiated to adipocytes as described above. 3T3-L1 adipocytes were serum-starved for 4 h and treated with 10 µM isoproterenol (Iso; catalog no. I6504, Sigma), 20 ng/ml NRG4, or Iso + NRG4 for 2 h. Lipolysis was measured by glycerol release with a glycerol assay kit (catalog no. MAK117-1KT, Sigma), and values were normalized to cell numbers.
Assessment of glucose uptake.
Insulin- or NRG4-stimulated glucose uptake on 3T3-L1 adipocytes or adipocytes isolated from eWAT was evaluated using a Glucose Uptake-Glo assay kit (Promega, Madison, WI) according to the manufacturer’s instruction. Briefly, adipocytes were serum-starved for 3 h, treated with insulin (10 µg/ml) or NRG4 (20 ng/ml) for 30 min, and then treated with 2-deoxy-d-glucose for 10 min. At the end of the assay, luminescence was measured using an Omega multimode microplate reader. Each experiment was performed in triplicate and was repeated three times.
Immunoblot analysis.
Tissues were homogenized on ice in lysis buffer [20 mM Tris·HCl (pH 7.5), 150 mM NaCl, 2 mM EDTA, 1% Nonidet P-40, and 10% glycerol] supplemented with protease and phosphatase inhibitors (5 μg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4, 5 mM NaF, 1 mM β-glycerophosphate, and 5 mM sodium pyrophosphate). Lysates were centrifuged at 10,000 g for 15 min at 4°C, and the resulting supernatants were subjected to immunoblot analysis, as previously described (32). Anti-EGFR, ErbB2, ErbB3, ErbB4, and anti-fatty acid-binding protein 4 antibodies were purchased from Cell Signaling Technology (Danvers, MA). Anti-GAPDH antibody was obtained from Sigma.
Data analysis and statistics.
Data are expressed as means ± SE. Differences among groups were analyzed by two-way ANOVA with Bonferroni’s corrections for multiple comparisons using GraphPad Prism 5. Statistical significance was defined as P < 0.05.
RESULTS
ErbB4 deletion induced obesity in MFD-fed mice.
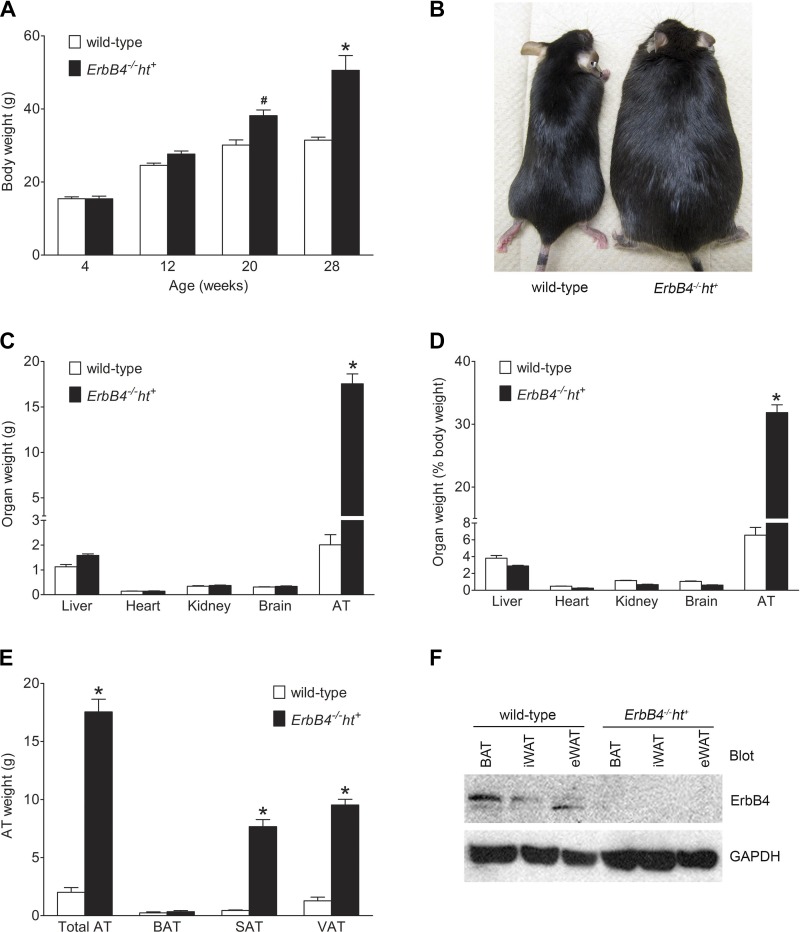
MFD-fed mice with ErbB4 deletion gradually became obese after 20 wk of age (P < 0.05 vs. wild-type), with continuously increased body weight during the subsequent 8 wk (P < 0.001 vs. wild-type), whereas over the same time period, the body weight of wild-type mice remained relatively stable (Fig. 1A). Figure 1B shows a representative image of the mice at 28 wk of age. The increased body weight of ErbB4 deletion mice was mainly due to the accumulation of adipose tissue, while the weights of other organs, such as liver, heart, kidney, and brain, were similar between groups (Fig. 1, C and D). Further analysis indicated a significant increase in the weight of subcutaneous AT and visceral AT in ErbB4 deletion compared with wild-type mice but no significant changes in the weight of BAT (Fig. 1E).
Fig. 1.
ErbB4 deletion induced obesity in mice fed the medium-fat diet (MFD). A: body weight during the MFD feeding period. Values are means ± SE (n = 9–10 in each group). #P < 0.05, *P < 0.001 vs. wild-type. B: representative images of 28-wk-old mice fed the MFD for 24 wk. C: weight of liver, heart, kidney, brain, and adipose tissue (AT) at 28 wk of age. D: organ weights in C expressed as percentage of body weight. E: weight of total AT, brown AT (BAT), subcutaneous AT (SAT), and visceral AT (VAT). F: representative image of ErbB4 expression levels in BAT, inguinal white AT (iWAT), and epididymal white AT (eWAT) in wild-type and ErbB4 deletion mice. GAPDH was used as loading control. Values are means ± SE (n = 9–10 in each group). *P < 0.001 vs. corresponding wild-type.
ErbB4 expression levels in the fat depots were analyzed by Western blotting. As shown on Fig. 1F, ErbB4 was expressed in BAT, iWAT, and eWAT in wild-type, but not ErbB4 deletion, mice.
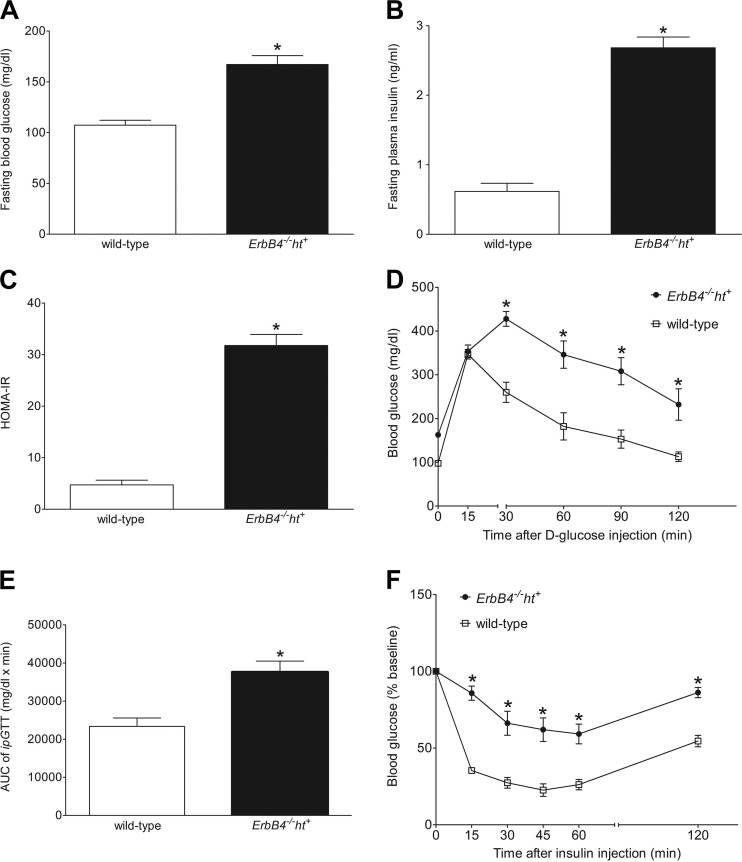
ErbB4 deletion induced hyperglycemia and insulin resistance.
After 24 wk on the MFD, fasting glucose and insulin levels were significantly increased in ErbB4 deletion compared with wild-type mice (Fig. 2, A and B). HOMA-IR, an indicator of insulin resistance, was significantly higher in ErbB4 deletion mice as well (Fig. 2C). Glucose tolerance was examined by intraperitoneal glucose tolerance test (ipGTT). After glucose injection, both the peak blood glucose and rate of decline were markedly different in ErbB4 deletion compared with wild-type mice (Fig. 2D). Significant differences (P < 0.001) were observed when area under the curve for all glucose readings during the course of the ipGTT was analyzed (Fig. 2E). To determine whether the glucose intolerance was the result of impaired insulin sensitivity, insulin tolerance tests (ipITT) were performed on nonfasting mice. After insulin injection, blood glucose levels were rapidly and significantly decreased in wild-type mice, but this effect was blunted in ErbB4 deletion mice at all time points (Fig. 2F). These results suggest that ErbB4 may play a potential role in the maintenance of postprandial glucose homeostasis.
Fig. 2.
ErbB4 deletion induced hyperglycemia and affected glucose homeostasis. A and B: blood glucose and insulin levels in ErbB4 deletion and wild-type mice after a 16-h fast. C: homeostatic model assessment of insulin resistance (HOMA-IR), calculated as follows: HOMA-IR = [glucose (mg/dl)] * [insulin (mU/l)]/405. D: blood glucose levels of ErbB4 deletion and wild-type mice during intraperitoneal glucose tolerance test (ipGTT). E: total area under the curve (AUC) for relative blood glucose levels during ipGTT. F: blood glucose levels during intraperitoneal insulin tolerance test (ipITT) expressed as percentage of initial (baseline) value. Values are means ± SE (n = 9–10 in each group). *P < 0.01 vs. wild-type (in A, B, C, and E) or vs. wild-type at the corresponding time point (D and F).
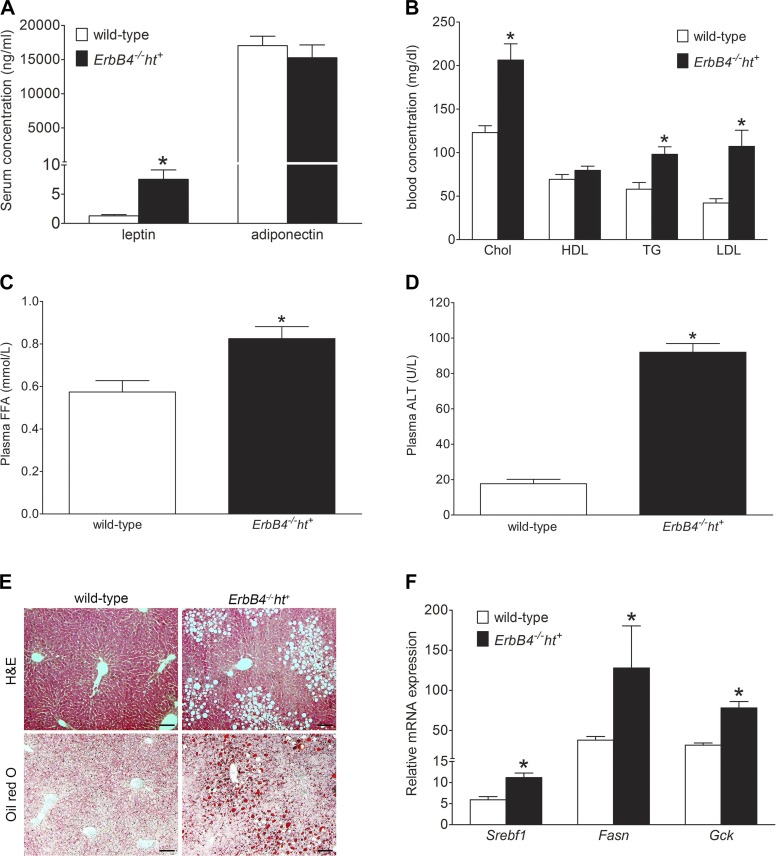
ErbB4 deletion mice had elevated blood leptin levels.
Leptin, a polypeptide hormone produced and secreted by WAT, plays a vital role in the regulation of energy and glucose homeostasis through inhibition of food intake and increasing energy expenditure (18). Serum leptin levels were significantly increased in ErbB4 deletion compared with wild-type mice (Fig. 3A), although no differences were detected in food intake between the two groups (data not shown). These results suggest that ErbB4 deletion mice may exhibit relative leptin resistance. The levels of adiponectin, another major adipocytokine that mediates insulin-sensitizing effects, were numerically lower in ErbB4 deletion than wild-type mice, but not significantly different (Fig. 3A).
Fig. 3.
Mice with ErbB4 deletion developed dyslipidemia and hepatic steatosis. A: serum leptin and adiponectin levels in ErbB4 deletion and wild-type mice fed the medium-fat diet for 24 wk. B: blood lipid profile indicating levels of cholesterol (Chol), high-density lipoprotein cholesterol (HDL), triglyceride (TG), and low-density lipoprotein cholesterol (LDL). C: plasma free fatty acid (FFA) levels. D: plasma alanine aminotransferase (ALT) levels. Values are means ± SE (n = 9–10 in each group). *P < 0.01 vs. wild-type. E: representative hematoxylin-eosin- and oil red O-stained sections of liver from wild-type and ErbB4 deletion mice. Scale bars = 100 µm. F: relative hepatic lipogenic gene [sterol regulatory element-binding transcription factor 1 (Srebf1), fatty acid synthase (Fasn), and glucokinase (Gck)] expression levels from real-time PCR assay. Values are means ± SE (n = 6 in each group). *P < 0.05 vs. wild-type.
ErbB4 deletion induced dyslipidemia and hepatic steatosis.
Levels of blood cholesterol, triglyceride, low-density lipoprotein cholesterol, and plasma free fatty acid after 24 wk on the MFD were higher in ErbB4 deletion than wild-type mice, while levels of blood high-density lipoprotein cholesterol were not significantly different (Fig. 3, B and C). At the same time, liver function was impaired in ErbB4 deletion mice, indicated by elevated plasma alanine aminotransferase levels, along with hepatic steatosis, assessed by hematoxylin-eosin or ORO staining (Fig. 3, D and E), and increased hepatic lipogenesis, indicated by elevated expression of lipogenic genes, including Srebpf1, Fasn, and Gck (Fig. 3F).
ErbB4 deletion resulted in adipocyte hypertrophy and elevated level of inflammation.
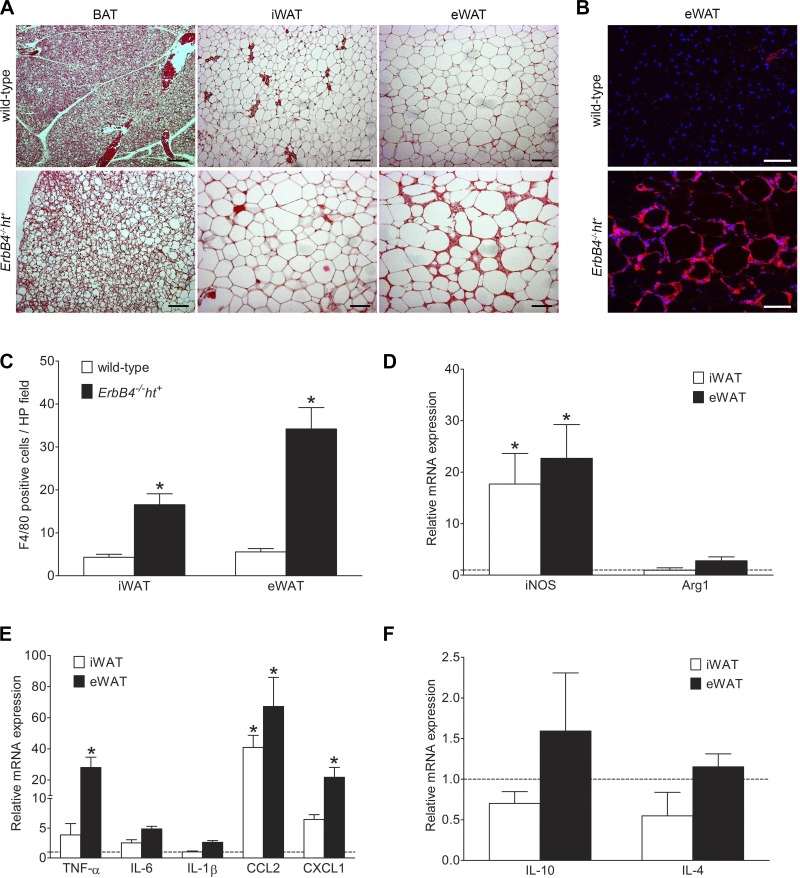
In wild-type mice, adipocytes were small, with little lipid accumulation in BAT and with regular sharp edges in iWAT and eWAT. In contrast, lipid accumulation in adipocytes was markedly increased, which caused them to lose their sharp edges and form balloon-like shapes in all fat depots in ErbB4 deletion mice. These changes were most striking in eWAT (Fig. 4A).
Fig. 4.
ErbB4 deletion resulted in adipocyte hypertrophy with inflammation. A: hematoxylin-eosin staining of brown adipose tissue (BAT), inguinal white adipose tissue (iWAT), and epididymal white adipose tissue (eWAT) from wild-type and ErbB4 deletion mice indicating hypertrophy with lipid accumulation in all fat depots with ErbB4 deletion compared with wild-type. Scale bars = 100 µm. B: representative image of F4/80 immunofluorescence staining. Red, F4/80; blue, DAPI. Scale bars = 100 µm. C: F4/80-positive cell quantification using ImageJ. HP, high-power. D–F: relative mRNA levels of inducible nitric oxide synthase (iNOS) and arginase 1 (Arg1), preinflammatory cytokines [TNF-α, IL-6, IL-1β, C-C motif chemokine ligand 2 (CCL2), and chemokine (C-X-C motif) ligand 1 (CXCL1)], and anti-inflammatory cytokines (IL-10 and IL-4) in iWAT and eWAT from ErbB4 deletion and wild-type (dashed line) mice. Values are means ± SE. n = 9–10. *P < 0.01 vs. wild-type.
Adipocyte tissue inflammation plays a key role in the development and progression of MetS (20). Accordingly, macrophage infiltration was investigated by F4/80 immunostaining, and cytokine levels were determined by quantitative PCR. There were only scattered F4/80-positive cells in all fat depots of wild-type mice and in the BAT of ErbB4 deletion mice. However, F4/80-positive immunostaining was significantly increased and clustered, with crown-like patterns, in the iWAT and eWAT of ErbB4 deletion mice and was most pronounced in eWAT (Fig. 4, B and C).
We also investigated the macrophage phenotype in the adipose tissue. mRNA expression of inducible nitric oxide synthase, a marker for M1 macrophages, was significantly higher in both iWAT and eWAT from ErbB4 deletion mice than in the corresponding depots from wild-type mice. In contrast, there were no significant differences in mRNA expression levels of arginase 1, a marker for M2 macrophages, in either eWAT or iWAT (Fig. 4D). Expression levels of mRNA for TNF-α and chemokine (C-X-C motif) ligand 1 (CXCL1) in eWAT and C-C motif chemokine ligand 2 (CCL2) in iWAT and eWAT were significantly increased in adipose tissue from ErbB4 deletion compared with wild-type mice (Fig. 4E). In contrast, IL-10 and IL-4 were decreased in iWAT and not significantly increased in eWAT from ErbB4 deletion compared with wild-type mice (Fig. 4F).
Effect of ErbB4 on 3T3-L1 preadipocyte differentiation.
To explore the effect of ErbB4 on adipogenesis and lipogenesis, both of which can contribute to obesity, we investigated the expression profile of ErbB4 and other EGFR family members during the process of 3T3-L1 preadipocyte maturation. As shown in Fig. 5C, compared with other EGFR family members, ErbB4 has a unique expression pattern: high expression levels in both preadipocytes and mature adipocytes but significantly reduced expression levels during induction and differentiation stages. EGFR and ErbB2 were mainly expressed in the preadipocytes and intermediate adipocytes, with low or minimal expression in mature 3T3-L1 adipocytes. In contrast, ErbB3 was only expressed in mature 3T3-L1 adipocytes. Fatty acid-binding protein 4 was used as a marker for adipocyte maturation. These results suggest that decreased ErbB4 expression may be required for 3T3-L1 adipogenesis and/or lipogenesis by increasing the differentiation of preadipocytes to adipocytes. To further investigate this hypothesis, 3T3-L1 cells were treated with NRG4 from the initiation stage of induction (d1) to the fully differentiated stage (d6). Our results showed that NRG4 treatment did not affect adipogenesis, as no significant differences in cell numbers were detected at d6 between NRG4- and vehicle-treated control groups (Fig. 5D). However, NRG4 treatment significantly decreased the lipid content in the 3T3-L1 adipocytes, indicating that NRG4-ErbB4 signaling may play an inhibiting role in lipogenesis (Fig. 5E).
Effect of ErbB4 signaling on glucose uptake.
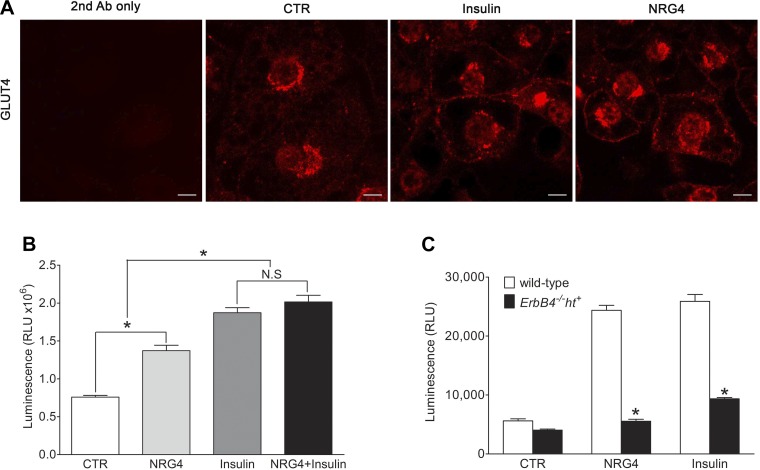
The high levels of ErbB4 expression in mature 3T3-L1 adipocytes prompted us to investigate its role in glucose uptake in the presence of NRG4 compared with insulin, an agent known to stimulate glucose uptake. Localization of GLUT4, the major glucose transporter for glucose uptake in adipose tissue, was studied by immunofluorescent staining with an anti-GLUT4 antibody (R&D Systems, Minneapolis, MN). GLUT4 was predominantly expressed in the cytoplasm in the unstimulated 3T3-L1 adipocytes and was redistributed to the plasma membrane in response to NRG4 treatment, which was comparable to the effect of insulin stimulation (Fig. 6A). NRG4 treatment also significantly increased 3T3-L1 adipocyte glucose uptake compared with the vehicle control, although not as potently as insulin treatment. The combination of NRG4 and insulin did not significantly increase glucose uptake compared with insulin alone (Fig. 6B).
Fig. 6.
Effect of ErbB4 signaling on glucose uptake. A: GLUT4 immunofluorescence in 3T3-L1 adipocytes treated for 15 min with insulin or neuroregulin 4 (NRG4) compared with vehicle-only controls (CTR). Secondary antibody (2nd Ab) only was used as negative control for immunofluorescent staining. Scale bars = 100 µm. B: glucose uptake of 3T3-L1 cells treated with NGR4, insulin, or NGR4 + insulin for 30 min. NRG4 treatment significantly stimulated glucose uptake by 3T3-L1 adipocytes, although not as potently as insulin. NRG4 did not show an additive effect on insulin-stimulated glucose uptake. RLU, relative light units; NS, not significant. Values are means ± SE. *P < 0.05. C: glucose uptake of isolated adipocytes from ErbB4 deletion or wild-type mice treated with NRG4 or insulin. Values are means ± SE. n = 5. *P < 0.01 vs. wild-type.
Next, we investigated glucose uptake ability in isolated adipocytes from eWAT of wild-type and ErbB4 deletion mice. As shown in Fig. 6C, the basal level of glucose uptake was slightly lower in adipocytes from ErbB4 deletion than wild-type mice. NRG4 significantly increased glucose uptake to levels comparable to insulin in eWAT from wild-type mice. However, NRG4 did not increase glucose uptake in adipocytes from ErbB4 deletion mice, and insulin-stimulated glucose uptake was significantly blunted.
NRG4 treatment of 3T3-L1 adipocytes increased browning but did not affect lipolysis.
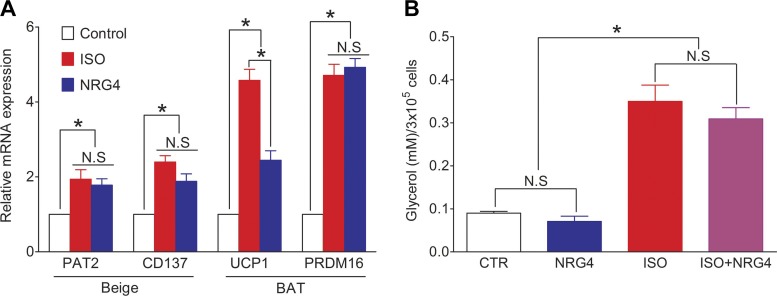
To test whether NRG4 has an effect on browning of 3T3-L1 adipocytes, 3T3-L1 adipocytes were treated with 20 ng/ml NRG4 or 10 µM Iso, a nonspecific adrenergic receptor agonist that has been shown to upregulate uncoupling proteins (UCP1) and thermogenesis (19). After treatment for 48 h, cells were washed twice with PBS and RNA was extracted and assayed by quantitative PCR. Proton/amino acid transporter 2 (PAT2) and cluster of differentiation 137 (CD137) were used as markers for beige cells, and UCP1 and PR domain zinc finger protein 16 (PRDM16) for brown adipocytes. Both PAT2 and CD137 mRNA expression levels were significantly increased after Iso or NRG4 treatment compared with control, with no significant difference between the two treatments. For the brown adipocyte markers, mRNA levels of both UCP1 and PRDM16 were significantly increased after Iso or NRG4 treatment, but NRG4 was a less potent inducer of UCP1 expression (Fig. 7A).
Fig. 7.
Effect of neuroregulin 4 (NRG4) on 3T3-L1 adipocyte browning and lipolysis. A: relative mRNA expression levels of markers for beige or brown adipocytes after treatment of 3T3-L1 cells with NRG4 or isoproterenol (Iso). PAT2, proton/amino acid transporter 2; CD137, cluster of differentiation 37; UCP1, uncoupling protein 1; PRDM16, PR domain zinc finger protein 16; NS, not significant. Values are means ± SE. *P < 0.05 vs. control. B: lipolysis assay of 3T3-L1 adipocytes treated with NRG4, Iso, or Iso + NGR4. Values are means ± SE. n = 5. *P < 0.01.
We also studied the effect of NRG4 on 3T3-L1 lipolysis, again using Iso as a positive control. Treatment with 10 µM Iso increased lipolysis nearly threefold. NRG4 (20 ng/ml) alone did not affect 3T3L1 lipolysis, nor did it significantly alter Iso-stimulated lipolysis (P > 0.05; Fig. 7B).
DISCUSSION
In the current study we found that mice with ErbB4 deletion gradually developed MetS when fed a MFD for 24 wk. To our knowledge, this is the first report to extend the results from the genome-wide association studies indicating linkage of the ErbB4 gene with obesity and diabetes (1, 13, 16, 23). Compared with the high-fat mouse diet (24–35 g% fat, or 45–60 kcal% fat), the MFD is more representative of the modern daily Western-style diet, which has been considered to contribute to the increasing prevalence of obesity and MetS (22).
Analysis of body composition revealed markedly increased body fat mass, with increased lipid accumulation in both white and brown adipocytes in ErbB4 deletion mice, a finding consistent with the in vitro studies in 3T3-L1 adipocytes, in which NRG4-ErbB4 signaling was associated with inhibition of lipogenesis. Those results were similar to the effects of HB-EGF on the adipocyte differentiation of C3H10T1/2 pluripotent mesenchymal cells, in which HB-EGF treatment during adipogenic induction inhibited lipid accumulation as well (12). Therefore, the disturbance of ErbB4 signaling through the binding of other ligands in addition to NRG4 may be also involved in the development of MetS in ErbB4 deletion mice.
In the current study we also found increased inflammation in adipose tissue, especially in eWAT from ErbB4 deletion mice with associated M1 macrophage polarization, which may contribute to the insulin resistance in the MetS (28). Similar to the eWAT, gastrocnemius muscle from ErbB4 deletion mice also showed decreased response to either insulin or NRG4 treatment, as indicated by phosphorylated AKT levels, compared with wild-type mice (data not shown), indicating that decreased NRG4-ErbB4 signaling may play a role in the insulin resistance. Studies have shown that increased serum NRG4 levels are associated with a reduced risk of MetS (2). NRG4 gene transfer, which increased NRG4 expression by 60% in the eWAT, prevented high-fat diet-induced obesity, with >50% reduction of adipocyte hypertrophy in eWAT in C57BL/6 mice (15).
In addition, NRG4 has been reported to preserve metabolic homeostasis through attenuation of hepatic lipogenesis (27), and the expression levels of NRG4 were significantly decreased in the liver and eWAT of obese mice. Consistent with these findings, in the present study, MFD-fed mice with ErbB4 deletion also developed liver steatosis, which might be due to increased lipogenesis.
Similar to the expression pattern of NRG4 (27), we found that ErbB4 is also expressed in all fat depots, including BAT. Recent studies have shown that transplantation of BAT can increase glucose tolerance in diabetic mice (10). NRG4, highly expressed in BAT and upregulated in WAT upon cold exposure, may play a pivotal role in this process (25). Consistent with this hypothesis, we found that NRG4 treatment of 3T3-L1 adipocytes, which have characteristics of white adipocytes, significantly increased expression levels of PAT2 and CD137, brite/beige cell markers, as well as UCP-1 and PRDM16, brown adipocyte markers, to an extent similar to Iso, a nonspecific adrenergic receptor agonist that can partially mimic cold exposure in vitro. Although ErbB4 deletion mice did not show decreased BAT mass compared with wild-type mice, the brown adipocytes in BAT with ErbB4 deletion were larger, with more lipid accumulation, indicating a transformation of BAT to WAT (whitening), which can impair the ability of BAT to maintain metabolic homeostasis.
In summary, we found that ErbB4 deletion mice challenged with a MFD gradually developed obesity, dyslipidemia, hyperglycemia, and insulin resistance, a constellation of signs of MetS. Increased lipogenesis, adipose inflammation, and “whitening” of brown adipocytes resulted from ErbB4 deletion. Therefore, our results indicate that ErbB4 may play an important role in glucose and lipid homeostasis and should be considered a novel pathway for future intervention for MetS.
GRANTS
These studies were supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-051265, DK-062794, and DK-095785 and US Department of Veterans Affairs VA Merit Award 00507969 (to R. C. Hund) and Vanderbilt Institute for Clinical and Translational Research Grant UL1 TR-002243 from the National Institutes of Health (to F. Zeng).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
F.Z. and R.C.H. conceived and designed research; F.Z., Y.W., L.A.K., and S.W. performed experiments; F.Z., Y.W., L.A.K., and S.W. analyzed data; F.Z., Y.W., L.A.K., and S.W. interpreted results of experiments; F.Z. prepared figures; F.Z. drafted manuscript; F.Z. and R.C.H. edited and revised manuscript; R.C.H. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Frank E Jones (Department of Cell and Molecular Biology, Tulane University, New Orleans, LA) for providing the ErbB4−/−ht+ mice and Dr. Martin Gassmann (Department of Biomedicine, Institute of Physiology, University of Basel, Switzerland) for the use agreement on those mice.
REFERENCES
- 1.Böger CA, Sedor JR. GWAS of diabetic nephropathy: is the GENIE out of the bottle? PLoS Genet 8: e1002989, 2012. doi: 10.1371/journal.pgen.1002989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai C, Lin M, Xu Y, Li X, Yang S, Zhang H. Association of circulating neuregulin 4 with metabolic syndrome in obese adults: a cross-sectional study. BMC Med 14: 165, 2016. doi: 10.1186/s12916-016-0703-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cantó C, Chibalin AV, Barnes BR, Glund S, Suárez E, Ryder JW, Palacín M, Zierath JR, Zorzano A, Gumà A. Neuregulins mediate calcium-induced glucose transport during muscle contraction. J Biol Chem 281: 21690–21697, 2006. doi: 10.1074/jbc.M600475200. [DOI] [PubMed] [Google Scholar]
- 4.Ceddia RP, Lee D, Maulis MF, Carboneau BA, Threadgill DW, Poffenberger G, Milne G, Boyd KL, Powers AC, McGuinness OP, Gannon M, Breyer RM. The PGE2 EP3 receptor regulates diet-induced adiposity in male mice. Endocrinology 157: 220–232, 2016. doi: 10.1210/en.2015-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen JC, Zeng FH, Forrester SJ, Eguchi S, Zhang MZ, Harris RC. Expression and function of the epidermal growth factor receptor in physiology and disease. Physiol Rev 96: 1025–1069, 2016. doi: 10.1152/physrev.00030.2015. [DOI] [PubMed] [Google Scholar]
- 6.Christian M. Transcriptional fingerprinting of “browning” white fat identifies NRG4 as a novel adipokine. Adipocyte 4: 50–54, 2014. doi: 10.4161/adip.29853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care 28: 1769–1778, 2005. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 8.Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med 119: 812–819, 2006. doi: 10.1016/j.amjmed.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher EJ, Leroith D, Karnieli E. Insulin resistance in obesity as the underlying cause for the metabolic syndrome. Mt Sinai J Med 77: 511–523, 2010. doi: 10.1002/msj.20212. [DOI] [PubMed] [Google Scholar]
- 10.Gunawardana SC, Piston DW. Insulin-independent reversal of type 1 diabetes in nonobese diabetic mice with brown adipose tissue transplant. Am J Physiol Endocrinol Metab 308: E1043–E1055, 2015. doi: 10.1152/ajpendo.00570.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harding J, Sooriyakumaran M, Anstey KJ, Adams R, Balkau B, Briffa T, Davis TM, Davis WA, Dobson A, Giles GG, Grant J, Knuiman M, Luszcz M, Mitchell P, Pasco JA, Reid C, Simmons D, Simons L, Tonkin A, Woodward M, Shaw JE, Magliano DJ. The metabolic syndrome and cancer: is the metabolic syndrome useful for predicting cancer risk above and beyond its individual components? Diabetes Metab 41: 463–469, 2015. doi: 10.1016/j.diabet.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Lee JS, Suh JM, Park HG, Bak EJ, Yoo YJ, Cha JH. Heparin-binding epidermal growth factor-like growth factor inhibits adipocyte differentiation at commitment and early induction stages. Differentiation 76: 478–487, 2008. doi: 10.1111/j.1432-0436.2007.00250.x. [DOI] [PubMed] [Google Scholar]
- 13.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J, Croteau-Chonka DC, Esko T, Fall T, Ferreira T, Gustafsson S, Kutalik Z, Luan J, Mägi R, Randall JC, Winkler TW, Wood AR, Workalemahu T, Faul JD, Smith JA, Zhao JH, Zhao W, Chen J, Fehrmann R, Hedman AK, Karjalainen J, Schmidt EM, Absher D, Amin N, Anderson D, Beekman M, Bolton JL, Bragg-Gresham JL, Buyske S, Demirkan A, Deng G, Ehret GB, Feenstra B, Feitosa MF, Fischer K, Goel A, Gong J, Jackson AU, Kanoni S, Kleber ME, Kristiansson K, Lim U, Lotay V, Mangino M, Leach IM, Medina-Gomez C, Medland SE, Nalls MA, Palmer CD, Pasko D, Pechlivanis S, Peters MJ, Prokopenko I, Shungin D, Stančáková A, Strawbridge RJ, Sung YJ, Tanaka T, Teumer A, Trompet S, van der Laan SW, van Setten J, Van Vliet-Ostaptchouk JV, Wang Z, Yengo L, Zhang W, Isaacs A, Albrecht E, Ärnlöv J, Arscott GM, Attwood AP, Bandinelli S, Barrett A, Bas IN, Bellis C, Bennett AJ, Berne C, Blagieva R, Blüher M, Böhringer S, Bonnycastle LL, Böttcher Y, Boyd HA, Bruinenberg M, Caspersen IH, Chen YI, Clarke R, Daw EW, de Craen AJM, Delgado G, Dimitriou M, Doney ASF, Eklund N, Estrada K, Eury E, Folkersen L, Fraser RM, Garcia ME, Geller F, Giedraitis V, Gigante B, Go AS, Golay A, Goodall AH, Gordon SD, Gorski M, Grabe HJ, Grallert H, Grammer TB, Gräßler J, Grönberg H, Groves CJ, Gusto G, Haessler J, Hall P, Haller T, Hallmans G, Hartman CA, Hassinen M, Hayward C, Heard-Costa NL, Helmer Q, Hengstenberg C, Holmen O, Hottenga JJ, James AL, Jeff JM, Johansson Å, Jolley J, Juliusdottir T, Kinnunen L, Koenig W, Koskenvuo M, Kratzer W, Laitinen J, Lamina C, Leander K, Lee NR, Lichtner P, Lind L, Lindström J, Lo KS, Lobbens S, Lorbeer R, Lu Y, Mach F, Magnusson PKE, Mahajan A, McArdle WL, McLachlan S, Menni C, Merger S, Mihailov E, Milani L, Moayyeri A, Monda KL, Morken MA, Mulas A, Müller G, Müller-Nurasyid M, Musk AW, Nagaraja R, Nöthen MM, Nolte IM, Pilz S, Rayner NW, Renstrom F, Rettig R, Ried JS, Ripke S, Robertson NR, Rose LM, Sanna S, Scharnagl H, Scholtens S, Schumacher FR, Scott WR, Seufferlein T, Shi J, Smith AV, Smolonska J, Stanton AV, Steinthorsdottir V, Stirrups K, Stringham HM, Sundström J, Swertz MA, Swift AJ, Syvänen AC, Tan ST, Tayo BO, Thorand B, Thorleifsson G, Tyrer JP, Uh HW, Vandenput L, Verhulst FC, Vermeulen SH, Verweij N, Vonk JM, Waite LL, Warren HR, Waterworth D, Weedon MN, Wilkens LR, Willenborg C, Wilsgaard T, Wojczynski MK, Wong A, Wright AF, Zhang Q, Brennan EP, Choi M, Dastani Z, Drong AW, Eriksson P, Franco-Cereceda A, Gådin JR, Gharavi AG, Goddard ME, Handsaker RE, Huang J, Karpe F, Kathiresan S, Keildson S, Kiryluk K, Kubo M, Lee J-Y, Liang L, Lifton RP, Ma B, McCarroll SA, McKnight AJ, Min JL, Moffatt MF, Montgomery GW, Murabito JM, Nicholson G, Nyholt DR, Okada Y, Perry JRB, Dorajoo R, Reinmaa E, Salem RM, Sandholm N, Scott RA, Stolk L, Takahashi A, Tanaka T, van ’t Hooft FM, Vinkhuyzen AAE, Westra H-J, Zheng W, Zondervan KT, Heath AC, Arveiler D, Bakker SJL, Beilby J, Bergman RN, Blangero J, Bovet P, Campbell H, Caulfield MJ, Cesana G, Chakravarti A, Chasman DI, Chines PS, Collins FS, Crawford DC, Cupples LA, Cusi D, Danesh J, de Faire U, den Ruijter HM, Dominiczak AF, Erbel R, Erdmann J, Eriksson JG, Farrall M, Felix SB, Ferrannini E, Ferrières J, Ford I, Forouhi NG, Forrester T, Franco OH, Gansevoort RT, Gejman PV, Gieger C, Gottesman O, Gudnason V, Gyllensten U, Hall AS, Harris TB, Hattersley AT, Hicks AA, Hindorff LA, Hingorani AD, Hofman A, Homuth G, Hovingh GK, Humphries SE, Hunt SC, Hyppönen E, Illig T, Jacobs KB, Jarvelin M-R, Jöckel K-H, Johansen B, Jousilahti P, Jukema JW, Jula AM, Kaprio J, Kastelein JJP, Keinanen-Kiukaanniemi SM, Kiemeney LA, Knekt P, Kooner JS, Kooperberg C, Kovacs P, Kraja AT, Kumari M, Kuusisto J, Lakka TA, Langenberg C, Marchand LL, Lehtimäki T, Lyssenko V, Männistö S, Marette A, Matise TC, McKenzie CA, McKnight B, Moll FL, Morris AD, Morris AP, Murray JC, Nelis M, Ohlsson C, Oldehinkel AJ, Ong KK, Madden PAF, Pasterkamp G, Peden JF, Peters A, Postma DS, Pramstaller PP, Price JF, Qi L, Raitakari OT, Rankinen T, Rao DC, Rice TK, Ridker PM, Rioux JD, Ritchie MD, Rudan I, Salomaa V, Samani NJ, Saramies J, Sarzynski MA, Schunkert H, Schwarz PEH, Sever P, Shuldiner AR, Sinisalo J, Stolk RP, Strauch K, Tönjes A, Trégouët D-A, Tremblay A, Tremoli E, Virtamo J, Vohl M-C, Völker U, Waeber G, Willemsen G, Witteman JC, Zillikens MC, Adair LS, Amouyel P, Asselbergs FW, Assimes TL, Bochud M, Boehm BO, Boerwinkle E, Bornstein SR, Bottinger EP, Bouchard C, Cauchi S, Chambers JC, Chanock SJ, Cooper RS, de Bakker PIW, Dedoussis G, Ferrucci L, Franks PW, Froguel P, Groop LC, Haiman CA, Hamsten A, Hui J, Hunter DJ, Hveem K, Kaplan RC, Kivimaki M, Kuh D, Laakso M, Liu Y, Martin NG, März W, Melbye M, Metspalu A, Moebus S, Munroe PB, Njølstad I, Oostra BA, Palmer CNA, Pedersen NL, Perola M, Pérusse L, Peters U, Power C, Quertermous T, Rauramaa R, Rivadeneira F, Saaristo TE, Saleheen D, Sattar N, Schadt EE, Schlessinger D, Slagboom PE, Snieder H, Spector TD, Thorsteinsdottir U, Stumvoll M, Tuomilehto J, Uitterlinden AG, Uusitupa M, van der Harst P, Walker M, Wallaschofski H, Wareham NJ, Watkins H, Weir DR, Wichmann H-E, Wilson JF, Zanen P, Borecki IB, Deloukas P, Fox CS, Heid IM, O’Connell JR, Strachan DP, Stefansson K, van Duijn CM, Abecasis GR, Franke L, Frayling TM, McCarthy MI, Visscher PM, Scherag A, Willer CJ, Boehnke M, Mohlke KL, Lindgren CM, Beckmann JS, Barroso I, North KE, Ingelsson E, Hirschhorn JN, Loos RJF, Speliotes EK; LifeLines Cohort Study; ADIPOGen Consortium; AGEN-BMI Working Group; CARDIOGRAMplusC4D Consortium; CKDGen Consortium; GLGC; ICBP; MAGIC Investigators; MuTHER Consortium; MIGen Consortium; PAGE Consortium; ReproGen Consortium; GENIE Consortium; International Endogene Consortium . Genetic studies of body mass index yield new insights for obesity biology. Nature 518: 197–206, 2015. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.López-Soldado I, Niisuke K, Veiga C, Adrover A, Manzano A, Martínez-Redondo V, Camps M, Bartrons R, Zorzano A, Gumà A. Neuregulin improves response to glucose tolerance test in control and diabetic rats. Am J Physiol Endocrinol Metab 310: E440–E451, 2016. doi: 10.1152/ajpendo.00226.2015. [DOI] [PubMed] [Google Scholar]
- 15.Ma Y, Gao M, Liu D. Preventing high fat diet-induced obesity and improving insulin sensitivity through neuregulin 4 gene transfer. Sci Rep 6: 26242, 2016. doi: 10.1038/srep26242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maeda S, Imamura M, Kurashige M, Araki S, Suzuki D, Babazono T, Uzu T, Umezono T, Toyoda M, Kawai K, Imanishi M, Hanaoka K, Maegawa H, Uchigata Y, Hosoya T. Replication study for the association of 3 SNP loci identified in a genome-wide association study for diabetic nephropathy in European type 1 diabetes with diabetic nephropathy in Japanese patients with type 2 diabetes. Clin Exp Nephrol 17: 866–871, 2013. doi: 10.1007/s10157-013-0797-5. [DOI] [PubMed] [Google Scholar]
- 17.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 18.Meek TH, Morton GJ. Leptin, diabetes, and the brain. Indian J Endocrinol Metab 16, Suppl 3: S534–S542, 2012. doi: 10.4103/2230-8210.105568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller CN, Yang JY, England E, Yin A, Baile CA, Rayalam S. Isoproterenol increases uncoupling, glycolysis, and markers of beiging in mature 3T3-L1 adipocytes. PLoS One 10: e0138344, 2015. doi: 10.1371/journal.pone.0138344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science 339: 172–177, 2013. doi: 10.1126/science.1230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J 19: 3159–3167, 2000. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rusli F, Boekschoten MV, Zubia AA, Lute C, Müller M, Steegenga WT. A weekly alternating diet between caloric restriction and medium fat protects the liver from fatty liver development in middle-aged C57BL/6J mice. Mol Nutr Food Res 59: 533–543, 2015. doi: 10.1002/mnfr.201400621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandholm N, Salem RM, McKnight AJ, Brennan EP, Forsblom C, Isakova T, McKay GJ, Williams WW, Sadlier DM, Mäkinen VP, Swan EJ, Palmer C, Boright AP, Ahlqvist E, Deshmukh HA, Keller BJ, Huang H, Ahola AJ, Fagerholm E, Gordin D, Harjutsalo V, He B, Heikkilä O, Hietala K, Kytö J, Lahermo P, Lehto M, Lithovius R, Osterholm AM, Parkkonen M, Pitkäniemi J, Rosengård-Bärlund M, Saraheimo M, Sarti C, Söderlund J, Soro-Paavonen A, Syreeni A, Thorn LM, Tikkanen H, Tolonen N, Tryggvason K, Tuomilehto J, Wadén J, Gill GV, Prior S, Guiducci C, Mirel DB, Taylor A, Hosseini SM, Parving HH, Rossing P, Tarnow L, Ladenvall C, Alhenc-Gelas F, Lefebvre P, Rigalleau V, Roussel R, Tregouet DA, Maestroni A, Maestroni S, Falhammar H, Gu T, Möllsten A, Cimponeriu D, Ioana M, Mota M, Mota E, Serafinceanu C, Stavarachi M, Hanson RL, Nelson RG, Kretzler M, Colhoun HM, Panduru NM, Gu HF, Brismar K, Zerbini G, Hadjadj S, Marre M, Groop L, Lajer M, Bull SB, Waggott D, Paterson AD, Savage DA, Bain SC, Martin F, Hirschhorn JN, Godson C, Florez JC, Groop PH, Maxwell AP; DCCT/EDIC Research Group . New susceptibility loci associated with kidney disease in type 1 diabetes. PLoS Genet 8: e1002921, 2012. doi: 10.1371/journal.pgen.1002921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stančáková A, Laakso M. Genetics of metabolic syndrome. Rev Endocr Metab Disord 15: 243–252, 2014. doi: 10.1007/s11154-014-9293-9. [DOI] [PubMed] [Google Scholar]
- 25.Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, Goodyear LJ. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 123: 215–223, 2013. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suárez E, Bach D, Cadefau J, Palacin M, Zorzano A, Gumá A. A novel role of neuregulin in skeletal muscle. Neuregulin stimulates glucose uptake, glucose transporter translocation, and transporter expression in muscle cells. J Biol Chem 276: 18257–18264, 2001. doi: 10.1074/jbc.M008100200. [DOI] [PubMed] [Google Scholar]
- 27.Wang GX, Zhao XY, Meng ZX, Kern M, Dietrich A, Chen Z, Cozacov Z, Zhou D, Okunade AL, Su X, Li S, Blüher M, Lin JD. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat Med 20: 1436–1443, 2014. doi: 10.1038/nm.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welty FK, Alfaddagh A, Elajami TK. Targeting inflammation in metabolic syndrome. Transl Res 167: 257–280, 2016. doi: 10.1016/j.trsl.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan PJ, Xu Y, Wan Q, Feng J, Li H, Gao CL, Yang J, Zhong HH, Zhang ZH. Decreased plasma neuregulin 4 concentration is associated with increased high-sensitivity C-reactive protein in newly diagnosed type 2 diabetes mellitus patients: a cross-sectional study. Acta Diabetol 54: 1091–1099, 2017. doi: 10.1007/s00592-017-1044-4. [DOI] [PubMed] [Google Scholar]
- 30.Zeng F, Miyazawa T, Kloepfer LA, Harris RC. Deletion of ErbB4 accelerates polycystic kidney disease progression in cpk mice. Kidney Int 86: 538–547, 2014. doi: 10.1038/ki.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng F, Miyazawa T, Kloepfer LA, Harris RC. ErbB4 deletion accelerates renal fibrosis following renal injury. Am J Physiol Renal Physiol 314: F773–F787, 2017. doi: 10.1152/ajprenal.00260.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng F, Zhang MZ, Singh AB, Zent R, Harris RC. ErbB4 isoforms selectively regulate growth factor induced Madin-Darby canine kidney cell tubulogenesis. Mol Biol Cell 18: 4446–4456, 2007. doi: 10.1091/mbc.e07-03-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]