Abstract
Many small mammals, such as the laboratory mouse, utilize the hypometabolic state of torpor in response to caloric restriction. The signals that relay the lack of fuel to initiate a bout of torpor are not known. Because the mouse will only enter a torpid state when calorically challenged, it may be that one of the inputs for initiation into a bout of torpor is the lack of the primary fuel (glucose) used to power brain metabolism in the mouse. Using glucose telemetry in mice, we tested the hypotheses that 1) circulating glucose (GLC), core body temperature (Tb), and activity are significantly interrelated; and 2) that the level of GLC at the onset of torpor differs from both GLC during arousal from torpor and during feeding when there is no torpor. To test these hypotheses, six C57Bl/6J mice were implanted with glucose telemeters and exposed to different feeding conditions (ad libitum, fasting, limited food intake, and refeeding) to create different levels of GLC and Tb. We found a strong positive and linear correlation between GLC and Tb during ad libitum feeding. Furthermore, mice that were calorically restricted entered torpor bouts readily. GLC was low during torpor entry but did not drop precipitously as Tb did at the onset of a torpor bout. GLC significantly increased during arousal from torpor, indicating the presence of endogenous glucose production. While low GLC itself was not predictive of a bout of torpor, hyperactivity and low GLC preceded the onset of torpor, suggesting that this may be involved in triggering torpor.
Keywords: caloric restriction, fasting, glucose, hibernation, torpor
INTRODUCTION
Under conditions of scarce food availability and low ambient temperature (Ta), the laboratory mouse (Mus musculus) readily enters into a state of torpor. Torpor is a state of transient metabolic suppression, characterized by the controlled lowering of metabolic rate, core body temperature (Tb), and physical activity (12, 15, 19, 28). In animals that hibernate, multiday periods of hypometabolism are separated by periodic arousals, whereas in animals that utilize daily torpor the hypometabolic state and the ensuing drop in Tb last <24 h (11). The knowledge of the physiological mechanisms of torpor is a critical prerequisite to explore the feasibility of safely inducing a torpor-like state in humans, with applications ranging from medical procedures to space travel (4).
Periods of food restriction are conducive for daily torpor induction in the mouse (16). However, the metabolic signals that can provoke a torpor bout and that arise from restricted caloric intake are not well understood. Given that daily torpor occurs in the mouse in response to decreased energy availability, potential contributors toward the torpid state in daily torpor include a drop in levels of energy-sensitive hormones such as leptin and insulin, as well as in the availability of fuels, such as glucose, free fatty acids, and ketones from the circulation (19). The idea that glucose might be involved with, or influence, torpor is not a new one. In animals that utilize daily torpor in response to caloric restriction, a gradual decline in plasma glucose precedes or is associated with daily torpor onset (5, 8, 14, 22). Interestingly, and similar to animals that use daily torpor, the fall of Tb and metabolic rate during deep hibernation is also accompanied by a reduction in plasma glucose (1, 10, 26), although this finding is not universal among hibernators (21, 38). The potential role of limited glucose metabolism as a contributor to hypometabolism onset also arises from pharmacological studies. 2-Deoxy-glucose (2-DG), a glucose analog that blocks glycolysis, causes a transient decrease in Tb in the deer mouse, the Siberian hamster, and the marsupial hibernator Cercartetus nanus, species that engage in daily torpor (6, 29, 36). However, it is important to note that 2-DG also induces a low Tb in animals that do not use torpor, such as the rat and humans (9, 25). Taken together, the available evidence thus raises the hypothesis that the lack of glucose utilization in the brain may play a role in the onset and/or maintenance of daily torpor.
Torpor induction and arousal depend on the activity of the autonomic nervous system (1, 3, 28, 30–32). Part of the autonomic changes during daily torpor likely involve the liver, with an impact on hepatic glucose dynamics. Under normal conditions, sympathetic activation of the liver leads to hepatic glucose release while suppressing glycogen synthesis, whereas parasympathetic activation via the vagus nerve exerts the opposite effect (20), even though parasympathetic output to the pancreas increases glucagon secretion (33). Presumably, the fall in blood glucose throughout a day of caloric restriction or fasting is a consequence of both 1) reduced carbohydrate intake, and 2) the widespread reduction of sympathetic outflow that accompanies daily torpor, including decreased sympathetic outflow to the liver.
Experiments to date that have examined glucose availability and torpor have been hampered by technical limitations of periodic and repeated blood sample collection, which may induce stress and influence physiological behavior. Recent technical developments have led to the commercial availability of a telemetry system for continuous and simultaneous monitoring of blood glucose concentration (GLC), Tb, and activity (ACT). A system like this allows for a much greater temporal resolution of changes in GLC, with minimal animal handling. Indeed, this system has been recently applied in a longitudinal study of a mouse model of diabetes mellitus (18). We took advantage of the high temporal resolution of GLC, Tb, and ACT from these telemeters to test: 1) whether GLC, Tb, and ACT are significantly interrelated, and 2) that the level of circulating glucose at the onset of torpor differs from both glucose during arousal from torpor and from feeding conditions that do not lead to torpor.
MATERIALS AND METHODS
Ethical approval.
All experiments were approved by the Williams College Institutional Animal Care and Use Committee (Protocol No. SS-N-17) and were performed in accordance with the guidelines described by the US National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Surgery.
C57BL/6J male mice (n = 6) were purchased from Jackson Laboratories (Bar Harbor, ME). Animals were maintained on a 12:12-h light-dark cycle, with lights on at 5 AM, which corresponded to Zeitgeiber time (ZT) = 0, whereas ZT = 12 corresponded to lights off (5 PM). Mice were housed at a Ta of 30°C until surgery. Mice were fed ad libitum a low-fat chow (10% kcal from fat; D12450B; Research Diets). Mouse age and body weight at surgery were 16.1 ± 2.0 wk and 27.7 ± 0.2 g, respectively. Surgery was performed on a heating pad and under isoflurane anesthesia (2.5% in O2) to implant telemeters for the measurement of blood glucose and peritoneal temperature (model HD-XG; Data Science International). We utilized adult male mice because we felt the likelihood of success would be greater due to the larger body mass compared with younger or female mice. The telemetry implant sensor was inserted in the carotid arch via a left carotid artery cannulation following manufacturer instructions. The catheter connected to the sensor was tunneled subcutaneously to the peritoneal cavity, where the telemeter body was implanted and sutured to the body wall. An analgesic was administered subcutaneously at the end of the procedure (Meloxicam at 5 mg/kg body wt; Henry Schein). Mice were maintained on the heating pad for 48 h after the end of surgery and then housed individually at 30°C and fed ad libitum with a standard diet for 7 more days to allow postoperative recovery.
Experimental protocol, recordings, and data acquisition.
The implanted telemetry devices were calibrated in vivo over the course of the study against measurements of plasma glucose obtained using a glucometer (Nova Stat-Strip blood glucose monitor; Data Sciences International). An initial two-point calibration was performed at the beginning of the recordings by measuring glucose in duplicate from a nick in the tail tip of the mouse, both before an intraperitoneal bolus injection of glucose (3 mg/g body wt at ~ZT10) and 5 min after the subsequent peak in the telemeter GLC signal. The intraperitoneal glucose injection increased GLC by at least 200 mg/dL. Single-point calibrations were then made with duplicate blood glucose measurements twice per week at the same time of the day (ZT10). The calibration algorithm in the acquisition software converted the raw telemetry data (recorded in nA to mg/dl).
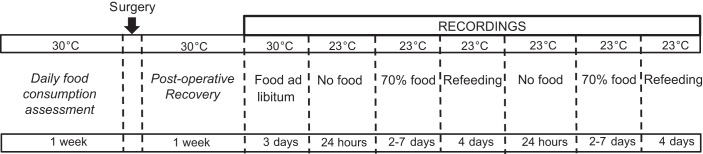
A baseline recording was performed for 3 days with mice fed ad libitum and exposed to Ta of 30°C (Fig. 1). Mice were then fasted for 24 h starting from ZT10, with free access to water and exposed to a Ta of 23°C, which was maintained constant for the rest of the experimental protocol. Then, mice underwent a caloric restriction protocol, with 2 g of diet administered at ZT10. This amount represents ~70% of normal intake for male mice at this Ta in our laboratory. Mice were calorically restricted until a bout of torpor was achieved between 2 and 7 days of caloric restriction. Mice were then refed ad libitum for 4 days. The experimental schedule of fasting, caloric restriction, and refeeding was repeated twice for each mouse to increase the recording database for analysis. During recordings, all interactions with the mice, consisting of food administration/removal, bedding change, and GLC calibration, were performed in the last 2 h of the light period (i.e., from ZT10 to ZT12). The GLC, Tb, and ACT signals were acquired (Dataquest ART; DSI, St. Paul, MN) typically every 10 s, with occasional sampling every 20 s if required when data acquisition was crowded with several data streams. The ACT signal was based on the change in telemeter signal strength as the mouse moved about in its cage.
Fig. 1.
Schedule of surgery and conditions during which the biosignals were recorded. See Experimental protocol, recordings, and data acquisition for details.
Data analysis.
Data analysis was performed with Matlab (Mathworks, Natick, MA). The values of GLC, Tb, and ACT were averaged over consecutive 5-min periods, and then, for analyses of day-night rhythm, further averaged over 2-h periods. ACT is expressed in arbitrary units (AU) normalized per minute of recording. The last 2 h of the light period of each recording day (i.e., from ZT10 to ZT12) were excluded from the analysis to avoid interference due to experimenter interaction with the mice.
To compare directly the day-night fluctuations in GLC with those in Tb during ad libitum feeding, a z-score value was calculated for each of the two variables on 2-h bins. In particular, the difference between each 2-h bin value and the average of the eleven 2-h bin values (the 2-h bin from ZT10 to ZT12 was excluded from analysis, see above) was divided by the standard deviation of the eleven 2-h bin values.
To assess the link between GLC and Tb in association with torpor bouts, we defined “cooling periods” as time periods during which Tb monotonically decreased (on 5-min average values) resulting in an overall Tb reduction of ≥5°C with respect to the starting point of each period. Similarly, “rewarming periods” were defined as time periods during which Tb monotonically increased (on 5-min average values) resulting in an overall Tb increase of ≥5°C. The dynamics of GLC, Tb, and ACT during torpor onset and arousal were analyzed on a 60-min window centered at the onset of the cooling period or the onset of the rewarming period, respectively. For each mouse, each instance of torpor onset or arousal was compared with the average of all 60-min windows recorded in different days at a Ta of 23°C, centered at the same ZT of the torpor onset/arousal instance, devoid of any cooling or rewarming sequence, and with Tb at ≥34°C for at least 2 h before and 2 h after the window center (i.e., during stable euthermia).
Estimates of the maximum and minimum values of GLC (GLC-MAX and GLC-MIN), including those associated with cooling and rewarming periods, were compared on 5-min average values over all recording days at Ta of 23°C, taking into account the value of Tb. To make these estimates robust to the sporadic occurrence of unusually high or low GLC readings, we defined GLC-MAX and GLC-MIN as the upper and lower adjacent values of GLC, which correspond to the GLC values indicated by Tukey’s boxplot whiskers. In particular, GLC-MAX was estimated as the highest GLC reading within the upper inner fence, which is 1.5 times the interquartile range (3rd quartile–1st quartile) above the 3rd quartile of GLC. GLC-MIN was estimated as the lowest GLC reading within the lower inner fence, which is 1.5 times the interquartile range (3rd quartile–1st quartile) below the first quartile of GLC. For each mouse, boxplots were computed for all 5-min average values of GLC and, separately, for 5-min average values of GLC included in cooling or rewarming periods, subdivided as a function of three arbitrarily defined Tb ranges: ≤30°C, 30–34°C, and ≥34°C.
Statistical analysis.
Statistical analysis was performed with SPSS (SPSS, Armonk, NY). Data were analyzed with a repeated measures ANOVA with Huynh-Feldt correction and with t-tests with statistical significance for P < 0.05. The linear correlation between the z-scores of GLC and Tb was determined with the Pearson’s correlation coefficient. Data were reported as means ± SE.
RESULTS
Daily rhythm of GLC, Tb, and ACT in baseline conditions of ad libitum feeding and thermoneutrality.
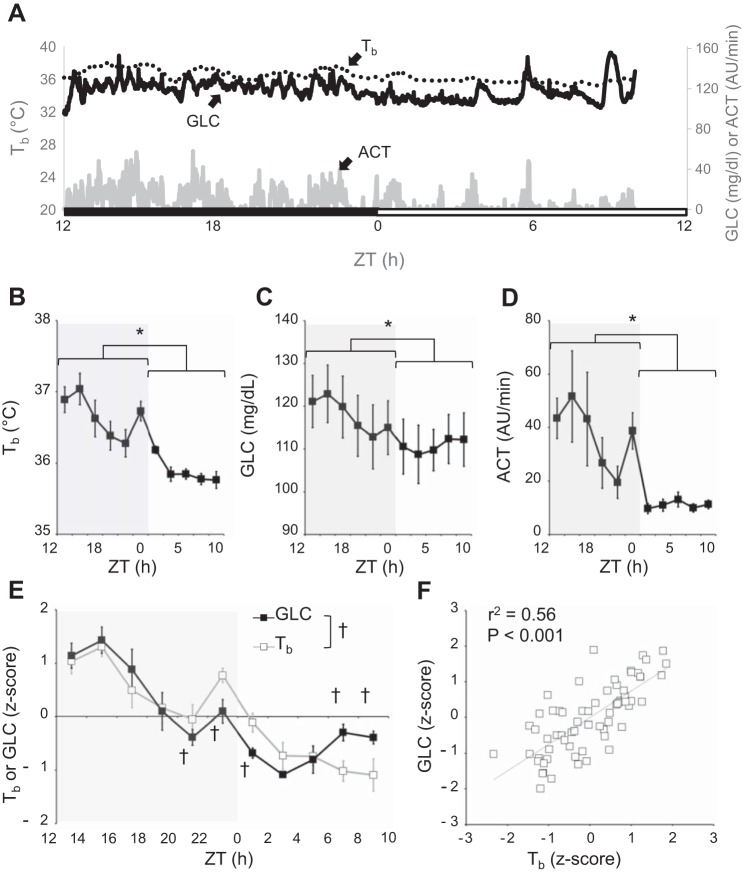
Figure 2A shows the raw data collected in a typical mouse in a 22-h period in baseline conditions of ad libitum feeding and at a Ta of 30°C. GLC, Tb, and ACT all varied significantly with time (P < 0.05, ANOVA), with values significantly higher during the dark period than during the light period (P < 0.05) (Fig. 2, B–D). Analyses of the z-scores of Tb and GLC revealed that the two variables significantly differed at specific times of the day (P = 0.001, ANOVA, variable × time interaction). In particular, GLC increased less than Tb at the dark-light transition and increased at the end of the light period in the face of a fall in Tb (P < 0.05, by t-tests). Nevertheless, Pearson’s correlation analysis indicated that the daily values of Tb were significantly (P < 0.001) and linearly correlated with those of GLC, with 56% of shared variance (Fig. 2E).
Fig. 2.
Blood glucose, core body temperature, and activity in ad libitum fed C57Bl/6J male mice in thermoneutrality. A: raw data collected in a 22-h period in a typical mouse in ad libitum feeding condition at 30°C Ta. ACT, activity, expressed in arbitrary units (AU) per 1-min recording; Tb, core body temperature; GLC, blood glucose concentration. Lights were on at 5 AM, which corresponded to Zeitgeiber time (ZT) = 0, whereas ZT = 12 corresponded to lights off (5 PM). The black bar indicates the dark period, and the white bar corresponds to the light period. B–D: core body temperature (Tb), GLC, and ACT, respectively, averaged over 2-h bins for the six mice under study. Data are reported as means ± SE. *P < 0.05, light vs. dark period (t-test). E: z-score values of Tb and GLC averaged over 2-h intervals in a 22-h period in ad libitum feeding condition at 30°C Ta. †P < 0.05, Tb z-score vs. GLC z-score. F: relationship between GLC and Tb z-scores calculated through Pearson’s linear correlation analysis; r2, coefficient of determination.
GLC, Tb, and ACT during fasting, caloric restriction, and refeeding at 23°C Ta.
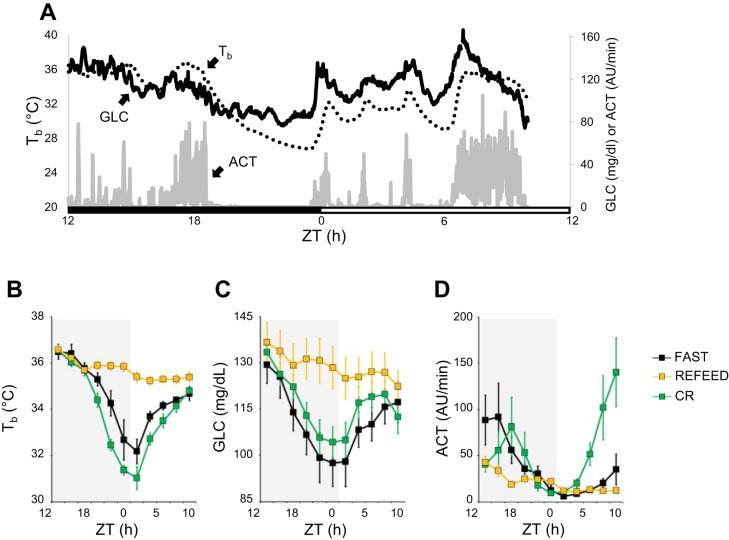
When mice were fasted or calorically restricted at a Ta of 23°C, they often engaged in a bout of torpor, defined for the purpose of this study as period from the onset of a cooling period to the end of the subsequent rewarming period. These torpor bouts started before the onset of the light phase and typically lasted through the first few hours of the light phase (Fig. 3A). As Fig. 3 shows, Tb and GLC dropped significantly during fasting and caloric restriction as compared with refeeding (P < 0.001, by t-tests). However, ACT was not significantly different among the three conditions until the second half of the light period where ACT was elevated in the circadian rhythm condition (P = 0.011, by t-tests), likely a result of food anticipatory behavior in these mice. Circadian rhythms of higher Tb, GLC, and ACT during the dark phase vs. the light phase were reestablished during the refeeding periods (P < 0.001, by t-test).
Fig. 3.
Blood glucose, core body temperature, and activity during fasting, caloric restriction, and refeeding. A: core body temperature (Tb), blood glucose concentration (GLC), and activity [ACT; expressed in arbitrary units (AU) per 1-min recording] on the 3rd day of caloric restriction for 1 of the mice. Lights were on at 5 AM, which corresponded to Zeitgeiber time (ZT) = 0, whereas ZT = 12 corresponded to lights off (5 PM). The black bar indicates the dark period, and the white bar corresponds to the light period. From tracings like these, the values of Tb (B), GLC (C), and ACT (D) were averaged over consecutive 2-h periods during fasts, over days of caloric restriction, and during refeeding. Data are reported as means ± SE; n = 6.
ACT, Tb and GLC during cooling/rewarming periods.
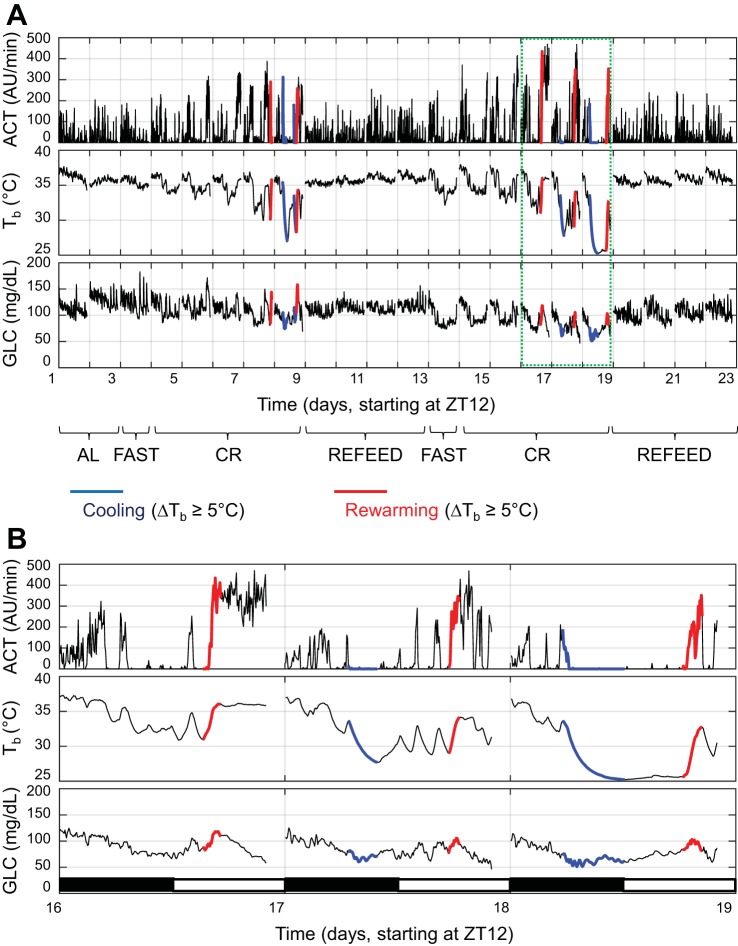
The recordings of ACT, Tb, and GLC during ad libitum feeding at a Ta of 30°C and then during fasting, caloric restriction, and refeeding periods at a Ta of 23°C in one representative mouse are shown in Fig. 4A, highlighting the cooling (blue lines) and rewarming (red lines) periods. As shown by this mouse, torpor bouts became prominent after 2 days of caloric restriction and ceased during the refeeding periods. Recordings during 3 days of caloric restriction with prominent torpor bouts are displayed at higher magnification in Fig. 4B.
Fig. 4.
Blood glucose, core body temperature, and activity over 23 days of continuous recording. A: recordings of activity [ACT; in arbitrary units (AU) per 1-min recording], core body temperature (Tb) and blood glucose concentration (GLC) during ad libitum (AL) feeding at 30°C Ta, and then, at 23°C Ta, during fasting (FAST), caloric restriction (CR; 70% of normal food intake), and ad libitum refeeding (REFEED) periods from one representative mouse. The cooling and rewarming periods (cf. materials and methods for details) are represented in blue and red, respectively. B: magnified view of 3 days of caloric restriction when torpor. AU, arbitrary units.
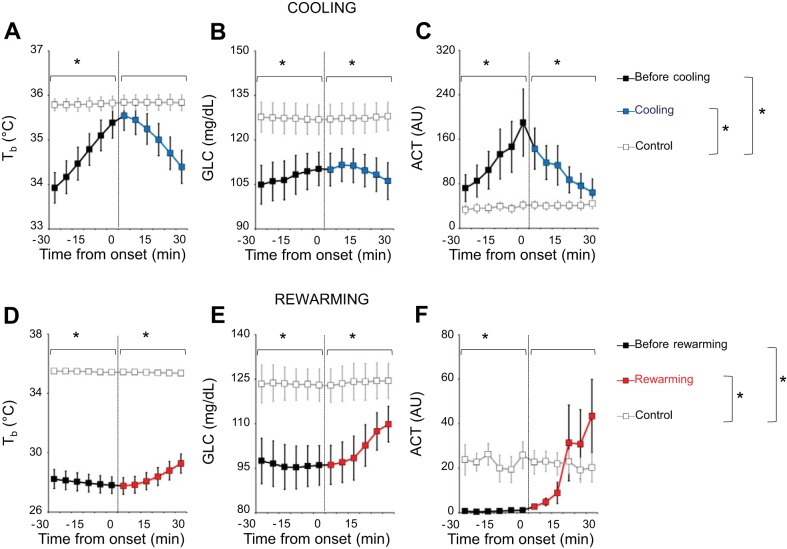
The results of the quantitative analysis of cooling and rewarming periods are shown in Fig. 5. The 60-min windows centered on the onset of the cooling periods showed significant time-dependent differences in ACT and Tb compared with control windows (P ≤ 0.009, ANOVA, time × cooling interaction). In particular, Tb was significantly lower with respect to the control windows in the 30 min preceding the onset of cooling (P = 0.028, by t-test) but not in the 30 min afterwards (P = 0.092, by t-test; Fig. 5A). On the other hand, GLC in the 60-min window centered on the onset of the cooling period (Fig. 5B) differed significantly from values in control windows (ANOVA, main effect of the cooling vs. control window, P = 0.010) but did not differ significantly as a function of time (ANOVA, P = 0.275 for the main effect of time; P = 0.193 for the time × condition interaction). Accordingly, GLC was significantly lower than in control sequences during the periods preceding and following cooling onset (P ≤ 0.019, by t-test; Fig. 5B). ACT values were significantly higher with respect to control windows both before and after the onset of the cooling period (P ≤ 0.041, by t-test; Fig. 5C).
Fig. 5.
Blood glucose, core body temperature, and activity before/after the onset of cooling and rewarming periods. A–C: comparison of core body temperature (Tb), blood glucose concentration (GLC), and activity [ACT; in arbitrary units (AU) per 1-min recording], averaged over 5-min intervals, between cooling periods (black lines before cooling onset, blue lines after cooling onset) and temporally matched periods of stable euthermia (control periods, gray lines). D–F: comparison the same variables between rewarming periods (black lines before rewarming onset, red lines after rewarming onset) and temporally matched periods of stable euthermia (control periods, gray lines). Time 0 indicates the onset of the cooling/rewarming periods. Data are reported as means ± SE; n = 6. *P < 0.05, cooling or rewarming vs. control periods (t-test).
The 60-min windows centered on the onset of the rewarming periods showed significant time-dependent differences in ACT, Tb, and GLC compared with control windows (P ≤ 0.009, ANOVA, time × rewarming interaction). Both Tb (Fig. 5D) and GLC (Fig. 5E) were significantly lower before and after the onset of the rewarming period than during the control windows (P ≤ 0.004, by t-test). During the 30 min before the onset of the rewarming period, ACT was virtually zero and significantly lower than in control windows (P = 0.006, by t-test). ACT levels then increased after the onset of rewarming to values similar to those in control windows (P = 0.759, by t-test; Fig. 5F).
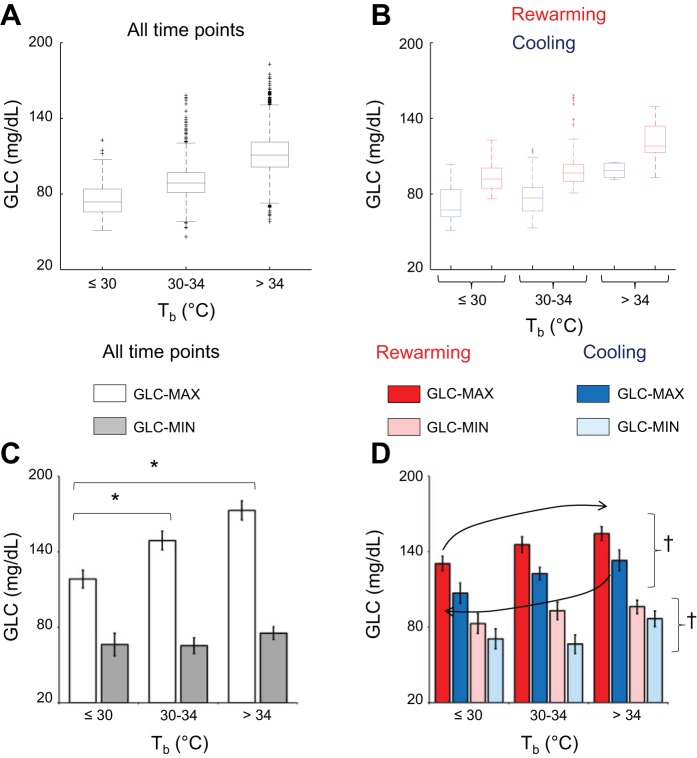
The results of the analysis of GLC-MAX and GLC-MIN associated with cooling and rewarming periods as a function of Tb are shown in Fig. 6. Figure 6, A and B, shows boxplots of GLC as a function in Tb taking into account all time points (i.e., 5-min average values, Fig. 6A) or only the time points included in cooling or rewarming periods (Fig. 6B). As detailed in materials and methods, the values of GLC-MAX and GLC-MIN for each mouse and Tb category were computed as those of the upper and lower boxplot whiskers, respectively. The values of GLC-MAX computed taking into account all time points differed significantly among Tb categories (≤30°C, 30–34°C, >34°C; P < 0.001, ANOVA), whereas those of GLC-MIN did not (P = 0.130, ANOVA; Fig. 6C). Accordingly, the values of GLC-MAX were significantly lower at values of Tb ≤30°C and 30–34°C than at values of Tb > 34°C (P ≤ 0.024, by t-test). The same analysis performed focusing only on the cooling and rewarming periods is represented in Fig. 6D. As expected from the results of Fig. 6C, the values of GLC-MAX differed significantly as a function of Tb, whereas those of GLC-MIN did not (ANOVA main effect, P = 0.015 and P = 0.062, respectively). Both the values of GLC-MAX and those of GLC-MIN were significantly higher during rewarming than during cooling periods (ANOVA main effect, P = 0.006 and P = 0.003, respectively), without significant interaction effects between cooling/rewarming and the Tb category (ANOVA, P = 0.918 and P = 0.233, respectively).
Fig. 6.
The relationship between blood glucose and core body temperature during periods of body cooling and rewarming. A and B: boxplots of the distribution of blood glucose concentration (GLC) values (averages over 5-min intervals) in 1 representative mouse throughout the whole recording period at 23°C, including all time points (A) or only the cooling (blue) and rewarming (red) periods (B; cf. materials and methods for details), as a function of core body temperature (Tb; ≤30°C, 30–34°C, >34°C). Boxplot whiskers were taken as estimates of the maximum and minimum values of GLC robust to the effect of extreme values in the distribution (MAX and MIN, respectively). C and D: means ± SE (n = 6 mice) of MAX and MIN of GLC on all time points (C) or only for cooling (blue) and rewarming (red) periods (D) as a function of Tb (≤30°C, 30–34°C, >34°C). The black arrows in panel D highlight the hysteresis in the relationship between GLC and Tb during cooling and rewarming periods. *P < 0.05, for values at 30–34°C Tb vs. values at ≤30°C or >34°C Tb (t-test). †P < 0.05, cooling vs. rewarming periods irrespective of Tb (ANOVA main effect).
DISCUSSION
Using a recent technical development of a telemetry system for continuous and simultaneous monitoring of GLC, Tb, and ACT, we tested: 1) whether and how day-night rhythms of GLC, Tb, and ACT are interrelated in mice at thermoneutrality; 2) whether GLC and ACT values at the onset of a torpor bout and arousal from the torpor bout differ from those in control conditions when torpor does not occur. We found a strong positive and linear correlation between circulating glucose and Tb during ad libitum feeding at thermoneutrality (Fig. 2). Furthermore, mice that were calorically restricted entered torpor bouts readily. Circulating blood glucose was low during torpor entry but did not drop precipitously as Tb did at the onset of a torpor bout. Blood glucose significantly increased during arousal from torpor during times when the mice were not feeding, indicating the presence of endogenous glucose production (Fig. 4). Finally, while low blood glucose itself was not predictive of a bout of torpor, the onset of torpor was associated with the combination of low blood glucose and hyperactivity (Fig. 5).
GLC showed the expected robust circadian rhythm in these mice as reported in the literature for humans (2, 35) and rodents (17). It should be noted that previous reports for GLC have been measured at standard laboratory temperatures, and the current work extends this finding to thermoneutrality. GLC peaks at the light-dark transition (Fig. 2), in agreement with previous research in rodents (17). However, because we handled the mice before the onset of the dark period, our findings are confounded by a potential sympathetic response to animal handling. We show here that GLC and Tb were clearly interrelated (Fig. 2). It is likely that the measurements made here at baseline (ACT, GLC, and Tb) are primarily influenced by the sleep-wake state of the mouse. Mice are feeding, are generally active, and have greater sympathetic drive when awake, all which would drive up Tb and GLC. However, we did not assess the sleep-wake state of these mice, although the temporal resolution of these telemeters will allow for future studies in just this area. In addition, we speculate that the higher increase in GLC at the end of the light-dark transition could be partly due to the decrease in glucose tolerance during the circadian rest period and during sleep. Studies on human subjects constantly infused with glucose to avoid endogenous glucose production have demonstrated that glucose tolerance varies across the day, reaching its minimum during the night, which corresponds to the rest period for humans (34). The reduced glucose utilization during sleep is also supported by the high levels of plasma glucose measured during nonrapid eye movement sleep (27).
We compared GLC, Tb, and ACT values during cooling and rewarming periods with those in temporally matched periods of stable euthermia. This analytical approach made it possible to take into account ZT and Tb in evaluating the relationship between GLC and torpor (Fig. 5). GLC did not precipitously drop before the onset of torpor (Figs. 3A and 4), suggesting that torpor is not preceded by a dramatic drop in GLC. On the other hand, we found that GLC was always lower in the cooling and rewarming periods with the respect to control conditions. It is intriguing that in the 30 min preceding the onset of the cooling period, when mice had low GLC, both Tb and ACT substantially increased (Fig. 5, A and C). This elevation in ACT and Tb parallels the finding in dormice where the pretorpor period is associated with elevated sympathetic and metabolic activity as evidenced by an increase in ventilation, heart rate and metabolic rate (7). Peaks in metabolic and respiratory rate prior entrance into torpor have also been observed in many hibernators like pocket mice, Djungarian hamsters, and alpine marmots (14, 24, 37). Accordingly, the peak metabolic rate was chosen for the definition of torpor onset in experiments performed in Djungarian hamsters (14). Taken together with this previous work, our present results raise the hypothesis that an increase in ACT in the face of hypoglycemia plays a role in torpor onset in calorically restricted mice.
During a fast, hepatic production of glucose is the primary source of circulating glucose and is under the control of the autonomic nervous system as well as circulating hormones such as glucagon and insulin. The autonomic nervous system is intimately involved with both torpor induction and arousal (1, 3, 28, 30–32). Our results suggest that mice, either fasted or calorically restricted, were metabolizing circulating glucose, or at least taking it up from circulation, without full hepatic replacement of GLC. Upon arousal from torpor, we found that GLC increased (Figs. 3, 4, and 5). The rise in GLC during arousal from torpor is coincident with changes in other physiological functions that are also under autonomic influence (28, 30). Hence, we can hypothesize that the rise in GLC upon arousal from torpor results from increased sympathetic drive to the liver. It is important to note that other pathways besides glycogenolysis can support metabolism during the arousal from torpor. Fat reserves may provide the necessary energy to arouse from torpor as suggested by the respiratory quotient (14, 23). Indeed, an increased production of ketones from the liver, heart, and plasma is observed during arousal from torpor in mice (22).
For any given category of Tb defined here, we found that GLC was higher during rewarming than during cooling (Fig. 6D). This relationship between GLC and Tb during the torpor bout has also been observed with other physiological parameters. For example, heart rate and metabolic rate are both much greater during arousal as compared with torpor entry (13, 30). This relationship between GLC and Tb may simply be a result of the thermal inertia of the animal and the telemeter. That is, the changes in Tb are slow relative to the changes in GLC because the mass of the animal/telemeter necessitates more time to be reflected in the measurement than does GLC. However, this hysteretic GLC/Tb relationship in torpor may also reflect substantially different physiological states of the organism as it enters and arouses from torpor. These possibilities are not mutually exclusive in that the animal may truly be in a much different physiological state and our perception of that difference is amplified due to the nature of the parameter being measured.
In conclusion, our results demonstrate that GLC and Tb are interrelated across the day-night period, both in baseline and in conditions predisposing to torpor onset. It may follow from this relationship between GLC and Tb that high-glucose levels are not permissive for torpor induction. Furthermore, high ACT and low GLC precede torpor onset, suggesting that this may be involved in triggering torpor.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant 1R15-HL-120072-01A1 (to S. J. Swoap).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
V.L.M., G.Z., A.S., and S.J.S. conceived and designed research; V.L.M., A.V., M.J.B., and S.J.S. performed experiments; V.L.M., A.V., M.J.B., and A.S. analyzed data; A.S. and S.J.S. prepared figures; V.L.M., A.V., G.Z., A.S., and S.J.S. interpreted results of experiments; V.L.M. drafted manuscript; V.L.M., A.V., G.Z., A.S., and S.J.S. edited and revised manuscript; V.L.M., A.V., M.J.B., G.Z., A.S., and S.J.S. approved final version of manuscript.
REFERENCES
- 1.Atgie C, Nibbelink M, Ambid L. Sympathoadrenal activity and hypoglycemia in the hibernating garden dormouse. Physiol Behav 48: 783–787, 1990. doi: 10.1016/0031-9384(90)90227-U. [DOI] [PubMed] [Google Scholar]
- 2.Boden G, Chen X, Urbain JL. Evidence for a circadian rhythm of insulin sensitivity in patients with NIDDM caused by cyclic changes in hepatic glucose production. Diabetes 45: 1044–1050, 1996. doi: 10.2337/diab.45.8.1044. [DOI] [PubMed] [Google Scholar]
- 3.Braulke LJ, Heldmaier G. Torpor and ultradian rhythms require an intact signalling of the sympathetic nervous system. Cryobiology 60: 198–203, 2010. doi: 10.1016/j.cryobiol.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Cerri M. The central control of energy expenditure: exploiting torpor for medical applications. Annu Rev Physiol 79: 167–186, 2017. doi: 10.1146/annurev-physiol-022516-034133. [DOI] [PubMed] [Google Scholar]
- 5.Dark J, Lewis DA, Zucker I. Hypoglycemia and torpor in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 276: R776–R781, 1999. doi: 10.1152/ajpregu.1999.276.3.R776. [DOI] [PubMed] [Google Scholar]
- 6.Dark J, Miller DR, Zucker I. Reduced glucose availability induces torpor in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 267: R496–R501, 1994. doi: 10.1152/ajpregu.1994.267.2.R496. [DOI] [PubMed] [Google Scholar]
- 7.Elvert R, Heldmaier G. Cardiorespiratory and metabolic reactions during entrance into torpor in dormice, Glis. J Exp Biol 208: 1373–1383, 2005. doi: 10.1242/jeb.01546. [DOI] [PubMed] [Google Scholar]
- 8.Franco M, Contreras C, Nespolo RF. Profound changes in blood parameters during torpor in a South American marsupial. Comp Biochem Physiol A Mol Integr Physiol 166: 338–342, 2013. doi: 10.1016/j.cbpa.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Freinkel N, Metzger BE, Harris E, Robinson S, Mager M. The hypothermia of hypoglycemia. Studies with 2-deoxy-D-glucose in normal human subjects and mice. N Engl J Med 287: 841–845, 1972. doi: 10.1056/NEJM197210262871702. [DOI] [PubMed] [Google Scholar]
- 10.Galster WA, Morrison P. Cyclic changes in carbohydrate concentrations during hibernation in the arctic ground squirrel. Am J Physiol 218: 1228–1232, 1970. doi: 10.1152/ajplegacy.1970.218.4.1228. [DOI] [PubMed] [Google Scholar]
- 11.Geiser F. Hibernation. Curr Biol 23: R188–R193, 2013. doi: 10.1016/j.cub.2013.01.062. [DOI] [PubMed] [Google Scholar]
- 12.Geiser F. Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu Rev Physiol 66: 239–274, 2004. doi: 10.1146/annurev.physiol.66.032102.115105. [DOI] [PubMed] [Google Scholar]
- 13.Geiser F, Currie SE, O’Shea KA, Hiebert SM. Torpor and hypothermia: reversed hysteresis of metabolic rate and body temperature. Am J Physiol Regul Integr Comp Physiol 307: R1324–R1329, 2014. doi: 10.1152/ajpregu.00214.2014. [DOI] [PubMed] [Google Scholar]
- 14.Heldmaier G, Klingenspor M, Werneyer M, Lampi BJ, Brooks SP, Storey KB. Metabolic adjustments during daily torpor in the Djungarian hamster. Am J Physiol Endocrinol Metab 276: E896–E906, 1999. doi: 10.1152/ajpendo.1999.276.5.E896. [DOI] [PubMed] [Google Scholar]
- 15.Heldmaier G, Ortmann S, Elvert R. Natural hypometabolism during hibernation and daily torpor in mammals. Respir Physiol Neurobiol 141: 317–329, 2004. doi: 10.1016/j.resp.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Hudson JW, Scott IM. Daily torpor in the laboratory mouse, Mus musculus var. albino. Physiol Zool 52: 205–218, 1979. doi: 10.1086/physzool.52.2.30152564. [DOI] [Google Scholar]
- 17.Kalsbeek A, la Fleur S, Fliers E. Circadian control of glucose metabolism. Mol Metab 3: 372–383, 2014. doi: 10.1016/j.molmet.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korstanje R, Ryan JL, Savage HS, Lyons BL, Kane KG, Sukoff Rizzo SJ. Continuous glucose monitoring in female nod mice reveals daily rhythms and a negative correlation with body temperature. Endocrinology 158: 2707–2712, 2017. doi: 10.1210/en.2017-00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melvin RG, Andrews MT. Torpor induction in mammals: recent discoveries fueling new ideas. Trends Endocrinol Metab 20: 490–498, 2009. doi: 10.1016/j.tem.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizuno K, Ueno Y. Autonomic nervous system and the liver. Hepatol Res 47: 160–165, 2017. doi: 10.1111/hepr.12760. [DOI] [PubMed] [Google Scholar]
- 21.Musacchia XJ, Deavers DR. The regulation of carbohydrate metabolism in hibernators In: Survival in the Cold, edited by Musacchia XJ, Jansky L. Amsterdam, The Netherlands: Elsevier North Holland, 1981, p. 55–75. [Google Scholar]
- 22.Nestler JR. Metabolic substrate change during daily torpor in deer mice. Can J Zool 69: 322–327, 1991. doi: 10.1139/z91-052. [DOI] [Google Scholar]
- 23.Nestler JR. Relationships between respiratory quotient and metabolic rate during entry to and arousal from daily torpor in deer mice (Peromyscus maniculatus). Physiol Zool 63: 504–515, 1990. doi: 10.1086/physzool.63.3.30156225. [DOI] [Google Scholar]
- 24.Ortmann S, Heldmaier G. Regulation of body temperature and energy requirements of hibernating alpine marmots (Marmota marmota). Am J Physiol Regul Integr Comp Physiol 278: R698–R704, 2000. doi: 10.1152/ajpregu.2000.278.3.R698. [DOI] [PubMed] [Google Scholar]
- 25.Pénicaud L, Thompson DA, Le Magnen J. Effects of 2-deoxy-D-glucose on food and water intake and body temperature in rats. Physiol Behav 36: 431–435, 1986. doi: 10.1016/0031-9384(86)90310-0. [DOI] [PubMed] [Google Scholar]
- 26.Sarajas HS. Blood Glucose Studies in Permanently Cannulated Hedgehogs during a Bout of Hibernation. Helsinki, Finland: Suomalainen Tiedeakatemia, 1967, p. 11. [Google Scholar]
- 27.Scheen AJ, Byrne MM, Plat L, Leproult R, Van Cauter E. Relationships between sleep quality and glucose regulation in normal humans. Am J Physiol Endocrinol Metab 271: E261–E270, 1996. doi: 10.1152/ajpendo.1996.271.2.E261. [DOI] [PubMed] [Google Scholar]
- 28.Silvani A, Cerri M, Zoccoli G, Swoap SJ. Is adenosine action common ground for NREM sleep, torpor, and other hypometabolic states? Physiology (Bethesda) 33: 182–196, 2018. doi: 10.1152/physiol.00007.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stamper JL, Dark J. Metabolic fuel availability influences thermoregulation in deer mice (Peromyscus maniculatus). Physiol Behav 61: 521–524, 1997. doi: 10.1016/S0031-9384(96)00495-7. [DOI] [PubMed] [Google Scholar]
- 30.Swoap SJ, Gutilla MJ. Cardiovascular changes during daily torpor in the laboratory mouse. Am J Physiol Regul Integr Comp Physiol 297: R769–R774, 2009. doi: 10.1152/ajpregu.00131.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swoap SJ, Gutilla MJ, Liles LC, Smith RO, Weinshenker D. The full expression of fasting-induced torpor requires beta 3-adrenergic receptor signaling. J Neurosci 26: 241–245, 2006. doi: 10.1523/JNEUROSCI.3721-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swoap SJ, Weinshenker D. Norepinephrine controls both torpor initiation and emergence via distinct mechanisms in the mouse. PLoS One 3: e4038, 2008. doi: 10.1371/journal.pone.0004038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taborsky GJ Jr, Mundinger TO. Minireview: The role of the autonomic nervous system in mediating the glucagon response to hypoglycemia. Endocrinology 153: 1055–1062, 2012. doi: 10.1210/en.2011-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Cauter E, Désir D, Decoster C, Féry F, Balasse EO. Nocturnal decrease in glucose tolerance during constant glucose infusion. J Clin Endocrinol Metab 69: 604–611, 1989. doi: 10.1210/jcem-69-3-604. [DOI] [PubMed] [Google Scholar]
- 35.Van Cauter E, Polonsky KS, Scheen AJ. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev 18: 716–738, 1997. doi: 10.1210/edrv.18.5.0317. [DOI] [PubMed] [Google Scholar]
- 36.Westman W, Geiser F. The effect of metabolic fuel availability on thermoregulation and torpor in a marsupial hibernator. J Comp Physiol B 174: 49–57, 2004. doi: 10.1007/s00360-003-0388-y. [DOI] [PubMed] [Google Scholar]
- 37.Withers PC. Metabolic, respiratory and haematological adjustments of the little pocket mouse to circadian torpor cycles. Respir Physiol 31: 295–307, 1977. doi: 10.1016/0034-5687(77)90073-1. [DOI] [PubMed] [Google Scholar]
- 38.Zimmerman ML. Carbohydrate and torpor duration in hibernating golden-mantled ground squirrels (Citellus lateralis). J Comp Physiol 147: 129–135, 1982. doi: 10.1007/BF00689301. [DOI] [Google Scholar]