Abstract
Renal iron recycling preserves filtered iron from urinary excretion. However, it remains debated whether ferroportin (FPN), the only known iron exporter, is functionally involved in renal iron recycling and whether renal iron recycling is required for systemic iron homeostasis. We deleted FPN in whole nephrons by use of a Nestin-Cre and in the distal nephrons and collecting ducts, using a Ksp-Cre, and investigated its impacts on renal iron recycling and systemic iron homeostasis. FPN deletion by Nestin-Cre, but not by Ksp-Cre, caused excess iron retention and increased ferritin heavy chain (FTH1) specifically in the proximal tubules and resulted in the reduction of serum and hepatic iron. The systemic iron redistribution was aggravated, resulting in anemia and the marked downregulation of hepatic hepcidin in elderly FPN knockout (KO)/Nestin-Cre mice. Similarly, in iron-deficient FPN KO/Nestin-Cre mice, the renal iron retention worsened anemia with the activation of the erythropoietin-erythroferrone-hepcidin pathway and the downregulation of hepatic hepcidin. Hence, FPN likely located at the basolateral membrane of the proximal tubules to export iron into the circulation and was required for renal iron recycling and systemic iron homeostasis particularly in elderly and iron-deficient mice. Moreover, FPN deletion in the proximal tubules alleviated ischemic acute kidney injury, possibly by upregulating FTH1 to limit catalytic iron and by priming antioxidant mechanisms, indicating that FPN could be deleterious in the pathophysiology of ischemic acute kidney injury (AKI) and thus may be a potential target for the prevention and mitigation of ischemic AKI.
Keywords: acute kidney injury, ferroportin, iron reabsorption, ischemia/reperfusion
INTRODUCTION
The kidney reabsorbs iron bound by specific proteins such as transferrin, lactoferrin, ferritin, and neutrophil gelatinase-associated lipocalin (Ngal) by employing megalin and cubulin receptors at the apical plasma membrane of the proximal tubular epithelial cells (6, 13, 21, 42). It is thought that the reabsorbed iron is subsequently transported into the circulation (33), which prevents the filtered iron from urinary excretion (24, 33). However, despite renal iron recycling being recognized, knowledge about the transport of the reabsorbed iron into the circulation and the roles of renal iron recycling in systemic iron homeostasis are still limited.
Ferroportin (FPN, SLC40A1) is the only known iron exporter in mammals. FPN is located at the basolateral plasma membrane of duodenal epithelial cells and exports the absorbed dietary iron into the circulation (1, 14, 25). FPN also exports iron at the surface of hepatocytes, macrophages, and other types of cells (47, 48). In contrast, the functions of FPN in the kidney remain controversial because of its confusing subcellular localization reported by different studies. Starzynski et al. (35) and Wolff et al. (43) reported that FPN protein located at the basolateral plasma membrane of the proximal tubular epithelial cells, and suggested that FPN might export the reabsorbed iron into the circulation. However, Veuthey et al. (39) and Zarjou et al. (46) thought that FPN located at the apical plasma membrane of the proximal tubular epithelial cells and functioned to import the luminal iron (46). Moreover, it was also reported that in hepcidin (HAMP1) knockout (KO) mice, FPN was highly expressed in the basolateral and intracellular membrane in the epithelial cells of the thick ascending limbs, but its subcellular localization in the proximal tubules was not clearly specified (26). Hence, functional analysis using FPN KO mouse models is required to clarify the roles of FPN protein in the kidney and the impacts of kidney FPN on systemic iron homeostasis.
Excess iron can directly injure renal tubular cells in vitro and in vivo (20, 24, 29, 32, 34), and consistently, catalytic iron is released and damages epithelia in both patients and animal models (3–5, 46). Indeed, iron chelators provide marked functional and histological protection in ischemia/reperfusion (I/R) and cisplatin-, rhabdomyolysis-, and contrast-induced acute kidney injury (AKI) in animal models (3–5), likely by removing poorly bound catalytic iron rather by depleting cellular iron stores. In fact, a recent study found that increased baseline levels of serum iron and iron overload alleviated ischemic AKI (38), suggesting a protective role for replete cellular stores of iron in ischemic AKI. However, it remains unknown whether the protection was primed by the preexisting systemic and/or renal iron and by the preexisting cellular stores of iron in specific renal tubular cells or iron in the extracellular compartments such as serum.
To genetically dissect the physiological functions of FPN in the kidney, we generated FPN KO models by using Nestin-Cre and Ksp-Cre drivers and analyzed the functional impacts of FPN deletion on renal iron recycling, systemic iron homeostasis, and ischemic AKI. Our data showed that FPN KO by Nestin-Cre but not Ksp-Cre resulted in excess iron retention and increased ferritin (FTH1) expression specifically in the proximal tubules and also caused the decrease of serum and hepatic iron, indicating that FPN was required for iron recycling and systemic iron homeostasis. FPN KO by Nestin-Cre also alleviated ischemic AKI possibly by increasing FTH1 and glutathione peroxidase-4 (GPx4) expression and by suppressing NADPH oxidase-4 (NOX4) expression and apoptosis, suggesting that FPN in the proximal tubules was deleterious to accelerate kidney damages.
MATERIALS AND METHODS
Animals.
Use of animals and corresponding protocols in this study were approved by the Institutional Animal Care and Use Committee at Tongji University (IACUC no. TJLAC-015-032), Shanghai, China. Rosa26R-LacZ (B6;129S4-Gt(ROSA)26Sortm1Sor/J; stock no: 003309; Jackson Laboratory) was mated with Nestin-Cre, B6.Cg-Tg(Nes-Cre)1Kln/J (3771; Jackson Laboratory) or Ksp-Cre (012237; Jackson Laboratory) drivers to verify Cre-mediated gene deletion in the kidneys, respectively. To delete the FPN gene in whole nephron or in the distal nephron and collecting ducts, FPN-floxed (FPNf/f with exons 6–7 floxed, gift from Dr. Fudi Wang, Zhejiang University, China) mice were crossed with Nestin-Cre or Ksp-Cre to generate FPNf/f/Nestin-Cre or FPNf/f/Ksp-Cre mice, respectively, and PCR-genotyping followed previously reported protocols (14).
To evaluate FPN deletion efficiency in the kidney, duodenum, liver, spleen, brain, and bone marrow (3 mo; n = 8, male: 3, female 5), total RNA was isolated with a RNAiso Plus kit (9109, Takara), and was reverse-transcribed to cDNA with a PrimeScript RT kit with gDNA Eraser (RR047A, Takara). Quantitative (q)PCR was then performed using primers (FPN forward: ACCTGGCTACGTCGAAAATG, reverse: CCAGGCATGAATACGGAGAT), and 18S rRNA was amplified as internal control using specific primers (forward: CCTGCGGATTAATTTGACTC, reverse: AACTAAG AACGGCCATGCAC), and regular PCR was performed using primers (forward: ATGACCAAGGCAAGAGATCA, reverse: TTAAGCGTAATCTGGAACATCGTGA).
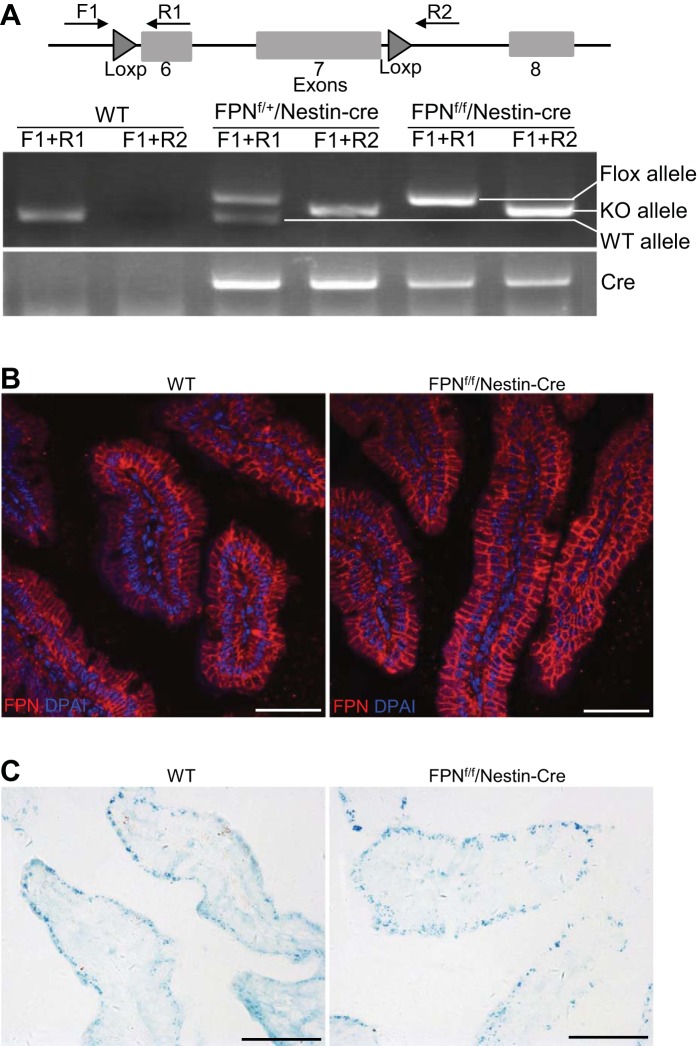
To identify wild-type (WT), floxed, and KO alleles of the FPN gene in the duodenums of WT and FPNf/f/Nestin-Cre mice, genomic DNA was extracted, and PCR was performed using primers forward 1 (CTACACGTGCTCTCTTGAGAT), reverse 1 (GGTTAAACTGCTTCAAAG G) and reverse 2 (CCTCATATGTGAGTCAAAGTATAG), as indicated in Fig. 2A. Cre recombinase was PCR genotyped using specific primers (forward: CAGCATTGCTGTCACTTG GTC; reverse: ATTTGCCTGCATTACCGGTCG).
Fig. 2.
Nestin-Cre did not cause ferroportin (FPN) deletion in duodenal epithelia of floxed FPN (FPNf/f)/Nestin-Cre mice. A: PCR genotyping showed that the FPN gene was deleted in some duodenal cells of FPNf/+/Nestin-Cre and FPNf/f/Nestin-Cre mice. B: immunofluorescence showed that expression of FPN protein in duodenal epithelia of FPNf/f/Nestin-Cre mice was intact and comparable to WT counterparts. C: Perl’s staining showed comparable iron accumulation in duodenal epithelia of WT and FPNf/f/Nestin-Cre mice. Scale bars, 50 μm.
To generate systemic iron deficiency or iron overload, FPNf/f/Nestin-Cre, FPNf/f/Ksp-Cre or WT mice (2 mo) were fed iron-deficient diets (6 ppm iron from Research Diets, NJ, but actual iron concentration is ~27 ± 9 ppm, n = 3) for 1 mo (n = 6, male 3, female 3) or were administered a single dose of iron dextran (10 mg iron) by intraperitoneal injection and then maintained on regular chow (iron replete; actual iron concentration is 250 ± 28 ppm, n = 3) for 1 mo (n = 7–17, male: 3–8, female: 4–10). The iron concentrations in iron-deficient and regular mouse chows were measured using an Optima 2100 atomic absorption spectrometer (PerkinElmer).
Detection of β-galactosidase (LacZ) activities.
Kidneys from Rosa26R-LacZ/Nestin-Cre and Rosa26R-LacZ/Ksp-Cre mice were sliced and fixed in 4% paraformaldehyde (PFA) in PBS for 40 min at room temperature. To detect β-galactosidase activities, kidney slices were permeabilized in a solution (0.02% Nonidet P-40, 0.01% sodium deoxycholate, and 2 mM MgCl2 in PBS) and then stained for β-galactosidase activities in an X-Gal solution (1 mg/ml X-gal, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, and 2 mM MgCl2 in PBS) overnight at 37°C. The stained kidney slices were washed, dehydrated, and embedded in paraffin by following standard procedures, and serial 4-μm sections were prepared, visualized, and photographed using a Nikon ECLIPSE 80i microscope.
Perl’s staining.
Deparaffinized duodenum and kidney sections were incubated in Perl’s staining solution (5% potassium ferrocyanide, pH 1.0) at room temperature for 60 min and subsequently washed for 15 min in distilled water and counterstained with neutral red. The staining was visualized and photographed using the Nikon ECLIPSE 80i microscope.
Measurement of hematocrit, serum iron, transferrin saturation, and tissue nonheme iron.
Hematocrit was measured using hematocrit tubes as previously reported (19). Serum iron, transferrin saturation (TSAT), and tissue nonheme iron were quantitated by following protocols as previously reported (13a).
Transient expression of mouse FPN in HEK-293 cells.
Mouse FPN open reading frame was amplified using primers (forward: CTAGAGATCTCACCATGACCAAGGCAAGAGATC; reverse: CTAGTCTAGATTAAGCGTAATCTGGAACATC GTGA) from duodenal cDNA by RT-PCR and cloned into a pcDNA3.1(+) plasmid to generate pcDNA3.1(+)-FPN. The pcDNA3.1(+)-FPN, and empty plasmids were transiently transfected into HEK-293T cells by using Lipofectamine 2000 (Thermofisher), and after 48 h the transfected cells were collected and lysed in RIPA buffer supplemented with protease inhibitors (Sigma-Aldrich) for evaluating the activity and specificity of anti-FPN antibodies using Western blot.
Immunoflurescence and immunohistochemistry.
The duodenum was fixed in 4% PFA, embedded in optimal cutting temperature compound, and cryosectioned for immunofluorescent staining by using anti-FPN antibodies (1:200, ab78066 from Abcam; 1:50, MTP11-S from Alpha Diagnostic International) and Alexa Fluor 594-conjugated donkey anti-rabbit secondary antibodies (Jackson Immunoresearch Laboratories). Nuclei were stained with DAPI (Sigma-Aldrich). The staining was visualized and photographed using a Leica TCS SP8 confocal microscope.
Paraffin sections of WT and FPNf/f/Nestin-Cre kidneys were dewaxed and hydrated for antigen retrieval in a boiling solution (0.01 mol/l sodium citrate, pH6.0) for 20 min, and then sequentially stained by using anti-FTH1 antibody (1:500, ab75973, Abcam) or anti-FPN antibodies (1:200, ab78066, Abcam; 1:50, MTP11-S from Alpha Diagnostic International) and a horseradish peroxidase (HRP)-conjugated anti-rabbit antibody (Jackson Immunoresearch Laboratories). The staining was colorized using a DAB substrate kit (SK-4105, Vector Laboratories) and visualized and photographed using the Nikon ECLIPSE 80i microscope.
RNA isolation and real-time qPCR.
Total RNA and cDNA were obtained as previously described. qPCR was performed in an ABI ViiA 7 real-time PCR system using a SYBR Green kit (RR820A, Takara) and gene-specific primers: EPO forward: CCAAGGAGGCAGAAA ATGTC, reverse: CCTCCATTCTTTTCCAAGCA; duodenal cytochrome B1 (Dcytb1) forward: CAATCGTCATG CCCATACAC, reverse: TACGAGGGGTGTTTCAGGAC; divalent metal transporter 1 (iron-responsive element [DMT1(IRE); NM_00114616 1] forward: GGGTTTTTGTTTGGTGCATT, reverse: GGCAGGAGGATCTCTGTGA G; transferrin receptor 1 (Tfr1) forward: TGCAGAAAAGGTTGCAAATG, reverse: TGAGCATGTCCAAAGA GTGC; HAMP1 forward: GCAGCACCACCTATCTCCAT, reverse: CAATGTCTGCC CTGCTTTCT; bone morphogenetic protein-6 (BMP6) forward: CAAGTCTTGCAGGAGCATCA, reverse: CCAGCCA ACCTTCTTCTGAG; SMAD7 forward: GCAGCAGTTACCCCATCTTC, reverse: TG ATGGAGAAACCAGGGAAC; erythroferrone (ERFE) forward: ATGGGGCTGGAGAACAGC, reverse: TGGCATTGTCCAAGAAGACA). 18S rRNA was used as an internal control using primers described above.
Generation of ischemia/reperfusion AKI.
FPNf/f/Nestin-Cre and WT mice (Male, 3 mo; n = 8) were anesthetized by intraperitoneally injecting pentobarbital sodium (75 mg/kg) and were then subjected to renal bilateral I/R injuries by using a protocol previously described (27). Briefly, incisions were made along the midline of the abdomen, and both renal pedicles were clamped for 20 min to induce bilateral ischemia. Clamping was then released to allow blood reperfusion for 48 h.
Measurement of serum creatinine and blood urea nitrogen.
Serum creatinine and blood urea nitrogen (BUN) were measured by using the automatic biochemistry assay CHRMI-X-180 (Japan Syemex) according to the manufacturer’s instructions.
Histological analysis of AKI.
Paraffin sections of mouse kidneys were prepared and stained with hematoxylin and eosin following a standard protocol. To quantify the severity of kidney injury, at least 10 different areas in each kidney section were randomly captured with ×200 magnification using a Leica microscope (Leica Microsystems). Severity of tubular injuries in the kidney was quantified by counting injured tubules that displayed necrotic lysis, tubular dilation, tubular brush border loss, sloughing of cellular debris into the tubular lumen, and cast formation and then calculating their percentage among all tubules. The injury score was defined the same way as previously described, i.e., 0, none; 1, <10%; 2 = 10–25%; 3 = 26–50%; 4 = 51–75%; 5, ≥75% (45).
Western blot.
Protein prepared from kidney lysates was immunoblotted following a standard protocol. Briefly, mouse kidney tissues were homogenized in RIPA buffer (150 mM NaCl, 50 mM Tris·HCl, pH 7.4, 5 mM EDTA, 1% Triton X-100, 0.5% deoxycholate, 0.1% SDS, and 1× protease inhibitor cocktail from Sigma-Aldrich), and the extracted proteins were fractionated by SDS-PAGE and subsequently transferred to PVDF membranes for immunodetection with specific primary antibodies and correspondent HRP-conjugated secondary antibodies. Primary antibodies used in this study were anti-FPN (1:1,000, ab78066 and Ab85370, Abcam; 1:1,000, sc-49668, Santa Cruz Biotechnology; 1:1,000, MTP11-S, Alpha Diagnostic International); anti-FTH1 (1:5,000, ab75973, Abcam), anti-NADPH oxidase-4 (NOX4) (1:5,000, A1528, Abclonal), anti-glutathione peroxidase-1 (GPx1) (1:2,000, A0873, Abclonal), anti-GPx4 (1:2,000, A1933, Abclonal), anti-GPx6 (1:1,000, sc292680, Santa Cruz Biotechnology), anti-catalase (1:2,000, ab16731, Abcam), anti-heme oxygenase-1 (HO-1; 1:2,000, A1346, Abclonal), anti-receptor-interacting Ser/Thr kinase-3 (RIPK3) (1:2,000, ab56164, Abcam), anti-p-RIPK3 (1:2,000, phosphorylation at Tyr231 and Ser232, ab201912, Abcam), anti-Bax (1:1,000, 556467, BD Biosciences), anti-cleaved caspase-3 (1:2,000, 9664, Cell Signaling), and anti-β-actin (1:5,000, HC201, Transgene). All HRP-conjugated secondary antibodies and ECL kits were purchased from Jackson Immunoreseach Laboratories and Thermo Scientific, respectively. After secondary antibodies had been applied, the proteins were visualized by ECL-based chemiluminescence using an ImageQuant LAS 4000 mini system (GE Healthcare). Protein abundance was quantified based on densitometry using Fiji software (Scion, Frederick, MD). Protein abundance was expressed relative to β-actin.
Statistical analyses.
All data are presented as means ± SE and were statistically analyzed using Student’s t-test or one-way ANOVA followed by Dunnett’s test or two-way ANOVA followed by post hoc Tukey’s honestly significnt difference test when applicable. A P value of <0.05 was considered statistically significant.
RESULTS
FPN KO by Nestin-Cre resulted in increased renal iron and decreased serum and hepatic iron.
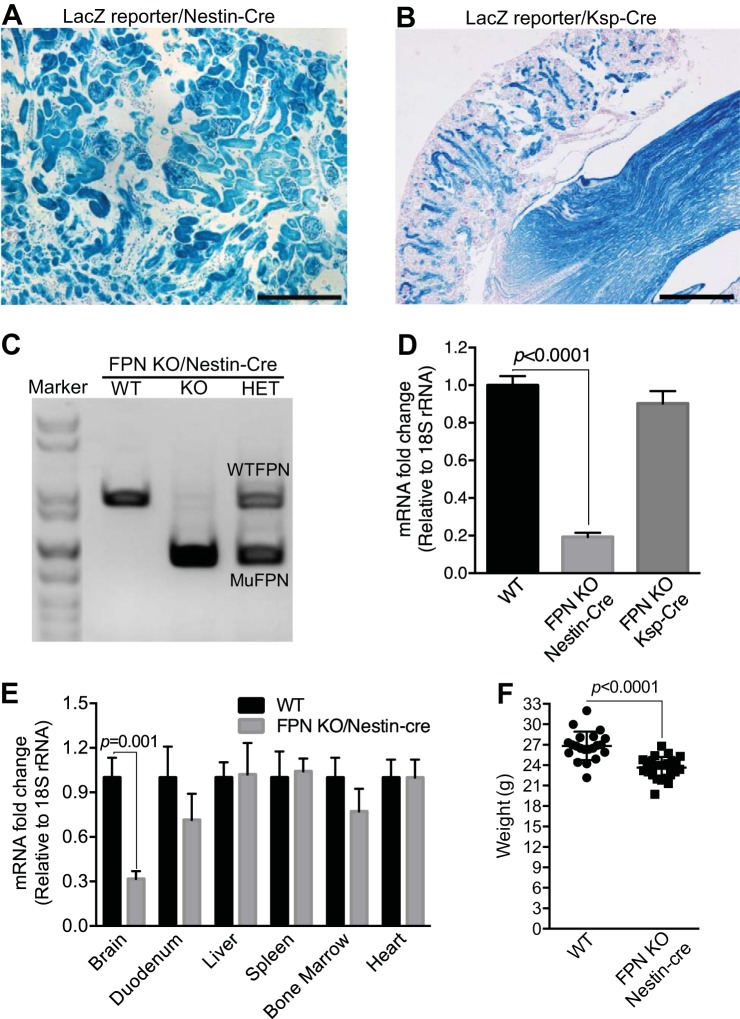
To dissect the physiological roles of FPN in the kidney, we genetically deleted the FPN gene by crossing a FPN floxed mouse (FPNf/f) (14) with Nestin-Cre (15, 30) and Ksp-Cre (41) drivers. Nestin-Cre was used because nestin is highly expressed in nephron progenitor cells in condensed cap mesenchyme of embryonic kidneys (12), and we and others showed that it activated the expression of LacZ in the descendent tissues of condensed cap mesenchyme including glomerulus, proximal tubules, loop of Henle, and distal tubules in a ROSA26-LacZ reporter mouse (Fig. 1A) (12). In contrast, we and others showed that Ksp-Cre was specifically expressed in the distal nephrons and collecting ducts (Fig. 1B), and it was therefore used to delete the FPN gene in these compartments (41). As shown in Fig. 1, C and D, both RT-PCR and qPCR analyses demonstrated the efficient reduction of FPN mRNA (~80%) in FPNf/f/Nestin-Cre kidneys but only a negligible decrease in FPNf/f/Ksp-Cre kidneys compared with WT counterparts. These results validated the efficient FPN deletion by Nestin-Cre and also suggested the dominant expression of FPN mRNA in the proximal tubules rather than in the distal nephrons and collecting ducts. Consistent with the expression pattern of Nestin-Cre in extrarenal tissues (15, 30), qPCR analyses showed the marked reduction of FPN mRNA due to its deletion in the brain but not in the duodenum, liver, spleen, bone marrow, and heart (Fig. 1E), which excluded the possible effects of FPN deletion by Nestin-Cre in the latter organs on systemic iron regulation. Furthermore, PCR genotyping, immunofluorescence, and Perl’s staining demonstrated that FPN was actually deleted in some cells in the duodenums of FPNf/+/Nestin-Cre and FPNf/f/Nestin-Cre mice (Fig. 2A), but the expression of FPN protein in the duodenal epithelia of FPNf/f/Nestin-Cre mice was intact and comparable to that in WT counterparts (Fig. 2B), and iron accumulation was also unchanged (Fig. 2C), which was consistent with the expression of nestin in the glial cells, interstitial cells of Cajal, endothelial cells, pericytes, and myenteric and submucosal ganglia, but not in the epithelial cells of the intestine (7, 10, 37).
Fig. 1.
Ferroportin (FPN) was efficiently deleted in the kidney by Nestin-Cre but not by Ksp-Cre. Nestin-Cre (A) and Ksp-Cre (B) were crossed with Rosa26R-LacZ reporter mice, and β-galactosidase activity was stained to show gene deletion in the kidneys. RT-PCR (C) and qPCR (D) showed FPN knockout (KO) efficiency in the kidneys of floxed FPN (FPNf/f)/Nestin-Cre and FPNf/f/Ksp-Cre vs. wild-type (WT) mice (n = 8; male: 3; female: 5), respectively. E: qPCR showed the marked reduction of FPN mRNA in brain but not in duodenum, liver, spleen, bone marrow, and heart of FPNf/f/Nestin-Cre vs. WT mice (n = 8; male: 3; female: 5). F: body weight was slightly reduced in FPNf/f/Nestin-Cre vs. WT mice (3 mo; n = 21–26; male: 10–13; female: 10–13). Data are presented as means ± SE. D was analyzed by one-way ANOVA followed by Dunnett’s test; E and F were analyzed using Student’s t-test. Scale bars: A, 200 μm; B, 500 μm.
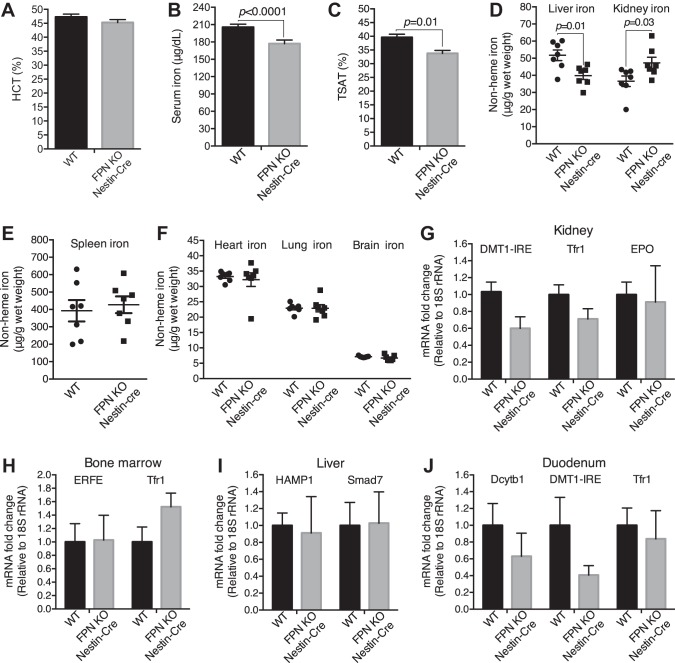
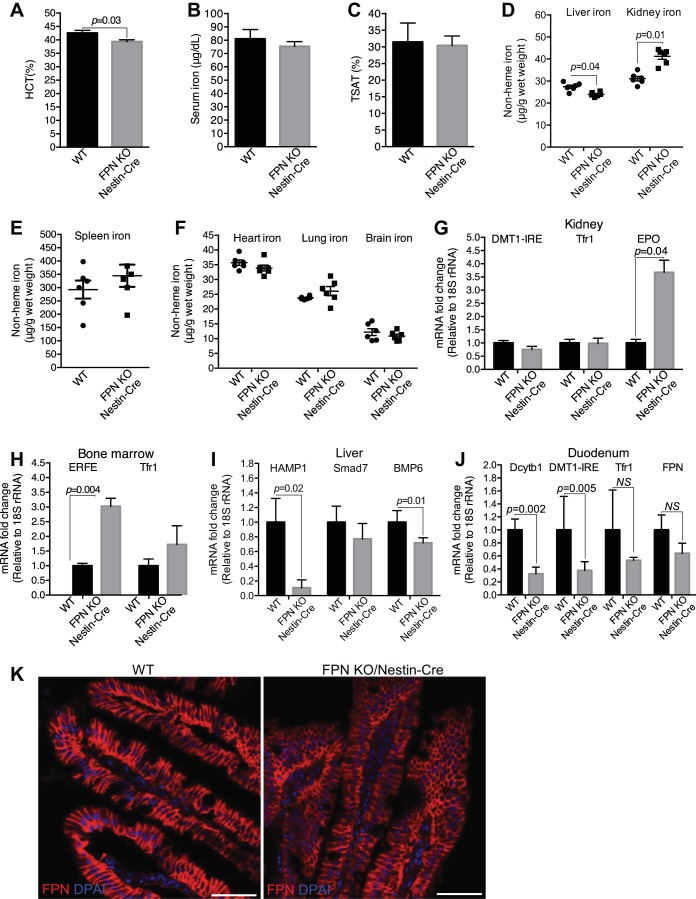
FPN KO by Nestin-Cre did not cause apparent morphological and behavioral defects in mice, but it indeed caused significant loss of body weight (~13%, 3 mo; Fig. 1F). Since FPN is the sole iron exporter so far reported (17), we assumed that, if FPN locates at the basolateral plasma membrane of the proximal tubular cells (35, 43) and exports the reabsorbed iron into the circulation, its deletion should result in excess iron retention in the kidney and consequent systemic iron reduction; but if FPN locates at the apical plasma membrane (39, 46) and imports iron from the lumen (46), its deletion might have negligible effects on renal iron accumulation, since almost all filtered iron in the proximal tubules is thought to be bound by specific proteins such as transferrin and Ngal in healthy conditions and is reabsorbed by megalin and cubulin receptors (6, 13, 21, 42). As shown in Fig. 3, A–D, although hematocrits were unchanged, FPN KO by Nestin-Cre (3 mo old) resulted in a significant decrease of serum iron, transferrin saturation, and hepatic iron by 14, 15, and 23%, respectively, and significant increase of renal iron by 29% in FPNf/f/Nestin-Cre compared with WT mice. In contrast, iron content in the spleen (Fig. 3E), heart, lung, and brain (Fig. 3F) was comparable. Surprisingly, the expression of DMT1(IRE), transferrin receptor 1 (Tfr1), and erythropoietin (EPO) in the kidney (Fig. 3G), ERFE and Tfr1 in the bone marrow (Fig. 3H), HAMP1 and Smad7 in the liver (Fig. 3I), and duodenal cytochrome B1 (Dcytb1), DMT1(IRE), and Tfr1 in the duodenum (Fig. 3J) were comparable in FPNf/f/Nestin-Cre and WT mice, indicating negligible impacts of hepcidin and intestinal iron transport on systemic iron redistribution. Hence, these results suggest that FPN probably located at the basolateral plasma membrane of the proximal tubular cells and exported iron into the circulation; thus, its deletion by Nestin-Cre blocked renal iron recycling into the circulation, resulting in renal iron retention and serum and hepatic iron reduction.
Fig. 3.
Ferroportin (FPN) knockout (KO) by Nestin-Cre resulted in the increase of renal iron and the decrease of serum and liver iron. A: hematocrit was comparable in floxed FPN (FPNf/f)/Nestin-Cre and WT mice (3 mo old; n = 37–40, male: 17–20, female: 17–20). Serum iron (B) and transferrin saturation (TSAT; C) were significantly decreased in FPNf/f/Nestin-Cre vs. WT mice (3 mo old). D: liver iron was decreased, whereas kidney iron was increased in FPNf/f/Nestin-Cre vs. WT mice (3 mo old). Iron contents in spleen (E) and heart, lung, and brain (F) were comparable in FPNf/f/Nestin-Cre and WT mice (3 mo old). G–J: qPCR showed that the expressions of divalent metal transporter 1(iron-responsive element) [DMT1(IRE)], transferrin receptor 1 (Tfr1), and erythropoietin (EPO) in kidney (G), erythroferrone (ERFE) and Tfr1 in bone marrow (H), hepcidin (HAMP1) and Smad7 in liver (I), and duodenal cytochrome B1 (Dcytb1), DMT-1(IRE), and Tfr1 in duodenum (J) were comparable in FPNf/f/Nestin-Cre and WT mice. Data are presented as means ± SE and were analyzed using Student’s t-test. B–J: n = 7 (male: 3, female: 4).
FPN in the proximal tubules exported iron into the circulation.
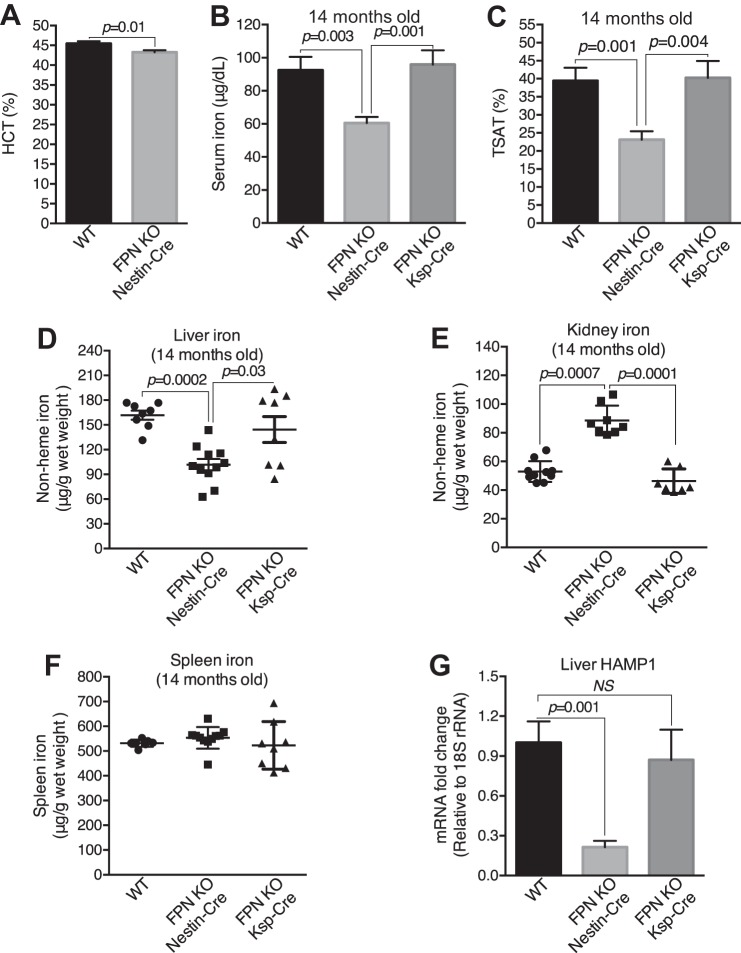
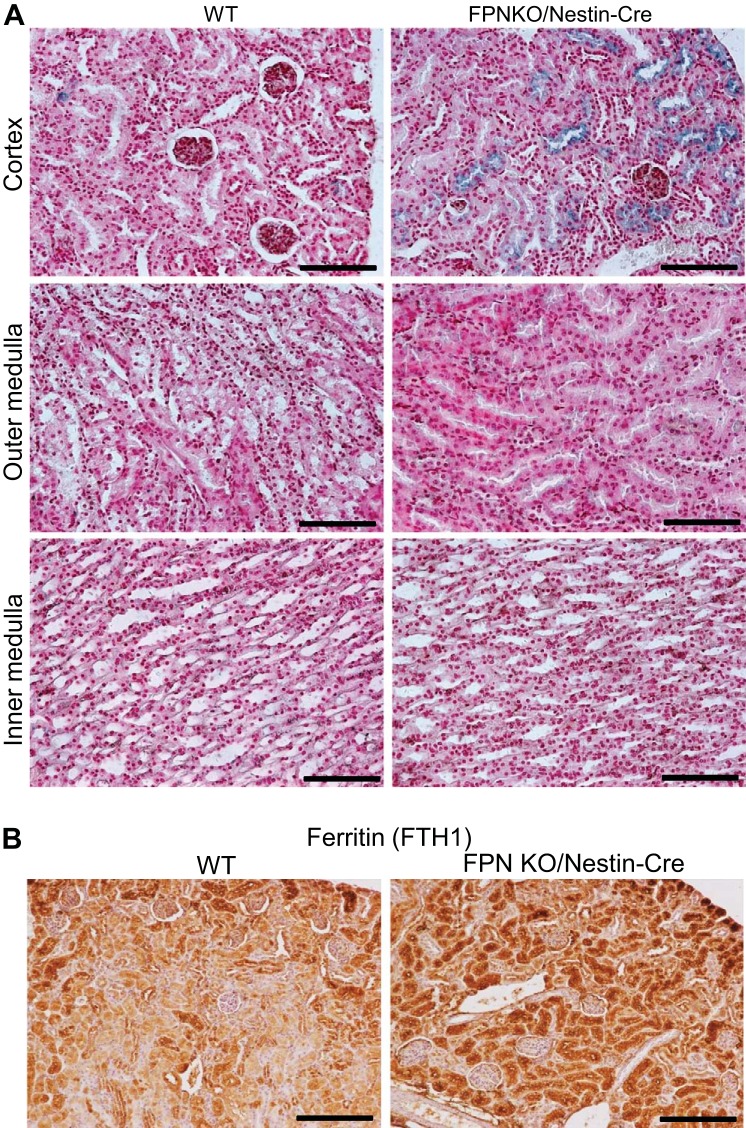
It is also possible that FPN locates at the apical plasma membrane of the proximal tubules (39, 46) and exports the reabsorbed iron into the lumen. In this case, the exported iron should be reabsorbed again in the distal nephrons and collecting ducts to prevent from urinary excretion in healthy conditions; therefore, FPN deletion by Ksp-Cre should result in excess iron retention in these compartments, and the increase of renal iron is expected. To test this possibility, we examined the iron distribution in elderly FPNf/f/Nestin-Cre, FPNf/f/Ksp-Cre and WT mice (14 mo old). As expected, serum iron (Fig. 4B), transferrin saturation (Fig. 4C), and liver iron (Fig. 4D) were significantly deceased, by 34, 41, and 39%, respectively, and kidney iron was markedly increased by 68% (Fig. 4E) in FPNf/f/Nestin-Cre compared with WT mice. As a result, hematocrits were reduced (~5%; Fig. 4A). In contrast, serum iron, transferrin saturation, and kidney and liver iron were comparable in FPNf/f/Ksp-Cre and WT mice, respectively (Fig. 4, B–E), and spleen iron was comparable in FPNf/f/Nestin-Cre, FPNf/f/Ksp-Cre and WT mice (Fig. 4F). Consistently, HAMP1 mRNA was markedly downregulated in FPNf/f/Nestin-Cre but not in FPNf/f/Ksp-Cre livers (Fig. 4G), and both excess iron deposition (Fig. 5A) and increased FTH1 expression (Fig. 5B) were observed specifically in the proximal tubules of FPNf/f/Nestin-Cre mice. Together, these results indicated that FPN likely located at the basolateral rather than apical plasma membrane of the proximal tubular cells and exported iron into the circulation to complete iron recycling.
Fig. 4.
Ferroportin (FPN) recycled renal iron in the proximal tubules. A: hematocrit was significantly decreased in floxed FPN (FPNf/f)/Nestin-Cre vs. WT mice (14 mo; n = 20–21, male: 10–11, female: 10–11. Serum iron (B) and transferrin saturation (TSAT; C) were decreased in FPNf/f/Nestin-Cre vs. WT and FPNf/f/Ksp-Cre mice (14 mo old). Liver iron was decreased (D) and kidney iron was markedly increased (E) in FPNf/f/Nestin-Cre vs. WT and FPNf/f/Ksp-Cre mice. F: iron content in the spleen was comparable in WT, FPNf/f/Nestin-Cre, and FPNf/f/Ksp-Cre mice. G: hepcidin (HAMP1) was markedly downregulated in livers of FPNf/f/Nestin-Cre vs. WT and FPNf/f/Ksp-Cre mice. Data are presented as means ± SE. A was analyzed using Student’s t-test; B–G (n = 8–11; male: 5, female: 6) were analyzed using one-way ANOVA followed by Dunnett’s test.
Fig. 5.
Ferroportin (FPN) knockout (KO) by Nestin-Cre caused excess iron deposition and increased FTH1 expression specifically in the proximal tubules. A: Perl’s staining showed excess iron deposition specifically in the proximal tubules but not in the loop of Henle, distal tubules, and collecting ducts in floxed FPN (FPNf/f)/Nestin-Cre mice. Scale bars: 100 μm. B: immunochemistry showed increased FTH1 specifically in proximal tubules. Scale bars, 200 μm.
FPN KO by Nestin-Cre resulted in the increase of renal iron and the decrease of serum and liver iron under systemic iron deficiency.
Nestin-Cre could also mediate the deletion of the FPN gene in extrarenal tissues, particularly in the central and peripheral nervous systems (Fig. 1E) (15, 30); therefore, we assumed that FPN KO in these tissues might regulate systemic iron balance. To evaluate this assumption, we fed FPNf/f/Nestin-Cre and WT mice (2 mo old) iron-deficient diets (FeD, ~27 ppm iron) for 1 mo to induce systemic iron deficiency. As shown in Fig. 6A, hematocrits were significantly decreased in FeD FPNf/f/Nestin-Cre compared with WT mice. Importantly, whereas serum iron (Fig. 6B) and transferrin saturation (Fig. 6C) were leveled, kidney iron was still significantly increased by ~31%, and liver iron was reduced by ~13% in FeD FPNf/f/Nestin-Cre compared with WT mice (Fig. 6D). Again, the iron content in the spleen (Fig. 6E), heart, lung, and brain (Fig. 6F) were comparable in FeD FPNf/f/Nestin-Cre and WT mice, respectively. Hence, together with the intact expression of FPN in the duodenal epithelium, liver, and spleen (Figs. 1E, 2B, and 6K) and the unchanged brain iron in FPNf/f/Nestin-Cre mice (Figs. 3F and 6F), these results indicated that the impacts of FPN KO in extrarenal tissues on systemic iron homeostasis should be minor.
Fig. 6.
Ferroportin (FPN) knockout (KO) by Nestin-Cre resulted in increased renal iron and decreased liver iron under systemic iron deficiency. A: hematocrit was significantly decreased in iron-deficient floxed FPN (FPNf/f)/Nestin-Cre vs. WT mice (3 mo). Serum iron (B) and transferrin saturation (TSAT; C) were leveled in iron-deficient FPNf/f/Nestin-Cre and WT mice (3 mo), respectively. D: liver iron was significantly decreased and kidney iron increased in iron-deficient FPNf/f/Nestin-Cre vs. WT mice. Iron contents in spleen (E) and heart, lung, and brain (F) were comparable in iron-deficient FPNf/f/Nestin-Cre and WT mice. G: erythropoietin (EPO) but not divalent metal transporter 1(iron-responsive element) [DMT1(IRE)] and transferrin receptor 1 (Tfr1) mRNA was markedly upregulated in kidneys of iron-deficient FPNf/f/Nestin-Cre mice. H: erythroferrone (ERFE) but not Tfr1 mRNA was markedly upregulated in bone marrows of iron-deficient FPNf/f/Nestin-Cre mice. I: hepcidin (HAMP1) and bone morphogenetic protein-6 (BMP6) mRNA were markedly downregulated in livers of iron-deficient FPNf/f/Nestin-Cre mice. J: duodenal cytochrome B1 (Dcytb1) and DMT-1(IRE) mRNA were significantly decreased in duodenums of iron-deficient FPNf/f/Nestin-Cre mice. K: immunofluorescence showed lower expression levels of FPN protein in duodenal epithelia of FPNf/f/Nestin-Cre than in WT mice. Data are presented as means ± SE (n = 6; male: 3, female: 3) and were analyzed using Student’s t-test. Scale bars, 50 μm.
Interestingly, EPO, but not DMT-1(IRE) and Tfr1, was upregulated fourfold in FPNf/f/Nestin-Cre compared with WT kidneys (Fig. 6G). Consistent with the regulatory cascades activated by EPO (18, 40), ERFE in bone marrow was upregulated threefold (Fig. 6H), and HAMP1 was downregulated ~10-fold in livers (Fig. 6I). Surprisingly, both Dcytb1 and DMT1 were downregulated in duodenums of FPNf/f/Nestin-Cre compared with WT mice, and whereas the downregulation of Tfr1 and FPN mRNA was not statistically significant (Fig. 6J), FPN protein was reduced in the duodenal epithelia (Fig. 6K), suggesting the downregulation of transepithelial iron transport in the duodenums of FPNf/f/Nestin-Cre mice.
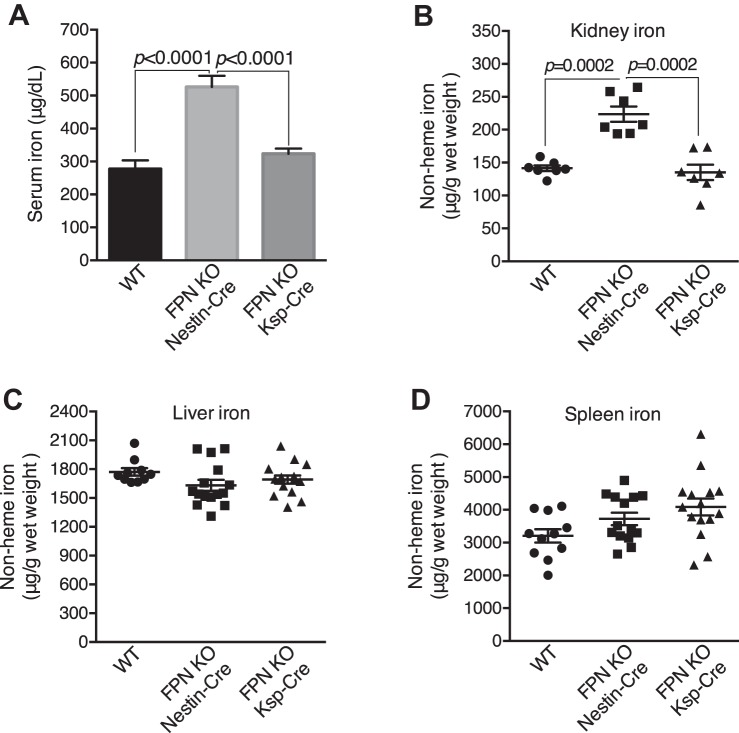
FPN KO by Nestin-Cre resulted in an increase of renal iron under systemic iron overload.
We next intraperitoneally injected a single dose of iron dextran (10 mg iron per mouse, 2 mo old) into FPNf/f/Nestin-Cre, FPNf/f/Ksp-Cre and WT mice to induce systemic iron overload and then evaluated iron distribution 1 mo later. As shown in Fig. 7A, serum iron was surprisingly increased by 75% in FPNf/f/Nestin-Cre compared with FPNf/f/Ksp-Cre and WT mice. As expected, kidney iron was markedly increased by 58% in FPNf/f/Nestin-Cre compared with FPNf/f/Ksp-Cre and WT mice (Fig. 7B), whereas liver and spleen iron were comparable (Fig. 7, C and D). In contrast, serum and kidney iron were comparable in FPNf/f/Ksp-Cre and WT mice (Fig. 7, A and B). Again, these results indicated that FPN KO in the proximal tubules caused renal iron retention.
Fig. 7.
Ferroportin (FPN) knockout (KO) by Nestin-Cre resulted in increased renal iron under systemic iron overload. A: serum iron was increased in iron overload floxed FPN (FPNf/f)/Nestin-Cre vs. WT and FPNf/f/KSP-Cre mice. Kidney iron was markedly increased in iron overload FPNf/f/Nestin-Cre vs. WT and FPNf/f/KSP-Cre mice (B) despite liver iron being leveled (C). D: spleen iron was comparable in iron overload FPNf/f/Nestin-Cre, FPNf/f/KSP-Cre and WT mice. Data are presented as means ± SE (n = 7–17; male: 3–8, female: 4–10) and were analyzed using one-way ANOVA followed by Dunnett’s test.
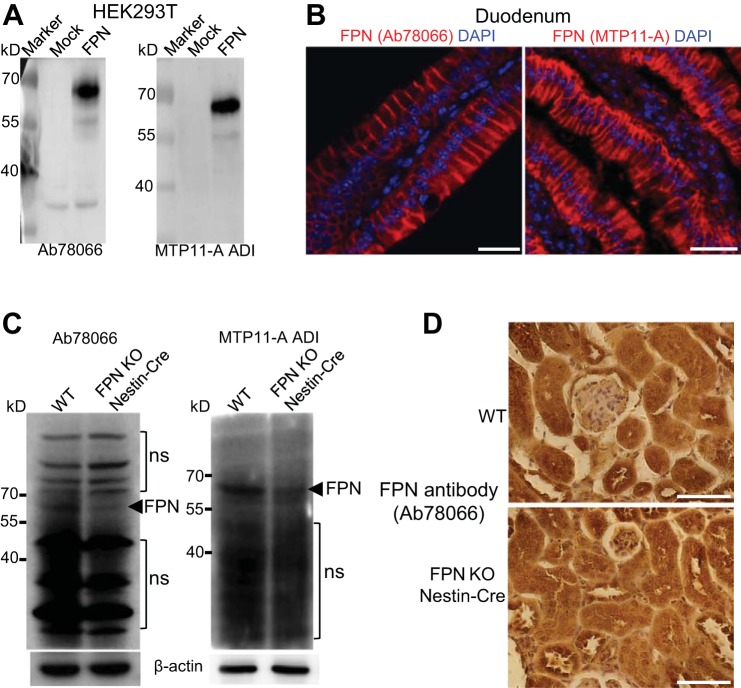
Evaluation of FPN antibodies for subcellular localization of FPN in the kidney.
To evaluate the subcellular localization of FPN protein in the kidney, we tested four different commercially available FPN antibodies (Ab78066 and Ab85370 from Abcam; sc-49668 from Santa Cruz Biotechnology; MTP11-A from Alpha Diagnostic International), and we found that only two (Ab78066 and MTP11-A) could detect mouse FPN protein overexpressed in HEK-293T cells (Fig. 8A) and recognize FPN protein at the basolateral plasma membrane of the duodenal epithelial cells in FeD mice (Fig. 8B), indicating their specificity to FPN protein in the duodenum. However, Western blot using these antibodies showed multiple abundant nonspecific proteins in both WT and FPNf/f/Nestin-Cre kidneys besides their recognition of FPN protein in WT kidneys with higher levels than in FPNf/f/Nestin-Cre (Fig. 8C). Indeed, immunochemistry using Ab78066 and MTP11-A showed nonspecific intracellular staining in the proximal tubules, loop of Henle, and collecting ducts in both WT and FPN KO/Nestin-Cre kidneys (Fig. 8D), indicating that antibodies solely specific to FPN are required for its subcellular localization in the kidney.
Fig. 8.
Evaluation of ferroportin (FPN) antibodies for subcellular localization of FPN in the kidney. A: FPN antibodies Ab78066 from Abcam and MTP11-A from ADI recognized mouse FPN protein overexpressed in HEK-293T cells. B: both Ab78066 and MTP11-A recognized FPN protein at the basolateral membrane of duodenal epithelial cells. Scale bars, 25 µm. C: Western blot using Ab78066 and MTP11-A showed decreased FPN protein in kidneys of FPNf/f/Nestin-Cre vs. WT mice but also detected multiple abundant nonspecific (ns) proteins. D: immunochemistry using Ab78066 showed nonspecific intracellular staining in both WT and FPNf/f/Nestin-Cre kidneys. Scale bars, 50 µm.
FPN KO by Nestin-Cre protected from ischemic AKI.
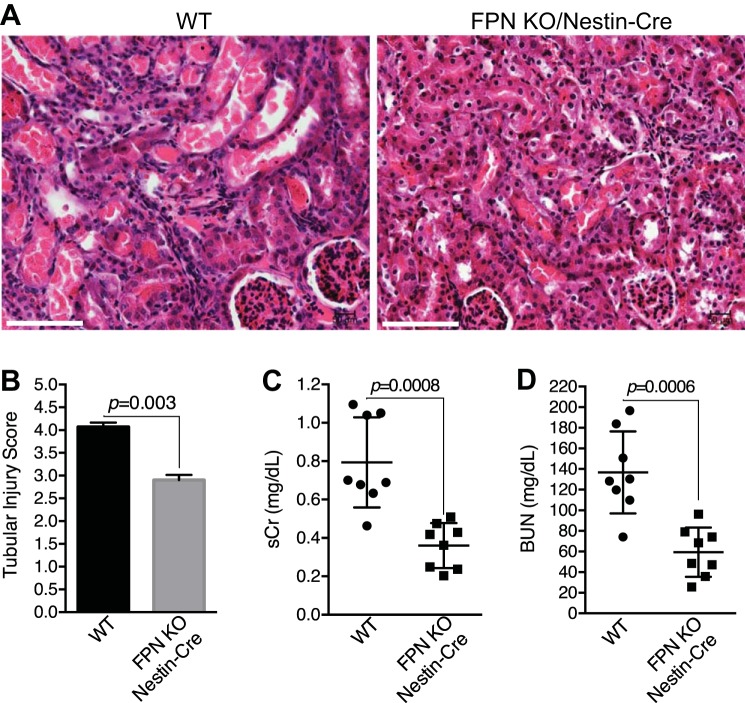
Preexisting iron repletion and iron overload were recently reported to alleviate ischemic AKI (38). Since FPN KO by Nestin-Cre specifically increased iron deposition in the proximal tubules (Fig. 5), we examined whether the increased stores of iron in the proximal tubules in FPNf/f/Nestin-Cre mice impacted ischemic AKI (I/R; 20-min ischemia followed by 48-h reperfusion). As shown in Fig. 9A, I/R incurred acute necrosis and tubular cell damage in all kidneys, but remarkably, damage was more severe in WT than in FPNf/f/Nestin-Cre mice, as evidenced by widespread cast accumulation and higher injury scores in the proximal tubules (Fig. 9, A and B). As a result, both serum creatinine (sCr) and BUN were markedly higher in WT than in FPNf/f/Nestin-Cre mice (Fig. 9, C and D).
Fig. 9.
Ferroportin (FPN) knockout (KO) by Nestin-Cre protected from ischemic acute kidney injury (AKI). Representative hematoxylin and eosin staining (A) and tubular injury score (B) showed less severe damage in ischemic kidneys of floxed FPN (FPNf/f)/Nestin-Cre vs. WT mice (ischemia, 20 min; reperfusion, 48 h). Scale bar, 100 µm. Both serum creatinine (sCr; C) and blood urea nitrogen (BUN; D) were lower in FPNf/f/Nestin-Cre than in WT mice with ischemic AKI. Data are presented as means ± SE (3 mo; n = 8, male) and were analyzed using Student’s t-test.
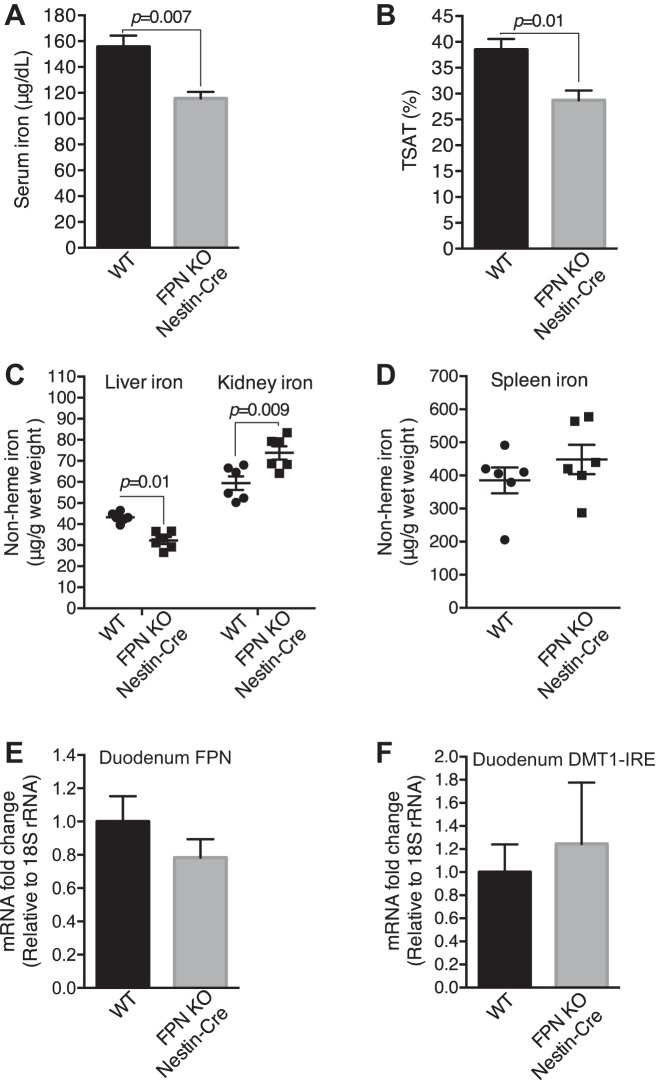
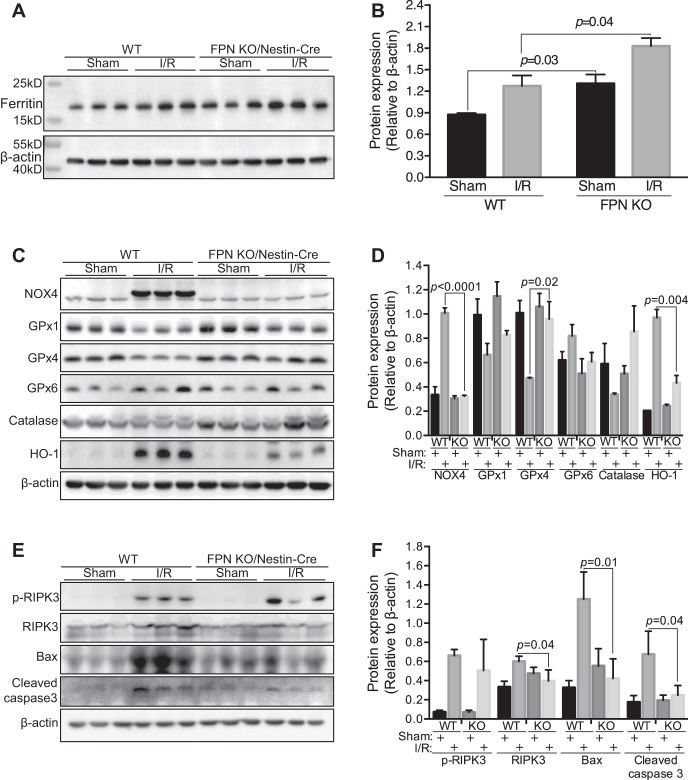
Tissue iron analysis showed lower serum and liver iron and transferrin saturation (Fig. 10, A–C), and higher kidney iron (Fig. 10C) in FPNf/f/Nestin-Cre than in WT I/R mice, whereas spleen iron was comparable (Fig. 10D). Further qPCR analysis showed negligible changes of FPN and DMT1(IRE) mRNA in the duodenums in FPNf/f/Nestin-Cre compared with WT mice (Fig. 10, E and F). Consistently, kidney FTH1 was significantly higher in FPNf/f/Nestin-Cre than in WT sham and I/R mice (Fig. 11, A and B). Given that FTH1 is required to mitigate AKI (46), these data suggested that the preexisting replete cellular iron stores in the proximal tubules contributed to alleviating ischemic AKI possibly by increasing FTH1 to limit catalytic iron production.
Fig. 10.
Ferroportin (FPN) knockout (KO) by Nestin-Cre resulted in decreased serum and liver iron and increased renal iron in ischemic acute kidney injury (AKI) mice. Serum iron (A), transferrin saturation (TSAT; B), and liver iron (C) were decreased, and kidney iron was increased (C) in floxed FPN (FPNf/f)/Nestin-Cre vs. WT ischemic AKI mice. D: spleen iron was comparable in FPNf/f/Nestin-Cre and WT ischemic AKI mice. E and F: FPN and divalent metal transporter 1(iron-responsive element) [DMT1(IRE)] mRNA were comparable in duodenums of FPNf/f/Nestin-Cre and WT ischemic AKI mice, respectively. Data are presented as means ± SE (3 mo; n = 6, male) and were analyzed using Student’s t-test.
Fig. 11.
Ferroportin (FPN) knockout (KO) by Nestin-Cre increased FTH1 and glutathione peroxidase-4 (GPx4) and decreased NADPH oxidase-4 (NOX4) and apoptosis in ischemia/reperfusion (I/R) kidneys. Western blot (A) and densitometric analysis (B) showed increased ferritin (FTH1) in both sham and I/R floxed FPN (FPNf/f)/Nestin-Cre vs. WT kidneys. C and D: NOX4 and heme oxygenase-1 (HO-1) proteins were decreased and GPx4 was increased in I/R kidneys of FPNf/f/Nestin-Cre vs. WT mice, and GPx1, GPx6, and catalase were comparable, respectively. E and F: phosphorylated receptor-interacting Ser/Thr kinase-3 (p-RIPK3) was comparable, and Bax and cleaved caspase-3 were decreased in I/R kidneys of FPNf/f/Nestin-Cre vs. WT mice. Data are presented as means ± SE (3 mo; n = 3, male) and were analyzed using two-way ANOVA followed by post hoc Tukey’s HSD test.
FPN KO by Nestin-Cre increased GPx4 and decreased NOX4 and apoptosis in I/R kidneys.
Overproduction of reactive oxygen species (ROS) is one of the major mechanisms in the pathophysiology of ischemic AKI (2, 9); thus, we analyzed major regulatory proteins for ROS production and degradation. NOX4 is a major source of ROS in the kidney under both physiological and pathophysiological conditions (28, 31), and antioxidant proteins such as GPx, catalase, and HO-1 are required to eliminate ROS and to protect cells from oxidative injuries (8, 31). As shown in Fig. 11, C and D, there were markedly lower levels of NOX4 and higher levels of GPx4 in FPNf/f/Nestin-Cre than in WT I/R kidneys. HO-1, a cell-protective protein that is increased in response to injury (8), was significantly lower in FPNf/f/Nestin-Cre than in WT I/R kidneys (Fig. 11, C and D). In contrast, GPx1, GPx6, and catalase were comparable in FPNf/f/Nestin-Cre compared with WT in ischemic kidneys (Fig. 11, C and D). Hence, these data indicated that FPN KO by Nestin-Cre resulted in the downregulation of NOX4 and the upregulation of GPx4, which possibly contributed to suppressing ROS accumulation.
A number of cell death pathways, including apoptosis, necroptosis, and ferroptosis are implicated in the pathophysiology of ischemic AKI (11, 16, 22, 23). We found that proapoptotic proteins Bax and cleaved forms of caspase-3 were markedly lower in FPNf/f/Nestin-Cre than in WT I/R kidneys, but the phosphorylation of an essential regulator of necroptosis (44), RIPK3, was comparable (Fig. 11, E and F). These data indicated that FPN KO by Nestin-Cre probably inhibited apoptosis in FPNf/f/Nestin-Cre compared with WT ischemic kidneys. In addition, since GPx4 is a key regulator in ferroptosis and loss of its function accelerates ischemic AKI by promoting ferroptosis (16, 23), the increase of GPx4 in FPNf/f/Nestin-Cre kidneys might also contribute to suppressing kidney damage by inhibiting ferroptosis.
DISCUSSION
Our present study demonstrated that FPN likely located at the basolateral membrane of the proximal tubules to export iron into the circulation and was required for renal iron recycling and systemic iron homeostasis particularly in elderly and iron-deficient mice and that its deletion in the kidney protected from ischemic AKI.
FPN in the proximal tubules was required for renal iron recycling to export iron into the circulation. The functions of FPN in the kidney remained debatable for many years due to its controversial subcellular localization (35, 39, 43, 46). Here, we provided direct functional evidence to demonstrate that FPN was predominantly expressed in the proximal tubules and functioned as an iron exporter rather than a previously reported iron importer (46). In HAMP1−/− and hemojuvelin-deficient (Hjv−/−) hemochromatotic mice, the excess iron was specifically deposited in the distal rather than the proximal nephrons (26), indicating that FPN unlikely imported iron at the apical membrane of the proximal tubules. Furthermore, FPN deletion by Ksp-Cre in the distal nephrons and collecting ducts failed to cause renal iron retention; thus, there seemed to be no significant amount of iron exported from the proximal tubules to the distal nephrons for reabsorption in healthy conditions, which indicated that FPN also unlikely exported iron at the apical membrane of the proximal tubules. In contrast, FPN KO by Nestin-Cre resulted in renal iron retention specifically in the proximal tubules and in the reduction of serum and hepatic iron. Hence, these results indicated that FPN likely located at the basolateral membrane of the proximal tubules and exported the reabsorbed iron into the circulation to complete renal iron recycling.
FPN KO in the proximal tubules accounted for serum and liver iron reduction in FPN KO/Nestin-Cre mice. Renal iron retention in FPN KO/Nestin-Cre mice was consistently observed under iron-deficient, -replete, and overload conditions despite different changes of serum and liver iron under different systemic iron conditions, indicating that the iron retention in the proximal tubules was likely the primary phenotype and also the major cause for systemic iron redistribution in FPN KO/Nestin-Cre mice. It was unlikely that FPN KO by Nestin-Cre in extrarenal tissues contributed significantly to systemic iron dyshomeostasis in FPN KO/Nestin-Cre mice. Consistent with its expression pattern (10, 15, 30, 37), Nestin-Cre did not mediate FPN deletion in the duodenal epithelium, liver, spleen, heart, and bone marrow, and the expression of FPN protein in the duodenal epithelia of FPN KO/Nestin-Cre mice was intact and comparable to WT counterparts under iron-replete conditions. Indeed, Nestin-Cre markedly reduced FPN mRNA expression in the brains, but brain iron remained unchanged under both iron-replete and -deficient conditions, and so did serum iron under iron-deficient conditions, indicating the negligible impacts of FPN KO in the brain on systemic iron homeostasis. Interestingly, under systemic iron-deficient conditions, the expressions of Dcytb1 and DMT1(IRE) mRNA and FPN protein were decreased in the duodenums of FPN KO/Nestin-Cre mice, indicating that there might be an unknown cross-talk between the kidney and duodenum that signaled the regulation of intestinal iron absorption by renal iron recycling, and the impaired duodenal iron absorption likely accounted for some of the systemic iron deficiency in these mice.
FPN in the proximal tubules was required for systemic iron homeostasis particularly in elderly and iron-deficient mice. There were only negligible changes of hematocrit and iron gene expression in the duodenum, liver, bone marrow, and kidney in young iron-replete FPN KO/Nestin-Cre mice (3 mo), indicating the minor roles of FPN in the proximal tubules in the regulation of systemic iron homeostasis. However, in elderly iron-replete FPNf/f/Nestin-Cre mice (14 mo), hematocrit and HAMP1 mRNA in the livers were significantly reduced, indicating worsened systemic iron dyshomeostasis. Similarly, hematocrit and HAMP1 mRNA in the livers were significantly reduced with the activation of the EPO-ERFE-HAMP1 pathway (18, 40) in young iron-deficient FPNf/f/Nestin-Cre mice (3 mo), also indicating worsened systemic iron dyshomeostasis. Hence, FPN in the proximal tubules was required for maintaining systemic iron homeostasis particularly in elderly and iron-deficient mice.
FPN KO in the proximal tubules protected from ischemic AKI likely by increasing the cellular stores of iron. A protective role for the preexisting replete cellular stores of iron in ischemic AKI was recently suggested (38). We found that FPN KO by Nestin-Cre increased the cellular stores of iron and FTH1 in the proximal tubules and protected from ischemic AKI. FTH1 in the proximal tubules limited catalytic iron and thus alleviated cisplatin- and rhabdomyolysis-induced AKI (46), indicating that the increased FTH1 in FPN KO/Nestin-Cre kidneys possibly contributed to mitigating ischemic AKI. It was also possible that the reduced serum and liver iron in FPNf/f/Nestin-Cre mice might limit systemic iron transport to the I/R kidneys and thus contribute to protecting from ischemic AKI. In fact, hepcidin treatment alleviated ischemic AKI probably by limiting the efflux of hepatic or splenic iron and by decreasing serum iron (29). In addition, FPN KO in the proximal tubules reduced NOX4 and apoptosis and increased GPx4 in the kidneys, indicating that the antioxidant mechanisms were activated. Hence, the increased iron in the proximal tubules of FPN KO/Nestin-Cre may serve to upregulate FTH1 to limit catalytic iron and also to prime the antioxidant mechanisms, thus it provided the preconditioning-type effects on ischemic AKI, rather than that the increased stores of iron per se were protective.
In sum, FPN in the proximal tubules regulated renal iron recycling and systemic iron homeostasis and was also a deleterious factor in the pathophysiology of ischemic AKI.
GRANTS
A. Qiu is supported by China National Science Foundation 31271551, Funding of Alpha Institute of Natural Medicine in Tongji and AHA Grant 14GRNT18840098. J. Barasch is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants 1U54 DK-104309-01, 2R01 DK-073462, and the RFA-DK-16-026, Kidney Precision Medicine Project.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.Q. conceived and designed research; X.W., X.Z., J.Z., and S.Z. performed experiments; X.W., X.Z., J.Z., Z.W., and W.S. analyzed data; X.W., F.W., W.S., and A.Q. interpreted results of experiments; X.W. and Z.W. prepared figures; A.Q. drafted manuscript; J.B. and A.Q. edited and revised manuscript; X.W., X.Z., J.Z., F.W., W.S., J.B., and A.Q. approved final version of manuscript.
REFERENCES
- 1.Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem 275: 19906–19912, 2000. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- 2.Aragno M, Cutrin JC, Mastrocola R, Perrelli MG, Restivo F, Poli G, Danni O, Boccuzzi G. Oxidative stress and kidney dysfunction due to ischemia/reperfusion in rat: attenuation by dehydroepiandrosterone. Kidney Int 64: 836–843, 2003. doi: 10.1046/j.1523-1755.2003.00152.x. [DOI] [PubMed] [Google Scholar]
- 3.Baliga R, Ueda N, Shah SV. Increase in bleomycin-detectable iron in ischaemia/reperfusion injury to rat kidneys. Biochem J 291: 901–905, 1993. doi: 10.1042/bj2910901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baliga R, Zhang Z, Baliga M, Shah SV. Evidence for cytochrome P-450 as a source of catalytic iron in myoglobinuric acute renal failure. Kidney Int 49: 362–369, 1996. doi: 10.1038/ki.1996.53. [DOI] [PubMed] [Google Scholar]
- 5.Baliga R, Zhang Z, Baliga M, Ueda N, Shah SV. Role of cytochrome P-450 as a source of catalytic iron in cisplatin-induced nephrotoxicity. Kidney Int 54: 1562–1569, 1998. doi: 10.1046/j.1523-1755.1998.00161.x. [DOI] [PubMed] [Google Scholar]
- 6.Barasch J, Hollmen M, Deng R, Hod EA, Rupert PB, Abergel RJ, Allred BE, Xu K, Darrah SF, Tekabe Y, Perlstein A, Wax R, Bruck E, Stauber J, Corbin KA, Buchen C, Slavkovich V, Graziano J, Spitalnik SL, Bao G, Strong RK, Qiu A. Disposal of iron by a mutant form of lipocalin 2. Nat Commun 7: 12973, 2016. doi: 10.1038/ncomms12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belkind-Gerson J, Carreon-Rodriguez A, Benedict LA, Steiger C, Pieretti A, Nagy N, Dietrich J, Goldstein AM. Nestin-expressing cells in the gut give rise to enteric neurons and glial cells. Neurogastroenterol Motil 25: 61–9.e7, 2013. doi: 10.1111/nmo.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolisetty S, Zarjou A, Agarwal A. Heme oxygenase 1 as a therapeutic target in acute kidney injury. Am J Kidney Dis 69: 531–545, 2017. doi: 10.1053/j.ajkd.2016.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 14: 2199–2210, 2003. doi: 10.1097/01.ASN.0000079785.13922.F6. [DOI] [PubMed] [Google Scholar]
- 10.Cantarero Carmona I, Luesma Bartolomé MJ, Lavoie-Gagnon C, Junquera Escribano C. Distribution of nestin protein: immunohistochemical study in enteric plexus of rat duodenum. Microsc Res Tech 74: 148–152, 2011. doi: 10.1002/jemt.20884. [DOI] [PubMed] [Google Scholar]
- 11.Castaneda MP, Swiatecka-Urban A, Mitsnefes MM, Feuerstein D, Kaskel FJ, Tellis V, Devarajan P. Activation of mitochondrial apoptotic pathways in human renal allografts after ischemiareperfusion injury. Transplantation 76: 50–54, 2003. doi: 10.1097/01.TP.0000069835.95442.9F. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Boyle S, Zhao M, Su W, Takahashi K, Davis L, Decaestecker M, Takahashi T, Breyer MD, Hao CM. Differential expression of the intermediate filament protein nestin during renal development and its localization in adult podocytes. J Am Soc Nephrol 17: 1283–1291, 2006. doi: 10.1681/ASN.2005101032. [DOI] [PubMed] [Google Scholar]
- 13.Christensen EI, Carone FA, Rennke HG. Effect of molecular charge on endocytic uptake of ferritin in renal proximal tubule cells. Lab Invest 44: 351–358, 1981. [PubMed] [Google Scholar]
- 13a.Cook JD. Iron. Methods in Hematology, vol. 1 New York, NY: Churchill Livingstone Inc, 1980, p. 15–115. [Google Scholar]
- 14.Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab 1: 191–200, 2005. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Dubois NC, Hofmann D, Kaloulis K, Bishop JM, Trumpp A. Nestin-Cre transgenic mouse line Nes-Cre1 mediates highly efficient Cre/loxP mediated recombination in the nervous system, kidney, and somite-derived tissues. Genesis 44: 355–360, 2006. doi: 10.1002/dvg.20226. [DOI] [PubMed] [Google Scholar]
- 16.Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, Basavarajappa D, Rådmark O, Kobayashi S, Seibt T, Beck H, Neff F, Esposito I, Wanke R, Förster H, Yefremova O, Heinrichmeyer M, Bornkamm GW, Geissler EK, Thomas SB, Stockwell BR, O’Donnell VB, Kagan VE, Schick JA, Conrad M. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol 16: 1180–1191, 2014. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganz T. Cellular iron: ferroportin is the only way out. Cell Metab 1: 155–157, 2005. doi: 10.1016/j.cmet.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet 46: 678–684, 2014. doi: 10.1038/ng.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keel SB, Doty RT, Yang Z, Quigley JG, Chen J, Knoblaugh S, Kingsley PD, De Domenico I, Vaughn MB, Kaplan J, Palis J, Abkowitz JL. A heme export protein is required for red blood cell differentiation and iron homeostasis. Science 319: 825–828, 2008. doi: 10.1126/science.1151133. [DOI] [PubMed] [Google Scholar]
- 20.Kovtunovych G, Eckhaus MA, Ghosh MC, Ollivierre-Wilson H, Rouault TA. Dysfunction of the heme recycling system in heme oxygenase 1-deficient mice: effects on macrophage viability and tissue iron distribution. Blood 116: 6054–6062, 2010. doi: 10.1182/blood-2010-03-272138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kozyraki R, Fyfe J, Verroust PJ, Jacobsen C, Dautry-Varsat A, Gburek J, Willnow TE, Christensen EI, Moestrup SK. Megalin-dependent cubilin-mediated endocytosis is a major pathway for the apical uptake of transferrin in polarized epithelia. Proc Natl Acad Sci USA 98: 12491–12496, 2001. doi: 10.1073/pnas.211291398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linkermann A, Bräsen JH, Darding M, Jin MK, Sanz AB, Heller JO, De Zen F, Weinlich R, Ortiz A, Walczak H, Weinberg JM, Green DR, Kunzendorf U, Krautwald S. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci USA 110: 12024–12029, 2013. doi: 10.1073/pnas.1305538110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linkermann A, Skouta R, Himmerkus N, Mulay SR, Dewitz C, De Zen F, Prokai A, Zuchtriegel G, Krombach F, Welz PS, Weinlich R, Vanden Berghe T, Vandenabeele P, Pasparakis M, Bleich M, Weinberg JM, Reichel CA, Bräsen JH, Kunzendorf U, Anders HJ, Stockwell BR, Green DR, Krautwald S. Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci USA 111: 16836–16841, 2014. doi: 10.1073/pnas.1415518111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martines AM, Masereeuw R, Tjalsma H, Hoenderop JG, Wetzels JF, Swinkels DW. Iron metabolism in the pathogenesis of iron-induced kidney injury. Nat Rev Nephrol 9: 385–398, 2013. doi: 10.1038/nrneph.2013.98. [DOI] [PubMed] [Google Scholar]
- 25.McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, Miret S, Bomford A, Peters TJ, Farzaneh F, Hediger MA, Hentze MW, Simpson RJ. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell 5: 299–309, 2000. doi: 10.1016/S1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- 26.Moulouel B, Houamel D, Delaby C, Tchernitchko D, Vaulont S, Letteron P, Thibaudeau O, Puy H, Gouya L, Beaumont C, Karim Z. Hepcidin regulates intrarenal iron handling at the distal nephron. Kidney Int 84: 756–766, 2013. doi: 10.1038/ki.2013.142. [DOI] [PubMed] [Google Scholar]
- 27.Paragas N, Qiu A, Zhang Q, Samstein B, Deng SX, Schmidt-Ott KM, Viltard M, Yu W, Forster CS, Gong G, Liu Y, Kulkarni R, Mori K, Kalandadze A, Ratner AJ, Devarajan P, Landry DW, D’Agati V, Lin CS, Barasch J. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat Med 17: 216–222, 2011. doi: 10.1038/nm.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol 24: R453–R462, 2014. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scindia Y, Dey P, Thirunagari A, Liping H, Rosin DL, Floris M, Okusa MD, Swaminathan S. Hepcidin mitigates renal ischemia-reperfusion injury by modulating systemic iron homeostasis. J Am Soc Nephrol 26: 2800–2814, 2015. doi: 10.1681/ASN.2014101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sclafani AM, Skidmore JM, Ramaprakash H, Trumpp A, Gage PJ, Martin DM. Nestin-Cre mediated deletion of Pitx2 in the mouse. Genesis 44: 336–344, 2006. doi: 10.1002/dvg.20220. [DOI] [PubMed] [Google Scholar]
- 31.Sedeek M, Nasrallah R, Touyz RM, Hébert RL. NADPH oxidases, reactive oxygen species, and the kidney: friend and foe. J Am Soc Nephrol 24: 1512–1518, 2013. doi: 10.1681/ASN.2012111112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheerin NS, Sacks SH, Fogazzi GB. In vitro erythrophagocytosis by renal tubular cells and tubular toxicity by haemoglobin and iron. Nephrol Dial Transplant 14: 1391–1397, 1999. doi: 10.1093/ndt/14.6.1391. [DOI] [PubMed] [Google Scholar]
- 33.Smith CP, Thévenod F. Iron transport and the kidney. Biochim Biophys Acta 1790: 724–730, 2009. doi: 10.1016/j.bbagen.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Sponsel HT, Alfrey AC, Hammond WS, Durr JA, Ray C, Anderson RJ. Effect of iron on renal tubular epithelial cells. Kidney Int 50: 436–444, 1996. doi: 10.1038/ki.1996.334. [DOI] [PubMed] [Google Scholar]
- 35.Starzyński RR, Canonne-Hergaux F, Lenartowicz M, Krzeptowski W, Willemetz A, Styś A, Bierła J, Pietrzak P, Dziaman T, Lipiński P. Ferroportin expression in haem oxygenase 1-deficient mice. Biochem J 449: 69–78, 2013. doi: 10.1042/BJ20121139. [DOI] [PubMed] [Google Scholar]
- 37.Vanderwinden JM, Gillard K, De Laet MH, Messam CA, Schiffmann SN. Distribution of the intermediate filament nestin in the muscularis propria of the human gastrointestinal tract. Cell Tissue Res 309: 261–268, 2002. doi: 10.1007/s00441-002-0590-3. [DOI] [PubMed] [Google Scholar]
- 38.Vaugier C, Amano MT, Chemouny JM, Dussiot M, Berrou C, Matignon M, Ben Mkaddem S, Wang PHM, Fricot A, Maciel TT, Grapton D, Mathieu JRR, Beaumont C, Peraldi MN, Peyssonnaux C, Mesnard L, Daugas E, Vrtovsnik F, Monteiro RC, Hermine O, Ginzburg YZ, Benhamou M, Camara NOS, Flamant M, Moura IC. Serum iron protects from renal postischemic injury. J Am Soc Nephrol 28: 3605–3615, 2017. doi: 10.1681/ASN.2016080926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veuthey T, D’Anna MC, Roque ME. Role of the kidney in iron homeostasis: renal expression of Prohepcidin, Ferroportin, and DMT1 in anemic mice. Am J Physiol Renal Physiol 295: F1213–F1221, 2008. doi: 10.1152/ajprenal.90216.2008. [DOI] [PubMed] [Google Scholar]
- 40.Wang CY, Core AB, Canali S, Zumbrennen-Bullough KB, Ozer S, Umans L, Zwijsen A, Babitt JL. Smad1/5 is required for erythropoietin-mediated suppression of hepcidin in mice. Blood 130: 73–83, 2017. doi: 10.1182/blood-2016-12-759423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wertz K, Herrmann BG. Kidney-specific cadherin (cdh16) is expressed in embryonic kidney, lung, and sex ducts. Mech Dev 84: 185–188, 1999. doi: 10.1016/S0925-4773(99)00074-X. [DOI] [PubMed] [Google Scholar]
- 42.Willnow TE, Goldstein JL, Orth K, Brown MS, Herz J. Low density lipoprotein receptor-related protein and gp330 bind similar ligands, including plasminogen activator-inhibitor complexes and lactoferrin, an inhibitor of chylomicron remnant clearance. J Biol Chem 267: 26172–26180, 1992. [PubMed] [Google Scholar]
- 43.Wolff NA, Liu W, Fenton RA, Lee WK, Thévenod F, Smith CP. Ferroportin 1 is expressed basolaterally in rat kidney proximal tubule cells and iron excess increases its membrane trafficking. J Cell Mol Med 15: 209–219, 2011. doi: 10.1111/j.1582-4934.2009.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu Y, Ma H, Shao J, Wu J, Zhou L, Zhang Z, Wang Y, Huang Z, Ren J, Liu S, Chen X, Han J. A role for tubular necroptosis in cisplatin-induced AKI. J Am Soc Nephrol 26: 2647–2658, 2015. doi: 10.1681/ASN.2014080741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamada K, Miwa T, Liu J, Nangaku M, Song WC. Critical protection from renal ischemia reperfusion injury by CD55 and CD59. J Immunol 172: 3869–3875, 2004. doi: 10.4049/jimmunol.172.6.3869. [DOI] [PubMed] [Google Scholar]
- 46.Zarjou A, Bolisetty S, Joseph R, Traylor A, Apostolov EO, Arosio P, Balla J, Verlander J, Darshan D, Kuhn LC, Agarwal A. Proximal tubule H-ferritin mediates iron trafficking in acute kidney injury. J Clin Invest 123: 4423–4434, 2013. doi: 10.1172/JCI67867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Z, Zhang F, An P, Guo X, Shen Y, Tao Y, Wu Q, Zhang Y, Yu Y, Ning B, Nie G, Knutson MD, Anderson GJ, Wang F. Ferroportin1 deficiency in mouse macrophages impairs iron homeostasis and inflammatory responses. Blood 118: 1912–1922, 2011. doi: 10.1182/blood-2011-01-330324. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Z, Zhang F, Guo X, An P, Tao Y, Wang F. Ferroportin1 in hepatocytes and macrophages is required for the efficient mobilization of body iron stores in mice. Hepatology 56: 961–971, 2012. doi: 10.1002/hep.25746. [DOI] [PubMed] [Google Scholar]