Abstract
The objective of this study was to examine theoretically how Ca2+ reabsorption in the proximal tubule (PT) is modulated by Na+ and water fluxes, parathyroid hormone (PTH), Na+-glucose cotransporter (SGLT2) inhibitors, and acetazolamide. We expanded a previously published mathematical model of water and solute transport in the rat PT (Layton AT, Vallon V, Edwards A. Am J Physiol Renal Physiol 308: F1343–F1357, 2015) that did not include Ca2+. Our results indicate that Ca2+ reabsorption in the PT is primarily driven by the transepithelial Ca2+ concentration gradient that stems from water reabsorption, which is itself coupled to Na+ reabsorption. Simulated variations in permeability or transporter activity elicit opposite changes in paracellular and transcellular Ca2+ fluxes, whereas a simulated decrease in filtration rate lowers both fluxes. The model predicts that PTH-mediated inhibition of the apical Na+/H+ exchanger NHE3 reduces Na+ and Ca2+ transport to a similar extent. It also suggests that acetazolamide- and SGLT2 inhibitor-induced calciuria at least partly stems from reduced Ca2+ reabsorption in the PT. In addition, backleak of phosphate (PO4) across tight junctions is predicted to reduce net PO4 reabsorption by ~20% under normal conditions. When transcellular PO4 transport is substantially reduced by PTH, paracellular PO4 flux is reversed and contributes significantly to PO4 reabsorption. Furthermore, PTH is predicted to exert an indirect impact on PO4 reabsorption via its inhibitory action on NHE3. This model thus provides greater insight into the mechanisms that modulate Ca2+ and PO4 reabsorption in the PT.
Keywords: calcium, kidney, mathematical model, parathyroid hormone, phosphate
INTRODUCTION
Sixty to 70% of the filtered load of Ca2+ is reabsorbed in the proximal tubule (PT), mostly across the paracellular route, via passive diffusion and convection (i.e., solvent drag). There is some evidence (reviewed in Ref. 18) that a small fraction (10–20%) of Ca2+ is reabsorbed across the transcellular route, but the underlying molecular transporters remain to be elucidated. The respective contribution of diffusion and convection to Ca2+ fluxes in the PT and the extent to which the transport of Ca2+ is coupled to that of Na+ and water have yet to be fully understood (27, 39). The objective of the present study was to provide a quantitative description of the forces that drive Ca2+ reabsorption in the PT and to examine how Ca2+ transport in this segment is modulated by filtration rates, Na+, parathyroid hormone (PTH), acetazolamide, and inhibitors of Na+-glucose transporters (SGLTs). We expanded a model of water and solute transport in the PT that we published previously (26) but that did not include Ca2+.
We also modified the model’s handling of anionic inorganic phosphate (PO4). In plasma, PO4 is mostly present as and in a 4:1 ratio. About 80% of the filtered load of PO4 is reabsorbed by the PT (27, 49). PO4 entry into the cell is mediated by three types of Na+-phosphate cotransporters: NaPi-IIa (SLC34A1), NaPi-IIc (SLC34A3), and PiT-2 (SLC20A2) (10). NaPi-IIa mediates cotransport of 3 Na+:1 , NaPi-IIc mediates cotransport of 2 Na+:1 , and PiT-2 mediates cotransport of 2 Na+:1 . Whereas the previous version of the PT model only considered a generic apical 1 Na+:1 cotransporter, in the present study we accounted for these specific Na+-PO4 cotransporters. We also compared the direct and indirect ways in which PTH affects PO4 transport in the PT.
The PT model incorporates flow-dependent transport, i.e., the observation that high flow velocity augments transepithelial fluxes by increasing transporter membrane abundance (34). Du et al. (14) demonstrated that Na+ and reabsorption varies proportionally to the microvillous torque. Following the approach of Weinstein and colleagues (62), the abundance of transporters in apical and basolateral membranes is taken to be proportional to the torque. Flow-dependent transport plays an important role in maintaining perfusion-absorption balance. It may also act to prevent large excursions in the transepithelial fluxes of water and Na+ at a given perfusion rate.
MODEL DESCRIPTION
Conservation equations.
The mathematical model of transport along the PT of a male rat is based on conservation equations, which are solved at steady state. The PT consists of two cortical (S1–S2) segments (with a combined length taken as 0.97 cm) and a medullary (S3) segment (0.13 cm). We assume that all segments of the PT express the same types of channels, pumps, and cotransporters, with the exception of glucose transporters (26). Membrane surface areas are reduced by a factor of 2 in the S3 segment to account for decreased membrane infolding. As described above, luminal and peritubular transporter density increases linearly with the relative microvillous torque (62); the proportionality constant is set to 1.8 in the S1–S2 segment and to 0.9 in the S3 segment in the present study, so that the predicted reabsorption of Na+ and K+ equals approximately two-thirds of the filtered load.
As shown in Fig. 1, the model represents four compartments: the lumen (L), the cell cytosol (C), the lateral intercellular space (I), and the peritubular fluid (P). Conservation of Ca2+ in the lumen, cells, and intercellular space is written as follows:
| (1) |
| (2) |
FV represents the luminal flow rate, SNM is the surface area per unit length at the interface between compartments N and M, and JNM is the flux across that interface. Note that at steady state the net flux of Ca2+ from intracellular stores to the cytosol and the net rate of Ca2+ binding to Ca2+ buffers in the cytosol and the sarcoplasmic reticulum are zero (for their dynamic expressions see Ref. 12).
Fig. 1.
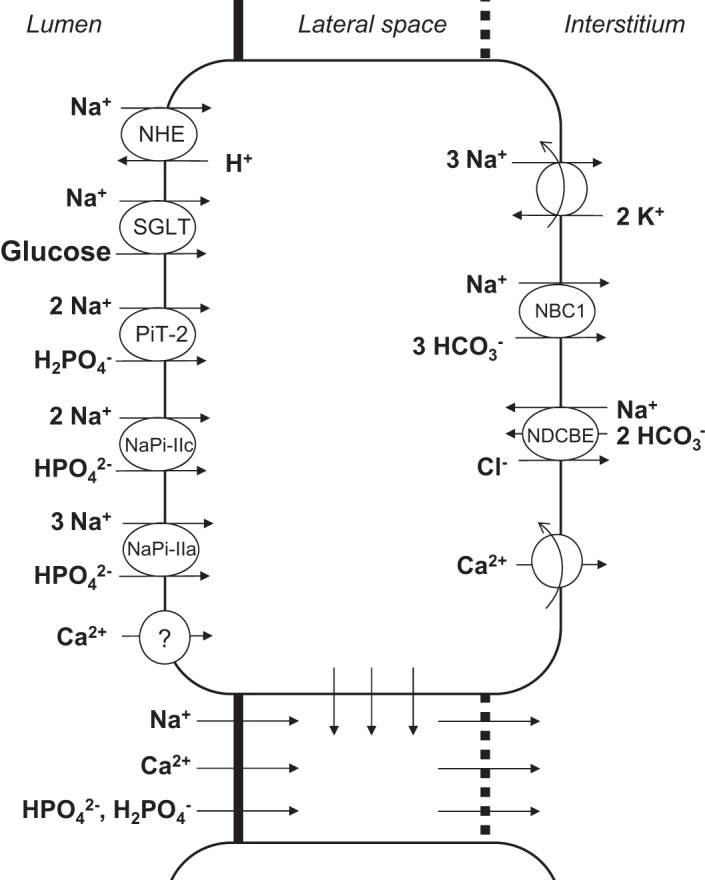
Model representation of a rat proximal tubule cell and the adjacent paracellular pathway. The model describes transport of water and 16 solutes. Only the main Na+ and Ca2+ transporters are shown. Ca2+ is assumed to enter the cell across an apical Ca2+ channel and exit via a plasma membrane Ca2+ pump. NHE, Na+/H+ exchanger; SGLT, Na+-glucose transporter; PiT-2, NaPi-IIc, and NaPi-IIa, Na+-PO4 transporters; NBC, Na+- cotransporter; NDCBE, Na+-dependent Cl−/ exchanger.
Ca2+ fluxes.
The Ca2+ flux from the lumen to compartment M (M = C, I) is computed as follows:
| (3a) |
| (3b) |
| (3c) |
The first term in Eq. 3a corresponds to the convective component of the flux: JV is the volume flux (superscripts are omitted for simplicity), is a (logarithmic) mean concentration, and σCa is the reflection coefficient of the membrane to Ca2+; σCa is set to 1 for cell membranes, 0.89 for the tight junction (TJ) (41), and 0 for the basement membrane (BM; at the interface between the lateral interspace and the peritubular fluid). The second term corresponds to the electrodiffusive component: PCa is the membrane permeability to Ca2+, and ζCa is a normalized electric potential difference, a function of the valence zCa of Ca2+ and the electric potential Ψ. R and F are the ideal gas and Faraday constants, respectively.
The permeability of the TJ to Ca2+ is taken as 20 × 10−5 cm/s based on the compilation in Ref. 18. The permeability of the BM to Ca2+ is computed based on the permeability to Na+, given a Ca2+-to-Na+ free diffusivity ratio of 7.93:13.3 (30). The permeability of the apical membrane to Ca2+ is set to 0.005 × 10−5 cm/s so as to yield a transcellular flux amounting to 15% of the overall flux.
The molecular mechanisms by which Ca2+ is extruded from PT cells remain to be identified. Based on recent transcriptomic data (28), we neglect the potential contribution of Na+/Ca2+ exchangers and assume that Ca2+ is pumped out of the cell by plasma membrane Ca2+-ATPase (PMCA) at rate given by
| (4) |
The affinity of the pump to Ca2+ () is taken as 75.6 nM (57) and the maximum flux () as 0.5 × 10−9 mmol·cm−2·s−1. Parameter values are summarized in Table 1.
Table 1.
Ca2+ and PO4 parameter values
| Parameter | Value | Reference |
|---|---|---|
| Permeability to Ca2+, cm/s | ||
| Tight junction | 20 × 10−5 | 18 |
| Basement membrane | 60 × 10−5 | Based on 30 |
| Apical cell membrane | 0.005 × 10−5 | Adjusted |
| PMCA maximum flux, mmol·cm−2·s−1 | 0.5 × 10−9 | Adjusted |
| PMCA affinity to Ca2+, nM | 75.6 | 57 |
| Reflection coefficient of tight junction to Ca2+ | 0.89 | 41 |
| Transporter density, mmol2·J−1·s−1·cm−2 | ||
| NaPi-IIa | 0.30 × 10−9 | Adjusted |
| NaPi-IIc | 0.25 × 10−9 (0 in S3) | Adjusted |
| PiT-2 | 0.10 × 10−9 (0 in S3) | Adjusted |
PMCA, plasma membrane Ca2+-ATPase; S3, medullary segment of the proximal tubule.
Interstitial concentration gradient.
Interstitial fluid concentrations are prescribed in this model: they are equal to plasma concentrations in the cortex and increase linearly along the corticomedullary axis in the medulla. Based on our macroscopic model of Ca2+ transport in different populations of nephrons, vasa recta, and the interstitium (56), which did not represent processes at the cell/molecular level, we assume that the interstitial Ca2+ concentration ([Ca2+]) increases linearly from 1.25 mM at the corticomedullary junction to 1.60 mM at the end of the S3 segment and to 2.50 mM at the junction between the outer medulla (OM) and inner medulla (IM). The single-nephron glomerular filtration rate (SNGFR) is taken as 30 nl/min, and the filtered load of Ca2+ is 37.5 pmol/min per nephron.
PO4 transport.
In previously published models of the PT (26, 62), the apical PO4 transporter was represented as a 1 Na+:1 cotransporter. It is now known that PO4 entry into PT cells is mediated by the cotransporters NaPi-IIa, NaPi-IIc, and PiT-2 (11, 27); their respective stoichiometries are as follows: 3 Na+:1 , 2 Na+:1 , and 2 Na+:1 . Hence, NaPi-IIa and PiT-2 are electrogenic, whereas NaPi-IIc is electroneutral. Fluxes across these cotransporters are computed using the nonequilibrium thermodynamic approach (60). Based on the immunochemistry findings of Picard et al. (42), we assume that NaPi-IIa is expressed along the full PT and that NaPi-IIc and PiT-2 are only present in the convoluted PT. The transport coefficient (a measure of density) of NaPi-IIa is set to 0.30 × 10−9 mmol2·J−1·s−1·cm−2 everywhere, and those of NaPi-IIc and PiT-2 are taken as 0.25 × 10−9 and 0.10 × 10−9 mmol2·J−1·s−1·cm−2, respectively, in the S1–S2 segment and zero in the S3 segment. No other PO4 cellular entry pathways are considered. The interstitial concentration of PO4 is taken to increase from 2.6 mM at the corticomedullary junction to 3.9 mM at the OM-IM junction (58), and the filtered load of PO4 is 78.0 pmol/min per nephron.
RESULTS
Forces driving Ca2+ reabsorption.
Under baseline conditions, the model predicts that the PT reabsorbs 68–70% of the filtered load of Na+, K+, and Cl−. Fractional Ca2+ reabsorption is 69.3%, and the tubular fluid-to-glomerular filtrate [Ca2+] ratio [(TF/GF)Ca] increases from 1.0 to 1.3, in accordance with reported measurements (reviewed in Ref. 18). As depicted in Fig. 2, the molar flow rate of Ca2+ in the lumen decreases in parallel with volume flow. As the rate of water reabsorption increases significantly in the medullary (S3) segment, owing to the interstitial osmolality gradient, so do [Ca2+] in the lumen and (TF/GF)Ca (Fig. 2).
Fig. 2.
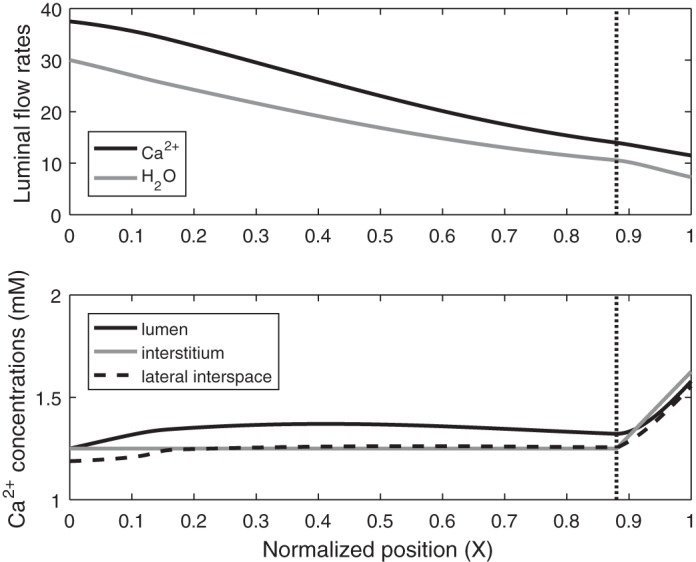
Base-case Ca2+ flows and concentrations along the proximal tubule. Top: molar flow rate of Ca2+ (in pmol/min per nephron) and H2O (in nl/min per nephron) in the lumen as a function of normalized position X (i.e., position divided by proximal tubule length). Bottom: Ca2+ concentration in lumen, interstitium, and lateral interspace as a function of X. Vertical line denotes the boundary between the cortex and the medulla, which marks the transition between the S2 and S3 segments.
Results are given on a per-nephron basis: JCa denotes the local or average Ca2+ flux (in pmol·min−1·mm−1) and TCa denotes the rate of Ca2+ reabsorption along the entire PT (in pmol/min). The base-case TCa is computed as 26.0 pmol/min, 85% of which is paracellular. Equivalently, the average JCa equals 2.36 pmol·min−1·mm−1 (Table 2). Paracellular transport of Ca2+ across the TJ, which separates the lumen from the lateral interspace, is followed by Ca2+ transport across the BM, which separates the lateral interspace from the interstitium (Fig. 1). Across the TJ, the Ca2+ flux is predominantly governed by electrodiffusion, rather than by convection (i.e., solvent drag). As water reabsorption raises [Ca2+] in the lumen above that in the lateral interspace and the interstitium (Fig. 2), the [Ca2+] gradient across the TJ drives Ca2+ reabsorption (Fig. 3). Since the reflection coefficient of the TJ to Ca2+ is 0.89 (41), i.e., close to 1, solvent drag across this barrier is limited (see Eq. 3a).
Table 2.
Predicted Ca2+ fluxes and fractional reabsorption
|
JCa, pmol·min−1·mm−1 per nephron |
||||
|---|---|---|---|---|
| Total | Paracellular | Transcellular | %Reabsorption | |
| Base case | 2.36 | 2.02 (1.77 + 0.25) | 0.34 | 69.3 |
| 5-fold decrease in | 2.35 | 2.28 (2.03 + 0.25) | 0.07 | 69.0 |
| 5-fold increase in | 2.41 | 0.86 (0.62 + 0.24) | 1.55 | 70.9 |
| 2-fold decrease in | 2.20 | 1.84 (1.58 + 0.26) | 0.36 | 64.8 |
| 10-fold decrease in | 1.40 | 0.99 (0.70 + 0.29) | 0.41 | 41.2 |
| 2-fold increase in | 2.42 | 2.10 (1.86 + 0.24) | 0.32 | 71.0 |
| 10-fold increase in | 2.46 | 2.16 (1.92 + 0.24) | 0.30 | 72.1 |
| 10-fold decrease in | 2.44 | 2.11 (1.87 + 0.24) | 0.33 | 71.7 |
| 10-fold increase in | 2.33 | 1.99 (1.74 + 0.25) | 0.34 | 68.5 |
| = 0 | 2.54 | 2.24 (0.09 + 2.15) | 0.30 | 74.6 |
| = 1 | 2.33 | 1.99 (1.99 + 0) | 0.34 | 68.6 |
| Interstitial [Ca2+] at the OM-IM junction = 3.75 mM | 2.27 | 1.93 (1.67 + 0.26) | 0.34 | 66.5 |
| Interstitial [Ca2+] at the OM-IM junction = 2.0 mM | 2.40 | 2.06 (1.81 + 0.25) | 0.34 | 70.4 |
| 40% increase in NHE3 and NBC1 expression and 10% increase in Na+-K+-ATPase expression | 2.48 | 2.21 (1.94 + 0.27) | 0.27 | 72.8 |
| CA inhibition in the PT lumen | 1.82 | 1.34 (1.17 + 0.17) | 0.48 | 53.3 |
| 90% inhibition of NBC1 | 1.79 | 1.21 (1.03 + 0.18) | 0.58 | 52.6 |
| 100% inhibition of SGLT2 | 2.07 | 1.66 (1.45 + 0.21) | 0.41 | 60.8 |
| 100% inhibition of SGLT2 + 15% decrease in SNGFR | 1.64 | 1.34 (1.17 + 0.17) | 0.30 | 56.6 |
Values in parentheses show decomposition of Ca2+ flux (JCa) into its diffusive and convective components, respectively. , , and , permeability of apical cell membrane, tight junction, and basement membrane to Ca2+; , reflection coefficient of the tight junction to Ca2+; [Ca2+], Ca2+ concentration; OM-IM, outer medulla-inner medulla; CA, carbonic anhydrase; NBC1, basolateral Na+- cotransporter; SGLT2, Na+-glucose cotransporter type 2; SNGFR, single-nephron glomerular filtration rate.
Fig. 3.
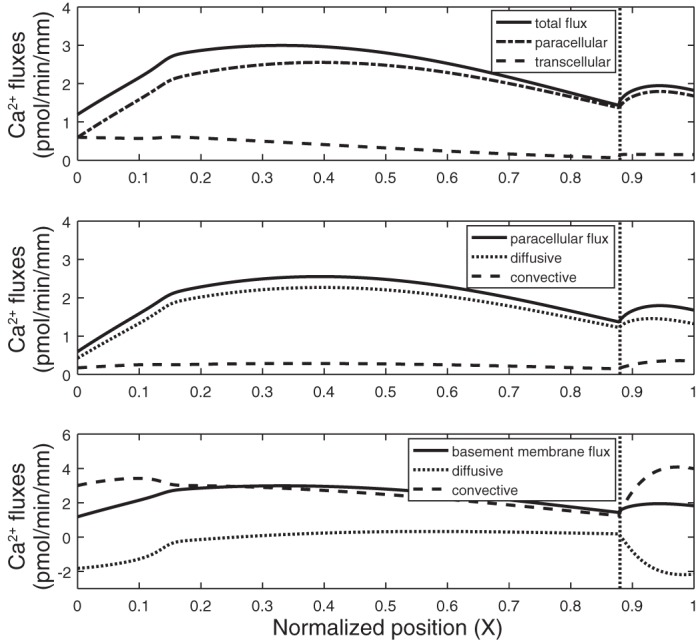
Ca2+ fluxes along the proximal tubule in the base case. Top: total transepithelial Ca2+ flux is the sum of Ca2+ fluxes across the tight junction (paracellular) and the apical cell membrane (transcellular). Middle: paracellular Ca2+ flux is the sum of its electrodiffusive and convective components. Bottom: Ca2+ flux across the basement membrane is mostly driven by convection. At steady state, it is the sum of the tight junction flux and flux from the lateral cell membrane into the lateral interspace. Length-averaged values are given in Table 1. Vertical line denotes the boundary between the cortex and the medulla.
At steady state, the flux of Ca2+ across the BM is the sum of the Ca2+ flux across the TJ and the Ca2+ flux from the lateral membrane of cells into the lateral interspace. As shown in Fig. 3, Ca2+ transport across the BM is primarily driven by convection. Solvent drag is significantly enhanced, relative to the TJ, because the reflection coefficient of the BM to Ca2+ (and all other electrolytes) is zero; moreover, there is significant water reabsorption from the cell into the lateral interspace, so that the water flux across the BM is larger than that across the TJ. The rapid convective transport of Ca2+ into the interstitium lowers [Ca2+] in the lateral interspace below that in the interstitium along parts of the tubule (Fig. 2), thereby eliciting backdiffusion of Ca2+ across the BM (Fig. 3).
Note that electrodiffusion is governed by both the transmembrane [Ca2+] gradient and the transmembrane voltage (ΔΨ). The electric potential in the lumen is predicted to rise from −0.22 mV at the PT inlet to a maximum of 1.31 mV about halfway through the tubule and to decrease to +0.94 mV at the outlet. The electric potential in the lateral interspace remains between −0.06 and +0.01 mV in the cortex and the medulla. As ΔΨ across the TJ changes sign and becomes lumen-positive, its contribution to the electrodiffusive flux increases. Nevertheless, our computations suggest that, overall, ΔΨ exerts a lower driving force than the lumen-to-lateral interspace [Ca2+] gradient.
In the renal medulla, the axial interstitial osmolarity gradient accelerates the rate of water removal from the PT lumen, thereby augmenting convective Ca2+ fluxes in the S3 segment (Fig. 3). The large increase in solvent drag across the BM is partly counteracted by enhanced Ca2+ diffusion in the opposite direction, as the lateral interspace [Ca2+] increasingly lags behind the interstitial [Ca2+] (Fig. 2).
Impact of transcellular Ca2+ permeability.
The contribution of transcellular Ca2+ transport in the PT and its underlying mechanisms are not understood. We chose the baseline value of the apical membrane Ca2+ permeability () so that transcellular TCa represents 15% of total TCa. The impact of varying is illustrated in Fig. 4. The maximum PMCA flux was varied by the same factor as to maintain intracellular [Ca2+] at ∼100 nM. The model predicts that increasing and, thus, the transcellular JCa conversely reduces paracellular JCa, because it accelerates Ca2+ removal from the lumen, thereby diminishing the lumen-to-interstitium [Ca2+] gradient. In fact, the paracellular JCa decreases to the extent that even though the net JCa is higher than in the base case in the early part of the PT, it is lower in the late PT. Conversely, decreasing the transcellular JCa augments the paracellular JCa, as the lumen-to-interstitium [Ca2+] gradient becomes larger. Consequently, overall Ca2+ reabsorption is not much affected by variations in (Table 2).
Fig. 4.
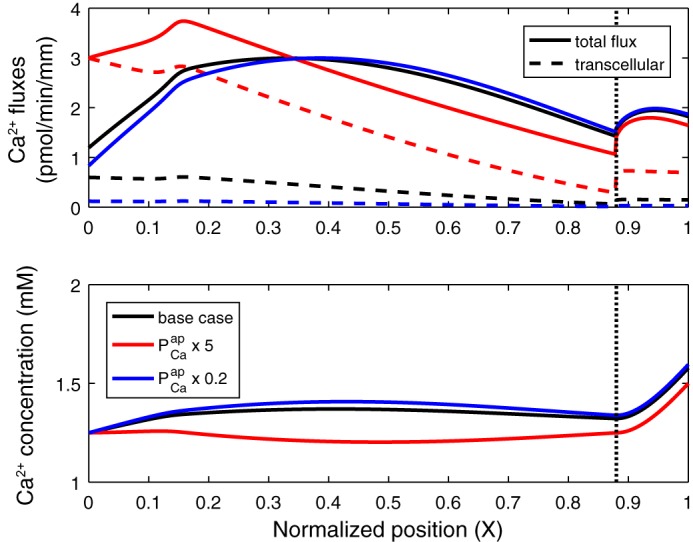
Impact of permeability of the apical membrane to Ca2+ () on Ca2+ fluxes and concentrations along the proximal tubule. Top: total and transcellular Ca2+ fluxes. Paracellular flux is the difference between the 2 curves. Bottom: luminal Ca2+ concentration. is set to its baseline value (base case), increased by a factor of 5 (× 5), or decreased by a factor of 5 (× 0.2). A 10-fold increase in is predicted to result in Ca2+ secretion via the paracellular route (not shown). Vertical line denotes the boundary between the cortex and the medulla.
Impact of solvent drag.
We examined the effects of solvent drag on JCa by varying the reflection coefficient of the TJ to Ca2+ () from 0 to 1. Decreasing from its baseline value of 0.89 to 0, that is, enhancing the convective transport of Ca2+ across the TJ (see Eq. 3a), is predicted to substantially increase the paracellular JCa in the first half of the PT, relative to the base case, and to lower it in the second half due to the resulting decrease in the lumen-to-interstitium [Ca2+] gradient (Fig. 5). Because of these counterbalancing effects, overall Ca2+ reabsorption is only 7.6% higher (28.0 pmol·min−1·mm−1) than in the base case. Conversely, increasing from 0.89 to 1.0 slightly reduces (by 1.1%) overall Ca2+ reabsorption.
Fig. 5.
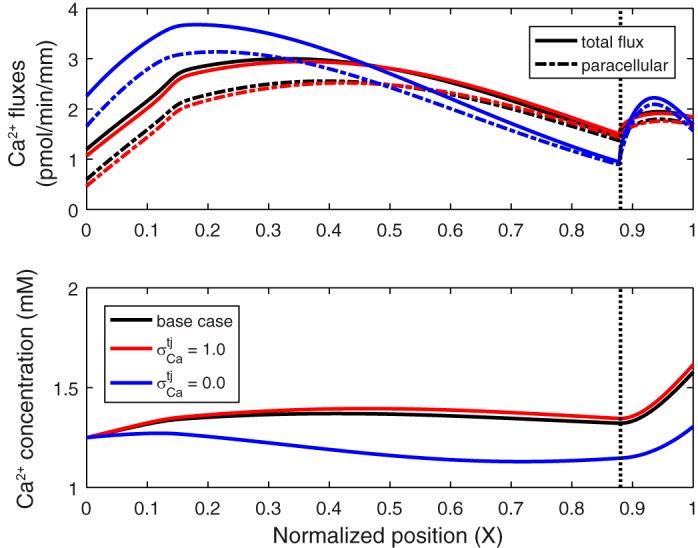
Ca2+ fluxes and concentrations along the proximal tubule for different values of the reflection coefficient of the tight junction to Ca2+ (): 0.89 (base case) 1.0, and 0.0. Top: total and paracellular Ca2+ fluxes. Bottom: luminal Ca2+ concentration. Vertical line denotes the boundary between the cortex and the medulla.
Impact of BM permeability.
Permeability of the BM to Ca2+ () has not been measured. As shown in Table 2, the model predicts that a 10-fold decrease in raises Ca2+ reabsorption by 3.4%, because it reduces the backdiffusion of Ca2+ across the BM. A 10-fold increase in has opposite, albeit smaller, effects: it lowers Ca2+ reabsorption by 1.2%.
Impact of axial interstitial gradient.
Another uncertainty relates to the interstitial axial [Ca2+] gradient in the medulla. In the base case, interstitial [Ca2+] is taken to double between the cortex and the OM-IM junction. If we assume, instead, a threefold increase, the lag between luminal [Ca2+] and interstitial [Ca2+] in the medulla increases (results not shown). Hence, paracellular Ca2+ reabsorption is reduced along the S3 segment, from 2.51 pmol/min in the base case to 1.46 pmol/min, and overall Ca2+ reabsorption decreases from 26.0 to 24.9 pmol/min. Conversely, a smaller medullary [Ca2+] gradient is predicted to enhance the diffusion of Ca2+ across the paracellular pathway in the S3 segment and augment its overall reabsorption (Table 2).
Effects of glomerular filtration rate variations.
To examine the coupling between the transport of Ca2+ and that of water and/or Na+, we first simulated a 20% increase in the SNGFR. As described above, the model accounts for flow-dependent transport (14, 34, 62). A high flow rate in the PT lumen acts via the microvillous torque to recruit more transporters to the cell membrane, thereby enhancing transcellular fluxes and maintaining perfusion-absorption balance. The model thus predicts that a 20% increase in the filtered load of fluid and solutes results in substantially higher absolute reabsorption rates, but the fractional reabsorption of water, Na+, and Ca2+ increases only by 3–4% (from 69.3% to 73.1% for Ca2+). More specifically, as the filtered load of Ca2+ is raised from 37.5 to 45.0 pmol/min per nephron, TCa increases from 26.0 to 32.9 pmol/min, and the length-averaged JCa increases from 2.36 to 2.99 pmol·min−1·mm−1. Ca2+ transport is elevated across both pathways: transcellular JCa increases owing to enhanced membrane abundance of Ca2+ transporters, and paracellular JCa increases because the higher rate of water reabsorption augments the lumen-to-interstitium [Ca2+] gradient.
Conversely a 20% decrease in the SNGFR reduces the microvillous torque in the PT lumen, subsequently lowering transporter abundance at the membrane and absolute reabsorption rates. The fractional reabsorption of water, Na+, and Ca2+ is predicted to then decrease by 5–7% (from 69.3% to 62.7% for Ca2+). With a Ca2+ filtered load of 30.0 pmol/min per nephron, TCa equals 18.8 pmol/min, and the length-averaged JCa equals 1.71 pmol·min−1·mm−1 (Table 3). Ca2+ transport is reduced both across cells, due to fewer Ca2+ transporters at the membrane, and between cells, due to the lower lumen-to-interstitium [Ca2+] gradient.
Table 3.
Predicted impact of parathyroid hormone on Ca2+ transport
|
JCa, pmol·min−1·mm−1 per nephron |
|||||
|---|---|---|---|---|---|
| Filtered Load, pmol/min per nephron | %Reabsorption | Net | Paracellular | Transcellular | |
| Base case | 37.5 | 69.3 | 2.36 | 2.02 | 0.34 |
| Reduced expression of Na+ transporters* | 37.5 | 49.2 | 1.68 | 1.13 | 0.55 |
| 20% decrease in SNGFR | 30.0 | 62.7 | 1.71 | 1.50 | 0.21 |
| 20% decrease in SNGFR and reduced expression of Na+ transporters* | 30.0 | 47.0 | 1.28 | 0.94 | 0.34 |
JCa, Ca2+ flux; SNGFR, single-nephron glomerular filtration rate.
Basal expression of Na+/H+ exchanger type 3, Na+-PO4 cotransporters (NaPi-IIa, NaPi-IIc, and PiT-2), and Na+-K+-ATPase is lowered by 75%, 75%, and 25%, respectively.
Apical Na+/H+ exchanger type 3-mediated PTH effects.
PTH acts to augment Ca2+ reabsorption in the thick ascending limb and below, but in the PT it has been found to reduce TCa, at least in dogs (2, 51). This may be explained by the coupling between Na+ and Ca2+ transport in the PT. PTH is known to inhibit apical Na+/H+ exchanger type 3 (NHE3), Na+-PO4 cotransporters, and the basolateral Na+-K+-ATPase pump (27); the resulting decrease in Na+ and water reabsorption in the PT likely reduces Ca2+ transport as well. To test this hypothesis, we examined the effects of a 30% decrease in PT Na+ reabsorption (as observed in Ref. 2) on TCa at constant SNGFR. Reducing Na+ entry into the PT cell lowers intracellular Na+ concentration ([Na+]), thereby augmenting basolateral Na+ secretion via the Na+-dependent Cl−/ exchanger NDCBE; a decrease in Na+-K+-ATPase activity partly counterbalances these effects. Moreover, as transcellular Na+ transport decreases, less water is reabsorbed, so that luminal flow decreases less rapidly; the higher microvillous torque then recruits more transporters to the membrane, which conversely enhances transcellular transport. In the following simulations, Na+ reabsorption was reduced by 30%, while intracellular [Na+] was maintained at >10 mM, by lowering the basal (without torque-mediated effects) expression of NHE3, Na+-PO4 transporters, and Na+-K+-ATPase by 75%, 75%, and 25%, respectively. Owing to the compensatory and torque-modulated effects described above, the Na+ fluxes across NHE3, Na+-PO4 transporters, and Na+-K+-ATPase were equal to 79%, 57%, and 92%, respectively, of their base-case values.
As summarized in Table 3, the 30% decrease in transepithelial Na+ transport is predicted to reduce TCa also by 30%, in accordance with experimental observations (2). As water reabsorption diminishes, luminal [Ca2+] is lowered, and both convection and diffusion of Ca2+ across TJs decrease. The 44% reduction in the paracellular JCa is, however, partially counterbalanced by a 62% increase in the transcellular JCa, stemming from a more negative electric potential within the cell cytosol (not shown). Overall, these results indicate that PTH indeed reduces TCa indirectly, via its effects on Na+ transport.
PTH is also known to reduce glomerular filtration rate (GFR), perhaps via tubuloglomerular feedback (27). Thus, in the next set of simulations, we both lowered SNGFR by 20% and reduced the basal expression of NHE3, apical Na+-PO4 transporters, and Na+-K+-ATPase by 75%, 75%, and 25%, respectively. As shown in Table 3, the effects of decreasing SNGFR and Na+ transporter activity on Ca2+ transport are almost additive; fractional Ca2+ reabsorption is computed as 47.0% vs. 69.3% in the base case.
Impact of paracellular Ca2+ permeability.
There is some evidence that PTH may also decrease paracellular permeability in the PT (23, 31). To our knowledge, the specific effects of PTH on permeability of the TJ to Ca2+ () have not been investigated. Our model suggests that large reductions in may have a substantial effect on overall Ca2+ reabsorption, in spite of counteracting effects (Fig. 6). Decreasing lowers paracellular TCa, thereby raising luminal [Ca2+] and, thus, the driving force for transcellular transport; increases in transcellular TCa are limited, however (Table 2). Moreover, if is diminished by a factor of ≤5, paracellular JCa becomes higher than in the base case toward the end of the PT, where the effects of the larger [Ca2+] gradient (namely, the greater driving force) are sufficient to overcome the effects of the permeability reduction. Nevertheless, overall TCa decreases by >10% if is reduced by a factor of >2 (Table 2).
Fig. 6.
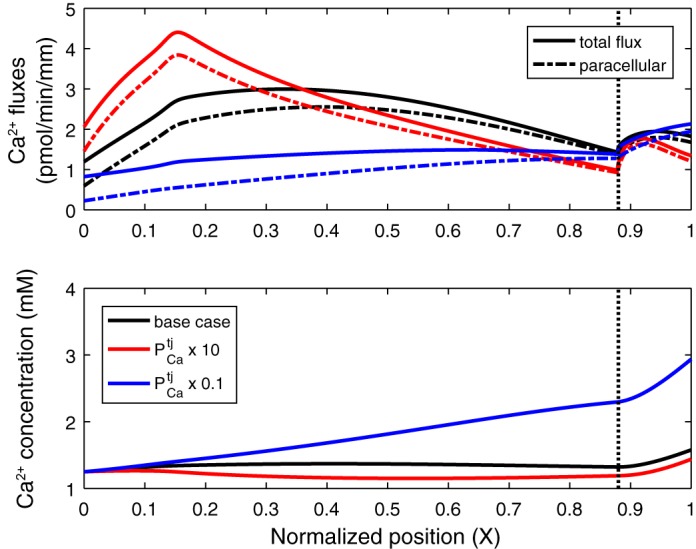
Impact of the permeability of the tight junction to Ca2+ () on Ca2+ fluxes and concentrations along the proximal tubule. Top: total and paracellular Ca2+ fluxes. Bottom: luminal Ca2+ concentration. is set to its baseline value (base case), multiplied by 10 (× 10), or divided by 10 (× 0.1). Vertical line denotes the boundary between the cortex and the medulla.
Increasing elicits opposite effects, namely, a reduction in luminal [Ca2+] and, therefore, in transcellular TCa. Furthermore, paracellular JCa becomes lower than in the base case beyond a certain point along the PT (Fig. 6), owing to the reduced lumen-to-interstitium [Ca2+] gradient. These compensating mechanisms substantially mitigate the effects of even large (e.g., 10-fold) increases in , as recapitulated in Table 2. Overall, these results suggest that decreasing and increasing induce asymmetric responses.
Ca2+-sensing mechanisms.
Several studies have suggested that the Ca2+-sensing-receptor (CaSR) or CaSR-like molecules may be expressed in the PT (6, 22, 43), but the impact of the CaSR on Ca2+ transport in that segment has not been examined and is a subject of debate (32). Capasso et al. demonstrated that treating PT segments with a calcimimetic agent activates NHE-mediated H+ extrusion (13). Together, their results suggest that Ca2+-sensing mechanisms increase NHE-mediated Na+ reabsorption, which in turn enhances fluid reabsorption. Thus these mechanisms and PTH appear to exert opposite effects, as also suggested by other studies (6). To examine the impact of Ca2+ sensing on TCa, we increased the basal (without torque-modulated effects) expression of NHE3 by 40%. Expression of Na+-K+-ATPase was raised concomitantly, albeit to a lesser extent (10%), so as to maintain intracellular [Na+] below 25 mM. The basal expression of basolateral Na+- cotransporters was also augmented (by 40%) to prevent large fluctuations in intracellular volume: adjustment of Na+- cotransport is one of the mechanisms underlying cell volume regulation in the PT (16, 59, 61).
Augmenting the Na+ flux across NHE3 raises intracellular [Na+], which then decreases Na+ entry via Na+-PO4 cotransporters. It also increases water reabsorption, thus reducing the luminal flow and microvillous torque, such that fewer transporters are recruited to the membrane. Specifically, upregulating Na+ transporter activity as described above is predicted to raise fractional Na+ reabsorption from 70.1% to 73.5%, which in turn elevates fractional Ca2+ reabsorption from 69.3% to 72.8% (Table 2). Together, our results suggest that variations in Na+ reabsorption induce nearly identical changes in TCa.
Impact of acid-base status.
Acetazolamide, a commonly prescribed inhibitor of carbonic anhydrase (CA), raises urinary Ca2+ excretion (3, 38). To investigate the impact of acetazolamide in the PT specifically, we reduced the rates of CO2-H2CO3 interconversion in the PT lumen by a factor of 10−4 (to uncatalyzed values). As previously described (62), luminal CA inhibition induces PT diuresis, because it abolishes the and Cl− gradients that normally drive paracellular water reabsorption. The present model predicts that Na+ reabsorption is then reduced by 20%, across both transcellular and paracellular pathways, as apical H+ recycling via NHE3 is significantly impaired and the lumen-to-interstitium [Na+] gradient is reduced. Ca2+ reabsorption is predicted to similarly decrease by 23% (Table 2); an identical 23% acetazolamide-induced decrease was observed in the dog PT (8).
Acetazolamide may also indirectly inhibit the basolateral Na+-HCO3 transporter NBC1 (50), mutations of which are associated with proximal renal tubular acidosis. Our model suggests that NBC1 inhibition reduces transcellular Na+ reabsorption, subsequently diminishing water reabsorption and paracellular Na+ fluxes, as well as Ca2+ reabsorption. An isolated 90% decrease in NBC1 expression is predicted to reduce TCa from 26.0 to 19.7 pmol/min (Table 2), a 24% decrease. Luminal pH at the PT outlet is computed as 7.34 vs. 7.28 in the base case.
Effects of SGLT2 inhibitors.
Inhibitors of SGLT2 are increasingly used to treat diabetes, but they have been linked to bone loss or increased risk of fracture, possibly as a result of altered Ca2+ and PO4 metabolism (1). SLGT2 inhibition increases urinary Ca2+ excretion in rats and mice (33, 35), but whether this is due to a direct effect on proximal Ca2+ reabsorption is unknown. We examined the effects of blocking SGLT2 on Ca2+ reabsorption in the PT under normoglycemia. SGLT2 inhibition elicits glucose-induced osmotic diuresis, which subsequently reduces the lumen-to-interstitium [Ca2+] gradient and, therefore, paracellular Ca2+ transport. On the other hand, the higher luminal flow increases microvillous torque, thereby upregulating the membrane abundance of transport proteins and enhancing transcellular Ca2+ transport. As a net result, the computed TCa decreases by 12% (Table 2), fractional Ca2+ reabsorption decreases by 8.5% (from 69.3% to 60.8%), and delivery of Ca2+ to the loop of Henle increases from 11.5 to 14.7 pmol/min per nephron.
By significantly reducing proximal reabsorption, SGLT2 blockers activate tubuloglomerular feedback, which in turn reduces SNGFR. Chronic SGLT2 blockade was found to lower GFR by 15% in diabetic rats (54). When we simulated a 15% decrease in SNGFR in combination with a 100% inhibition of SGLT2, fractional Ca2+ reabsorption decreased even further (to 56.6%), and the distal delivery of Ca2+ remained higher than in the base case (13.8 pmol/min per nephron). Together, these simulations suggest that SGLT2 blockers may significantly affect renal handling of Ca2+ (see below).
PO4 reabsorption.
The baseline expression of NaPi-IIa, NaPi-IIc, and PiT-2 was chosen so that NaPi-IIa mediates 70% of PO4 reabsorption in the PT (27), with the remainder arbitrarily divided equally between NaPi-IIc and PiT-2. In the base case, the model predicts that the PT reabsorbs 79.2% of the filtered load of PO4, entirely across transcellular pathways. The tubular fluid-to-glomerular filtrate PO4 concentration ratio [(TF/GF)PO4] is <1.0 and is comparable to micropuncture measurements in rats and dogs (4, 63). Specifically, the computed value of (TF/GF)PO4 decreases from 1.0 to 0.53 along the S1–S2 segment and increases to 0.86 in the S3 segment due to the interstitial medullary concentration gradient.
If it is assumed that the TJ permeability to PO4 equals 4 × 10−5 cm/s (62), the model predicts significant PO4 backleak across the TJ (Fig. 7). Net PO4 reabsorption (TPO4) is 61.8 pmol/min, with 78.5 pmol/min reabsorbed via transcellular routes and 16.7 pmol/min secreted via paracellular routes. The average PO4 flux (JPO4) is computed as 5.6 pmol·min−1·mm−1.
Fig. 7.

Base-case PO4 fluxes and concentrations along the proximal tubule. Top: total, paracellular, and transcellular PO4 fluxes. Bottom: PO4 concentration in the lumen and interstitium. Vertical line denotes the boundary between the cortex and the medulla.
The contribution of NaPi-IIa to PO4 reabsorption in mice is thought to be ~70%, based on gene knockout studies (7, 46, 53). To assess the importance of compensatory mechanisms, we simulated the effects of inhibiting each type of Na+-PO4 transporter in turn. Results are summarized in Table 4. Full inhibition of NaPi-IIa is predicted to reduce net PO4 reabsorption by 26% (to 45.7 pmol/min) and net Na+ reabsorption by 3%. The lower PO4 transport rate means that (TF/GF)PO4 remains above 1.0, backleak across the TJ into the lumen is abolished, and the paracellular pathway mediates PO4 reabsorption instead of secretion; it is predicted to represent 16% of the total flux. The contribution of NaPi-IIc to TPO4 then exceeds that of PiT-2 (Table 4), likely because NaPi-IIc carries (as does NaPi-IIa), which is more abundant than , the species carried by PiT-2.
Table 4.
Predicted PO4 fluxes following transporter inhibition
|
JPO4, pmol·min−1·mm−1 per nephron |
||||||
|---|---|---|---|---|---|---|
| Net | Paracellular | Transcellular | Across NaPi-IIa | Across NaPi-IIc | Across PiT-2 | |
| Base case | 5.62 | −1.52 | 7.14 | 4.98 (69.7%) | 1.05 (14.7%) | 1.11 (15.5%) |
| 100% inhibition of NaPi-IIa | 4.16 | +0.65 | 3.51 | 0 | 1.97 (56.2%) | 1.54 (43.8%) |
| 100% inhibition of NaPi-IIc | 5.38 | −1.04 | 6.42 | 5.24 (81.5%) | 0 | 1.18 (18.5%) |
| 100% inhibition of PiT-2 | 5.38 | −1.06 | 6.44 | 5.23 (81.2%) | 1.21 (18.8%) | 0 |
JPO4, flux of PO4 ( + ); NaPi-IIa, NaPi-IIc, and PiT-2, Na+-PO4 cotransporters.
When either NaPi-IIc or PiT-2 is fully inhibited, the activity of NaPi-IIa increases by ~5% in compensation. The lumen-to-interstitium PO4 concentration ([PO4]) gradient still favors paracellular PO4 secretion, as in the base case, but the PO4 flux across the TJ is nevertheless reduced. As a result of both effects, net TPO4 is predicted to decrease only slightly, by 4% (Table 4). Net Na+ reabsorption diminishes by <1%.
Note that as a baseline, the model assumes that NaPi-IIc and PiT-2 are only expressed in the convoluted PT (42). When we assumed a uniform distribution of NaPi-IIc and PiT-2 along the full PT, the computed TPO4 increased from 61.8 to 62.6 pmol/min, a 1.3% difference.
Effects of PTH on PO4 transport.
PTH decreases PO4 reabsorption in the PT in a direct manner, by reducing the membrane abundance of NaPi-IIa, NaPi-IIc, and PiT-2 via different mechanisms (10, 24, 37, 42, 47). PTH stimulates the internalization of NaPi-IIa by activating PKA and PKC (10), two kinases that are also involved in NHE3 inhibition (25, 27). We surmised that PTH may also affect TPO4 indirectly, via its inhibitory action on NHE3. We examined each of these effects, first separately and then simultaneously.
Shown in Table 5 are the computed average PO4 fluxes for different degrees of Na+-PO4 cotransporter inhibition. As expected, PO4 reabsorption is predicted to decrease significantly with increasing inhibition; 90% inhibition of all Na+-PO4 transporters reduces TPO4 from 61.8 to 36.1 pmol/min. Conversely, NHE3 inhibition by itself is predicted to enhance TPO4, because it lowers intracellular [Na+], thereby stimulating the activity of Na+-PO4 transporters. This means that the direct effect of PTH on PO4 transporter abundance and its indirect effect on PO4 transporter activity counteract each other. Consequently, when the abundance of apical PO4 transporters is reduced by ≤75%, the predicted TPO4 is higher when NHE3 inhibition is taken into account than when it is not (Table 5).
Table 5.
Predicted impact of parathyroid hormone on PO4 transport
|
JPO4, pmol·min−1·mm−1 per nephron |
|||||
|---|---|---|---|---|---|
| Filtered Load, pmol/min per nephron | %Reabsorption | Net | Transcellular | Paracellular | |
| Base case | 78.0 | 79.2 | 5.62 | 7.14 | −1.52 |
| 75% inhibition of Na+-PO4 transporters | 78.0 | 54.7 | 3.88 | 2.69 | +1.19 |
| 90% inhibition of Na+-PO4 cotransporters | 78.0 | 46.2 | 3.27 | 1.26 | +2.01 |
| NHE3 inhibition* | 78.0 | 94.8 | 6.73 | 9.92 | −3.19 |
| NHE3 inhibition* and 75% inhibition of Na+-PO4 cotransporters | 78.0 | 55.5 | 3.93 | 4.16 | −0.23 |
| NHE3 inhibition* and 90% inhibition of Na+-PO4 cotransporters | 78.0 | 38.6 | 2.73 | 1.97 | +0.76 |
| 20% reduction in SNGFR | 62.4 | 67.1 | 3.81 | 4.47 | −0.66 |
| 20% reduction in SNGFR, NHE3 inhibition* and 75% inhibition of Na+-PO4 cotransporters | 62.4 | 47.7 | 2.70 | 2.59 | +0.11 |
| 20% reduction in SNGFR, NHE3 inhibition* and 90% inhibition of Na+-PO4 cotransporters | 62.4 | 35.8 | 2.03 | 1.22 | +0.81 |
JPO4, flux of PO4 (+ ); NHE3, Na+/H+ exchanger type 3; SNGFR, single-nephron glomerular filtration rate. Fractional inhibition of Na+-PO4 cotransporters NaPi-IIa, NaPi-IIc, and PiT-2 is taken to be the same.
Parathyroid hormone is assumed to reduce basal expression of NHE3 and Na+-K+-ATPase by 75% and 25%, respectively.
Interestingly, the model predicts that when the abundance of apical PO4 transporters is reduced by ≥80%, the predicted TPO4 is lower when NHE3 inhibition is taken into account than when it is not (Table 5). This occurs because, in the latter case (i.e., no indirect, counterbalancing effects via NHE3), transcellular JPO4 is so small that luminal [PO4] increases steeply along the PT, which strongly stimulates PO4 reabsorption across the paracellular route. In the former case (i.e., in the presence of counterbalancing effects via NHE3), transcellular JPO4 remains higher, and paracellular PO4 reabsorption is significantly lower (Table 5).
As described above, PTH also reduces GFR. By itself, a 20% decrease in SNGFR is predicted to reduce fractional PO4 reabsorption by 12% (from 79.2% to 67.1%). When combined with PTH-mediated inhibition of Na+-PO4 transporters and NHE3, fractional PO4 reabsorption is further reduced (Table 5).
DISCUSSION
Determinants of Ca2+ transport in the PT.
The main objective of this study was to elucidate the physical mechanisms underlying Ca2+ reabsorption and its regulation in the PT. We expanded a previously published model of transport across the PT, which did not account for Ca2+ (26). Our model suggests that the lumen-to-interstitium [Ca2+] gradient, which results from water reabsorption, is the main force driving Ca2+ transport across the PT epithelium. The transepithelial electric potential difference contributes to the Ca2+ flux to a lesser extent, and only in the distal PT, where it is lumen-positive. When we set the valence of Ca2+ to zero in our simulations, the computed TCa decreased by only 5% (from 26.0 to 24.8 pmol/min). If it is assumed that the reflection coefficient of the TJ to Ca2+ is equal to 0.89 (41), the convective JCa across the TJ is predicted to be approximately seven times lower than the electrodiffusive JCa (Table 2). As previously suggested (17), our model indicates that Ca2+ reabsorption in the PT is dominated by passive diffusion.
The nature and contribution of transcellular Ca2+ fluxes in the PT are poorly understood. Apical L-type Ca2+ channels were identified in cultured rabbit PT cells (65), and colocalization of transient receptor potential channel 1 and aquaporin 1 was observed in rat kidneys (21), but the specific Ca2+ molecular transporters in rat PT cells remain to be determined. Interestingly, our results suggest that enhancing transcellular Ca2+ transport has a small impact on overall TCa, because it induces a counteracting decrease in paracellular Ca2+ transport (by reducing the lumen-to-interstitium [Ca2+] gradient). Conversely, reducing transcellular Ca2+ transport elicits a compensatory increase in paracellular Ca2+ transport, such that the overall TCa also does not vary much (Table 2). Hence, it may be difficult to parse the contribution of each pathway unless experiments are carefully designed.
Besides the contribution of transcellular TCa, other uncertainties include the magnitude of the corticomedullary interstitial [Ca2+] gradient in the medulla (which controls Ca2+ reabsorption in the S3 segment), the reflection coefficient of the TJ to Ca2+, and the permeability of the BM to Ca2+. Our results suggest that the latter three parameters have only a moderate impact on TCa (Table 2).
Regulation of Ca2+ transport.
Whereas PTH augments Ca2+ reabsorption in the thick ascending limb and the distal tubule, studies in dogs suggest that it paradoxically reduces TCa in the PT (2, 51). This reduction is thought to result from PTH-mediated inhibition of NHE3, which diminishes transepithelial Na+ and Ca2+ fluxes (and fractional reabsorption) by the same factor according to our simulations. PTH is also known to affect GFR. Per se, a PTH-induced decrease in GFR reduces the absolute transport rates of Na+ and Ca2+, but fractional reabsorption is somewhat maintained by flow-dependent (torque-mediated) transport mechanisms. Since the largest proportion (nearly two-thirds) of the filtered load of Ca2+ is reabsorbed in the PT, small variations in transepithelial Ca2+ transport in the PT may have a considerable impact on final calciuria, even if Ca2+ reabsorption increases downstream via tubular cross talk between segments.
In addition, the model predicts that a (putative) inhibitory action of PTH on TJ permeability to Ca2+ may also lower TCa substantially (Table 2); indeed, large decreases in paracellular JCa cannot be compensated for by comparable increases in transcellular Ca2+ transport, owing to its limited capacity. The model generally predicts opposite (i.e., counteracting) changes in paracellular and transcellular Ca2+ fluxes (Table 2). One exception is when GFR is varied: in this case, Ca2+ transport increases (or decreases) both across and between cells, as described above.
Increasing luminal [Ca2+] has a demonstrable impact on water and Na+ fluxes in the PT (13), but its effects on JCa have not been examined to our knowledge. If it is assumed that Ca2+-sensing mechanisms exert only indirect, NHE3-mediated effects on Ca2+ transport in that segment, they are predicted to affect Na+ and Ca+ reabsorption in the same proportion. The model suggests that increases in JNa are limited in vivo, owing to flow-dependent transport and the tight coupling between water and Na+ transport; thus, Ca2+-sensing-induced increases in JCa may be similarly restricted.
The mechanisms by which acetazolamide augments urinary Ca2+ excretion remain to be fully characterized. The present study suggests that acetazolamide-induced inhibition of CA in the PT lumen decreases transcellular Na+ reabsorption, which in turn lowers paracellular Ca2+ fluxes. Our results thus support the hypothesis that acetazolamide-induced calciuria at least partly stems from reduced Ca2+ reabsorption in the PT (3).
SLGT2 inhibitors, which are increasingly used to treat diabetes (55), are associated with disturbances in bone metabolism, higher plasma PO4 levels, and elevated urinary Ca2+ excretion (1, 33, 35). In particular, the mechanisms underlying hypercalciuria remain to be elucidated. Our model predicts that, by itself, blocking SGLT2 in the PT reduces Ca2+ reabsorption by 12% in that segment, thereby lowering fractional Ca2+ reabsorption from 69.3% to 60.8%. SGLT2 inhibition is also known to decrease SNGFR via tubuloglomerular feedback (54). Even when SNGFR (i.e., the filtered load) is concomitantly reduced by 15–25%, Ca2+ delivery to the loop of Henle remains 15–20% higher than in the base case, according to our simulations. This significant increase in Ca2+ delivery may not be fully compensated for downstream, given the limited Ca2+ transport capacity of distal segments. In other words, our results suggest that the effects of SGLT2 inhibition on Ca2+ transport in the PT may contribute to SGLT2 blocker-induced hypercalciuria and bone disease.
In addition, it has been postulated that the increase in plasma [PO4] induced by SGLT2 inhibition may stem from increased tubular PO4 reabsorption (52). However, our model suggests that TPO4 may not increase if SNGFR is reduced. Per se, blocking SGLT2 is predicted to raise TPO4 (by 5.8%) via two mechanisms: not only is the activity of Na-PO4 cotransporters stimulated by the decrease in intracellular [Na+], but their membrane abundance (and that of other transcellular transporters) is also upregulated in response to the higher luminal flow. Nevertheless, even a small (5%) SGLT2 inhibition-induced decrease in SNGFR more than counterbalances these effects and lowers TPO4 below its baseline value. If it is assumed that blocking SGLT2 lowers SNGFR by 15% (54), the computed TPO4 is 20% lower than in the absence of SGLT2 inhibitors.
Impact of claudin-2 deletion.
Fractional Ca2+ excretion (FECa) is increased threefold, from 0.13 to 0.40% (40), in claudin-2 knockout mice, likely as a result of impaired Ca2+ reabsorption in the PT (19). Claudin-2 is the main cation- and water-permeable channel in the PT (19, 40, 44); its Ca2+-to-Na+ permeability ratio has been estimated as 1:4 (64). In PT segments specifically, Na+, Cl−, and water reabsorption is reduced by 20–40% following claudin-2 gene deletion (40, 45). Whether the FECa increase stems from altered claudin-2-dependent paracellular Ca2+ transport in the PT or is only an indirect consequence of impaired paracellular Na+ and water reabsorption remains to be ascertained. To shed light on this question, we simulated a 90% decrease in the paracellular permeability of PT TJs to Na+ and water, with or without a concomitant 90% decrease in .
A 10-fold decrease in the paracellular permeability to Na+ and water is predicted to lower their reabsorption by 25% and 24%, respectively. By itself, this reduces TCa from 26.0 to 18.3 pmol/min and fractional Ca2+ reabsorption from 69.3% to 48.7%. If is reduced by 90% in tandem, the computed fractional Ca2+ reabsorption decreases further, to 28.8%; in other words, the load delivered to the thick ascending limb is then 2.3 times higher than under normal conditions. Under these circumstances, it seems unlikely that Ca2+ reabsorption mechanisms downstream from the PT (namely, passive reabsorption in the thick ascending limb and active transport in the distal convoluted and connecting tubules) can be sufficiently upregulated to maintain urinary Ca2+ excretion at ∼1%. This suggests that the higher FECa observed in claudin-2 KO mice might only be an indirect effect, the consequence of reduced Na+ and water reabsorption in the PT. A model of transport along the entire nephron would help fully elucidate this question. Experiments designed to block Ca2+ reabsorption specifically in the thick ascending limb and/or the distal convoluted tubule of claudin-2 knockout mice would also yield a better understanding of the relative contribution of these segments in compensating for the loss of Ca2+ in the PT due to the absence of claudin-2.
Determinants of PO4 transport.
The model was also expanded to account for the specific stoichiometry of each apical Na+-PO4 transporter in the PT. In mice, but not in humans, the contribution of NaPi-IIa predominates (9, 27), and we assumed in the base case that NaPi-IIa mediates 70% of TPO4 vs. 15% each for NaPi-IIc and PiT-2. In addition, permeability of the TJ to and was taken as 4.0 × 10−5 cm/s (62). With these hypotheses, the model predicts that, under normal conditions, the passive backleak of PO4 across the TJ reduces net PO4 reabsorption by 21% (Table 4). However, when transcellular PO4 transport is substantially impaired, the paracellular PO4 flux switches direction and may contribute significantly to PO4 reabsorption (Tables 4 and 5). Moreover, when transport via NaPi-IIa is fully blocked, NaPi-IIc flux increases significantly more than PiT-2 flux. Together, these results suggest that it may be difficult to extrapolate measurements in knockout animal models to quantify the contribution of each Na+-PO4 transporter.
PTH is known to reduce PO4 reabsorption in the PT by lowering the membrane expression of NaPi-IIa, NaPi-IIc, and PiT-2 (27). PTH may also impact TPO4 indirectly by inhibiting NHE3. According to our simulations, inhibition of NHE3 per se stimulates the activity of Na+-PO4 transporters, suggesting that the direct and indirect effects of PTH on TPO4 counteract each other. Moreover, when transcellular PO4 reabsorption is severely reduced, paracellular PO4 reabsorption may increase very significantly in compensation. PTH-induced decreases in GFR may also contribute to lowering TPO4 (Table 5).
Possible model extensions.
Since the molecular transporters that mediate transcellular Ca2+ reabsorption in the PT remain to be identified, our model assumes that the transcellular JCa is solely driven by the Ca2+ electrochemical potential gradient: this assumption is valid if Ca2+ entry into the cell occurs via a Ca2+ channel, but not if it is mediated by a cotransporter. Further experimental studies are needed to clarify this. Additionally, the current model could be expanded in several ways. It does not account for the binding between Ca2+ and or , the rate of which is pH-dependent. Nor does it include phospho- and calcitropic hormones other than PTH, such as fibroblast growth factor 23 (FGF23) and vitamin D3. Vitamin D3 enhances the intestinal absorption of both Ca2+ and PO4, and its synthesis in PT cells is activated by PTH (5) and, conversely, inhibited by FGF23 (48). However, whether vitamin D3 directly modulates Ca2+ and PO4 fluxes in the PT remains to be ascertained (27). FGF23 is an important regulator of PO4 metabolism; in the PT, it reduces PO4 reabsorption by decreasing NaPi-IIa and NaPi-IIc expression (20). FGF23 requires α-klotho as a cofactor, which is expressed mainly in the distal tubule and, to a much lower extent, in the PT (29). Thus the actions of FGF23 in the PT may be indirect and may involve a distal-to-proximal tubular feedback mechanism that has yet to be elucidated (36). Finally, this PT model should be linked to our models of Ca2+ transport in the distal nephron (12, 15) to yield an integrated understanding of renal Ca2+ handling.
In conclusion, we have developed the first model of Ca2+ transport in the PT. Our results indicate that Ca2+ reabsorption in that segment is principally driven by the lumen-to-interstitium [Ca2+] gradient that is generated by water reabsorption. Our model also provides greater insight into the different mechanisms by which the reabsorption of Ca2+ and PO4 is regulated in that segment.
GRANTS
O. Bonny is supported by a special program of the Swiss National Foundation (National Centre of Competence in Research, Kidney, Control of Homeostasis) and Swiss National Foundation Grant 310030-163340.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.E. and O.B. conceived and designed research; A.E. performed experiments; A.E. analyzed data; A.E. and O.B. interpreted results of experiments; A.E. prepared figures; A.E. drafted manuscript; A.E. and O.B. edited and revised manuscript; A.E. and O.B. approved final version of manuscript.
REFERENCES
- 1.Adil M, Khan RA, Kalam A, Venkata SK, Kandhare AD, Ghosh P, Sharma M. Effect of anti-diabetic drugs on bone metabolism: evidence from preclinical and clinical studies. Pharmacol Rep 69: 1328–1340, 2017. doi: 10.1016/j.pharep.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Agus ZS, Gardner LB, Beck LH, Goldberg M. Effects of parathyroid hormone on renal tubular reabsorption of calcium, sodium, and phosphate. Am J Physiol 224: 1143–1148, 1973. [DOI] [PubMed] [Google Scholar]
- 3.Alexander RT, Dimke H. Effect of diuretics on renal tubular transport of calcium and magnesium. Am J Physiol Renal Physiol 312: F998–F1015, 2017. doi: 10.1152/ajprenal.00032.2017. [DOI] [PubMed] [Google Scholar]
- 4.Amiel C, Kuntziger H, Richet G. Micropuncture study of handling of phosphate by proximal and distal nephron in normal and parathyroidectomized rat. Evidence for distal reabsorption. Pflügers Arch 317: 93–109, 1970. doi: 10.1007/BF00592495. [DOI] [PubMed] [Google Scholar]
- 5.Armbrecht HJ, Hodam TL, Boltz MA. Hormonal regulation of 25-hydroxyvitamin D3-1α-hydroxylase and 24-hydroxylase gene transcription in opossum kidney cells. Arch Biochem Biophys 409: 298–304, 2003. doi: 10.1016/S0003-9861(02)00636-7. [DOI] [PubMed] [Google Scholar]
- 6.Ba J, Brown D, Friedman PA. Calcium-sensing receptor regulation of PTH-inhibitable proximal tubule phosphate transport. Am J Physiol Renal Physiol 285: F1233–F1243, 2003. doi: 10.1152/ajprenal.00249.2003. [DOI] [PubMed] [Google Scholar]
- 7.Beck L, Karaplis AC, Amizuka N, Hewson AS, Ozawa H, Tenenhouse HS. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Natl Acad Sci USA 95: 5372–5377, 1998. doi: 10.1073/pnas.95.9.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck LH, Goldberg M. Effects of acetazolamide and parathyroidectomy on renal transport of sodium, calcium, and phosphate. Am J Physiol 224: 1136–1142, 1973. doi: 10.1152/ajplegacy.1973.224.5.1136. [DOI] [PubMed] [Google Scholar]
- 9.Biber J, Hernando N, Forster I. Phosphate transporters and their function. Annu Rev Physiol 75: 535–550, 2013. doi: 10.1146/annurev-physiol-030212-183748. [DOI] [PubMed] [Google Scholar]
- 10.Biber J, Hernando N, Forster I, Murer H. Regulation of phosphate transport in proximal tubules. Pflügers Arch 458: 39–52, 2009. doi: 10.1007/s00424-008-0580-8. [DOI] [PubMed] [Google Scholar]
- 11.Blaine J, Chonchol M, Levi M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin J Am Soc Nephrol 10: 1257–1272, 2015. doi: 10.2215/CJN.09750913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonny O, Edwards A. Calcium reabsorption in the distal tubule: regulation by sodium, pH, and flow. Am J Physiol Renal Physiol 304: F585–F600, 2013. doi: 10.1152/ajprenal.00493.2012. [DOI] [PubMed] [Google Scholar]
- 13.Capasso G, Geibel PJ, Damiano S, Jaeger P, Richards WG, Geibel JP. The calcium sensing receptor modulates fluid reabsorption and acid secretion in the proximal tubule. Kidney Int 84: 277–284, 2013. doi: 10.1038/ki.2013.137. [DOI] [PubMed] [Google Scholar]
- 14.Du Z, Yan Q, Duan Y, Weinbaum S, Weinstein AM, Wang T. Axial flow modulates proximal tubule NHE3 and H-ATPase activities by changing microvillus bending moments. Am J Physiol Renal Physiol 290: F289–F296, 2006. doi: 10.1152/ajprenal.00255.2005. [DOI] [PubMed] [Google Scholar]
- 15.Edwards A. Regulation of calcium reabsorption along the rat nephron: a modeling study. Am J Physiol Renal Physiol 308: F553–F566, 2015. doi: 10.1152/ajprenal.00577.2014. [DOI] [PubMed] [Google Scholar]
- 16.Edwards A, Layton AT. Cell volume regulation in the proximal tubule of rat kidney: proximal tubule cell volume regulation. Bull Math Biol 79: 2512–2533, 2017. doi: 10.1007/s11538-017-0338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman PA. Mechanisms of renal calcium transport. Exp Nephrol 8: 343–350, 2000. doi: 10.1159/000020688. [DOI] [PubMed] [Google Scholar]
- 18.Friedman PA. Renal calcium metabolism. In The Kidney: Physiology and Pathology (3rd ed.), edited by Seldin DD, Giebisch G. Philadelphia: Lippincott Williams & Wilkins, 2000, p. 1749–1790. [Google Scholar]
- 19.Fromm M, Piontek J, Rosenthal R, Günzel D, Krug SM. Tight junctions of the proximal tubule and their channel proteins. Pflügers Arch 469: 877–887, 2017. doi: 10.1007/s00424-017-2001-3. [DOI] [PubMed] [Google Scholar]
- 20.Gattineni J, Bates C, Twombley K, Dwarakanath V, Robinson ML, Goetz R, Mohammadi M, Baum M. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am J Physiol Renal Physiol 297: F282–F291, 2009. doi: 10.1152/ajprenal.90742.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goel M, Sinkins WG, Zuo C-D, Estacion M, Schilling WP. Identification and localization of TRPC channels in the rat kidney. Am J Physiol Renal Physiol 290: F1241–F1252, 2006. doi: 10.1152/ajprenal.00376.2005. [DOI] [PubMed] [Google Scholar]
- 22.Graca JAZ, Schepelmann M, Brennan SC, Reens J, Chang W, Yan P, Toka H, Riccardi D, Price SA. Comparative expression of the extracellular calcium-sensing receptor in the mouse, rat, and human kidney. Am J Physiol Renal Physiol 310: F518–F533, 2016. doi: 10.1152/ajprenal.00208.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobson HR. Altered permeability in the proximal tubule response to cyclic AMP. Am J Physiol Renal Fluid Electrolyte Physiol 236: F71–F79, 1979. doi: 10.1152/ajprenal.1979.236.1.F71. [DOI] [PubMed] [Google Scholar]
- 24.Kempson SA, Lötscher M, Kaissling B, Biber J, Murer H, Levi M. Parathyroid hormone action on phosphate transporter mRNA and protein in rat renal proximal tubules. Am J Physiol Renal Fluid Electrolyte Physiol 268: F784–F791, 1995. doi: 10.1152/ajprenal.1995.268.4.F784. [DOI] [PubMed] [Google Scholar]
- 25.Kurashima K, Yu FH, Cabado AG, Szabó EZ, Grinstein S, Orlowski J. Identification of sites required for down-regulation of Na+/H+ exchanger NHE3 activity by cAMP-dependent protein kinase: phosphorylation-dependent and -independent mechanisms. J Biol Chem 272: 28672–28679, 1997. doi: 10.1074/jbc.272.45.28672. [DOI] [PubMed] [Google Scholar]
- 26.Layton AT, Vallon V, Edwards A. Modeling oxygen consumption in the proximal tubule: effects of NHE and SGLT2 inhibition. Am J Physiol Renal Physiol 308: F1343–F1357, 2015. doi: 10.1152/ajprenal.00007.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JJ, Plain A, Beggs MR, Dimke H, Alexander RT. Effects of phospho- and calciotropic hormones on electrolyte transport in the proximal tubule. F1000 Res 6: 1797, 2017. doi: 10.12688/f1000research.12097.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JW, Chou C-L, Knepper MA. Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol 26: 2669–2677, 2015. doi: 10.1681/ASN.2014111067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li S-A, Watanabe M, Yamada H, Nagai A, Kinuta M, Takei K. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct 29: 91–99, 2004. doi: 10.1247/csf.29.91. [DOI] [PubMed] [Google Scholar]
- 30.Li Y-H, Gregory S. Diffusion of ions in sea water and in deep-sea sediments. Geochim Cosmochim Acta 38: 703–714, 1974. doi: 10.1016/0016-7037(74)90145-8. [DOI] [Google Scholar]
- 31.Lorentz WB. Effect of parathyroid hormone on renal tubular permeability. Am J Physiol 231: 1401–1407, 1976. doi: 10.1152/ajplegacy.1976.231.5.1401. [DOI] [PubMed] [Google Scholar]
- 32.Loupy A, Ramakrishnan SK, Wootla B, Chambrey R, de la Faille R, Bourgeois S, Bruneval P, Mandet C, Christensen EI, Faure H, Cheval L, Laghmani K, Collet C, Eladari D, Dodd RH, Ruat M, Houillier P. PTH-independent regulation of blood calcium concentration by the calcium-sensing receptor. J Clin Invest 122: 3355–3367, 2012. doi: 10.1172/JCI57407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ly JP, Onay T, Sison K, Sivaskandarajah G, Sabbisetti V, Li L, Bonventre JV, Flenniken A, Paragas N, Barasch JM, Adamson SL, Osborne L, Rossant J, Schnermann J, Quaggin SE. The Sweet Pee model for Sglt2 mutation. J Am Soc Nephrol 22: 113–123, 2011. doi: 10.1681/ASN.2010080888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maddox DA, Fortin SM, Tartini A, Barnes WD, Gennari FJ. Effect of acute changes in glomerular filtration rate on Na+/H+ exchange in rat renal cortex. J Clin Invest 89: 1296–1303, 1992. doi: 10.1172/JCI115715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mamidi RNVS, Proctor J, De Jonghe S, Feyen B, Moesen E, Vinken P, Ma JY, Bryant S, Snook S, Louden C, Lammens G, Ways K, Kelley MF, Johnson MD. Carbohydrate malabsorption mechanism for tumor formation in rats treated with the SGLT2 inhibitor canagliflozin. Chem Biol Interact 221: 109–118, 2014. doi: 10.1016/j.cbi.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Martin A, David V, Quarles LD. Regulation and function of the FGF23/klotho endocrine pathways. Physiol Rev 92: 131–155, 2012. doi: 10.1152/physrev.00002.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsumoto N, Hemmi A, Yamato H, Ohnishi R, Segawa H, Ohno S, Miyamoto K. Immunohistochemical analyses of parathyroid hormone-dependent downregulation of renal type II Na-Pi cotransporters by cryobiopsy. J Med Invest 57: 138–145, 2010. doi: 10.2152/jmi.57.138. [DOI] [PubMed] [Google Scholar]
- 38.McIntosh HW, Seraglia M, Uhlemann I, Kore R. Effect of acetazolamide and triple sulfonamide on citrate and calcium excretion. Can Med Assoc J 89: 1332–1333, 1963. [PMC free article] [PubMed] [Google Scholar]
- 39.Moor MB, Bonny O. Ways of calcium reabsorption in the kidney. Am J Physiol Renal Physiol 310: F1337–F1350, 2016. doi: 10.1152/ajprenal.00273.2015. [DOI] [PubMed] [Google Scholar]
- 40.Muto S, Hata M, Taniguchi J, Tsuruoka S, Moriwaki K, Saitou M, Furuse K, Sasaki H, Fujimura A, Imai M, Kusano E, Tsukita S, Furuse M. Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc Natl Acad Sci USA 107: 8011–8016, 2010. doi: 10.1073/pnas.0912901107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ng RC, Rouse D, Suki WN. Calcium transport in the rabbit superficial proximal convoluted tubule. J Clin Invest 74: 834–842, 1984. doi: 10.1172/JCI111500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Picard N, Capuano P, Stange G, Mihailova M, Kaissling B, Murer H, Biber J, Wagner CA. Acute parathyroid hormone differentially regulates renal brush border membrane phosphate cotransporters. Pflügers Arch 460: 677–687, 2010. doi: 10.1007/s00424-010-0841-1. [DOI] [PubMed] [Google Scholar]
- 43.Riccardi D, Traebert M, Ward DT, Kaissling B, Biber J, Hebert SC, Murer H. Dietary phosphate and parathyroid hormone alter the expression of the calcium-sensing receptor (CaR) and the Na+-dependent Pi transporter (NaPi-2) in the rat proximal tubule. Pflügers Arch 441: 379–387, 2000. doi: 10.1007/s004240000436. [DOI] [PubMed] [Google Scholar]
- 44.Rosenthal R, Milatz S, Krug SM, Oelrich B, Schulzke J-D, Amasheh S, Günzel D, Fromm M. Claudin-2, a component of the tight junction, forms a paracellular water channel. J Cell Sci 123: 1913–1921, 2010. doi: 10.1242/jcs.060665. [DOI] [PubMed] [Google Scholar]
- 45.Schnermann J, Huang Y, Mizel D. Fluid reabsorption in proximal convoluted tubules of mice with gene deletions of claudin-2 and/or aquaporin1. Am J Physiol Renal Physiol 305: F1352–F1364, 2013. doi: 10.1152/ajprenal.00342.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Segawa H, Onitsuka A, Furutani J, Kaneko I, Aranami F, Matsumoto N, Tomoe Y, Kuwahata M, Ito M, Matsumoto M, Li M, Amizuka N, Miyamoto K. Npt2a and Npt2c in mice play distinct and synergistic roles in inorganic phosphate metabolism and skeletal development. Am J Physiol Renal Physiol 297: F671–F678, 2009. doi: 10.1152/ajprenal.00156.2009. [DOI] [PubMed] [Google Scholar]
- 47.Segawa H, Yamanaka S, Onitsuka A, Tomoe Y, Kuwahata M, Ito M, Taketani Y, Miyamoto K. Parathyroid hormone-dependent endocytosis of renal type IIc Na-Pi cotransporter. Am J Physiol Renal Physiol 292: F395–F403, 2007. doi: 10.1152/ajprenal.00100.2006. [DOI] [PubMed] [Google Scholar]
- 48.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113: 561–568, 2004. doi: 10.1172/JCI200419081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silve C, Friedlander G. Renal regulation of phosphate excretion. In: The Kidney: Physiology and Pathology (3rd ed.), edited by Seldin DD, Giebisch G. Philadelphia: Lippincott Williams & Wilkins, 2000, p. 1885–1904. [Google Scholar]
- 50.Soleimani M. Na+: cotransporters (NBC): expression and regulation in the kidney. J Nephrol 15, Suppl 5: S32–S40, 2002. [PubMed] [Google Scholar]
- 51.Sutton RAL, Wong NLM, Dirks JH. Effects of parathyroid hormone on sodium and calcium transport in the dog nephron. Clin Sci Mol Med 51: 345–351, 1976. [DOI] [PubMed] [Google Scholar]
- 52.Taylor SI, Blau JE, Rother KI. Possible adverse effects of SGLT2 inhibitors on bone. Lancet Diabetes Endocrinol 3: 8–10, 2015. doi: 10.1016/S2213-8587(14)70227-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tenenhouse HS, Martel J, Gauthier C, Segawa H, Miyamoto K. Differential effects of Npt2a gene ablation and X-linked Hyp mutation on renal expression of Npt2c. Am J Physiol Renal Physiol 285: F1271–F1278, 2003. doi: 10.1152/ajprenal.00252.2003. [DOI] [PubMed] [Google Scholar]
- 54.Thomson SC, Rieg T, Miracle C, Mansoury H, Whaley J, Vallon V, Singh P. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol 302: R75–R83, 2012. doi: 10.1152/ajpregu.00357.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thrasher J. Pharmacologic management of type 2 diabetes mellitus: available therapies. Am J Med 130, 6S: S4–S17, 2017. doi: 10.1016/j.amjmed.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 56.Tournus M, Seguin N, Perthame B, Thomas SR, Edwards A. A model of calcium transport along the rat nephron. Am J Physiol Renal Physiol 305: F979–F994, 2013. doi: 10.1152/ajprenal.00696.2012. [DOI] [PubMed] [Google Scholar]
- 57.Tsukamoto Y, Saka S, Saitoh M. Parathyroid hormone stimulates ATP-dependent calcium pump activity by a different mode in proximal and distal tubules of the rat. Biochim Biophys Acta 1103: 163–171, 1992. doi: 10.1016/0005-2736(92)90070-3. [DOI] [PubMed] [Google Scholar]
- 58.Weinstein AM. A mathematical model of rat proximal tubule and loop of Henle. Am J Physiol Renal Physiol 308: F1076–F1097, 2015. doi: 10.1152/ajprenal.00504.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weinstein AM. Modeling epithelial cell homeostasis: steady-state analysis. Bull Math Biol 61: 1065–1091, 1999. doi: 10.1006/bulm.1999.0127. [DOI] [PubMed] [Google Scholar]
- 60.Weinstein AM. Nonequilibrium thermodynamic model of the rat proximal tubule epithelium. Biophys J 44: 153–170, 1983. doi: 10.1016/S0006-3495(83)84287-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weinstein AM, Sontag ED. Modeling proximal tubule cell homeostasis: tracking changes in luminal flow. Bull Math Biol 71: 1285–1322, 2009. doi: 10.1007/s11538-009-9402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weinstein AM, Weinbaum S, Duan Y, Du Z, Yan Q, Wang T. Flow-dependent transport in a mathematical model of rat proximal tubule. Am J Physiol Renal Physiol 292: F1164–F1181, 2007. doi: 10.1152/ajprenal.00392.2006. [DOI] [PubMed] [Google Scholar]
- 63.Wen S-F. Micropuncture studies of phosphate transport in the proximal tubule of the dog. The relationship to sodium reabsorption. J Clin Invest 53: 143–153, 1974. doi: 10.1172/JCI107532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu ASL, Cheng MH, Angelow S, Günzel D, Kanzawa SA, Schneeberger EE, Fromm M, Coalson RD. Molecular basis for cation selectivity in claudin-2-based paracellular pores: identification of an electrostatic interaction site. J Gen Physiol 133: 111–127, 2009. doi: 10.1085/jgp.200810154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang MI, O’Neil RG. Regulated calcium channel in apical membranes renal proximal tubule cells. Am J Physiol Cell Physiol 271: C1757–C1764, 1996. doi: 10.1152/ajpcell.1996.271.5.C1757. [DOI] [PubMed] [Google Scholar]


