Abstract
Acute kidney injury can be caused by multiple factors, including sepsis, respiratory failure, heart failure, trauma, or nephrotoxic medications, among others. Here, a mouse model was used to investigate potential urinary metabolic biomarkers of hypoxia-induced AKI. Urine metabolic profiles of 48 Swiss Webster mice were assessed using nuclear magnetic resonance spectroscopy (NMR) for 7 days following 72 h exposure to a hypoxic 6.5% oxygen environment. Histological analyses indicated a lack of gross nephron structural changes in the aftermath of hypoxia. Immunohistochemical (IHC) analyses, however, indicated elevated expression of protein injury biomarkers in distal and proximal tubules but not glomeruli. Kidney injury molecule-1 levels peaked in distal tubules at 72 h and were still increasing in proximal tubules at 7 days posthypoxia, whereas cystatin C levels were elevated at 24 h but decreased thereafter, and were elevated and still increasing in proximal tubules at 7 days posthypoxia. Neutrophil gelatinase-associated lipocalin levels were modestly elevated from 24 h to 7 days posthypoxia. NMR-based metabolic profiling revealed that urine metabolites involved in energy metabolism and associated biosynthetic pathways were initially decreased at 24 h posthypoxia, consistent with metabolic suppression as a mechanism for cell survival, but were significantly elevated at 48 and 72 h posthypoxia, indicating a burst in organism metabolism associated with reactivation of cellular energetics during recovery after cessation of hypoxia and return to a normoxic environment. The IHC results indicated that kidney injury persists long after plasma and urine biomarkers of hypoxia return to normal values.
Keywords: acute kidney injury, hypoxia, immunohistochemistry, metabolic profiling, NMR
INTRODUCTION
Acute kidney injury (AKI), previously known as acute renal failure, is characterized by an abrupt loss or drop in kidney function (32, 40, 53). AKI can be caused by multiple conditions, including sepsis, heart failure, and nephrotoxic medications (32, 40, 43). AKI results in complications in 7% of hospitalizations with up to 20% of intensive care unit patients suffering an episode of AKI (40). Assessment of possible renal etiologies of AKI must consider the four main structures of the kidney (tubules, glomeruli, interstitium, and blood vessels) (3). Toxic and ischemic reperfusion injury (IRI)-induced AKI has been reported to be mainly due to tubular epithelial cell dysfunction (3, 13), although our recent investigations indicated that AKI causes injury to both proximal and distal tubules as well as glomeruli (7).
The current clinical diagnostic measures of AKI include a significant decrease in glomerular filtration rate and a significant increase in serum creatinine (SCr) (5, 48). Other diagnostic biomarkers include an increase in urinary neutrophil gelatinase-associated lipocalin (NGAL) and blood urea nitrogen (BUN) (33). Risk, Injury, Failure, Loss, and End-State Kidney Disease criteria provide guidelines for assessing kidney function (37). Although diagnostic approaches exist for AKI, they generally lack the desired sensitivity and diagnostic specificity following immediate onset of AKI. SCr levels only change significantly following a 30–50% loss in kidney function, whereas BUN levels are unreliable because they are known to vary strongly with diet and liver function (28). Recently, several more reliable protein biomarkers of AKI have been discovered, including NGAL, cystatin C, and kidney injury molecule-1 (KIM-1), all of which are upregulated following an incidence of AKI (28, 36, 37, 44). KIM-1 is a transmembrane glycoprotein expressed in renal proximal tubule epithelial cells that has been widely used as a biomarker for kidney injury. It has the advantage that it is minimally expressed in normal healthy kidneys but is upregulated in urine and in the proximal tubules of the kidney following injury (44, 51). Cystatin C is a nonglycosylated polypeptide that is filtered and reabsorbed by the tubules but following injury is not reabsorbed by the proximal tubules and therefore shows up in the blood and the proximal tubules (28, 49). NGAL, a small glycoprotein found in granules of neutrophils thought to scavenge siderophores when inflammation occurs (32, 36, 37), is expressed by injured kidney epithelial cells and shows up at elevated levels in the urine shortly after onset of AKI. Although these biomarkers have been identified and validated for assessment of AKI, it is not known whether or not they are useful for differentiating between different possible causes of AKI.
Metabonomics is the study of changes in small molecule metabolic profiles in biological fluids as a result of disease or injury (21, 23, 55, 70). Metabonomics has the potential to provide information that directly relates to the pathophysiology of human diseases (27, 46). Nuclear magnetic resonance spectroscopy (NMR)-based metabonomics has proven to be a fast, reliable, noninvasive diagnostic tool for pathological metabolic and toxicological processes in humans and mouse models of human disease (26, 27, 45), and it has been used to identify potential metabolic biomarkers for human diseases, including cancer (2), gestational diabetes (10), neonatal AKI (42), and endometriosis (12). Metabonomics has been used in recent years to study AKI induced by nephrotoxins (50), sepsis (54), and IRI (7, 24). Despite recent progress, there is a paucity of metabonomics data available specifically examining hypoxia-induced AKI.
In this study, we used a mouse model of hypoxia 1) to measure plasma creatinine levels associated with defined conditions of hypoxia, 2) to probe the expression of three protein biomarkers of AKI in kidney tissue, namely cystatin C, Kim-1, and NGAL, 3) to assess the effects of hypoxia on gross kidney structure, and 4) to establish urine metabolic profile changes that occur in response to hypoxia and subsequent recovery following hypoxia. Our measurements indicated an initial decrease in all urinary metabolites at 24 h posthypoxia, consistent with metabolic suppression as a mechanism for cell survival, followed by a burst in organism metabolism at 48 and 72 h posthypoxia, associated with reactivation of cellular energetics and metabolism during a period of recovery in response to return to a normoxic environment, and eventually a return to a near prehypoxia urinary metabolic profile at 1 wk posthypoxia. Interestingly, the protein kidney injury biomarkers KIM-1 and cystatin C remained at extremely elevated levels in the proximal and/or distal tubules even at 1 wk posthypoxia.
MATERIALS AND METHODS
Institutional Approval of Mouse Studies
All procedures involving mice in this study were approved by the Institutional Animal Care and Use Committee at Miami University (Project Nos. 921_2018_Feb and 921_2018_Feb).
Mouse Model of Hypoxia-Induced AKI
Forty-eight female Swiss Webster mice (22–24 g) (Charles River Laboratory, Wilmington, MA) were used to study hypoxia-induced AKI. Mice were placed in metabolic cages for urine collection 3 days before exposure to hypoxic environment to obtain control urine samples. The mice were transferred directly to a hypoxic environment with the oxygen set to ~6.5% and constantly monitored with a ProOx model 110 analyzer (BioSpherix, Parish, NY). The choice of 6.5% to define the hypoxic environment was determined empirically based on the lowest atmospheric oxygen concentration that the mice could generally survive for at least 72 h. Tests at 4.5% resulted in some mice deaths. The precise conditions that define hypoxia are not definitively defined (38, 57). Moreover, the partial pressure of oxygen varies within biological fluids, biological tissues, and organs even for a controlled atmospheric concentration of oxygen (57). An atmospheric oxygen concentration of 6.5% corresponds to a partial pressure pO2 = 49.7 mmHg, which is on the order of arterial blood pO2 values found in severe chronic obstructive pulmonary lung disease (COPD) patients (<55 mmHg) who require supplemental oxygen for palliative care (29). By comparison, normoxia is considered equivalent to 20.9% O2 found at sea level (159 mmHg), and the normal arterial blood partial pressure corresponds to a pO2 equal to 90 mmHg (57). The hypoxic environment was regulated using a controlled flow of nitrogen and oxygen. Mice were housed in the chamber for 72 h under hypoxic conditions with ad libitum access to water and food. No metabolic samples were collected while mice were in the hypoxia chamber. This was in part due to the restricted space available in the hypoxia chamber. It was also noted that mice tended to huddle together during exposure to hypoxia, and it was deemed that isolation of the mice in addition to the extreme hypoxia may have caused unendurable trauma. Mice were allowed to recover in regular housing cages, then mice were transferred to metabolic cages, and urine samples were collected 24, 48, 72, and 168 h following injury. Each time point contained 12 mice. At each time point, the animals were anesthetized, the abdominal cavity was opened, and blood was obtained via a cardiac puncture. The blood collected at the time of sacrifice was transferred to BD microtainer tubes with K2 EDTA (Fisher Scientific, Waltham, MA) to enable isolation of blood plasma samples. The plasma sample was used for measurement of plasma creatinine using a quantitative colorimetric assay kit (Sigma, St. Louis, MO) (36, 37) and for NMR analysis. Mice were euthanized, and both kidneys were harvested and stored. Samples of kidney tissues were paraffin embedded for subsequent histological and immunohistochemical analyses.
Histology
Sagittal sections (5 μm) of the kidneys were stained using standard hematoxylin and eosin (H&E) stain and periodic acid-Schiff (PAS) staining. All micrographs were recorded using an Olympus AX70 light microscope with a Nikon digital camera (Photometrics, Tucson, AZ).
Immunohistochemistry
NGAL.
NGAL immunohistochemical analysis was performed as follows. Mouse kidney sections were deparaffinized, and endogenous peroxidase activity was ablated by incubation in hydrogen peroxide in methanol. Primary NGAL antibody (1:100) (rabbit anti-mouse antibody; Biorbyt, Atlanta, GA) was then added to sections and incubated overnight at 4°C in blocking solution. After application of primary antibody, NGAL was detected using a commercially available ImmPRESS reagent kit (Vector Laboratories) with DAB Substrate (Vector Laboratories) kit for peroxidase staining. The sections were counterstained with hematoxylin.
KIM-1.
KIM-1 expression was assessed as previously described by Chihanga et al. (7). In brief, mouse kidney sections were deparaffinized, and endogenous peroxidase activity was ablated by incubation. Sections were heated in a microwave oven in 0.1 mol/l sodium citrate buffer, pH 6.0 for 10 min, and were blocked with 2.5% normal horse serum (Vector Laboratories, Burlingame, CA) at room temperature for 1 h. After application of the primary antibody, KIM-1, was detected using the ImmPRESS reagent kit (Vector Laboratories) with the DAB Substrate (Vector Laboratories) kit for peroxidase staining.
Cystatin C.
Chihanga et al. (7) previously described cystatin C expression in the kidney tissue. In summary, mouse kidney sections were deparaffinized, and endogenous peroxidase activity was ablated by incubation in hydrogen peroxide in methanol. Primary antibody cystatin C (1:250) (rabbit anti-mouse antibody; Abcam, Cambridge, MA) was then added to sections and incubated overnight at 4°C in blocking solution. After application of primary antibody, cystatin C was detected using a commercially available ImmPRESS reagent kit (Vector Laboratories) with DAB Substrate (Vector Laboratories) kit for peroxidase staining. The sections were counterstained with hematoxylin.
NMR Data Collection and Analysis
NMR sample preparation was completed as previously described by Romick-Rosendale et al. (41) and Chihanga et al. (7) with modifications to the trimethylsilylpropanoic acid (TSP) concentration. Briefly, urine and plasma samples were thawed on ice before preparation for NMR analysis. A 500-μl aliquot of each sample was pH corrected to 7.4, centrifuged, and buffered to a TSP final concentration of 10 mM. Plasma samples were prepared with D2O, EDTA, and TSP to a final concentration of 10%, 10 mM, and 10 mM, respectively.
NMR spectra were recorded as previously described by Romick-Rosendale et al. (41) and Chihanga et al. (7) on a Bruker AVANCE III spectrometer operating at 850 MHz. All experiments were conducted at 298 K using 5-mm NMR tubes (Norell, Morganton, NC). Standard 1H 1D presaturation (zgpr) and the 1D 1H CPMG-presat (cpmgpr1d) experiments were recorded and processed as previously reported by Romick Rosendale et al. (41). Standard 2D 1H-13C HSQC experiments from the TopSpin 2.1.1. were used for data collection (7).
Identification and Quantification of Metabolites
Metabolite identification and quantification were assessed as previously described by Chihanga et al. (7). In summary, metabolites were assigned using Bruker AMIX software version 3.9 (Analysis of MIXtures software; Bruker Biospin), Chenomx version 8.1 (Chenomx, Alberta, Canada), and COLMAR (4) by matching experimental spectra to reference databases of metabolites (7).
Principal Components Analysis, Partial Least-Squared Discriminant Analysis, and Statistical Significance Analysis
Spectral resonances were bucketed using the manual bucketing function using the AMIX software package (Bruker BioSpin, Billerica, MA). Bucketing yields a table of intensities for each NMR peak. Buckets were not scaled or normalized before principal components analysis (PCA). The significance of the PCA loadings plots data was evaluated according to Goodpaster et al. (15). The Bonferroni-corrected alpha value, αBC, equal to 0.05 divided by the number of buckets, was the same for all urine comparisons (αBC = 0.000323) since the same manual bucketing pattern was used for all spectra. Metabolite concentrations were measured relative to the TSP internal standard concentration. Mahalanobis, F value, and F-critical values were calculated as previously described by Goodpaster and Kennedy (14). Partial least-squares-discriminant analyses (PLS-DA) were performed using the SIMCA-P version 11.0 software package (Umetrics, Umea, Sweden).
Metabolic Pathway Analysis
Analysis of the metabolic pathways implicated by the NMR-based urine metabolic profiling experiments was conducted using the MetaboAnalyst software (8, 60–69). Briefly, the lists of metabolites identified from the metabolic profiling analysis were uploaded to the MetaboAnalyst Pathway Analysis module, and the mouse organism was selected for analysis. The resulting output was used to identify the pathways involved, which metabolites were involved in each pathway, and the significance of the effect on the pathway based on the MetaboAnalyst algorithms.
RESULTS
Clinical Assays for AKI
Analysis of plasma creatinine levels indicated hypoxia-induced AKI in the 24-h posthypoxia mice, based on P value analysis assuming a critical α value = 0.05 that indicated that the plasma creatinine levels were statistically significantly higher in the 24-h posthypoxia group compared with the control group (Table 1). However, the 48-, 72-, and 168-h groups showed no significant difference in plasma creatinine levels compared with control samples (Table 1).
Table 1.
Summary of serum creatinine assays for AKI
| Serum Average | P Value | |
|---|---|---|
| Control | 0.29 ± 0.06 | |
| 24 h | 0.40 ± 0.1 | 0.04 |
| 48 h | 0.33 ± 0.04 | 0.20 |
| 72 h | 0.35 ± 0.05 | 0.09 |
| 168 h | 0.32 ± 0.05 | 0.37 |
n = 6 Experiments. Units are mg/dl.
Histological Analysis of the Effect of Hypoxia on Kidney Tissue
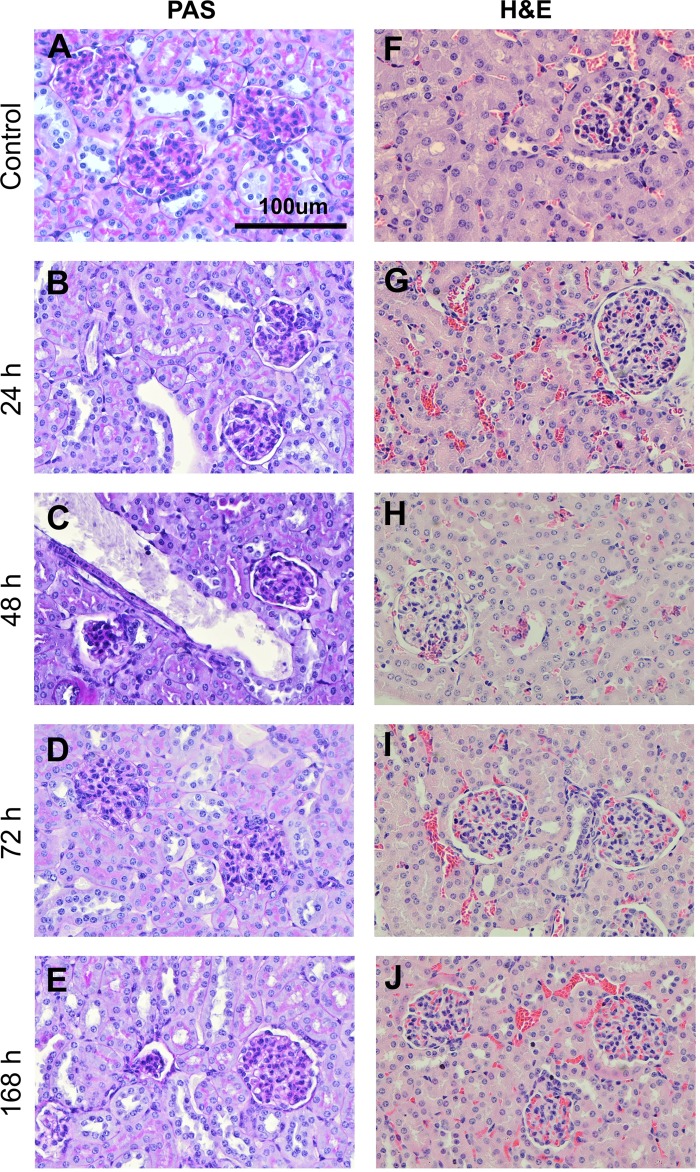
Histology was used to determine if there were any gross structural changes to the kidney tissue caused by exposure to hypoxia. A comparison between control mice and hypoxia-exposed mice showed no significant changes to glomerular or tubular structure (Fig. 1). H&E staining of cortical kidney tissue of the control samples and hypoxic tissue at all time points showed normal dense packing of renal corpuscles and proximal and distal tubules (Fig. 1, right). Hallmarks of structural damage, such as dilation of tubular lumens, hyaline casts in tubular lumens, and vacuolization (7), were absent in all hypoxic tissue, indicating lack of gross structural damage to the kidneys associated with hypoxia. In PAS-stained sections, the proximal tubule brush borders, which stained bright pink, remained easily identifiable in all hypoxic tissue, also indicating lack of gross structural damage associated with hypoxia (Fig. 1, left).
Fig. 1.
Histology analysis of cortical kidney tissue in control mice and mice subjected to hypoxia. Cortical tissue sections stained with periodic acid-Schiff (PAS) for control (A), 24 h posthypoxia (B), 48 h posthypoxia (C), 72 h posthypoxia (D), and 7 days posthypoxia (E) and stained with hematoxylin and eosin (H&E) for control (F), 24 h posthypoxia (G), 48 h posthypoxia (H), 72 h posthypoxia (I), and 7 days posthypoxia (J).
Immunohistochemical Analysis of the Effect of Hypoxia on Kidney Tissue
Immunohistochemical (IHC) staining was used to probe the expression of three protein biomarkers of AKI, including KIM-1, cystatin C, and NGAL. Normal cortical kidney tissue lacked any detectable IHC staining for KIM-1 (Fig. 2A). In contrast, after hypoxia, the kidney tissue revealed an increase in expression of KIM-1 throughout the distal tubules at the 24- and 48-h time points (Fig. 2, D and G). By 72 h posthypoxia, strong KIM-1 staining began to show up in the proximal tubules in addition to strong staining in the distal tubules, and at 168 h posthypoxia both the proximal and distal tubules had increased expression of KIM-1 (Fig. 2, J and M). Glomeruli were free from KIM-1 staining at all time points.
Fig. 2.
Immunohistochemical analysis of cortical kidney tissue in control mice and mice subjected to hypoxia. A: control cortical tissue probed with kidney injury molecule-1 (KIM-1). B: control cortical tissue probed with cystatin C. C: control cortical tissue probed with neutrophil gelatinase-associated lipocalin (NGAL). 24 h posthypoxia: hypoxia cortical tissue probed with KIM-1 (D), hypoxia cortical tissue probed with cystatin C (E), and hypoxia cortical tissue probed with NGAL (F). 48 h posthypoxia: hypoxia cortical tissue probed with KIM-1 (G), hypoxia cortical tissue probed with cystatin C (H), and hypoxia cortical tissue probed with NGAL (I). 72 h posthypoxia: hypoxia cortical tissue probed with KIM-1 (J), hypoxia cortical tissue probed with cystatin C (K), and hypoxia cortical tissue probed with NGAL (L). 7 days posthypoxia: hypoxia cortical tissue probed with KIM-1 (M), hypoxia cortical tissue probed with cystatin C (N), and hypoxia cortical tissue probed with NGAL (O).
IHC staining for cystatin C in the normal cortical tissue exhibited only weak nonspecific staining (Fig. 2B). Interestingly, the pattern of cystatin C staining as a function of time after hypoxia was distinctly different compared with KIM-1. Cystatin C staining was moderate in the distal tubules at 24 h posthypoxia, and there was almost no cystatin C staining in the proximal tubules, although some weak staining was observed in the proximal tubule brush borders (Fig. 2E). By 48 h posthypoxia, cystatin C staining in the distal tubules began to fade, and cystatin C staining started to become detectable in the cytoplasm of the proximal tubules. At 72 h posthypoxia, cystatin C staining of the distal tubules was no longer detectable, and hotspots of strong cystatin C staining of the proximal tubules emerged (Fig. 2K). At 168 h posthypoxia, cystatin C staining was absent from distal tubules, and cystatin C staining was localized in hotspots in proximal tubules (Fig. 2N). Glomeruli were free from cystatin C staining at all times posthypoxia.
Finally, the control tissue exhibited no detectable staining for NGAL (Fig. 2C). At 24 h posthypoxia, moderate NGAL staining was observed in the distal tubules, and weak NGAL staining was apparent in the proximal tubules. At 48 h posthypoxia, NGAL staining remained moderate in distal tubules and faded in proximal tubules (Fig. 2F). At 72 h posthypoxia, the NGAL staining faded even further in both distal and proximal tubules (Fig. 2I), and this trend continued at 168 h posthypoxia (Fig. 2O). Again, no NGAL staining was apparent in glomeruli at any time point posthypoxia.
Overview of 1H-NMR-Based Metabolic Profiling of Urine Samples
Urine samples were collected from 48 mice for 3 days before being placed in the hypoxia chamber and at 24, 48, 72, and 168 h after 72 h in the hypoxia chamber. 1H-NMR data collected for all samples show a general reduction in metabolite concentrations 24 h posthypoxia followed by an increase in metabolite concentrations at 48 and 72 h posthypoxia and a return to near normal levels at 7 days posthypoxia. NMR spectra were subjected to PCA and partial least-squares-discriminant analyses (PLS-DA) to identify resonances whose intensities changed significantly after hypoxia. Vetting of potentially important metabolites was evaluated based on the following four independent measures: P value <0.05, fold change >2, PLS-DA variable importance in projection (VIP) score >1, and accuracy as defined by the area under the receiver operator characteristic curve (AUROC) analysis >80%.
1H-NMR-Based Metabolic Profiling of Urine Samples Collected 24 h Posthypoxia
Statistical analysis indicated that 48 out of 155 resonances experienced a statistically significant change in intensity based on P values for comparison of prehypoxia and 24 h posthypoxia urine spectra, which corresponded to a significant decrease in 15 metabolites, including 2-oxoisocaproate, valine, ethanol, alanine, putrescine, acetate, trimethylamine, myo-inositol, fructose, trans-aconitate, benzoate, hippurate, trigonelline, Sumiki’s acid, and methyl nicotinamide (Table 2). Potentially important resonances that could not be identified were evaluated using a volcano plot analysis that yielded 27 unidentified buckets with P values <0.05 and fold changes >2 (Table 3). The PLS-DA scores plot (Fig. 3A) exhibited visible and statistically significant separation between groups supported by a Mahalanobis distance of 4.23 and an F value of 46.35 compared with an F-critical value of 3.52 with 59.4% of the variance explained by the first component and 18.6% explained by the second component. Cross-validation indicated good model quality [R2Y (cum) = 0.90] and good predictive power [Q2 (cum) value = 0.78]. PLS-DA indicated 50 significant buckets based on VIP scores >1, corresponding to 14 identified metabolites (Table 2), which included benzoate, trigonelline, 2-oxoisocaproate, acetate, isethionic acid, trimethylamine, putrecine, citrate, cis-aconitate, d-fructose, alanine, dimethylbiguanidine, valine, and creatinine. There were 27 additional potentially important unassigned NMR resonances with VIP scores >1 that could not be identified, and these are listed in Table 3.
Table 2.
List of assigned resonances that undergo a significant change in intensity at 24 h posthypoxia
| Metabolites | ppm | 24-h P Value | Fold Change | VIP | AUC |
|---|---|---|---|---|---|
| Benzoate* | 7.879 | 0.000129774 | 5.196 | 1.656 | 0.992 |
| Benzoate* | 7.487 | 0.000192433 | 4.572 | 1.608 | 0.983 |
| Benzoate | 7.554 | 0.382495751 | 1.344 | 0.731 | 0.633 |
| Trigonelline* | 8.837 | 0.00102535 | 2.150 | 1.454 | 0.908 |
| Trigonelline | 9.124 | 0.021876408 | 1.615 | 0.977 | 0.725 |
| Trigonelline | 4.477 | 0.980868399 | −1.006 | 0.867 | 0.558 |
| 2-Oxoisocaproate* | 0.907 | 0.001829616 | 10.378 | 1.397 | 0.967 |
| Acetate* | 1.922 | 0.00215223 | 4.670 | 1.320 | 0.925 |
| Isethionic acid | 3.161 | 0.537653893 | −1.196 | 1.250 | 0.508 |
| Isethionic acid | 3.963 | 0.66395565 | −1.222 | 1.066 | 0.617 |
| Trimethylamine* | 2.878 | 0.013939829 | 2.534 | 1.213 | 0.825 |
| Putrescine* | 1.753 | 0.005927442 | 4.313 | 1.183 | 0.942 |
| Citrate* | 2.539 | 0.761292586 | −1.139 | 1.180 | 0.650 |
| Citrate* | 2.673 | 0.721105489 | 1.149 | 1.025 | 0.700 |
| cis-Aconitate | 3.114 | 0.741309659 | −1.133 | 1.157 | 0.525 |
| cis-Aconitate | 5.693 | 0.946142207 | −1.020 | 1.066 | 0.542 |
| d-Fructose* | 3.605 | 0.005127687 | 3.937 | 1.155 | 0.825 |
| d-Fructose | 3.905 | 0.675923815 | 1.156 | 0.980 | 0.650 |
| d-Fructose | 4.016 | 0.648574738 | 1.222 | 0.980 | 0.717 |
| d-Fructose | 3.733 | 0.015974906 | 2.627 | 0.938 | 0.783 |
| d-Fructose | 3.699 | 0.124643849 | 1.549 | 0.775 | 0.675 |
| Alanine* | 1.482 | 0.002830962 | 2.534 | 1.145 | 0.867 |
| Dimethylbiguanide | 3.005 | 0.988145223 | −1.005 | 1.096 | 0.550 |
| Valine* | 0.993 | 0.003510802 | 2.109 | 1.069 | 0.842 |
| Valine | 1.016 | 0.018352586 | 1.969 | 0.919 | 0.808 |
| Creatinine | 3.048 | 0.819584103 | 1.055 | 1.039 | 0.592 |
| Creatinine | 4.059 | 0.414477642 | 1.254 | 0.888 | 0.667 |
| myo-Inositol | 3.300 | 0.013814655 | 2.495 | 0.958 | 0.833 |
| myo-Inositol | 3.546 | 0.015628801 | 2.088 | 0.981 | 0.792 |
| threo-Isocitric acid | 2.451 | 0.609018184 | 1.157 | 0.952 | 0.633 |
| threo-Isocitric acid | 2.504 | 0.175061158 | 1.486 | 0.846 | 0.708 |
| Sumiki's acid | 6.485 | 0.015428112 | 1.433 | 0.918 | 0.808 |
| Sumiki's acid | 6.970 | 0.210507117 | 1.274 | 0.822 | 0.675 |
| Ethanol | 1.209 | 0.032839702 | 2.107 | 0.898 | 0.783 |
| Ethanol | 3.653 | 0.312984805 | 1.355 | 0.859 | 0.583 |
| Guanidineacetic acid | 3.789 | 0.450583756 | 1.271 | 0.893 | 0.625 |
| Betaine | 3.243 | 0.427832243 | 1.301 | 0.878 | 0.633 |
| Indoxyl sulfate | 7.213 | 0.559705542 | 1.150 | 0.868 | 0.658 |
| Indoxyl sulfate | 7.271 | 0.183586981 | 1.448 | 0.797 | 0.700 |
| Indoxyl sulfate | 7.707 | 0.089806766 | 1.610 | 0.796 | 0.708 |
| trans-Aconitate | 6.590 | 0.03401648 | 1.672 | 0.848 | 0.783 |
| trans-Aconitate | 3.487 | 0.084240561 | 1.734 | 0.813 | 0.708 |
| Acetylphosphate | 2.046 | 0.294207904 | 1.358 | 0.847 | 0.683 |
| Hippurate | 7.781 | 0.03247755 | 1.668 | 0.836 | 0.750 |
| Hippurate | 7.680 | 0.262278287 | 1.870 | 0.696 | 0.525 |
| Trimethylamine N-oxide | 3.269 | 0.049777474 | 2.341 | 0.834 | 0.800 |
| Succinate | 2.405 | 0.761416962 | 1.160 | 0.795 | 0.592 |
| Dimethylamine | 2.721 | 0.138797706 | 1.420 | 0.788 | 0.692 |
| 1-Methylnicotinamide | 8.898 | 0.813283665 | 1.059 | 0.778 | 0.583 |
| 1-Methylnicotinamide | 8.941 | 0.705590071 | 1.188 | 0.388 | 0.583 |
| 3-Hydroxyisobutyric | 1.126 | 0.277114782 | 1.714 | 0.769 | 0.700 |
| Lactate | 1.332 | 0.116858519 | 4.494 | 0.723 | 0.717 |
| Lactate | 4.122 | 0.304698201 | 1.802 | 0.716 | 0.625 |
AUC, area under the curve; ppm, parts/million; VIP, variable importance in projection.
Resonances for this metabolite were in an overlapped region of the spectrum.
Table 3.
List of unassigned resonances that undergo a significant change in intensity at 24 h posthypoxia
| ppm | P Value | Fold Change | VIP | AUC |
|---|---|---|---|---|
| 3.036 | 0.006509137 | −2.681 | 1.862 | 0.792 |
| 3.935 | 0.018094533 | −2.038 | 1.805 | 0.758 |
| 4.564† | 0.02051477 | −2.118 | 1.622 | 0.808 |
| 3.228 | 0.10803403 | −1.893 | 1.501 | 0.692 |
| 6.191 | 0.45752589 | −1.119 | 1.431 | 0.592 |
| 1.572† | 0.00206267 | 7.335 | 1.358 | 0.917 |
| 1.367† | 0.002159925 | 6.279 | 1.354 | 0.917 |
| 4.524† | 0.03276429 | −2.778 | 1.349 | 0.842 |
| 7.336 | 0.39043062 | −1.275 | 1.317 | 0.583 |
| 6.205 | 0.93605235 | −1.012 | 1.252 | 0.525 |
| 1.145† | 0.002633984 | 2.635 | 1.249 | 0.875 |
| 5.381 | 0.63172448 | −1.222 | 1.167 | 0.583 |
| 5.980 | 0.92362738 | −1.011 | 1.162 | 0.508 |
| 1.184 | 0.18285475 | 1.530 | 1.153 | 0.675 |
| 2.910 | 0.005657174 | 1.940 | 1.074 | 0.850 |
| 7.637 | 0.54223718 | −1.370 | 1.074 | 0.617 |
| 1.053† | 0.004891165 | 2.435 | 1.072 | 0.850 |
| 5.836 | 0.63089122 | −1.102 | 1.059 | 0.517 |
| 3.195 | 0.98245888 | 1.007 | 1.056 | 0.542 |
| 8.082† | 0.016031552 | 2.183 | 1.053 | 0.850 |
| 2.607 | 0.92198483 | 1.027 | 1.048 | 0.533 |
| 3.313† | 0.008472165 | 2.863 | 1.039 | 0.842 |
| 7.971† | 0.003837156 | 1.887 | 1.038 | 0.867 |
| 8.967 | 0.99173706 | −1.003 | 1.029 | 0.717 |
| 4.218 | 0.94951869 | 1.017 | 1.017 | 0.508 |
| 6.292 | 0.69913899 | 1.060 | 1.011 | 0.517 |
| 7.513 | 0.008037577 | 1.942 | 1.003 | 0.783 |
| 1.847 | 0.011342844 | 2.252 | 0.996 | 0.850 |
| 2.377 | 0.012736841 | 2.721 | 0.971 | 0.817 |
| 0.965 | 0.018665457 | 1.962 | 0.931 | 0.792 |
| 1.401 | 0.020984854 | 2.036 | 0.922 | 0.800 |
| 3.422 | 0.016040094 | 1.931 | 0.914 | 0.800 |
| 6.868 | 0.025973991 | 1.737 | 0.904 | 0.783 |
| 5.198 | 0.83113497 | −1.028 | 0.902 | 0.525 |
| 1.822 | 0.022141927 | 1.916 | 0.901 | 0.783 |
| 1.409 | 0.031080587 | 1.930 | 0.893 | 0.767 |
| 1.251 | 0.036190105 | 2.261 | 0.879 | 0.783 |
| 7.165 | 0.039511743 | 1.788 | 0.878 | 0.808 |
| 1.829 | 0.034341414 | 1.869 | 0.876 | 0.750 |
| 1.835 | 0.039010562 | 1.857 | 0.868 | 0.742 |
| 6.559 | 0.030646663 | 1.327 | 0.863 | 0.708 |
AUC, area under the curve; ppm, parts/million; VIP, variable importance in projection.
Change in the intensity of the resonance was significant by all four criteria.
Fig. 3.
Two-dimensional partial least-squares-discriminant analyses (PLS-DA) scores plots for comparisons of urine samples from hypoxic mice and control mice. PLS-DA scores plots for comparison of the 24 (A)-, 48 (B)-, 72 (C)-, and 168 (D)-h urine samples from control mice (blue) and hypoxia mice (red).
Accuracy of Potential Biomarkers of AKI at 24 h Posthypoxia
Resonance peaks for all identified metabolites were subjected of AUROC analysis, and the results are presented in Table 2. Ten peaks had >80% accuracy, >2-fold change, VIP score >1, and P value <0.05 (Table 2) corresponding to benzoate, trigonelline, 2-oxoisocaproate, acetate, trimethylamine, putrecine, fructose, alanine, and valine. There were nine additional potentially important unidentified NMR resonances that satisfied all four of the vetting criteria (Table 3). These metabolites and unassigned NMR resonances that passed all four vetting criteria represent the most reliable collection of metabolites and unidentified NMR peaks that experienced a significant change in concentration at 24 h posthypoxia and represent potential biomarkers of exposure to hypoxia.
1H-NMR-Based Metabolic Profiling of Urine Samples Collected 48 h Posthypoxia
P value analysis indicated that 100 out of 155 resonances changed significantly between the prehypoxia and 48 h posthypoxia spectra. Whereas most metabolites that experienced a significant change in concentration at 24 h posthypoxia decreased compared with the controls, at 48 h posthypoxia 21 metabolites had higher concentrations compared with the controls, including 3-hydroxyisobutyric, putrescine, Sumiki’s acid, betaine, lactate, alanine, acetylphosphate, succinate, citrate, cis/trans-aconitate, threo-isocitric acid, dimethylamine, dimethylbiguanide, creatinine, isethionic acid, trimethylamine N-oxide, myo-inositol, fructose, guanidine, hippurate, and trigonelline (Table 4). Volcano plot analysis identified 36 unassigned resonances with P values <0.05 and fold changes greater than two (Table 5). The PLS-DA scores plot (Fig. 3B) exhibited visible and statistically significant group separation supported by a Mahalanobis distance of 4.57 and an F value of 54.09 compared with an F-critical value of 3.52 with 56.7% of the variance explained by the first component and 6.8% explained by the second component. Cross-validation indicated good model quality [R2Y (cum) = 0.85] and good predictive power [Q2 (cum) = 0.75]. PLS-DA indicated 21 peaks with VIP scores >1 corresponding to 15 metabolites, including 1-methylnicotinamide, trimethylamine, isethionic acid, Sumiki’s acid, ethanol, trimethylamine N-oxide, guanidineacetic acid, d-fructose, acetate, indoxyl sulfate, creatinine, acetylphosphate, benzoate, trans-aconitate, and myo-inositol (Table 4). There were 35 additional potentially important NMR resonances with VIP scores >1 that could not be identified (Table 5).
Table 4.
List of assigned resonances that undergo a significant change in intensity at 48 posthypoxia
| Metabolites | ppm | 48-h P value | Fold Change | VIP | AUC |
|---|---|---|---|---|---|
| 1-Methylnicotinamide* | 8.941 | 0.003844944 | 3.302 | 2.090 | 0.908 |
| 1-Methylnicotinamide | 9.280 | 0.778583498 | 1.057 | 1.351 | 0.583 |
| 1-Methylnicotinamide | 8.898 | 0.763495448 | 1.054 | 1.254 | 0.533 |
| Trimethylamine* | 2.878 | 0.004661616 | 6.012 | 1.935 | 0.975 |
| Isethionic acid* | 3.161 | 2.88606E-06 | −2.853 | 1.414 | 0.975 |
| Isethionic acid | 3.963 | 0.023251964 | −4.311 | 0.752 | 0.867 |
| Sumiki's acid* | 6.485 | 3.06303E-05 | −1.915 | 1.325 | 0.967 |
| Sumiki's acid | 6.970 | 0.000627532 | −1.923 | 0.997 | 0.917 |
| Sumiki's acid | 4.609 | 0.699998939 | −1.277 | 0.169 | 0.900 |
| Ethanol* | 3.653 | 3.81521E-05 | −2.898 | 1.248 | 0.942 |
| Ethanol | 1.209 | 0.00534028 | −1.869 | 0.844 | 0.883 |
| Trimethylamine N-oxide | 3.269 | 0.666734899 | −1.164 | 1.246 | 0.683 |
| Guanidineacetic acid* | 3.789 | 8.14835E-05 | −2.761 | 1.227 | 0.933 |
| d-Fructose* | 3.699 | 8.83168E-05 | −2.427 | 1.218 | 0.908 |
| d-Fructose* | 4.016 | 0.001448695 | −3.829 | 1.163 | 0.950 |
| d-Fructose* | 3.733 | 0.00099795 | −2.359 | 1.053 | 0.875 |
| d-Fructose | 3.905 | 0.001902041 | −2.385 | 0.998 | 0.875 |
| d-Fructose | 3.605 | 0.002199952 | −1.782 | 0.917 | 0.825 |
| d-Fructose | 4.641 | 0.417020478 | −1.431 | 0.353 | 0.867 |
| Acetate | 1.922 | 0.928983367 | 1.034 | 1.214 | 0.550 |
| Indoxyl sulfate | 7.213 | 0.131943866 | −1.401 | 1.108 | 0.650 |
| Indoxyl sulfate | 7.271 | 0.195070307 | −1.420 | 1.014 | 0.758 |
| Indoxyl sulfate | 7.707 | 0.032505264 | −1.606 | 0.999 | 0.767 |
| Indoxyl sulfate | 7.358 | 0.039977346 | −2.141 | 0.907 | 0.750 |
| Creatinine | 4.059 | 0.000119288 | −1.988 | 1.104 | 0.925 |
| Creatinine | 3.048 | 0.004234508 | −1.588 | 0.893 | 0.850 |
| Acetylphosphate* | 2.046 | 0.000100454 | −2.149 | 1.097 | 0.942 |
| Benzoate | 7.879 | 0.348310348 | −1.355 | 1.055 | 0.558 |
| Benzoate | 7.487 | 0.326052159 | −1.367 | 1.048 | 0.583 |
| Benzoate | 7.554 | 0.061066625 | −2.404 | 0.633 | 0.633 |
| trans-Aconitate* | 3.487 | 0.000284649 | −2.563 | 1.054 | 0.917 |
| trans-Aconitate | 6.590 | 0.013049016 | −2.290 | 0.857 | 0.833 |
| myo-Inositol | 3.546 | 0.00037499 | −1.989 | 1.048 | 0.908 |
| myo-Inositol | 3.300 | 0.022962094 | −2.118 | 0.956 | 0.775 |
| cis-Aconitate | 3.114 | 0.001062787 | −3.284 | 0.979 | 0.875 |
| cis-Aconitate | 5.693 | 0.001059784 | −2.469 | 0.980 | 0.875 |
| threo-Isocitric acid | 2.451 | 0.001729813 | −2.064 | 0.975 | 0.867 |
| threo-Isocitric acid | 2.504 | 0.007515865 | −1.984 | 0.887 | 0.808 |
| Lactate | 4.122 | 0.00287931 | −4.209 | 0.940 | 0.867 |
| Lactate | 1.332 | 0.032150893 | −4.074 | 0.756 | 0.792 |
| Hippurate | 7.680 | 0.002778232 | −4.557 | 0.928 | 0.858 |
| Hippurate | 7.781 | 0.037346743 | −1.607 | 0.901 | 0.717 |
| Hippurate | 7.824 | 0.086121069 | −6.016 | 0.558 | 0.608 |
| Citrate | 2.539 | 0.003212165 | −6.721 | 0.908 | 0.958 |
| Citrate | 2.673 | 0.007362784 | −6.151 | 0.843 | 0.917 |
| Putrescine | 1.753 | 0.030132847 | −1.505 | 0.906 | 0.742 |
| Dimethylbiguanide | 3.005 | 0.00448999 | −2.659 | 0.881 | 0.867 |
| Betaine | 3.243 | 0.004160391 | −2.132 | 0.878 | 0.833 |
| Trigonelline | 9.124 | 0.03106121 | −2.159 | 0.869 | 0.725 |
| Trigonelline | 8.837 | 0.027832498 | −2.155 | 0.854 | 0.758 |
| Trigonelline | 4.477 | 0.144127826 | −1.529 | 0.527 | 0.850 |
| Alanine | 1.482 | 0.004375133 | −2.204 | 0.860 | 0.850 |
| 3-Hydroxyisobutyric | 1.126 | 0.009498393 | −4.175 | 0.803 | 0.917 |
| Dimethylamine | 2.721 | 0.019743272 | −1.457 | 0.803 | 0.783 |
| 2-Oxoisocaproate | 0.907 | 0.272802099 | −1.878 | 0.457 | 0.508 |
AUC, area under the curve; ppm, parts/million; VIP, variable importance in projection.
Resonances for this metabolite were in an overlapped region of the spectrum.
Table 5.
List of unassigned resonances that undergo a significant change in intensity at 48 h posthypoxia
| Unidentified ppm | 48-h P value | Fold Change | VIP | AUC |
|---|---|---|---|---|
| 8.256 | 0.05430041 | 1.538 | 2.340 | 0.725 |
| 8.051 | 0.36303373 | 1.148 | 1.658 | 0.583 |
| 8.967 | 0.89060261 | 1.025 | 1.614 | 0.567 |
| 8.460† | 0.006423272 | −2.602 | 1.524 | 0.858 |
| 1.572 | 0.84955116 | 1.035 | 1.502 | 0.575 |
| 1.777 | 0.28718951 | −1.280 | 1.396 | 0.642 |
| 4.099† | 2.19197E-05 | −2.332 | 1.318 | 0.942 |
| 3.036 | 0.22836342 | −1.456 | 1.282 | 0.625 |
| 7.971 | 0.30745416 | −1.223 | 1.249 | 0.608 |
| 3.422 | 0.96821436 | 1.014 | 1.249 | 0.567 |
| 3.858† | 5.70015E-05 | −3.287 | 1.248 | 0.942 |
| 4.218† | 2.5511E-05 | −2.768 | 1.241 | 0.933 |
| 4.156† | 6.03375E-05 | −3.427 | 1.209 | 0.950 |
| 3.313 | 0.48105468 | −1.250 | 1.134 | 0.675 |
| 6.191† | 0.000664383 | −1.615 | 1.131 | 0.900 |
| 7.087 | 0.0754395 | −1.286 | 1.097 | 0.683 |
| 5.460 | 0.001418612 | −1.714 | 1.096 | 0.867 |
| 6.205 | 0.001234151 | −1.599 | 1.085 | 0.883 |
| 3.089† | 0.000123442 | −2.314 | 1.084 | 0.933 |
| 2.768† | 0.000145721 | −2.168 | 1.080 | 0.933 |
| 2.910 | 0.62277646 | −1.092 | 1.071 | 0.558 |
| 6.868 | 0.12713178 | −1.406 | 1.067 | 0.667 |
| 3.098† | 0.000162808 | −2.350 | 1.067 | 0.933 |
| 2.321† | 0.00019409 | −2.021 | 1.066 | 0.925 |
| 1.822† | 0.000184919 | −2.404 | 1.066 | 0.908 |
| 1.829† | 0.00018501 | −2.350 | 1.064 | 0.917 |
| 7.513 | 0.06341882 | −1.612 | 1.050 | 0.675 |
| 6.559 | 0.20988375 | −1.158 | 1.045 | 0.683 |
| 2.621† | 0.000344016 | −2.538 | 1.041 | 0.942 |
| 7.386 | 0.06521209 | −1.504 | 1.039 | 0.675 |
| 1.835† | 0.000318562 | −2.272 | 1.031 | 0.908 |
| 8.332 | 0.7394379 | −1.046 | 1.030 | 0.558 |
| 2.607 | 0.000414066 | −1.912 | 1.020 | 0.917 |
| 1.184 | 0.017224093 | −1.940 | 1.017 | 0.792 |
| 6.916 | 0.034406931 | −1.441 | 1.004 | 0.725 |
| 4.292 | 0.000582588 | −2.823 | 0.996 | 0.900 |
| 2.181 | 0.000736743 | −1.782 | 0.988 | 0.892 |
| 8.113 | 0.001367306 | −2.612 | 0.980 | 0.925 |
| 1.789 | 0.001403549 | −2.239 | 0.975 | 0.875 |
| 4.376 | 0.007354679 | −1.837 | 0.951 | 0.833 |
| 3.195 | 0.002947806 | −2.161 | 0.949 | 0.842 |
| 2.271 | 0.002631495 | −1.762 | 0.949 | 0.875 |
| 2.823 | 0.001539265 | −1.747 | 0.949 | 0.858 |
| 1.847 | 0.012424051 | −1.768 | 0.942 | 0.792 |
| 7.336 | 0.010485388 | −1.762 | 0.941 | 0.808 |
| 3.131 | 0.018010538 | −1.611 | 0.935 | 0.808 |
| 3.228 | 0.001949273 | −3.860 | 0.925 | 0.883 |
| 7.130 | 0.007712232 | −1.647 | 0.921 | 0.775 |
| 7.044 | 0.003265269 | −1.640 | 0.917 | 0.850 |
| 3.356 | 0.013167695 | −1.646 | 0.917 | 0.800 |
| 1.081 | 0.003215373 | −2.366 | 0.913 | 0.908 |
| 1.099 | 0.004303299 | −3.002 | 0.889 | 0.942 |
| 7.456 | 0.012594605 | −1.917 | 0.885 | 0.742 |
| 1.251 | 0.004016398 | −2.601 | 0.876 | 0.900 |
| 3.205 | 0.006980481 | −2.457 | 0.861 | 0.817 |
| 8.082 | 0.005524891 | −1.933 | 0.845 | 0.875 |
| 6.525 | 0.016814629 | −1.489 | 0.843 | 0.758 |
| 8.140 | 0.008510142 | −3.526 | 0.838 | 0.883 |
| 7.735 | 0.006672847 | −2.257 | 0.834 | 0.833 |
| 7.591 | 0.007409575 | −2.617 | 0.828 | 0.800 |
| 1.464 | 0.00939605 | −2.060 | 0.813 | 0.825 |
| 1.425 | 0.013194283 | −2.518 | 0.799 | 0.917 |
| 7.424 | 0.013508336 | −2.063 | 0.782 | 0.842 |
| 8.488 | 0.24975498 | −1.420 | 0.768 | 0.800 |
| 7.762 | 0.035293812 | −1.811 | 0.757 | 0.733 |
| 5.836 | 0.015957669 | −1.718 | 0.742 | 0.742 |
| 5.381 | 0.022978214 | −2.880 | 0.735 | 0.692 |
| 1.409 | 0.022136789 | −2.801 | 0.725 | 0.875 |
| 1.401 | 0.025340424 | −2.893 | 0.711 | 0.875 |
| 5.980 | 0.023149341 | −1.762 | 0.710 | 0.758 |
| 0.938 | 0.03380896 | −3.334 | 0.667 | 0.867 |
| 0.965 | 0.036755429 | −2.311 | 0.655 | 0.800 |
| 1.053 | 0.038983287 | −1.686 | 0.651 | 0.775 |
| 4.440 | 0.14139003 | −2.043 | 0.616 | 0.825 |
| 1.367 | 0.07658471 | −2.106 | 0.599 | 0.692 |
| 7.637 | 0.08519504 | −5.814 | 0.561 | 0.642 |
| 4.564 | 0.71467401 | −1.284 | 0.190 | 0.883 |
| 4.524 | 0.81120693 | −1.228 | 0.168 | 0.883 |
AUC, area under the curve; ppm, parts/million; VIP, variable importance in projection.
Change in the intensity of the resonance was significant by all four criteria.
Accuracy of Potential Biomarkers of AKI at 48 h Posthypoxia
Eleven peaks had >80% accuracy, >2-fold change, VIP score >1, and P value <0.05 (Table 4) corresponding to benzoate, trigonelline, 2-oxoisocaproate, acetate, trimethylamine, putrecine, fructose, alanine, and valine. Fourteen additional NMR peaks also satisfied all four of the vetting criteria, and these are listed in Table 5. These metabolites and unassigned NMR resonances represent the most reliable collection of metabolites and unidentified NMR peaks that experienced a significant change in concentration at 48 h posthypoxia and represent potential biomarkers of exposure to hypoxia.
1H-NMR-Based Metabolic Profiling of Urine Samples Collected 72 h Posthypoxia
Statistical analysis indicated that 36 out of 155 resonances experienced statistically significant intensity changes between prehypoxia and 72 h posthypoxia spectra corresponding to lactate, alanine, acetate, dimethylamine, creatinine, indoxyl-sulfate, benzoate, and methyl-nicotinamide (Table 6). Volcano plot analysis identified eight additional potentially important unassigned NMR resonances with P values <0.05 and fold changes >2 (Table 7). The PLS-DA scores plot (Fig. 3C) exhibited modest but statistically insignificant separation with a Mahalanobis distance of 2.91 and an F value of 18.43 compared with an F-critical value of 3.63 with 56.9% of the variance explained by the first component and 15.8% explained by the second component. Cross-validation indicated good model quality [R2Y (cum) = 0.87] but only moderate predictive power [Q2 (cum) = 0.66]. PLS-DA identified 27 NMR resonances with VIP scores >1 corresponding to 2-oxoisocaproate, trimethylamine-N-oxide, benzoate, betaine, d-fructose, trimethylamine, alanine, cis-aconitate, 1-methylnicotinamide, Sumiki’s acid, indoxyl sulfate, hippurate, lactate, acetate, isethionic acid, trans-aconitate, guanidine acetic acid, 3-hydroxybutyric acid, and creatinine (Table 6). There were 32 additional potentially important NMR resonances with VIP scores >1 that could not be identified (Table 7).
Table 6.
List of assigned resonances that undergo a significant change in intensity at 72 h posthypoxia
| Metabolites | ppm | 72-h P value | Fold Change | VIP | AUC |
|---|---|---|---|---|---|
| 2-Oxoisocaproate | 0.907 | 0.665200686 | 1.145 | 1.563 | 0.511 |
| Trimethylamine N-oxide | 3.269 | 0.683757724 | 1.154 | 1.519 | 0.511 |
| Benzoate | 7.879 | 0.027736072 | −3.057 | 1.414 | 0.830 |
| Benzoate | 7.487 | 0.025178825 | −2.701 | 1.411 | 0.807 |
| Benzoate | 7.554 | 0.495765072 | −1.250 | 1.041 | 0.545 |
| Betaine | 3.243 | 0.634824836 | 1.189 | 1.379 | 0.523 |
| d-Fructose | 3.905 | 0.50873211 | 1.276 | 1.324 | 0.534 |
| d-Fructose | 4.016 | 0.451011233 | 1.339 | 1.312 | 0.591 |
| d-Fructose | 3.733 | 0.624044729 | 1.185 | 1.262 | 0.523 |
| d-Fructose | 4.641 | 0.303251846 | 1.476 | 1.122 | 0.568 |
| Trimethylamine | 2.878 | 0.127594079 | −1.979 | 1.315 | 0.727 |
| Alanine | 1.482 | 0.006418194 | −2.423 | 1.262 | 0.864 |
| cis-Aconitate | 5.693 | 0.153824949 | −1.477 | 1.238 | 0.682 |
| cis-Aconitate | 3.114 | 0.319643873 | −1.407 | 0.824 | 0.670 |
| 1-Methylnicotinamide | 8.205 | 0.006816209 | −2.142 | 1.225 | 0.864 |
| 1-Methylnicotinamide | 8.941 | 0.410475738 | −1.307 | 1.206 | 0.727 |
| 1-Methylnicotinamide | 9.280 | 0.673375581 | −1.149 | 0.759 | 0.511 |
| 1-Methylnicotinamide | 8.898 | 0.424973825 | −1.359 | 0.669 | 0.602 |
| Sumiki's acid | 4.609 | 0.13751331 | 2.022 | 1.203 | 0.659 |
| Sumiki's acid | 6.485 | 0.829412829 | 1.053 | 1.102 | 0.500 |
| Indoxyl sulfate | 7.358 | 0.86197806 | 1.061 | 1.190 | 0.545 |
| Indoxyl sulfate | 7.707 | 0.353053752 | −1.280 | 0.804 | 0.670 |
| Indoxyl sulfate | 7.213 | 0.043564136 | −1.781 | 0.799 | 0.773 |
| Indoxyl sulfate | 7.271 | 0.077814986 | −1.609 | 0.785 | 0.761 |
| Hippurate | 7.824 | 0.857729839 | −1.076 | 1.185 | 0.557 |
| Hippurate | 7.680 | 0.879563072 | −1.054 | 0.895 | 0.545 |
| Hippurate | 7.781 | 0.15570119 | −1.479 | 0.850 | 0.727 |
| Lactate | 1.332 | 0.012295895 | −3.296 | 1.181 | 0.852 |
| Acetate | 1.922 | 0.02028213 | −2.594 | 1.177 | 0.807 |
| myo-Inositol | 3.300 | 0.857086379 | −1.069 | 1.149 | 0.568 |
| myo-Inositol | 3.546 | 0.198287503 | −1.391 | 0.672 | 0.705 |
| Isethionic acid | 3.963 | 0.793373292 | −1.104 | 1.142 | 0.511 |
| Isethionic acid | 3.161 | 0.195158898 | −1.512 | 0.782 | 0.716 |
| trans-Aconitate | 3.487 | 0.832702286 | −1.070 | 1.081 | 0.614 |
| trans-Aconitate | 6.590 | 0.329370704 | −1.483 | 0.623 | 0.602 |
| Guanidineacetic acid | 3.789 | 0.940239682 | −1.023 | 1.038 | 0.545 |
| 3-Hydroxyisobutyric | 1.126 | 0.854154735 | −1.081 | 1.013 | 0.659 |
| Creatinine | 3.048 | 0.02215601 | −1.646 | 1.010 | 0.795 |
| Creatinine | 4.059 | 0.073006945 | −1.515 | 0.821 | 0.750 |
| Dimethylamine | 2.721 | 0.040216322 | −1.663 | 0.917 | 0.750 |
| Putrescine | 1.753 | 0.177510646 | −1.407 | 0.910 | 0.739 |
| Ethanol | 1.209 | 0.442675863 | −1.237 | 0.893 | 0.648 |
| Ethanol | 3.653 | 0.276054143 | −1.328 | 0.813 | 0.636 |
| Acetylphosphate | 2.046 | 0.159000279 | −1.477 | 0.813 | 0.705 |
| Succinate | 2.405 | 0.063856472 | −1.975 | 0.800 | 0.705 |
| Valine | 1.016 | 0.260379445 | −1.442 | 0.768 | 0.705 |
| Valine | 0.993 | 0.137121106 | −1.733 | 0.747 | 0.818 |
| threo-Isocitric acid | 2.451 | 0.065367075 | −1.819 | 0.742 | 0.750 |
| threo-Isocitric acid | 2.504 | 0.150216366 | −1.565 | 0.717 | 0.739 |
| Trigonelline | 4.477 | 0.588856426 | −1.135 | 0.723 | 0.591 |
| Trigonelline | 8.837 | 0.394839552 | −1.416 | 0.693 | 0.614 |
| Trigonelline | 9.124 | 0.3552089 | −1.457 | 0.665 | 0.602 |
| Dimethylbiguanide | 3.005 | 0.232931607 | −1.578 | 0.705 | 0.682 |
| Citrate | 2.673 | 0.124007035 | −2.687 | 0.668 | 0.795 |
| Citrate | 2.539 | 0.179350784 | −2.532 | 0.621 | 0.773 |
AUC, area under the curve; ppm, parts/million; VIP, variable importance in projection.
Table 7.
List of unassigned resonances that undergo a significant change in intensity at 72 h posthypoxia
| Unidentified ppm |
72-h P Value | Fold Change | VIP | AUC |
|---|---|---|---|---|
| 5.836† | 0.001177288 | −2.328 | 2.010 | 0.864 |
| 5.804 | 0.003163907 | −1.612 | 1.514 | 0.909 |
| 6.191 | 0.006967516 | −1.633 | 1.406 | 0.841 |
| 8.140 | 0.018983458 | −2.257 | 1.393 | 0.784 |
| 7.424 | 0.40468555 | 1.507 | 1.384 | 0.523 |
| 1.777 | 0.83071445 | 1.079 | 1.358 | 0.614 |
| 8.082 | 0.92235448 | 1.033 | 1.307 | 0.511 |
| 2.898† | 0.004070401 | −2.347 | 1.291 | 0.841 |
| 5.980† | 0.026473942 | −2.149 | 1.279 | 0.864 |
| 7.971 | 0.046694814 | −1.668 | 1.210 | 0.761 |
| 5.252 | 0.39846061 | 1.298 | 1.196 | 0.568 |
| 3.205 | 0.8486154 | 1.062 | 1.192 | 0.500 |
| 8.113 | 0.90918318 | 1.037 | 1.192 | 0.568 |
| 7.637 | 0.82420843 | −1.095 | 1.178 | 0.557 |
| 7.130† | 0.0079266 | −2.130 | 1.161 | 0.852 |
| 4.564 | 0.25177995 | 1.567 | 1.154 | 0.602 |
| 0.938 | 0.93626578 | 1.035 | 1.148 | 0.636 |
| 3.313 | 0.78382599 | −1.104 | 1.140 | 0.614 |
| 2.851† | 0.006590004 | −2.334 | 1.112 | 0.864 |
| 4.440 | 0.92998582 | 1.027 | 1.104 | 0.500 |
| 4.156 | 0.81059932 | −1.078 | 1.095 | 0.636 |
| 7.929 | 0.84729202 | −1.068 | 1.087 | 0.523 |
| 8.332 | 0.94065354 | 1.023 | 1.082 | 0.602 |
| 8.256 | 0.7482656 | −1.158 | 1.070 | 0.636 |
| 6.205† | 0.031837241 | −1.525 | 1.064 | 0.807 |
| 3.858 | 0.9303192 | −1.027 | 1.061 | 0.580 |
| 4.292 | 0.77356006 | −1.089 | 1.041 | 0.591 |
| 6.386 | 0.043776052 | −1.484 | 1.040 | 0.739 |
| 6.292 | 0.12695237 | −1.371 | 1.018 | 0.716 |
| 6.916 | 0.010081087 | −1.804 | 1.014 | 0.875 |
| 5.460 | 0.033312526 | −1.576 | 1.013 | 0.761 |
| 1.464 | 0.014643153 | −1.892 | 1.002 | 0.841 |
| 2.910 | 0.013912417 | −1.859 | 0.945 | 0.841 |
| 1.367 | 0.014669784 | −1.831 | 0.941 | 0.818 |
| 1.847 | 0.025676098 | −1.759 | 0.932 | 0.864 |
| 6.559 | 0.016439639 | −1.750 | 0.929 | 0.864 |
| 7.307 | 0.026630695 | −1.814 | 0.920 | 0.818 |
| 1.145 | 0.033046408 | −2.534 | 0.914 | 0.841 |
| 6.525 | 0.030635844 | −2.188 | 0.901 | 0.864 |
| 7.513 | 0.038071269 | −1.567 | 0.881 | 0.795 |
| 6.868 | 0.023114784 | −1.966 | 0.865 | 0.818 |
| 7.456 | 0.04257945 | −1.617 | 0.846 | 0.795 |
| 7.044 | 0.036338081 | −1.660 | 0.833 | 0.773 |
| 3.098 | 0.044720211 | −1.712 | 0.829 | 0.784 |
| 1.184 | 0.047181535 | −1.838 | 0.828 | 0.750 |
AUC, area under the curve; ppm, parts/million; VIP, variable importance in projection.
Change in the intensity of the resonance was significant by all four criteria.
Accuracy of Potential Biomarkers of AKI at 72 h Posthypoxia
Six peaks had >80% accuracy, >2-fold change, VIP score >1, and P value <0.05 (Table 6) corresponding benzoate, alanine, 1-methylnicotinamide, lactate, and acetate. Six additional NMR peaks also satisfied all four of the vetting criteria, and these are listed in Table 7. These metabolites and unassigned NMR resonances represent the most reliable collection of metabolites and unidentified NMR peaks that experienced a significant change in concentration at 72 h posthypoxia and represent potential biomarkers of exposure to hypoxia.
1H-NMR-Based Metabolic Profiling of Urine Samples Collected 168 h Posthypoxia
Statistical analysis indicated that six NMR resonances experienced significant changes corresponding to alanine, trimethylamine, creatinine, d-fructose, isethionic acid, and 1-methylnicotinamide (Table 8). Volcano plot analysis identified five additional potentially important unassigned NMR resonances with P values <0.05 and fold changes >2 (Table 9). The PLS-DA scores plot (Fig. 3D) exhibited marginal separation with a Mahalanobis distance of 3.51 and an F value of 32.19 compared with an F-critical value of 3.46 with 47.6% of the variance explained by the first component and 20.1% explained by the second component. Cross-validation indicated good model quality [R2Y (cum) = 0.77] and good predictive power [Q2 (cum) = 0.75]. PLS-DA identified 19 NMR resonances with VIP scores >1 corresponding to trimethylamine, alanine, hippurate, creatinine, trigonelline, d-fructose, 3-hydroxyisobutyric acid, 2-oxoisocaproate, isethionic acid, myo-inositol, cis-aconitate, dimethylbiguanidine, 1-methylnicotinamide, and betaine. There were 33 additional potentially important NMR resonances with VIP scores >1 that could not be identified (Table 9).
Table 8.
List of assigned resonances that undergo a significant change in intensity at 168 h posthypoxia
| Metabolites | ppm | 168-h P Value | Fold Change | VIP | AUC |
|---|---|---|---|---|---|
| Trimethylamine* | 2.878 | 0.005237464 | 2.990 | 1.623 | 0.826 |
| Alanine | 1.482 | 0.004733452 | −1.907 | 1.496 | 0.876 |
| Hippurate | 7.824 | 0.383418902 | 1.919 | 1.387 | 0.669 |
| Hippurate | 7.781 | 0.924060435 | −1.021 | 1.079 | 0.529 |
| Hippurate | 7.680 | 0.986256144 | −1.008 | 1.041 | 0.562 |
| Creatinine | 3.048 | 0.010147793 | −1.393 | 1.226 | 0.777 |
| Creatinine | 4.059 | 0.055712789 | −1.393 | 1.008 | 0.752 |
| Trigonelline | 9.124 | 0.276302446 | 1.623 | 1.224 | 0.612 |
| Trigonelline | 8.837 | 0.267466063 | 1.553 | 1.215 | 0.645 |
| d-Fructose* | 4.641 | 0.026678948 | −2.125 | 1.195 | 0.851 |
| d-Fructose | 3.605 | 0.802115334 | −1.073 | 1.032 | 0.537 |
| d-Fructose | 3.733 | 0.51166055 | −1.305 | 0.910 | 0.628 |
| d-Fructose | 3.699 | 0.170264542 | −1.500 | 0.893 | 0.711 |
| d-Fructose | 3.905 | 0.448886254 | −1.386 | 0.890 | 0.645 |
| d-Fructose | 4.016 | 0.498782251 | −1.404 | 0.867 | 0.653 |
| 3-Hydroxyisobutyric | 1.126 | 0.736324646 | 1.153 | 1.178 | 0.636 |
| 2-Oxoisocaproate | 0.907 | 0.870378952 | 1.047 | 1.171 | 0.512 |
| Isethionic acid | 3.161 | 0.020442884 | −1.761 | 1.127 | 0.818 |
| myo-Inositol | 3.300 | 0.960711134 | 1.016 | 1.108 | 0.529 |
| myo-Inositol | 3.546 | 0.09395062 | −1.485 | 0.957 | 0.694 |
| cis-Aconitate | 3.114 | 0.946809292 | 1.020 | 1.107 | 0.579 |
| cis-Aconitate | 5.693 | 0.689561323 | −1.092 | 0.888 | 0.579 |
| Dimethylbiguanide | 3.005 | 0.951493279 | 1.015 | 1.063 | 0.529 |
| 1-Methylnicotinamide | 8.941 | 0.043055172 | 1.997 | 1.029 | 0.702 |
| 1-Methylnicotinamide | 9.280 | 0.735239408 | 1.071 | 0.749 | 0.537 |
| 1-Methylnicotinamide | 8.898 | 0.754563498 | 1.068 | 0.447 | 0.562 |
| Betaine | 3.243 | 0.909095455 | −1.036 | 1.024 | 0.711 |
| Succinate | 2.405 | 0.879224753 | 1.066 | 0.954 | 0.653 |
| Acetylphosphate | 2.046 | 0.188565114 | −1.270 | 0.928 | 0.727 |
| Lactate | 1.332 | 0.085813792 | −2.587 | 0.922 | 0.686 |
| Lactate | 4.122 | 0.210007164 | −1.795 | 0.813 | 0.669 |
| Sumiki's acid | 6.970 | 0.588884304 | −1.103 | 0.911 | 0.694 |
| Sumiki's acid | 4.609 | 0.098209184 | −2.110 | 0.895 | 0.777 |
| Sumiki's acid | 6.485 | 0.679355218 | −1.110 | 0.821 | 0.752 |
| Valine | 0.993 | 0.071519276 | −1.266 | 0.907 | 0.736 |
| Valine | 1.016 | 0.186895842 | −1.199 | 0.861 | 0.686 |
| Ethanol | 3.653 | 0.283334914 | −1.348 | 0.906 | 0.653 |
| Ethanol | 1.209 | 0.174038668 | −1.307 | 0.880 | 0.736 |
| Guanidineacetic acid | 3.789 | 0.284697335 | −1.443 | 0.885 | 0.694 |
| Dimethylamine | 2.721 | 0.1009823 | −1.265 | 0.872 | 0.686 |
| Indoxyl sulfate | 7.271 | 0.207652298 | −1.409 | 0.823 | 0.653 |
| Indoxyl sulfate | 7.707 | 0.34065528 | −1.280 | 0.808 | 0.645 |
| Indoxyl sulfate | 7.358 | 0.265068631 | −1.607 | 0.779 | 0.653 |
| Citrate | 2.539 | 0.161666014 | −1.579 | 0.762 | 0.711 |
| Citrate | 2.673 | 0.418746589 | −1.266 | 0.663 | 0.645 |
| Putrescine | 1.753 | 0.48871098 | −1.114 | 0.721 | 0.628 |
| Benzoate | 7.879 | 0.165257291 | −1.494 | 0.719 | 0.661 |
| Benzoate | 7.487 | 0.16570031 | −1.478 | 0.710 | 0.653 |
| Acetate | 1.922 | 0.519496953 | 1.238 | 0.360 | 0.537 |
AUC, area under the curve; ppm, parts/million; VIP, variable importance in projection.
Resonances for this metabolite were in an overlapped region of the spectrum.
Table 9.
List of unassigned resonances that undergo a significant change in intensity at 168 h posthypoxia
| Unidentified ppm | 168-h P Value | Fold Change | VIP | AUC |
|---|---|---|---|---|
| 3.036† | 0.009082233 | −2.065 | 1.468 | 0.826 |
| 8.051 | 0.11092471 | 1.317 | 1.430 | 0.653 |
| 7.637 | 0.37675145 | 1.911 | 1.392 | 0.620 |
| 4.376 | 0.003025501 | −1.804 | 1.382 | 0.851 |
| 8.460 | 0.02486285 | −8.053 | 1.349 | 0.769 |
| 3.205 | 0.55776239 | 1.215 | 1.332 | 0.554 |
| 5.460 | 0.004509224 | −1.598 | 1.330 | 0.793 |
| 3.422† | 0.004752437 | −2.034 | 1.321 | 0.826 |
| 4.524† | 0.025250348 | −3.985 | 1.298 | 0.876 |
| 2.910 | 0.17192662 | 1.240 | 1.259 | 0.702 |
| 7.971 | 0.010619589 | −1.412 | 1.247 | 0.793 |
| 7.929 | 0.59710698 | 1.181 | 1.243 | 0.521 |
| 8.256 | 0.06857343 | 1.339 | 1.239 | 0.669 |
| 4.564† | 0.029556548 | −2.947 | 1.232 | 0.860 |
| 2.851 | 0.017136172 | −1.332 | 1.197 | 0.810 |
| 7.762 | 0.63891295 | 1.139 | 1.196 | 0.603 |
| 4.099 | 0.013056691 | −1.790 | 1.196 | 0.835 |
| 0.938 | 0.75117021 | 1.127 | 1.181 | 0.628 |
| 3.935 | 0.014337673 | −1.624 | 1.174 | 0.810 |
| 5.980 | 0.037113267 | −1.421 | 1.172 | 0.777 |
| 7.044 | 0.84014584 | 1.031 | 1.171 | 0.521 |
| 7.735 | 0.84096137 | 1.060 | 1.157 | 0.512 |
| 1.425 | 0.019558034 | −1.412 | 1.152 | 0.810 |
| 3.228 | 0.86609577 | 1.079 | 1.145 | 0.570 |
| 1.099 | 0.8935225 | 1.041 | 1.134 | 0.587 |
| 5.105 | 0.09849226 | −1.605 | 1.131 | 0.678 |
| 7.087 | 0.44809571 | 1.248 | 1.128 | 0.504 |
| 2.621 | 0.8996603 | 1.047 | 1.111 | 0.678 |
| 4.218 | 0.029162409 | −1.644 | 1.091 | 0.818 |
| 7.165 | 0.72609993 | 1.085 | 1.083 | 0.562 |
| 3.195 | 0.99123466 | −1.003 | 1.067 | 0.504 |
| 5.381 | 0.89440174 | −1.051 | 1.018 | 0.504 |
| 6.205 | 0.038392384 | −1.257 | 1.012 | 0.769 |
| 7.336 | 0.06190944 | −1.568 | 0.942 | 0.802 |
AUC, area under the curve; ppm, parts/million; VIP, variable importance in projection.
Change in the intensity of the resonance was significant by all four criteria.
Accuracy of Potential Biomarkers of AKI at 168 h Posthypoxia
Two peaks had >80% accuracy, >2-fold change, VIP score >1, and P value <0.05 (Table 8) corresponding to trimethylamine and d-fructose. Four additional NMR peaks also satisfied all four of the vetting criteria, and these are listed in Table 9. These metabolites and unassigned NMR resonances represent the most reliable collection of metabolites and unidentified NMR peaks that experienced a significant change in concentration at 168 h posthypoxia and represent potential biomarkers of exposure to hypoxia.
Altered Metabolic Pathways
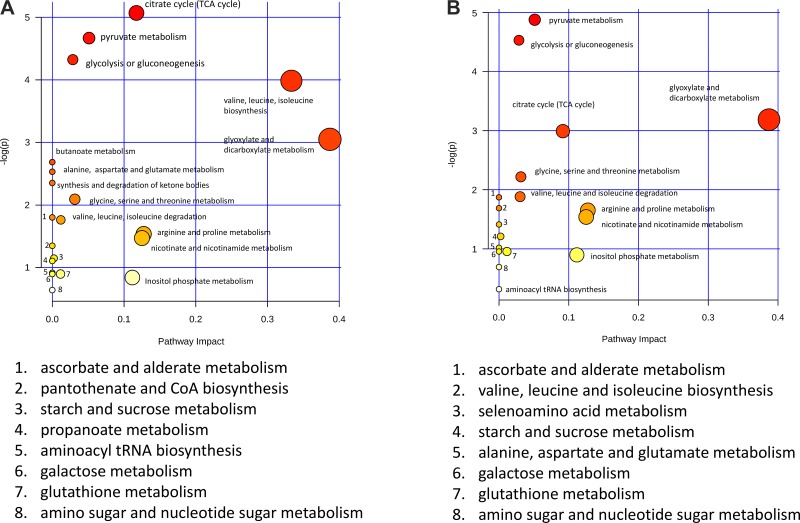
The initial response to hypoxia measured at 24 h posthypoxia resulted in a decrease in the urinary concentrations of 30 metabolites. A pathway analysis of these metabolites (Fig. 4A) revealed that these metabolites belonged to 20 metabolic pathways. Of these, six of the affected metabolic pathways had P values <0.05 based on the pathway enrichment algorithm (62) (Fig. 4A). Of these pathways, the tricarboxylic acid (TCA) cycle, pyruvate metabolism, and glycolysis or gluconeogenesis pathways all directly refer to energy metabolism pathways. The remaining three pathways, valine, leucine, and isoleucine biosynthesis and degradation and glyoxylate and dicarboxylate metabolism, reflect energy utilization. Because the metabolites associated with these pathways all decreased 24 h posthypoxia, this reflected decreased activity of these pathways at this time point.
Fig. 4.
Pathway analysis based on metabolic profiling comparisons at 24 and 48 h posthypoxia. A: MetaboAnalyst pathway analysis for the 24-h sample comparison calculated using the 30 metabolites identified from the nuclear magnetic resonance spectroscopy (NMR)-based metabolic profiling analysis. The metabolic pathways are either labeled in the plot or indicated on bottom. The pathway significance (y-axis) is based on the pathway enrichment algorithm and is indicated by intensity of the color shading, and the pathway impact (x-axis) is determined by pathway topology analysis and is indicated by the diameter of the circle representing the pathway. TCA, tricarboxylic acid. B: MetaboAnalyst pathway analysis for the 48-h sample comparison calculated using the 28 metabolites identified from the nuclear magnetic resonance spectroscopy (NMR)-based metabolic profiling analysis.
At 48 h posthypoxia, six of the top seven most significant pathways identified in the pathway analysis matched those identified at 24 h posthypoxia, but, at 48 h posthypoxia, these metabolites were at elevated concentrations relative to the control mice (Fig. 4B), whereas the same metabolites involved in these top-ranked metabolic pathways were observed at decreased concentrations. The fact that these same metabolites associated with energy and related metabolism pathways were increased at 48 and 72 h posthypoxia compared with the prehypoxia controls indicated that the organism energy metabolism was elevated compared with the prehypoxia state, indicating a burst of energy metabolism associated with kidney injury and a period of organism recovery at these time points following cessation of hypoxia.
DISCUSSION
The goal of this study was to determine hallmarks of hypoxia-induced kidney injury using a combination of histology, immunohistochemistry staining for protein kidney injury biomarkers, and urinary metabolic profiling. For urinary metabolic profiling, urine samples were collected from mice for 3 days before hypoxic insult, mice were then housed in a hypoxia chamber at 6.5% O2 concentration for 72 h, and the state of hypoxia was then ceased as the mice were reintroduced to a normoxic environment. Urine samples were collected at 24 h, 48 h, 72 h, or 7 days after reintroduction to a normoxic environment. After overnight urine collections in metabolic cages, the mice were euthanized to enable harvesting of their kidneys for histology and immunohistochemistry analysis and harvesting of blood samples for clinical assays of AKI. Clinical assays indicated mild AKI in the first 24 h posthypoxia based on elevated plasma creatinine levels. Plasma creatinine levels returned to normal 48 h posthypoxia, suggesting recovery of kidney function based on plasma creatinine levels.
Conventional histological analysis of H&E- and PAS-stained cortical sections of the kidneys exposed to hypoxia showed no significant changes in nephron structures compared with the controls. However, IHC staining revealed distinct and interesting spatial and transient patterns of expression of protein AKI biomarkers. For example, at 24 h posthypoxia, KIM-1was strongly expressed in distal tubule epithelia but not expressed in proximal tubule epithelia or glomeruli. KIM-1 staining at 48 h posthypoxia was similar to that at 24 h posthypoxia. By 72 h posthypoxia, however, KIM-1 staining was very strong in distal tubule epithelia and emerging in proximal tubule epithelia, but still absent in glomeruli. By 7 days posthypoxia (168 h) KIM-1 staining was strong and evenly distributed in both distal and proximal tubule epithelia, but completely absent from glomeruli. In striking contrast, cystatin C staining at 24 h posthypoxia was initially strong in distal tubule epithelia but increasingly dissipated at 48 and 72 h posthypoxia and was completely absent in distal tubules by 7 days posthypoxia. Cystatin C staining in proximal tubule epithelia mirrored that of KIM-1, initially absent in proximal tubule epithelia but increasing with time until it reached a maximum at 7 days posthypoxia. As with KIM-1, cystatin C staining was absent in glomeruli. NGAL staining mirrored that of KIM-1, except that the staining intensity was weaker at all time points.
These results reveal an intriguing pattern of expression of kidney injury biomarkers in that cystatin C expression was maximum in distal tubule epithelia at the initial 24 h posthypoxia point and disappeared by 7 days posthypoxia, whereas KIM-1 and NGAL expression in distal tubules was initially absent and increased to its maximum value at 7 days. All markers were expressed at very low or undetectable levels in proximal tubule epithelia at 24 h posthypoxia and continued to increase out to 7 days. These patterns were distinct from those observed in our mouse model study of ischemia reperfusion injury-induced AKI where all of the biomarkers were expressed strongly in proximal tubule epithelia but completely absent distal tubule epithelia (7).
NMR-based metabolic profiling indicated a general decrease in urine metabolites at 24 h posthypoxia, followed by a surge in metabolite concentrations at 48 and 72 h posthypoxia and return to near normal concentrations at 7 days posthypoxia. Our previous study of IRI-induced AKI indicated ultrastructural and functional changes to the kidney that caused urine dilution (7). The urine dilution was due to a combination of loss of water reabsorption by damaged proximal tubule brush borders and by obstructed flow from the distal tubules in the ureter by a widespread distribution of protein casts in distal tubules (7). This combination led to increased water retention in the nephrons that caused nephron distension and reduced volume of urine output that was also diluted compared with normal urine. In the study reported here, histological analysis indicated no evidence of brush border damage, no evidence of proteinaceous casts in the distal tubules, and no evidence of nephron distension. Consequently, urine dilution was ruled out as an explanation for decreased urinary metabolite concentrations at 24 h posthypoxia.
Decreased urinary concentrations of many metabolites was, however, consistent with systemic metabolic suppression in response to hypoxia. It is well established that hypoxia causes suppression of cellular metabolism in response to oxygen accessibility limitations (16, 39, 58). Another physiological response to limited oxygen access has been referred to as oxygen conformance (20), which describes a physiological adaptation to low oxygen access that results in reduced mitochondrial oxygen consumption, i.e., respiration rate, to reduce cellular ATP demands in an attempt to avoid apoptosis (58). At the cellular level, oxygen conformance and reduced respiration involve activation of hypoxia-inducible factor-1 (HIF-1) (9, 47, 58). Under normoxic conditions, HIF-1α is destined for degradation, following a hydroxylation process that requires oxygen. Hypoxia results in decreased available oxygen in cells that disrupts the hydroxylation process, resulting in activation of HIF-1α. HIF-1α prevents cells from anoxic cell death by altering multiple metabolic processes. Activation of HIF-1α reduces the carbon supply for the TCA cycle, disrupting many biosynthetic pathways. HIF-1α recruits pyruvate dehydrogenase kinase (PDK1) (9) and lactate dehydrogenase A (LDH-A) (11, 58). PDK1 disrupts the conversion of pyruvate to acetyl-CoA, and LDH-A actively increases pyruvate to lactate turnover. LDH-A conversion of pyruvate to lactate is also coupled with NADH oxidation, increasing the NAD+ concentration, which increases glycolysis (11, 58). HIF-1 recruitment of PDK1 and LDH-A reduces the amount of pyruvate entering the TCA cycle. Reduced TCA cycle activity would greatly reduce the concentration of several metabolites normally found in the urine metabolome, including those observed at 24 h posthypoxia in this study, i.e., succinate, lactate, and citrate. Other metabolites produced in the mitochondria of kidney cells, such as hippurate, trimethylamine-N-oxide, trimethylamine, and n-nitrosodimethylamine dimethylamine, were also at lower concentrations in the urine 24 h posthypoxia. Waste products such as hippurate are normally produced and secreted by the cortical cells of the kidney, which are the primary locations of hippurate synthesis (30). Hippurate synthesis takes place in the mitochondrial matrix, and, therefore, the significant decrease of hippurate in urine of hypoxic mice could be caused by the dramatic decrease in mitochondrial function in the distal tubules of the kidney.
HIF-1α also impairs electron transport chain (ETC) activity and subsequently ATP generation because of reduced TCA cycle activity (58). Under normoxic conditions, the TCA cycle produces the reducing equivalents NADH and FADH2. The ETC uses these reducing equivalents to create a proton gradient that supports ATP generation (58). Hypoxia results in reduced cellular ATP availability because of oxygen conformance, but also in a significantly higher concentration of reactive oxygen species (ROS) (6, 31). ROS trigger multiple gene activation mechanisms to reduce cellular ATP demands to avoid cell death (6, 31) and have been reported to regulate an integrated stress response to hypoxia (31). Cellular ATP demands during hypoxia have been shown to be reduced by the activation of pancreatic eIF2α kinase (PERK) (20) and AMP-activated protein kinase (AMPK) (17). PERK and AMPK both downregulate protein translation, and AMPK is known to inhibit Na-K-ATPase, all mechanisms that require ATP (17, 25).
Return to a normoxic environment would result in destabilization of HIF-1, leading to its subsequent degradation. Because HIF-1 was responsible for the downregulation of metabolism in response to hypoxia, its degradation in response to normoxia would reactivate normal cellular metabolism, resulting in a burst of metabolic activity detected by increased urinary concentrations of metabolic intermediates. Indeed, several urinary metabolites increased in concentration at 48 and 72 h posthypoxia compared with control samples. Consistent with this hypothesis, creatinine, hippurate, and trigonelline waste products increased in urine 48 and 72 h posthypoxia. Other urinary metabolic intermediates directly related to the TCA cycle, such as citrate, acetate, and succinate lactate, were also increased at 48 and 72 h posthypoxia, again consistent with this model. Other pathways suppressed during hypoxia evident in the 24-h posthypoxia samples, such as the methylamine cycle and those related to kidney function and specifically arginine metabolism, had significantly higher activity following exposure to normoxic environment. Trimethylamine-N-oxide, trimethylamine, and n-nitrosodimethylamine, putrescine, and creatine are metabolites related to arginine and methylamine metabolism (19). Putrescine, also significantly lower in normoxic conditions in the first 24 h following hypoxia, was increased at the 48- and 72-h time points. Putrescine is a product of urea cycle and arginine metabolism (34). Increased metabolism 48 and 72 h following hypoxia apparently resulted in an increase in the synthesis of arginine and all other metabolites related to arginine metabolism such as trimethylamine-N-oxide, trimethylamine, n-nitrosodimethylamine, and putrescine (56, 59, 71). The kidneys are also a major source of arginine synthesis in the body, which is responsible for the production of creatine in the liver (52, 59). The creatine-to-creatinine reaction that occurs in mitochondria of muscle cells provides stability for cellular ATPase (59). Creatinine was also significantly decreased in urine 24 h posthypoxia. Loss of the organic cation transporter OTC2 has been used to explain a decrease in creatinine levels in AKI and cancer (18). OTC2 transporters are located in the proximal tubule epithelia of the kidney and are responsible for up to 40% of creatinine clearance from the blood. At 48 and 72 h, creatinine output was significantly higher than both the 24-h posthypoxia and control urine samples, implying that the OTC2 transporter was active and functional. Therefore, the OTC2 transporter activity was decreased as a function of reduced creatinine synthesis 24 h posthypoxia and not due to loss of filtration capability. This was also supported by the increase in creatinine clearance 48 and 72 h posthypoxia. Our previous study of IRI-induced AKI also showed clinical evidence of compromised creatinine filtration 24 h postinjury and histological evidence of damage to the proximal tubule cells that are known to have creatinine transporters (7).
In our previous study of IRI-induced AKI, dramatic increases in urinary glucose were observed caused by loss of reabsorption by damaged proximal tubule epithelia (7). In contrast, elevated glucose levels were not observed in the urine in any of the time points posthypoxia, indicating lack of similar damage to the proximal tubule epithelia brush borders, which was consistent with our PAS-stained cortical histology sections. Interestingly, fructose was present in urine 48 and 72 h posthypoxia at significantly elevated concentrations. Although our previous study (7) did not identify appearance of fructose due to ischemia, other studies that specifically looked at fructose as a marker of AKI did show increased urinary levels of this metabolite. Activation of the enzyme aldose reductase, in the kidney, which activates the polyol pathway and leads to conversion of glucose to sorbitol, and then to fructose by the enzyme sorbitol dehydrogenase, has previously been identified (1, 22). Our study appears to provide evidence of polyol pathway activation in the absence of ischemia, supported by the increase in fructose 48 and 72 h posthypoxia.
Because hypoxia affected all cells, tissues, and organs in the mouse, this could be responsible for the general significant decrease in urinary metabolites at 24 h posthypoxia to suppressed metabolism and subsequent elevated levels of metabolites since normal cellular function was restored during the 48- to 72-h posthypoxia period as mitochondrial function returned to normal in the whole mouse. The 168 h posthypoxia showed the least number of significant buckets between the control and the hypoxia-exposed samples, indicating a near return to normally regulated metabolism. This is understandable, since cells and tissue are not under hypoxic distress, and the mechanisms of adaption and survival become less active. Despite the metabolic profiling results indicating recovery and a return to a normally regulated metabolism, the protein biomarkers of AKI were still significantly overexpressed in the proximal tubules, even at 7 days posthypoxia.
In closing, our combined histology, IHC, and metabolic profiling analysis shed interesting and new insight into kidney injury caused by hypoxia. The IHC data revealed distinct spatial and temporal expression patterns for different protein biomarkers for hypoxia-induced AKI, and these patterns were distinct from our prior studies of IRI-induced AKI. Likewise, the metabolic profiling data also revealed distinct temporal patterns of changes that could be explained by a cycle of initial suppression of cellular metabolism caused by hypoxia, followed by a burst of elevated metabolism indicative of cellular repair after return to normoxia, and finally followed by a return to near normal urine metabolic profile as the mice experienced full recovery. The NMR-based metabolic profiling data, vetted by four independent measured, yielded lists of specific metabolites and unidentified NMR resonances that can be used as panels of urinary metabolic biomarkers to detect effects of hypoxia and evidence of its subsequent stage of recovery. Interestingly, although the NMR data indicated that the urine metabolic profiles largely returned to their prehypoxia state at 7 days posthypoxia, the KIM-1 and cystatin C protein kidney injury biomarkers increased from 24 h posthypoxia and reached their maximum levels at 7 days posthypoxia when the study was terminated, indicating that more research is necessary to understand this complex and surprising temporal pattern. Finally, the IHC and metabolic profiling data provide evidence that it should be possible to use these technical approaches to differentiate between different causes of AKI.
GRANTS
P. Devarajan is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant P50-DK-096418. The instrumentation used in this work was obtained with the support of Miami University and the Ohio Board of Regents with funds used to establish the Ohio Eminent Scholar Laboratory where the work was performed.
DISCLOSURES
P. Devarajan is the discoverer of NGAL and has submitted patents for use of NGAL as a biomarker of AKI. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
T.C. and M.A.K. conceived and designed research; T.C., H.N.R., Q.M., and S.B. performed experiments; T.C., Q.M., and M.A.K. analyzed data; T.C. and M.A.K. interpreted results of experiments; T.C., Q.M., and M.A.K. prepared figures; T.C. and M.A.K. drafted manuscript; T.C., P.D., and M.A.K. edited and revised manuscript; T.C., H.N.R., Q.M., P.D., and M.A.K. approved final version of manuscript.
REFERENCES
- 1.Andres-Hernando A, Li N, Cicerchi C, Inaba S, Chen W, Roncal-Jimenez C, Le MT, Wempe MF, Milagres T, Ishimoto T, Fini M, Nakagawa T, Johnson RJ, Lanaspa MA. Protective role of fructokinase blockade in the pathogenesis of acute kidney injury in mice. Nat Commun 8: 14181, 2017. doi: 10.1038/ncomms14181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armitage EG, Barbas C. Metabolomics in cancer biomarker discovery: current trends and future perspectives. J Pharm Biomed Anal 87: 1–11, 2014. doi: 10.1016/j.jpba.2013.08.041. [DOI] [PubMed] [Google Scholar]
- 3.Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol 2: 1303–1353, 2012. doi: 10.1002/cphy.c110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bingol K, Li DW, Bruschweiler-Li L, Cabrera OA, Megraw T, Zhang F, Brüschweiler R. Unified and isomer-specific NMR metabolomics database for the accurate analysis of (13)C-(1)H HSQC spectra. ACS Chem Biol 10: 452–459, 2015. doi: 10.1021/cb5006382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 14: 2199–2210, 2003. doi: 10.1097/01.ASN.0000079785.13922.F6. [DOI] [PubMed] [Google Scholar]
- 6.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci USA 95: 11715–11720, 1998. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chihanga T, Ma Q, Nicholson JD, Ruby HN, Edelmann RE, Devarajan P, Kennedy MA. NMR spectroscopy and electron microscopy identification of metabolic and ultrastructural changes to the kidney following ischemia-reperfusion injury. Am J Physiol Renal Physiol 314: F154–F166, 2018. doi: 10.1152/ajprenal.00363.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chong J, Soufan O, Li C, Caraus I, Li S, Bourque G, Wishart DS, Xia J. MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Res 46, W1: W486–W494, 2018. doi: 10.1093/nar/gky310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Comellas AP, Dada LA, Lecuona E, Pesce LM, Chandel NS, Quesada N, Budinger GR, Strous GJ, Ciechanover A, Sznajder JI. Hypoxia-mediated degradation of Na,K-ATPase via mitochondrial reactive oxygen species and the ubiquitin-conjugating system. Circ Res 98: 1314–1322, 2006. doi: 10.1161/01.RES.0000222418.99976.1d. [DOI] [PubMed] [Google Scholar]
- 10.Diaz SO, Pinto J, Graça G, Duarte IF, Barros AS, Galhano E, Pita C, Almeida MC, Goodfellow BJ, Carreira IM, Gil AM. Metabolic biomarkers of prenatal disorders: an exploratory NMR metabonomics study of second trimester maternal urine and blood plasma. J Proteome Res 10: 3732–3742, 2011. doi: 10.1021/pr200352m. [DOI] [PubMed] [Google Scholar]
- 11.Firth JD, Ebert BL, Ratcliffe PJ. Hypoxic regulation of lactate dehydrogenase A. Interaction between hypoxia-inducible factor 1 and cAMP response elements. J Biol Chem 270: 21021–21027, 1995. doi: 10.1074/jbc.270.36.21021. [DOI] [PubMed] [Google Scholar]
- 12.Ghazi N, Arjmand M, Akbari Z, Mellati AO, Saheb-Kashaf H, Zamani Z. (1)H NMR- based metabolomics approaches as non- invasive tools for diagnosis of endometriosis. Int J Reprod Biomed (Yazd) 14: 1–8, 2016. doi: 10.29252/ijrm.14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gill N, Nally JV Jr, Fatica RA. Renal failure secondary to acute tubular necrosis: epidemiology, diagnosis, and management. Chest 128: 2847–2863, 2005. doi: 10.1378/chest.128.4.2847. [DOI] [PubMed] [Google Scholar]
- 14.Goodpaster AM, Kennedy MA. Quantification and statistical significance analysis of group separation in NMR-based metabonomics studies. Chemom Intell Lab Syst 109: 162–170, 2011. doi: 10.1016/j.chemolab.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodpaster AM, Romick-Rosendale LE, Kennedy MA. Statistical significance analysis of nuclear magnetic resonance-based metabonomics data. Anal Biochem 401: 134–143, 2010. doi: 10.1016/j.ab.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Gorr TA. Hypometabolism as the ultimate defence in stress response: how the comparative approach helps understanding of medically relevant questions. Acta Physiol (Oxf) 219: 409–440, 2017. doi: 10.1111/apha.12747. [DOI] [PubMed] [Google Scholar]
- 17.Gusarova GA, Dada LA, Kelly AM, Brodie C, Witters LA, Chandel NS, Sznajder JI. Alpha1-AMP-activated protein kinase regulates hypoxia-induced Na,K-ATPase endocytosis via direct phosphorylation of protein kinase C zeta. Mol Cell Biol 29: 3455–3464, 2009. doi: 10.1128/MCB.00054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He Q, Johnston J, Zeitlinger J. ChIP-nexus enables improved detection of in vivo transcription factor binding footprints. Nat Biotechnol 33: 395–401, 2015. doi: 10.1038/nbt.3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hershberger KA, Martin AS, Hirschey MD. Role of NAD+ and mitochondrial sirtuins in cardiac and renal diseases. Nat Rev Nephrol 13: 213–225, 2017. doi: 10.1038/nrneph.2017.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hochachka PW, Buck LT, Doll CJ, Land SC. Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc Natl Acad Sci USA 93: 9493–9498, 1996. doi: 10.1073/pnas.93.18.9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang GS, Yang JY, Ryu DH, Kwon TH. Metabolic profiling of kidney and urine in rats with lithium-induced nephrogenic diabetes insipidus by (1)H-NMR-based metabonomics. Am J Physiol Renal Physiol 298: F461–F470, 2010. doi: 10.1152/ajprenal.00389.2009. [DOI] [PubMed] [Google Scholar]
- 22.Hwang YC, Sato SN, Tsai JY, Yan SD, Bakr S, Zhang HP, Oates PJ, Ramasamy R. Aldose reductase activation is a key component of myocardial response to ischemia. FASEB J 16: 243–245, 2002. doi: 10.1096/fj.01-0368fje. [DOI] [PubMed] [Google Scholar]
- 23.Jiang CY, Yang KM, Yang L, Miao ZX, Wang YH, Zhu HB. A (1)H NMR-based metabonomic investigation of time-related metabolic trajectories of the plasma, urine and liver extracts of hyperlipidemic hamsters. PLoS One 8: e66786, 2013. doi: 10.1371/journal.pone.0066786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jouret F, Leenders J, Poma L, Defraigne JO, Krzesinski JM, de Tullio P. Nuclear magnetic resonance metabolomic profiling of mouse kidney, urine and serum following renal ischemia/reperfusion injury. PLoS One 11: e0163021, 2016. doi: 10.1371/journal.pone.0163021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koumenis C, Naczki C, Koritzinsky M, Rastani S, Diehl A, Sonenberg N, Koromilas A, Wouters BG. Regulation of protein synthesis by hypoxia via activation of the endoplasmic reticulum kinase PERK and phosphorylation of the translation initiation factor eIF2alpha. Mol Cell Biol 22: 7405–7416, 2002. doi: 10.1128/MCB.22.21.7405-7416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lane AN, Fan TW, Bousamra M II, Higashi RM, Yan J, Miller DM. Stable isotope-resolved metabolomics (SIRM) in cancer research with clinical application to nonsmall cell lung cancer. OMICS 15: 173–182, 2011. doi: 10.1089/omi.2010.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larive CK, Barding GA Jr, Dinges MM. NMR spectroscopy for metabolomics and metabolic profiling. Anal Chem 87: 133–146, 2015. doi: 10.1021/ac504075g. [DOI] [PubMed] [Google Scholar]
- 28.Laterza OF, Price CP, Scott MG. Cystatin C: an improved estimator of glomerular filtration rate? Clin Chem 48: 699–707, 2002. [PubMed] [Google Scholar]
- 29.Lau J, Chew PW, Wang C, White AC. Long-Term Oxygen Therapy for Severe COPD. Rockville, MD: Department of Health and Human Services, 2004. [PubMed] [Google Scholar]
- 30.Lees HJ, Swann JR, Wilson ID, Nicholson JK, Holmes E. Hippurate: the natural history of a mammalian-microbial cometabolite. J Proteome Res 12: 1527–1546, 2013. doi: 10.1021/pr300900b. [DOI] [PubMed] [Google Scholar]
- 31.Liu L, Wise DR, Diehl JA, Simon MC. Hypoxic reactive oxygen species regulate the integrated stress response and cell survival. J Biol Chem 283: 31153–31162, 2008. doi: 10.1074/jbc.M805056200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X, Guan Y, Xu S, Li Q, Sun Y, Han R, Jiang C. Early predictors of acute kidney injury: a narrative review. Kidney Blood Press Res 41: 680–700, 2016. doi: 10.1159/000447937. [DOI] [PubMed] [Google Scholar]
- 33.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A; Acute Kidney Injury Network . Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minois N, Carmona-Gutierrez D, Madeo F. Polyamines in aging and disease. Aging (Albany NY) 3: 716–732, 2011. doi: 10.18632/aging.100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mishra J, Ma Q, Kelly C, Mitsnefes M, Mori K, Barasch J, Devarajan P. Kidney NGAL is a novel early marker of acute injury following transplantation. Pediatr Nephrol 21: 856–863, 2006. doi: 10.1007/s00467-006-0055-0. [DOI] [PubMed] [Google Scholar]
- 36.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol 14: 2534–2543, 2003. doi: 10.1097/01.ASN.0000088027.54400.C6. [DOI] [PubMed] [Google Scholar]
- 37.Mishra J, Mori K, Ma Q, Kelly C, Yang J, Mitsnefes M, Barasch J, Devarajan P. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 15: 3073–3082, 2004. doi: 10.1097/01.ASN.0000145013.44578.45. [DOI] [PubMed] [Google Scholar]
- 38.Nikinmaa M. What is hypoxia? Acta Physiol (Oxf) 209: 1–4, 2013. doi: 10.1111/apha.12146. [DOI] [PubMed] [Google Scholar]
- 39.Pugh CW. Modulation of the hypoxic response. Adv Exp Med Biol 903: 259–271, 2016. doi: 10.1007/978-1-4899-7678-9_18. [DOI] [PubMed] [Google Scholar]
- 40.Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol 10: 193–207, 2014. doi: 10.1038/nrneph.2013.282. [DOI] [PubMed] [Google Scholar]
- 41.Romick-Rosendale LE, Goodpaster AM, Hanwright PJ, Patel NB, Wheeler ET, Chona DL, Kennedy MA. NMR-based metabonomics analysis of mouse urine and fecal extracts following oral treatment with the broad-spectrum antibiotic enrofloxacin (Baytril). Magn Reson Chem 47, Suppl 1: S36–S46, 2009. doi: 10.1002/mrc.2511. [DOI] [PubMed] [Google Scholar]
- 42.Romick-Rosendale LE, Schibler KR, Kennedy MA. A potential biomarker for acute kidney injury in preterm infants from metabolic profiling. J Mol Biomark Diagn pii, Suppl 3: 001, 2012. doi: 10.4172/2155-9929.S3-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saat TC, van den Akker EK, IJzermans JN, Dor FJ, de Bruin RW. Improving the outcome of kidney transplantation by ameliorating renal ischemia reperfusion injury: lost in translation? J Transl Med 14: 20, 2016. doi: 10.1186/s12967-016-0767-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sabbisetti VS, Ito K, Wang C, Yang L, Mefferd SC, Bonventre JV. Novel assays for detection of urinary KIM-1 in mouse models of kidney injury. Toxicol Sci 131: 13–25, 2013. doi: 10.1093/toxsci/kfs268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sellers K, Fox MP, Bousamra M II, Slone SP, Higashi RM, Miller DM, Wang Y, Yan J, Yuneva MO, Deshpande R, Lane AN, Fan TWM. Pyruvate carboxylase is critical for non-small-cell lung cancer proliferation. J Clin Invest 125: 687–698, 2015. doi: 10.1172/JCI72873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smolinska A, Blanchet L, Buydens LMC, Wijmenga SS. NMR and pattern recognition methods in metabolomics: from data acquisition to biomarker discovery: a review. Anal Chim Acta 750: 82–97, 2012. doi: 10.1016/j.aca.2012.05.049. [DOI] [PubMed] [Google Scholar]
- 47.Solaini G, Baracca A, Lenaz G, Sgarbi G. Hypoxia and mitochondrial oxidative metabolism. Biochim Biophys Acta 1797: 1171–1177, 2010. doi: 10.1016/j.bbabio.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 48.Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int 62: 1539–1549, 2002. doi: 10.1046/j.1523-1755.2002.00631.x. [DOI] [PubMed] [Google Scholar]
- 49.Togashi Y, Imura N, Miyamoto Y. Urinary cystatin C as a renal biomarker and its immunohistochemical localization in anti-GBM glomerulonephritis rats. Exp Toxicol Pathol 65: 1137–1143, 2013. doi: 10.1016/j.etp.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 50.Uehara T, Horinouchi A, Morikawa Y, Tonomura Y, Minami K, Ono A, Yamate J, Yamada H, Ohno Y, Urushidani T. Identification of metabolomic biomarkers for drug-induced acute kidney injury in rats. J Appl Toxicol 34: 1087–1095, 2014. doi: 10.1002/jat.2933. [DOI] [PubMed] [Google Scholar]
- 51.Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V, Troth S, Muniappa N, Thudium D, Gerhold D, Holder DJ, Bobadilla NA, Marrer E, Perentes E, Cordier A, Vonderscher J, Maurer G, Goering PL, Sistare FD, Bonventre JV. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol 28: 478–485, 2010. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol 20: 672–679, 2009. doi: 10.1681/ASN.2008070669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol 3: 844–861, 2008. doi: 10.2215/CJN.05191107. [DOI] [PubMed] [Google Scholar]
- 54.Waltz P, Carchman E, Gomez H, Zuckerbraun B. Sepsis results in an altered renal metabolic and osmolyte profile. J Surg Res 202: 8–12, 2016. doi: 10.1016/j.jss.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 55.Wei Q, Xiao X, Fogle P, Dong Z. Changes in metabolic profiles during acute kidney injury and recovery following ischemia/reperfusion. PLoS One 9: e106647, 2014. doi: 10.1371/journal.pone.0106647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei TT, Zhao LC, Jia JM, Xia HH, Du Y, Lin QT, Lin XD, Ye XJ, Yan ZH, Gao HC. Metabonomic analysis of potential biomarkers and drug targets involved in diabetic nephropathy mice. Sci Rep 5: 11998, 2015. doi: 10.1038/srep11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wenger RH, Kurtcuoglu V, Scholz CC, Marti HH, Hoogewijs D. Frequently asked questions in hypoxia research. Hypoxia (Auckl) 3: 35–43, 2015. doi: 10.2147/HP.S92198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wheaton WW, Chandel NS. Hypoxia. 2. Hypoxia regulates cellular metabolism. Am J Physiol Cell Physiol 300: C385–C393, 2011. doi: 10.1152/ajpcell.00485.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev 80: 1107–1213, 2000. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- 60.Xia J, Broadhurst DI, Wilson M, Wishart DS. Translational biomarker discovery in clinical metabolomics: an introductory tutorial. Metabolomics 9: 280–299, 2013. doi: 10.1007/s11306-012-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xia J, Mandal R, Sinelnikov IV, Broadhurst D, Wishart DS. MetaboAnalyst 2.0–a comprehensive server for metabolomic data analysis. Nucleic Acids Res 40: W127–W133, 2012. doi: 10.1093/nar/gks374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xia J, Psychogios N, Young N, Wishart DS. MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res 37: W652–W660, 2009. doi: 10.1093/nar/gkp356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xia J, Sinelnikov IV, Han B, Wishart DS. MetaboAnalyst 3.0–making metabolomics more meaningful. Nucleic Acids Res 43: W251–W257, 2015. doi: 10.1093/nar/gkv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xia J, Sinelnikov IV, Wishart DS. MetATT: a web-based metabolomics tool for analyzing time-series and two-factor datasets. Bioinformatics 27: 2455–2456, 2011. doi: 10.1093/bioinformatics/btr392. [DOI] [PubMed] [Google Scholar]
- 65.Xia J, Wishart DS. Metabolomic data processing, analysis, and interpretation using MetaboAnalyst. Curr Protoc Bioinformatics 14: 14.10, 2011. doi: 10.1002/0471250953.bi1410s34. [DOI] [PubMed] [Google Scholar]
- 66.Xia J, Wishart DS. MetPA: a web-based metabolomics tool for pathway analysis and visualization. Bioinformatics 26: 2342–2344, 2010. doi: 10.1093/bioinformatics/btq418. [DOI] [PubMed] [Google Scholar]
- 67.Xia J, Wishart DS. MSEA: a web-based tool to identify biologically meaningful patterns in quantitative metabolomic data. Nucleic Acids Res 38: W71–W77, 2010. doi: 10.1093/nar/gkq329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xia J, Wishart DS. Using MetaboAnalyst 3.0 for comprehensive metabolomics data analysis. Curr Protoc Bioinformatics 55: 14.10.1–14.10.91, 2016. doi: 10.1002/cpbi.11. [DOI] [PubMed] [Google Scholar]
- 69.Xia J, Wishart DS. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat Protoc 6: 743–760, 2011. doi: 10.1038/nprot.2011.319. [DOI] [PubMed] [Google Scholar]
- 70.Ye L, Mao W. Metabonomic biomarkers for risk factors of chronic kidney disease. Int Urol Nephrol 48: 547–552, 2016. doi: 10.1007/s11255-016-1239-6. [DOI] [PubMed] [Google Scholar]
- 71.Zhao LC, Dong MJ, Liao SX, Du Y, Zhou Q, Zheng H, Chen MJ, Ji JS, Gao HC. Identification of key metabolic changes in renal interstitial fibrosis rats using metabonomics and pharmacology. Sci Rep 6: 27194, 2016. doi: 10.1038/srep27194. [DOI] [PMC free article] [PubMed] [Google Scholar]