Abstract
Changes in the expression of Na transport proteins were measured in the kidneys of mice with increased dietary K intake for 1 wk. The epithelial Na channel (ENaC) was upregulated, with enhanced expression of full-length and cleaved forms of α-ENaC and cleaved γ-ENaC. At the same time, the amount of the NaCl cotransporter NCC and its phosphorylated form decreased by ~50% and ~80%, respectively. The expression of the phosphorylated form of the Na-K-2Cl cotransporter NKCC2 also decreased, despite an increase in overall protein content. The effect was stronger in males (80%) than in females (40%). This implies that less Na+ is reabsorbed in the thick ascending limb of Henle’s loop and distal convoluted tubule along with Cl−, whereas more is reabsorbed in the aldosterone-sensitive distal nephron in exchange for secreted K+. The abundance of the proximal tubule Na/H exchanger NHE3 decreased by ~40%, with similar effects in males and females. Time-course studies indicated that NCC and NHE3 proteins decreased progressively over 7 days on a high-K diet. Expression of mRNA encoding these proteins increased, implying that the decreased protein levels resulted from decreased rates of synthesis or increased rates of degradation. The potential importance of changes in NHE3, NKCC2, and NCC in promoting K+ excretion was assessed with a mathematical model. Simulations indicated that decreased NHE3 produced the largest effect. Regulation of proximal tubule Na+ transport may play a significant role in achieving K homeostasis.
Keywords: ENaC, NCC, NHE3, NKCC2, mathematical modeling, urinary K excretion
INTRODUCTION
The kidneys adjust the rate of excretion of K+ to match the dietary intake of the cation. Renal handling of filtered K+ is a complex process that entails both reabsorption in early nephron segments, such as the proximal tubule, and secretion in distal segments, particularly the connecting tubule (CNT) (20). Urinary excretion of K+ may be enhanced by upregulation of specific K+ transporters, such as the K+ channel ROMK (12, 23, 32, 33). It may also be influenced by systems that do not transport K+ directly but alter the driving force for K+ movement. For example, K+ reabsorption in the proximal tubule depends on transport of Na+ and fluid, which creates a chemical driving force for paracellular reabsorption of K+. In distal segments such as the CNT, K+ movement is also coupled to that of Na+ but in the opposite sense; Na+ uptake from the lumen through epithelial Na channels (ENaCs) depolarizes the apical membrane, increasing the driving force for K+ secretion into the luminal fluid.
Finally, Na+ transport in one part of the nephron can influence K+ transport downstream. This may be mediated by increased Na+ delivery to K+-secreting segments, enhancing K+ secretion through electrical coupling (28); by enhanced fluid delivery, increasing the volume available to carry the secreted K+; or directly through luminal membrane shear stress which may activate K+ channels (22, 37).
To understand regulation of K+ excretion, it is therefore necessary to consider all segments of the nephron and their interactions. Here we assess changes in expression of Na+ transporters along the nephron in response to increased dietary K+ intake. The major new finding is that elevated dietary K intake decreases the expression of the proximal tubule Na/H exchanger NHE3 and the phosphorylated form of the Na-K-2Cl cotransporter NKCC2 in the thick ascending limb of Henle’s loop (TALH), as well as that of the NaCl cotransporter NCC in the distal convoluted tubule (DCT). We have used mathematical modeling to assess the overall significance of these changes on K+ excretion.
METHODS
Animals.
All work with animals was conducted according to Institutional Animal Care and Use Committee-approved protocols at Yale School of Medicine. C57BL/6J mice of both genders were purchased from the Jackson Laboratory (Bar Harbor, ME). Experiments were performed on adult mice weighing 25–35 g. Animals were divided into four groups (two male and two female, n = 4 per group) and fed control diet 1% KCI (CK) or diet supplemented with 5% KCI (HK) purchased from Harlan Teklad Laboratory (Madison, WI). In most cases, animals were maintained on the indicated diets for 7 days. As shown in Figs. 6 and 7, the diets were administered for 1, 3, and 7 days. All animals had free access to tap water. Animals were anesthetized with an intraperitoneal injection (100–150 mg/kg body weight) of Inactin (thiobutabarbital sodium salt hydrate; Sigma, St. Louis, MO), and kidneys were collected and stored at −80°C for later analysis.
Fig. 6.

Time course of changes in NHE3 and NCC protein with dietary K loading. Kidneys were harvested from male mice fed control-K (CK) or high-K (HK) diets for 1–7 days. A and C: representative Western blots for NHE3 (A) and NCC (C). Protein (25μg) from a kidney homogenate of a different animal were loaded into each lane of the Western blots. B and D: densitometric analysis of NHE3 (B) and NCC (D) abundance. Data were normalized to densities of β-actin and then renormalized to the mean value for the CK mice. Data represent means ± SE for four animals. *P < 0.05 HK vs. CK.
Fig. 7.

Time course of changes in NHE3 (A) and NCC (B) mRNA in response to dietary K loading. Kidneys were harvested from male mice fed control-K (CK) or high-K (HK) diets for 1–7 days. mRNA levels were assessed by quantitative PCR (Cyclophilin A was the internal control). NHE3 and NCC were normalized to values under CK conditions. Data represent means ± SE for four animals. ns: no significant difference; *P < 0.05; **P < 0.01; ***P < 0.001 HK vs. CK.
Western blot and densitometric analysis.
Kidney tissues were homogenized with Mem-PER Mammalian Protein Extraction Reagent containing Halt protease inhibitor cocktail (Thermo Scientific, Rockford, IL). Membrane protein was isolated from mouse kidney, and protein concentration was determined by Bradford assay. Equal amounts of protein samples were separated by SDS-PAGE using 4%–20% precast gels (Bio-Rad Laboratories, Inc., Hercules, CA) and transferred to nitrocellulose membranes. Antibody dilutions were as follows: NHE3 (1:1,000), NHE2 (1:200), NaPi-2a (1:500), NKCC2 (1:1,000), phosphorylated (p)NKCC2 (1:200), NCC (1:5,000), pNCC (1:1,000), α-ENaC (1:1,000), β-ENaC (1:500), γ-ENaC (1:500), and β-actin (1:5,000). The immune complexes were detected with the enhanced chemiluminescence reagent kit (Thermo Scientific). Bound complexes were visualized on autoradiography film (HyBlot CL, Denville Scientific, Holliston, MA). Immunoblotting data were quantified using ImageJ and Gel Band Fitter (University of Kentucky, Lexington, KY). Signals were normalized to those of β-actin, a loading control gene, or compared with staining of all proteins with Coomassie blue.
Antibodies.
Polyclonal primary antibodies against NHE3 either were obtained from Chemicon International (Millipore Corp., Billerica, MA) or were kindly provided by Dr. Daniel Biemesdorfer (Yale University) (7). Both antibodies gave similar results. The antibody against NHE2 was purchased from Alomone Laboratories (Jerusalem, Israel). Anti-NKCC2 was purchased from Chemicon International. The antibody against NCC was a gift from Prof. Alicia McDonough (University of Southern California) (19). The antibody against the phospho-T53 form of NCC was described previously (6). Polyclonal antibodies against the β and γ subunits of rat ENaC were based on short peptide sequences in the carboxy termini as described previously (9, 21). The antibody against the NH2-terminus of mouse α-ENaC (26) was a gift of Prof. Johannes Loffing (University of Zurich). The antibody against NaPi-2 (16) was a gift of Dr. Mark Knepper (National Institutes of Health). The anti-pT96NKCC2 antibody (39) was a gift of Prof. Sung-Sen Yang (National Taiwan University).
RT-PCR.
Total RNA was isolated from mouse kidney by using TRIzol reagent (Invitrogen, Carlsbad, CA) and further purified by NucleoSpin RNA kit (Clontech). cDNA synthesis from total RNA was carried out using the high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions. Expression of specific mRNA species was quantified by real-time RT-PCR using Power SYBR Green PCR Master Mix (Applied Biosystems). Real-time PCR was performed on a STRATAGENE Mx3005p QPCR system (Agilent Technologies, Santa Clara, CA). The cycling conditions for all genes were 95°C for 5 min, followed by 40 cycles at 94°C for 15 s and 60°C for 60 s. The final mRNA abundance of each gene was normalized to a housekeeping gene, Cyclophilin A. The primers of mouse NCC, NHE3, and Cyclophilin A for RT-PCR were synthesized by Oligo Synthesis Resource (W. M. Keck Foundation at Yale School of Medicine), and primer sequences are listed as follows: NCC, gggtttgtgtcatgaggatg (forward) and agatggtggtggcctgctc (reverse); NHE3, gctccccaagtacggacaatat (forward) and acagcactgacattttccctcaa (reverse); and Cyclophilin A, ttgcagacaaagttccaaagaca (forward) and aagtcaccaccctggcacat (reverse). Cyclophilin A expression was consistent and not affected by gender or diet. The levels (in arbitrary units) were as follows: male/CK: 15.4 ± 0.1; male/HK: 15.5 ± 0.1; female/CK: 15.2 ± 0.1; female/HK: 15.1 ± 0.1 (n = 8). Similar results were obtained using 18s rRNA to normalize the data.
Mathematical modeling.
The mathematical model used in this work is the simulation of rat kidney nephrons (one kidney) presented in Weinstein (36). Baseline interstitial (peritubular) conditions for cortex and medulla are those displayed in Table 3 of the companion work (34). In the calculations here, the condition of high dietary K+ was represented as a 10% increase of interstitial K+ concentration in all regions (with a compensating reduction in interstitial Na+).
Statistics.
All experimental data are presented as means ± SE. At least four kidneys from four mice were contained in Western blotting experiments. Student’s t-test was used to compare control and experimental groups. The difference between the mean values of an experimental group and a control group was considered significant if P < 0.05.
RESULTS
Mice were fed a high-K+ (HK) diet supplemented with 5% KCl or a control diet (CK) with 1% KCl. After 1 wk, analysis of plasma revealed no significant differences in the concentrations of Na (CK: 143 ± 2 mM; HK 145 ± 1 mM, n = 6) or K (CK: 4.2 ± 0.3 mM; HK 4.3 ± 0.1 mM, n = 6). Animals were euthanized, and kidneys were excised and homogenized for analysis of Na+-transport proteins by Western blot. Figures 1–5 illustrate these results.
Figure 1 shows the effects of an HK diet on the expression of the apical Na/H exchanger NHE3, which accounts for much of Na+ reabsorption in the proximal tubule. Somewhat to our surprise, NHE3 protein consistently diminished in response to increased K intake. The decreases were similar in male (to 61% of controls) and female (to 56% of controls) mice. In NHE3 knockout mice, the Na-phosphate cotransporter NaPi-2 is upregulated, apparently to compensate partially for the decrease in Na transport capacity in the proximal tubule (4). With the HK diet, the abundance of NaPi-2 did not change significantly and, if anything, was decreased.
Fig. 1.
Effects of a dietary K on expression of NHE3 and NaPi2 in mouse kidney. Kidneys were harvested from mice fed control-K (CK) or high-K (HK) diets for 7 days. A and C: protein (60 μg) from a kidney lysate of a different animal was loaded into each lane of the Western blots. Blots were stained with anti-NHE3 (A) or anti-NaPi2 (C). B and D: densitometry values were normalized to the mean value for the CK mice. Results were similar for male and female mice. Data represent means ± SE for four animals. **P < 0.01 HK vs. CK.
As shown in Fig. 2, the closely related exchanger NHE2 did not change in abundance in response to K+ loading. Like NHE3, this transporter mediates Na+ reabsorption from tubular fluid but is mainly expressed in the luminal membrane of the DCT (31). The cotransporter NKCC2, which reabsorbs Na from the urine in the TALH, had a similar abundance in females on the CK and HK diets, whereas in males the expression was modestly elevated with high K intake (Fig. 3). We also assessed the abundance of the phosphorylated form of the transporter using an antibody directed against the phospho-Thr96 region of the protein (39). In males, the abundance of the phosphoprotein decreased sharply by 70% despite the higher level of total NKCC2. In females, the abundance of pNKCC decreased less (by 40%) with the HK diet, and the effect was not statistically significant. Averaged over all animals, the pNKCC2 content in K-adapted mice decreased to 0.45 ± 0.07% of controls (P = 0.003).
Fig. 2.
Effects of dietary K on expression of NHE2 in mouse kidney. Kidneys were harvested from mice fed control-K (CK) or high-K (HK) diets for 7 days. A: protein (25 μg) from each kidney lysate of a different animal was loaded into each lane of the Western blots. B: densitometry values were normalized to the mean value for the CK mice. Results were similar for male and female mice. Data represent means ± SE for four animals. No significant differences between HK and CK diets were detected.
Fig. 3.
Effects of dietary K on expression of NKCC2 in mouse kidney. Kidneys were harvested from mice fed control-K (CK) or high-K (HK) diets for 7 days. A and C: protein (40 μg) from a kidney lysate of a different animal were loaded into each lane of the Western blots. Blots were stained with anti-NKCC2 (A) or anti-pT96NKCC2 (C). B and D: densitometry values were normalized to the mean value for the CK mice. Data represent means ± SE for four animals. **P < 0.01 HK vs. CK.
Figure 4 illustrates the effects of K+ intake on expression of the NaCl cotransporter NCC, the major apical Na+ transport protein in the DCT. Expression decreased with an HK diet in both male (to 57% of controls) and female (to 54% of controls) animals. We also measured the abundance of pNCC using an antibody directed against an epitope of the protein that includes phospho-T53. This is believed to reflect the active form of the transporter (14, 28). The amount of pNCC decreased in animals on a HK diet to 36% of control (males) and to 21% of controls (females), if anything to a greater extent than total NCC. These findings agree with previous results in mice (5, 30, 38) and rats (11).
Fig. 4.
Effects of dietary K on expression of NCC in mouse kidney. Kidneys were harvested from mice fed control-K (CK) or high-K (HK) diets for 7 days. A and C: protein (60 μg) from a kidney lysate of a different animal were loaded into each lane of the Western blots. B and D: densitometry values were normalized to the mean value for the CK mice. Blots probed with anti-NCC antibody (A and B). Blots probed with anti-p53NCC antibody (C and D). Results were similar for male and female mice. Data represent means ± SE for four animals. *P < 0.05; **P < 0.01; ***P < 0.001 HK vs. CK.
Finally, high K+ intake altered the expression and processing of ENaC (Fig. 5). In particular, the amounts of both full-length and cleaved forms of the α-ENaC subunit increased. The expression of β-ENaC increased modestly in females only. Here, the most significant change was an increase in the amount of a higher molecular mass form of the subunit that presumably is more heavily glycosylated (Fig. 5C, arrow). We did not attempt to quantitate this effect. For γ-ENaC, the abundance of the cleaved form of the subunit increased markedly, whereas that of the 80 kDa full-length form decreased. These changes are typical for animals with increased levels of circulating aldosterone and reflect increased ENaC trafficking to the apical membrane (10). Similar findings have been reported previously (11).
Fig. 5.
Effects of dietary K on expression of ENaC in mouse kidney. Kidneys were harvested from mice fed control-K (CK) or high-K (HK) diets for 7 days. A, C, and E: equal amounts (40–60 μg) of protein from a kidney lysate of a different animal were loaded into each lane of the Western blots. B, D, and F: densitometry values were normalized to the mean value for the CK mice. α-ENaC (A and B); β-ENaC (C and D), the arrow indicates the heavily glycosylated form of the β subunit; and γ-ENaC (E and F). Results were similar for male and female mice. Data represent means ± SE for four animals. *P < 0.05; **P < 0.01; ***P < 0.001 HK vs. CK.
We next defined the time course of the decreases in NHE3 and NCC proteins in response to increased K+ intake. As shown in Fig. 6, NCC protein decreased after 1 day on the HK diet and continued to decline over the next 6 days. NHE3 showed a similar time course, except that the changes were not statistically significant until 7 days of K loading.
We then asked if the observed changes in protein expression could be mediated through altered gene expression. We assayed for mRNA encoding NCC and NHE3 in mouse kidney extracts using quantitative PCR. As shown in Fig. 7, we detected no changes in either NCC or NHE3 message after 1 day on the HK diet. However, after 3 or 7 days of K loading, mRNA for both of these transporters increased significantly. Thus, the decreases in transporter protein cannot be explained by mRNA expression and must entail decreased rates of protein synthesis and/or increased rates of degradation.
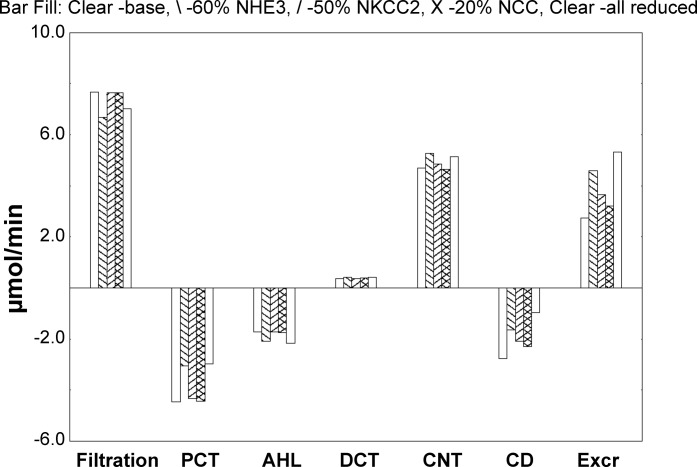
Model calculations were undertaken to estimate the impact of the observed changes in transporter density on fluid and electrolyte handling. Specifically, starting from baseline, simulations were done with 3 parameter variations: NHE3 density reduced by 40%, NKCC2 density reduced by 50%, and NCC density reduced by 80%. These were examined individually and then concurrently; calculations were repeated with all peritubular K+ concentrations increased by 10%. For each simulation, Table 1 shows whole-kidney handling of Na+ and K+ and volume. The first column is filtration; the middle columns are reabsorptive fluxes in proximal convoluted tubules (PCTs), ascending Henle limbs, distal tubules, CNTs, and total collecting ducts (CDs) (negative fluxes are secretory); and the final column is urinary excretion. Figure 8 displays K+ fluxes under baseline peritubular conditions for four parameter sets; positive deflections are luminal additions (filtration or secretion), and negative deflections are reabsorption. What is most evident from the figure is that overall K+ excretion is increased by over 60%, when NHE3 density is reduced by 40%. This increase derives in part from an increase in CNT K+ secretion but also substantially from decreased K+ reabsorption within proximal tubule and collecting duct. The increase in K+ excretion occurs despite a decrease in filtered K+ and enhanced reabsorption in ascending Henle’s limb. With the NHE3 decrease, there is increased fluid delivery to the loop of Henle, which increases solute transport there and also activates tubuloglomerular feedback (reducing glomerular filtration).
Table 1.
Calculations using the full nephron model (tubuloglomerular feedback in place)
| Absolute Reabsorption |
|||||||
|---|---|---|---|---|---|---|---|
| Filtration | PCT | AHL | DCT | CNT | CD | Excretion | |
| Volume, μl/min | |||||||
| Baseline | 1,535 | 966 | 2 | 18 | 92 | 84 | 17 |
| NHE3 at 60% | 1,336 | 683 | −5 | 13 | 72 | 129 | 47 |
| NKCC2 at 50% | 1,527 | 943 | −4 | 14 | 75 | 103 | 30 |
| NCC at 20% | 1,531 | 960 | 2 | 16 | 78 | 94 | 24 |
| All transporter reductions | 1,404 | 684 | −13 | 6 | 35 | 161 | 92 |
| High K* all transporter reductions | 1,352 | 648 | −14 | 6 | 34 | 159 | 90 |
| Sodium, μmol/min | |||||||
| Baseline | 221.1 | 138.3 | 42.5 | 5.7 | 8.9 | 1.6 | 1.5 |
| NHE3 at 60% | 192.4 | 97.2 | 44.7 | 6.6 | 10.3 | 3.2 | 7.5 |
| NKCC2 at 50% | 220.0 | 134.9 | 39.7 | 5.9 | 9.6 | 2.7 | 4.0 |
| NCC at 20% | 220.4 | 137.4 | 42.5 | 3.5 | 9.2 | 2.3 | 2.9 |
| All transporter reductions | 202.2 | 97.4 | 41.7 | 4.9 | 10.9 | 4.0 | 18.0 |
| High K* all transporter reductions | 194.0 | 91.8 | 40.2 | 5.1 | 11.4 | 4.1 | 17.1 |
| Potassium, μmol/min | |||||||
| Baseline | 7.7 | 4.4 | 1.7 | −0.4 | −4.7 | 2.8 | 2.8 |
| NHE3 at 60% | 6.7 | 3.0 | 2.1 | −0.4 | −5.3 | 1.6 | 4.6 |
| NKCC2 at 50% | 7.6 | 4.3 | 1.7 | −0.4 | −4.9 | 2.1 | 3.7 |
| NCC at 20% | 7.7 | 4.4 | 1.7 | −0.4 | −4.7 | 2.3 | 3.2 |
| All transporter reductions | 7.0 | 3.0 | 2.2 | −0.4 | −5.1 | 1.0 | 5.3 |
| High K* all transporter reductions | 7.4 | 3.1 | 2.3 | −0.5 | −5.5 | 1.0 | 5.8 |
AHL, medullary plus cortical thick ascending Henle limb; CD, cortical plus outer medullary plus inner medullary collecting duct; CNT, connecting tubule; DCT, distal convoluted tubule; PCT, proximal convoluted tubule.
High K is a 10% increase in peritubular K concentrations in all regions (5.0 to 5.5 in cortex).
Fig. 8.
Effects of decreasing the abundance of NHE3, NKCC2, and NCC on K+ transport in a mathematical model of the rat nephron. The calculations are summarized in Table 1. Bars show K+ transport along the nephron under baseline peritubular concentrations. The first set of bars (left) is glomerular K+ filtration; in the five middle sets, segmental K+ addition (secretion) is positive, whereas reabsorption is a negative deflection; the final set of bars (right) is K+ excretion. Each set contains 5 bars corresponding to the following: baseline parameters (open bar, left), NHE3 density reduced by 40% (back slash), NKCC2 density reduced by 50% (forward slash), and NCC density reduced by 80% (crosshatch); the open bar on the right in each set corresponds to concurrent reductions in all 3 transporter densities.
With reference to Table 1, 50% NKCC2 reduction reduced Na+ reabsorption in PCT by 2.5% and in AHL by 7.6%, so that overall Na+ excretion was 2.6-fold greater than baseline. (The drop in PCT transport derives from increased distal volume flow, higher tubule pressures, and the impact on microvillous drag.) The 32% increase in K+ excretion derives largely from diminished reabsorption in CD.
By itself, 80% NCC reduction produced only a 40% decrease in DCT Na+ reabsorption; in CNT this resulted in a 3% increase in Na+ reabsorption and no perceptible change in K+ secretion. With just NCC inhibition, the 14% increase in overall K+ excretion derived from the fall in collecting duct K+ reabsorption, attendant to the increase in fluid flow.
Simultaneous reductions in all 3 transporter densities produced a fivefold increase in urine flow, a 12-fold increase in Na+ excretion, and a near doubling of K+ excretion, all despite a 10% decline in glomerular filtration rate (GFR) mediated by tubuloglomerular feedback. When added to the reductions in transporter densities, the 10% increase in interstitial K+ concentrations produced only a 10% increase in K+ excretion, because of enhanced CNT K+ secretion; this mild hyperkalemia produced little change in urine flow or Na+ excretion.
DISCUSSION
Comparison with previous results.
The most significant experimental findings of this study are the decrease in the abundance of NCC, pNCC, pNKCC2, and NHE3 protein when animals are fed an HK diet. The effects on NCC were expected based on previous reports. Acute increases in plasma K resulting from rapid K intake (26), intravenous infusion of K (25), or inhibition of K excretion (13) lead to decreases in NCC phosphorylation but not total protein content. This effect has also been demonstrated in vitro using isolated perfused kidneys and kidney slices (24). More chronic elevation in dietary K decreases total NCC protein, whereas a low-K diet enhances expression (5, 11, 29, 30, 38). It should be noted, however, that when K citrate was used to increase K intake, the content of pNCC actually increased (5). This suggests that the anion also plays a role in regulating transporter expression.
Na+ transport by the TALH may also be inhibited by a high interstitial K+ concentration during K+ loading (27). To our knowledge, our data show for the first time a decrease in NKCC phosphorylation that could contribute to this phenomenon and enhance Na+ delivery to K-secreting segments. One caveat to this interpretation is that the effect appears to be gender dependent. Another is that the antibody used to detect the phosphorylated cotransporter does not distinguish pNKCC2 from pNKCC1 (39).
The decrease in NHE3 content was more surprising. In a previous study, we reported similar responses to K loading in both wild-type and SGK1−/− mice (38). However, in that case dietary K was given as a combination of citrate and carbonate as well as chloride salts. As NHE3 abundance is increased by chronic metabolic acidosis (2), it is possible that the observed decrease resulted at least in part from metabolic alkalosis caused by the ingestion of the organic anions. In the current study, K+ was given in the form of KCl, eliminating the possibility of alkalosis as a signal. These results are consistent with the idea that K+ intake per se leads to NHE3 downregulation. However, in both cases, both dietary K+ and Cl− were increased, and in principle either of these ions could have mediated the observed effects. We presume that the diminished NHE3 content decreases reabsorption of Na+ mainly by the proximal tubule. However, the exchanger is also found in the thin descending and thick ascending limbs of Henle’s loop, and we cannot rule out the possibility that changes in these segments contribute to the overall effects.
Acute inhibition of proximal tubule Na+ reabsorption by hyperkalemia has been appreciated for a long time (3). The more chronic effects on protein abundance make a striking parallel with the changes in NCC expression described above. Decreased NHE3 abundance is a robust result of K+ loading in the mouse. We have documented the effect with different salts of K+, with both genders, and in two genetically modified mouse strains including knockouts of SGK1 (38) and the AT1aR (data not shown). Others have reported the opposite effect of increased NHE3 with dietary K deprivation (8).
Mechanism for decreased protein content.
One straightforward mechanism for decreasing the expression of any protein is through inhibition of gene transcription. However, we found for both NCC and NHE3 that mRNA abundance was increased, rather than decreased, by high K+ intake. This implies that inhibition of protein synthesis and/or stimulation of protein degradation must play a role in suppressing the overall protein content of the transporters. At present, we have no way to distinguish these two possibilities. In addition, we do not know what signals are involved in the increase in NCC and NHE3 mRNA. To our knowledge, this is the first report of changes in these transcript levels in response to changes in K+ intake.
High extracellular K concentrations can promote dephosphorylation of NCC in the kidney directly, without the need for humoral, neural, or paracrine mediators (24). It is not clear whether long-term changes in NCC protein are also directly mediated by plasma K levels or if other factors might be involved. Whether NHE3 protein responds directly to hyperkalemia or whether other mediators may be involved is also unknown. Because mRNA levels changed in the opposite direction, the effect is not mediated through changes in transcription of the gene encoding NHE3. A similar dissociation was reported for OK cells, a model of the proximal tubule, in which long-term exposure to high levels of dopamine decreased NHE3 protein content without changing mRNA levels (15).
Physiological significance.
Under conditions of high K+ intake, decreased Na+ reabsorption by the proximal tubule will lead to parallel decreases in proximal tubule K+ reabsorption through the paracellular pathway. In addition, delivery of both Na+ and fluid to downstream segments will promote K+ secretion. In cells expressing ENaC, increased luminal Na+ concentration will stimulate luminal Na+ entry through the channels; the ensuing apical membrane depolarization promotes K+ exit from the cell into the lumen through K+ channels. Furthermore, increased volume flow through the K+-secreting segments will reduce the concentration of K+ in the luminal fluid, sustaining K+ secretion and inhibiting the backflux of K+ through leak pathways. Finally, increased flow is believed to activate both Na+ reabsorption and K+ secretion in distal segments through increased mechanical shear stress (22, 37).
We used a mathematical model to assess these effects more quantitatively; the model selected for these calculations was the nephron population of a whole kidney. The simulations of this work included reductions of NHE3, NKCC2, and NCC activities, commensurate with our observed changes in these transporters. ENaC increases were not explored, as the baseline model parameters already assumed briskly transporting CNT and collecting ducts, compatible with aldosterone effects. In broad terms, the impact of NHE3 and NKCC2 reductions dwarfed the effect of decreasing NCC density. Specifically, reduction in NHE3 altered fluxes along the entire nephron: reducing proximal K+ reabsorption, enhancing CNT K+ secretion, and blunting CD K+ reabsorption by increasing luminal flow. K+ excretion increased, despite tubuloglomerular feedback-mediated reduction in glomerular filtration.
This focus on NHE3 echoes conclusions from a recent examination of mild hyperkalemia in a full kidney simulation (35). In that work, renal blood vessels were included, and medullary interstitial K+ concentrations were computed rather than specified. In the kidney model, increased cortical K+ reduced PCT Na+ reabsorption and produced both kaliuresis and natriuresis. The difference between the two model calculations derives from the fact that in the full kidney model, medullary ray K+ was computed and did not change with the increase in cortical K+, so that GFR was unchanged and the reduction of proximal Na+ reabsorption increased distal Na+ delivery. In the calculations of this work, an increase in medullary ray interstitial K+ concentration was part of the global change in interstitial conditions. This reduced GFR, thus blunting the impact of decreased PCT Na+ reabsorption.
The translational significance of the natriuresis of a high K+ diet has had considerable attention among workers in hypertension and heart failure (1, 17). Micropuncture studies (3, 18) in proximal tubule have identified acute effects of K+ infusions to blunt PCT Na+ transport. Those acute observations were captured by the PCT of these models, in which peritubular K+ alkalinized the cell and thus depressed NHE3 transport. Here, we assume frank reduction in NHE3 density under conditions of chronic elevation of K+ intake. Whether the acute impact of K+ on cell pH plays a role in resetting luminal membrane NHE3 function is an open question.
In summary, mice eating a diet rich in K+ will excrete the greater load through a combination of direct activation of the K+ secretory system in principal cells of the aldosterone-sensitive distal nephron and by inhibiting salt and water transport in more proximal segments. Of these, the most important may be the proximal tubule, where diminished Na+ reabsorption through Na/H exchangers will strongly influence net K+ secretion in downstream segments, leading to significant enhancement of overall K+ excretion.
GRANTS
This work was supported by NIH Grants RO1-DK-099284 and RO1-DK-1111380.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.Y., S.X., A.M.W., T.W., and L.G.P. conceived and designed research; L.Y., S.X., X.G., and A.M.W. performed experiments; L.Y., S.X., A.M.W., T.W., and L.G.P. analyzed data; L.Y., S.X., S.U., A.M.W., T.W., and L.G.P. interpreted results of experiments; L.Y., S.X., A.M.W., T.W., and L.G.P. prepared figures; A.M.W. and L.G.P. drafted manuscript; L.Y., A.M.W., T.W., and L.G.P. edited and revised manuscript; L.Y., S.X., X.G., S.U., A.M.W., T.W., and L.G.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Daniel Biemesdorfer, Mark Knepper, Alicia McDonough, and Sung-Sen Yang for gifts of antibodies.
REFERENCES
- 1.Adrogué HJ, Madias NE. Sodium surfeit and potassium deficit: keys to the pathogenesis of hypertension. J Am Soc Hypertens 8: 203–213, 2014. doi: 10.1016/j.jash.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Bobulescu IA, Moe OW. Luminal Na(+)/H (+) exchange in the proximal tubule. Pflugers Arch 458: 5–21, 2009. doi: 10.1007/s00424-008-0595-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandis M, Keyes J, Windhager EE. Potassium-induced inhibition of proximal tubular fluid reabsorption in rats. Am J Physiol 222: 421–427, 1972. doi: 10.1152/ajplegacy.1972.222.2.421. [DOI] [PubMed] [Google Scholar]
- 4.Brooks HL, Sorensen AM, Terris J, Schultheis PJ, Lorenz JN, Shull GE, Knepper MA. Profiling of renal tubule Na+ transporter abundances in NHE3 and NCC null mice using targeted proteomics. J Physiol 530: 359–366, 2001. doi: 10.1111/j.1469-7793.2001.0359k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castañeda-Bueno M, Cervantes-Perez LG, Rojas-Vega L, Arroyo-Garza I, Vázquez N, Moreno E, Gamba G. Modulation of NCC activity by low and high K(+) intake: insights into the signaling pathways involved. Am J Physiol Renal Physiol 306: F1507–F1519, 2014. doi: 10.1152/ajprenal.00255.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiga M, Rai T, Yang SS, Ohta A, Takizawa T, Sasaki S, Uchida S. Dietary salt regulates the phosphorylation of OSR1/SPAK kinases and the sodium chloride cotransporter through aldosterone. Kidney Int 74: 1403–1409, 2008. doi: 10.1038/ki.2008.451. [DOI] [PubMed] [Google Scholar]
- 7.Du Z, Wan L, Yan Q, Weinbaum S, Weinstein AM, Wang T. Regulation of glomerulotubular balance: II: impact of angiotensin II on flow-dependent transport. Am J Physiol Renal Physiol 303: F1507–F1516, 2012. doi: 10.1152/ajprenal.00277.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elkjaer ML, Kwon TH, Wang W, Nielsen J, Knepper MA, Frøkiaer J, Nielsen S. Altered expression of renal NHE3, TSC, BSC-1, and ENaC subunits in potassium-depleted rats. Am J Physiol Renal Physiol 283: F1376–F1388, 2002. doi: 10.1152/ajprenal.00186.2002. [DOI] [PubMed] [Google Scholar]
- 9.Ergonul Z, Frindt G, Palmer LG. Regulation of maturation and processing of ENaC subunits in the rat kidney. Am J Physiol Renal Physiol 291: F683–F693, 2006. doi: 10.1152/ajprenal.00422.2005. [DOI] [PubMed] [Google Scholar]
- 10.Frindt G, Gravotta D, Palmer LG. Regulation of ENaC trafficking in rat kidney. J Gen Physiol 147: 217–227, 2016. doi: 10.1085/jgp.201511533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frindt G, Palmer LG. Effects of dietary K on cell-surface expression of renal ion channels and transporters. Am J Physiol Renal Physiol 299: F890–F897, 2010. doi: 10.1152/ajprenal.00323.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frindt G, Shah A, Edvinsson J, Palmer LG. Dietary K regulates ROMK channels in connecting tubule and cortical collecting duct of rat kidney. Am J Physiol Renal Physiol 296: F347–F354, 2009. doi: 10.1152/ajprenal.90527.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frindt G, Yang L, Uchida S, Weinstein AM, Palmer LG. Responses of distal nephron Na+ transporters to acute volume depletion and hyperkalemia. Am J Physiol Renal Physiol 313: F62–F73, 2017. doi: 10.1152/ajprenal.00668.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gamba G. The thiazide-sensitive Na+-Cl- cotransporter: molecular biology, functional properties, and regulation by WNKs. Am J Physiol Renal Physiol 297: F838–F848, 2009. doi: 10.1152/ajprenal.00159.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu MC, Di Sole F, Zhang J, McLeroy P, Moe OW. Chronic regulation of the renal Na(+)/H(+) exchanger NHE3 by dopamine: translational and posttranslational mechanisms. Am J Physiol Renal Physiol 304: F1169–F1180, 2013. doi: 10.1152/ajprenal.00630.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim GH, Martin SW, Fernández-Llama P, Masilamani S, Packer RK, Knepper MA. Long-term regulation of renal Na-dependent cotransporters and ENaC: response to altered acid-base intake. Am J Physiol Renal Physiol 279: F459–F467, 2000. doi: 10.1152/ajprenal.2000.279.3.F459. [DOI] [PubMed] [Google Scholar]
- 17.Krishna GG, Kapoor SC. Potassium depletion exacerbates essential hypertension. Ann Intern Med 115: 77–83, 1991. doi: 10.7326/0003-4819-115-2-77. [DOI] [PubMed] [Google Scholar]
- 18.Levine DZ, Walker T, Nash LA, Raman S. Effects of KCl infusions on proximal tubular function in normal and potassium-depleted rats. Kidney Int 4: 318–325, 1973. doi: 10.1038/ki.1973.123. [DOI] [PubMed] [Google Scholar]
- 19.Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM. Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol 285: F998–F1012, 2003. doi: 10.1152/ajprenal.00067.2003. [DOI] [PubMed] [Google Scholar]
- 20.Malnic G, Muto S, Giebisch G. Regulation of Potassium Excretion. In: The Kidney; Physiology and Pathophysiology, edited by Alpern RJ, Hebert SC (4th ed.). Burlington, MA: Academic Press, 2007. [Google Scholar]
- 21.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest 104: R19–R23, 1999. doi: 10.1172/JCI7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morimoto T, Liu W, Woda C, Carattino MD, Wei Y, Hughey RP, Apodaca G, Satlin LM, Kleyman TR. Mechanism underlying flow stimulation of sodium absorption in the mammalian collecting duct. Am J Physiol Renal Physiol 291: F663–F669, 2006. doi: 10.1152/ajprenal.00514.2005. [DOI] [PubMed] [Google Scholar]
- 23.Palmer LG, Antonian L, Frindt G. Regulation of apical K and Na channels and Na/K pumps in rat cortical collecting tubule by dietary K. J Gen Physiol 104: 693–710, 1994. doi: 10.1085/jgp.104.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Penton D, Czogalla J, Wengi A, Himmerkus N, Loffing-Cueni D, Carrel M, Rajaram RD, Staub O, Bleich M, Schweda F, Loffing J. Extracellular K+ rapidly controls NaCl cotransporter phosphorylation in the native distal convoluted tubule by Cl− -dependent and independent mechanisms. J Physiol 594: 6319–6331, 2016. doi: 10.1113/JP272504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rengarajan S, Lee DH, Oh YT, Delpire E, Youn JH, McDonough AA. Increasing plasma [K+] by intravenous potassium infusion reduces NCC phosphorylation and drives kaliuresis and natriuresis. Am J Physiol Renal Physiol 306: F1059–F1068, 2014. doi: 10.1152/ajprenal.00015.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J. Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83: 811–824, 2013. doi: 10.1038/ki.2013.14. [DOI] [PubMed] [Google Scholar]
- 27.Stokes JB. Consequences of potassium recycling in the renal medulla. Effects of ion transport by the medullary thick ascending limb of Henle’s loop. J Clin Invest 70: 219–229, 1982. doi: 10.1172/JCI110609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Subramanya AR, Ellison DH. Distal convoluted tubule. Clin J Am Soc Nephrol 9: 2147–2163, 2014. doi: 10.2215/CJN.05920613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terker AS, Zhang C, Erspamer KJ, Gamba G, Yang CL, Ellison DH. Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int 89: 127–134, 2016. doi: 10.1038/ki.2015.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vallon V, Schroth J, Lang F, Kuhl D, Uchida S. Expression and phosphorylation of the Na+-Cl− cotransporter NCC in vivo is regulated by dietary salt, potassium, and SGK1. Am J Physiol Renal Physiol 297: F704–F712, 2009. doi: 10.1152/ajprenal.00030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang T, Hropot M, Aronson PS, Giebisch G. Role of NHE isoforms in mediating bicarbonate reabsorption along the nephron. Am J Physiol Renal Physiol 281: F1117–F1122, 2001. doi: 10.1152/ajprenal.2001.281.6.F1117. [DOI] [PubMed] [Google Scholar]
- 32.Wang WH, Schwab A, Giebisch G. Regulation of small-conductance K+ channel in apical membrane of rat cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 259: F494–F502, 1990. doi: 10.1152/ajprenal.1990.259.3.F494. [DOI] [PubMed] [Google Scholar]
- 33.Wei Y, Bloom P, Lin D, Gu R, Wang WH. Effect of dietary K intake on apical small-conductance K channel in CCD: role of protein tyrosine kinase. Am J Physiol Renal Physiol 281: F206–F212, 2001. doi: 10.1152/ajprenal.2001.281.2.F206. [DOI] [PubMed] [Google Scholar]
- 34.Weinstein AM. A mathematical model of rat proximal tubule and loop of Henle. Am J Physiol Renal Physiol 308: F1076–F1097, 2015. doi: 10.1152/ajprenal.00504.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinstein AM. A mathematical model of the rat kidney: K+-induced natriuresis. Am J Physiol Renal Physiol 312: F925–F950, 2017. doi: 10.1152/ajprenal.00536.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weinstein AM. A mathematical model of the rat nephron: glucose transport. Am J Physiol Renal Physiol 308: F1098–F1118, 2015. doi: 10.1152/ajprenal.00505.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol 280: F786–F793, 2001. doi: 10.1152/ajprenal.2001.280.5.F786. [DOI] [PubMed] [Google Scholar]
- 38.Yang L, Frindt G, Lang F, Kuhl D, Vallon V, Palmer LG. SGK1-dependent ENaC processing and trafficking in mice with high dietary K intake and elevated aldosterone. Am J Physiol Renal Physiol 312: F65–F76, 2017. doi: 10.1152/ajprenal.00257.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang SS, Lo YF, Wu CC, Lin SW, Yeh CJ, Chu P, Sytwu HK, Uchida S, Sasaki S, Lin SH. SPAK-knockout mice manifest Gitelman syndrome and impaired vasoconstriction. J Am Soc Nephrol 21: 1868–1877, 2010. doi: 10.1681/ASN.2009121295. [DOI] [PMC free article] [PubMed] [Google Scholar]