Abstract
With no lysine kinase 4 (WNK4) is essential to activate the thiazide-sensitive NaCl cotransporter (NCC) along the distal convoluted tubule, an effect central to the phenotype of familial hyperkalemic hypertension. Although effects on potassium and sodium channels along the connecting and collecting tubules have also been documented, WNK4 is typically believed to have little role in modulating sodium chloride reabsorption along the thick ascending limb of the loop of Henle. Yet wnk4−/− mice (knockout mice lacking WNK4) do not demonstrate the hypocalciuria typical of pure distal convoluted tubule dysfunction. Here, we tested the hypothesis that WNK4 also modulates bumetanide-sensitive Na-K-2Cl cotransporter (NKCC2) function along the thick ascending limb. We confirmed that wnk4−/− mice are hypokalemic and waste sodium chloride, but are also normocalciuric. Results from Western blots suggested that the phosphorylated forms of both NCC and NKCC2 were in lower abundance in wnk4−/− mice than in controls. This finding was confirmed by immunofluorescence microscopy. Although the initial response to furosemide was similar in wnk4−/− mice and controls, the response was lower in the knockout mice when reabsorption along the distal convoluted tubule was inhibited. Using HEK293 cells, we showed that WNK4 increases the abundance of phosphorylated NKCC2. More supporting evidence that WNK4 may modulate NKCC2 emerges from a mouse model of WNK4-mediated familial hyperkalemic hypertension in which more phosphorylated NKCC2 is present than in controls. These data indicate that WNK4, in addition to modulating NCC, also modulates NKCC2, contributing to its physiological function in vivo.
Keywords: calcium, NKCC2, sodium, thick ascending limb, WNK kinase
INTRODUCTION
WNK kinases activate cation chloride transporters via the intermediary kinases SPAK and OxSR1 (also called OSR1), which directly phosphorylate the transporters. Mutations in WNK kinases, or in proteins that regulate their abundances, are known to cause familial hyperkalemic hypertension (FHHt, also known as pseudohypoaldosteronism type 2 or Gordon syndrome) by stimulating renal NaCl reabsorption. As patients with FHHt are especially sensitive to thiazide diuretics (19), it has long been recognized that activation of the thiazide-sensitive NaCl cotransporter (NCC) plays a central role in the pathogenesis of this disease. The centrality of NCC, which is expressed along the distal convoluted tubule (DCT), was supported when transgenic or knock-in models introducing disease-causing WNK4 mutations were found to manifest abundant and activated NCC, along with hypertrophy of the DCT. Further, the disease phenotype in these animals could be abrogated with either thiazide administration or by deleting NCC genetically (15, 48).
WNK4 is expressed along the thick ascending limb (TAL), the DCT, and into the connecting and collecting tubule. O’Reilly and colleagues (28) reported that WNK4 mRNA is expressed from the medullary thick ascending limb into the connecting tubule and collecting duct. Ohno and colleagues (29) found that WNK4 protein colocalizes with NKCC2 at the macula densa and cortical TAL, but did not detect WNK4 along the medullary TAL, a finding largely confirmed by McCormick and colleagues (22). As WNK kinases can activate both NKCC2 and NCC ex vivo (40), one might suspect that WNK4 would play a role in stimulating NKCC2 along the TAL. Yet, although it was shown that WNK4 can activate NKCC2 in Xenopus oocytes (26), studies in animals have been interpreted to suggest that WNK4 does not modulate NKCC2 phosphorylation or activity. NKCC2 abundance was reported to be normal by Castañeda-Bueno and colleagues (3) in WNK4 knockout mice, and Takahashi and colleagues (42) reported that neither total nor phosphorylated NKCC2 abundances were altered. Both groups concluded that WNK4 regulates NCC but not NKCC2 in the mammalian kidney.
Yet several characteristics of the WNK4 knockout models suggest that WNK4 deletion affects more than just NCC and the DCT. Although the phenotypes of WNK4 knockout and hypomorphic mice vary somewhat, hypokalemia may be more prominent in WNK4 knockout mice than in NCC knockout mice (3, 16, 39). Further, WNK4 knockout mice have been reported to be normocalciuric (3, 30), whereas low urine calcium concentration is striking in NCC knockout mice (16, 39), and is a cardinal diagnostic criterion of Gitelman syndrome, which is caused by loss of NCC function in humans (1). These differences suggest that transport defects in FHHt may include segments other than the DCT, and led us to test the hypothesis that WNK4 deletion reduces NKCC2 activity and TAL transport function in vivo.
METHODS
Animals.
wnk4−/− mice were rederived from cryopreserved sperm (3) at Charles River onto a C57Bl/6NCrl background. The transgenic FHHt WNK4 Q562E mice (15) were generously provided by Richard Lifton. Animal studies were approved by Oregon Health and Science University Institutional Animal Care and Use Committee (Protocol IP00286).
Antibodies.
Antibody sources, species, and dilutions are provided in Table 1.
Table 1.
List of antibodies used
| Antibody | Species | Dilution for WB | Dilution for IF | Source | Ref. No. | Used in Figure No. |
|---|---|---|---|---|---|---|
| pT53-NCC* | Rabbit | 1:2,000 | 1:10,0000 | Ellison Laboratory, R40 | 21 | 1, 4 |
| NCC* | Rabbit | 1:6,000 | 1:7,500 | Ellison Laboratory | 2 | 1, 4 |
| pT96, pT101-NKCC2 | Rabbit | 1:1,000 | 1:1,000 | Bachmann Laboratory | 25 | 1, 4, 7 |
| NKCC2 | Guinea Pig | 1:3,000 | 1:8,000 | Bachmann Laboratory | 38 | 1, 3, 4, 7 |
| pS91-NKCC2 | Sheep | 3 µg/ml | 1 µg/ml | MRC PPU, Dundee Univ. S838B | 36 | 4, 5 |
| NKCC2 | Sheep | 3 µg/ml | MRC PPU, Dundee Univ. S451C | 34 | 5 | |
| WNK4* | Rabbit | 1:1,000 | Ellison Laboratory | 46 | 3 | |
| AQP2 | Goat | 1:1,000 | Santa Cruz sc-9882 | 3 | ||
| Parvalbumin (PV)* | Guinea Pig | 1:2,000 | Swant GP72 | 7 | 4 | |
| Calbindin D-28K (C28) | Mouse | 1:2,000 | Swant 300 | 4 | 4 |
Validated in knockout mice.
Kidney Western blot.
Kidneys were harvested and immediately snap-frozen in liquid nitrogen and stored at −80°C until homogenization. They were homogenized in 1 ml cold homogenization buffer containing lysis buffer (300 mM sucrose, 50 mM Tris·HCl, pH 7.4, 1 mM EDTA, 1 mM EGTA, 1 mM NaVO4, 50 mM NaF, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 1 mg/ml aprotinin and 4 mg/ml leupeptin) using a 10 ml Potter-Elvehjem homogenizer. Homogenate was centrifuged at 6,000 g for 15 min at 4°C and supernatant was transferred to a new tube and stored at −80°C. Twenty micrograms protein was separated on a 4–12% Bis-tris acetate gel (Invitrogen, Carlsbad, CA) and transferred to a polyvinylidene fluoride membrane using Trans-Blot Turbo Transfer System (Bio-Rad). The membrane was blocked with 5% nonfat milk in PBS with 0.1% Tween, followed by incubation with primary antibody as indicated in figure legends and detailed in Table 1 for either 1 h at room temperature or overnight at 4°C. Membranes were washed, incubated with HRP-coupled secondary antibody (1:7,500, Invitrogen, HRP-goat anti-rabbit IgG 65-6120 or HRP-rabbit anti-guinea pig IgG 61-4620; 1:7,500, ThermoFisher, HRP-donkey anti-sheep A16047), washed again, and incubated with Western Lightning ECL (Perkin Elmer, Waltham, MA). ECL signal was detected with a Syngene Pxi4 imager, and densitometry was performed with ImageJ. Protein abundance was normalized by densitometric quantitation to β-actin.
Immunofluorescence.
Animals were injected with anesthesia cocktail (ketamine/xylazine/acepromazine, 50/5/0.5 mg/kg), and under deep anesthesia animals were perfusion fixed with 4% paraformaldehyde. After cryoprotection in 800 mosmol/l sucrose and freezing in Optimal Temperature Cutting (OCT) compound, 5-μm sections were cut. Sections were incubated overnight at 4°C with anti-tNCC, anti-pT53-NCC, anti-tNKCC2, anti-pT96, pT101-NKCC2, anti-WNK4, anti-AQP2, anti-parvalbumin, and/or anti-calbindin (Table 1). Sections were incubated in secondary antibody at 1:2,000 for 1 h at room temperature [Alexa Fluor 647 donkey anti rabbit (Life Technologies A31573), Alexa Fluor 488 goat anti-guinea pig (Life Technologies A11073), Alexa Fluor 555 donkey anti-goat (Life Technologies A21432), Alexa Fluor 555 donkey anti-sheep (Life Technologies A21436), Cy3-goat anti-rabbit (Zymed 81-6115), and Cy3-goat anti-mouse (Invitrogen A10521)]. All sections were stained with DAPI (Sigma-Aldrich D9542) and mounted with ProLong Diamond Antifade Mountant (ThermoFisher Scientific P36970). Images were captured with a ZEISS AXIO Imager M2 fluorescent microscope.
Dietary manipulation.
For baseline urine collection, animals were fed normal diet (TestDiet AIN-93G 0.36% K+, 0.51% Ca2+ and adjusted to 0.49% Na+). For high-sodium/normal-potassium (HS/NK) and high-sodium/low-potassium (HS/LK) urine collection, animals were fed Teklad potassium-deficient diet (TD.88239, Envigo, 1% Ca2+) adjusted to 6% sodium (HS/LK) or 6% sodium and 1% potassium (HS/NK).
Urine collection.
Animals were acclimated to metabolic cages (Hatteras Instruments MMC100) for 2 days before urine collection. Animals were fed a gelled diet (baseline diet, HS/NK, or HS/LK as described above) and had free access to water. Urine was collected under water-saturated light mineral oil after 24 h. Urine Ca2+ was assayed using the o-cresolphthalein method (Pointe Scientific C7503).
Blood analysis.
Blood was collected via cardiac puncture under isoflurane anesthesia and transferred into heparinized tubes; 80 μl was loaded into a Chem8+ cartridge for electrolyte measurement by i-STAT analyzer (Abbot Point of Care, Princeton, NJ).
Furosemide response test.
Animals were injected intraperitoneally with vehicle (1.2% ethanolamine in normal saline), then placed in metabolic cages and urine was collected for 3 h. On a different day, the same animals were injected with furosemide (25 mg/kg body wt) in vehicle, followed by 3 h urine collection. Urine Na+ was determined by flame photometry (Cole-Parmer Instrument 2655–10). Hydrochlorothiazide (HCTZ) was injected daily at 25 mg/kg for 5 consecutive days. On day 5, the furosemide response test was performed as above with either vehicle or furosemide (25 mg/kg) injected 1 h following the HCTZ injection.
NKCC2 phosphorylation assays.
HEK293 cells (ATCC CRL-1573) were used for transient transfection of hNKCC2-Flag (DU30183, MRC-PPU, Dundee Univ.) (1), hSPAK-HA (DU2999, MRC-PPU, Dundee Univ.) (2), and mWNK4-HA (2, 3), or mWNK4-L319F (2). All clones have been used and described previously. Cells were grown to 70–80% confluence and transfected with Lipofectamine 2000 (Life Technologies). Forty eight hours after transfection, cells were lysed with lysis buffer [50 mM Tris·HCl (pH 7.5), 1 mM EGTA, 1 mM EDTA, 50 mM sodium fluoride, 5 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 1% (wt/vol) Nonidet P-40, 270 mM sucrose, 0.1% (vol/vol) 2-mercaptoethanol, and Roche complete tablet protease inhibitors], and protein concentration was quantified. hNKCC2-Flag was immunoprecipitated using FLAG Immunoprecipitation Kit (FLAGIPT1 SIGMA). Immunoprecipitated proteins were separated via SDS-PAGE and Western blotted for NKCC2 (MRC PPU, Dundee Univ. S838B) and pS91-NKCC2 (MRC PPU, Dundee Univ. S451C).
Statistical analyses.
The null hypothesis was tested using unpaired t-tests, Mann Whitney U-test, or two-way analysis of variance (ANOVA) using GraphPad Prism 7 as indicated in the figure legends.
RESULTS
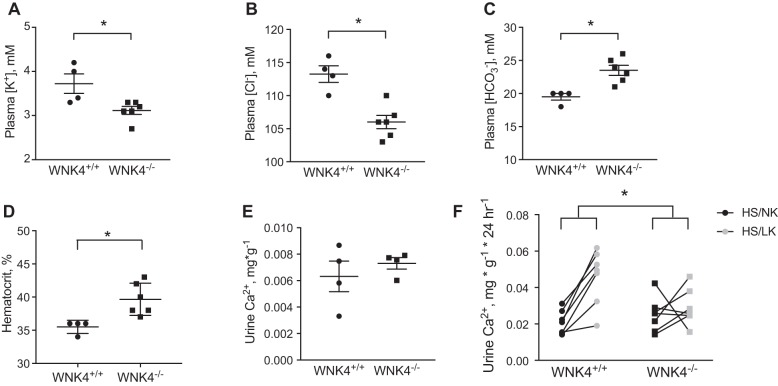
Plasma and urine electrolytes (Fig. 1), confirmed the wnk4−/− phenotype reported previously (3), with hypokalemic hypochloremic alkalosis. There also appeared to be mild extracellular fluid volume contraction, based on differences in hematocrit (Fig. 1). Similar to prior reports (3), 24-h urinary calcium excretion did not differ between controls and wnk4−/− mice (Fig. 1). Although the plasma electrolyte values resemble those reported for mice or humans lacking NCC activity, the urine calcium excretion is normal in the wnk4−/− mice; low urine calcium is a well-known characteristic of mice (39) and humans (1) that lack functional NCC; and it is also observed in mice lacking the kinase SPAK, which has predominant effects along the DCT (13, 20, 47).
Fig. 1.
Plasma and urine electrolytes and hematocrit in control (wnk4+/+) and knockout (wnk4−/−) mice. A: plasma [K+]. B: plasma [Cl−]. C: plasma []. D: hematocrit. E: urine calcium excretion. F: effects of diet on urine calcium excretion. Black symbols indicate high-salt/normal-K+ diet (HS/NK), and gray symbols indicate high-salt/low-K+ diet (HS/LK). A–E: effects compared with Mann-Whitney U-test. *P < 0.05. For F, 2-way analysis of variance was performed.
To uncover possible mechanisms responsible for the unexpected calcium results, we investigated the renal calcium response to another intervention that differs between control mice and mice lacking NCC. When dietary NaCl is high, low dietary potassium (HS/LK diet) activates NCC and leads to substantial hypercalciuria in normal mice (44). Slc12a3−/− animals (NCC knockouts) not only lack this hypercalciuric response to a low-potassium diet, but they also experience a paradoxical reduction in calcium excretion when challenged with a HS/LK diet (44). If the tubulopathy caused by WNK4 ablation was mediated only by NCC dysfunction, then we would expect wnk4−/− animals to show a similar reduction in calcium excretion on a HS/LK diet. Here, control mice exhibited the expected calciuresis after consuming such a diet for 4 days, whereas urine calcium remained unchanged in wnk4−/− animals (Fig. 1F).
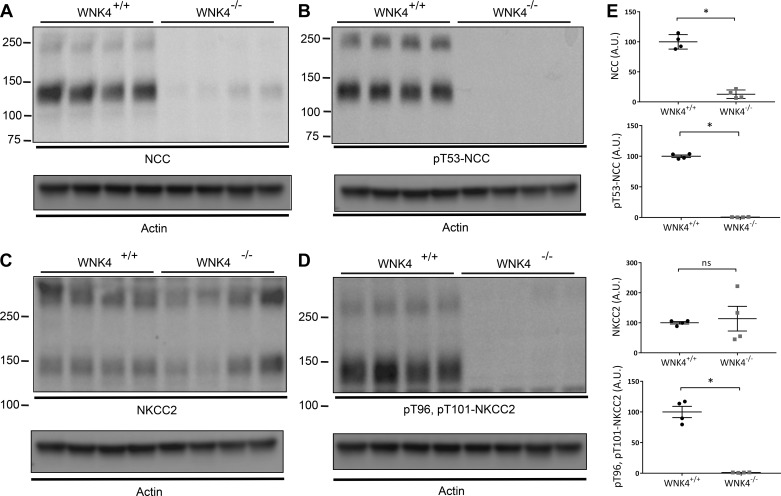
Data thus far suggested that the phenotype of wnk4−/− animals with respect to urine calcium excretion differs from that of slc12a3−/− animals. In fact, the wnk4−/− phenotype resembles Bartter syndrome type 3 more than Gitelman syndrome (1, 17). Bartter syndrome type 3 results from transport defects along both the TAL and DCT (14); thus we postulated that absence of WNK4 might be reducing NKCC2 activity, as well as NCC activity. When examined by Western blot, both total and phosphorylated (active) NCC abundances were strikingly lower in wnk4−/− mice than in controls (Fig. 2, A and B), a finding that has been reported previously by others (3, 42). We also support previous reports (3, 42) indicating that total NKCC2 abundance was similar in wnk4−/− and control mice (Fig. 2C). Surprisingly, however, and in contrast to a prior finding (42), phosphorylated NKCC2 was also lower in the wnk4−/− mice than in controls (Fig. 2D). As antibodies against phosphorylated NCC and NKCC2 may cross react, as phosphorylation sites are homologous, this result likely reflects the decrease in both transport proteins.
Fig. 2.
Abundance of NCC and NKCC2 in control and wnk4−/− mice. A: abundance of total NCC. B: abundance of phosphorylated NCC (pT53-NCC). C: abundance of total NKCC2. D: abundance of phosphorylated NKCC2 (pT96, pT101-NKCC2). In each case, presumed monomers and multimeric forms are shown. Actin loading controls are shown for each blot. E: abundances of NCC, pT53-NCC, and pT96, pT101-NKCC2 were highly significantly different between controls and wnk4−/−. *P < 0.001. In the case of NKCC2, there were no significant differences. Note that this anti-phospho-NKCC2 antibody is not specific, as suggested by the overlapping apparent molecular weight of the band in D (compare with B and C). Densitometric quantitation to actin was performed using ImageJ.
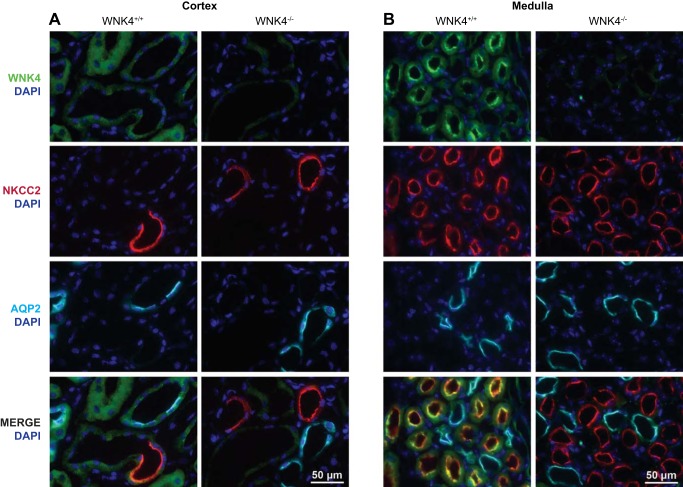
We therefore examined the expression and localization of WNK4 and cation chloride cotransporters in control and wnk4−/− mice using perfusion fixed kidneys. As we have described (44), WNK4 is expressed along the DCT, but the expression is low by immunofluorescence when mice consume a control diet (Fig. 3A). WNK4 was also expressed at a relatively low, but easily detected level in cortical TAL (Fig. 3A). In contrast, WNK4 was abundant along medullary TALs, where it had a strong apical orientation (Fig. 3B). Consistent with TAL localization in the medulla, it colocalized extensively with NKCC2 (Fig. 3B), but not with aquaporin 2 (AQP2), a marker of collecting ducts. WNK4 was not apparent in the papilla. In wnk4−/− mice, WNK4 staining was essentially absent, confirming the specificity of our antibody.
Fig. 3.
Localization of WNK4 (green) and NKCC2 (red) in kidney cortex and medulla of control and wnk4−/− mice. A shows the cortical region from control and wnk4−/− mice, showing WNK4 (green), NKCC2 (red), and aquaporin 2 (blue), and a merged image. B shows the medullary region. In the medulla, there is more prominent apical and subapical WNK4, which strikingly colocalizes with NKCC2 (merged image). DAPI (4′,6-diamidino-2-phenylindole) is a nuclear stain.
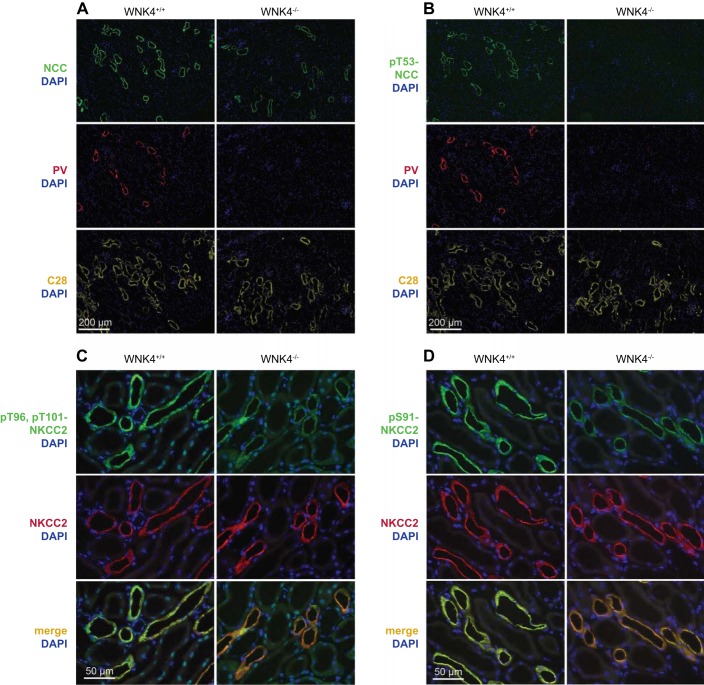
Figure 4 shows the effects of WNK4 deletion on NCC and NKCC2 abundances and sites of expression. Total NCC was strikingly low in wnk4−/− mice, consistent with the previously mentioned Western blot results, although NCC was still easily detectable along calbindin-positive segments (Fig. 4A). This indicates that NCC is still expressed along the DCT2 in the knockout mice [as indicated by colocalization with calbindin (27)]. Further, and as seen in other models in which NaCl transport along the distal tubule is disrupted genetically (16), the DCT1 was essentially absent, as indicated by the near absence of tubules positive for both parvalbumin [a marker of DCT1 (10)] and NCC in the knockout mice (Fig. 4, A and B). Phosphorylated (activated) NCC, however, was not detected anywhere in the wnk4−/− mice (Fig. 4B).
Fig. 4.
Localization and abundance of total and phosphorylated transporters in control and wnk4−/− mice. A: abundance and localization of NCC in control and wnk4−/− mice. Parvalbumin (PV) is expressed along the DCT1. Calbindin (C28) is expressed along the DCT2 and CNT. DAPI is a nuclear marker. B: abundance and localization of pT53-NCC in control and wnk4−/− mice. C: abundance and localization of NKCC2 and pT96, pT101-NKCC2 in control and wnk4−/− mice. Note that only pT96, pT101-NKCC2 is substantially reduced by WNK4 knockout and that total NKCC2 abundance appears unchanged. D: abundance and localization of NKCC2 and pS91-NKCC2 in control and wnk4−/− mice. Note that again, only pS91-NKCC2 is reduced by WNK4 knockout.
As noted above, antibodies against amino-terminal phosphorylation motifs of cation chloride cotransporters may not be specific, because the phosphorylation motifs are similar among members (35). Owing to this limitation, it is likely that the results of the pT96, pT101-NKCC2 Western blots described above represent phosphorylated species of both NCC and NKCC2. Thus we confirmed the reduction in pT96, pT101-NKCC2 using immunofluorescence, where localization would afford specificity. Figure 4C shows that the abundance of total NKCC2 did not appear to differ between control and wnk4−/− mice, confirming the results of the Western blot. In contrast, pT96, pT101-NKCC2 abundance was strikingly reduced along the TAL in the wnk4−/− mice, compared with controls. Additionally, Fig. 4D shows a similar reduction in phospho-NKCC2 using an antibody at a different phospho-site, S91.
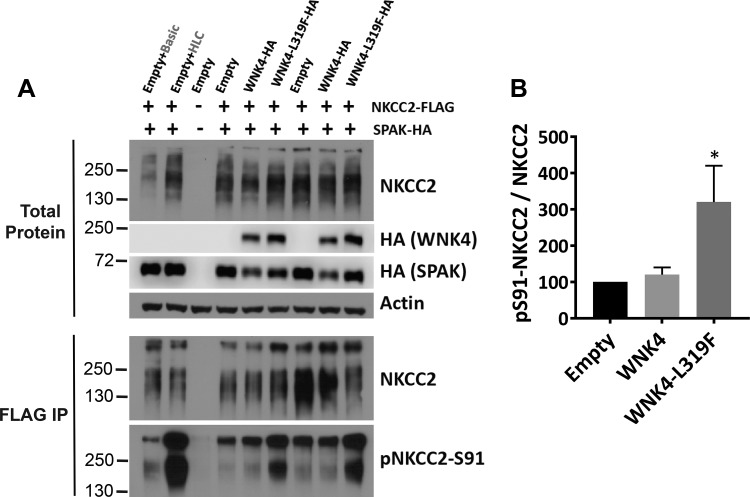
Although SPAK and OxSR1 both stimulate NKCC2 phosphorylation and activity in cells (32), the roles of WNK4 in this process in vivo are not as clear; two groups suggested that WNK4 does not affect this transporter in vivo (3, 42). To test whether WNK4 can activate NKCC2, we analyzed the ability of WNK4 to phosphorylate NKCC2 in HEK293 cells. As it is recognized that WNK4 kinase activity may be inhibited by chloride in cultured cells in which intracellular chloride concentration is high (43), we utilized a mutant WNK4 that is resistant to chloride inhibition (43). Figure 5 shows that this mutant WNK4-L319F, when cotransfected with HA-SPAK and FLAG-NKCC2, significantly increased the abundance of phosphorylated NKCC2 in HEK293 cells.
Fig. 5.
WNK4 promotes NKCC2 phosphorylation in HEK293 cells. A: HEK293 cells were transiently transfected with NKCC2-Flag and SPAK-HA with either WNK4-HA or WNK4-L319F-HA (constitutively active). The effect of WNK4 coexpression on NKCC2 phosphorylation was assessed by Western blot using anti-NKCC2 (MRC PPU, Dundee Univ. S838B) and anti-pS91-NKCC2 (MRC PPU, Dundee Univ. S451C). Prior to blotting, NKCC2 was immunoprecipitated to ensure the specificity of the signal obtained with the phosphoantibody. As a control, a set of HEK293 cells transfected with NKCC2-Flag and SPAK-HA were treated with basic medium (isotonic, normal-[Cl−] medium) or hypotonic/low-chloride medium (HLC), which induces NKCC2 phosphorylation (1). B: results of quantitation. Densitometric analysis was performed using ImageJ. *P < 0.05 vs. empty; n = 4.
Diuretic tests are frequently employed to assess the function of ion transport proteins in vivo. Here, acute treatment with furosemide induced a natriuresis of equal magnitude in wnk4−/− and control mice (Fig. 6A). Although this initially suggested that TAL function was similar in the two groups, we reasoned that the furosemide effect may have been blunted in control mice by load-induced increases in NaCl reabsorption along the distal nephron, which would not have occurred in the wnk4−/− mice. To determine the effects of furosemide in the absence of reabsorption along the DCT, we repeated this experiment in mice treated with hydrochlorothiazide to block NCC activity. In this case, the furosemide response was significantly smaller in the wnk4−/− mice than in controls (Fig. 6B). Coupled with the data described above, these results suggest that WNK4 plays a basal role in stimulating NKCC2 along the TAL.
Fig. 6.
Effects of furosemide on urinary sodium excretion. A: effects on sodium excretion in control and wnk4−/− mice. B: effects of furosemide on sodium excretion in control and wnk4−/− mice treated with hydrochlorothiazide. *Effects of furosemide were different in the two strains, when analyzed by two-way ANOVA.
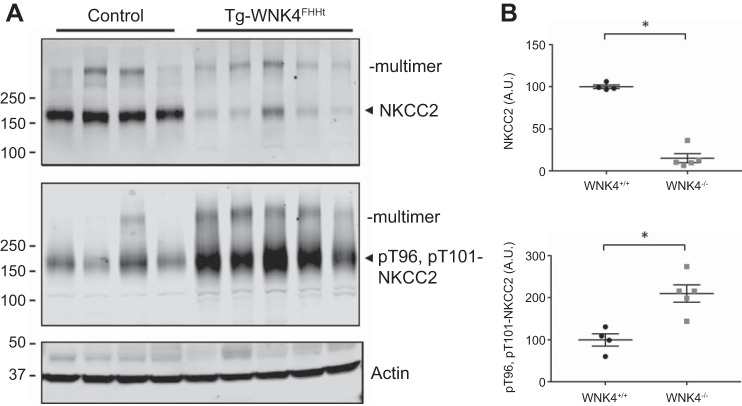
WNK4 overactivity causes FHHt, which is characterized by salt-sensitive hypertension, hyperkalemia, and hyperchloremic metabolic acidosis (45), the opposite of Gitelman syndrome. FHHt is believed to result primarily from inappropriate activation of NCC resulting in increased DCT sodium reabsorption; multiple animal studies provide evidence that correction is possible by treatment with HCTZ (15, 48). Recently, the phenotype has been mimicked when NCC is activated only along the DCT1 (10). Data from the current work, however, suggest that WNK4 regulates ion transport along both the DCT and TAL. Therefore, we hypothesized that FHHt-causing mutations in WNK4, which activate DCT sodium transport, may also activate NKCC2. To test this, we used a previously reported mouse model that expresses an FHHt-causing WNK4Q562E transgene (15). Compared with control animals, Western blots of FHHt WNK4 transgenic mice had increased abundance of pT96, pT101-NKCC2, although total NKCC2 was actually reduced (Fig. 7).
Fig. 7.
Comparison of total and phosphorylated NKCC2 in control mice and mice transgenic for WNK4 carrying the Q562E mutation (15). A: note that mice carrying the mutation exhibit more pT96, pT101-NKCC2 than control mice. B: densitometric quantitation to actin. *P < 0.05.
DISCUSSION
Much has been learned during the past 16 years about the essential roles played by WNK kinases in electrolyte homeostasis and human disease. An important remaining question, however, is how signaling specificity is achieved. In kidney cells, WNK kinases facilitate the phosphorylation of the cation chloride cotransporters, NCC, NKCC, and likely the potassium chloride cotransporters. In each case, the actual phosphorylation appears to be mediated, at least in part, by the intermediary kinases SPAK and OxSR1. It is known that NCC and NKCC are activated by phosphorylation along their amino-terminal domains (8). It seems reasonable, therefore, that the specificity of WNK action in the kidney is determined primarily by sites of specific WNK expression, and sites of coexpression with SPAK or OxSR1. Shortly after WNKs were discovered, WNK4 mRNA was detected along thick ascending limbs, as well as distal convoluted tubules and collecting ducts (28). More recently, two groups, using antibodies validated with knockout mice, also found WNK4 along the TAL, connecting tubule (CNT), and cortical collecting duct as well as the DCT (22, 29). This expression pattern raised the possibility that WNK4 dysfunction, such as occurs in FHHt, might alter ion transport along nephron segments in addition to the DCT. Yet most evidence has favored a predominant effect of WNK4 along the DCT, and specifically on NCC (10, 24).
This focus on WNK4 regulation of NCC along the DCT derived originally from observations that thiazides are remarkably effective in correcting both the hyperkalemia and hypertension in patients with FHHt, but furosemide has also reportedly been effective, at least in some cases (9). In mice in which WNK4 has been deleted, however, the abundance of NKCC2 was found to be similar to that in controls (37, 42), suggesting that, despite its expression along TAL, WNK4 did not regulate NKCC2. Yet there were some inconsistencies with this view. Gamba and colleagues (3) found that wnk4−/− mice wasted sodium at baseline and had elevated urine volume, findings that have not been detected in slc12a3−/− mice (23, 39, 41). Further, the urine calcium/creatinine ratio was similar to control values in wnk4−/− mice (3). Hypocalciuria is a signature feature of Gitelman syndrome (1), and has been detected in slc12a3−/− mice (16, 39), as well as stk39−/− mice lacking SPAK, which also manifest dysfunction primarily of the DCT. The wnk4−/−phenotype, including salt and water wasting and normocalciuria (3 and above) instead resembles Bartter syndrome type 3 in humans (1), as well as the phenotype recently described to accompany deletion of the chloride channel, CLC-k2, which is expressed along the TAL and DCT in mice (14). The current phenotypic data, therefore, coupled with the evidence for alterations in NKCC2 abundance, point strongly to a combined NKCC2/NCC defect in wnk4−/− mice.
Despite an apparent reduction in NKCC2 activity in the wnk4−/− mice, the TAL-specific phenotype appears to be mild. This suggests that, even without WNK4, NKCC2 remains active. Several considerations may explain this. First, other WNK kinases, such as WNK1, may stimulate SPAK and OxSR1 along TAL. Second, other kinases may play an important role in activating NKCC2. Ferdaus and colleagues (6) found evidence for substantial NKCC2 phosphorylation even in mice lacking both SPAK and OxSR1, and suggested that other kinase pathways must be involved. Yet the lack of phosphorylated NKCC2 in the wnk4−/− mice was striking, and although not compared directly, appeared much more extreme that that observed in mice lacking both SPAK and OxSR1 (6). This raises the possibility that WNK4 is mediating NKCC2 phosphorylation independent of SPAK/OxSR1, a possibility raised by the work of Delpire and colleagues (33). This group noted that NKCC2 phosphorylation could occur independent of SPAK/OxSR1, when both WNK4 and Cab39 (calcium binding protein 39 also called mouse protein 25) were present. Although the reduction in pT96, pT101-NKCC2 abundance detected by Western blot was striking, the results likely demonstrate both NCC and NKCC2 abundance, owing to antibody cross reactivity. As the reduction in pT53-NCC abundance is so striking, the magnitude in pT96, pT101-NKCC2 reduction likely appears greater than it is, as suggested by the clear evidence shown by immunofluorescence that pT96, pT101-NKCC2 is present in the wnk4−/− mice. To assay NKCC2 phosphorylation directly in cultured cells, we employed an antibody directed at phosphor-serine 91. Although phosphorylation at this site is not essential for transporter activation, it is strongly phosphorylated in the presence of hypotonic low-chloride stimulation and therefore reflects overall SPAK/OSR1 activity (36). Thus the current results suggest the relevance of WNK4/SPAK/OSR1 along the TAL.
If NKCC2 activity is decreased by WNK4 knockout, then one would expect that NKCC2 activity should be increased in patients with FHHt caused by WNK4 mutations. It seems surprising, therefore, that thiazide diuretics are more effective than loop diuretics in treating hyperkalemia (31). Yet recent work has clearly shown that the DCT plays a unique role in determining potassium secretion along the CNT and collecting duct (5), likely reflecting its proximity to potassium-secreting downstream segments. Although ensuing effects on distal salt delivery have traditionally been invoked to explain this phenomenon, more recent findings suggest that this relationship may be more complicated and involve remodeling of the distal nephron. The current results provide another example of the remarkable plasticity of the DCT. Deletion of WNK4 caused a near-complete absence of DCT1. This is the opposite structural effect of that observed by Grimm and colleagues (10) in mice with constitutively active SPAK expressed in DCT1 segments. Thus it appears that an increased ratio of DCT1/DCT2 is associated with potassium retention, and a decreased ratio is associated with increased potassium secretion. As noted previously (15), WNK4 activity plays a central role in this phenomenon.
Thus the current data indicate that WNK4 along the thick ascending limb modulates NKCC2 phosphorylation status and activity. Deletion of WNK4 generates a phenotype that is closer to human Bartter syndrome type 3 (also called classic Bartter syndrome), which results from combined TAL and DCT dysfunction. This likely explains the failure to detect hypocalciuria in wnk4−/− mice previously. Conversely, a component of increased NKCC2 activity likely contributes to the FHHt phenotype in patients who inherit mutations in WNK4. As patients with FHHt resulting from WNK4 mutations do exhibit hypercalciuria (18), however, effects along the DCT likely predominate in humans. Finally, the current results provide additional evidence for the diverse effects of WNK kinases on multiple transport pathways.
GRANTS
This work was supported by NIH Grants DK51496, DK54983, and T32DK067864, and Department of Veterans Affairs Merit Review 1IOBX002228.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.S.T., M.C.-B., J.A.M., W.-H.W., G.G., C.-L.Y., and D.H.E. conceived and designed research; A.S.T., M.C.-B., M.Z.F., R.J.C., K.J.E., X.-T.S., and L.N.M. performed experiments; A.S.T., M.C.-B., M.Z.F., R.J.C., K.J.E., X.-T.S., L.N.M., J.A.M., W.-H.W., G.G., C.-L.Y., and D.H.E. analyzed data; A.S.T., M.C.-B., K.J.E., X.-T.S., L.N.M., J.A.M., W.-H.W., G.G., C.-L.Y., and D.H.E. interpreted results of experiments; A.S.T., M.C.-B., X.-T.S., L.N.M., W.-H.W., and D.H.E. prepared figures; A.S.T. and D.H.E. drafted manuscript; A.S.T., L.N.M., J.A.M., W.-H.W., G.G., C.-L.Y., and D.H.E. edited and revised manuscript; A.S.T., M.C.-B., M.Z.F., R.J.C., K.J.E., X.-T.S., L.N.M., J.A.M., W.-H.W., G.G., C.-L.Y., and D.H.E. approved final version of manuscript.
REFERENCES
- 1.Blanchard A, Bockenhauer D, Bolignano D, Calò LA, Cosyns E, Devuyst O, Ellison DH, Karet Frankl FE, Knoers NV, Konrad M, Lin SH, Vargas-Poussou R. Gitelman syndrome: consensus and guidance from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 91: 24–33, 2017. doi: 10.1016/j.kint.2016.09.046. [DOI] [PubMed] [Google Scholar]
- 2.Bostanjoglo M, Reeves WB, Reilly RF, Velázquez H, Robertson N, Litwack G, Morsing P, Dørup J, Bachmann S, Ellison DH. 11Beta-hydroxysteroid dehydrogenase, mineralocorticoid receptor, and thiazide-sensitive Na-Cl cotransporter expression by distal tubules. J Am Soc Nephrol 9: 1347–1358, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Castañeda-Bueno M, Cervantes-Pérez LG, Vázquez N, Uribe N, Kantesaria S, Morla L, Bobadilla NA, Doucet A, Alessi DR, Gamba G. Activation of the renal Na+:Cl− cotransporter by angiotensin II is a WNK4-dependent process. Proc Natl Acad Sci USA 109: 7929–7934, 2012. doi: 10.1073/pnas.1200947109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celio MR, Baier W, Schärer L, Gregersen HJ, de Viragh PA, Norman AW. Monoclonal antibodies directed against the calcium binding protein Calbindin D-28k. Cell Calcium 11: 599–602, 1990. doi: 10.1016/0143-4160(90)90014-L. [DOI] [PubMed] [Google Scholar]
- 5.Ellison DH, Terker AS, Gamba G. Potassium and its discontents: new insight, new treatments. J Am Soc Nephrol 27: 981–989, 2016. doi: 10.1681/ASN.2015070751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferdaus MZ, Barber KW, López-Cayuqueo KI, Terker AS, Argaiz ER, Gassaway BM, Chambrey R, Gamba G, Rinehart J, McCormick JA. SPAK and OSR1 play essential roles in potassium homeostasis through actions on the distal convoluted tubule. J Physiol 594: 4945–4966, 2016. doi: 10.1113/JP272311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filice F, Celio MR, Babalian A, Blum W, Szabolcsi V. Parvalbumin-expressing ependymal cells in rostral lateral ventricle wall adhesions contribute to aging-related ventricle stenosis in mice. J Comp Neurol 525: 3266–3285, 2017. doi: 10.1002/cne.24276. [DOI] [PubMed] [Google Scholar]
- 8.Gamba G. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev 85: 423–493, 2005. doi: 10.1152/physrev.00011.2004. [DOI] [PubMed] [Google Scholar]
- 9.Gordon RD. Syndrome of hypertension and hyperkalemia with normal glomerular filtration rate. Hypertension 8: 93–102, 1986. doi: 10.1161/01.HYP.8.2.93. [DOI] [PubMed] [Google Scholar]
- 10.Grimm PR, Coleman R, Delpire E, Welling PA. Constitutively active SPAK causes hyperkalemia by activating NCC and remodeling distal tubules. J Am Soc Nephrol 28: 2597–2606, 2017. doi: 10.1681/ASN.2016090948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimm PR, Taneja TK, Liu J, Coleman R, Chen YY, Delpire E, Wade JB, Welling PA. SPAK isoforms and OSR1 regulate sodium-chloride co-transporters in a nephron-specific manner. J Biol Chem 287: 37673–37690, 2012. doi: 10.1074/jbc.M112.402800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hennings JC, Andrini O, Picard N, Paulais M, Huebner AK, Cayuqueo IK, Bignon Y, Keck M, Cornière N, Böhm D, Jentsch TJ, Chambrey R, Teulon J, Hübner CA, Eladari D. The ClC-K2 chloride channel is critical for salt handling in the distal nephron. J Am Soc Nephrol 28: 209–217, 2017. doi: 10.1681/ASN.2016010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, Nelson-Williams C, Ellison DH, Flavell R, Booth CJ, Lu Y, Geller DS, Lifton RP. Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet 38: 1124–1132, 2006. doi: 10.1038/ng1877. [DOI] [PubMed] [Google Scholar]
- 16.Loffing J, Vallon V, Loffing-Cueni D, Aregger F, Richter K, Pietri L, Bloch-Faure M, Hoenderop JG, Shull GE, Meneton P, Kaissling B. Altered renal distal tubule structure and renal Na+ and Ca2+ handling in a mouse model for Gitelman’s syndrome. J Am Soc Nephrol 15: 2276–2288, 2004. doi: 10.1097/01.ASN.0000138234.18569.63. [DOI] [PubMed] [Google Scholar]
- 17.Matsunoshita N, Nozu K, Shono A, Nozu Y, Fu XJ, Morisada N, Kamiyoshi N, Ohtsubo H, Ninchoji T, Minamikawa S, Yamamura T, Nakanishi K, Yoshikawa N, Shima Y, Kaito H, Iijima K. Differential diagnosis of Bartter syndrome, Gitelman syndrome, and pseudo-Bartter/Gitelman syndrome based on clinical characteristics. Genet Med 18: 180–188, 2016. doi: 10.1038/gim.2015.56. [DOI] [PubMed] [Google Scholar]
- 18.Mayan H, Munter G, Shaharabany M, Mouallem M, Pauzner R, Holtzman EJ, Farfel Z. Hypercalciuria in familial hyperkalemia and hypertension accompanies hyperkalemia and precedes hypertension: description of a large family with the Q565E WNK4 mutation. J Clin Endocrinol Metab 89: 4025–4030, 2004. doi: 10.1210/jc.2004-0037. [DOI] [PubMed] [Google Scholar]
- 19.Mayan H, Vered I, Mouallem M, Tzadok-Witkon M, Pauzner R, Farfel Z. Pseudohypoaldosteronism type II: marked sensitivity to thiazides, hypercalciuria, normomagnesemia, and low bone mineral density. J Clin Endocrinol Metab 87: 3248–3254, 2002. doi: 10.1210/jcem.87.7.8449. [DOI] [PubMed] [Google Scholar]
- 20.McCormick JA, Mutig K, Nelson JH, Saritas T, Hoorn EJ, Yang C-L, Rogers S, Curry J, Delpire E, Bachmann S, Ellison DH. A SPAK isoform switch modulates renal salt transport and blood pressure. Cell Metab 14: 352–364, 2011. doi: 10.1016/j.cmet.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCormick JA, Nelson JH, Yang CL, Curry JN, Ellison DH. Overexpression of the sodium chloride cotransporter is not sufficient to cause familial hyperkalemic hypertension. Hypertension 58: 888–894, 2011. doi: 10.1161/HYPERTENSIONAHA.110.167809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCormick JA, Yang CL, Zhang C, Davidge B, Blankenstein KI, Terker AS, Yarbrough B, Meermeier NP, Park HJ, McCully B, West M, Borschewski A, Himmerkus N, Bleich M, Bachmann S, Mutig K, Argaiz ER, Gamba G, Singer JD, Ellison DH. Hyperkalemic hypertension-associated cullin 3 promotes WNK signaling by degrading KLHL3. J Clin Invest 124: 4723–4736, 2014. doi: 10.1172/JCI76126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris RG, Hoorn EJ, Knepper MA. Hypokalemia in a mouse model of Gitelman’s syndrome. Am J Physiol Renal Physiol 290: F1416–F1420, 2006. doi: 10.1152/ajprenal.00421.2005. [DOI] [PubMed] [Google Scholar]
- 24.Murthy M, Kurz T, O’Shaughnessy KM. WNK signalling pathways in blood pressure regulation. Cell Mol Life Sci 74: 1261–1280, 2017. doi: 10.1007/s00018-016-2402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mutig K, Paliege A, Kahl T, Jöns T, Müller-Esterl W, Bachmann S. Vasopressin V2 receptor expression along rat, mouse, and human renal epithelia with focus on TAL. Am J Physiol Renal Physiol 293: F1166–F1177, 2007. doi: 10.1152/ajprenal.00196.2007. [DOI] [PubMed] [Google Scholar]
- 26.Na T, Wu G, Zhang W, Dong WJ, Peng JB. Disease-causing R1185C mutation of WNK4 disrupts a regulatory mechanism involving calmodulin binding and SGK1 phosphorylation sites. Am J Physiol Renal Physiol 304: F8–F18, 2013. doi: 10.1152/ajprenal.00284.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nijenhuis T, Hoenderop JG, van der Kemp AW, Bindels RJ. Localization and regulation of the epithelial Ca2+ channel TRPV6 in the kidney. J Am Soc Nephrol 14: 2731–2740, 2003. doi: 10.1097/01.ASN.0000094081.78893.E8. [DOI] [PubMed] [Google Scholar]
- 28.O’Reilly M, Marshall E, Macgillivray T, Mittal M, Xue W, Kenyon CJ, Brown RW. Dietary electrolyte-driven responses in the renal WNK kinase pathway in vivo. J Am Soc Nephrol 17: 2402–2413, 2006. doi: 10.1681/ASN.2005111197. [DOI] [PubMed] [Google Scholar]
- 29.Ohno M, Uchida K, Ohashi T, Nitta K, Ohta A, Chiga M, Sasaki S, Uchida S. Immunolocalization of WNK4 in mouse kidney. Histochem Cell Biol 136: 25–35, 2011. doi: 10.1007/s00418-011-0827-x. [DOI] [PubMed] [Google Scholar]
- 30.Ohta A, Rai T, Yui N, Chiga M, Yang SS, Lin SH, Sohara E, Sasaki S, Uchida S. Targeted disruption of the Wnk4 gene decreases phosphorylation of Na-Cl cotransporter, increases Na excretion and lowers blood pressure. Hum Mol Genet 18: 3978–3986, 2009. doi: 10.1093/hmg/ddp344. [DOI] [PubMed] [Google Scholar]
- 31.Pathare G, Hoenderop JG, Bindels RJ, San-Cristobal P. A molecular update on pseudohypoaldosteronism type II. Am J Physiol Renal Physiol 305: F1513–F1520, 2013. doi: 10.1152/ajprenal.00440.2013. [DOI] [PubMed] [Google Scholar]
- 32.Piechotta K, Lu J, Delpire E. Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1). J Biol Chem 277: 50812–50819, 2002. doi: 10.1074/jbc.M208108200. [DOI] [PubMed] [Google Scholar]
- 33.Ponce-Coria J, Markadieu N, Austin TM, Flammang L, Rios K, Welling PA, Delpire E. A novel Ste20-related proline/alanine-rich kinase (SPAK)-independent pathway involving calcium-binding protein 39 (Cab39) and serine threonine kinase with no lysine member 4 (WNK4) in the activation of Na-K-Cl cotransporters. J Biol Chem 289: 17680–17688, 2014. doi: 10.1074/jbc.M113.540518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rafiqi FH, Zuber AM, Glover M, Richardson C, Fleming S, Jovanović S, Jovanović A, O’Shaughnessy KM, Alessi DR. Role of the WNK-activated SPAK kinase in regulating blood pressure. EMBO Mol Med 2: 63–75, 2010. doi: 10.1002/emmm.200900058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richardson C, Alessi DR. The regulation of salt transport and blood pressure by the WNK-SPAK/OSR1 signalling pathway. J Cell Sci 121: 3293–3304, 2008. doi: 10.1242/jcs.029223. [DOI] [PubMed] [Google Scholar]
- 36.Richardson C, Sakamoto K, de los Heros P, Deak M, Campbell DG, Prescott AR, Alessi DR. Regulation of the NKCC2 ion cotransporter by SPAK-OSR1-dependent and -independent pathways. J Cell Sci 124: 789–800, 2011. doi: 10.1242/jcs.077230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.San-Cristobal P, Pacheco-Alvarez D, Richardson C, Ring AM, Vazquez N, Rafiqi FH, Chari D, Kahle KT, Leng Q, Bobadilla NA, Hebert SC, Alessi DR, Lifton RP, Gamba G. Angiotensin II signaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK-dependent pathway. Proc Natl Acad Sci USA 106: 4384–4389, 2009. doi: 10.1073/pnas.0813238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmitt R, Klussmann E, Kahl T, Ellison DH, Bachmann S. Renal expression of sodium transporters and aquaporin-2 in hypothyroid rats. Am J Physiol Renal Physiol 284: F1097–F1104, 2003. doi: 10.1152/ajprenal.00368.2002. [DOI] [PubMed] [Google Scholar]
- 39.Schultheis PJ, Lorenz JN, Meneton P, Nieman ML, Riddle TM, Flagella M, Duffy JJ, Doetschman T, Miller ML, Shull GE. Phenotype resembling Gitelman’s syndrome in mice lacking the apical Na+-Cl− cotransporter of the distal convoluted tubule. J Biol Chem 273: 29150–29155, 1998. doi: 10.1074/jbc.273.44.29150. [DOI] [PubMed] [Google Scholar]
- 40.Shekarabi M, Zhang J, Khanna AR, Ellison DH, Delpire E, Kahle KT. WNK kinase signaling in ion homeostasis and human disease. Cell Metab 25: 285–299, 2017. doi: 10.1016/j.cmet.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 41.Sinning A, Radionov N, Trepiccione F, Lopez-Cayuqueo KI, Jayat M, Baron S, Corniere N, Alexander RT, Hadchouel J, Eladari D, Hubner CA, Chambrey R. Double knockout of the Na+-driven Cl−/HCO3− exchanger and Na+/Cl− cotransporter induces hypokalemia and volume depletion. J Am Soc Nephrol 28: 130–139, 2017. doi: 10.1681/ASN.2015070734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takahashi D, Mori T, Nomura N, Khan MZ, Araki Y, Zeniya M, Sohara E, Rai T, Sasaki S, Uchida S. WNK4 is the major WNK positively regulating NCC in the mouse kidney. Biosci Rep 34: e00107, 2014. doi: 10.1042/BSR20140047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terker AS, Zhang C, Erspamer KJ, Gamba G, Yang CL, Ellison DH. Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int 89: 127–134, 2016. doi: 10.1038/ki.2015.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, Siler DA, Park HJ, Fu Y, Cohen DM, Weinstein AM, Wang WH, Yang CL, Ellison DH. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 21: 39–50, 2015. doi: 10.1016/j.cmet.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson FH, Disse-Nicodème S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 46.Yang CL, Liu X, Paliege A, Zhu X, Bachmann S, Dawson DC, Ellison DH. WNK1 and WNK4 modulate CFTR activity. Biochem Biophys Res Commun 353: 535–540, 2007. doi: 10.1016/j.bbrc.2006.11.151. [DOI] [PubMed] [Google Scholar]
- 47.Yang SS, Lo YF, Wu CC, Lin SW, Yeh CJ, Chu P, Sytwu HK, Uchida S, Sasaki S, Lin SH. SPAK-knockout mice manifest Gitelman syndrome and impaired vasoconstriction. J Am Soc Nephrol 21: 1868–1877, 2010. doi: 10.1681/ASN.2009121295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang SS, Morimoto T, Rai T, Chiga M, Sohara E, Ohno M, Uchida K, Lin SH, Moriguchi T, Shibuya H, Kondo Y, Sasaki S, Uchida S. Molecular pathogenesis of pseudohypoaldosteronism type II: generation and analysis of a Wnk4(D561A/+) knockin mouse model. Cell Metab 5: 331–344, 2007. doi: 10.1016/j.cmet.2007.03.009. [DOI] [PubMed] [Google Scholar]