Abstract
Renovascular disease (RVD), which is prevalent in the elderly, significantly increases cardiovascular risk and can progressively deteriorate renal function. The loss of renal function in patients with RVD is associated with a progressive dysfunction, damage, and loss of renal microvessels, which can be combined with decreased renal bioavailability of vascular endothelial growth factor (VEGF) and a defective vascular repair and proliferation. This association has been the impetus for recent efforts that have focused on developing methods to stop the progression of renal injury by protecting the renal microvasculature. This mini-review focuses on recent studies supporting potential applications of VEGF therapy for the kidney and discusses underlying mechanisms of renoprotection.
Keywords: angiogenesis, drug delivery, intervention, microcirculation, renovascular disease
INTRODUCTION
The kidney is a highly vascularized organ in which the renal microvasculature not only serves the metabolic demands of the kidney, but also plays a key role for whole body homeostasis of blood pressure, body fluid and electrolyte balance, pH, calcium metabolism, and erythropoiesis. Microvascular (MV) rarefaction is observed in renal pathologies and can be structural or functional: anatomical loss of the microvessels or functional loss of the microvessels with no anatomical change, respectively (33). Several studies have shown that renal MV rarefaction, which entails dysfunction and remodeling, are universal features in acute and chronic renal disease from different etiologies (19, 20) that progress and evolve along with renal dysfunction and injury (20). Clinical and experimental studies have also shown that damage of the small renal vessels may precede and predict the decline in renal function in hypertension, diabetes, and obesity (2, 9, 28, 35), which are major causes of chronic renal disease. Altogether, these compelling data support a plausible cause-and-effect relationship between MV rarefaction and renal injury and offer a rationale for MV protection as a therapeutic approach.
Chronic renovascular disease (RVD) is a progressive condition initially caused by unilateral or bilateral obstruction of the main renal artery or primary bifurcations. RVD is caused by atherosclerotic renal artery stenosis in more than 90% of cases (45), affects 9–11% of the adults in the United States (41, 46, 47), and may lead to or intensify cardiovascular and renal pathologies. Indeed, patients with RVD usually suffer from hypertension and higher cardiovascular morbidity and mortality, and they are more prone to develop chronic kidney disease (CKD) by up to 25% (45). Using a clinically relevant model of RVD in swine, studies from our laboratory showed that the progressive decline in the function of the stenotic kidney (e.g., reduced renal blood flow and glomerular filtration) and renal injury in RVD associates with a progressive renal MV rarefaction that is exacerbated by defective mechanisms of MV repair and proliferation in the kidney (10–12, 14, 15). Furthermore, we showed that structural and functional renal MV rarefaction in RVD is largely driven by progressively reduced renal bioavailability of vascular endothelial growth factor (VEGF), an endogenous angiogenic cytokine with prominent roles in MV proliferation and repair that maintains vascular networks in every tissue, including the kidney (18). The loss of VEGF is followed by altered downstream angiogenic signaling (14, 29, 52), which subsequently contributes to the defective renal recruitment and homing of cell progenitors in RVD. Our previous work (14, 15) supports the idea that disruption of this endogenous mechanism of tissue healing in RVD significantly contributes to progression of the disease.
Recent studies support the potential for VEGF therapy as a tool to restore renal MV architecture and function and slow the progression of renal injury in different models of renal disease (3, 10–13, 31, 32). This concise review will focus on potential mechanisms and novel applications of VEGF therapy for the kidney.
VEGF in the kidney.
VEGF is a proangiogenic and prosurvival factor for endothelial cells that promotes cell division, survival (22, 23), mobilization of endothelial progenitor cells (EPCs) (53), and vascular proliferation. VEGF plays a central role in angiogenesis, an important process not only during organ development, but also in the response to tissue ischemia (18), during which it maintains and repairs MV networks in virtually every organ. A functional vasculature is vital for maintaining the kidney’s normal physiological functions, and the importance of VEGF in this process is highlighted by studies showing that VEGF inhibition associated with the development of hypertension, podocyte loss (25), and renal injury (17). Furthermore, a significant reduction in VEGF availability is observed in renal pathologies associated with chronic reductions in blood flow, including RVD (29), CKD of different etiologies (e.g., diabetes and hypertension) (19, 20), and progressive glomerulopathies (42).
VEGF therapy: recent studies.
To counteract tissue hypoxia, the microvasculature adapts to local metabolic conditions partly through angiogenesis, which entails sprouting of new vessels from preexisting ones (20). Unlike in conditions of acute ischemia, tissues exposed to chronic ischemia not only often have significant MV damage, but also an impaired angiogenic response that results, in part, from decreased availability of VEGF and altered downstream signaling (29, 38). On the basis of these observations, the idea of VEGF therapy to recover MV proliferation and repair has been tried in different pathological conditions, such as neurodegenerative diseases (16), placental abnormalities (24), peripheral neuropathies (49), peripheral artery disease (44), myocardial ischemia (1, 40), and renal ischemia, as occurs in RVD (30, 39, 51). However, not until recently has more attention turned toward the potential of VEGF therapy for the kidney.
Studies have reported the benefits of VEGF therapy in several experimental models of renal disease (3, 31, 32). For example, in both the remnant kidney model and a rodent model of CKD, systemic or subcutaneous injection of the VEGF successfully preserved the MV structure and density by stimulating EPCs, which subsequently stabilized renal function and attenuated disease progression (3, 31, 32). These studies support the feasibility of VEGF therapy as a tool to preserve renal vascular integrity. We recently demonstrated the feasibility and therapeutic efficacy of intrarenal administration of recombinant-human (rh)-VEGF through the main renal artery, distal to the vascular obstruction (10, 11) in a clinically relevant swine model of chronic RVD. Both preventive (10) and interventional (11) intrarenal administration of rh-VEGF produced similar renoprotective effects on MV rarefaction and stenotic kidney hemodynamics and function, indicating that newly formed vessels are likely functional. Along with improved vessel structure and function, fibrosis was also attenuated, suggesting that intrarenal VEGF therapy may attenuate the progression of renal injury in RVD, even when administered at an advanced stage of the disease (Fig. 1). However, one limitation of this approach is that intravenous, intrarenal, or subcutaneous VEGF delivery may potentially affect other organ systems besides the kidney. In addition, the short half-life and high susceptibility to degradation of VEGF in vivo may require repeated intrarenal injections to achieve significant therapeutic benefits. Indeed, previous studies (1, 16, 24, 40) showed that repeated or continuous VEGF therapy directly to the target site was required for efficacy, underscoring the need for sustained VEGF release (21). Repeated VEGF therapy may further increase the potential for untoward side effects in other organ systems. Therefore, to improve efficacy and reduce potential side effects, new strategies that target VEGF specifically to the kidney may address these concerns and help to advance the field.
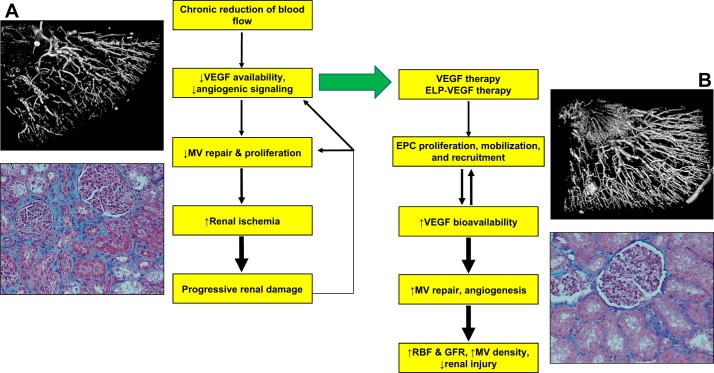
Fig. 1.
Schematic flow chart describing mechanisms of renal injury in renovascular disease and proposed mechanism of renoprotection by vascular endothelial growth factor (VEGF) therapy. Accompanied by a three-dimensional micro-CT reconstruction of the renal microcirculation (A and B, top) and a representative renal cross section showing renal fibrosis in the stenotic kidney (trichrome, ×20, A and B, bottom). MV, microvascular; ELP, elastin-like polypeptides; EPC, endothelial progenitor cells; RBF, renal blood flow; GRF, glomerular filtration rate.
Elastin-like polypeptides: a novel tool for VEGF delivery.
Elastin-like polypeptides (ELPs) are synthetic proteins based on human elastin that can be used as efficient drug delivery vectors because of their long plasma half-life (5, 34) and low immunogenicity (37, 43). Additionally, ELPs can be modified to the desired size and transition temperature (48) to improve tissue penetration and targeting, and they can be linked to virtually any therapeutic proteins or peptides (5–8, 36, 37).
Recently, Chade et al. (12, 13) developed a novel biopolymer-delivered VEGF construct by fusing VEGF to an ELP carrier (ELP-VEGF). The ELP-VEGF construct is active in vitro and in vivo, accumulates mostly in the kidney regardless of the administration route (12, 13), and is detectable in the circulation for a longer period of time with a slower renal clearance than unconjugated VEGF (21). In addition, we demonstrated that a single intrarenal (12) or systemic (13) injection of ELP-VEGF in a swine model of RVD induced renoprotective effects that were superior to unconjugated VEGF. These renoprotective effects were associated with improved renal VEGF signaling and augmented expression of factors involved in mobilization and homing of cell progenitors (13) (Fig. 1). Therefore, it is possible that the long-term, sustained effects of ELP-VEGF therapy were largely driven by stimulating progenitor cell recruitment to the kidney, leading to a proangiogenic milieu that restored endogenous mechanisms of vascular repair and proliferation, consequently resulting in a prolonged renoprotection in RVD. Importantly, intrarenal or systemic ELP-VEGF therapy did not induce any off-the-kidney effects (13). Overall, these studies demonstrated the therapeutic potential of using ELP as a renal vector for delivery of VEGF, and highlighted the success of ELP-VEGF therapy to target the kidney, protect the renal microcirculation, and preserve renal function more efficiently than unconjugated VEGF.
More recently, Bidwell et al. (4) developed a novel kidney-targeted version of an ELP carrier (KTP-ELP) to achieve even greater renal specificity (4). Uptake by human renal cells (glomerular microvascular endothelial cells, podocytes, and proximal tubular epithelial cells) of the KTP-ELP construct in vitro was higher than untargeted ELP, and acute or chronic administration of KTP-ELP in vivo resulted in greater renal accumulation than ELP with no adverse effects on renal function in rodent or swine models (4), demonstrating the specificity and safety of the KTP-ELP carrier regardless of the species. Ongoing efforts aim to fuse KTP-ELP to VEGF to augment renal VEGF targeting; future studies will determine whether KTP-ELP-VEGF improves renal function in RVD as efficiently as ELP-VEGF or more, but with the advantage of minimizing the potential for off-target effects regardless of the route of administration.
Conclusions.
VEGF therapy for the kidney is a feasible strategy that may be honed and built upon. It is promising that many of the findings and new methods of delivering VEGF to the injured kidney have been characterized in a clinically relevant swine model of RVD, whose similarities to humans may increase the potential for a “bench-to-bedside” transition of therapeutic angiogenesis in the near future. Furthermore, the success of VEGF therapy in inducing protective effects on the microvasculature in several clinical pathologies (26, 27, 50) supports the notion that it may be beneficial for clinical use in other diseases. Potential clinical applications of VEGF therapy will be further supported by future preclinical studies on outcomes of VEGF therapy in relation to biological characteristics, such as animal age and sex. Finally, since damage of the renal microcirculation is universally present in chronic renal disease (20), the road ahead may offer opportunities for application to renal diseases of different etiologies, and, ideally, at different stages of severity, although future studies are necessary to determine the effectiveness of VEGF therapy in all renal disease. Such studies may help to identify the “window of opportunity” by which therapeutic angiogenesis can successfully halt or reverse the progression of renal injury.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R41 DK-109737 from the National Institutes of Health, Grant 18490005 from the American Heart Association, and a grant from intramural research support program from the University of Mississippi Medical Center (A. R. Chade).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.G. and A.R.C. prepared figures; E.G. and A.R.C. drafted manuscript; E.G. and A.R.C. edited and revised manuscript; E.G. and A.R.C. approved final version of manuscript.
REFERENCES
- 1.Banai S, Jaklitsch MT, Shou M, Lazarous DF, Scheinowitz M, Biro S, Epstein SE, Unger EF. Angiogenic-induced enhancement of collateral blood flow to ischemic myocardium by vascular endothelial growth factor in dogs. Circulation 89: 2183–2189, 1994. doi: 10.1161/01.CIR.89.5.2183. [DOI] [PubMed] [Google Scholar]
- 2.Basile DP. Rarefaction of peritubular capillaries following ischemic acute renal failure: a potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens 13: 1–7, 2004. doi: 10.1097/00041552-200401000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Basile DP, Fredrich K, Chelladurai B, Leonard EC, Parrish AR. Renal ischemia reperfusion inhibits VEGF expression and induces ADAMTS-1, a novel VEGF inhibitor. Am J Physiol Renal Physiol 294: F928–F936, 2008. doi: 10.1152/ajprenal.00596.2007. [DOI] [PubMed] [Google Scholar]
- 4.Bidwell GL III, Mahdi F, Shao Q, Logue OC, Waller JP, Reese C, Chade AR. A kidney-selective biopolymer for targeted drug delivery. Am J Physiol Renal Physiol 312: F54–F64, 2017. doi: 10.1152/ajprenal.00143.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bidwell GL III, Perkins E, Hughes J, Khan M, James JR, Raucher D. Thermally targeted delivery of a c-Myc inhibitory polypeptide inhibits tumor progression and extends survival in a rat glioma model. PLoS One 8: e55104, 2013. doi: 10.1371/journal.pone.0055104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bidwell GL III, Perkins E, Raucher D. A thermally targeted c-Myc inhibitory polypeptide inhibits breast tumor growth. Cancer Lett 319: 136–143, 2012. doi: 10.1016/j.canlet.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bidwell GL III, Raucher D. Application of thermally responsive polypeptides directed against c-Myc transcriptional function for cancer therapy. Mol Cancer Ther 4: 1076–1085, 2005. doi: 10.1158/1535-7163.MCT-04-0253. [DOI] [PubMed] [Google Scholar]
- 8.Bidwell GL III, Whittom AA, Thomas E, Lyons D, Hebert MD, Raucher D. A thermally targeted peptide inhibitor of symmetrical dimethylation inhibits cancer-cell proliferation. Peptides 31: 834–841, 2010. doi: 10.1016/j.peptides.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bobik A. The structural basis of hypertension: vascular remodelling, rarefaction and angiogenesis/arteriogenesis. J Hypertens 23: 1473–1475, 2005. doi: 10.1097/01.hjh.0000174970.56965.4f. [DOI] [PubMed] [Google Scholar]
- 10.Chade AR, Kelsen S. Renal microvascular disease determines the responses to revascularization in experimental renovascular disease. Circ Cardiovasc Interv 3: 376–383, 2010. doi: 10.1161/CIRCINTERVENTIONS.110.951277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chade AR, Kelsen S. Reversal of renal dysfunction by targeted administration of VEGF into the stenotic kidney: a novel potential therapeutic approach. Am J Physiol Renal Physiol 302: F1342–F1350, 2012. doi: 10.1152/ajprenal.00674.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chade AR, Tullos NA, Harvey TW, Mahdi F, Bidwell GL III. Renal therapeutic angiogenesis using a bioengineered polymer-stabilized vascular endothelial growth factor construct. J Am Soc Nephrol 27: 1741–1752, 2016. doi: 10.1681/ASN.2015040346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chade AR, Williams ML, Guise E, Vincent LJ, Harvey TW, Kuna M, Mahdi F, Bidwell GL III. Systemic biopolymer-delivered vascular endothelial growth factor promotes therapeutic angiogenesis in experimental renovascular disease. Kidney Int 93: 842-854, 2018. doi: 10.1016/j.kint.2017.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chade AR, Zhu X, Lavi R, Krier JD, Pislaru S, Simari RD, Napoli C, Lerman A, Lerman LO. Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation 119: 547–557, 2009. doi: 10.1161/CIRCULATIONAHA.108.788653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chade AR, Zhu XY, Krier JD, Jordan KL, Textor SC, Grande JP, Lerman A, Lerman LO. Endothelial progenitor cells homing and renal repair in experimental renovascular disease. Stem Cells 28: 1039–1047, 2010. doi: 10.1002/stem.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cvetanovic M, Patel JM, Marti HH, Kini AR, Opal P. Vascular endothelial growth factor ameliorates the ataxic phenotype in a mouse model of spinocerebellar ataxia type 1. Nat Med 17: 1445–1447, 2011. doi: 10.1038/nm.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eremina V, Jefferson JA, Kowalewska J, Hochster H, Haas M, Weisstuch J, Richardson C, Kopp JB, Kabir MG, Backx PH, Gerber HP, Ferrara N, Barisoni L, Alpers CE, Quaggin SE. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med 358: 1129–1136, 2008. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 9: 669–676, 2003. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 19.Futrakul N, Butthep P, Futrakul P. Altered vascular homeostasis in chronic kidney disease. Clin Hemorheol Microcirc 38: 201–207, 2008. [PubMed] [Google Scholar]
- 20.Futrakul N, Butthep P, Laohareungpanya N, Chaisuriya P, Ratanabanangkoon K. A defective angiogenesis in chronic kidney disease. Ren Fail 30: 215–217, 2008. doi: 10.1080/08860220701813335. [DOI] [PubMed] [Google Scholar]
- 21.George EM, Liu H, Robinson GG, Mahdi F, Perkins E, Bidwell GL III. Growth factor purification and delivery systems (PADS) for therapeutic angiogenesis. Vasc Cell 7: 1, 2015. doi: 10.1186/s13221-014-0026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerber HP, Dixit V, Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem 273: 13313–13316, 1998. doi: 10.1074/jbc.273.21.13313. [DOI] [PubMed] [Google Scholar]
- 23.Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 273: 30336–30343, 1998. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert JS, Verzwyvelt J, Colson D, Arany M, Karumanchi SA, Granger JP. Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placentalischemia-induced hypertension. Hypertension 55: 380–385, 2010. doi: 10.1161/HYPERTENSIONAHA.109.141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harvey TW, Engel JE, Chade AR. Vascular endothelial growth factor and podocyte protection in chronic hypoxia: effects of endothelin-A receptor antagonism. Am J Nephrol 43: 74–84, 2016. doi: 10.1159/000444719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hendel RC, Henry TD, Rocha-Singh K, Isner JM, Kereiakes DJ, Giordano FJ, Simons M, Bonow RO. Effect of intracoronary recombinant human vascular endothelial growth factor on myocardial perfusion: evidence for a dose-dependent effect. Circulation 101: 118–121, 2000. doi: 10.1161/01.CIR.101.2.118. [DOI] [PubMed] [Google Scholar]
- 27.Henry TD, Rocha-Singh K, Isner JM, Kereiakes DJ, Giordano FJ, Simons M, Losordo DW, Hendel RC, Bonow RO, Eppler SM, Zioncheck TF, Holmgren EB, McCluskey ER. Intracoronary administration of recombinant human vascular endothelial growth factor to patients with coronary artery disease. Am Heart J 142: 872–880, 2001. doi: 10.1067/mhj.2001.118471. [DOI] [PubMed] [Google Scholar]
- 28.Iliescu R, Chade AR. Progressive renal vascular proliferation and injury in obese Zucker rats. Microcirculation 17: 250–258, 2010. doi: 10.1111/j.1549-8719.2010.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iliescu R, Fernandez SR, Kelsen S, Maric C, Chade AR. Role of renal microcirculation in experimental renovascular disease. Nephrol Dial Transplant 25: 1079–1087, 2010. doi: 10.1093/ndt/gfp605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juillard L, Lerman LO, Kruger DG, Haas JA, Rucker BC, Polzin JA, Riederer SJ, Romero JC. Blood oxygen level-dependent measurement of acute intra-renal ischemia. Kidney Int 65: 944–950, 2004. doi: 10.1111/j.1523-1755.2004.00469.x. [DOI] [PubMed] [Google Scholar]
- 31.Kang DH, Hughes J, Mazzali M, Schreiner GF, Johnson RJ. Impaired angiogenesis in the remnant kidney model: II. Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J Am Soc Nephrol 12: 1448–1457, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Leonard EC, Friedrich JL, Basile DP. VEGF-121 preserves renal microvessel structure and ameliorates secondary renal disease following acute kidney injury. Am J Physiol Renal Physiol 295: F1648–F1657, 2008. doi: 10.1152/ajprenal.00099.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levy BI, Schiffrin EL, Mourad JJ, Agostini D, Vicaut E, Safar ME, Struijker-Boudier HA. Impaired tissue perfusion: a pathology common to hypertension, obesity, and diabetes mellitus. Circulation 118: 968–976, 2008. doi: 10.1161/CIRCULATIONAHA.107.763730. [DOI] [PubMed] [Google Scholar]
- 34.Liu W, Dreher MR, Furgeson DY, Peixoto KV, Yuan H, Zalutsky MR, Chilkoti A. Tumor accumulation, degradation and pharmacokinetics of elastin-like polypeptides in nude mice. J Control Release 116: 170–178, 2006. doi: 10.1016/j.jconrel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 35.Maric-Bilkan C, Flynn ER, Chade AR. Microvascular disease precedes the decline in renal function in the streptozotocin-induced diabetic rat. Am J Physiol Renal Physiol 302: F308–F315, 2012. doi: 10.1152/ajprenal.00421.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Massodi I, Moktan S, Rawat A, Bidwell GL III, Raucher D. Inhibition of ovarian cancer cell proliferation by a cell cycle inhibitory peptide fused to a thermally responsive polypeptide carrier. Int J Cancer 126: 533–544, 2010. doi: 10.1002/ijc.24725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massodi I, Thomas E, Raucher D. Application of thermally responsive elastin-like polypeptide fused to a lactoferrin-derived peptide for treatment of pancreatic cancer. Molecules 14: 1999–2015, 2009. doi: 10.3390/molecules14061999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olszewska-Pazdrak B, Hein TW, Olszewska P, Carney DH. Chronic hypoxia attenuates VEGF signaling and angiogenic responses by downregulation of KDR in human endothelial cells. Am J Physiol Cell Physiol 296: C1162–C1170, 2009. doi: 10.1152/ajpcell.00533.2008. [DOI] [PubMed] [Google Scholar]
- 39.Palm F, Connors SG, Mendonca M, Welch WJ, Wilcox CS. Angiotensin II type 2 receptors and nitric oxide sustain oxygenation in the clipped kidney of early Goldblatt hypertensive rats. Hypertension 51: 345–351, 2008. doi: 10.1161/HYPERTENSIONAHA.107.097832. [DOI] [PubMed] [Google Scholar]
- 40.Pearlman JD, Hibberd MG, Chuang ML, Harada K, Lopez JJ, Gladstone SR, Friedman M, Sellke FW, Simons M. Magnetic resonance mapping demonstrates benefits of VEGF-induced myocardial angiogenesis. Nat Med 1: 1085–1089, 1995. doi: 10.1038/nm1095-1085. [DOI] [PubMed] [Google Scholar]
- 41.Ritchie J, Green D, Chrysochou C, Chalmers N, Foley RN, Kalra PA. High-risk clinical presentations in atherosclerotic renovascular disease: prognosis and response to renal artery revascularization. Am J Kidney Dis 63: 186–197, 2014. doi: 10.1053/j.ajkd.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 42.Rudnicki M, Perco P, Enrich J, Eder S, Heininger D, Bernthaler A, Wiesinger M, Sarközi R, Noppert SJ, Schramek H, Mayer B, Oberbauer R, Mayer G. Hypoxia response and VEGF-A expression in human proximal tubular epithelial cells in stable and progressive renal disease. Lab Invest 89: 337–346, 2009. doi: 10.1038/labinvest.2008.158. [DOI] [PubMed] [Google Scholar]
- 43.Shamji MF, Betre H, Kraus VB, Chen J, Chilkoti A, Pichika R, Masuda K, Setton LA. Development and characterization of a fusion protein between thermally responsive elastin-like polypeptide and interleukin-1 receptor antagonist: sustained release of a local antiinflammatory therapeutic. Arthritis Rheum 56: 3650–3661, 2007. doi: 10.1002/art.22952. [DOI] [PubMed] [Google Scholar]
- 44.Takeshita S, Zheng LP, Brogi E, Kearney M, Pu LQ, Bunting S, Ferrara N, Symes JF, Isner JM. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Invest 93: 662–670, 1994. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Textor SC. Ischemic nephropathy: where are we now? J Am Soc Nephrol 15: 1974–1982, 2004. doi: 10.1097/01.ASN.0000133699.97353.24. [DOI] [PubMed] [Google Scholar]
- 46.Textor SC, Lerman LO. Reality and renovascular disease: when does renal artery stenosis warrant revascularization? Am J Kidney Dis 63: 175–177, 2014. doi: 10.1053/j.ajkd.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 47.Textor SC, Misra S, Oderich GS. Percutaneous revascularization for ischemic nephropathy: the past, present, and future. Kidney Int 83: 28–40, 2013. doi: 10.1038/ki.2012.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Urry DW, Luan CH, Parker TM, Gowda DC, Prasad KU, Reid MC, Safavy A. Temperature of polypeptide inverse temperature transition depends on mean residue hydrophobicity. J Am Chem Soc 113: 4346–4348, 1991. doi: 10.1021/ja00011a057. [DOI] [Google Scholar]
- 49.Verheyen A, Peeraer E, Lambrechts D, Poesen K, Carmeliet P, Shibuya M, Pintelon I, Timmermans JP, Nuydens R, Meert T. Therapeutic potential of VEGF and VEGF-derived peptide in peripheral neuropathies. Neuroscience 244: 77–89, 2013. doi: 10.1016/j.neuroscience.2013.03.050. [DOI] [PubMed] [Google Scholar]
- 50.Wafai R, Tudor EM, Angus JA, Wright CE. Vascular effects of FGF-2 and VEGF-B in rabbits with bilateral hind limb ischemia. J Vasc Res 46: 45–54, 2009. doi: 10.1159/000139132. [DOI] [PubMed] [Google Scholar]
- 51.Warner L, Gomez SI, Bolterman R, Haas JA, Bentley MD, Lerman LO, Romero JC. Regional decreases in renal oxygenation during graded acute renal arterial stenosis: a case for renal ischemia. Am J Physiol Regul Integr Comp Physiol 296: R67–R71, 2009. doi: 10.1152/ajpregu.90677.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu XY, Chade AR, Rodriguez-Porcel M, Bentley MD, Ritman EL, Lerman A, Lerman LO. Cortical microvascular remodeling in the stenotic kidney: role of increased oxidative stress. Arterioscler Thromb Vasc Biol 24: 1854–1859, 2004. doi: 10.1161/01.ATV.0000142443.52606.81. [DOI] [PubMed] [Google Scholar]
- 53.Zisa D, Shabbir A, Mastri M, Suzuki G, Lee T. Intramuscular VEGF repairs the failing heart: role of host-derived growth factors and mobilization of progenitor cells. Am J Physiol Regul Integr Comp Physiol 297: R1503–R1515, 2009. doi: 10.1152/ajpregu.00227.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]