Abstract
The secretion of the protease renin from renal juxtaglomerular cells is enhanced by subnormal extracellular calcium concentrations. The mechanisms underlying this atypical effect of calcium have not yet been unraveled. We therefore aimed to characterize the effect of extracellular calcium concentration on calcium handling of juxtaglomerular cells and on renin secretion in more detail. For this purpose, we used a combination of experiments with isolated perfused mouse kidneys and direct calcium measurements in renin-secreting cells in situ. We found that lowering of the extracellular calcium concentration led to a sustained elevation of renin secretion. Electron-microscopical analysis of renin-secreting cells exposed to subnormal extracellular calcium concentrations revealed big omega-shaped structures resulting from the intracellular fusion and subsequent emptying of renin storage vesicles. The calcium concentration dependencies as well as the kinetics of changes were rather similar for renin secretion and for renovascular resistance. Since vascular resistance is fundamentally influenced by myosin light chain kinase (MLCK), myosin light chain phosphatase (MLCP), and Rho-associated protein kinase (Rho-K) activities, we examined the effects of MLCK-, MLCP-, and Rho-K inhibitors on renin secretion. Only MLCK inhibition stimulated renin secretion. Conversely, inhibition of MCLP activity lowered perfusate flow and strongly inhibited renin secretion, which could not be reversed by lowering of the extracellular calcium concentration. Renin-secreting cells and smooth muscle cells of afferent arterioles showed immunoreactivity of MLCK. These findings suggest that the inhibitory effect of calcium on renin secretion could be explained by phosphorylation-dependent processes under control of the MLCK.
Keywords: calcium, MLCK, renin
INTRODUCTION
The protease renin is produced, stored, and secreted from renal juxtaglomerular cells. These are pericyte/smooth muscle-like cells located at the vascular poles of glomeruli of the kidney. It has already been observed almost four decades ago that perfusion of kidneys with solutions containing low calcium concentrations evokes an increase of renin secretion. This finding of a stimulation of renin secretion by subnormal calcium concentrations could be reproduced not only in isolated kidneys but also in kidney slices, isolated glomeruli, and in isolated juxtaglomerular cells with some variation in the strength of the effect (9, 16, 43, 48, 54). From these findings, it was inferred that, opposite to other secretory cells, calcium plays a negative rather than positive role for renin secretion in juxtaglomerular cells. It was this unusual behavior which coined the expression “calcium paradox of renin secretion.” The intracellular calcium concentration is regulated by calcium influx into and calcium extrusion from the cell. In this context the involvement of voltage-gated calcium channels (VOCCs), which were shown to exist in renin-producing cells (13, 14), has been controversially discussed in the literature. Evidence for a role of VOCCs for renin secretion has been obtained in several studies in which pharmacological inhibitors of L-type calcium channels stimulated renin release, whereas activators of this channels attenuated renin secretion (13, 36, 38, 57). However, other studies challenge the importance of L-type channels in this context (7, 31, 59). The reasons for the inconsistency of these studies are currently unclear. Aside from the still unclear role of calcium channels, several hypotheses have been developed which concentrate on the calcium paradox of renin secretion itself. The concept that the calcium-sensing receptor could provide the essential link between extracellular calcium and renin secretion still awaits proof (1, 46). Other concepts suggested an interference of calcium with the cyclic AMP (cAMP) signaling pathway, which so far is the best-established and physiologically most relevant stimulatory trigger for renin secretion. Such a link between calcium and cAMP signaling could result from the existence of calcium inhibitable adenylate cyclase (AC) isoforms and calcium activatable cAMP-phosphodiesterase (PDE) isoforms in renin-secreting cells (16, 43–45). It is therefore conceivable that calcium could lower cAMP levels and thus attenuate renin secretion. A direct intracellular inhibitory effect of calcium is supported by the observations that calmodulin antagonists stimulate renin secretion similarly as does a lowering of the extracellular calcium concentration (6, 10, 19, 24, 49). However, calmodulin seems to be unlikely to control intracellular cAMP levels, because both the inhibition of AC and the activation of PDE by calcium are considered as calmodulin independent (37, 51). Thus an additional calmodulin-dependent pathway is likely to exist. Calcineurin, a calcium-calmodulin-dependent phosphatase, appears to influence renin secretion in a cAMP-independent way (34). In line, several studies showed that the calcineurin inhibitor cyclosporine A significantly enhances renin secretion in juxtaglomerular cells independent of cellular cAMP levels and PKA activity (28, 34). Another well-known target of the calcium-calmodulin complex is the myosin light chain kinase (MLCK). In this context, it has already been suggested that MLCK might be involved in the regulation of renin secretion, because inhibitors of MLCK were found to stimulate renin secretion from isolated kidney slices (25, 48). Despite these hypotheses a generally accepted concept for this unusual and striking effect of calcium on a secretion process has not yet been developed.
A major obstacle for a definite understanding of the calcium paradox of renin secretion is the lack of a clear and controllable experimental system that allows a biochemical analysis of the secretory process in renin-secreting cells. So far isolated cell preparations, isolated glomeruli, kidney slices, and isolated perfused kidneys have been used to study the control of renin secretion (16, 43, 48, 54). All of these models contain only minor portions of renin-secreting cells. Moreover, it appears that upon isolation of cells and disruption of cell-to-cell coupling, important features of calcium-regulated renin secretion get lost. In this context several studies showed that intercellular communication via Cx40-dependent gap junctions plays an important role in relaying the calcium-dependent inhibitor effects of angiotensin II and of intrarenal pressure on renin secretion (33, 66, 67).
As a more systematic approach to understand the calcium paradox, we have now combined several methods such as the pressure-controlled isolated perfused mouse kidney to study renin secretion in situ, the isolation of glomeruli with labeled renin-producing cells to measure intracellular calcium in juxtaglomerular cells in situ and electron microscopy to study the ultrastructure of juxtaglomerular cells. With these methods we aimed to determine the kinetics and the changes of renin secretion and of intracellular calcium concentration upon changes of the extracellular calcium concentration. We further considered potential downstream signaling pathways along which calcium could affect renin secretion.
MATERIALS AND METHODS
Ethical approval.
All experiments were performed according to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH) and were approved by local authorities.
Animals.
All experiments were performed on isolated kidneys of mice in which homologous recombination was used to introduce a green fluorescent protein (GFP) cassette into exon one of the renin gene contained within a 240-kb bacterial artificial chromosome (BAC) to create a construct that has GFP expression controlled by the renin regulatory region (RenGFP BAC). Thus all cells actively expressing the renin gene could be identified by GFP fluorescence (15). A total number of 50 animals was used in our experiments. Animals were maintained on standard rodent chow (0.6% NaCl; Ssniff, Soest, Germany) with free access to tap water. Animals used in this study were 4–6 wk old. All animal experiments were performed according to the Guide for the Care and Use of Laboratory Animals, published by the NIH, and approved by the local ethics committee.
Isolated perfused mouse kidney and measurement of renin secretion.
The isolated perfused mouse kidney model has been described in detail elsewhere (66). Briefly, the animals were anesthetized with an intraperitoneal injection of ketamine (50 mg/kg body wt, Curamed, Karlsruhe, Germany) and xylazine (80 mg/kg body wt, Ratiopharm, Ulm, Germany); the abdominal aorta was cannulated, and the right kidney was excised, placed in a thermostatted moistening chamber, and perfused at constant pressure (90 mmHg). Using an electronic feedback control, perfusion pressure could be changed and held constant in a pressure range between 40 and 140 mmHg. Finally, the renal vein was cannulated and the venous effluent was collected for the determination of renin activity and venous blood flow. The basic perfusion medium consisted of a modified Krebs-Henseleit solution supplemented with 6 g/100 ml bovine serum albumin and with freshly washed human red blood cells (10% hematocrit). For the determination of renin secretion rates, samples of the venous effluent were taken in intervals of 2 min during each experimental period. Renin concentration in plasma samples was measured on the basis of the generation of angiotensin I after the addition of plasma from bilaterally nephrectomized male rats as excess renin substrate. The generated angiotensin I (ng angiotensin I·h−1·ml−1) was determined by an angiotensin I (plasma renin activity) ELISA kit (IBL International, Hamburg, Germany). Renin secretion was modulated by adding defined concentrations of isoproterenol, EGTA, 8-bromo-cAMP, ML-7, Y-27632, or calyculin A (all from Sigma, Deisenhofen, Germany) to the perfusate.
Glomeruli isolation and calcium measurement.
Calcium measurement was performed on isolated single glomeruli. For this purpose, 4- to 6-wk-old RenGFP BAC mice were euthanized by cervical dislocation. The kidneys were excised and the kidney cortex was isolated and minced, followed by a 20-min digestion with a modified Ringer solution containing collagenase Type 2 (Worthington, Lakewood, NJ), trypsin inhibitor Type II-S, and glycine (all from Sigma, Deisenhofen Germany). After digestion, single glomeruli with attached renin-producing cells were identified by GFP-fluorescence using a Stereo Discovery V8 microscope, a HXP 120 V lamp and the filter set 38 HE (all from Carl Zeiss GmbH, Oberkochen, Germany) and collected for calcium measurements. Prior to measurements, single glomeruli were placed in a perfusion bath, secured with a manipulator, and loaded with fura 2-AM (5 µM, 30 min at 37°C). Washout was performed by constant perfusion (3 ml/min) with modified Ringer solution. Different concentrations of EGTA were directly added to the perfusion solution. The concentration of EGTA unbound calcium was calculated using the initial calcium concentration of the used Ringer perfusion medium (2.5 mM) and the graded concentrations of EGTA added to the perfusion medium (0.25, 0.5, 0.75, 1.0, 3.0 mM, respectively) with the help of the Ca-EGTA Calculator v1.3 software (web version available at maxchelator.stanford.edu). Calcium measurements were performed by placing regions of interest (ROI) directly on the renin-producing cells, which could be clearly identified by GFP fluorescence. Fura 2 emission intensity at 510 nm was detected in response to excitation at 340 nm (excitation maximum of Ca2+-bound state) and 380 nm (excitation maximum of Ca2+-unbound state), providing the ratio (340/380). Fura 2 emission was detected using a VisiFluor Calcium Imaging System and analyzed using the Visiview Software (all from Visitron Systems, Puchheim, Germany).
Transmission electron microscopy.
Kidneys were fixated with constant pressure (90 mmHg) for 3 min by perfusion with phosphate-buffered saline (PBS) buffer containing 2% glutaraldehyde. The kidney was cut in half and stored at 4°C in 2% glutaraldehyde/PBS until embedment for TEM. Then the kidney tissue was cut in 1-mm3-wide blocks and embedded in epoxide resin (epoxy embedment kit, Fluka, Neu-Ulm, Germany) using an automatic microwave (Leica EM AMV, Leica, Wetzlar, Germany). The embedded tissue was cut into 70-nm-thick serial slices using an ultramicrotome (EM UC7, Leica), which were then placed on copper grids coated with pioloform. The serial slices were contrasted using a 4% uranyl acetate solution and a 0.5% lead citrate solution. For the acquisition of the images of a juxtaglomerular cell, a transmission electron microscope (Phillips CM12 TEM, Fei, Eindhoven, Netherlands) with a Laboratory5 cathode and an acceleration voltage of 120 keV was used. The digitalization was carried out with a TEM-1000 slow-scan CCD camera and the program EM-Menu 4.0 (both from TVIPS-Tietz, Gauting, Germany).
Immunohistochemistry.
As described previously (58), kidneys were perfusion fixed with 3% paraformaldehyde, dehydrated, and embedded in paraffin. Immunolabeling was performed on 5-μm sagittal paraffin sections. After blocking with 10% horse serum and 1% BSA in PBS, sections were incubated with chicken anti-mouse renin (generated by Davids Immunotechnologie, Regensburg, Germany), anti-α-smooth muscle actin (anti-α-SMA; Beckman Coulter, Immunotech, Marseille, France), and anti-MLCK (Abcam) antibodies overnight at 4°C, followed by incubation with Cy5-, Cy2-, and TRITC-labeled secondary antibodies (Dianova). Slices were mounted with Dako Cytomation Glycergel mounting medium and viewed with an Axiovert Microscope or analyzed with a confocal microscope (LSM 710; Zeiss, Göttingen, Germany), using sequential scanning (Plan Apochromat × 63/1.4 oil objective, excitation at 488 and 543 nm, and emission at 505 to 530 and 560 to 615 nm).
Statistics.
Values are given as means ± SE. Differences between experimental groups were analyzed by ANOVA and Bonferroni’s adjustment for multiple comparisons. Otherwise, Student’s t-test was used to test significance of difference between two groups. P < 0.05 was considered statistically significant.
RESULTS
Isolated mouse kidneys were perfused at a constant pressure of 90 mmHg. Addition of 3 mM of the calcium chelating agent EGTA to the perfusate which contained 2.5 mM calcium led to an effective reduction of free calcium to the micromolar range. EGTA led to a rapid increase of perfusate flow indicating vasodilatation (Fig. 1B). After removal of EGTA, flow rates rapidly returned to Pre values. The vasodilatation induced by EGTA was paralleled by a marked increase of renin secretion which developed over a few minutes and then reached a plateau. After removal of EGTA from the perfusate, renin secretion returned within 2 min to values measured before the addition of EGTA. The β-adrenergic agonist isoproterenol, which activates the cyclic AMP pathway, caused a moderate vasodilatation and led to an increase of renin secretion to levels similar to that elicited by EGTA alone. Addition of EGTA in the presence of isoproterenol increased perfusate flow rates to similar levels as with EGTA alone, while the response of renin secretion was stronger. Within 1 min after the addition of EGTA, renin secretion increased to a transient peak phase followed by a phase sustained elevation of renin secretion (Fig. 1A). This plateau corresponded to a renin secretion rate that was about threefold to fourfold higher than with isoproterenol alone.
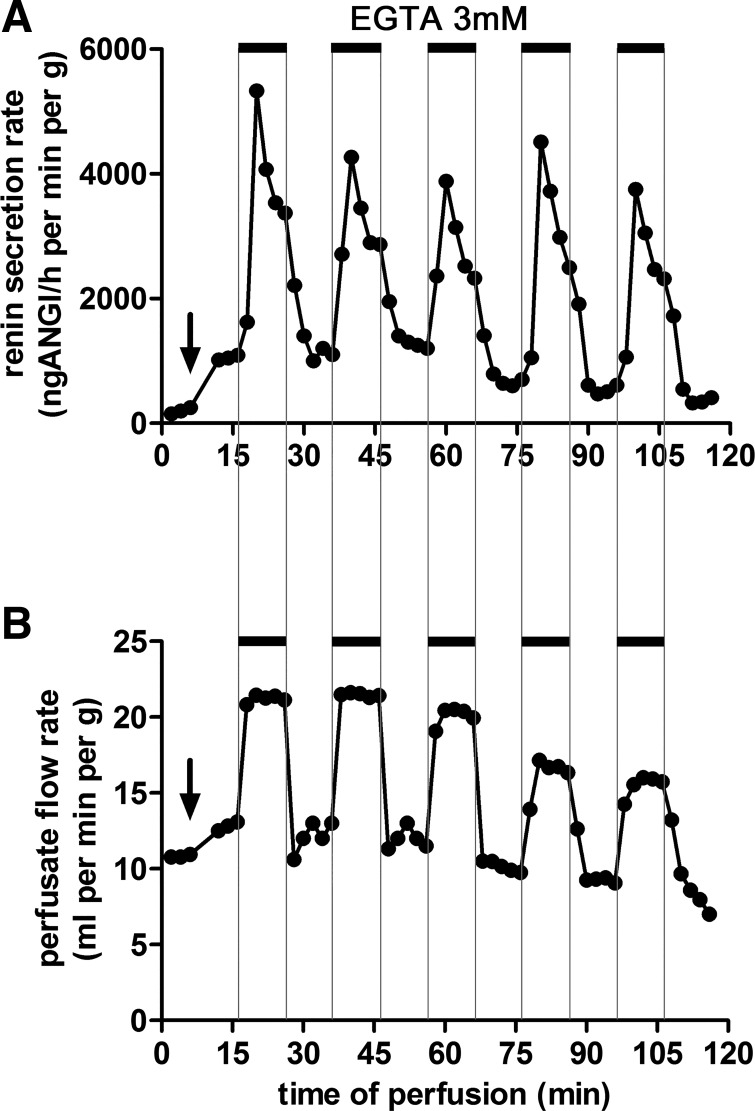
Fig. 1.
Effects of addition of EGTA (3 mM) to the perfusate on renin secretion (A) and flow (B) of isolated mouse kidneys perfused at a constant pressure of 90 mmHg. After a control period, isoproterenol (10 nM) was added to the perfusate (arrows), which led to a moderate increase of flow and to a fivefold increase of renin secretion. Further addition of EGTA to the perfusate in the presence of isoproterenol led to rapid overshooting increase of renin secretion followed by a plateau of renin secretion that was about fourfold higher than with isoproterenol alone. After removal of EGTA from the perfusate, renin secretion rates rapidly returned to previous values. The temporal changes of renin secretion induced by EGTA were rather similar to the changes of perfusate flow. Data are means ± SE of 4 kidneys.
The stimulatory effect of EGTA renin secretion did not show signs of desensitization or exhaustion. When EGTA was repeatedly added to the perfusate for periods lasting for 10 min, respectively, a stereotypic response pattern of renin secretion was observed (Fig. 2A), suggesting no major desensitization of the process by which lowering extracellular calcium enhances the release of renin. Similar stereotypic responses to repeated additions of EGTA to the perfusate were also noted for perfusate flow (Fig. 2B).
Fig. 2.
Effects of repeated addition of EGTA (3 mM) to the perfusate on renin secretion (A) and flow (B) of an isolated mouse kidney perfused at a constant pressure of 90 mmHg. After a control period, isoproterenol (10 nM) was added to the perfusate (arrows), which led to a moderate increase of flow and to a fivefold increase of renin secretion. During isoproterenol stimulation, repeated and subsequent 10-min treatment with EGTA led to similar responses of both renin secretion rate and perfusate flow rate without signs of desensitization. Shown are the data of a representative experiment.
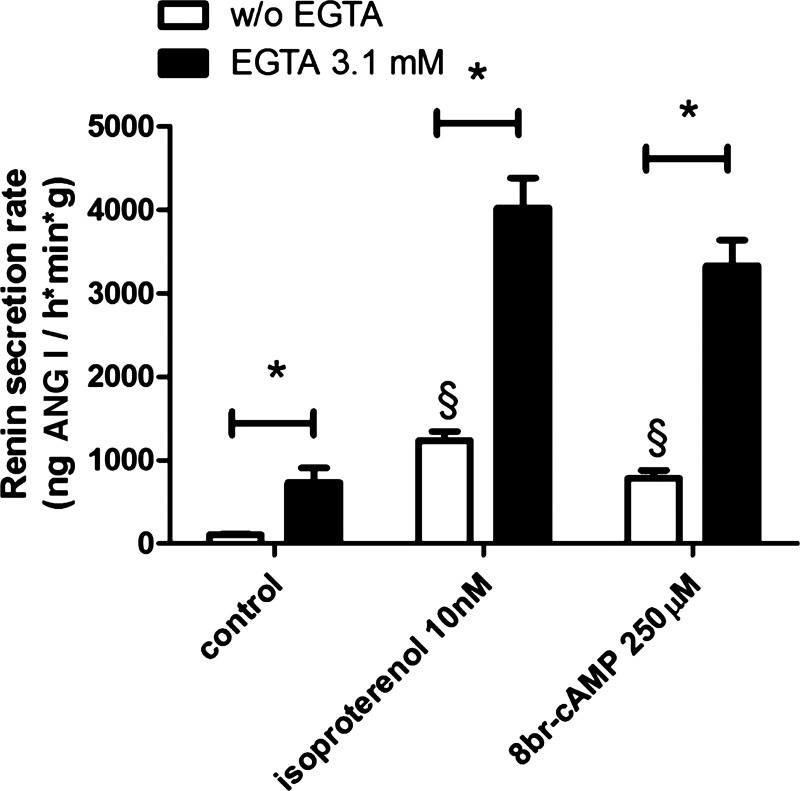
The classic physiological pathway triggering renin secretion is activation of the cAMP-signaling pathway (3, 29, 44, 45). Since both calcium inhibitable adenylate-cyclases and calcium activatable cAMP-phosphodiesterase isoforms have been described to exist in renin producing cells (16, 43–45), we examined if interference with intracellular cAMP levels could contribute to the strong enhancement of renin secretion induced by lowering extracellular calcium in the presence of isoproterenol. For this purpose we compared the effect of EGTA on renin secretion elicited by isoproterenol with that elicited by the membrane permeable and degradation resistant cAMP analog 8-bromo cAMP. For the evaluation of the renin secretion rate, we disregarded the transient peak phase occurring after EGTA addition (shown in Fig. 1) and used values which had been measured after a stable plateau phase was reached. As shown in Fig. 3, 8-bromo-cAMP led to a similar stimulation of renin secretion as did isoproterenol which activates the cAMP signaling via activation of the adenylate cyclase. Notably, EGTA enhanced renin secretion both in the presence of isoproterenol and in the presence of 8-bromo cAMP to a similar extent suggesting that EGTA enhances renin secretion primarily by mechanisms not related to modulation of intracellular cAMP levels.
Fig. 3.
Effects of addition of different stimulators on renin secretion rates of isolated mouse kidneys perfused at a constant pressure of 90 mmHg. Data were obtained after a stable plateau phase had been reached. Isoproterenol at 10 nM, which activates AC isoforms leading to increased cAMP levels, elicited a similar stimulation of renin secretion as 250 µM 8-bromo-cAMP, a degradation-resistant cAMP analog (§). EGTA (3.1 mM) further enhanced renin secretion to a similar extent in the presence of both 10 nM isoproterenol and 250 µM 8-bromo-cAMP, indicating mechanisms of action not related to modulation of intracellular cAMP levels (*P < 0.05; data are means ± SE of 4 kidneys).
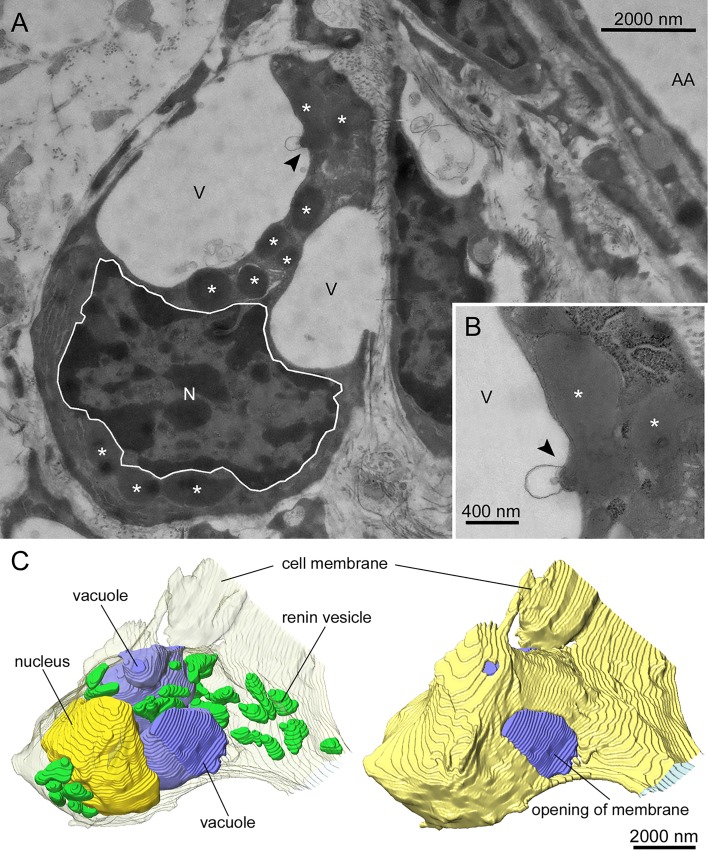
Addition of EGTA to the perfusate caused a striking change of the ultrastructure of renin cells. Transmission electron microscopical (TEM) images of kidneys perfused with isoproterenol (10 nM) and EGTA (3 mM) for 20 min revealed big omega-like structures, which imposed as big holes with only a few openings to the extracellular space (Fig. 4A). Occasionally it could be observed that electron-dense vesicles, which are presumably renin storage vesicles, fused with vacuole-like structures and released the stored material into these vacuoles (Fig. 4B). A 3D reconstruction of those cells confirmed these observations. The stimulated renin-producing cells featured several very large vacuole-like structures, which took a large quantity of intracellular volume and showed at least one opening to the extracellular space per vacuole. Several of the renin storage vesicles were found to be either in very close proximity or already fused to these vacuoles, partially releasing their contents into the vacuolar space (Fig. 4C). Activation of renin secretion by isoproterenol alone did not lead to the appearance of such vacuolar structures.
Fig. 4.
Transmission electron microscopical image (original magnification × 3,800) of a renin-producing cell of a mouse kidney perfused at 90 mmHg after 20 min of stimulation with 10 nM isoproterenol and 3 mM EGTA (A). The observed cell lies in close proximity but not adjacent to an afferent arteriole (AA). In addition, the nucleus (N), renin-containing vesicles (*), and large vacuole-like structures (V) are shown. Several renin-containing vesicles release their vesicular content into the vacuolar space (arrow). A detailed image (original magnification ×13,000) of a release of vesicular content is shown in B. 3-Dimensional reconstructions of stimulated renin-producing cells confirmed the above findings. The cell membrane is represented as a transparent layer, containing the nucleus (yellow), large vacuoles (blue), and renin-containing vesicles (green), some of them in very close proximity to the vacuoles (C, left). Additionally, the cell membrane is presented nontransparent yellow to show that the vacuoles are connected to the extracellular space (C, right).
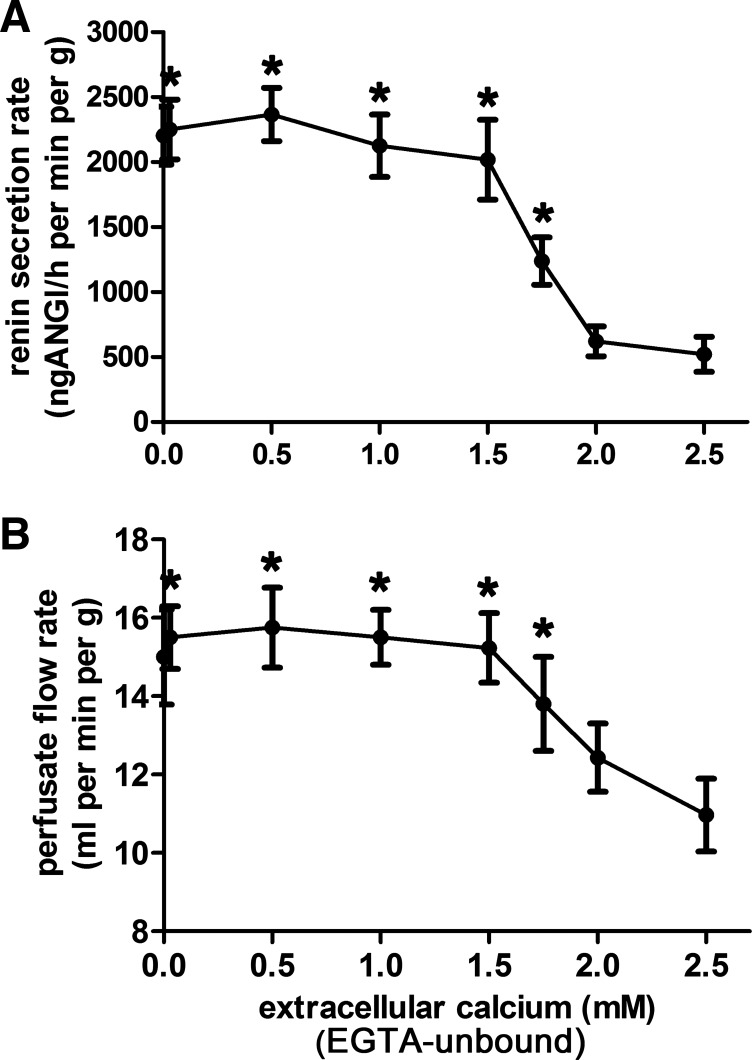
To narrow down possible mechanisms along which lowering of the extracellular calcium concentration could enhance renin secretion, we determined the dependency of renin secretion on the extracellular concentration of calcium. For this purpose, either graded concentrations of EGTA at a constant concentration of total calcium (2.5 mM) were added to the perfusate or graded concentrations of CaCl2 at a constant concentration of EGTA (3 mM) were added to the perfusate of isolated kidneys. The resulting curves were rather similar for both experimental protocols, supporting the inference that the effect of EGTA on renin secretion is indeed calcium dependent. Therefore the curves for the two protocols were combined to one curve (Fig. 5A). For the sake of clarity, the extracellular concentration of EGTA-unbound calcium ions is referred to as “extracellular calcium concentration” in the following text. Renin secretion began to increase when extracellular (EGTA-unbound) calcium was lowered below 2.0 mM and reached its maximum already at an extracellular calcium concentration of 1.5 mM, indicating a steep effector-response curve (Fig. 5A). Further lowering of calcium even into the micromolar range neither further enhanced nor attenuated renin secretion. Notably the concentration dependency of renin secretion on extracellular calcium was rather similar to the dependency of perfusate flow (at constant perfusion pressure of 90 mmHg) on extracellular calcium (Fig. 5B).
Fig. 5.
Effects of lowering the extracellular EGTA-unbound calcium concentration on renin secretion (A) and flow (B) of isolated mouse kidneys perfused at a constant pressure of 90 mmHg. An increase in renin secretion was observable when the concentration of extracellular EGTA-unbound calcium was decreased below 2 mM and reached a plateau at a concentration of 1.5 mM. This concentration dependency of renin secretion is mirrored by the perfusate flow. *Significant difference from control values (2.5 mM EGTA), P < 0.05; data are means ± SE of 4 kidneys.
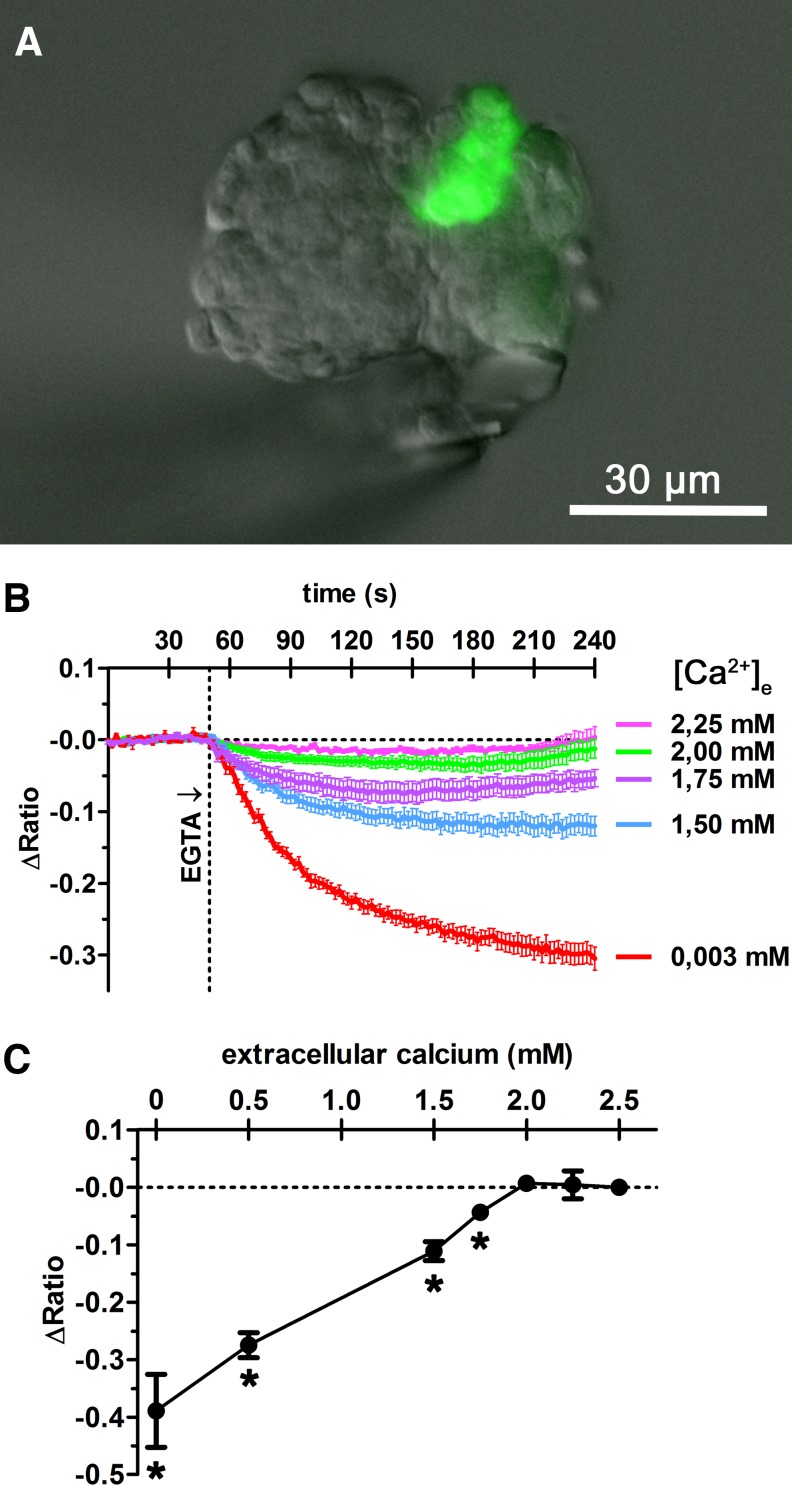
To investigate how changes of the extracellular calcium concentration affect the intracellular concentration of calcium in renin-secreting cells, fura-2 calcium measurements were performed in fluorescence-labeled renin-secreting cells of isolated glomeruli (Fig. 6A). Lowering extracellular calcium from 2.5 mM to 2.25 or 2.0 mM led to transient decreases of cytosolic calcium concentrations which recovered to basal values within 4 min (Fig. 6B). Lowering extracellular calcium below 2.0 mM led to decreased cytosolic steady-state levels, which further decreased with decreasing extracellular concentrations (Fig. 6, B and C).
Fig. 6.
For intracellular calcium measurement, an isolated glomerulus was placed in a perfusion bath and perfused at constant flow of 3 ml/min. To lower the extracellular calcium concentration, different concentrations of EGTA were added to the perfusion medium. The renin-producing cells could be identified by green GFP fluorescence at 63× magnification (A). Fura-2 calcium measurements were performed by placing regions of interest (ROI) on renin-producing cells. Fura-2 emission intensity at 510 nm was detected in response to excitation at 340 nm (excitation maximum of Ca2+-bound state) and 380 nm (excitation maximum of Ca2+-unbound state), providing the ratio (340/380). ΔRatio describes the ratio deviation from average baseline ratio measured in the unstimulated cells. Lowering of the extracellular calcium concentration [Ca2+]e from 2.5 to 2.25 or 2 mM led to transient decreases of intracellular calcium concentration, which recovered to basal values within 4 min (B). Further decreasing extracellular calcium concentration led to a concentration-dependent decrease of cytosolic calcium steady-state levels, measured after 15 min (B, C). *Significant difference from control values (2.5 mM EGTA), P < 0.05; data are means ± SE of 7 renin-producing cells.
The concentration dependency to acute changes of the extracellular calcium concentration of vascular resistance on the one hand and of renin secretion on the other led us to consider if both processes could have a common mechanistic denominator. Vascular tone as determined by the contraction state of smooth muscle cells is mainly determined by the balance of myofilament phosphorylation and dephosphorylation. Key enzymes for these processes are the myosin light chain kinase (MLCK) and the myosin light chain phosphatase (MLCP). The MLCP can in part be inhibited via the Rho-associated protein kinase (Rho-K) pathway. Inhibition of MLCK by ML-7 increased perfusate flow rates and renin secretion rates to levels comparable to lowering of extracellular calcium by EGTA alone (control) or EGTA in the presence of ML-7 (Fig. 7, A and B). Inhibition of Rho-K by Y-27316 also markedly increased perfusate flow but did not change renin secretion. Lowering extracellular calcium by EGTA in the presence of Y-27316 had no further effect on perfusate flow but enhanced renin secretion to similar levels as seen in the absence of Y-27316 (Fig. 7). The phosphatase inhibitor calyculin A caused a strong decrease of perfusate flow and of renin secretion (Fig. 7). In the presence of calyculin A, lowering of the extracellular calcium concentration by EGTA exerted only moderate vasodilation effects as indicated by the increase of perfusate flow while renin secretion remained suppressed. Summing up these findings suggest that myosin light kinase inhibition exerts rather similar effects as does lowering of the extracellular calcium concentration while inhibition of dephosphorylating phosphatases exerts opposite effects, and more notable, abolished the effect of EGTA on renin secretion.
Fig. 7.
Effects of addition of the pharmacological inhibitor of MLCK (ML-7), the inhibitor of MLCP (calyculin A), and a Rho-K-Inhibitor (Y-27632) to the perfusate on renin secretion (A) and flow (B) of isolated mouse kidneys perfused at a constant pressure of 90 mmHg. Data were obtained after a stable plateau phase had been reached. ML-7 inhibits MLCK and leads to an increase in flow rate and renin secretion rate comparable to the effects of EGTA alone (control) and EGTA in presence of ML-7. Inhibition of Rho-K by Y-27632 also caused a strong increase in perfusate flow but no increase in renin secretion. Lowering of the extracellular calcium concentration by EGTA in the presence of Y-27632 led to no further increase in perfusate flow but to an increase in renin secretion to levels seen in the absence of Y-27632 (control). Inhibition of the MLCP by calyculin A caused a strong decrease of both perfusate flow and renin secretion. In the presence of calyculin A, lowering of the extracellular calcium concentration by EGTA exerted only moderate vasodilatation effects as indicated by the increase of perfusate flow while renin secretion remained suppressed. *P < 0.05; data are means ± SE of 4 kidneys.
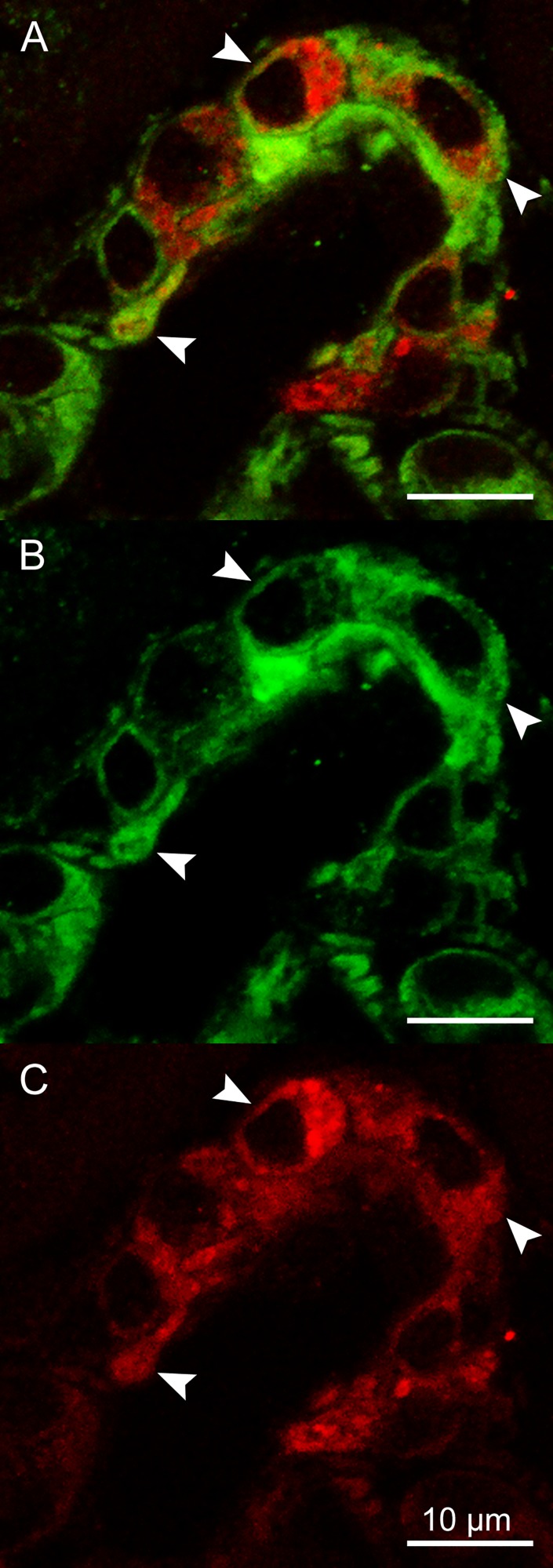
To support the assumption that the MLCK inhibitor influenced renin secretion by a direct effect on renin-secreting cells, we examined MLCK expression in smooth muscle cells and in renin-secreting cells of afferent arterioles. MLCK immunoreactivity was well detectable in ~70% of renin cells at an expression level similar to vascular smooth muscle cells. In these cells, we observed MLCK immunoreactivity located in the cytoplasm, as indicated by arrows (Fig. 8).
Fig. 8.
Immunohistochemical staining of renin (red) and MLCK (green) in an afferent arteriole at 63× magnification. Colocalization of renin and MLCK immunoreactivity was detectable in the cytoplasm of ~70% of observed renin-producing cells and is indicated by arrows (A). Additionally, both renin and MLCK fluorescence are shown in separate images (B, C).
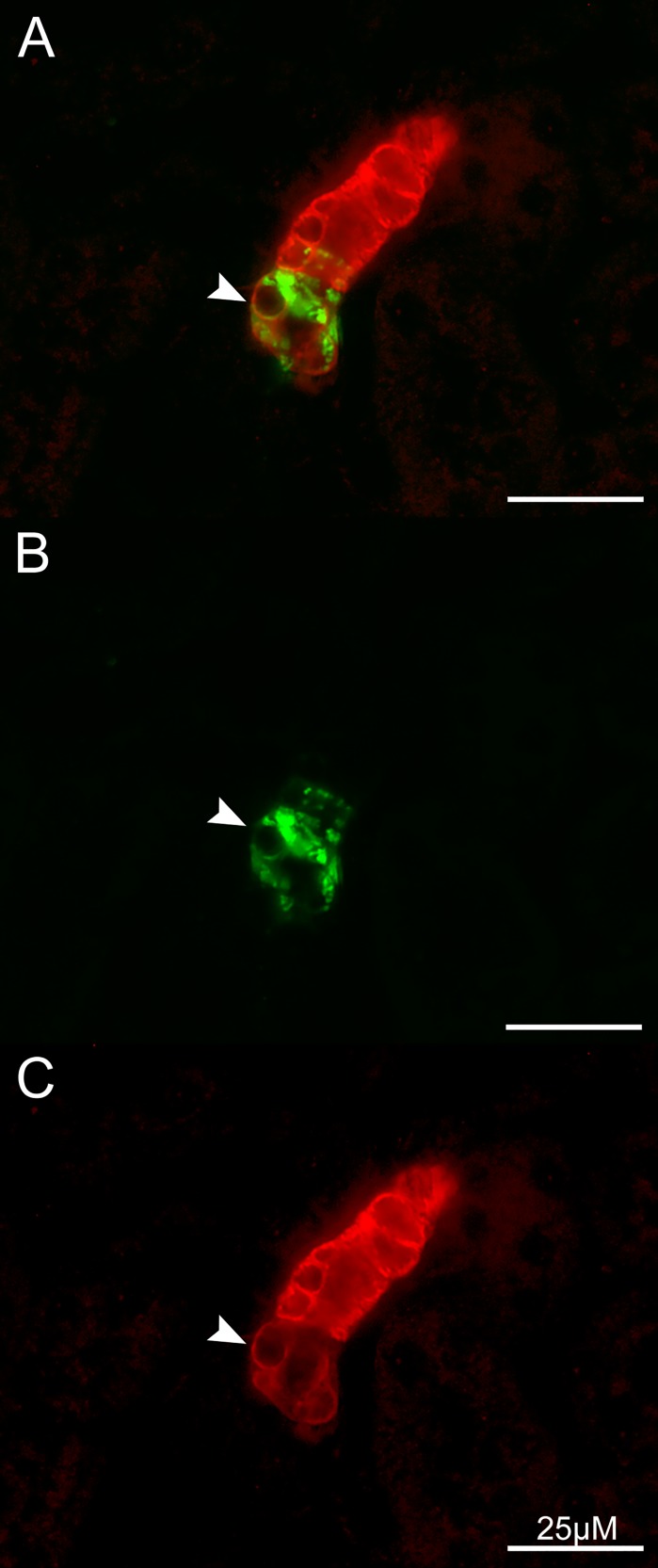
Similarly, immunoreactivity for the smooth muscle myosin heavy chain Myh11 was found in the entire vasculature and in 60% of renin-producing cells, although at a significantly lower intensity and localized at the cell membrane (Fig. 9).
Fig. 9.
Immunohistochemical staining of renin (green) and Myh11 (red) in an afferent arteriole at 40× magnification. Myh 11 immunoreactivity was observed in ~60% of renin-producing cells; an arrow points to localization at the cell membrane (A). Additionally, both renin and Myh11 fluorescence are shown in separate images (B, C).
DISCUSSION
It has been observed almost four decades ago that lowering of the extracellular calcium concentration causes a prominent enhancement of renin secretion from the kidneys. Despite numerous attempts to find an explanation for this very unusual effect of calcium on a secretion process, the real knowledge about this process remains still rather scarce. We have therefore concentrated our investigations on renin secretion from the whole kidney, in which the original data were obtained (11, 52, 64). The whole kidney approach raises the question if changes of the intracellular calcium concentration of other cell types of the juxtaglomerular apparatus, such as macula densa (MD) cells or endothelial cells, could indirectly influence renin secretion and thus explain the effects of extracellular calcium on renin secretion. Both cell types seem to influence renin secretion by modulating cAMP levels in renin-producing cells (14, 29, 53). An increase of intracellular calcium in endothelial cells leads to activation of NO synthase (eNOS). Effects on of NO on renin secretion are mediated by a cGMP-induced inhibition of cAMP degradation, leading to increased cAMP levels and stimulation of renin secretion (29). Conversely, reducing the intracellular calcium concentration in endothelial cells would be expected to lower NO production and in consequence to lower rather than to stimulate renin secretion. In MD cells, increased calcium levels likely stimulate the release of prostaglandins, which would stimulate renin release via the cAMP pathway (14, 53). Therefore, reducing the intracellular calcium concentration in MD cells would be expected to lower rather than to stimulate renin secretion. An essential mediator role of endothelial and of macula densa cells for the effect of extracellular calcium on renin secretion appears therefore unlikely. The concentration of extracellular calcium is tightly regulated and ranges from 2.2 to 2.6 mM, with 1.1 to 1.4 mM existing in an unbound ionized state (1). Our data show that the calcium concentration range, in which extracellular calcium exerts its inhibitory effect on renin secretion, is close to this physiological range. Moreover, it is in the same range in which extracellular calcium influences the tone of renal arterioles, i.e., the contraction state of smooth muscle cells per se, as well as in response to pressure- and stretch-dependent stimuli (22, 32, 62).
Our measurements show that the dependency of the intracellular calcium concentration in renin-secreting cells on extracellular calcium is very similar to the dependency of renin secretion on extracellular calcium. This similarity may be taken as a further indirect piece of support that the cytosolic concentration of calcium within renin-secreting cells strongly depends on the extracellular calcium concentration and somehow impedes renin secretion. Other findings already showed that reducing the intracellular calcium concentration in isolated juxtaglomerular cells with the membrane-permeable calcium chelating agent BAPTA-AM leads to a strong increase in renin secretion (44, 45). Additionally, numerous in vitro studies where intracellular calcium levels were altered in juxtaglomerular cells using calcium channel agonists, calcium channel blockers, and hormones known to increase intracellular calcium such as angiotensin II, vasopressin, or endothelin, also support this theory (4, 12, 30, 56).
Our findings suggest that myosin light chain kinase (MLCK), the activity state of which is determined by calcium-calmodulin (27, 39, 40), could be a likely mediator of the inhibitory effect of cytosolic calcium on renin secretion. This conclusion would be in accordance with results obtained with primary cultures of renin cells, kidney slices, and kidney perfusion experiments suggesting that calmodulin antagonists cause a disinhibition of renin secretion (6, 10, 24, 49) and would confirm a previous hypothesis derived from kidney slice experiments that MLCK activity exerts a negative effect on renin secretion (25, 48, 50). In support of this, we also found that MLCK is expressed in renin-secreting cells similar to vascular smooth muscle cells of afferent arterioles. Furthermore, other characteristic components of the smooth muscle contractile apparatus, such as myosin heavy chain 11 (Myh11), are also expressed in the majority of renin-producing cells. Myh11 immunoreactivity was limited to the cell membrane and did not colocalize with renin vesicles. In addition, also inhibition of Rho-associated protein kinase (Rho-K) showed quite different effects on renovascular resistance and on renin secretion. Rho-K is considered to inhibit myosin phosphatases and thus to enhance the vasoconstrictor effect of myosin light chain phosphorylation induced by MLCK, which has been described as the so-called calcium-sensitizing effect (63). That this myosin phosphorylation could indeed play a role for renin secretion is suggested by the effects of the inhibitor of PP1 and of PP2A phosphatases which not only induced well-known vasoconstriction (2, 23, 26) but also strong inhibition of renin secretion that could not be reversed by lowering of the extracellular calcium concentration. It should be mentioned in this context that inhibition of renin secretion by the phosphatase inhibitor calyculin A has previously been reported for incubated kidney slices (25, 50).
This control of renin vesicle exocytosis by calcium/MLCK appears as a rapid, reversible, and reproducible process similar to the contraction of vascular smooth muscle cells. TEM analysis revealed that lowering extracellular calcium led to big vacuole-like structures which likely resulted from the intracellular fusion of renin storage vesicles very similar to the process of compound exocytosis (20, 21, 55, 60). Those big vacuoles usually show one or only a few openings to the extracellular space. One could imagine that calcium/MLCK blocks the intracellular fusion of storage vesicles by a phosphorylation process that somehow leads to physical inhibition of the contact between vesicles. These phosphorylation processes could also influence both the fusion probability of big vacuole-like structures with the cell membrane and fusion pore opening, as indicated by the Myh 11 immunoreactivity located at the cell membrane of the majority of renin-producing cells. There are already numerous studies which point to the actin-myosin network and the MLCK playing a significant role in the regulation of vesicle exocytosis. There, they act either as a positive or negative regulator, depending on the examined secretory system (8, 17, 35, 41, 65). In fact, it has already been hypothesized that myofilament-related processes could sterically inhibit renin secretion (18, 42). Such an indirect negative effect of calcium on vesicle fusion would also be in accordance with the basic concepts of vesicle fusion, for which a direct inhibitory effect of calcium is not foreseen (5, 47). However, so far, electron microscopical studies of renin-producing cells have not provided direct evidence for an association of renin vesicles with actin or myosin filaments (61). In addition, drugs known to disrupt actin filaments were reported to exert no effect on renin secretion from isolated perfused kidneys (60). Since it is well established that activation of the cAMP-signaling pathway is a trigger for renin secretion and since cAMP-triggered vesicle fusion is well known, we would hypothesize that the cAMP signaling pathway provides a direct fusogenic trigger for renin storage vesicles among each other and with the plasma membrane. The efficacy of this cAMP-dependent fusogenic trigger would then be controlled by calcium which would finally determine if vesicles come close enough to each other and to the cell membrane to allow the fusogenic trigger to become active. Such a concept would be in accordance with our observations that lowering calcium potentiates the stimulatory effect of cAMP, without directly altering the cAMP metabolism (51, 60).
GRANTS
This work was supported by Roswell Park Cancer Institute and National Cancer Institute (NCI) Grant P30CA016056 and German Research Council Grant SFB 699/B6.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.S. and A.K. conceived and designed research; D.S. and L.P. performed experiments; D.S. and A.K. analyzed data; D.S., L.P., K.W.G., and A.K. interpreted results of experiments; D.S. prepared figures; D.S. and A.K. drafted manuscript; D.S., K.W.G., and A.K. edited and revised manuscript; D.S., L.P., K.W.G., and A.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge the expert technical assistance of R. Steppan, R. Götz, and A. Zügner.
REFERENCES
- 1.Atchison DK, Beierwaltes WH. The influence of extracellular and intracellular calcium on the secretion of renin. Pflügers Arch 465: 59–69, 2013. doi: 10.1007/s00424-012-1107-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baek I, Jeon SB, Kim J, Seok YM, Song MJ, Chae SC, Jun JE, Park WH, Kim IK. A role for Rho-kinase in Ca-independent contractions induced by phorbol-12,13-dibutyrate. Clin Exp Pharmacol Physiol 36: 256–261, 2009. doi: 10.1111/j.1440-1681.2008.05045.x. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Kim SM, Oppermann M, Faulhaber-Walter R, Huang Y, Mizel D, Chen M, Lopez ML, Weinstein LS, Gomez RA, Briggs JP, Schnermann J. Regulation of renin in mice with Cre recombinase-mediated deletion of G protein Gsalpha in juxtaglomerular cells. Am J Physiol Renal Physiol 292: F27–F37, 2007. doi: 10.1152/ajprenal.00193.2006. [DOI] [PubMed] [Google Scholar]
- 4.Churchill PC, Churchill MC. BAY K 8644, a calcium channel agonist, inhibits renin secretion in vitro. Arch Int Pharmacodyn Ther 285: 87–97, 1987. [PubMed] [Google Scholar]
- 5.Cohen Y, Rahamimov R, Naveh-Many T, Silver J, Rahamimoff R. Where is the “inverting factor” in hormone secretion from parathyroid cells? Am J Physiol Endocrinol Metab 273: E631–E637, 1997. doi: 10.1152/ajpendo.1997.273.3.E630. [DOI] [PubMed] [Google Scholar]
- 6.Della Bruna R, Pinet F, Corvol P, Kurtz A. Calmodulin antagonists stimulate renin secretion and inhibit renin synthesis in vitro. Am J Physiol Renal Fluid Electrolyte Physiol 262: F397–F402, 1992. doi: 10.1152/ajprenal.1992.262.3.F397. [DOI] [PubMed] [Google Scholar]
- 7.Dietz JR. Effects of a calcium channel agonist on renin release from perfused rat kidneys. Ren Physiol 9: 279–286, 1986. [DOI] [PubMed] [Google Scholar]
- 8.Eitzen G. Actin remodeling to facilitate membrane fusion. Biochim Biophys Acta 1641: 175–181, 2003. doi: 10.1016/S0167-4889(03)00087-9. [DOI] [PubMed] [Google Scholar]
- 9.Fray JC. Mechanism by which renin secretion from perfused rat kidneys is stimulated by isoprenaline and inhibited by high perfusion pressure. J Physiol 308: 1–13, 1980. doi: 10.1113/jphysiol.1980.sp013457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fray JC, Lush DJ, Share DS, Valentine AN. Possible role of calmodulin in renin secretion from isolated rat kidneys and renal cells: studies with trifluoperazine. J Physiol 343: 447–454, 1983. doi: 10.1113/jphysiol.1983.sp014903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fray JS. Stimulation of renin release in perfused kidney by low calcium and high magnesium. Am J Physiol Renal Fluid Electrolyte Physiol 232: F377–F382, 1977. doi: 10.1152/ajprenal.1977.232.4.F377. [DOI] [PubMed] [Google Scholar]
- 12.Friis UG, Jørgensen F, Andreasen D, Jensen BL, Skøtt O. Membrane potential and cation channels in rat juxtaglomerular cells. Acta Physiol Scand 181: 391–396, 2004. doi: 10.1111/j.1365-201X.2004.01310.x. [DOI] [PubMed] [Google Scholar]
- 13.Friis UG, Jørgensen F, Andreasen D, Jensen BL, Skøtt O. Molecular and functional identification of cyclic AMP-sensitive BKCa potassium channels (ZERO variant) and L-type voltage-dependent calcium channels in single rat juxtaglomerular cells. Circ Res 93: 213–220, 2003. doi: 10.1161/01.RES.0000085041.70276.3D. [DOI] [PubMed] [Google Scholar]
- 14.Friis UG, Stubbe J, Uhrenholt TR, Svenningsen P, Nüsing RM, Skøtt O, Jensen BL. Prostaglandin E2 EP2 and EP4 receptor activation mediates cAMP-dependent hyperpolarization and exocytosis of renin in juxtaglomerular cells. Am J Physiol Renal Physiol 289: F989–F997, 2005. doi: 10.1152/ajprenal.00201.2005. [DOI] [PubMed] [Google Scholar]
- 15.Glenn ST, Jones CA, Pan L, Gross KW. In vivo analysis of key elements within the renin regulatory region. Physiol Genomics 35: 243–253, 2008. doi: 10.1152/physiolgenomics.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grünberger C, Obermayer B, Klar J, Kurtz A, Schweda F. The calcium paradoxon of renin release: calcium suppresses renin exocytosis by inhibition of calcium-dependent adenylate cyclases AC5 and AC6. Circ Res 99: 1197–1206, 2006. doi: 10.1161/01.RES.0000251057.35537.d3. [DOI] [PubMed] [Google Scholar]
- 17.Gutiérrez LM. New insights into the role of the cortical cytoskeleton in exocytosis from neuroendocrine cells. Int Rev Cell Mol Biol 295: 109–137, 2012. doi: 10.1016/B978-0-12-394306-4.00009-5. [DOI] [PubMed] [Google Scholar]
- 18.Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, physiology, and molecular biology of renin secretion. Physiol Rev 70: 1067–1116, 1990. doi: 10.1152/physrev.1990.70.4.1067. [DOI] [PubMed] [Google Scholar]
- 19.Hackenthal E, Schwertschlag U, Taugner R. Cellular mechanisms of renin release. Clin Exp Hypertens A 5: 975–993, 1983. [DOI] [PubMed] [Google Scholar]
- 20.Hafez I, Stolpe A, Lindau M. Compound exocytosis and cumulative fusion in eosinophils. J Biol Chem 278: 44921–44928, 2003. doi: 10.1074/jbc.M306013200. [DOI] [PubMed] [Google Scholar]
- 21.Hartmann J, Scepek S, Hafez I, Lindau M. Differential regulation of exocytotic fusion and granule-granule fusion in eosinophils by Ca2+ and GTP analogs. J Biol Chem 278: 44929–44934, 2003. doi: 10.1074/jbc.M306014200. [DOI] [PubMed] [Google Scholar]
- 22.Hwa JJ, Bevan JA. A nimodipine-resistant Ca2+ pathway is involved in myogenic tone in a resistance artery. Am J Physiol Heart Circ Physiol 251: H182–H189, 1986. doi: 10.1152/ajpheart.1986.251.1.H182. [DOI] [PubMed] [Google Scholar]
- 23.Itoh H, Shimomura A, Okubo S, Ichikawa K, Ito M, Konishi T, Nakano T. Inhibition of myosin light chain phosphatase during Ca2+-independent vasocontraction. Am J Physiol Cell Physiol 265: C1319–C1324, 1993. doi: 10.1152/ajpcell.1993.265.5.C1319. [DOI] [PubMed] [Google Scholar]
- 24.Kawamura M, Inagami T. Calmodulin antagonists stimulate renin release from isolated rat glomeruli. Endocrinology 112: 1857–1859, 1983. doi: 10.1210/endo-112-5-1857. [DOI] [PubMed] [Google Scholar]
- 25.Kim MH, Kim SH, Kim HS, Chang JW, Hong YS, Kim HW, Park CS. Regulation of renin secretion through reversible phosphorylation of myosin by myosin light chain kinase and protein phosphatase type 1. J Pharmacol Exp Ther 285: 968–974, 1998. [PubMed] [Google Scholar]
- 26.Knapp J, Aleth S, Balzer F, Schmitz W, Neumann J. Calcium-independent activation of the contractile apparatus in smooth muscle of mouse aorta by protein phosphatase inhibition. Naunyn Schmiedebergs Arch Pharmacol 366: 562–569, 2002. doi: 10.1007/s00210-002-0635-x. [DOI] [PubMed] [Google Scholar]
- 27.Krueger JK, Gallagher SC, Zhi G, Geguchadze R, Persechini A, Stull JT, Trewhella J. Activation of myosin light chain kinase requires translocation of bound calmodulin. J Biol Chem 276: 4535–4538, 2001. doi: 10.1074/jbc.C000857200. [DOI] [PubMed] [Google Scholar]
- 28.Kurtz A, Della Bruna R, Kühn K. Cyclosporine A enhances renin secretion and production in isolated juxtaglomerular cells. Kidney Int 33: 947–953, 1988. doi: 10.1038/ki.1988.92. [DOI] [PubMed] [Google Scholar]
- 29.Kurtz A, Götz KH, Hamann M, Wagner C. Stimulation of renin secretion by nitric oxide is mediated by phosphodiesterase 3. Proc Natl Acad Sci USA 95: 4743–4747, 1998. doi: 10.1073/pnas.95.8.4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurtz A, Pfeilschifter J, Hutter A, Bührle C, Nobiling R, Taugner R, Hackenthal E, Bauer C. Role of protein kinase C in inhibition of renin release caused by vasoconstrictors. Am J Physiol Cell Physiol 250: C563–C571, 1986. doi: 10.1152/ajpcell.1986.250.4.C563. [DOI] [PubMed] [Google Scholar]
- 31.Kurtz A, Skott O, Chegini S, Penner R. Lack of direct evidence for a functional role of voltage-operated calcium channels in juxtaglomerular cells. Pflugers Arch 416: 281–287, 1990. doi: 10.1007/BF00392064. [DOI] [PubMed] [Google Scholar]
- 32.Laher I, Hwa J, Bevan JA. Calcium and vascular myogenic tone. Ann N Y Acad Sci 522: 216–225, 1988. doi: 10.1111/j.1749-6632.1988.tb33359.x. [DOI] [PubMed] [Google Scholar]
- 33.Machura K, Neubauer B, Müller H, Tauber P, Kurtz A, Kurtz L. Connexin 40 is dispensable for vascular renin cell recruitment but is indispensable for vascular baroreceptor control of renin secretion. Pflugers Arch 467: 1825–1834, 2015. doi: 10.1007/s00424-014-1615-y. [DOI] [PubMed] [Google Scholar]
- 34.Madsen K, Friis UG, Gooch JL, Hansen PB, Holmgaard L, Skøtt O, Jensen BL. Inhibition of calcineurin phosphatase promotes exocytosis of renin from juxtaglomerular cells. Kidney Int 77: 110–117, 2010. doi: 10.1038/ki.2009.418. [DOI] [PubMed] [Google Scholar]
- 35.Malacombe M, Bader MF, Gasman S. Exocytosis in neuroendocrine cells: new tasks for actin. Biochim Biophys Acta 1763: 1175–1183, 2006. doi: 10.1016/j.bbamcr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Marre M, Misumi J, Raemsch KD, Corvol P, Menard J. Diuretic and natriuretic effects of nifedipine on isolated perfused rat kidneys. J Pharmacol Exp Ther 223: 263–270, 1982. [PubMed] [Google Scholar]
- 37.Matsumura Y, Koyama Y, Shinyama H, Uriu T, Ichihara T, Morimoto S. Calmodulin-independent stimulation of renin release by exposure of rat kidney cortical slices to calcium. Clin Exp Pharmacol Physiol 15: 585–590, 1988. doi: 10.1111/j.1440-1681.1988.tb01117.x. [DOI] [PubMed] [Google Scholar]
- 38.Matsumura Y, Sasaki Y, Shinyama H, Morimoto S. The calcium channel agonist, Bay K 8644, inhibits renin release from rat kidney cortical slices. Eur J Pharmacol 117: 369–372, 1985. doi: 10.1016/0014-2999(85)90011-1. [DOI] [PubMed] [Google Scholar]
- 39.Means AR, Bagchi IC, VanBerkum MF, Kemp BE. Regulation of smooth muscle myosin light chain kinase by calmodulin. Adv Exp Med Biol 304: 11–24, 1991. doi: 10.1007/978-1-4684-6003-2_3. [DOI] [PubMed] [Google Scholar]
- 40.Means AR, George SE. Calmodulin regulation of smooth-muscle myosin light-chain kinase. J Cardiovasc Pharmacol 12, Suppl 5: S25–S29, 1988. doi: 10.1097/00005344-198800125-00005. [DOI] [PubMed] [Google Scholar]
- 41.Nightingale TD, Cutler DF, Cramer LP. Actin coats and rings promote regulated exocytosis. Trends Cell Biol 22: 329–337, 2012. doi: 10.1016/j.tcb.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 42.Ogawa K, Yamasato M, Taniguchi K. Exocytosis of secretory granules in the juxtaglomerular granular cells of kidneys. Anat Rec 243: 336–346, 1995. doi: 10.1002/ar.1092430308. [DOI] [PubMed] [Google Scholar]
- 43.Ortiz-Capisano MC, Liao TD, Ortiz PA, Beierwaltes WH. Calcium-dependent phosphodiesterase 1C inhibits renin release from isolated juxtaglomerular cells. Am J Physiol Regul Integr Comp Physiol 297: R1469–R1476, 2009. doi: 10.1152/ajpregu.00121.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ortiz-Capisano MC, Ortiz PA, Harding P, Garvin JL, Beierwaltes WH. Adenylyl cyclase isoform v mediates renin release from juxtaglomerular cells. Hypertension 49: 618–624, 2007. doi: 10.1161/01.HYP.0000255172.84842.d2. [DOI] [PubMed] [Google Scholar]
- 45.Ortiz-Capisano MC, Ortiz PA, Harding P, Garvin JL, Beierwaltes WH. Decreased intracellular calcium stimulates renin release via calcium-inhibitable adenylyl cyclase. Hypertension 49: 162–169, 2007. doi: 10.1161/01.HYP.0000250708.04205.d4. [DOI] [PubMed] [Google Scholar]
- 46.Ortiz-Capisano MC, Reddy M, Mendez M, Garvin JL, Beierwaltes WH. Juxtaglomerular cell CaSR stimulation decreases renin release via activation of the PLC/IP3 pathway and the ryanodine receptor. Am J Physiol Renal Physiol 304: F248–F256, 2013. doi: 10.1152/ajprenal.00451.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pang ZP, Südhof TC. Cell biology of Ca2+-triggered exocytosis. Curr Opin Cell Biol 22: 496–505, 2010. doi: 10.1016/j.ceb.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park CS, Chang SH, Lee HS, Kim SH, Chang JW, Hong CD. Inhibition of renin secretion by Ca2+ through activation of myosin light chain kinase. Am J Physiol Cell Physiol 271: C242–C247, 1996. doi: 10.1152/ajpcell.1996.271.1.C242. [DOI] [PubMed] [Google Scholar]
- 49.Park CS, Honeyman TW, Chung ES, Lee JS, Sigmon DH, Fray JC. Involvement of calmodulin in mediating inhibitory action of intracellular Ca2+ on renin secretion. Am J Physiol Renal Fluid Electrolyte Physiol 251: F1055–F1062, 1986. doi: 10.1152/ajprenal.1986.251.6.F1055. [DOI] [PubMed] [Google Scholar]
- 50.Park CS, Kim MH, Leem CH, Jang YJ, Kim HW, Kim HS, Hong YS. Inhibitory effect of calyculin A, a Ser/Thr protein phosphatase type I inhibitor, on renin secretion. Am J Physiol Renal Fluid Electrolyte Physiol 275: F664–F670, 1998. [DOI] [PubMed] [Google Scholar]
- 51.Park CS, Sigmon DH, Han DS, Honeyman TW, Fray JC. Control of renin secretion by Ca2+ and cyclic AMP through two parallel mechanisms. Am J Physiol Regul Integr Comp Physiol 251: R531–R536, 1986. doi: 10.1152/ajpregu.1986.251.3.R531. [DOI] [PubMed] [Google Scholar]
- 52.Peart WS, Quesada T, Tenyi I. The effects of EDTA and EGTA on renin secretion. Br J Pharmacol 59: 247–252, 1977. doi: 10.1111/j.1476-5381.1977.tb07486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Persson AE, Ollerstam A, Liu R, Brown R. Mechanisms for macula densa cell release of renin. Acta Physiol Scand 181: 471–474, 2004. doi: 10.1111/j.1365-201X.2004.01320.x. [DOI] [PubMed] [Google Scholar]
- 54.Peti-Peterdi J, Fintha A, Fuson AL, Tousson A, Chow RH. Real-time imaging of renin release in vitro. Am J Physiol Renal Physiol 287: F329–F335, 2004. doi: 10.1152/ajprenal.00420.2003. [DOI] [PubMed] [Google Scholar]
- 55.Pickett JA, Edwardson JM. Compound exocytosis: mechanisms and functional significance. Traffic 7: 109–116, 2006. doi: 10.1111/j.1600-0854.2005.00372.x. [DOI] [PubMed] [Google Scholar]
- 56.Ritthaler T, Della Bruna R, Krämer BK, Kurtz A. Endothelins inhibit cyclic-AMP induced renin gene expression in cultured mouse juxtaglomerular cells. Kidney Int 50: 108–115, 1996. doi: 10.1038/ki.1996.293. [DOI] [PubMed] [Google Scholar]
- 57.Roy MW, Guthrie GP Jr, Holladay FP, Kotchen TA. Effects of verapamil on renin and aldosterone in the dog and rat. Am J Physiol Endocrinol Metab 245: E410–E416, 1983. doi: 10.1152/ajpendo.1983.245.4.E410. [DOI] [PubMed] [Google Scholar]
- 58.Sauter A, Machura K, Neubauer B, Kurtz A, Wagner C. Development of renin expression in the mouse kidney. Kidney Int 73: 43–51, 2008. doi: 10.1038/sj.ki.5002571. [DOI] [PubMed] [Google Scholar]
- 59.Scholz H, Kurtz A. Differential regulation of cytosolic calcium between afferent arteriolar smooth muscle cells from mouse kidney. Pflügers Arch 431: 46–51, 1995. doi: 10.1007/BF00374376. [DOI] [PubMed] [Google Scholar]
- 60.Steppan D, Zügner A, Rachel R, Kurtz A. Structural analysis suggests that renin is released by compound exocytosis. Kidney Int 83: 233–241, 2013. doi: 10.1038/ki.2012.392. [DOI] [PubMed] [Google Scholar]
- 61.Taugner R, Rosivall L, Bührle CP, Gröschel-Stewart U. Myosin content and vasoconstrictive ability of the proximal and distal (renin-positive) segments of the preglomerular arteriole. Cell Tissue Res 248: 579–588, 1987. doi: 10.1007/BF00216486. [DOI] [PubMed] [Google Scholar]
- 62.Uchida E, Bohr DF. Myogenic tone in isolated perfused resistance vessels from rats. Am J Physiol 216: 1343–1350, 1969. doi: 10.1152/ajplegacy.1969.216.6.1343. [DOI] [PubMed] [Google Scholar]
- 63.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 389: 990–994, 1997. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 64.Van Dongen R, Peart WS. Calcium dependence of the inhibitory effect of angiotensin on renin secretion in the isolated perfused kidney of the rat. Br J Pharmacol 50: 125–129, 1974. doi: 10.1111/j.1476-5381.1974.tb09599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Villanueva J, Torregrosa-Hetland CJ, García-Martínez V, del Mar Francés M, Viniegra S, Gutiérrez LM. The F-actin cortex in chromaffin granule dynamics and fusion: a minireview. J Mol Neurosci 48: 323–327, 2012. doi: 10.1007/s12031-012-9718-4. [DOI] [PubMed] [Google Scholar]
- 66.Wagner C, de Wit C, Kurtz L, Grünberger C, Kurtz A, Schweda F. Connexin40 is essential for the pressure control of renin synthesis and secretion. Circ Res 100: 556–563, 2007. doi: 10.1161/01.RES.0000258856.19922.45. [DOI] [PubMed] [Google Scholar]
- 67.Wagner C, Jobs A, Schweda F, Kurtz L, Kurt B, Lopez ML, Gomez RA, van Veen TA, de Wit C, Kurtz A. Selective deletion of Connexin 40 in renin-producing cells impairs renal baroreceptor function and is associated with arterial hypertension. Kidney Int 78: 762–768, 2010. doi: 10.1038/ki.2010.257. [DOI] [PMC free article] [PubMed] [Google Scholar]