Abstract
Diabetic kidney disease (DKD) is a chronic kidney pathology that leads to end-stage renal disease. Previous studies from our laboratory indicate that there is an association between the development of DKD and the transient receptor potential canonical 6 (TRPC6) channel. Trpc6 expression and activity were increased in the streptozotocin (STZ)-treated Dahl Salt-sensitive (Dahl SS) rat, an established model of type 1 diabetes. Here, using a Trpc6 knockout created on the Dahl SS rat background (SSTrpc6−/−), we test the hypothesis that the absence of Trpc6 will protect podocytes and kidney function during the development of DKD. Four groups of animals (control SSWT, SSTrpc6−/−, STZ-treated SSWT, and STZ-SSTrpc6−/−) were utilized in this study. Diabetes development was monitored for 11 wk after STZ injection with periodic weight, glucose, and urinary output measurements. There was an increase in albuminuria and glomerular injury following STZ treatment, which was not different between Dahl SS and SSTrpc6−/− groups. Western blot analysis revealed elevated levels of nephrin in urine samples of STZ-SSWT rats, which was higher compared with STZ-SSTrpc6−/− rats. Furthermore, pathological increases in basal [Ca2+]i levels and foot process damage of podocytes during the development of DKD was attenuated in the STZ-SSTrpc6−/− compared with STZ-SSWT rats. Overall, our data indicate that TRPC6 channel inhibition may have at least partial renoprotective effects, which could lead to the development of new pharmacological tools to treat or prevent the progression of DKD.
Keywords: diabetic kidney disease, intracellular calcium, podocytes, Trpc6
INTRODUCTION
Diabetic kidney disease (DKD) is a renal complication of diabetes that is becoming one of the leading causes of end-stage renal disease. According to the National Kidney Foundation, ~660,000 Americans have been diagnosed with kidney failure, and the annual Medicare spending to treat kidney failure in the United States is over $31 billion. With the unmet medical need of DKD prevention, understanding every facet of the disease is essential to developing targeted therapies. Hyperglycemia from diabetes causes progressive damage to the glomerular cells and capillaries of the kidneys. Podocytes are specialized glomerular epithelial cells with foot processes that form a major part of the renal filtration barrier. Apoptosis of podocytes disrupts the integrity of the glomerular filtration barrier, resulting in the development of proteinuria and renal injury (6, 23). Considering the dramatic effect that the apoptosis of podocytes has on renal function, identifying their pathogenesis is imperative.
Research has shown that members of the transient receptor potential canonical (TRPC) channel family are important in normal glomerular function, and their overactivation can lead to podocyte and mesangial cell apoptosis (14, 21). TRPC channels belong to a superfamily of cation channels that are nonselectively permeable to calcium (1, 39). Activation of some of the TRPC channels was shown to cause glomerular damage because of their role in podocytopenia (podocyte deficiency); however, human mutations are only identified in TRPC 6 (TRPC6) channel, which has been proven to be a major player in podocyte depletion during renal disease. A gain-of-function mutation of the human TRPC6 channel has been identified as a genetic impetus for focal segmental glomerulosclerosis (FSGS) (7, 28, 38).
The TRPC6 channel is located on the membrane of the podocyte as part of a signaling complex interacting with nephrin and podocin and other key players within the slit diaphragm (14, 28). Under normal physiological conditions, the channel remains predominantly dormant until a stimulus causes its activation (14). We and others have identified that angiotensin II (Ang II) signaling mechanisms, which are increased during the progression of DKD, activate TRPC6 channels (2, 8, 9, 11, 12, 26, 32). Using the Trpc6−/− mouse model, we found that their podocytes had a decreased calcium influx in response to Ang II compared with wild-type littermates. This indicates the importance of TRPC6 channel in podocyte calcium handling and its potential role in podocyte damage (11). It was reported that hyperglycemia in combination with Ang II causes an increase in Trpc6 promoter activity and an overexpression of the TRPC6 channel, accompanied by an increase in Ca2+ influx within podocytes (14, 32). Furthermore, this pathway is implicated in the regulation of glomerular volume dynamics (12). These findings make Trpc6 an attractive target for potential benefits in treating DKD.
The streptozotocin (STZ)-induced diabetic Dahl salt-sensitive (Dahl SS) rat, as a type 1 diabetes mellitus model of DKD, develops the necessary pathological characteristics of DKD (31). Körner et al. (17) initially found that the changes in the glomerular morphology cannot only be attributed to this strain’s genetic impetus for salt-induced hypertension but lie more in the effects of the hyperglycemia. Slaughter et al. (31) discovered that treating Dahl SS rats with STZ induces increases in renal fibrosis, glomerular injury, proteinuria and hypertension with changes in renal hemodynamics such as elevated renal vascular resistance and hyperfiltration. The uniqueness of the diabetic Dahl SS rat lies in that unlike other models of STZ-induced diabetes, it develops the comparable functional and morphological renal abnormalities observed in patients with DKD. Our previous studies using the STZ-SS rats showed an increase in glomerular damage, elevated podocyte basal calcium levels, and Ang II-mediated calcium influx through the TRPC6 channel (14). To investigate the importance of Trpc6 signaling in podocytes and the implications of related mechanisms in DKD further, we created the Trpc6 knockout (SSTrpc6−/−) on the Dahl SS rat background. We hypothesized that the deletion of Trpc6 will protect podocytes and glomeruli during the development of DKD. Our results demonstrate that loss of Trpc6 leads to some renoprotective benefits with blunting of an increase in basal calcium in podocytes, reduced foot process damage, and a reduction in nephrin shedding in animals with DKD. These studies are important to further our understanding of the role of TRPC6 in podocyte damage and advance our understanding of the pathogenesis of DKD.
MATERIALS AND METHODS
Animals.
All animal-related procedures have been approved by the Medical College of Wisconsin Institutional Animal Care Use Committee and adhere to the NIH “Guide for the Care and Use of Laboratory Animals.” SSTrpc6−/− rats (SS-Trpc6em1Mcwi) were created at the MCW Gene Editing Rat Resource Center using the CRISPR/Cas9 technology on the SS/JrHsdMcwi background. Seven-week-old male SSTrpc6−/− and wild-type littermates (SSWT) were used for these experiments and fed a 0.4% NaCl AIN diet since weaning and during the 11-wk protocol. SSWT or SSTrpc6−/− rats were injected with STZ (65 mg/kg ip total body weight) or vehicle (sodium citrate solution, pH 4.5) and simultaneously given an insulin or blank (not containing insulin) subcutaneous implant (LinShin Canada) to maintain blood glucose at a stable level (300–400 mg/dl) (9). Blood glucose levels and urine collections were measured at 7, 8, 10, 16, and 18 wk of age using tail pricks, a glucometer, and metabolic cages. Urine samples were used to determine microalbumin, creatinine, and nephrin levels (ELISA assay and Western blotting; ab58968, Abcam, Cambridge, UK).
Measurement of glomerular filtration rate in conscious rats.
The glomerular filtration rate (GFR) was measured in unrestrained conscious rats using a high-throughput method featuring detection of fluorescent FITC-labeled inulin (TdB Consultancy AB, Uppsala, Sweden) clearance from blood. The method was adapted for rats (27) from a protocol previously described for mice by Rieg (29). GFR was calculated using the following equation: GFR = n/(A1/K1 + A2/K2), where n = injected amount (n = c × V, where c = FITC-inulin concentration, V = injected volume); A1 = Span1, y-intercept of elimination; K1 = 1/time 1; A2 = Span2, y-intercept of distribution; K2 = 1/time 2.
Histochemical analysis.
Kidney damage was assessed by automatic glomerular localization approach developed by Dr. John Bukowy (3). Kidneys were fixed in 10% formalin, embedded, and cut at 4-µm-thick slices, and then deparaffinized for histochemistry. Slices were stained using Masson’s Trichrome staining to assess glomerular damage. At random, over 100 glomeruli from kidneys of rats from each group were blindly scored for glomerular injury on a scale of 0 to 4. On the scale, 0 represents a normal healthy kidney, 1 being 1–25% of mesangial expansion and sclerosis (mesangial expansion, thickening of the basement membrane, and/or irregular lumina of capillaries), 2 for 26%–50% (mild segmental hyalinosis involving 50% of the glomerular tuft), 3 being for 51%–75% (diffuse sclerosis involving 50% of the glomerular capillaries), and a score of 4 is given for 76%–100% mesangial expansion and presence of sclerosis (extensive sclerosis with obliteration of the glomerular capillary tuft). MetaMorph image analysis program of Nikon-NIE microscopically scanned images at ×10 and Nikon Super Coolscan 9000 images of slides was used to analyze the distribution of the representative staining colors of the Masson’s Trichrome.
Electron microscopy.
For the electron microscopy experiments, glomeruli were fixed in 2% glutaraldehyde buffered in 0.1 M cacodylate (pH 7.4) and processed as described previously (10). Sections were viewed in a Hitachi HS600 transmission electron microscope and images recorded via an AMT 1K digital imaging camera.
Basal [Ca2+]i measurements in the podocytes.
The Nikon A1-R laser scanning confocal microscope system was used as detailed in previous publications to determine basal calcium levels in podocytes (9, 13). Briefly, decapsulated, freshly isolated glomeruli of perfused kidneys were incubated with FuraRed (5 µM) and Fluo-4 (5 µM) for ~40 min at room temperature on a rotating shaker. Incubated glomeruli were attached to poly-l-lysine-coated coverslips and mounted into the Nikon A1-R imaging chamber containing a 2 mM Ca2+ bath solution (contents detailed in previous publication) (9, 13). Ionomycin and MnCl2 were used according to a standard protocol to calibrate fluorescence to [Ca2+]. Image analysis was done using ImageJ open software equipped with the ND Utility plugin (9, 13). Fluorescence was converted to [Ca2+]i by the following equation:
where Kd is a standard dissociation constant for Fluo-4, F is the baseline fluorescence, Fmax is the maximum fluorescence after ionomycin application, and Fmin is the quenched fluorescence after MnCl2 application.
Statistical analysis.
Figures were prepared using the Origin 6.0 (MicroCal Software). Summarized data are presented as line graphs and box plots (box indicating standard deviation and whiskers indicating standard error mean). Statistical analysis consisted of Student’s t-test and one-way ANOVA, and P < 0.05 was considered significant.
RESULTS
Development of DKD in Trpc6 knockout rats after an injection of STZ.
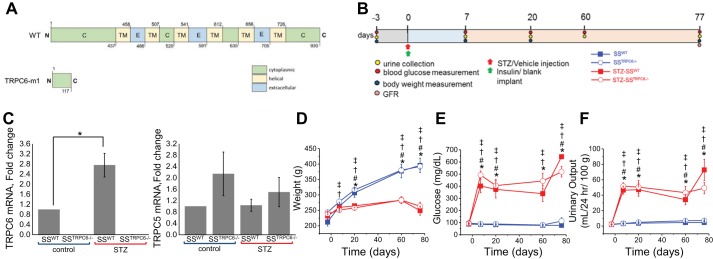
The SSTrpc6−/− rat was created on the Dahl SS rat background using a frameshift insertion in exon 2 that resulted in a premature stop codon. A scheme of resulting 117AA protein (wild type is 930AA) (CTAGCATCTTCCGCACaaCACTGGGATGTT) is shown on Fig. 1A. The knockout of Trpc6 in SSTrpc6−/− animals was confirmed by Sanger sequencing and fluorescent genotyping (data not shown). Diabetes was induced via an intraperitoneal injection of STZ in 7-wk-old SSTrpc6−/− and SS rats. Animals simultaneously received insulin pellets subcutaneously. to maintain glucose levels at survivable conditions (Fig. 1B). qRT-sPCR was performed to probe for Trpc6 and Trpc5 mRNA expression under normal conditions and following development of type 1 DKD. As predicted, Trpc6 mRNA levels were negligible in SSTrpc6−/− rats and were significantly elevated after the development of DKD as it was previously described (8, 9, 35, 41). In contrast, Trpc5 levels did not change neither in SSTrpc6−/− rats nor following STZ treatment (Fig. 1C). After hyperglycemia was confirmed 3 days following injections, blood glucose levels, urinary excretion, and weight were measured at days 7, 20, 60, and 77 (end point of 11-wk protocol). STZ-treated animals overall (STZ-SSTrpc6−/− and STZ- SSWT animals) had significantly greater increases in their blood glucose levels (Fig. 1E) and urinary excretion (Fig. 1F) with a significant decline in body weight (Fig. 1D) in comparison to the control animals (SSTrpc6−/− and SSWT animals). Interestingly, though not statistically significant, there was a slight blunting of an increase in glucose and urinary excretion levels for the STZ-SSTrpc6−/− compared with the STZ-SSWT animals. These data indicate that loss of Trpc6 function in STZ-treated animals results in a dampening of characteristic markers of DKD such as the dramatic increase in blood glucose and urinary output.
Fig. 1.
Development of STZ-induced DKD in transient receptor potential canonical 6 (Trpc6) knockout and wild-type (WT) rats during the 11-wk protocol. A: scheme of WT TRPC6 and truncated TRPC6-m1 (SSTrpc6−/−), which resulted from a 2-bp insertion leading to a frameshift mutation. B: protocol used for all four experimental groups (vehicle or STZ-treated WT and SSTrpc6−/− rats). C: RT-PCR quantification of Trpc6 and Trpc5 mRNA expression in WT and SSTrpc6−/− rats treated and not treated with STZ (values normalized to 18S); n = 4 rats for SSWT, SSTrpc6−/−, STZ-SSWT, and STZ-SSTrpc6−/−. D–F: development of diabetes in SS and SSTrpc6−/− rats during the 11 wk after STZ injection observed by weight (D), blood glucose (E), and urinary output changes (F). n = 5, 18, 9, and 17 rats for SSWT, SSTrpc6−/−, STZ-SSWT, and STZ-SSTrpc6−/−, respectively. *P < 0.05 for SSWT versus STZ-SSWT, #P < 0.05 for SSWT versus STZ-SSTrpc6−/−, †P < 0.05 for SSTrpc6−/− versus STZ-SSTrpc6−/−, ‡P < 0.05 for SSTrpc6−/− versus STZ-SSWT. DKD, diabetic kidney disease; GFR, glomerular filtration rate; SS, salt-sensitive; STZ, streptozotocin.
Assessment of renal morphology and function in Trpc6 knockout rats after STZ treatment.
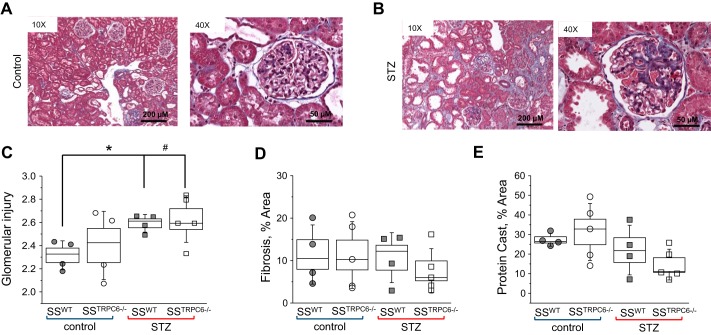
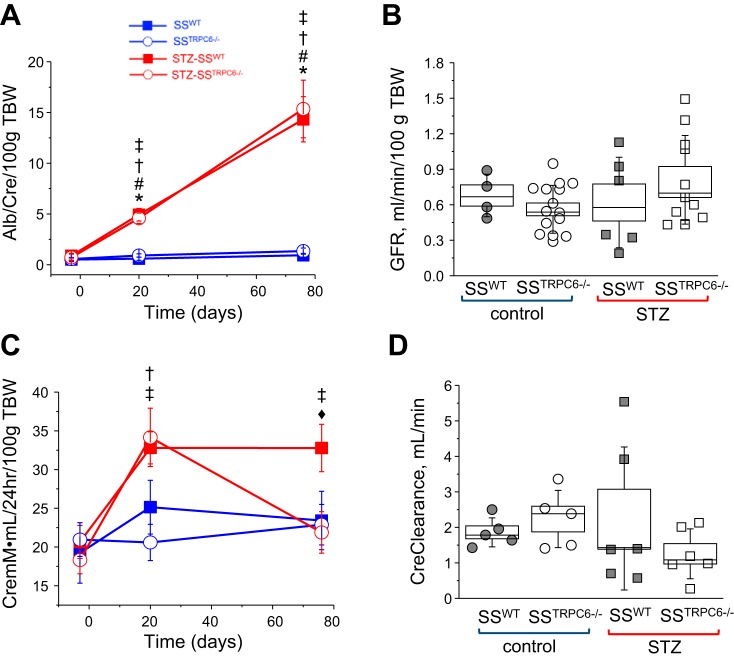
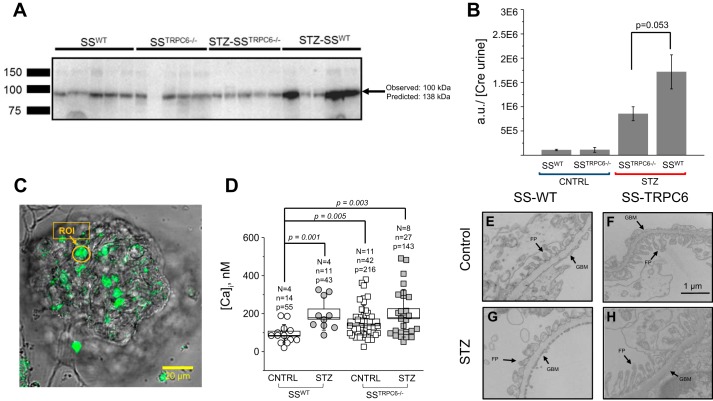
Tissue samples collected at end point were processed for histological analysis of renal morphology using Masson’s Trichrome (Fig. 2). Glomerulosclerosis was apparent in the STZ-treated animals in comparison to control with glomerular injury scores being significantly higher (Fig. 2, A–C). There was no difference in the level of interstitial fibrosis and protein cast formation in the STZ-treated animal versus the control animals; however, there does appear to be a trend for decreases in both abnormalities in the STZ-SSTrpc6−/− versus STZ-SSWT (Fig. 2, D and E). Additionally, urine samples collected at the time points indicated in Fig. 1B were used for measuring microalbumin and creatinine outputs to assess renal function. STZ-treated animals had significantly increased excretion of albumin in comparison to control animals (Fig. 3A). Creatinine levels showed that STZ-treated rats had higher excretion levels in comparison to vehicle-treated animals. Importantly, the STZ-SSTrpc6−/− animals had significantly lower creatinine excretion than the STZ-SSWT animals after 11 wk of STZ treatment (Fig. 3C). In assessment of glomerular function, GFR was determined using the previously described method of conscious blood sampling of FITC-Inulin infused animals (Fig. 3B) (29). There was a trend for hyperfiltration in STZ-treated rats, but overall there was no significant difference between the four groups. Creatinine clearance showed a trend for a decrease in the STZ-SSTrpc6−/− versus STZ-SSWT, but as a whole there was no significant difference between any of the groups (Fig. 3D). Furthermore, we tested levels of nephrin, a key component of the podocyte slit pore membrane, in the urine of all four groups of animals. Western blotting for nephrin shedding comparing the baseline (pretreatment with STZ) to the final time point revealed reduction in nephrin shedding in the STZ-SSTrpc6−/− versus STZ-SSWT (Fig. 4, A and B).
Fig. 2.
Evaluation of kidney damage during the development of DKD in SS and SSTrpc6−/− rats. A and B: representative images of kidney injury with and without STZ treatment. C–E: Immunohistochemical analyses of kidney injury. Quantification of glomerular injury (C), interstitial fibrosis (D), and protein casts (E) in the vehicle- and STZ-treated WT and SSTrpc6−/− rats. For all histological analysis n = 4 for SSWT and STZ-SSWT; n = 5 for SSTrpc6−/− and STZ-SSTrpc6−/−. DKD, diabetic kidney disease; SS, salt-sensitive; STZ, streptozotocin; WT, wild type.
Fig. 3.
Evaluation of kidney function. A and C: microalbuminuria and urinary creatinine excretion in STZ-SSWT and STZ-SSTrpc6−/− rats compared with control animals; urine samples from 3 days before STZ injection and 20 and 76 days after STZ-injection. B: glomerular filtration rate in control- and STZ-treated rats. D: creatinine clearance in control versus STZ-treated SSWT and SSTrpc6−/− rats. *P < 0.05 for SSWT versus STZ-SSWT, #P < 0.05 for SSWT versus STZ-SSTrpc6−/−, †P < 0.05 for SSTrpc6−/− versus STZ-SSTrpc6−/−, ‡P < 0.05 for SSTrpc6−/− versus STZ-SSWT, ♦P < 0.05 for STZ-SSTrpc6−/− versus STZ-SSWT. GFR, glomerular filtration rate; SS, salt-sensitive; STZ, streptozotocin; TBW, total body weight; WT, wild type.
Fig. 4.
Assessment of podocyte damage. A: Western blot analysis of urinary nephrin levels. B: quantification of Western blot shown in A. Urine samples collected at the end of experiments (day 76) were used in Western blot analysis for nephrin shedding in all experimental groups. Each line represents one rat. Arrow indicates nephrin band and weight. C: representative image of confocal fluorescent microscopy. Region of interest (ROI) and arrow indicating an example of podocyte used for analysis. D: box plot summarizing the concentration of intracellular calcium ([Ca2+]i) comparing SSWT versus STZ-SSWT versus SSTrpc6−/− versus STZ-SSTrpc6−/−. N = no. of rats, n = no. of glomeruli, P = no. of podocytes. E–H: representative electron microscopy images of freshly isolated glomeruli. Foot process (FP) and glomerular basement membrane (GBM) are shown. STZ-SSTrpc6−/− have a blunting in FP damage in comparison to STZ-SSWT. n = 3 for SSTrpc6−/− and STZ-SSTrpc6−/− and n = 2 for SSWT and STZ-SSWT. SS, salt-sensitive; STZ, streptozotocin; WT, wild type.
Basal calcium measurements and morphology of podocytes.
Freshly isolated glomeruli were used in confocal fluorescent microscopy with Fluo-4 AM intensity levels in response to ionomycin and MnCl2 to determine basal calcium levels (Fig. 4D). As we previously reported (9), STZ-SSWT rats had a significant increase in their basal intracellular calcium levels ([Ca2+]i) compared with control animals (Fig. 4D). However, when evaluating basal calcium levels in SSTrpc6−/−, there was no significant difference between control and STZ-treated animals (Fig. 4D). Interestingly, basal level was significantly elevated in SSTrpc6−/− compared with SSWT rats, which was also shown for Trpc6 knockout mice (8) and might represent some compensatory mechanisms. Using electron microscopy, we showed that there was a reduction in foot process effacement for STZ-SSTrpc6−/− podocytes in comparison to STZ-SSWT (Fig. 4, E–H). However, quantification of these structural changes was not performed because of low number of samples, and no definite conclusions could be made. These results in combination with those shown in Figs. 2 and 3 reveal that loss of Trpc6 function in DKD conditions has a podocyte semiprotective phenotype.
DISCUSSION
DKD is a devastating disease that is overwhelming patients suffering from diabetes and is one of the chief causes of end-stage renal disease. In DKD, podocyte loss caused by damage and dysfunction was found to be a major factor in DKD disease progression. Podocyte cell death leads to further degradation of the glomerular filtration barrier, causing proteinuria and renal injury. The present study aimed to investigate the role of Trpc6 in the progression of DKD and to determine the effects of its loss. It was found that in the absence of Trpc6 there is a blunting of the development of DKD in the STZ-SSTrpc6−/− evident by the slight attenuation in glucose, urinary output, and creatinine clearance levels in comparison to the STZ-SSWT, though not statistically significant. Also, this study shows an attenuation of an increase in creatinine and nephrin shedding levels in urine samples, an increase in basal calcium levels for STZ-SSTrpc6−/−, and a decrease in foot process effacement for STZ-SSTrpc6−/− rats.
There is synergistic interaction of mild diabetes mellitus and hypertension in regulation of renal function and promotion of kidney injury (33, 37). As described by the Center for Disease Control and Prevention, the percentage of adults with diabetes who have hypertension is rapidly growing. However, most of the available diabetic research models do not develop elevated blood pressure and kidney injury. Thus, we used the STZ-treated type 1 diabetic Dahl SS rat model. The STZ model is well established and offers the advantage of quickly and reproducibly inducing hyperglycemia and renal hypertrophy in rats. However, the STZ-treated mouse or rat models typically do not develop glomerular disease unless hypertension is induced. As reported by Williams’s laboratory (31) and us (9), SS rats treated with STZ develop hyperfiltration and progressive proteinuria and display renal histological lesions characteristic to those seen in patients with DKD. Therefore, although this model has some limitations, it mimics the development of DKD in human. Furthermore, as we have shown previously (9), STZ-SS rats develop diabetes after STZ treatment, but DKD lesions start only after 11 wk of treatment (DKD was not pronounced after 7 wk of STZ). Importantly, here the effect of Trpc6 knockout is observed at 11 wk after STZ when DKD is already developed.
Previous genetic studies have established a link between Trpc6 and FSGS, providing the foundation for recent studies into the potential role Trpc6 may have in other glomerular diseases (28, 38). Trpc6 is an integral player in not only the calcium handling of the podocyte, but also the confirmation of its cellular structure (4, 5, 20, 24, 25). As an example, Möller et al. reported that when Trpc6 is overexpressed in healthy mice, it leads to restructuring of the podocytes actin cytoskeleton with alterations in calcium flux as well. Additionally, they found that overexpression of Trpc6 in these healthy mice causes proteinuria (25). Multiple findings from various investigators delineating the connection between the TRPC6 channel’s involvement in the podocyte function and the pathogenesis of glomerular diseases identify Trpc6 as a potential clinical target for kidney disease, including DKD (2, 4, 8, 9, 11, 12, 14, 18, 19, 22, 32, 35, 40, 41). As an example, a connection between Trpc6 and permeability factors such as TNF and suPAR in cases of FSGS was reported. A correlation between increases in these factors and the quantity of TRPC6 on the cell surface was found (15). Hyperglycemia along with elevation in Ang II levels are sufficient to cause overexpression of Trpc6, which results in increased calcium influx and eventual podocyte dysfunction and death (32). The increases in basal calcium levels and foot process effacement evident in our STZ-SSWT animals were signs of declining podocyte function. Losing the function of the TRPC6 channel attenuated these increases, showing that Trpc6 plays a pivotal role in the progression of podocyte dysfunction in DKD. Therefore, our data provide additional insight into the role of Trpc6 in DKD. Importantly, Kim et al. (16) tested contribution of Trpc6 deletion in puromycin aminonucleoside nephrosis model. Sprague-Dawley rats with deletion of Trpc6 had reduced urine albumin excretion, serum cholesterol, and triglycerides, markedly attenuated glomerulosclerosis, and tubulointerstitial fibrosis.
Only Trpc6 has been strongly linked to a glomerular disease; however, several other TRPC channels, including Trpc3 and 5 have been proposed to be involved in podocyte calcium handling. As an example, while investigating the mechanisms underlying the Trpc6 gain-of-function mutation contribution to glomerular dysfunction, it was found that Trpc5 plays an important role in the podocyte cytoskeleton. Tian et al. (34) elucidated the role of Trpc5 in calcium-induced remodeling of the podocyte cytoskeleton and found that it triggers a Rac1 pathway that leads to foot process effacement. The investigators found that efficient podocyte calcium handling and function is dependent on a balance between “homeostatic” Trpc6 and “inducible” Trpc5 functions (34). To test the hypothesis that Trpc5 might be upregulated under disease condition or compensate for Trpc6 knockout, we have performed qRT-PCR of Trpc5 mRNA expression in control and STZ-treated SSTrpc6−/− and SSWT tissue samples. However, no significant difference was apparent between any groups (Fig. 1C). Similarly, the abundance of TRPC5 channels did not change in puromycin aminonucleoside model with Trpc6 deletion (16). The recent literature presents a contradictory opinion on the topic of Trpc5 or Trpc6. Data from Greka and colleagues (30, 42) proposed Trpc5 being the driving force in the development of proteinuria, suggesting that inhibiting Trpc5 protects against breakdown of the filtration barrier, thus preventing the progression of kidney disease. These findings are in contradiction to those mentioned previously and more recently to the findings of Wang et al. (36), whose data indicate that overexpression or gain-of-function in Trpc5 has no effect on the filtration barrier. Furthermore, inhibition of Trpc6, but not Trpc5 channels, attenuated Ang II-induced calcium flux and glomerular permeability (12). These data suggest that Trpc5 may not be playing a role in the progression of DKD and that the podocyte is not attempting to compensate for the loss of Trpc6.
In conclusion, our current findings suggest that targeting and inhibiting Trpc6 may have some renoprotective effects in DKD, preventing severe damage to the podocytes but failing to protect the glomerulus as a whole. This could be due to the possibility that the complications of this disease are so intense that protecting the podocyte alone may not be sufficient to inhibit disease progression. Further studies of a more detailed look into the signaling pathway for this channel are needed, and more attempts should be made to elucidate the relationship between Trpc6 and DKD. The conclusions derived from this study shed some light on the topic and give further evidence for the importance of Trpc6 in DKD.
GRANTS
This research was supported by the NIH Grants HL-135749, HL-116264 (to A. Staruschenko), HL-114474 (to A. M. Geurts), DK-020595 Pilot & Feasibility project (to O. Palygin); Juvenile Diabetes Research Foundation Grant 1-INO-2016–223-A-N; and American Diabetes Association Grant 1-15-BS-172 (to A. Staruschenko).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.S., D.V.I., and A.S. conceived and designed research; D.S., D.V.I., V.L., A.M.G., and O.P. performed experiments; D.S., D.V.I., V.L., P.E.N., A.M.G., and O.P. analyzed data; D.S., D.V.I., O.P., and A.S. interpreted results of experiments; D.S., D.V.I., and O.P. prepared figures; D.S., D.V.I., and A.S. drafted manuscript; D.S., D.V.I., V.L., O.P., and A.S. edited and revised manuscript; D.S., D.V.I., V.L., P.E.N., A.M.G., O.P., and A.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge Clive Wells (MCW Electron Microscopy Core), Christine Duris (Histology core), Lisa Henderson, Jenifer Phillips, and Camille Taylor (MCW Physiology Biochemistry Core) for excellent technical assistance. Drs. John Bukowy and Allen W. Cowley, Jr., are appreciated for help with glomeruli scoring. We also appreciate Dr. Christine Klemens for helpful reading and critical suggestions.
REFERENCES
- 1.Abramowitz J, Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J 23: 297–328, 2009. doi: 10.1096/fj.08-119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson M, Roshanravan H, Khine J, Dryer SE. Angiotensin II activation of TRPC6 channels in rat podocytes requires generation of reactive oxygen species. J Cell Physiol 229: 434–442, 2014. doi: 10.1002/jcp.24461. [DOI] [PubMed] [Google Scholar]
- 3.Bukowy JD, Dayton A, Cloutier D, Manis AD, Staruschenko A, Lombard JH, Solberg Woods LC, Beard DA, Cowley AW Jr. Region-based convolutional neural nets for localization of glomeruli in trichrome-stained whole kidney sections. J Am Soc Nephrol 29: 2081–2088, 2018. doi: 10.1681/ASN.2017111210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dryer SE, Reiser J. TRPC6 channels and their binding partners in podocytes: role in glomerular filtration and pathophysiology. Am J Physiol Renal Physiol 299: F689–F701, 2010. doi: 10.1152/ajprenal.00298.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Endlich K, Kliewe F, Endlich N. Stressed podocytes-mechanical forces, sensors, signaling and response. Pflugers Arch 469: 937–949, 2017. doi: 10.1007/s00424-017-2025-8. [DOI] [PubMed] [Google Scholar]
- 6.Fogo AB. Causes and pathogenesis of focal segmental glomerulosclerosis. Nat Rev Nephrol 11: 76–87, 2015. doi: 10.1038/nrneph.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heeringa SF, Möller CC, Du J, Yue L, Hinkes B, Chernin G, Vlangos CN, Hoyer PF, Reiser J, Hildebrandt F. A novel TRPC6 mutation that causes childhood FSGS. PLoS One 4: e7771, 2009. doi: 10.1371/journal.pone.0007771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ilatovskaya DV, Blass G, Palygin O, Levchenko V, Pavlov TS, Grzybowski MN, Winsor K, Shuyskiy LS, Geurts AM, Cowley AW, Birnbaumer L, Staruschenko A. A NOX4/TRPC6 pathway in podocyte calcium regulation and renal damage in diabetic kidney disease. J Am Soc Nephrol 29: 1917–1927, 2018. doi: 10.1681/ASN.2018030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ilatovskaya DV, Levchenko V, Lowing A, Shuyskiy LS, Palygin O, Staruschenko A. Podocyte injury in diabetic nephropathy: implications of angiotensin II-dependent activation of TRPC channels. Sci Rep 5: 17637, 2015. doi: 10.1038/srep17637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ilatovskaya DV, Levchenko V, Ryan RP, Cowley AW Jr, Staruschenko A. NSAIDs acutely inhibit TRPC channels in freshly isolated rat glomeruli. Biochem Biophys Res Commun 408: 242–247, 2011. doi: 10.1016/j.bbrc.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ilatovskaya DV, Palygin O, Chubinskiy-Nadezhdin V, Negulyaev YA, Ma R, Birnbaumer L, Staruschenko A. Angiotensin II has acute effects on TRPC6 channels in podocytes of freshly isolated glomeruli. Kidney Int 86: 506–514, 2014. doi: 10.1038/ki.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ilatovskaya DV, Palygin O, Levchenko V, Endres BT, Staruschenko A. The role of angiotensin II in glomerular volume dynamics and podocyte calcium handling. Sci Rep 7: 299, 2017. doi: 10.1038/s41598-017-00406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ilatovskaya DV, Palygin O, Levchenko V, Staruschenko A. Single-channel analysis and calcium imaging in the podocytes of the freshly isolated glomeruli. J Vis Exp 100: e52850, 2015. doi: 10.3791/52850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ilatovskaya DV, Staruschenko A. TRPC6 channel as an emerging determinant of the podocyte injury susceptibility in kidney diseases. Am J Physiol Renal Physiol 309: F393–F397, 2015. doi: 10.1152/ajprenal.00186.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim EY, Roshanravan H, Dryer SE. Changes in podocyte TRPC channels evoked by plasma and sera from patients with recurrent FSGS and by putative glomerular permeability factors. Biochim Biophys Acta 1863: 2342–2354, 2017. doi: 10.1016/j.bbadis.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim EY, Yazdizadeh Shotorbani P, Dryer SE. Trpc6 inactivation confers protection in a model of severe nephrosis in rats. J Mol Med (Berl) 96: 631–644, 2018. doi: 10.1007/s00109-018-1648-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Körner A, Jaremko G, Eklöf A-C, Aperia A. Rapid development of glomerulosclerosis in diabetic Dahl salt-sensitive rats. Diabetologia 40: 367–373, 1997. doi: 10.1007/s001250050689. [DOI] [PubMed] [Google Scholar]
- 18.Krall P, Canales CP, Kairath P, Carmona-Mora P, Molina J, Carpio JD, Ruiz P, Mezzano SA, Li J, Wei C, Reiser J, Young JI, Walz K. Podocyte-specific overexpression of wild type or mutant trpc6 in mice is sufficient to cause glomerular disease. PLoS One 5: e12859, 2010. doi: 10.1371/journal.pone.0012859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu B, He X, Li S, Xu B, Birnbaumer L, Liao Y. Deletion of diacylglycerol-responsive TRPC genes attenuates diabetic nephropathy by inhibiting activation of the TGFβ1 signaling pathway. Am J Transl Res 9: 5619–5630, 2017. [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Z, Yang J, Zhang X, Xu P, Zhang T, Yang Z. Developmental changes in the expression and function of TRPC6 channels related the F-actin organization during differentiation in podocytes. Cell Calcium 58: 541–548, 2015. doi: 10.1016/j.ceca.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Ma R, Chaudhari S, Li W. Canonical transient receptor potential 6 channel: a new target of reactive oxygen species in renal physiology and pathology. Antioxid Redox Signal 25: 732–748, 2016. doi: 10.1089/ars.2016.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma R, Liu L, Jiang W, Yu Y, Song H. FK506 ameliorates podocyte injury in type 2 diabetic nephropathy by down-regulating TRPC6 and NFAT expression. Int J Clin Exp Pathol 8: 14063–14074, 2015. [PMC free article] [PubMed] [Google Scholar]
- 23.Mallipattu SK, He JC. The podocyte as a direct target for treatment of glomerular disease? Am J Physiol Renal Physiol 311: F46–F51, 2016. doi: 10.1152/ajprenal.00184.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markó L, Mannaa M, Haschler TN, Krämer S, Gollasch M. Renoprotection: focus on TRPV1, TRPV4, TRPC6 and TRPM2. Acta Physiol (Oxf) 219: 589–612, 2017. doi: 10.1111/apha.12828. [DOI] [PubMed] [Google Scholar]
- 25.Möller CC, Wei C, Altintas MM, Li J, Greka A, Ohse T, Pippin JW, Rastaldi MP, Wawersik S, Schiavi S, Henger A, Kretzler M, Shankland SJ, Reiser J. Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J Am Soc Nephrol 18: 29–36, 2007. doi: 10.1681/ASN.2006091010. [DOI] [PubMed] [Google Scholar]
- 26.Nijenhuis T, Sloan AJ, Hoenderop JG, Flesche J, van Goor H, Kistler AD, Bakker M, Bindels RJ, de Boer RA, Möller CC, Hamming I, Navis G, Wetzels JF, Berden JH, Reiser J, Faul C, van der Vlag J. Angiotensin II contributes to podocyte injury by increasing TRPC6 expression via an NFAT-mediated positive feedback signaling pathway. Am J Pathol 179: 1719–1732, 2011. doi: 10.1016/j.ajpath.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palygin O, Levchenko V, Ilatovskaya DV, Pavlov TS, Pochynyuk OM, Jacob HJ, Geurts AM, Hodges MR, Staruschenko A. Essential role of Kir5.1 channels in renal salt handling and blood pressure control. JCI Insight 2: e92331, 2017. doi: 10.1172/jci.insight.92331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reiser J, Polu KR, Möller CC, Kenlan P, Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C, McGee M, Sugimoto H, Brown D, Kalluri R, Mundel P, Smith PL, Clapham DE, Pollak MR. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet 37: 739–744, 2005. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rieg T. A High-throughput method for measurement of glomerular filtration rate in conscious mice. J Vis Exp 75: e50330, 2013. doi: 10.3791/50330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaldecker T, Kim S, Tarabanis C, Tian D, Hakroush S, Castonguay P, Ahn W, Wallentin H, Heid H, Hopkins CR, Lindsley CW, Riccio A, Buvall L, Weins A, Greka A. Inhibition of the TRPC5 ion channel protects the kidney filter. J Clin Invest 123: 5298–5309, 2013. doi: 10.1172/JCI71165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slaughter TN, Paige A, Spires D, Kojima N, Kyle PB, Garrett MR, Roman RJ, Williams JM. Characterization of the development of renal injury in type-1 diabetic Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 305: R727–R734, 2013. doi: 10.1152/ajpregu.00382.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sonneveld R, van der Vlag J, Baltissen MP, Verkaart SA, Wetzels JF, Berden JH, Hoenderop JG, Nijenhuis T. Glucose specifically regulates TRPC6 expression in the podocyte in an AngII-dependent manner. Am J Pathol 184: 1715–1726, 2014. doi: 10.1016/j.ajpath.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Staruschenko A. Hypertension and diabetes mellitus: the chicken and egg problem. Hypertension 69: 787–788, 2017. doi: 10.1161/HYPERTENSIONAHA.117.08671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian D, Jacobo SM, Billing D, Rozkalne A, Gage SD, Anagnostou T, Pavenstädt H, Hsu HH, Schlondorff J, Ramos A, Greka A. Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Sci Signal 3: ra77, 2010. doi: 10.1126/scisignal.2001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, Jirka G, Rosenberg PB, Buckley AF, Gomez JA, Fields TA, Winn MP, Spurney RF. Gq signaling causes glomerular injury by activating TRPC6. J Clin Invest 125: 1913–1926, 2015. doi: 10.1172/JCI76767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X, Dande RR, Yu H, Samelko B, Miller RE, Altintas MM, Reiser J. TRPC5 does not cause or aggravate glomerular disease. J Am Soc Nephrol 29: 409–415, 2018. doi: 10.1681/ASN.2017060682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z, do Carmo JM, Aberdein N, Zhou X, Williams JM, da Silva AA, Hall JE. Synergistic interaction of hypertension and diabetes in promoting kidney injury and the role of endoplasmic reticulum stress. Hypertension 69: 879–891, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S, Burchette JL, Pericak-Vance MA, Howell DN, Vance JM, Rosenberg PB. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308: 1801–1804, 2005. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
- 39.Woudenberg-Vrenken TE, Bindels RJ, Hoenderop JG. The role of transient receptor potential channels in kidney disease. Nat Rev Nephrol 5: 441–449, 2009. doi: 10.1038/nrneph.2009.100. [DOI] [PubMed] [Google Scholar]
- 40.Yu H, Kistler A, Faridi MH, Meyer JO, Tryniszewska B, Mehta D, Yue L, Dryer S, Reiser J. Synaptopodin limits TRPC6 podocyte surface expression and attenuates proteinuria. J Am Soc Nephrol 27: 3308–3319, 2016. doi: 10.1681/ASN.2015080896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang X, Song Z, Guo Y, Zhou M. The novel role of TRPC6 in vitamin D ameliorating podocyte injury in STZ-induced diabetic rats. Mol Cell Biochem 399: 155–165, 2015. doi: 10.1007/s11010-014-2242-9. [DOI] [PubMed] [Google Scholar]
- 42.Zhou Y, Castonguay P, Sidhom EH, Clark AR, Dvela-Levitt M, Kim S, Sieber J, Wieder N, Jung JY, Andreeva S, Reichardt J, Dubois F, Hoffmann SC, Basgen JM, Montesinos MS, Weins A, Johnson AC, Lander ES, Garrett MR, Hopkins CR, Greka A. A small-molecule inhibitor of TRPC5 ion channels suppresses progressive kidney disease in animal models. Science 358: 1332–1336, 2017. doi: 10.1126/science.aal4178. [DOI] [PMC free article] [PubMed] [Google Scholar]