Abstract
Acid-sensing ion channels (ASICs) are trimeric proton-activated, cation-selective neuronal channels that are considered to play important roles in mechanosensation and nociception. Here we investigated the role of ASIC3, a subunit primarily expressed in sensory neurons, in bladder sensory signaling and function. We found that extracellular acidification evokes a transient increase in current, consistent with the kinetics of activation and desensitization of ASICs, in ~25% of the bladder sensory neurons harvested from both wild-type (WT) and ASIC3 knockout (KO) mice. The absence of ASIC3 increased the magnitude of the peak evoked by extracellular acidification and reduced the rate of decay of the ASIC-like currents. These findings suggest that ASICs are assembled as heteromers and that the absence of ASIC3 alters the composition of these channels in bladder sensory neurons. Consistent with the notion that ASIC3 serves as a proton sensor, 59% of the bladder sensory neurons harvested from WT, but none from ASIC3 KO mice, fired action potentials in response to extracellular acidification. Studies of bladder function revealed that ASIC3 deletion reduces voiding volume and the pressure required to trigger micturition. In summary, our findings indicate that ASIC3 plays a role in the control of bladder function by modulating the response of afferents to filling.
Keywords: acid-sensing ion channel, afferent signaling, ASIC3, bladder function, sensory neurons
INTRODUCTION
The processes of urine storage and micturition are coordinated by a complex neuronal system that receives sensory input from structures located in the bladder mucosa and musculature (20, 21, 27, 29). Despite the importance of the afferent pathways in the control of bladder function and nociception, there is limited understanding of the mechanisms by which sensory neurons sense bladder filling and of the factors that control their excitability. In the present study, we examined the role of the acid-sensing ion channel 3 (ASIC3) in bladder sensory signaling.
ASICs are neuronal cation selective channels that are activated by extracellular acidification. In rodents, four genes have been identified (ACCN1, ACCN2, ACCN3, and ACCN4) that encode for six ASIC subunits and splice variants (ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3, and ASIC4) (1, 4, 7, 13, 22, 30, 32, 42, 47, 53). ASIC3 is primarily expressed in sensory neurons where it was proposed to contribute to mechanosensation and nociception (13, 46). Although ASIC subunits share a high degree of identity, only ASIC1a, ASIC1b, and ASIC3 form channels by themselves that respond to extracellular acidification in the physiological range (37). In heterologous expression systems, ASIC subunits can associate to form homo- and heterotrimers with distinctive biophysical properties including agonist affinity, single-channel conductance, rate of desensitization, rate of recovery after desensitization, and cation selectivity (see 37, 38 for details). Despite the broad knowledge of the structure and function of ASICs in vitro systems, little is known about the assembly, subunit composition, and function of these channels in vivo.
The afferent innervation of the bladder consists of thinly myelinated Aδ fibers and unmyelinated C fibers that convey information from the urothelium and musculature to the spinal cord (11, 20, 21, 29). The general consensus is that normal micturition depends on mechanosensitive Aδ fiber afferents that respond to bladder distension in the physiological range (11, 20, 21, 27, 29). The role of C fibers on bladder function is less clear. Initial reports described bladder afferents with the conduction velocity of C fibers and high thresholds that responded only to high intravesical pressures (6, 33, 35). However, late reports described a subpopulation of bladder C fibers that responded to distension in the physiological range (5, 24, 26, 51, 55). Bladder afferent fibers are carried by the pelvic nerve with cell bodies located in lumbosacral (L6–S2) dorsal root ganglia (DRG), and by the hypogastric nerve with cell bodies located in thoracolumbar (T13–L2) DRG (21). Afferent signaling activates spinal tract neurons that project supraspinally to the brain stem reticular formation, parabrachial nucleus, thalamus, and periaqueductal gray ventrolateral region (PAG) (20, 27). The latter is known to innervate directly the pontine micturition center, which sends excitatory signals back to the spinal cord.
The goal of this study was to investigate the role of ASIC3 in bladder sensory signaling and control of normal bladder function. Results indicate that ASIC3 constitutes a proton sensor in a subset of bladder sensory neurons. Analysis of bladder function in awake and anesthetized mice revealed that the deletion of ASIC3 reduces the pressure required to trigger micturition and voiding volume. The significance of these findings on bladder function is discussed.
MATERIALS AND METHODS
Reagents.
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO), unless otherwise specified. Rabbit polyclonal antibody to ASIC3 was purchased from Millipore (cat. no. AB5678P), goat polyclonal antibody to ASIC3 was purchased from Novus Biologicals (cat. no. NBP1–46288), and guinea pig polyclonal antibody to ASIC3 was purchased from Neuromics (cat. no. GP1405).
Animals.
All experimental procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. ASIC3 knockout (ASIC3 KO) mice were obtained from Jackson Laboratories. Age-matched C57BL/6J [wild-type (WT)] were used as controls. WT and ASIC3 KO mice were bred at the University of Pittsburgh. Mice were housed under 12:12-h light-dark cycles with free access to food and water. Physiological studies were conducted with male and female mice between the ages of 12 and 24 wk, unless stated otherwise. Animals were euthanized by CO2 inhalation, followed by a thoracotomy.
Genotyping.
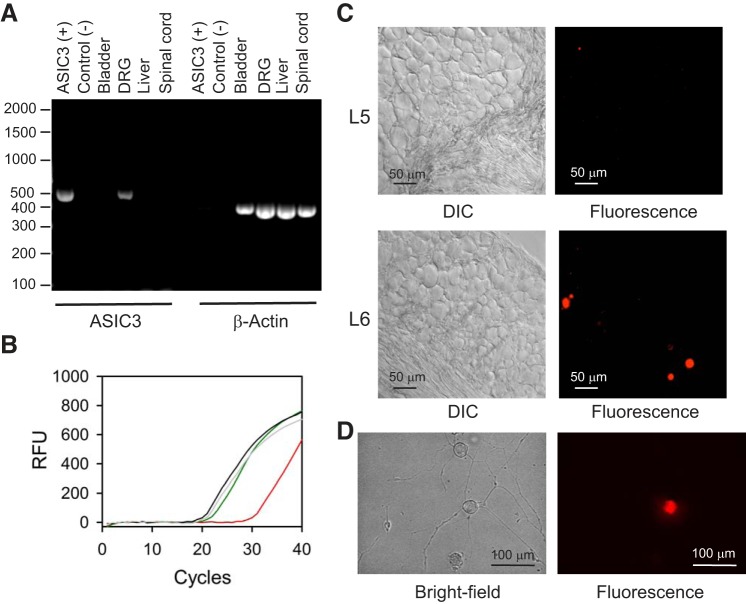
Tissue harvested in a 1.5-ml tube from an ear punch was suspended in 200 μl of Donghuis’s buffer [50 mM Tris-base pH 8.0, 50 mM KCl, 2.5 mM EDTA, 0.45% IGEPAL CA-630 (NP40), 0.45% Tween-20] containing 1.6 units of proteinase K and incubated overnight at 55°C. Thereafter, the tube was incubated at 94°C for 10 min, centrifuged at 21,000 g for 10 min, and the supernatant was saved for qPCR analysis. Since the ASIC3 KO mouse was generated by deletion of ASIC3 exon 1 by homologous recombination (46), for genotyping we designed primers targeting exon 1 of ASIC3 with Primer-Blast (NIH) (56). The following primers were used for quantification of DNA copies by qPCR: ASIC3 exon 1 forward, CAGCTGTACTCCTGTCGCTG; ASIC3 exon 1 reverse, GGCGCAGGGGATTGATGTTA; β-actin forward, GGCTGTATTCCCCTCCATCG; and β-actin reverse, CCAGTTGGTAACAATGCCATGT. qPCR amplification reactions included 1 μl of sample, 5 nmol of each primer, 5 μl of 2X iTaq Universal SYBR Green Supermix (Bio-Rad) in a total reaction mix volume of 10 μl. qPCR amplification was performed in a GFX Connect real-time PCR (Bio-Rad). Cycling conditions were 95°C for 2 min, followed by 39 cycles of 95°C for 15 s and 60°C for 15 s, and a melting step from 65°C to 95°C in 0.5°C increments per 5 s. qPCR were run in duplicate. Genotype was determined using the ΔCT method and β-actin as the reference gene. Representative amplification curves for ASIC3 and β-actin are shown in Fig. 1B.
Fig. 1.
Acid-sensing ion channel 3 (ASIC3) is expressed in sensory neurons. A: reverse transcription-PCR (RT-PCR) demonstrates the presence of transcripts for ASIC3 in dorsal root ganglia (DRG), but not urinary bladder or spinal cord. A construct coding for murine ASIC3 was used as positive control. RNA was isolated with the RNAqueous-4PCR kit and reverse transcription was done with the AccuScript PfuUltra II RT-PCR kit as indicated in materials and methods. The expected size of the PCR products for ASIC3 and β-actin was 437 and 359 bp, respectively. B: representative amplification plots for ASIC3 and β-actin. DNA was extracted from an ear punch collected from wild-type and ASIC3 KO mice as described in materials and methods. Curves indicate amplification from genomic DNA of ASIC3 (wild type, green; ASIC3 KO, red) and β-actin (wild type, gray; ASIC3 KO, black). C: differential interference contrast (DIC) and epifluorescence micrographies captured from cryosections of a paraformaldehyde-fixed DRG [lumbar 5 (L5) and L6] harvested from a mouse injected in the bladder wall with DiI. Note that DiI-labeled cells are present in L6, but not in L5. Representative of 4 independent experiments. D: bright-field and epifluorescence micrographies of isolated sensory neurons harvested from mice injected with DiI in the bladder wall.
Analysis of gene expression by RT-PCR.
RNA from spinal cord, dorsal root ganglia (DRG), urinary bladder, and liver were isolated with the RNAqueous-4PCR kit (Thermo Fisher) according to the manufacturer instructions. Reverse transcription was performed with AccuScript PfuUltra II RT-PCR kit (Agilent) using random primers and following the manufacturer instructions. The following primers were used for cDNA amplification by PCR: ASIC3 forward, CAGCTCTGGACGCTATGCT; ASIC3 reverse, AACATGTGTTCGATGCCCATT; β-actin forward, GCCTTCCTTCTTGGGTATGGAA; and β-actin reverse, CAGCTCAGTAACAGTCCGCC. PCR reactions included 2 μl of sample, 10 μl of 2X iProof (Bio-Rad), 10 nmol of each primer in a total reaction mix volume of 20 μl. Cycling conditions were 95°C for 1 min, followed by 35 cycles of 94°C for 30 s, 58°C for 1 min and 72°C for 30 s, and an extension step of 72°C for 5 min. PCR reactions were run in 2% agarose gels with GelRed (1:10,000) (Biotium).
Tissue processing and image capture.
Briefly, DRG and bladders were fixed with 4% (vol/vol) paraformaldehyde in phosphate-buffered saline (PBS) buffer for 30 min at 37°C. Fixed tissue was incubated in 30% (wt/vol) sucrose dissolved in PBS at 4°C until it lost its buoyancy and sank to the bottom of the tube. Tissue was embedded in a 50/50 mixture of O.C.T. and sucrose (30% wt/vol in PBS), before storage at −80°C. Frozen tissue blocks were sectioned with a CM1950 cryostat (Leica). Bladder and DRG sections were processed for immunofluorescence with primary antibodies against ASIC3 as previously described (44). Images from fixed DRG were captured with a Leica DM6000B upright microscope (fitted with a 40X HCX PL-APO, 1.25 NA objective) equipped with a QImaging Retiga 4000R color digital camera interfaced with an Apple iMac computer running Volocity Acquisition software (version 6.3). Live images were captured with a Nikon inverted microscope model Ti (Nikon) equipped with a digital camera model C11440-10C (Hamamatsu) and optical filter exchanger (Sutter Instrument). The captured images were assembled in Adobe Illustrator.
Retrograde labeling of bladder sensory neurons.
Bladder afferent neurons were labeled with the fluorescent dye DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate, Invitrogen) as reported previously (44). Briefly, mice were anesthetized with isoflurane, and the bladder was exposed through an abdominal incision (∼1 cm in length). DiI (5% wt/vol in DMSO) was injected at three to four sites (total volume, 15–20 μl) in the bladder wall with a syringe. At each injection site, the needle was kept in place for 20–30 s after inoculation. Any visible leakage of dye was removed by application of a cotton swab and rinsed with saline. The muscle layer and skin incision were individually closed with 5.0 PDO absorbable monofilament surgical suture (AD Surgical). Postoperative analgesia was provided by subcutaneous administration of ketoprofen (5 mg/kg) (Zoetis). Ampicillin (10 mg/kg) (Boehringer Ingelheim Vetmedica) was administrated to prevent infections. Mice were housed under the conditions described above between 7 and 10 days before any further procedure was performed.
Isolation of bladder sensory neurons.
Lumbosacral (L6–S2) DRG collected from two to three mice were transferred to a cell culture dish containing DMEM media (Invitrogen). DRG were minced and agitated in a cell culture flask containing 5 ml of DMEM media supplemented with 10 mg of collagenase type 4 (Worthington Biochemical) and 5 mg of trypsin (Worthington Biochemical) for 30 min at 37°C. Tissue fragments were gently triturated with a fire-polished glass pipette and the cell suspension was centrifuged at 420 g for 5 min. The pellet, containing DRG somas, was resuspended in DMEM media supplemented with 10% FBS, 100 U/ml of penicillin and 100 μg/ml of streptomycin (Invitrogen). The centrifugation and resuspension steps were repeated three times. The pellet from the last centrifugation was resuspended in 1.5 ml of neurobasal media [Neuro-A medium supplemented with 5% of B27 supplements, 0.5 mM l-glutamine, 100 U/ml of penicillin, 100 μg/ml of streptomycin and 100 ng/ml nerve growth factor (Invitrogen)]. The suspension was plated on coverslips coated with ornithine (Sigma) and laminin (Invitrogen) inside a six-well tissue culture plate. After an incubation of 2 h at 37°C with 5% CO2, 3 ml of warm neurobasal media was added to each well and the tissue culture plate was returned to the incubator. Electrophysiological studies were performed within 2–4 days after plating.
Patch-clamp studies.
Whole cell patch-clamp recordings from isolated bladder sensory neurons were obtained with the perforated patch technique using Amphotericin B. Voltage-clamp and current-clamp recordings were performed at room temperature with an Axopatch 200B patch-clamp amplifier (Molecular Devices), and data were captured with a Digidata 1440A acquisition system and pClamp 10 (Molecular Devices). Signals were low-pass filtered at 1 kHz (4-pole Bessel filter) and digitized at 5 kHz. Micropipettes were pulled from borosilicate glass capillary tubes (Warner Instruments) with a PP-81 puller (Narishige). Fire-polished micropipettes with a tip resistance of 1.5–3 mΩ were used for patch-clamp recordings. The pipette filling solution contained (in mM) 145 KCl, 1 MgCl2, 0.1 CaCl2, 1 EGTA, and 10 HEPES (pH 7.2). Amphotericin B was added to the pipette solution to a final concentration of 120 μg/ml. The extracellular bath solution contained (in mM) 135 NaCl, 5 KCl, 1 MgCl2, 2.5 CaCl2, 10 glucose and 10 HEPES pH 8. Acidic test solutions of pH 6.0 were buffered with MES. After establishing whole cell configuration in voltage-clamp mode, the membrane potential was clamped at −60 mV and the cell capacitance was obtained by reading the value for input capacitance neutralization directly from the amplifier. A gravity-feed perfusion system (Automate Scientific) and a perfusion pencil positioned near the cell were used for rapid fluid delivery.
Patch-clamp data analysis.
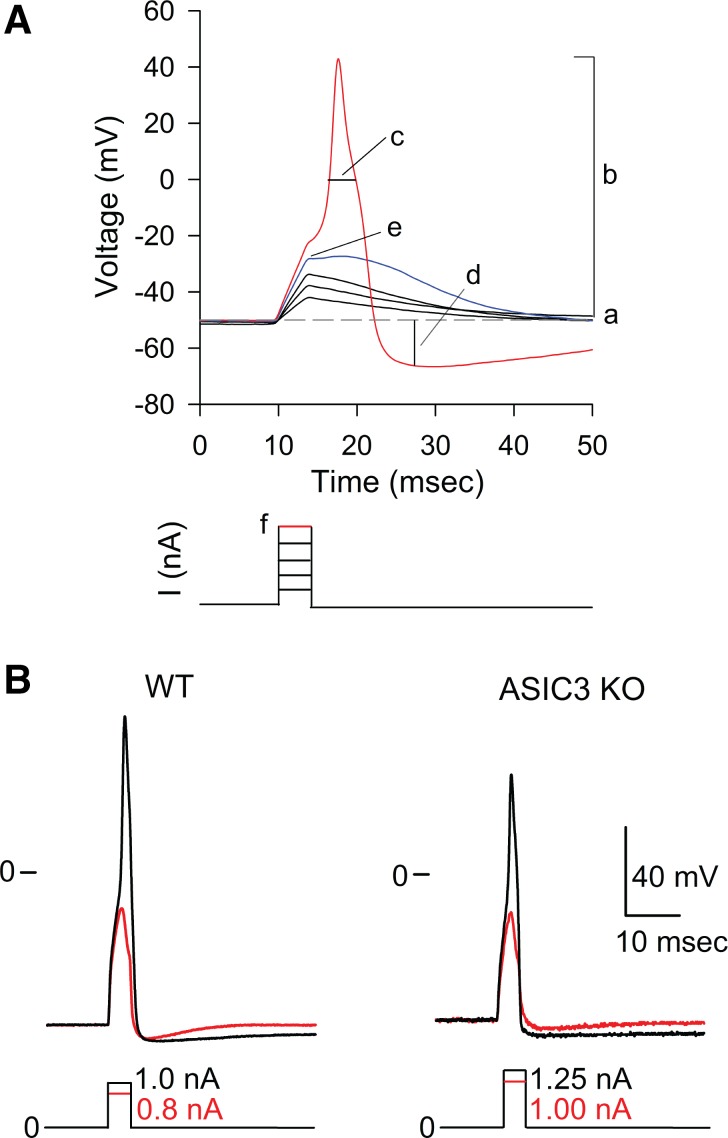
Patch-clamp experiments were analyzed with Clampfit (Molecular Devices). Time constant of current decay (desensitization) was estimated by fitting experimental data to a single-exponential function. All the neurons studied showed a resting membrane potential more negative than −40 mV. In some experiments, action potentials were evoked by injecting a series of 4-ms rectangular depolarizing current pulses of increasing intensity. The following passive and active membrane properties were measured for bladder sensory neurons: resting membrane potential, action potential amplitude, action potential duration at 0 mV, magnitude of hyperpolarization below the resting membrane potential, action potential threshold, and rheobase (see Fig. 3). The action potential rheobase and threshold are defined as the minimum depolarizing current injection necessary to evoke an action potential and the maximum membrane potential depolarization observed in the absence of an action potential, respectively.
Fig. 3.
Absence of acid-sensing ion channel 3 (ASIC3) does not alter the passive or active membrane properties of bladder sensory neurons. A: analysis of passive and active membrane properties of bladder sensory neurons. Representative voltage recordings obtained in the current-clamp configuration with the perforated patch-clamp technique from a DiI-labeled bladder sensory neuron. Action potentials were evoked by 4-ms depolarizing current pulses through the recording electrode. The current injection protocol is shown beneath the voltage traces. Letters refer to the passive and active membrane properties examined: a, resting membrane potential; b, action potential amplitude; c, action potential duration at 0 mV; d, magnitude of after hyperpolarization below resting membrane potential (AHP) (in mV); e, action potential threshold, which is defined as the greatest membrane potential (mV) achieved in response to a current pulse that does not trigger an action potential; f, rheobase, which is defined as the smallest amount of depolarizing current (nA) required to trigger an action potential. B: absence of ASIC3 from bladder sensory neurons does not alter action potential properties. The response of bladder sensory neurons to extracellular acidification was examined in the voltage-clamp configuration of patch clamp. Only neurons that displayed ASIC-like currents were further studied in the current-clamp configuration. To define action potential properties, a series of 4-ms rectangular depolarizing current pulses of increasing intensity were injected until an action potential was evoked. Representative action potentials evoked in response to electrical stimulation in bladder sensory neurons from WT and ASIC3 KO mice are shown. Current-pulse protocols are shown at the bottom of the tracings.
Void spot assay.
Mice were placed in a cage with chromatography paper (21 cm × 17 cm) adhered to the bottom for 1 h with free access to water and without food. Afterwards, animals were returned to their home cage and papers were placed in individual plastic bags for further analysis. Void spot assays were performed at the same time of the circadian day for all groups (5:30 PM). In a few instances, mice did not void during the assay, which lasted an hour. In that case, the filters were omitted from the analysis, and the assay was repeated. Images of void spot assay were collected in a Gel Doc XR+ Imaging system (Bio-Rad) and saved as a TIFF file. Images were analyzed in ImageJ Fiji (49, 50). To measure the spot area, the lower and upper threshold values were set to visualize the void spots and then the area for each individual spot was calculated with the analyze particles command.
Bladder weight.
Urinary bladders were harvested through an abdominal incision, carefully dried with a Kimwipe paper to eliminate the urine, and weighed to obtain the wet mass.
Assessment of bladder function by continuous cystometry.
Female mice were anesthetized with urethane (subcutaneous 1.2 g/kg), and the bladders were exposed through an abdominal incision. Upon being exteriorized, shallow purse-string sutures were made around the dome of the bladder using a 6-0 silk suture. The area within the boundary of the sutures was punctured with an 18-gauge needle, and a flame-flanged PE-50 tube was inserted into the hole. The suture was tightened around the tubing and the tube was gently retracted until the flange was flush with the mucosa. The bladder was returned to the peritoneal cavity, and the surgical incision was closed around the PE-50 tubing in two layers using 6-0 silk suture. The PE-50 tubing was connected to a three-way port: one branch led to a pressure transducer (ADInstruments), while another was connected to a syringe pump (Harvard Apparatus) for continuous infusion with saline solution. The pressure transducer was connected to a Quad Bridge Amplifier and Powerlab 4/30 (ADInstruments), which was interfaced to a computer running LabChart 8.0 (ADInstruments). Saline solution was infused into the bladder at a rate of 20 μl/min. Data for successive bladder voiding cycles were collected and analyzed as previously described (44). For each animal the following parameters were estimated: 1) basal pressure, the lowest pressure recorded after a void; 2) threshold pressure, the pressure recorded just before voiding was triggered; 3) peak pressure, the maximum pressure recorded during voiding; 4) trigger pressure, the difference between the threshold and basal pressure; 5) intercontraction interval (ICI), the time between two consecutive voids; and 6) voiding volume. The voiding volume was estimated from three collections. The values of basal pressure, threshold pressure, peak pressure, and ICI for each animal represent the average from at least six data points.
Statistical analysis.
Data are expressed as means ± SE (n), where n equals the number of independent experiments analyzed. Parametric or nonparametric tests were employed as appropriate. P < 0.05 was considered statistically significant. Fitting and statistical comparisons were performed with Clampfit (Molecular Device, Sunnyvale, CA), Sigmaplot 12.5 (Systat Software, Chicago, IL), and GraphPad 7 (GraphPad Software, San Diego, CA).
RESULTS
ASIC3 is expressed in sensory neurons.
ASIC3 is primarily expressed in a population of nociceptors, and probably some non-nociceptors, in dorsal root ganglia (DRG) (13, 43). Previous studies showed that the expression of ASIC3 is negligible in the bladder mucosa and detrusor muscle of control animals (15, 39). However, in the face of chemical cystitis induced by cyclophosphamide, the expression of this subunit is significantly upregulated in the urothelium (15). To determine whether ASIC3 is expressed in the urinary bladder and sensory neurons, we isolated RNA from urinary bladder, DRG, spinal cord, and liver of WT mice and performed RT-PCR. cDNA coding for murine ASIC3 served as a positive control in these experiments. As shown in Fig. 1A, message for ASIC3 was detected in DRG, but not in the urinary bladder, liver, or spinal cord. Because proteins are synthesized in the soma of sensory neurons, mRNA is expected to be found in the DRG, but not in the innervated organ. Thus RT-PCR analysis indicates ASIC3 is primarily expressed in sensory neurons. In addition, we examined ASIC3 expression in urinary bladders and DRG harvested from WT and ASIC3 KO mice by immunofluorescence. Using our protocols, the antibodies against ASIC3 appeared to lack specificity. We were not able to detect differences between WT and ASIC3 KO mice (data not shown).
ASICs are assembled as heteromers in bladder sensory neurons.
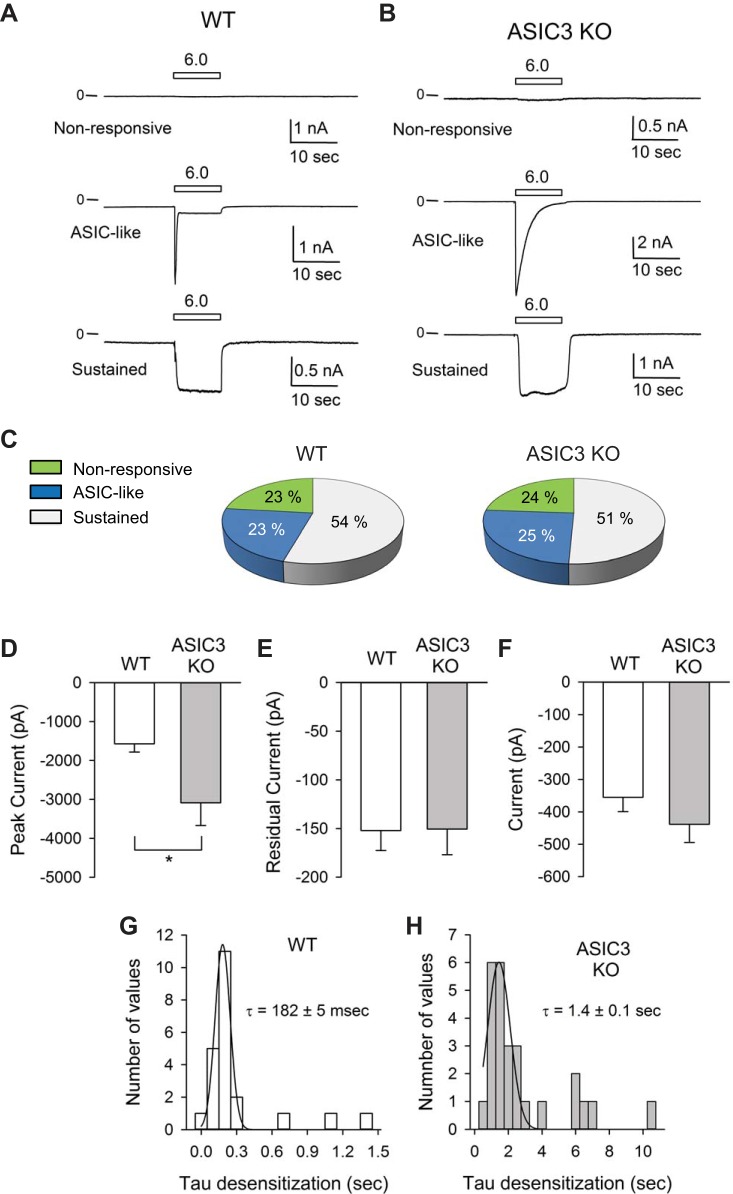
To examine the role of ASIC3 in bladder afferent signaling, we used retrograde tracing to label bladder sensory neurons and the patch-clamp technique to evaluate their response to extracellular acidification. To label bladder sensory neurons, DiI was injected in the bladder wall as previously described (44). The dye, which is transported in a retrograde fashion, reaches somas in the lumbosacral (LS) DRG 7–10 days after injection. Images of a lumbar 5 (L5) (negative control) and a lumbar 6 (L6) DRG harvested from a mouse injected with DiI in the bladder wall are shown in Fig. 1C. As expected, no labeled cells were observed in cryosections from L5. DiI-labeled cells were primarily found in L6 and S1. Representative bright-field and epifluorescence micrographies of isolated sensory neurons harvested from mice injected with DiI in the bladder wall are shown in Fig. 1D. To assess ASIC function in sensory neurons, proton-activated currents were measured with the perforated patch-clamp technique in the voltage-clamp configuration 2–4 days after plating. The amphotericin B-perforated patch-clamp technique has the advantage of maintaining the cytosolic composition without altering endogenous levels of Ca2+ and signaling molecules. For these experiments, a perfusion pencil was positioned near the cell being studied. To apply a pH pulse, a solution of pH 6.0 was delivered using a software-controlled gravity-fed perfusion system. Representative recordings of currents evoked in response to extracellular acidification in bladder sensory neurons isolated from WT and ASIC3 KO mice are shown in Fig. 2, A and B. Based on the response to extracellular acidification, sensory neurons were classified as nonresponsive or responsive with a transient or sustained response. Neurons with a transient response (ASIC-like) exhibited a rapid increase in current in response to extracellular acidification, which was followed by a rapid current decay (desensitization). Neurons with a sustained response responded to extracellular acidification with an increase in current that did not decay over time. A significant proportion (23–24%) of the bladder sensory neurons from both WT and ASIC3 KO mice did not respond to a drop in extracellular pH (Fig. 2C). The proportion of sensory neurons that responded to extracellular acidification with ASIC-like and sustained currents was similar in control and ASIC3 KO mice (Fig. 2C). Of major significance, the peak current evoked by extracellular acidification in the subset of neurons with ASIC-like response was significantly larger in ASIC3 KO than WT mice (Fig. 2D). No difference in the residual current (after the transient response) was observed between neurons with ASIC-like currents from WT and ASIC3 KO mice. The time constant of desensitization (τ) of the ASIC-like current was significantly faster in sensory neurons from WT than ASIC3 KO mice (Fig. 2, G and H) (n = 22–26, P < 0.0001). Taken together, these findings suggest that ASICs are assembled as heteromers in bladder sensory neurons and that the absence of ASIC3 alters the composition of these channels. Of note, the time constant of decay of the ASIC-like current in neurons harvested from ASIC3 KO mice is consistent with the τ of desensitization of ASIC1a (9, 23, 40, 52). No significant changes in the magnitude of the sustained current evoked by pH were noticed between neurons from WT and ASIC3 KO mice (Fig. 2F). This last finding indicates that ASIC3 is not part of the ion channel that mediates the sustained response to extracellular acidification.
Fig. 2.
Proton-evoked currents in bladder sensory neurons. Dorsal root ganglia (DRG) were harvested from mice 7–10 days after injection of DiI in the bladder wall. Sensory neurons were isolated and cultured as indicated in materials and methods. Whole cell currents were evoked by a change in extracellular pH from 8.0 to 6.0. A and B: representative tracings showing the 3 distinctive responses to extracellular acidification seen in bladder sensory neurons from wild-type (WT) (A) and acid-sensing ion channel 3 (ASIC3) knockout (KO) (B) mice. Sensory neurons were classified on the basis of their response to extracellular acidification as nonresponsive, responsive with a transient increase in current (ASIC-like), and responsive with sustained current increase. C: percentage of nonresponsive sensory neurons, responsive with ASIC-like currents or with a sustained response to extracellular acidification. Data were collected from 125–134 sensory neurons from WT and ASIC3 KO mice. D: peak current evoked by extracellular acidification in bladder sensory neurons with ASIC-like currents from WT and ASIC3 KO mice (n = 22–26, Mann-Whitney test, *P < 0.05). E: steady-state (residual) current at pH 6.0 for bladder sensory neurons with ASIC-like response from WT and ASIC3 KO mice (n = 22–26). F: proton-evoked current in bladder sensory neurons with sustained response to extracellular acidification (n = 46–54). G: distribution of τ of desensitization for neurons with ASIC-like currents from WT mice. Data were fitted to a Gaussian distribution (mean ± SE, n = 22). H: distribution of τ of desensitization for neurons with ASIC-like currents from ASIC3 KO mice. Data were fitted to a Gaussian distribution (mean ± SE, n = 26).
ASIC3 functions as a proton sensor in bladder sensory neurons.
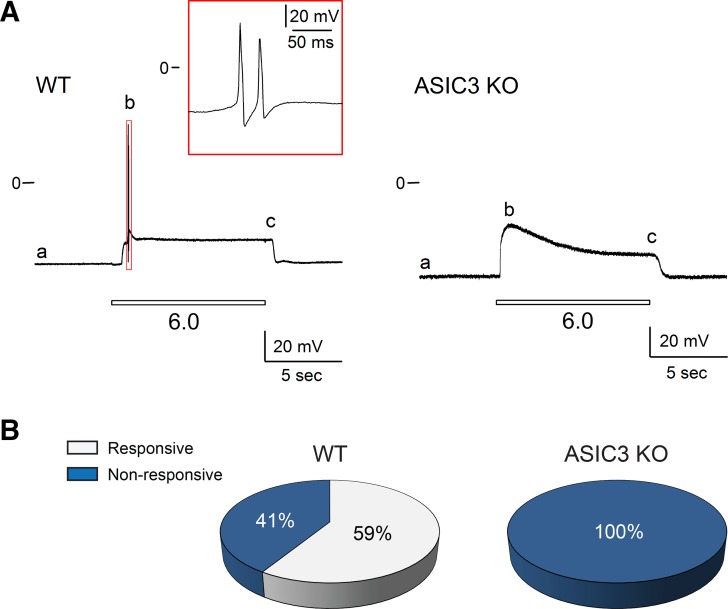
To determine whether the expression of ASIC3 alters the passive or active membrane properties of the bladder sensory neurons, we examined the response to electrical stimulation of neurons harvested from WT and ASIC3 KO mice. In these experiments only bladder sensory neurons that displayed ASIC-like currents were studied. Figure 3A shows a representative voltage trace obtained in the current-clamp mode with the perforated patch-clamp technique from a DiI-labeled bladder sensory neuron. Action potentials were evoked by 4-ms depolarizing current pulses through the recording electrode. Letters refer to the active and passive membrane properties examined (see legend for details). Figure 3B shows representative tracings of action potentials evoked in response to an electrical square pulse with a duration of 4 ms in neurons from WT and ASIC3 KO mice. No significant differences in the passive or active membrane properties were observed between sensory neuron with ASIC-like currents from WT and ASIC3 KO mice (Table 1). To determine whether extracellular acidification can trigger action potential firing, bladder sensory neurons with ASIC-like currents from WT and ASIC3 KO mice were exposed to a solution of pH 6.0 in the current-clamp mode. Of major significance, 59% of the bladder sensory neurons from WT mice, but none of those harvested from ASIC3 KO mice, fired action potentials in response to extracellular acidification (Fig. 4). Significantly, extracellular acidification depolarized the membrane potential of sensory neurons from ASIC3 KO mice to a similar value to the required to trigger an action potential [action potential threshold (AP threshold)] in sensory neurons from WT mice (Table 2). Likewise, sensory neurons with a sustained response to extracellular acidification from WT and ASIC3 KO mice did not fire action potentials in response to a drop in extracellular pH (n = 21–25, data not shown). Together, these findings suggest that ASIC3 provides proton sensitivity to a group of bladder sensory neurons.
Table 1.
Passive and active membrane properties of lumbosacral bladder sensory neurons with ASIC-like currents
| WT | ASIC3 KO | |
|---|---|---|
| Number of cells | 8 | 19 |
| Cm, pF | 34 ± 5 | 34 ± 3 |
| RMP, mV | −55 ± 3 | −59 ± 2 |
| Rheobase, nA | 1.3 ± 0.3 | 1.0 ± 0.2 |
| AP threshold, mV | −33 ± 2 | −31 ± 1 |
| AP duration, ms | 3.2 ± 0.4 | 2.9 ± 0.1 |
| Peak amplitude, mV | 111 ± 6 | 112 ± 6 |
| AHP mag, mV | −10 ± 4 | −8 ± 2 |
Values are means ± SE. Cm, membrane capacitance; RMP, resting membrane potential; AP, action potential; AHP mag, magnitude of after hyperpolarization below resting membrane potential.
Fig. 4.
Extracellular acidification triggers action potential firing in bladder sensory neurons from wild-type (WT) mice. Lumbosacral (L6–S2) dorsal root ganglia (DRG) were harvested from WT and acid-sensing ion channel 3 (ASIC3) knockout (KO) mice. The response of bladder sensory neurons to extracellular acidification was evaluated in the voltage-clamp configuration of patch clamp. Neurons that displayed ASIC-like currents were further studied in the current-clamp mode. A: representative voltage recordings illustrating the effect of extracellular acidification on bladder sensory neurons from WT and ASIC3 KO mice. Note that bladder sensory neurons from WT, but not ASIC3 KO mice, fire action potentials in response to extracellular acidification. Letters: a, resting membrane potential (RMP); b, amplitude of membrane potential change in response to a drop in extracellular pH to 6.0 (amplitude); c, sustained membrane potential at pH 6.0 (MP at pH 6.0) (see Table 2). B: percentage of bladder sensory neurons from WT and ASIC3 KO mice that fired action potentials in response to extracellular acidification (n = 17–28).
Table 2.
Effect of extracellular acidification on the membrane properties of bladder sensory neurons with ASIC-like currents
| WT |
ASIC3 KO | ||
|---|---|---|---|
| Responsive | Nonresponsive | Nonresponsive | |
| No. of cells | 10 | 7 | 24 |
| Cm, pF | 36 ± 4 | 35 ± 5 | 32 ± 3 |
| RMP, mV | −55 ± 3 | −63 ± 2 | −61 ± 2 |
| AP threshold, mV | −26 ± 3 | ||
| AP duration, ms | 3.2 ± 0.4 | ||
| Amplitude, mV | 103 ± 11 | 23 ± 2*** | 31 ± 2** |
| MP (at pH 6.0), mV | −43 ± 3 | −52 ± 2 | −39 ± 2† |
Values are means ± SE. Bladder sensory neurons with ASIC-like currents from wild-type (WT) and ASIC3 knockout (KO) mice were exposed to a solution of pH 6.0 in the current-clamp mode of patch clamp. Neurons that fired action potentials in response to extracellular acidification were classified as responsive. Cm, membrane capacitance; RMP, resting membrane potential; AP, action potential; Amplitude, difference between the resting membrane potential and maximal potential evoked in response to a drop in extracellular pH to 6.0; MP at pH 6.0, sustained membrane potential at pH 6.0 (see Fig. 5). Statistically significant differences compared with WT responsive:
P < 0.01,
P < 0.001.
Statistically significant difference compared with WT nonresponsive:
P < 0.01
(Kruskal-Wallis test following by Dunn’s multiple comparisons test).
ASIC3 regulates bladder function.
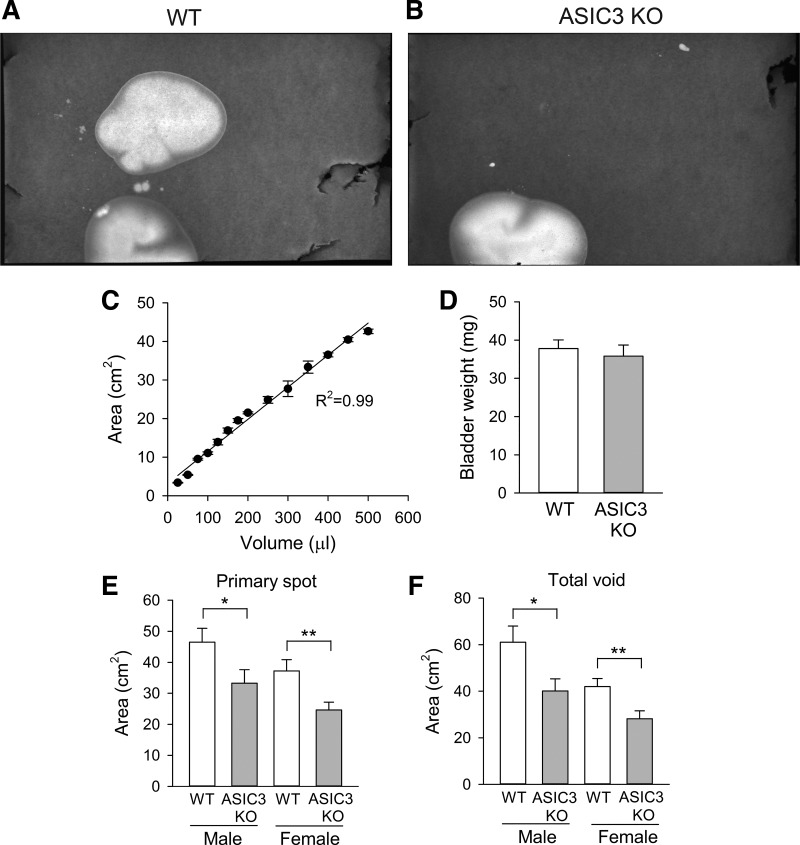
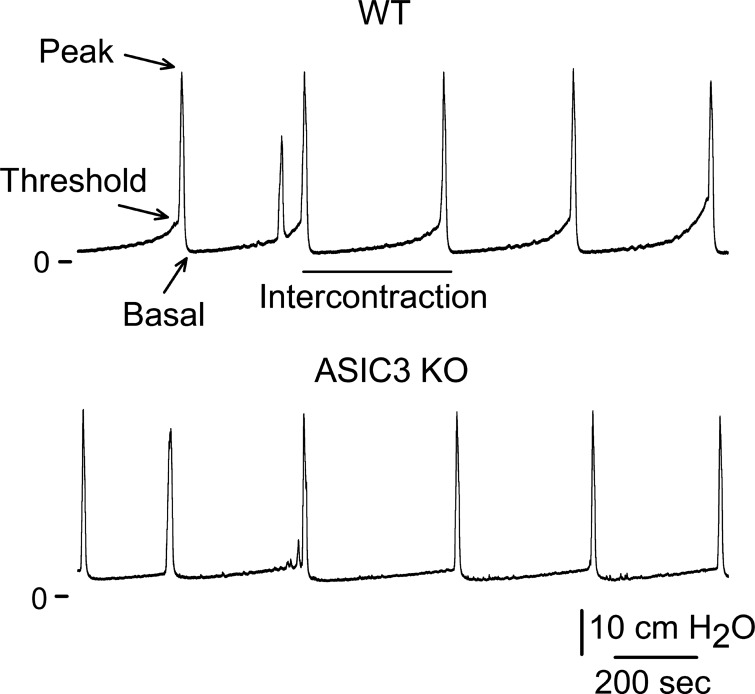
To assess the role of ASIC3 in bladder function, we performed a void spot assay in awake mice. Mice were housed in individual cages with a filter paper adhered to the bottom for 1 h. Representatives images of void spot assay are shown in Fig. 5, A and B. Void spot area show a linear correlation with urine volume (Fig. 5C). Significantly, primary spot area and total void area were significantly smaller in ASIC3 KO than WT mice (Fig. 5, E and F). Consistent with previous studies, this result might reflect either a change in bladder capacity or in the mechanism that senses bladder volume. To determine whether the deletion of ASIC3 alters bladder capacity, we harvested bladders from WT and ASIC3 KO mice and measured bladder weight. As shown in Fig. 5D, the weight of bladders harvested from WT and ASIC3 KO mice was similar (n = 5–6, P = 0.61). To assess whether the deletion of ASIC3 alters the mechanism that sense bladder filling, we performed continuous cystometry in WT and ASIC3 KO mice under urethane anesthesia. The cystometogram records the pressure response during continuous bladder filling, providing a functional measurement of urinary bladder function. During the filling phase, sympathetic postganglionic nerves activate β-adrenergic receptors that relax the detrusor muscle. Consequently, the bladder maintains a fairly constant pressure during filling, but once the bladder reaches capacity the nervous system triggers detrusor contraction, which is followed by relaxation of the urethral sphincter and micturition. Representative recordings of cystometrograms obtained from WT and ASIC3 KO mice are shown in Fig. 6. No significant changes in basal pressure, peak pressure, intercontraction interval, and voiding volume were noticed between WT and ASIC3 KO mice (Table 3). Significantly, the change in intravesical pressure during the filling phase (Δ threshold pressure − basal pressure) was significantly smaller in ASIC3 KO than WT mice. Although the threshold pressure was lower in ASIC3 KO than WT mice, the values did not reach statistical significance.
Fig. 5.
Void spot assay data for male and female wild-type (WT) and acid-sensing ion channel 3 (ASIC3) knockout (KO) mice. A and B: representative images of void spot assays from female WT (A) and ASIC3 KO mice (B). C: linearity of the spot area quantification. Urine aliquots were dropped in a filter paper and spot area was quantified as described in materials and methods (n = 3–4). D: bladder weight of WT and ASIC3 KO mice (n = 5–6, t-test, P = 0.61). E: primary void spot area for male and female WT and ASIC3 KO mice (n = 11–16 individual mice per group, *P < 0.05, **P < 0.01, t-test). F: total void area for male and female WT and ASIC3 KO mice (n = 11–16 individual mice per group, *P < 0.05, ** P < 0.01, t-test).
Fig. 6.
Acid-sensing ion channel 3 (ASIC3) deletion reduces the threshold pressure for voiding. Bladder function was evaluated by continuous cystometry in wild-type (WT) and ASIC3 knockout (KO) mice as describe in materials and methods. Representative tracings of cystometrograms performed in WT and ASIC3 KO mice are shown (n = 7–8).
Table 3.
Cystometry parameters for WT and ASIC3 KO mice
| WT | ASIC3 KO | |
|---|---|---|
| Basal pressure, cmH2O | 5.39 ± 1.21 | 5.31 ± 1.26 |
| Threshold pressure, cmH2O | 12.9 ± 1.96 | 9.30 ± 1.39 |
| Peak pressure, cmH2O | 42.7 ± 1.90 | 44.3 ± 1.72 |
| ΔThreshold − basal pressure, cmH2O | 7.48 ± 1.26 | 3.99 ± 0.63* |
| Intercontraction interval, s | 220 ± 36 | 226 ± 45 |
| Voiding volume, µl | 67 ± 13 | 70 ± 12 |
Values are means ± SE. A statistically significant difference between WT and ASIC3 KO mice:
P < 0.05 (n = 6–8) (t-test).
DISCUSSION
The goal of this study was to gain insight into the role of ASIC3 in bladder sensory signaling. Although a solid body of evidence suggests that ASIC3 plays important roles in mechanosensation and nociception, there is little understanding of how this channel is activated in vivo, and therefore, how changes in its activity contribute to these processes. We studied the response of bladder sensory neurons to extracellular acidification, a stimulus that activates ASICs in heterologous expression systems. Our studies defined three distinct groups of bladder sensory neurons based on the response to extracellular acidification: 1) a non-responsive group, 2) a group that showed a transient increase in current (ASIC-like) in response to acidification, and 3) a group that displayed a sustained current increase in response to extracellular acidification. Consistent with these findings, Dang and colleagues (18) previously reported that 78% of the lumbosacral bladder sensory neurons harvested from rats respond to a drop in extracellular pH of 5 with a current increase of mixed kinetics including fast, intermediate, slow, and sustained. Unexpectedly, the absence of ASIC3 did not alter the percentage of nonresponsive and responsive neurons with transient and sustained responses, but it changed the magnitude and decay of the current evoked in response to extracellular acidification in the group of neurons with ASIC-like currents. Consistent with previous studies (46), the peak current evoked in response to extracellular acidification was larger in neurons with transient response from ASIC3 KO than WT mice. The decay of the ASIC-like current in neurons harvested from WT mice resembles the desensitization of ASIC3 (9), whereas the slow desensitization of the ASIC-like current in neurons from ASIC3 KO mice is consistent with the desensitization of ASIC1a (9, 23, 40, 52). Although the time constant of decay of the ASIC-like current in neurons from ASIC3 KO mice is in the same order of magnitude of the desensitization of ASIC1a when studied in heterologous expression systems, we cannot rule out the possible contribution ASIC2 to the proton-sensitive component. For instance, Gautam and Benson (31) reported that ASIC-like currents were absent in mice lacking ASIC1a, ASIC2, and ASIC3, but not on those lacking individual or two subunits. Based on these results, the authors concluded that ASICs in muscle afferents are assembled as heteromers of ASIC1a, ASIC2, and ASIC3 subunits (31). Taking this into consideration, our studies suggest that in bladder sensory neurons of WT mice, ASICs are assembled as heteromers of ASIC1a-ASIC3, or ASIC1a-ASIC2-ASIC3 subunits.
Studies conducted with ASIC3 KO mice have provided conflictive results regarding the role of this channel in mechanosensation and nociception. ASIC3 KO mice showed reduced sensitivity to noxious skin pinch and attenuated response to acid and noxious heat, but increased sensitivity to light touch (46). Moreover, using an in vitro skin preparation, Moshourab and colleagues (45) showed that the absence of ASIC3 increases the mechanosensitivity of rapidly adapting mechanoreceptors and decreases the mechanosensitivity of both Aδ- and C-fiber nociceptors. ASIC3 KO mice also present deficits in neurosensory mechanotransduction of stomach, colon, and blood volume control (28, 36, 41), but increased sensitivity to chemical-, heat-, and mechanically induced pain (14). Together, these findings suggest that ASIC3 is not per se a mechanosensor, but it plays an important role modulating sensory signaling. Since ASICs are believed to serve as proton sensors in vivo, we assessed whether extracellular acidification can evoke action potentials in bladder sensory neurons and whether ASIC3 is required for this. Significantly, our study shows that a significant proportion of bladder sensory neurons with ASIC-like currents from WT, but none from ASIC3 KO mice, fire action potentials in response to a drop in extracellular pH. This result was unexpected, particularly because ASIC-like currents were larger in neurons from ASIC3 KO than WT mice. We posit that a change in the ion selectivity of ASICs or the kinetics of activation/desensitization of the proton-evoked current in sensory neurons from ASIC3 KO accounts for the observed result. This finding has two important implications: first, it indicates ASIC3 is an essential part of a proton sensor in bladder afferents, and second, it supports the notion that protons might play a central role modulating sensory signaling in the urinary bladder. In this regard, protons have been recently considered to function as neurotransmitters in the lateral amygdala where they regulate synaptic plasticity (25). Compelling evidence suggests that the urothelium, the epithelial layer that covers the interior of the bladder, functions as a “sensory web” that transmits information about the filling state of the bladder to underlying sensory terminals using a finely tuned machinery of signaling molecules (e.g., ATP) (2, 3, 10). ATP is released from vesicular pools and through membrane-associated channels (8, 54) and is rapidly hydrolyzed in the bladder interstitium (58, 59). We posit that the hydrolysis of ATP released from the urothelium could produce enough protons to transiently drop extracellular pH in the vicinity of afferent terminals and active ASICs.
To define the contribution of ASIC3 to bladder function, we performed void spot assays and continuous cystometry in WT and ASIC3 KO mice. Consistent with the notion that ASIC3 is a central part of the sensory system that controls bladder function, voiding volume was significantly smaller in ASIC3 KO than WT mice. Furthermore, the Δ threshold − basal pressure, a measurement of the pressure required to trigger voiding, was also significantly smaller in ASIC3 KO than WT mice. Because ASIC3 deletion reduced voiding volume and the pressure threshold to trigger micturition, we conclude that ASIC3 does not work in bladder afferents as a mechanosensor. Our findings are consistent with ASIC3 playing a modulatory role in bladder sensory signaling. Peak pressure was comparable in WT and ASIC3 KO mice, indicating that deletion of ASIC3 does not affect detrusor muscle function. Unexpectedly, ICI and voiding volume during continuous cystometry were similar in ASIC3 KO mice and WT mice. We posit that the infusion rate during continuous cystometry and/or the anesthesia might account for the differences observed in functional bladder assays in awake and anesthetized mice. In this regard, previous studies have shown that the results of void spot assay and cystometry are not necessarily comparable (12). Although urethane anesthesia is commonly used to study micturition in rodents, a detailed comparison of cystometry parameters between awake and anesthetized rodents has not been performed.
In conclusion, the present study revealed that ASIC3 plays a role in the control of bladder function by tuning sensory signaling during filling. Since aberrant afferent signaling is considered to play an important role in symptom generation in a number of bladder conditions including interstitial cystitis (16, 21, 48), chemical cystitis (17, 19, 57), and chronic bacterial cystitis (34), understanding the mechanisms by which bladder afferents sense storage and trigger micturition has important implications for treatment of these conditions. Further studies are necessary to identify the proteins involved in neurosensory mechanotransduction in the bladder and to define how the molecular components function together to control bladder function.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-099196 and R01-DK-084060 and by the Cellular Physiology and Kidney Imaging Cores of the Pittsburgh Center for Kidney Research (P30-DK-079307).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.M., J.G.R., A.L.M., and M.D.C. performed experiments; N.M., J.G.R., and M.D.C. analyzed data; N.M., J.G.R., and M.D.C. interpreted results of experiments; N.M., J.G.R., and M.D.C. prepared figures; N.M., J.G.R., and M.D.C. edited and revised manuscript; N.M., J.G.R., and M.D.C. approved final version of manuscript; M.D.C. conceived and designed research; M.D.C. drafted manuscript.
REFERENCES
- 1.Akopian AN, Chen CC, Ding Y, Cesare P, Wood JN. A new member of the acid-sensing ion channel family. Neuroreport 11: 2217–2222, 2000. doi: 10.1097/00001756-200007140-00031. [DOI] [PubMed] [Google Scholar]
- 2.Apodaca G. The uroepithelium: not just a passive barrier. Traffic 5: 117–128, 2004. doi: 10.1046/j.1600-0854.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- 3.Apodaca G, Balestreire E, Birder LA. The uroepithelial-associated sensory web. Kidney Int 72: 1057–1064, 2007. doi: 10.1038/sj.ki.5002439. [DOI] [PubMed] [Google Scholar]
- 4.Babinski K, Lê KT, Séguéla P. Molecular cloning and regional distribution of a human proton receptor subunit with biphasic functional properties. J Neurochem 72: 51–57, 1999. doi: 10.1046/j.1471-4159.1999.0720051.x. [DOI] [PubMed] [Google Scholar]
- 5.Bahns E, Ernsberger U, Jänig W, Nelke A. Functional characteristics of lumbar visceral afferent fibres from the urinary bladder and the urethra in the cat. Pflugers Arch 407: 510–518, 1986. doi: 10.1007/BF00657509. [DOI] [PubMed] [Google Scholar]
- 6.Bahns E, Halsband U, Jänig W. Responses of sacral visceral afferents from the lower urinary tract, colon and anus to mechanical stimulation. Pflugers Arch 410: 296–303, 1987. doi: 10.1007/BF00580280. [DOI] [PubMed] [Google Scholar]
- 7.Bässler EL, Ngo-Anh TJ, Geisler HS, Ruppersberg JP, Gründer S. Molecular and functional characterization of acid-sensing ion channel (ASIC) 1b. J Biol Chem 276: 33782–33787, 2001. doi: 10.1074/jbc.M104030200. [DOI] [PubMed] [Google Scholar]
- 8.Beckel JM, Daugherty SL, Tyagi P, Wolf-Johnston AS, Birder LA, Mitchell CH, de Groat WC. Pannexin 1 channels mediate the release of ATP into the lumen of the rat urinary bladder. J Physiol 593: 1857–1871, 2015. doi: 10.1113/jphysiol.2014.283119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci USA 99: 2338–2343, 2002. doi: 10.1073/pnas.032678399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birder L, Andersson KE. Urothelial signaling. Physiol Rev 93: 653–680, 2013. doi: 10.1152/physrev.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birder L, de Groat W, Mills I, Morrison J, Thor K, Drake M. Neural control of the lower urinary tract: peripheral and spinal mechanisms. Neurourol Urodyn 29: 128–139, 2010. doi: 10.1002/nau.20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjorling DE, Wang Z, Vezina CM, Ricke WA, Keil KP, Yu W, Guo L, Zeidel ML, Hill WG. Evaluation of voiding assays in mice: impact of genetic strains and sex. Am J Physiol Renal Physiol 308: F1369–F1378, 2015. doi: 10.1152/ajprenal.00072.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen CC, England S, Akopian AN, Wood JN. A sensory neuron-specific, proton-gated ion channel. Proc Natl Acad Sci USA 95: 10240–10245, 1998. doi: 10.1073/pnas.95.17.10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen CC, Zimmer A, Sun WH, Hall J, Brownstein MJ, Zimmer A. A role for ASIC3 in the modulation of high-intensity pain stimuli. Proc Natl Acad Sci USA 99: 8992–8997, 2002. doi: 10.1073/pnas.122245999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corrow K, Girard BM, Vizzard MA. Expression and response of acid-sensing ion channels in urinary bladder to cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol 298: F1130–F1139, 2010. doi: 10.1152/ajprenal.00618.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daly DM, Collins VM, Chapple CR, Grundy D. The afferent system and its role in lower urinary tract dysfunction. Curr Opin Urol 21: 268–274, 2011. doi: 10.1097/MOU.0b013e3283476ea2. [DOI] [PubMed] [Google Scholar]
- 17.Dang K, Bielefeldt K, Gebhart GF. Cyclophosphamide-induced cystitis reduces ASIC channel but enhances TRPV1 receptor function in rat bladder sensory neurons. J Neurophysiol 110: 408–417, 2013. doi: 10.1152/jn.00945.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dang K, Bielefeldt K, Gebhart GF. Differential responses of bladder lumbosacral and thoracolumbar dorsal root ganglion neurons to purinergic agonists, protons, and capsaicin. J Neurosci 25: 3973–3984, 2005. doi: 10.1523/JNEUROSCI.5239-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dang K, Lamb K, Cohen M, Bielefeldt K, Gebhart GF. Cyclophosphamide-induced bladder inflammation sensitizes and enhances P2X receptor function in rat bladder sensory neurons. J Neurophysiol 99: 49–59, 2008. doi: 10.1152/jn.00211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Groat WC, Wickens C. Organization of the neural switching circuitry underlying reflex micturition. Acta Physiol (Oxf) 207: 66–84, 2013. doi: 10.1111/apha.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Groat WC, Yoshimura N. Afferent nerve regulation of bladder function in health and disease. Handb Exp Pharmacol 194: 91–138, 2009. doi: 10.1007/978-3-540-79090-7_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Weille JR, Bassilana F, Lazdunski M, Waldmann R. Identification, functional expression and chromosomal localisation of a sustained human proton-gated cation channel. FEBS Lett 433: 257–260, 1998. doi: 10.1016/S0014-5793(98)00916-8. [DOI] [PubMed] [Google Scholar]
- 23.Della Vecchia MC, Rued AC, Carattino MD. Gating transitions in the palm domain of ASIC1a. J Biol Chem 288: 5487–5495, 2013. doi: 10.1074/jbc.M112.441964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dmitrieva N, McMahon SB. Sensitisation of visceral afferents by nerve growth factor in the adult rat. Pain 66: 87–97, 1996. doi: 10.1016/0304-3959(96)02993-4. [DOI] [PubMed] [Google Scholar]
- 25.Du J, Reznikov LR, Price MP, Zha XM, Lu Y, Moninger TO, Wemmie JA, Welsh MJ. Protons are a neurotransmitter that regulates synaptic plasticity in the lateral amygdala. Proc Natl Acad Sci USA 111: 8961–8966, 2014. doi: 10.1073/pnas.1407018111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Floyd K, Hick VE, Morrison JF. Mechanosensitive afferent units in the hypogastric nerve of the cat. J Physiol 259: 457–471, 1976. doi: 10.1113/jphysiol.1976.sp011476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci 9: 453–466, 2008. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fromy B, Lingueglia E, Sigaudo-Roussel D, Saumet JL, Lazdunski M. Asic3 is a neuronal mechanosensor for pressure-induced vasodilation that protects against pressure ulcers. Nat Med 18: 1205–1207, 2012. doi: 10.1038/nm.2844. [DOI] [PubMed] [Google Scholar]
- 29.Gabella G, Davis C. Distribution of afferent axons in the bladder of rats. J Neurocytol 27: 141–155, 1998. doi: 10.1023/A:1006903507321. [DOI] [PubMed] [Google Scholar]
- 30.García-Añoveros J, Derfler B, Neville-Golden J, Hyman BT, Corey DP. BNaC1 and BNaC2 constitute a new family of human neuronal sodium channels related to degenerins and epithelial sodium channels. Proc Natl Acad Sci USA 94: 1459–1464, 1997. doi: 10.1073/pnas.94.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gautam M, Benson CJ. Acid-sensing ion channels (ASICs) in mouse skeletal muscle afferents are heteromers composed of ASIC1a, ASIC2, and ASIC3 subunits. FASEB J 27: 793–802, 2013. doi: 10.1096/fj.12-220400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gründer S, Geissler HS, Bässler EL, Ruppersberg JP. A new member of acid-sensing ion channels from pituitary gland. Neuroreport 11: 1607–1611, 2000. doi: 10.1097/00001756-200006050-00003. [DOI] [PubMed] [Google Scholar]
- 33.Häbler HJ, Jänig W, Koltzenburg M. Myelinated primary afferents of the sacral spinal cord responding to slow filling and distension of the cat urinary bladder. J Physiol 463: 449–460, 1993. doi: 10.1113/jphysiol.1993.sp019604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hibbing ME, Conover MS, Hultgren SJ. The unexplored relationship between urinary tract infections and the autonomic nervous system. Auton Neurosci 200: 29–34, 2016. doi: 10.1016/j.autneu.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jänig W, Morrison JF. Functional properties of spinal visceral afferents supplying abdominal and pelvic organs, with special emphasis on visceral nociception. Prog Brain Res 67: 87–114, 1986. doi: 10.1016/S0079-6123(08)62758-2. [DOI] [PubMed] [Google Scholar]
- 36.Jones RC III, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J Neurosci 25: 10981–10989, 2005. doi: 10.1523/JNEUROSCI.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 82: 735–767, 2002. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- 38.Kellenberger S, Schild L. International Union of Basic and Clinical Pharmacology. XCI. structure, function, and pharmacology of acid-sensing ion channels and the epithelial Na+ channel. Pharmacol Rev 67: 1–35, 2015. doi: 10.1124/pr.114.009225. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi H, Yoshiyama M, Zakoji H, Takeda M, Araki I. Sex differences in the expression profile of acid-sensing ion channels in the mouse urinary bladder: a possible involvement in irritative bladder symptoms. BJU Int 104: 1746–1751, 2009. doi: 10.1111/j.1464-410X.2009.08658.x. [DOI] [PubMed] [Google Scholar]
- 40.Krauson AJ, Rued AC, Carattino MD. Independent contribution of extracellular proton binding sites to ASIC1a activation. J Biol Chem 288: 34375–34383, 2013. doi: 10.1074/jbc.M113.504324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee CH, Sun SH, Lin SH, Chen CC. Role of the acid-sensing ion channel 3 in blood volume control. Circ J 75: 874–883, 2011. doi: 10.1253/circj.CJ-10-0607. [DOI] [PubMed] [Google Scholar]
- 42.Lingueglia E, Champigny G, Lazdunski M, Barbry P. Cloning of the amiloride-sensitive FMRFamide peptide-gated sodium channel. Nature 378: 730–733, 1995. doi: 10.1038/378730a0. [DOI] [PubMed] [Google Scholar]
- 43.Molliver DC, Immke DC, Fierro L, Paré M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol Pain 1: 35, 2005. doi: 10.1186/1744-8069-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montalbetti N, Rued AC, Clayton DR, Ruiz WG, Bastacky SI, Prakasam HS, Eaton AF, Kullmann FA, Apodaca G, Carattino MD. Increased urothelial paracellular transport promotes cystitis. Am J Physiol Renal Physiol 309: F1070–F1081, 2015. doi: 10.1152/ajprenal.00200.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moshourab RA, Wetzel C, Martinez-Salgado C, Lewin GR. Stomatin-domain protein interactions with acid-sensing ion channels modulate nociceptor mechanosensitivity. J Physiol 591: 5555–5574, 2013. doi: 10.1113/jphysiol.2013.261180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR, Welsh MJ. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron 32: 1071–1083, 2001. doi: 10.1016/S0896-6273(01)00547-5. [DOI] [PubMed] [Google Scholar]
- 47.Price MP, Snyder PM, Welsh MJ. Cloning and expression of a novel human brain Na+ channel. J Biol Chem 271: 7879–7882, 1996. doi: 10.1074/jbc.271.14.7879. [DOI] [PubMed] [Google Scholar]
- 48.Rosamilia A, Igawa Y, Higashi S. Pathology of interstitial cystitis. Int J Urol 10, Suppl: S11–S15, 2003. doi: 10.1046/j.1442-2042.10.s1.4.x. [DOI] [PubMed] [Google Scholar]
- 49.Schindelin J, Rueden CT, Hiner MC, Eliceiri KW. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol Reprod Dev 82: 518–529, 2015. doi: 10.1002/mrd.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sengupta JN, Gebhart GF. Mechanosensitive properties of pelvic nerve afferent fibers innervating the urinary bladder of the rat. J Neurophysiol 72: 2420–2430, 1994. doi: 10.1152/jn.1994.72.5.2420. [DOI] [PubMed] [Google Scholar]
- 52.Tolino LA, Okumura S, Kashlan OB, Carattino MD. Insights into the mechanism of pore opening of acid-sensing ion channel 1a. J Biol Chem 286: 16297–16307, 2011. doi: 10.1074/jbc.M110.202366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waldmann R, Champigny G, Voilley N, Lauritzen I, Lazdunski M. The mammalian degenerin MDEG, an amiloride-sensitive cation channel activated by mutations causing neurodegeneration in Caenorhabditis elegans. J Biol Chem 271: 10433–10436, 1996. doi: 10.1074/jbc.271.18.10433. [DOI] [PubMed] [Google Scholar]
- 54.Wang EC, Lee JM, Ruiz WG, Balestreire EM, von Bodungen M, Barrick S, Cockayne DA, Birder LA, Apodaca G. ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. J Clin Invest 115: 2412–2422, 2005. doi: 10.1172/JCI24086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wen J, Morrison JF. The effects of high urinary potassium concentration on pelvic nerve mechanoreceptors and ‘silent’ afferents from the rat bladder. Adv Exp Med Biol 385: 237–239, 1995. doi: 10.1007/978-1-4899-1585-6_29. [DOI] [PubMed] [Google Scholar]
- 56.Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics 13: 134, 2012. doi: 10.1186/1471-2105-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci 19: 4644–4653, 1999. doi: 10.1523/JNEUROSCI.19-11-04644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu W. Polarized ATP distribution in urothelial mucosal and serosal space is differentially regulated by stretch and ectonucleotidases. Am J Physiol Renal Physiol 309: F864–F872, 2015. doi: 10.1152/ajprenal.00175.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu W, Robson SC, Hill WG. Expression and distribution of ectonucleotidases in mouse urinary bladder. PLoS One 6: e18704, 2011. doi: 10.1371/journal.pone.0018704. [DOI] [PMC free article] [PubMed] [Google Scholar]