Abstract
The synthesis of methionine is critical for most bacteria. It is known that cellular methionine has a feedback effect on the expression of met genes involved in de novo methionine biosynthesis. Previous studies revealed that Gram-negative bacteria control met gene expression at the transcriptional level by regulator proteins, while most Gram-positive bacteria regulate met genes at post-transcriptional level by RNA regulators (riboregulators) located in the 5′UTR of met genes. However, despite its importance, the methionine biosynthesis pathway in the Gram-negative Xanthomonas genus that includes many important plant pathogens is completely uncharacterized. Here, we address this issue using the crucifer black rot pathogen Xanthomonas campestris pv. campestris (Xcc), a model bacterium in microbe–plant interaction studies. The work identified an operon (met) involved in de novo methionine biosynthesis in Xcc. Disruption of the operon resulted in defective growth in methionine-limited media and in planta. Western blot analysis revealed that the expression of the operon is dependent on methionine levels. Further molecular analyses demonstrated that the 5′UTR, but not the promoter of the operon, is involved in feedback regulation on operon expression in response to methionine availability, providing an example of a Gram-negative bacterium utilizing a 5′UTR region to control the expression of the genes involved in methionine biosynthesis.
Keywords: Xanthomonas, methionine biosynthesis, 5’UTR, post-transcriptional regulation, virulence
Introduction
To maintain regular protein synthesis, bacterial cells require a constant supply of amino acids. Most achieve this by taking up amino acids from the surrounding environment and/or synthesizing amino acids from simpler compounds (i.e. de novo synthesis). The synthesis of amino acids is considered a complicated and biologically expensive process and is therefore strictly regulated. It is known that among the efficient mechanisms of regulation is feedback control at the transcriptional or post-transcriptional level [1]. Of the 20 standard amino acids which are needed for bacterial cell viability, the sulfur-containing methionine [(formyl-)methionine] is somewhat unique. This is because it is the first amino acid at the N-terminus of most proteins and plays an irreplaceable role in the initiation of protein biosynthesis. In addition, the methionine derivative S-adenosylmethionine (SAM) serves as a universal methyl group donor in a variety of methyltransferase reactions [2].
Most bacterial species, with the exception of some endosymbionts, are able to synthesize methionine using the trans-sulfuration pathway and/or the direct sulfhydrylation pathway from homoserine [3]. The trans-sulfuration pathway in Escherichia coli has been extensively characterized as having four steps: homoserine → O-succinyltransferase → cystathionine → homocysterine → methionine, which are catalysed by homoserine O-succinyltransferase (encoded by metA), cystathionine γ-synthase (encoded by metB), cystathionine β-lyase (encoded by metC) and methionine synthase (encoded by metH or metE), respectively [4] (Fig. 1). Furthermore, it is defined that the homoserine is derived from aspartate in three steps: aspartate → aspartyl phosphate → aspartate semialdehyde → homoserine, which are catalysed by bi-functional aspartate kinase/homoserine dehydrogenase (encoded by metL), aspartate-β-semialdehyde dehydrogenase (encoded by asd) and homoserine dehydrogenase (encoded by hom), respectively [5] (Fig. 1).
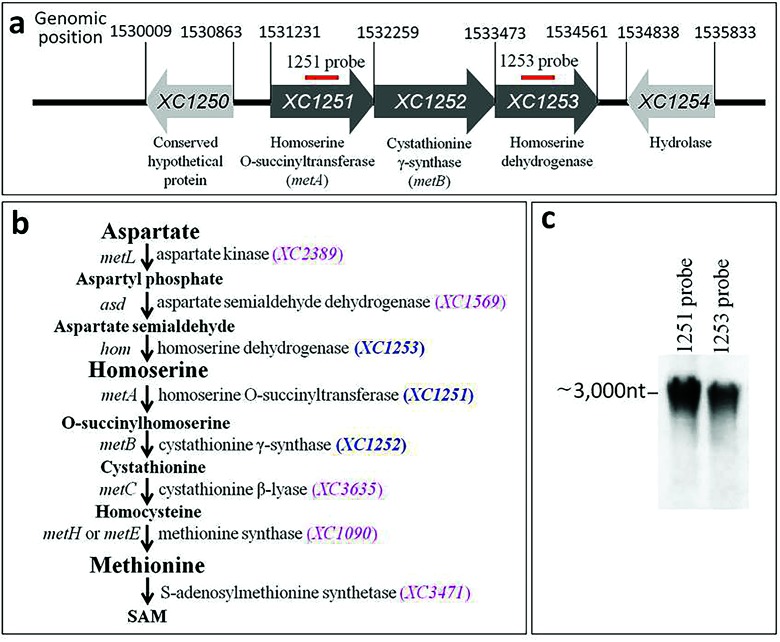
Fig. 1.
Genetic organization and identification of Xcc met operon. (a) A schematic diagram showing the genetic organization of the met operon (XC1251-XC1252-XC1253). The sequences matched to the probes used in the Northern blotting analysis are indicated by thick, red lines above the genes. (b) Predicted methionine biosynthesis pathway in Xcc. (c) Detection of transcripts of the met operon by Northern blotting.
The way in which methionine biosynthesis is regulated has also been characterized in certain Gram-negative and -positive bacteria. The general principle for this is similar to the biosynthesis of most other amino acids, where methionine biosynthesis is inactive when methionine is available in the surroundings and active when methionine is limited. The availability of cellular methionine has an effect on the expression of the genes involved in methionine biosynthesis (most of these were called met genes). However, the detailed molecular basis of met gene regulation differs among bacteria, most likely due to the environment in whichthey are found. In general, Gram-positive bacteria utilize RNA regulators (riboregulators) that function at the post-transcriptional level, for example the S-box and T-box riboswitches. Conversely, Gram-negative bacteria employ protein regulators that function at the transcriptional level [6–9].
Although extensive studies have been carried out on E. coli and some other Gram-negative bacteria [4, 10], little is known about methionine biosynthesis and its regulation in Gram-negative phytopathogenic bacteria [11–13]. Xantomonas campestris pv. campestris (Xcc) is a member of the large genus Xanthomonas, which comprises 27 species of Gram-negative bacteria, most of which are plant pathogens. As well as being an important plant pathogen, Xcc is considered a model pathogen for studying the molecular basis in microbe–plant interactions [14]. In the current study, we reconstructed the methionine biosynthesis pathway in an Xcc strain by integrating the genome information from Xcc and what is known regarding the E. coli methionine biosynthesis route. Using this information, we demonstrated that the synthesis of methionine is critical for Xcc to attain regular growth and full virulence in host plant tissues. Additionally, we identified the met operon consisting of three open reading frames (ORFs) that encode enzymes potentially involved in catalysing methionine biosynthesis. More importantly, we obtained direct evidence showing that the 5′UTR, but not the promoter of the met operon, is involved in feedback regulation of its expression in response to methionine availability. To the best of our knowledge, this provides the first example of a Gram-negative bacterium using 5′UTR to regulate methionine biosynthesis.
Methods
Bacterial strains, plasmids and growth conditions
The bacterial strains and plasmids used in this work are listed in Table S1 (available in the online version of this article). Xcc and E. coli strains were grown under conditions described previously [15].
Construction of the mutant strain 1201PK2
The mutant 1201PK2 was constructed by the homologous suicide plasmid integration method as described by Windgassen and associates [16]. A 392 bp internal fragment of XC1251 was amplified using the DNA of the Xcc wild-type strain 8004 as template and the primer pair 1251 M-F/1251 M-R (Table S2). The DNA fragment was cloned into the suicide plasmid pK18mob, creating pK1251, which was introduced into strain 8004 by conjugation. The mutant transconjugants obtained with an insertion in XC1251 were confirmed by PCR using the primer pair P18con-F/1251con-R (Table S2). One of the confirmed mutants, named 1201PK2, was used for further study.
Determination of the transcription start site (TSS) of the met operon (XC1251-XC1253) by 5′-RACE
Total RNA was extracted from the wild-type strain 8004 and treated with DNase I. Five micrograms of the DNA-free RNA and 15 pmol of the gene-specific primer 1251GSP1 (CAACCAGCGTGTGCAGAC) were incubated at 70 °C for 5 min and then 42 °C for 1 h in the presence of 1×M-MuLV-RT buffer, 1 mM dNTPs, 20 U RNase inhibitor and 200 U M-MuLV Reverse Transcriptase. After treatment with 2 U µl−1 RNase H for 30 min (to remove the remaining RNAs), the reaction product (cDNA) was purified using S.N.A.P.Column and finally resolved in 50 µl sterilized dH2O. Then, 10 µl purified cDNA was incubated at 37 °C for 10 min in the presence of 0.2 mM dCTP and 20 U of TDT (terminal deoxynucleotidyl transferase) to add a poly(C) tail to the 3′-end of the cDNA. Finally, 5 µl poly(C) tailed cDNA was used as template for PCR. To improve sensitivity, two rounds of semi-nested PCR were performed. The first of these was performed using 5 µl poly(C) tailed cDNA as template, and AAP (GGCCACGCGTCGACTAGTACGGGIIGGGIIGGGIIG) and an internal oligonucleotide, 1251GSP2 (CGCCGCATGTCGGCTGGCGAATTGC) as primers; the second was performed using the product from the first PCR as template, and AUAP (GGCCACGCGTCGACTAGTAC) and another internal oligonucleotide, 1251GSP3 (ATGCCGCCCGCCACGAACACCAC) as primers. After gel purification, the final PCR products were cloned into the pMD18-T vector and sequenced.
Construction of strain XC1251-3F expressing the recombinant protein XC1251-3FLAG
The strain XC1251-3F (Table S1) expressing a recombinant XC1251 protein (XC1251-3FLAG) with a 3×Flag tag at its C-terminus was constructed by in-frame insertion of the 3×Flag-coding sequence into the 3′-end (just before the stop codon TGA) of the XC1251 coding sequence in strain 8004, using the suicide vector-mediated unmarked allelic replacement method described by Patey et al. [17]. Briefly, a 781 bp DNA upstream flanking sequence of XC1251 and a 607 bp DNA fragment containing the 3×Flag-coding sequence, the stop codon TGA and downstream flanking sequence of XC1251 were generated by PCR amplification using the DNA of strain 8004 as template and the primer pairs 1251-3F-U-F/1251-3F-U-R and 1251–3F-D-F/1251–3F-D-R (Table S2), respectively. The DNA fragments thus obtained were linked by fusion PCR and cloned into the suicide plasmid pK18mobsacB, generating the recombinant plasmid pK1251-3F, which was then transferred into strain 8004 by conjugation. Single-crossover integration transconjugants were selected and confirmed by sucrose-sensitive phenotype. A confirmed single-crossover transconjugant was checked for the in-frame insertion of 3×Flag-coding sequence before the stop codon of XC1251 by sequencing analysis of the PCR products generated by amplification of its genomic DNA with the primer pair 1251-3F-U-F/1251-3F-D-R (Table S2). The expression of XC1251-3FLAG protein was further confirmed by Western blotting.
Construction of promoter-gusA and 5′UTR-gusA fusion reporters
Two promoter-gusA transcriptional fusion reporter plasmids, named pP1251L and pP1251S, were constructed by fusing 109 and 52 bp DNA sequences upstream of the TSS with the 1826 bp DNA segment of the promoterless but SD-containing gusA gene of E. coli, respectively, and cloning the 1935 and 1878 bp fused DNA fragments thus obtained into the promoterless cloning sites of the vector pLAFR6, respectively. The 1935 and 1878 bp fused DNA fragments were obtained by PCR amplification using E. coli K12 genomic DNA as template and the primer pairs P1251L-SDgusA-F/gusA-R and P1251S-SDgusA-F/gusA-R (Table S2), respectively.
The 5′UTR of XC1251-XC1253 operon-gusA transcriptional fusion reporter p5UTR-SD+ and translational fusion reporter p5UTR-SD- were constructed by cloning the Plac-5′UTR-SD+gusA and Plac-5′UTR-SD-gusA fragment into the vector pLAFR6, respectively. Plac-5′UTR-SD+gusA and Plac-5′UTR-SD-gusA fragments were generated by ligation of the 235 bp DNA fragment Plac-5′UTR with the 1847 bp DNA fragment SD+gusA and 1829 bp DNA fragment SD-gusA by fusion PCR, respectively. Plac-5′UTR was obtained by PCR amplification using the DNA of strain 8004 as template and the primer pair Plac-5′UTR-F/5′UTR-R (Table S2), and SD+gusA and SD-gusA were obtained by PCR amplification using the DNA of E. coli K12 as template and the primer pairs SD+-gusA-F/gusA-R and SD--gusA-F/gusA-R (Table S2), respectively.
Northern blotting and Western blotting
Xcc cells were cultured under specific growth conditions and collected at 24 h post-inoculation. Total RNA was isolated using the PureLink RNA Mini kit (Thermo Fisher Scientific, Shanghai, China), and 3–5 µg RNA was separated on 6 % denature (8 M urea) polyacrylamide gel and transferred to a positively charged nylon membrane (Roche Applied Science, Mannheim, Germany). After UV-crosslinking, the membrane was hybridized with a DIG-labelled RNA probe [prepared using a DIG RNA labelling kit (Roche Applied Science)] at 68 °C for 8 h. Signal bands were detected using the DIG-Northern Starter Kit (Roche Applied Science) and visualized with an ImageQuant LAS 500 imager (GE Healthcare, Beijing, China). Western blotting was performed as described previously [15].
GUS activity assay and plant test
GUS activity was determined by measurement of the OD415 using ρ-nitrophenyl-β-d-glucuronide as a substrate, as described previously [18]. The virulence of Xcc strains was tested on the leaves of Chinese radish (Raphanus sativus L. var. radiculus Pers.) using the leaf-clipping method [15].
Results
Xcc encodes a met (XC1251-XC1253) operon that is essential for growth in the absence of methionine
Here, using the genome annotation of the Xcc strain 8004 [19] and the methionine biosynthetic pathway of E. coli [4], we constructed an in silico methionine biosynthetic pathway for Xcc. As shown in Fig. 1, all of the genes encoding the enzymes that accomplish the trans-sulfuration pathway from homoserine to methionine and the process from aspartate to homoserine are present in the genome of Xcc strain 8004. However, the gene (named metY in E. coli) encoding O-acetylhomoserine sulfhydrylase, which is indispensable for the direct sulfhydrylation pathway, is absent in the genome. This in silico model implies that Xcc synthesizes methionine via the trans-sulfuration pathway but not via a direct sulfhydrylation pathway. Notably, the genes encoding the enzymes converting aspartate semialdehyde to cystathionine, i.e. XC1251 (metA), XC1252 (metB) and XC1253 (hom), are located together, while the genes encoding other enzymes involved in the biosynthesis of methionine from aspartate are scattered on the chromosome of Xcc strain 8004 (Fig. 1).
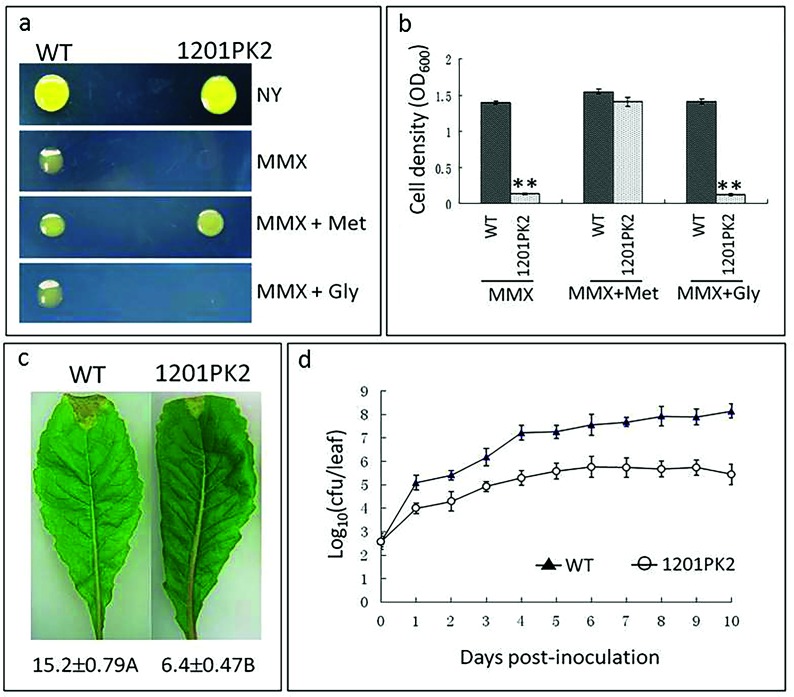
Bioinformatic analysis of the ORFs XC1251-XC1253 suggests that these genes are transcribed in the same direction and potentially co-transcribed as a single operon (Fig. 1). This was verified by Northern blotting analysis (Fig. 1). We named this the met (XC1251-XC1253) operon for simplification. To validate the involvement of the met operon in methionine biosynthesis, an insertion mutant strain named 1201PK2 (Table S1) was constructed. This mutant has an insertion in the first ORF (i.e. XC1251) of the operon, thus blocking the transcription of the whole operon. As illustrated in Fig. 2, the mutant strain grew well in the nutrient-rich medium (NYG) but was unable to grow in the nutrient-limited medium (MMX), while the wild-type strain 8004 grew well in both media. However, supplementing MMX with methionine restored the mutant’s ability to grow as well as the wild-type (Fig. 2). Interestingly, none of the other 19 standard amino acids, which were used as supplements under the same test conditions, could rescue the growth of the mutant in MMX. These results demonstrated that the mutant is methionine auxotroph and the met operon is essential for the growth of Xcc in the absence of methionine.
Fig. 2.
Inactivation of the met operon has an impact on Xcc virulence and growth in methionine-deficient medium and in planta. (a) and (b) Plate assay and quantitative liquid culture assay for detection of the growth of Xcc strains. NYG, nutrien- rich medium; MMX, minimal medium; MMX+Met or Gly, MMX supplemented with 1 mM of methionine or glycine. The photographs were taken at either 48 h (for NYG) or 72 h (for MMX and MMX+Met or Gly) post-incubation at 28 °C. The cell density was measured 72 h after incubation at 28 °C. (c) Black rot symptoms caused by Xcc strains on Chinese radish leaves. Images were taken at day 10 post-inoculation. Values under each leaf are the average lesion length (in mm) (mean±sd) from three repeats, each with 50 leaves. Different letters following the values indicate significant difference (t-test, P=0.01). (d) Growth rates of Xcc strains in inoculated leaves of Chinese radish. Bacterial cells were recovered from the inoculated leaves every day over a period of 10 days post-inoculation. Data are the mean±sd from a representative experiment, and similar results were obtained in two other independent experiments. WT, the wild-type strain 8004; 1201PK2, the met operon mutant strain.
The met operon is required for full virulence and in planta growth of Xcc
To investigate whether the met operon (and by inference methionine biosynthesis) is important for the pathogenicity of Xcc, we tested the virulence of the mutant strain 1201PK2 on the host plant Chinese radish by leaf-clipping assay [15]. The results showed that the disease symptoms caused by the mutant were significantly weaker compared to the wild-type. As shown in Fig. 2(c), at 10 days post-inoculation the wild-type produced disease symptoms with a mean lesion length of 15.2 mm, while the mean lesion length caused by the mutant was only 6.4 mm. This finding indicates that the met operon is required for full virulence of Xcc.
The mutant grew poorly in environments lacking methionine (Fig. 2a, b). For this reason, we further assessed the growth of the mutant in planta. A group of five inoculated leaves were homogenized and plated on NYG medium supplemented with appropriate antibiotics. Growth was recorded after incubation at 28 °C for 10 days. During the observation period, the number of mutant colonies recovered from the infected leaves was 10- to 100-fold lower than that of the wild-type (Fig. 2d). This finding demonstrates that the met operon (and by inference methionine biosynthesis) is important for Xcc to propagate regularly in host plants.
met operon expression is controlled in a methionine-dependent manner
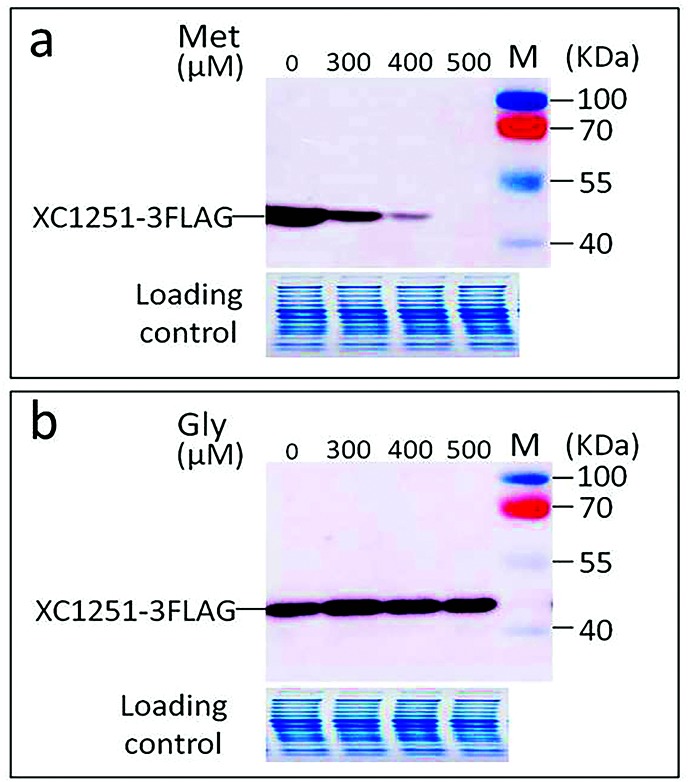
To gain an insight into how the expression of the met operon is controlled, a wild-type strain expressing a recombinant XC1251 protein (XC1251-3FLAG) was generated and named XC1251-3F (Table S1). This was achieved by adding 3×Flag tag to the C-terminus of XC1251 by in-frame fusing a DNA segment encoding 3×Flag tag to the 3′-end (just before the stop codon TGA) of XC1251, and introducing it into the genome of strain 8004 using a suicide vector-mediated unmarked allelic replacement method [17]. The expression level of the recombinant protein XC1251-3FLAG was assessed using Western blotting when the Xcc strain was grown in MMX with or without methionine. The result showed that XC1251-3FLAG protein expression was elevated in cells grown in MMX without methionine (Fig. 3a). However, the level of XC1251-3FLAG was reduced dramatically in the presence of methionine. No detectable XC1251-3FLAG protein was observed when the concentration of methionine was increased to 500 µM (Fig. 3a). Moreover, the addition of other standard amino acids (such as 500 µM glycine) did not effect XC1251-3FLAG expression (Fig. 3b), indicating that the repression of XC1251-3FLAG expression by methionine was specific. These results suggest that the met operon is expressed in a methionine-dependent manner, where activation and inactivation of the operon are dependent on the level of methionine in the surrounding environment.
Fig. 3.
Western blotting detection of the effect of methionine on the expression of XC1251-3FLAG protein. Strain XC1251-3F was grown in minimal medium MMX or MMX supplemented with methionine (Met) (a) or glycine (Gly) (b) to a final concentration of 300, 400 and 500 µM, respectively. Cells were collected 24 h post-inoculation and total proteins were isolated from the cells. The level of XC1251-3FLAG protein in the total protein samples was detected by Western blotting analysis using anti-Flag monoclonal antibody as probe.
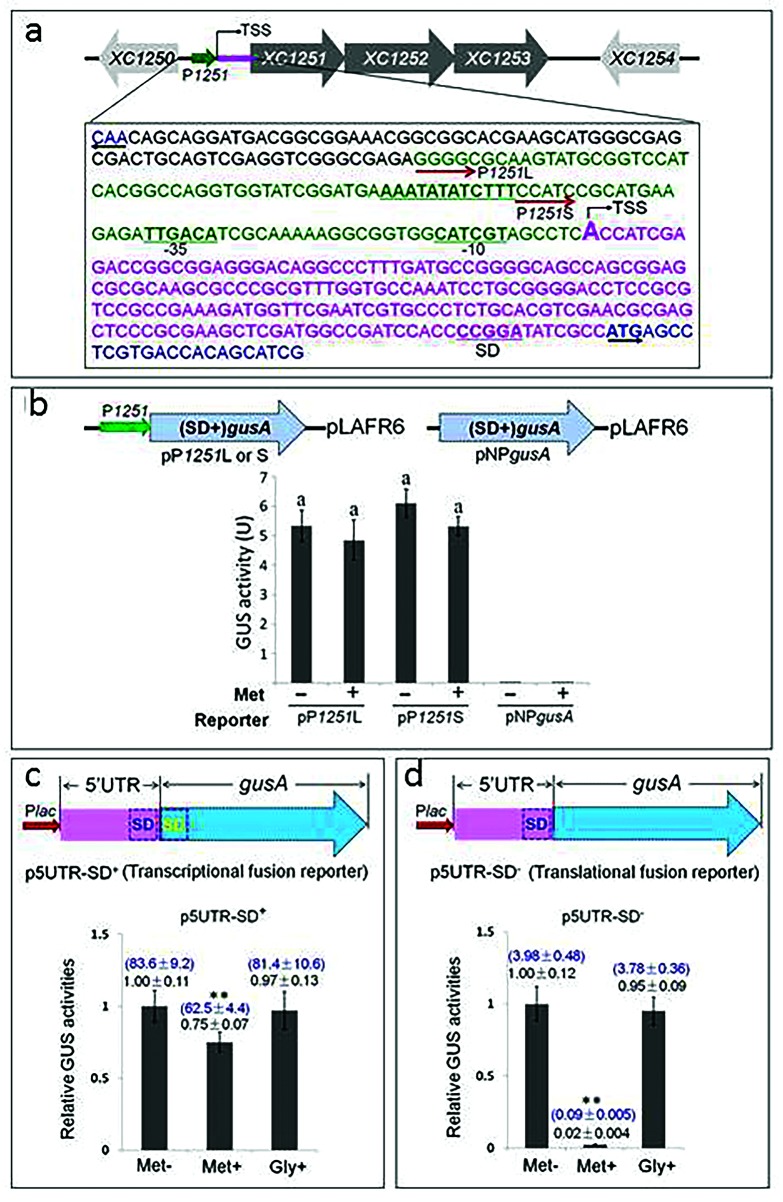
The 5′UTR, but not the promoter of the met operon, is involved in controlling expression in response to methionine availability
The above findings suggest that Xcc may regulate the expression of the met operon by a feedback inhibition manner in response to methionine. To investigate whether feedback inhibition by methionine plays a role in controlling the transcriptional level of the met operon, we first determined the transcriptional start site (TSS) of the operon by 5’RACE (rapid amplification of cDNA ends) (Fig. S1). This analysis predicted putative −10 and −35 elements of the core promoter of the operon based on the identified TSS (Fig. 4a). Based on this, two promoter-gusA transcriptional fusion reporter plasmids, named pP1251L and pP1251S, were constructed by fusing 109 and 52 bp DNA sequences upstream of the TSS with the promoterless gusA gene (which still retained its Shine–Dalgano sequence) and cloning into the vector pLAFR6 (Fig. 4b). Subsequently, these reporter plasmids were introduced into strain 8004, generating reporter strains named 8004/pP1251L and 8004/pP1251S (Table S1). Simultaneously, a recombinant plasmid named pNPgusA (Table S1) was constructed, in which the promoterless gusA gene was cloned into the promoterless cloning sites of the vector pLAFR6 and introduced into strain 8004 as a negative control. GUS activity produced by the obtained reporter strains was determined after incubation for 72 h in MMX with or without methionine. The result demonstrated that the recombinant strains 8004/pP1251L and 8004/pP1251S produced similar GUS activities in MMX (Fig. 4b). This suggests that the 52 and 109 bp DNA segments upstream of the TSS of the operon have similar promoter activities. Contrary to expectations, both strains produced similar GUS activities in MMX with and without methionine supplementation (Fig. 4b). However, the control strain 8004/pNPgusA (Table S1) did not produce any detectable GUS activity (Fig. 4b). These data imply that the promoter of the met operon is not involved in the feedback regulation of methionine biosynthesis in response to methionine availability.
Fig. 4.
Characterization and functional analysis of the promoter region and the 5′UTR of the met operon. (a) Characterization of the promoter. The small green arrow represents the promoter (P1251) of the operon. TSS represents the identified transcriptional start site. The promoter-containing DNA sequences used for the construction of reporter plasmids are shown in green, while the 5’UTR sequence is in magenta. The −10/–35 region and the Shine-Dalgarno sequence (SD) are indicated. (b) Functional analysis of the promoter. The upper part shows the genetic organization of the reporter plasmids pP1251L and pP1251L. The lower part shows the GUS activities produced by the reporter strains 8004/pP1251L (pP1251L) and 8004/pP1251S (pP1251S), as well as the negative control strain 8004/pNPgusA (pNPgusA) grown for 72 h in MMX alone (Met−) and MMX with 1 mM methionine (Met+). Data are from a representative experiment, and similar results were obtained in two other independent experiments. The letters above the columns represent no significant difference at P=0.05 by t-test. (c) and (d) Functional analysis of the 5′UTR. The upper part shows the genetic organization of the 5′UTR-gusA transcriptional fusion reporter plasmid (p5UTR-SD+) and the translational fusion reporter plasmid (p5UTR-SD−). The lower part shows the GUS activities produced by the reporter strains 8004/p5′UTR-SD+ (p5UTR-SD+) and 8004/p5′UTR-SD− (p5UTR-SD−) grown in MMX alone (Met−) and MMX with the addition of 1 mM methionine (Met+) or 1 mM glycine (Gly+). The asterisks above the columns represent significant difference at P<0.05 by t-test. The data in blue and black above the columns are the absolute and relative values, respectively. One unit of GUS activity was defined as milligrams (mg) of ρ-nitrophenol released from ρ-nitrophenyl β-d-glucuronide per minute per ml of bacterial culture (cell density: OD600=1.0). Data presented are from a representative experiment, and similar results were obtained in two other independent experiments.
The fact that the promoter of the met operon did not respond to methionine availability suggests that the feedback control of this operon may be at the post-transcriptional level. Notably, the operon has a very long (190 nt) 5′UTR (Fig. 4a), implying that the 5′UTR may be responsible for feedback control. To test this possibility, 5′UTR-gusA transcriptional and translational fusion reporter plasmids, named p5′UTR-SD+ and p5′UTR-SD-, respectively, were constructed (Fig. 4c, d), where both fusions were driven by the E. coli lac promoter whose expression is constitutive in Xcc. The reporter plasmids were introduced into strain 8004, generating the reporter strains named 8004/p5′UTR-SD+ and 8004/p5′UTR-SD− (Table S1). The GUS activities produced by the reporter strains grown in MMX with or without methionine were measured. The results showed that the GUS activity produced by the translational reporter was reduced by 98 % when methionine was added (Fig. 4d). Addition of glycine to the medium had no effect on the GUS activity produced by either of the reporters (Fig. 4c, d), indicating that the reduction in GUS activity by methionine was specific. These data denote that the 5’UTR plays a key role in the feedback regulation of the met operon, whose regulation appears to be mainly at the translational level. Interestingly, only 75 % GUS activity was produced by the transcriptional reporter when methionine was added (Fig. 4c), suggesting that the 5′UTR may also employ a transcription attenuation mechanism to regulate the met operon.
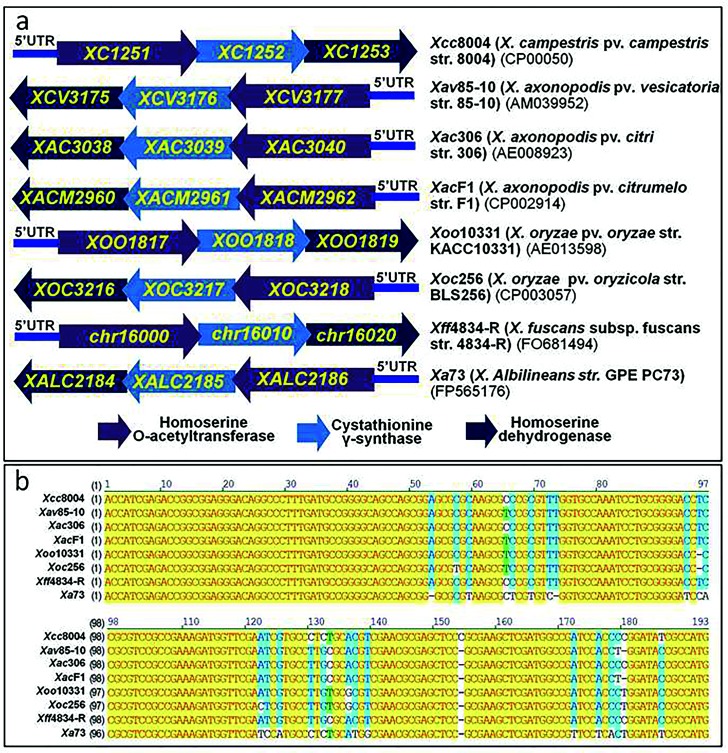
The met operon is widely distributed among Xanthomonas species
A blast analysis revealed that the met operon containing metA, metB and hom genes is present in all completely sequenced Xanthomonas species, subspecies and pathovars (i.e. X. campestris pv. campestris, X. axonopodis pv. vesicatoria, X. axonopodis pv. citri, X. axonopodis pv. citrumelo, X. oryzae pv. oryzae, X. oryzae pv. oryzicola, X. fuscans subsp. fuscans and X. albilineans) (Fig. 5). On the contrary, the location of the metA, metB and hom genes is scattered among the genomes of other bacteria. Interestingly, a further blast search showed that the 5′UTR of the met operon is highly conserved among all sequenced Xanthomonas genomes, including those with a draft sequence present in the NCBI sequence database (https://blast.ncbi.nlm.nih.gov/) (Fig. 5, Table S3). In addition to Xanthomoas species, the highly conserved 5′UTR sequence is also found in Pseudoxanthomonas suwonensis and Stenotrophomonas acidaminiphila (Table S3).
Fig. 5.
The met operon is highly conserved among sequenced Xanthomonas species. The arrows represent genes annotated in the genomes of Xanthomonas strains. The numbers in parentheses below the strain names are the access numbers in the NCBI database. The numbers above the arrows are the ORF names annotated.
Discussion
To establish a successful infection, a plant-parasitic bacterium must be capable of utilizing plant compounds as nutrients for growth in addition to overcoming plant defences. However, the infected plant tissue is generally a nutrient-deficient environment where nutritional compounds such as nucleotides and amino acids are insufficient for bacterial growth. Thus, having the ability to synthesize nucleotides and amino acids from simpler compounds is critical for plant-parasitic pathogens to proliferate efficiently and invade hosts successfully. Xcc is an intercellular parasite that generally invades, colonizes and multiplies in the vascular tissues of cruciferous plants. In this work, we showed that an Xcc mutant strain defective for the met operon (and by inference methionine biosynthesis) caused a severe reduction in virulence and growth rate in the host plant. Given that the mutant grew as well as the wild-type in nutrient-rich medium, this result suggests that there was insufficient methionine in the vascular tissues of the host plant tested for Xcc growth, and that de novo synthesis of methionine is crucial for Xcc to proliferate and establish an infection in the host.
As illustrated in Fig. 1, Xcc possesses genes encoding a complete set of enzymes required for methionine synthesis from aspartate via the trans-sulfuration pathway. Certain bacteria have developed an alternative pathway, called the direct sulfhydrylation pathway, to synthesize methionine, in which O-acetylhomoserine is directly converted to homocysteine by O-acetylhomoserine sulfhydrylase, encoded by metY [3]. However, it seems that members of the Xanthomonas genus do not employ the direct sulfhydrylation pathway to synthesize methionine, as the metY gene is missing in the genomes of all sequenced Xanthomonas strains as revealed by a blast search against the NCBI sequence database (https://blast.ncbi.nlm.nih.gov/).
Previous studies reported that in Gram-negative bacteria the expression of the genes involved in methionine synthesis is regulated by a mechanism of transcriptional-level feedback inhibition [4]. In E. coli the met genes involved in methionine biosynthesis are regulated by the activator MetR and the repressor MetJ, which bind directly to the MetR- and MetJ-binding motifs present in the promoters of met genes [20]. The Xcc strain 8004 encodes a MetR homologue (XC0327) that shares 37 % identity with the E. coli MetR protein, although metJ is missing in its genome [19]. However, bioinformatics analysis revealed that there is no typical MetR-binding motif present in any of the promoters of the genes involved in methionine biosynthesis, as illustrated in Fig. 1. Given that the promoter of the Xcc met operon does not respond to methionine availability (Fig. 4b), the MetR homologue in Xcc may not have a direct effect on the expression of met genes. It was reported that Ralstonia solanacearum MetR regulates the expression of metE but not metH [13], both of which are regulated by MetR in E. coli and Salmonella typhimurium [21]. Interestingly, R. solanacearum metE, metH and metR mutants are not auxotrophic for methionine [13]. Whether the MetR homologue in Xcc plays a role in methionine biosynthesis needs to be further investigated.
As mentioned above, Gram-positive bacteria generally utilize RNA regulators such as the S-box and T-box riboswitches to control the expression of genes involved in de novo methionine biosynthesis at the post-transcriptional level. Based on the results of promoter-gusA assay and 5′UTR-gusA fusion reporter analysis, we conclude that the Xcc met operon is constitutively transcribed regardless of whether methionine is sufficient or deficient in the environment, but that translation initiation of the operon mRNA is tightly controlled by its 5′UTR in response to methionine availability. Consistently, the predicted secondary structure of the 5′UTR shows that the SD sequence of the first ORF XC1251 is blocked (Fig. S2), suggesting that the 5’UTR can regulate the expression of XC1251 at the translational level. To the best of our knowledge, this is the first work showing that the 5’UTR of the met operon mRNA in a Gram-negative bacterium plays a key role in the regulation of de novo methionine biosynthesis. It is known that methionine’s downstream product SAM can be recycled. During methylation, SAM is converted to S-adenosylhomocysteine (SAH), which is then hydrolysed by SAH hydrolase to form the upstream metabolite of methionine, homocysteine [2]. It was demonstrated that a riboswitch at the 5′UTR of the gene encoding SAH hydrolase senses SAH and activates genes involved in co-enzyme recycling in Gram-negative bacteria, such as Pseudomonas syringae [22]. The riboswitch is highly conserved in the 5′UTRs of the genes predicted to encode SAH hydrolase in Xcc strains, suggesting that Xcc may also use the 5′UTR of SAH hydrolase-encoding gene to regulate SAM recycling [22].
Interestingly, an Rfam search (http://rfam.xfam.org/) showed that the 1–120 nt region in the 190 nt 5′UTR of the Xcc met operon displays 52 % sequence identity with the aptamer domain of the yitJ SAM-I riboswitch of Bacillus subtilis. However, there is no such SAM-I riboswitch-like sequence present in the 5′UTR regions of other genes involved in Xcc methionine biosynthesis. This suggests that the expression of the met operon and other met genes is differentially regulated in Xcc. Given that the met operon and its 5′UTR are highly conserved in Xanthomonas genus, to further investigate the detailed molecular mechanism by which the 5′UTR controls the expression of the met operon in Xcc will be very valuable in regard to our understanding of methionine biosynthesis regulation in Xanthomonas.
Supplementary Data
Funding information
This work was supported by the National Natural Science Foundation of China (31470237), the 973 Program of the Ministry of Science and Technology of China (2015CB150600) and the Ba Gui Scholar Program of Guangxi Zhuang Autonomous Region of China (2014A002).
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: BLAST, basic local alignment search tool; bp, base pair; GUS, β-glucuronidase; SD, Shine-Dalgarno sequence; 5’UTR, 5’-untranslated region.
Three supplementary tables and two supplementary figures are available with the online version of this article.
Edited by: I. J. Oresnik and G. H. Thomas
References
- 1.Serganov A, Patel DJ. Amino acid recognition and gene regulation by riboswitches. Biochim Biophys Acta. 2009;1789:592–611. doi: 10.1016/j.bbagrm.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fontecave M, Atta M, Mulliez E. S-adenosylmethionine: nothing goes to waste. Trends Biochem Sci. 2004;29:243–249. doi: 10.1016/j.tibs.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 3.McCutcheon JP, Moran NA. Extreme genome reduction in symbiotic bacteria. Nat Rev Microbiol. 2012;10:13–26. doi: 10.1038/nrmicro2670. [DOI] [PubMed] [Google Scholar]
- 4.Ferla MP, Patrick WM. Bacterial methionine biosynthesis. Microbiology. 2014;160:1571–1584. doi: 10.1099/mic.0.077826-0. [DOI] [PubMed] [Google Scholar]
- 5.Viola RE. The central enzymes of the aspartate family of amino acid biosynthesis. Acc Chem Res. 2001;34:339–349. doi: 10.1021/ar000057q. [DOI] [PubMed] [Google Scholar]
- 6.Sherwood AV, Henkin TM. Riboswitch-mediated gene regulation: novel RNA architectures dictate gene expression responses. Annu Rev Microbiol. 2016;70:361–374. doi: 10.1146/annurev-micro-091014-104306. [DOI] [PubMed] [Google Scholar]
- 7.Breaker RR. Prospects for riboswitch discovery and analysis. Mol Cell. 2011;43:867–879. doi: 10.1016/j.molcel.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodionov DA, Vitreschak AG, Mironov AA, Gelfand MS. Comparative genomics of the methionine metabolism in Gram-positive bacteria: a variety of regulatory systems. Nucleic Acids Res. 2004;32:3340–3353. doi: 10.1093/nar/gkh659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutiérrez-Preciado A, Henkin TM, Grundy FJ, Yanofsky C, Merino E. Biochemical features and functional implications of the RNA-based T-box regulatory mechanism. Microbiol Mol Biol Rev. 2009;73:36–61. doi: 10.1128/MMBR.00026-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leyn SA, Suvorova IA, Kholina TD, Sherstneva SS, Novichkov PS, et al. Comparative genomics of transcriptional regulation of methionine metabolism in Proteobacteria. PLoS One. 2014;9:e113714. doi: 10.1371/journal.pone.0113714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cubitt MF, Hedley PE, Williamson NR, Morris JA, Campbell E, et al. A metabolic regulator modulates virulence and quorum sensing signal production in Pectobacterium atrosepticum. Mol Plant Microbe Interact. 2013;26:356–366. doi: 10.1094/MPMI-09-12-0210-R. [DOI] [PubMed] [Google Scholar]
- 12.Andersen GL, Beattie GA, Lindow SE. Molecular characterization and sequence of a methionine biosynthetic locus from Pseudomonas syringae. J Bacteriol. 1998;180:4497–4507. doi: 10.1128/jb.180.17.4497-4507.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plener L, Boistard P, González A, Boucher C, Genin S. Metabolic adaptation of Ralstonia solanacearum during plant infection: a methionine biosynthesis case study. PLoS One. 2012;7:e36877. doi: 10.1371/journal.pone.0036877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mansfield J, Genin S, Magori S, Citovsky V, Sriariyanum M, et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol Plant Pathol. 2012;13:614–629. doi: 10.1111/j.1364-3703.2012.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Li RF, Ming ZH, Lu GT, Tang JL. Identification of a novel type III secretion-associated outer membrane-bound protein from Xanthomonas campestris pv. campestris. Sci Rep. 2017;7:42724. doi: 10.1038/srep42724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Windgassen M, Urban A, Jaeger KE. Rapid gene inactivation in Pseudomonas aeruginosa. FEMS Microbiol Lett. 2000;193:201–205. doi: 10.1111/j.1574-6968.2000.tb09424.x. [DOI] [PubMed] [Google Scholar]
- 17.Patey G, Qi Z, Bourg G, Baron C, O'Callaghan D. Swapping of periplasmic domains between Brucella suis VirB8 and a pSB102 VirB8 homologue allows heterologous complementation. Infect Immun. 2006;74:4945–4949. doi: 10.1128/IAI.00584-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jefferson RA, Burgess SM, Hirsh D. beta-glucuronidase from Escherichia coli as a gene-fusion marker. Proc Natl Acad Sci USA. 1986;83:8447–8451. doi: 10.1073/pnas.83.22.8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qian W, Jia Y, Ren SX, He YQ, Feng JX, et al. Comparative and functional genomic analyses of the pathogenicity of phytopathogen Xanthomonas campestris pv. campestris. Genome Res. 2005;15:757–767. doi: 10.1101/gr.3378705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weissbach H, Brot N. Regulation of methionine synthesis in Escherichia coli. Mol Microbiol. 1991;5:1593–1597. doi: 10.1111/j.1365-2958.1991.tb01905.x. [DOI] [PubMed] [Google Scholar]
- 21.Urbanowski ML, Stauffer LT, Plamann LS, Stauffer GV. A new methionine locus, metR, that encodes a trans-acting protein required for activation of metE and metH in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1987;169:1391–1397. doi: 10.1128/jb.169.4.1391-1397.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang JX, Lee ER, Morales DR, Lim J, Breaker RR. Riboswitches that sense S-adenosylhomocysteine and activate genes involved in coenzyme recycling. Mol Cell. 2008;29:691–702. doi: 10.1016/j.molcel.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.