Abstract
Magnocellular oxytocin (OT) and vasopressin (VP) neurons express an afterhyperpolarization (AHP) following spike trains that attenuates firing rate and contributes to burst patterning. This AHP includes contributions from an apamin-sensitive, medium-duration AHP (mAHP) and from an apamin-insensitive, slow-duration AHP (sAHP). These AHPs are Ca2+ dependent and activated by Ca2+ influx through voltage-gated Ca2+ channels. Across central nervous system neurons that generate Ca2+-dependent AHPs, the Ca2+ channels that couple to the mAHP and sAHP differ greatly, but for magnocellular neurosecretory cells this relationship is unknown. Using simultaneous whole cell recording and Ca2+ imaging, we evaluated the effect of specific high-voltage-activated (HVA) Ca2+ channel blockers on the mAHP and sAHP. Block of all HVA channels via 400 μM Cd2+ inhibited almost the entire AHP. We tested nifedipine, conotoxin GVIA, agatoxin IVA, and SNX-482, specific blockers of L-, N-, P/Q-, and R-type channels, respectively. The N-type channel blocker conotoxin GVIA (1 μM) was the only toxin that inhibited the mAHP in either OT or VP neurons although the effect on VP neurons was weaker by comparison. The sAHP was significantly inhibited by N-type block in OT neurons and by R-type block in VP neurons although neither accounted for the entirety of the sAHP. Thus the mAHP appears to be elicited by Ca2+ from mostly N-type channels in both OT and VP neurons, but the contributions of specific Ca2+ channel types to the sAHP in each cell type are different. Alternative sources to HVA channels may contribute Ca2+ for the sAHP.
NEW & NOTEWORTHY Despite the importance of afterhyperpolarization (AHP) mechanisms for regulating firing behavior of oxytocin (OT) and vasopressin (VP) neurons of supraoptic nucleus, which types of high-voltage-activated Ca2+ channels elicit AHPs in these cells was unknown. We found that N-type channels couple to the medium AHP in both cell types. For the slow AHP, N-type channels contribute in OT neurons, whereas R-type contribute in VP neurons. No single Ca2+ channel blocker abolished the entire AHP, suggesting that additional Ca2+ sources are involved.
Keywords: medium-duration afterhyperpolarization, slow-duration afterhyperpolarization, oxytocin, vasopressin, calcium
INTRODUCTION
High-voltage-activated (HVA) Ca2+ channels were classically categorized by their pharmacology and their subunit composition (Birnbaumer et al. 1994). Many neuronal cell types express L-, N-, P/Q-, and R-type HVA Ca2+ channels (Castelli and Magistretti 2006; Fisher and Bourque 1995, 1996; Foehring and Armstrong 1996; Lorenzon and Foehring 1995; Mermelstein et al. 1999; Pineda et al. 1998; Quinlan et al. 2008; Simms and Zamponi 2014; Stewart and Foehring 2000; Wang and Fisher 2014). This includes oxytocin (OT) and vasopressin (VP) magnocellular neurosecretory cells (MNCs). These cells control milk ejection and parturition during the reproductive cycle (OT) as well as water balance and vasoconstriction (VP) (Poulain et al. 1977). Depolarization attributable to action potentials (APs) triggers the voltage-dependent activation of these channels, allowing Ca2+ ions to flow inward. The existence of different HVA channel subtypes reflects a need to meet the complex Ca2+ requirements of neurons and other excitable cells (Snutch et al. 2013). A classic example in neurons is specificity in Ca2+ channel coupling to transmitter release in certain neurons (Catterall 2011; Cohen-Kutner et al. 2010; Satake and Imoto 2014; Snutch et al. 2013; Wheeler et al. 1994).
HVA Ca2+ channels are also involved in the activation of Ca2+-dependent K+ conductances underlying spike afterhyperpolarizations (AHPs) following spike trains. AHPs shape firing behavior and cause spike frequency adaptation in many cell types (Andrade et al. 2012; Meech 1978; Pineda et al. 1998; Sah 1996). MNCs demonstrate three distinct AHPs after spiking. The fast AHP (fAHP) follows a single spike and is involved in repolarization of the cell after an AP. We do not address the fAHP here. In MNCs, the medium AHP (mAHP) is a Ca2+-dependent conductance activated after one to three spikes, has an inactivation time constant (τ) of ~500 ms, and is blocked by the SK channel toxin apamin (Armstrong et al. 1994; Bourque and Brown 1987; Kirkpatrick and Bourque 1996; Teruyama and Armstrong 2005). The mAHP contributes strongly to spike frequency adaptation in MNCs but also contributes to burst length in VP neurons (Kirkpatrick and Bourque 1996). The slow AHP (sAHP) is also Ca2+ dependent but requires longer spike trains, lasts for seconds, is insensitive to apamin, and is markedly attenuated by muscarinic receptor activation (Ghamari-Langroudi and Bourque 2004); the underlying channel is unknown. The sAHP contributes to phasic patterning in VP neurons (Ghamari-Langroudi and Bourque 2004). MNCs fire in phasic bursting patterns, maximizing hormone release and minimizing secretory fatigue (Dutton and Dyball 1979). This phasic bursting is characterized by seconds of repetitive firing followed by seconds of quiescence.
Which Ca2+ channels couple to AHPs differs among neuronal cell types and is often different for the mAHP and sAHP within the same neuron. In myenteric neurons and vagal motoneurons, N-type currents are coupled to both mAHPs and sAHPs (Sah 1995; Vogalis et al. 2001). In contrast, L-type channels contribute to the sAHP in CA1, CA3, and sympathetic neurons (Martínez-Pinna et al. 2000; Moyer et al. 1992; Tanabe et al. 1998). An extensive review of the HVA Ca2+ channels coupling to sAHPs can be found in Andrade et al. (2012).
Although both OT and VP MNCs exhibit prominent mAHPs and sAHPs (Teruyama and Armstrong 2005), the associated Ca2+ channels contributing to their activation are unknown. Previous work in our laboratory has demonstrated a stark mechanistic difference in that both the mAHP and sAHP are dependent on phosphatidylinositol 4,5-bisphosphate (PIP2) in OT but not VP neurons (Kirchner et al. 2017). It is possible that some of the observed differences could be explained by a difference in coupling of AHPs to Ca2+ channels between the cell types. Here we present a direct comparison of the Ca2+ channels involved in the generation of AHPs between OT and VP neurons.
METHODS
Slice preparation.
These data were obtained from 56 cells from 30 wild-type Sprague-Dawley rats’ supraoptic neurons (SONs). Coronal brain slices (250 µm) were prepared from randomly cycling, virgin adult Sprague-Dawley female rats (Harlan Laboratories, Indianapolis, IN) weighing between 150 and 220 g. We used randomly cycling females as in our previous studies. This ensured that any phenomena uncovered would not be dependent on estrous state. We recorded from between one and four cells per rat. We restricted these studies to females because of the laboratory’s extensive characterization of the differences between VP and OT neurons in this sex (Kirchner et al. 2017; Stern and Armstrong 1996; Teruyama and Armstrong 2005, 2007). The University of Tennessee Health Science Center institutional animal care and use committee review board approved all experiments. Animals were on an ad libitum diet. For use in experiments, rats were deeply anesthetized with ketamine (100 mg/kg)/xylazine (10%) and perfused through the heart with cold artificial cerebrospinal fluid (aCSF) with NaCl replaced by 210 mM sucrose. The rats were decapitated via guillotine. The brains were then removed and subsequently sliced in ice-cold aCSF with 210 mM sucrose replacing NaCl, using a Leica VT1000S vibrating microtome. After being sliced, the tissue was transferred to an aCSF-filled holding chamber and warmed for 15–20 min at 32°C. aCSF was continuously bubbled with 95% O2-5% CO2 and contained (in mM) 20.00 D-glucose, 0.45 ascorbic acid, 2.50 KCl, 1.00 MgSO4, 1.25 NaH2PO4.H2O, 26.00 NaHCO3, 125.00 NaCl, and 2.00 CaCl2. Slices were then transferred to aCSF at room temperature, where they remained for at least 40 min before the recording.
Electrophysiology.
Slices were placed in the well of a Plexiglas chamber attached to a modified stage on an Olympus BX51WI upright microscope and perfused with aCSF containing 5 mM CsCl to block the slow depolarizing afterpotential (Ghamari-Langroudi and Bourque 1998; Teruyama and Armstrong 2005, 2007) and 10 µM 6,7-dinitroquinoxaline-2,3-dione (DNQX; Sigma-Aldrich, St. Louis, MO), 40 µM 2R-amino-5-phosphonovaleric acid (Tocris, Minneapolis, MN), and 100 µM picrotoxin (Tocris) to block fast synaptic currents. The aCSF was bubbled constantly with 95% O2-5% CO2, warmed to 32°C ± 1°C, and flowed at ~2 ml/min. For recordings in which we applied Cd2+ to the cells, we used phosphate-free aCSF consisting of the following (in mM): 121.0 NaCl, 1.3 MgCl2, 3.0 KCl, 26.0 NaHCO3, 20.0 D-glucose, and 2.5 CaCl2 to prevent Cd2+ precipitation. Whole cell and current-clamp recordings were obtained using an Axon Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA). Traces were digitized using an Axon 1440A Digitizer at 10 kHz on a Dell desktop computer running Clampex 9 software (Molecular Devices).
Recording pipettes (4–8 MΩ) were pulled from borosilicate glass (1.5-mm OD) using a P-1000 flaming/brown horizontal micropipette puller (Sutter Instruments, Novato, CA). The pipette internal solution consisted of the following (in mM): 135.0 KMeSO4, 8.0 NaCl, 10.0 HEPES, 2.0 Mg-ATP, 0.3 Na-GTP, 0.1 leupeptin, 6.0 phosphocreatine, and 0.2 EGTA with pH 7.2–7.4 and 285–295.0 mOsm (kg/H2O). Biocytin (0.1%) (Sigma-Aldrich) was added on the day of the experiment for visualization during immunochemical identification of cell type (Horikawa and Armstrong 1988). The liquid junction potential for the KMeSO4 internal was approximately −10 mV and was not corrected.
AHPs measured in current clamp were evoked from a resting membrane potential of −55 mV by injecting 300 pA of current via a 20-spike, 20-Hz pulse train. AHP amplitudes correlate with spike count, so, to isolate the mAHP, we injected a shorter train of 5 spikes of the same amplitude and frequency (Ghamari-Langroudi and Bourque 2004). For Ca2+ channels, reagents included 5.0 µM nifedipine (Nif; Sigma-Aldrich) to block L-type channels, 1.0 µM ω-conotoxin GVIA (CnTx GVIA; Peptides International, Louisville, KY or Alomone Laboratories, Jerusalem, Israel) to block N-type channels, 500.0 nM agatoxin-IVA (AgTx IVA; Alomone Laboratories) to block P/Q-type channels, and 0.3 μM SNX-482 (Alomone Laboratories) to block R-type channels. Because SNX-482 also blocks A-type K+ currents (Liu and Bean 2014), 4 mM 4-aminopyridine (4-AP; Sigma-Aldrich) was used to block A-current (IA) and other voltage-gated K+ channels contributing to spike width before administering SNX-482. Cells whose series resistance exceeded 20 MΩ and/or changed by >20% during the recording were discarded. Traces were averaged over two or more runs for each individual cell.
Immunochemistry.
Slices were fixed in 4.0% paraformaldehyde and 0.2% picric acid in PBS and stored at 4°C. Biocytin-labeled SONs were processed for double labeling with either anti-OT or anti-VP neurophysins. The anti-VP neurophysin is a rabbit polyclonal antibody provided by Alan Robinson (UCLA, Professor Emeritus) and was used at 1:20,000. The anti-OT neurophysin antibody (PS36) is a mouse monoclonal antibody provided by Harold Gainer (National Institutes of Health, Emeritus) and was used at 1:500. All antibodies and labeling reagents were dissolved in PBS + 0.5% Triton X-100 (PBST). After 36–72 h of incubation at 4°C, the slices were washed with PBST and incubated in a cocktail of secondary antibodies including Alexa Fluor 488 goat anti-rabbit immunoglobulin G (IgG) and Alexa Fluor 594 goat anti-mouse IgG (1:200) along with Avidin-AMCA (1:200) for reaction with the biocytin. Double staining of biocytin and one antibody complemented the negative staining of the other identified neurons as OT or VP.
Calcium imaging.
Ca2+ imaging was performed alongside patch-clamp electrophysiology on a single computer using a Polychrome V monochromater (TILL Photonics, Planegg, Germany) or on two computers using a Zeiss LSM 7MP 2 photon system (Carl Zeiss, Oberkochen, Germany). Regardless of imaging hardware, the pipette internal solution for analyzing AHP tail currents was as previously described, except that 0.2 mM EGTA was replaced with 0.1 mM fura-2. Measurements were made from the soma, excluding the nucleus. We excited fura-2 at 380 nm and report all data as %ΔF/F (Callaway et al. 1993; Teruyama and Armstrong 2005). Fura-2 is a highly sensitive indicator that has little toxicity, unlike other fluorescein-based indicators. Although fura-2 allows rationing to determine [Ca2+]i, single-wavelength imaging allows greater temporal resolution, and the changes we measure represent proportional changes in [Ca2+]i as we start from a hyperpolarized state (resting) and allow time for [Ca2+]i to recover between trials (Roper et al. 2003). These data were analyzed either with the custom acquisition program described below or with Igor Pro 7.0 (Wavemetrics, Portland, OR).
In the case of the Polychrome V, Ca2+ transients were acquired using a custom Windows-based program (CCD32; written by Dr. J. Callaway, UTHSC, Memphis, TN) based on software developed by Lasser-Ross et al. (1991). Images were obtained with a CCD Imago Sensicam camera at 380 nm using a USHIO UXL-150MO 150 W Xenon arc lamp. We measured fluorescence changes at an emission wavelength of 520 ± 40 nm. Photobleaching was corrected by subtracting a Ca2+ signal from a control sweep of equal length, in which the cell was not stimulated and held at a hyperpolarized holding potential (−70 mV) to minimize Ca2+ entry. We subtracted background autofluorescence by using a reference area near the cell.
When we used the two-photon system, Ca2+ transients were acquired with Windows-based software, Zeiss Zen 2010. Cells were imaged on a Zeiss LSM 7MP 2 photon system equipped with a Coherent Chameleon Vision-S laser (Titanium-Sapphire). We excited fura-2 at 800 nm. Cells were imaged using a raster scan at 30–40 Hz depending on the region of interest size. This equipment was used to supplement data obtained on the polychrome V while the latter was under repair.
Statistics and analysis.
In MNCs, the fAHP is a brief event lasting <15 ms (Dopico et al. 1999) and was not evaluated here. The mAHP decay τ is ~500 ms in MNCs (Teruyama and Armstrong 2005), whereas the sAHP has a decay τ of 1–2 s (Ghamari-Langroudi and Bourque 2004). With these considerations, we operationally defined the mAHP and sAHP in a 20-pulse current-clamp protocol as the amplitude of the AHP at peak (~100 ms after train) and 1,000 ms after the stimulus (sAHP), respectively. Although measurement of the AHP at the peak likely contains contributions from the sAHP, it is dominated by the mAHP because of the slower-onset kinetics of the sAHP (Ghamari-Langroudi and Bourque 2004; Kirchner et al. 2017; Teruyama and Armstrong 2007). To further evaluate the mAHP, we isolated the peak hyperpolarization after a shorter (5 pulses) train in current clamp that activates the mAHP without the sAHP. We operationally define the sAHP as the current amplitude 1 s after the pulse, a time point when the mAHP has mostly inactivated (Teruyama and Armstrong 2005).
All electrophysiological traces were analyzed in ClampFit 10.2 (Molecular Devices) or Igor Pro (Wavemetrics). Measurements of amplitude were averaged over a 30-ms segment of current (30 points). All statistics were performed in PRISM or SPSS. Data were evaluated using a two-way repeated-measures ANOVA to evaluate the within-sample effects of channel blockers on the cells and between-sample effects of OT vs. VP neurons. We used a Sidak multiple-comparisons test to evaluate toxin effects on specific cell types (OT or VP). The exception to this is the 20-spike Ca2+ data for the SNX-482 experiments, which did not meet the criteria for normality (Fig. 6). Therefore, we used a Friedman’s test for this data. We used a Bonferroni-Dunn post hoc analysis to make comparisons in the Friedman’s test. We used a repeated-measures ANOVA with a Bonferroni post hoc analysis to evaluate AP half-width (Fig. 5C), as well as a one-way ANOVA to evaluate differences in spike broadening during trains (Fig. 5D). APs are truncated in the figures. Data in the figures are marked as statistically significant depending on the number of asterisks associated with a difference between groups, *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
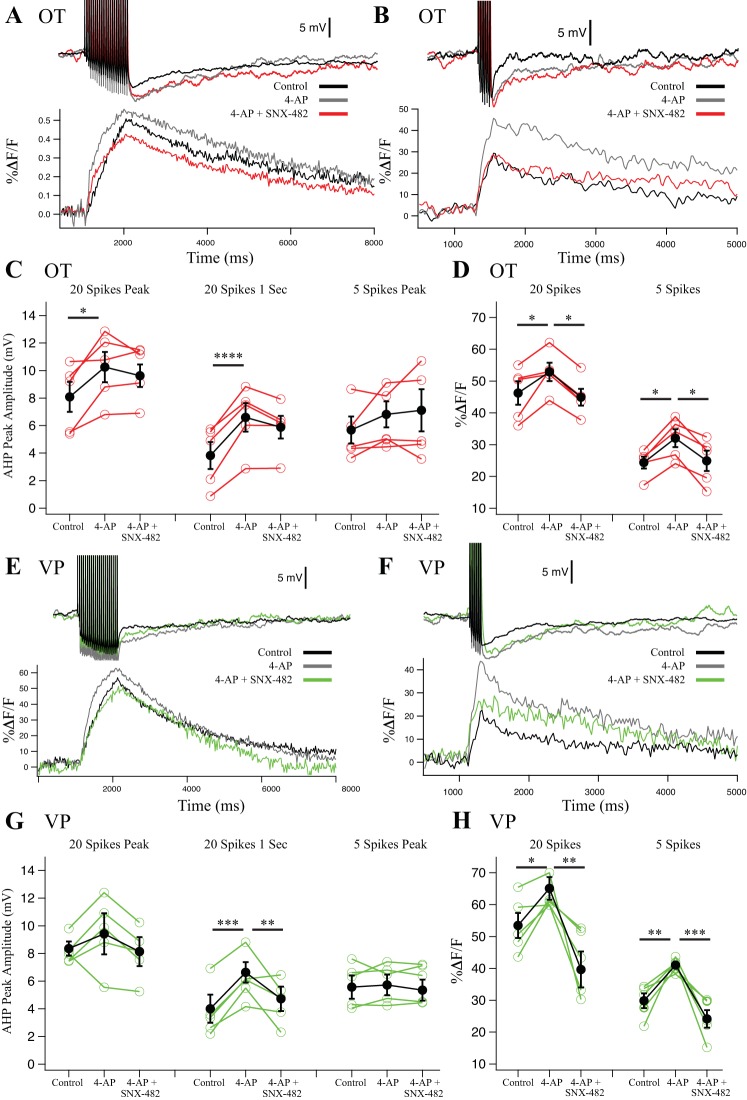
Fig. 6.
Effect of R-type blocker SNX-482 (0.3 μM) on afterhyperpolarizations (AHPs) and their corresponding Ca2+ transients in oxytocin (OT) (n = 5) (A–D) and vasopressin (VP) (n = 5) (E–H) neurons. 4-Aminopyridine (4-AP) was preapplied to block Kv4 channels and isolate the effect of SNX482 on R-type Ca2+ channels. A: example AHP after a 20-Hz, 20-spike train from an OT neuron and its corresponding somatic Ca2+ signal. B: example AHP after a 20-Hz, 5-spike train from an OT neuron and its corresponding somatic Ca2+ signal. C: summary data for OT neuron AHP measurements at the 20-spike peak amplitude (medium and slow AHP, mAHP + sAHP), at 1 s after the (sAHP), and 5-spike AHPs at the peak amplitude (mAHP). Red points are individual cells, and black points are group averages. After 20 spikes, 4-AP significantly increased the AHP at peak (*P < 0.05) and 1 s (****P < 0.0001) in 20-spike AHPs. Subsequent application of SNX-482 did not significantly reduce the AHP compared with 4-AP. 5-spike AHPs were unaffected. D: summary data for OT neuron peak Ca2+ transients during AHP stimulation in 20-spike AHPs and in 5-spike AHPs. 4-AP increased Ca2+ transients significantly in 20-spike and 5-spike trains (*P < 0.05), and successive SNX-482 significantly inhibited the Ca2+ signal compared with 4-AP after both 20 and 5 spikes (*P < 0.05). E: example AHP after a 20-spike train from a VP neuron and its corresponding somatic Ca2+ signal. F: example AHP after a 5-spike train from a VP neuron and its corresponding somatic Ca2+ signal. G: summary data for VP neuron AHP measurements at the 20-spike peak amplitude (mAHP + sAHP), at 1 s after the train (sAHP), and 5-spike AHPs at the peak amplitude (mAHP). Green points are individual cells, and black points are group averages. 4-AP significantly increased the 20-spike AHP at 1 s (***P < 0.001). Subsequent SNX-482 significantly reduced the 20-spike peak and 1-s AHP compared with 4-AP alone (**P < 0.01). 5-spike AHPs were unaffected. H: summary data for VP neuron peak Ca2+ transients in 20-spike AHPs and 5-spike AHPs. 4-AP significantly increased Ca2+ in the 20-spike (*P < 0.05) and 5-spike (**P < 0.01) protocol. Subsequent SNX-482 significantly decreased Ca2+ transients in both 20- (**P < 0.01) and 5-spike AHPs (***P < 0.001). All data were evaluated with a 2-way repeated-measures ANOVA with the exception of the 20-spike Ca2+ imaging data. For this data, we used a Friedman’s test because this data did not present a normal distribution.
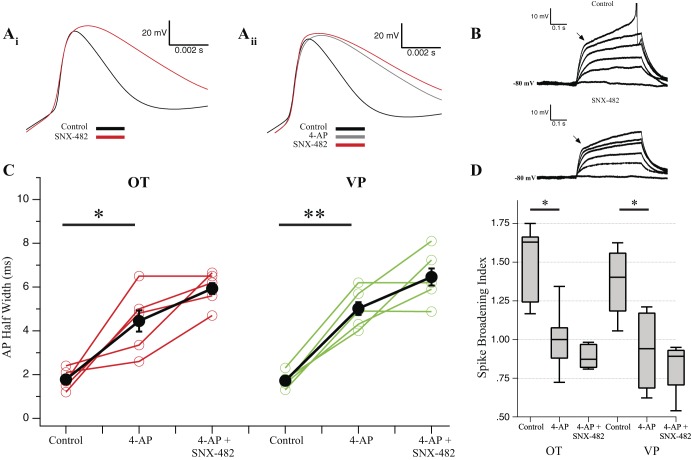
Fig. 5.
SNX-482 blocks transient outward rectification and broadens spikes [oxytocin (OT) and vasopressin (VP) n = 5]. A, i: example single action potential (AP) from an OT neuron treated with SNX-482 (0.3 μM) showing obvious broadening of the AP. A, ii: example single AP from a different OT neuron than Ai, treated first with 4 mM 4-aminopyridine (4-AP), followed by subsequent application of SNX-482. B: step injections of depolarizing current reveal a fast transient outward rectifier that is reduced by SNX-482 in the same cell as A, i. Positive steps (25 pA) were generated from cells at −80 mV. C: in 20-spike trains, we measured AP half-width of the 1st spike. In OT and VP neurons, 4-AP results in significant spike broadening (OT, *P < 0.05; VP, **P < 0.01). Subsequent application of SNX-482 produced no further broadening (P > 0.05) (repeated-measures ANOVA). D: changes in spike broadening between the 1st and 20th spike in a train represented as the ratio of AP half-width between the 2 spikes. Values >1 indicate spike-dependent broadening, whereas values <1 indicate narrowing. Application of 4-AP significantly reduced spike-dependent broadening within the 20-spike train in both cell types (*P < 0.05). Subsequent application of SNX-482 does not significantly reduce the spike-broadening index compared with 4-AP alone (1-way ANOVA).
RESULTS
To determine which specific Ca2+ channel subtypes contributed Ca2+ that elicited the mAHP and sAHP in SONs, we measured AHPs in current clamp before and after the application of pharmacological channel blockers. We used a train of 5 or 20 spikes stimulated at 20 Hz from a resting potential of −55 mV to control for effects on spike frequency adaptation and ensure all neurons received the same stimuli. We simultaneously monitored changes in bulk somatic Ca2+ ([Ca2+]i) during stimulation. This measurement reflects Ca2+ entry plus extrusion and buffering. Measuring this allowed us to confirm the efficacy of toxin block on Ca2+ entry through its respective Ca2+ channel.
Cd2+ block of HVA Ca2+ channels inhibits most of the AHP.
We first used the inorganic Ca2+ channel blocker Cd2+ to test whether activation of the AHP in both MNC cell types required Ca2+ entry. Cd2+ (400 µM) blocked 80 ± 18% of the mAHP and 81 ± 7% of the sAHP in OT neurons (n = 6) (Fig. 1). In VP neurons, it blocked 79 ± 17% of the mAHP and 89 ± 2% of the sAHP (n = 6). These data are consistent with previous reports that inorganic blockers such as Cd2+, Mn2+, and Co2+ block most of the AHP in MNCs (Bourque et al. 1985; Ghamari-Langroudi and Bourque 2004; Kirkpatrick and Bourque 1996). Even stronger block can be achieved by chelating intracellular Ca2+, highlighting that activation of AHPs is dependent on elevated intracellular Ca2+ (Andrew and Dudek 1984).
Fig. 1.
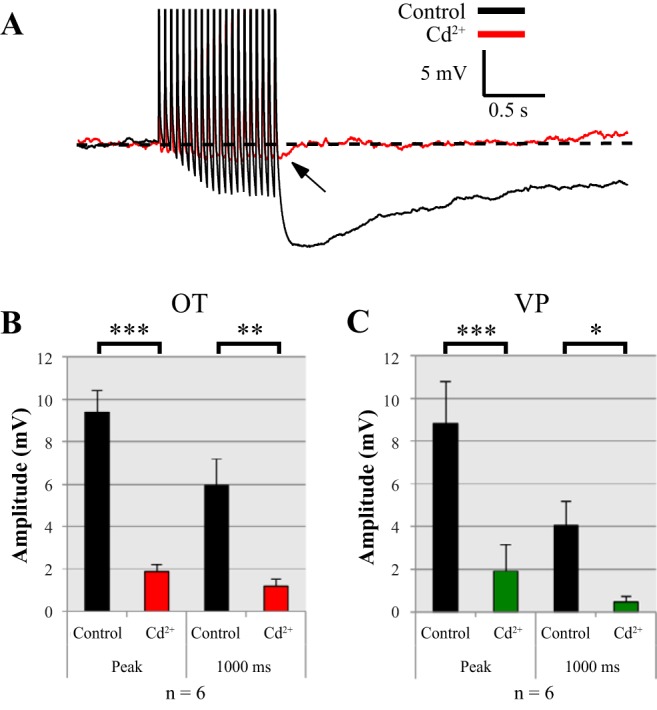
400 µM Cd2+ application to magnocellular neurosecretory cells inhibited most of the afterhyperpolarization (AHP) in current clamp. AHPs were evoked using a pulse train of 20 spikes and measured at peak (medium and slow AHP, mAHP + sAHP) and 1 s (sAHP) following the pulse. A: example of an oxytocin (OT) neuron before and after Cd2+ application of an AHP in current clamp. The only remaining portion of the AHP is small (B and C). Summary data for Cd2+ inhibition at the peak current and 1 s following the pulse train in both OT (n = 6) (B) and vasopressin (VP) (n = 6) (C) neurons are shown. Cd2+ significantly inhibits AHPs in OT neurons at peak (***P < 0.001) and 1,000 ms (**P < 0.01). Cd2+ significantly inhibits AHPs in VP neurons at peak (***P < 0.001) and 1,000 ms (*P < 0.05). All data were evaluated with a 2-way repeated-measure ANOVA.
L-type channels.
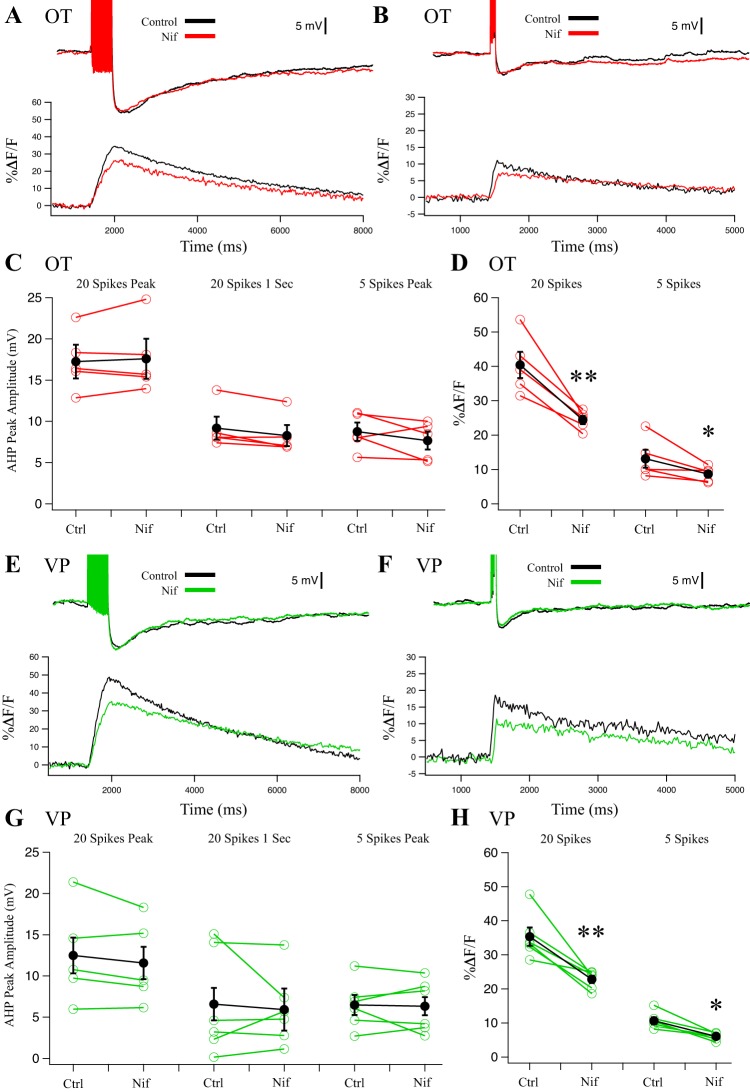
Application of the dihydropyridine L-type channel blocker Nif (5 µM) did not alter either the mAHP or sAHP in OT (Fig. 2, A–C; n = 6, P > 0.05) and VP (Fig. 2, E–G; n = 6, P > 0.05) neurons. However, Nif reduced the peak [Ca2+]i, suggesting reduced Ca2+ entry. In OT neurons, we observed a 40 ± 1% reduction in the peak Ca2+ signal after 20 spikes (Fig. 2D; n = 5, P < 0.01) and a 34 ± 4% reduction after 5 spikes (Fig. 2D; n = 5, P < 0.05). In VP neurons, we observed a 36 ± 2% reduction in the peak Ca2+ signal after 20 spikes (Fig. 2H; n = 5, P < 0.01) and a 43 ± 1% reduction after 5 spikes (Fig. 2H; n = 5, P < 0.05). The reduction in Ca2+ signal is consistent with previous observations in which 5–10 µM Nif blocked a similar proportion of whole cell Ca2+ currents in acutely dissociated SONs (Fisher and Bourque 1995; Foehring and Armstrong 1996).
Fig. 2.
Effect of L-type blocker 5 µM nifedipine (Nif) on afterhyperpolarizations (AHPs) and corresponding Ca2+ transients in oxytocin (OT) (n = 5) (A–D) and vasopressin (VP) (n = 5) (E–H) neurons. A: example AHP after a 20-Hz, 20-spike train from an OT neuron treated with Nif and the corresponding somatic Ca2+ signal. B: example AHP after a 20-Hz, 5-spike train from an OT neuron treated with Nif and its corresponding somatic Ca2+ signal. C: summary data for OT neuron AHP measurements at the 20-spike peak amplitude (medium and slow AHP, mAHP + sAHP), at 1 s after the train (sAHP), and 5-spike AHPs at the peak amplitude (mAHP). Red points are individual cells, and black points are group averages (paired t-test; P > 0.05 for all 3 measurements). D: summary data for OT neuron Ca2+ transients. Nif significantly reduced peak %ΔF/F in 20-spike AHPs (**P < 0.01) and 5-spike AHPs (*P < 0.05). E: example AHP after a 20-Hz, 20-spike train from a VP neuron treated with Nif and its corresponding somatic Ca2+ signal. F: example AHP after a 20-Hz, 5-spike train from a VP neuron treated with Nif and its corresponding somatic Ca2+ signal. G: summary data for VP neuron AHP measurements at the 20-spike peak amplitude (mAHP + sAHP), at 1 s after the train (sAHP), and 5-spike AHPs at the peak amplitude (mAHP). Green points are individual cells, and black points are group averages (paired t-test; P > 0.05 for all 3 measurements). H: Nif significantly reduced peak %ΔF/F from 20-spike trains (**P < 0.01) and 5-spike trains (*P < 0.05). All data were evaluated with a 2-way repeated-measures ANOVA.
N-type channels.
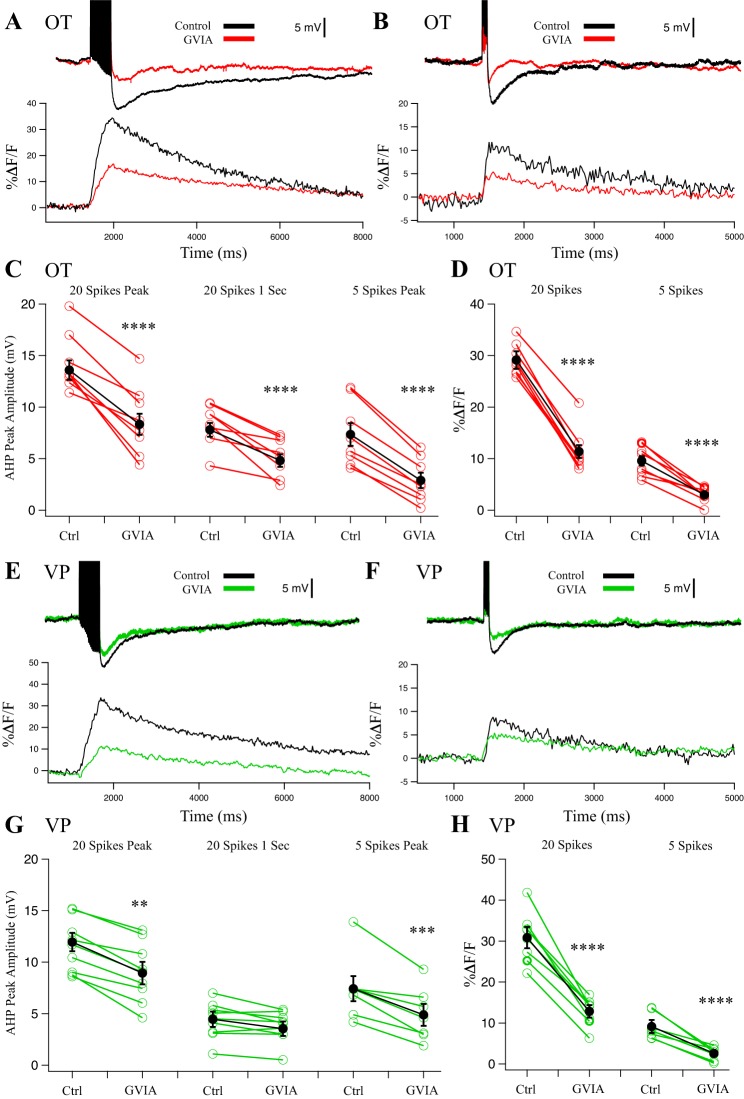
We next tested for effects of the N-type channel blocker GVIA (1 µM). In OT neurons, GVIA strongly reduced AHP amplitude (Fig. 3, A–D; n = 8). GVIA inhibited the 20-spike peak AHP by 39 ± 7% (P < 0.0001), at 1 s after the pulse by 38 ± 7% (P < 0.0001), and the 5-spike peak AHP by 61 ± 11% (P < 0.0001). GVIA inhibited the corresponding peak Ca2+ signals by 61 ± 4% for 20 spikes (P < 0.0001) and 69 ± 5% for 5 spikes (P < 0.0001).
Fig. 3.
Effect of N-type blocker 1 µM conotoxin GVIA (CnTx GVIA) on afterhyperpolarizations (AHPs) and corresponding Ca2+ transients in oxytocin (OT) (n = 8) (A–D) and vasopressin (VP) (n = 7) (E–H) neurons. A: example AHP after a 20-Hz, 20-spike train from an OT neuron treated with CnTx GVIA and its corresponding somatic Ca2+ signal. B: example AHP after a 20-Hz, 5-spike train from an OT neuron treated with CnTx GVIA and its corresponding somatic Ca2+ signal. C: summary data for OT neuron AHP measurements at the 20-spike peak (medium and slow AHP, mAHP + sAHP; ****P < 0.0001), at 1 s after the train (sAHP; ****P < 0.0001), and 5-spike AHPs at the peak amplitude (mAHP; ****P < 0.0001). Red points are individual cells, and black points are group averages. D: summary data for OT neuron Ca2+ transients during AHP stimulation. CnTx GVIA significantly reduced peak %ΔF/F in 20-spike AHPs (****P < 0.0001) and reduced %ΔF/F in 5-spike AHPs (****P < 0.0001). E: example AHP after a 20-Hz, 20-spike train from a VP neuron treated with CnTx GVIA and its corresponding somatic Ca2+ signal. F: example AHP after a 20-Hz, 5-spike train from a VP neuron treated with CnTx GVIA and its corresponding somatic Ca2+ signal. G: summary data for VP neuron AHP measurements at the 20-spike peak amplitude (mAHP + sAHP; **P < 0.01), at 1 s after the train (sAHP; P > 0.05), and 5-spike AHPs at the peak amplitude (mAHP; ***P < 0.001). Green points are individual cells, and black points are group averages. H: CnTx GVIA significantly reduced peak %ΔF/F in 20-spike AHPs (paired t-test; ****P < 0.0001) and 5-spike AHPs (****P < 0.0001). All data were evaluated with a 2-way repeated-measures ANOVA.
In VP neurons (n = 7), GVIA inhibited the 20-spike peak AHP by 25 ± 9% (P < 0.01), at 1 s after the pulse by 29 ± 5% (P > 0.05), and the 5-spike peak AHP by 25 ± 12% (P < 0.001). GVIA strongly inhibited the corresponding peak Ca2+ signals by 60 ± 4% for 20 spikes (P < 0.0001) and 67 ± 6% for 5 spikes (P < 0.0001) in VP neurons.
Furthermore, there was a significant interaction between cell type and GVIA effect for AHP measurements at 20-spike peaks (P < 0.001), 20-spike 1-s (P < 0.01), and 5-spike peaks (P < 0.05). There was no interaction for [Ca2+]i peaks by GVIA between OT and VP neurons (P > 0.05). Thus, although N-type channels were found to be a major contributor to the AHP in both cell types, the N-type contribution was stronger for OT neuron AHPs. This happens despite no differences in decrease in [Ca2+]i by GVIA between OT and VP neurons.
P/Q-type channels.
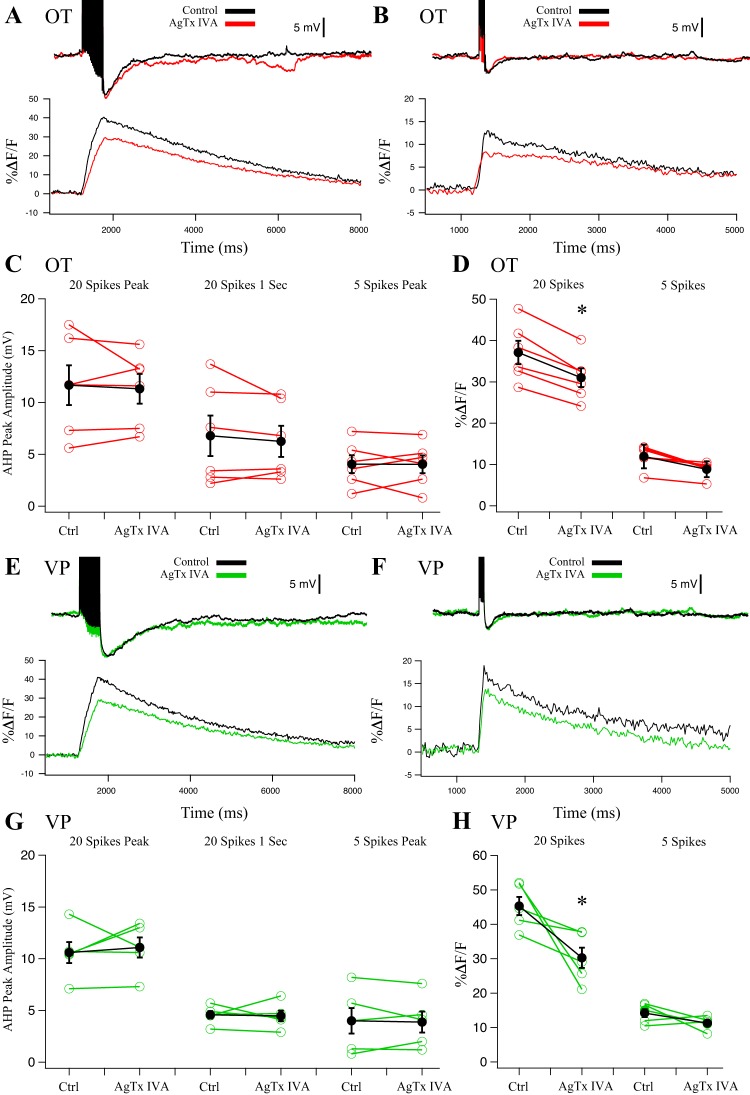
To evaluate P/Q-type channel contribution, we used AgTx IVA (0.5 µM). P- and Q-type variants are often distinguished by their sensitivity to AgTx IVA (Mintz et al. 1992); lower concentrations of AgTx IVA (10–100 nM) have been shown to block only P-type variants, whereas 0.5 µM blocks both P- and Q-type (Foehring and Armstrong 1996; Zhang et al. 1993). Both P- and Q-type currents are generated by the CACNA1A gene, and splice variants may explain the phenotypic differences (Bourinet et al. 1999; Nimmrich and Gross 2012). We evaluated AgTx IVA at 0.5 µM to determine contribution by P/Q channels. In OT (n = 6) and VP (n = 5) neurons, application of 0.5 µM AgTx IVA had no significant effect on either the mAHP or sAHP (Fig. 4; P > 0.05), suggesting that P/Q-type currents do not couple to the AHPs. Simultaneous Ca2+ imaging revealed a decrease in peak somatic Ca2+. In OT neurons, AgTx IVA inhibited the 20-spike Ca2+ peak by 16 ± 6% (P < 0.05) and the 5-spike Ca2+ peak by 25 ± 6% (P > 0.05) (Fig. 4D). In VP neurons, AgTx IVA significantly inhibited the 20-spike Ca2+ peak by 33 ± 5% (P < 0.05). The 5-spike Ca2+ peak decreased by 20 ± 5%, but this was not significant (P > 0.05) (Fig. 4H). Given the lack of effect at 0.5µM, we did not test lower concentrations of AgTx IVA to sort P from Q channel contributions.
Fig. 4.
Effect of P/Q-type blocker 0.5 µM agatoxin-IVA (AgTx IVA) on afterhyperpolarizations (AHPs) and corresponding Ca2+ transients in oxytocin (OT) (n = 6) (A–D) and vasopressin (VP) (n = 5) (E–H) neurons. A: example AHP after a 20-Hz, 20-spike train from an OT neuron treated with AgTx IVA and its corresponding somatic Ca2+ signal. B: example AHP after a 20-Hz, 5-spike train from an OT neuron treated with AgTx IVA and its corresponding somatic Ca2+ signal. C: summary data for OT neuron AHP measurements at the 20-spike peak amplitude (medium and slow AHP, mAHP + sAHP), at 1 s after the train (sAHP), and 5-spike AHPs at the peak amplitude (mAHP). Red points are individual cells, and black points are group averages (paired t-test; P > 0.05 for all 3 measurements). D: summary data for OT neuron Ca2+ transients. AgTx IVA significantly reduced peak %ΔF/F after 20-spike AHPs (*P < 0.05) but not 5-spike AHPs (P > 0.05). E: example AHP after a 20-Hz, 20-spike train from a VP neuron treated with AgTx IVA and its corresponding somatic Ca2+ signal. F: example AHP after a 20-Hz, 5-spike train from a VP neuron treated with AgTx IVA and its corresponding somatic Ca2+ signal. G: summary data for VP neuron AHP measurements at the 20-spike peak amplitude (mAHP + sAHP), at 1 s after the train (sAHP), and 5-spike AHPs at the peak amplitude (mAHP). Green points are individual cells, and black points are group averages (paired t-test; P > 0.05 for all 3 measurements). H: AgTx IVA significantly reduced peak %ΔF/F in 20-spike AHPs (*P < 0.05) but not 5-spike AHPs (P > 0.05). All data were evaluated with a 2-way repeated-measures ANOVA.
R-type channels.
We also tested the R-type blocker SNX-482 (0.3 µM). Although a potent blocker of R-type channels, SNX-482 also blocks IA with high affinity (1–10 nM) (Liu and Bean 2014). Block of IA increases AP width in MNCs, increasing Ca2+ influx during spike trains, and consequently altering the AHP amplitude and time course (Bourque 1988; Hlubek and Cobbett 2000). We initially observed that SNX-482 greatly broadened single spikes (Fig. 5A, i). This effect mimicked that of 4-AP (4 mM), which blocks IA, as well as other Kv channels. After prior 4-AP (4 mM) application, SNX-482 did not widen spikes further (Fig. 5A, ii). Second, we observed a decrease in the transient outward rectifier (Fig. 5B) after SNX-482, which has previously been shown to be blocked by 4-AP application and attributable to IA in MNCs (Bourque 1988; Fisher and Bourque 1998; Stern and Armstrong 1997). With these considerations, we quantified the spike broadening of spikes by measuring the half-width of the first AP of each 20-spike train (OT n = 5; VP n = 5; Fig. 5C). Neurons dosed with 4-AP (4 mM) showed significantly larger AP half-widths in both OT (P < 0.05) and VP (P < 0.01) neurons. Subsequent SNX-482 did not further increase spike width (OT P > 0.05; VP P > 0.05). These data demonstrate that 4-AP blocks almost all of IA and that SNX-482 effects on AHPs cannot be attributed to changes in spike width. Additionally, 4-AP application increased spike widths more in VP neurons, consistent with previous reports of VP neurons having a larger IA (Fisher and Bourque 1998; Stern and Armstrong 1997).
Finally, we evaluated spike broadening from the first to the 20th spike during the train. Repetitive activity produces spike frequency-dependent broadening (Andrew and Dudek 1985; Bourque and Renaud 1985; Hlubek and Cobbett 2000), which has been shown to increase Ca2+ influx, further complicating SNX-482 effects (Jackson et al. 1991). We measured the ratio of the 20th and 1st spike in each train as an index of frequency-dependent broadening (Fig. 5D). In both OT and VP neurons, spike broadening we observed during the train was significantly inhibited by 4-AP (OT and VP P < 0.05). In 4-AP, the spike-broadening index was 1.00 ± 0.10 for OT and 0.96 ± 0.10 for VP, indicating that there was almost no change in AP half-width during trains in 4-AP. This suggests that a majority of the spike broadening in MNCs is the result of IA inactivation. Further application of SNX-482 to block R-type channels did not significantly alter the spike-broadening index (P > 0.05). Because changes in AHP amplitude could reflect both changes in spike broadening and consequent Ca2+ entry as well as reduction of Ca2+ channels, we tested the R-type channel effects of SNX-482 on the AHP after preapplied 4-AP.
In AHP measurements from both OT and VP neurons, 4-AP enhanced 20-spike AHP size, amplitude, spike width, and the corresponding Ca2+ transients (Figs. 5 and 6), suggesting that the broader spikes increased Ca2+ entry. In OT neurons, the AHP enhancement was significant for 20 spikes at peak (20 ± 10% increase; P < 0.05) and 1 s (71 ± 11% increase; P < 0.0001) (Fig. 6C). In VP neurons, the measurement at 1 s after 20 spikes was significant (60 ± 12% increase; P < 0.001; Fig. 6G), whereas the peak measurement was not significant (16 ± 26% increase; P > 0.05; Fig. 6G). The 5-spike AHP was unaffected by 4-AP in either cell type (P > 0.05; Fig. 6).
The corresponding OT Ca2+ transients were significantly increased by 4-AP application in 20-spike AHPs by 15 ± 5% (control vs. 4-AP, P < 0.05; Fig. 6D) and 5-spike AHPs by 31+ 8% (control vs. 4-AP, P < 0.05; Fig. 6D). Likewise, the corresponding VP Ca2+ transients in 20- and 5-spike peaks significantly increased after 4-AP by 21 ± 6% and 38 ± 2%, respectively (20 spikes: control vs. 4-AP, P < 0.05; 5 spikes: control vs. 4-AP, P < 0.01; Fig. 6H). These data were also consistent with the notion that broader spikes after 4-AP increased Ca2+ entry.
Whereas OT neurons demonstrated no significant inhibition of the AHP after subsequent application of SNX-482, the response of VP neurons was different (Fig. 6, C and D). Here SNX-482 reduced 20-spike AHPs at peak by 15 ± 8% and 1 s by 26 ± 11% after 4-AP application (20-spike peak: 4-AP vs. 4-AP + SNX, P > 0.05; 20-spike 1 s: 4-AP vs. 4-AP + SNX, P < 0.01; Fig. 6G). This was despite the further increase in AP width in SNX-482 after 4-AP (see above). SNX-482 did not significantly affect 5-spike peaks (P > 0.05; Fig. 6).
These differential responses may be due to different Ca2+ channel contribution to Ca2+ entry. Application of SNX-482 in OT neurons inhibited the 20-spike Ca2+ peak by only 15 ± 5% (4-AP vs. 4-AP + SNX, P < 0.05; Fig. 6D) and the 5-spike Ca2+ peak by only 22 ± 10% (4-AP vs. 4-AP + SNX, P < 0.05; Fig. 6D). In contrast, in VP neurons, SNX-482 inhibited the 20-spike Ca2+ peak by 45 ± 6% (4-AP vs. 4-AP + SNX, P < 0.01; Fig. 6H) and the 5-spike Ca2+ peak by 45 ± 5% (4-AP vs. 4-AP + SNX, P < 0.001; Fig. 6H).
DISCUSSION
We tested which HVA Ca2+ channels elicit the medium and slow phases of AHPs in OT- and VP-releasing neurons of the SON. Our laboratory has previously observed distinct mechanistic differences in AHP generation between OT and VP neurons (Kirchner et al. 2017), spurring interest in the possibility of cell-type differences in coupling of Ca2+ channel types to AHPs. The present study demonstrates that Ca2+ channel contributions differ between OT and VP cells.
We observed no significant differences in baseline AHP size or [Ca2+]i signals between OT and VP neurons in any of the experiments in which a Ca2+ channel blocker was evaluated. Furthermore, complete block of HVA Ca2+ channels with Cd2+ drastically inhibits the AHP in both cell types, as has been shown previously. The small remaining AHP after Cd2+ may reflect a Ca2+-independent K+ current, such as voltage-gated K+ current or the Na+-activated K+ current recently reported (Bansal and Fisher 2016).
The major contribution of N-type channels to the AHP.
In both cell types studied, N-type channels appear to be the most influential of the HVA types for Ca2+-dependent AHP generation. OT neurons show especially strong reduction in the mAHP and sAHP after GVIA application. The coupling to the mAHP was the strongest, but there is also a major contribution to the sAHP. Thus N-type channels provide the primary source of Ca2+ for both the mAHP and sAHP in OT neurons. It is important to note that some sAHP remains in OT neurons after N-type channel block, suggesting that other sources of Ca2+ exist for OT sAHPs as well. Block of the other HVA Ca2+ channel types had no effect on the AHP in these cells, however.
N-type channels also couple to mAHPs in VP neurons; GVIA significantly reduces the mAHP. However, the inhibition by GVIA is significantly less in VP neurons compared with OT despite similar inhibition of Ca2+ transients between the cell types. Like OT neurons, GVIA is the only HVA channel toxin that we tested that affected VP mAHPs. These results suggest that N-type Ca2+ entry is a primary source of Ca2+ for mAHPs in both OT and VP neurons.
Corresponding [Ca2+]i signals in MNCs.
Regardless of whether or not an HVA Ca2+ channel blocker was able to affect the AHP, we consistently observed a significant reduction in cytoplasmic [Ca2+]i after each application, indicating that the toxins were effectively blocking the channels. We acknowledge that summation of toxin effects on [Ca2+]i from all experiments exceeds 100%. There are a few reasons for this. First, although these toxins are reasonably specific (Pringos et al. 2011), the most parsimonious explanation based on our previous studies of calcium currents in dissociated cells with good space clamp is that these agents are not perfectly selective (Fisher and Bourque 1995; Lorenzon and Foehring 1995). This is consistent with other studies showing that, although Ca2+ channel toxins are highly selective, there is some overlap in targets (Foehring and Armstrong 1996; Gandía et al. 1995). Second, we compared populations of cells with variation in expression levels of HVA channel types among cells. Third, because preapplication of 4-AP increases [Ca2+]i, percent reduction by SNX-482 will be skewed because Ca2+ inhibition is not being compared with the cell at the control peak [Ca2+]i value. Finally, it is possible that toxin application lessens the dendritic depolarization caused by the evoked APs, thereby diminishing activation of Ca2+ channels not targeted by the toxin. However, it is highly unlikely that proximal dendritic Ca2+ itself diffuses far along the dendrite enough to influence our somatic measurements (Wilson and Callaway 2000).
The reduction in [Ca2+]i after N-type block is the greatest by any of the blockers in both cell types, consistent with MNCs containing a large population of N-type channels, as reported previously (Fisher and Bourque 1996; Foehring and Armstrong 1996; Lemos et al. 2012).
As discussed earlier, SNX-482 blocks IA in addition to R-type channels, resulting in larger AP half-widths. This AP broadening resulted in increased in [Ca2+]i and posed a potential confound for our evaluation of SNX-482 alone on the Ca2+-dependent AHP. After 4-AP block of IA, we observed a significant reduction in [Ca2+]i after SNX-482 despite no change in the AP half-width or spike-broadening index (Fig. 5), demonstrating its ability to block R-type channels independent of changes in spike width.
Strict coupling of mAHPs to Ca2+ microdomains.
mAHPs in other cell types are often exclusively coupled to a specific HVA Ca2+ channel (Sah 1995; Vogalis et al. 2001). This strict coupling is consistent with SK channel activation by microdomains of Ca2+, suggesting that Ca2+ channels must be in relatively close proximity to the SK channels and that bulk cytoplasmic Ca2+ would be a poor indicator of [Ca2+]i at the membrane (Fakler and Adelman 2008; Hallworth et al. 2003; Neher 1998). In neocortical pyramidal neurons, the relationship between the mAHP and bulk Ca2+ is poor (Abel et al. 2004), consistent with its activation from microdomains concentrated near the membrane. Our results are also consistent with this idea, as GVIA is the only toxin to inhibit mAHPs in OT and VP neurons, despite the ability of the other toxins to reduce [Ca2+]i.
Coupling of Ca2+ to the sAHP in VP neurons.
Cd2+ effectively blocks both mAHPs and sAHPs in both cell types. However, of subtype-specific HVA blockers, only block of N-type channels resulted in a decisive reduction in OT mAHPs or sAHPs. N-type channels are coupled to mAHPs in VP neurons as well, but only after R-type block (Fig. 6) was the sAHP inhibited in VP neurons. Although N-type (OT) or R-type (VP) channels may be the preferred Ca2+ source for the sAHP, it is possible that block of the preferred Ca2+ channels could reveal other Ca2+ channels with looser coupling to the sAHP. This could be facilitated by the slow kinetics of the sAHP. In other cell types, there is lower specificity in the coupling of Ca2+ channels to the sAHP compared with the mAHP (Ghamari-Langroudi and Bourque 2004; Lorenzon and Foehring 1992). This may also explain why Cd2+ is such a potent blocker of the AHP compared with GVIA or SNX-482.
It has been proposed that there is an intermediate step between Ca2+ entry and activation of the sAHP [i.e., Ca2+ does not directly bind to the K+ channel, reviewed in Andrade et al. (2012)]. In this model, cytoplasmic Ca2+ binding proteins act as the calcium sensor for the sAHP. Evidence consistent with a cytoplasmic Ca2+ sensor for the sAHP includes experiments in which pharmacological and genetic downregulation of cytoplasmic calcium-binding proteins (e.g., visinin-like calcium binding and calcineurin) resulted in substantial sAHP inhibition of myenteric and hippocampal neurons (Tzingounis et al. 2007; Villalobos and Andrade 2010; Vogalis et al. 2004). Besides HVA Ca2+ channels, another possible source for Ca2+ is from intracellular stores, which contribute to sAHPs in hippocampal neurons (Torres et al. 1996; van de Vrede et al. 2007) and immature neocortical pyramidal neurons (Pineda et al. 1998). It is possible that the sAHP in VP neurons is activated by Ca2+-induced Ca2+ release from internal stores, resulting in AHP resistance to block by specific Ca2+ channel blockers. Another possible source could be transient, low-voltage-activated (LVA) T-type Ca2+ channels. Although the presence of T-channels in MNCs has been reported (Erickson et al. 1993; Fisher and Bourque 1995), others report little low-threshold Ca2+ current (Foehring and Armstrong 1996; Luther and Tasker 2000; Luther et al. 2002). An LVA-type current sensitive to Ni2+ was shown to contribute to the depolarizing afterpotential in guinea pig SONs (Erickson et al. 1993). Finally, the differences in the response of these cells to Ca2+ could be differences in the sensitivity of the AHP channel to Ca2+ or to the differential expression of K+ AHP channels themselves.
Conclusions.
These studies revealed the HVA channel sources of Ca2+ to the AHP in MNCs. Briefly, in OT and VP neurons of the SON, it appears that mAHP generation reflects activation of only N-type channels although to a significantly lesser degree in VP compared with OT neurons. We observed cell type-specific preferential coupling of the sAHP with specific Ca2+ channels, N-type for OT neurons and R-type for VP neurons. This reinforces mechanistic differences of AHP generation between cell types, some of which might underlie the differential modulation of PIP2 of AHPs by affecting HVA currents (Kirchner et al. 2017).
GRANTS
This work was supported by grants R01NS-044163 and R01HD-072056.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.K.K., R.C.F., and W.E.A. conceived and designed research; M.K.K. and J.C.C. performed experiments; M.K.K. analyzed data; M.K.K., R.C.F., J.C.C., and W.E.A. interpreted results of experiments; M.K.K. prepared figures; M.K.K., R.C.F., and W.E.A. drafted manuscript; M.K.K., R.C.F., J.C.C., and W.E.A. edited and revised manuscript; M.K.K., R.C.F., J.C.C., and W.E.A. approved final version of manuscript.
REFERENCES
- Abel HJ, Lee JCF, Callaway JC, Foehring RC. Relationships between intracellular calcium and afterhyperpolarizations in neocortical pyramidal neurons. J Neurophysiol 91: 324–335, 2004. doi: 10.1152/jn.00583.2003. [DOI] [PubMed] [Google Scholar]
- Andrade R, Foehring RC, Tzingounis AV. The calcium-activated slow AHP: cutting through the Gordian knot. Front Cell Neurosci 6: 47, 2012. doi: 10.3389/fncel.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew RD, Dudek FE. Intrinsic inhibition in magnocellular neuroendocrine cells of rat hypothalamus. J Physiol 353: 171–185, 1984. doi: 10.1113/jphysiol.1984.sp015330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew RD, Dudek FE. Spike broadening in magnocellular neuroendocrine cells of rat hypothalamic slices. Brain Res 334: 176–179, 1985. doi: 10.1016/0006-8993(85)90583-9. [DOI] [PubMed] [Google Scholar]
- Armstrong WE, Smith BN, Tian M. Electrophysiological characteristics of immunochemically identified rat oxytocin and vasopressin neurones in vitro. J Physiol 475: 115–128, 1994. doi: 10.1113/jphysiol.1994.sp020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal V, Fisher TE. Na(+) -activated K(+) channels in rat supraoptic neurones. J Neuroendocrinol 28: 28, 2016. doi: 10.1111/jne.12394. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L, Campbell KP, Catterall WA, Harpold MM, Hofmann F, Horne WA, Mori Y, Schwartz A, Snutch TP, Tanabe T, Tsien RW. The naming of voltage-gated calcium channels. Neuron 13: 505–506, 1994. doi: 10.1016/0896-6273(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Bourinet E, Soong TW, Sutton K, Slaymaker S, Mathews E, Monteil A, Zamponi GW, Nargeot J, Snutch TP. Splicing of alpha 1A subunit gene generates phenotypic variants of P- and Q-type calcium channels. Nat Neurosci 2: 407–415, 1999. doi: 10.1038/8070. [DOI] [PubMed] [Google Scholar]
- Bourque CW. Transient calcium-dependent potassium current in magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol 397: 331–347, 1988. doi: 10.1113/jphysiol.1988.sp017004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque CW, Brown DA. Apamin and d-tubocurarine block the afterhyperpolarization of rat supraoptic neurosecretory neurons. Neurosci Lett 82: 185–190, 1987. doi: 10.1016/0304-3940(87)90127-3. [DOI] [PubMed] [Google Scholar]
- Bourque CW, Renaud LP. Calcium-dependent action potentials in rat supraoptic neurosecretory neurones recorded in vitro. J Physiol 363: 419–428, 1985. doi: 10.1113/jphysiol.1985.sp015719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque CW, Randle JC, Renaud LP. Calcium-dependent potassium conductance in rat supraoptic nucleus neurosecretory neurons. J Neurophysiol 54: 1375–1382, 1985. doi: 10.1152/jn.1985.54.6.1375. [DOI] [PubMed] [Google Scholar]
- Callaway JC, Lasser-Ross N, Stuart AE, Ross WN. Dynamics of intracellular free calcium concentration in the presynaptic arbors of individual barnacle photoreceptors. J Neurosci 13: 1157–1166, 1993. doi: 10.1523/JNEUROSCI.13-03-01157.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli L, Magistretti J. High-voltage-activated Ca2+ currents show similar patterns of expression in stellate and pyramidal cells from rat entorhinal cortex layer II. Brain Res 1090: 76–88, 2006. doi: 10.1016/j.brainres.2006.03.037. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Voltage-gated calcium channels. Cold Spring Harb Perspect Biol 3: a003947, 2011. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Kutner M, Nachmanni D, Atlas D. CaV2.1 (P/Q channel) interaction with synaptic proteins is essential for depolarization-evoked release. Channels (Austin) 4: 266–277, 2010. doi: 10.4161/chan.4.4.12130. [DOI] [PubMed] [Google Scholar]
- Dopico AM, Widmer H, Wang G, Lemos JR, Treistman SN. Rat supraoptic magnocellular neurones show distinct large conductance, Ca2+-activated K+ channel subtypes in cell bodies versus nerve endings. J Physiol 519: 101–114, 1999. doi: 10.1111/j.1469-7793.1999.0101o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton A, Dyball REJ. Phasic firing enhances vasopressin release from the rat neurohypophysis. J Physiol 290: 433–440, 1979. doi: 10.1113/jphysiol.1979.sp012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KR, Ronnekleiv OK, Kelly MJ. Role of a T-type calcium current in supporting a depolarizing potential, damped oscillations, and phasic firing in vasopressinergic guinea pig supraoptic neurons. Neuroendocrinology 57: 789–800, 1993. doi: 10.1159/000126438. [DOI] [PubMed] [Google Scholar]
- Fakler B, Adelman JP. Control of K(Ca) channels by calcium nano/microdomains. Neuron 59: 873–881, 2008. doi: 10.1016/j.neuron.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Fisher TE, Bourque CW. Voltage-gated calcium currents in the magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol 486: 571–580, 1995. doi: 10.1113/jphysiol.1995.sp020835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher TE, Bourque CW. Calcium-channel subtypes in the somata and axon terminals of magnocellular neurosecretory cells. Trends Neurosci 19: 440–444, 1996. doi: 10.1016/0166-2236(96)10034-5. [DOI] [PubMed] [Google Scholar]
- Fisher TE, Bourque CW. Properties of the transient K+ current in acutely isolated supraoptic neurons from adult rat. Adv Exp Med Biol 449: 97–106, 1998. doi: 10.1007/978-1-4615-4871-3_9. [DOI] [PubMed] [Google Scholar]
- Foehring RC, Armstrong WE. Pharmacological dissection of high-voltage-activated Ca2+ current types in acutely dissociated rat supraoptic magnocellular neurons. J Neurophysiol 76: 977–983, 1996. doi: 10.1152/jn.1996.76.2.977. [DOI] [PubMed] [Google Scholar]
- Gandía L, Borges R, Albillos A, García AG. Multiple calcium channel subtypes in isolated rat chromaffin cells. Pflugers Arch 430: 55–63, 1995. doi: 10.1007/BF00373839. [DOI] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Bourque CW. Caesium blocks depolarizing after-potentials and phasic firing in rat supraoptic neurones. J Physiol 510: 165–175, 1998. doi: 10.1111/j.1469-7793.1998.165bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Bourque CW. Muscarinic receptor modulation of slow afterhyperpolarization and phasic firing in rat supraoptic nucleus neurons. J Neurosci 24: 7718–7726, 2004. doi: 10.1523/JNEUROSCI.1240-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallworth NE, Wilson CJ, Bevan MD. Apamin-sensitive small conductance calcium-activated potassium channels, through their selective coupling to voltage-gated calcium channels, are critical determinants of the precision, pace, and pattern of action potential generation in rat subthalamic nucleus neurons in vitro. J Neurosci 23: 7525–7542, 2003. doi: 10.1523/JNEUROSCI.23-20-07525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlubek MD, Cobbett P. Differential effects of K(+) channel blockers on frequency-dependent action potential broadening in supraoptic neurons. Brain Res Bull 53: 203–209, 2000. doi: 10.1016/S0361-9230(00)00335-X. [DOI] [PubMed] [Google Scholar]
- Horikawa K, Armstrong WE. A versatile means of intracellular labeling: injection of biocytin and its detection with avidin conjugates. J Neurosci Methods 25: 1–11, 1988. doi: 10.1016/0165-0270(88)90114-8. [DOI] [PubMed] [Google Scholar]
- Jackson MB, Konnerth A, Augustine GJ. Action potential broadening and frequency-dependent facilitation of calcium signals in pituitary nerve terminals. Proc Natl Acad Sci USA 88: 380–384, 1991. doi: 10.1073/pnas.88.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner MK, Foehring RC, Wang L, Chandaka GK, Callaway JC, Armstrong WE. Phosphatidylinositol 4,5-bisphosphate (PIP2) modulates afterhyperpolarizations in oxytocin neurons of the supraoptic nucleus. J Physiol 595: 4927–4946, 2017. doi: 10.1113/JP274219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick K, Bourque CW. Activity dependence and functional role of the apamin-sensitive K+ current in rat supraoptic neurones in vitro. J Physiol 494: 389–398, 1996. doi: 10.1113/jphysiol.1996.sp021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasser-Ross N, Miyakawa H, Lev-Ram V, Young SR, Ross WN. High time resolution fluorescence imaging with a CCD camera. J Neurosci Methods 36: 253–261, 1991. doi: 10.1016/0165-0270(91)90051-Z. [DOI] [PubMed] [Google Scholar]
- Lemos JR, Ortiz-Miranda SI, Cuadra AE, Velázquez-Marrero C, Custer EE, Dad T, Dayanithi G. Modulation/physiology of calcium channel sub-types in neurosecretory terminals. Cell Calcium 51: 284–292, 2012. doi: 10.1016/j.ceca.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PW, Bean BP. Kv2 channel regulation of action potential repolarization and firing patterns in superior cervical ganglion neurons and hippocampal CA1 pyramidal neurons. J Neurosci 34: 4991–5002, 2014. doi: 10.1523/JNEUROSCI.1925-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzon NM, Foehring RC. Relationship between repetitive firing and afterhyperpolarizations in human neocortical neurons. J Neurophysiol 67: 350–363, 1992. doi: 10.1152/jn.1992.67.2.350. [DOI] [PubMed] [Google Scholar]
- Lorenzon NM, Foehring RC. Characterization of pharmacologically identified voltage-gated calcium channel currents in acutely isolated rat neocortical neurons. II. Postnatal development. J Neurophysiol 73: 1443–1451, 1995. doi: 10.1152/jn.1995.73.4.1443. [DOI] [PubMed] [Google Scholar]
- Luther JA, Tasker JG. Voltage-gated currents distinguish parvocellular from magnocellular neurones in the rat hypothalamic paraventricular nucleus. J Physiol 523: 193–209, 2000. doi: 10.1111/j.1469-7793.2000.t01-1-00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther JA, Daftary SS, Boudaba C, Gould GC, Halmos KC, Tasker JG. Neurosecretory and non-neurosecretory parvocellular neurones of the hypothalamic paraventricular nucleus express distinct electrophysiological properties. J Neuroendocrinol 14: 929–932, 2002. doi: 10.1046/j.1365-2826.2002.00867.x. [DOI] [PubMed] [Google Scholar]
- Martínez-Pinna J, Davies PJ, McLachlan EM. Diversity of channels involved in Ca2+ activation of K+ channels during the prolonged AHP in guinea-pig sympathetic neurons. J Neurophysiol 84: 1346–1354, 2000. doi: 10.1152/jn.2000.84.3.1346. [DOI] [PubMed] [Google Scholar]
- Meech RW. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng 7: 1–18, 1978. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Mermelstein PG, Foehring RC, Tkatch T, Song W-J, Baranauskas G, Surmeier DJ. Properties of Q-type calcium channels in neostriatal and cortical neurons are correlated with β subunit expression. J Neurosci 19: 7268–7277, 1999. doi: 10.1523/JNEUROSCI.19-17-07268.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz IM, Venema VJ, Swiderek KM, Lee TD, Bean BP, Adams ME. P-type calcium channels blocked by the spider toxin omega-Aga-IVA. Nature 355: 827–829, 1992. doi: 10.1038/355827a0. [DOI] [PubMed] [Google Scholar]
- Moyer JR Jr, Thompson LT, Black JP, Disterhoft JF. Nimodipine increases excitability of rabbit CA1 pyramidal neurons in an age- and concentration-dependent manner. J Neurophysiol 68: 2100–2109, 1992. doi: 10.1152/jn.1992.68.6.2100. [DOI] [PubMed] [Google Scholar]
- Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron 20: 389–399, 1998. doi: 10.1016/S0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- Nimmrich V, Gross G. P/Q-type calcium channel modulators. Br J Pharmacol 167: 741–759, 2012. doi: 10.1111/j.1476-5381.2012.02069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda JC, Waters RS, Foehring RC. Specificity in the interaction of HVA Ca2+ channel types with Ca2+-dependent AHPs and firing behavior in neocortical pyramidal neurons. J Neurophysiol 79: 2522–2534, 1998. doi: 10.1152/jn.1998.79.5.2522. [DOI] [PubMed] [Google Scholar]
- Poulain DA, Wakerley JB, Dyball RE. Electrophysiological differentiation of oxytocin- and vasopressin-secreting neurones. Proc R Soc Lond B Biol Sci 196: 367–384, 1977. doi: 10.1098/rspb.1977.0046. [DOI] [PubMed] [Google Scholar]
- Pringos E, Vignes M, Martinez J, Rolland V. Peptide neurotoxins that affect voltage-gated calcium channels: a close-up on ω-agatoxins. Toxins (Basel) 3: 17–42, 2011. doi: 10.3390/toxins3010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan ME, Alberto CO, Hirasawa M. Short-term potentiation of mEPSCs requires N-, P/Q- and L-type Ca2+ channels and mitochondria in the supraoptic nucleus. J Physiol 586: 3147–3161, 2008. doi: 10.1113/jphysiol.2007.148957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper P, Callaway J, Shevchenko T, Teruyama R, Armstrong W. AHP’s, HAP’s and DAP’s: how potassium currents regulate the excitability of rat supraoptic neurones. J Comput Neurosci 15: 367–389, 2003. doi: 10.1023/A:1027424128972. [DOI] [PubMed] [Google Scholar]
- Sah P. Different calcium channels are coupled to potassium channels with distinct physiological roles in vagal neurons. Proc Biol Sci 260: 105–111, 1995. doi: 10.1098/rspb.1995.0066. [DOI] [PubMed] [Google Scholar]
- Sah P. Ca(2+)-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci 19: 150–154, 1996. doi: 10.1016/S0166-2236(96)80026-9. [DOI] [PubMed] [Google Scholar]
- Satake S, Imoto K. Cav2.1 channels control multivesicular release by relying on their distance from exocytotic Ca2+ sensors at rat cerebellar granule cells. J Neurosci 34: 1462–1474, 2014. doi: 10.1523/JNEUROSCI.2388-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms BA, Zamponi GW. Neuronal voltage-gated calcium channels: structure, function, and dysfunction. Neuron 82: 24–45, 2014. doi: 10.1016/j.neuron.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Snutch TP, Peloquin J, Mathews E, McRory JE. Molecular Properties of Voltage-Gated Calcium. Landes Bioscience, 2013. [accessed 16 Oct 2017] https://www.ncbi.nlm.nih.gov/books/NBK6181/. [Google Scholar]
- Stern JE, Armstrong WE. Changes in the electrical properties of supraoptic nucleus oxytocin and vasopressin neurons during lactation. J Neurosci 16: 4861–4871, 1996. doi: 10.1523/JNEUROSCI.16-16-04861.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE, Armstrong WE. Sustained outward rectification of oxytocinergic neurones in the rat supraoptic nucleus: ionic dependence and pharmacology. J Physiol 500: 497–508, 1997. doi: 10.1113/jphysiol.1997.sp022036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A, Foehring RC. Calcium currents in retrogradely labeled pyramidal cells from rat sensorimotor cortex. J Neurophysiol 83: 2349–2354, 2000. doi: 10.1152/jn.2000.83.4.2349. [DOI] [PubMed] [Google Scholar]
- Tanabe M, Gähwiler BH, Gerber U. L-type Ca2+ channels mediate the slow Ca2+-dependent afterhyperpolarization current in rat CA3 pyramidal cells in vitro. J Neurophysiol 80: 2268–2273, 1998. doi: 10.1152/jn.1998.80.5.2268. [DOI] [PubMed] [Google Scholar]
- Teruyama R, Armstrong WE. Enhancement of calcium-dependent afterpotentials in oxytocin neurons of the rat supraoptic nucleus during lactation. J Physiol 566: 505–518, 2005. doi: 10.1113/jphysiol.2005.085985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teruyama R, Armstrong WE. Calcium-dependent fast depolarizing afterpotentials in vasopressin neurons in the rat supraoptic nucleus. J Neurophysiol 98: 2612–2621, 2007. doi: 10.1152/jn.00599.2007. [DOI] [PubMed] [Google Scholar]
- Torres GE, Arfken CL, Andrade R. 5-Hydroxytryptamine4 receptors reduce afterhyperpolarization in hippocampus by inhibiting calcium-induced calcium release. Mol Pharmacol 50: 1316–1322, 1996. [PubMed] [Google Scholar]
- Tzingounis AV, Kobayashi M, Takamatsu K, Nicoll RA. The diffusible calcium sensor, hippocalcin, gates the calcium activation of the slow afterhyperpolarization in hippocampal pyramidal neurons. Neuron 53: 487–493, 2007. doi: 10.1016/j.neuron.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Vrede Y, Fossier P, Baux G, Joels M, Chameau P. Control of IsAHP in mouse hippocampus CA1 pyramidal neurons by RyR3-mediated calcium-induced calcium release. Pflugers Arch 455: 297–308, 2007. doi: 10.1007/s00424-007-0277-4. [DOI] [PubMed] [Google Scholar]
- Villalobos C, Andrade R. Visinin-like neuronal calcium sensor proteins regulate the slow calcium-activated afterhyperpolarizing current in the rat cerebral cortex. J Neurosci 30: 14361–14365, 2010. doi: 10.1523/JNEUROSCI.3440-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogalis F, Furness JB, Kunze WA. Afterhyperpolarization current in myenteric neurons of the guinea pig duodenum. J Neurophysiol 85: 1941–1951, 2001. doi: 10.1152/jn.2001.85.5.1941. [DOI] [PubMed] [Google Scholar]
- Vogalis F, Harvey JR, Furness JB. Suppression of a slow post-spike afterhyperpolarization by calcineurin inhibitors. Eur J Neurosci 19: 2650–2658, 2004. doi: 10.1111/j.0953-816X.2004.03369.x. [DOI] [PubMed] [Google Scholar]
- Wang D, Fisher TE. Expression of CaV 2.2 and splice variants of CaV 2.1 in oxytocin- and vasopressin-releasing supraoptic neurones. J Neuroendocrinol 26: 100–110, 2014. doi: 10.1111/jne.12127. [DOI] [PubMed] [Google Scholar]
- Wheeler DB, Randall A, Tsien RW. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science 264: 107–111, 1994. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Callaway JC. Coupled oscillator model of the dopaminergic neuron of the substantia nigra. J Neurophysiol 83: 3084–3100, 2000. doi: 10.1152/jn.2000.83.5.3084. [DOI] [PubMed] [Google Scholar]
- Zhang JF, Randall AD, Ellinor PT, Horne WA, Sather WA, Tanabe T, Schwarz TL, Tsien RW. Distinctive pharmacology and kinetics of cloned neuronal Ca2+ channels and their possible counterparts in mammalian CNS neurons. Neuropharmacology 32: 1075–1088, 1993. doi: 10.1016/0028-3908(93)90003-L. [DOI] [PubMed] [Google Scholar]