Abstract
The purpose of our study was to examine the associations between the performance of older adults on four tests of mobility and the physical capabilities of the lower leg muscles. The assessments included measures of muscle strength, muscle activation, and perceived fatigability. Muscle activation was quantified as the force fluctuations—a measure of force steadiness—and motor unit discharge characteristics of lower leg muscles during submaximal isometric contractions. Perceived fatigability was measured as the rating of perceived exertion achieved during a test of walking endurance. Twenty participants (73 ± 4 yr) completed one to four evaluation sessions that were separated by at least 3 wk. The protocol included a 400-m walk, a 10-m walk at maximal and preferred speeds, a chair-rise test, and the strength, force steadiness, and discharge characteristics of motor units detected by high-density electromyography of lower leg muscles. Multiple-regression analyses yielded statistically significant models that explained modest amounts of the variance in the four mobility tests. The variance explained by the regression models was 39% for 400-m walk time, 33% for maximal walk time, 42% for preferred walk time, and 27% for chair-rise time. The findings indicate that differences in mobility among healthy older adults were partially associated with the level of perceived fatigability (willingness of individuals to exert themselves) achieved during the test of walking endurance and the discharge characteristics of soleus, medial gastrocnemius, and tibialis anterior motor units during steady submaximal contractions with the plantar flexor and dorsiflexor muscles.
NEW & NOTEWORTHY Differences among healthy older adults in walking endurance, walking speed, and ability to rise from a chair can be partially explained by the performance capabilities of lower leg muscles. Assessments comprised the willingness to exert effort (perceived fatigability) and the discharge times of action potentials by motor units in calf muscles during submaximal isometric contractions. These findings indicate that the nervous system contributes significantly to differences in mobility among healthy older adults.
Keywords: aging, force steadiness, mobility, motor units, walking endurance
INTRODUCTION
The decline in mobility that accompanies advancing age has a profound negative impact on quality of life (Ferrucci et al. 2016). Longitudinal studies have found that decreases in performance on tests of mobility are predictive of subsequent disability and survival. For example, the time it takes older adults to walk 400 m—a test of walking endurance—is associated with the risk of incident cardiovascular disease, mobility disability, and mortality (Newman et al. 2006; Vestergaard et al. 2009b). Similarly, the slowing of gait speed over short distances is predictive of subsequent declines in cognitive function (Best et al. 2016) and increased risk of dependency, institutionalization, and mortality (Cesari et al. 2009; Woo et al. 1999).
Cross-sectional studies have identified a range of physiological variables that can explain significant amounts of the variance in mobility scores of older adults before the onset of perceived limitations (Guralnik et al. 1995; Hicks et al. 2012; Lord et al. 2002; Schmitz et al. 2009; Stenholm et al. 2015). Moreover, baseline measurements of the time to complete a heel-shin coordination test and the peak force produced by the knee extensors during a maximal isometric contraction in adults (>65 yr) were predictive of self-reported and observed time to walk 400 m 3 yr later (Beauchamp et al. 2014). Similarly, low values for grip strength, knee extension strength, and lower extremity power in adults > 65 yr were predictive of clinically meaningful declines in gait speed 3 yr later (Hicks et al. 2012).
A consensus conference on mobility limitations (“Aging, the CNS, and Mobility”) concluded that adaptations within the central nervous system contribute significantly to mobility limitations in older adults who do not exhibit overt neurological disease symptoms (Rosso et al. 2013). One of the more profound neural adaptations influencing mobility in older adults is the proximal shift in the distribution of joint torques and powers during the stance phase of walking. When walking at the same speed, for example, the percentage of total positive work performed by the muscles around the hip, knee, and ankle joints during the stance phase was 16%, 11%, and 73%, respectively, for young adults and 44%, 5%, and 51%, respectively, for older adults (DeVita and Hortobágyi 2000). However, older adults are able to perform 44% more positive work about the ankle joint when walking uphill than when walking on a level surface (Franz and Kram 2014), which suggests that at least some of the redistribution of joint work is attributable to the preference of older adults not to engage the plantar flexor muscles during level walking.
Consistent with this interpretation, Stenholm et al. (2015) reported that two of the four most important predictor variables for the loss of mobility (400-m walk, stair ascent) over 9 yr for adults >65 yr were neurological signs (primitive reflexes and tremor). Similarly, poor sensorimotor nerve function in older adults (77 ± 3 yr) at baseline predicted incident mobility disability (30% of participants) within the subsequent 9 yr (Ward et al. 2014). Moreover, perceived fatigability, which was quantified as the rating of perceived exertion (RPE) after walking on a treadmill at 0.67 m/s for 5 min, was negatively correlated with daily levels of physical activity (Wanigatunga et al. 2018) and was predictive of an impending decline in mobility of older adults (Simonsick et al. 2016).
Declines in motor function exhibited by older adults are also evident at the level of single-motor unit activity. For example, the lower rate of increase in force produced by older adults during ballistic isometric contractions is strongly associated with lesser peak discharge rates of single motor units (Klass et al. 2008). Similarly, the peak discharge rate of motor units in the vastus lateralis muscle of older adults during maximal voluntary contractions (MVCs) is less than that observed in young adults but can be increased after strength training (Kamen and Knight 2004). Moreover, the amplitude of the force fluctuations during steady, submaximal contractions, which are often greater in older adults (Galganski et al. 1993; Laidlaw et al. 2000), is strongly associated with the variance in common synaptic input received by motor neurons (Farina and Negro 2015; Feeney et al. 2018; Negro et al. 2009).
Because of the critical role of lower extremity muscles in preserving mobility in older adults (Clark et al. 2013; Guralnik et al. 1995; Muehlbauer et al. 2018; Stenroth et al. 2015), our study examined the associations between the performances of older adults on four tests of mobility and the physical capabilities of the lower leg muscles. The assessments included measures of muscle strength, muscle activation, and perceived fatigability. Muscle activation was quantified as the amplitude of the fluctuations in force and the discharge characteristics of motor units during steady isometric contractions with lower leg muscles, whereas perceived fatigability was measured as the RPE during the 400-m walk test. In light of the results of a previous study on individuals with multiple sclerosis (Almuklass et al. 2018), we hypothesized that statistically significant amounts of the variance in performance on each of the mobility tests could be explained by different combinations of muscle strength, muscle activation during steady isometric contractions (force steadiness and motor unit discharge characteristics of lower extremity muscles), and perceived fatigability.
METHODS
Twenty healthy older adults (73 ± 4 yr; 12 women, 8 men) completed one to four evaluation sessions that were each separated by 3 wk. All procedures were approved by the Institutional Review Board (Protocol No. 13-0687) and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all subjects. Subject characteristics are listed in Table 1.
Table 1.
Subject characteristics
| No. of subjects | 20 (12 women, 8 men) |
|---|---|
| Age, yr | 73 ± 4 |
| 400-m walk time, s | 211 ± 47 |
| Maximal 10-m walk time, s | 4.9 ± 0.9 |
| Preferred 10-m walk time, s | 8.2 ± 2.6 |
| Chair-rise time, s | 7.0 ± 1.3 |
| MVC force, N | |
| Plantar flexion | 173 ± 64 |
| Dorsiflexion | 122 ± 45 |
| Force steadiness at 10% MVC force, % | |
| Plantar flexion | 4.3 ± 3.1 |
| Dorsiflexion | 7.1 ± 5.0 |
| Force steadiness at 20% MVC force, % | |
| Plantar flexion | 2.8 ± 2.5 |
| Dorsiflexion | 4.4 ± 2.7 |
Values are means ± SD. Force steadiness values are coefficient of variation for force (%). MVC, maximal voluntary contraction.
Subjects participated in a 6-wk intervention that involved 18 treatment sessions (3 sessions/wk) in which neuromuscular electrical stimulation (NMES) was applied to the calf muscles. Details of the protocol and the results of the NMES intervention are described elsewhere (Mani et al. 2018). The outcomes were assessed in evaluation sessions performed before the start of the intervention (week 0), between weeks 3 and 4 (here referred to as week 4), after the completion of the 6-wk intervention (week 7), and 3 wk after the end of the intervention (week 10). The evaluations included a 400-m walk, a 10-m walk at maximal and preferred speeds, and the chair-rise test. The same investigator measured all performances throughout the study. The NMES intervention elicited statistically significant improvements in all four tests of mobility but with different time courses: 400-m and maximal 10-m walk times were improved at week 4, with no further changes at weeks 7 and 10, whereas chair-rise time was improved at week 7, with no additional change at week 10 (Mani et al. 2018). Although most participants contributed data from more than one session to the data set, their performance on the mobility tests invariably differed across sessions and added to the overall variance in the outcome metrics. In the present report, therefore, each evaluation session is treated as an independent observation to examine the explanatory power of the outcome variables for the variance in the four tests of mobility. The intent of our present report is not to examine the progression of changes across the intervention.
Evaluation sessions.
The number of evaluation sessions completed by each participant ranged from one to four, but all dependent (the 4 mobility tests) and independent (muscle strength, muscle activation, and perceived fatigability) variables were measured in every session. The time to complete each mobility test was measured with a stopwatch. Walking endurance was characterized as the time taken to walk 400 m. Subjects walked 2.5 laps around an indoor track at a brisk pace while they were accompanied by one of the investigators. RPE was measured before and after the 400-m walk and at every half-lap (80 m) during the test (Vestergaard et al. 2009a). Participants were asked, “How hard are you exerting yourself?” at every half-lap (80-m increments) during the 400-m walk and responded with a number on a scale that ranged from 0 (rest) to 10 (maximal).
Walking speed was quantified as the time to walk 10 m along a 20-m indoor walkway. Subjects were asked to walk as quickly as possible (maximal walking speed) and at their usual, self-selected pace for preferred walking speed. Time was measured from the middle 10 m during each of three trials and averaged across trials (Clark et al. 2013). Subjects rested for at least 1 min between trials. Some subjects, however, had difficulty understanding the difference between walking quickly and jogging, which resulted in times <4.0 s being excluded from the analysis of maximal walking speed (24% of observations).
The chair-rise test provides a measure of dynamic balance, with the time it takes older adults to complete the test being moderately associated with the strength of leg muscles (including the ankle dorsiflexors), sensorimotor function, and psychological attributes (Lord et al. 2002). We were interested in investigating whether differences in the activation characteristics of lower leg muscles might also be associated with differences in the time to complete the chair-rise test. The chair-rise test was performed on a standard-height chair with no armrests (Bohannon et al. 2007). Starting in the seated position, each participant was instructed to keep the arms placed across the chest while standing up and sitting down five times as quickly as possible. Subjects rested for at least 1 min between each of the three trials. The average time of three trials was used in data analysis.
Muscle strength was measured for both legs separately as the peak force achieved during two to five maximal isometric contractions with the ankle dorsiflexors and plantar flexors. Each muscle group was tested in isolation with the subject in a supine position and the ankle held at a constant 90° angle (Almuklass et al. 2018; Mani et al. 2018). A strain gauge transducer (MLP-300; Transducer Techniques, Temecula, CA) was placed in series with a strap wrapped around the forefoot to measure the force exerted by the ankle muscles during each MVC. The MVC task required the subject to increase force from 0 to maximum over 3 s and to hold the maximal force for ~3 s. Subjects were encouraged verbally during the task and rested for at least 1 min between trials. Visual feedback of the MVC force was displayed on a monitor placed ~1 m in front of the participants (visual angle = ~0.25°). Force was sampled at 2 kHz with an analog-to-digital converter (Power 1401; Cambridge Electronic Design, Cambridge, UK). The maximal force reached in the first two trials in which peak force differed by ≤5% was denoted as the MVC force.
Force steadiness was assessed during submaximal isometric contractions with the dorsiflexors and plantar flexors of the dominant leg (Spike2 version 6; Cambridge Electronic Design, Cambridge, UK). The force signals were low-pass filtered (≤50 Hz; Coulbourn Instruments, Allentown, PA) and digitized at 1 kHz. The protocol involved two target forces to quantify the relative modulation of motor unit activity among the lower leg muscles: 10% MVC (Holmes et al. 2015; Tracy and Enoka 2002) and 20% MVC (Justice et al. 2014; Oshita et al. 2011). The target force and the applied force were displayed on the monitor, and subjects were asked to match the indicated target and then maintain a steady contraction for ~30 s. Two trials were performed at each target force. The digitized force signals were filtered with a 20-Hz low-pass, second-order Butterworth filter. To avoid aberrant fluctuations in the force signal, such as those due to corrective actions, the duration of each trial from which force steadiness was analyzed ranged from 10 s to 30 s based on visual inspection (Fig. 1D). Force steadiness was quantified as the amplitude of the normalized force fluctuations {coefficient of variation = [(standard deviation/mean) × 100]}.
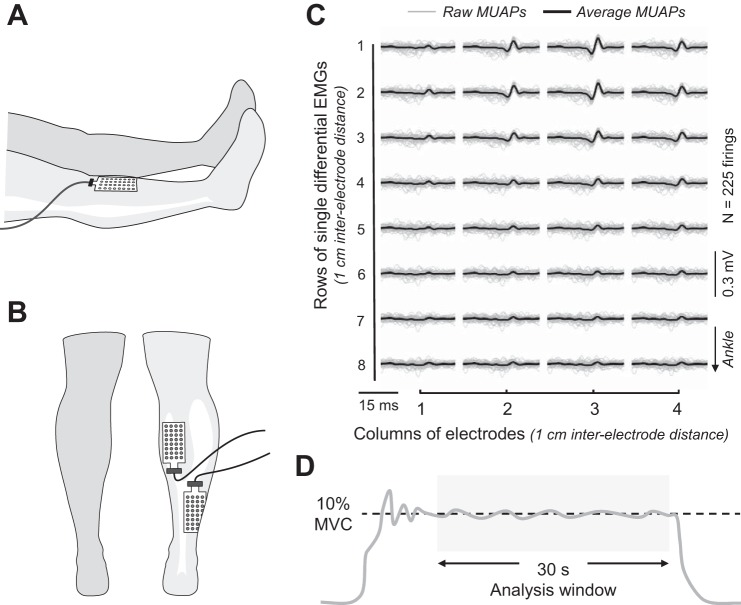
Fig. 1.
A and B: placement of high-density electrodes over tibialis anterior (A) and medial gastrocnemius and soleus (B) muscles. C: representative motor unit action potential (MUAP) waveforms from medial gastrocnemius during 30-ms intervals in each of the 4 columns. D: an example force signal during a steady isometric contraction. MVC, maximal voluntary contraction.
EMG signals were recorded during the steadiness tasks with high-density grid electrodes placed over medial gastrocnemius, the lateral half of soleus, and tibialis anterior of the dominant leg (Waterloo footedness score: 9.6 ± 5.9; Chapman et al. 1987). The high-density electrodes (W-EMG; Bitron, Turin, Italy) comprised two grids with 32 electrodes each (8 rows × 4 columns; interelectrode distance = 10 mm). The surface of the skin was prepared with an abrasive gel and isopropyl rubbing alcohol before placement of the electrodes. The position of the grid electrode for medial gastrocnemius was set at 2–4 cm distal from the popliteal fossa and centered over the medial head of gastrocnemius. The electrode for soleus was placed 2–4 cm proximal from the most superior portion of the Achilles tendon over the lateral aspect of muscle (Fig. 1B). The electrode for tibialis anterior was placed ~1 cm lateral to the center of the tibia over the belly of the muscle (Fig. 1A). Electrode placements were photographed during the first session with each subject and used as a reference in subsequent evaluation sessions.
To ensure an optimal electrical contact between the skin and the electrode and to minimize movement artifact, the grids were held in place with bioadhesive foam and conductive paste (Ten20; Weaver, Aurora, CO). Two reference electrodes (2 × 3.5 cm, conductive hydrogel, Kendall; Covidien, Mansfield, MA) were placed over the patella, tibia, or malleoli based on the accessibility of the landmark.
Data analysis.
Motor unit action potentials were discriminated off-line via a custom decomposition algorithm (Holobar et al. 2010; Holobar and Zazula 2007) written in MATLAB code (version R2013b; The MathWorks, Natick, MA) (Fig. 1C). Each decomposed signal was examined to exclude transient waveforms based on a series of criteria derived from previous work on single motor units (Barry et al. 2007; Moritz et al. 2005; Pascoe et al. 2014). First, any interspike interval (ISI) <25 ms or >400 ms was removed, and then any motor unit with a coefficient of variation for ISI <10% or >50% was rejected. The waveforms of all remaining motor units were visually inspected to ensure that they were consistent with expected shapes for motor unit action potentials. From an original 2,545 decomposed signals, 476 (19%) waveforms were discarded and 2,069 motor units were retained for analysis (see Tables 3 and 4). Most of the discarded waveforms (n = 342) were recorded during the steady contraction at the higher target force (20% MVC).
Table 3.
Motor unit numbers and discharge characteristics for the three test muscles at two target forces across the 6-wk NMES intervention
| Motor Units, n |
Mean ISI, ms |
CV for ISI, % |
ISI Skewness |
ISI Kurtosis |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10% | 20% | 10% | 20% | 10% | 20% | 10% | 20% | 10% | 20% | |
| Tibialis anterior | ||||||||||
| Week 0 | 159 | 138 | 101 ± 17 | 91 ± 20 | 27 ± 7 | 31 ± 7 | 1.8 ± 1.2 | 1.8 ± 1.0 | 9 ± 7 | 9 ± 7 |
| Week 4 | 137 | 132 | 93 ± 31 | 89 ± 17 | 28 ± 7 | 30 ± 7 | 1.9 ± 1.1 | 2.0 ± 1.3 | 9 ± 7 | 10 ± 7 |
| Week 7 | 129 | 123 | 97 ± 23 | 83 ± 20 | 27 ± 5 | 31 ± 9 | 1.7 ± 1.0 | 2.0 ± 1.3 | 8 ± 6 | 9 ± 7 |
| Week 10 | 80 | 83 | 89 ± 15 | 81 ± 15 | 28 ± 7 | 30 ± 6 | 2.0 ± 1.2 | 2.2 ± 1.1 | 9 ± 7 | 10 ± 7 |
| Medial gastrocnemius | ||||||||||
| Week 0 | 46 | 67 | 140 ± 44 | 131 ± 26 | 34 ± 7 | 32 ± 8 | 1.3 ± 0.9 | 1.6 ± 1.2 | 5 ± 6 | 5 ± 5 |
| Week 4 | 57 | 83 | 145 ± 21 | 125 ± 22 | 28 ± 9 | 30 ± 8 | 1.4 ± 1.1 | 1.7 ± 1.3 | 6 ± 6 | 8 ± 8 |
| Week 7 | 53 | 69 | 132 ± 7 | 118 ± 34 | 28 ± 9 | 30 ± 11 | 1.4 ± 1.4 | 1.5 ± 1.3 | 6 ± 6 | 7 ± 8 |
| Week 10 | 18 | 39 | 112 ± 51 | 119 ± 35 | 35 ± 9 | 32 ± 11 | 1.5 ± 0.7 | 2.1 ± 1.5 | 6 ± 4 | 10 ± 9 |
| Soleus | ||||||||||
| Week 0 | 94 | 104 | 149 ± 33 | 147 ± 31 | 28 ± 4 | 29 ± 6 | 1.3 ± 1.3 | 1.3 ± 1.2 | 6 ± 6 | 5 ± 6 |
| Week 4 | 73 | 89 | 138 ± 43 | 133 ± 32 | 29 ± 5 | 31 ± 6 | 1.3 ± 1.0 | 1.3 ± 1.3 | 5 ± 5 | 7 ± 6 |
| Week 7 | 69 | 119 | 147 ± 23 | 137 ± 14 | 30 ± 7 | 29 ± 4 | 1.4 ± 1.4 | 1.5 ± 1.1 | 6 ± 5 | 7 ± 6 |
| Week 10 | 41 | 77 | 119 ± 24 | 135 ± 22 | 28 ± 6 | 27 ± 6 | 1.3 ± 1.2 | 1.3 ± 1.2 | 7 ± 6 | 6 ± 6 |
Values are means ± SD. Targets are at % maximal voluntary contraction (MVC) force. CV, coefficient of variation; ISI, interspike interval; NMES, neuromuscular electrical stimulation.
Table 4.
Discharge characteristics of motor units in tibialis anterior, medial gastrocnemius, and soleus muscles during steady, isometric contractions at two target forces
| Tibialis Anterior | Medial Gastrocnemius | Soleus | Effect Size [P value] | |
|---|---|---|---|---|
| Motor units, n | 981 | 422 | 666 | |
| Mean ISI, ms | ||||
| 10% | 101 ± 25 | 143 ± 38* | 146 ± 36* | 0.61 [<0.001] |
| 20% | 92 ± 21 | 122 ± 28* | 141 ± 30*† | 0.68 [<0.001] |
| Effect size [P value] | 0.20 [<0.001] | 0.30 [<0.001] | 0.07 [0.067] | |
| Coefficient of variation for ISI (%) | ||||
| 10% | 27 ± 11 | 28 ± 11 | 28 ± 11 | 0.04 [0.57] |
| 20% | 28 ± 11 | 27 ± 12 | 28 ± 11 | 0.06 [0.10] |
| Effect size [P value] | 0.04 [0.21] | 0.02 [0.63] | 0.04 [0.36] | |
| ISI distribution skewness | ||||
| 10% | 1.8 ± 1.1 | 1.1 ± 1.1* | 1.3 ± 1.2* | 0.23 [<0.001] |
| 20% | 2.0 ± 1.2 | 1.6 ± 1.3* | 1.3 ± 1.2*† | 0.25 [<0.001] |
| Effect size [P value] | 0.07 [0.023] | 0.01 [0.047] | 0.016 [0.68] | |
| ISI distribution kurtosis | ||||
| 10% | 9 ± 10 | 6 ± 8* | 7 ± 8* | 0.23 [<0.001] |
| 20% | 11 ± 11 | 9 ± 11* | 7 ± 8* | 0.23 [<0.001] |
| Effect size [P value] | 0.06 [0.06] | 0.20 [0.001] | 0.018 [0.65] |
Values are means ± SD. Targets are at % maximal voluntary contraction forces indicated. ISI, interspike interval.
P < 0.05 relative to tibialis anterior.
P < 0.05 relative to medial gastrocnemius.
Measurements acquired in each of the evaluation sessions were treated as independent outcomes to assess the strength of the associations between motor unit discharge characteristics and force steadiness and the performance times on the mobility tests. Although average discharge characteristics across two trials at each target force indicated that different sets of motor units were recorded during each trial, some motor units were likely recorded during both trials at each target force (Martinez-Valdes et al. 2017) and at the two target forces. Consequently, only motor units recorded during the first trial at each target force were used in the analysis.
Statistics.
Normality was assessed with the Kolmogorov-Smirnov (≥50 observations) and Shapiro-Wilk (<50 observations) tests. The data for all variables were not normally distributed and were evaluated with nonparametric tests. Data obtained from the four evaluation sessions were examined with Friedman’s test, values for the three muscles were compared with the Kruskal-Wallis test and Dunn’s test with Bonferroni adjustments for post hoc analysis, and the results for two target forces were evaluated with the Mann-Whitney U-test. The effect size for the Mann-Whitney U-test was calculated with (where z = Z value and n1 and n2 = number of observations in each group) and that for the Kruskal-Wallis test was determined with (where n = number of observations). Effect sizes of 0.2 were considered small, and those ≥ 0.8 were considered large (Cohen 1988).
Spearman correlations evaluated associations between the four measures of mobility (400-m walk time, maximal walk time, preferred walk time, and chair-rise time) and the performance characteristics (dorsiflexion and plantar flexion MVC forces, force steadiness, motor unit discharge characteristics, and perceived fatigability). The variables that were significantly correlated (P < 0.05; Table 5) were entered into a stepwise, linear, multiple-regression analysis to identify those characteristics most strongly associated with the variance in each mobility outcome. The number of explanatory variables depended on the unique amount of variance explained by each variable. When the regression analysis suggested a single explanatory variable, the strength of the association was reported as a Pearson’s r correlation. Multicollinearity was estimated with variance inflation factor (VIF). Normality tests and Cook’s distance criteria were performed to remove up to three outliers (Cook 1977) in order to ensure that the residuals were normally distributed.
Table 5.
Correlation coefficients between times for mobility tests and performance characteristics
| 400-m Walk, s | Max Walk, s | Preferred Walk, s | Chair Rise, s | |
|---|---|---|---|---|
| Tibialis anterior | ||||
| Mean ISI, ms | 0.024 | −0.011 | −0.003 | 0.267† |
| Mean ISI, %∆ | −0.108 | −0.027 | 0.148 | −0.065 |
| CV for ISI, % | −0.109 | −0.121 | −0.-67 | −0.193 |
| Skewness | −0.047 | −0.061 | −0.087 | −0.005 |
| Kurtosis | −0.252 | −0.079 | −0.293† | −0.040 |
| Medial gastrocnemius | ||||
| Mean ISI, ms | 0.061 | 0.378† | −0.023 | 0.172 |
| Mean ISI, %∆ | 0.053 | 0.228 | 0.248 | 0.259 |
| CV for ISI, % | 0.088 | 0.206 | 0.334 | −0.019 |
| Skewness | −0.217 | −0.360* | −0.099 | −0.294 |
| Kurtosis | −0.221 | −0.363* | −0.183 | −0.157 |
| Soleus | ||||
| Mean ISI, ms | 0.396† | 0.495* | 0.223 | 0.447* |
| Mean ISI, %∆ | 0.193 | 0.318 | 0.213 | 0.197 |
| CV for ISI, % | −0.193 | 0.035 | −0.035 | −0.030 |
| Skewness | −0.344* | −0.359* | −0.266 | −0.344* |
| Kurtosis | −0.285 | −0.252 | −0.305 | −0.285 |
| Plantar flexion | ||||
| MVC force, N | 0.077 | 0.041 | −0.071 | −0.063 |
| Force steadiness, % | 0.088 | 0.087 | 0.050 | 0.175 |
| Dorsiflexion | ||||
| MVC force, N | −0.082 | −0.036 | −0.043 | 0.155 |
| Force steadiness, % | 0.035 | 0.137 | 0.119 | −0.082 |
| RPE | ||||
| Start | 0.124 | 0.203 | 0.171 | 0.172 |
| 80 m | −0.001 | 0.048 | 0.007 | −0.107 |
| 160 m | −0.175 | −0.173 | −0.131 | −0.174 |
| 240 m | −0.261† | −0.207 | −0.248† | −0.207 |
| 320 m | −0.426* | −0.314* | −0.392* | −0.484* |
| End | −0.389* | −0.379* | −0.385* | −0.484* |
| Difference (start − end) | −0.366* | −0.376* | −0.276† | −0.515* |
Motor unit discharge characteristics are from the 10% maximal voluntary contraction (MVC) target force. Rating of perceived exertion (RPE) was acquired every half-lap during the 400-m walk test. Force steadiness values are coefficient of variation (CV) for force (%) at 10% MVC force. ISI, interspike interval; mean ISI (%∆), difference in mean ISI between the values at the 2 target forces (10% and 20% MVC).
P < 0.01.
P < 0.05.
Motor unit ISI distributions were characterized with measurements of skewness (degree of symmetry) and kurtosis (breadth). A normal distribution has a skewness value of 0 and a kurtosis of 3; a skewness of 1.2 denotes a greater than normal proportion of longer ISIs (positively skewed), whereas a kurtosis of 3.5 indicates a narrow distribution with values clustering about a central value. The reason for measuring both skewness and kurtosis is that we have previously found different adjustments in the coefficient of variation for ISI and the ISI distribution metrics during long-duration contractions performed by older adults (Pascoe et al. 2014). All statistical procedures were performed with SPSS (version 24.0, SPSS, Chicago, IL) with α set to 0.05.
RESULTS
The data were obtained from 20 subjects in 69 successful evaluation sessions. The number of evaluation sessions (weeks 0, 4, 7, and 10) from which acceptable motor unit recordings were obtained ranged from one to four for the three muscles. Also, the number of subjects from whom detectable motor unit potentials could be recorded varied across the intervention because of technical difficulties, and five of the subjects were not able to perform the evaluation session at week 10 (Table 2).
Table 2.
Number of subjects from whom acceptable motor unit recordings were obtained
| Week 0 | Week 4 | Week 7 | Week 10 | |
|---|---|---|---|---|
| Tibialis anterior | 18 | 17 | 13 | 10 |
| Medial gastrocnemius | 13 | 12 | 11 | 10 |
| Soleus | 14 | 12 | 11 | 9 |
Recordings were obtained for 3 lower leg muscles in the 4 evaluation sessions (weeks 0, 4, 7, and 10) before, during, and after the 6-wk intervention.
Motor unit characteristics.
There were no statistically significant differences in discharge characteristics across evaluation time points or between target forces (Table 3). However, there were some small differences in the discharge characteristics of motor units at the two target forces across the three muscles (Table 4). Within muscles, mean ISI was briefer at 20% than 10% MVC force for tibialis anterior (effect size = 0.20; P < 0.001) and medial gastrocnemius (effect size = 0.30; P < 0.001) but not soleus (effect size = 0.07; P = 0.067). Across muscles, post hoc analysis indicated that mean ISI was longer for medial gastrocnemius and soleus than tibialis anterior at both 10% (effect size = 0.61; P < 0.001) and 20% (effect size = 0.68; P < 0.001) target forces. Also, mean ISI was longer for soleus than medial gastrocnemius at 20% MVC force (effect size = 0.07; P < 0.05).
Although the coefficient of variation for ISI was not significantly different for any given muscle at either the 10% (effect size = 0.04; P = 0.57) or the 20% (effect size = 0.06; P = 0.10) MVC target force, ISI skewness was less in the medial gastrocnemius and soleus muscles than in tibialis anterior at both 10% (effect size = 0.23; P < 0.001) and 20% (effect size = 0.25; P < 0.001) target forces. Also, ISI kurtosis was significantly less in the medial gastrocnemius and soleus muscles than in tibialis anterior at both the 10% (effect size = 0.23; P < 0.001) and the 20% (effect size = 0.23; P < 0.001) target forces. Moreover, ISI skewness was greater for medial gastrocnemius than for lateral soleus at 20% MVC force (P < 0.05).
Associations between outcome variables.
Mean ISI and skewness for soleus during isometric plantar flexion at 10% MVC force were positively correlated with the time to walk 400 m (r = 0.396, r = −0.344), maximal 10-m walk time (r = 0.495, r = −0.359), and time to complete the chair-rise test (r = 0.447, r = −0.344) (Table 5). Mean ISI (r = 0.378), skewness (r = −0.360), and kurtosis (r = −0.363) for medial gastrocnemius at 10% MVC force were correlated with maximal 10-m walk time. Mean ISI (r = 0.267) and kurtosis (r = −0.293) for tibialis anterior at 10% MVC force were correlated with chair-rise time and 10-m preferred walk time, respectively. The modulation of mean ISI from 10% to 20% MVC force, the coefficient of variation for ISI at 10% MVC force, MVC force, and force steadiness were not significantly correlated with any of the mobility tests (Table 5).
RPE after completion of the first 1.5 laps of the 400-m walk test (240-m mark) was negatively correlated with the 400-m time (r = −0.261) and preferred 10-m walk time (r = −0.248). RPE values were significantly correlated for all remaining half-laps of all four mobility measures (Table 5). The negative correlations were interpreted to indicate that individuals who completed the testing of walking endurance more quickly were willing to exert a greater level of effort.
Regression models.
The correlation results (Table 5) were used to perform a stepwise, multiple-regression analysis to construct models that explained significant amounts of the variance in the mobility tests. Because of the absence of statistically significant correlations for the modulation of mean ISI between the two target forces (10% and 20% MVC) with any of the mobility tests, the initial regression analysis was performed with only the data from the lower target force (10% MVC). To assess the reliability of the regression models, however, we repeated the analysis with the data from both target forces. With the inclusion of discharge characteristics of motor units at both target forces, the amount of variance explained by the models was increased for maximal and preferred 10-m walk times but not 400-m walk time or chair-rise time. Moreover, two of the models (preferred 10-m walk and chair rise) included motor unit data from the isometric contractions at 20% MVC force. The reported regression models contain the sets of outcome variables that could explain most of the variance in each mobility measurement.
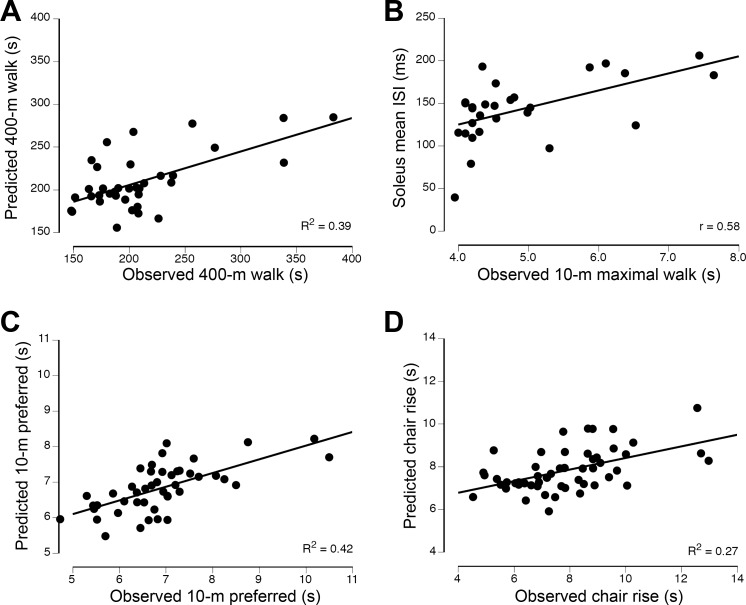
The outcome variables explained 39% of the variance (P = 0.049) in the 400-m walk time (Fig. 2A) with two explanatory variables: RPE recorded at the end of the 400-m walk test (partial r = −0.370, VIF = 1.455, β = −0.374, P = 0.029) and mean ISI for soleus during plantar flexion at 10% MVC force (partial r = 0.336, VIF = 1.455, β = 0.335, P = 0.049). Two variables explained 42% of the variance in the preferred 10-m walk time (Fig. 2C): RPE recorded at the end of the 400-m walk test (partial r = −0.571, VIF = 1.034, β = −0.538, P < 0.001) and coefficient of variation for ISI of motor units in medial gastrocnemius during plantar flexion at 20% MVC force (partial r = 0.339, VIF = 1.034, β = 0.279, P = 0.028). The regression analysis identified a single variable to explain 33% of the variance in maximal 10-m walk time (Fig. 2B): mean ISI for soleus during plantar flexion at 10% MVC force (Pearson’s r = 0.558, P = 0.002). The regression model for the chair-rise test explained 27% of the variance and comprised two explanatory variables (Fig. 2D): RPE recorded at the end of the 400-m walk test (partial r = −0.455, VIF = 1.003, β = −0.436, P = 0.001) and mean ISI for motor units in tibialis anterior during dorsiflexion at 20% MVC force (partial r = 0.296, VIF = 1.003, β = 0.265, P = 0.03).
Fig. 2.
Associations between the observed times to complete the 400-m walk (A), maximal 10-m walk (B), preferred 10-m walk (C), and chair-rise (D) tests and the physical capabilities of the lower leg muscles. The explanatory variables (R2 = 0.39) for 400-m walk time were the rating of perceived exertion at the end of the 400-m walk and mean interspike interval (ISI) for motor units in the soleus during 10% maximal voluntary contraction (MVC) force steadiness contractions. The explanatory variable (r = 0.558) for 10-m maximal walk time was mean ISI for motor units in the soleus during 10% MVC force steadiness task. The explanatory variables (R2 = 0.42) for 10-m preferred walk time were rating of perceived exertion at the end of the 400-m walk and the coefficient of variation for ISI of motor units in medial gastrocnemius during the 20% MVC force steadiness task. The explanatory variables (R2 = 0.27) for chair-rise time were rating of perceived exertion at the end of the 400-m walk test and mean ISI for motor units in tibialis anterior during the 20% MVC force steadiness task.
DISCUSSION
The key findings of our study were that the outcome variables explained modest amounts of the variance (27–42%) in the performance of healthy older adults on four standard tests of mobility. Partially consistent with our hypothesis, the explanatory variables comprised measures of muscle activation (discharge characteristics of motor units in soleus, medial gastrocnemius, and tibialis anterior) and perceived fatigability but not the strength of the lower leg muscles. Significantly, one of the explanatory variables for all four mobility tests was a measure of the discharge characteristics of motor units in a lower leg muscle during steady isometric contractions.
The time it took the participants enrolled in our study (73 ± 4 yr) to complete each mobility test was often faster than the values reported in other studies. For example, our participants completed the 400-m walk in 211 ± 47 s, which was less than the average time (270 s) it took the fastest group (n = 196) of older adults (>65 yr) to walk 400 m in a study that enrolled 948 healthy participants (Vestergaard et al. 2009b). Similarly, our subjects completed five repetitions of the sit-to-stand task in 7.0 ± 1.3 s, which was faster than the average values of 11.4 s for individuals aged 60–69 yr, 12.6 s for those aged 70–79 yr, and 14.8 s for those aged 80–89 yr, which were derived from a meta-analysis (Bohannon 2006). Consistent with these differences, the maximal walking speed of our participants (2.0 ± 0.1 m/s) was within the fast (2.2 ± 0.2 m/s) and slow (1.8 ± 0.2 m/s) speeds for a group (n = 20; 65–80 yr) of well-functioning older adults (Clark et al. 2013).
As we found in a study on persons with multiple sclerosis (Almuklass et al. 2018), the variance in walking performance is partially related to the discharge characteristics of motor units in lower leg muscles during submaximal isometric contractions. In our present study, mean ISI in soleus exhibited positive partial r values indicating that briefer mean ISIs—greater discharge rates—during the isometric contraction were associated with a faster time to complete two of the walking tests of mobility (400-m walk and maximal 10-m walk). Similarly, mean ISI for soleus (partial r = 0.51) was one of the explanatory variables for the variance in maximal gait speed (25-ft walk) for persons with multiple sclerosis who had walking limitations, whereas it was mean ISI for medial gastrocnemius (partial r = –0.48) that emerged as an explanatory variable for the variance in walking endurance (6-min walk) for persons with multiple sclerosis (Almuklass et al. 2018). The dominant role of soleus during tests of mobility was underscored by the finding that its absolute EMG amplitude, as measured with high-density surface electrodes, was greater during walking than that for medial gastrocnemius (Mani et al. 2017).
Our finding of a strong influence of motor unit activity in soleus on tests of mobility is consistent with fine-wire recordings of single-motor unit activity in calf muscles during standing balance (Héroux et al. 2014). The single-motor unit recordings indicated that balance was maintained with little motor unit activity in lateral gastrocnemius, intermittent motor unit activity in medial gastrocnemius, and continuous motor unit activity in soleus. The coefficient of variation for ISI averaged 41% for the medial gastrocnemius motor units and 19% for the soleus motor units, and these values were ~8% greater than those observed during a steady isometric contraction. Consistent with these differences in discharge characteristics of single motor units during standing, there were marked differences in the recruitment thresholds as measured from the net plantar flexor muscle torque during an isometric ramp-and-hold contraction. Average recruitment thresholds measured during isometric ramp-and-hold contractions for the active motor units were 67% for lateral gastrocnemius, 3% for medial gastrocnemius, and 2% for soleus relative to the maximal plantar flexor torque observed during standing balance (Héroux et al. 2014). Although the muscle actions involved in maintaining standing balance differ from those engaged during the mobility tests used in our study, it seems that greater reliance is placed on the soleus motor units during both tasks.
Despite these differences in average discharge characteristics and recruitment thresholds for motor units in the three lower leg muscles, the key question is why does between-subject variance in mean ISI during a steady, submaximal contraction explain statistically significant amounts of the variance in 400-m and 10-m walk times of older adults? Over most of the operating range of a muscle, the force it generates for a specific task is controlled by the concurrent recruitment and rate coding of the motor units in the muscle (Enoka and Duchateau 2017). One of the more significant consequences of aging for the motor system is the marked remodeling of motor unit territories, including a reduction in the number of motor neurons and an increase in the innervation number of the surviving motor units (Hepple and Rice 2016). Significantly, the remodeling rate differs across individuals and is at least partially dependent on the lifetime level of physical activity (Power et al. 2016). Given that our participants exhibited a range of preferred (0.9–2.0 m/s) and maximal (1.3–2.5 m/s) walking speeds, their lower leg muscles likely comprised variable amounts of motor unit remodeling. To achieve the specified target force (10% MVC) for the steady contraction, therefore, our participants would have used different combinations of recruitment and rate coding (Piasecki et al. 2016; Watanabe et al. 2016).
As we observed in a study on individuals with multiple sclerosis (Almuklass et al. 2018), several of the discharge characteristics (mean and coefficient of variation for ISI, skewness, and kurtosis) measured during steady isometric contractions with the plantar flexors (medial gastrocnemius and soleus) and dorsiflexors (tibialis anterior) were associated with times to complete the mobility tests. The regression analyses indicated there was considerable overlap in the variance of the performance times explained by the motor unit discharge characteristics. Our findings were that individuals with faster discharge rates (shorter ISIs) in soleus motor units during the steady contraction at 10% MVC force performed two of the walking tests (400 m and maximal 10 m) more quickly. However, it was the discharge characteristics at 20% MVC force that explained significant amounts of the variance in the other two mobility tests (preferred 10 m and chair rise): greater variability (coefficient of variation) in the ISIs for motor units in medial gastrocnemius was associated with slower preferred walking speeds, and longer mean ISIs in tibialis anterior were associated with slower times to stand up and sit down five times. When participants were asked to walk at a brisk pace, therefore, those who employed greater discharge rates of soleus motor units during the steady isometric contraction had faster times for the walking tasks. When walking at a preferred speed, however, it was the regularity of the discharge times for medial gastrocnemius motor units that was more strongly associated with the time to walk 10 m. Although previous work has demonstrated that greater fatigability of the dorsiflexors is associated with lower levels of mobility in older adults (Justice et al. 2014), our study appears to be the first to report an association between the discharge characteristics of tibialis anterior motor units during an isometric contraction and the performance of older adults on a test of mobility (chair-rise test).
Perceived fatigability, an explanatory variable for three of the four mobility tests, is strongly associated with mobility limitations in older adults (Simonsick et al. 2016; Vestergaard et al. 2009a, 2009b; Wanigatunga et al. 2018). For example, Simonsick et al. (2016) found that the Borg rating of perceived exertion (RPE)—an index of perceived fatigability—after 5 min of walking on a treadmill at 1.5 mph predicted clinically meaningful declines in gait speed and walking ability ~2.1 yr later in older adults. Consistent with this result, we found that RPE after completion of the 400-m distance was significantly associated with the variance in the time it took the older adults to complete the test of walking endurance. The negative correlation between RPE and 400-m time indicates that individuals who achieved a greater level of exertion performed the test more quickly. Similarly, Simonsick et al. (2014) found that older adults who reported an RPE > 10 (6–20 scale) after the 5-min treadmill walk performed significantly worse on a chair-rise test than those with lower RPE scores.
Perceived fatigability is one of two domains that influence the self-reported level of fatigue (Enoka and Duchateau 2016). It can be modulated either by adjustments related to the maintenance of homeostasis or by the psychological state of the individual. The key outcome in our study was that the RPEs were negatively correlated with time to complete the 400-m walk, preferred walking speed, and the chair-rise test. The RPEs were obtained during the test of walking endurance (400-m walk), with the associated values arising at the end of the test. The associations indicate that individuals who were willing to achieve a greater level of exertion during the 400-m walk also preferred to walk more quickly and performed the chair-rise test more quickly. The most unexpected outcome was the strength of the association between RPE and chair-rise time, which is typically assumed to be limited by the strength of the knee extensor and hip flexor muscles (Corrigan and Bohannan 2001; Dehail et al. 2007; Gross et al. 1998). However, Lord et al. (2002) found that the strength of the knee extensors accounted for only one-half of the explained variance in sit-to-stand performance of older adults (n = 669; >75 yr) and that the other explanatory variables included two other strength measures, four sensorimotor variables, and three psychological attributes. Of relevance to our study, the psychological attributes associated with sit-to-stand performance were anxiety, vitality, and pain, which were interpreted as providing measures of motivation and apprehension. The regression model reported by Lord et al. (2002), however, only explained 35% of the variance in sit-to-stand performance, which suggests that performance on this test by older adults is not primarily dependent on the strength of the knee extensor muscles. Among our outcomes, the relative willingness of participants to exert themselves (perceived fatigability) emerged as the one of the explanatory variables for the variance in chair-rise times, and the other was mean ISI of motor units in tibialis anterior at 20% MVC force.
Although our study identified several variables that could explain moderate amounts of the variance in the time it took older adults to complete four mobility tests, a significant portion of the variability in the performance of these tests remains unsolved, possibly because of limitations in our experimental protocol. One limitation of our study was the technical difficulties we experienced during the intervention with the measurements of muscle strength, which reduced the number of observations that could be included in the analysis. Also, some of the participants had difficulty not jogging when we asked them to walk quickly. In addition, the approach could be broadened to evaluate the potential explanatory power of the strength and force steadiness of the muscles around the knee and hip joints, especially given the adjustments in joint power that have been observed in older adults.
In summary, our findings indicate that modest amounts of the variance in clinical tests of mobility performed by older adults could be explained by perceived fatigability (RPE) as assessed during the test of walking endurance (400-m walk) and the discharge characteristics of motor units in the soleus, medial gastrocnemius, and tibialis anterior muscles during submaximal isometric contractions. RPEs were negatively correlated with time to complete three of the mobility tests, which suggests that the willingness of participants to exert themselves was significantly associated with walking endurance, preferred walking speed, and standing from a seated position. The motor unit results indicate that faster discharge rates (soleus and tibialis anterior) and greater ISI variability (medial gastrocnemius) during the steady contractions were associated with faster times to complete the walking tests of mobility. Of the two sets of variables that were correlated with three of the mobility tests, the explanatory power of the RPE was more strongly associated with the time to complete each test.
GRANTS
This work was supported by a National Institute on Aging T32 Grant (AG-000279) awarded to Robert S. Schwartz.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.M., A.M.A., and R.M.E. conceived and designed research; D.M. performed experiments; D.M., A.M.A., T.M.V., and A.B. analyzed data; D.M., A.M.A., and R.M.E. interpreted results of experiments; D.M. prepared figures; D.M. and R.M.E. drafted manuscript; D.M., A.M.A., and R.M.E. edited and revised manuscript; D.M., A.M.A., T.M.V., A.B., and R.M.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank collaborators at LISiN, Department of Electronics and Telecommunications, Politecnico di Torino, for loaning us a high-density EMG system. We also thank Melissa Mazzo for assisting us with Fig. 1.
REFERENCES
- Almuklass AM, Davis L, Hamilton LD, Vieira TM, Botter A, Enoka RM. Motor unit discharge characteristics and walking performance of individuals with multiple sclerosis. J Neurophysiol 119: 1273–1282, 2018. doi: 10.1152/jn.00598.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry BK, Pascoe MA, Jesunathadas M, Enoka RM. Rate coding is compressed but variability is unaltered for motor units in a hand muscle of old adults. J Neurophysiol 97: 3206–3218, 2007. doi: 10.1152/jn.01280.2006. [DOI] [PubMed] [Google Scholar]
- Beauchamp MK, Leveille SG, Patel KV, Kiely DK, Phillips CL, Bandinelli S, Ferrucci L, Guralnik J, Bean JF. What physical attributes underlie self-reported vs. observed ability to walk 400 m in later life? An analysis from the InCHIANTI Study. Am J Phys Med Rehabil 93: 396–404, 2014. doi: 10.1097/PHM.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best JR, Liu-Ambrose T, Boudreau RM, Ayonayon HN, Satterfield S, Simonsick EM, Studenski S, Yaffe K, Newman AB, Rosano C; Health, Aging and Body Composition Study . An evaluation of the longitudinal, bidirectional associations between gait speed and cognition in older women and men. J Gerontol A Biol Sci Med Sci 71: 1616–1623, 2016. doi: 10.1093/gerona/glw066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohannon RW. Reference values for the five-repetition sit-to-stand test: a descriptive meta-analysis of data from elders. Percept Mot Skills 103: 215–222, 2006. doi: 10.2466/pms.103.1.215-222. [DOI] [PubMed] [Google Scholar]
- Bohannon RW, Shove ME, Barreca SR, Masters LM, Sigouin CS. Five-repetition sit-to-stand test performance by community-dwelling adults: a preliminary investigation of times, determinants, and relationship with self-reported physical performance. Isokinet Exerc Sci 15: 77–81, 2007. [Google Scholar]
- Cesari M, Pahor M, Lauretani F, Zamboni V, Bandinelli S, Bernabei R, Guralnik JM, Ferrucci L. Skeletal muscle and mortality results from the InCHIANTI Study. J Gerontol A Biol Sci Med Sci 64A: 377–384, 2009. doi: 10.1093/gerona/gln031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman JP, Chapman LJ, Allen JJ. The measurement of foot preference. Neuropsychologia 25: 579–584, 1987. doi: 10.1016/0028-3932(87)90082-0. [DOI] [PubMed] [Google Scholar]
- Clark DJ, Manini TM, Fielding RA, Patten C. Neuromuscular determinants of maximum walking speed in well-functioning older adults. Exp Gerontol 48: 358–363, 2013. doi: 10.1016/j.exger.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences (2nd ed.). Hillsdale, NJ: Erlbaum, 1988. [Google Scholar]
- Cook RD. Detection of influential observation in linear regression. Technometrics 19: 15–18, 1977. doi: 10.2307/1268249 [DOI] [Google Scholar]
- Corrigan D, Bohannon RW. Relationship between knee extension force and stand-up performance in community-dwelling elderly women. Arch Phys Med Rehabil 82: 1666–1672, 2001. doi: 10.1053/apmr.2001.26811. [DOI] [PubMed] [Google Scholar]
- Dehail P, Bestaven E, Muller F, Mallet A, Robert B, Bourdel-Marchasson I, Petit J. Kinematic and electromyographic analysis of rising from a chair during a “sit-to-walk” task in elderly subjects: role of strength. Clin Biomech (Bristol, Avon) 22: 1096–1103, 2007. doi: 10.1016/j.clinbiomech.2007.07.015. [DOI] [PubMed] [Google Scholar]
- DeVita P, Hortobágyi T. Age causes a redistribution of joint torques and powers during gait. J Appl Physiol (1985) 88: 1804–1811, 2000. doi: 10.1152/jappl.2000.88.5.1804. [DOI] [PubMed] [Google Scholar]
- Enoka RM, Duchateau J. Translating fatigue to human performance. Med Sci Sports Exerc 48: 2228–2238, 2016. doi: 10.1249/MSS.0000000000000929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoka RM, Duchateau J. Rate coding and the control of muscle force. Cold Spring Harb Perspect Med 7: a029702, 2017. doi: 10.1101/cshperspect.a029702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina D, Negro F. Common synaptic input to motor neurons, motor unit synchronization, and force control. Exerc Sport Sci Rev 43: 23–33, 2015. doi: 10.1249/JES.0000000000000032. [DOI] [PubMed] [Google Scholar]
- Feeney DF, Mani D, Enoka RM. Variability in common synaptic input to motor neurons modulates both force steadiness and pegboard time in young and older adults. J Physiol 596: 3793–3806, 2018. doi: 10.1113/JP275658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Cooper R, Shardell M, Simonsick EM, Schrack JA, Kuh D. Age-related change in mobility: perspectives from life course epidemiology and geroscience. J Gerontol A Biol Sci Med Sci 71: 1184–1194, 2016. doi: 10.1093/gerona/glw043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz JR, Kram R. Advanced age and the mechanics of uphill walking: a joint-level, inverse dynamic analysis. Gait Posture 39: 135–140, 2014. doi: 10.1016/j.gaitpost.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galganski ME, Fuglevand AJ, Enoka RM. Reduced control of motor output in a human hand muscle of elderly subjects during submaximal contractions. J Neurophysiol 69: 2108–2115, 1993. 10.1152/jn.1993.69.6.2108. [DOI] [PubMed] [Google Scholar]
- Gross MM, Stevenson PJ, Charette SL, Pyka G, Marcus R. Effect of muscle strength and movement speed on the biomechanics of rising from a chair in healthy elderly and young women. Gait Posture 8: 175–185, 1998. doi: 10.1016/S0966-6362(98)00033-2. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 332: 556–562, 1995. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepple RT, Rice CL. Innervation and neuromuscular control in ageing skeletal muscle. J Physiol 594: 1965–1978, 2016. doi: 10.1113/JP270561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Héroux ME, Dakin CJ, Luu BL, Inglis JT, Blouin JS. Absence of lateral gastrocnemius activity and differential motor unit behavior in soleus and medial gastrocnemius during standing balance. J Appl Physiol (1985) 116: 140–148, 2014. doi: 10.1152/japplphysiol.00906.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks GE, Shardell M, Alley DE, Miller RR, Bandinelli S, Guralnik J, Lauretani F, Simonsick EM, Ferrucci L. Absolute strength and loss of strength as predictors of mobility decline in older adults: the InCHIANTI study. J Gerontol A Biol Sci Med Sci 67A: 66–73, 2012. doi: 10.1093/gerona/glr055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MR, Gould JR, Peña-González I, Enoka RM. Force steadiness during a co-contraction task can be improved with practice, but only by young adults and not by middle-aged or old adults. Exp Physiol 100: 182–192, 2015. doi: 10.1113/expphysiol.2014.083741. [DOI] [PubMed] [Google Scholar]
- Holobar A, Minetto MA, Botter A, Negro F, Farina D. Experimental analysis of accuracy in the identification of motor unit spike trains from high-density surface EMG. IEEE Trans Neural Syst Rehabil Eng 18: 221–229, 2010. doi: 10.1109/TNSRE.2010.2041593. [DOI] [PubMed] [Google Scholar]
- Holobar A, Zazula D. Multichannel blind source separation using a convolution kernel compensation. IEEE Trans Signal Process 55: 4487–4496, 2007. doi: 10.1109/TSP.2007.896108. [DOI] [Google Scholar]
- Justice JN, Mani D, Pierpoint LA, Enoka RM. Fatigability of the dorsiflexors and associations among multiple domains of motor function in young and old adults. Exp Gerontol 55: 92–101, 2014. doi: 10.1016/j.exger.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen G, Knight CA. Training-related adaptations in motor unit discharge rate in young and older adults. J Gerontol A Biol Sci Med Sci 59: 1334–1338, 2004. doi: 10.1093/gerona/59.12.1334. [DOI] [PubMed] [Google Scholar]
- Klass M, Baudry S, Duchateau J. Age-related decline in rate of torque development is accompanied by lower maximal motor unit discharge frequency during fast contractions. J Appl Physiol (1985) 104: 739–746, 2008. doi: 10.1152/japplphysiol.00550.2007. [DOI] [PubMed] [Google Scholar]
- Laidlaw DH, Bilodeau M, Enoka RM. Steadiness is reduced and motor unit discharge is more variable in old adults. Muscle Nerve 23: 600–612, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- Lord SR, Murray SM, Chapman K, Munro B, Tiedemann A. Sit-to-stand performance depends on sensation, speed, balance, and psychological status in addition to strength in older people. J Gerontol A Biol Sci Med Sci 57: M539–M543, 2002. doi: 10.1093/gerona/57.8.M539. [DOI] [PubMed] [Google Scholar]
- Mani D, Almuklass AM, Amiridis IG, Enoka RM. Neuromuscular electrical stimulation can improve mobility in older adults but the time course varies across tasks: double-blind, randomized trial. Exp Gerontol 108: 269–275, 2018. doi: 10.1016/j.exger.2018.04.018. [DOI] [PubMed] [Google Scholar]
- Mani D, Almuklass AM, Hamilton LD, Vieira T, Botter A, Enoka RM. High-density surface EMG recordings of calf muscle activity in older adults during walking. Annual Meeting of the American Society of Biomechanics, Boulder, CO, August 2017. [Google Scholar]
- Martinez-Valdes E, Negro F, Laine CM, Falla D, Mayer F, Farina D. Tracking motor units longitudinally across experimental sessions with high-density surface electromyography. J Physiol 595: 1479–1496, 2017. doi: 10.1113/JP273662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz CT, Barry BK, Pascoe MA, Enoka RM. Discharge rate variability influences the variation in force fluctuations across the working range of a hand muscle. J Neurophysiol 93: 2449–2459, 2005. doi: 10.1152/jn.01122.2004. [DOI] [PubMed] [Google Scholar]
- Muehlbauer T, Granacher U, Borde R, Hortobágyi T. Non-discriminant relationships between leg muscle strength, mass and gait performance in healthy young and old adults. Gerontology 64: 11–18, 2018. doi: 10.1159/000480150. [DOI] [PubMed] [Google Scholar]
- Negro F, Holobar A, Farina D. Fluctuations in isometric muscle force can be described by one linear projection of low-frequency components of motor unit discharge rates. J Physiol 587: 5925–5938, 2009. doi: 10.1113/jphysiol.2009.178509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, Pahor M, Satterfield S, Brach JS, Studenski SA, Harris TB. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA 295: 2018–2026, 2006. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- Oshita K, Yano S. Low-frequency force steadiness practice in plantar flexor muscle reduces postural sway during quiet standing. J Physiol Anthropol 30: 233–239, 2011. doi: 10.2114/jpa2.30.233. [DOI] [PubMed] [Google Scholar]
- Pascoe MA, Holmes MR, Stuart DG, Enoka RM. Discharge characteristics of motor units during long-duration contractions. Exp Physiol 99: 1387–1398, 2014. doi: 10.1113/expphysiol.2014.078584. [DOI] [PubMed] [Google Scholar]
- Piasecki M, Ireland A, Stashuk D, Hamilton-Wright A, Jones DA, McPhee JS. Age-related neuromuscular changes affecting human vastus lateralis. J Physiol 594: 4525–4536, 2016. doi: 10.1113/JP271087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power GA, Allen MD, Gilmore KJ, Stashuk DW, Doherty TJ, Hepple RT, Taivassalo T, Rice CL. Motor unit number and transmission stability in octogenarian world class athletes: can age-related deficits be outrun? J Appl Physiol (1985) 121: 1013–1020, 2016. doi: 10.1152/japplphysiol.00149.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso AL, Studenski SA, Chen WG, Aizenstein HJ, Alexander NB, Bennett DA, Black SE, Camicioli R, Carlson MC, Ferrucci L, Guralnik JM, Hausdorff JM, Kaye J, Launer LJ, Lipsitz LA, Verghese J, Rosano C. Aging, the central nervous system, and mobility. J Gerontol A Biol Sci Med Sci 68: 1379–1386, 2013. doi: 10.1093/gerona/glt089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz A, Silder A, Heiderscheit B, Mahoney J, Thelen DG. Differences in lower-extremity muscular activation during walking between healthy older and young adults. J Electromyogr Kinesiol 19: 1085–1091, 2009. doi: 10.1016/j.jelekin.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsick EM, Glynn NW, Jerome GJ, Shardell M, Schrack JA, Ferrucci L. Fatigued, but not frail: perceived fatigability as a marker of impending decline in mobility-intact older adults. J Am Geriatr Soc 64: 1287–1292, 2016. doi: 10.1111/jgs.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsick EM, Schrack JA, Glynn NW, Ferrucci L. Assessing fatigability in mobility-intact older adults. J Am Geriatr Soc 62: 347–351, 2014. doi: 10.1111/jgs.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenholm S, Shardell M, Bandinelli S, Guralnik JM, Ferrucci L. Physiological factors contributing to mobility loss over 9 years of follow-up—results from the InCHIANTI study. J Gerontol A Biol Sci Med Sci 70: 591–597, 2015. doi: 10.1093/gerona/glv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenroth L, Sillanpää E, McPhee JS, Narici MV, Gapeyeva H, Pääsuke M, Barnouin Y, Hogrel JY, Butler-Browne G, Bijlsma A, Meskers CG, Maier AB, Finni T, Sipilä S. Plantarflexor muscle-tendon properties are associated with mobility in healthy older adults. J Gerontol A Biol Sci Med Sci 70: 996–1002, 2015. doi: 10.1093/gerona/glv011. [DOI] [PubMed] [Google Scholar]
- Tracy BL, Enoka RM. Older adults are less steady during submaximal isometric contractions with the knee extensor muscles. J Appl Physiol (1985) 92: 1004–1012, 2002. doi: 10.1152/japplphysiol.00954.2001. [DOI] [PubMed] [Google Scholar]
- Vestergaard S, Nayfield SG, Patel KV, Eldadah B, Cesari M, Ferrucci L, Ceresini G, Guralnik JM. Fatigue in a representative population of older persons and its association with functional impairment, functional limitation, and disability. J Gerontol A Biol Sci Med Sci 64A: 76–82, 2009a. doi: 10.1093/gerona/gln017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard S, Patel KV, Bandinelli S, Ferrucci L, Guralnik JM. Characteristics of 400-meter walk test performance and subsequent mortality in older adults. Rejuvenation Res 12: 177–184, 2009b. doi: 10.1089/rej.2009.0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanigatunga AM, Simonsick EM, Zipunnikov V, Spira AP, Studenski S, Ferrucci L, Schrack JA. Perceived fatigability and objective physical activity in mid- to late-life. J Gerontol A Biol Sci Med Sci 73: 630–635, 2018. doi: 10.1093/gerona/glx181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RE, Boudreau RM, Caserotti P, Harris TB, Zivkovic S, Goodpaster BH, Satterfield S, Kritchevsky SB, Schwartz AV, Vinik AI, Cauley JA, Simonsick EM, Newman AB, Strotmeyer ES; Health, Aging and Body Composition Study . Sensory and motor peripheral nerve function and incident mobility disability. J Am Geriatr Soc 62: 2273–2279, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Holobar A, Kouzaki M, Ogawa M, Akima H, Moritani T. Age-related changes in motor unit firing pattern of vastus lateralis muscle during low-moderate contraction. Age (Dordr) 38: 48, 2016. doi: 10.1007/s11357-016-9915-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J, Ho SC, Yu AL. Walking speed and stride length predicts 36 months dependency, mortality, and institutionalization in Chinese aged 70 and older. J Am Geriatr Soc 47: 1257–1260, 1999. doi: 10.1111/j.1532-5415.1999.tb05209.x. [DOI] [PubMed] [Google Scholar]