Abstract
Many sensory systems are tuned to specific parameters of behaviors and have effects that are task-specific. We have studied how force feedback contributes to activation of synergist muscles in serially homologous legs of stick insects. Forces were applied using conventional half-sine or ramp and hold functions. We also utilized waveforms of joint torques calculated from experiments in freely walking animals. In all legs, forces applied to either the tarsus (foot) or proximal leg segment (trochanter) activated synergist muscles that generate substrate grip and support, but coupling of the depressor muscle to tarsal forces was weak in the front legs. Activation of trochanteral receptors using ramp and hold functions generated positive feedback to the depressor muscle in all legs when animals were induced to seek substrate grip. However, discharges of the synergist flexor muscle showed adaptation at moderate force levels. In contrast, application of forces using torque waveforms, which do not have a static hold phase, produced sustained discharges in muscle synergies with little adaptation. Firing frequencies reflected the magnitude of ground reaction forces, were graded to changes in force amplitude, and could also be modulated by transient force perturbations added to the waveforms. Comparison of synergist activation by torques and ramp and hold functions revealed a strong influence of force dynamics (dF/dt). These studies support the idea that force receptors can act to tune muscle synergies synchronously to the range of force magnitudes and dynamics that occur in each leg according to their specific use in behavior.
NEW & NOTEWORTHY The effects of force receptors (campaniform sensilla) on leg muscles and synergies were characterized in stick insects using both ramp and hold functions and waveforms of joint torques calculated by inverse dynamics. Motor responses were sustained and showed reduced adaptation to the more “natural” and nonlinear torque stimuli. Calculation of the first derivative (dF/dt) of the torque waveforms demonstrated that this difference was correlated with the dynamic sensitivities of the system.
Keywords: load, posture, sensory encoding, walking
INTRODUCTION
The effects of proprioceptive sense organs on motor actions are not constant but context-dependent, as their effects are modified by the nervous system to be task-specific (Gervasio et al. 2013; Hellekes et al. 2012; Pearson 2008; Prochazka 1989; Ritzmann and Büschges 2007; Tuthill and Wilson 2016). Proprioceptive inputs can function as elements in pattern generators, adapt motor activities to load and variations in the environment, and aid in the activation of groups of muscles as synergists (Bidaye et al. 2018; Duysens et al. 2013; Hagio and Kouzaki 2014; Ritzmann and Zill 2017). However, analysis of these effects can be problematic as a number of experiments have shown that neural circuits that utilize sensory signals are tuned to the specific parameters that occur in behavior (French et al. 2016; Lettvin et al. 1959; Pearson et al. 2006; Pfeiffer and French 2015). As noted by Pfeiffer and French (2015), many stimuli “contain limited frequency bands and predictable components [for which dynamics] may be crucial for nonlinear systems.”
The determination of appropriate stimulus parameters is particularly challenging in considering the effects of receptors that encode forces (Duysens et al. 2000; Nichols 2018; Zill et al. 2004). Forces are usually applied experimentally in ranges that are estimates derived from measurements of ground reaction forces (Gruhn et al. 2016). Although some experiments have directly monitored forces at muscle tendons and joints, these data have not been systematically used in studies of afferent responses or motor effects (Fleming and Beynnon 2004; Gregor and Abelew 1994; Komi 1990; Newland and Emptage 1996). Furthermore, discharges of receptors that indicate forces in legs in posture and walking can be affected by a number of parameters, including muscle properties, the specific patterns of motor neuron firing, and the variations resulting from the patterns of coordination of leg movements (Dallmann et al. 2017; Ross and Nichols 2009; Zill et al. 2009).
We (Schmitz 1993) have studied how force feedback from campaniform sensilla, receptors that encode forces as strains in the exoskeleton, is incorporated into the generation and control of posture and walking in insects. Campaniform sensilla of legs can be excited by imposed forces, but they specifically encode muscle forces that act against external loads (Zill et al. 2011, 2013). In the proximal leg, the receptors are concentrated on the trochanteral segment. This location permits detection of the net forces generated by groups of extrinsic and intrinsic muscles that act on the appendage. Some groups of receptors can monitor forces acting in the plane of movement of the coxotrochanteral joint (i.e., the joint torque; Kaliyamoorthy et al. 2006; Zill et al. 2012). Previous studies demonstrated that, when stick insects grasp a substrate after being induced to make searching movements, feedback from sense organs detecting forces in the middle legs can activate groups of muscles that are used to establish and maintain substrate grip (Akay et al. 2001; Bässler et al. 1991; Zill et al. 2014). Receptors that encode these forces in diverse leg segments (tarsus, trochanter) reinforce the same synergist muscles (Zill et al. 2017).
The present study was undertaken to examine the effects of forces in front and hindlegs to test the hypothesis that force detecting sense organs have similar effects on muscle synergies in all serially homologous legs. In stick insects, the front, middle, and hindlegs are differentially used in behavior and generate different patterns of ground reaction forces in walking (Dallmann et al. 2016). As in other insects, the hindlegs provide the major propulsive forces in forward walking, whereas the middle legs stabilize the animal and exert forces that first brake and then enhance progression (Cruse 1976). Forces generated by front legs show considerable variation in walking as they can function both in support and propulsion and also act as sensory appendages to explore the environment under visual guidance (Cruse and Bartling 1995; Full and Tu 1991). However, the available evidence indicates that similar neural and muscular activities generate substrate grip in all serially homologous legs, suggesting that there may be common mechanisms in the control and regulation of forces.
We also tested the hypothesis that sensory signals of force can contribute both to the initiation and the ongoing modulation of motor outputs. In the present study, we applied forces using conventional waveforms (half-sine and ramp and hold functions; Zill et al. 2012). We also examined the effects of using waveforms of joint torques as stimuli. These waveforms were calculated from a study that recorded ground reaction forces and three-dimensional kinematics of animals traversing a walkway containing a series of force plates (Dallmann et al. 2016). Although the torque waveforms were mean values derived by the method of inverse dynamics (rather than measured directly), their magnitude and dynamic characteristics more closely reflect the parameters of forces that occur in the animal’s behavior (Cruse 1976). The present experiments demonstrate that forces applied to the trochanter using waveforms of joint torques are highly effective in activating leg muscles that act as synergists in substrate grip and support/propulsion of body weight. Furthermore, synergist muscles show little adaptation to torque waveforms, as occurs with ramp and hold functions. In all legs, motor activities reflect the dynamics and magnitudes of joint torques, and discharges can be modulated by transient imposed force perturbations.
METHODS
Experiments were performed on adult, female stick insects (Carausius morosus) obtained from colonies at Bielefeld University or the University of Cologne. Activities of tibial campaniform were recorded from adult, male cockroaches (Periplaneta americana) obtained commercially (Carolina Biological Supply).
Physiological studies.
Animals were first securely restrained with staples over the mesothorax and abdomen. The coxa of the leg to be mechanically stimulated was firmly fixed with cyanoacrylate adhesive to small staples placed above and below the segment. The distal leg segments of that leg were only constrained by staples. Other legs were also initially loosely restrained with staples that permitted some movement. These staples were subsequently removed to induce extensive searching movements. Myographic activities were recorded with pairs of 50-μm silver wires (AG005825; Goodfellow) that were insulated to within 500 μm of their tips. The wires were placed in the proximal coxa to record the trochanteral depressor muscle, in the midfemur to monitor the tibial flexor, and proximal tibial to record the retractor unguis (pretarsal flexor) muscle (Fig. 1C; Zill et al. 2015). Electromyographic signals were amplified and filtered (50-Hz notch, 250-Hz high-pass, 3.5-kHz low-pass) using a custom-built amplifier (MA102; Electronics Workshop, Institute of Zoology, Cologne, Germany). Filtered signals were converted from analog to digital (Power1401 mk II) and recorded with a Spike2 interface [Cambridge Electronic Design (CED), Cambridge, United Kingdom].
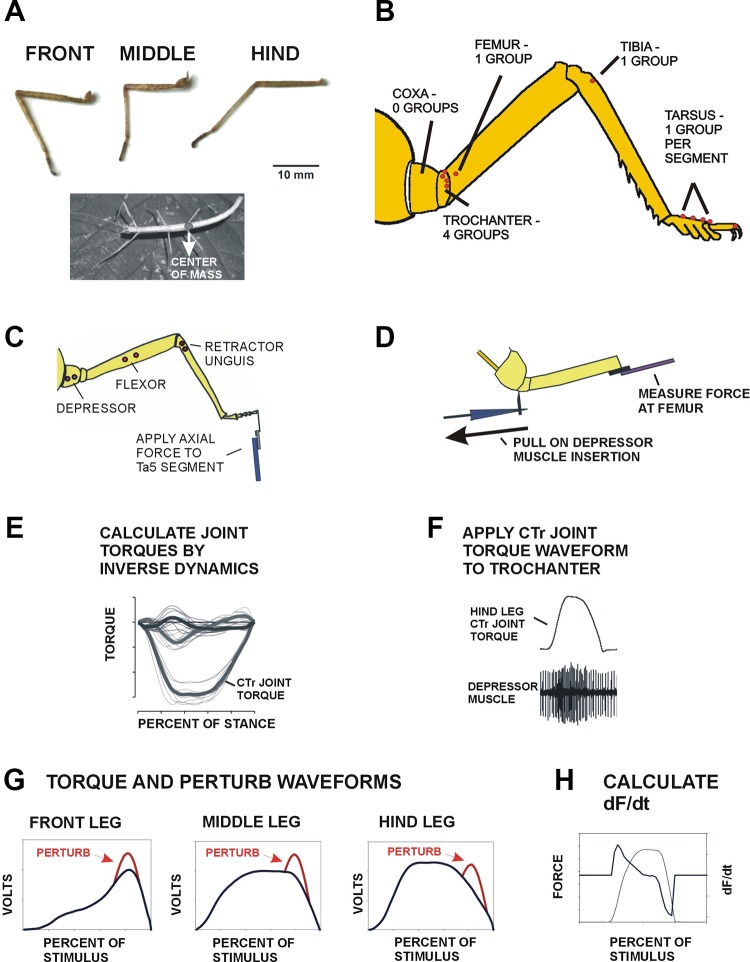
Fig. 1.
Preparations and generation of torque waveforms. A: serially homologous legs of stick insects. Front, middle, and hindlegs of stick insects have similar segments and overall size. Forces exerted by the legs differ as the center of mass is located over the hindleg coxa. B: diagram of locations of campaniform sensilla. Similar groups of campaniform sensilla are present on the trochanter and tarsus in all legs. [Adapted from Arthropod Structure & Development (Zill et al. 2015), with permission from Elsevier.] C: preparation for stimulation of tarsal sensilla. Axial forces were applied to the end of the 5th tarsal segment (Ta5) to stimulate tarsal campaniform sensilla. Motor activities were recorded in leg muscles that act as synergists in substrate grip (trochanteral depressor, tibial flexor, and retractor unguis). [Adapted from Arthropod Structure & Development (Zill et al. 2015), with permission from Elsevier.] D: stimulation of trochanteral force receptors. Forces were applied at the insertion of the depressor muscle and measured in the distal femur. Searching was induced in other legs by removal of support/contact. [Adapted from Zill et al. (2012).] E: use of joint torques as mechanical stimuli. In a previous study (Dallmann et al. 2016), ground reaction forces and leg kinematics were measured in freely moving animals. Joint torques were calculated by inverse dynamics. F: in the present study, the coxotrochanteral (CTr) torque waveforms were applied as stimuli to the trochanter while recordings were taken from leg muscles. G: torque waveforms and perturbations. Mean values of joint torques were applied to the trochanteral depressor insertion or distal femur (shown in black). In some tests, transient force perturbations (shown in red) were added to the waveforms as brief half-sine increases (160-ms duration at phase 0.72). H: rate of change of force (dF/dt) was calculated from the waveform or the applied force.
Forces applied to the tarsus.
The methods of mechanical stimulation of tarsal campaniform sensilla have been previously described (Zill et al. 2014). The proximal tarsal segments were restrained with staples, and adhesive was applied to the proximal three segments. Forces were imposed to the distal end of the tarsus (after removal of the claws and arolium) using a probe with strain gauges attached to a piece of shim steel that was mounted on a piezoelectric crystal (Fig. 1C). Half-sine and ramp and hold voltage waveforms were generated from prerecorded sequences using the CED interface.
Forces applied to the trochanter.
In these tests, the distal segments of the leg were amputated in the proximal tibia at a level that preserved recording of the tibial (RUII) portion of the retractor unguis (Zill et al. 2015). Cyanoacrylate adhesive was applied to immobilize the femorotibial joint. In other preparations, the leg was cut in the distal femur to eliminate the possibility of feedback from movements of the femorotibial joint (as prolonged mechanical stimulation activated the flexor, which produced loosening of the adhesive at the joint). Forces were applied to the insertion of the trochanteral depressor muscle using a computer-controlled motor (Fig. 1D; details in Zill et al. 2012). The shaft of a minuten pin was attached to the armature of the motor, and the sharp end was inserted into the cuticle distal to the attachment of the depressor muscle tendon. This region is reinforced by an internal cuticular buttress (Zill et al. 2000, 2012), creating a small compartment distal to the muscle insertion. Voltage waveforms were applied to the motor from the CED interface to mimic depressor muscle contractions, and the resultant forces were resisted and monitored by placing the force probe next to the femur. The plane of pull of the motor was carefully adjusted so that it produced smooth depression movements of the femur when the force probe at the femur was transiently removed. The stability of the coxa (restrained by staples) was visually monitored during the experiments, and tests were terminated if movement was observed (this was particularly problematic in recordings of front legs, which are flexibly coupled to the body, a factor that also limited the use of repetitive mechanical stimuli that could contribute to decrease in stability when the front legs actively grasped the substrate). Forces in the direction of levation of the coxotrochanteral (CTr) joint could also be applied through the piezoelectric crystal in the probe and resisted by the pin in the trochanter. A previous study of the responses and effects of stick insect trochanteral campaniform sensilla showed that the effects of forces applied to the depressor insertion are generally equivalent to and can sum with forces applied as loads to the distal femur (Zill et al. 2012).
Torque waveforms.
Torque waveforms were obtained from a previous study that measured ground reaction forces in freely walking animals via force plates (Dallmann et al. 2016). In that study, animals walked on a long, narrow platform in which three-dimensional force transducers were embedded (Fig. 1E). Body and leg motions were captured and reconstructed using a marker-based Vicon Motion Capture System with high-speed cameras (Vicon MX10 with 8 T10 cameras, controlled by software Nexus version 1.4.1; Vicon, Oxford, United Kingdom). Data from the force plates were low-pass filtered at 12.5 or 25 Hz. Torques about the intrinsic leg joints were determined by inverse dynamics using a three-dimensional rigid link model, and mean torques were calculated from pooled data (Fig. 1E).
The mean CTr joint torque waveforms were converted to output voltages by importing their numerical values into sequencer files in the CED software (Fig. 1F). We also introduced transient increases into the waveforms to test the effects of force variations (Fig. 1G), as the waveforms represented mean values (see discussion). The increases were uniformly added to the torque values as half-sine waveforms for 20% of the waveform duration (~160-ms duration) that were initiated at 72% duration. The perturbations resulted in a mean peak increase of 37.9%, but this varied for different legs (front leg 30.1%, middle leg 28.1%, and hindleg 55.4%) depending on the values of the torque waveforms in that period. The sequencer files were output as voltages using the interface and rerecorded in hardware with low-pass filters to smooth voltage “steps” in the file output. Those recorded files were then played as “templates” and applied to the leg through motor and pin at the trochanter or through the piezoelectric crystal to apply forces directly to the distal femur. The gain of the outputs was adjusted (via a voltage attenuator in series with the CED output) during the experiment to approximate the mean levels of force calculated from freely moving animals.
Inducing active searching movements.
Forces were first applied to the trochanter in restrained, “resting” animals. The restraints on the remaining legs were then removed to induce searching and attempted righting responses (Zill et al. 2017). In searching, legs show repeated movements to establish a grip with an object or substrate (Berg et al. 2013). These bouts often occurred repeatedly in stick insects between rest periods of 3 min in which the animal was given a surface (rod or large insect pin) to grasp. Searching movements could also be induced by gently touching the abdomen or cerci.
Data storage and analysis.
Neurophysiological and force data were recorded using the CED interface and analyzed using custom scripts in the CED software (Spike2 version 7.01). The value of the rate of change of force (Fig. 1H; dF/dt) was calculated offline in the software using channel processing or custom scripts. Spike sorting was used to differentiate unit activities according to potential amplitude in extracellular recordings. Although many recordings (flexor, retractor) showed multiunit activities, plots of the firing frequency reflected the amplitude and rate of forces applied as sustained stimuli, and rectification/integration of signals was not used in analysis of the data. Data were plotted in SigmaPlot (Systat Software). In all plots and analyses, N indicates the number of animals and n the number of tests.
RESULTS
Leg structure and detection of forces in serially homologous legs.
The front, middle, and hindlegs of stick insects (Fig. 1A) have similar segments and, in contrast to many other insects, the same overall size (Bässler 1983). However, forces exerted by the legs differ as the center of mass is located just posterior to the hindleg thoracocoxal joint, largely due to the substantial length of the abdomen. This effectively unloads the front legs in upright walking and frees them to function as tactile appendages or “arms,” although the front legs can also provide support and propulsion when traversing nonhorizontal substrates (Cruse 1976).
Similar groups of campaniform sensilla are present on all legs (Fig. 1B). Although not extensively characterized, recordings of tarsal and trochanteral sensilla show similar sensitivities in encoding the rate and magnitude of applied forces (S. N. Zill, unpublished observations) as has been extensively studied in middle legs of stick insects (S. N. Zill, unpublished observations; Zill et al. 2012, 2014).
Effects of tarsal forces in front and hindlegs.
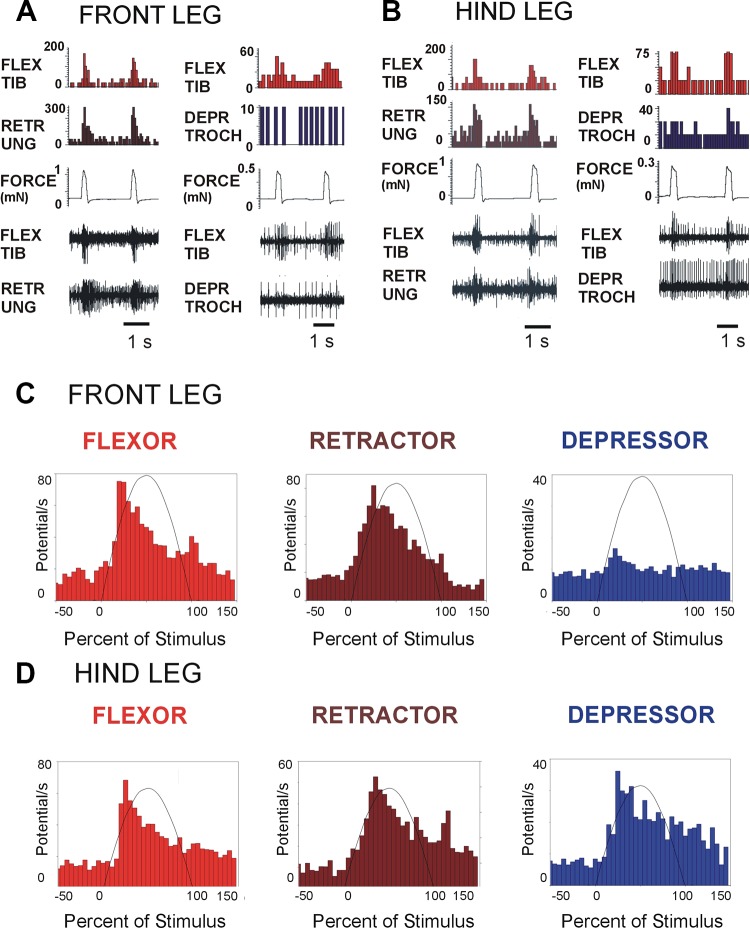
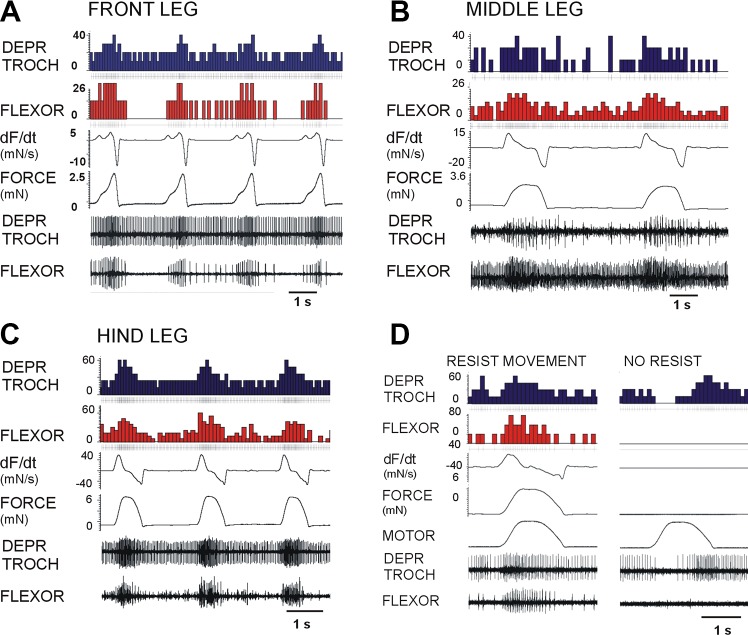
Mechanical forces that stimulated the tarsal sensilla of the front and hindlegs produced rapid activation of synergist muscles that generate substrate grip and support in different leg segments (Fig. 2, A and B). The tibial flexor and retractor unguis were consistently and rapidly activated (histograms: Fig. 3C: front leg: flexor, N = 4, n = 357; retractor, N = 3, n = 238; depressor, N = 4, n = 428; Fig. 3D: hindleg: flexor, N = 3, n = 202; retractor, N = 3, n = 224; depressor, N = 4, n = 254), as was found in the middle legs (Zill et al. 2015). However, one major difference was that activation of the depressor was elicited in the hindlegs (Fig. 2B, right, and Fig. 2D, right) but was weak or absent in the front legs (Fig. 2A, right, and Fig. 2C, right). Inputs from distal force receptors appear to be less strongly coupled to proximal leg musculature in front legs, consistent with their flexible use in walking and climbing.
Fig. 2.
Effects of tarsal forces on muscle synergies in front and hindlegs. A and B: stimulation of the tarsal campaniform sensilla of the front (A) and hind (B) legs produced activation of the muscles that generate substrate grip [tibial flexor (FLEX TIB) and retractor unguis (RETR UNG)], as previously found in the middle legs. However, activation of the trochanteral depressor (DEPR TROCH) was vigorous in hindlegs (B, right) but only weak or absent in the front legs (A, right). C and D: cumulative histograms of muscle responses to stimulation of tarsal receptors. These plots show firing during the force application (indicated by the half-sine waveform) as well as periods (50% stimulus duration) before and after mechanical stimuli were applied to the tarsus. Inputs from tarsal campaniform sensilla in front (C) and hind (D) legs strongly excited distal leg muscles but were only weakly coupled to proximal leg musculature in front legs, consistent with their flexible use in walking and climbing.
Fig. 3.
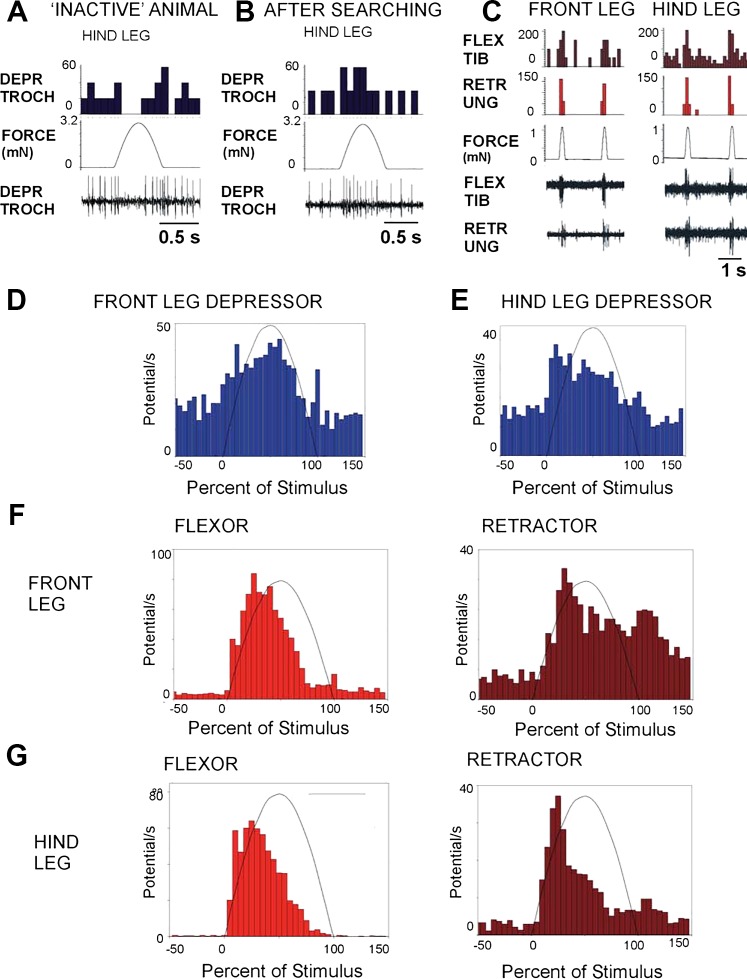
Effects of trochanteral depressor (DEPR TROCH) force are state-dependent and activate the same synergies in all legs. A: changes in effects of force stimuli. Forces applied to the hindleg depressor produced inhibition of tonic firing in the depressor in a restrained, undisturbed preparation. B: after induction of searching by release of restraints on front and middle legs, the same forces produced excitation of the depressor. Similar responses were obtained in the depressor of the front legs (data not shown) during searching of the middle and hindlegs. C: recordings of activities of flexor (FLEX TIB) and retractor (RETR UNG) muscles to forces applied to the depressor muscle insertion in front and hindlegs following induced searching movements. D and E: cumulative histograms of depressor firing in animals that were actively pushing against the probe after searching show rapid increases in both front (D) and hind (E) legs following the onset of mechanical stimulation (indicated by the half-sine waveform). F and G: force application to trochanter produced activation of the flexor and retractor unguis muscles as synergists in both front and hindlegs.
Effects of forces on the trochanter in front and hindlegs.
The trochanteral/femoral force receptors were activated by mimicking contractions of the depressor muscle through a motor linked to a pin at the depressor insertion or by forces applied to the distal femur (Fig. 1D). The effects of receptors indicating forces on the depressor muscle insertion were strongly dependent on the state of the animal. In preparations that showed tonic motoneuron activity at rest (before inducing searching movements), forces applied to the depressor insertion as half-sine functions produced inhibition of depressor firing (Fig. 3A). Immediately following bouts of searching or in animals that were actively pushing against the force probe, the same forces produced excitation of the depressor (Fig. 3B). Depressor firing was consistently enhanced in active animals in both the front (Fig. 3D) and hindlegs (Fig. 3E), and bursts in the hindleg depressor were often prolonged after the termination of the stimulus (histograms: front leg, N = 4, n = 258; hindleg, N = 3, n = 147). The same stimuli also activated the flexor and retractor unguis muscles as synergists in both the front (Fig. 3, C and F) and hindlegs (Fig. 3, C and G; histograms: front leg: flexor, N = 4, n = 474; retractor, N = 3, n = 384; hindleg: flexor, N = 3, n = 349; retractor, N = 3, n = 203).
Forces applied as ramp and hold functions also produced vigorous, sustained bursting in the depressor of both the front (Fig. 4A) and hindlegs (Fig. 4B) following searching movements. However, the depressor firing frequencies in the front and hindlegs depended both on the level of force application and the baseline levels of activity. Cumulative histograms of depressor firing to forces applied to the depressor insertion typically showed lower rates of discharge in front legs (Fig. 4C, top) than in hindlegs (Fig. 4C, bottom; histograms: hindleg, N = 1, n = 57; front leg, N = 1, n = 24). However, preliminary tests in which forces were applied at both similar levels in force and comparable baseline firing frequencies showed that the increase in firing frequency (discharge baseline) was similar in both legs (data not shown). Further experiments are needed to support these observations, but the differences in effects in the front and hindlegs were, therefore, not consistent with a simple change in gain (output/input).
Fig. 4.
Effects of forces applied as ramp and hold functions and adaptation in synergist muscles. A and B: after periods of leg searching, forces applied as ramp and hold functions produced sustained bursting in the depressor muscle (DEPR TROCH) of both the front (A) and hindlegs (B). C: histograms of depressor firing to forces applied to the depressor insertion showed lower rates of discharge in front legs (top) than in hindlegs (bottom; see main text for discussion). D: adaptation. Forces applied as ramp and hold functions at the depressor insertion in a front leg produced sustained firing in the depressor but bursts in the synergist flexor (FLEXOR TIB) adapted rapidly to the stimulus. E: histogram of front leg flexor bursts applied at moderate levels (mean amplitude: 1.94 mN). Firing rapidly adapted to sustained forces. F: adaptation depends on force amplitude. Application of forces in another preparation showed adaptation in flexor firing (left) that was decreased when the force amplitude was increased (right).
Adaptation in synergist muscle activation.
Forces applied as ramp and hold functions could show differential effects in the depressor and flexor muscles. Figure 4D shows a recording of the trochanteral depressor and tibial flexor muscles when forces were applied to the front leg of an animal following a bout of searching. The depressor fires in bursts that are sustained throughout the stimulus, but firing in the tibial flexor rapidly adapts after the onset of the stimulus. Figure 4E is a cumulative histogram of the flexor discharges that occurred after application of a ramp and hold stimulus of a mean amplitude of 1.9 mN (N = 5, n = 480). However, a number of factors could contribute to this finding, including the stimulus waveform and amplitude. Figure 4F shows recordings of a front leg flexor when two levels of force were applied to the femur with ramp and hold waveforms: the discharge rapidly adapts to the lower force level (Fig. 4F, left), but firing is more sustained to a higher amplitude of force (Fig. 4F, right). Similar characteristics are seen in force encoding by campaniform sensilla (Ridgel et al. 2000). Thus determining the appropriate force amplitudes and waveforms are critical in evaluating the effects of force receptors.
Application of torque waveforms as mechanical stimuli.
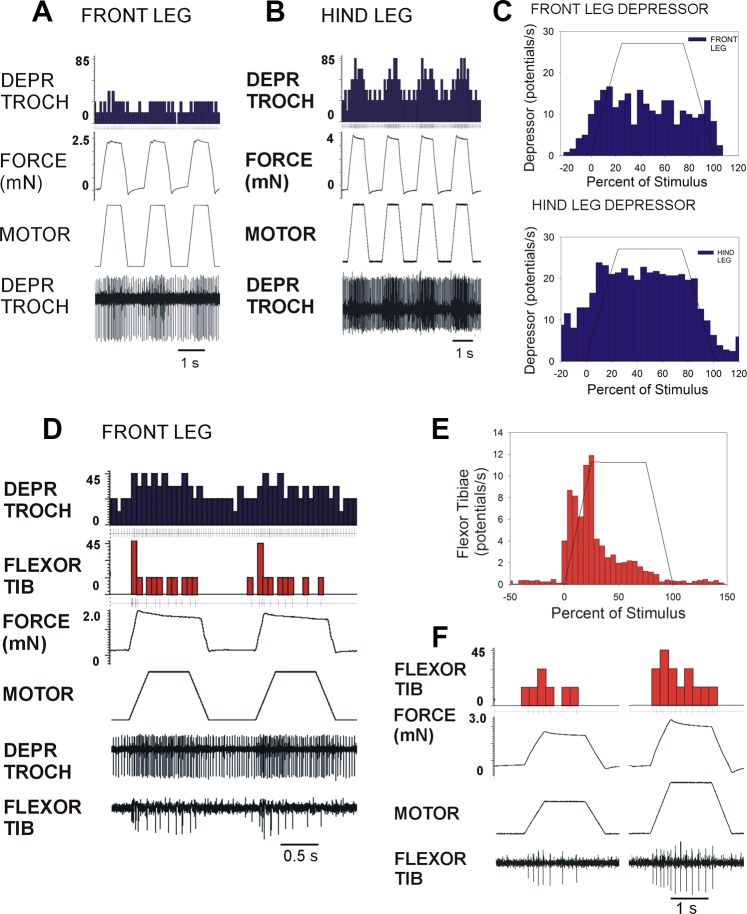
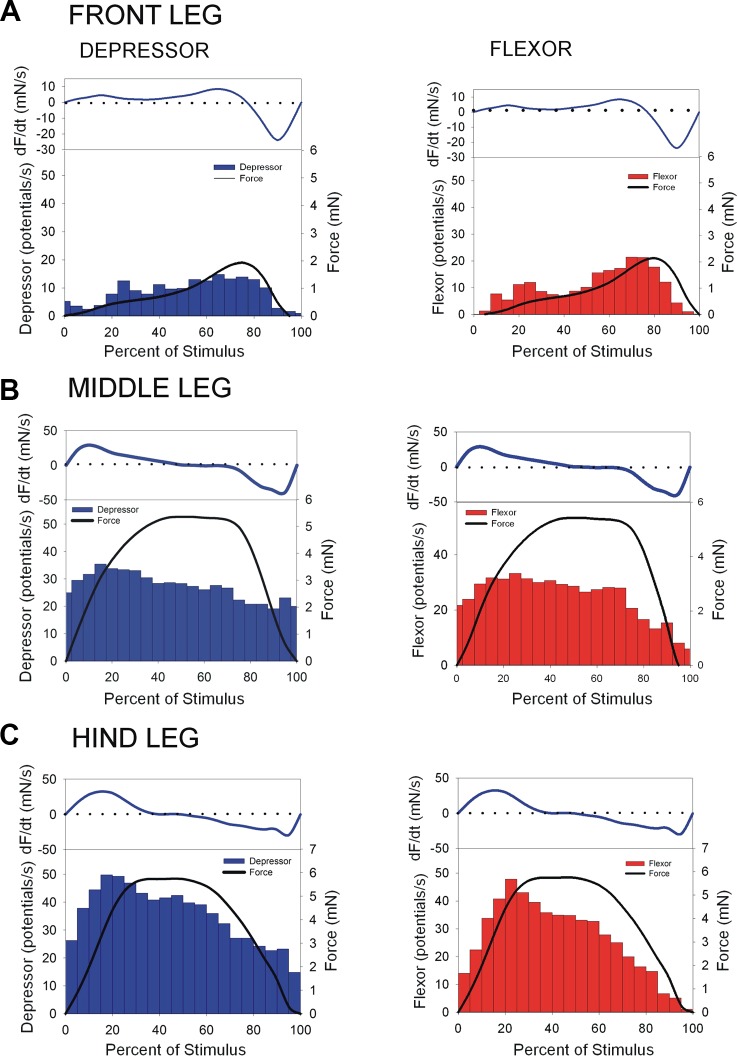
Application of forces to the depressor muscle insertion or distal femur using torque waveforms at amplitudes similar to those occurring in freely walking animals produced activation of the same muscle synergies in the front (Fig. 5A), middle (Fig. 5B), and hindlegs (Fig. 5C). Discharges of the flexor muscle were sustained in all legs and showed little of the adaptation seen to application of ramp and hold functions. These discharges depended on generation of resisted forces as they only occurred when forces were resisted (Fig. 5D, left) but not when movement was permitted by displacing the force probe (Fig. 5D, right).
Fig. 5.
Use of torque waveforms as mechanical stimuli. A–C: recordings of activities in depressor (DEPR TROCH) and flexor muscles of the front (A), middle (B), and hind (C) legs when forces were applied to the trochanter with torque waveforms at levels similar to those calculated to occur during walking. These tests produced activation of the same depressor-flexor synergy in all legs, and discharges of the flexor muscle were more sustained. D: muscle activation depends on resisted force. Discharges in both the hindleg depressor and flexor occurred when forces applied to hindleg were resisted (left) but not when the force probe was displaced (right) and joint movement occurred. dF/dt, rate of change of force.
Pooled histograms of firing during tests using torque waveforms are shown in Fig. 6 for the front (Fig. 6A), middle (Fig. 6B), and hindlegs (Fig. 6C). Forces applied with torque waveforms produced vigorous and sustained activation of muscles in all legs (histograms: Fig. 6A: front leg: depressor, N = 3, n = 317; flexor, N = 3, n = 282; Fig. 6B: middle leg: depressor, N = 4, n = 80; flexor, N = 4, n = 222; Fig. 6C: hindleg: depressor, N = 4, n = 169; flexor, N = 4, n = 281).
Fig. 6.
Muscle activation depends on force amplitude and rate (dF/dt). These graphs show the mean responses of depressor (in blue) and flexor muscles (in red) to application of forces using coxotrochanteral (CTr) joint torque waveforms in front (A), middle (B), and hindlegs (C). Plots of the force magnitude are overlaid, whereas the mean rates of change of force (dF/dt) are plotted above the histograms. Histograms of muscle activities are set to the same scale. A: front legs. CTr torque increased gradually (positive values of dF/dt) and then rapidly declined. Activation of both the depressor and flexor muscles was sustained and generally accelerated throughout the period of force application. B: middle legs. CTr torque increased during the 1st half of the stimulus to a much higher level than in the front legs and then declined (beginning at ~80% of the waveform). dF/dt remained positive through the period of force increase. Firing in both the depressor and flexor was sustained and only gradually decreased when the force declined. C: hindlegs. CTr torques showed the largest initial rate of increase (dF/dt) and had the largest magnitude in the hindlegs. Forces reached a brief plateau (approximately 40–60%, 0 level in dF/dt) and then declined, decreasing more rapidly toward the end of the stimulus. Firing in both the depressor and flexor increased throughout the rising phase and was sustained, only gradually declining in the period of force decrease. Thus responses to torque stimuli were sustained and reflected both the magnitude and rate of change of the applied forces.
These plots also show the torque waveform and rate of change of force (dF/dt) and provide insight into the relative contributions of the dynamic characteristics of the waveforms and the amplitude of the forces. In the front legs, the CTr joint torque increased gradually before reaching a peak at ~80% of the duration of the waveform (Fig. 6A). Despite the modest amplitude of the waveform, force application produced a continuous increase in discharge frequency in both the depressor (Fig. 6A, left) and flexor (Fig. 6A, right) motor neurons as the waveform acted as a prolonged ramp phase (dF/dt > 0). The middle leg torque was considerably larger and rose asymptotically to reach a peak at ~50% duration (Fig. 6B) and then began a slow decline at ~75% of the waveform. The rate of change remained positive throughout the first half of force application. Discharges of both the depressor (Fig. 6B, left) and flexor (Fig. 6B, right) were sustained with only a gradual decrease until the forces declined. The hindleg joint torque was the largest and rose rapidly to a high peak at ~40% duration and was then sustained (until ~60% duration). The rate of change of force reached an early maximum and then declined below 0 after the peak force was attained. The mean discharge frequencies of both the depressor (Fig. 6C, left) and flexor (Fig. 6C, right) reflected the rate of rise and subsequently declined slowly as the force magnitude was high. Thus effects of imposed forces depended both on the magnitude and dynamic nature of the waveforms.
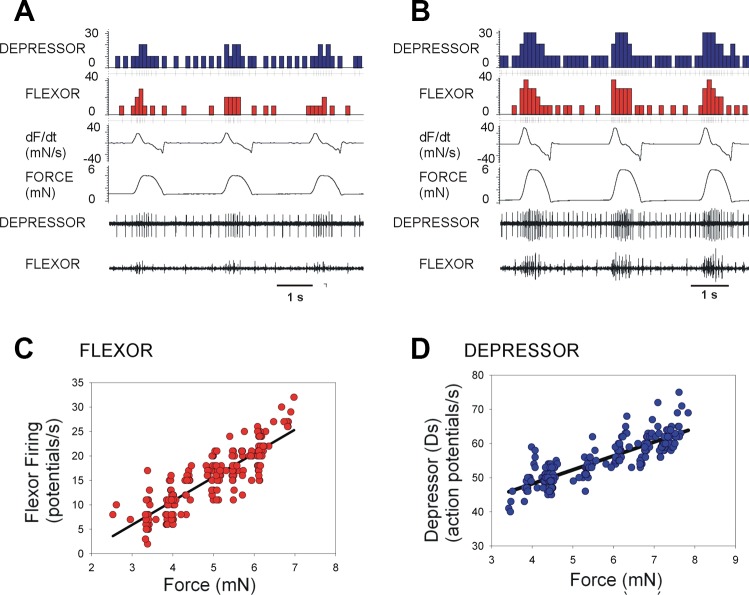
Scaling of responses to force amplitude.
The torque waveforms used in these studies represent mean values of the range of forces developed in walking. These forces can exhibit substantial variations, even in successive steps (Dallmann et al. 2016). We, therefore, applied tests in which the amplitude of the waveform was systematically varied. Figure 7, A and B, are recordings from the depressor and flexor muscles of a hindleg in an animal that showed tonic activity in slow depressor motor neuron firing following an episode of searching movements. Application of forces at different levels elicited motor discharges that were graded and reflected the force magnitude. Figure 7, C and D, are plots of the mean discharge frequencies of the flexor (Fig. 7C) and depressor (Fig. 7D) muscles during a series of application of forces at different levels in experiments in which the animals sustained discharges for long periods (plots: Fig. 7C: flexor, N = 3, n = 213; Fig. 7D: depressor, N = 1, n = 260). Each point is the mean discharge frequency during a single trial. The effects on motor discharges were a continuum, and the mean firing was graded and reflected the magnitude of the imposed torque (regression flexor, r2 = 0.75; depressor, r2 = 0.74). These findings are consistent with the idea that force receptors can act to tune muscle synergies synchronously to the levels of load.
Fig. 7.
Scaling of effects of torque waveform amplitude. A and B: recordings of the hindleg depressor and flexor in an animal that showed sustained activity following leg searching. Application of forces at lower (A) and higher (B) levels produced activation of both muscles. Both the force and rate of change of force (dF/dt) are simultaneously and proportionately increased. C and D: plot of the mean discharge frequencies of flexor (C) and depressor (D) muscles during series in which forces were applied at different amplitudes. Effects on motor discharges in both muscles were graded and reflected the torque increase.
Introduction of small perturbations in torque stimuli.
Ground reaction forces recorded in freely walking animals could show transient variations in individual steps (Dallmann et al. 2016). Some of these variations may be significant and indicate lifting and placement of other legs to support the body load (Dallmann et al. 2017). As a preliminary investigation of the effects of these variations, we introduced small perturbation (see methods) into the torque waveforms. The perturbations occurred at the same phase, but the proportional increase differed among the serially homologous legs due to variations in shape of the torque waveforms and the magnitude of the forces applied.
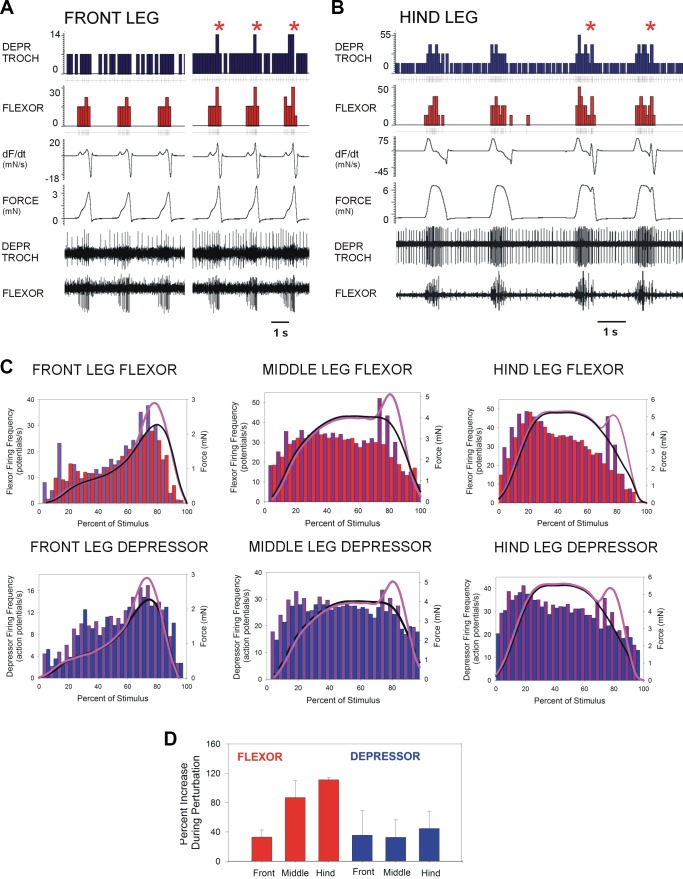
Figure 8A is a recording taken from the front leg of an animal after searching movements. The first three tests show the effects of the torque waveform without the perturbation followed by three successive force applications in which the perturbation was included. Although the effects are transient, there was a brief increase in the firing of flexor and depressor motor neurons following the onset of the perturbation. Figure 8B shows a similar recording from the depressor and flexor muscles of the hindleg of another preparation in which two tests without the perturbation are followed by two others in which the force was transiently elevated. The firing of the depressor increased and the flexor showed a small additional burst following the onset of the perturbation.
Fig. 8.
Effects of force perturbations. A and B: forces were applied to the trochanter using torque waveforms (at left in each set) and waveforms that included a small transient force increase (at right, marked with asterisks). Transient perturbations produced brief increases in the level and rate of change of force followed by larger and more rapid decreases. Each perturbation elicited brief changes in firing frequencies in both the depressor (DEPR TROCH) and flexor muscles in the front (A) and hind (B) legs. C: cumulative histograms of tests of responses to normal and perturbed forces. Perturbations (shown in purple) elicited brief, rapid increases in motor outputs in all legs. D: percentage change in firing frequencies during perturbations. Percentage change in firing frequencies was calculated (perturbed/unperturbed) during the periods of perturbations in the experiments plotted in C. Increases in discharges occurred in both flexor (red) and depressor (blue) motor neurons in all legs. dF/dt, rate of change of force.
Figure 8C shows cumulative histograms and waveforms of tests of responses to normal and perturbed forces in animals that showed sustained motor firing at moderate frequencies. The perturbations could elicit rapid, transient changes in motor outputs in all legs (histograms: flexor: front leg, N = 3, n = 256 torque, 223 perturbation; middle leg, N = 3, n = 158 torque, 165 perturbation; hindleg, N = 3, n = 227 torque, 316 perturbation; depressor: front leg, N = 3, n = 317 torque, 285 perturbation; middle leg, N = 3, n = 51 torque, 46 perturbation; hindleg, N = 3, n = 115 torque, 147 perturbation). The mean firing frequencies in these tests were comparable [front leg depressor: unperturbed 8.8 (4.5 SD), perturbed 9.3 (4.7 SD); front leg flexor: unperturbed 13.9 (8.0 SD), perturbed 15.7 (10.6 SD); middle leg depressor: unperturbed 24.4 (4.0 SD), perturbed: 26.0 (5.5 SD); middle leg flexor: unperturbed 26.4 (7.3 SD), perturbed 30.4 (10.0 SD); hindleg depressor: unperturbed 26.4 (7.3 SD), perturbed 30.4 (10.0 SD); hindleg flexor: unperturbed 27.2 (13.5 SD), perturbed 31.4 (13.4 SD)]. We also quantified these effects by calculating the mean percentage change in motoneuron firing during the periods of perturbations in the experiments. The histogram in Fig. 8D plots the percentage change [mean (SD)] in firing frequencies (perturbed/unperturbed) of motor neurons in front, middle, and hindlegs (data set same as Fig. 8C). Firing increased in motor neuron discharge in muscles of all legs, but the percentage changes were highest in flexor discharges in the middle and hindlegs (reflecting the absolute magnitude of the perturbations). Small force variations could, therefore, elicit rapid and simultaneous changes in output in both muscles.
Comparison of effects of ramp and hold and torque waveforms.
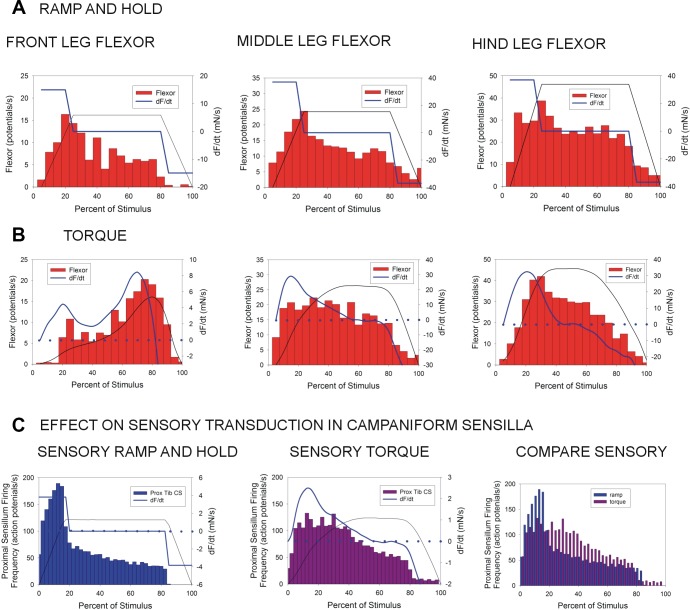
To gain insight into the effects of use of different waveforms as stimuli, we compared synergist activation in the flexor muscle in tests in which forces were applied as ramp and hold functions with responses to torque stimuli. Data were compared from trials that were initiated at similar baseline levels of flexor activity. As our studies indicated that responses depended both on force amplitude and waveform, data were plotted from tests in which the hold phase reached maximum values that were similar to the magnitudes of joint torques calculated from freely walking animals (although the difference in magnitude between the middle and hindlegs was not preserved in this data set).
The largest difference in flexor discharges to the two forms of mechanical stimulation was seen in the front legs [Fig. 9A, left: histogram: ramp, N = 3, n = 148, maximum (max.) amplitude = 1.99 mN; Fig. 9B, left: torque, N = 3, n = 187, max. amplitude = 1.84 mN]. Ramp and hold forces elicited firing that increased rapidly during the ramp and then showed substantial adaptation in the hold phase, when force was held at a low level (relative to other legs). In contrast, the torque waveform had a prolonged rise in which the rate of change remained positive and a rapid decline, with no static hold phase. The flexor discharge frequency followed the rate of change of force (dF/dt) and first increased gradually and then finally accelerated to reach a peak late in the phase of force application.
Fig. 9.
Comparison of tests using ramp and hold and torque waveforms. These histograms plot the mean discharge frequencies of flexor motoneurons during tests in which forces were applied to the trochanter using ramp and hold (A) and torque waveforms (B) at similar peak magnitudes and baseline motor neuron firing frequencies. Force waveform (black line) and rate of change (dF/dt; blue line) are overlaid. Scales of dF/dt in the torque plots are adjusted to show the initial period of increasing rates of force application. A: ramp and hold waveforms. In the front and middle legs, forces applied as ramp and hold waveforms produced firing during the rising phase that adapted during hold phase. Discharges were more sustained in hindlegs as the applied force had the largest magnitude. B: torque waveforms. In front legs (left), the flexor firing frequency closely reflected dF/dt. In middle legs (middle), flexor firing was more sustained during the period of gradual force increase in the torque waveform (corresponding to the hold phase in A). Discharge rate was sustained in the hindleg flexor (right) at the high level of forces applied with both torque and ramp and hold waveforms. C: effects of waveform on sensory transduction. “Generic” effects of waveform shape, independent of other leg specializations, were tested by applying ramp and hold and torque waveforms to the cockroach tibia while monitoring activities of tibial campaniform sensilla (CS; highly accurate unit identification is possible in these recordings). These histograms plot the discharge of proximal tibial sensilla (Prox Tib CS) to ramp and hold (left) and torque (middle) waveforms. Comparison of tests (right) showed substantially reduced adaptation in discharges to the torque waveform in the period corresponding to the static phase of ramp and hold stimulation.
In the middle legs, ramp and hold functions produced rapid, intense firing that also adapted considerably during the hold phase, even though the forces were much larger than those applied to front legs (Fig. 9A, middle: histogram: ramp, N = 4, n = 129, max. amplitude = 4.81 mN). The mean flexor firing during the hold phase (middle 50% of the ramp and hold waveform) in this data set was 12.2 (2.5 SD) potentials per second. Flexor firing to torque waveforms initially increased and then was sustained through the first half of the stimulus (Fig. 9B, middle: torque, N = 4, n = 126, max. amplitude = 4.35 mN). The prolongation of discharge reflected the continued slow rise in force level, which was also apparent in the sustained positive value of dF/dt. Consequently, the mean discharge during the period corresponding to the hold phase (middle 50% of the waveform) was higher 17.6 (3.3) potentials per second, a difference that was statistically significant (Student’s t-test, P = 0.003).
The highest rates of flexor discharge were elicited in the hindlegs (Fig. 9A, right: ramp, N = 3, n = 124, max. amplitude = 4.42 mN; Fig. 9B, right: torque, N = 3, n = 91, max. amplitude = 4.17 mN). The mean discharge during the hold phase of the ramp and hold function was 25.8 (3.2 SD) potentials per second and somewhat higher during the corresponding period using the torque waveform [mean 28.0 (7.84 SD)], but this difference was not statistically significant. Thus flexor firing in the serially homologous legs depended on both the magnitude and dynamics of the forces that were applied.
Does the difference between ramp and hold and torque waveforms affect the discharges of leg campaniform sensilla? This question has not yet been addressed for the trochanteral campaniform sensilla due to technical constraints, as unit activities can only be recorded (from the main leg nerve) using low amplitudes of force (Zill et al. 2012). To examine whether the effects on adaptation were dependent on the transduction mechanism of campaniform sensilla, independent of specializations in structure of stick insect legs, we tested the effects of simple changes in waveform shape on adaptation in the transduction mechanism of cockroach tibial campaniform sensilla (Ridgel et al. 2000). Activities of individual proximal tibial sensilla can readily and accurately be determined in cockroaches, even at higher levels of applied force (Ridgel et al. 2000), due, in part, to the fact that axons of the receptors course in a separate nerve (n5r8) in the femur. Identification of sensory units is somewhat problematic in recordings of stick insect tibial sensilla due to the diverse amplitudes and variable number of receptors that are monitored in extracellular recordings, which can only be taken from the main leg nerve (nervus cruris; Zill et al. 2011, p. 854).
Figure 9C shows plots of the firing of tibial (Gp6) proximal sensilla to forces applied as a ramp and hold function to the distal tibia (Ridgel et al. 2000; Fig. 9C, left) and when the more dynamic waveform of the stick insect middle leg CTr torque was applied to the same maximum level (Fig. 9C, middle). Overlay of the sensory discharges (Fig. 9C, right) shows that the torque waveform elicited sustained discharge and reduced adaptation (histograms: ramp and hold, N = 2 animals, n = 283 tests; torque, N = 2 same animals, n = 218). These preliminary findings suggest that the dynamic sensitivity of campaniform sensilla could substantially contribute to the differences seen in motor activities to forces applied using torque waveforms.
DISCUSSION
These experiments have shown that forces applied to each of the serially homologous legs of stick insects can have strong and widespread effects in leg muscles. These data support our first hypothesis, that force detecting sense organs have similar effects in all serially homologous legs in providing positive feedback to the depressor muscle and activating synergist muscles used in substrate grip and support. In the following, we will discuss the effects of force feedback on motor control and activation of muscle synergies and how the system may be tuned to the parameters and dynamics of specific forces generated by the animal.
Forces on the tarsus and trochanter activate muscle synergies in substrate grip.
Mechanical forces applied to the tarsus in the front and hindlegs activated the retractor unguis and tibial flexor muscles, as was previously demonstrated in the middle legs (Zill et al. 2014). The retractor unguis acts to establish substrate grip directly by flexing the pretarsus (claws and arolium) and by pressing the adhesive pads of the tarsal segments against the substrate. Similar tarsal mechanisms are apparently present in all segmentally homologous legs of stick insects (Labonte and Federle 2013). Although the response properties of the tarsal campaniform sensilla of stick insect front and hindlegs have not been fully characterized, preliminary experiments indicate that they also discharge to axial forces, which, in the animal, are produced by resisted contractions of the retractor muscle. Thus, in all legs, the tarsal receptors can provide positive force feedback to enhance activity in the muscle that directly generates substrate grip.
Engagement with the substrate also requires activation of synergist muscles at different leg joints. The pretarsal claws of insects are anatomically anisotropic and can only exert concentric, pulling forces (Gorb 2008; Ichikawa et al. 2016). Concentric forces also enhance adhesion of the pretarsal arolium (Labonte and Federle 2013). Within the leg, pulling forces are generated by the tibial flexor muscle in a mechanism of distributed inward grip (Wile et al. 2008). The synergistic activation of the retractor unguis and tibial flexor by sensory signals of force, therefore, reflects a common anatomic mechanism for generating initial substrate adhesion. Similar mechanisms are present in other insects, and the constraints of distributed inward grip during the stance phase of walking may contribute to the patterns of coordination in gait (Ramdya et al. 2017).
Mechanical forces acting on the tarsus produced activation of the trochanteral depressor in the middle and hindlegs but only weakly affected firing in the front legs (Zill et al. 2015). This disparity may reflect the flexible use of the front legs, which can act to brake or pull the animal in posture and in the early part of stance (Cruse 1976; Dallmann et al. 2016). The depressor muscle enhances grip by pressing the leg against the substrate during the stance phase of upright walking (Dallmann et al. 2017), but flexibility in coupling may be essential as other muscles are activated in support during climbing or traversing inverted surfaces (Duch and Pflüger 1995). The front leg motor circuits are also more directly dependent on descending activity from higher centers (Schaefer and Ritzmann 2001), and leg use can be guided by visual or antennal inputs (Blaesing and Cruse 2004; Watson et al. 2002), although the effects of those systems were not systematically tested in the present studies.
Forces applied to the trochanter also enhanced the same synergists, but this depended on the behavior of the animal. Synergists were activated when force was resisted but not when the leg was free to move, confirming that activation was mediated by sensory signals of force, not joint movement (Fig. 5D). These effects probably occur via pathways separate from those producing positive feedback as activities in synergist muscles could be modulated independent of changes in the depressor firing (e.g., Fig. 4D).
Flexor bursts were substantially enhanced after leg searching movements. The facilitation often persisted for 10–30 s (see Zill et al. 2015, Fig. 7) but could also continue for longer periods (up to several minutes). This finding is similar to the results of previous studies of the motor effects of campaniform sensilla (Zill et al. 2012), but the specific mechanisms underlying facilitation of muscle synergies have not been determined (Akay et al. 2001; see also Bässler 1983). Studies using intracellular recordings have shown that leg searching is mediated by premotor nonspiking interneurons (Berg et al. 2013, 2015). One nonspiking premotor interneuron, I4, can excite the muscle synergy that produces substrate grip (retractor, depressor, flexor) as well as providing inhibitory synaptic drive to the tibial extensor (Büschges 1995). Other interneurons active during searching can show slow repolarization after termination of bouts of leg movements (Berg 2014). Recent studies have also demonstrated that activation of neurosecretory DUM cells can facilitate reflexes of leg chordotonal organs (Stolz and Schmidt 2015). These results suggest that both circuit properties and neuromodulation can contribute to the enhancement of the motor effects of proprioceptive sense organs, but further studies are needed to define the mechanisms underlying synergist facilitation.
Force feedback that enhances motor outputs is a common mechanism in insect legs.
In all legs, the effects of forces applied to the depressor muscle insertion or distal femur strongly depended on the behavior of the animal: forces imposed in “quiescent” animals inhibited the depressor, but the same forces enhanced depressor activities during and after bouts of searching movements in other legs (Zill et al. 2012). Previous studies in stick insect middle legs showed that “sign reversal” occurred in the effects of trochanteral campaniform sensilla on the depressor muscle when animals were made active by touching the abdomen (Zill et al. 2012). The occurrence of reflex “reversals” in effects of proprioceptive sense organs is now well-documented in stick insects (Bässler et al. 1991; Hellekes et al. 2012), and reversals of the motor effects of the trochanteral campaniform sensilla also occur during walking when the direction of progression is changed (Akay et al. 2007).
Forces imposed on the trochanter elicited higher rates of tonic depressor firing in hindlegs than in front legs after searching movements were elicited. This suggested that the reflex gain (ratio of output to input) may be greater in hindlegs (Pearson and Misiaszek 2000). However, preliminary experiments that compared the effects of forces at equivalent levels and initial firing frequencies in the front and hindlegs showed that this effect depended on the baseline firing frequencies (see comparable analysis of vertebrate stretch reflexes: Gritsenko et al. 2001; Pantall et al. 2016). This finding argues against a simple increase in reflex gain independent of centrally generated drive, but further experiments are needed to characterize the segmental differences in force sensitivity.
The enhancement of depressor firing by forces mimicking resisted depressor muscle contractions strongly suggests that the system uses positive force feedback to reinforce active muscle contractions and generate support and propulsion in walking, as has now been demonstrated in a number of vertebrate and invertebrate animals (Grey et al. 2007; Nichols 2018; Pearson 1972, 2008; Prochazka et al. 1997; Prochazka 2015). In addition, measurements of ground reaction forces in upright walking indicate that the largest forces generated in all of the serially homologous legs are in the direction of leg depression (vertical forces z-axis; Cruse 1976; Dallmann et al. 2016; Full et al. 1991). Vertical forces are also generated in walking by stick insect front legs, even though the center of mass is located posteriorly (at the level of the hindlegs). Studies in other insects have also shown discharges in front leg depressor muscles in stance (Quimby et al. 2006; Tryba and Ritzmann 2000), and this may be required in the tripod gait, when front legs provide supportive forces after middle legs are lifted in swing.
Effects of joint torques and tuning of muscle synergies.
The major rationale for use of torque waveforms is that they provide an indication of the magnitude and dynamic characteristics of force encountered in the animal’s behavior. These waveforms lacked a prolonged, static hold phase as joint torques are not constant even in standing stick insects (Lévy and Cruse 2008). Although these waveforms were applied in animals attempting to maintain substrate grip rather than walking, previous studies have shown the sensory signals used to determine adequacy of substrate contact are similar in both behaviors and the same group of muscle synergists are activated (Bässler et al. 1991). However, it is important to note that there are limitations in the use of inverse dynamics (Simpson et al. 2015; Zajac and Gordon 1989). Analysis of linked segment models assumes that joints are frictionless and limbs are composed of rigid segments. Use of the coxotrochanteral joint torque has also been advantageous, as previous studies indicated that there is no extensive cocontraction of the coxal trochanteral depressor and levator muscles (Dallmann et al. 2017; Rosenbaum et al. 2010), although activities of other portions of the muscles in the body wall have not been documented.
These tests showed that waveforms of joint torques could produce vigorous driving of motor neuron activities in both the depressor and synergist flexor muscles. The motor firing frequencies in individual legs generally depended on the torque amplitude. The effects were highest in the hindlegs, which exert large forces in propulsion, and smallest in the front legs, which only exert large vertical ground reaction forces toward the end of the step cycle (Cruse 1976; Dallmann et al. 2016). In the animal, this means simply that the effect of force feedback depends on the magnitude of forces generated by a leg in behavior. This conclusion was also supported by experiments in which the magnitude of the torque waveform was systematically varied (Fig. 8): firing frequencies of both the depressor and synergist flexor muscles varied according to the magnitude of the applied forces.
The dynamics (dF/dt) of the waveform were also important factors in determining the frequency of motor outputs and in decreasing adaptation in some legs. Unlike ramp and hold functions, the torque waveforms continuously change over a range of frequencies, as do movements of the animal. Comparison of torque and ramp and hold functions showed the greatest difference in the front legs: rather than rising rapidly to a static level, forces rose gradually and then rapidly declined. Although the magnitude was small relative to other legs, the firing frequencies of both the depressor and flexor followed the waveform dynamics and showed gradual and continuous acceleration to a late peak followed by a rapid decline. Discharge of the flexor did not show the adaptation seen to ramp and hold functions. The middle leg torque waveform was generally similar to a ramp and hold function, but the force continued to increase asymptotically rather than abruptly attaining a static level. Firing frequency of the flexor was significantly higher in middle legs, and adaptation was also significantly reduced. In hindlegs, the effect of the large magnitude of the forces predominated, and both the discharges to ramp and hold and torque waveforms showed reduced adaptation during the period of force application. Thus relatively small differences in the rate of change of force (dF/dt) can have significant effects on sensory and motor activities in the legs.
Effects of perturbations: evidence for continuous modulation of output by force signals.
Transient variations in forces may be significant and indicate lifting and placement of other legs, which could be important factors in establishing leg coordination (Dallmann et al. 2017; Zill et al. 2009). We tested whether variations in force could affect motor outputs by introducing small perturbations that represented modulations of ongoing activities rather than large sudden increases that could evoke compensatory reactions (Bartling and Schmitz 2000; see also Mazzaro et al. 2005).
These perturbations regularly elicited brief changes in discharge frequencies in both the depressor and synergist flexor muscles when animals actively pressed against the force probe. In the depressor, which was tonically active before the tests, the transient increments in force elicited similar (~20%) increases in firing frequencies in all legs. The percentage increase in activation of the synergist flexor varied considerably among segmental legs (Fig. 9D) and was highest in the middle and hindlegs, which generate the largest forces in locomotion. Previous studies have shown that mechanical stimuli that activated trochanteral campaniform sensilla modulated activities in the protractor and retractor coxae muscles in walking animals (Schmitz 1993). However, it is important to note that present experiments were “open loop” and muscle contractions did not generate leg movements, whereas behaving animals use both position/velocity and force control (Szczecinski et al. 2015). Experiments in which perturbations were imposed as changes in body height also elicited compensatory changes in motor output (Cruse et al. 1993, 2009).
Use of force signals independently or in multimodal control apparently requires comparator elements to differentiate external loads from self-generated forces that occur during normal movements (Flanders 2011; Kistemaker et al. 2013). These elements have been formulated as efference copy (von Holst and Mittelstaedt 1971), referent control (Feldman 2016; Feldman and Levin 2016), and neuronal replicas for predictive internal signaling (Straka et al. 2018). Premotor interneurons that could provide this information have been identified in cats (Krutki et al. 2011), although the specific mechanisms underlying these comparisons have not been defined in insect thoracic ganglia. Task-dependent effects, such as those described in the present study, may also result from descending signals that alter the influence of sense organs as components of specific behaviors (Mu and Ritzmann 2008). However, the present experiments support our second hypothesis, that signals of force play a role in the continuous modulation of motor outputs, as has been demonstrated and modeled in other systems (af Klint et al. 2010; Ekeberg and Pearson 2005; von Twickel et al. 2011).
Sensitivities of sense organs that monitor forces.
Many of the characteristics of the motor responses to forces parallel sensitivities of sense organs that detect forces. Resisted depressor forces are known to excite leg campaniform sensilla (Zill et al. 2012), but other internal sense organs, such as multipolar receptors, may also be activated (Guthrie 1967). Campaniform sensilla, which encode muscle forces as cuticular strains, are extremely sensitive to force dynamics and show adaptation to low levels of force (Ridgel et al. 2000). We did not extensively test the effects of joint torques on the stick insect trochanteral campaniform sensilla largely due to technical limitations. Activities of those receptors have, to date, only been monitored through recordings of the main leg nerve, and unit activities can only be reliably obtained at very low levels of force (this problem may be resolved by use of other recording techniques). We tested the effects of the use of dynamic vs. ramp and hold waveforms on transduction in cockroach tibial campaniform sensilla due to the accuracy of unit identification (see methods) and found that adaptation was substantially reduced. Further studies on the encoding of forces that are nonlinear and reach high levels would aid in determining whether sensory responses are specifically tuned to forces generated by the animal. Discharges of campaniform sensilla recorded in freely moving animals show continuous modulation of high-frequency discharges in walking that reflect the level of load (Noah et al. 2001), and large load increase can modify walking in other insects (Mendes et al. 2014).
Are proprioceptive systems tuned to the dynamics of stimuli generated by the animal?
The present study has shown that there are significant differences in the effects of forces imposed using torque waveforms vs. more conventional waveforms. Joint torques elicited discharges that modulated motor firing, and adaptation of discharges in synergist muscles (Zill et al. 2015) was greatly reduced or eliminated, largely as a consequence of the dynamic nature of the stimulus. Although joint torques determined by inverse dynamics are not “natural” stimuli, they more closely reflect a variable that, to date, has proven otherwise largely inaccessible. Joint torques have previously been used in studies that modeled the discharges of trochanteral receptors in walking (Kaliyamoorthy et al. 2006). We do not yet know the relative contributions of sensory properties vs. central nervous processing to dynamic sensitivities in stick insects, although data, so far, indicate that the response properties of receptors are similar in groups in homologous legs. Subtle but significant changes in the dynamic responses of sensory receptors have been found in other systems using exteroceptive stimuli similar to those encountered in behavior in spider slit sensilla (Pfeiffer and French 2015) and fly photoreceptors (Juusola and de Polavieja 2003). It is also not known whether similar changes are seen in the properties of other sense organs that monitor forces (Jami 1992).
Potential advantages of incorporating force dynamics in control systems.
These findings also have potential implications and benefits for simulations of walking and for controllers of legged robots and prosthetic devices. Joint torques are used as components in many simulations and robotic controllers, but the effects of varying force dynamics have rarely been considered. The present study has shown that, although the torque stimuli were low-frequency waveforms, introductions of small perturbations in forces could modulate motor activities, suggesting the sensory signals of force can also be used to detect variation in force levels as has been evidenced in vertebrate systems in walking (af Klint et al. 2010) and grip (White et al. 2008). Although some models have included force sensors (Szczecinski et al. 2015) or elements integrating force information (Cruse et al. 2009; Tóth and Daun-Gruhn 2016), in many models force inputs are considered only to function as switches that can mediate phase transitions (Prochazka 2015). Current prosthetic devices for leg amputees incorporate force signals in microprocessor regulation of joint stiffness but are limited in adaptability in not providing time varying dynamic control (Rouse et al. 2015). Other simulations have incorporated dynamic changes in forces (Owaki et al. 2013), but the variations (sinusoidal) only generally reflect the forces or torques seen in the animal’s behavior. Accurate replication of force dynamics may be necessary in modeling locomotory systems since, as noted by Pearson et al. (2006), “examination of the role of sensory signals does not require detailed simulations of central neuronal networks, provided that the influences of the sensory signals can be captured by modifying the timing and magnitude of muscle activity in a manner that mimics the physiological data.” The findings of the present study suggest that use of stimuli that more closely approximate the dynamics of forces generated and encountered by the animal may be useful in understanding how the system regulates motor outputs, including the activation of muscle synergies (Gopalakrishnan et al. 2014).
GRANTS
This study was supported by Embodied Interaction as a Core of Cognitive Interaction from the Cluster of Excellence 227, German Research Foundation (DFG); DFG Grant BU 857-14; National Science Foundation Major Research Instrumentation Program Grant MRI 0959012; National Institute of General Medical Sciences Grant U54-GM-104942 to the West Virginia Clinical and Translational Science Institute; and travel grants from the University of Cologne.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.N.Z., C.J.D., A.B., and J.S. conceived and designed research; S.N.Z. performed experiments; S.N.Z. and S.C. analyzed data; S.N.Z., C.J.D., A.B., and J.S. interpreted results of experiments; S.N.Z. prepared figures; S.N.Z. drafted manuscript; S.N.Z., C.J.D., A.B., S.C., and J.S. edited and revised manuscript; S.N.Z., C.J.D., A.B., S.C., and J.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We warmly thank Andrew French and Nicholas Szczecinski for their excellent insights and comments and Michael Dübbert for invaluable technical support and advice.
REFERENCES
- af Klint R, Mazzaro N, Nielsen JB, Sinkjaer T, Grey MJ. Load rather than length sensitive feedback contributes to soleus muscle activity during human treadmill walking. J Neurophysiol 103: 2747–2756, 2010. doi: 10.1152/jn.00547.2009. [DOI] [PubMed] [Google Scholar]
- Akay T, Bässler U, Gerharz P, Büschges A. The role of sensory signals from the insect coxa-trochanteral joint in controlling motor activity of the femur-tibia joint. J Neurophysiol 85: 594–604, 2001. doi: 10.1152/jn.2001.85.2.594. [DOI] [PubMed] [Google Scholar]
- Akay T, Ludwar BC, Göritz ML, Schmitz J, Büschges A. Segment specificity of load signal processing depends on walking direction in the stick insect leg muscle control system. J Neurosci 27: 3285–3294, 2007. doi: 10.1523/JNEUROSCI.5202-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartling C, Schmitz J. Reaction to disturbances of a walking leg during stance. J Exp Biol 203: 1211–1223, 2000. [DOI] [PubMed] [Google Scholar]
- Bässler U. Neural Basis of Elementary Behavior in Stick Insects. Berlin: Springer, 1983. doi: 10.1007/978-3-642-68813-3. [DOI] [Google Scholar]
- Bässler U, Rohrbacher J, Karg G, Breutel G. Interruption of searching movements of partly restrained front legs of stick insects, a model situation for the start of a stance phase? Biol Cybern 65: 507–514, 1991. doi: 10.1007/BF00204664. [DOI] [Google Scholar]
- Berg E. Adaptive Motor Control: Neuronal Mechanisms Underlying (Targeted) Searching Movements (PhD thesis) Cologne, Germany: Univ. of Cologne, 2014. [Google Scholar]
- Berg E, Büschges A, Schmidt J. Single perturbations cause sustained changes in searching behavior in stick insects. J Exp Biol 216: 1064–1074, 2013. doi: 10.1242/jeb.076406. [DOI] [PubMed] [Google Scholar]
- Berg EM, Hooper SL, Schmidt J, Büschges A. A leg-local neural mechanism mediates the decision to search in stick insects. Curr Biol 25: 2012–2017, 2015. doi: 10.1016/j.cub.2015.06.017. [DOI] [PubMed] [Google Scholar]
- Bidaye SS, Bockemühl T, Büschges A. Six-legged walking in insects: how CPGs, peripheral feedback, and descending signals generate coordinated and adaptive motor rhythms. J Neurophysiol 119: 459–475, 2018. doi: 10.1152/jn.00658.2017. [DOI] [PubMed] [Google Scholar]
- Blaesing B, Cruse H. Stick insect locomotion in a complex environment: climbing over large gaps. J Exp Biol 207: 1273–1286, 2004. doi: 10.1242/jeb.00888. [DOI] [PubMed] [Google Scholar]
- Büschges A. Role of local nonspiking interneurons in the generation of rhythmic motor activity in the stick insect. J Neurobiol 27: 488–512, 1995. doi: 10.1002/neu.480270405. [DOI] [PubMed] [Google Scholar]
- Cruse H. The function of the legs in the free walking stick insect Carausius morosus. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 112: 235–262, 1976. doi: 10.1007/BF00606541. [DOI] [Google Scholar]
- Cruse H, Bartling C. Movement of joint angles in the legs of a walking insect, Carausius morosus. J Insect Physiol 41: 761–771, 1995. doi: 10.1016/0022-1910(95)00032-P. [DOI] [Google Scholar]
- Cruse H, Dürr V, Schilling M, Schmitz J. Principles of insect locomotion. Spat Temporal Patterns Action Oriented Percept Roving Robots 1: 43–96, 2009. doi: 10.1007/978-3-540-88464-4_2. [DOI] [Google Scholar]
- Cruse H, Schmitz J, Braun U, Schweins A. Control of body height in a stick insect walking on a treadwheel. J Exp Biol 181: 141–155, 1993. [Google Scholar]
- Dallmann CJ, Dürr V, Schmitz J. Joint torques in a freely walking insect reveal distinct functions of leg joints in propulsion and posture control. Proc Biol Sci 283: 20151708, 2016. doi: 10.1098/rspb.2015.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallmann CJ, Hoinville T, Dürr V, Schmitz J. A load-based mechanism for inter-leg coordination in insects. Proc Biol Sci 284: 20171755, 2017. doi: 10.1098/rspb.2017.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duch C, Pflüger HJ. Motor patterns for horizontal and upside-down walking and vertical climbing in the locust. J Exp Biol 198: 1963–1976, 1995. [DOI] [PubMed] [Google Scholar]
- Duysens J, Clarac F, Cruse H. Load-regulating mechanisms in gait and posture: comparative aspects. Physiol Rev 80: 83–133, 2000. doi: 10.1152/physrev.2000.80.1.83. [DOI] [PubMed] [Google Scholar]
- Duysens J, De Groote F, Jonkers I. The flexion synergy, mother of all synergies and father of new models of gait. Front Comput Neurosci 7: 14, 2013. doi: 10.3389/fncom.2013.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekeberg O, Pearson K. Computer simulation of stepping in the hind legs of the cat: an examination of mechanisms regulating the stance-to-swing transition. J Neurophysiol 94: 4256–4268, 2005. doi: 10.1152/jn.00065.2005. [DOI] [PubMed] [Google Scholar]
- Feldman AG. Active sensing without efference copy: referent control of perception. J Neurophysiol 116: 960–976, 2016. doi: 10.1152/jn.00016.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman AG, Levin MF. Spatial control of reflexes, posture and movement in normal conditions and after neurological lesions. J Hum Kinet 52: 21–34, 2016. doi: 10.1515/hukin-2015-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanders M. What is the biological basis of sensorimotor integration? Biol Cybern 104: 1–8, 2011. doi: 10.1007/s00422-011-0419-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming BC, Beynnon BD. In vivo measurement of ligament/tendon strains and forces: a review. Ann Biomed Eng 32: 318–328, 2004. doi: 10.1023/B:ABME.0000017542.75080.86. [DOI] [PubMed] [Google Scholar]
- French AS, Immonen EV, Frolov RV. Static and dynamic adaptation of insect photoreceptor responses to naturalistic stimuli. Front Physiol 7: 477, 2016. doi: 10.3389/fphys.2016.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Full RJ, Blickhan R, Ting LH. Leg design in hexapedal runners. J Exp Biol 158: 369–390, 1991. [DOI] [PubMed] [Google Scholar]
- Full RJ, Tu MS. Mechanics of a rapid running insect: two-, four- and six-legged locomotion. J Exp Biol 156: 215–231, 1991. [DOI] [PubMed] [Google Scholar]
- Gervasio S, Farina D, Sinkjær T, Mrachacz-Kersting N. Crossed reflex reversal during human locomotion. J Neurophysiol 109: 2335–2344, 2013. doi: 10.1152/jn.01086.2012. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan A, Modenese L, Phillips AT. A novel computational framework for deducing muscle synergies from experimental joint moments. Front Comput Neurosci 8: 153, 2014. doi: 10.3389/fncom.2014.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorb SN. Biological attachment devices: exploring nature’s diversity for biomimetics. Philos Trans A Math Phys Eng Sci 366: 1557–1574, 2008. doi: 10.1098/rsta.2007.2172. [DOI] [PubMed] [Google Scholar]
- Gregor RJ, Abelew TA. Tendon force measurements and movement control: a review. Med Sci Sports Exerc 26: 1359–1372, 1994. doi: 10.1249/00005768-199411000-00011. [DOI] [PubMed] [Google Scholar]
- Grey MJ, Nielsen JB, Mazzaro N, Sinkjaer T. Positive force feedback in human walking. J Physiol 581: 99–105, 2007. doi: 10.1113/jphysiol.2007.130088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritsenko V, Mushahwar V, Prochazka A. Adaptive changes in locomotor control after partial denervation of triceps surae muscles in the cat. J Physiol 533: 299–311, 2001. doi: 10.1111/j.1469-7793.2001.0299b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruhn M, Rosenbaum P, Bockemühl T, Büschges A. Body side-specific control of motor activity during turning in a walking animal. eLife 5: e13799, 2016. doi: 10.7554/eLife.13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie DM. Multipolar stretch receptors and the insect leg reflex. J Insect Physiol 13: 1637–1644, 1967. doi: 10.1016/0022-1910(67)90159-X. [DOI] [Google Scholar]
- Hagio S, Kouzaki M. The flexible recruitment of muscle synergies depends on the required force-generating capability. J Neurophysiol 112: 316–327, 2014. doi: 10.1152/jn.00109.2014. [DOI] [PubMed] [Google Scholar]
- Hellekes K, Blincow E, Hoffmann J, Büschges A. Control of reflex reversal in stick insect walking: effects of intersegmental signals, changes in direction, and optomotor-induced turning. J Neurophysiol 107: 239–249, 2012. doi: 10.1152/jn.00718.2011. [DOI] [PubMed] [Google Scholar]
- Ichikawa T, Toh Y, Sakamoto H. Structure and function of the elastic organ in the tibia of a tenebrionid beetle. Naturwissenschaften 103: 41, 2016. doi: 10.1007/s00114-016-1363-2. [DOI] [PubMed] [Google Scholar]
- Jami L. Golgi tendon organs in mammalian skeletal muscle: functional properties and central actions. Physiol Rev 72: 623–666, 1992. doi: 10.1152/physrev.1992.72.3.623. [DOI] [PubMed] [Google Scholar]
- Juusola M, de Polavieja GG. The rate of information transfer of naturalistic stimulation by graded potentials. J Gen Physiol 122: 191–206, 2003. doi: 10.1085/jgp.200308824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliyamoorthy S, Zill SN, Quinn RD. Force sensors in hexapod locomotion. In: Mobile Robotics: Moving Intelligence, edited by Buchli J. Rijeka, Croatia: InTech, 2006, p. 495–512. doi: 10.5772/4736. [DOI] [Google Scholar]
- Kistemaker DA, Van Soest AJ, Wong JD, Kurtzer I, Gribble PL. Control of position and movement is simplified by combined muscle spindle and Golgi tendon organ feedback. J Neurophysiol 109: 1126–1139, 2013. doi: 10.1152/jn.00751.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komi PV. Relevance of in vivo force measurements to human biomechanics. J Biomech 23, Suppl 1: 23–34, 1990. doi: 10.1016/0021-9290(90)90038-5. [DOI] [PubMed] [Google Scholar]
- Krutki P, Jelen S, Jankowska E. Do premotor interneurons act in parallel on spinal motoneurons and on dorsal horn spinocerebellar and spinocervical tract neurons in the cat? J Neurophysiol 105: 1581–1593, 2011. doi: 10.1152/jn.00712.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labonte D, Federle W. Functionally different pads on the same foot allow control of attachment: stick insects have load-sensitive “heel” pads for friction and shear-sensitive “toe” pads for adhesion. PLoS One 8: e81943, 2013. doi: 10.1371/journal.pone.0081943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lettvin JY, Maturana HR, McCulloch WS, Pitts WH. What the frog’s eye tells the frog’s brain. Proc IRE Inst Radio Eng 47: 1940–1951, 1959. doi: 10.1109/JRPROC.1959.287207. [DOI] [Google Scholar]
- Lévy J, Cruse H. Controlling a system with redundant degrees of freedom. I. Torque distribution in still standing stick insects. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 194: 719–733, 2008. doi: 10.1007/s00359-008-0343-1. [DOI] [PubMed] [Google Scholar]
- Mazzaro N, Grey MJ, Sinkjaer T. Contribution of afferent feedback to the soleus muscle activity during human locomotion. J Neurophysiol 93: 167–177, 2005. doi: 10.1152/jn.00283.2004. [DOI] [PubMed] [Google Scholar]
- Mendes CS, Rajendren SV, Bartos I, Márka S, Mann RS. Kinematic responses to changes in walking orientation and gravitational load in Drosophila melanogaster. PLoS One 9: e109204, 2014. doi: 10.1371/journal.pone.0109204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu L, Ritzmann RE. Interaction between descending input and thoracic reflexes for joint coordination in cockroach: I. Descending influence on thoracic sensory reflexes. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 194: 283–298, 2008. doi: 10.1007/s00359-007-0307-x. [DOI] [PubMed] [Google Scholar]
- Newland PL, Emptage NJ. The central connections and actions during walking of tibial campaniform sensilla in the locust. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 178: 749–762, 1996. doi: 10.1007/BF00225823. [DOI] [PubMed] [Google Scholar]
- Nichols TR. Distributed force feedback in the spinal cord and the regulation of limb mechanics. J Neurophysiol 119: 1186–1200, 2018. doi: 10.1152/jn.00216.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noah JA, Quimby L, Frazier SF, Zill SN. Force detection in cockroach walking reconsidered: discharges of proximal tibial campaniform sensilla when body load is altered. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 187: 769–784, 2001. doi: 10.1007/s00359-001-0247-9. [DOI] [PubMed] [Google Scholar]
- Owaki D, Kano T, Nagasawa K, Tero A, Ishiguro A. Simple robot suggests physical interlimb communication is essential for quadruped walking. J R Soc Interface 10: 20120669, 2013. doi: 10.1098/rsif.2012.0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantall A, Hodson-Tole EF, Gregor RJ, Prilutsky BI. Increased intensity and reduced frequency of EMG signals from feline self-reinnervated ankle extensors during walking do not normalize excessive lengthening. J Neurophysiol 115: 2406–2420, 2016. doi: 10.1152/jn.00565.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson K, Ekeberg O, Büschges A. Assessing sensory function in locomotor systems using neuro-mechanical simulations. Trends Neurosci 29: 625–631, 2006. doi: 10.1016/j.tins.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Pearson KG. Central programming and reflex control of walking in the cockroach. J Exp Biol 56: 173–193, 1972. [Google Scholar]
- Pearson KG. Role of sensory feedback in the control of stance duration in walking cats. Brain Res Brain Res Rev 57: 222–227, 2008. doi: 10.1016/j.brainresrev.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Misiaszek JE. Use-dependent gain change in the reflex contribution to extensor activity in walking cats. Brain Res 883: 131–134, 2000. doi: 10.1016/S0006-8993(00)02880-8. [DOI] [PubMed] [Google Scholar]
- Pfeiffer K, French AS. Naturalistic stimulation changes the dynamic response of action potential encoding in a mechanoreceptor. Front Physiol 6: 303, 2015. doi: 10.3389/fphys.2015.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A. Sensorimotor gain control: a basic strategy of motor systems? Prog Neurobiol 33: 281–307, 1989. doi: 10.1016/0301-0082(89)90004-X. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Sensory control of normal movement and of movement aided by neural prostheses. J Anat 227: 167–177, 2015. doi: 10.1111/joa.12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A, Gillard D, Bennett DJ. Positive force feedback control of muscles. J Neurophysiol 77: 3226–3236, 1997. doi: 10.1152/jn.1997.77.6.3226. [DOI] [PubMed] [Google Scholar]
- Quimby LA, Amer AS, Zill SN. Common motor mechanisms support body load in serially homologous legs of cockroaches in posture and walking. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 192: 247–266, 2006. doi: 10.1007/s00359-005-0062-9. [DOI] [PubMed] [Google Scholar]
- Ramdya P, Thandiackal R, Cherney R, Asselborn T, Benton R, Ijspeert AJ, Floreano D. Climbing favours the tripod gait over alternative faster insect gaits. Nat Commun 8: 14494, 2017. doi: 10.1038/ncomms14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgel AL, Frazier SF, DiCaprio RA, Zill SN. Encoding of forces by cockroach tibial campaniform sensilla: implications in dynamic control of posture and locomotion. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 186: 359–374, 2000. doi: 10.1007/s003590050436. [DOI] [PubMed] [Google Scholar]
- Ritzmann RE, Büschges A. Adaptive motor behavior in insects. Curr Opin Neurobiol 17: 629–636, 2007. doi: 10.1016/j.conb.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Ritzmann RE, Zill SN. Control of locomotion in hexapods. In: The Oxford Handbook of Invertebrate Neurobiology, edited by Byrne JH. Oxford, UK: Oxford Univ. Press, 2017, p. 1–28. doi: 10.1093/oxfordhb/9780190456757.013.20. [DOI] [Google Scholar]
- Rosenbaum P, Wosnitza A, Büschges A, Gruhn M. Activity patterns and timing of muscle activity in the forward walking and backward walking stick insect Carausius morosus. J Neurophysiol 104: 1681–1695, 2010. doi: 10.1152/jn.00362.2010. [DOI] [PubMed] [Google Scholar]
- Ross KT, Nichols TR. Heterogenic feedback between hindlimb extensors in the spontaneously locomoting premammillary cat. J Neurophysiol 101: 184–197, 2009. doi: 10.1152/jn.90338.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse EJ, Villagaray-Carski NC, Emerson RW, Herr HM. Design and testing of a bionic dancing prosthesis. PLoS One 10: e0135148, 2015. doi: 10.1371/journal.pone.0135148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer PL, Ritzmann RE. Descending influences on escape behavior and motor pattern in the cockroach. J Neurobiol 49: 9–28, 2001. doi: 10.1002/neu.1062. [DOI] [PubMed] [Google Scholar]
- Schmitz J. Load-compensating reactions in the proximal leg joints of stick insects during standing and walking. J Exp Biol 183: 15–33, 1993. [Google Scholar]
- Simpson CS, Sohn MH, Allen JL, Ting LH. Feasible muscle activation ranges based on inverse dynamics analyses of human walking. J Biomech 48: 2990–2997, 2015. doi: 10.1016/j.jbiomech.2015.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz T, Schmidt J. Subesophageal modulation of thoracic motor activity in the stick insect (Abstract) 11th Göttingen Meeting of the German Neuroscience Society March 18–21, 2015, p. T21-3D. [Google Scholar]
- Straka H, Simmers J, Chagnaud BP. A new perspective on predictive motor signaling. Curr Biol 28: R232–R243, 2018. doi: 10.1016/j.cub.2018.01.033. [DOI] [PubMed] [Google Scholar]
- Szczecinski NS, Martin JP, Bertsch DJ, Ritzmann RE, Quinn RD. Neuromechanical model of praying mantis explores the role of descending commands in pre-strike pivots. Bioinspir Biomim 10: 065005, 2015. doi: 10.1088/1748-3190/10/6/065005. [DOI] [PubMed] [Google Scholar]
- Tóth TI, Daun-Gruhn S. A three-leg model producing tetrapod and tripod coordination patterns of ipsilateral legs in the stick insect. J Neurophysiol 115: 887–906, 2016. doi: 10.1152/jn.00693.2015. [DOI] [PubMed] [Google Scholar]
- Tryba AK, Ritzmann RE. Multi-joint coordination during walking and foothold searching in the Blaberus cockroach. I. Kinematics and electromyograms. J Neurophysiol 83: 3323–3336, 2000. doi: 10.1152/jn.2000.83.6.3323. [DOI] [PubMed] [Google Scholar]
- Tuthill JC, Wilson RI. Mechanosensation and adaptive motor control in insects. Curr Biol 26: R1022–R1038, 2016. doi: 10.1016/j.cub.2016.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Holst E, Mittelstaedt H. The principle of reafference: interactions between the central nervous system and the peripheral organs. In: Perceptual Processing: Stimulus Equivalence and Pattern Recognition, edited by Dodwell PC. New York: Appleton-Century-Crofts, 1971. [Google Scholar]
- von Twickel A, Büschges A, Pasemann F. Deriving neural network controllers from neuro-biological data: implementation of a single-leg stick insect controller. Biol Cybern 104: 95–119, 2011. doi: 10.1007/s00422-011-0422-1. [DOI] [PubMed] [Google Scholar]
- Watson JT, Ritzmann RE, Pollack AJ. Control of climbing behavior in the cockroach, Blaberus discoidalis. II. Motor activities associated with joint movement. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 188: 55–69, 2002. doi: 10.1007/s00359-002-0278-x. [DOI] [PubMed] [Google Scholar]
- White O, Dowling N, Bracewell RM, Diedrichsen J. Hand interactions in rapid grip force adjustments are independent of object dynamics. J Neurophysiol 100: 2738–2745, 2008. doi: 10.1152/jn.90593.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wile GD, Daltorio KA, Diller ED, Palmer LR, Gorb SN, Ritzmann RE, Quinn RD. Screenbot: walking inverted using distributed inward gripping. In: Proceedings of the IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS'08). Nice, France: IEEE/RSJ, 2008, p. 1513–1518. doi: 10.1109/IROS.2008.4651045. [DOI] [Google Scholar]
- Zajac FE, Gordon ME. Determining muscle’s force and action in multi-articular movement. Exerc Sport Sci Rev 17: 187–230, 1989. [PubMed] [Google Scholar]
- Zill S, Frazier SF, Neff D, Quimby L, Carney M, DiCaprio R, Thuma J, Norton M. Three-dimensional graphic reconstruction of the insect exoskeleton through confocal imaging of endogenous fluorescence. Microsc Res Tech 48: 367–384, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- Zill S, Schmitz J, Büschges A. Load sensing and control of posture and locomotion. Arthropod Struct Dev 33: 273–286, 2004. doi: 10.1016/j.asd.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Zill SN, Büschges A, Schmitz J. Encoding of force increases and decreases by tibial campaniform sensilla in the stick insect, Carausius morosus. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 197: 851–867, 2011. doi: 10.1007/s00359-011-0647-4. [DOI] [PubMed] [Google Scholar]
- Zill SN, Chaudhry S, Büschges A, Schmitz J. Directional specificity and encoding of muscle forces and loads by stick insect tibial campaniform sensilla, including receptors with round cuticular caps. Arthropod Struct Dev 42: 455–467, 2013. doi: 10.1016/j.asd.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Zill SN, Chaudhry S, Büschges A, Schmitz J. Force feedback reinforces muscle synergies in insect legs. Arthropod Struct Dev 44: 541–553, 2015. doi: 10.1016/j.asd.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Zill SN, Chaudhry S, Exter A, Büschges A, Schmitz J. Positive force feedback in development of substrate grip in the stick insect tarsus. Arthropod Struct Dev 43: 441–455, 2014. doi: 10.1016/j.asd.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Zill SN, Keller BR, Duke ER. Sensory signals of unloading in one leg follow stance onset in another leg: transfer of load and emergent coordination in cockroach walking. J Neurophysiol 101: 2297–2304, 2009. doi: 10.1152/jn.00056.2009. [DOI] [PubMed] [Google Scholar]
- Zill SN, Neff D, Chaudhry S, Exter A, Schmitz J, Büschges A. Effects of force detecting sense organs on muscle synergies are correlated with their response properties. Arthropod Struct Dev 46: 564–578, 2017. doi: 10.1016/j.asd.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zill SN, Schmitz J, Chaudhry S, Büschges A. Force encoding in stick insect legs delineates a reference frame for motor control. J Neurophysiol 108: 1453–1472, 2012. doi: 10.1152/jn.00274.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]