Abstract
Correlations and inferred causal interactions among local field potentials (LFPs) simultaneously recorded in distinct visual brain areas can provide insight into how visual and cognitive signals are communicated between neuronal populations. Based on the known anatomical connectivity of hierarchically organized visual cortical areas and electrophysiological measurements of LFP interactions, a framework for interareal frequency-specific communication has emerged. Our goals were to test the predictions of this framework in the context of the early visual pathways and to understand how attention modulates communication between the visual thalamus and primary visual cortex. We recorded LFPs simultaneously in retinotopically aligned regions of the visual thalamus and primary visual cortex in alert and behaving macaque monkeys trained on a contrast-change detection task requiring covert shifts in visual spatial attention. Coherence and Granger-causal interactions among early visual circuits varied dynamically over different trial periods. Attention significantly enhanced alpha-, beta-, and gamma-frequency interactions, often in a manner consistent with the known anatomy of early visual circuits. However, attentional modulation of communication among early visual circuits was not consistent with a simple static framework in which distinct frequency bands convey directed inputs. Instead, neuronal network interactions in early visual circuits were flexible and dynamic, perhaps reflecting task-related shifts in attention.
NEW & NOTEWORTHY Attention alters the way we perceive the visual world. For example, attention can modulate how visual information is communicated between the thalamus and cortex. We recorded local field potentials simultaneously in the visual thalamus and cortex to quantify the impact of attention on visual information communication. We found that attentional modulation of visual information communication was not static, but dynamic over the time course of trials.
Keywords: coherence, Granger causality, LGN, local field potential, V1
INTRODUCTION
A fundamental goal in systems neuroscience is to understand how sensory and cognitive information is communicated across brain areas. Simultaneous measurements of local field potentials (LFPs) in different brain areas have aided in our understanding of interareal communication. Because LFPs represent the activity of localized populations of neurons (Katzner et al. 2009; Lashgari et al. 2012; Xing et al. 2009), correlations and inferred causal interactions between LFPs recorded across brain areas can provide insight into how information is communicated between neuronal populations (Buzsáki and Schomburg 2015; Fries 2005; 2009; Friston et al. 2015). Much is known about the hierarchical organization of visual brain areas (Briggs 2017; Callaway 2005; Felleman and Van Essen 1991; Sincich and Horton 2005). Accordingly, the visual system is a useful model in which to study signal interactions among simultaneously recorded LFPs to explore the principles governing feedforward and feedback interareal communication and whether cognitive processes such as attention alter corticocortical communication (Bastos et al. 2012; Jensen et al. 2015).
Visual information travels in the feedforward direction from the retina to the lateral geniculate nucleus (LGN) of the thalamus to primary visual cortex (V1) (Briggs 2017; Callaway 2005). From V1, visual signals are transmitted in the feedforward direction to a variety of low- and mid-level extrastriate visual cortical areas including V2, MT, and V4 (Briggs 2017; Felleman and Van Essen 1991; Sincich and Horton 2005). Neurons in low- and mid-level extrastriate areas project to progressively higher level visual cortical areas (Felleman and Van Essen 1991). Generally, feedforward connections arise from afferent projection neurons located in the superficial and deep layers of a lower cortical area with axons targeting the middle layers, usually layer 4, of the recipient higher cortical area. Feedback connections arise from efferent projection neurons located in the superficial and deep layers of a higher cortical area with axons targeting superficial and deep layers of the recipient lower cortical area (Felleman and Van Essen 1991). A notable exception to this feedforward connection rule is the pathway from LGN to V1. LGN axons mainly target layer 4C; however, additional axons target both deep (layer 6) and superficial (layers 2/3 and 4A) layers (Fitzpatrick et al. 1983; Hendrickson et al. 1978; Hendry and Reid 2000). Feedback connections from V1 to the LGN arise entirely from efferent projection neurons, termed corticogeniculate neurons, located in layer 6 (Briggs et al. 2016; Fitzpatrick et al. 1994).
By combining known anatomical connectivity with electrophysiological measurements of functional connectivity, such as correlation or coherence and directed interactions between LFPs simultaneously recorded in different visual areas, a framework has emerged for describing interareal communication of visual and attention signals through synchronized LFP oscillations in distinct frequency bands. In this framework, feedforward visual signals between the LGN and V1 are expressed as synchronized LFP oscillations in the beta-frequency band (~15–30 Hz) (Bastos et al. 2014), while corticocortical feedforward signals are indicated by synchronized LFPs in the gamma-frequency band (~30–80 Hz) (Bastos et al. 2015; Bollimunta et al. 2011; Michalareas et al. 2016; Roberts et al. 2013; van Kerkoerle et al. 2014). Feedback visual signals are expressed as synchronized LFP oscillations in the alpha-frequency band (~7–15 Hz) (Bastos et al. 2015; Michalareas et al. 2016; van Ede et al. 2015; van Kerkoerle et al. 2014). Interestingly, synchronized gamma-frequency band LFP oscillations have been implicated in both bottom-up/feedforward and top-down/feedback corticocortical communication (Gregoriou et al. 2009; Gregoriou et al. 2015; Michalareas et al. 2016; Richter et al. 2017; van Kerkoerle et al. 2014). Furthermore, gamma-frequency LFPs are also modulated by visual attention, especially in extrastriate visual cortical areas (Bauer et al. 2014; Bosman et al. 2012; Buffalo et al. 2010; Chalk et al. 2010; Fries et al. 2001, 2008; Gregoriou et al. 2009, 2015; Siegel et al. 2008; Taylor et al. 2005; Vinck et al. 2013). Visual attention modulates neuronal activity across the visual hierarchy with less modulation in LGN and V1 and greater modulation in higher extrastriate visual cortical areas (Ito and Gilbert 1999; Luck et al. 1997; McAdams and Reid 2005; McAlonan et al. 2006; 2008; Motter 1993; O’Connor et al. 2002; Vanduffel et al. 2000). Some have proposed that attention signals are propagated from higher extrastriate visual cortical areas to progressively lower visual cortical areas in the top-down/feedback direction (Buffalo et al. 2010; Gregoriou et al. 2009). Importantly, the only direct route by which attention signals can reach the LGN from the cortex is through corticogeniculate circuits. Whether attention signals are propagated in feedforward or feedback directions, and whether distinct frequency bands convey these attention signals, remain open questions.
In addition to frequency-specific interareal communication, a number of studies have identified differences in visually evoked LFP power and attentional modulation across the layers of visual cortex (Buffalo et al. 2011; Maier et al. 2010, 2011; Welle and Contreras 2016; Xing et al. 2012). The overall consensus among these studies is that visually evoked gamma activity is greatest in the superficial and middle layers while lower frequency power is greater in deeper cortical layers (Hansen and Dragoi 2011; Maier et al. 2010; Spaak et al. 2012; Welle and Contreras 2016; Xing et al. 2012). Attentional modulation of LFP power also differs across the cortical depth with stronger attentional modulation in superficial and deep layers (Buffalo et al. 2010; Nandy et al. 2017; van Kerkoerle et al. 2017). Few studies have examined directed interactions among LFPs recorded across the cortical layers. Whether interactions within a cortical area influence interareal communication also remains unknown.
We sought to test the predictions of the frequency-specific interareal communication framework in the early visual pathways for which circuit connectivity is well established. We recorded LFPs simultaneously in retinotopically aligned regions of the LGN and V1 in alert and behaving monkeys performing an attention-demanding contrast-change detection task (Hembrook-Short et al. 2017). Based on known circuit connectivity between the LGN and V1 and the frequency-specific interareal communication framework, we generated four testable hypotheses about attentional modulation of feedforward and feedback communication in the early visual pathways. First, based on initial coherence measurements, we hypothesized that attentional modulation of communication between the LGN and V1 would be greatest in beta- and gamma-frequency bands. Second, based on predictions of the framework that feedforward visual signals are conveyed by beta-frequency oscillations (Bastos et al. 2014), we hypothesized that attentional enhancement of feedforward communication would be selective for beta frequencies. Third, also based on predictions of the framework that top-down attention signals are carried by alpha- and/or gamma-frequency oscillations (Bastos et al. 2012; Gregoriou et al. 2015; van Kerkoerle et al. 2014), we hypothesized that attentional enhancement of feedback communication would be selective for alpha or gamma frequencies. Fourth, based on predictions of the framework and the fact that feedback from V1 to the LGN is mediated by corticogeniculate neurons in layer 6, we hypothesized that attentional enhancement of feedback communication from V1 to the LGN would be greatest for alpha- and/or gamma-band interactions between the deep layers of V1 and the LGN. Our findings were supportive of some, but not all, of the hypotheses proposed. Overall, attention mostly facilitated beta- and gamma-band interactions, supporting the first hypothesis. Consistent with the second hypothesis, attention enhanced feedforward communication for beta frequencies; however, this effect was not selective for beta frequencies or feedforward interactions. Attention consistently facilitated feedback interactions in the gamma band for connections between the deep cortical layers of V1 and the LGN, consistent with the third and fourth hypotheses. In contrast, attentional enhancement of alpha-band feedback was only observed during the cue period, before visual stimulus presentation, and was distributed across V1 layers. Our observations deviated from the predictions of the framework in part because attentional modulation of feedforward, feedback, and local circuit interactions varied dynamically over different trial periods. Together, our results suggest that dynamic interactions among early visual circuits cannot be adequately described by a simple static framework in which distinct frequency bands convey directed inputs. More flexible and dynamic frameworks are likely required to accurately reflect neuronal network activity during perceptual and cognitive tasks.
MATERIALS AND METHODS
This study involved novel analyses of neurophysiological data collected previously as a part of separate studies (Bastos et al. 2014; Briggs et al. 2013; Hembrook-Short et al. 2017). Therefore, all of the procedures involving monkeys have been described (Hembrook-Short et al. 2017). All procedures performed for this study conformed to the guidelines set forth by the National Institutes of Health and were approved by the Institutional Animal Care and Use Committees at the Geisel School of Medicine at Dartmouth and the University of California, Davis.
Surgical preparation, maintenance, recording, and behavioral task.
Three adult female macaque monkeys (Macacca mulatta) were used for this study. Surgical preparation, maintenance, electrophysiological recording, eye tracking, visual stimulation, and behavioral tasks have been described in detail previously (Briggs et al. 2013; Hembrook-Short et al. 2017). Briefly, under full surgical anesthesia and in aseptic conditions, monkeys received head posts, small craniotomies for recording access, and/or chronically implanted electrodes. Craniotomies were encircled by recording chambers that were flushed at least three times per week. Single-electrodes or multielectrode arrays were inserted into retinotopically aligned regions of LGN and V1, and continuous voltage data were amplified and digitized (10,000 Hz) with referencing to guide tubes containing electrodes. Continuous voltage data were low-pass filtered at 200 Hz and downsampled to 1,000 Hz to generate local field potentials (LFPs) for all recorded electrodes/contacts. Two different techniques were used to assign LFP recording contacts to the supragranular (SG), granular (G), or infragranular (IG) laminar compartments. In monkeys B and O, proximity in vertical distance of LFP recording contacts to identified orthodromically activated layer 4C neurons was used (Briggs et al. 2013). In monkey E, the border between layer 4C and layer 5 was estimated based on the first polarity reversal in the current source density spectrum (Maier et al. 2010; Maier et al. 2011) generated from average LFP responses to flashed stimuli and LFP recording contacts were assigned to SG, G, and IG laminar compartments based on vertical distance to this border. Proper laminar compartment assignment of LFP recording contacts was also independently confirmed based on physiological response properties of single neurons recorded during the same sessions (Hembrook-Short et al. 2017).
LFPs were recorded while monkeys performed a contrast-change detection task in which spatial attention was allocated to one of two identical drifting sinusoidal grating stimuli according to the fixation dot color cue (Fig. 1A). Gratings were 2–4° in diameter and had a fixed temporal frequency of 4Hz, starting contrast between 20 and 70%, and orientation matching the preferred orientation of recorded neurons (Briggs et al. 2013; Hembrook-Short et al. 2017). All trials followed the same timeline: a 2-s intertrial period; a fixation dot appeared prompting monkeys to obtain fixation; once fixation was obtained, the 0.3-s cue period began; following the cue period, two drifting gratings were displayed and remained on the monitor for 1.2–3 s (grating display duration varied per trial according to a hazard function with an average of 1.7 s); contrast of 1 grating increased by 10%; and monkeys maintained fixation and indicated detection of contrast change by pushing a button or releasing a lever within a 1-s answer period. Trials were run in blocks of 10–20 trials in which monkeys directed attention toward or away from the stimulus overlapping recorded neuronal receptive fields. Within each block of trials, 95% of trials were validly cued. On invalidly cued trials, monkeys were rewarded for correct detection of the contrast change at the uncued location. Monkeys' accuracy and reaction times indicated correct shifts in spatial attention according to the fixation dot cue color (Hembrook-Short et al. 2017).
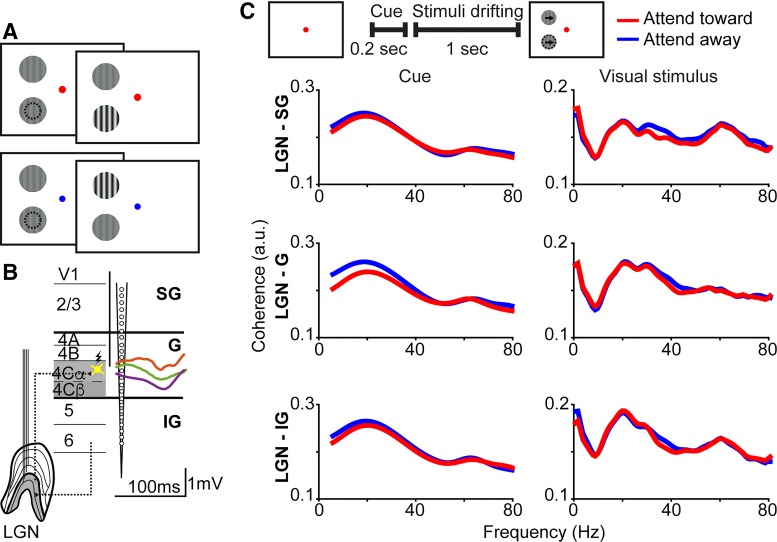
Fig. 1.
Lateral geniculate nucleus (LGN) of the thalamus and primary visual cortex (V1) coherence by cortical laminar compartment. A: schematic screen shots of the contrast-change detection task. A red fixation dot (top) cued monkeys to attend toward the stimulus in the receptive field of recorded neurons (indicated by dashed circles, not shown to monkeys) while a blue fixation dot (bottom) cued monkeys to attend away. B: schematic representation of single- and multielectrode arrays placed in LGN and V1, feedforward/feedback circuits connecting LGN and V1 (dashed lines), and methods used to assign recording contacts to supragranular (SG), granular (G), or infragranular (IG) laminar compartments (additional laminar borders illustrated as black lines and labeled on left). Yellow spiny stellate neuron in layer 4C represents an orthodromically stimulated neuron, from which proximity to recording contacts (represented by vertical black line) was measured in vertical distance. Alternatively, average local field potential (LFP) responses to flashed stimuli were recorded across linear array contacts to assign laminar location. Orange, green, and purple lines are single-session average layer 4 LFPs recorded in monkeys B, O, and E, respectively, with scale bar underneath; stimulus onset is start of each curve. C: schematic screen shot icons at the top illustrate the visual stimuli present on the monitor and the analysis window durations for the cue and visual stimulus display periods of the attention task. Arrows and dashed circle in the right schematic screen shot represent the motion in the stimuli and the stimulus overlapping the receptive fields of recorded neurons, respectively, and were not shown to the monkeys. Grand average (all trials, all 3 monkeys) coherence between LGN and supragranular (SG; top), granular (G; middle), or infragranular (IG; bottom) laminar compartments of V1 during the cue period (left column) and the visual stimulus display period (right column). Red and blue curves indicate coherence on attend-toward and attend-away trials respectively. There were no statistically significant differences in coherence across attention conditions at any frequency.
Data analysis: preprocessing.
Analyses were performed only on sessions in which monkeys completed at least 40 correct trials for each attention condition: attend-toward or attend-away from the stimulus in the receptive field of recorded neurons. Monkey B performed 54 to 55 ± 12 trials in each attention condition per session; monkey O performed 59 ± 8 trials in each attention condition per session; and monkey E performed 105 to 108 ± 24 trials in each attention condition per session. Across three monkeys, a total of 25 sessions with simultaneous LGN and V1 recordings were analyzed (monkey B = 13 sessions; monkey O = 6 sessions; and monkey E = 6 sessions). For LGN-V1 interactions, average coherence and Granger-causal interactions were computed from the following numbers of trials per monkey: monkey B: 178–318 trials per laminar compartment and attention condition; monkey O: 56–179 trials per laminar compartment and attention condition; and monkey E: 627–645 trials per laminar compartment and attention condition. An additional 34 sessions from monkey E were used for analyses of Granger-causal interactions across the laminar compartments within V1. V1-only trials from monkey E ranged from 544 to 600 trials per laminar compartment and attention condition.
LFP data were separated by attention condition and then three nonoverlapping trial periods were analyzed: intertrial period (0.5-s window) to provide a baseline measurement of LGN-V1 coherence; cue period (0.3-s window) in which attention was cued by the fixation dot color but no grating stimuli were displayed; and visual stimulus display period (1-s window) including the last four complete visual stimulus cycles before the contrast change per trial. Individual trial period LFPs containing noise artifacts (e.g., due to movement) were excluded from further analysis by identifying unusual spikes in the integral power spectrum calculated per trial period and per contact.
All LFPs were linearly detrended (best-fit line per 1-s window subtracted) and z-scored per trial period and per contact. To avoid introducing bias into the vector autoregressive (VAR) model estimation of Granger-causality, z-scoring involved subtracting the temporal ensemble mean (mean across all trials concatenated together) instead of the per trial mean and dividing by the standard deviation of all trials in a session. Because Granger-causality analysis requires stationary time-series data, a Kwiatkowski-Phillips-Schmidt-Shin (KPSS) unit root test with an alpha value of 0.05 was applied to all LFPs (per trial period, per contact) using the mvgc_kpss function from the Multivariate Granger-causality (MVGC) Toolbox (Barnett and Seth 2014; Seth et al. 2015) for MATLAB (MathWorks, Natick MA). Any trial period LFPs that did not meet the criteria of the KPSS stationarity test were excluded from further analysis. In rare cases where no contacts within a laminar compartment passed the KPSS stationarity test (<1% of trials), the entire trial was excluded from further analysis. For each trial (of each period type), LFPs were then averaged across contacts in the LGN and in the SG, G, and IG laminar compartments of V1.
Data analysis: coherence.
The mscohere function (periodic Hamming window = 1,000 ms, 1,024 discrete Fourier transform points) from the Signal Processing Toolbox for MATLAB was used to calculate the per-trial magnitude-squared coherence between the LGN and each laminar compartment for each trial period type. To avoid potential changes in coherence due to cue onset, coherence during the cue period was analyzed during the final 200 ms of the 300-ms cue period. However, cue period coherence did not differ across measurements from the full 300 ms or final 200 ms of the cue period (P = 0.5). For display purposes only, coherence during the visual stimulus display period was smoothed with a 10-Hz smoothing window. Grand average coherence, the average of all sessions across all three monkeys, and individual monkey average coherence are displayed in Figs. 1C and 2, respectively.
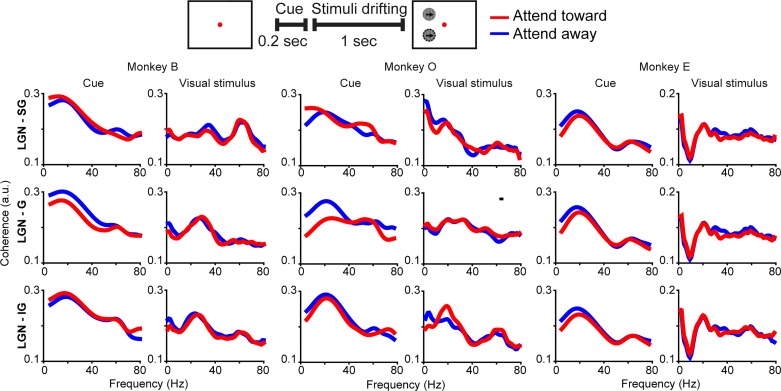
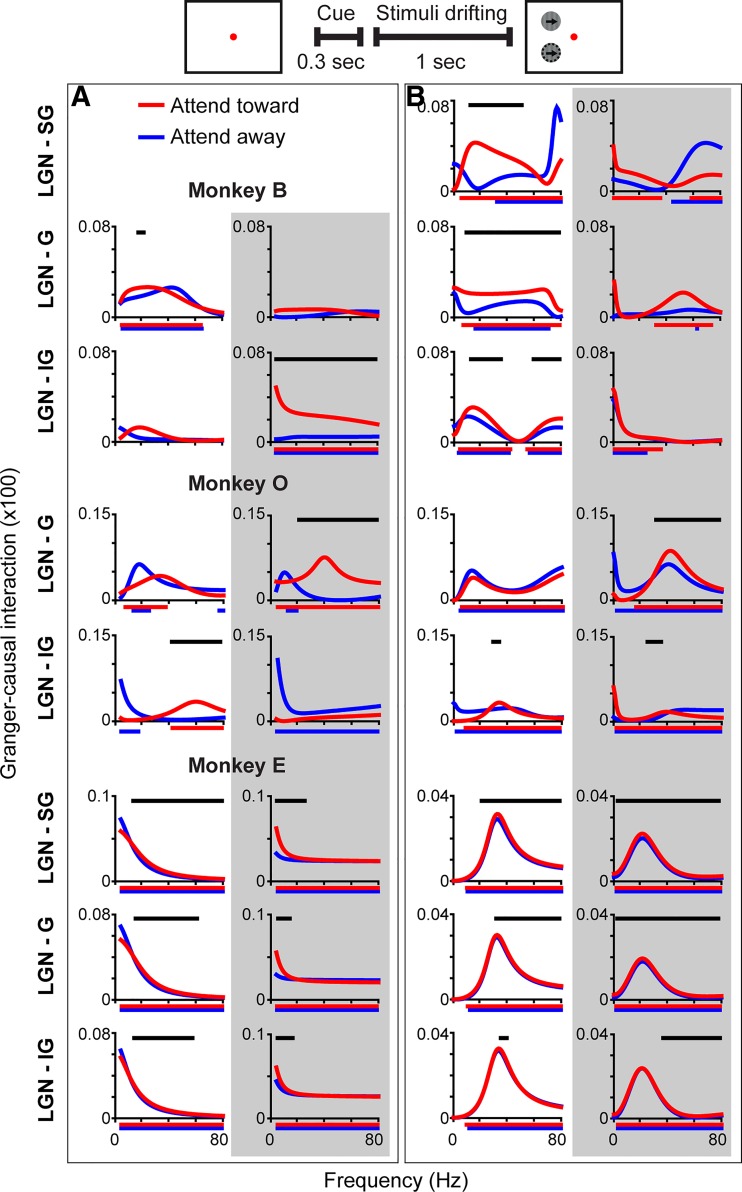
Fig. 2.
Lateral geniculate nucleus (LGN) of the thalamus and primary visual cortex (V1) coherence by cortical laminar compartment in individual monkeys Schematic at top as in Fig. 1C. Average coherence between the LGN and the supragranular (SG; top row), granular (G; middle row), and infragranular (IG; bottom row) laminar compartments of V1 during the cue period (left columns) and the visual stimulus display period (right columns) illustrated separately for monkey B, monkey O, and monkey E (labeled at top). Red and blue curves indicate coherence on attend-toward and attend-away trials, respectively. Black bar above monkey O LGN-G visual stimulus period coherence indicates frequencies at which coherence was significantly different across attention conditions (Bonferroni corrected P < 0.05).
Statistical analysis: coherence.
Three statistical tests were performed on coherence data. First, coherence data from each monkey individually, measured during the cue and visual stimulus display periods, were compared with coherence measured during the baseline period. A Bonferroni-corrected t-test [corrected for up to 80 frequencies (1–80 Hz) and 2 comparisons per monkey (cue and visual stimulus display period comparisons to baseline), alpha = 0.05] was used to compare cue and visual stimulus display period coherence to baseline period coherence per monkey. Second, coherence data from each monkey individually were compared across attention conditions using a Bonferroni-corrected t-test (corrected for up to 80 frequencies, alpha = 0.05). Third, grand average coherence data from all sessions in all monkeys were compared across attention conditions using a Bonferroni-corrected t-test (corrected for up to 80 frequencies, alpha = 0.05).
Data analysis: Granger-causality.
For the MVGC analysis, we used the open source MVGC Toolbox for MATLAB (Barnett and Seth 2014) following the steps described previously (Barnett and Seth 2011). We did not find it beneficial to re-reference, filter, or window LFP data as a part of the preprocessing procedure. We performed side-by-side comparisons of analyses of re-referenced and non-re-referenced data and observed no overall differences in the results, so we opted to use non-re-referenced data. Spectral pairwise conditional MVGC analyses were performed per trial on LFPs recorded in the LGN paired with LFPs recorded in the SG, G, or IG laminar compartments of V1. Separate analyses were performed on the following trial periods: full cue period (300 ms), visual stimulus period (1 s, corresponding to the last 4 complete cycles of the visual stimulus per trial), first and second halves (150 ms each) of the cue period, and first and second halves (500 ms each) of the visual stimulus period (Fig. 3). An additional analysis was performed using seven (150 ms each) segments of the visual stimulus period (Fig. 5). Finally, spectral pairwise conditional MVGC analyses were performed per trial on LFPs recorded in the SG, G, and IG laminar compartments of V1 using LFPs recorded from monkey E (Fig. 6).
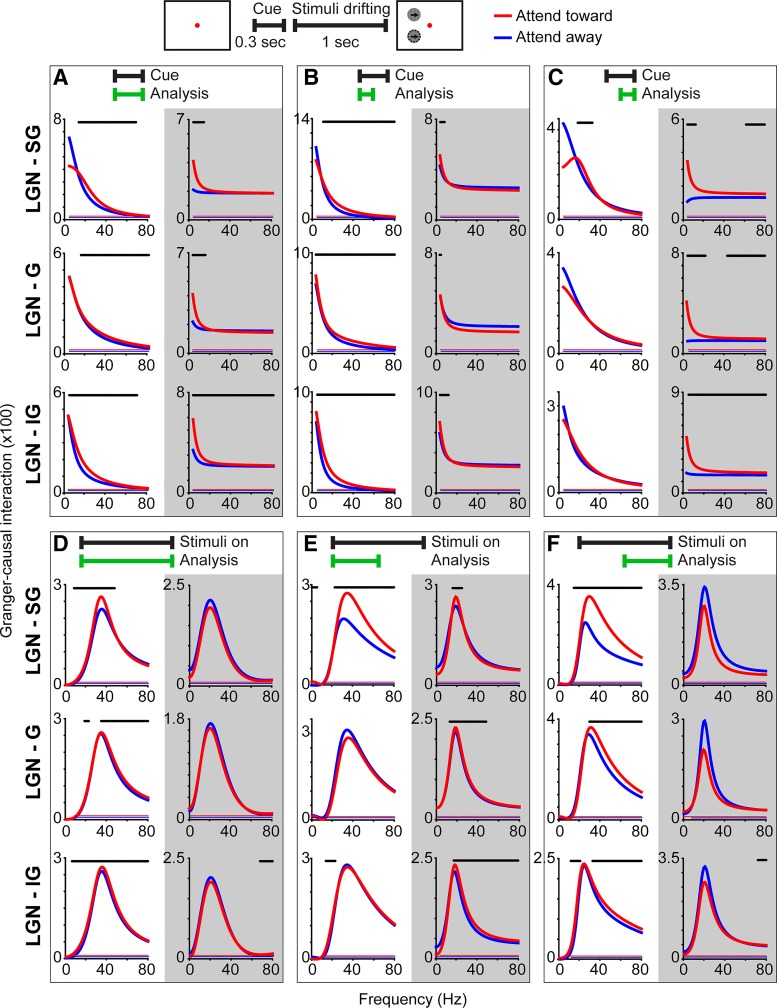
Fig. 3.
Granger-causal interactions between the lateral geniculate nucleus (LGN) of the thalamus and primary visual cortex (V1) laminar compartments. Schematic at top illustrates the cue and visual stimulus display periods analyzed in A–C and D–F, respectively. A–C: Granger-causal interactions (data combined from all 3 monkeys) between the LGN and supragranular (SG), granular (G), or infragranular (IG) laminar compartments of V1 for attend-toward (red) and attend-away (blue) conditions during the full (A), 1st half (B), and 2nd half (C) of the cue period (analysis windows indicated by green bars at top). White backgrounds (1st columns in each) illustrate feedforward interactions from LGN to V1 and gray backgrounds (2nd columns in each) illustrate feedback interactions from V1 to LGN. Red and blue lines just above the x-axis indicate frequencies at which Granger-causal interactions were significantly greater than noise (Bonferroni corrected P < 0.05). Black lines above curves indicate frequencies at which Granger-causal interactions were significantly different across attention conditions (P < 0.05, see materials and methods). Note differences in y-axis scaling. Analyses were truncated at 80 Hz because power was low for frequencies greater than 80 Hz in all 3 monkeys. D–F: Granger-causal interactions (data combined from all 3 monkeys) between the LGN and SG, G, and IG laminar compartments of V1 during the visual stimulus display period. Conventions as in A–C.
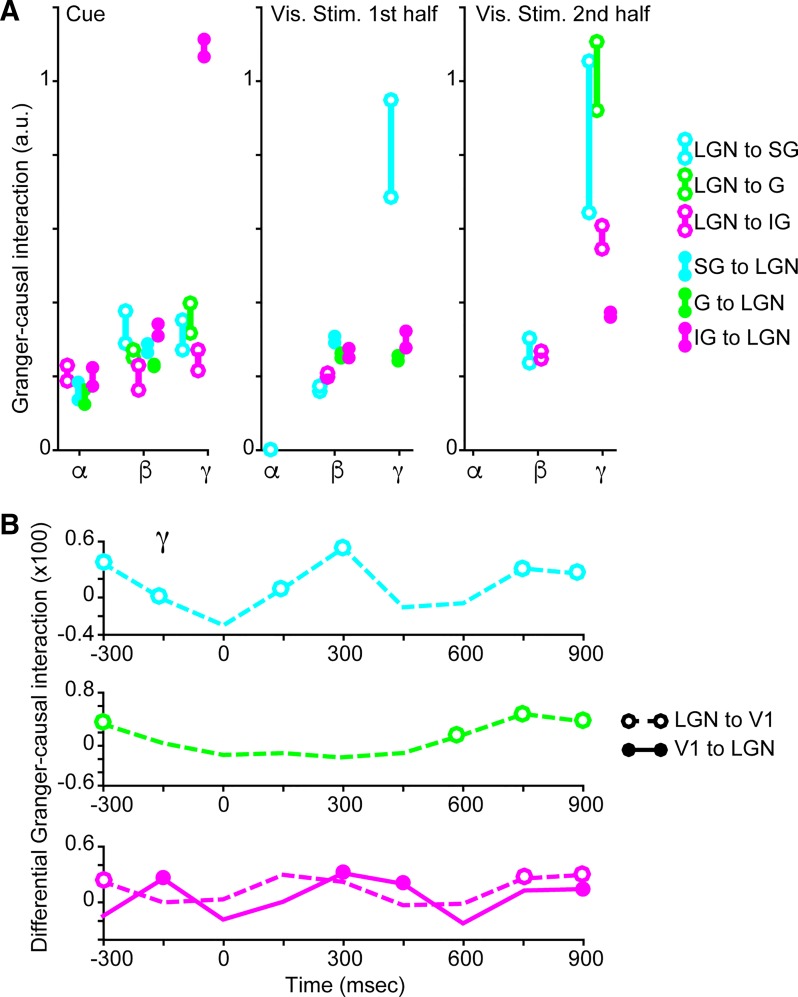
Fig. 5.
Quantified Granger-causal interactions between the lateral geniculate nucleus (LGN) of the thalamus and primary visual cortex (V1) and time course of gamma-band Granger-causal interactions. A: Granger-causal interaction quantified per alpha (α; 7–14 Hz)-, beta (β; 15–29 Hz)-, and gamma (γ; 30–80 Hz)-frequency bands, illustrated separately for feedforward (open circles) and feedback (closed circles) interactions between LGN and the supragranular (SG; cyan), granular (G; green), or infragranular (IG; magenta) laminar compartments. Only Granger-causal interactions per frequency that were significantly enhanced with attention (P < 0.05, see materials and methods) are illustrated: quantified interactions on attend-toward and attend-away trials are top and bottom circles, respectively, linked by solid lines. Quantified Granger-causal interactions are also illustrated separately for the cue period (left), the 1st half of the visual stimulus display period (middle), and the 2nd half of the visual stimulus display period (right). B: time course of average differential Granger-causal interactions (attend-toward minus attend-away) in the gamma frequency band. Dashed lines indicate feedforward interactions from LGN to SG (top, cyan) and LGN to G (middle, green) laminar compartments, and open circles indicate significant attentional enhancement of Granger-causal interaction (P < 0.05, see materials and methods) at a given time bin (150-ms bins; −300–0 ms represents the cue period and 0–1,000 ms represents the visual stimulus display period). For interactions between the LGN and the IG laminar compartment (bottom, magenta), both feedforward (dashed lines/open circles) and feedback (solid line/closed circles) differential Granger-causal interactions are shown. Note differences in y-axis scaling.
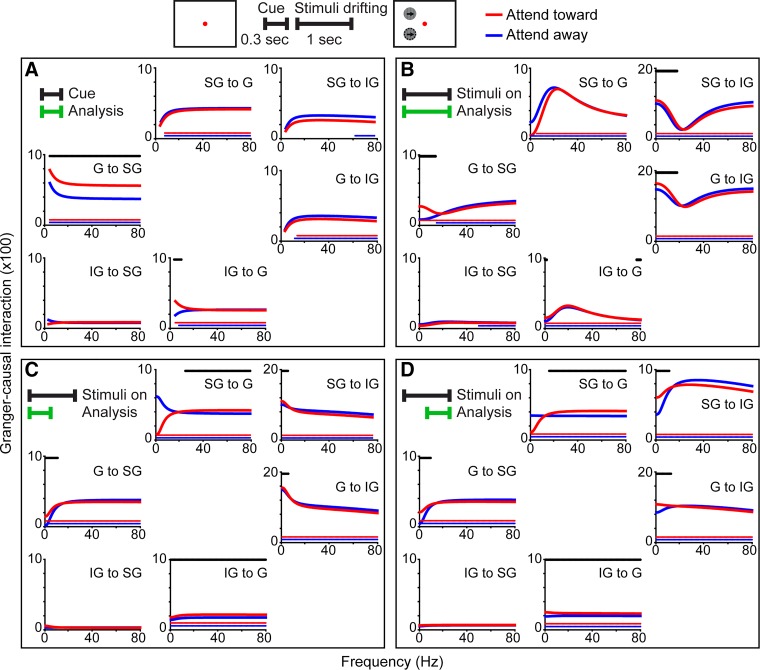
Fig. 6.
Granger-causal interactions among laminar compartments within primary visual cortex (V1). Schematic at top illustrates the cue and visual stimulus display periods analyzed in A and B–D, respectively. A: Granger-causal interactions (data from monkey E) between supragranular (SG), granular (G), or infragranular (IG) laminar compartments of V1 for attend-toward (red) and attend-away (blue) conditions during the full cue period (analysis window indicated by green bar at top left). Directed interactions indicated for each plot. Red and blue lines just above the x-axis indicate frequencies at which Granger-causal interactions were significantly greater than noise (Bonferroni corrected P < 0.05). Black lines above curves indicate frequencies at which Granger-causal interactions were significantly different across attention conditions (P < 0.05, see materials and methods). B–D: Granger-causal interactions between laminar compartments during the full length (B), 1st half (C), and 2nd half (D) of the visual stimulus display period. Conventions as in A. Note differences in y-axis scaling.
The following steps were performed for each analysis. First, the optimal model order, or number of lags, for the VAR model was chosen using the tsdata_to_infocrit function. Selection of the optimal model was based on Bayesian criterion information using the ordinary least squares regression model. This model calculates the solution to the regression with QR decomposition. From the optimal VAR model, a residuals covariance matrix was determined using the tsdata_to_var and var_to_autocovar functions. The frequency-domain MVGC was calculated per trial using the autocov_to_spwcgc function as:
where Sxx is the spectral density, Hxy is the transfer function, and Σx|y are the conditional covariance residuals (Barnett and Seth 2011). Frequency-domain MVGCs were then concatenated for each trial period type across all sessions and all monkeys and grand averages calculated. To quantify MVGCs in the alpha-, beta-, and gamma-frequency bands, integrals of the grand average frequency-domain MVGCs within each frequency band (alpha: 7–14 Hz; beta: 15–29 Hz; and gamma: 30–80 Hz) were calculated. This quantification was performed for MVGCs measured during the full cue period, the first and second halves of the visual stimulus display period, and nine 150-ms bins spanning the cue and visual stimulus display periods. Differential Granger-causal interaction per frequency and per LGN-V1 laminar compartment interaction was computed as the quantified MVGC on attend-toward trials minus the quantified MVGC on attend-away trials.
Statistical analysis: Granger-causality.
Two separate statistical analyses were performed on each set of Granger-causal interaction data. First, to determine whether frequency-domain MVGCs were significantly different from noise, permutation sampling was performed separately for attend-toward and attend-away conditions for each trial period type. This same statistical analysis was performed on Granger-causal interaction data from individual monkeys and on data combined from all three monkeys. The null distribution for the frequency-domain MVGCs was based on 500 random permutations of a block size equal to the optimal VAR model order (computed using the permtest_tsdata_to_spwcgc function). Based on the estimated null distribution, empirical P values were determined using a Bonferroni-correction (corrected for 80 frequencies, alpha = 0.05), computed using the empirical_pval function.
Second, to determine whether frequency-domain MVGCs were significantly different across attention conditions, the following steps were performed for each trial period type within each set of Granger-causal interaction data. As above, the same statistical analysis was performed on data from individual monkeys and on data combined from all three monkeys. In the first step of this statistical analysis, a random subset of 250 null array distributions from the attend-toward and attend-away arrays were concatenated and randomly shuffled. This step was repeated to produce two separate, random distributions of length 500. These two distributions were then subtracted to produce a difference null distribution that could be used to estimate p-values for the ‘observed difference’. The observed difference was calculated as the frequency-domain MVGCs for the attend-toward condition minus those for the attend-away condition. P < 0.05 was considered significant.
RESULTS
To explore how attention signals are communicated between the LGN and V1, we examined coherence and Granger-causality among LFPs recorded simultaneously in the two visual brain areas of three monkeys performing contrast-change detection tasks requiring covert shifts in visual spatial attention. To further explore how attention alters the propagation of signals among local cortical circuits, we examined attentional modulation of Granger-causal interactions among LFPs recorded across the cortical layers within V1 in one monkey. New analyses were performed on neurophysiological data collected as a part of prior studies for which behavioral results have been reported (Bastos et al. 2014; Briggs et al. 2013; Hembrook-Short et al. 2017). Three monkeys performed a contrast-change detection task in which a colored fixation dot cued monkeys to shift the focus of attention either toward or away from a drifting sinusoidal grating placed within the receptive fields of recorded neurons (Fig. 1A). While monkeys performed the attention task, LFPs were recorded simultaneously in retinotopically aligned regions of the LGN and V1, including multiple contacts spanning the cortical layers of V1. V1 recording contacts were assigned to SG, G, or IG laminar compartments based on proximity to LGN-recipient neurons in layer 4C (Briggs et al. 2013) or the first polarity reversal in average LFP responses to flashed stimuli. Average LFPs recorded in layer 4 were similar across monkeys irrespective of laminar contact localization method (Fig. 1B).
LGN-V1 coherence during cue and visual stimulus display periods.
Coherence between the LGN and V1 was similar across the SG, G, and IG laminar compartments; this was the case for coherence measured during both the cue period and the visual stimulus display period of the attention task (Fig. 1C). During the cue period, coherence was consistently enhanced in the beta- and low-gamma-frequency bands across laminar compartments, while during the visual stimulus display period, coherence was consistently enhanced in beta- and low- and high-gamma-frequency bands across laminar compartments (Fig. 1C). Coherence between the LGN and the laminar compartments of V1 was similar across individual monkeys, peaking at lower frequencies during the cue period and with more higher frequency coherence during the visual stimulus display period (Fig. 2). Importantly, in all three monkeys individually, coherence in beta- and gamma-frequency bands during the cue and visual stimulus display periods was significantly greater than coherence during the baseline period (P < 0.05, see materials and methods). Increases in coherence during the cue and visual stimulus display periods were not due to cue or visual stimulus onset as analysis windows for each began at least 100 ms following cue or visual stimulus onset. Interestingly, there were no significant differences in coherence across attention conditions during the cue or visual stimulus display period, with the exception of a significant attentional enhancement of gamma-band coherence between the LGN and the G laminar compartment during the visual stimulus display period for monkey O (Fig. 2, black line above middle row, 4th column plot indicates frequencies at which coherence was significantly enhanced with attention). Overall, there was a significant increase in coherence between the LGN and V1 during both the cue and visual stimulus display periods of attention trials that was consistent across laminar compartments and monkeys. There was little significant attentional modulation of coherence; however, this could be due to directed attentional modulation of selective LGN-V1 circuits or differential attentional modulation over the course of trials. To gain insight into the directionality of interactions in early visual circuits and the temporal dynamics of attentional modulation of circuit communication, we applied MVGC analyses to the LFP data set.
Attentional modulation of Granger-causal interactions between LGN and V1.
While feedforward anatomical connections exist between the LGN and all three laminar compartments of V1 (Fitzpatrick et al. 1983; Hendrickson et al. 1978; Hendry and Reid 2000), feedback connections from V1 to LGN arise from corticogeniculate neurons restricted to layer 6 in the IG laminar compartment (Briggs et al. 2016; Fitzpatrick et al. 1994). Motivated by the known circuitry of the early visual pathways and the emerging framework for frequency-specific interareal communication, we tested the following four hypotheses. First, based on our coherence results, we hypothesized that attentional modulation of communication between the LGN and V1 may be largest in the beta- and gamma-frequency bands. Second, based on the predictions of the framework, we hypothesized that attention enhances feedforward communication selectively at beta frequencies. Third, also based on predictions of the framework, we tested whether attention enhances feedback communication at alpha or gamma frequencies. Fourth, because feedback from V1 to the LGN is mediated by corticogeniculate neurons in layer 6, we hypothesized that attentional modulation of feedback may be greatest for IG to LGN communication at alpha or gamma frequencies.
To test these four hypotheses, we utilized MVGC analysis to study the timing and directionality of signal communication between the LGN and V1 laminar compartments and compared Granger-causal interactions across attention conditions. Figure 3 illustrates feedforward (left columns per box with white backgrounds) and feedback (right columns per box with gray backgrounds) Granger-causal interactions between the LGN and V1 laminar compartments during the full duration (Fig. 3A) and first and second halves of the cue period (Fig. 3, B and C) and during the full 1-s duration (Fig. 3D) and first and second halves of the visual stimulus display period of trials (Fig. 3, E and F). Granger-causal interactions are illustrated separately for attend-toward (red) and attend-away (blue) trials. We applied two separate statistical analyses to the Granger-causal interaction data. Permutation sampling was performed separately for attend-toward and attend-away trials to determine whether Granger-causal interactions were significantly different from noise: significant signal per frequency (P < 0.05, see materials and methods) is indicated in each plot as red and blue lines just above the x-axis. All Granger-causal interactions between the LGN and V1 laminar compartments were significantly above noise for each attention condition (Fig. 3; data combined from 3 monkeys). To test whether Granger-causal interactions were significantly different across attention conditions, actual distributions of interaction values across attention conditions were compared with randomly sampled and shuffled distributions: significant attentional modulation per frequency (P < 0.05, see materials and methods) is indicated in plots as black lines above curves. Notably, we always observed statistically significant facilitation, and never suppression, of Granger-causal interactions with attention; this was the case across frequencies and laminar compartments (Fig. 3).
Broadly, the Granger-causal interaction findings matched the coherence results in that Granger-causal interactions were greater at lower frequencies during the cue period and displayed peaks in beta- and/or gamma-frequency bands during the visual stimulus display period. Thus, in support of the first hypothesis, we observed significant attentional modulation of Granger-causal interactions between the LGN and V1 mainly in beta- and gamma-frequency bands (Fig. 3, A–F, black lines above curves indicate frequencies at which Granger-causal interactions were significantly different across attention conditions). Both of these overall patterns, low-frequency vs. beta- and/or gamma-frequency peaks in cue vs. visual stimulus display periods and attentional modulation of interactions in beta- and gamma-frequency bands, were also apparent in Granger-causal interactions measured for each monkey individually, especially when Granger-causal interactions were significantly above noise (Fig. 4; same statistical analyses described above were utilized to measure significance from noise and significant attention effects). Thus Granger-causal interactions broadly reflected the coherence results. Furthermore, attention had a larger influence on interactions in beta- and gamma-frequency bands, consistent with the first hypothesis proposed above.
Fig. 4.
Granger-causal interactions between the lateral geniculate nucleus (LGN) of the thalamus and primary visual cortex (V1) laminar compartments in individual monkeys Schematic at top illustrates analysis periods in A and B. Granger-causal interactions between the LGN and supragranular (SG), granular (G), or infragranular (IG) laminar compartments of V1 for attend-toward (red) and attend-away (blue) conditions during the cue period (A) and the visual stimulus display period (B) illustrated separately for monkey B, monkey O, and monkey E (labeled above plots in A box). White backgrounds (1st columns in each box) illustrate feedforward interactions from LGN to V1 and gray backgrounds (2nd columns in each) illustrate feedback interactions from V1 to LGN. Red and blue lines just below the x-axis indicate frequencies at which Granger-causal interactions were significantly greater than noise (Bonferroni-corrected P < 0.05). Black lines above curves indicate frequencies at which Granger-causal interactions were significantly different across attention conditions (P < 0.05, see materials and methods). Note differences in y-axis scaling. For monkey O and monkey B (cue period only), there were insufficient trials to compute Granger-causal interactions between the LGN and the SG laminar compartment.
Examination of Granger-causal interactions in different trial segments revealed that attentional modulation of communication between the LGN and V1 varied over the course of trials. During the full cue period, attention significantly enhanced feedforward interactions between the LGN and V1, mainly in beta and/or gamma frequencies (Fig. 3A, left column with white background). This effect was consistent across laminar compartments and was mainly driven by attentional facilitation of feedforward interactions during the first half of the cue period (Fig. 3B, left column with white background), since attention had little impact on feedforward interactions during the second half of the cue period (Fig. 3C, left column with white background). Attentional facilitation of feedforward interactions in beta- and gamma-frequency bands was also observed in all three monkeys individually (Fig. 4A, left column with white background).
Attention enhanced feedback interactions at lower frequencies consistently across laminar compartments and throughout the segments of the cue period (Fig. 3, A–C, right columns with gray backgrounds). In contrast, attentional enhancement of feedback interactions in the gamma-frequency band was limited to IG-to-LGN communication and occurred during the second half of the cue period (Fig. 3C, bottom right with gray background). Significant attentional enhancement of feedback communication between SG and G laminar compartments and the LGN at gamma frequencies was observed during the second half of the cue period (Fig. 3C, top and middle right with gray background), but these interactions were not sufficiently strong to produce significant attentional enhancement at the same frequencies over the course of the full cue period (Fig. 3A, top and middle right with gray background). Attentional modulation of feedback interactions in individual monkeys was variable, mostly because interactions did not always reach statistical significance above noise. However, in two monkeys, attention significantly facilitated feedback interactions between the IG laminar compartment and LGN across frequencies (Fig. 4A, right column with gray background).
Overall, LGN-V1 interactions during the cue period when attention had been cued but no visual stimuli were displayed suggested: 1) during the earliest point in the trial, attention facilitated feedforward communication from the LGN to all laminar compartments in V1, partially supporting the second hypothesis that attention enhances feedforward communication at beta frequencies; 2) attention facilitated feedback communication from V1 to the LGN at alpha frequencies throughout the cue period (in 2 of 3 monkeys), supporting the third hypothesis that attention enhances feedback communication at alpha frequencies; and 3) toward the end of the cue period, attention enhanced communication of feedback signals from the IG laminar compartment to the LGN at gamma frequencies, supporting the third and fourth hypotheses that attention enhances gamma-band feedback in IG-to-LGN circuits.
Attentional modulation of Granger-causal interactions during the visual stimulus display period also demonstrated temporal dynamics. While attention facilitated feedforward interactions in the beta- and gamma-frequency bands somewhat consistently across laminar compartments and throughout the visual stimulus display period, attentional facilitation of beta- and gamma- band interactions was greater during the second half of the visual stimulus display period (Fig. 3, D–F, right columns with white background). This trend was more pronounced for LGN-to-G and -IG laminar compartment communications, as attentional enhancement of LGN-to-SG interactions was quite robust during both first and second halves of the visual stimulus display period (Fig. 3, E and F, top right with white background). Attentional facilitation of feedforward interactions in beta- and gamma-frequency bands during the visual stimulus display period was also observed in all three monkeys individually (Fig. 4B, left column with white background).
Attentional enhancement of feedback Granger-causal interactions during the visual stimulus display period was again most apparent for communication between the IG laminar compartment and the LGN (Fig. 3, D–F, bottom right with gray background). Significant facilitation of IG-to-LGN interactions in the gamma-frequency band during the full visual stimulus display period (Fig. 3D, bottom right with gray background) was mostly driven by attentional enhancement of IG-to-LGN feedback during the first half of the visual stimulus display period (Fig. 3E, bottom right with gray background). Some significant attentional enhancement of feedback interactions between the SG and G laminar compartments and the LGN in beta and gamma frequencies was observed during the first half of the visual stimulus display period (Fig. 3E, top and middle right with gray background), however these interactions were suppressed by attention during the second half of the visual stimulus display period (Fig. 3F, top and middle right with gray background). Significant attentional enhancement of feedback interactions in beta and gamma frequency bands during the visual stimulus display period was observed in two monkeys individually (Fig. 4B, right column with gray background).
Over the course of the visual stimulus display period, feedforward interactions in beta- and gamma-frequency bands were facilitated by attention, with robust facilitation of gamma-band interactions toward the end of the visual stimulus display period. Attention facilitated feedback interactions in beta- and gamma-frequency bands, mainly during the first half of the visual stimulus display period, with more frequencies showing significant attentional modulation for IG-to-LGN communication. Interestingly, there was little attentional modulation of interactions, feedforward or feedback, in the alpha-frequency band, perhaps because Granger-causal interactions were weaker at lower frequencies during the visual stimulus display period (Fig. 3, D–F). Together these results partially support the first and second hypotheses: that attention facilitates beta- and gamma-frequency interactions and that beta oscillations are associated with feedforward signal communication, although attentional enhancement of feedforward communication was not restricted to the beta band. The findings are also consistent with the fourth hypothesis that attention enhances feedback interactions between the deep layers and the LGN. Notably, we observed very little attentional modulation of feedback communication in the alpha band during the visual stimulus display period, inconsistent with the predictions of the framework.
To more clearly visualize Granger-causal interactions that were significantly modulated by attention (significance assessed as described above and illustrated in Fig. 3), we quantified LGN-V1 interactions in the alpha-, beta-, and gamma-frequency bands during the cue and first and second halves of the visual stimulus display period (Fig. 5A). Open and closed circles indicate significantly modulated feedforward and feedback Granger-causal interactions, respectively, with values for attend-toward (top circles) and attend-away trials (bottom circles) linked by vertical lines. As illustrated in Fig. 3 and described above, attention facilitated Granger-causal interactions in the alpha-frequency band mostly in the feedback direction and mainly during the cue period (Fig. 5A, closed circles in alpha bins). There was a significant attentional facilitation of alpha-band feedforward interaction between the LGN and the SG laminar compartment during the first half of the visual stimulus display period (Fig. 5A, middle, cyan open circle in alpha bin; and Fig. 3E, top left with white background); however, quantified interaction values were near zero in both attention conditions. Therefore, our results provided limited support for the prediction that alpha-band oscillations convey top-down attention signals, since significant attentional facilitation was observed almost exclusively during the cue period.
Attention facilitated beta-band interactions in both feedforward and feedback directions for LGN communications with all laminar compartments during the cue period, for most feedforward and feedback communications during the first half of the visual stimulus display period, and for LGN-to-G and -IG feedforward communications during the second half of the visual stimulus display period (Fig. 5A, open and closed circles in beta bins). Additionally, beta-band Granger-causal interaction values were relatively similar across trial periods. These findings support the first and second hypotheses in part, because attentional modulation of beta-band interactions was significant for feedforward communications throughout the course of the trial. However, significant attentional enhancement of feedback interactions in the beta band was also observed during the cue and first half of the visual stimulus display period, so beta oscillations did not selectively convey feedforward signals.
The strongest Granger-causal interaction values and the largest attentional modulations were observed for interactions in the gamma-frequency band (Fig. 5A, open and closed circles in gamma bins), consistent with the first hypothesis. Attention enhanced feedforward gamma-band interactions between the LGN and all laminar compartments during the cue period and during the second half of the visual stimulus display period but only between the LGN and the G laminar compartment during the first half of the visual stimulus display period (Fig. 5A, open circles in gamma bins). Attentional enhancement of feedback gamma-band interactions was mainly restricted to IG-to-LGN communication and was largest during the cue period (Fig. 5A, magenta closed circles in gamma bins). This latter result is consistent with the third and fourth hypotheses that attentional facilitation of feedback interactions is greatest for gamma-band IG-to-LGN communication.
Because Granger-causal interactions in the gamma-frequency band had the largest values and were most facilitated by attention, we further explored the dynamics of attentional modulation of interactions in the gamma band with higher temporal resolution. We performed a separate MVGC analysis on LFPs in 150-ms bins spanning the cue and visual stimulus display periods. Attentional modulation of gamma-band Granger-causal interactions as a function of time is displayed for feedforward communication between the LGN and the SG (Fig. 5B, top, cyan), G (Fig. 5B, middle, green), and IG laminar compartments (Fig. 5B, bottom, magenta dashed line) and for feedback communication between the IG laminar compartment and the LGN (Fig. 5B, bottom, magenta solid line). Curves represent differential Granger-causal interactions (attend-toward minus attend-away), and open and closed circles indicate statistically significant attentional facilitation of Granger-causal interaction at a given time bin (P < 0.05, see materials and methods). Feedforward LGN-to-SG interactions in the gamma band were significantly facilitated by attention at many time points but underwent fluctuations throughout the trial (Fig. 5B, top). Feedforward LGN-to-G interactions in the gamma band were significantly enhanced by attention during the early part of the cue and toward the end of the visual stimulus display period but were not enhanced during the middle of the trial (Fig. 5B, middle). Feedforward LGN-to-IG interactions in the gamma band followed a similar pattern as feedforward LGN-to-G interactions (Fig. 5B, bottom, dashed magenta line). In contrast, feedback IG-to-LGN interactions in the gamma band were significantly enhanced by attention later during the cue, during the middle, and toward the end of the trial (Fig. 5B, bottom, solid magenta line). Together these findings illustrate that the most robust Granger-causal interactions that were most facilitation by attention were not constantly facilitated throughout the duration of the trial. Instead, feedforward and feedback interactions were modulated by attention in a temporally dynamic manner.
Attentional modulation of Granger-causal interactions within V1.
It is possible that local circuit interactions within V1 contributed to the observed temporal dynamics in attentional modulation of communication between LGN and V1. For example, local circuits connecting the G to SG laminar compartments in the feedforward direction provide driving input about visual stimulus features to output projection neurons in the SG laminar compartment (Callaway 2004; Douglas and Martin 2004). Accordingly, these driving circuits could be responsible for relatively consistent feedforward interactions between LGN and G and SG laminar compartments during the visual stimulus display period (Fig. 3, D–F). To determine whether attention modulates the communication of visual information among local circuits in V1, we performed MVGC analyses on a data set of LFPs recorded simultaneously across the cortical depth in monkey E. Figure 6 illustrates the results of the MVGC analyses examining interactions among the SG, G, and IG laminar compartments during the full cue period (Fig. 6A), the full visual stimulus display period (Fig. 6B), and the first (Fig. 6C) and second (Fig. 6D) halves of the visual stimulus display period. The same statistical analyses were applied to within-V1 interaction data: red and blue lines just above the x-axis of plots indicate statistical significance above noise for attend-toward and attend-away trials, respectively, while black lines above curves indicate frequencies at which Granger-causal interactions were significantly different across attention conditions (P < 0.05 for each, see materials and methods). Encouragingly, Granger-causal interactions for IG-to-SG communication were not above noise (Fig. 6, A–D, bottom left plots per box), consistent with a lack of driving connections from deep to superficial cortical layers in primate V1 (Callaway 2004).
Overall, attentional modulation of local circuit interactions within V1 was aligned with attentional modulation of LGN-V1 interactions. Attention enhanced feedforward communication across frequencies during the cue period (e.g., G-to-SG interaction; Fig. 6A, middle row, left column) and in alpha- and beta-frequency bands during the visual stimulus display period (e.g., G-to-SG, SG-to-IG, and G-to-IG interactions; Fig. 6, B–D, middle row, left columns, and top and middle rows, right columns per box). Attention enhanced feedback communication in beta- and gamma-frequency bands during the visual stimulus display period (e.g., SG-to-G and IG-to-G interactions; Fig. 6, C and D, top and bottom rows, middle column per box). This enhancement was present in the first and second halves of the visual stimulus display period but did not reach statistical significance for the full visual stimulus period (compare plots indicated above to those in Fig. 6B), likely due to temporal dynamics of attentional modulation of within-V1 interactions and/or interactions with the LGN (as shown in Fig. 5B).
DISCUSSION
Analyses of correlations and inferred causal interactions among LFPs recorded across visual brain areas have generated a framework through which distinct visual and cognitive signals are indicated by synchronized LFP oscillations at various frequencies. A general consensus has emerged in which interareal feedforward communication of visual information is indicated by synchronized beta- and gamma-frequency oscillations, while feedback communication of visual information is expressed as synchronized alpha-frequency oscillations (Bastos et al. 2014, 2015; Bastos et al. 2015; Bollimunta et al. 2011; Michalareas et al. 2016; Roberts et al. 2013; van Ede et al. 2015; van Kerkoerle et al. 2014). Synchronized gamma oscillations have been implicated in communicating attention signals along corticocortical circuits in both the bottom-up and top-down directions (Bosman et al. 2012; Buffalo et al. 2010; Gregoriou et al. 2009; Siegel et al. 2008; Zhou and Desimone 2011). It is more challenging to examine local circuit interactions in vivo to determine whether specific local feedforward or feedback circuits within a visual cortical area convey information in different frequency bands. However multiple studies have identified differences in power for visually evoked responses across the cortical layers (Buffalo et al. 2011; Hansen and Dragoi 2011; Maier et al. 2010; Maier et al. 2011; Spaak et al. 2012; Welle and Contreras 2016; Xing et al. 2012), and experimental and theoretical studies suggest that local cortical circuits generate gamma oscillations (Cardin 2016; Otte et al. 2010; Sohal 2016; Tiesinga and Sejnowski 2009; Veit et al. 2017). Our objective was to integrate the predictions of the framework for frequency-specific interareal communication with the known anatomical connections of the early visual system and test whether attention modulates feedforward, feedback, and local circuit interactions in a manner consistent with the framework.
We generated four testable hypotheses: 1) based on our coherence results, we predicted that attention modulates Granger-causal interactions between the LGN and V1 most at beta and gamma frequencies; 2) based on the framework, we predicted that attention enhances feedforward communication between the LGN and V1 selectively at beta frequencies; 3) also based on the framework, we predicted that attention enhances feedback communication from V1 to the LGN at alpha or gamma frequencies; and 4) based on the anatomy of corticogeniculate feedback, we predicted that attentional modulation of feedback is greatest for alpha- or gamma-band communication from the IG laminar compartment to the LGN. Additionally, we examined whether local interactions within V1 followed similar patterns as interactions between the LGN and V1. While some of our findings were partially consistent with the framework and the hypotheses outlined above, many deviations from the four predictions, and thus the framework, were also evident in our dataset. One key reason for these deviations was that attentional modulation of communication in early visual circuits was not static, but dynamic, over the time course of trials in the attention task.
The coherence and Granger-causal interaction results we obtained were largely consistent with one another (Figs. 1C and 3), and the results of each analysis were also consistent across individual monkeys (Figs. 2 and 4). While attention did not significantly modulate coherence, this was likely due to the fact that attention facilitated directed interactions in specific frequency bands with unique temporal dynamics. These directed and dynamic modulations were not apparent in the broader analysis window used to assess coherence but were evident in Granger-causality analyses of LGN-V1 interactions. Importantly, coherence between the LGN and V1 measured during the cue and visual stimulus display periods was significantly greater than baseline, providing a foundation for Granger-causality assessments. Overall, attention facilitated Granger-causal interactions between the LGN and V1 most in beta and gamma frequencies (Fig. 5A), supporting the first hypothesis. While attention also facilitated Granger-causal interactions within V1 at beta and gamma frequencies, attention often facilitated local V1 interactions at alpha frequencies as well (Fig. 6).
Supporting the second hypothesis and consistent with the framework, attention facilitated feedforward interactions at beta frequencies between the LGN and V1 (Figs. 3 and 5A) and within V1 (Fig. 6) in a relatively consistent manner across trial periods. These observations are compatible with anatomical evidence for feedforward connections between the LGN and all three laminar compartments within V1 (Fitzpatrick et al. 1983; Hendrickson et al. 1978; Hendry and Reid 2000) as well as evidence for attentional facilitation of geniculocortical input to layer 4 (Briggs et al. 2013). However, attentional modulation of beta-band interactions was not restricted to feedforward communication. For example, attention enhanced feedback communication between V1 and the LGN at beta frequencies during the cue and first half of the visual stimulus display period (Fig. 3, A and E). Similarly, attention enhanced beta-band feedback communication from the IG to G laminar compartments within V1 (Fig. 6, C and D). Additionally, attention facilitated feedforward communication between the LGN and V1 in beta- and gamma-frequency bands (Fig. 5A). This particular observation is at odds with a prior study that reported feedforward communication of visual information from the LGN to V1 selectively in beta, but not gamma, frequencies (Bastos et al. 2014). While overlapping data were used in both studies, there were a number of important differences in recording conditions and analysis methods. In the Bastos et al. (2014) study, LFPs were recorded only with single metal electrodes yielding a smaller sample. LFPs were analyzed without consideration of laminar compartment location or attention condition. Additionally, LFPs were measured during the full visual stimulus display period only, with no alignment of LFPs to the grating cycle per trial. The lack of grating cycle alignment in particular could have led to an underestimate of gamma band contributions in the prior study. Importantly, here we observed consistent attentional modulation of feedforward communication in the beta-frequency band, even though communication of feedforward information about attention was not selective for beta frequencies. Taken together, the results of the two studies suggest that feedforward beta-band oscillations reflect a mixture of visual stimulus and attention information, leading to strong feedforward Granger-causal interactions and significant facilitation by attention.
Parts of the third and fourth hypotheses were predicated on the framework prediction that alpha-band oscillations convey feedback signals (Bastos et al. 2015; Michalareas et al. 2016; van Ede et al. 2015; van Kerkoerle et al. 2014). We observed significant attentional enhancement of alpha-band feedback between all laminar compartments of V1 and the LGN but only during the cue period (Figs. 3 and 5A). Importantly, attentional modulation of feedback communication during the cue period was not restricted to the alpha band and included enhancement of beta- and gamma-band feedback communication as well (Figs. 3 and 5A). Thus the framework prediction that alpha frequencies convey feedback signals was minimally supported by our findings. It is worth considering that the cue period corresponded to a time window when monkeys’ attention was cued but no visual stimuli were displayed on the monitor. Thus attentional enhancement during the cue period of feedback Granger-causal interactions spanning laminar compartments and frequencies could reflect expectation or anticipation of stimulus onset (Bauer et al. 2014; Thut et al. 2006; Zhang et al. 2012). Attention trials were run in blocks of 10–20 trials per attended location, so there was little uncertainty at the start of each trial about the location of directed spatial attention. Broadband attentional enhancement of feedback signals could therefore reflect priming, expectation of stimulus presentation or reward, and/or attention (Bauer et al. 2014; Stănişor et al. 2013; Thut et al. 2006; Zhang et al. 2012). Finally, attentional enhancement of alpha-band interactions within V1 was evident during both the cue and visual stimulus display periods (Fig. 6). Therefore, it is possible that facilitation of alpha-band feedback is a cortical rather than a corticothalamic phenomenon.
A number of prior studies have demonstrated that attention increases gamma-band interactions between visual cortical areas (Bosman et al. 2012; Gregoriou et al. 2009; Siegel et al. 2008). Some of these studies suggest gamma-band modulations represent feedforward corticocortical communication of attention signals (Bosman et al. 2012; Siegel et al. 2008), while others suggest they represent feedback corticocortical communication of attention signals (Gregoriou et al. 2009). Interareal synchronization of gamma oscillations could reflect attentional modulation, invariant to the feedforward or feedback direction of communication. We aimed to test whether attention enhanced feedforward and/or feedback interactions at gamma frequencies (3rd hypothesis) and whether attentional enhancement of gamma-band feedback would be greatest for IG-to-LGN communication (fourth hypothesis) since corticogeniculate circuits are the only anatomical pathway through which attention signals can travel directly from the visual cortex to the LGN (Briggs et al. 2016; Fitzpatrick et al. 1994). Attention enhanced gamma-band interactions between the LGN and V1 in both feedforward and feedback directions (Figs. 3 and 5A). Furthermore, gamma-band Granger-causal interactions in both directions were the most robust interactions observed and displayed the greatest attentional enhancement (Fig. 5A). Attention enhanced feedforward gamma-band interactions between the LGN and all laminar compartments but with unique temporal dynamics for each (Fig. 5B). Only feedforward G-to-SG local V1 circuit interactions were facilitated by attention at gamma frequencies and this occurred only during the cue period (Fig. 6A). Thus, although attentional facilitation of feedforward gamma-band interactions was distributed across networks, each displayed unique temporal dynamics.
Across all of the predictions that we tested, our results provided the strongest support for the fourth hypothesis. Specifically, attentional enhancement of feedback Granger-causal interactions was greatest for IG-to-LGN communication at gamma frequencies (Figs. 3 and 5A). Attentional facilitation of gamma-band feedback between the IG laminar compartment and the LGN also displayed temporal dynamics and was robust during the cue and visual stimulus display periods (Fig. 5B). Interestingly, attention also facilitated gamma-band feedback interactions locally within V1, especially from SG-to-G and IG-to-G laminar compartments during the visual stimulus display period (Fig. 6, C and D). Together, our results pertaining to attentional modulation of gamma-band interactions suggest that attention facilitates both feedforward and feedback gamma-band interactions in the early visual system. However, attentional enhancement of feedback gamma-band interactions between V1 and LGN is restricted to the corticogeniculate circuit.
In summary, our observations only partially supported the framework for interareal frequency-specific communication of visual and attention signals and deviated from it in a number of important ways. We observed consistent attentional facilitation of feedforward beta-band interactions between the LGN and V1 throughout the course of trials; however, attentional facilitation of feedforward interactions was not selective for beta frequencies and attention enhanced feedback interactions in the beta band as well. We observed attentional facilitation of feedback alpha-band interactions, but this was restricted to the cue period. Additionally, cue period feedback was facilitated by attention in a broadband manner, perhaps reflecting global expectation. Within V1, attention enhanced alpha-band interactions throughout the course of trials. Since we found little evidence for attentional enhancement of selective alpha-band feedback among corticothalamic circuits, selective alpha-band feedback may be a cortical phenomenon. We observed attentional facilitation of gamma-band interactions in both feedforward and feedback directions, supporting the notion that attention regulates bidirectional interareal communication at gamma frequencies. Importantly, attentional enhancement of feedback gamma-band interactions was selective for the laminar compartment containing corticogeniculate neurons. Finally, attentional enhancement of communication between the LGN and V1 and within V1 was not static but dynamic over the course of trials. Previous studies have demonstrated that subjects’ attention is not static over the course of trials (Cohen and Maunsell 2011). The dynamics that we observed could reflect changes in attention state over time and/or reverberations in signal communication across interareal and local circuits. As a whole, our results suggest that a simple static framework in which distinct frequency bands convey directed inputs is not adequate to describe communication in early visual circuits. During visual attention tasks, neuronal network interactions among early visual circuits are flexible and dynamic, similar to patterns of activity among individual neurons. Future work will determine whether these dynamics reflect shifts in network activity according to attention task demands.
GRANTS
This work was funded by National Eye Institute Grants EY-018683, EY-013588, and EY-025219 (to F. Briggs) and EY-023165 to J. R. Hembrook-Short), National Science Foundation Grant EPSCoR 1632738, and grants from the Whitehall Foundation and Hitchcock Foundation. V. L. Mock was supported by a Graduate Fellowship from the Albert J. Ryan Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
V.L.M. and F.B. conceived and designed research; V.L.M. and J.R.H.-S. performed experiments; V.L.M., K.L.L., and F.B. analyzed data; V.L.M., K.L.L., and F.B. interpreted results of experiments; V.L.M., K.L.L., and F.B. prepared figures; V.L.M. and F.B. drafted manuscript; V.L.M., K.L.L., J.R.H.-S., and F.B. edited and revised manuscript; V.L.M., K.L.L., J.R.H.-S., and F.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Elise Bragg for expert technical assistance and Drs. Karen Moodie and Kirk Maurer for veterinary assistance. We thank Dr. Matthijs van der Meer for consultation on statistical analyses. We also thank Dr. W. Martin Usrey for helpful comments on the manuscript.
REFERENCES
- Barnett L, Seth AK. Behaviour of Granger causality under filtering: theoretical invariance and practical application. J Neurosci Methods 201: 404–419, 2011. doi: 10.1016/j.jneumeth.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Barnett L, Seth AK. The MVGC multivariate Granger causality toolbox: a new approach to Granger-causal inference. J Neurosci Methods 223: 50–68, 2014. doi: 10.1016/j.jneumeth.2013.10.018. [DOI] [PubMed] [Google Scholar]
- Bastos AM, Briggs F, Alitto HJ, Mangun GR, Usrey WM. Simultaneous recordings from the primary visual cortex and lateral geniculate nucleus reveal rhythmic interactions and a cortical source for γ-band oscillations. J Neurosci 34: 7639–7644, 2014. doi: 10.1523/JNEUROSCI.4216-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos AM, Usrey WM, Adams RA, Mangun GR, Fries P, Friston KJ. Canonical microcircuits for predictive coding. Neuron 76: 695–711, 2012. doi: 10.1016/j.neuron.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos AM, Vezoli J, Bosman CA, Schoffelen JM, Oostenveld R, Dowdall JR, De Weerd P, Kennedy H, Fries P. Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron 85: 390–401, 2015. [Comment in: Neuron 85: 236–237, 2015. doi: 10.1016/j.neuron.2014.12.067. ] doi:. [DOI] [PubMed] [Google Scholar]
- Bauer M, Stenner M-P, Friston KJ, Dolan RJ. Attentional modulation of alpha/beta and gamma oscillations reflect functionally distinct processes. J Neurosci 34: 16117–16125, 2014. doi: 10.1523/JNEUROSCI.3474-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollimunta A, Mo J, Schroeder CE, Ding M. Neuronal mechanisms and attentional modulation of corticothalamic α oscillations. J Neurosci 31: 4935–4943, 2011. doi: 10.1523/JNEUROSCI.5580-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman CA, Schoffelen JM, Brunet N, Oostenveld R, Bastos AM, Womelsdorf T, Rubehn B, Stieglitz T, De Weerd P, Fries P. Attentional stimulus selection through selective synchronization between monkey visual areas. Neuron 75: 875–888, 2012. doi: 10.1016/j.neuron.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs F. Mammalian Visual System Organization. In: Oxford Research Encyclopedia of Neuroscience, edited by Sherman SM. Oxford, UK: Oxford University Press, 2017, p. 1–20. [Google Scholar]
- Briggs F, Kiley CW, Callaway EM, Usrey WM. Morphological substrates for parallel streams of corticogeniculate feedback originating in both V1 and V2 of the macaque monkey. Neuron 90: 388–399, 2016. doi: 10.1016/j.neuron.2016.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs F, Mangun GR, Usrey WM. Attention enhances synaptic efficacy and the signal-to-noise ratio in neural circuits. Nature 499: 476–480, 2013. doi: 10.1038/nature12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalo EA, Fries P, Landman R, Buschman TJ, Desimone R. Laminar differences in gamma and alpha coherence in the ventral stream. Proc Natl Acad Sci USA 108: 11262–11267, 2011. doi: 10.1073/pnas.1011284108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalo EA, Fries P, Landman R, Liang H, Desimone R. A backward progression of attentional effects in the ventral stream. Proc Natl Acad Sci USA 107: 361–365, 2010. doi: 10.1073/pnas.0907658106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Schomburg EW. What does gamma coherence tell us about inter-regional neural communication? Nat Neurosci 18: 484–489, 2015. doi: 10.1038/nn.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway EM. Cell types and local circuits in primary visual cortex of the macaque monkey. In: The Visual Neurosciences, edited by Chalupa L, Werner J. Cambridge, MA: MIT Press, 2004, p. 680–694. [Google Scholar]
- Callaway EM. Structure and function of parallel pathways in the primate early visual system. J Physiol 566: 13–19, 2005. doi: 10.1113/jphysiol.2005.088047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA. Snapshots of the brain in action: local circuit operations through the lens of gamma oscillations. J Neurosci 36: 10496–10504, 2016. doi: 10.1523/JNEUROSCI.1021-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalk M, Herrero JL, Gieselmann MA, Delicato LS, Gotthardt S, Thiele A. Attention reduces stimulus-driven gamma frequency oscillations and spike field coherence in V1. Neuron 66: 114–125, 2010. doi: 10.1016/j.neuron.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JH. When attention wanders: how uncontrolled fluctuations in attention affect performance. J Neurosci 31: 15802–15806, 2011. doi: 10.1523/JNEUROSCI.3063-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas RJ, Martin KA. Neuronal circuits of the neocortex. Annu Rev Neurosci 27: 419–451, 2004. doi: 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex 1: 1–47, 1991. doi: 10.1093/cercor/1.1.1. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick D, Itoh K, Diamond IT. The laminar organization of the lateral geniculate body and the striate cortex in the squirrel monkey (Saimiri sciureus). J Neurosci 3: 673–702, 1983. doi: 10.1523/JNEUROSCI.03-04-00673.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick D, Usrey WM, Schofield BR, Einstein G. The sublaminar organization of corticogeniculate neurons in layer 6 of macaque striate cortex. Vis Neurosci 11: 307–315, 1994. doi: 10.1017/S0952523800001656. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci 9: 474–480, 2005. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci 32: 209–224, 2009. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science 291: 1560–1563, 2001. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Fries P, Womelsdorf T, Oostenveld R, Desimone R. The effects of visual stimulation and selective visual attention on rhythmic neuronal synchronization in macaque area V4. J Neurosci 28: 4823–4835, 2008. doi: 10.1523/JNEUROSCI.4499-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Bastos AM, Pinotsis D, Litvak V. LFP and oscillations-what do they tell us? Curr Opin Neurobiol 31: 1–6, 2015. doi: 10.1016/j.conb.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science 324: 1207–1210, 2009. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriou GG, Paneri S, Sapountzis P. Oscillatory synchrony as a mechanism of attentional processing. Brain Res 1626: 165–182, 2015. doi: 10.1016/j.brainres.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Hansen BJ, Dragoi V. Adaptation-induced synchronization in laminar cortical circuits. Proc Natl Acad Sci USA 108: 10720–10725, 2011. doi: 10.1073/pnas.1102017108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hembrook-Short JR, Mock VL, Briggs F. Attentional modulation of neuronal activity depends on neuronal feature selectivity. Curr Biol 27: 1878–1887.e5, 2017. doi: 10.1016/j.cub.2017.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson AE, Wilson JR, Ogren MP. The neuroanatomical organization of pathways between the dorsal lateral geniculate nucleus and visual cortex in Old World and New World primates. J Comp Neurol 182: 123–136, 1978. doi: 10.1002/cne.901820108. [DOI] [PubMed] [Google Scholar]
- Hendry SH, Reid RC. The koniocellular pathway in primate vision. Annu Rev Neurosci 23: 127–153, 2000. doi: 10.1146/annurev.neuro.23.1.127. [DOI] [PubMed] [Google Scholar]
- Ito M, Gilbert CD. Attention modulates contextual influences in the primary visual cortex of alert monkeys. Neuron 22: 593–604, 1999. doi: 10.1016/S0896-6273(00)80713-8. [DOI] [PubMed] [Google Scholar]
- Jensen O, Bonnefond M, Marshall TR, Tiesinga P. Oscillatory mechanisms of feedforward and feedback visual processing. Trends Neurosci 38: 192–194, 2015. doi: 10.1016/j.tins.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Katzner S, Nauhaus I, Benucci A, Bonin V, Ringach DL, Carandini M. Local origin of field potentials in visual cortex. Neuron 61: 35–41, 2009. doi: 10.1016/j.neuron.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashgari R, Li X, Chen Y, Kremkow J, Bereshpolova Y, Swadlow HA, Alonso JM. Response properties of local field potentials and neighboring single neurons in awake primary visual cortex. J Neurosci 32: 11396–11413, 2012. doi: 10.1523/JNEUROSCI.0429-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol 77: 24–42, 1997. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- Maier A, Adams GK, Aura C, Leopold DA. Distinct superficial and deep laminar domains of activity in the visual cortex during rest and stimulation. Front Syst Neurosci 4: 1–11, 2010. doi: 10.3389/fnsys.2010.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier A, Aura CJ, Leopold DA. Infragranular sources of sustained local field potential responses in macaque primary visual cortex. J Neurosci 31: 1971–1980, 2011. doi: 10.1523/JNEUROSCI.5300-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams CJ, Reid RC. Attention modulates the responses of simple cells in monkey primary visual cortex. J Neurosci 25: 11023–11033, 2005. doi: 10.1523/JNEUROSCI.2904-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Cavanaugh J, Wurtz RH. Attentional modulation of thalamic reticular neurons. J Neurosci 26: 4444–4450, 2006. doi: 10.1523/JNEUROSCI.5602-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Cavanaugh J, Wurtz RH. Guarding the gateway to cortex with attention in visual thalamus. Nature 456: 391–394, 2008. doi: 10.1038/nature07382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalareas G, Vezoli J, van Pelt S, Schoffelen JM, Kennedy H, Fries P. Alpha-beta and gamma rhythms subserve feedback and feedforward influences among human visual cortical areas. Neuron 89: 384–397, 2016. doi: 10.1016/j.neuron.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motter BC. Focal attention produces spatially selective processing in visual cortical areas V1, V2, and V4 in the presence of competing stimuli. J Neurophysiol 70: 909–919, 1993. doi: 10.1152/jn.1993.70.3.909. [DOI] [PubMed] [Google Scholar]
- Nandy AS, Nassi JJ, Reynolds JH. Laminar organization of attentional modulation in macaque visual area V4. Neuron 93: 235–246, 2017. doi: 10.1016/j.neuron.2016.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor DH, Fukui MM, Pinsk MA, Kastner S. Attention modulates responses in the human lateral geniculate nucleus. Nat Neurosci 5: 1203–1209, 2002. doi: 10.1038/nn957. [DOI] [PubMed] [Google Scholar]
- Otte S, Hasenstaub A, Callaway EM. Cell type-specific control of neuronal responsiveness by gamma-band oscillatory inhibition. J Neurosci 30: 2150–2159, 2010. doi: 10.1523/JNEUROSCI.4818-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter CG, Thompson WH, Bosman CA, Fries P. Top-down beta enhances bottom-up gamma. J Neurosci 37: 6698–6711, 2017. doi: 10.1523/JNEUROSCI.3771-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MJ, Lowet E, Brunet NM, Ter Wal M, Tiesinga P, Fries P, De Weerd P. Robust gamma coherence between macaque V1 and V2 by dynamic frequency matching. Neuron 78: 523–536, 2013. doi: 10.1016/j.neuron.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Seth AK, Barrett AB, Barnett L. Granger causality analysis in neuroscience and neuroimaging. J Neurosci 35: 3293–3297, 2015. doi: 10.1523/JNEUROSCI.4399-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel M, Donner TH, Oostenveld R, Fries P, Engel AK. Neuronal synchronization along the dorsal visual pathway reflects the focus of spatial attention. Neuron 60: 709–719, 2008. doi: 10.1016/j.neuron.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Sincich LC, Horton JC. The circuitry of V1 and V2: integration of color, form, and motion. Annu Rev Neurosci 28: 303–326, 2005. doi: 10.1146/annurev.neuro.28.061604.135731. [DOI] [PubMed] [Google Scholar]
- Sohal VS. How close are we to understanding what (if anything) gamma oscillations do in cortical circuits? J Neurosci 36: 10489–10495, 2016. doi: 10.1523/JNEUROSCI.0990-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaak E, Bonnefond M, Maier A, Leopold DA, Jensen O. Layer-specific entrainment of γ-band neural activity by the α rhythm in monkey visual cortex. Curr Biol 22: 2313–2318, 2012. doi: 10.1016/j.cub.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stănişor L, van der Togt C, Pennartz CM, Roelfsema PR. A unified selection signal for attention and reward in primary visual cortex. Proc Natl Acad Sci USA 110: 9136–9141, 2013. doi: 10.1073/pnas.1300117110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K, Mandon S, Freiwald WA, Kreiter AK. Coherent oscillatory activity in monkey area v4 predicts successful allocation of attention. Cereb Cortex 15: 1424–1437, 2005. doi: 10.1093/cercor/bhi023. [DOI] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascual-Leone A. Alpha-band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci 26: 9494–9502, 2006. doi: 10.1523/JNEUROSCI.0875-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiesinga P, Sejnowski TJ. Cortical enlightenment: are attentional gamma oscillations driven by ING or PING? Neuron 63: 727–732, 2009. doi: 10.1016/j.neuron.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ede F, van Pelt S, Fries P, Maris E. Both ongoing alpha and visually induced gamma oscillations show reliable diversity in their across-site phase-relations. J Neurophysiol 113: 1556–1563, 2015. doi: 10.1152/jn.00788.2014. [DOI] [PubMed] [Google Scholar]
- van Kerkoerle T, Self MW, Dagnino B, Gariel-Mathis M-A, Poort J, van der Togt C, Roelfsema PR. Alpha and gamma oscillations characterize feedback and feedforward processing in monkey visual cortex. Proc Natl Acad Sci USA 111: 14332–14341, 2014. doi: 10.1073/pnas.1402773111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kerkoerle T, Self MW, Roelfsema PR. Layer-specificity in the effects of attention and working memory on activity in primary visual cortex. Nat Commun 8: 13804, 2017. [Erratum in: Nat Commun 8: 15555, 2017. doi: 10.1038/ncomms15555. ] doi:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanduffel W, Tootell RB, Orban GA. Attention-dependent suppression of metabolic activity in the early stages of the macaque visual system. Cereb Cortex 10: 109–126, 2000. doi: 10.1093/cercor/10.2.109. [DOI] [PubMed] [Google Scholar]
- Veit J, Hakim R, Jadi MP, Sejnowski TJ, Adesnik H. Cortical gamma band synchronization through somatostatin interneurons. Nat Neurosci 20: 951–959, 2017. doi: 10.1038/nn.4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinck M, Womelsdorf T, Buffalo EA, Desimone R, Fries P. Attentional modulation of cell-class-specific gamma-band synchronization in awake monkey area v4. Neuron 80: 1077–1089, 2013. doi: 10.1016/j.neuron.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welle CG, Contreras D. Sensory-driven and spontaneous gamma oscillations engage distinct cortical circuitry. J Neurophysiol 115: 1821–1835, 2016. doi: 10.1152/jn.00137.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing D, Yeh CI, Burns SP, Shapley R. Laminar analysis of visually evoked activity in the primary visual cortex. Proc Natl Acad Sci USA 109: 13871–13876, 2012. doi: 10.1073/pnas.1201478109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing D, Yeh CI, Shapley RM. Spatial spread of the local field potential and its laminar variation in visual cortex. J Neurosci 29: 11540–11549, 2009. doi: 10.1523/JNEUROSCI.2573-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhaoping L, Zhou T, Fang F. Neural activities in v1 create a bottom-up saliency map. Neuron 73: 183–192, 2012. doi: 10.1016/j.neuron.2011.10.035. [DOI] [PubMed] [Google Scholar]
- Zhou H, Desimone R. Feature-based attention in the frontal eye field and area V4 during visual search. Neuron 70: 1205–1217, 2011. doi: 10.1016/j.neuron.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]