Abstract
Successful motor performance relies on our ability to adapt to changes in the environment by learning novel mappings between motor commands and sensory outcomes. Such adaptation is thought to involve two distinct mechanisms: an implicit, error-based component linked to slow learning and an explicit, strategic component linked to fast learning and savings (i.e., faster relearning). Because behavior, at any given moment, is the resultant combination of these two processes, it has remained a challenge to parcellate their relative contributions to performance. The explicit component to visuomotor rotation (VMR) learning has recently been measured by having participants verbally report their aiming strategy used to counteract the rotation. However, this procedure has been shown to magnify the explicit component. Here we tested whether task-specific eye movements, a natural component of reach planning, but poorly studied in motor learning tasks, can provide a direct readout of the state of the explicit component during VMR learning. We show, by placing targets on a visible ring and including a delay between target presentation and reach onset, that individual differences in gaze patterns during sensorimotor learning are linked to participants’ rates of learning and their expression of savings. Specifically, we find that participants who, during reach planning, naturally fixate an aimpoint rotated away from the target location, show faster initial adaptation and readaptation 24 h later. Our results demonstrate that gaze behavior cannot only uniquely identify individuals who implement cognitive strategies during learning but also how their implementation is linked to differences in learning.
NEW & NOTEWORTHY Although it is increasingly well appreciated that sensorimotor learning is driven by two separate components, an error-based process and a strategic process, it has remained a challenge to identify their relative contributions to performance. Here we demonstrate that task-specific eye movements provide a direct read-out of explicit strategies during sensorimotor learning in the presence of visual landmarks. We further show that individual differences in gaze behavior are linked to learning rate and savings.
Keywords: eye movements, motor adaptation, motor learning, reaching visuomotor rotation
INTRODUCTION
Skilled motor behavior requires the ability to adapt to changes in the environment that alter the mapping between motor commands and their sensory consequences (Shadmehr et al. 2010; Wolpert et al. 2011). Such adaptation has been extensively investigated using reaching or throwing tasks with displacing prisms (Bedford 1999; Fernández-Ruiz and Díaz 1999; Martin et al. 1996; Redding and Wallace 2006) and reaching tasks under a visuomotor rotation (VMR), in which the viewed position of the hand (or cursor representing the hand) is rotated about the hand start location (e.g., Cunningham 1989; Krakauer et al. 2000, 2005; Wigmore et al. 2002). Traditionally, learning in such tasks was presumed to be driven by an implicit process involving the gradual updating of an internal model, which links motor commands and sensory outcomes, based on errors between predicted and viewed consequences of action (Shadmehr et al. 2010; Wolpert et al. 2011). Several studies, however, have demonstrated that learning can also be augmented by (or interfered with) the use of cognitive strategies (Benson et al. 2011; Bock et al. 2003; Fernandez-Ruiz et al. 2011; Heuer and Hegele 2008; Martin et al. 1996; Mazzoni and Krakauer 2006; Redding and Wallace 1993, 2002; Taylor and Ivry 2011). To dissociate the implicit and strategic components of VMR learning, Taylor and colleagues (2014) recently developed a task in which participants, before each reaching movement, verbally reported their aiming direction, used to counteract the rotation, via numbers placed on a circle surrounding the hand start position. They demonstrated that learning is the resultant combination of two separate processes: A fast explicit process reflecting strategic aiming and a more gradual, implicit process reflecting updating of an internal model.
More recently, this verbal reporting task has also been used to probe the mechanisms underlying savings, which refers to faster relearning of a previously forgotten (or “washed out”) memory (Brashers-Krug et al. 1996; Ebbinghaus 1913; Krakauer et al. 2005). Morehead et al. (2015) showed that improvements in aiming strategy underlie the faster rate of learning observed when individuals reencounter the VMR following washout of initial learning. This result, along with the finding that fast learning and relearning are not observed when the expression of the explicit component is mitigated by limiting preparation time (Fernandez-Ruiz et al. 2011; Haith et al. 2015; Leow et al. 2017), suggests that savings are largely driven by the recall of previously implemented strategies. However, because the declarative nature of the verbal reporting task has been shown to influence the explicit (Taylor et al. 2014) or implicit (Leow et al. 2017) contributions to learning, alternative measures may be critical to parcelling out their unique contributions to learning and how they shape individual performance.
Eye movements are a fundamental component to the planning and control of visually guided actions (Johansson et al. 2001; Land and Furneaux 1997). During reach planning, gaze is naturally directed to the target before initiation of the hand movement to improve spatial localization of the target and help guide the hand to the target using visual feedback (Paillard 1982; Prablanc et al. 1979). Since the explicit component of VMR adaptation involves strategically reaiming the hand toward an aimpoint that is rotated away from the target, it is plausible that eye movements are used to identify this aimpoint location. While there is some evidence to suggest that gaze behavior may be linked to the explicit component of learning (Rand and Rentsch 2015, 2016), this relationship has not been directly examined nor has it been explored how the time course of gaze behavior during learning may be linked to individual differences in learning rates and the expression of savings. Here we tested the novel hypothesis that task-specific eye movements, during a VMR task in which targets are presented on a ring of visual landmarks, can provide a direct “readout” of both the implementation and state of the explicit component over the time course of sensorimotor learning and relearning following washout. Specifically, we hypothesized that, during a delay period between target presentation and reach onset, during which we assume reach planning occurs, participants will naturally direct their gaze to a location on the landmark ring corresponding to the point they intend to reaim toward.
MATERIALS AND METHODS
Participants
A total of 56 young right-handed adults participated in one of three experiments. Twenty-one people took part in the intermittent report experiment (experiment 1; 5 men and 16 women; age: 18–25 yr), after exclusion of two participants due to technical problems. The no report experiment (experiment 2) was performed by 21 different participants (8 men and 13 women, age 18–22 yr). Twelve participants were recruited for the no preview experiment (experiment 3; 5 men and 7 women; age: 19–24 yr). Participants had normal or corrected-to-normal vision and provided written informed consent before participation. The experiment was part of a research project that was approved by the general research ethics board from Queen’s University.
Apparatus
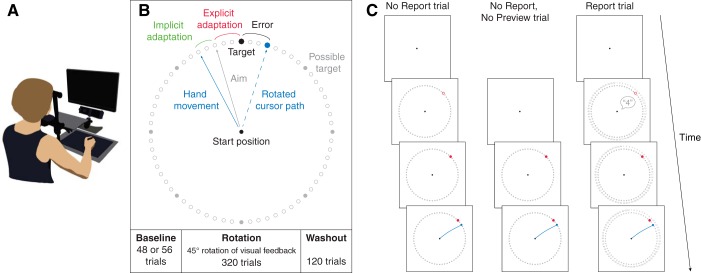
Participants were seated at a table and performed center-out reaching movements to visual targets by sliding a stylus across a digitizing tablet (Fig. 1A). Stimuli were presented on a vertical LCD monitor (display size: 47.5 × 26.5 cm; resolution: 1,920 × 1,080 pixels; refresh rate: 60 Hz) placed ~50 cm in front of a chin and forehead rest. Vision of the tablet and hand was occluded by a rectangular piece of black styrofoam attached horizontally below the chin rest. Movement trajectories were sampled at 100 Hz by the digitizing tablet (active area: 311 × 216 mm; Wacom Intuous). The ratio between movement of the tip of the stylus and movement of the cursor presented on the screen was set to 1:2, so that a movement of 5 cm on the tablet corresponded to a 10-cm movement of the cursor. Eye movements were tracked at 500 Hz using a video-based eye tracker (Eyelink 1000; SR Research) placed beneath the monitor.
Fig. 1.
Experimental setup and procedures. A: experimental setup. Participants performed fast reaching movements by sliding a pen across a digitizing tablet, without vision of the hand. Visual stimuli and the cursor representing the hand position were presented on a monitor. B: task. A target was presented in 1 of 8 locations and flanked by a ring of landmark circles. Veridical cursor feedback was provided in the baseline and washout blocks. In the rotation block, participants were exposed to a 45° rotation of the cursor feedback. C: trial types. In no report trials, participants were given a 2-s preview of the target and landmarks before the response was cued. In report trials, participants reported their aiming direction via the numbered visual landmarks.
Procedure
Each trial started with the participant moving the cursor (4-mm radius cyan circle) into the starting position (5-mm radius white circle) using the stylus. The cursor became visible when its center was within 2 cm of the center of the start position. After the cursor was held within the start position for 500 ms, a red target circle (5-mm radius) and 64 outlined gray “landmark” circles (3-mm radius, spaced 5.625° apart) were presented on a ring with a radius of 10 cm (Fig. 1B) after a 100-ms delay. The target was presented at one of eight locations, separated by 45° (0, 45, 90, 135, 180, 225, 270, and 315°), in randomized sets of eight trials. As outlined below, the subsequent trial events depended on the trial type.
In no report trials (used in experiments 1 and 2), the target initially appeared as an outlined circle, and participants were given a target preview of 2 s before the target filled in, which served as the cue for participants to initiate their reach. In no report, no preview trials (used in experiment 3), the target appeared as a filled circle and participants were instructed to initiate their reach immediately when the target appeared. In report trials (used in experiment 1), the target was an outlined circle and the visual landmarks were numbered. Participants were required to verbally report the number of the landmark they planned to reach toward for the cursor to hit the target (as in Taylor et al. 2014), and the experimenter recorded the number using a keyboard. The target turned red 2 s after its appearance, or immediately after the experimenter recorded the response if the response took longer than 2 s, providing the go signal for the participant to initiate their reach.
In all trials, participants were instructed to hit the target with their cursor by making a fast reaching movement on the tablet. They were instructed to “slice” the cursor through the target to minimize online corrections during the reach. If the movement was initiated (i.e., the cursor had moved fully out of the start circle) before the go cue, the trial was aborted and a feedback text message “too early” appeared centrally on the screen. If the movement was initiated more than 600 ms (2 s in experiment 3) after the go cue, the trial was aborted and a feedback text message “too late” appeared on the screen. In trials with correct timing, the cursor was visible during the movement to the ring (at 10-cm distance) and then became stationary for 1 s when it reached the ring, providing the participant with visual feedback of their end point reach error. If any part of the stationary cursor overlapped with any part of the target, the target was colored green and the participant received one point. Points were displayed on the screen every 80 trials in the rotation and washout blocks, followed by a 30-s break.
Each testing session took ~75 min to complete and consisted of a baseline block with veridical cursor feedback, a rotation block in which feedback of the cursor during the reach was rotated clockwise by 45°, and a washout block in which veridical cursor feedback was restored. Participants in experiments 1 and 2 completed two sessions, separated by a day, whereas participants in experiment 3 completed a single session. Participants were not informed about nature or presence of the VMR before or during the experiment.
Experiment 1: intermittent report.
In the baseline block, participants first completed 48 no report trials followed by 8 report trials. In the rotation block, participants completed 320 trials (40 sets of 8 trials). To test whether gaze fixations before executing a reach movement can provide a readout of the explicit component of visuomotor adaptation, in the rotation block we randomly intermixed two report trials and six no report trials in each set of eight trials. This intermittent reporting was introduced after an initial set of eight no report trials. At this moment, participants were told by the experimenter that “they had probably noticed something strange is going on” and they were instructed to report the direction of their hand movement (not the cursor movement) required to hit the target when the numbers are displayed. In the washout block following the rotation block, participants completed 120 no report trials without a rotation. To examine savings when reexposed to the VMR, and its relation to gaze patterns, participants performed two identical testing sessions separated by 24 h.
Experiment 2: no report.
The second experiment was designed to test the extent to which the implementation of an aiming strategy, and the occurrence of fixations at the aimpoint, is influenced by having participants report their aiming direction. This experiment was identical to the intermittent report experiment (experiment 1) except that the baseline block only included 48 no report trials, and all 320 trials in the rotation block were no report trials. To examine savings when reexposed to the VMR, participants performed two identical testing sessions separated by 24 h.
Experiment 3: no preview.
We tested a third group of participants to examine the extent to which the implementation of an aiming strategy, and the occurrence of fixations at the aimpoint, depends on having a target preview period, as previous studies have shown strategic aiming is effortful, especially at short preparation times (Leow et al. 2017). The experiment was the same as the no report experiment (experiment 2) except that all of the trials in the baseline, rotation, and washout blocks were no report, no preview trials and participants only performed a single testing session. Our instructions did not stress reaction time, but they emphasized that participants had to make a single, fast, uncorrected reaching movement slicing through the target. If the duration of the reach was longer than 400 ms, the trial was aborted and a text feedback message “too slow” appeared centrally on the screen.
Data Analysis
Hand movements.
Trials in which the reach was initiated too early or too late (as detected online) were excluded from the offline analysis of hand and eye movements (~5% and ~6% of trials in experiment 1 and 2, respectively). We also excluded trials in which the movement time, defined as the time between the moment the cursor had fully moved out of the start position until the cursor reached the 10-cm target distance, was longer than 400 ms (<1% of trials in experiment 1 and 2; ~2% in experiment 3). To assess task performance on each trial, we calculated the hand angle with respect to the target angle at the moment the cursor reached the target distance. To do this, we first linearly interpolated the position of the pen on the tablet to 1,000 Hz and then converted its x and y position at the moment the cursor reached 10-cm distance from the start position to an angle and finally subtracted the target angle. The end point hand angles were averaged across sets of eight trials, containing one repetition of each target direction. As a measure of early learning, we averaged the hand angle across sets 2–10 of the rotation block, excluding the first set in which participants often showed highly variable behavior.
Eye movements.
For the intermittent report experiment (experiment 1), we first excluded report trials from the analysis of gaze data, since in these trials participants would naturally direct their eyes to the number they want to report. For all experiments, we excluded trials in which there was missing gaze data during at least 50% of the time from the onset of the target until the cursor crossed the ring (i.e., the preview and movement phases; ~7% of trials in experiment 1; ~8% of trials in experiment 2; and ~4% of trials in experiment 3). This was done to obtain a complete picture of the time course of gaze fixations over the preview and movement phase. Our analysis focused on participants’ fixation locations during the preview and movement phases. For each trial, we first detected and removed blinks from the x and y gaze positions that were provided by the eye tracker. Gaze data were low-pass filtered using a second order recursive Butterworth filter with a cutoff frequency of 50 Hz. The filtered x and y gaze positions were used to calculate horizontal, vertical, and resultant gaze velocity. To obtain fixations, we first identified saccades as having a resultant velocity of 20 cm/s for five or more consecutive samples (10 ms). Saccade onset was defined as the last of five samples below the threshold of 20 cm/s, and saccade offset was defined as the first of five samples below this threshold. Next, fixations were defined as periods of 50 or more consecutive samples (100 ms) in which a saccade with a minimal displacement of 0.5 cm did not occur. We computed the mean x and y gaze positions for each fixation and converted this to a distance from the start position and an angle relative to the target.
We used the resulting fixation locations to quantify gaze patterns 1) over the time course of a single trial, and 2) over the course of each testing session. To examine gaze patterns over the time course of a trial, we first normalized time by scaling each phase (target preview, reaction time, reach, and feedback) of each trial to the mean duration of that phase across all subjects. Next, we computed, for each participant and each sample of all valid trials in the rotation block, the probability that a fixation occurred in three areas: 1) the start point area (<75% of target distance), 2) the visual target area (75–125% of target distance and within 8.4° of the target angle), and 3) a wide “aim area” between the visual target area and −45°, i.e., the hand angle that would fully counteract the rotation, hereafter called the “hand target.” The visual target area included one landmark on each side of the target, so that the maximum width of the target area spanned ~3.4° of visual angle. Fixations at locations outside these three areas were very rare.
To examine task-relevant gaze fixations over the course of the testing session, we only used fixation angles between 75 and 125% of the target distance. During the preview period on rotation trials, gaze typically shifted to the visual target briefly after its appearance, and from there gaze shifted, often over two or three saccades, toward the hand target (see Fig. 2, A and B for an example). Therefore, we selected the fixation angle closest to the hand target, discarding fixations within the target area, to obtain a single measure of the putative “aimpoint fixation angle” for each trial. (Note that report trials were excluded from this analysis.) The darker colored dots in Fig. 3, 3rd column, show the fixations selected using this procedure. For group analyses, the resulting fixation angles were averaged across sets of eight trials (or 6 no report trials in experiment 1), for each set that contained at least two “aimpoint fixations.”
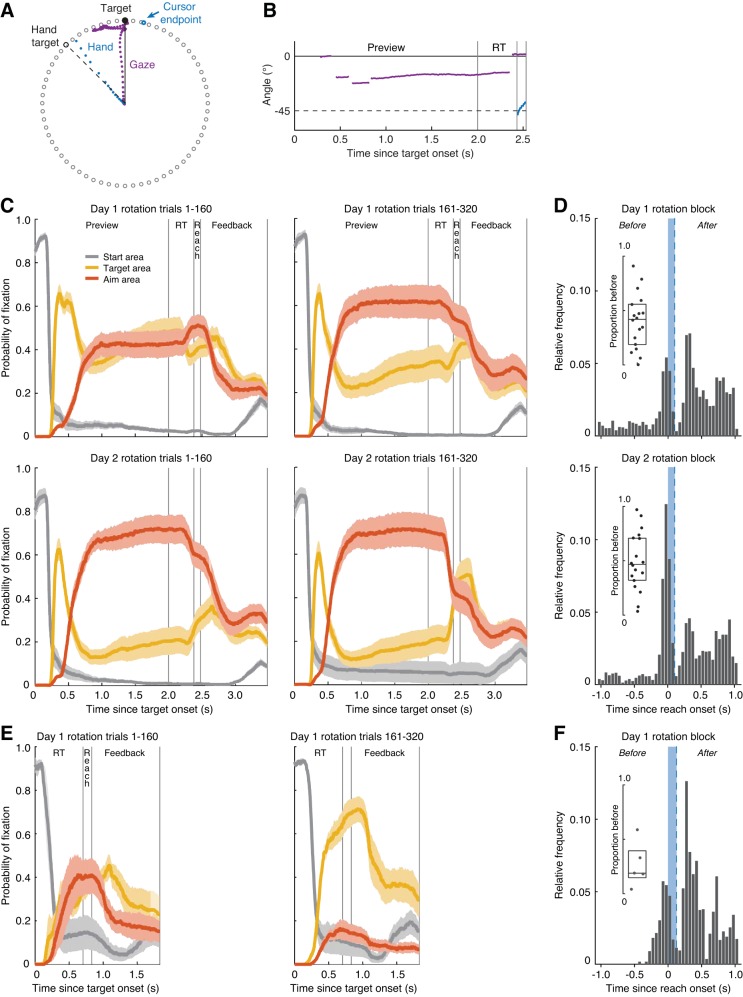
Fig. 2.
Gaze behavior in rotation trials. A: typical behavior in a no report trial in the rotation block. This participant first moved their gaze to the visual target, then in the direction of the hand target, and back to the visual target before executing the reach movement. B: time course of fixations (75–125% of target distance; purple), and hand movement (blue) during the target preview, hand reaction time (RT), and reach for the trial shown in A. C: probability of fixation in the start area (<75% of target distance; gray trace), target area (75–125% of target distance and <8.4° of the visual target; yellow trace), and aim area (75–125% of target distance and −8.4° to −45° from the visual target; orange trace) as a function of normalized within trial timing, averaged across the subgroup of aimpoint fixators in experiment 1 (n = 18). Shaded areas represent means ± SE. Separate graphs are shown for the 1st (left) and 2nd (right) half of the rotation block on day 1 (top) and day 2 (bottom). D: timing of fixation in the visual target area, following a fixation in the aimpoint area, in the rotation block of experiment 1 (51% of correct no report trials). The blue area indicates the mean duration of the reach. Inset: proportion of trials in which a fixation at the visual target started before the offset of the reach (blue dashed line), relative to the total number of selected trials. The dots indicate this proportion for each aimpoint fixator; the boxplot indicates the median and interquartile range across subjects. E: probability plots averaged across aimpoint fixators (n = 5) in experiment 3, organized and computed the same as in C. F: as in D, containing 21% of rotation trials, averaged across aimpoint fixators in experiment 3.
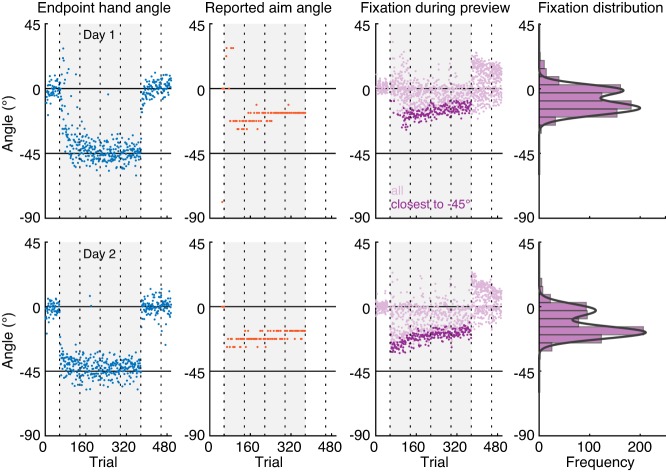
Fig. 3.
Raw data and experimental approach of classifying participants. Raw end point hand angles (blue), reported aim angles (orange) and fixation angles (purple) during the 2-s target preview period in no report trials of a representative participant in the intermittent report Experiment on day 1 (top) and day 2 (bottom). The gray background indicates when a 45° rotation was applied to the cursor feedback. Vertical dotted lines indicate the timing of 30 s breaks during the experiment. The darker purple dots show, for each trial, the selected fixation angle closest to the hand target, used to compute the group average aimpoint fixation angle. Rightmost column: histogram of all fixation angles in the rotation block. This participant was classified as an aimpoint fixator because their gaze distribution was well fit by a mixture of 2 Gaussian curves.
Gaussian curve fitting.
Our hypothesis that gaze patterns can provide a readout of the explicit component predicts that the distribution of each participant’s fixation locations should be bimodal, with a peak at the angle of the visual target, and a second peak at the participant’s putative aiming angle. A peak at the aiming angle occurred in the majority but not all participants. To test for possible differences in learning curves between participants that did or did not exhibit aimpoint fixations, we divided our participants into subgroups of “aimpoint fixators” and “target-only fixators.” To do this, we first created, for each participant, a histogram of all fixation angles at 75 to 125% target distance during the preview phase of the trials in the rotation block (see Fig. 3), excluding the first 40 trials wherein the explicit component changes rapidly (see Fig. 4; Taylor et al. 2014). The center of the histogram bins corresponded to the angles of the landmarks, and the width of the bins corresponded to the angular distance in between each two landmarks, such that each bin was 5.625° wide. We used the “fit” function in the MATLAB curve fitting toolbox to perform a nonlinear least squares fit of a mixture of two Gaussian curves to the bin counts y, according to:
where a1 and a2 are the amplitudes of the Gaussians, b1 and b2 are the means of the Gaussians, and c1 and c2 are related to the width of the Gaussians. The lower bounds of the a, b, and c parameters were set to [0 −180 0], and the upper bounds were set to [Inf 180 Inf]. The starting value for a was set to half of the total bin count, and the starting value for c was set to 6 based on initial, unconstrained fits. We set the starting value for b1 to 0 (i.e., the visual target), and for b2 we used starting values around the mean of the reported aiming direction in the intermittent report experiment (means ± SD: −23.3 ± 7.6; starting values [−30, −28, −26, −24, −22, −20, −18, −16]). We selected the fit with the highest variance explained by the model. Participants were categorized as aimpoint fixators if the fitting procedure returned two significant Gaussians (see Fig. 3); that is, the 95% of the confidence interval (CI) of the means b1 and b2 did not overlap, and the CI of b2 was outside of the center histogram bin. Otherwise, participants were categorized as target-only fixators, in which case a single Gaussian curve was fit to the bin counts. For three participants in experiments 1 and 2, and one participant in experiment 3, the CI of the mean of the best fit unimodal curve for one of the days was outside of the center bin. These participants were categorized as aimpoint fixators.
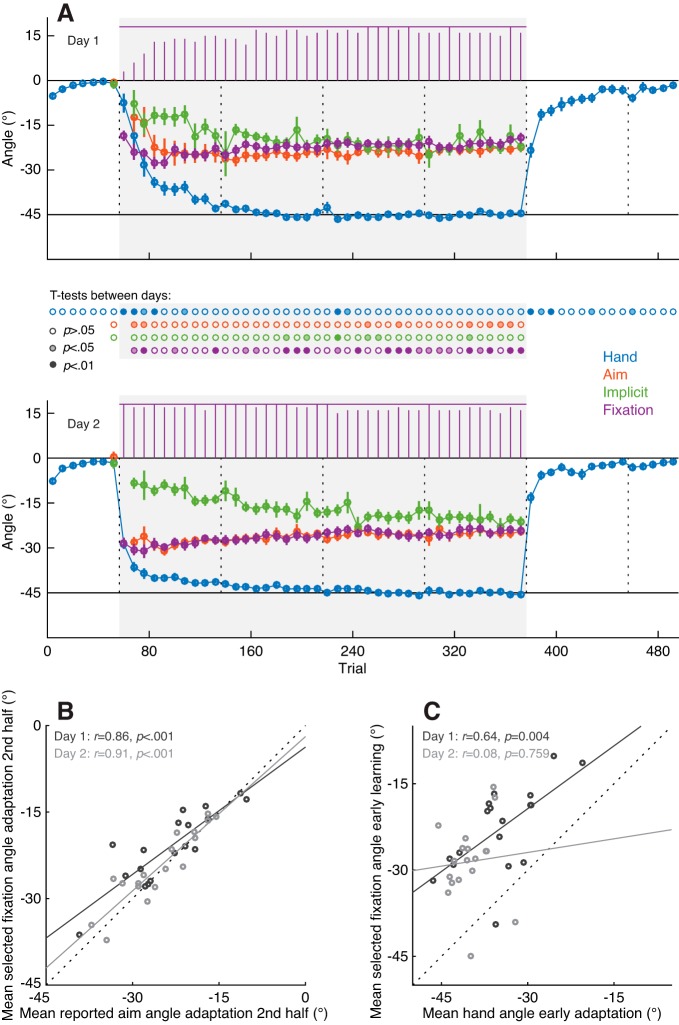
Fig. 4.
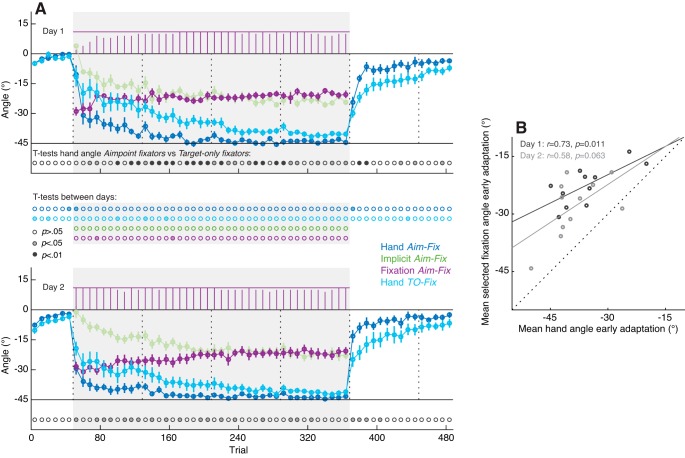
Results intermittent report experiment. A: end point hand angles (blue), reported aim angles (orange), implicit angles (green), and selected fixation angles (purple) on day 1 (top) and day 2 (bottom), averaged across aimpoint fixators (n = 18) in experiment 1. Each data point represents the average of a set of 8 trials, with error bars showing ± 1 SE across subjects. Purple bars at the top of each graph depict the number of participants contributing to the average selected fixation angle in each trial set. The gray background indicates when the 45° rotation was applied to the cursor feedback. Vertical dotted lines indicate the timing of 30-s breaks during the experiment. The rows of dots in between the top and bottom graphs show the results of uncorrected paired t-tests between each of the data points on days 1 and 2, with the color saturation indicating the significance level. B: relation between the reported aim angle and the selected fixation angle, averaged across the 2nd half of the rotation block of days 1 and 2. Dashed line indicates the unity line. C: relation between hand angle and selected fixation angle during early adaptation (trial sets 2–10 of the rotation block). R and P values in B and C show Pearson’s correlation coefficient and its significance value, respectively.
Estimating the explicit and implicit component.
For experiment 1 (intermittent report experiment), we estimated the explicit component of visuomotor adaptation using the verbally reported aiming direction (Taylor et al. 2014). We converted the verbally reported landmark number to an angle relative to the target. The reported aiming angles were averaged across sets of eight trials. As such, each value per eight-trial set represents the average of two report trials. Subsequently, implicit adaptation was estimated for each set by subtracting the averaged explicit angle from the averaged hand angle (Taylor et al. 2014). Because, in experiment 1, we found that the aimpoint fixation angle closely matched the explicit, verbally reported aimpoint angle (see results), for experiment 2 we estimated implicit adaptation for each trial set by subtracting the averaged aimpoint fixation angle from the averaged hand angle.
Statistical analyses.
To assess differences in task performance between days 1 and 2, we performed paired t-tests on the hand angles, reported aiming angles, implicit angles, and fixation angles averaged across sets of eight trials. To assess differences in adaptation between subgroups of aimpoint and target-only fixators, we performed unpaired t-tests on the hand angles averaged across sets. We computed Pearson’s correlation coefficients to assess, across participants, the relationship between variables.
RESULTS
The goal of our study was to assess whether gaze behavior, a natural component of reach planning, can be reliably used to probe both the implementation and state of cognitive strategy use during VMR learning and relearning 24 h later. We predicted that gaze fixations, before reaching on each trial, would closely track participants’ verbally reported aiming direction, as assayed on separate trials (experiment 1). Upon establishing this link, we further predicted that gaze fixations, in the absence of any verbal reporting, would provide a unique means of identifying individuals using cognitive strategies (experiments 2 and 3). In all three experiments, we predicted that gaze behavior would be directly related to individuals’ rate of visuomotor adaptation and expression of savings.
Characterization of Within-Trial Gaze behavior
Figure 2, A and B, shows gaze behavior in an example no report trial in the rotation block of experiment 1. Typical gaze behavior involved first shifting gaze from the start position to the visual target at ~200 to 300 ms following target onset. Next, gaze often shifted to a position somewhere in between the visual target and the hand target for two to three fixations. Thereafter, gaze either remained in the aimpoint area during the reach or shifted back to the visual target before the onset of the reach. This behavior is consistent with our prediction that the distribution of each participant’s fixation locations should be bimodal, with a peak at the angle of the visual target and a second peak at the participant’s putative aiming angle. A peak at the aiming angle occurred in the majority but not all participants. Therefore, we first divided participants into groups based on their distribution of fixation angles in the rotation block. In the intermittent report experiment, 18 out of 21 participants showed a bimodal distribution of fixations that was well fit by a mixture of two Gaussians (see Fig. 3 for an example) and were therefore classified as aimpoint fixators. In the no report experiment, 11 out of 21 participants were classified as aimpoint fixators. In the no preview experiment, 5 out of 12 participants were aimpoint fixators. Here, we will first describe the within-trial gaze behavior of these subgroups of participants.
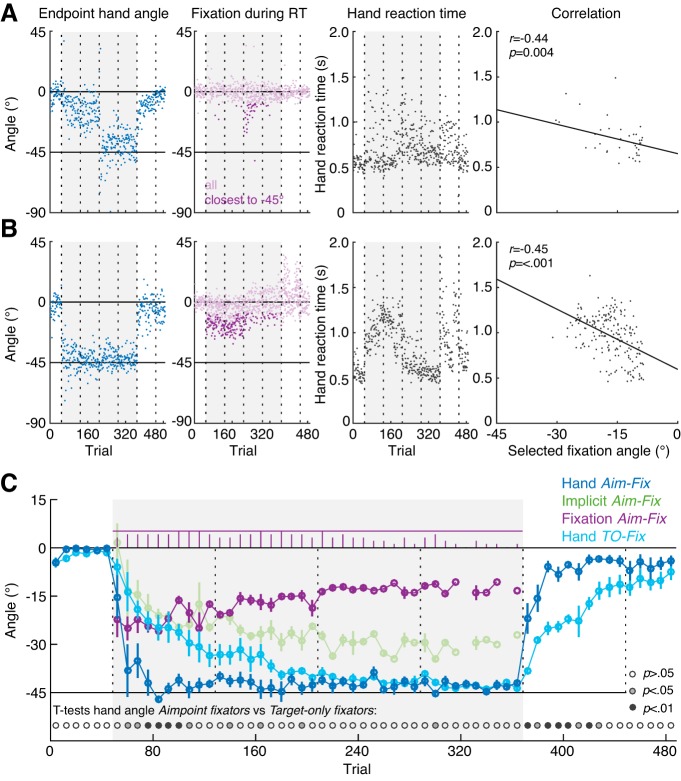
Figure 2C shows how gaze behavior unfolds over the time course of a single trial in terms of the probability of fixation in the start point area, visual target area, and the area in between the visual target and the hand target at −45° (aim area), averaged across aimpoint fixators. To examine how gaze behavior changed over the course of learning, we computed the probabilities separately for the first and second half of the rotation block on days 1 and 2. In all of these intervals, there was initially a high probability of fixation in the start area when the target appeared, which was followed by a quick increase in probability of fixation at the visual target. Next, fixations occurred in the visual target or aim area, with a decrease in probability in the target area and an increase of probability in the aim area between the first and second half of the rotation block on day 1. On average, fixations in the aim area occurred in 73 ± 5 and 88 ± 4% of the correct no report trials in the rotation block on the first and second day, respectively, in the subgroup of aimpoint fixators. During the reach, we observed a slightly higher probability of fixation in the aimpoint area compared with the target area, which leveled out in the second half of the rotation block on day 2. The individual data revealed that, during the reach, 12 out of the 18 aimpoint fixators fixated in the aim area in a portion of trials and in the target area in another portion of trials, 5 aimpoint fixators predominantly fixated the aimpoint, and 1 aimpoint fixator predominantly fixated the target. Figure 2D shows the timing of the onset of fixations at the visual target for trials in which the target fixation was preceded by a fixation in the aim area. As can been seen in this graph, participants showed two patterns of gaze behavior. They either shifted their gaze to the visual target right before the onset of the reach, likely to use visual feedback during the reach, or they shifted their gaze to the visual target after the offset of the reach, likely to obtain visual feedback about the error. They tended not to shift their gaze to the target during the reach, explaining the dip in fixation onset frequency around the offset of the reach. As shown in the inset, the occurrence of these two patterns varied across participants but many participants exhibited both patterns.
One plausible explanation of the experiment 1 findings is that because we asked participants to verbally report (and thus, presumably fixate) their aimpoint on a minority (25%) of trials, this may have biased their gaze patterns on the remaining majority (75%) of trials. To assess whether the nature of the verbal reporting task biased the resulting eye movement patterns, a second group of participants performed the same two sessions of the VMR task but without the requirement to report their aiming direction. Here, 11 out of our 21 participants were classified as aimpoint fixators based on the fitted Gaussian curves. The time course of the probability of fixation in the start and target and aim area averaged across the first and second half of the rotation blocks (data not shown) appeared strikingly similar to that shown for experiment 1 (see Fig. 2C). Aimpoint fixations occurred in 71 ± 4 and 81 ± 5% of correct trials in the rotation block on the first and second day, respectively. Thus, although the proportion of aimpoint fixators was affected by the task of verbal reporting, the gaze behavior of aimpoint fixators was highly consistent across experiments.
To assess whether a brief (2 s) preview period of the target is necessary for aimpoint fixations to occur, we performed a third experiment. This no preview experiment was identical to the no report experiment (experiment 2), with the exception that, on each trial, participants were instructed to initiate a reach movement upon appearance of the target (i.e., no preview period) and participants performed only a single session of the VMR task. Despite the lack of a target preview period, 5 out of 12 participants still showed fixations in the area between the visual target and the hand target during of the rotation block, resulting in a bimodal distribution of fixation angles. Figure 2E shows the probability of fixation in the start point area, visual target area, and aim area in the first and second half of the rotation block. In contrast to the first and second experiment, aimpoint fixations did generally not persist throughout the rotation block, resulting in a lower probability of fixation in the aim area and a higher probability of fixation in the target area in the second compared with the first half of the rotation block. Figure 2F shows the timing of target fixations in trials with aimpoint fixations. As in experiment 1, participants shifted their gaze to the target before or after the reach, with a low frequency of target fixation onset around the offset of the reach. In most trials, participants fixated the visual target after the reach, as shown by the low proportion “before” in Fig. 2F, inset, suggesting that in trials with a fixation in the aim area, this fixation occurred during the reach.
Experiment 1: Intermittent Report
The first experiment contained two separate sessions, separated by 24 h, of baseline reaches with veridical cursor feedback, adaptation to a 45° VMR of the cursor feedback, and washout with veridical feedback. During the rotation block, in 25% of trials, participants were asked to report the number of the landmark they planned to aim their hand to for the cursor to hit the target. We extracted patterns of gaze fixations in the remaining 75% of trials. Figure 3 shows, for an example participant, the raw end point hand angles, reported aiming angles, and the angles of all fixations during the target preview period of no report trials. The participant shows rapid adaptation of the end point hand angle from 0° to −45°, with quicker adaptation on the second day compared with the first day (i.e., savings). Furthermore, their verbally reported aiming angle shows a similarly fast change toward −45° in the beginning of the rotation block and then very slowly drifts back toward about −20° by the end of the rotation block. This participant shows gaze fixations both at the visual target and at an angle in between the visual target and the hand target. The tail of the distribution, denoted by the darker colored purple dots that show the selected fixation angle closest to the hand target at −45° in each trial, very closely mimics the temporal evolution of verbally reported aiming angles during the task. Notably, during the washout block, this participant seems to fixate an additional aimpoint location at the diametrically opposite side of the target, as if the rotation were reversed rather than turned off. Many participants exhibited this same behavior, suggesting that reversion to baseline during washout involves the implementation of a reverse strategy. Specifically, 16 aimpoint fixators showed fixations at the opposite side of the target during washout, although 3 of these participants only showed this behavior on one of the days. Figure 3A, right, shows that the histogram of fixation angles during the rotation block for this participant was well fit by a mixture of two Gaussian curves. When we applied this same approach to the histogram of fixation angles of each participant (see materials and methods), we found that for 18 out of our 21 participants the histogram was better fit by a mixture of two Gaussian curves than one Gaussian curve, and we thus classified these individuals as aimpoint fixators. Only two participants showed fixations almost exclusively at the visual target location in the rotation block (i.e., were poorly fit by a mixture of two Gaussian curves), thus classifying these individuals as target-only fixators. Notably, both of these target-only fixators reported nonzero values and showed rather fast changes in the hand angle suggesting that they did implement an explicit strategy. The one remaining participant switched from a unimodal distribution of fixation angles on day 1 to a bimodal distribution on the second day. To verify our approach of describing the time course of aimpoint fixations by selecting, on each trial, the fixation angle closest to the hand angle, we performed a linear regression between the mean of the selected fixation angles and the mean of the Gaussian curve in the aimpoint area. This analysis revealed a linear relationship across aimpoint fixators, with a slope close to one on day 1 (slope = 1.19, 95% CI = [1.02 1.36]; intercept = 8.82, 95% CI = [5.06, 12.58]) and day 2 of testing (slope = 0.99, 95% CI = [0.84 1.13]; intercept = 2.13, 95% CI = [−1.62 5.86]).
Figure 4A shows the end point hand angles, reported aiming angles, implicit adaptation angles obtained by subtraction of the reported aiming angles from the hand angles, and fixation angles closest to the hand target during the target preview period. All angles are averaged across sets of eight trials and across subjects classified as aimpoint fixators (i.e., 18 out of 21 participants). The time course of gaze fixations closely overlapped with that of the reported aiming angle, confirming our initial hypothesis that gaze fixations would closely track participants’ verbally reported aiming direction. To directly assess the relationship between these two variables, we computed correlation coefficients on the reported aiming angles and the fixation angles on each day, averaged across the trial sets in the second half of the rotation block (i.e., sets 21–40) where the explicit component is fairly stable. We observed a strong linear relationship between mean reported aiming angles and mean fixation angles on both days (Fig. 4B).
As a descriptive analysis of differences between testing days, we performed paired t-tests between each of the data points on days 1 and 2 (uncorrected for multiple comparisons). Consistent with prior work (Krakauer et al. 2005; Morehead et al. 2015), we found faster adaptation of the hand angle in the rotation block and washout block of day 2 compared with day 1 (i.e., savings). Notably, faster changes in hand angle following the onset of the rotation on day 2 were accompanied by a larger (i.e., more negative) reported aiming angle, as well as a larger fixation angle, without significant differences in the implicit angle. This suggests that savings were mainly driven by recall of an aiming strategy, possibly facilitated by gaze fixations. (Note that fixation angle differed significantly between days in several bins of the rotation block; however, there was no clear pattern in these differences with the exception that they generally reflected the tendency for aimpoint fixations to be magnified on day 2.) If aimpoint fixations reflect an explicit strategy, greater fixation angles should correspond to faster learning in the beginning of the rotation block, where the contribution of the implicit component is small. To directly assess the relationship between learning and fixation angle during early learning, we computed a correlation, across participants, between the mean hand angle and mean fixation angle in trial sets 2–10 of the rotation block on both days. As shown in Fig. 4C, this revealed a positive linear relationship on day 1 but not on day 2. We suspect that the lack of a correlation on day 2 might reflect the fact that, due to day 1 learning, the variability across subjects in hand angles was much smaller on day 2 than on day 1.
Taken together, the main results of this intermittent report experiment are that 1) the vast majority of participants fixated an internal aimpoint, used to counteract the rotation, before executing the reach movement; 2) the magnitude and time course of these aimpoint fixations closely overlapped with that of the verbally reported aiming angle; and 3) a greater aimpoint fixation angle during early learning on day 1 was related to greater changes in hand angle.
Experiment 2: No Report
To assess whether the nature of the verbal reporting task biased the resulting eye movement patterns, in the no report experiment participants performed two sessions of the VMR task without the requirement to report their aiming direction. Here, we found that two subgroups of participants clearly emerged. Eleven out of our 21 participants were now classified as aimpoint fixators based on the fitted Gaussian curves. As in experiment 1, we again observed a strong linear relationship between the mean of the Gaussian curve in the aimpoint area and the mean selected fixation angle (day 1: r = 0.82, P = 0.002; day 2: r = 0.93, P < .001). Notably, the proportion of aimpoint fixators in this experiment was significantly less than in experiment 1 [Pearson χ2-test (1) = 5.27, P = 0.022]. In addition, we now found that eight participants exhibited fixations only around the visual target (target-only fixators) and two participants switched from only fixating the target on day 1 to fixating both the target and an aimpoint on day 2 (excluded from the analysis). In the washout block, all of the aimpoint fixators showed fixations to the opposite side of the target as in the learning block, as judged by eye, although one participant only showed this behavior on the second day. None of the target-only fixators showed fixations to the opposite side of the target in the washout block.
Figure 5A shows the end point hand angles, fixation angles, and the implicit angles estimated by subtracting the fixation angles from the hand angles, averaged across the subgroup of 11 aimpoint fixators, as well as the hand angles averaged across the subgroup of eight target-only fixators. Whereas the subgroup of aimpoint fixators exhibited adaptation rates that were very similar to those of the aimpoint fixators in experiment 1 (see Comparison Between Experiments 1 and 2), adaptation rates in the subgroup of target-only fixations were considerably slower, with significant between-group differences in hand angles in several sets of trials in the rotation block and early in the washout block. Moreover, whereas the subgroup of aimpoint fixators showed savings, as indicated by significantly faster adaptation on day 2 compared with day 1 in the first trial set of the rotation [t(10) = 4.33, uncorrected P = 0.002] and washout blocks [t(10) = −3.48, uncorrected P = 0.006], the subgroup of target-only fixators failed to show significant savings [1st set in rotation block: t(7) = 1.87, uncorrected P = 0.104; 1st set in washout block: t(7) = −1.76, uncorrected P = 0.122]. This suggests that learning in the target-only fixators group was largely implicit and did not involve an aiming strategy. To test our prediction that gaze behavior is directly related to individuals’ performance during early learning, we computed a correlation, across participants, on the mean hand angle and mean fixation angle during early learning (i.e., sets 2–10 of the rotation block). We observed a strong positive correlation on day 1 and a moderate, marginally significant correlation on day 2 (Fig. 5B).
Fig. 5.
Results no report experiment. A: end point hand angles, implicit angles (estimated through subtraction of fixation angles from hand angles), and selected fixation angles, averaged across aimpoint fixators (Aim-Fix, n = 11), as well as end point hand angles averaged across target-only fixators (TO-Fix, n = 8) in experiment 2. Each data point represents the average of a set of eight trials, with error bars showing ± 1 SE across subjects. Purple bars at the top of each graph show the number of aimpoint fixators contributing to the average selected fixation angle. The gray background indicates when the 45° rotation was applied to the cursor feedback. Vertical dotted lines indicate the timing of 30 s breaks during the experiment. The row of dots at the bottom of each graph shows the result of unpaired t-tests between the aimpoint fixators and the target-only fixators. The rows of dots in between the top and bottom graphs show the results of uncorrected paired t-tests between each of the data points on days 1 and 2, with the color saturation indicating the significance level. B: relation between hand angle and selected fixation angle across aimpoint fixators during early adaptation (trial sets 2–10 of the rotation block). R and P values show Pearson’s correlation coefficient and its significance value, respectively.
Taken together, the results from this second experiment suggest that 1) the use of verbal reporting measures increases the proportion of participants that implement cognitive strategies, and 2) participants who naturally exhibit aimpoint fixations in this task show fast adaptation and savings whereas those participants who only ever exhibit target fixations show comparably slow adaptation, with no evidence for savings.
Comparison Between Experiments 1 and 2
Across the first two experiments, we found that a significantly larger proportion of participants fixated an aimpoint before reaching under a VMR when the task involved verbally reporting the aiming direction on a subset of trials. When we compared the hand angle of all subjects in the intermittent report experiment (experiment 1) to the hand angle of all subjects in the no report experiment (experiment 2; data not shown), we found significant differences in a large part of the rotation block, especially on the first day of testing. On average, participants in the intermittent reporting experiment showed faster adaptation and deadaptation and a greater asymptotic adaptation level than participants in the no report experiment. However, when we compared the subgroups of aimpoint fixators in both experiments (18 participants in experiment 1 and 11 participants in experiment 2), there were no significant differences in hand angle, except in 2 out of the 55 bins across the entirety of the rotation and washout blocks of each day. These results suggest that the declarative nature of verbal reporting increases the proportion of participants that implement an aiming strategy, resulting in faster learning, but does not affect the magnitude of the explicit component.
Experiment 3: No Preview
To assess the influence of a brief (2 s) target preview on gaze behavior and learning, in the no preview experiment participants were instructed to initiate a reach movement directly upon appearance of the target. Participants performed only a single session of the VMR task. Despite the lack of a target preview period, 5 out of 12 participants still showed fixations in the area between the visual target and the hand target during of the rotation block, resulting in a bimodal distribution of fixation angles. Figure 6, A and B, shows the raw hand angles, fixation angles, and hand reaction times of two example participants. The participant in Fig. 6A appeared to implement an aiming strategy about half way through the rotation block, as judged by the sudden change in hand angle and a brief period of aimpoint fixations. Although this participant was classified as a target-only fixator based on the distribution of fixation angles, we manually classified this participant as a switcher. Figure 6B shows an example aimpoint fixator. As for this example participant, aimpoint fixations generally did not persist throughout the entire rotation block, unlike in the first two experiments. Rather, aimpoint fixations were only expressed at what appears to be the start of the implementation of a aiming strategy, as judged from corresponding fast changes in hand angle. Note that the lack of persistence of aimpoint fixations does not imply that the explicit component has reduced back to zero (see discussion). As can be seen in Fig. 6, A and B, 3rd column, fixating an aimpoint came at the cost of a higher reaction time. Fig. 6, A and B, right column, shows the relation between the selected fixation angle and the hand reaction time for both participants. On average, the aimpoint fixators showed a significant negative relationship between selected fixation angle and reaction time of the hand movement [means ± SE, r = −0.29 ± 0.07, one-sample t-test against 0, t(4) = −4.18, P = 0.014].
Fig. 6.
Results no preview experiment. A and B: raw end point hand angles (blue), fixation angles during the hand reaction time interval (RT; purple), and hand reaction times (black) of 2 example participants in experiment 3. The gray background indicates when a 45° rotation was applied to the cursor feedback. Vertical dotted lines indicate the timing of 30 s breaks during the experiment. The darker purple dots show, for each trial, the selected fixation angle closest to the hand target. Rightmost column: the relation between selected fixation angles and hand reaction time. R and P values show Pearson’s correlation coefficient and its significance value, respectively. C: end point hand angles, implicit angles (estimated through subtraction of fixation angles from hand angles), and selected fixation angles, averaged across aimpoint fixators (Aim-Fix, n = 5), as well as end point hand angles averaged across target-only fixators (TO-Fix, n = 6) in experiment 3. Each data point represents the average of a set of 8 trials, with error bars showing ± 1 SE across subjects. Purple bars at the top of each graph show the number of aimpoint fixators contributing to the average selected fixation angle. The row of dots at the bottom of the graph shows the result of unpaired t-tests between the aimpoint fixators and the target-only fixators. Also shown in C, end point hand angles averaged across the subgroup of five aimpoint fixators and six target-only fixators. The participant shown in A was excluded from the group average because of the sudden change in hand angle. As in experiment 2, adaptation and washout were faster for the aimpoint fixators than for the target-only fixators.
To summarize, we found that even without a brief, instructed preview period of the visual target, nearly half the participants still fixated an internal aimpoint location, which, while resulting in faster adaptation, came at the cost of longer reaction times. Notably, in these aimpoint fixators, fixations further away from the visual target (i.e., a greater aiming angle) were associated with longer hand reaction times, consistent with the idea that explicit aiming may involve the mental rotation of a movement end point or trajectory (Anguera et al. 2010; Fernandez-Ruiz et al. 2011; McDougle and Taylor 2016). This experiment reinforces the findings from the first two experiments that trial-to-trial gaze behavior during adaptation is linked to the implementation of a cognitive strategy.
DISCUSSION
Here we explored the idea that task-specific gaze fixations over the time course of sensorimotor adaptation and readaptation 24 h later can provide a covert means of identifying individuals who use explicit strategies during learning, as well as how the contribution of the explicit component to learning evolves over time. We show, across three experiments, that gaze behavior during VMR learning parcellates the explicit and implicit components to learning, is linked to individual differences in learning rates, and can predict the expression of savings.
Previous research has examined free gaze behavior during adaptation to a VMR in the presence of visual landmarks. Rand and Rentsch (2016) investigated gaze location at the time of reach onset during adaptation to 30, 75, and 150° rotations without online cursor feedback and without a delay period. They showed that, for the 30 and 75° rotations, participants fixated the visual target during early learning but, in subsequent trials, often fixated the “hand target” (i.e., the location of the hand when the cursor was on the visual target). In their task, learning appeared to be almost entirely explicit, as limited aftereffects were observed, and therefore, the hand target was effectively the aimpoint. Thus this previous study provided evidence that gaze behavior can reflect the explicit component of learning (Rand and Rentsch 2016). The current study both supports and extends this previous work. First, by using a paradigm in which, both within and across days, the relative contributions of the implicit and explicit components vary markedly, we could show that changes in the explicit component were matched by changes in gaze behavior. Second, because the hand target and the aimpoint were clearly dissociated in our paradigm, we could unequivocally show that gaze is frequently directly to the aimpoint. Third, we provide additional evidence, based on individual differences, for the close mapping between gaze behavior and the explicit component of learning. Finally, the fact that we observed a similar correspondence between gaze behavior and reach performance in experiments 1 and 2 indicates that the magnitude of the explicit component, per se, is not influenced by requiring participants to provide verbal reports of their aiming direction. Our results, in combination with the previous work of Rand and Rentsch (2016), indicate that gaze behavior can provide a useful tool for assessing the explicit component of visuomotor adaptation across a range of paradigms.
Gaze Behavior as a Substitute to Verbal Reporting
The large participant groups tested in the current study allowed us to divide individuals into two main subgroups: 1) a group that only fixated the visual target (i.e., target-only fixators), and 2) a group that fixated both the visual target and a separate aimpoint (i.e., aimpoint fixators). When not being probed about their aiming strategy, we found that target-only fixators adapted more gradually and did not exhibit savings, indicative of implicit processes governing their learning and relearning of the VMR (Morehead et al. 2015). By contrast, aimpoint fixators exhibited fast adaptation and savings, indicating the use of explicit strategies. Previous research has already shown a relationship between the use of explicit strategies and learning (Heuer and Hegele 2008; Taylor et al. 2014; Werner and Bock 2007); here we show that this relation is also present when we use gaze behavior to assess strategy use. Our results indicate that group membership is affected by verbal reporting, such that the requirement to declare aiming direction on a subset of trials increases the number of aimpoint fixators rather than the magnitude of the explicit component, as previously assumed (Leow et al. 2017; Taylor et al. 2014). We further noticed that several participants were quite rigid in their verbal reporting; that is, they consistently tended to report, across trials, a fixed number of landmarks counterclockwise to the visual target as their aimpoint. The declarative nature and rigidness of reporting suggest an advantage to using gaze to assess the contribution of explicit processes to learning. First, the lack of aimpoint fixations may identify participants who, in the absence of being prompted by verbal reporting, would not spontaneously implement an aiming strategy. Second, in participants who do implement such strategies, gaze can provide a covert, yet sensitive, measure of the magnitude of the explicit component.
We recognize, however, that there may also be some shortcomings in using gaze fixations to assess the explicit component. First, the absence of aimpoint fixations does not preclude the possibility that explicit strategies are still being implemented. However, the gradual nature of learning and absence of savings in nonaimpoint fixators in the no report experiment suggest that their learning is largely implicit (Morehead et al. 2015). Second, adding landmarks to the visual scene is an essential modification to elicit aimpoint fixations in participants who naturally implement a cognitive strategy. Without providing this scaffolding, it is highly likely that gaze would solely be attracted by the saliency of the visual target, as gaze is not frequently, nor reliably, directed toward blank spaces. Indeed, it is for this very reason that we added landmarks to the visual scene. Third, we showed that providing a brief target preview is helpful in eliciting a robust pattern of aimpoint fixations. Of course, the occurrence of aimpoint fixations will also depend on other factors and will likely become more robust in conditions in which the explicit component is large (e.g., Rand and Rentsch 2016). Nevertheless, given that the primary method used for assessing the time course of explicit and implicit components to learning involves declarative reporting (Taylor et al. 2014), which itself enhances the probability that cognitive strategies are implemented and which also necessitates the use of landmarks and increases reaction times, we believe that the use of gaze behavior has inherent advantages.
The Role of Aimpoint Fixations During Visuomotor Learning
During a trial, aimpoint fixators typically shifted their gaze from the start position to the visual target shortly after its appearance and then shifted their gaze (in one or a series of saccades) to the aimpoint. Presumably, aimpoint fixations assist participants in performing a mental rotation of the motor goal location or movement direction (McDougle and Taylor 2016). This suggestion is supported by our observation that in the no preview experiment, aimpoint fixators’ hand reaction times were correlated with the magnitude of their fixation angles. This relationship between rotation magnitude and reaction time bears strong similarity to previous observations in studies of visually guided reaching and object rotation (Pellizzer and Georgopoulos 1993; Shepard and Metzler 1971; see also Fernandez-Ruiz et al. 2011). However, aimpoint fixations are not necessary in applying an aiming strategy. In the no preview experiment, most aimpoint fixators stopped fixating an aimpoint during the rotation block but this did not result in a sudden increase in hand error. Furthermore, in the intermittent report experiment, the two participants who did not show aimpoint fixations nevertheless reported aiming in the direction to counter the rotation, and showed fast learning, suggesting that they implemented an aiming strategy. Finally, as discussed above, it is unlikely that participants would fixate an aimpoint when visual landmarks are not present, yet they can still implement a strategy. Nevertheless, the majority of participants showed a robust pattern of aimpoint fixations in the experiments that used a brief target preview. When participants were not asked to report their aiming direction, their fixation pattern could distinguish between faster learners that implemented an aiming strategy and slower, more implicit learners.
When reaching under normal visual feedback conditions, humans naturally direct their gaze to the reach target before moving their hand (e.g., Neggers and Bekkering 2000; Prablanc et al. 1979;). In the current study, we observed that participants who showed aimpoint fixations exhibited two dominant fixation patterns around the time of the reach when reaching under a VMR. They either shifted their gaze from the aim area to the visual target before the reach and kept their gaze on the target during the reach, or they fixated in the aim area during the reach, which was often followed by a gaze shift to the visual target after completing the reach. Fixating the visual target during the reach optimizes the use of peripheral visual feedback in automatically correcting for errors in the reach trajectory (e.g., Carlton 1981; Paillard 1996; de Brouwer et al. 2017; Land et al. 1999; Saunders and Knill 2003). Although in the current study participants were instructed to make ballistic, uncorrected reaching movements, it is unlikely that participants fully ignored peripheral visual information of the cursor, which provides an important reason for fixating the target. Surprisingly, however, aimpoint fixators were slightly more likely to fixate in the aim area than at the visual target during the execution of the reach (0.5 vs. 0.4 probability, respectively, when averaged over rotation blocks). In fact, several participants almost exclusively fixated the aimpoint during the reach. These aimpoint fixations were often followed by a gaze shift to the visual target after the offset of the reach, likely to obtain visual feedback about the target error. One explanation for fixating the aimpoint during the reach is that this could improve reach accuracy through the use of extraretinal signals, that is, proprioceptive signals or an efference copy of oculomotor commands (e.g., Prablanc et al. 1986). However, in the setup used in the current study, this would also require a transformation from the vertical plane, in which the eye movements were made, to the horizontal plane, in which the hand movements were made. Furthermore, it is important to recognize that participants did not actually direct their gaze to the hand target (which would, in principle, provide the most spatially accurate extraretinal information to hit the target), but rather a strategic location to counteract the rotation that could change from trial to trial. One intriguing possibility, which may explain why gaze often remained at this location, is that the trial-by-trial state of the implicit component during learning is directly built into the transformation from gaze proprioceptive coordinates to the hand movement. Although previous work has examined reference frame transformations from gaze-centered to hand-centered coordinates (Buneo and Andersen 2006; Crawford et al. 2004), it has not directly explored how this mapping might be affected by implicit learning.
Gaze Behavior During Washout
Strikingly, we observed that almost all participants who fixated an aimpoint during the rotation block also appeared to fixate an aimpoint, in the opposite direction, in the deadaptation (washout) blocks on both days. That is, even though veridical visual feedback was restored during washout, the distribution of gaze angles appeared to be bimodal with a second peak in between the visual target and +45°, as if the rotation were reversed rather than extinguished. This indicates that deadaptation itself also involves an explicit component and not just the gradual reduction of the implicit component, for which it is commonly used (Krakauer et al. 2005). This finding is consistent with recent work showing that deadapting to an instantaneously removed rotation, A, results in savings when subsequently experiencing rotation −A (Herzfeld et al. 2014). The idea that deadaptation involves an explicit component appears to contradict recent findings from Morehead and colleagues (2015) who asked participants to verbally report their aiming direction during deadaptation and found that participants aimed toward the target rather than an opposite aimpoint. This discrepancy might be due to differences in the magnitude of the implicit component at the time the rotation was removed. Namely, when the implicit component at the end of the rotation block is small, as in the Morehead study (~10°), extinguishing the rotation will produce only a small error between the target and the cursor position, which is less likely to drive an aiming strategy (Bond and Taylor 2015). Notably, for many participants in our study, gaze remained at an “opposite” aimpoint throughout the full 120 trials of deadaptation, suggesting that the implicit component was not, in fact, washed out (as explicit aiming was being used to counteract it). Further research is needed to carefully unravel the complete time course of the explicit and implicit components to deadaptation.
Brain Mechanisms Linking Gaze and Explicit Processes
Whereas there is extensive evidence that implicit, error-based sensorimotor adaptation is reliant on cerebellar mechanisms (Morton and Bastian 2006; Smith and Shadmehr 2005; Tseng et al. 2007), the neural systems associated with the explicit component of learning remain largely unknown. The verbal reporting task employed by Taylor and colleagues (2014) showed that the use of explicit strategies in VMR learning can be declarative. Although experiment 2 did not involve verbal reporting, we suspect that aimpoint fixators, if queried, would similarly acknowledge use of such strategies. Perhaps not surprisingly, evidence from neuroimaging, aging, and lesion studies has implicated prefrontal cortex in explicit strategies (Taylor and Ivry 2014). Several studies have implicated dorsolateral prefrontal cortex, in particular, in contributing to sensorimotor adaptation and savings (Della-Maggiore 2005; Floyer-Lea and Matthews 2004; Shadmehr and Holcomb 1997), likely through its known role in working memory processes (Curtis and D’Esposito 2003; Seidler et al. 2012) and mental rotation (Cohen et al. 1996). With respect to the current results, we expect the frontal eye fields, located in prefrontal cortex and a key hub in the oculomotor network associated with target selection (Thompson and Bichot 2005), to be involved in the selection of aimpoints as saccade targets. The role of declarative processes in strategic reaiming further suggests that regions in the medial temporal lobe (MTL) might also be partly responsible for the reported oculomotor behavior. MTL regions appear integral to guiding gaze to strategic locations in a visual scene (Meister and Buffalo 2016), and the neuroanatomical connectivity of the MTL makes it well poised to interface with oculomotor regions in prefrontal cortex (Shen et al. 2016).
GRANTS
This research was funded by the Natural Sciences and Engineering Research Council of Canada and the Canadian Institutes of Health Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.J.d.B., M.A., J.R.F., and J.P.G. conceived and designed research; A.J.d.B. and M.A. performed experiments; A.J.d.B. analyzed data; A.J.d.B., J.R.F., and J.P.G. interpreted results of experiments; A.J.d.B. prepared figures; A.J.d.B. and M.A. drafted manuscript; A.J.d.B., J.R.F., and J.P.G. edited and revised manuscript; A.J.d.B., M.A., J.R.F., and J.P.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Martin York for technical support.
REFERENCES
- Anguera JA, Reuter-Lorenz PA, Willingham DT, Seidler RD. Contributions of spatial working memory to visuomotor learning. J Cogn Neurosci 22: 1917–1930, 2010. doi: 10.1162/jocn.2009.21351. [DOI] [PubMed] [Google Scholar]
- Bedford FL. Keeping perception accurate. Trends Cogn Sci 3: 4–11, 1999. doi: 10.1016/S1364-6613(98)01266-2. [DOI] [PubMed] [Google Scholar]
- Benson BL, Anguera JA, Seidler RD. A spatial explicit strategy reduces error but interferes with sensorimotor adaptation. J Neurophysiol 105: 2843–2851, 2011. doi: 10.1152/jn.00002.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock O, Abeele S, Eversheim U. Human adaptation to rotated vision: interplay of a continuous and a discrete process. Exp Brain Res 152: 528–532, 2003. doi: 10.1007/s00221-003-1643-x. [DOI] [PubMed] [Google Scholar]
- Bond KM, Taylor JA. Flexible explicit but rigid implicit learning in a visuomotor adaptation task. J Neurophysiol 113: 3836–3849, 2015. doi: 10.1152/jn.00009.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brashers-Krug T, Shadmehr R, Bizzi E. Consolidation in human motor memory. Nature 382: 252–255, 1996. doi: 10.1038/382252a0. [DOI] [PubMed] [Google Scholar]
- Buneo CA, Andersen RA. The posterior parietal cortex: sensorimotor interface for the planning and online control of visually guided movements. Neuropsychologia 44: 2594–2606, 2006. doi: 10.1016/j.neuropsychologia.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Carlton LG. Visual information: the control of aiming movements. Quarterly J Exp Psychol Sect A 33: 87–93, 1981. doi: 10.1080/14640748108400771. [DOI] [Google Scholar]
- Cohen MS, Kosslyn SM, Breiter HC, DiGirolamo GJ, Thompson WL, Anderson AK, Brookheimer SY, Rosen BR, Belliveau JW. Changes in cortical activity during mental rotation. A mapping study using functional MRI. Brain 119: 89–100, 1996. doi: 10.1093/brain/119.1.89. [DOI] [PubMed] [Google Scholar]
- Crawford JD, Medendorp WP, Marotta JJ. Spatial transformations for eye-hand coordination. J Neurophysiol 92: 10–19, 2004. doi: 10.1152/jn.00117.2004. [DOI] [PubMed] [Google Scholar]
- Cunningham HA. Aiming error under transformed spatial mappings suggests a structure for visual-motor maps. J Exp Psychol Hum Percept Perform 15: 493–506, 1989. doi: 10.1037/0096-1523.15.3.493. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci 7: 415–423, 2003. doi: 10.1016/S1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- de Brouwer AJ, Jarvis T, Gallivan JP, Flanagan JR. Parallel specification of visuomotor feedback gains during bimanual reaching to independent goals. eNeuro 4: ENEURO.0026-17.2017, 2017. doi: 10.1523/ENEURO.0026-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Maggiore V, McIntosh AR. Time course of changes in brain activity and functional connectivity associated with long-term adaptation to a rotational transformation. J Neurophysiol 93: 2254–2262, 2005. doi: 10.1152/jn.00984.2004. [DOI] [PubMed] [Google Scholar]
- Ebbinghaus H. Memory: a Contribution to Experimental Psychology. New York: Teachers College Press, 1913. doi: 10.1037/10011-000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Ruiz J, Díaz R. Prism adaptation and aftereffect: specifying the properties of a procedural memory system. Learn Mem 6: 47–53, 1999. [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Wong W, Armstrong IT, Flanagan JR. Relation between reaction time and reach errors during visuomotor adaptation. Behav Brain Res 219: 8–14, 2011. doi: 10.1016/j.bbr.2010.11.060. [DOI] [PubMed] [Google Scholar]
- Floyer-Lea A, Matthews PM. Changing brain networks for visuomotor control with increased movement automaticity. J Neurophysiol 92: 2405–2412, 2004. doi: 10.1152/jn.01092.2003. [DOI] [PubMed] [Google Scholar]
- Haith AM, Huberdeau DM, Krakauer JW. The influence of movement preparation time on the expression of visuomotor learning and savings. J Neurosci 35: 5109–5117, 2015. doi: 10.1523/JNEUROSCI.3869-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzfeld DJ, Vaswani PA, Marko MK, Shadmehr R. A memory of errors in sensorimotor learning. Science 345: 1349–1353, 2014. doi: 10.1126/science.1253138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer H, Hegele M. Adaptation to visuomotor rotations in younger and older adults. Psychol Aging 23: 190–202, 2008. doi: 10.1037/0882-7974.23.1.190. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G, Bäckström A, Flanagan JR. Eye-hand coordination in object manipulation. J Neurosci 21: 6917–6932, 2001. doi: 10.1523/JNEUROSCI.21-17-06917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Ghez C, Ghilardi MF. Adaptation to visuomotor transformations: consolidation, interference, and forgetting. J Neurosci 25: 473–478, 2005. doi: 10.1523/JNEUROSCI.4218-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Pine ZM, Ghilardi MF, Ghez C. Learning of visuomotor transformations for vectorial planning of reaching trajectories. J Neurosci 20: 8916–8924, 2000. doi: 10.1523/JNEUROSCI.20-23-08916.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land M, Mennie N, Rusted J. The roles of vision and eye movements in the control of activities of daily living. Perception 28: 1311–1328, 1999. doi: 10.1068/p2935. [DOI] [PubMed] [Google Scholar]
- Land MF, Furneaux S. The knowledge base of the oculomotor system. Philos Trans R Soc Lond B Biol Sci 352: 1231–1239, 1997. doi: 10.1098/rstb.1997.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leow LA, Gunn R, Marinovic W, Carroll TJ. Estimating the implicit component of visuomotor rotation learning by constraining movement preparation time. J Neurophysiol 118: 666–676, 2017. doi: 10.1152/jn.00834.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. II. Specificity and storage of multiple gaze-throw calibrations. Brain 119: 1199–1211, 1996. doi: 10.1093/brain/119.4.1199. [DOI] [PubMed] [Google Scholar]
- Mazzoni P, Krakauer JW. An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci 26: 3642–3645, 2006. doi: 10.1523/JNEUROSCI.5317-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougle SD, Taylor JA. Mental rotation as a behavioural and neural model of explicit aiming during visuomotor learning. Advances in Motor Learning & Motor Control. San Diego, CA, November 11, 2016. Abstract 160. [Google Scholar]
- Meister ML, Buffalo EA. Getting directions from the hippocampus: the neural connection between looking and memory. Neurobiol Learn Mem 134: 135–144, 2016. doi: 10.1016/j.nlm.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morehead JR, Qasim SE, Crossley MJ, Ivry R. Savings upon re-aiming in visuomotor adaptation. J Neurosci 35: 14386–14396, 2015. doi: 10.1523/JNEUROSCI.1046-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci 26: 9107–9116, 2006. doi: 10.1523/JNEUROSCI.2622-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neggers SF, Bekkering H. Ocular gaze is anchored to the target of an ongoing pointing movement. J Neurophysiol 83: 639–651, 2000. doi: 10.1152/jn.2000.83.2.639. [DOI] [PubMed] [Google Scholar]
- Paillard SF. The contribution of peripheral and central vision to visually guided reaching. In: Analysis of Visual Behaviour, edited by Ingle DJ, Goodale MA, Mansfield RJ. Cambridge, MA: MIT Press, 1982, p. 367–385. [Google Scholar]
- Paillard J. Fast and slow feedback loops for the visual correction of spatial errors in a pointing task: a reappraisal. Can J Physiol Pharmacol 74: 401–417, 1996. doi: 10.1139/y96-033. [DOI] [PubMed] [Google Scholar]
- Pellizzer G, Georgopoulos AP. Common processing constraints for visuomotor and visual mental rotations. Exp Brain Res 93: 165–172, 1993. doi: 10.1007/BF00227791. [DOI] [PubMed] [Google Scholar]
- Prablanc C, Echallier JF, Komilis E, Jeannerod M. Optimal response of eye and hand motor systems in pointing at a visual target. I. Spatio-temporal characteristics of eye and hand movements and their relationships when varying the amount of visual information. Biol Cybern 35: 113–124, 1979. doi: 10.1007/BF00337436. [DOI] [PubMed] [Google Scholar]
- Prablanc C, Pélisson D, Goodale MA. Visual control of reaching movements without vision of the limb. I. Role of retinal feedback of target position in guiding the hand. Exp Brain Res 62: 293–302, 1986. doi: 10.1007/BF00238848. [DOI] [PubMed] [Google Scholar]
- Rand MK, Rentsch S. Gaze locations affect explicit process but not implicit process during visuomotor adaptation. J Neurophysiol 113: 88–99, 2015. doi: 10.1152/jn.00044.2014. [DOI] [PubMed] [Google Scholar]
- Rand MK, Rentsch S. Eye-hand coordination during visuomotor adaptation with different rotation angles: effects of terminal visual feedback. PLoS One 11: e0164602, 2016. doi: 10.1371/journal.pone.0164602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redding GM, Wallace B. Adaptive coordination and alignment of eye and hand. J Mot Behav 25: 75–88, 1993. doi: 10.1080/00222895.1993.9941642. [DOI] [PubMed] [Google Scholar]
- Redding GM, Wallace B. Strategic calibration and spatial alignment: a model from prism adaptation. J Mot Behav 34: 126–138, 2002. doi: 10.1080/00222890209601935. [DOI] [PubMed] [Google Scholar]
- Redding GM, Wallace B. Generalization of prism adaptation. J Exp Psychol Hum Percept Perform 32: 1006–1022, 2006. doi: 10.1037/0096-1523.32.4.1006. [DOI] [PubMed] [Google Scholar]
- Saunders JA, Knill DC. Humans use continuous visual feedback from the hand to control fast reaching movements. Exp Brain Res 152: 341–352, 2003. doi: 10.1007/s00221-003-1525-2. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Bo J, Anguera JA. Neurocognitive contributions to motor skill learning: the role of working memory. J Mot Behav 44: 445–453, 2012. doi: 10.1080/00222895.2012.672348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Holcomb HH. Neural correlates of motor memory consolidation. Science 277: 821–825, 1997. doi: 10.1126/science.277.5327.821. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Smith MA, Krakauer JW. Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci 33: 89–108, 2010. doi: 10.1146/annurev-neuro-060909-153135. [DOI] [PubMed] [Google Scholar]
- Shen K, Bezgin G, Selvam R, McIntosh AR, Ryan JD. An Anatomical Interface between Memory and Oculomotor Systems. J Cogn Neurosci 28: 1772–1783, 2016. doi: 10.1162/jocn_a_01007. [DOI] [PubMed] [Google Scholar]
- Shepard RN, Metzler J. Mental rotation of three-dimensional objects. Science 171: 701–703, 1971. doi: 10.1126/science.171.3972.701. [DOI] [PubMed] [Google Scholar]
- Smith MA, Shadmehr R. Intact ability to learn internal models of arm dynamics in Huntington’s disease but not cerebellar degeneration. J Neurophysiol 93: 2809–2821, 2005. doi: 10.1152/jn.00943.2004. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Ivry RB. Flexible cognitive strategies during motor learning. PLOS Comput Biol 7: e1001096, 2011. doi: 10.1371/journal.pcbi.1001096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Ivry RB. Cerebellar and prefrontal cortex contributions to adaptation, strategies, and reinforcement learning. Prog Brain Res 210: 217–253, 2014. doi: 10.1016/B978-0-444-63356-9.00009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Krakauer JW, Ivry RB. Explicit and implicit contributions to learning in a sensorimotor adaptation task. J Neurosci 34: 3023–3032, 2014. doi: 10.1523/JNEUROSCI.3619-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP. A visual salience map in the primate frontal eye field. Prog Brain Res 147: 251–262, 2005. doi: 10.1016/S0079-6123(04)47019-8. [DOI] [PubMed] [Google Scholar]
- Tseng Y-W, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol 98: 54–62, 2007. doi: 10.1152/jn.00266.2007. [DOI] [PubMed] [Google Scholar]
- Werner S, Bock O. Effects of variable practice and declarative knowledge on sensorimotor adaptation to rotated visual feedback. Exp Brain Res 178: 554–559, 2007. doi: 10.1007/s00221-007-0925-0. [DOI] [PubMed] [Google Scholar]
- Wigmore V, Tong C, Flanagan JR. Visuomotor rotations of varying size and direction compete for a single internal model in motor working memory. J Exp Psychol Hum Percept Perform 28: 447–457, 2002. doi: 10.1037/0096-1523.28.2.447. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Diedrichsen J, Flanagan JR. Principles of sensorimotor learning. Nat Rev Neurosci 12: 739–751, 2011. doi: 10.1038/nrn3112. [DOI] [PubMed] [Google Scholar]