Abstract
Stroke often involves primary motor cortex (M1) and its corticospinal projections (CST). As hand function is critically dependent on these structures, its recovery is often incomplete. The neuronal substrate supporting affected hand function is not well understood but likely involves reorganized M1 and CST of the lesioned hemisphere (M1IL and CSTIL). We hypothesized that affected hand function in chronic stroke is related to structural and functional reorganization of M1IL and CSTIL. We tested 18 patients with chronic ischemic stroke involving M1 or CST. Their hand function was compared with 18 age-matched healthy subjects. M1IL thickness and CSTIL fractional anisotropy (FA) were determined with MRI and compared with measures of the other hemisphere. Transcranial magnetic stimulation (TMS) was applied to M1IL to determine its input-output function [stimulus response curve (SRC)]. The plateau of the SRC (MEPmax), inflection point, and slope parameters of the curve were extracted. Results were compared with measures in 12 age-matched healthy controls. MEPmax of M1IL was significantly smaller (P = 0.02) in the patients, indicating reduced CSTIL motor output, and was correlated with impaired hand function (P = 0.02). M1IL thickness (P < 0.01) and CSTIL-FA (P < 0.01) were reduced but did not correlate with hand function. The results indicate that employed M1IL or CSTIL structural measures do not explain the extent of impairment in hand function once M1 and CST are sufficiently functional for TMS to evoke a motor potential. Instead, impairment of hand function is best explained by the abnormally low output from M1IL.
NEW & NOTEWORTHY Hand function often remains impaired after stroke. While the critical role of the primary motor cortex (M1) and its corticospinal output (CST) for hand function has been described in the nonhuman primate stroke model, their structure and function have not been systematically evaluated for patients after stroke. We report that in chronic stroke patients with injury to M1 and/or CST an abnormally reduced M1 output is related to impaired hand function.
Keywords: human, rehabilitation, stroke
INTRODUCTION
In humans, most ischemic strokes occur in the territory of the middle cerebral artery and impact the integrity of the primary motor system [primary motor cortex (M1) and its corticospinal projections (CST)] (Corbetta et al. 2015). As demonstrated in nonhuman primate studies, hand function is dependent on the M1 neurons with direct connections to the spinal α-motoneuron (corticomotoneurons) (Bennett and Lemon 1996; Porter 1985). These corticomotoneuronal fibers make up a functional component of the CST. Lesions to M1, referred to as ipsilesional M1 (M1IL), and/or lesions to CST, referred to as ipsilesional CST (CSTIL), result in a detrimental effect on the function of the hand contralateral to the lesion (Lang and Schieber 2003; Lemon 1997). Despite rehabilitation treatment, compromised hand function often persists and is one of the most common long-term deficits after stroke in humans (Dromerick et al. 2006).
Rodent and nonhuman primate studies on paw/hand motor recovery indicate that the primary motor system of the lesioned hemisphere is critical in supporting hand motor function (Dancause and Nudo 2011). Specifically, expansion of the distal forelimb representation in M1IL is associated with the normalization of hand function in nonhuman primate stroke models (Nudo et al. 1996b) and recovery of independent finger movements is not observed after lesion of the CSTIL (Zaaimi et al. 2012). Previous studies on hand motor task-related brain activation in chronic stroke patients show a positive correlation between greater task-related M1IL activation and extent of impaired hand function (Nair et al. 2007). However, the details of M1 and CST structure and function as they relate to impaired hand function have not been studied in humans. Identifying these critical aspects of the primary motor system could guide the development of new neuromodulation therapies.
One reason why the association between the primary motor system and hand motor function is not understood in greater detail is that stroke recovery research in humans has focused on overall upper extremity (UE) function (Bestmann et al. 2010; Burke Quinlan et al. 2015; Feng et al. 2015; Gauthier et al. 2012; Park et al. 2016; Puig et al. 2010; Schaechter et al. 2006; Sterr et al. 2013; Stinear et al. 2007, 2014; Ward et al. 2003a, 2003b; Zhu et al. 2010). Common tests of UE function include the Frenchay Arm Test, Action Research Arm Test, Motricity Index, Upper-Extremity Fugl-Meyer, and Wolf Motor Function Test (WMFT). The composite scores derived from these tests more closely describe overall UE function rather than hand function because the majority of their subtests require only proximal arm function. This distinction is important because the neural substrates supporting the hand and the UE are different. Specifically, results from nonhuman primate studies demonstrate that muscles of the UE are innervated by multiple motor tracts such as the CST (Lemon 1997), the reticulospinal tract (Riddle et al. 2009; Zaaimi et al. 2012), and the vestibulospinal tract (Markham 1987), while muscles of the hand and finger are predominately innervated by only the CST, which contains the projections from corticomotoneurons (Lemon 1997; Zaaimi et al. 2012). When the CST is permanently lesioned, the monkeys recover the ability to use the upper limb but are left with an inability to move the fingers independently (Lawrence and Kuypers 1968; Zaaimi et al. 2012). Consequently, conclusions drawn from studies on UE function cannot be generalized to hand and finger function.
Of the few human studies that have evaluated the substrates of hand motor function after stroke, most evaluated only M1IL and CSTIL function stimulus response curve (SRC; and/or intracortical inhibition; Nair et al. 2007; Ward et al. 2006) or M1IL and CSTIL structure (lesion load and/or M1 thickness (Jones and Jefferson 2011; Riley et al. 2011). It is difficult to interpret the results of previous studies that have evaluated both the function and structure of M1 and CST as the contributions of M1IL and CSTIL were not evaluated independently from the contributions of the contralesional M1 (M1CL) and CST (CSTCL) (Borich et al. 2015). Since the motor representations in M1IL and M1CL reorganize differently after stroke, the role of M1IL and CSTIL cannot be determined from analyses that collapse both hemispheres into a single measure (Allred and Jones 2008; Touvykine et al. 2016).
Because of the established relationship between anatomy and function in nonhuman primates, we hypothesized that the extent of injury to the function and structure of M1IL and CSTIL is correlated with the extent of impairment in hand and finger function (referred to as hand function) in humans with chronic stroke. Eighteen patients with chronic stroke involving either M1 and/or the CST were studied. Hand motor function was quantified with the Jebsen-Taylor Test (JTT; a standardized test of hand function) and peak acceleration of wrist extension movements (a kinematic measure of hand function). To determine abnormality of this kinematic measure, peak acceleration of the affected hand was compared with measures of the dominant hand from 18 age-matched healthy subjects. The JTT score and peak acceleration were the main outcome measures for affected hand function. Measures of M1IL and CSTIL function and structure were determined using transcranial magnetic stimulation (TMS) and MRI techniques. TMS of M1 results in a synchronized discharge of CST neurons (Amassian and Cracco 1987; Day et al. 1987). When TMS is applied at increasing intensities, a SRC can be plotted. We used the Boltzmann function to calculate three parameters of the SRC that describe the input-output function of M1 in great detail (Capaday 1997; Devanne et al. 1997) and form the main outcome measures in the present analysis: the plateau (MEPmax), inflection point (S50), and the slope. We also used the paired-pulse TMS technique to explore inhibitory neuronal activity in M1(Kujirai et al. 1993). To determine abnormality in M1IL and CSTIL function, TMS measures were compared with measures obtained from a second group of 12 age-matched healthy subjects. M1 and CST structure were characterized by the cortical thickness of M1 and fractional anisotropy (FA) of the CST (Basser 1995; Han et al. 2006). The correlations between affected hand function (JTT score and peak acceleration) and M1IL and CSTIL structure (M1 thickness and FA value) and function (SRC-derived curve parameter) were tested to determine whether affected hand function in chronic stroke is related to structural and functional reorganization of M1IL and CSTIL. Specifically we hypothesized that affected hand function in chronic stroke is impaired when compared with healthy age-matched controls and that extent of impaired hand function in the stroke population is related to M1IL and CSTIL structure and function.
MATERIALS AND METHODS
Subjects
Over a 3-yr period we screened patients of three major hospitals with a total of more than 10,000 admissions for stroke per year to identify, consent, and study 18 patients (10 males; 61.78 ± 11.89 yr; Table 1; Fig. 1A) that met the following inclusion criteria: 1) single ischemic infarction affecting M1 and/or CST more than 6 mo before study enrollment, 2) motor deficit in the hand contralateral to the infarct, 3) no other neurological disorder or aphasia, 4) no contradiction to TMS or MRI, 5) no intake of medication that interfered with TMS measures, 6) a measurable motor-evoked potential (MEP) with TMS of the extensor carpi ulnaris (ECU) hot spot of the affected hemisphere (see below for details), 7) normal cognition, and 8) the ability to give informed consent. Patients were classified as suffering from either cortical or subcortical stroke. Based on visual inspection by a board-certified neurologist (C. Buetefisch) of the T2-weighted MRI of the brain, a cortical stroke was defined as a lesion that involved the cortex with or without involvement of subcortical structures while a subcortical stroke was defined as a lesion affecting the CST without any cortical involvement. Comorbidity was determined from medical records and interview by a board certified neurologist who also determined UE muscle strength and tone using the Medical Research Council Scale (Matthews 1977) and the modified Ashworth Scale (Bohannon and Smith 1987). Normal cognition was confirmed with the Repeatable Battery for the Assessment of Neuropsychological Status (Randolph et al. 1998). Prestroke handedness was determined by the Edinburgh Handedness Inventory (Oldfield 1971). Subjects completed written informed consent before entering the study. The Institutional Review Board of Emory University approved the study.
Table 1.
Characteristics of stroke patients
| Subject | Age | Sex | PSD, mo | Stroke | Edinburgh (LQ) | Dominant Hand | Affected Hand | RBANS (total scale) | MRC (affected UE) | Ashworth | PMH |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 60 | M | 133 | s | −40 | L | L | 78 | 4+ | 3 | HTN |
| 2 | 76 | M | 18 | c | 100 | R | R | 54 | 4+ | 0 | HLD |
| 3 | 63 | F | 18 | c | 100 | R | R | 89 | 4+ | 0 | HTN |
| 4 | 51 | F | 9 | s | 100 | R | R | 89 | 4+ | 1 | |
| 5 | 61 | M | 13 | c | 25 | R | R | 96 | 4+ | 0 | |
| 6 | 67 | F | 7 | s | 100 | R | R | 118 | 4+ | 0 | HTN |
| 7 | 63 | M | 14 | s | 100 | R | R | 89 | 4+ | 1 | |
| 8 | 62 | F | 10 | s | −100 | L | L | 83 | 4 | 0 | HTN, DM |
| 9 | 76 | F | 17 | c | 78 | R | R | 95 | 4+ | 0 | HTN, HLD, DM |
| 10 | 78 | M | 17 | c | 100 | R | L* | 108 | 4 | 1 | |
| 11 | 72 | F | 66 | s | 100 | R | L* | 105 | 3 | 2 | |
| 12 | 55 | F | 10 | s | 100 | R | L* | 86 | 3 | 2 | HTN |
| 13 | 44 | M | 18 | s | 100 | R | R | 104 | 4+ | 0 | |
| 14 | 66 | M | 8 | c | 80 | R | L* | 94 | 4+ | 0 | |
| 15 | 68 | M | 53 | s | −100 | L | L | 100 | 4+ | 0 | |
| 16 | 68 | M | 65 | s | 80 | R | R | 72 | 4+ | 1 | |
| 17 | 32 | M | 84 | c | 71 | R | L* | 104 | 4+ | 0 | |
| 18 | 50 | F | 16 | c | 100 | R | R | 85 | 4+ | 0 | HTN, HLD, pre-DM |
F, female; M, male; PSD, poststroke duration; R, right; L, left; LQ, laterality quotient; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; MRC, Medical Research Council, the MRC for wrist extension movement is reported; UE, upper extremity; c, stroke involved cortex (cortical); s, stroke spared the cortex (subcortical); PMH, past medical history; HTN, hypertension; HLD, hyperlipidemia; DM, diabetes mellitus.
Nondominant hand was affected; dominance of hand refers to the time before the stroke.
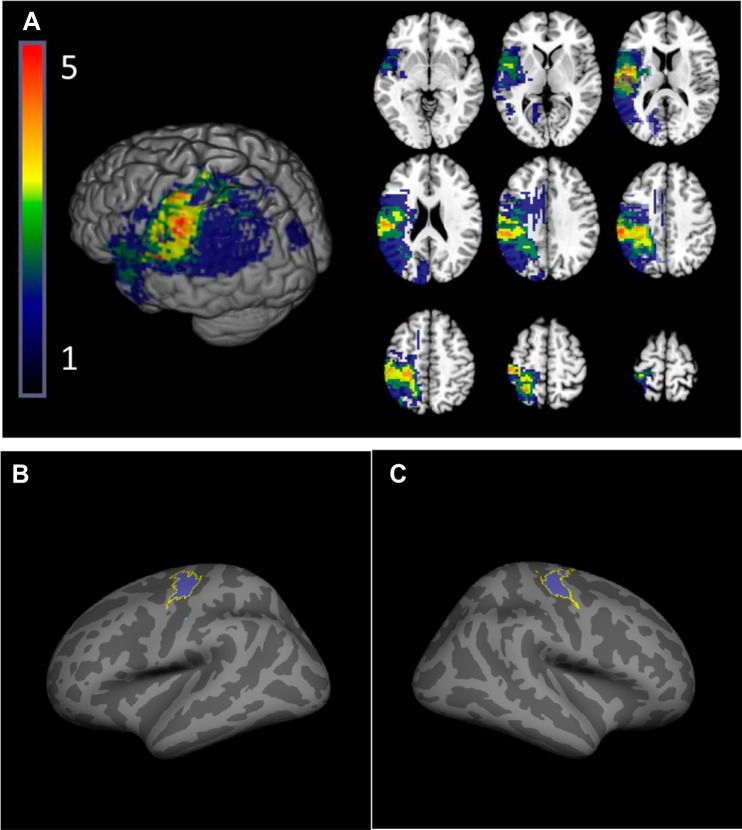
Fig. 1.
Stroke lesion overlap. A: participants’ lesions were normalized to standard space and flipped if necessary to the left hemisphere for display purposes, shown overlaid on the ch2.better.nii brain distributed with MRIcron. Color indicates the number of participants with lesions in each voxel. B and C: for association between primary motor cortex of the lesioned hemisphere (M1IL) structure and hand function, we restricted the extraction of cortical thickness values to the hand area of M1IL using the intersection of anatomically defined BA4 ex vivo label supplied by the FreeSurfer toolkit (Fischl et al. 2008) and a hand task-based functional MRI mask.
Stroke data were compared with data from two groups of age-matched healthy subjects collected in two separate studies. The first group’s (n = 18, aged 62.94 ± 6.98 yr) data were used to determine abnormality in the measures of hand function, and the second group’s (n = 12, aged 61.33 ± 5.47 yr) data were used to determine abnormality for TMS measures of M1 function. The healthy subjects met the same inclusion criteria as the patients except that they had not suffered a stroke and had no motor deficit.
Measures of Affected Hand Function
Affected hand function was quantified using the JTT, a standardized timed measure of hand function (Jebsen et al. 1969), and peak acceleration of wrist extension movements (Buetefisch et al. 2015; Bütefisch et al. 1995; Kesar et al. 2017). Because stroke patients may have difficulty executing selective thumb or finger movements for kinematic measures, wrist movements were chosen to allow testing across a larger range of hand motor impairment. Furthermore, wrist extension is essential for wrist stabilization, which is a prerequisite for hand and finger function, and is weak and recovers poorly in patients and nonhuman primates after stroke involving M1 and CST (Zaaimi et al. 2012). These methodological and clinical considerations are further supported by evidence from nonhuman primate studies of M1 where digit and wrist representation are localized in proximity to one another (Nudo et al. 1996a; Park et al. 2001), have monosynaptic CST projections (Zaaimi et al. 2012), and reorganize in response to a skilled hand training after lesion of the M1 hand area (Nudo et al. 1996b). Peak acceleration was chosen because it depends on rapid recruitment of M1 pyramidal tract neurons and signaling through the CST (Fromm and Evarts 1981). In addition to the main outcome measures of JTT and peak acceleration, patients were also assessed with the WMFT (Wolf et al. 1989, 2001) to characterize proximal and distal UE function and with the Motor Activity Log (MAL) (Uswatte et al. 2006) to determine the impact of compromised hand function on activities of daily living (ADL).
Motor function.
During the JTT, patients completed seven motor tasks as quickly as possible (capped at 120 s) with each hand (Jebsen et al. 1969). For the WMFT, patients completed 15 timed subtests (capped at 120 s) with each arm (Whitall et al. 2006). When the affected arm was tested, movement quality was recorded on a five-point functional ability scale (FAS), where five indicated normal movement. Movement quality was rated by a trained research physical therapist. Maximum grip strength of each hand was also tested using a hand dynamometer.
Movement kinematics.
The kinematic assessment has been described in detail before (Buetefisch et al. 2015; Kesar et al. 2017). Briefly, patients executed five wrist extension movements with the affected hand as quickly as possible in response to an auditory cue. Acceleration in two movement planes (extension/flexion; abduction/adduction) was recorded by an accelerometer mounted on the hand. EMG activity (bandpass: 3 Hz to 1 kHz) was recorded from the ECU muscle, which acts as an agonist in the wrist extension movement. Kinematic and EMG signals were sampled at 1 kHz.
Activities of daily living.
The “How Well” subtest of the MAL was used to assess hand function during ADL (Uswatte et al. 2006). The highest score (5) of the “How Well” subtest indicated that the quality of affected hand movement was the same at the time of testing as before the stroke.
Measures of M1 and CST Function
EMG activity (bandpass: 3 Hz to 1 kHz) was recorded from the ECU muscle with surface electrodes (9-mm diameter) in a belly-tendon montage with a 3-cm distance between electrodes and the active electrode positioned over the motor point and the reference electrode proximal to the motor point. LabVIEW was used for data acquisition (National Instruments). Raw EMG was sampled and digitized at a frequency of 5 kHz and stored for offline analysis.
TMS was applied over the ECU muscle hotspot of M1IL through a figure-of-eight coil (7-cm wing diameter) using two Magstim stimulators connected via a Bistim module (Magstim). To ensure accurate positioning of the coil, the TMS coil was registered to an MRI image of the participant’s brain using a frameless neuronavigation system (BrainSight software; Rogue Research, Montreal, Canada).
The detailed description of data collection for the SRC and short-interval intracortical inhibition (SICI) has been previously reported (Buetefisch et al. 2015; Bütefisch et al. 2008; Kesar et al. 2017). Briefly, at the hot spot of the ECU muscle, the resting motor threshold (rMT), defined as the minimum stimulus intensity to evoke an MEP of >0.05 mV in at least 5 of 10 trials (Rossini et al. 1994), was determined to the nearest 1% of the maximum stimulator output (MSO). For the SRC, TMS pulses were applied at increasing intensities that ranged from at least the nearest 5% of MSO below resting motor threshold (rMT) up to at least 80% MSO in increments of 5% MSO. Blocks of 10 TMS pulses were applied at each intensity with an interstimulus interval (ISI) of 5 s. For SICI, 10 single pulses (TS) at an intensity of 120% rMT were interspersed with 20 paired pulses (pp) where the TS was preceded by a conditioning stimulus (CS) intensity of either 60 or 80% rMT (10 pp for each CS intensity, ISI of 2 ms). SICI was only collected in patients who had MEPs of ~200-μV amplitude or greater (Daskalakis et al. 2002).
Measures of M1 and CST Structure
Cortical thickness and FA served as measures of M1 and CST structure, respectively. Images were obtained on a Siemens 3T Trio scanner using a 12-channel head coil. For T1-weighted imaging, an MPRAGE sequence with the following parameters was used: TR = 2250 ms, TE = 4.18ms, TI = 900 ms, flip angle = 9°, matrix = 256 × 256, FOV = 256 mm, and 176 sagittal slices, resulting in 1 mm3 isotropic voxels. These images were also used to estimate the stroke volume in addition to measuring the cortical thickness of M1. Diffusion tensor imaging data were acquired using a diffusion-weighted EPI sequence with TR = 7.7 s, TE = 90 ms, 60 slices, matrix size = 102 × 102, and FOV = 204 × 204 mm, with an isotropic voxel size of 2.0 mm3. Two averages of 30 noncollinear diffusion directions were collected with a b value of 1,000 s/mm2, along with a reference B0 image. MRI images were collected from 17 out of 18 stroke patients.
Data Analysis
Measures of affected hand function.
motor function.
The JTT raw score (RAW) was calculated by summing the time to complete all but two subtests (writing and simulated feeding). These subtests were omitted because of low test-retest reliability (Stern 1992). The RAW score was normalized to age- and sex-matched standard scores (STD) that accounted for hand dominance using the formula: (RAW – STD)/(RAW + STD) (Hackel et al. 1992; Jebsen et al. 1969). A normalized score greater than zero indicated abnormal hand function. For the WMFT, the total performance time, mean FAS, and mean grip strength were calculated (Wolf et al. 2001).
kinematic measures.
The peak acceleration and direction of the ballistic wrist extension movements were derived from the first-peak acceleration in the two major movement axes (Buetefisch et al. 2015; Bütefisch et al. 2000). Reaction time was defined as time between the movement cue and onset of movement-related EMG in the ECU muscle. The onset of movement-related EMG was defined as the time when mean EMG of a moving 20-ms time window exceeded mean resting EMG by 3 SD (50-ms time window following the movement cue). Trials were discarded when the reaction time was greater than 1,000 ms or movement began before the movement cue.
activities of daily living.
The score for the “How Well” subtest was calculated by averaging the scores for each activity. If participants denied using their affected arm for an activity, it was omitted from the “How Well” subtest score (Uswatte et al. 2006).
Measures of M1 and CST function.
Data were analyzed in LabView. Trials with increased EMG background were excluded from further data analysis. Increased EMG background was defined as EMG amplitude exceeding 50 µV in the 50 ms before the TMS pulse. This criteria resulted in the exclusion of 9.4% of the patients’ SRC measurement trials and 9.6% of the SICI trials. For healthy subjects, 2.9% of trials were excluded for SRC and 5.3% for SICI measures. A minimum of five included trials were required for calculation of the mean and SD at each intensity for each subject; otherwise, the data point was coded as missing data (Buetefisch et al. 2011; Bütefisch et al. 2003, 2008).
The mean peak-to-peak MEP amplitudes for each TMS intensity were plotted for the SRC. The area under the SRC (AUC) was calculated by summing the mean MEP amplitudes evoked by TMS intensities between 35 and 80% MSO. A three-parameter Boltzmann function was fitted to all SRCs that reached a plateau using the Levenberg-Marquard least-squares algorithm to extract three curve parameters: MEPmax (plateau of SRC), S50 (TMS intensity needed to elicit an MEP of an amplitude corresponding to the inflection point), and M (slope) parameter. The M parameter is proportional to the maximum slope of the SRC independent of MEPmax (Devanne et al. 1997). For SICI, the mean peak-to-peak MEP amplitude evoked by the paired pulse (CS/TS) was expressed as a percentage of the mean peak-to-peak MEP amplitude evoked by the TS alone (%MEP). A smaller %MEP indicated greater intracortical inhibition. This was done for CS of 60 and 80% MT separately.
Measures of M1 and CST structure.
m1 thickness.
Cortical reconstruction and volumetric segmentation were conducted for the T1-weighted images using the “recon-all” function of the Freesurfer toolkit version 5.3.0 (http://surfer.nmr.mgh.harvard.edu) (Dale et al. 1999; Fischl et al. 1999). This process includes motion correction, removal of nonbrain tissue, automated Talairach transformation, segmentation of cortical and subcortical structures, intensity normalization, model construction of boundary between cortical grey matter-white matter and pial surface, and topology correction, through which the surface area, volume, Gaussian curvature, mean curvature, folding index, thickness, and thickness standard deviation of the cortical and subcortical structures were derived. The processed images were manually inspected and edited for accuracy. Lesions were accounted for by applying edits to the white matter volume to ensure surfaces conformed to the gray-white boundary. Cortical thickness values were calculated using a series of procedures with the FreeSurfer (FS) imaging toolkit (Dale et al. 1999; Fischl et al. 1999). For comparison of M1 thickness of the affected and nonaffected hemisphere of stroke patients, we used the average cortical thickness for anatomically defined BA4 combining the anterior and posterior portions (Fischl et al. 2008) for each hemisphere (ipsi- and contralesional). For association between M1 structure and hand function, we used the intersection of anatomically defined M1 as described above and a functionally defined region of interest (ROI) (Fig. 1, B and C). First, a voxel-wise mask based on group activation from functional imaging data collected while participants performed a cued wrist extension task (two runs/participant, eleven 15-s task/15-s rest blocks per run, six cued wrist extensions per task block, with functional data from participants with right hemisphere lesions were flipped across the midline axis so that the functional ROI resulted from data from all participants regardless of lesion location) was extracted using a P threshold of 0.001 and was registered to the FS common atlas space (fsaverage). Active voxels from the mask (“1”) were then mapped to the cortical surface of the atlas on a per hemisphere basis. With the use of embedded FS algorithms, surface-based ROIs were generated by selecting vertices that overlapped between the task-based functional (f)MRI mask and the BA4 exvivo label supplied by the FS toolkit. These overlap ROIs were then mapped to each subject and used as labels to restrict extraction of cortical thickness values more specific to the hand area of M1.
fractional anisotropy.
Diffusion-weighted images (DWI) were processed using TRACULA, an automated method available through FS 5.3.0 (http://surfer.nmr.mgh.harvard.edu). While TRACULA can be used to reconstruct 18 major white matter pathways, this study focused on the CST. After the initial reconstruction and labeling of the cortical and subcortical regions through FS on the T1-weighted images, the tractography consists of three steps: 1) preprocessing, 2) pathway diffusion model validation, and 3) three-dimensional pathway reconstruction (Yendiki et al. 2011). The preprocessing step uses the anatomical segmentation obtained in the reconstruction step to correct for motion, eddy current effects, and B0 distortion in the DWI. The second step of TRACULA uses FSL’s (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) bedpost tool to fit the ball-and-stick model of diffusion to the DWI data, returning the reconstructed white matter pathways as outputs. The final step of TRACULA is performed through the fitting of the tracts’ shape to the outputs from the second step, and an atlas of healthy manually labeled subjects. We used the weighted mean FA value over the entire CST for each hemisphere.
lesion volume.
Lesion volume was calculated by dividing the volume of the lesion mask, hand drawn in MRIcron (http://people.cas.sc.edu/rorden/mricron/index.html), by the volume of the whole brain mask extracted during skull stripping in AFNI (Cox 1996).
Statistical Analysis
The specifics of statistical testing are listed with each measure in results. According to our stated hypothesis, one-sided t-tests were used to determine whether the kinematic measures (peak acceleration) or were impaired relative to healthy control’s hand performance. One-sided, one-sample t-tests was used to compare the clinical scores (JJT) of affected hand of stroke patients to healthy, age- and gender-matched scores. Other kinematic and clinical measures were explored in a similar way. We used the F-test to explore the variances of these measures. Simple regression analyses were conducted to explore the association between hand kinematics (peak acceleration) and hand motor function (JTT score). According to our stated hypothesis, one-sided t-tests were used to determine whether the TMS measures MEPmax, S50, and M parameter differed from normal. Other TMS measures were explored in a similar way. Bivariate correlation analysis and multiple linear regression analyses were conducted to test our hypothesis of an association between main outcome measures of hand kinematics (peak acceleration), hand motor function (JTT score), and measures of M1 and CST function (MEPmax, S50, and slope) and structure (M1 thickness and CST FA). Associations between hand function and other secondary measures (SICI, rMT, stroke volume, and MAL) were also explored in secondary analyses using multiple linear regression. Separate repeated measures ANOVAs and t-tests were performed to determine the effect of stroke location on measures of M1 and CST structure. According to our stated hypothesis, one-sided t-tests were used to determine whether the M1 thickness and CST FA measures were smaller in the affected compared with the nonaffected hemisphere of stroke patients. One- sided t-tests were used to test whether M1IL was thinner with cortical stroke location compared with subcortical. Alpha was set to 0.05.
RESULTS
The specifics of statistical testing and the number of subjects included in each analysis are listed with each measure in the result section and Tables 2–4.
Table 2.
Summary of measures describing hand motor function
| Motor Function, Motor Kinematics, and Activities of Daily Livging |
||||||
|---|---|---|---|---|---|---|
| Stroke aff hand | Stroke nonaff hand | Healthy dominant hand | Standard score | Student's t-test aff/nonaff | Student's t-test aff/standard score | |
| Motor function | ||||||
| JTT, normalized total time | 0.49 ± 0.24 (18) | 0.16 ± 0.11 (18) | 0 = normal function | P < 0.01; t(17) = 5.84* | P < 0.01; t(17) = 8.66* | |
| WMFT, total time, s | 179.60 ± 260.10 (18) | 34.91 ± 29.44 (18) | P = 0.01; t(17) = 2.42* | |||
| WMFT, FAS | 3.91 ± 0.67 (18) | 5 = normal function | P < 0.01; t(15) = 6.54* | |||
| WMFT, grip strength, kg | 21.15 ± 11.62 (18) | 33.37 ± 14.92 (18) | P < 0.01; t(17) = 6.19* | |||
| Stroke aff hand | Stroke nonaff hand | Healthy dominant hand | Standard score | Student's t-test aff/healthy | F-test aff/healthy | |
| Motor kinematics | ||||||
| Peak wrist acceleration, g | 0.66 ± 0.40 (18) | 1.38 ± 0.30 (18) | P < 0.01; t(31.43) = 6.02* | P = 0.24; F(17,17) = 180 | ||
| Angle,° | 124.20 ± 24.19 (18) | 128.10 ± 9.10 (18) | P = 0.26; t(21.95) = 0.648 | P < 0.01; F(17,16) = 7.065* | ||
| Reaction time, ms | 221.30 ± 70.41 (18) | 184.60 ± 34.98 (18) | P = 0.03; t(34) = 1.98* | P < 0.01; F(17,17) = 4.05* | ||
| Stroke aff hand | Stroke nonaff hand | Healthy dominant hand | Standard score | Student's t-test aff/nonaff | Student's t-test aff/standard score | |
| Activites of daily living | ||||||
| MAL, “How Well” subtest | 3.80 ± 0.96 (18) | 5 = normal function | P < 0.01; t(17) = 7.58* | |||
| 1 = no contribution | P < 0.01 t(17) = 14.45* | |||||
Measures are reported as means ± SD (n); t-tests are reported as t(df) = t-value; and F-tests are reported as F(Dfn, DFd) = F-value. WMFT, Wolf Motor Function Test; MAL, Motor Activity Log; Dfn, the degree of freedom for the stroke subjects; DFd, for the healthy subjects; aff, affected; JTT, Jebsen-Taylor Test; FAS, functional ability scale;
indicates significant results.
Table 4.
Multiple linear regression analysis testing the association between the measures of M1 and CST structure and function with the measures of affected hand function
| Covariate |
|||||
|---|---|---|---|---|---|
| Dependent/Independent Variable | n | None | Age | PSD | Lesion Volume |
| Peak wrist acc. | |||||
| rMT | 18 | −0.002 [−0.012, 0.008] (0.754) | −0.001 [−0.013, 0.010] (0.812) | −0.001 [−0.012, 0.009] (0.799) | 0.002 [−0.010, 0.014] (0.730) |
| AUC | 18 | 0.030 [−0.064, 0.124] (0.544) | 0.028 [−0.084, 0.141] (0.596) | 0.024 [−0.078, 0.127] (0.619) | 0.033 [−0.102, 0.167] (0.610) |
| MEPmax | 15 | 0.048 [−0.529, 0.624] (0.874) | 0.047 [−0.638, 0.732] (0.884) | 0.036 [−0.606, 0.677] (0.905) | −0.052 [−0.732, 0.627] (0.868) |
| M parameter | 13 | 0.745 [−1.215, 2.705] (0.472) | 0.926 [−1.447, 3.299] (0.405) | 0.396 [−1.958, 2.749] (0.716) | 0.622 [−1.523, 2.767] (0.528) |
| S50 | 15 | −0.012 [−0.029, 0.006] (0.213) | −0.013 [−0.034, 0.009] (0.217) | −0.011 [−0.031, 0.008] (0.231) | −0.004 [−0.025, 0.017] (0.691) |
| SICI CS60 | 10 | 0.001 [−0.007, 0.010] (0.746) | −0.000 [−0.015, 0.014] (0.943) | 0.001 [−0.010, 0.011] (0.867) | 0.001 [−0.009, 0.011] (0.818) |
| SICI CS80 | 10 | 0.004 [−0.007, 0.015] (0.449) | 0.005 [−0.018, 0.027] (0.618) | 0.004 [−0.010, 0.018] (0.536) | 0.004 [−0.011, 0.019] (0.565) |
| M1 hand | 17 | 0.004 [−0.638, 0.647] (0.989) | 0.017 [−0.744, 0.778] (0.962) | 0.109 [−0.640, 0.859] (0.759) | 0.157 [−0.611, 0.924] (0.668) |
| FA | 15 | 1.138 [−2.284, 4.560] (0.526) | 1.435 [−2.570, 5.439] (0.450) | 3.412 [−1.234, 8.058] (0.136) | 0.588 [−3.308, 4.484] (0.748) |
| Jebsen-Taylor Test | |||||
| rMT | 18 | 0.001 [−0.004, 0.007] (0.654) | 0.002 [−0.005, 0.009] (0.582) | 0.001 [−0.005, 0.008] (0.684) | 0.004 [−0.004, 0.012] (0.299) |
| AUC | 18 | −0.016 [−0.072, 0.039] (0.575) | −0.022 [−0.087, 0.044] (0.491) | −0.015 [−0.077, 0.048] (0.623) | −0.073 [−0.150, 0.004] (0.060) |
| MEPmax | 15 | −0.216 [−0.533, 0.102] (0.206) | −0.245 [−0.613, 0.123] (0.173) | −0.212 [−0.575, 0.151] (0.227) | −0.459 [−0.829, −0.090] (0.019)* |
| M parameter | 13 | 0.345 [−0.671, 1.360] (0.520) | 0.071 [−0.947, 1.090] (0.879) | 0.584 [−0.587, 1.755] (0.293) | 0.118 [−1.169, 1.404] (0.841) |
| S50 | 15 | 0.003 [−0.008, 0.014] (0.589) | 0.004 [−0.009, 0.017] (0.506) | 0.003 [−0.010, 0.015] (0.617) | 0.004 [−0.010, 0.019] (0.528) |
| SICI CS60 | 10 | 0.000 [−0.004, 0.004] (0.984) | −0.002 [−0.008, 0.004] (0.523) | 0.000 [−0.005, 0.005] (0.958) | −0.001 [−0.004, 0.003] (0.661) |
| SICI CS80 | 10 | −0.000 [−0.005, 0.005] (0.954) | −0.004 [−0.014, 0.005] (0.351) | −0.000 [−0.007, 0.007] (0.976) | −0.002 [−0.007, 0.002] (0.239) |
| M1 hand | 17 | −0.260 [−0.647, 0.128] (0.209) | −0.253 [−0.713, 0.206] (0.257) | −0.320 [−0.774, 0.133] (0.152) | −0.240 [−0.720, 0.240] (0.302) |
| FA | 15 | −0.267 [−2.596, 2.062] (0.826) | −0.220 [−2.993, 2.553] (0.866) | −1.626 [−4.870, 1.619] (0.296) | −0.461 [−3.218, 2.297] (0.722) |
The regression coefficient, the confidence interval, and the P values are given {coefficient [CI]; (P)}. Each P value corresponds to the significance of a simple linear regression analysis (no covariate) or multiple linear regression analysis (single covariate) testing the association between the dependent and independent variable. The sample size (n) used for the linear regression analyses of each dependent and independent variable pair is reported. SRC parameters were maximum motor-evoked potential [MEPmax (plateau)], M parameter (proportional for the maximum slope), and S50 (transcranial magnetic stimulation intensity that evokes an MEP amplitude of half MEPmax). rMT, resting motor threshold; AUC, area under the SRC; SICI, short-interval intracortical inhibition; M1 hand, hand area of primary motor cortex; FA, fractional anisotropy of the ipsilesional corticospinal tract; PSD, poststroke duration. CS60 and CS80, conditioning stimulus intensity of either 60 or 80% rMT.
P < 0.05, significant results.
Measures of Hand Function
Motor kinematics.
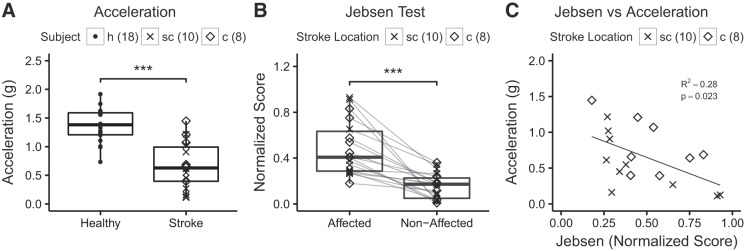
Patients’ kinematic data from the affected hand were compared with the dominant hand performance of 18 age-matched healthy subjects using unpaired one-tailed t-tests (Table 2). The mean peak wrist acceleration was reduced in patients when compared with healthy subjects (P < 0.01, Fig. 2A).
Fig. 2.
Measures of hand motor function. A: peak acceleration of wrist extension movements was significantly lower in stroke patients [displayed separately for subcortical (sc) and cortical (c) location of stroke] when compared with healthy subjects (h) (***P < 0.01, unpaired one-tailed t-tests). B: normalized Jebsen-Taylor Test score data for the affected hand was significantly higher when compared with the nonaffected hand (***P < 0.01, unpaired one-tailed t-tests). Note that this is a time based test where higher scores indicate worse performance. C: relationship between peak acceleration and normalized Jebsen-Taylor Test score. For the correlation analysis, the R2 and P value are given. The number of subjects is indicated. Location of stroke is indicated but statistical testing was done across all stroke patients and healthy subjects.
Motor function.
The results of the statistical analyses for hand/arm motor function are summarized in Table 2. As expected, in one-tailed t-tests the normalized JTT scores for the affected hand were greater than 0 (P < 0.01, Fig. 2B). Deficits in the WMFT were determined by comparing performance of the affected and nonaffected arm using paired one-tailed t-tests because standard scores did not encompass our patients’ age range. Grip strength (P < 0.01) and performance speed (P = 0.01) in the affected hand were reduced and FAS was abnormal (P < 0.01).
Activities of daily living (MAL).
Using one-sample, one-tailed t-tests, we determined that patients used the affected arm less effectively during ADL as indicated by a “How Well” subtest score less than 5 (Table 2, P < 0.01). Even though patients self-reported motor deficits, the affected arm still had functional contribution since the “How Well” subtest scores were greater than one, the minimum score indicating use (Table 2, P < 0.01).
Bivariate correlation analyses were conducted to test the association between hand kinematics, hand motor function, and use in ADL. There was a correlation between peak wrist acceleration and the JTT scores suggesting that this kinematic measure of wrist extension movement is related to functional aspects of hand use (P = 0.02, Fig. 2C). JTT scores were also associated with self-reported function of the affected hand for ADL as they were correlated with the “How Well” (P < 0.01) subtest of the MAL.
TMS Measures of M1 and CST Function
To determine the impact of stroke on M1 function, the TMS measures of M1IL function (rMT, SRC, AUC, MEPmax, M parameter, S50, and SICI) of the stroke patients were compared with measures collected from M1 of the dominant hemisphere of 12 healthy age-matched subjects (Table 3). In 3 out of 18 stroke subjects and 1 out of 12 healthy subjects, TMS elicited only small MEPs at high stimulator output intensities. In addition, the M parameter of 2 of the 15 stroke patients and 1 of the 11 healthy subjects exceeded 3 SD of the mean and were subsequently excluded (Table 3). Data from these four subjects were not included in the analysis of the curve parameters (Table 3). For SICI measures, 10 of 18 stroke subjects and 11 of 12 healthy subjects had MEP amplitudes >0.1 mV in response to a single test pulse and were included in the final analysis (Daskalakis et al. 2002). According to our hypothesis, we expected the rMT, S50, and SICI expressed as %MEP to be greater and the AUC, MEPmax, and M parameter to be smaller for the stroke than healthy subjects, so unpaired one-tailed t-tests were used. Unpaired one-tailed t-tests were also used for the remaining comparisons.
Table 3.
Measures of M1 and CST function and structure
| Stroke, All (Ipsi-) | Stroke, All (Contra-) | Healthy | t-Test Ispi-/Contra- | t-Test Ipsi-/Healthy | F-Test Ipsi-/Healthy | Stroke, Cort (Ipsi-) | Stroke, Subcort (ipsi-) | t-Test Cort/Subcort | |
|---|---|---|---|---|---|---|---|---|---|
| M1 function | |||||||||
| rMT, %MSO | 67.44 ± 19.88 (18) | 53.17 ± 8.310 (12) | P = 0.01; t(28) = 2.34* | P < 0.01; F(17,11) = 5.72* | 60.50 ± 18.61 | 73.00 ± 20.01 | P = 0.19; t(16) = 1.36 | ||
| AUC, mV | 1.63 ± 2.075 (18) | 3.65 ± 3.11 (12) | P = 0.02; t(28) = 2.14* | P = 0.13; F(11,17) = 0.13 | 60.50 ± 18.61 | 73.00 ± 20.01 | P = 0.15; t(15) = 1.34 | ||
| MEPmax, mV | 0.40 ± 0.399 (15) | 0.87 ± 0.70 (11) | P = 0.02; t(24) = 2.21* | P = 0.06; F(10,14) = 3.05 | 0.327 ± 0.349 | 0.47 ± 0.47 | P = 0.25; t(13) = 0.70 | ||
| M parameter | 0.26 ± 0.13 (13) | 0.28 ± 0.13 (10) | P = 0.36; t(21) = 0.36 | P = 0.93; F(9,12) = 1.03 | 0.303 ± 0.158 | 0.23 ± 0.09 | P = 0.15; t(11) = 1.08 | ||
| S50, %MSO | 65.07 ± 12.29 (15) | 58.66 ± 9.62 (11) | P = 0.07; t(24) = 1.50 | P = 0.44; F(14,10) = 1.63 | 61.16 ± 12.73 | 68.49 ± 11.60 | P = 0.13; t(13) = 1.17 | ||
| SICI, CS60, %MEP | 82.79 ± 31.40 (10) | 62.23 ± 30.70 (12) | P = 0.08; t(20) = 1.47 | P = 0.54; F(11,9) = 0.96 | 81.21 ± 38.93 | 85.15 ± 20.50 | P = 0.43; t(8) = 0.18 | ||
| SICI CS80, %MEP | 78.51 ± 22.30 (10) | 59.73 ± 27.17 (12) | P = 0.05; t(20) = 1.71 | P = 0.29; F(11,9) = 2.05 | 75.89 ± 28.51 | 81.43 ± 10.60 | P = 0.36; t(8) = 0.37 | ||
| Ml structure | |||||||||
| Lesion volume, % | 1.11 ± 1.45 (17) | N/A | N/A | 2.39 ± 1.49 | 0.21 ± 0.33 | P < 0.01; t(15) = 4.51* | |||
| Ml thickness, mm | 2.31 ± 0.23 (17) | 2.43 ± 0.19 (17) | P < 0.04;t(16) = 1.84* | 2.13 ± 0.22 | 2.44 ± 0.12 | P < 0.01; t(15) = 3.64* | |||
| FA of CST | 0.46 ± 0.06 (15) | 0.48 ± 0.04 (15) | P < 0.01;t(14) = 3.09* | 0.47 ± 0.06 | 0.44 ± 0.05 | — |
Measures are reported as means ± SD (n). Stimulus response curve (SRC) parameters were maximum motor-evoked potential [MEPmax (plateau)], M parameter (proportional for the maximum slope), and S50 (transcranial magnetic stimulation intensity that evokes an MEP amplitude of half MEPmax). Ipsi, ipsilesional; Contra, contralesional; rMT, resting motor threshold; AUC, area under the SRC; SICI, short-interval intracortical inhibition; M1, primary motor cortex; FA, fractional anisotropy, CST, corticospinal tract; CS60 and CS80, conditioning stimulus intensity of either 60 or 80% rMT; MSO, maximum stimulator output;
indicates significant results.
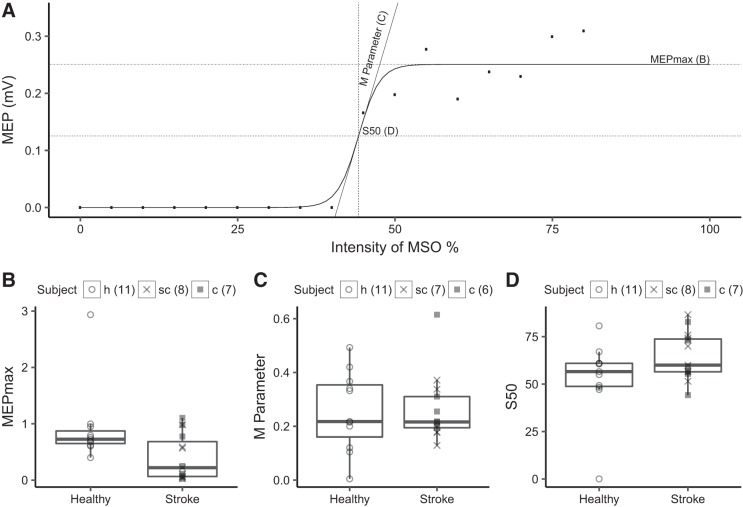
Analysis of the curve parameters demonstrated differences in the input-output properties of CST between M1IL and healthy M1 (Fig. 3). Specifically, the calculated MEPmax was statistically significantly smaller (P = 0.02) for M1IL than healthy M1. The rMT was higher (P = 0.01) and the AUC was lower (P = 0.02) for M1IL when compared with healthy M1 indicating a reduction in corticospinal excitability after stroke.
Fig. 3.
Transcranial magnetic stimulation (TMS) measures of primary motor cortex of the lesioned hemisphere (M1IL). A: the stimulus response curve (SRC) evoked by TMS of extensor carpi ulnaris hot spot at 35–80% maximum stimulator output (MSO) intensity for a single stroke patient. We extracted 3 SRC parameters: the plateau of the curve, which is the maximum motor-evoked potential (MEPmax; B), the M parameter (C), and the inflection point of the curve (S50; D) for 15 of the 18 stroke and 11 of the 12 healthy subjects. The number of subjects for each parameter is indicated. B: MEPmax was statistically significant lower in stroke than in healthy subjects (P = 0.02) indicating that maximum corticospinal projection output of surviving neurons was reduced. C and D: there was no statistically significant difference in M parameter and S50 between stroke and healthy subjects (see Table 3 for details of the statistical testing).
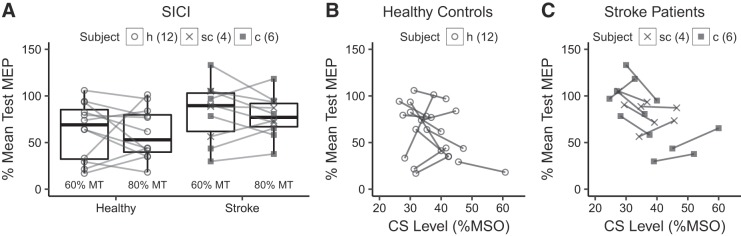
SICI was significantly weaker in M1IL than healthy M1 when tested at CS of 80% (P = 0.05). This tendency was also seen when tested at CS of 60% but did not reach the level of statistically significance (P = 0.08, Fig. 4A). When intensity of CS is plotted against its inhibitory effect on the test MEP patients with high rMT (corresponding to higher CS intensity), inspection of the graph demonstrates less inhibitory effect at CS of 80% MT when compared with CS at 60% MT. The lower CS intensity of 60% MT corresponded to stimulator output intensities of 35–45% MSO (Fig. 4B). This is consistent with the notion that after stroke the relationship between rMT and CS intensity that produces maximum inhibition is weak and that CS intensity of 80% MT may be too high to capture lower threshold inhibitory interneurons (Bütefisch et al. 2003). None of the measures of M1IL function differed between cortical and subcortical stroke patients (Table 3).
Fig. 4.
A: short-interval intracortical inhibition (SICI) was weaker in primary motor cortex of the lesioned hemisphere (M1IL) than healthy M1 when tested at a conditioning stimulus (CS) of 80% (P = 0.051) but not at CS of 60% (P = 0.08). B and C: in these scatterplots of healthy controls (B) and stroke patients (C) SICI is plotted against the CS intensity expressed as %maximum stimulator output (MSO). For each subject, the 2 measures of SICI are shown corresponding the CS of 60 and 80% motor threshold (MT). Note that in stroke patients higher CS intensities result in less inhibition consistent with the notion that after stroke, the relationship between MT and CS intensity that produces maximum inhibition is weak and that CS intensity of 80% MT may be too high to capture the effects of lower threshold inhibitory interneurons (Bütefisch et al. 2003). The number of subjects is indicated. Location of stroke is indicated, but statistical testing was done across all stroke patients and healthy subjects. MEP, motor-evoked potential; c, cortical stroke location; sc, subcortical location; h, healthy.
MRI Measures of M1 and CST Structure
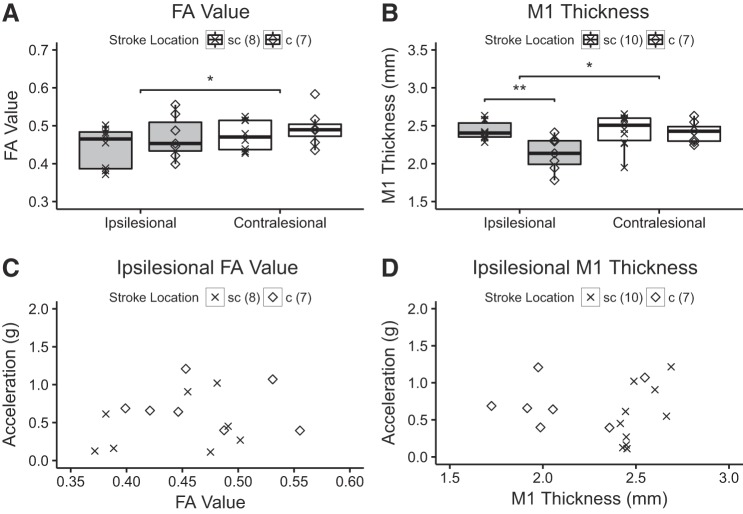
To determine whether stroke had an impact on the MRI-based measures of M1 and CST structure, the effects of stroke location on measures of M1 and CST structure were determined using two separate repeated measures ANOVAs with effect of hemisphere (ipsilesional/contralesional) and stroke location (cortical/subcortical) as independent variables and M1 thickness or CST-FA as the dependent variable (Fig. 5). There was an effect of hemisphere [P = 0.01, F(1,13) = 8.79] but not stroke location [P = 0.34, F(1,13) = 0.98] or interaction between hemisphere and stroke location [P = 0.72, F(1,13) = 0.14] on the CST-FA value. Post hoc testing with a one-tailed t-test found that the CSTIL-FA value was lower than the CSTCL-FA value (P < 0.01), indicating that this measure was sensitive in detecting the stroke related structural changes on CST. For M1 thickness, the effect of hemisphere was close to significance [P = 0.054, F(1,15) = 4.34] and statistically significant for stroke location [P = 0.03, F(1,15) = 5.72]. The interaction between hemisphere and stroke location was statistically significant [P = 0.03, F(1,15) = 5.75]. Post hoc testing with a one-tailed t-test demonstrated that M1IL was thinner than M1CL when collapsed across stroke location (P < 0.04). In the affected hemisphere, M1IL was significantly thinner in patients with cortical than subcortical stroke locations (P < 0.01). The measures of M1IL and CSTIL structure are summarized in Table 3. As expected, the lesion volume was smaller in patients with subcortical stroke than cortical stroke (one-tailed t-test, P < 0.01).
Fig. 5.
Measures of primary motor cortex (M1) and corticospinal projection (CST) structure and hand function. A: the fractional anisotropy (FA) value of stroke patients is significantly lower when compared with healthy subjects (*P < 0.01, one-tailed t-test). The location of stroke is indicated, but comparison was done across the entire group. There was no difference for FA value between cortical and subcortical location of the stroke. B: the M1 of the affected hemisphere was significantly thinner when compared with M1 of the nonaffected hemisphere (**P < 0.04, one-tailed t-test). For the cortical location of the stroke, M1 was significantly thinner when compared with M1 of patients with subcortical stroke healthy subjects (*P < 0.01, one-tailed t-test). C: ipsilesional FA value. D: ipsilesional M1 thickness. c, Cortical stroke location, sc, subcortical location.
Association Between M1 and CST Function and Structure
The results of the exploratory linear regression analysis did not show any statistically significantly association between the abnormalities in the TMS measures of M1IL function (AUC, M parameter, S50, and MEPmax) and MRI-based measures of thickness of M1IL in the region activated during wrist extension or with CSTIL-FA.
Association Between M1 and CST Function and Affected Hand Function
For the hypothesis testing analysis, SRC-derived curve parameters (MEPmax, S50, and slope) from 15 stroke patients and AUC from 18 stroke patients were available to test the relationship between the measures of M1IL and CSTIL function and the motor function (JTT score) and kinematics (peak acceleration) of the affected hand. We also explored the relationship with other TMS measures (rMT and SICI). Table 4 contains the specific number of subjects used in each test and a summary of the results. Linear regression analysis demonstrated that measures of M1IL function were not statistically significantly linearly correlated with either peak acceleration or the normalized JTT score.
As age (Kelly-Hayes et al. 2003), poststroke duration (Jørgensen et al. 1995), and lesion volume (Schiemanck et al. 2005) affect the variability in hand function after stroke, we explored whether the associations between peak wrist acceleration or JTT score and measures of M1IL function became stronger when these variables were controlled for. Separate exploratory multiple linear regression analysis were performed with measures of M1IL function as the independent variables, JTT or peak acceleration as the dependent variable, and age, poststroke duration, and lesion volume as covariates. The JTT score was correlated with greater MEPmax when lesion volume was controlled for alone (P = 0.02, R2 = 0.41). There were no other significant associations (Table 4).
Association Between M1 and CST Structure and Affected Hand Function
To test the hypothesized relationship between MRI measures of M1IL and CSTIL structure and affected hand function, we examined the association between the thickness of cortex in the portion of M1IL that is active during wrist extension and CSTIL-FA with peak acceleration or JTT score of the affected hand with simple linear correlation analyses. There was no correlation between these measures (Table 4). Exploratory multiple linear regression analysis failed to demonstrate a correlation between peak acceleration or normalized JTT score and M1IL thickness or CSTIL-FA even when age, poststroke duration, and lesion volume were controlled for separately (Table 4).
DISCUSSION
In this study, we tested the hypothesis that chronic stroke-related injury to M1IL or its CSTIL projections is correlated with the extent of impairment in hand and finger function. We found that more normal functional M1 output as quantified with the TMS-derived measure of MEPmax was correlated with more normal hand motor function (normalized JTT score) when stroke volume was controlled. We did not find any strong associations between hand function and primary motor system structural integrity as measured by M1 thickness or CST FA. While this seems to be in contrast to our stated hypothesis that was generated from evidence of nonhuman primate experiments for the importance of M1 and its corticospinal projection for hand function (Lemon 2008; Nudo and Milliken 1996), we consider another explanation for these results. In the present study, all patients had measurable MEPs in response to TMS of M1IL. Considering that the presence of an MEP in response to TMS of M1IL is dependent on the temporal and spatial summation of descending volleys at the level of the α-motoneuron pool (Di Lazzaro et al. 2004), the studied patient sample was biased toward more favorable M1IL function and structure. More specifically, the presence of an MEP in response to TMS indicates that sufficient number of pyramidal tract neurons and their corticospinal projections are present to propagate D and I waves along the M1 output in the CST. As the employed approach measures the FA of the entire CST, the measure may not be sensitive in detecting more subtle changes in the motor CST output for the hand. Furthermore, the employed M1 thickness measure was sensitive in detecting differences in M1 structure between the affected and nonaffected hemisphere but M1 thickness for the affected hemisphere did not relate to the extent of impaired hand function, even when limited to the regions of M1 that are activated by hand and wrist movement during fMRI. We would therefore argue that in chronic stroke patients with a measurable evoked MEP response to TMS, M1IL output, as measured with the TMS-derived parameter MEPmax, best explains affected hand functions while the current M1IL and CSTIL structural measures do not add to the explanation of the extent of impaired hand function. This is consistent with the evidence from nonhuman primate work where M1 and its projection to hand function are the major contributors to hand function while projections from other brain areas, for example, posterior parietal cortex and premotor areas, contribute to a lesser extent (Lemon and Griffiths 2005). These areas also project along the CST and were therefore captured by the FA measure of the present study, limiting the specificity of the FA measure as fibers specifically relevant to M1 function could not be independently isolated. While direct comparison to UE studies is limited, a lack of relationship between M1IL thickness and UE function was also reported for patients suffering from chronic stroke (Jones et al. 2016). In longitudinal studies CSTIL-FA also lacked additional predictive value in the presence of an M1IL TMS-evoked MEP response (Stinear et al. 2007).
Measures of Affected Hand Function
In the present study, patients’ affected hands were impaired in all three categories: movement kinematics, motor function, and function in ADL. These results are consistent with evidence derived from nonhuman primate stroke models where lesions to the M1 hand area result in abnormal movement kinematics and a loss of function in the hand contralateral to the infarct (Dancause et al. 2006; Nudo and Milliken 1996). It is also in line with the notion that the integrity of M1 and CST is important for normal hand function in humans (Lang and Schieber 2003; Schieber et al. 2009). Correlation of peak acceleration of wrist extension movements with measures of hand function and function in ADL suggests kinematic measures are functionally relevant and justifies their use when examining the relationship to brain structure or function.
TMS Measures of M1 and CST Function
We found that rMT was higher in M1IL than in healthy M1, which is consistent with other reports comparing rMT between chronic stroke patients with impaired hand function and lesions to M1 and its corticospinal projections and a healthy population (Bütefisch et al. 2008). Other studies have also reported higher rMT of the ipsilesional M1 when compared with the contralesional M1, but the lack of a healthy control group does not allow identification of the site of abnormality (Borich et al. 2015; Liepert et al. 2000). The previously reported hemispheric difference in rMT with relative higher rMT in M1IL when compared with rMT of M1CL could be attributed to an abnormal decrease in M1IL excitability or an abnormal increase in M1CL excitability because unilateral stroke results in bihemispheric neural reorganization in rodents (Allred and Jones 2008; Touvykine et al. 2016) and humans (Schaechter et al. 2008) (for review, see Dancause et al. 2015). Our observation that M1IL rMT was abnormally high as compared with healthy M1 rMT suggests that the hemispheric difference in rMT found in previous studies is at least partially the result of an abnormal decrease in M1IL excitability. The mechanistic explanation for this finding is unclear. There is no evidence in the structural measures of the present study to support loss of pyramidal tract neurons and their corticospinal projections as an underlying cause because we found no relationship between M1IL thickness or CSTIL-FA and rMT. However, our FA and M1 thickness measures may not be sensitive enough to capture subtle change in the corticomotoneuron fibers and dispersion of I wave propagation along the CST could therefore account for a lower rMT.
When measured at a constant level of motor activity (here, at rest), the three SRC curve parameters (S50, M parameter, and MEPmax) completely characterize the input-output relationship of the M1 corticospinal output. (Devanne et al. 1997). Therefore, a change in one or more parameters indicates a change in the input-output relationship in M1IL and its corticospinal output. The abnormally low MEPmax found here suggests that CST output from M1IL was reduced after stroke. The smaller AUC in M1IL when compared with healthy M1 also supports this notion. Reduced CST output with older age has been reported (Talelli et al. 2008) but cannot explain our findings as the reduction of CST output observed in our sample was statistically different from the CST output in our age-matched control population. As the balance between excitatory and inhibitory activity evoked by high TMS intensities determines maximum CST output, a lower MEPmax in M1IL could be associated with either the reduction of excitatory activity or an increase of inhibitory transmission after stroke (Devanne et al. 1997). Although changes in the curve parameters do not allow discrimination of the location of the change in excitability along the M1 corticospinal projections, the size of the MEP amplitude is related to the generation of TMS-induced I waves (Di Lazzaro et al. 2004). Similar to previous studies (Bütefisch et al. 2003; Manganotti et al. 2008), inhibitory transmission was reduced as evidenced by abnormally lower SICI in M1IL and would therefore not support altered GABAAergic inhibition-related control of I waves (Di Lazzaro et al. 2012) as a mechanism underlying reduced MEPmax. Loss or reduced excitability of pyramidal tract neurons with related reduction in the number of descending volleys may be the more likely cause of the depressed CST output (Devanne et al. 1997). While M1IL was significantly thinner than M1CL, we did not find evidence in the current study to support this notion as there was no significant association between M1IL thickness and amplitude of MEPmax. As temporal summation of successive descending volleys is needed to bring resting α-motoneurones to discharge (Di Lazzaro et al. 2004), the reduction of the number of corticomotoneurons and their projections with subsequent dispersion of I wave propagation along the CST could also account for a decrease in the MEPmax. While FAIL was reduced when compared with FACL there was no evidence for an association between reduced FAIL and MEPmax or any of the other curve parameters. There was no statistically significant difference between M1IL and healthy M1 in the remaining parameters (M parameter and S50) indicating that the threshold and gain of the most excitable elements at the cortical, subcortical, and spinal level were closer to normal (Devanne et al. 1997).
Association Between M1 and CST Function and Affected Hand Function
We found that a greater MEPmax, reflecting more CST output from M1IL, was positively correlated with better hand motor function (JTT) when stroke volume was controlled. As MEPmax is lower in stroke patients than in healthy controls, our results indicate that a more normal CST output from M1IL is associated with better hand motor function. The other TMS measures (rMT, S50, M parameter, and SICI) were not associated with hand function. Comparison with prior TMS studies in chronic stroke patients is limited by the fact that MEPmax was not measured in prior studies (Borich et al. 2015; Thickbroom et al. 2002). While not directly comparable, the results of fMRI studies demonstrated more normal task-related blood oxygenation level-dependent response in M1 in chronic patients with better UE function (Ward et al. 2003b). However, this relationship was not observed consistently as other UE studies did not report the same relationship (Schaechter et al. 2006).
MRI Measures of M1 and CST Structure
FA is a well-established measure of CST integrity that quantifies white matter microstructure (Basser 1995) and is commonly used to detect damage to the CST in chronic stroke (Lindberg et al. 2007) (Schaechter et al. 2008). In the current study, we used FA to assess the integrity of the CST in the studied population and derived two major results.
First, we found that CST integrity was lower in the ipsilesional than contralesional hemisphere as indicated by a lower FA value for CSTIL than CSTCL. Even though many of the patients in the studied population suffered from comorbid diseases (hypertension, diabetes) that could affect white matter integrity, these diseases would have affected the CST bilaterally (Kodl et al. 2008; Stenset et al. 2006). Therefore, these diseases cannot explain the difference in FA between CSTIL and CSTCL. It is also unlikely that dominance of the affected hemisphere accounted for the difference in FA values since dominance-related asymmetries in CST-FA have not been detected in healthy adults (Westerhausen et al. 2007). Consequently, it is most probable that the hemispheric difference in FA reflects stroke-related changes to the CST. As stroke-related changes to the brain are the result of both degeneration and regeneration processes, the mechanisms underlying differences in FA values are likely mixed but cannot be further delineated in the current study (Jones and Jefferson 2011).
Second, CST-FA was similar between subcortical and cortical stroke patients indicating that the integrity of the CSTIL was damaged regardless of lesion location. Impaired structure of CSTIL could have been caused by either the initial cerebrovascular insult or by subsequent related processes such as Wallerian degeneration and retrograde degeneration, processes by which myelin surrounding the axon degrades in response to cell body or axonal injury such as stroke to M1 or CST (Lindberg et al. 2007; Thomalla et al. 2004). Notably, the majority of patients in the current study experienced direct damage to the CSTIL as the ischemic core involved the CSTIL for most patients, even those classified with cortical stroke. The finding is consistent with reports from large-scale studies that most cortical strokes extend subcortically and may explain why cortical and subcortical stroke patients experienced similar levels of CSTIL damage (Corbetta et al. 2015; Kang et al. 2003) and reduced FA along the CST distal to the lesions in patients after stroke (Lindberg et al. 2007; Pierpaoli et al. 2001; Thomalla et al. 2004; Werring et al. 2000).
An alternative measure of CST integrity is CST lesion load. Greater CSTIL lesion load, calculated from the overlap between the infarct and the CSTIL, is associated with poorer UE function after stroke (Feng et al. 2015; Zhu et al. 2010) and has been proposed as a biomarker for UE recovery (Feng et al. 2015). However, given that CSTIL lesion load primarily measures direct CST damage (Feng et al. 2015; Zhu et al. 2010) and CSTIL-FA captures both direct and indirect CST damage (Lindberg et al. 2007), CSTIL-FA was a more appropriate measure of CSTIL integrity for the current study.
Finally, we used cortical thickness to assess M1 structure because M1 thickness is a reliable and replicable measure of M1 neuroanatomy (Han et al. 2006). Consistent with our hypothesis, M1IL was thinner when compared with M1CL, which was more pronounced in patients with cortical stroke than for patients with subcortical stroke. As the ischemic core involved M1IL in patients with cortical stroke, it is not surprising that patients suffering from cortical stroke demonstrated thinner cortex when compared with M1 of the intact hemisphere and the group with subcortical stroke location. As M1IL thickness was smaller in both cortical and subcortical location of the stroke, it is unlikely that the findings are explained by mechanical traction of scar tissue on cortex. For patients with subcortical stroke, thinner M1IL may reflect atrophy in this functionally connected but distant, brain region from the ischemic core (Bidmon et al. 1997; Li et al. 1998). In line with this, a study that compared M1IL thickness to M1 thickness of a healthy brain found reduced M1IL thickness in patients suffering from chronic subcortical stroke (Zhang et al. 2014).
Association Between M1 and CST Structure and Affected Hand Function
We did not find evidence to support our hypothesis that MRI-based measures of M1IL structure were associated with the function of the affected hand. To our knowledge this is the first study to test for this association using hand function. While a positive correlation between bilateral precentral gyrus thickness and hand dexterity was reported for patients in the chronic phase of stroke, the thickness of M1IL and M1CL was not tested separately (Borich et al. 2015). Our results would suggest that this reported correlation was not driven by variation in M1IL thickness. Our conclusion is similar to reports on UE function where M1IL thickness is also not associated with UE function (Jones et al. 2016; Schaechter et al. 2006). The lack of an association may also be related to the presence of a TMS-evoked MEP in all of the studied patients. Because a TMS-evoked MEP can only occur when sufficient number of corticomotoneurons are excited by the TMS pulse, we biased our sample toward patients with relatively preserved corticomotoneurons in M1. Another possible explanation for the lack of correlation between cortical output as measured with TMS of M1 and hand function and cortical thickness is related to the poor correlation between neuronal density and cortical thickness (la Fougère et al. 2011).
CSTIL structure as measured by FA was also not associated with the function of the affected hand. There are two main explanations for this result. First, although we hypothesized that the integrity of CSTIL fibers originating from the M1IL hand area to be associated with function of the hand, the FA measure was taken from the entire CST, which is composed of topographically organized groups of fibers descending from multiple cortical motor structures and projecting to the spinal cord (Dum and Strick 1991). The current FA measure evaluates the integrity of all CST fibers without isolating those that originate in M1 innervate α-motor neurons of the hand (Yendiki et al. 2011). It is therefore conceivable that the same FA value is associated with different hand functions depending on the amount of spared CST fibers originating from the M1 hand area. Therefore, the association between CSTIL structure and hand function may have been lost as a result of the nonspecificity of the applied FA measure.
Second, the lack of an association may also be related to the presence of a TMS-evoked MEP in all of the studied patients. Because a TMS-evoked MEP can only occur when the CST fibers originating from M1 hand area have sufficient integrity, we biased our sample toward patients with relatively preserved CST fibers from M1. We speculate that increases in CSTIL integrity are therefore not proportionally related to increases in affected hand function once the CST fibers originating in M1 hand area are sufficiently intact. This notion would support the aforementioned statement that the same extent of injury to the CST as measured with FA may be associated with the presence or absence of TMS-evoked MEP and different hand function depending on the amount of spared CST fibers originating from the M1 hand area.
This notion is consistent with the finding of one study in chronic stroke patients that the presence of an MEP is predictive of motor recovery and that including FA measures in these patients does not add predictive value (Stinear et al. 2007). In the absence of the knowledge about MEP status (presence or absence of MEP with M1 TMS of the affected hemisphere), a positive relationship between UE function and CSTIL structure was demonstrated (Park et al. 2016). It seems that the FA measure is sensitive enough to the effects of stroke on CSTIL structure as indicated by the significantly smaller FA value when compared with CSTCL but a more detailed analysis of the CST components is necessary to determine the relationship between M1 structure and hand function.
Limitations
There are few limitations to the current study. First, we were unable to distinguish between dominant and nondominant hemispheres due to the sample size. Despite this limitation, we identified abnormality in M1IL and CSTIL function and structure, which is consistent with the literature and supports the notion that the applied methods are sensitive enough to pick up differences between the two study populations. Second, measurements obtained at a single time point do not allow us to distinguish between the regenerative and degenerative processes supporting the manifestation of observed CSTIL and M1IL abnormalities (Jones and Jefferson 2011).
Conclusions
We demonstrate that variability in the extent of impaired hand function in stroke affecting M1 or the CST where there is a measurable MEP is best explained by the abnormally low output from M1IL. Although M1IL and CSTIL structures were impaired, there was no correlation with the extent of impaired hand function. As all study patients had a measurable muscle response to TMS applied to M1IL, we argue that structural measures do not explain variability of hand function once M1 and the pyramidal tract function is sufficient for TMS to evoke a MEP. The generation of a measurable MEP requires temporal and spatial summation of TMS-evoked descending volleys that seem to depend on neuronal circuitries that are also crucial for normal hand function. It remains to be determined whether the variability in hand function may be more strongly related to the M1IL and CSTIL structure in individuals without measurable MEP responses to TMS.
GRANTS
This research was supported by National Institutes of Health Grants R01-HD-052753, R56-NS-070879, R21-HD-067906, and R01-NS-090677 and American Heart Association Grant 15PRE25760023.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.M.B., K.P.R., M.W.H., S.R.B., X.H., D.D., and G.H. conceived and designed research; C.M.B., K.P.R., G.M.K., M.W., and S.R.B. performed experiments; C.M.B., K.P.R., M.W.H., M.W., M.P., S.R.B., F.N., D.J.C., X.H., D.D., and G.H. interpreted results of experiments; C.M.B., K.P.R., D.J.C., and D.D. prepared figures; C.M.B., M.W.H., D.J.C., D.D., and G.H. drafted manuscript; C.M.B., K.P.R., M.W.H., F.N., D.J.C., and G.H. edited and revised manuscript; K.P.R., M.W.H., G.M.K., M.W., M.P., D.J.C., and D.D. analyzed data; C.M.B., K.P.R., M.W.H., G.M.K., M.W., M.P., S.R.B., F.N., D.J.C., X.H., D.D., and G.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank our subjects for participation in this study, Aimee Reiss for assistance with patient recruitment, Marsha Bidgood for assistance with patient assessment, Farrah Rink for assistance with the experiments and Dr. Sebastian Buetefisch for technical support.
REFERENCES
- Allred RP, Jones TA. Maladaptive effects of learning with the less-affected forelimb after focal cortical infarcts in rats. Exp Neurol 210: 172–181, 2008. doi: 10.1016/j.expneurol.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amassian VE, Cracco RQ. Human cerebral cortical responses to contralateral transcranial stimulation. Neurosurgery 20: 148–155, 1987. doi: 10.1097/00006123-198701000-00031. [DOI] [PubMed] [Google Scholar]
- Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed 8: 333–344, 1995. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Bennett KM, Lemon RN. Corticomotoneuronal contribution to the fractionation of muscle activity during precision grip in the monkey. J Neurophysiol 75: 1826–1842, 1996. doi: 10.1152/jn.1996.75.5.1826. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Swayne O, Blankenburg F, Ruff CC, Teo J, Weiskopf N, Driver J, Rothwell JC, Ward NS. The role of contralesional dorsal premotor cortex after stroke as studied with concurrent TMS-fMRI. J Neurosci 30: 11926–11937, 2010. doi: 10.1523/JNEUROSCI.5642-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidmon HJ, Oermann E, Schleicher A, Kato K, Kinscherf R, Buchkremer-Ratzmann I, Witte OW, Zilles K. Copper-zinc superoxide dismutase and isolectin B4 binding are markers for associative and transhemispheric diaschisis induced by focal ischemia in rat cortex. Neurosci Lett 228: 163–166, 1997. doi: 10.1016/S0304-3940(97)00389-3. [DOI] [PubMed] [Google Scholar]
- Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 67: 206–207, 1987. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- Borich MR, Neva JL, Boyd LA. Evaluation of differences in brain neurophysiology and morphometry associated with hand function in individuals with chronic stroke. Restor Neurol Neurosci 33: 31–42, 2015. doi: 10.3233/RNN-140425. [DOI] [PubMed] [Google Scholar]
- Buetefisch C, Heger R, Schicks W, Seitz R, Netz J. Hebbian-type stimulation during robot-assisted training in patients with stroke. Neurorehabil Neural Repair 25: 645–655, 2011. doi: 10.1177/1545968311402507. [DOI] [PubMed] [Google Scholar]
- Buetefisch CM, Howard C, Korb C, Haut MW, Shuster L, Pergami P, Smith C, Hobbs G. Conditions for enhancing the encoding of an elementary motor memory by rTMS. Clin Neurophysiol 126: 581–593, 2015. doi: 10.1016/j.clinph.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke Quinlan E, Dodakian L, See J, McKenzie A, Le V, Wojnowicz M, Shahbaba B, Cramer SC. Neural function, injury, and stroke subtype predict treatment gains after stroke. Ann Neurol 77: 132–145, 2015. doi: 10.1002/ana.24309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bütefisch C, Hummelsheim H, Denzler P, Mauritz KH. Repetitive training of isolated movements improves the outcome of motor rehabilitation of the centrally paretic hand. J Neurol Sci 130: 59–68, 1995. doi: 10.1016/0022-510X(95)00003-K. [DOI] [PubMed] [Google Scholar]
- Bütefisch CM, Davis BC, Wise SP, Sawaki L, Kopylev L, Classen J, Cohen LG. Mechanisms of use-dependent plasticity in the human motor cortex. Proc Natl Acad Sci USA 97: 3661–3665, 2000. doi: 10.1073/pnas.97.7.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bütefisch CM, Netz J, Wessling M, Seitz RJ, Hömberg V. Remote changes in cortical excitability after stroke. Brain 126: 470–481, 2003. doi: 10.1093/brain/awg044. [DOI] [PubMed] [Google Scholar]
- Bütefisch CM, Wessling M, Netz J, Seitz RJ, Hömberg V. Relationship between interhemispheric inhibition and motor cortex excitability in subacute stroke patients. Neurorehabil Neural Repair 22: 4–21, 2008. doi: 10.1177/1545968307301769. [DOI] [PubMed] [Google Scholar]
- Capaday C. Neurophysiological methods for studies of the motor system in freely moving human subjects. J Neurosci Methods 74: 201–218, 1997. doi: 10.1016/S0165-0270(97)02250-4. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Ramsey L, Callejas A, Baldassarre A, Hacker CD, Siegel JS, Astafiev SV, Rengachary J, Zinn K, Lang CE, Connor LT, Fucetola R, Strube M, Carter AR, Shulman GL. Common behavioral clusters and subcortical anatomy in stroke. Neuron 85: 927–941, 2015. doi: 10.1016/j.neuron.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29: 162–173, 1996. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9: 179–194, 1999. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, Zoubina EV, Plautz EJ, Mahnken JD, Nudo RJ. Effects of small ischemic lesions in the primary motor cortex on neurophysiological organization in ventral premotor cortex. J Neurophysiol 96: 3506–3511, 2006. doi: 10.1152/jn.00792.2006. [DOI] [PubMed] [Google Scholar]
- Dancause N, Nudo RJ. Shaping plasticity to enhance recovery after injury. Prog Brain Res 192: 273–295, 2011. doi: 10.1016/B978-0-444-53355-5.00015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancause N, Touvykine B, Mansoori BK. Inhibition of the contralesional hemisphere after stroke: reviewing a few of the building blocks with a focus on animal models. Prog Brain Res 218: 361–387, 2015. doi: 10.1016/bs.pbr.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R. The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol 543: 317–326, 2002. doi: 10.1113/jphysiol.2002.017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Thompson PD, Dick JP, Nakashima K, Marsden CD. Different sites of action of electrical and magnetic stimulation of the human brain. Neurosci Lett 75: 101–106, 1987. doi: 10.1016/0304-3940(87)90083-8. [DOI] [PubMed] [Google Scholar]
- Devanne H, Lavoie BA, Capaday C. Input-output properties and gain changes in the human corticospinal pathway. Exp Brain Res 114: 329–338, 1997. doi: 10.1007/PL00005641. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, Rothwell JC. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol 115: 255–266, 2004. doi: 10.1016/j.clinph.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Profice P, Ranieri F, Capone F, Dileone M, Oliviero A, Pilato F. I-wave origin and modulation. Brain Stimul 5: 512–525, 2012. doi: 10.1016/j.brs.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Dromerick AW, Lang CE, Birkenmeier R, Hahn MG, Sahrmann SA, Edwards DF. Relationships between upper-limb functional limitation and self-reported disability 3 months after stroke. J Rehabil Res Dev 43: 401–408, 2006. doi: 10.1682/JRRD.2005.04.0075. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci 11: 667–689, 1991. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Wang J, Chhatbar PY, Doughty C, Landsittel D, Lioutas VA, Kautz SA, Schlaug G. Corticospinal tract lesion load: An imaging biomarker for stroke motor outcomes. Ann Neurol 78: 860–870, 2015. doi: 10.1002/ana.24510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Rajendran N, Busa E, Augustinack J, Hinds O, Yeo BT, Mohlberg H, Amunts K, Zilles K. Cortical folding patterns and predicting cytoarchitecture. Cereb Cortex 18: 1973–1980, 2008. doi: 10.1093/cercor/bhm225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 9: 195–207, 1999. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fromm C, Evarts EV. Relation of size and activity of motor cortex pyramidal tract neurons during skilled movements in the monkey. J Neurosci 1: 453–460, 1981. doi: 10.1523/JNEUROSCI.01-05-00453.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier LV, Taub E, Mark VW, Barghi A, Uswatte G. Atrophy of spared gray matter tissue predicts poorer motor recovery and rehabilitation response in chronic stroke. Stroke 43: 453–457, 2012. doi: 10.1161/STROKEAHA.111.633255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackel ME, Wolfe GA, Bang SM, Canfield JS. Changes in hand function in the aging adult as determined by the Jebsen Test of Hand Function. Phys Ther 72: 373–377, 1992. doi: 10.1093/ptj/72.5.373. [DOI] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Busa E, Pacheco J, Albert M, Killiany R, Maguire P, Rosas D, Makris N, Dale A, Dickerson B, Fischl B. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage 32: 180–194, 2006. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Arch Phys Med Rehabil 50: 311–319, 1969. [PubMed] [Google Scholar]
- Jones PW, Borich MR, Vavsour I, Mackay A, Boyd LA. Cortical thickness and metabolite concentration in chronic stroke and the relationship with motor function. Restor Neurol Neurosci 34: 733–746, 2016. doi: 10.3233/RNN-150623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Jefferson SC. Reflections of experience-expectant development in repair of the adult damaged brain. Dev Psychobiol 53: 466–475, 2011. doi: 10.1002/dev.20557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen HS, Nakayama H, Raaschou HO, Vive-Larsen J, Støier M, Olsen TS. Outcome and time course of recovery in stroke. Part II: Time course of recovery. The Copenhagen Stroke Study. Arch Phys Med Rehabil 76: 406–412, 1995. doi: 10.1016/S0003-9993(95)80568-0. [DOI] [PubMed] [Google Scholar]
- Kang DW, Chalela JA, Ezzeddine MA, Warach S. Association of ischemic lesion patterns on early diffusion-weighted imaging with TOAST stroke subtypes. Arch Neurol 60: 1730–1734, 2003. doi: 10.1001/archneur.60.12.1730. [DOI] [PubMed] [Google Scholar]
- Kelly-Hayes M, Beiser A, Kase CS, Scaramucci A, D’Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: the Framingham study. J Stroke Cerebrovasc Dis 12: 119–126, 2003. doi: 10.1016/S1052-3057(03)00042-9. [DOI] [PubMed] [Google Scholar]
- Kesar TM, Belagaje SR, Pergami P, Haut MW, Hobbs G, Buetefisch CM. Effects of monoaminergic drugs on training-induced motor cortex plasticity in older adults. Brain Res 1670: 106–117, 2017. doi: 10.1016/j.brainres.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodl CT, Franc DT, Rao JP, Anderson FS, Thomas W, Mueller BA, Lim KO, Seaquist ER. Diffusion tensor imaging identifies deficits in white matter microstructure in subjects with type 1 diabetes that correlate with reduced neurocognitive function. Diabetes 57: 3083–3089, 2008. doi: 10.2337/db08-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol 471: 501–519, 1993. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- la Fougère C, Grant S, Kostikov A, Schirrmacher R, Gravel P, Schipper HM, Reader A, Evans A, Thiel A. Where in-vivo imaging meets cytoarchitectonics: the relationship between cortical thickness and neuronal density measured with high-resolution [18F]flumazenil-PET. Neuroimage 56: 951–960, 2011. doi: 10.1016/j.neuroimage.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Lang CE, Schieber MH. Differential impairment of individuated finger movements in humans after damage to the motor cortex or the corticospinal tract. J Neurophysiol 90: 1160–1170, 2003. doi: 10.1152/jn.00130.2003. [DOI] [PubMed] [Google Scholar]
- Lawrence DG, Kuypers HG. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain 91: 1–14, 1968. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- Lemon R. Mechanisms of cortical control of hand function. Neuroscientist 3: 389–398, 1997. doi: 10.1177/107385849700300612. [DOI] [Google Scholar]
- Lemon RN. Descending pathways in motor control. Annu Rev Neurosci 31: 195–218, 2008. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Griffiths J. Comparing the function of the corticospinal system in different species: organizational differences for motor specialization? Muscle Nerve 32: 261–279, 2005. doi: 10.1002/mus.20333. [DOI] [PubMed] [Google Scholar]
- Li Y, Jiang N, Powers C, Chopp M, Johansson BB. Neuronal damage and plasticity identified by microtubule-associated protein 2, growth-associated protein 43, and cyclin D1 immunoreactivity after focal cerebral ischemia in rats. Stroke 29: 1972–1980, 1998. doi: 10.1161/01.STR.29.9.1972. [DOI] [PubMed] [Google Scholar]
- Liepert J, Bauder H, Wolfgang HR, Miltner WH, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke 31: 1210–1216, 2000. doi: 10.1161/01.STR.31.6.1210. [DOI] [PubMed] [Google Scholar]
- Lindberg PG, Skejø PH, Rounis E, Nagy Z, Schmitz C, Wernegren H, Bring A, Engardt M, Forssberg H, Borg J. Wallerian degeneration of the corticofugal tracts in chronic stroke: a pilot study relating diffusion tensor imaging, transcranial magnetic stimulation, and hand function. Neurorehabil Neural Repair 21: 551–560, 2007. doi: 10.1177/1545968307301886. [DOI] [PubMed] [Google Scholar]
- Manganotti P, Acler M, Zanette GP, Smania N, Fiaschi A. Motor cortical disinhibition during early and late recovery after stroke. Neurorehabil Neural Repair 22: 396–403, 2008. doi: 10.1177/1545968307313505. [DOI] [PubMed] [Google Scholar]
- Markham CH. Vestibular control of muscular tone and posture. Can J Neurol Sci 14, Suppl: 493–496, 1987. doi: 10.1017/S0317167100037975. [DOI] [PubMed] [Google Scholar]
- Matthews WB. Aids to examination of the peripheral nervous system. Medical Research Council Memorandum No. 45. J Neurol Sci 33: 299, 1977. doi: 10.1016/0022-510X(77)90205-2. [DOI] [Google Scholar]
- Nair DG, Hutchinson S, Fregni F, Alexander M, Pascual-Leone A, Schlaug G. Imaging correlates of motor recovery from cerebral infarction and their physiological significance in well-recovered patients. Neuroimage 34: 253–263, 2007. doi: 10.1016/j.neuroimage.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol 75: 2144–2149, 1996. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci 16: 785–807, 1996a. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science 272: 1791–1794, 1996b. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Park CH, Kou N, Ward NS. The contribution of lesion location to upper limb deficit after stroke. J Neurol Neurosurg Psychiatry 87: 1283–1286, 2016. doi: 10.1136/jnnp-2015-312738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MC, Belhaj-Saïf A, Gordon M, Cheney PD. Consistent features in the forelimb representation of primary motor cortex in rhesus macaques. J Neurosci 21: 2784–2792, 2001. doi: 10.1523/JNEUROSCI.21-08-02784.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, Basser P. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage 13: 1174–1185, 2001. doi: 10.1006/nimg.2001.0765. [DOI] [PubMed] [Google Scholar]
- Porter R. The corticomotoneuronal component of the pyramidal tract: corticomotoneuronal connections and functions in primates. Brain Res 357: 1–26, 1985. doi: 10.1016/0165-0173(85)90005-0. [DOI] [PubMed] [Google Scholar]
- Puig J, Pedraza S, Blasco G, Daunis-I-Estadella J, Prats A, Prados F, Boada I, Castellanos M, Sánchez-González J, Remollo S, Laguillo G, Quiles AM, Gómez E, Serena J. Wallerian degeneration in the corticospinal tract evaluated by diffusion tensor imaging correlates with motor deficit 30 days after middle cerebral artery ischemic stroke. AJNR Am J Neuroradiol 31: 1324–1330, 2010. doi: 10.3174/ajnr.A2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol 20: 310–319, 1998. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- Riddle CN, Edgley SA, Baker SN. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci 29: 4993–4999, 2009. doi: 10.1523/JNEUROSCI.3720-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley JD, Le V, Der-Yeghiaian L, See J, Newton JM, Ward NS, Cramer SC. Anatomy of stroke injury predicts gains from therapy. Stroke 42: 421–426, 2011. doi: 10.1161/STROKEAHA.110.599340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijević MR, Hallett M, Katayama Y, Lücking CH, Maertens de Noordhout A, Marsden CD, Murray NMF, Rothwell J, Swash M, Tomberg C. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 91: 79–92, 1994. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Schaechter JD, Moore CI, Connell BD, Rosen BR, Dijkhuizen RM. Structural and functional plasticity in the somatosensory cortex of chronic stroke patients. Brain 129: 2722–2733, 2006. doi: 10.1093/brain/awl214. [DOI] [PubMed] [Google Scholar]
- Schaechter JD, Perdue KL, Wang R. Structural damage to the corticospinal tract correlates with bilateral sensorimotor cortex reorganization in stroke patients. Neuroimage 39: 1370–1382, 2008. doi: 10.1016/j.neuroimage.2007.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber MH, Lang CE, Reilly KT, McNulty P, Sirigu A. Selective activation of human finger muscles after stroke or amputation. Adv Exp Med Biol 629: 559–575, 2009. doi: 10.1007/978-0-387-77064-2_30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemanck SK, Post MW, Witkamp TD, Kappelle LJ, Prevo AJ. Relationship between ischemic lesion volume and functional status in the 2nd week after middle cerebral artery stroke. Neurorehabil Neural Repair 19: 133–138, 2005. doi: 10.1177/154596830501900207. [DOI] [PubMed] [Google Scholar]
- Stenset V, Johnsen L, Kocot D, Negaard A, Skinningsrud A, Gulbrandsen P, Wallin A, Fladby T. Associations between white matter lesions, cerebrovascular risk factors, and low CSF Abeta42. Neurology 67: 830–833, 2006. doi: 10.1212/01.wnl.0000234030.77831.5a. [DOI] [PubMed] [Google Scholar]
- Stern EB. Stability of the Jebsen-Taylor Hand Function Test across three test sessions. Am J Occup Ther 46: 647–649, 1992. doi: 10.5014/ajot.46.7.647. [DOI] [PubMed] [Google Scholar]
- Sterr A, Dean PJ, Vieira G, Conforto AB, Shen S, Sato JR. Cortical thickness changes in the non-lesioned hemisphere associated with non-paretic arm immobilization in modified CI therapy. Neuroimage Clin 2: 797–803, 2013. doi: 10.1016/j.nicl.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain 130: 170–180, 2007. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Byblow WD, Ward SH. An update on predicting motor recovery after stroke. Ann Phys Rehabil Med 57: 489–498, 2014. doi: 10.1016/j.rehab.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Talelli P, Ewas A, Waddingham W, Rothwell JC, Ward NS. Neural correlates of age-related changes in cortical neurophysiology. Neuroimage 40: 1772–1781, 2008. doi: 10.1016/j.neuroimage.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thickbroom GW, Byrnes ML, Archer SA, Mastaglia FL. Motor outcome after subcortical stroke: MEPs correlate with hand strength but not dexterity. Clin Neurophysiol 113: 2025–2029, 2002. doi: 10.1016/S1388-2457(02)00318-8. [DOI] [PubMed] [Google Scholar]
- Thomalla G, Glauche V, Koch MA, Beaulieu C, Weiller C, Röther J. Diffusion tensor imaging detects early Wallerian degeneration of the pyramidal tract after ischemic stroke. Neuroimage 22: 1767–1774, 2004. doi: 10.1016/j.neuroimage.2004.03.041. [DOI] [PubMed] [Google Scholar]
- Touvykine B, Mansoori BK, Jean-Charles L, Deffeyes J, Quessy S, Dancause N. The effect of lesion size on the organization of the ipsilesional and contralesional motor cortex. Neurorehabil Neural Repair 30: 280–292, 2016. doi: 10.1177/1545968315585356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uswatte G, Taub E, Morris D, Light K, Thompson PA. The Motor Activity Log-28: assessing daily use of the hemiparetic arm after stroke. Neurology 67: 1189–1194, 2006. doi: 10.1212/01.wnl.0000238164.90657.c2. [DOI] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain 126: 2476–2496, 2003a. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain 126: 1430–1448, 2003b. doi: 10.1093/brain/awg145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Newton JM, Swayne OB, Lee L, Thompson AJ, Greenwood RJ, Rothwell JC, Frackowiak RS. Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain 129: 809–819, 2006. doi: 10.1093/brain/awl002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werring DJ, Toosy AT, Clark CA, Parker GJ, Barker GJ, Miller DH, Thompson AJ. Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J Neurol Neurosurg Psychiatry 69: 269–272, 2000. doi: 10.1136/jnnp.69.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhausen R, Huster RJ, Kreuder F, Wittling W, Schweiger E. Corticospinal tract asymmetries at the level of the internal capsule: is there an association with handedness? Neuroimage 37: 379–386, 2007. doi: 10.1016/j.neuroimage.2007.05.047. [DOI] [PubMed] [Google Scholar]
- Whitall J, Savin DN Jr, Harris-Love M, Waller SM. Psychometric properties of a modified Wolf Motor Function test for people with mild and moderate upper-extremity hemiparesis. Arch Phys Med Rehabil 87: 656–660, 2006. doi: 10.1016/j.apmr.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A. Assessing Wolf motor function test as outcome measure for research in patients after stroke. Stroke 32: 1635–1639, 2001. doi: 10.1161/01.STR.32.7.1635. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Lecraw DE, Barton LA, Jann BB. Forced use of hemiplegic upper extremities to reverse the effect of learned nonuse among chronic stroke and head-injured patients. Exp Neurol 104: 125–132, 1989. doi: 10.1016/S0014-4886(89)80005-6. [DOI] [PubMed] [Google Scholar]
- Yendiki A, Panneck P, Srinivasan P, Stevens A, Zöllei L, Augustinack J, Wang R, Salat D, Ehrlich S, Behrens T, Jbabdi S, Gollub R, Fischl B. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front Neuroinform 5: 23, 2011. doi: 10.3389/fninf.2011.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaaimi B, Edgley SA, Soteropoulos DS, Baker SN. Changes in descending motor pathway connectivity after corticospinal tract lesion in macaque monkey. Brain 135: 2277–2289, 2012. doi: 10.1093/brain/aws115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Meng L, Qin W, Liu N, Shi FD, Yu C. Structural damage and functional reorganization in ipsilesional m1 in well-recovered patients with subcortical stroke. Stroke 45: 788–793, 2014. doi: 10.1161/STROKEAHA.113.003425. [DOI] [PubMed] [Google Scholar]
- Zhu LL, Lindenberg R, Alexander MP, Schlaug G. Lesion load of the corticospinal tract predicts motor impairment in chronic stroke. Stroke 41: 910–915, 2010. doi: 10.1161/STROKEAHA.109.577023. [DOI] [PMC free article] [PubMed] [Google Scholar]