Abstract
Background
Data collection has gained a great importance in numerous areas in the last years and also in the medical field. Collecting data is the key to knowledge and consequently improving data quality is fundamental, as the results of the data analysis can have a large impact on the clinical practice.
Methods
Collected data can be employed to assess the performance of surgeons or institutions and to implement hospital´s performance and productivity. The chest wall database is one of the satellites composing the European Society of Thoracic Surgery (ESTS) database and includes data on risk factors, surgical techniques, processes of care and outcomes related to chest wall pathologies. The participation to the registry is free and voluntary for the ESTS members. The ESTS chest wall database includes data on risk factors, surgical techniques, processes of care and outcomes related to chest wall pathologies. The collected data are designed for quality control and performance audit. Acquired data are anonymous, independently accessed and encrypted on a Dendrite platform, which provides data security and regular backups. The registry is managed by an external company (KData Clinicak Srl), which works together with the database committee in revising and updating periodically the database.
Results
The ESTS chest wall database is structured in four main sections: preoperative, intraoperative, postoperative and follow up. For each procedure registered in the database are collected a number of different variables regarding the patients’ characteristics, the surgical technique, the postoperative course until the discharge and also follow up data. Correction of pectus excavatum is the most common procedures registered in 2017 (392 patients, 67% of all data), followed by pectus bar removal (159 patients, 27% of all procedures).
Conclusions
The ESTS chest wall database is an ambitious European project, which aims to standardize all chest wall procedures in all their aspects.
Keywords: European Society of Thoracic Surgery database (ESTS database), ESTS chest wall, big data
Introduction
Data collection has gained a great importance in numerous areas in the last years and also in the medical field. Collecting data is the key to knowledge and consequently improving data quality is fundamental, as the results of the data analysis can have a large impact on the clinical practice (1,2). Collected data can be employed to assess the performance of surgeons or institutions and to implement hospital’s performance and productivity.
The European Society of Thoracic Surgery (ESTS) database is a multi-institutional and international registry, where the data are collected using a protected online platform (https://ests.kdataclinical.it) (3). To date, up to 15,000 new cases are registered in the database annually from 24 different countries, in details from 170 European and 15 non-European thoracic surgery units (4).
The chest wall database is one of the satellites composing the ESTS database and it collects data about the whole spectrum of chest wall diseases, like tumors, traumas or malformations (Table 1).
Table 1. Spectrum of diseases managed in the ESTS chest wall database.
| Congenital chest wall defects |
| Pectus excavatum (Nuss and Ravitch procedure) |
| Pectus carinatum (Abramson and modified Ravitch procedure) |
| Pectus arcuatum |
| Mixed defects |
| Chest wall tumors |
| Primary tumors of ribs/sternum |
| Metastatic disease |
| Traumas |
| Rib/Sternal resection and reconstructions |
ESTS, European Society of Thoracic Surgery.
Methods
Aim and characteristics of the chest wall database
The ESTS chest wall database includes data on risk factors, surgical techniques, processes of care and outcomes related to chest wall pathologies. These data are designed for quality control and performance audit. The registry comprehends the whole spectrum of the chest wall diseases in the form of a detailed database with the aim to find out the best practice at European (and non-European) level in order to develop guidelines and establish a standard to improve the outcome. A composite performance score (CPS) was created to assess the outcomes in different aspects of surgical practice of the participating thoracic surgery units (5,6). Monitoring of implants durability, possible complications and bad reactions in patients undergoing correction of chest wall deformities are highlights for the data collection and open some research possibilities. Data on patients’ surveillance after a chest wall procedure are also collected in the registry.
In the last 20 years, chest wall surgery has undergone a considerable growth in technique and material used for reconstruction (7). In fact, many techniques and materials are currently used from different thoracic surgeons in different areas, as so far there are no guidelines for the management of this kind of diseases. The chest wall database is determined to fulfill this purpose.
Another main objective of the database is to endorse the cooperation between international societies. The Society of Thoracic Surgeons General Thoracic Surgery Database (STS GTSD) and the ESTS Registry Task Force already have a cooperation since 2012 (8). The two societies database task forces meet annually to plan future research projects. In the last years were published some studies from the joint cooperation of the two registries (4), after the data harmonization and standardization between the databases.
Participation
The participation to the registry is free and voluntary for the ESTS members. At least one staff member should retain an ESTS membership and the participants have to request and obtain a personal login account completing the specific application form, which can be downloaded from the ESTS homepage (http://www.ests.org/collaboration/database_registration_form.aspx).
Every single contributor/unit has several benefits besides the obvious advantages for the medical community. In fact, every thoracic surgery unit participating to the database can access its own data collected in a standardized ESTS-endorsed dataset, which can be downloaded and used for internal analysis, statistics or research. Furthermore, the participants will receive a feedback regarding the quality of their data and performance compared to the international benchmarks. Every participating thoracic surgery unit can access the ESTS certification program (http://www.ests.org/collaboration/ests_quality_certification_programme.aspx) and can submit a research project to the ESTS database task force to access data derived from the entire database (http://www.ests.org/collaboration/ests_database_rules_for_publications_and_presentations.aspx).
Data collection
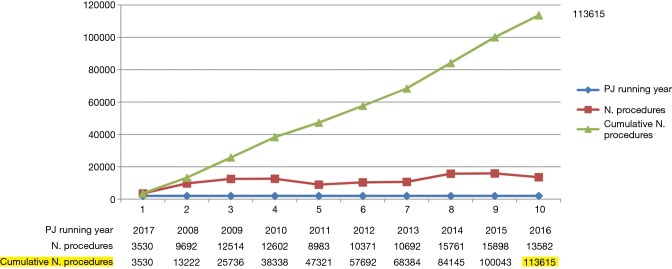
Acquired data are anonymous, independently accessed and encrypted on a Dendrite platform, which provides data security and regular backups. The registry is managed by an external company (KData Clinicak Srl), which works together with the Database Committee in revising and updating periodically the database. Single institutions and national registries can upload data in the database (9,10). Every year the ESTS Registry Annual Report (Silver Book) is published on the ESTS homepage (http://www.ests.org/collaboration/database_reports.aspx) including all data collected during the year (Figure 1).
Figure 1.
Data collected in the chest wall database (source from the Silver Book 2016).
Results
The ESTS chest wall database is structured in four main sections: preoperative, intraoperative, postoperative and follow up. For each procedure registered in the database are collected a number of different variables regarding the patients’ characteristics, the surgical technique, the postoperative course until the discharge and also follow up data (Tables 2,3). Figure 2 shows the number of collected procedures for chest wall deformities until November 2017. In 2016, 2,534 procedures in total were registered in the database according to the silver book and the vast majority of these procedures consisted of surgery for correction of chest wall deformities. Correction of pectus excavatum is the most common procedures registered in 2017 (392 patients, 67% of all data), followed by pectus bar removal (159 patients, 27% of all procedures). Figure 3 shows the correlation between age and gender. Males are generally more affected than females and most patients undergo the procedure in a young age (<40). Figure 4 shows the data regarding the materials used for reconstruction in patients undergoing surgery for pectus excavatum. Figure 5 describes the completeness of the collected data. The data have been collected from 32 hospitals from many European and non-European countries, in particular Brazil. In the supplementary is described in details the core dataset of the chest wall database in all its sections.
Table 2. Structure of the ESTS chest wall database.
| Preoperative |
| General patients’ characteristics |
| Diagnosis |
| Neoadjuvant chemo/radiotherapy |
| How defect affects patients (for congenital chest wall diseases) |
| Lung function and blood gas analysis |
| Comorbidities |
| Intraoperative |
| Chest wall subgroup (chest wall, costal cartilage, chest wall incision, reconstruction, rib, thoracoplasty) |
| Type of procedure |
| Reconstruction (technique and material) |
| Margins |
| Analgesia (epidural, localanesthetic, pericostal block) |
| Postoperative |
| Complication |
| Outcome at discharge, at 30 and 90 days |
| Length of hospital stay |
| Patients satisfaction at discharge |
| Length of epidural analgesia |
| Time to return to work |
| Follow up |
| Dead/alive |
| For Nuss procedures: (I) bar allergic reaction; (II) bar displacement and degree of displacement |
| Required reoperations |
| Wound infections |
| For rib fixation/chest wall reconstruction: reaction to different materials (allogenic/biologic better than artificial?) |
| Chronic pain syndrome |
| Other long-time complication |
ESTS, European Society of Thoracic Surgery.
Table 3. Intraoperative characteristics in details.
| Congenital defects |
| Correction of pectus carinatum (open/minimally invasive) |
| Correction of pectus excavatum (open/Nuss) |
| Pectus silicon implant |
| Correction of pectus arcuatum |
| Mixed deformity |
| Pectus bar removal |
| Technique & materials |
| With/without sternal fixation |
| Number and type of bars/stabilizators (in case of removal: end of planned treatment, allergy to metal, repeated dislodgement, chronic pain) |
| Type of silicone implants |
| Rib and sternal procedures (traumas) |
| Resection |
| Fixation (flail chest) |
| Details |
| Indications (acute trauma, malunion, post surgical fixation, chronic pain, inability to wean from ventilator) |
| System used (abiomet, synthes, stratos/stracos, acute innovation rib lock, gunze absorbable pins, orthopedic non-thoracic specific devices) |
| Chest wall tumors (primary malignant/metastatic) |
| Resection with reconstruction |
| Resection without reconstruction (no needed, covered by scapula) |
| Details |
| Size and position of resection |
| Number and site of resected ribs |
| Technique/material of reconstruction (prostesis, muscle flap, myocutaneus flap, omentum) |
Figure 2.
Total number of chest wall procedures registered in 2017 (updated until 11/2017).
Figure 3.
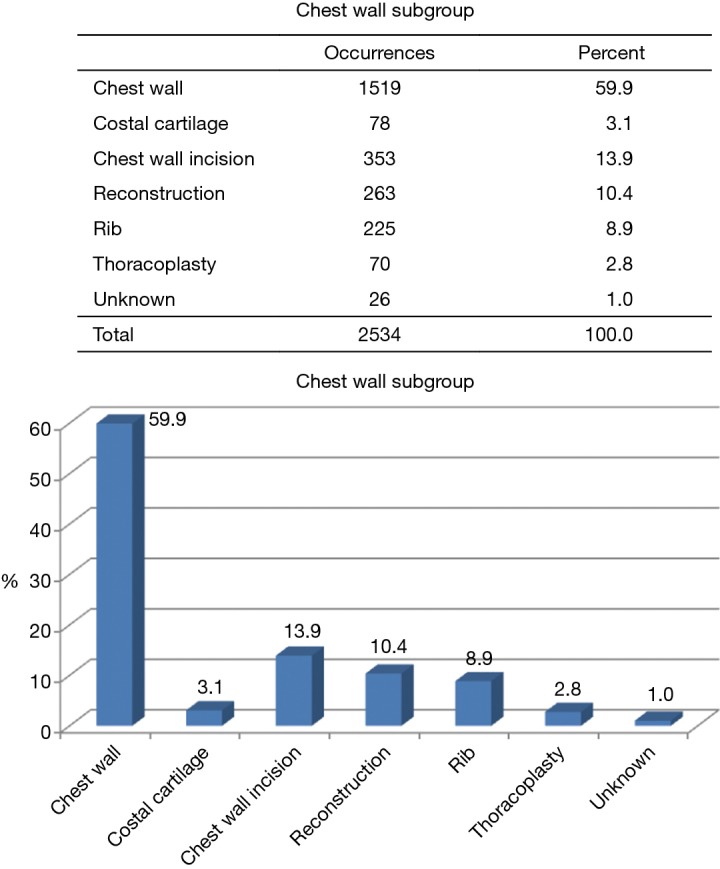
Chest wall procedures related to gender and age (updated until 11/2017).
Figure 4.

Data about material used to reconstruction in patients undergoing pectus excavatum.
Figure 5.
Completeness of the collected data.
Conclusions
The ESTS chest wall database is an ambitious European project, which aims to standardize all chest wall procedures in all their aspects, starting from the preoperative preparation, continuing with the surgical technique and helping treating complications. It has the potential to increase the number of collected data within the next years, taking account of the fact that currently only 15% of the European thoracic surgery units are contributing to the registry. Each thoracic surgery department should understand the advantages that imply joining the database, as single unit and as part of the whole group to improve the thoracic surgery practice around Europe.
ESTS preoperative chest wall.
| Date of thorsurgprocs | |
|---|---|
| Age at surgery | |
| Height | |
| Weight | |
| BMI | |
| ASA | 1: normal healthy individual, 2: mild systemic disease, 3: severe systemic disease not incapacitating, 4: incapacitating systemic disease—constant threat to life, 5: patient moribund—not expected to survive 24 hours |
| MRC score | 0: 1, 1: 2, 2: 3, 3: 4, 4: 5 |
| Cardiac comorbidity 1 | 0: none, 1: coronary artery disease, 2: any previous cardiac surgery, 3: current treatment for hypertension, 4: current treatment for arrhythmia, 5: current treatment for cardiac failure |
| Cardiac comorbidity 2 | 0: none, 1: coronary artery disease, 2: any previous cardiac surgery, 3: current treatment for hypertension, 4: current treatment for arrhythmia, 5: current treatment for cardiac failure |
| Cardiac comorbidity 3 | 0: none, 1: coronary artery disease, 2: any previous cardiac surgery, 3: current treatment for hypertension, 4: current treatment for arrhythmia, 5: current treatment for cardiac failure |
| Other comorbidities 1 | 0: none, 1: insulin-dependent diabetes, 2: serum creatinine >2 mg/dL, 3: CVA, 4: chronic kidney failure, 5: COPD, 6: gastric ulcer, 7: liver disease, 8: connective tissue disease, 9: myasthenia gravis, 10: previous malignancy, 11: other, 12: gastro-esophageal reflux |
| Other comorbidities 2 | 0: none, 1: insulin-dependent diabetes, 2: serum creatinine >2 mg/dL, 3: CVA, 4: chronic kidney failure, 5: COPD, 6: gastric ulcer, 7: liver disease, 8: connective tissue disease, 9: myasthenia gravis, 10: previous malignancy, 11: other, 12: gastro-esophageal reflux |
| Other comorbidities 3 | 0: none, 1: insulin-dependent diabetes, 2: serum creatinine >2 mg/dL, 3: CVA, 4: chronic kidney failure, 5: COPD, 6: gastric ulcer, 7: liver disease, 8: connective tissue disease, 9: myasthenia gravis, 10: previous malignancy, 11: other, 12: gastro-esophageal reflux |
| Urgency | 0: elective, 1: urgent, 2: emergency |
| ECOG | 0: fully active, 1: light work only, 2: mobile >50% waking hours, 3: mobile <50% waking hours, 4: immobile & unable to self-care |
| FEV1, litres | |
| FEV1, percent | |
| FEVC, litres | |
| PpoFEV1, percent | |
| FVC, percent | |
| FEV1, percent | |
| DLCO, percent | |
| PpoDLCO, percent | |
| Other diagnosis | |
| Diagnosis | 0: lung cancer (NSCLC), 6: empyema (acute: phase I/II), 1: lung cancer (SCLC), 2: oesophageal cancer, 3: mesothelioma, 4: lymphoma, 5: thymic tumors, 7: empyema (chronic), 8: chronic pleural inflammation, 9: pulmonary TB, 10: COPD, 11: interstitial lung disease, 12: pneumothorax, 13: trauma, 14: achalasia, 15: gastro-oesophageal reflux, 16: paraoesophageal hernia, 17: emphysema, 18: Zenker’s diverticulum, 19: pulmonary metastasis, 20: carcinaoid, 21: other, 22: chest wall condition |
| Morphology | 0: Non-neoplastic, 1: neoplastic benign, 2: neoplastic malignant primary, 3: neoplastic malignant secondary |
| Smokinghistory | 0: never smoked, 1: past smoker (stopped >1 month prior to surgery), 2: current smoker, 3: unknown |
| Haller index value | |
| CT scan | 0: no, 1: yes |
| Shortness of breath | 0: no, 1: yes |
| Chest pain | 0: no, 1: yes |
| Arrhythmias | 0: no, 1: yes |
| Palpitations | 0: no, 1: yes |
| Low selfesteem | 0: no, 1: yes |
| Psychological | 0: no, 1: yes |
| Other symptom | |
| Scoliosis | 0: no, 1: yes |
| Marfan | 0: no, 1: yes |
| Ehlers danlos | 0: no, 1: yes |
| Cardiac disease | 0: no, 1: yes |
| Previous cardiac surg | 0: no, 1: yes |
| Previous chest surg | 0: no, 1: yes |
ESTS operative chest wall.
| Group definition | 0: lung, 1: pleura, 2: chest wall, 3: trachea: bronchus, 4: mediastinum, 5: upper GI, 6: diaphragm |
|---|---|
| Group other procedure | |
| VATS | 0: no, 1: yes |
| Chest wall subgroup | 0: chest wall, 1: costal cartilage, 2: chest wall incision, 3: reconstruction, 4: rib, 5: thoracoplasty |
| Chest wall procedures | 0: biopsy of chest wall lesion, 1: creation of thoracic stoma, 2: excision of chest wall lesion, 3: repair of chest wall, 4: excision/repair of chest wall, 5: correction of chest wall defects |
| Qualifier excision of chest wall lesion | 0: distant flap, 1: local flap, 2: microvascular transferred flap |
| Qualifier for repair of chest wall | 0: plugging flail chest, 1: suture, 2: osteosynthesis |
| Excision repair qualifier | 0: prosthesis, 1: muscle flap, 2: myocutaneous flap, 3: omentum |
| Costal cartilage procedure | 1: excision of costal cartilage, 2: excision of xifisternum, 3: fixation of costal cartilage |
| Chest wall incision procedures | 0: exploratory median sternotomy, 1: exploratory thoracotomy, 2: mini thoracotomy, 3: previous chest wall incision |
| Correction of chest wall defects qualifier | 0: pectus bar removal, 1: pectus carinatum correction, 2: pectus excavatum correction, 3: pectus silicon implant, 4: pectus arcuatum, 5: mixed deformity, 6: pectus repair |
| Rib procedures | 0: rib resection, 1: rib resection for drainage, 2: rib fixation |
| Qualifier for rib resection | 0: biopsy, 1: for pain, 2: fracture |
| Thoracoplasty procedures | 0: plombage procedure, 1: thoracoplasty procedure |
| Qualifier for plombage | 0: insertion of plomb, 1: removal of plomb |
| Qualifier for thoracoplasty | 0: limited thoracoplasty, 1: schede thoracoplasty, 2: total thoracoplasty |
| Costal cartilage procedures | 1: excision of costal cartilage, 2: excision of xifisternum, 3: fixation of costal cartilage |
| Qualifier pectus carenatum | 0: with internal fixation, 1: without internal fixation |
| Rib sternal fixation | 1: referral for surgery, 2: acute trauma, 3: malunion, 4: post-surgical fixation, 5: chronic pain, 6: inability to wean from ventilator |
| Flail chest | 0: no, 1: yes |
| Surgery | 0: no, 1: yes |
| System used | 1: abiomet, 2: synthes, 3: stratos/stracos, 4: acute innovation rib lock, 5: gunze absorbable pins, 6: orthopedic non-thoracic specific devices |
| Number of plates used | |
| Number of screws used | |
| Acute trauma and fixation within 48 h | 0: no, 1: yes |
| Type of surgery | 1: talc pleurodesis, 2: local resection, 3: other |
| Reoperation | 0: no, 1: yes |
| Metallic implants | 0: no, 1: yes |
| System adopted correction chest wall defects | 1: abiomet, 2: synthes, 3: 3D medical, 4: other proprietary, country specific device |
| Number of bars | 1: 1, 2: 2, 3: 3 |
| Number of stabilizers | 1: 1 per bar, 2: 2 per bar, 3: no stabilizers, 4: additional sutures |
| Titanium bars | 0: no, 1: yes |
| Lactosorb | 0: no, 1: yes |
| Carinatum stabilization | 0: no, 1: yes |
| Time from first operation | |
| Cause for removal | 1: end of planned treatment, 2: allergy to metal, 3: repeated dislodgement, 4: chronic pain |
| Chest wall resection with or without repair | 1: primary chest wall tumour, 2: secondary malignancy invading the chest wall |
| Site of resection/chest wall resection repair | 1: 1st to 3rd rib, 2: 4th to 9th rib |
| Location resection | 1: anterior location, 2: posterior location |
| Covered by scapula | 0: no, 1: yes |
| Extended resection | 0: no, 1: yes |
| Reconstruction performed | 0: no, 1: yes |
| Device bars | 1: stratos, 2: synthes, 3: other |
| Margins | 1: positive margins, 2: less than 1 cm, 3: 1 to 3 cm, 4: >4 cm |
| Operative technique nuss | 0: no, 1: yes |
| Operative technique park | 0: no, 1: yes |
| Operative technique pillegard | 0: no, 1: yes |
| Operative technique other | 0: no, 1: yes |
| Comments operative technique | |
| Stabilizer used | |
| Comments stabilizer | |
| Crane technique | 0: no, 1: yes |
| Vacuum bell | 0: no, 1: yes |
| Other sternal elevation | 0: no, 1: yes |
| Comments sternal elevation | |
| Epidural | 0: no, 1: yes |
| Local anesthetic | 0: no, 1: yes |
| Pericostal block | 0: no, 1: yes |
| Comments adjuvant to anesthesia | |
| Correction method | |
| Type surgery carinatum | |
| Pectus brace | |
| Associate physio therapy manoeuvres | 0: no, 1: yes |
| Reabsorbable pericardium | 0: no, 1: yes |
| Patch | 0: no, 1: yes |
| Titanium bars excision repair chest wall | 0: no, 1: yes |
| Number of ribs resected | |
| Pleurectomy pleurodesis* | 0: pleurectomy, 1: chemical pleurodesis, 2: mechanical pleurodesis |
| Prev chest wall incqualifier* | 0: debridement, 1: procedure for sinus, 2: removal of wires, 3: reopening, 4: repair with flap, 5: resuture |
| Reason no surgery* | |
| Type of reconstruction* | 1: mesh, 2: pericardial patch, 3: absorbable pericardial patch, 4: metylmethacrylate sandwich, 5: titanium bars, 6: titanium plus pericardium, 7: custom made patient-matched titanium implants |
*, denotes fields multichoice.
ESTS postoperative chest wall.
| Date of discharge | |
|---|---|
| Complication 1 | 0: none, 1: air leak >5 days, 2: anastomotic leak (conservative), 3: anastomotic leak (requiring surgery), 4: ARDS, 5: atrial arrhythmia RX postop, 6: bronchopleural fistula, 8: atelectasis, 9: cardiac failure, 10: cerebro-vascular complications, 11: chylothorax, 12: conduit ischaemia, 13: delirium, 14: DVT, 15: empyema, 16: initial ventilation >48 hours, 17: multisystem failure, 18: myocardial infarct, 19: phrenic nerve injury, 20: pneumonia, 21: pulmonary embolism, 22: pulmonary oedema, 23: recurrent nerve palsy, 25: renal failure, 24: reintubate, 28: unexpected admission to ICU, 26: reoperation for bleeding, 27: tracheostomy, 29: ventricular arrhythmia RX postop, 30: wound infection, 31: other |
| Complication 2 | 0: none, 1: air leak >5 days, 2: anastomotic leak (conservative), 3: anastomotic leak (requiring surgery), 4: ARDS, 5: atrial arrhythmia RX postop, 6: bronchopleural fistula, 8: atelectasis, 9: cardiac failure, 10: cerebro-vascular complications, 11: chylothorax, 12: conduit ischaemia, 13: delirium, 14: DVT, 15: empyema, 16: initial ventilation >48 hours, 17: multisystem failure, 18: myocardial infarct, 19: phrenic nerve injury, 20: pneumonia, 21: pulmonary embolism, 22: pulmonary oedema, 23: recurrent nerve palsy, 25: renal failure, 24: reintubate, 28: unexpected admission to ICU, 26: reoperation for bleeding, 27: tracheostomy, 29: ventricular arrhythmia RX postop, 30: wound infection, 31: other |
| Complication3 | 0: none, 1: air leak >5 days, 2: anastomotic leak (conservative), 3: anastomotic leak (requiring surgery), 4: ARDS, 5: atrial arrhythmia RX postop, 6: bronchopleural fistula, 8: atelectasis, 9: cardiac failure, 10: cerebro-vascular complications, 11: chylothorax, 12: conduit ischaemia, 13: delirium, 14: DVT, 15: empyema, 16: initial ventilation >48 hours, 17: multisystem failure, 18: myocardial infarct, 19: phrenic nerve injury, 20: pneumonia, 21: pulmonary embolism, 22: pulmonary oedema, 23: recurrent nerve palsy, 25: renal failure, 24: reintubate, 28: unexpected admission to ICU, 26: reoperation for bleeding, 27: tracheostomy, 29: ventricular arrhythmia RX postop, 30: wound infection, 31: other |
| Major cardiopulmonary complications | 0: no, 1: yes |
| Date of death | |
| Cause of death | 0: death related to this operation, 1: death related to another operation, 3: death after discharge clearly unrelated to this operation |
| Outcome at discharge | 0: alive at discharge, 1: died in hospital |
| Outcome at 30 days | 0: alive at 30 days, 1: dead at 30 days |
| Notes | |
| unexpected return | 0: no, 1: yes |
| Re-admission to any hospital within 30 days discharge | 0: no, 1: yes, 2: unknown |
| Outcome at 90 days | 0: death, 1: alive, 3: unknown |
| Length of hospital stay | |
| Length of surgery min | |
| VAS score | 1: 1, 2: 2, 3: 3, 4: 4, 5: 5, 6: 6, 7: 7, 8: 8, 9: 9, 10: 10 |
| Patients satisfaction at discharge | 1: 1, 2: 2, 3: 3, 4: 4, 5: 5, 6: 6, 7: 7, 8: 8, 9: 9, 10: 10 |
| Length of epidural analgesia | |
| Time to return to work | |
| Entryid | |
| Any pectus recurrence with bar removal | 0: no, 1: yes |
| Mortality with bar removal surgery | 0: no, 1: yes |
| Bar allergic reaction | 0: no, 1: yes |
| Bar displacement | 0: no, 1: yes |
| Degree displacement | |
| Required reoperation | 0: no, 1: yes |
| Comments bar displacement | |
| Pneumothorax requiring chest tube | 0: no, 1: yes |
| Pleural effusion | 0: no, 1: yes |
| Cardiac injury | 0: no, 1: yes |
| Pericardial injury | 0: no, 1: yes |
| Major vascular injury | 0: no, 1: yes |
| Lung injury | 0: no, 1: yes |
| Trocar related injury | 0: no, 1: yes |
| Describe anest related injury | |
| Other complication | 0: no, 1: yes |
| Comments complications | |
| Anesthesia related injury | 0: no, 1: yes |
| Thoracic outlet syndrome | 0: no, 1: yes |
| Pericarditis | 0: no, 1: yes |
| Specify complication |
ESTS follow up chest wall.
| Date last followup | |
|---|---|
| Date of death | |
| Alive | 0: no, 1: yes |
| Cause of death | 0: cardiac, 1: neurological, 2: renal, 3: respiratory, 4: pulmonary embolism, 5: GI, 6: infection, 7: cancer recurrence, 8: other cancer, 9: others, 10: not known |
| Entryid | |
| Bar allergic reaction flow | 0: no, 1: yes |
| Bar displacement flow | 0: no, 1: yes |
| Degree displacement | |
| Required reoperation | 0: no, 1: yes |
| Comments bar displacement | |
| Developed pectus carinatum flow | 0: no, 1: yes |
| Recurrent pectus excavatum flow | 0: no, 1: yes |
| Cardiac injury flow | 0: no, 1: yes |
| Aortic or vascular injury flow | 0: no, 1: yes |
| Thoracic outlet syndrome flow | 0: no, 1: yes |
| Worsening scoliosis flow | 0: no, 1: yes |
| Chronic pain syndrome flow | 0: no, 1: yes |
| Other complication flow | 0: no, 1: yes |
| Wound infection flow | 0: no, 1: yes |
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Salati M, Brunelli A, Dahan M, et al. Task-independent metrics to assess the data quality of medical registries using the European Society of Thoracic Surgeons (ESTS) Database. Eur J Cardiothorac Surg 2011;40:91-8. 10.1016/j.ejcts.2010.11.004 [DOI] [PubMed] [Google Scholar]
- 2.Salati M, Falcoz PE, Decaluwe H, et al. The European thoracic data quality project: An Aggregate Data Quality score to measure the quality of international multi-institutional databases. Eur J Cardiothorac Surg 2016;49:1470-5. 10.1093/ejcts/ezv385 [DOI] [PubMed] [Google Scholar]
- 3.Seder CW, Falcoz PE, Salati M, International General Thoracic Surgery Database Collaboration Thorac Surg Clin 2017;27:303-13. 10.1016/j.thorsurg.2017.03.010 [DOI] [PubMed] [Google Scholar]
- 4.Salati M, Brunelli A, Decaluwe H, et al. Report from the European Society of Thoracic Surgeons Database 2017: patterns of care and perioperative outcomes of surgery for malignant lung neoplasm. Eur J Cardiothorac Surg 2017;52:1041-8. 10.1093/ejcts/ezx272 [DOI] [PubMed] [Google Scholar]
- 5.Brunelli A, Berrisford RG, Rocco G, et al. The European Thoracic Database project: composite performance score to measure quality of care after major lung resection. Eur J Cardiothorac Surg 2009;35:769-74. 10.1016/j.ejcts.2009.01.037 [DOI] [PubMed] [Google Scholar]
- 6.Brunelli A, Rocco G, Van Raemdonck D, et al. Lessons learned from the European thoracic surgery database: the Composite Performance Score. Eur J Surg Oncol 2010;36 Suppl 1:S93-9. 10.1016/j.ejso.2010.06.023 [DOI] [PubMed] [Google Scholar]
- 7.Sanna S, Brandolini J, Pardolesi A, et al. Materials and techniques in chest wall reconstruction: a review. J Vis Surg 2017;3:95. 10.21037/jovs.2017.06.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez FG, Falcoz PE, Kozower BD, et al. The Society of Thoracic Surgeons and the European Society of Thoracic Surgeons general thoracic surgery databases: joint standardization of variable definitions and terminology. Ann Thorac Surg 2015;99:368-76. 10.1016/j.athoracsur.2014.05.104 [DOI] [PubMed] [Google Scholar]
- 9.Salati M. Reasons to participate in European Society of Thoracic Surgeons database. J Thorac Dis 2015;7:S112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klepetko W, Aberg TH, Lerut AE, et al. Structure of general thoracic surgery in Europe. Eur J Cardiothorac Surg 2001;20:663-8. 10.1016/S1010-7940(01)00942-3 [DOI] [PubMed] [Google Scholar]