Abstract
Background
Hepatectomy is a widely accepted curative treatment for hepatocellular carcinoma (HCC). However, the disease frequently recurs after a curative hepatectomy. The objective of this study is to provide a better understanding of the pattern of disease recurrence and the risk factors involved so as to improve the postoperative surveillance.
Methods
A retrospective study for all patients receiving hepatectomy for HCC between 2003 and 2014 was performed. Emphasis was made on the timing and pattern of recurrent disease, and type of treatment given.
Results
There were 506 patients in the study. Median follow-up was 43.7 months. The 1-, 3-, 5-, 10-year overall and disease free survival were 89.5%, 74.1%, 63.9%, 49.0% and 69.5%, 54.3%, 43.4%, 30.9% respectively. Recurrent disease occurred in 267 patients, 47.2% occurred within 9 months of hepatectomy and 80.1% recurred only in liver. Median survival was shorter for recurrence occurring within 9 months compared with those occurring between 10 months and 2 years postoperatively (36.2 vs. 65.7 months, P<0.01) whilst less curative treatment was offered for patients with early (within 9 months) intrahepatic alone recurrence (22.2% vs. 51.7%, P<0.01). Multivariate analysis revealed tumor size >3.5 cm and history of rupture were risk factors for recurrence within 9 months.
Conclusions
These findings suggest that recurrent diseases are common after curative hepatectomy for HCC and most recurrences occur in the remnant liver. Since almost half of recurrences occurred within first 9 months after hepatectomy, a more stringent postoperative surveillance with target imaging of liver in this period is needed. Early diagnosis of recurrent disease and curative retreatment hopefully can bring about a longer survival.
Keywords: Hepatectomy, hepatocellular carcinoma (HCC), recurrence
Introduction
Liver resection is the mainstay of curative treatment for hepatocellular carcinoma (HCC). However, recurrent disease remains a major problem after hepatectomy which exceeds 70% at 5 years (1), and this is also the major cause of compromised survival. According to the literature, most recurrences occur early after hepatectomy and most only recur in the residual liver (2-5). Selected cases of intrahepatic recurrent diseases are still amendable for curative treatments like repeated hepatectomy or local ablation. Successful retreatment hopefully can provide a longer survival.
To our understanding, there is no recommended standardised surveillance program for patients after curative hepatectomy for HCC. Contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) is the recommended imaging for post-hepatectomy surveillance but the ideal imaging interval is unknown (1). Many centres employed an imaging interval of 3 months in the first 2 years and 6 months thereafter (6). Inadequate postoperative surveillance imaging may delay diagnosis of recurrent disease and deprive patients chance of curative retreatment. In this study, an analysis was made on the timing and pattern of recurrent diseases after curative hepatectomy for HCC, and factors involved in recurrences. The objective of this study is to provide a better understanding of the pattern of disease recurrence and the risk factors involved so as to improve the postoperative surveillance.
Methods
The patient demographics, tumour characteristics, peri-operative data, histological findings and long term outcomes of patients receiving hepatectomy in our institute were all recorded prospectively in a hepatectomy database. The database was updated periodically with the information came from follow up visits and investigation results. In the period between 1st January 2003 and 31st December 2014, all patients who received their first time hepatectomy with histologically confirmed HCC constituted the cohort of patients for the present study.
In our institute, the selection of patients with HCC for hepatectomy was based on the consideration of tumour factor, liver functional status and patient factor. Resection would not be offered in the presence of extrahepatic metastasis (except for limited porta lymph node metastasis), disease beyond margin clear resection, major macrovascular invasion beyond confine of resection (except when the tumour thrombus can be extracted, e.g., from main portal vein).
Patient would have complete work-up for liver function before surgery. Routine blood counts, liver function, renal function, coagulation profile, alpha-feto protein (AFP) would be checked. Besides, indocyanine green (ICG) test would be done for each patient and in cases when volume of residual liver was deemed marginal after resection a CT volumetry would be determined. Pre-operative portal vein embolization (PVE) would be performed for small residual liver volume which might be combined with pre-operative transarterial chemo-embolization (TACE). TACE was also occasionally used to downstage the tumour before resection. In general, major hepatectomy could be done for Child’s A patients, and for Child’s B patients, only minor hepatectomy would be performed. For the ICG test, a dye retention rate at 15 minutes (R15) more than 15% would preclude a major hepatectomy whilst more than 20% preclude any liver resection. In some cases, concomitant radiofrequency ablation (RFA) or microwave ablation (MWA) were allowed for destruction of synchronous tumours which were not favourable for simultaneous resection.
There was no age limit for hepatectomy, especially for minor resection and for those who were suitable for minimally invasive approach. Choice of alternative curative treatment like local ablation or TACE if appropriate would be offered if patient had substantial risk due to extreme old age or multiple comorbidities or due to patients’ wish.
For ruptured resectable localized HCC, emergency hepatectomy would be offered without prior transarterial embolization (TAE). Operation would also be offered for patients who failed TAE control of bleeding. Other patients would have TAE first as done by referring hospital or other surgical on call teams. Interval hepatectomy would then be offered for those resectable HCC. For unresectable disease, TACE would be offered once bleeding has stopped and liver function has recovered. For more advanced disease, only supportive treatment was offered.
Operative technique for liver resection has been reported previously (7). Open hepatectomy was performed via a right subcostal incision with upward midline extension. Operative ultrasound (USG) was performed to confirm extent of disease and to guide resection. Pringle maneuver was generally not applied except in study cases. Liver transection was carried out with the use of cavitron ultrasonic surgical aspirator (CUSATM, Valley-Lab, Boulder, Colorado, USA) in combination with a saline coupled dissecting sealer (TissueLinkTM, TissueLink Medical, Dover, Delaware, USA). A resection margin of 1 cm was aimed, but in case it was not possible the requisite was a negative resection margin. Towards the latter period, laparoscopic and robotic approach for liver resection were introduced to our institute for selected cases of patients. Liver was divided with the use of LigasureTM (ValleyLab, Boulder, Colorado, USA) or Harmonic AceTM (Ethicon Endo-surgery, Cincinnati, Ohio, USA) with TissueLinkTM. The same oncological principle applied for tumour resection as open approach. Adjuvant TACE would be offered to patients with involved resection margin or high risk histological finding (multiple satellite nodules and microvascular invasion) or for study purpose.
After surgery, patients were followed up in outpatient clinics 3 monthly within first year and then half yearly afterwards. Imaging (USG or CT) was offered 3 monthly in first year and then around half yearly afterwards. Chest X-ray would be done half yearly. AFP would be checked before each follow-up. Further investigation might be offered if patient had unexplained increase in AFP or suspicious lesion on USG or CT scan. These included MRI or dual tracer positron emission tomography (PET). Recurrence was diagnosed at time for the first occurrence of recurrence on imaging (USG, CT, MRI or PET).
Patients with proven recurrent diseases would subject to assessment for possibility of repeat hepatectomy or local ablation. Hepatectomy was preferred compared to local ablation unless tumor was small and suitable for percutaneous local ablation or patient was high risk for liver resection under general anaesthesia. TACE would be offered for those multi-focal intrahepatic recurrence or those who were not suitable for resection or ablation. Systemic chemotherapy or target therapy would be offered for those with extrahepatic recurrent diseases.
Overall survival (OS) was calculated from time of hepatectomy to date of death or last available follow-up. Disease free survival (DFS) was calculated from time of hepatectomy to date of diagnosis of first recurrence or last available follow-up.
Results
There were 506 patients in this study. The patient demographics, clinicopathological features and tumour characteristics were shown in Table 1. Vast majority were male patients (85.4%) with a median age of 57 years and predominantly hepatitis B carrier (82.6%). Cirrhosis was noted in more than half of the patients. Around one quarter of tumors were multinodular. The median size of tumors was 4 cm. Major hepatectomy accounted for 41.7% of cases and most adopted the open approach (84.2%). Majority of tumors were moderately differentiated with one quarter of tumors had either micro or macro vascular invasion.
Table 1. Patient demographics, clinicopathological findings and operative outcomes.
| Characters | Median [range] or number (%), N=506 |
|---|---|
| Age | 57 [21–85] |
| Male gender | 432 (85.4) |
| Child’s grading | |
| A | 489 (96.6) |
| B | 16 (3.2) |
| C | 1 (0.2) |
| Hepatitis B carrier | 418 (82.6) |
| Hepatitis C carrier | 30 (5.9) |
| Pre-operative AFP (µg/L) | 40 [1–258,000] |
| Pre-operative TACE | 47 (9.3) |
| Pre-operative PVE | 25 (4.9) |
| Number of tumour | |
| Solitary | 382 (75.5) |
| Multinodular | 124 (24.5) |
| 2 | 56 (11.1) |
| 3 | 17 (3.4) |
| 4 | 9 (1.8) |
| ≥5 | 42 (8.3) |
| Size of largest tumour (cm) | 4 [0.7–24.0] |
| Recurrent tumour (after previous treatment except hepatectomy) | 16 (3.2) |
| Ruptured tumour | 34 (6.7) |
| Degree of differentiation | |
| Well | 62 (12.3) |
| Moderate | 390 (77.1) |
| Poor | 45 (8.9) |
| Not available | 9 (1.8) |
| Satellite nodules | 58 (11.5) |
| Macrovascular invasion | 37 (7.3) |
| Microvascular invasion | 98 (19.4) |
| Resection margin (cm) | 1.3 [0–6.5] |
| Involved margin | 14 (2.8) |
| Cirrhosis | 289 (57.1) |
| Major hepatectomy | 211 (41.7) |
| Concomitant RFA or MWA | 13 (2.6) |
| Surgical approach | |
| Open | 426 (84.2) |
| Laparoscopic | 41 (8.1) |
| Robotic | 39 (7.7) |
| 90-day mortality | 7 (1.4) |
| Morbidity | 142 (28.1) |
| Blood loss (mL) | 350 [5–11,300] |
| Blood transfusion | 44 (8.7) |
| Postoperative hospital stay (days) | 7 [2–55] |
| Adjuvant TACE | 81 (16.0) |
AFP, alpha-feto protein; TACE, transarterial chemoembolization; PVE, portal vein embolization; RFA, radiofrequency ablation; MWA, microwave ablation.
The operative outcomes of this group of patients were also shown in Table 1. Seven patients died within 90 days of operation, five were in-hospital mortality due to postoperative liver failure while the remaining two patients were readmitted after discharge, one died of liver failure and the other died of portal vein thrombosis and systemic sepsis. Post-operative complications occurred in 28.1% of patients, with median blood loss 350 mL and blood transfusion rate 8.7%. The median postoperative hospital stay was 7 days.
The median follow-up for this group of patients were 43.7 months (range, 0.2–157.1 months). The 1-, 3-, 5-, 10-year OS and DFS were 89.5%, 74.1%, 63.9%, 49.0% and 69.5%, 54.3%, 43.4%, 30.9% respectively (Figure 1). Recurrent disease occurred in 267 patients (52.8%), out of which 214 patients (80.1%) had intrahepatic recurrence only, 31 patients (11.6%) had extrahepatic recurrence only and 22 patients (8.3%) had both intrahepatic and extrahepatic recurrences. Sites of extrahepatic recurrence in decreasing order of frequency (with patient number in parentheses) were lung [31], lymph node [12], bone [11], peritoneum [11], adrenal [5], spleen [2], pancreas [1] and Gerota’s fascia [1].
Figure 1.
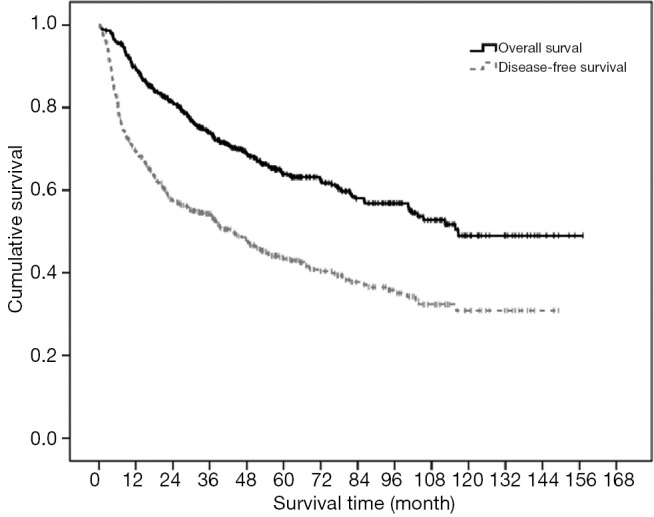
The overall and disease free survival curves for the study patients
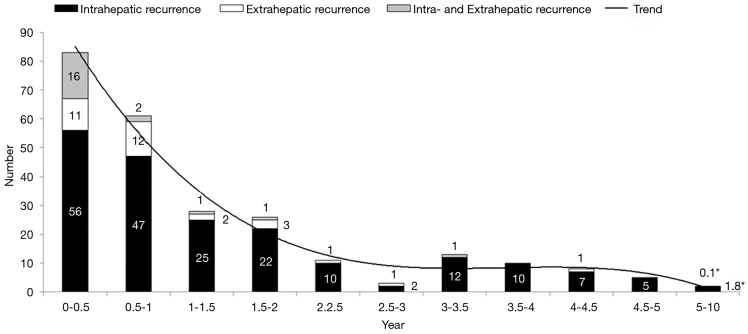
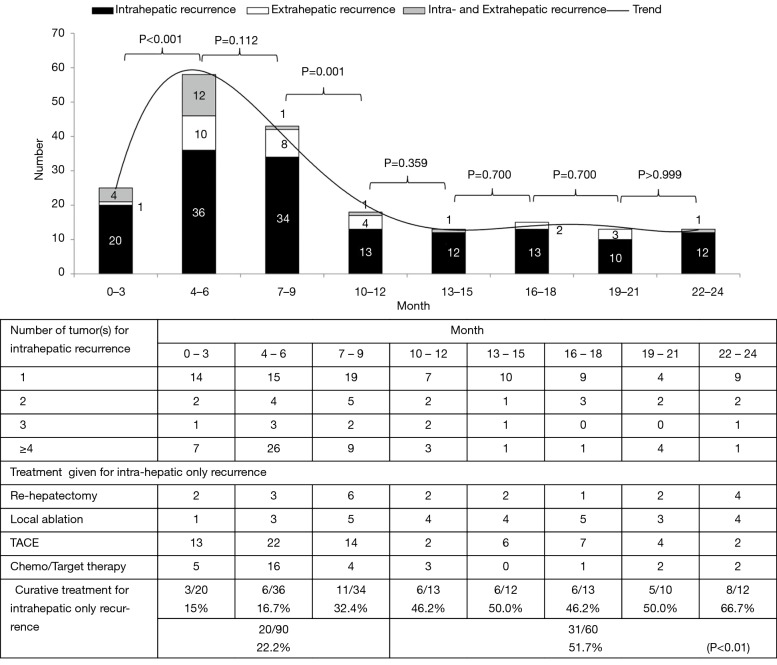
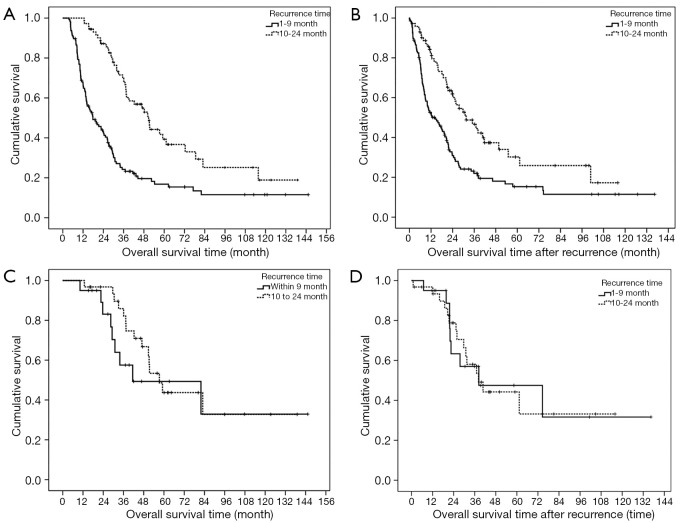
The 1-, 3-, 5-, and 10-year disease recurrence rate were 28.9%, 43.8%, 54.4% and 67.0% respectively. Figure 2 showed the distribution of different types of recurrence with time after hepatectomy. Recurrence rate was the highest soon after hepatectomy and it showed a gradual decline with time after hepatectomy. The incidence became static after 2 years postoperatively. Figure 3 showed the distribution of recurrence in terms of 3-month period for the first 2 years after hepatectomy. Highest incidence of recurrence was in the period of postoperative 4- to 6-month period. Overall, the 9-month period postoperatively represented the riskiest period with 47.2% of all recurrence occurred in this period. The median survival was significantly shorter if patients had recurrence within 9 months after operation versus those who had recurrence within 10 to 24 months postoperatively (36.2 versus 65.7 months, log-rank test P<0.01) (Figure 4A). The difference remained significant when survival was calculated from the time of first recurrence (Figure 4B). On the other hand, less curative treatment was offered to patients with liver alone recurrence in this period compared with recurrence between 10 and 24 months postoperatively (22.2% versus 51.7%, P<0.01) (Figure 3). If only the survival of patients who received curative treatment for recurrent diseases was determined, there was no difference in the median survival between patients who recurred within 9 months or who recurred between 10 and 24 months postoperatively (41.5 versus 57.3 months, log-rank test P=0.386) (Figure 4C), the survival remained similar when survival was calculated from time of first recurrence (Figure 4D). For the compliance of the imaging surveillance, 357 patients (70.6%) took surveillance imaging in the period 0–3 months. Fifty-eight patients experienced recurrence in the period 4–6 months, and 41 out of these 58 patients (70.7%) had surveillance imaging in the period 0–3 months.
Figure 2.
Distribution of recurrence with time after hepatectomy. *, for the period 5–10 years, the figure represents the average number of recurrences over half year period.
Figure 3.
Details of distribution of early recurrence with time after hepatectomy and treatment given. TACE, transarterial chemoembolization.
Figure 4.
The survival of patients with or without receiving curative treatment according to time of recurrence and calculated from time of operation or time of diagnosed first recurrence. (A) Survival according to different time of recurrence (Log-rank test: P<0.001); (B) survival calculated from time of recurrence and according to different time of Recurrence (Log-rank test: P<0.001); (C) survival of patients who have received curative treatment for recurrences; and according to different time of recurrence (Log-rank test: P=0.386); (D) survival of patients who have received curative treatment for recurrences, calculated from time of recurrence and according to different time of recurrence (Log-rank test: P=0.996).
There were 37 and 45 patients received hepatectomy and local ablation respectively for their recurrences in the study period. Reasons for curative treatment not given included multifocal recurrences beyond hepatectomy or local ablation, poor liver reserve for repeat hepatectomy or local ablation, patient refusal and concomitant extrahepatic recurrence. As for illustration, a 63-year-old man, who had multinodular tumors up to 11 cm all confined to right lobe of liver, underwent a right hepatectomy. CT at 3-month post operation revealed multifocal recurrence in left liver, TACE was given as the recurrent tumor nodules scattered in the remaining liver beyond liver resection or local ablation. Patient developed impaired liver function afterwards and so further TACE was not possible. Follow up USG revealed portal vein thrombosis. Patient was given palliative care and succumbed 6 months after operation.
Univariate logistic regression for recurrence within 9 months showed that tumor size, multiple tumors, satellite nodules, macro or micro vascular invasion, involved margin, AFP, blood loss, blood transfusion and history of rupture were significant factors. From the analysis of receiver operating characteristic (ROC) curves of 9 months recurrence for size of lesions, blood loss and AFP, the cut-off points using Youden index were 3.55 cm, 605 mL and 94 µg/L respectively. So, it was reasonable to use 3.5 cm, 600 mL and 90 µg/L as cut-off points for size of lesions, blood loss and AFP in logistic regression. On multivariate analysis, only tumor ≥3.5 cm (P=0.024) and history of rupture (P=0.013) were risk factors for early recurrence within 9 months (Table 2).
Table 2. Univariate and multivariate logistic regression for recurrence within 9 months.
| Variable | Univariate regression | Multivariate regression | |||
|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | ||
| Age | 0.99 (0.97–1.01) | 0.298 | – | – | |
| Sex | – | – | |||
| M | 0.81 (0.45–1.44) | 0.469 | |||
| F | – | – | |||
| Child’s grading | – | – | |||
| A | 0.48 (0.17–1.32) | 0.155 | |||
| B/C | – | – | |||
| Hepatitis B carrier | 0.83 (0.48–1.43) | 0.493 | – | – | |
| Cirrhosis | 0.95 (0.62–1.46) | 0.807 | – | – | |
| No. of comorbidities | 0.91 (0.76–1.08) | 0.275 | – | – | |
| ICG (R15) | 1.01 (0.97–1.05) | 0.638 | – | – | |
| Fibroscan | 1.01 (0.98–1.04) | 0.739 | – | – | |
| AFP ≥90 ug/L | 1.87 (1.20–2.91) | 0.006* | 1.55 (0.96–2.50) | 0.073 | |
| Pre-op TACE | 1.01 (0.48–2.10) | 0.982 | – | – | |
| Pre-op PVE | 0.88 (0.33–2.40) | 0.806 | – | – | |
| For those hepatitis B carrier pre-op antiviral | 0.76 (0.46–1.26) | 0.283 | – | – | |
| Size of tumour ≥3.5 cm | 2.80 (1.73–4.55) | <0.001* | 1.79 (1.05–3.05) | 0.033* | |
| Number of tumour | |||||
| Solitary | 0.36 (0.23–0.57) | <0.001* | 0.64 (0.33–1.25) | 0.190 | |
| Multinodular | – | – | – | – | |
| Recurrent tumour | 1.73 (0.59–5.09) | 0.320 | – | – | |
| Degree of differentiation | – | – | |||
| Well | 0.59 (0.21–1.68) | 0.325 | |||
| Moderate | 1.15 (0.53–2.48) | 0.724 | |||
| Poor | – | – | |||
| Satellite nodules | 3.68 (2.08–6.51) | <0.001* | 1.52 (0.66–3.50) | 0.327 | |
| Macro vascular invasion | 2.79 (1.40–5.60) | 0.004* | 1.45 (0.62–3.37) | 0.393 | |
| Micro vascular invasion | 2.15 (1.32–3.52) | 0.002* | 1.43 (0.80–2.54) | 0.226 | |
| Resection margin (cm) | 0.83 (0.67–1.03) | 0.088 | – | – | |
| Involve margin | 3.92 (1.34–11.43) | 0.012* | 1.63 (0.41–6.41) | 0.488 | |
| Major/minor hepatectomy | – | – | |||
| Major | 0.88 (0.57–1.36) | 0.563 | |||
| Minor | – | – | |||
| Surgical approach | – | – | |||
| Open | 1.46 (0.77–2.76) | 0.245 | |||
| Lap/robotic | – | – | |||
| Hilar clamp applied | 0.96 (0.51–1.81) | 0.904 | – | – | |
| Concomitant RFA or MWA | 2.40 (0.77–7.48) | 0.133 | – | – | |
| Blood loss ≥600 mL | 2.30 (1.47–3.60) | <0.001* | 1.51 (0.89–2.54) | 0.125 | |
| Morbidity | 1.06 (0.66–1.70) | 0.814 | – | – | |
| Blood transfusion | 2.33 (1.21–4.49) | 0.011* | 1.30 (0.59–2.87) | 0.513 | |
| Re-operation | 0.93 (0.20–4.45) | 0.929 | – | – | |
| Post-op hospital stay (days) | 1.00 (0.97–1.04) | 0.966 | – | – | |
| Adjuvant TACE | 1.38 (0.80–2.39) | 0.252 | – | – | |
| Ruptured tumor | 4.84 (2.38–9.87) | <0.001* | 2.48 (1.09–5.61) | 0.030* | |
*, statistically significant. ICG (R15), indocyanine green test (retention rate at 15 minutes). AFP, alpha-feto protein; TACE, transarterial chemoembolization; PVE, portal vein embolization; RFA, radiofrequency ablation; MWA, microwave ablation.
Discussion
With the advancement of surgical techniques and dissecting instruments, hepatectomy once thought to be a high risk surgery is now considered to be a safe operation with acceptable mortality and morbidity. In the present series, the mortality rate was 1.4%, morbidity rate was 28.1%, median blood loss was 350 mL with a blood transfusion rate of 8.7%. This was comparable with the result of contemporary series in the literature (8,9). Despite the much improved short term outcomes of hepatectomy for HCC, the long term result remains disappointed due to high tumor recurrence rate and compromised survival. Reports on long term outcomes more than 5 years after hepatectomy were scarce in literature, a 49.0% and 30.9% 10-year overall and disease-free survival in this study probably represented a very favourable outcome already (10-12). Certainly there is room for further improvement. The lack of an effective adjuvant measure to prevent recurrence after surgery appears to be a contributing factor. TACE has been tried as adjuvant treatment after curative hepatectomy but so far there is no evidence for its routine use (13,14). While efforts are attempted to reduce the incidence of recurrence, survival may also be prolonged if early detection and timely treatment of recurrent disease can be achieved. Hence, an understanding of the pattern and timing of recurrence after hepatectomy is important.
Intrahepatic recurrent HCC originates either from intrahepatic metastasis of primary tumor or from a metachronous multicentric occurrence (15,16). It is believed that early recurrence may represent intrahepatic metastasis whereas late recurrence is most likely due to a multicentric recurrence. There is no consensus yet regarding the dividing line between early and late recurrence. A period ranged from 6 months to 2 years after hepatectomy had been used in previous studies (17-19). In a recent paper, 17 months was claimed to be the best cut-off value between early and late recurrence based on the difference in overall survival after initial recurrence (16). Nevertheless, in regard to the timing of recurrence, it probably occurs earlier than it was thought to be 1 or 2 years after hepatectomy. In a study in which digital subtraction angiography was used for detection of recurrent disease, high risk period was the first half year with the highest incidence of recurrence occurred 3-4 months after hepatectomy (3).
The present study showed that more than half of the patients would develop recurrent disease after hepatectomy for HCC (52.8%) and most recurrences occurred in liver only (80.1%). As majority of patients had liver only recurrence, chance of cure was possible by repeated hepatectomy or local ablation. In Figure 3, we found a peak of recurrence centered at 4–6 months. When we compared two consecutive 3-month periods within the first 2 years, the number of recurrence dropped significantly after 9 months and the trend remained static, so the cut-off was set at 9 months. Detailed analysis in this study showed that most recurrence occurred within first 9 months after hepatectomy and it carried significantly shorter survival. The worse prognosis in this group of patients with early recurrence might be related to a more aggressive biological behaviour of tumors (1). However, in patients with liver alone recurrence, significantly less curative treatment was offered in this period. Curative treatment included rehepatectomy or local ablation (RFA or MWA). It has been shown that TACE had significantly inferior result than rehepatectomy or local ablation for intrahepatic recurrence (20). Actually when only patients who could receive curative treatment were analysed, there was no difference in survival between early recurrence within 9 months or recurrence within 10 to 24 months after hepatectomy. Thus the shorter survival in early recurrent disease could also be due to less timely curative treatment offered to this group of patient, which in turn could be due to delayed diagnosis of recurrent disease.
The peak incidence of recurrence in postoperative 4–6 months might just reflect the lag behind imaging to pick up a lesion that had occurred earlier, as around 30% of patients did not take any surveillance imaging in the period 0–3 months. Fifty-eight patients experienced recurrence in the period 4–6 months, and 17 out of these 58 patients (29.3%) did not have surveillance imaging in the period 0–3 months. For HCC treated by local ablation, an imaging within 1 month was advocated for determination of completeness of ablation (21). Similarly, an earlier imaging regime after hepatectomy may help to pick up early intrahepatic recurrent disease. This is especially true for tumors ≥3.5 cm or ruptured tumors as they are the risk factors for early recurrence within 9 months by multivariate analysis. Study has shown that a bimonthly imaging for the first 8 months after liver directed therapy for HCC was an optimal imaging schedule (22). Thereafter a 6 monthly imaging should be adequate for disease surveillance. CT or MRI would be the ideal choice of imaging but radiation hazard is a concern for CT in addition to the cost concern of both examinations. USG alternate with CT/MRI appears to be a reasonable substitute. Thus in high risk patients a more intense surveillance program with liver imaging one month after hepatectomy followed by bimonthly examinations in first 9 months of operation seems necessary. Whether the proposed more frequent imaging with higher probability of retreatment bring about longer survival needs to be validated by further studies.
Many studies have attempted to identify the risk factors for recurrence after hepatectomy for HCC. Validated predictors of recurrence were tumor size, multifocality, macroscopic and microscopic vascular invasion and poor differentiation (23). The present study just identified tumor size ≥3.5 cm or ruptured tumors as risk factors on multivariate analysis. However, these two factors were only relevant for early recurrence within 9 months after hepatectomy, other factors might play a role in late recurrence. According to our finding, early recurrence was uncommon for small size tumor and those without history of rupture despite the presence of other adverse histological factors.
The limitation of this study lies on its retrospective nature. Besides, the cause of HCC in this study is largely due to hepatitis B virus, thus the biological behaviour of tumour may be different from HCC caused by hepatitis C or alcoholism. Furthermore, liver transplant service is not available in our institute, this may affect the result of survival of patients who are eligible for primary transplant therapy or for salvage transplant after recurrence.
Conclusions
The findings of this study suggest that recurrent diseases are common after curative hepatectomy for HCC and most recurrences occur in the remnant liver. Since almost half of recurrences occurred within first 9 months after hepatectomy, a more stringent postoperative surveillance with target imaging of liver in this period is needed. Early diagnosis of recurrent disease and curative retreatment hopefully can bring about a longer survival.
Acknowledgements
The authors thank Mr. Philip Ip for his assistance with data processing and statistical analysis.
Ethical Statement: Since this was a retrospective study in which patients received standard operative care, the application to the local ethics board was waived. All patients had written informed consent before operations.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005;42:1208-36. 10.1002/hep.20933 [DOI] [PubMed] [Google Scholar]
- 2.Poon RT, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg 2000;232:10-24. 10.1097/00000658-200007000-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu X, Zhao H, Yang H, et al. A prospective clinical study on early recurrence of hepatocellular carcinoma after hepatectomy. J Surg Oncol 2009;100:488-93. 10.1002/jso.21354 [DOI] [PubMed] [Google Scholar]
- 4.Arnaoutakis DJ, Mavros MN, Shen F, et al. Recurrence patterns and prognostic factors in patients with hepatocellular carcinoma in noncirrhotic liver: a multi-institutional analysis. Ann Surg Oncol 2014;21:147-54. 10.1245/s10434-013-3211-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirokawa F, Hayashi M, Asakuma M, et al. Risk factors and patterns of early recurrence after curative hepatectomy for hepatocellular carcinoma. Surg Oncol 2016;25:24-9. 10.1016/j.suronc.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 6.Liu D, Fong DY, Chan AC, et al. Hepatocellular carcinoma: surveillance CT schedule after hepatectomy based on risk stratification. Radiology 2015;274:133-40. 10.1148/radiol.14132343 [DOI] [PubMed] [Google Scholar]
- 7.Lee KF, Wong J, Ng W, et al. Feasibility of liver resection without the use of the routine Pringle manoeuver: an analysis of 248 consecutive cases. HPB (Oxford) 2009;11:332-8. 10.1111/j.1477-2574.2009.00053.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Y, Lei X, Wu L, et al. Outcomes of hepatectomy for noncirrhotic hepatocellular carcinoma: a systematic review. Surg Oncol 2014;23:236-42. 10.1016/j.suronc.2014.11.001 [DOI] [PubMed] [Google Scholar]
- 9.Waller LP, Deshpande V, Pyrsopoulos N. Hepaocellular carcinoma: a comprehensive review. World J Hepatol 2015;7:2648-63. 10.4254/wjh.v7.i26.2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimada K, Sano T, Sakamoto Y, et al. A long-term follow-up and management study of hepatocellular carcinoma patients surviving for 10 years or longer after curative hepatectomy. Cancer 2005;104:1939-47. 10.1002/cncr.21461 [DOI] [PubMed] [Google Scholar]
- 11.Han DH, Choi GH, Park JY, et al. Lesson from 610 liver resections of hepatocellular carcinoma in a single center over 10 years. World J Surg Oncol 2014;12:192. 10.1186/1477-7819-12-192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang YJ, Chung KP, Chang YJ, et al. Long-term survival of patients undergoing liver resection for very large hepatocellular carcinomas. Br J Surg 2016;103:1513-20. 10.1002/bjs.10196 [DOI] [PubMed] [Google Scholar]
- 13.Jiang JH, Guo Z, Lu HF, et al. Adjuvant transarterial chemoembolization after curative resection of hepatocellular carcinoma: propensity score analysis. World J Gastroenterol 2015;21:4627-34. 10.3748/wjg.v21.i15.4627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao M, Zhu Z, Wang H, et al. Adjuvant transarterial chemoembolization for patients after curative resection of hepatocellular carcinoma: a meta-analysis. Scand J Gastroenterol 2017;52:624-34. 10.1080/00365521.2017.1292365 [DOI] [PubMed] [Google Scholar]
- 15.Colecchia A, Schiumerini R, Cucchetti A, et al. Prognostic factors for hepatocellular carcinoma recurrence. World J Gastroenterol 2014;20:5935-50. 10.3748/wjg.v20.i20.5935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamoto Y, Ikoma H, Morimura R, et al. Optimal duration of the early and late recurrence of hepatocellular carcinoma after hepatectomy. World J Gastroenterol 2015;21:1207-15. 10.3748/wjg.v21.i4.1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poon RT, Fan ST, Ng IO, et al. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer 2000;89:500-7. [DOI] [PubMed] [Google Scholar]
- 18.Shah SA, Greig PD, Gallinger S, et al. Factors associated with early recurrence after resection for hepatocellular carcinoma and outcomes. J Am Coll Surg 2006;202:275-83. 10.1016/j.jamcollsurg.2005.10.005 [DOI] [PubMed] [Google Scholar]
- 19.Ibrahim S, Roychowdhury A, Hean TK. Risk factors for intrahepatic recurrence after hepatectomy for hepatocellular carcinoma. Am J Surg 2007;194:17-22. 10.1016/j.amjsurg.2006.06.051 [DOI] [PubMed] [Google Scholar]
- 20.Wang K, Liu G, Li J, et al. Early intrahepatic recurrence of hepatocellular carcinoma after hepatectomy treated with re-hepatectomy, ablation or chemoembolization: a prospective cohort study. Eur J Surg Oncol 2015;41:236-42. 10.1016/j.ejso.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 21.Liu LN, Xu HX, Zhang YF, et al. Hepatocellular carcinoma after ablation: the imaging follow-up scheme. World J Gastroenterol 2013;19:797-801. 10.3748/wjg.v19.i6.797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boas FE, Do B, Louie JD, et al. Optimal imaging surveillance schedules after liver-directed therapy for hepatocellular carcinoma. J Vasc Interv Radiol 2015;26:69-73. 10.1016/j.jvir.2014.09.013 [DOI] [PubMed] [Google Scholar]
- 23.Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology 2016;150:835-53. 10.1053/j.gastro.2015.12.041 [DOI] [PubMed] [Google Scholar]