Abstract
Preeclampsia, a hypertensive syndrome occurring in 3–5% of human pregnancies, has lifelong health consequences for fetuses. Cognitive ability throughout life is altered, and adult stroke risk is increased. One potential etiological factor for altered brain development is low concentrations of proangiogenic placental growth factor (PGF). Impaired PGF production may promote an antiangiogenic fetal environment during neural and cerebrovascular development. We previously reported delayed vascularization of the hindbrain, altered retinal vascular organization, and less connectivity in the circle of Willis in Pgf−/− mice. We hypothesized Pgf−/− mice would have impaired cognition and altered brain neuroanatomy in addition to compromised cerebrovasculature. Cognitive behavior was assessed in adult Pgf−/− and Pgf+/+ mice by four paradigms followed by postmortem high-resolution MRI of neuroanatomy. X-ray microcomputed tomography imaging investigated the three-dimensional cerebrovascular geometry in another cohort. Pgf−/− mice exhibited poorer spatial memory, less depressive-like behavior, and superior recognition of novel objects. Significantly smaller volumes of 10 structures were detected in the Pgf−/− compared with Pgf+/+ brain. Pgf−/− brain had more total blood vessel segments in the small-diameter range. Lack of PGF altered cognitive functions, brain neuroanatomy, and cerebrovasculature in mice. Pgf−/− mice may be a preclinical model for the offspring effects of low-PGF preeclampsia gestation.
Keywords: brain development, magnetic resonance imaging, microcomputed tomography imaging, preeclampsia, pregnancy
INTRODUCTION
Preeclampsia (PE), an acute hypertensive syndrome of human pregnancy, affects 3–5% of pregnancies and has short- and long-term health impacts for mothers and their offspring. Offspring from PE pregnancies (PE-F1s) are more likely to experience cognitive and cerebrovascular disorders than age-matched offspring from non-PE pregnancies. PE-F1 infants have smaller head circumferences compared with offspring from uncomplicated pregnancies (39) and 3 yr old PE-F1s have lower IQ scores than offspring who were similarly growth restricted but did not experience PE (54). Adult PE-F1s have higher depressive symptom scores (75), lower cognitive ability as young adults (76), and greater self-reported cognitive impairment at 70 yr of age (77). Experiencing a PE gestation has also been associated with 32% higher risk of autism spectrum disorder (19), increased risk of attention deficit/hyperactivity disorder (53), lower neuromuscular development index scores in adolescence (34), and a higher risk of epilepsy (80). In the first magnetic resonance imaging (MRI) study reported for PE-F1s, altered neuroanatomy and cerebral vessels were identified in 7–10 yr old PE-F1s (62). Additionally, as older adults, PE-F1s had a greater risk of stroke independent of weight and gestational age at birth (39). Cognitive, neuroanatomical, and vascular alterations coexist in this cohort, likely stemming from PE-induced developmental insult.
PE pregnancies have an altered balance of pro- and antiangiogenic factors including placental growth factor (PGF, previously PlGF), a member of the vascular endothelial growth factor (VEGF) family. PGF concentration in maternal plasma normally rises over pregnancy to peak at the end of the second trimester in women (43, 72, 74) and at gestational day (GD)10–14 in mice (1) but is markedly low in women diagnosed with PE (4, 27, 70, 72, 74). In early-onset PE, circulating maternal PGF levels are low in first trimester during a critical period of fetal development (4, 37, 46, 68, 74). Circulating maternal PGF is largely placentally derived and reflected by fetal cord blood (70, 74) and amniotic fluid concentrations (70). In humans, maternal PGF concentrations have been associated with fetal growth (5, 38) and retinal arteriolar caliber in childhood (33). Extremely low gestational age neonates delivered for fetal or maternal indications including PE have a greater risk of low PGF concentrations in the blood (47), suggesting that PE-related PGF deficiency is not restricted to the mother. However, the impact of PGF deficiency on brain development is unknown. Here, the effect of PGF deficiency on neural and cerebrovascular development and behavior is investigated in adult Pgf−/− mice.
Pgf−/− mice are hypertensive and exhibit histopathological changes in the kidney, but these anomalies are not exaggerated by pregnancy despite impaired decidual angiogenesis (1, 61). Fetal umbilical artery resistance and heart rate are similar in Pgf−/− and Pgf+/+ pregnancies (1). Although Pgf−/− fetuses weigh less on GD14.5 and 18.5, there is no difference in pup weight at postnatal day 4 between Pgf−/− and Pgf+/+ mice (1). In wild-type mice, PGF is expressed in neuronal and vascular components of developing forebrain, midbrain, and hindbrain along with VEGF receptor 1 (VEGFR1, previously FLT1) and VEGF receptor 2 (VEGFR2) (49). Circulating murine placental PGF affects brain development since silencing placental PGF production altered fetal cortical vessels (44). In adult mice, PGF is increased in neurons and vessels after ischemic insult (3, 22, 35), while lack of PGF delays the angiogenic response to brain hypoxia (28). In adult humans, PGF is expressed in neurons, found in the cerebrospinal fluid, and increased in epilepsy or traumatic brain injury (82, 83). In vitro, PGF, like VEGF, protects neurons from oxygen and glucose deprivation (22). PGF is capable of signaling directly through the neuropilin-1 (NRP1) receptor (52, 55), which has an important role in brain angiogenesis and neural development (11, 73, 79). We previously compared cerebral vessels of Pgf−/− to Pgf+/+ mice with fetal and neonatal whole mount immunostaining, adult brain vascular casts, and stroke induction protocols. These studies identified altered retinal vascularization (40), delayed hindbrain vascularization during development, altered connectivity of the circle of Willis, and increased vulnerability to stroke in Pgf−/− mice (49). Here, our studies are extended to assess whether murine PGF deficiency alters adult cognitive behavioral function or brain neuroanatomy and to describe in greater detail impacts on the matured cerebrovasculature with unbiased analysis of three-dimensional (3D) images.
MATERIALS AND METHODS
Animals
Pgf−/− mice on a C57BL/6 background were bred under barrier husbandry at Queen’s University from foundation stock provided by Dr. Peter Carmeliet, Vesalius Research Centre, Leuven, Belgium. Male and female C57BL/6 (Pgf+/+) mice were purchased from Charles River Canada (St-Constant, QC, Canada) and acclimatized at least 2 wk before study. Pgf−/− by Pgf−/− matings were used to avoid any influence of circulating PGF from heterozygous or wild-type fetuses on the Pgf−/− offspring. All mice were maintained under a 12 h:12 h light-dark cycle and with ad libitum access to food and water. All procedures were conducted under protocols approved by the Queen’s University Animal Care Committee and compliant with Canadian Council on Animal Care guidelines. Experiments are reported according to the ARRIVE guidelines. Adult mice (n = 20 Pgf−/− and n = 23 Pgf+/+; 4–5 mo old) were used for cognitive behavioral testing; 32 of these 43 animals (for a predetermined sample size of n = 8 per sex per genotype for imaging analyses, 5–6 mo) were then prepared for MRI as described below. An additional 32 mice that did not undergo cognitive behavioral testing (n = 8 per sex per genotype, 5–6 mo) were prepared for microcomputed tomography (µCT) imaging, described below.
Cognitive Behavioral Testing
Each cognitive behavioral test was performed on the same study set of animals (n = 9 male Pgf−/−, 11 female Pgf−/−, 10 male Pgf+/+, and 13 female Pgf+/+ mice) with the aim of having at least eight animals per sex per genotype after exclusion of animals on the tail suspension test as described below. Testing was carried out in a darkened, 22°C, dedicated testing room, to which animals were introduced and habituated in their home cages for 1 h before dark cycle initiation and testing. Testing began at the start of the dark cycle and was completed in the first three-quarters of this time period. The testing room had minimal background noise and was dimly lit for the investigator by a 60 W light bulb. Mice performed each test once. Male and female mice were tested on the same days in no particular order. All equipment was cleaned between each trial to prevent any carryover of odors. The testing session was video recorded for blinded offline analysis.
Y-maze spontaneous alternation test.
To assess spatial learning, we had mice perform the Y-maze spontaneous alternation test (YMSAT). The Y-maze apparatus consisted of three identical opaque plastic arms (40 × 8 × 12 cm), with each arm oriented 120° apart. The walls of each arm were decorated with a unique design of masking tape to provide visual cues. Mice were individually placed in the center of the maze and allowed to freely explore the three arms of the maze for 10 min. Arm entries were scored, and successful alternation was defined as consecutive entries into a new arm before returning to the two previously visited arms. Percent alternation was calculated as: % Alternation = (number of successful alternations/total arm entries – 2) × 100.
Tail suspension test.
To assess depressive-like behavior of the mice, the tail suspension test (TST) was performed as described previously (10, 71). Mice were individually suspended by the tail in an opaque chamber (25 × 25 × 30 cm). Each mouse was suspended for a total of 6 min. Mice were considered to be immobile when they hung passively. The initial time to immobility, total time spent immobile, and number of immobile episodes were recorded. Plastic tubing was placed over the mouse tails to minimize climbing; however, four Pgf+/+ females, three Pgf−/− females, and one Pgf−/− male climbed their tails during testing and were excluded from analysis of total time immobile and number of immobile episodes. Latency to immobility was quantifiable for two of these Pgf+/+ females, and the Pgf−/− male as the first immobile episode occurred before tail climbing and these mice were therefore included in that analysis.
Novel object recognition test.
To test object recognition memory, mice performed the novel object recognition (NOR) test as described previously (6, 23). The arena was a Plexiglas open-field apparatus (45 × 45 × 25 cm), and the objects were unique constructions of Lego blocks, varying both in color and shape. On day 1 of testing, each mouse was individually released into the middle of the arena and allowed to explore for 10 min. On day 2, the sample phase, each mouse was released into the middle of the arena containing two identical objects. On day 3, the choice phase, each mouse was again released into the middle of the arena, which contained a familiar object and a novel object. The right/left location of the novel object was alternated between mice to control for side biases. Each mouse was defined as exploring an object if its nose was oriented in the direction of the object and the mouse was within a 4 cm zone around the object. The total number of visits to each object and total time spent exploring each object were analyzed. Preference for the novel object was calculated as the percentage of time spent exploring the novel object as a function of the total amount of exploration time [duration spent with novel object/(duration spent with novel object + duration spent with familiar object) × 100].
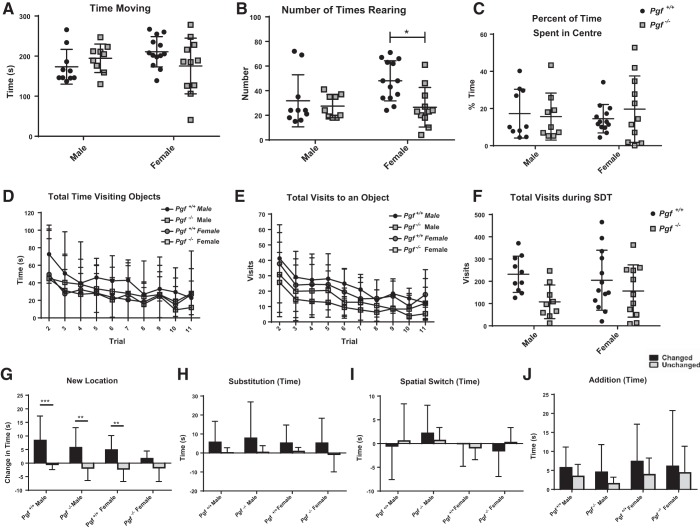
Serial dishabituation test.
To assess sex-specific strategies for object recognition, the serial dishabituation test (SDT) was performed as described by Bettis and Jacobs (6). The arena was a Plexiglas open-field apparatus (45 × 45 × 25 cm), and the objects were unique constructions of Lego blocks and dissimilar from the objects used in the NOR test. Each mouse performed 11 consecutive 6 min trials, separated by 3 min intertrial periods during which the mouse was returned to its home cage. Tests were video recorded for offline analysis. In trial 1 (Open Arena) the mouse is habituated to the open arena and activity of the mouse was assessed. Time moving, number of times rearing, and percent of time spent in the center of the arena were recorded. In trials 2–4 (Habituation) the mouse is exposed to an x-shaped array of five objects. In trial 5 (New Location), the center object in the array is moved to a position outside the square formed by the other four objects in the x-shaped array. In trial 7 (Spatial Switch), two objects are switched in location but the shape of the array remains unchanged. In trial 9 (Substitution), one familiar object is substituted with a novel object. In trial 11 (Addition), a new object is added to the array, for a total of six objects. The direction of object displacement (up, down, right, left) and subsequent array directionality alternated between mice of the same genotype and sex to minimize the effect of side preferences. Each new configuration of the array is repeated in the next trial so that the repeated trial can serve as a baseline, control trial for the next new configuration. As such, trial 5 is compared with trial 4, trial 7 to trial 6, trial 9 to trial 8, and trial 11 to trial 10. Each mouse was scored as exploring an object if its nose was oriented in the direction of the object and within a 4 cm zone around the object. The total number of visits to each object and total time spent exploring each object were analyzed. The difference in number of visits and time exploring the changed object vs. the unchanged objects were compared relative to number of visits and time exploring in the previous trial.
MRI
Sample preparation and imaging.
The first 32 mice to complete behavioral testing (n = 8 males and 8 females per genotype with 1 Pgf−/− male subsequently excluded for poor image quality) were prepared by a method optimized for MRI (9). Mice were anaesthetized with sodium pentobarbital (75 mg/kg ip) and perfused through the heart with 30 ml PBS (10010023, Life Technologies, ThermoFisher Scientific) containing 10 U/ml heparin and 2 mM ProHance (Bracco Diagnostics, Princeton, NJ) followed by 30 ml 4% paraformaldehyde (PFA, 25°C; Electron Microscopy Sciences, Hatfield, PA) containing 2 mM ProHance at a rate of 1 ml/min. Perfused mice were decapitated, and the skin, lower jaw, ears, eyes, cartilaginous nose tip, and body were removed from the head. The brain within the skull was postfixed in 4% PFA containing 2 mM ProHance at 4°C overnight. Samples were stored in PBS containing 0.01% sodium azide (SAZ001; Bioshop, Burlington, ON, Canada) and 2 mM ProHance at 4°C for a minimum of 28 days before imaging (20). A multichannel 7.0 Tesla MRI scanner (Agilent, Palo Alto, CA) was used to image the brain within the skull. We used 16 custom-built solenoid coils to image 16 brains concurrently with samples randomized to scan session (8). Parameters for the anatomical MRI scans were as follows: T2-weighted, 3D fast spin-echo sequence using a cylindrical k-space acquisition, with a repetition time of 350 ms and echo times of 12 ms per echo for six echoes, four averages, field-of-view of 20 × 20 × 25 mm and matrix size = 504 × 504 × 630 giving an image with 0.040 mm isotropic voxels (69).
MRI analysis.
To visualize and compare changes in the mouse brain, all of the images (both Pgf+/+ and Pgf−/− mice) were linearly (6 parameter followed by a 12 parameter) and nonlinearly registered together to create a consensus average (58). This allowed for the analysis of the deformations needed to take each individual mouse’s anatomy into this final average image, with the goal of modeling how the deformation fields related to genotype (45, 59). The Jacobian determinants of the deformation fields were then calculated as measures of volume at each voxel. Using the results of the linear alignment, we created multiple templates of a segmented anatomical atlas with 62 labeled structures (21) including the cortical lobes, corpus callosum, ventricles, cerebellum, brain stem, and olfactory bulbs (the MAGeT procedure) (16). From the final voted segmentation, volume changes were calculated and expressed in absolute (mm3) volumes. In addition to computing the absolute volume for each structure and voxel, we computed a relative volume where the volume of the given structure is reported after the effect of overall brain volume differences among mice is removed.
μCT Imaging
Sample preparation and imaging.
A separate cohort of Pgf−/− and Pgf+/+ mice that had not experienced behavioral testing (n = 8 males and 8 females per genotype) were anesthetized with an overdose of sodium pentobarbital (75 mg/kg ip). Mice were perfused via a Servo pressure pump (PS-200-P, Living Systems Instrumentation) according to the protocol previously published by Ghanavati et al. (32). In brief, mice were perfused through the heart with 5 U/ml heparinized PBS at 50 mmHg after the inferior vena cava was tied off and the descending aorta clamped. The common carotid arteries were clamped for a 2 min perfusion at 150 mmHg with Microfil (MV-122; Flow Tech, Carver, MA) to fill the posterior circulation of the brain. Then the carotid arteries were unclamped, and the perfusion continued for 20 min. The Microfil was allowed to polymerize for 90 min at 30 mmHg, and then the head was removed and dissected to isolate the brain within the skull. The brain was fixed inside the skull overnight in 10% formalin. The skull was decalcified in 8% formic acid (F0507; Sigma-Aldrich, Oakville, ON, Canada) over 48 h. Finally, the samples were rinsed, embedded in 1% agarose gel (AGA001-100, Bioshop), and stored at 4°C until imaging. Images were acquired with a Bruker Skyscan 1272 micro-CT scanner (Bruker Skyscan). The scanning protocol was as follows: with the X-ray source at 80 kV and 125 μA, the specimen was rotated 180° in 0.2° increments, generating 900 views that were reconstructed into data blocks with a 12 μm voxel size.
µCT image analysis.
Reconstructed images were normalized so the voxel intensities were between 0 and 1 and registered to a common space with the MRI anatomical brain atlas as previously described (17). Each image was manually segmented to exclude extracerebral vessels with Display software (Montreal Neurological Institute). The structure of the vasculature was identified automatically by a previously described segmentation algorithm (64). Vessel segments with diameter <0.04 mm were removed because of low imaging reliability for vessels this size. Total vessel segment numbers and diameters were extracted for comparison between genotypes. The μCT images were registered to the previously published MR mouse brain atlas (21) with nine rotational, translation, and scale parameters to determine total brain volume, which was used to calculate vessel segment density, vessel length density, and cerebral blood volume as a percent of total brain volume.
Statistical Analysis
All data are expressed as means ± SD unless stated otherwise. Data were checked for normal distribution with the D’Agostino and Pearson normality test. For performance on behavioral testing, unpaired t- or Mann-Whitney tests were used to examine genotype-dependent differences. Sex differences and a sex/genotype interaction were tested for with two-way ANOVAs and Tukey’s honestly significant difference post hoc tests. Parameters that required comparing the time a mouse spent exploring novel/familiar or changed/unchanged objects were examined with repeated-measures two-way ANOVAs and Sidak post hoc tests. For these parameters, sex differences and sex*genotype interactions were examined with linear mixed effects models including object, genotype, and sex as fixed effects and mouse as a random effect. Multiple comparisons when comparing absolute and relative volumes from MR analysis were controlled for by the false discovery rate (FDR) (31). Absolute brain volumes were correlated with performance on the cognitive behavioral tests with Spearman rank order correlation and an FDR of 0.1 for Benjamini-Hochberg (BH) adjusted P values. Number of vessel segments from the µCT analysis was compared between genotypes by cumulative distributions of vessel diameters with fitted spline models with knots at 100, 200, 300, and 400 µm. The spline coefficients were compared with a linear model incorporating genotype, sex, contrast level in the vessel tracking algorithm and coefficient. Vessel segment density, vessel length density, and cerebral blood volume were compared between genotypes with unpaired, two-tailed t-tests. Two-way ANOVA was used to test for a sex*genotype interaction. GraphPad Prism (version 6.07) and R Studio (version 1.1.423) were used for statistical analysis.
RESULTS
Cognitive Behavioral Testing
Impaired spatial learning on the YMSAT in Pgf−/− mice.
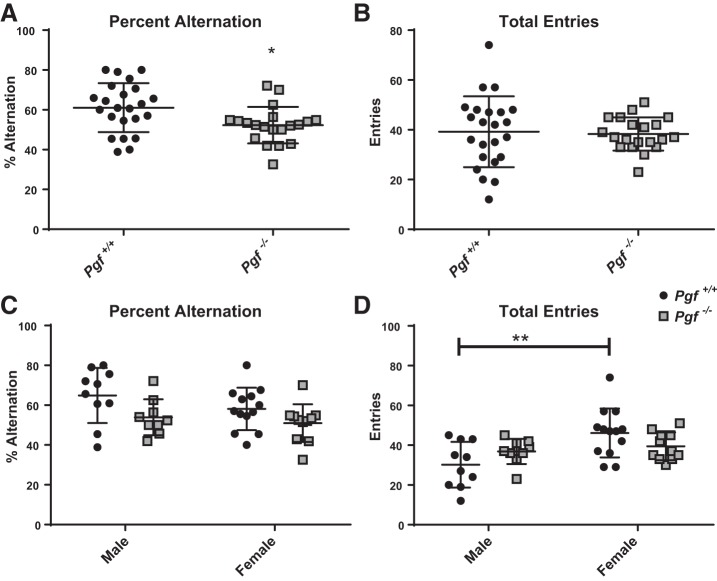
Percent alternation on the YMSAT, a measure of spatial learning, was lower in Pgf−/− mice compared with Pgf+/+ mice (P = 0.0124, Fig. 1A), suggesting impairment. No difference was seen in the total number of arm entries (Fig. 1B), suggesting the difference was not due to altered exploratory behavior. When males and females were analyzed separately, there was no sex*genotype interaction in percent alternation (Fig. 1C). Total arm entries were not different between genotypes when males and females were compared separately. However, sex (F = 9.219, P = 0.0043) and the sex*genotype interaction (F = 4.818, P = 0.0342) were significant as Pgf+/+ females had significantly more arm entries than Pgf+/+ males (P = 0.0025, Fig. 1D). This difference was not present between Pgf−/− males and Pgf−/− females.
Fig. 1.
Spatial learning in Pgf−/− mice. Compared with Pgf+/+ mice, Pgf−/− mice exhibited a significantly lower percent alternation (A) despite no difference in the total number of arm entries (B) in the Y-maze spontaneous alternation test (YMSAT). When stratified by sex, there was no significant difference in percent alternation (C), although female Pgf+/+ mice made significantly more entries than Pgf+/+males (D). Each mouse completed the 10 min YMSAT once. Data were analyzed with unpaired, two-tailed t-tests. Graphs show means ± SD. Black circles represent Pgf+/+ mice, while gray squares represent Pgf−/− mice; n = 23 Pgf+/+ and 20 Pgf−/− male and female mice. *P < 0.05.
Decreased depressive-like behavior on the TST in Pgf−/− mice.
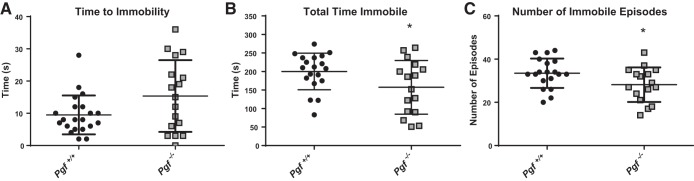
Time to immobility in the TST, a measure of depressive-like behavior, was similar in Pgf−/− and Pgf+/+ mice (Fig. 2A). However, Pgf−/− mice spent significantly less time immobile overall (P = 0.0466; Fig. 2B) and had significantly fewer immobile episodes (P = 0.042; Fig. 2C) compared with Pgf+/+, suggesting less depressive-like behavior. Stratification of males and females did not reveal a sex*genotype interaction difference in time to immobility, total time immobile, or number of immobile episodes (data not shown).
Fig. 2.
Depressive-like behavior in Pgf−/− mice. Compared with Pgf+/+ mice, Pgf−/− mice showed no difference in time to immobility (A), although they spent less time immobile (B) and had fewer immobile episodes (C) on the tail suspension test (TST). Each mouse completed the 6 min TST once. Unpaired two-tailed t-tests or Mann-Whitney tests for data that were not normally distributed were used for analysis. Graphs show means ± SD. Black circles represent Pgf+/+ mice, while gray squares represent Pgf−/− mice; n = 23 Pgf+/+ and 20 Pgf−/− male and female mice. *P < 0.05.
Greater preference for novel objects in Pgf−/− mice.
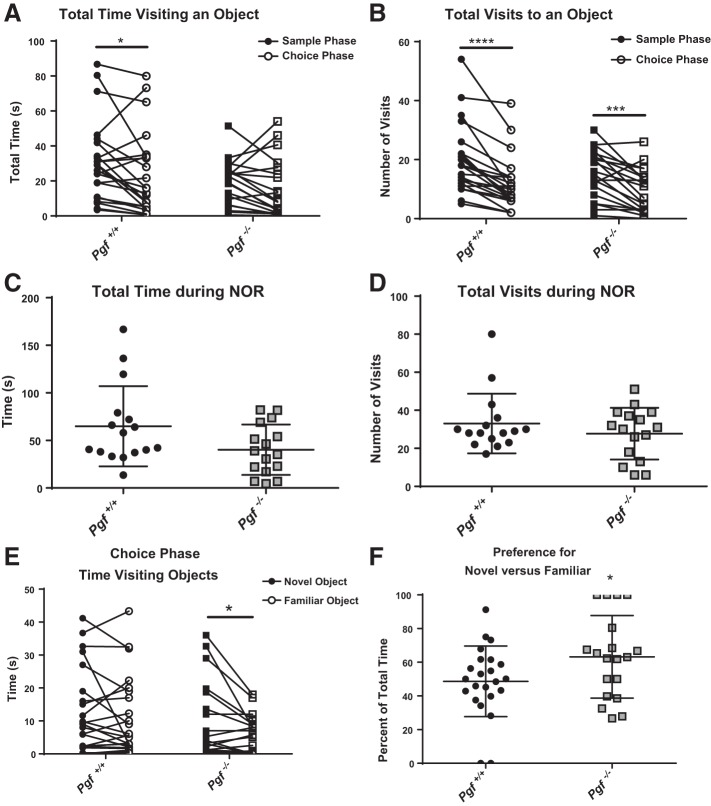
In the NOR test of object recognition memory, both Pgf−/− and Pgf+/+ mice acclimatized to the objects between the sample phase and the choice phase. Although there was no significant difference between the sample and choice phase for Pgf−/− mice, Pgf+/+ mice spent more time visiting objects in the sample phase compared with the choice phase (P = 0.0291, Fig. 3A). Both Pgf−/− and Pgf+/+ mice made significantly fewer visits to objects in the choice phase compared with the sample phase (P = 0.0004 for Pgf−/− mice and P < 0.0001 for Pgf+/+ mice, Fig. 3B). There was no significant difference between Pgf−/− and Pgf+/+ mice with respect to total time visiting or total number of visits during the entire NOR (Fig. 3, C and D). No sex difference or sex*genotype interaction was identified (data not shown).
Fig. 3.
Pgf−/− mouse performance on the novel object recognition (NOR) test. On the NOR test, Pgf+/+ but not Pgf−/− mice spent less time exploring the objects during the choice phase compared with the sample phase, although there was no difference between genotypes in either phase (A). Both Pgf+/+ and Pgf−/− mice made fewer visits to the objects during the choice phase compared with the sample phase with no significant difference between genotypes in either phase (B). There was no significant difference in time spent exploring objects (C) or number of visits to the objects (D) over the entire NOR. In the choice phase, Pgf−/− mice were able to differentiate between the novel object and familiar objects and spent more time exploring the novel object (E). Correspondingly, Pgf−/− mice exhibited greater preference for the novel object compared with Pgf+/+ mice as measured by the percent of total time spent exploring the novel object (F). Each mouse completed three 10 min trials. In the first trial, the arena was empty. In the second trial, two identical objects were present for the sample phase. In the third trial, one familiar object and one novel object were present for the choice phase. Data were analyzed with repeated-measures two-way ANOVA, unpaired, two-tailed t-tests, or Mann-Whitney tests as appropriate. Graphs show values for individual mice in the sample and choice phases (A, B, E), or means ± SD (C, D, F). Circles represent Pgf+/+ mice, while squares represent Pgf−/− mice; n = 23 Pgf+/+ and 20 Pgf−/− male and female mice. *P < 0.05, ***P < 0.001, ****P < 0.0001.
To determine if the mice could differentiate between the novel and familiar objects, the time visiting the novel and familiar objects during the choice phase was compared. Pgf−/− but not Pgf+/+ mice spent significantly more time investigating the novel object than the familiar object (P = 0.0479, Fig. 3E), although there was no difference in the number of visits to the novel object compared with the familiar object for either Pgf−/− or Pgf+/+ mice (data not shown). Pgf−/− showed a higher level of preference for the novel object with respect to proportion of time visiting compared with Pgf+/+ mice (P = 0.0446, Fig. 3F). Pgf−/− mice also exhibited a greater proportion of total visits to the novel object than Pgf+/+ mice although the difference was not statistically significant (data not shown). There was no difference between Pgf−/− and Pgf+/+ mice in time visiting or number of visits when the right and left objects were compared during the sample phase meaning no side preference was present. There was no sex*genotype interaction.
Reduced exploration but no difference in object memory in Pgf−/− mice.
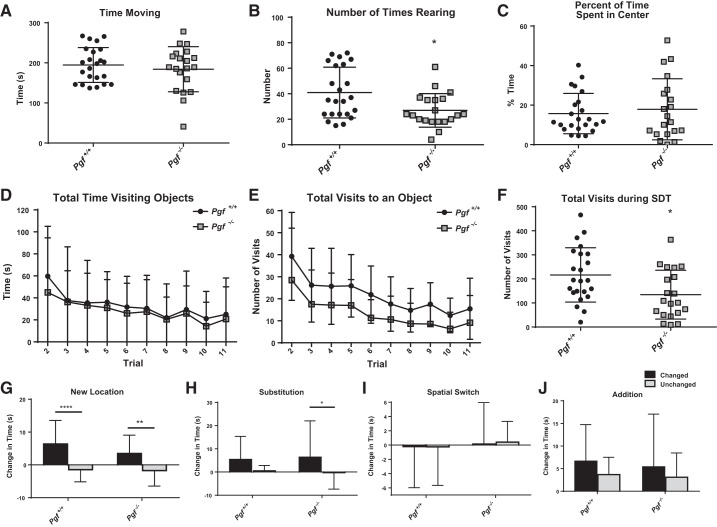
Activity and anxiety-like behavior were examined in the Open Arena trial of the SDT. Pgf−/− and Pgf+/+ mice exhibited no difference in the total time spent moving (Fig. 4A), suggesting no difference in activity levels. However, Pgf−/− mice reared fewer times, suggesting an impairment (P = 0.0204, Fig. 4B). Pgf−/− and Pgf+/+ mice spent a similar percentage of the time in the center of the arena, suggesting no difference in anxiety-like behavior (Fig. 4C). No sex difference was present as Pgf−/− and Pgf+/+ males and females were similar with respect to total time spent moving (Fig. 5A). Although Pgf−/− females reared significantly fewer times than Pgf+/+ females (P = 0.0143) and Pgf−/− and Pgf+/+ males did not differ (P = 0.9383), the sex*genotype interaction was not significant (Fig. 5B). Percent of time spent in the center, a measure of anxiety-like behavior, also did not show a sex difference (Fig. 5C).
Fig. 4.
Pgf−/− mouse performance on the serial dishabituation test (SDT). In the first trial of the SDT, Pgf+/+ and Pgf−/− mice spent similar amounts of time moving (A), although Pgf−/− mice reared fewer times (B). The time spent in the center of the arena as a percentage of total time was also similar between Pgf+/+ and Pgf−/− mice (C). Pgf+/+ and Pgf−/− mice spent similar amounts of time exploring the objects in each trial (D), although Pgf−/− mice tended to make fewer visits to the objects (E). Pgf−/− mice had a lower total number of visits over the entire SDT (F). Pgf+/+ and Pgf−/− mice exhibited a significantly greater increase in time investigating the changed object compared with the unchanged objects during the New Location trial (G). Only Pgf−/− mice spent significantly greater time investigating the changed object in the Substitution trial (H). Neither the Pgf+/+or Pgf−/− had significantly greater increases in exploration of the changed objects during the Spatial Switch (I) or Addition (J) trials. Each mouse underwent 11 consecutive trials in the SDT. The first trial allowed accommodation to the empty arena and was used to analyze activity and anxiety-like behavior. The second to fourth trials familiarized the mice with an array of objects. The geometry of the array was distorted (New Location, Addition) or objects were exchanged without altering the geometry (Spatial Switch, Substitution) in later trials. Changes in the time spent investigating changed and unchanged objects relative to the baseline trial experienced directly previously are presented. Data were analyzed with unpaired, two-tailed t-tests, Mann-Whitney tests and two-way repeated-measures ANOVAs. Graphs show means ± SD. Circles represent Pgf+/+ mice, while squares represent Pgf−/− mice. Black bars show the change in time spent investigating the changed object(s), while light gray bars show the change in time investigating the unchanged objects; n = 23 Pgf+/+ and 20 Pgf−/− male and female mice. *P < 0.05, **P < 0.01, ****P < 0.0001.
Fig. 5.
Pgf−/− mouse performance on the SDT by sex. In the first trial of the SDT, there was no difference in time spent moving (A) between Pgf+/+ and Pgf−/− males and females, although Pgf+/+ females reared significantly more times than Pgf−/− females (B). There was also no difference in percent of time spent in the center between groups (C). Time spent exploring (D) and number of visits made (E) to the objects decreased over the trials but with no significant difference between groups. Likewise, total number of visits made to the objects over the entire SDT was not different between groups (F). Pgf+/+ males, Pgf+/+ females and Pgf−/− males spent significantly more time exploring the changed object than the unchanged objects in the New Location trial (G). However, none of the groups spent significantly more time exploring the changed object(s) relative to the unchanged objects in the Substitution (H), Spatial Switch (I), or Addition (J) trials. No clear sex difference in object recognition memory was apparent. Data were analyzed by two-way ANOVAs and linear mixed models as appropriate. Graphs show means ± SD. Circles represent Pgf+/+ mice, while squares represent Pgf−/− mice. Black bars show the change in time spent investigating the changed object(s), while light gray bars show the change in time investigating the unchanged objects; n = 23 Pgf+/+ and 20 Pgf−/− male and female mice. *P < 0.05, **P < 0.01, ***P < 0.001.
Both Pgf−/− and Pgf+/+ mice spent less time investigating the objects in each subsequent trial with no difference between the genotypes on any trial (Fig. 4D) or with respect to total time investigating the objects over the entire SDT (data not shown). Number of visits to the objects also declined over the trials, indicating acclimatization (Fig. 4E). Although no significant difference between Pgf−/− and Pgf+/+ mice was identified on any individual trial, Pgf−/− mice tended to make fewer visits each trial and made significantly fewer visits to the objects over the entire SDT (P = 0.017, Fig. 4F), suggesting reduced exploratory behavior. No sex*genotype interaction was present (Fig. 5, D–F).
Object memory and attention were examined in the New Location, Substitution, Spatial Switch, and Addition trials of the SDT. Both Pgf−/− and Pgf+/+ mice discriminated between the changed object in a New Location and the unchanged objects, showing a greater increase in time investigating the changed object (P = 0.0076 for Pgf−/− mice and P < 0.0001 for Pgf+/+ mice, Fig. 4G) and a greater number of visits (P = 0.0009 for Pgf−/− mice and P = 0.0001 for Pgf+/+ mice, data not shown). Only Pgf−/− mice significantly discriminated between the changed and unchanged objects in the Substitution trial (P = 0.0362, Fig. 4H). Neither the Pgf−/− or the Pgf+/+ mice discriminated between the changed and unchanged objects in the Spatial Switch (Fig. 4I) or Addition trials, although both nonsignificantly tended to spend more time with the changed object in the Addition trial (Fig. 4J). Pgf−/− mice also made significantly more visits to the Addition object than to the unchanged objects (P = 0.0392, data not shown). There was no sex*genotype interaction for any of the trials (Fig. 5, G–J).
Smaller Brain Structure Volumes in Pgf−/− Mice
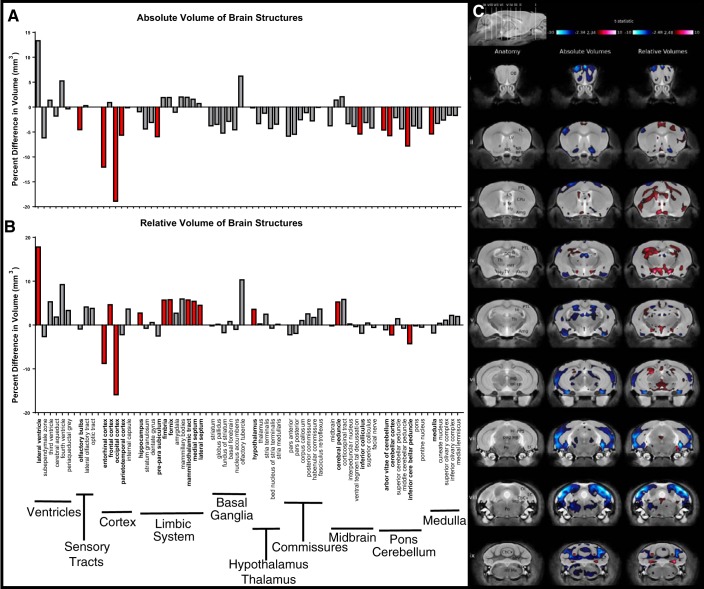
MRI structural analysis revealed significant changes to the absolute and relative volumes of Pgf−/− brain structures. The Pgf−/− brain was 3.6% smaller in volume than the Pgf+/+ brain. Absolute volume of 10 of 62 specific structures (the arbor vitae of cerebellum, cerebellar cortex, inferior cerebellar peduncle, entorhinal cortex, occipital lobe, parieto-temporal lobe, inferior colliculus, medulla, olfactory bulbs, and preparasubiculum) were smaller in Pgf−/− compared with Pgf+/+ mice (Fig. 6A). The occipital cerebral cortex and entorhinal cerebral cortex in particular had large percent differences between Pgf−/− and Pgf+/+ mice. The fourth and lateral ventricles had nonsignificantly larger absolute volumes, supporting an overall decrease in brain size. Relative volume analysis identified significantly smaller relative volume in four of 62 brain structures (the cerebellar cortex, inferior cerebellar peduncle, entorhinal cortex, and occipital lobe) and significantly larger relative volume in 10 of 62 structures: the frontal lobe, cerebral peduncle, fimbria, fornix, hippocampus, hypothalamus, lateral septum, lateral ventricle, mammilothalamic tract, and medial septum (Fig. 6B). Similarly, according to voxel-wise analysis, significant decreases in absolute volume throughout the Pgf−/− brain were counterbalanced by voxel-wise increases in relative volume in adjacent regions (Fig. 6C). Males and females were not examined separately as there was no significant genotype*sex interaction with respect to brain structure volume. Absolute volumes of 62 structures were tested for significant correlations with performance on behavioral testing (Table 1). Volume of areas in the hippocampus correlated with time immobile during the TST, and the volume of the habenular commissure and pontine nucleus correlated with performance on the NOR and SDT. No correlations were significant after correction for multiple comparisons.
Fig. 6.
Brain structure volumes in Pgf−/− mice. MR structural analysis of mice that underwent behavioral testing (n = 8 males and females of each genotype with 1 Pgf−/− male excluded for poor image quality) revealed significant differences in the absolute volume of 10/62 brain structures between the Pgf+/+ and Pgf−/− mice (A). The difference in relative volume was significant in 14/62 areas with 4 areas smaller and 10 areas larger in the Pgf−/− mice (B). Graphs show percent difference with significance indicated by filled red bars. Voxel-wise comparison revealed size differences in widespread areas of the Pgf−/− brain seen in coronal sections (i–ix) of the MR scan (C). Significantly smaller (blue) absolute volume is complimented by significantly larger (red) relative volumes in other areas. Structures are identified as: abv, arbor vita of cerebellum; Amg, amygdala; BFB, basal forebrain; CbCx, cerebellar cortex; ml, corpus callosum; cp, cerebral peduncle; CPu, caudate/putamen; DG, dentate gyrus; EC, entorhinal cortex; fi, fimbria; FL, frontal lobe; fx, fornix; Hi, hippocampus; Hy, hypothalamus; icp, inferior cerebellar peduncle; LS, lateral septum; LV, lateral ventricle; MB, midbrain; mtt, mammillothalamic tract; Me, medulla, ml, medial lemniscus; MS, medial septum; NA, nucleus accumbens; OB, olfactory bulb; OL, occipital lobe; pag, periaqueductal gray matter; Po, pons; PPS, pre-parasubiculum; PTL, parietotemporal lobe; sc, superior colliculus; scp, superior cerebellar peduncle; sm, stria medullaris; Th, thalamus; TV, third ventricle. Data were analyzed with a false discovery rate of 0.1 for multiple comparisons.
Table 1.
Significant correlations between brain structure absolute volumes and performance on behavioral testing
| Behavioral Test | Brain Region | R | P Value | BH-adjusted P Value |
|---|---|---|---|---|
| YMSAT - percent alternation | mammillary bodies | −0.375 | 0.0376 | >0.999 |
| TST - time immobile | cerebellar peduncle: inferior | 0.593 | 0.00288 | 0.179 |
| dentate gyrus of hippocampus | 0.492 | 0.0171 | 0.531 | |
| medulla | 0.466 | 0.0251 | 0.520 | |
| stratum granulosum of hippocampus | 0.458 | 0.0279 | 0.432 | |
| NOR test - preference for novel object | basal forebrain | −0.358 | 0.0480 | 0.743 |
| habenular commissure | −0.465 | 0.00846 | 0.525 | |
| pontine nucleus | −0.385 | 0.0323 | 0.668 | |
| third ventricle | −0.407 | 0.0230 | 0.714 | |
| SDT trial 1 - total time moving | amygdala | 0.355 | 0.0499 | 0.442 |
| cerebral cortex: frontal lobe | 0.443 | 0.0125 | 0.387 | |
| habenular commissure | 0.426 | 0.0167 | 0.259 | |
| mammillary bodies | 0.489 | 0.00521 | 0.323 | |
| olfactory tubercle | 0.368 | 0.0415 | 0.429 | |
| pontine nucleus | 0.429 | 0.0160 | 0.331 | |
| subependymale zone: rhinocele | 0.377 | 0.0368 | 0.456 | |
| SDT trial 1 - percent of time spent in center of arena | cerebellar peduncle: middle | 0.396 | 0.0275 | 0.244 |
| cerebral peduncle | 0.411 | 0.0218 | 0.225 | |
| habenular commissure | 0.420 | 0.0188 | 0.291 | |
| lateral olfactory tract | 0.395 | 0.0279 | 0.216 | |
| olfactory tubercle | 0.490 | 0.00518 | 0.321 | |
| optic tract | 0.412 | 0.0213 | 0.264 | |
| pontine nucleus | 0.480 | 0.00624 | 0.193 | |
| stria terminalis | 0.429 | 0.0161 | 0.333 | |
| SDT - total time visiting objects | anterior commissure: pars anterior | 0.463 | 0.00944 | 0.195 |
| cerebellar peduncle: middle | 0.368 | 0.0426 | 0.377 | |
| cerebral peduncle | 0.398 | 0.0274 | 0.283 | |
| habenular commissure | 0.462 | 0.00889 | 0.276 | |
| olfactory tubercle | 0.412 | 0.0220 | 0.341 | |
| pontine nucleus | 0.530 | 0.00248 | 0.154 | |
| Stria terminalis | 0.408 | 0.0236 | 0.293 |
B-H, Benjamini-Hochberg; YMSAT, Y-maze spontaneous alternation test; TST, tail suspension test; NOR, novel object recognition; SDT, serial dishabituation test.
Greater Number of Cerebral Vessels in Pgf−/− Mice
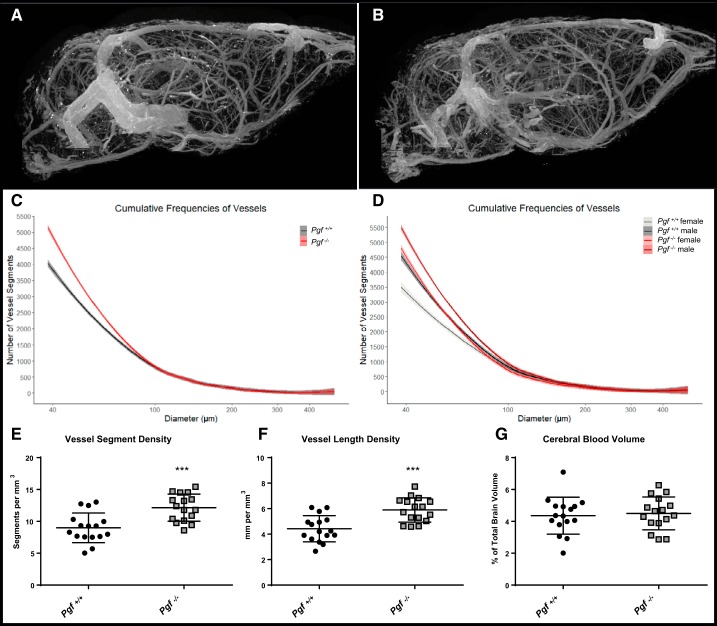
Representative 2D images of the 3D µCT scans of the cerebral vessels are shown in Fig. 7, A and B. The effect of genotype on the spline model coefficients was significant (F = 60.3615, P = 2.982(10−12). Pgf−/− mice had a greater number of small-diameter vessels than Pgf+/+ mice (Fig. 7C), although there was no difference in the number of large-diameter vessels. There was a significant genotype*sex interaction (F = 4.3828, P = 0.0384), although both male and female Pgf−/− mice tended to have more small-diameter vessels than Pgf+/+ male and female mice (Fig. 7D). Vessel segment density was significantly greater in Pgf−/− mice (P = 0.002, Fig. 7E) as was vessel length density (P = 0.004, Fig. 7F). However, there was no significant difference in cerebral blood volume between the two genotypes (P = 0.719, Fig. 7G). There was no significant genotype*sex interaction for either vessel segment density, vessel length density, or cerebral blood volume.
Fig. 7.
Cerebrovasculature in Pgf−/− mice. Microcomputed tomography (µCT) imaging of the cerebral vasculature in Pgf+/+ (A) and Pgf−/− (B) mice (n = 8 males and 8 females of each genotype) revealed Pgf−/− mice had greater numbers of 40–100 µm diameter vessel segments in the brain (C). Both male and female Pgf−/− mice had more small-diameter vessels relative to Pgf+/+ controls (D). Vessel segment density (E) and vessel length density (F) were greater in Pgf−/− mice, but cerebral blood volume was not different (G). Cumulative frequency histograms were analyzed with fitted spline models and linear modeling of the spline coefficients. Vessel segment density, vessel length density, and cerebral blood volume were compared by unpaired two-tail t-tests. Graphs show averaged cumulative frequency histograms with 95% confidence interval or means ± SD. In the cumulative frequency histograms, Pgf+/+ mice are represented by gray and Pgf−/− mice represented by red with females and males are differentiated by darker and lighter shades, respectively. Black circles represent Pgf+/+, while gray squares represent Pgf−/− mice. ***P < 0.001.
DISCUSSION
Our study examined cognitive function, neuroanatomy and cerebral vessels in mice lacking PGF. Cognitive alterations seen in Pgf−/− mice included impairments in spatial working memory, less depressive-like behavior, and less exploratory behavior. Genotype*sex interactions were not observed during cognitive behavioral testing in our study, despite the use of the NOR test and the SDT, which are reported to be sensitive to sex differences (6). Sex differences are reported in humans after in utero insults with respect to the “brain-sparing” phenomenon (2). Developmental insults appear to affect cognitive function in males to a greater extent than in females (65, 78). Furthermore, maternal circulating PGF is known to be higher in normal human pregnancies with a male rather than female fetus (24). Because neuroanatomical alterations also lacked sex*genotype interactions, total lack of PGF in the mouse model likely has a sex-independent effect that does not fully model the in utero effects of a low-PGF PE gestation and respective adaptation of the male or female fetus.
Two measurements were calculated to compare the brain volume in the Pgf−/− and Pgf+/+ mice. The absolute volume is straightforward to interpret, while the relative volume removes the overall brain volume differences, which should make the measurements more sensitive to subtle effects. A reduction in total brain volume and reduced absolute volume in 10 of 62 brain structures, including relatively large reductions in occipital lobe (−19.8% difference), and entorhinal cortex (−12.1% difference) accompanied the cognitive changes seen in Pgf−/− mice. These volume reductions may relate to impairments in visual processing (object recognition) and spatial navigation (7, 29, 36). In particular, the entorhinal cortex volume is a brain area intricately linked with the hippocampus for spatial memory and navigation (29, 36). Alterations to these structures may correspond to impaired performance on the YMSAT and less exploration of objects on the NOR test and SDT. However, no correlations between the absolute volume of brain structures and performance on behavioral testing were significant after we corrected for multiple comparisons. Altered relative numbers of glia and neurons could also explain cognitive behavioral differences that do not correlate to brain regional volumes if an increased number of glial cells obscures the reduced number of neurons when structure size is examined. One of the strengths of MRI-based brain morphology is high sensitivity; however, specificity is poor, with a range of potential cellular changes explaining these increases in volume. Previous studies by our group and others have shown that MRI is sensitive to changes in cell number, dendrite density, and expansion of extracellular space. Despite this, volume may not correspond to function.
When volumes relative to total brain volume were compared between genotypes, 10/62 structures were larger relative to total brain volume, likely resulting from smaller absolute volume of other structures and the brain overall. In Pgf−/− mice, the hippocampus, fimbria, fornix, and mammilothalamic tract relative volumes were larger, seeming to correspond to larger relative volume of the amygdala in our pilot study of PE-F1 children. Similarly, in Pgf−/− mice, the cerebral peduncle relative volume was larger, perhaps corresponding to larger brain-stem size in PE-F1s (62). However, the other brain areas identified to have larger size in these two studies, the hypothalamus, frontal lobe, medial septum, lateral septum, and lateral ventricle in Pgf−/− mice and the cerebellum and temporal lobe in PE-F1s (62), did not correspond. In fact, relative volumes of the cerebellar cortex and inferior cerebellar peduncle were smaller rather than larger in Pgf−/− mice. In PE-F1s, altered cerebellar architecture links to impaired performance on eye-tracking studies with increased velocity, decreased accuracy, and a greater number of saccades (63). PE-F1s also demonstrated impaired memory for names but improved performance on the memory-guided eye-tracking task (63). Although none of the cognitive behavioral tests in mice directly parallel these outcomes in children, Pgf−/− mice exhibit altered spatial memory. In both Pgf−/− mice and PE-F1s, altered behavior and cognitive performance may relate to disrupted balance between regions of the brain rather than to a difference in one specific structure.
In PE-F1 children, vessel radius was significantly smaller in the occipital and parietal lobes (62). Pgf−/− mice also display altered cerebrovasculature with a significant increase in the number of small-diameter vessels. This increase in the number of small-diameter vessels translates to an increase in vessel segment and vessel length density in the brain. However, cerebral blood volume was similar in Pgf−/− and Pgf+/+ mice, suggesting that the greater number of small vessels in the Pgf−/− brain does not result in increased blood supply. PGF is well-known as an arteriogenic and vessel maturation factor during angiogenesis (30, 60). In the Pgf−/− antimesometrial decidua during early pregnancy, thin, unbranched vessels are present instead of mature well-branched vessels (61). This is consistent with our observation of an increase in the number of small vessels in the brain. VEGF expression has previously been reported as upregulated in Pgf−/− mice, perhaps compensating for the loss of PGF (12). In this case, decreased vessel maturation and enlargement could be compensated for by an increase in VEGF-driven angiogenesis, resulting in no overall difference in blood supply. Since PGF has both angiogenic (12) and neuroprotective abilities (22), it is difficult to temporally separate the neural effects of PGF deficiency from the vascular changes. Altered vascularity may affect neural development, or, alternatively, altered neural development may impair vascularization. PGF is known to bind the NRP1 receptor (52, 55), a receptor important in neural and vascular organization (26, 56, 79). Both Pgf−/− mice and mice lacking the cytoplasmic domain of the NRP1 receptor exhibit higher frequencies of arteriovenous crossovers in the retina (25, 40). Although NRP1 has other ligands including semaphorin 3A and VEGF164, the PGF/NRP1 interaction may be important for neural and vascular organization during development.
We have suggested that PE-F1 cognitive and neuroanatomical alterations result from globally low production of PGF in the fetus that coincides with decreased placental production. PGF is expressed by human and mouse oocytes and embryos from the 1-cell to blastocyst stages [Gene Expression Omnibus (GEO) accession GDS12034 (41), GEO accession GDS18290 (81), GEO accession GDS1749 (84)], thus low placental PGF expression may reflect low PGF expression by embryonic cell lineages after epigenetic downregulation of PGF before or during blastocyst formation. Altered placental and embryonic angiogenic factors have previously been linked in the context of congenital heart defects. Maternal circulating PGF is low when fetuses have a congenital heart defect in the first (50), second (51), and third trimesters (18). In these pregnancies with abnormal embryonic development, maternal risk of PE is also higher (66). Brain expression of angiogenic players including Vegfr1, Hif2α, and Vegf but not Hif1α or Pgf is altered in fetuses with congenital heart defects (67). Similarly, greater nuchal translucency by ultrasound at 10–11 wk of human gestation has been linked to differing mRNA expression of 101 genes in placental chorionic villi (27). Placental dysfunction in PE may result from inappropriate maternal vascular adaptation, pre-existing impairments in the blastocyst, or a combination of these factors from each contributing individual. Where placental dysfunction and altered fetal development are linked, mechanisms like decreased PGF expression in the placenta may offer clues into compromised fetal development.
Other animal models of PE also demonstrate brain structural anomalies and cognitive dysfunction in the offspring. In a soluble FLT-1 (sFLT)-induced model of PE in mice, sex-specific changes in neuroanatomy were found by MRI, including smaller volume of the fimbria and greater volume of the neocortex in males (13). Developmental exposure to sFlt-modeled PE also altered motor outcomes in male and female offspring (14). These deficits were partially corrected through pravastatin administration (13, 14), which has been shown to induce placental PGF expression in a similar model (42). Another model using NG-nitro-l-arginine methyl ester (L-NAME) to induce a PE-like phenotype in rats reported decreased offspring cortical thickness at postnatal day 0, as well as an increased number of glial cells and decreased hippocampal neurogenesis in adulthood (48). L-NAME model offspring showed impaired spatial memory and navigation in the water maze test (48, 85) as well as impaired performance on a conditional discrimination Y-maze task (15). In these antiangiogenic models and in the Pgf−/− mouse, neuroanatomy of the hippocampus and associated structures appears to be vulnerable. Spatial memory and learning are also impaired on behavioral testing in both the Pgf−/− and L-NAME models. A limitation of the current knockout mouse model is its lifelong absence of PGF. Developmental insult would be initiated by the PGF deficiency, but subsequent fetal and or postnatal angiogenic and neurological compensation may occur. Thus, it is impossible to differentiate between the developmental effects of PGF deficiency from chronic, life-long outcomes from PGF deficiency. Future use of a developmentally regulated PGF gene deletion model would aid in discrimination between gestational and life-long effects of PGF deficiency. This should be combined with clinical studies in which levels of PGF are measured at critical postnatal times in individuals confirmed to have been born to preeclamptic women with low gestational maternal plasma PGF concentrations. Although no current animal model of PE is fully representative of the human syndrome, we propose the Pgf−/− mouse as a strong genetic model of impaired offspring neural development in PE. The current study provides baseline data for the Pgf−/− mouse by advanced imaging technologies. As a next step, PGF supplementation of Pgf−/− offspring should be assessed during the neonatal window of brain plasticity to determine if this time period is suitable for interventions to normalize behavioral, neuroanatomical, and vascular deficits that arose during fetal development.
Conclusion
Our data provide new insights into the importance of PGF expression during pregnancy and development, particularly in relation to cognitive function, brain neuroanatomy, and the cerebral vasculature of offspring. We propose the Pgf−/− mouse as a preclinical model for offspring effects of low-PGF PE gestation and for testing potential neonatal therapeutics.
GRANTS
This work was supported by awards from the Natural Sciences and Engineering Research Council of Canada (B. A. Croy) and the Canadian Foundation for Innovation (B. A. Croy). M. T. Rätsep and V. R. Kay are supported by Frederick Banting and Charles Best Canada Graduate Scholarships. B. Zavan was supported by training fellowships from CAPES’ Science without Borders Program (B. Zavan). B. A. Croy is supported by the Canada Research Chairs program. The work of P. Carmeliet is supported by the Belgian Science Policy of the Federal Government BELSPO – IUAP P7/03, long-term structural funding by the Flemish Government – Methusalem funding, and the Flemish Science Fund - FWO grants.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
V.R.K., M.T.R., A.F.H., B.Z., and C.L.L. performed experiments; V.R.K., M.T.R., L.S.C., A.F.H., M.E.N., and J.E. analyzed data; V.R.K., M.T.R., L.S.C., and B.A.C. interpreted results of experiments; V.R.K., M.T.R., and L.S.C. prepared figures; V.R.K. and M.T.R. drafted manuscript; V.R.K., M.T.R., J.N.R., C.T., J.G.S., and B.A.C. edited and revised manuscript; V.R.K., M.T.R., L.S.C., A.F.H., B.Z., M.E.N., J.E., J.N.R., P.C., C.T., J.G.S., and B.A.C. approved final version of manuscript; M.T.R., J.G.S., and B.A.C. conceived and designed research.
ACKNOWLEDGMENTS
The authors thank Dr. Brian Bennett and Ahmed Elharram, Queen’s University, Kingston, Canada, for provision of equipment and expertise used in the behavioral testing experiments.
REFERENCES
- 1.Aasa KL, Zavan B, Luna RL, Wong PG, Ventura NM, Tse MY, Carmeliet P, Adams MA, Pang SC, Croy BA. Placental growth factor influences maternal cardiovascular adaptation to pregnancy in mice. Biol Reprod 92: 44, 2015. doi: 10.1095/biolreprod.114.124677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker DJ, Lampl M, Roseboom T, Winder N. Resource allocation in utero and health in later life. Placenta 33, Suppl 2: e30–e34, 2012. doi: 10.1016/j.placenta.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Beck H, Acker T, Püschel AW, Fujisawa H, Carmeliet P, Plate KH. Cell type-specific expression of neuropilins in an MCA-occlusion model in mice suggests a potential role in post-ischemic brain remodeling. J Neuropathol Exp Neurol 61: 339–350, 2002. doi: 10.1093/jnen/61.4.339. [DOI] [PubMed] [Google Scholar]
- 4.Benovská M, Opluštilová A, Pinkavová J, Hodická Z, Cermáková Z. The new possibilities in early diagnosis of preeclampsia by soluble fms-like tyrosine kinase-1 and placental growth factor in 16–20 weeks gestation. Lab Med 49: 112–117, 2017. doi: 10.1093/labmed/lmx076. [DOI] [PubMed] [Google Scholar]
- 5.Bergen NE, Bouwland-Both MI, Steegers-Theunissen RP, Hofman A, Russcher H, Lindemans J, Jaddoe VW, Steegers EA. Early pregnancy maternal and fetal angiogenic factors and fetal and childhood growth: the Generation R Study. Hum Reprod 30: 1302–1313, 2015. doi: 10.1093/humrep/dev070. [DOI] [PubMed] [Google Scholar]
- 6.Bettis TJ, Jacobs LF. Sex differences in memory for landmark arrays in C57BL/J6 mice. Anim Cogn 16: 873–882, 2013. doi: 10.1007/s10071-013-0619-x. [DOI] [PubMed] [Google Scholar]
- 7.Boccara CN, Sargolini F, Thoresen VH, Solstad T, Witter MP, Moser EI, Moser MB. Grid cells in pre- and parasubiculum. Nat Neurosci 13: 987–994, 2010. doi: 10.1038/nn.2602. [DOI] [PubMed] [Google Scholar]
- 8.Bock NA, Nieman BJ, Bishop JB, Mark Henkelman R. In vivo multiple-mouse MRI at 7 Tesla. Magn Reson Med 54: 1311–1316, 2005. doi: 10.1002/mrm.20683. [DOI] [PubMed] [Google Scholar]
- 9.Cahill LS, Laliberté CL, Ellegood J, Spring S, Gleave JA, Eede MC, Lerch JP, Henkelman RM. Preparation of fixed mouse brains for MRI. Neuroimage 60: 933–939, 2012. doi: 10.1016/j.neuroimage.2012.01.100. [DOI] [PubMed] [Google Scholar]
- 10.Can A, Dao DT, Terrillion CE, Piantadosi SC, Bhat S, Gould TD. The tail suspension test. J Vis Exp: e3769, 2012. doi: 10.3791/3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cariboni A, Davidson K, Dozio E, Memi F, Schwarz Q, Stossi F, Parnavelas JG, Ruhrberg C. VEGF signalling controls GnRH neuron survival via NRP1 independently of KDR and blood vessels. Development 138: 3723–3733, 2011. doi: 10.1242/dev.063362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, Wu Y, Bono F, Devy L, Beck H, Scholz D, Acker T, DiPalma T, Dewerchin M, Noel A, Stalmans I, Barra A, Blacher S, VandenDriessche T, Ponten A, Eriksson U, Plate KH, Foidart JM, Schaper W, Charnock-Jones DS, Hicklin DJ, Herbert JM, Collen D, Persico MG. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med 7: 575–583, 2001. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 13.Carver AR, Andrikopoulou M, Lei J, Tamayo E, Gamble P, Hou Z, Zhang J, Mori S, Saade GR, Costantine MM, Burd I. Maternal pravastatin prevents altered fetal brain development in a preeclamptic CD-1 mouse model. PLoS One 9: e100873, 2014. doi: 10.1371/journal.pone.0100873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carver AR, Tamayo E, Perez-Polo JR, Saade GR, Hankins GD, Costantine MM. The effect of maternal pravastatin therapy on adverse sensorimotor outcomes of the offspring in a murine model of preeclampsia. Int J Dev Neurosci 33: 33–40, 2014. doi: 10.1016/j.ijdevneu.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Cauli O, Herraiz S, Pellicer B, Pellicer A, Felipo V. Treatment with sildenafil prevents impairment of learning in rats born to pre-eclamptic mothers. Neuroscience 171: 506–512, 2010. doi: 10.1016/j.neuroscience.2010.08.065. [DOI] [PubMed] [Google Scholar]
- 16.Chakravarty MM, Steadman P, van Eede MC, Calcott RD, Gu V, Shaw P, Raznahan A, Collins DL, Lerch JP. Performing label-fusion-based segmentation using multiple automatically generated templates. Hum Brain Mapp 34: 2635–2654, 2013. doi: 10.1002/hbm.22092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chugh BP, Lerch JP, Yu LX, Pienkowski M, Harrison RV, Henkelman RM, Sled JG. Measurement of cerebral blood volume in mouse brain regions using micro-computed tomography. Neuroimage 47: 1312–1318, 2009. doi: 10.1016/j.neuroimage.2009.03.083. [DOI] [PubMed] [Google Scholar]
- 18.Curti A, Zucchini C, De Maggio I, Ismail YS, Morano D, Falcone V, Meriggiola MC, Farina A. Fetal cardiac defects and third-trimester maternal serum placental growth factor. Ultrasound Obstet Gynecol 45: 751–752, 2015. doi: 10.1002/uog.14748. [DOI] [PubMed] [Google Scholar]
- 19.Dachew BA, Mamun A, Maravilla JC, Alati R. Pre-eclampsia and the risk of autism-spectrum disorder in offspring: meta-analysis. Br J Psychiatry 212: 142–147, 2018. doi: 10.1192/bjp.2017.27. [DOI] [PubMed] [Google Scholar]
- 20.de Guzman AE, Wong MD, Gleave JA, Nieman BJ. Variations in post-perfusion immersion fixation and storage alter MRI measurements of mouse brain morphometry. Neuroimage 142: 687–695, 2016. doi: 10.1016/j.neuroimage.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 21.Dorr AE, Lerch JP, Spring S, Kabani N, Henkelman RM. High resolution three-dimensional brain atlas using an average magnetic resonance image of 40 adult C57Bl/6J mice. Neuroimage 42: 60–69, 2008. doi: 10.1016/j.neuroimage.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 22.Du H, Li P, Pan Y, Li W, Hou J, Chen H, Wang J, Tang H. Vascular endothelial growth factor signaling implicated in neuroprotective effects of placental growth factor in an in vitro ischemic model. Brain Res 1357: 1–8, 2010. doi: 10.1016/j.brainres.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res 31: 47–59, 1988. doi: 10.1016/0166-4328(88)90157-X. [DOI] [PubMed] [Google Scholar]
- 24.Enninga EA, Nevala WK, Creedon DJ, Markovic SN, Holtan SG. Fetal sex-based differences in maternal hormones, angiogenic factors, and immune mediators during pregnancy and the postpartum period. Am J Reprod Immunol 73: 251–262, 2015. doi: 10.1111/aji.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fantin A, Schwarz Q, Davidson K, Normando EM, Denti L, Ruhrberg C. The cytoplasmic domain of neuropilin 1 is dispensable for angiogenesis, but promotes the spatial separation of retinal arteries and veins. Development 138: 4185–4191, 2011. doi: 10.1242/dev.070037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fantin A, Vieira JM, Plein A, Denti L, Fruttiger M, Pollard JW, Ruhrberg C. NRP1 acts cell autonomously in endothelium to promote tip cell function during sprouting angiogenesis. Blood 121: 2352–2362, 2013. doi: 10.1182/blood-2012-05-424713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farina A, Volinia S, Arcelli D, Francioso F, Desanctis P, Zucchini C, Pilu G, Carinci P, Morano D, Pittalis MC, Calderoni P, Vagnoni S, Rizzo N. Evidence of genetic underexpression in chorionic villi samples of euploid fetuses with increased nuchal translucency at 10-11 weeks’ gestation. Prenat Diagn 26: 128–133, 2006. doi: 10.1002/pd.1373. [DOI] [PubMed] [Google Scholar]
- 28.Freitas-Andrade M, Carmeliet P, Stanimirovic DB, Moreno M. VEGFR-2-mediated increased proliferation and survival in response to oxygen and glucose deprivation in PlGF knockout astrocytes. J Neurochem 107: 756–767, 2008. doi: 10.1111/j.1471-4159.2008.05660.x. [DOI] [PubMed] [Google Scholar]
- 29.Fyhn M, Molden S, Witter MP, Moser EI, Moser MB. Spatial representation in the entorhinal cortex. Science 305: 1258–1264, 2004. doi: 10.1126/science.1099901. [DOI] [PubMed] [Google Scholar]
- 30.Gaál EI, Tammela T, Anisimov A, Marbacher S, Honkanen P, Zarkada G, Leppänen VM, Tatlisumak T, Hernesniemi J, Niemelä M, Alitalo K. Comparison of vascular growth factors in the murine brain reveals placenta growth factor as prime candidate for CNS revascularization. Blood 122: 658–665, 2013. doi: 10.1182/blood-2012-07-441527. [DOI] [PubMed] [Google Scholar]
- 31.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15: 870–878, 2002. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 32.Ghanavati S, Yu LX, Lerch JP, Sled JG. A perfusion procedure for imaging of the mouse cerebral vasculature by X-ray micro-CT. J Neurosci Methods 221: 70–77, 2014. doi: 10.1016/j.jneumeth.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Gishti O, Jaddoe VW, Felix JF, Reiss I, Hofman A, Ikram MK, Steegers EA, Gaillard R. Influence of maternal angiogenic factors during pregnancy on microvascular structure in school-age children. Hypertension 65: 722–728, 2015. doi: 10.1161/HYPERTENSIONAHA.114.05008. [DOI] [PubMed] [Google Scholar]
- 34.Grace T, Bulsara M, Pennell C, Hands B. Maternal hypertensive diseases negatively affect offspring motor development. Pregnancy Hypertens 4: 209–214, 2014. doi: 10.1016/j.preghy.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Hayashi T, Noshita N, Sugawara T, Chan PH. Temporal profile of angiogenesis and expression of related genes in the brain after ischemia. J Cereb Blood Flow Metab 23: 166–180, 2003. doi: 10.1097/01.WCB.0000041283.53351.CB. [DOI] [PubMed] [Google Scholar]
- 36.Jacobs J, Kahana MJ, Ekstrom AD, Mollison MV, Fried I. A sense of direction in human entorhinal cortex. Proc Natl Acad Sci USA 107: 6487–6492, 2010. doi: 10.1073/pnas.0911213107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jadli A, Ghosh K, Satoskar P, Damania K, Bansal V, Shetty S. Combination of copeptin, placental growth factor and total annexin V microparticles for prediction of preeclampsia at 10-14 weeks of gestation. Placenta 58: 67–73, 2017. doi: 10.1016/j.placenta.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 38.James-Todd T, Cohen A, Wenger J, Brown F. Time-specific placental growth factor (PlGF) across pregnancy and infant birth weight in women with preexisting diabetes. Hypertens Pregnancy 35: 436–446, 2016. doi: 10.3109/10641955.2016.1172085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kajantie E, Eriksson JG, Osmond C, Thornburg K, Barker DJ. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki birth cohort study. Stroke 40: 1176–1180, 2009. doi: 10.1161/STROKEAHA.108.538025. [DOI] [PubMed] [Google Scholar]
- 40.Kay VR, Tayade C, Carmeliet P, Croy BA. Influences of placental growth factor on mouse retinal vascular development. Dev Dyn 246: 700–712, 2017. doi: 10.1002/dvdy.24540. [DOI] [PubMed] [Google Scholar]
- 41.Kocabas AM, Crosby J, Ross PJ, Otu HH, Beyhan Z, Can H, Tam WL, Rosa GJ, Halgren RG, Lim B, Fernandez E, Cibelli JB. The transcriptome of human oocytes. Proc Natl Acad Sci USA 103: 14027–14032, 2006. doi: 10.1073/pnas.0603227103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumasawa K, Ikawa M, Kidoya H, Hasuwa H, Saito-Fujita T, Morioka Y, Takakura N, Kimura T, Okabe M. Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proc Natl Acad Sci USA 108: 1451–1455, 2011. doi: 10.1073/pnas.1011293108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lecarpentier E, Gris JC, Cochery-Nouvellon E, Mercier E, Touboul C, Thadhani R, Karumanchi SA, Haddad B. Angiogenic factor profiles in pregnant women with a history of early-onset severe preeclampsia receiving low-molecular-weight heparin prophylaxis. Obstet Gynecol 131: 63–69, 2018. doi: 10.1097/AOG.0000000000002380. [DOI] [PubMed] [Google Scholar]
- 44.Lecuyer M, Laquerrière A, Bekri S, Lesueur C, Ramdani Y, Jégou S, Uguen A, Marcorelles P, Marret S, Gonzalez BJ. PLGF, a placental marker of fetal brain defects after in utero alcohol exposure. Acta Neuropathol Commun 5: 44, 2017. doi: 10.1186/s40478-017-0444-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lerch JP, Carroll JB, Spring S, Bertram LN, Schwab C, Hayden MR, Henkelman RM. Automated deformation analysis in the YAC128 Huntington disease mouse model. Neuroimage 39: 32–39, 2008. doi: 10.1016/j.neuroimage.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 46.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350: 672–683, 2004. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 47.Leviton A, Ryan S, Allred EN, Fichorova RN, Michael O’Shea T, Kuban K, Dammann O; ELGAN Study Investigators . Antecedents and early correlates of high and low concentrations of angiogenic proteins in extremely preterm newborns. Clin Chim Acta 471: 1–5, 2017. doi: 10.1016/j.cca.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 48.Liu X, Zhao W, Liu H, Kang Y, Ye C, Gu W, Hu R, Li X. Developmental and functional brain impairment in offspring from preeclampsia-like rats. Mol Neurobiol 53: 1009–1019, 2016. doi: 10.1007/s12035-014-9060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luna RL, Kay VR, Rätsep MT, Khalaj K, Bidarimath M, Peterson N, Carmeliet P, Jin A, Croy BA. Placental growth factor deficiency is associated with impaired cerebral vascular development in mice. Mol Hum Reprod 22: 130–142, 2016. doi: 10.1093/molehr/gav069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Llurba E, Syngelaki A, Sánchez O, Carreras E, Cabero L, Nicolaides KH. Maternal serum placental growth factor at 11-13 weeks’ gestation and fetal cardiac defects. Ultrasound Obstet Gynecol 42: 169–174, 2013. doi: 10.1002/uog.12346. [DOI] [PubMed] [Google Scholar]
- 51.Llurba E, Sánchez O, Ferrer Q, Nicolaides KH, Ruíz A, Domínguez C, Sánchez-de-Toledo J, García-García B, Soro G, Arévalo S, Goya M, Suy A, Pérez-Hoyos S, Alijotas-Reig J, Carreras E, Cabero L. Maternal and foetal angiogenic imbalance in congenital heart defects. Eur Heart J 35: 701–707, 2014. doi: 10.1093/eurheartj/eht389. [DOI] [PubMed] [Google Scholar]
- 52.Mamluk R, Gechtman Z, Kutcher ME, Gasiunas N, Gallagher J, Klagsbrun M. Neuropilin-1 binds vascular endothelial growth factor 165, placenta growth factor-2, and heparin via its b1b2 domain. J Biol Chem 277: 24818–24825, 2002. doi: 10.1074/jbc.M200730200. [DOI] [PubMed] [Google Scholar]
- 53.Mann JR, McDermott S. Are maternal genitourinary infection and pre-eclampsia associated with ADHD in school-aged children? J Atten Disord 15: 667–673, 2011. doi: 10.1177/1087054710370566. [DOI] [PubMed] [Google Scholar]
- 54.Many A, Fattal A, Leitner Y, Kupferminc MJ, Harel S, Jaffa A. Neurodevelopmental and cognitive assessment of children born growth restricted to mothers with and without preeclampsia. Hypertens Pregnancy 22: 25–29, 2003. doi: 10.1081/PRG-120016791. [DOI] [PubMed] [Google Scholar]
- 55.Migdal M, Huppertz B, Tessler S, Comforti A, Shibuya M, Reich R, Baumann H, Neufeld G. Neuropilin-1 is a placenta growth factor-2 receptor. J Biol Chem 273: 22272–22278, 1998. doi: 10.1074/jbc.273.35.22272. [DOI] [PubMed] [Google Scholar]
- 56.Mukouyama YS, Gerber HP, Ferrara N, Gu C, Anderson DJ. Peripheral nerve-derived VEGF promotes arterial differentiation via neuropilin 1-mediated positive feedback. Development 132: 941–952, 2005. doi: 10.1242/dev.01675. [DOI] [PubMed] [Google Scholar]
- 58.Nieman BJ, van Eede MC, Spring S, Dazai J, Henkelman RM, Lerch JP. MRI to Assess Neurological Function. Curr Protoc Mouse Biol 8: e44, 2018. doi: 10.1002/cpmo.44. [DOI] [PubMed] [Google Scholar]
- 59.Nieman BJ, Flenniken AM, Adamson SL, Henkelman RM, Sled JG. Anatomical phenotyping in the brain and skull of a mutant mouse by magnetic resonance imaging and computed tomography. Physiol Genomics 24: 154–162, 2006. doi: 10.1152/physiolgenomics.00217.2005. [DOI] [PubMed] [Google Scholar]
- 60.Pipp F, Heil M, Issbrücker K, Ziegelhoeffer T, Martin S, van den Heuvel J, Weich H, Fernandez B, Golomb G, Carmeliet P, Schaper W, Clauss M. VEGFR-1-selective VEGF homologue PlGF is arteriogenic: evidence for a monocyte-mediated mechanism. Circ Res 92: 378–385, 2003. doi: 10.1161/01.RES.0000057997.77714.72. [DOI] [PubMed] [Google Scholar]
- 61.Rätsep MT, Carmeliet P, Adams MA, Croy BA. Impact of placental growth factor deficiency on early mouse implant site angiogenesis Placenta 35: 772–775, 2014. [Erratum in: Placenta 36: 614, 2015]. doi: 10.1016/j.placenta.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 62.Rätsep MT, Paolozza A, Hickman AF, Maser B, Kay VR, Mohammad S, Pudwell J, Smith GN, Brien D, Stroman PW, Adams MA, Reynolds JN, Croy BA, Forkert ND. Brain structural and vascular anatomy is altered in offspring of pre-eclamptic pregnancies: A pilot study. AJNR Am J Neuroradiol 37: 939–945, 2016. doi: 10.3174/ajnr.A4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rätsep MT, Hickman AF, Maser B, Pudwell J, Smith GN, Brien D, Stroman PW, Adams MA, Reynolds JN, Croy BA, Paolozza A. Impact of preeclampsia on cognitive function in the offspring. Behav Brain Res 302: 175–181, 2016. doi: 10.1016/j.bbr.2016.01.030. [DOI] [PubMed] [Google Scholar]
- 64.Rennie MY, Detmar J, Whiteley KJ, Yang J, Jurisicova A, Adamson SL, Sled JG. Vessel tortuousity and reduced vascularization in the fetoplacental arterial tree after maternal exposure to polycyclic aromatic hydrocarbons. Am J Physiol Heart Circ Physiol 300: H675–H684, 2011. doi: 10.1152/ajpheart.00510.2010. [DOI] [PubMed] [Google Scholar]
- 65.Reynolds SA, Roberts JM, Bodnar LM, Haggerty CL, Youk AO, Catov JM. Newborns of preeclamptic women show evidence of sex-specific disparity in fetal growth. Gend Med 9: 424–435, 2012. doi: 10.1016/j.genm.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 66.Ruiz A, Ferrer Q, Sánchez O, Ribera I, Arévalo S, Alomar O, Mendoza M, Cabero L, Carrerras E, Llurba E. Placenta-related complications in women carrying a foetus with congenital heart disease. J Matern Fetal Neonatal Med 29: 3271–3275, 2016. doi: 10.3109/14767058.2015.1121480. [DOI] [PubMed] [Google Scholar]
- 67.Sánchez O, Ruiz-Romero A, Domínguez C, Ferrer Q, Ribera I, Rodríguez-Sureda V, Alijotas J, Arévalo S, Carreras E, Cabero L, Llurba E. Brain angiogenic gene-expression in congenital heart disease. Ultrasound Obstet Gynecol [in press] 2017. doi: 10.1002/uog.18977. [DOI] [PubMed] [Google Scholar]
- 68.Sonek J, Krantz D, Carmichael J, Downing C, Jessup K, Haidar Z, Ho S, Hallahan T, Kliman HJ, McKenna D. First-trimester screening for early and late preeclampsia using maternal characteristics, biomarkers, and estimated placental volume. Am J Obstet Gynecol 218: 126.e1–126.e13, 2018. doi: 10.1016/j.ajog.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 69.Spencer Noakes TL, Henkelman RM, Nieman BJ. Partitioning k-space for cylindrical three-dimensional rapid acquisition with relaxation enhancement imaging in the mouse brain. NMR Biomed 30: e3802, 2017. doi: 10.1002/nbm.3802. [DOI] [PubMed] [Google Scholar]
- 70.Staff AC, Braekke K, Harsem NK, Lyberg T, Holthe MR. Circulating concentrations of sFlt1 (soluble fms-like tyrosine kinase 1) in fetal and maternal serum during pre-eclampsia. Eur J Obstet Gynecol Reprod Biol 122: 33–39, 2005. doi: 10.1016/j.ejogrb.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 71.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology (Berl) 85: 367–370, 1985. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 72.Tidwell SC, Ho HN, Chiu WH, Torry RJ, Torry DS. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. Am J Obstet Gynecol 184: 1267–1272, 2001. doi: 10.1067/mob.2001.113129. [DOI] [PubMed] [Google Scholar]
- 73.Tillo M, Erskine L, Cariboni A, Fantin A, Joyce A, Denti L, Ruhrberg C. VEGF189 binds NRP1 and is sufficient for VEGF/NRP1-dependent neuronal patterning in the developing brain. Development 142: 314–319, 2015. doi: 10.1242/dev.115998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Torry DS, Wang HS, Wang TH, Caudle MR, Torry RJ. Preeclampsia is associated with reduced serum levels of placenta growth factor. Am J Obstet Gynecol 179: 1539–1544, 1998. doi: 10.1016/S0002-9378(98)70021-3. [DOI] [PubMed] [Google Scholar]
- 75.Tuovinen S, Räikkönen K, Kajantie E, Pesonen AK, Heinonen K, Osmond C, Barker DJ, Eriksson JG. Depressive symptoms in adulthood and intrauterine exposure to pre-eclampsia: the Helsinki Birth Cohort Study. BJOG 117: 1236–1242, 2010. doi: 10.1111/j.1471-0528.2010.02634.x. [DOI] [PubMed] [Google Scholar]
- 76.Tuovinen S, Räikkönen K, Kajantie E, Leskinen JT, Henriksson M, Pesonen AK, Heinonen K, Osmond C, Barker D, Eriksson JG. Hypertensive disorders in pregnancy and intellectual abilities in the offspring in young adulthood: the Helsinki Birth Cohort Study. Ann Med 44: 394–403, 2012. doi: 10.3109/07853890.2011.573497. [DOI] [PubMed] [Google Scholar]
- 77.Tuovinen S, Eriksson JG, Kajantie E, Lahti J, Pesonen AK, Heinonen K, Osmond C, Barker DJ, Räikkönen K. Maternal hypertensive disorders in pregnancy and self-reported cognitive impairment of the offspring 70 years later: the Helsinki Birth Cohort Study. Am J Obstet Gynecol 208: 200.e1–200.e9, 2013. doi: 10.1016/j.ajog.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 78.van Wassenaer AG, Westera J, van Schie PE, Houtzager BA, Cranendonk A, de Groot L, Ganzevoort W, Wolf H, de Vries JI. Outcome at 4.5 years of children born after expectant management of early-onset hypertensive disorders of pregnancy. Am J Obstet Gynecol 204: 510.e1–510.e9, 2011. doi: 10.1016/j.ajog.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 79.Vieira JM, Schwarz Q, Ruhrberg C. Selective requirements for NRP1 ligands during neurovascular patterning. Development 134: 1833–1843, 2007. doi: 10.1242/dev.002402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu CS, Sun Y, Vestergaard M, Christensen J, Ness RB, Haggerty CL, Olsen J. Preeclampsia and risk for epilepsy in offspring. Pediatrics 122: 1072–1078, 2008. doi: 10.1542/peds.2007-3666. [DOI] [PubMed] [Google Scholar]
- 81.Xie D, Chen CC, Ptaszek LM, Xiao S, Cao X, Fang F, Ng HH, Lewin HA, Cowan C, Zhong S. Rewirable gene regulatory networks in the preimplantation embryonic development of three mammalian species. Genome Res 20: 804–815, 2010. doi: 10.1101/gr.100594.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xu Y, Luo J, Yue Z, Wu L, Zhang X, Zhou C, Zhao F, Wang X, Chen G. Increased expression of placental growth factor in patients with temporal lobe epilepsy and a rat model. Brain Res 1429: 124–133, 2012. doi: 10.1016/j.brainres.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 83.Xu Y, Zhang Y, Guo Z, Yin H, Zeng K, Wang L, Luo J, Zhu Q, Wu L, Zhang X, Chen D. Increased placental growth factor in cerebrospinal fluid of patients with epilepsy. Neurochem Res 37: 665–670, 2012. doi: 10.1007/s11064-011-0646-4. [DOI] [PubMed] [Google Scholar]
- 84.Zeng F, Baldwin DA, Schultz RM. Transcript profiling during preimplantation mouse development. Dev Biol 272: 483–496, 2004. doi: 10.1016/j.ydbio.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 85.Zhu H, Zhu W, Hu R, Wang H, Ma D, Li X. The effect of pre-eclampsia-like syndrome induced by L-NAME on learning and memory and hippocampal glucocorticoid receptor expression: A rat model. Hypertens Pregnancy 36: 36–43, 2017. doi: 10.1080/10641955.2016.1228957. [DOI] [PubMed] [Google Scholar]