Abstract
Gut microbiota are associated with a variety of complex polygenic diseases. The usage of broad-spectrum antibiotics by patients affected by such diseases is an important environmental factor to consider, because antibiotics, which are widely prescribed to curb pathological bacterial infections, also indiscriminately eliminate gut commensal microbiota. However, the extent to which antibiotics reshape gut microbiota and per se contribute to these complex diseases is understudied. Because genetics play an important role in predisposing individuals to these modern diseases, we hypothesize that the extent to which antibiotics influence complex diseases depends on the host genome and metagenome. The current study tests this hypothesis in the context of hypertension, which is a serious risk factor for cardiovascular diseases. A 3 × 2 factorial design was used to test the blood pressure (BP) and microbiotal effects of three different antibiotics, neomycin, minocycline, and vancomycin, on two well-known, preclinical, genetic models of hypertension, the Dahl salt-sensitive (S) rat and the spontaneously hypertensive rat (SHR), both of which develop hypertension, but for different genetic reasons. Regardless of the class, oral administration of antibiotics increased systolic blood pressure of the S rat, while minocycline and vancomycin, but not neomycin, lowered systolic blood pressure in the SHR. These disparate BP effects were accompanied by significant alterations in gut microbiota. Our study highlights the need to consider an individualized approach for the usage of antibiotics among hypertensives, as their BP could be affected differentially based on their individual genetic and microbiotal communities.
Keywords: blood pressure, microbiota, minocycline, neomycin, vancomycin
INTRODUCTION
The discovery and use of antibiotics played a dominant role in protecting humans from infectious diseases, which were a leading cause of death in the 19th and 20th centuries. As a result, human life expectancy has significantly climbed over the centuries (46). However, this rise in human lifespan is accompanied by a surge in diseases of the modern industrialized society such as hypertension, diabetes, colitis, several neurological disorders and cancer. Recent studies indicate that there are strong associations between gut microbiotal communities and each one of these modern illnesses that plaque humanity (1–4, 8, 10, 12–16, 18, 20, 24–26, 28–30, 34, 35, 37–39, 41, 43, 45). Because antibiotics not only eliminate pathogenic bacteria but also get rid of beneficial commensal bacteria, especially in the gut, this raises the question of safety in the usage of antibiotics by patients with such modern ailments.
Host genetics are an important factor contributing to complex polygenic diseases, which are the same modern illnesses mentioned above as also being impacted by microbiota. Together, the host genome and the collective genomes of the microbiota that reside within the host represent a holobiont, wherein the variation of the host genome along with the alterations presented by its metagenome act in concert to determine an individualized level of response to environmental factors influencing host health. Viewed from the context of usage of antibiotics as an environmental factor, this leads to the possibility that individual responses to antibiotics may vary depending on the host and its microbiota.
In this study, we test this possibility by using two distinct, widely used, genetically well-defined preclinical models of hypertension to simulate two individual populations with disparate genetic predisposition to the development of hypertension. The Dahl salt-sensitive (S) rat is a genetic model of hypertension that mimics features of essential hypertension with increased sensitivity to dietary salt and renal disease as especially noted in African American populations (7). Gut transplantation studies in this model have highlighted the contributions of microbiota in the regulation of hypertension (24). Compared with the S rat, the second preclinical model, the spontaneously hypertensive rat (SHR), is relatively salt insensitive and presents with hypertension in the absence of renal complications. The genetic factors driving hypertension in these two models are identified to be different, whereby we examined the null hypothesis that regardless of these variations on the host genomes, three different classes of antibiotics represented by neomycin (aminoglycoside), minocycline (tetracycline), and vancomycin (glycopeptide) would impart a similar direction of change on their blood pressure (BP). Our results disproved this null hypothesis. We found that in the S rat, antibiotic administration causes an elevation in BP, while in the SHR administration of the same antibiotic causes either a reduction in BP or no change. These disparate effects were correlated by distinct alterations in gut microbiotal compositions. Based on these observations, we propose that antibiotic usage could have individualized effects on hypertensive patients, which is determined by their own unique genetic and microbiota.
METHODS
Animals and Diet
All animals were from our colony maintained at the University of Toledo College of Medicine and Life Sciences. All animal procedures and protocols used were approved by the University of Toledo Institutional Animal Care and Use Committee. Experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The inbred Dahl salt-sensitive (SS/Jr or S) rat strain was from the animal colony maintained at The University of Toledo College of Medicine and Life Sciences. The SHR strain was originally obtained from Harlan Sprague Dawley (Indianapolis, IN). Rats were bred and maintained on a low-salt diet (0.3% NaCl, Harlan Teklad diet TD 7034; Madison, WI). The Harlan Teklad diet (TD94217) was used for experiments involving a high-salt regimen (2% NaCl).
BP Measurements by Radiotelemetry
All rats were weaned at 30 days of age and maintained on a low-salt diet (0.3% NaCl, Harlan Teklad) until 6 wk of age. At this time, S rats were switched to a high-salt diet (2% NaCl) for an additional period of 38 days, while SHR were maintained on a low-salt diet. After 38 days on a high-salt diet regimen for S rats (low-salt diet for SHR), rats were surgically implanted with radiotelemetry transmitters (HDS10) (Data Science International, St Paul, MN) as previously described by our laboratory (23). Rats were individually housed and allowed to recover from surgery for 3 days before BP was recorded. All rats were the same age at the time of surgery.
Antibiotic Administration
After the 3-day recovery period from the radiotelemetry surgery, the systolic blood pressures (SBP) were taken, and the rats were grouped. They received normal drinking water or water supplemented with neomycin (0.5 g/l, GIBCO), vancomycin [50 mg·kg−1·day−1 (Hospira)], or minocycline [50 mg·kg−1·day−1 (Novaplus)]. The water bottles were replaced once a week.
Collection of Fecal Content
Prior to sacrifice, fecal contents were collected from the rats. The fecal content of each animal was snap-frozen on dry ice and was stored at −80°C to be used at a later time.
Genomic DNA Isolation, 16S rRNA Gene Sequencing, and Analysis of Microbiotal Composition
Fecal DNA was extracted from one fecal pellet (~0.2 g) using QIAamp PowerFecal DNA kit (Qiagen). We followed the Illumina User Guide: 16S Metagenomic Sequencing Library Preparation-Preparing 16S Ribosomal RNA Gene Amplicons for the Illumina MiSeq System (part # 15044223 Rev. B). The 16S rRNA gene V3-V4 region was amplified by PCR using Illumina sequencing primers: 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG and 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGGACTACHVGGGTWTCTAAT. For index PCR, Nextera XT index kit (FC-131-1002) was used to attach dual indexes. For the 25 μl reaction mixture, each reaction was prepared using 1× reaction buffer (Invitrogen; Thermo Fisher Scientific, Waltham, MA), 0.5 U Taq polymerase (Invitrogen), 200 nM of each primer, 2 mM MgCl2, 0.2 mM dNTPs, and 2 μl of 5 ng/μl DNA (1st PCR) or purified PCR product (2nd PCR). The thermocycling was performed in a Bio-Rad ThermoCycler, and the cycling conditions were as follows: initial denaturation at 95°C for 5 min, followed by 25 cycles of 95°C for 30 s, 58°C for 30 s, 72°C for 30 s, and a final extension at 72°C for 5 min. Each PCR amplicon was purified in two rounds using AMPure XP beads (Beckman Coulter, Brea, CA). Concentration of purified index PCR products was measured with the Qubit dsDNA HS Assay kit with QubitR 3.0 fluorometer (LifeTechnologies, Carlsbad, CA). The 4 nM of each amplicon was pooled equally, and the pooled amplicon size was checked on a 2100 Bioanalyzer (Agilent). Following the Illumina User Guide Illumina MiSeq System, 10 pM denatured and diluted library was mixed with 10 pM PhiX control spike-in to be 10% PhiX in the final volume. Then, it was loaded on Illumina MiSeq Reagent Kit v3 with 2× 300 cycles.
Raw 16S sequencing data were processed and analyzed with a bioinformatics pipeline of multiple software including USEARCH (11) and Quantitative Insights Into Microbial Ecology (QIIME) software package (version 1.9.1) (5). Raw paired-end reads were merged to create consensus sequences and then quality filtered with USEARCH (11) (version 9). Chimeric sequences were identified and filtered with QIIME (5) combined with the USEARCH (version 6) algorithm. Open reference operational taxonomic units were subsequently picked with QIIME combined with the USEARCH (version 6) algorithm, and taxonomy assignment was performed with Greengenes (9) as reference database at the 97% similarity threshold. Alpha-diversity (PD_whole_tree) and beta-diversity (unweighted UniFrac metrics) were calculated with the QIIME package.
Short Chain Fatty Acid Analysis
We extracted 25 µl of plasma with acetonitrile spiked with internal standards, and the supernatant was then mixed with 200 mM 3-nitrophenylhydrazine and 120 mM N-(3-dimethylaminopropyl)-N1-ethylcarbodiimide in a 2:1:1 (vol/vol/vol) ratio. The samples were derivatized at 40°C for 30 min and then injected into an Agilent 6410 triple quadrupole mass spectrometer equipped with an electrospray ionization (22) source in negative-ion mode coupled to an Agilent 1290 infinity HPLC system with an Acquity UPLC BEH C18 column (2.1 × 100 mm, 1.7 µm; Waters, Milford MA). Solvent A was formic acid (0.01%, vol/vol) in water, and solvent B was formic acid (0.01%, vol/vol) in acetonitrile. Quantitation was performed by calibration to internal standards and standard curves on the Mass Hunter quantitative suite version B.06.00 (Santa Clara). All levels are expressed in µM (17, 24, 40).
Statistical Analysis
All statistical analysis was performed by a one-way ANOVA on GraphPad Prism 5.02, followed by either a Dunnett’s post hoc test for significance or a Tukey test for comparison, as noted in the text. A P value of <0.05 was considered to be significant.
RESULTS
Blood Pressure and Heart Rate
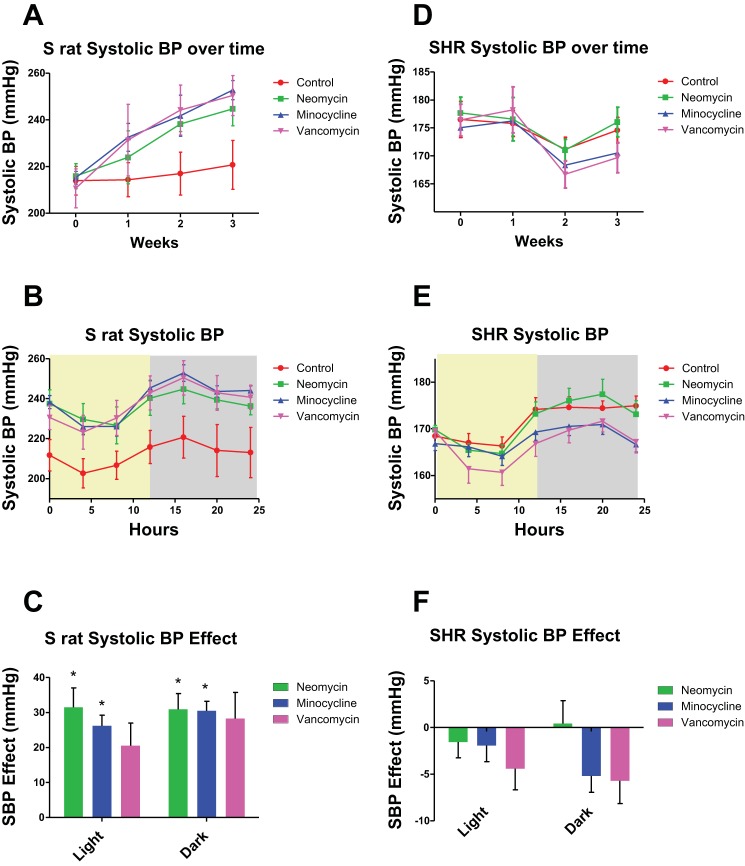
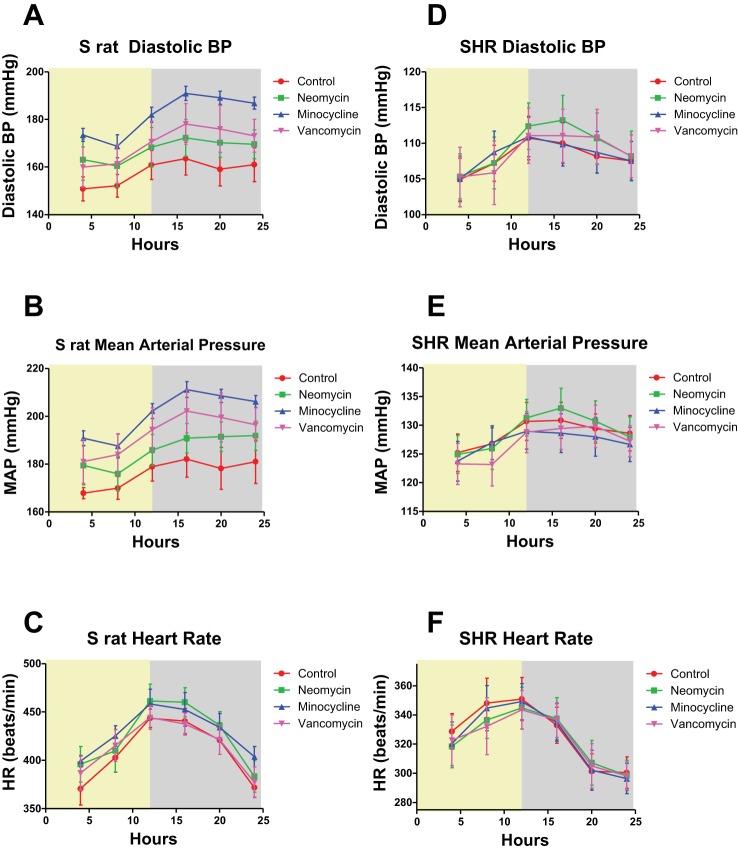
As seen in Fig. 1A, the S rats treated with neomycin, minocycline, or vancomycin had elevated SBP compared with controls over the 3 wk period. Importantly, the diurnal rhythms of the S rats given any of the three antibiotics were unchanged, as shown by the last 24 h BP recording in Fig. 1B. Moreover, when we compared the SBP of the S rats in the light and dark phases by a Dunnett’s post hoc test for significance, BP of animals receiving minocycline and neomycin were significantly increased compared with controls in both the light and dark phase. Animals treated with vancomycin were, however, not significantly different in either phase, despite the strong trend for an increased SBP (Fig. 1C). When comparing the BP effect in S rats and SHR by a Tukey test, we found significant differences for all three antibiotics in both the light and dark phases, indicating that the null hypothesis is indeed proven wrong. The diastolic BP (DBP) in the dark phase of S rats given minocycline was increased compared with the S rats without antibiotics; however, the other two antibiotics did not change the dark-phase DBP, and there were no changes in the light phase DBP (Fig. 2A). The mean arterial pressures (MAP) in the dark phase were significantly elevated in S rats given minocycline compared with controls but were not changed in the rats given vancomycin or neomycin, or any of the rats in the light phase (Fig. 2B). The heart rates (HR) were unchanged in any of the antibiotic treatment groups (Fig. 2C). In contrast, as seen in Fig. 1D, the SHR given minocycline or vancomycin had reduced SBP compared with controls, while neomycin did not alter the SBP. Again, the diurnal rhythms were not altered with antibiotic treatment (Fig. 1E). In SHR, none of the antibiotics caused a significant change in SBP in the light and dark phases, however minocycline and vancomycin had a strong trend towards decreasing SBP in both phases (Fig. 1F). In the SHR, the DBP, MAP, and HR were not changed (Fig. 2, D–F).
Fig. 1.
Dahl salt-sensitive (S) rats on antibiotics have an increased blood pressure (BP), while spontaneously hypertensive rats (SHR) on antibiotics do not. A: the systolic blood pressure (SBP) of S rats (n = 5) over 3 wk. Antibiotics were administered at week zero. Means and SEs are shown. There was no significance at time points 0 and 1 wk, while minocycline was significant at weeks 2 and 3. B: the 24 h recording from the last time point of A. The light phase is shown with a yellow background, while the dark phase has a gray background. Each data point represents a 4 h moving average. C: the SBP effect of each antibiotic on S rats. Means were calculated by subtracting the average SBP of the control rats from the average of each rat in the antibiotic groups for either the light or dark phase. Neomycin and minocycline were significantly (P < 0.05) higher than the control animals as calculated by ANOVA, followed by Dunnett’s post hoc test. D: the SBP of SHR (n = 6) over 3 wk. Antibiotics were administered at week zero. Means and SEs are shown. E: the 24 h recording from the last time point of D. The light phase is shown with a yellow background, while the dark phase has a gray background. Each data point represents a 4 h moving average. F: the SBP effect of each antibiotic on SHR. Means were calculated by subtracting the average SBP of the control rats from the average of each rat in the antibiotic groups for either the light or dark phase.
Fig. 2.
Diastolic blood press (DBP), mean arterial pressure (MAP), and heart rate (HR). A: the DBP recordings over the last 24 h in the 3 wk period of the same S rats as above. Each data point represents a 4 h moving average. The light phase is shown with a yellow background, while the dark phase has a gray background. Rats treated with minocycline had increased DBP in the dark phase only. B: the MAP of each of these S rats over the same 24 h period. Only minocycline showed significance and only in the dark phase. C: the HR of S rats over the 24 h period at the end of the 3 wk antibiotic treatment, and none of the rats showed significance. Means and SEs are shown. D: the DBP of SHR over 24 h from the end of the 3 wk period. E: the MAP of each of these SHR over the same 24 h period. F: the HR of these SHR in the same 24 h period. SHR had no significant differences.
Gut Microbiotal Diversity
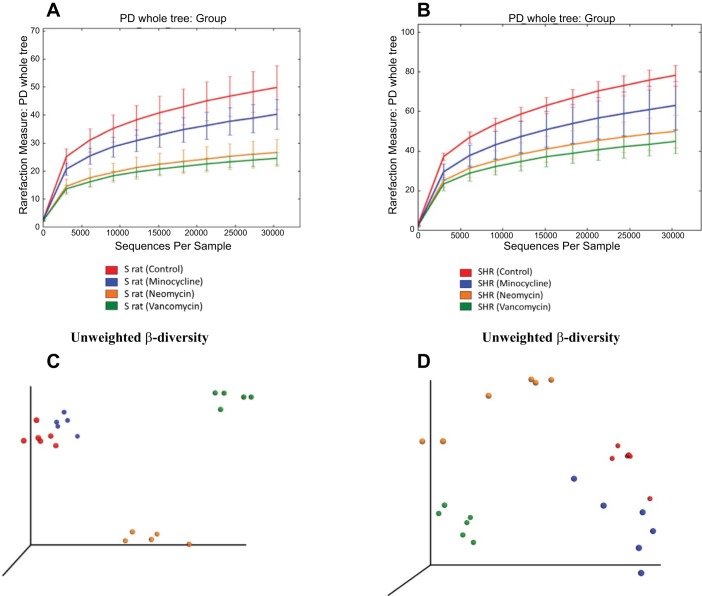
To determine the effect that gut microbiotal diversity has on BP with antibiotic treatment, both the alpha- and beta-diversities were analyzed. First, when comparing the alpha-diversity of controls, we found an increased diversity noted in the SHR compared with the S rat (Fig. 3, A and B). Second, each of the antibiotics has an effect on diversity in both strains. As seen in Fig. 3A, the S rats treated with neomycin had significantly reduced alpha-diversity (P = 0.002, P values for the last data points only) when compared with the control group. However, the S rats treated with minocycline did not have a significant difference in alpha-diversity. The S rats treated with vancomycin did have a significant reduction in alpha-diversity (P = 0.002, P values for the last data points only). In contrast, all three antibiotic treatment groups had significantly reduced alpha-diversities compared with controls in the SHR (Fig. 3B) with P values of 0.001, 0.028, and 0.002 (P values for the last data points only), respectively, for the neomycin, minocycline, and vancomycin treatment groups. Despite slight differences in significance levels, all three antibiotics caused a similar pattern of reducing diversity, regardless of the strain. The unweighted beta-diversities in the S rats are plotted in the principal coordinate analysis plots shown in Fig. 3C. The S rats treated with any antibiotic had distinct bacterial communities. The unweighted beta-diversities in the SHR are plotted in Fig. 3D. As shown, the SHR again showed significant changes in the bacterial communities.
Fig. 3.
Microbiotal diversity is reduced in S rats and SHR with antibiotic treatment. A: the alpha-diversity (PD_whole_tree) of the same S rats, both control and with antibiotics, whose BP are recorded in Fig. 1. Both neomycin and vancomycin had significantly reduced diversity (P = 0.002, P values for the last data points only) compared with controls, while minocycline was not significant. B: the alpha-diversity of SHR. All three antibiotics had a significantly reduced diversity than controls in SHR (neomycin P = 0.001, minocycline P = 0.028, and vancomycin P = 0.002). This analysis was conducted on fecal samples collected at the 3 wk time point from Fig. 1. Line colors: red, controls; blue, rats with minocycline; orange, rats with neomycin; green, rats with vancomycin. C: a principal coordinate analysis (PCoA) plot of the unweighted beta-diversity in S rats. The S rats treated with any antibiotic had a different unweighted beta-diversity. D: a PCoA plot of the unweighted beta-diversity in SHR. As shown, the SHR had significant changes in beta-diversity. Line colors: same as in A and B.
Taxonomic Comparisons
Phyla.
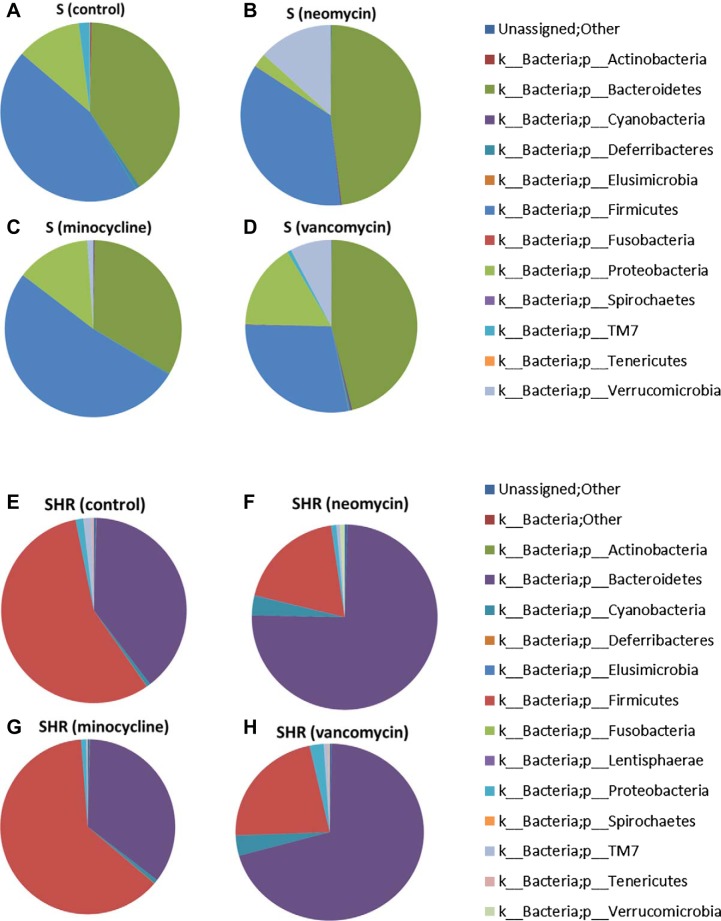
To determine the specific differences in the gut microbiota, each group was analyzed and plotted in Fig. 4. Importantly, S rats without any treatment (Fig. 4A) have different bacterial communities compared with SHR (Fig. 4E). While Bacteroidetes levels are similar between the two strains, there is a greater amount of Firmicutes in SHR than S rats. Additionally, S rats have greater amounts of Proteobacteria compared with SHR. These changes suggest that the genome selects for gut microbiota. However, these two strains of hypertensive rats were developed in the past in two different environments (Japan for the SHR and the US for S rats, at the time they were originally selected), so it is possible that the differences in gut microbiota were originally due to the varying environments. In addition, the S rats in this study were maintained on a high-salt diet, while the SHR were not. Salt is a known regulator of gut microbiota (44), and therefore, the possibility of salt having a role in the bacterial differences of the S rat and SHR should not be ignored. Antibiotics altered the phyla of both S rats and SHR. In the S rats treated with neomycin, there was an increase in the phyla Bacteroidetes, Cyanobacteria, Fusobacteria, and Verrucomicrobia, accompanied with a decrease in the phyla Actinobacteria, Deferribacteres, Firmicutes, Proteobacteria, TM7, and Tenericutes (Fig. 4, A and B). In the minocycline-treated S rats, there was an increase in Firmicutes, Proteobacteria, and Verrucomicrobia, while there was a decrease in Actinobacteria, Bacteroidetes, Cyanobacteria, Deferribacteres, TM7, and Tenericutes (Fig. 4, A and C). The vancomycin-treated S rats had an increase in the phyla Bacteroidetes, Cyanobacteria, Elusimicrobia, Fusobacteria, Proteobacteria, and Verrucomicrobia and a decrease in the phyla Actinobacteria, Deferribacteres, Firmicutes, TM7, and Tenericutes (Fig. 4, A and D). In SHR treated with neomycin, there was an increase in the phyla Bacteroidetes, Cyanobacteria, Elusimicrobia, and Verrucomicrobia, while there was a decrease in the phyla Firmicutes, Proteobacteria, TM7, and Tenericutes (Fig. 4, E and F). In the minocycline-treated SHR, there was an increase in the phyla Actinobacteria, Cyanobacteria, Deferribacteres, and Firmicutes, while there was a decrease in Bacteroidetes, Proteobacteria, TM7, and Tenericutes (Fig. 4, E and G). In the vancomycin-treated SHR, there was an increase in the phyla Bacteroidetes, Cyanobacteria, Proteobacteria, and Verrucomicrobia, and a decrease in Firmicutes, TM7, and Tenericutes (Fig. 4, E and H).
Fig. 4.
Phylum changes with antibiotic administration. The bacteria from fecal samples collected at 3 wk after antibiotic administration (n = 6/group) were identified by 16S sequencing. Shown are the phylum abundances. A–D: the S rats; E–H: the SHR.
Short Chain Fatty Acids
In the S rats, there were no significant changes in any of the short chain fatty acid (SCFA) levels measured (Fig. 5A). However, in SHR, proprionate levels were decreased with vancomycin and neomycin treatment (P < 0.05). Isovalerate was also reduced, but only with vancomycin treatment (Fig. 5B).
Fig. 5.
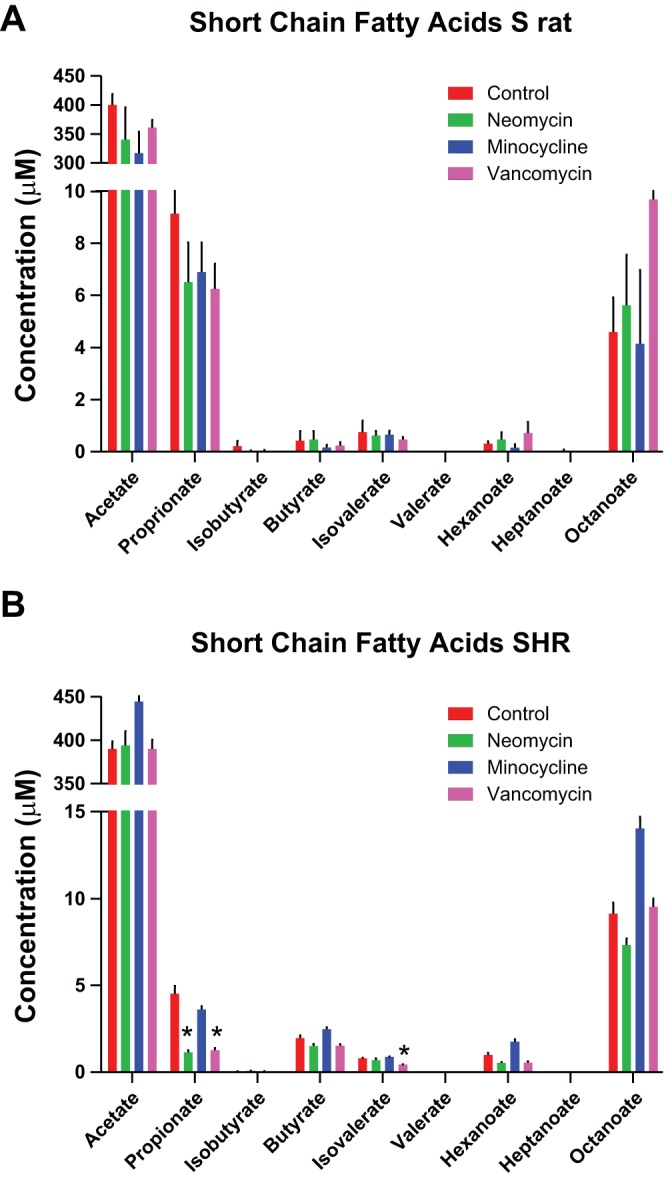
Short chain fatty acids (SCFAs) are altered in SHR with antibiotics. The levels of SCFAs in plasma at 3 wk after antibiotic administration (n = 6/group) were calculated and are shown. A: S rat SCFA levels; B: SCFA levels in SHR. SCFA levels are shown in μM (*P < 0.05).
DISCUSSION
This study aimed to determine if antibiotic administration affects BP, and we have found that this does indeed happen. However, surprisingly, each antibiotic does not alter BP in the same manner: the genetic background and gut microbiome of the rat determined how the BP was altered with antibiotics. One of the strains used in this study is the S rat, which is a salt-sensitive model of hypertension and is reported to represent features of the African American hypertensive patient population (7). For this study, we also used SHR, which are spontaneously hypertensive and do not exhibit a salt-sensitive hypertensive phenotype. In addition to the different types of hypertension these rats develop, there are also differences in other disease pathways. For example, S rats typically develop renal disease, while SHR are more prone to stroke. We found that in S rats the SBP are significantly elevated in both the light and dark cycle when they are treated with neomycin or minocycline. While the vancomycin did not cause a significant increase in SBP in either the light or dark phase, there is an evident trend for an increase in BP, with the average increase being 20–30 mmHg. This increase is substantial, and despite the lack of statistical significance, it is important to note for hypertensive patients. This increase in SBP was accompanied by an increase in DBP and MAP in rats treated with minocycline. The HR of these rats was not affected by any antibiotic.
In contrast, the light- and dark-phase SBP of SHR are not increased when the rats are treated with any of the three oral antibiotics. Minocycline and vancomycin caused a trend for a reduced SBP, with vancomycin having the largest effect. Again, while these changes were not significantly different, even a trend for reduction is important to note for hypertensive patients. The DBP and the MAP were not significant for any of the antibiotics tested. Additionally, the HR was not altered with any treatment. The discrepancy in these BP effects between S rats and SHR emphasizes the importance of studying the host genome-gut microbiome interactions because the divergent effects may be due to either the genome, the microbiome, or the interaction between the two. Moreover, reports in the literature have shown that minocycline can lower BP; however, this was found in Sprague Dawley rats that had angiontensin II-induced hypertension (45). This is a different strain of rats than what was used in this study, indicating again that the host genome has an effect on BP response to antibiotics. Additionally, minocycline is known to cross the blood-brain barrier, providing evidence that there may be confounding neurological variables acting in concert with the gut microbiota to prompt the disparate BP effects of this antibiotic.
Minocycline caused an increase in both the SBP and DBP of S rats; however, the other two antibiotics only caused an increase in SBP of S rats. While we do not know the exact mechanism of this primarily systolic hypertension, it is possible that metabolites from the gut microbiota are contributing to arterial stiffening and, therefore, the evident systolic hypertension. SCFAs are known metabolites of gut microbiota that contribute to systolic hypertension (27). However, in this study, there were no changes in SCFA levels in S rats with any antibiotic treatment. The only significant changes noted were in SHR treated with vancomycin and neomycin. Propionate levels were decreased with these two antibiotics, and isovalerate was reduced with vancomycin only (Fig. 5). However, in SHR there were no significant changes in BP. Therefore, there may be other bacterial metabolites that are affecting the BP changes noted.
Despite the discrepancies in BP effects of the antibiotics, the change in diversity in the gut microbiota is similar dependent on which antibiotic was administered. This is expected, as each antibiotic is most effective against a certain population of bacteria. Neomycin, an aminoglycoside, is most efficacious against gram-negative aerobes or facultative anaerobes, such as Proteobacteria (19), which was indeed the case in this study (Fig. 4). Minocycline, a tetracycline, is active against both gram-positive and gram-negative bacteria (6). Vancomycin, a glycopeptide antibiotic, inhibits cell wall synthesis and is therefore only active against gram-positive bacteria (33). This was seen in our study with a reduction in Firmicutes, which are gram-positive bacteria (Fig. 4). What is surprising is that similar alterations in these bacteria affect the host BP in different ways. This again points to the importance of assessing the host genome-gut microbiome interaction, rather than confining the assessment of such contributions to the microbiome alone.
Antibiotics are being progressively studied for their role in drug resistance. Therefore, more care is being taken when prescribing antibiotics to patients. While this is very important, perhaps a more important reason to avoid the prolific use of antibiotics is because they disturb gut microbiotal homeostasis and thereby contribute to pathophysiological consequences, depending on the patient genome and metagenome. There is already evidence for a role of antibiotics in human BP control. One case study showed that vancomycin treatment for an infection caused hypotension in a hypertensive patient, despite temporary discontinuation of her antihypertensive medications (31).
The Firmicutes/Bacteroidetes (F/B) ratio is often considered to be an indicator of gut dysbiosis and disease: a lower ratio is correlated with health, while a higher ratio is correlated with dysbiosis and disease. This ratio has been reported to correlate with the BP of the SHR and its normotensive counterpart, the Wistar Kyoto rat (WKY) (45). However, previously we have shown that the F/B ratio is not a good indicator of disease in the S rats, as the R rat has a higher F/B ratio than the S rat does, despite the reduced BP (24). In this study, we further confirmed that the F/B ratio does not correlate with elevated BP, in either the S rat or the SHR (Fig. 4, A–H). An increased F/B ratio was found in both S rats and SHR treated with minocycline, despite the opposite effects of minocycline on BP. Moreover, neomycin and vancomycin both caused a decreased F/B ratio, again despite the opposite BP effects observed in the two rat strains. These data suggest that the F/B ratio can be altered by antibiotics but does not have any correlation with BP.
In addition to the F/B ratio, there are reports that bacterial diversity is correlated with BP (45). Yang et al. (45) reported that administration of minocycline increased bacterial diversity in the SHR compared with WKY. However, the BP-lowering effect as a result of minocycline treatment in this study was not reported in the SHR, but with angiotensin II-infused Sprague Dawley rats. Our study indicates that in both in the S rat and SHR, bacterial diversity is reduced with any antibiotic (Fig. 3) and therefore not correlated with alterations in BP.
Specific bacteria have also been found to be regulators of health and disease (8, 15, 21, 32, 44). Some of the beneficial bacteria noted in the literature are Akkermansia and Lactobacillus. Akkermansia has been found to be beneficial for metabolic health, including protection from obesity and insulin resistance (8), and Lactobacillus has been found to prevent salt-induced hypertension (44). However, in this study, Akkermansia and Lactobacillus were not found to correlate with BP.
In addition to Lactobacillus, we have previously reported a negative correlation between S rat BP and Veillonellaceae (24). In this study, we saw differential changes of Veillonellaceae levels in the S rats; however, these changes were not associated with the BP changes in the S rat or SHR.
Sulfate-reducing bacteria, by increasing hydrogen sulfide levels, have also been implicated in BP regulation (36, 41, 42), and are therefore associated with host health. One of the sulfate-reducing bacteria is Desulfovibrio. In this study, S rats treated with any antibiotic had a reduction in Desulfovibrio, which could be one mechanism by which the BP of S rats is increased with antibiotic treatment. However, in SHR, Desulfovibrio levels are not associated with BP changes. Therefore, even though the sulfate-reducing bacteria might play a role in the S rat BP changes with antibiotics, they do not play a role in the SHR.
In conclusion, by using two strains of rats in this study, the S rat and SHR, we have shown that the host genome plays an important role in how BP will be affected differentially by antibiotic treatment. This highlights the importance of further studies to determine the mechanism behind these differing effects. Our study has important translational implications and serves as a basis for exploring similar disparities in human hypertensive patients.
GRANTS
This work was supported by Institutional funding from the University of Toledo College of Medicine to the University of Toledo Microbiome Consortium. A. V. Mathew acknowledges support from the National Institutes of Health (K08HL-130944), as does M. Vijay-Kumar (RO1 Grant CA-219144).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.G. and B.J. conceived and designed research; S.G., S.C., X.C., J.Y., B.M., H.Z., and A.V.M. performed experiments; S.G., S.C., X.C., J.Y., and A.V.M. analyzed data; S.G., S.C., X.C., M.V.-K., and B.J. interpreted results of experiments; S.G. prepared figures; S.G. drafted manuscript; S.G. and B.J. approved final version of manuscript; S.C., X.C., J.Y., B.M., H.Z., A.V.M., M.V.-K., and B.J. edited and revised manuscript.
REFERENCES
- 1.Adnan S, Nelson JW, Ajami NJ, Venna VR, Petrosino JF, Bryan RM Jr, Durgan DJ. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics 49: 96–104, 2017. doi: 10.1152/physiolgenomics.00081.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaut M. Gut microbiota and energy balance: role in obesity. Proc Nutr Soc 74: 227–234, 2015. doi: 10.1017/S0029665114001700. [DOI] [PubMed] [Google Scholar]
- 3.Boulangé CL, Neves AL, Chilloux J, Nicholson JK, Dumas ME. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med 8: 42, 2016. doi: 10.1186/s13073-016-0303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, Muccioli GG, Delzenne NM. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58: 1091–1103, 2009. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7: 335–336, 2010. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev 65: 232–260, 2001. doi: 10.1128/MMBR.65.2.232-260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowley AW., Jr Genetic and nongenetic determinants of salt sensitivity and blood pressure. Am J Clin Nutr 65, Suppl: 587S–593S, 1997. doi: 10.1093/ajcn/65.2.587S. [DOI] [PubMed] [Google Scholar]
- 8.Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L, Dumas ME, Rizkalla SW, Doré J, Cani PD, Clément K; MICRO-Obes Consortium . Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 65: 426–436, 2016. doi: 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- 9.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol 72: 5069–5072, 2006. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durgan DJ, Ganesh BP, Cope JL, Ajami NJ, Phillips SC, Petrosino JF, Hollister EB, Bryan RM Jr. Role of the Gut Microbiome in Obstructive Sleep Apnea-Induced Hypertension. Hypertension 67: 469–474, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26: 2460–2461, 2010. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 12.Festi D, Schiumerini R, Eusebi LH, Marasco G, Taddia M, Colecchia A. Gut microbiota and metabolic syndrome. World J Gastroenterol 20: 16079–16094, 2014. doi: 10.3748/wjg.v20.i43.16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galla S, Chakraborty S, Mell B, Vijay-Kumar M, Joe B. Microbiotal-Host Interactions and Hypertension. Physiology (Bethesda) 32: 224–233, 2017. doi: 10.1152/physiol.00003.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gérard P. Gut microbiota and obesity. Cell Mol Life Sci 73: 147–162, 2016. doi: 10.1007/s00018-015-2061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gkolfakis P, Dimitriadis G, Triantafyllou K. Gut microbiota and non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int 14: 572–581, 2015. doi: 10.1016/S1499-3872(15)60026-1. [DOI] [PubMed] [Google Scholar]
- 16.Goto Y, Kurashima Y, Kiyono H. The gut microbiota and inflammatory bowel disease. Curr Opin Rheumatol 27: 388–396, 2015. doi: 10.1097/BOR.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 17.Han J, Lin K, Sequeira C, Borchers CH. An isotope-labeled chemical derivatization method for the quantitation of short-chain fatty acids in human feces by liquid chromatography-tandem mass spectrometry. Anal Chim Acta 854: 86–94, 2015. doi: 10.1016/j.aca.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 18.Jose PA, Raj D. Gut microbiota in hypertension. Curr Opin Nephrol Hypertens 24: 403–409, 2015. doi: 10.1097/MNH.0000000000000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotra LP, Haddad J, Mobashery S. Aminoglycosides: perspectives on mechanisms of action and resistance and strategies to counter resistance. Antimicrob Agents Chemother 44: 3249–3256, 2000. doi: 10.1128/AAC.44.12.3249-3256.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lankelma JM, Nieuwdorp M, de Vos WM, Wiersinga WJ. The gut microbiota in internal medicine: implications for health and disease. Neth J Med 73: 61–68, 2015. [PubMed] [Google Scholar]
- 21.Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, Ballet V, Claes K, Van Immerseel F, Verbeke K, Ferrante M, Verhaegen J, Rutgeerts P, Vermeire S. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 63: 1275–1283, 2014. doi: 10.1136/gutjnl-2013-304833. [DOI] [PubMed] [Google Scholar]
- 22.Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM, Thomas LV, Zoetendal EG, Hart A. The gut microbiota and host health: a new clinical frontier. Gut 65: 330–339, 2016. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mell B, Abdul-Majeed S, Kumarasamy S, Waghulde H, Pillai R, Nie Y, Joe B. Multiple blood pressure loci with opposing blood pressure effects on rat chromosome 1 in a homologous region linked to hypertension on human chromosome 15. Hypertens Res 38: 61–67, 2015. doi: 10.1038/hr.2014.134. [DOI] [PubMed] [Google Scholar]
- 24.Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, Haribabu B, Vijay-Kumar M, Pennathur S, Joe B. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics 47: 187–197, 2015. doi: 10.1152/physiolgenomics.00136.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miele L, Giorgio V, Alberelli MA, De Candia E, Gasbarrini A, Grieco A. Impact of Gut Microbiota on Obesity, Diabetes, and Cardiovascular Disease Risk. Curr Cardiol Rep 17: 120, 2015. doi: 10.1007/s11886-015-0671-z. [DOI] [PubMed] [Google Scholar]
- 26.Nagao-Kitamoto H, Kitamoto S, Kuffa P, Kamada N. Pathogenic role of the gut microbiota in gastrointestinal diseases. Intest Res 14: 127–138, 2016. doi: 10.5217/ir.2016.14.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Natarajan N, Hori D, Flavahan S, Steppan J, Flavahan NA, Berkowitz DE, Pluznick JL. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein-coupled receptor 41. Physiol Genomics 48: 826–834, 2016. doi: 10.1152/physiolgenomics.00089.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pekkala S, Munukka E, Kong L, Pöllänen E, Autio R, Roos C, Wiklund P, Fischer-Posovszky P, Wabitsch M, Alen M, Huovinen P, Cheng S. Toll-like receptor 5 in obesity: the role of gut microbiota and adipose tissue inflammation. Obesity (Silver Spring) 23: 581–590, 2015. doi: 10.1002/oby.20993. [DOI] [PubMed] [Google Scholar]
- 29.Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, Petersen KF, Kibbey RG, Goodman AL, Shulman GI. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature 534: 213–217, 2016. doi: 10.1038/nature18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan L-X, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA 110: 4410–4415, 2013. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi Y, Aranda JM, Rodriguez V, Raizada MK, Pepine CJ. Impact of antibiotics on arterial blood pressure in a patient with resistant hypertension - A case report. Int J Cardiol 201: 157–158, 2015. doi: 10.1016/j.ijcard.2015.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ray A, Dittel BN. Interrelatedness between dysbiosis in the gut microbiota due to immunodeficiency and disease penetrance of colitis. Immunology 146: 359–368, 2015. doi: 10.1111/imm.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reynolds PE. Structure, biochemistry and mechanism of action of glycopeptide antibiotics. Eur J Clin Microbiol Infect Dis 8: 943–950, 1989. doi: 10.1007/BF01967563. [DOI] [PubMed] [Google Scholar]
- 34.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology 134: 577–594, 2008. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 35.Sekirov I, Russell SL, Antunes LCM, Finlay BB. Gut microbiota in health and disease. Physiol Rev 90: 859–904, 2010. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 36.Shen X, Carlström M, Borniquel S, Jädert C, Kevil CG, Lundberg JO. Microbial regulation of host hydrogen sulfide bioavailability and metabolism. Free Radic Biol Med 60: 195–200, 2013. doi: 10.1016/j.freeradbiomed.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh V, Chassaing B, Zhang L, San Yeoh B, Xiao X, Kumar M, Baker MT, Cai J, Walker R, Borkowski K, Harvatine KJ, Singh N, Shearer GC, Ntambi JM, Joe B, Patterson AD, Gewirtz AT, Vijay-Kumar M. Microbiota-Dependent Hepatic Lipogenesis Mediated by Stearoyl CoA Desaturase 1 (SCD1) Promotes Metabolic Syndrome in TLR5-Deficient Mice. Cell Metab 22: 983–996, 2015. doi: 10.1016/j.cmet.2015.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh V, Kumar M, San Yeoh B, Xiao X, Saha P, Kennett MJ, Vijay-Kumar M. Inhibition of Interleukin-10 Signaling Induces Microbiota-dependent Chronic Colitis in Apolipoprotein E Deficient Mice. Inflamm Bowel Dis 22: 841–852, 2016. doi: 10.1097/MIB.0000000000000699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341: 569–573, 2013. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Theriot CM, Koenigsknecht MJ, Carlson PE Jr, Hatton GE, Nelson AM, Li B, Huffnagle GB, Li JZ, Young VB. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat Commun 5: 3114, 2014. doi: 10.1038/ncomms4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomasova L, Dobrowolski L, Jurkowska H, Wrobel M, Huc T, Ondrias K, Ostaszewski R, Ufnal M. Intracolonic hydrogen sulfide lowers blood pressure in rats. Nitric Oxide 60: 50–58, 2016. doi: 10.1016/j.niox.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 42.Tomasova L, Drapala A, Jurkowska H, Wrobel M, Ufnal M. Na2S, a fast-releasing H2S donor, given as suppository lowers blood pressure in rats. Pharmacol Rep 69: 971–977, 2017. doi: 10.1016/j.pharep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 43.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 328: 228–231, 2010. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, Haase S, Mähler A, Balogh A, Markó L, Vvedenskaya O, Kleiner FH, Tsvetkov D, Klug L, Costea PI, Sunagawa S, Maier L, Rakova N, Schatz V, Neubert P, Frätzer C, Krannich A, Gollasch M, Grohme DA, Côrte-Real BF, Gerlach RG, Basic M, Typas A, Wu C, Titze JM, Jantsch J, Boschmann M, Dechend R, Kleinewietfeld M, Kempa S, Bork P, Linker RA, Alm EJ, Müller DN. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 551: 585–589, 2017. doi: 10.1038/nature24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, Sahay B, Pepine CJ, Raizada MK, Mohamadzadeh M. Gut dysbiosis is linked to hypertension. Hypertension 65: 1331–1340, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zaffiri L, Gardner J, Toledo-Pereyra LH. History of antibiotics: from fluoroquinolones to daptomycin (Part 2). J Invest Surg 26: 167–179, 2013. doi: 10.3109/08941939.2013.808461. [DOI] [PubMed] [Google Scholar]