Abstract
The following review summarizes the pro-con debate about current controversies regarding the pathogenesis of pulmonary arterial hypertension (PAH) that took place at the American Thoracic Society Conference in May 2017. Leaders in the field of PAH research discussed the importance of the immune system, the role of hemodynamic stress and endothelial apoptosis, as well as bone morphogenetic protein receptor-2 signaling in PAH pathogenesis. Whereas this summary does not intend to resolve obvious conflicts in opinion, we hope that the presented arguments entice further discussions and draw a new generation of enthusiastic researchers into this vibrant field of science to bridge existing gaps for a better understanding and therapy of this fatal disease.
INTRODUCTION
Medicine is not only a science, it is also an art; it deals with the very processes of life, which must be understood before they may be guided. ~Philippus Aureolus Paracelsus (1494–1541)
Over the past 20 years, research in pulmonary arterial hypertension (PAH) has skyrocketed and has resulted in a much-appreciated, deeper insight into the genetics, risk factors, cellular players, signaling pathways, and disease mechanisms of PAH. As commonly observed in science, “schools of thought” have developed with proponents and opponents providing experimental evidence for their respective beliefs without having the opportunity to debate and refute publicly each others’ ideas. Therefore, we aimed to give leaders in the field of PAH research the opportunity to debate each other during the “Pro-Con Debate: Current Controversies in PAH pathogenesis,” which was held at the American Thoracic Society International Conference in Washington, DC, in May 2017.
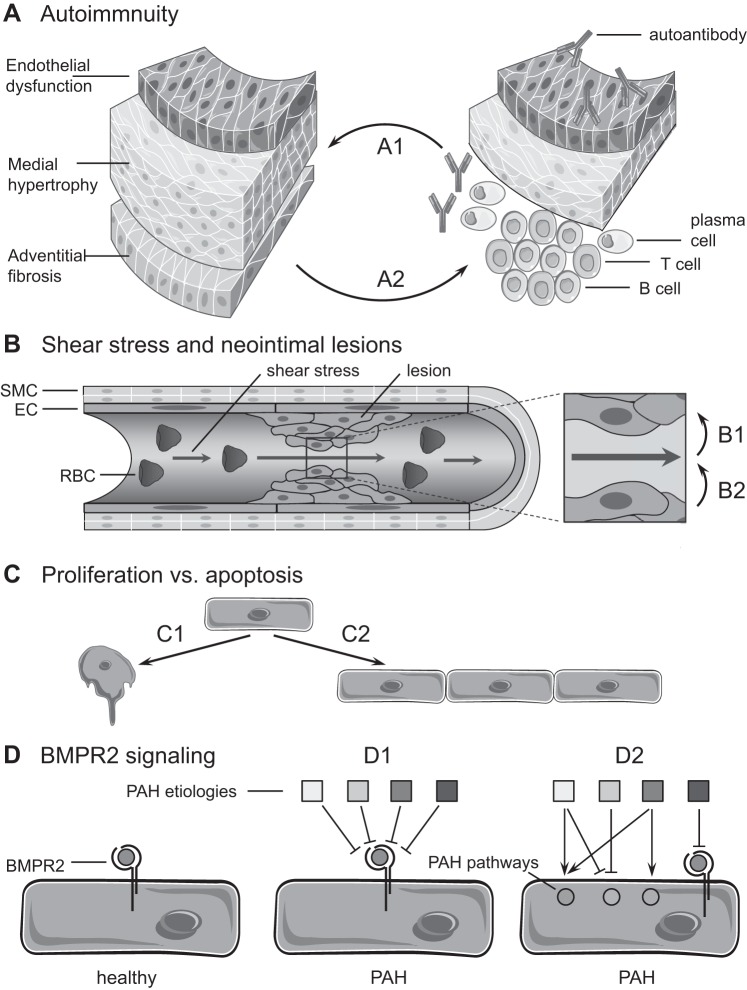
Topics that were addressed were the importance of the immune system, the role of hemodynamic stress and endothelial apoptosis, as well as bone morphogenetic protein receptor-2 (BMPR2) signaling in PAH pathogenesis (Fig. 1).
Fig. 1.
Schematic illustration of the conceptual controversies covered in the pro-con debate. Discussions focused on 4 key questions, namely the following: A: Is pulmonary arterial hypertension (PAH) an autoimmune disease (A1) or not (A2)? B: Does hemodynamic stress drive the formation of occlusive neointimal lesions in PAH (B1) or not (B2)? C: Is PAH a disease of endothelial cell (EC) apoptosis (C1) or proliferation (C2)? D: Is bone morphogenetic protein receptor type-2 (BMPR2) signaling the central therapeutic target in PAH (D1) or not (D2)? RBC, red blood cell; SMC, smooth muscle cell.
The debaters fought passionately with those in the audience, who were the final judges to declare debate winners. As we believe that this discourse is very much needed to advance our understanding of PAH pathogenesis and identify reliable and promising treatment targets (36, 42), here, we summarize the main points and counterpoints for each topic to make them available to the broader community with the goal to stimulate further discussion and research. Each argument is prefaced by brief summary of the key points, which are subsequently elaborated in a concise narrative. Enjoy!
THE ARGUMENTS
PAH Is an Autoimmune Disease, by Mark R. Nicolls
The key points of the argument are that several experimental pulmonary hypertension (PH) models fulfill the definition of an autoimmune disease, exhibiting dysfunctional regulatory T-cell (Treg) populations and autoantibodies; certain connective tissue diseases [CTDs; systemic lupus erythematosus (SLE) and systemic sclerosis] are considered autoimmune disorders and are strongly associated with PAH; even idiopathic PAH (IPAH) exhibits stigmata of an autoimmune disease with Treg disturbances, pulmonary inflammatory infiltrates, and high autoantibody titers; and furthermore, the lack of responsiveness to immune modulation is not proof that PAH is not an autoimmune disease yet may mean that the inflammatory injury is complete by the time PAH is detected.
PAH is an obliterative microangiopathy, strongly associated with activated lung and systemic immunity (11, 66), but whether it is fair to consider PAH an autoimmune process requires additional attributes beyond the presence of “bland” inflammation. To complicate matters, PAH is not a single disease but more likely, a syndrome with multiple triggers, culminating in a pulmonary vasculopathy. With these caveats, it seems likely that some forms of PAH may be the result of self-directed immune injury (i.e., autoimmunity).
Rules to help define autoimmune disease, modeled after Koch’s postulates, were developed by Witebsky in 1957 (122) and subsequently modified by Rose and Bona (92). These so-called “Witebsky postulates” include the following: the demonstration of disease transfer with inoculation of pathogenic antibodies or T cells into healthy animals, autologous reactivity of T cells in mixed lymphocyte reactions, indirect evidence based on the experimental creation of autoimmune disease in healthy animals, as well as circumstantial evidence in clinical disease.
In practice, some diseases are called “autoimmune” without fulfilling all of these postulates. The loss of protective immunity, such as a missing or dysfunctional Treg population, can similarly result in systemic autoimmunity (31, 32). PAH, as a cluster of conditions, does achieve some, if not all, of these criteria (18, 75, 84) and also occurs with Treg anomalies (41, 111). The passive transfer of autoantibodies directed against bronchus-associated lymphoid tissue produces pulmonary vascular remodeling and PAH in previously healthy animals (18). Treg-deficient rats develop severe inflammatory pulmonary vascular disease and PAH under conditions in which Treg-replete rats do not develop this disease (112).
Certain CTDs, such as SLE and systemic sclerosis, are considered autoimmune disorders and are strongly associated with PAH. Other associated disease features (e.g., lupus nephritis, autoimmune hemolytic anemia) are considered manifestations of autoimmunity, and so, in these less ambiguous cases, it appears reasonable to attribute PAH to the underlying autoimmune disorder. Even IPAH exhibits the stigmata of an autoimmune disease with the aforementioned Treg disturbances, pulmonary inflammatory infiltrates, and high autoantibody titers (78).
The fact that PAH does not always respond to immunosuppression is not, by itself, proof that PAH is not an autoimmune disease. The lack of responsiveness to immune modulation may mean that the inflammatory injury is complete by the time PAH is first detected. By analogy, immunotherapy does not typically work for type I diabetes, an autoimmune disease in which significant pancreatic β-cell destruction occurs before the condition is detected. Like type I diabetes, PAH may begin as a result of autoimmune injury but may progress due to nonautoimmune factors. The fact that PAH sometimes responds dramatically to immunosuppression in certain autoimmune conditions, such as lupus, is highly suggestive that PAH in these patients is autoimmune in origin.
Whereas the recent Study of Ubenimex in Patients with Pulmonary Arterial Hypertension (World Health Organization Group 1; or LIBERTY) trial, targeting leukotriene B4, was negative, an early study evaluating the safety of IL-1 antagonism with Anakinra for PAH is presently being conducted, and the results of the placebo-controlled, randomized B cell-depleting rituximab study for systemic sclerosis PAH, as well as the open-label IL-6-blocking Toclizumab trial, are particularly promising. These early phase clinical trials face significant obstacles, as the immunotherapies are being added to patients already on maximal vasodilation, a factor that makes these small studies inherently underpowered. However, even in the face of negative results, thoughtful analyses of patient subsets, biomarkers, and clinical trends should provide valuable information about the disease (and therapies), even if the primary outcomes are not achieved.
In summary, PAH is unquestionably an inflammatory disease, but the labeling of PAH as an autoimmune disease requires additional proof. The best argument in favor of PAH being an autoimmune manifestation is when it occurs in conditions that are undeniably autoimmune, such as the CTDs. As in other diseases considered to be autoimmune, PAH fulfills some (but not all) of the Witebsky postulates. Even IPAH exhibits features of an autoimmune disease. In some patients, PAH can be reversed [even cured (12, 47, 73)] with immunosuppression. Autoimmunity has a strong, explanatory force as a driver of certain types of PAH. This property facilitates experimentation, which capitalizes on known models of autoimmunity alleviation (such as the adoptive transfer of Tregs into immunodeficient animals) or causation (such as the inoculation of putatively pathogenic autoantibodies). These preclinical models mirror what is observed clinically, such as Treg anomalies and potentially, pathogenic autoantibodies in PAH patients. The cumulative evidence strongly points to autoimmune injury as a critical factor in the pathogenesis of PAH.
PAH Is Not an Autoimmune Disease, by Andrea Olschewski
The key points of the argument are that PAH does not fulfill the hallmarks of autoimmune diseases, as the autoantibody status fails to predict mortality risk in systemic sclerosis with PAH, no autoantibody reliably predicts the development of PAH, no cytokine reliably predicts the development of PAH, the female sex is a PAH-susceptibility factor only in younger age groups, and steroids are not effective in PAH except when the underlying disease is highly steroid responsive, such as SLE.
PAH is a complex and multifactorial disease with various phenotypes (99). PAH may develop in the context of several autoimmune diseases, and the association is clearly strongest in patients with scleroderma (SSc) and mixed CTD. Beyond increased perivascular immune cell accumulation and signs of vascular infiltration, circulating cytokines and chemokines are elevated in some PAH patients. The prevalence of PAH in subjects with CTD is ~25%, including patients with mixed CTD and SSc (38). In most cases of PAH that are associated with an autoimmune disease, the pathophysiology appears similar to that seen in IPAH, which clearly represents the largest patient group within group 1 PAH patients (28, 38). Although in both groups, circulating autoantibodies have been detected, analyses failed to find an association between serum autoantibodies and patients’ survival (37, 91). Moreover, a most recent study investigated a new type of autoantibodies directed against angiotensin type 1 and endothelin 1 receptors and found elevated levels of antibodies only in CTD-PAH and SSc-PAH patients (8). In all other forms, such as in IPAH, in PH associated with congenital heart disease or in chronic thromboembolic PH, the autoantibody level remained low, indicating that CTD-PAH or SSc-PAH but not IPAH might have an autoimmune origin. Furthermore, accumulation of circulating autoantibodies in SSc-PAH suggests that they may contribute to increased vascular endothelial reactivity; however, their relevance as predictive or even prognostic biomarkers has not been shown.
Histopathological evaluation demonstrates nearly identical pulmonary vascular lesions in patients with IPAH and in those with SSc-PAH, characterized by varying degrees of perivascular inflammatory infiltrates, comprising T and B lymphocytes, macrophages, dendritic cells, and mast cells (107). An interesting exception to this observation pertains to the plexiform lesions that appear to be monoclonal endothelial cell (EC) proliferations in IPAH but polyclonal in SSc-associated disease (116).
Inflammation precedes vascular remodeling in experimental PH, suggesting that inflammation plays a central role in the vascular disease. Several cytokines can directly control cell proliferation, migration, and differentiation of pulmonary vascular cells. Recent analyses of cytokine and chemokine levels in PAH support the notion that an overabundance of circulating cytokines or chemokines may exist (64, 102, 125). However, the results are contradictory and more importantly, no significant correlations between cytokine levels and hemodynamic parameters have been found.
In autoimmune diseases, there are two major clinical observations related to sex: first, females are more susceptible than males, and second, pregnancy reduces relapses in females (120). In IPAH, however, only a weak sex difference can be detected, especially if we look at the most prevalent age groups above 60 yr of age (38). In addition, only few preclinical models have shown autoimmunity, according to the hallmarks of an autoimmune disease, and none of them in an estrogen-related model of the disease, as they lack elevated levels of circulating autoantibodies, or cytokines, and they lack a response to steroids (123).
Finally, if PAH were such an inflammatory or autoimmune process, why is the mainstay therapy so clearly vasodilators (30)? Recent evidence indicates that both pulmonary vascular cells and inflammatory cells are important local sources of chemokines and cytokines that may lead to pulmonary vascular remodeling in PAH. It is still unclear whether such inflammatory processes are integral to the initiation and propagation of vascular remodeling or are just bystanders. Autoimmune diseases, such as systemic sclerosis or SLE, are acknowledged as causes of PAH. However, this PAH is not responsive to any treatment of the underlying disease. Thus strategies focusing on anti-inflammatory approaches or on alteration of immunity have strongly limited chances to treat PAH effectively.
Hemodynamic Stress Is the Most Important Driver in the Pathogenesis of Occlusive Neointimal Lesions in PAH, by Kohtaro Abe
The key points of the argument are that clinically meaningful pulmonary vasodilation has not been tested in patients yet due to systemic side effects; hemodynamic stress is sufficient to cause PAH; as the lung histopathology of all PAH patients is similar, independent of the PAH etiology, there must be a common mechanism that eventually leads to the similar vascular phenotype—hemodynamic stress; hemodynamic stress is necessary to cause PAH; and furthermore, hemodynamic stress leads to perivascular inflammation and occlusive neointimal lesion formation, creating a vicious cycle to decrease further the vascular luminal area and worsen PAH.
Is “vasodilators do not work in PAH” a definite conclusion? It is widely believed that vasoconstriction and subsequent hemodynamic factors play only a minor, if any, role in the pathophysiology of established PAH. This general belief stems from the following clinical findings: 1) no chronic treatment with currently available pulmonary vasodilators can cure or even halt PAH, and 2) merely a minority of patients with PAH at the time of diagnosis shows a positive response to acute vasodilator testing. There is, however, a pitfall in this seemingly solid conclusion; i.e., the most potent and effective pulmonary vasodilators have never been tested in PAH patients, largely due to their systemic side effects. In other words, we do not know what happens to pulmonary arterial pressure when the maximum dose of a potent vasodilator is administered to eliminate fully pulmonary vascular tone. Experimentally, we have found that a novel class of potent vasodilators—the Rho kinase inhibitor fasudil—markedly decreased (>50% reduction) the high right ventricular systolic pressure and total pulmonary resistance index at established stages in SU5416-hypoxia-exposed PAH rats, which closely simulate human PAH (2, 80, 115). The marked pulmonary vasodilation was observed only with higher doses of fasudil that also caused severe systemic hypotension. These experimental observations strongly suggest that an active pulmonary vascular tone exists in PAH that is clinically undetectable due to limitations in drug doses and/or potency. If this is the case in human PAH, then selective delivery of a high concentration of an unconventional vasodilator, such as fasudil, to the pulmonary circulation would work very effectively. Development of a pulmonary-selective drug delivery system is obviously needed to achieve this.
Hemodynamic stress is sufficient for development of occlusive neointimal lesions in PAH. It is true that not many patients with a congenital left-to-right shunt develop PAH. However, if the shunt is nonrestricted and post-tricuspid, then most (80–100%) of these patients develop PAH (22). This fact suggests that a single hemodynamic factor that satisfies certain criteria (a congenital left-to-right nonrestricted and post-tricuspid shunt) is sufficient to cause occlusive neointimal lesions and PAH. This notion is supported by animal models with a systemic-to-pulmonary shunt that develop occlusive neointimal lesions (96).
Is hemodynamic stress necessary for development of occlusive neointimal lesions in PAH? PAH, classified as group 1 PH, is apparently not a single disease but a group of diverse diseases that has three common major features, i.e., progressively severe PH with right heart failure, plexogenic arteriopathy on autopsy, and refractoriness to currently available pharmacological therapies. Among these features, plexogenic arteriopathy is a key feature, because it distinguishes this group of PH from others, and the development of small pulmonary occlusive neointimal lesions is likely the reason why this group of PH is progressive and refractory to therapies. Multiple “hits” are now considered a key requirement for development of plexogenic arteriopathy and PAH. There are numerous potential hits, including genetic and environmental factors. One important fact here is that all PAH patients exhibit a very similar, complex vascular phenotype independent of the cause or the combination of hits. This led us to think that there must be a certain mechanism that all forms have in common, which eventually leads to the similar vascular phenotype in this group of diverse diseases. With the consideration that hemodynamic stress alone is sufficient to cause PAH, we hypothesized that increased hemodynamic stress might be the common mechanism for the development of occlusive neointimal lesions and progressive PAH. We tested this hypothesis by using a unique combination of the SU5416-hypoxia model of PAH and a left pulmonary artery-banding technique to reduce hemodynamic stress only in the left lung (1). Results of this study clearly demonstrated that hemodynamic stress is necessary for the development and maintenance of occlusive neointimal lesions and is an upstream stimulus of perivascular inflammation. Based on these observations, we propose that hemodynamic stress and the following perivascular inflammation and occlusive neointimal lesion formation create a vicious cycle to decrease further the vascular luminal area and worsen PAH. We believe that this vicious cycle could be the common mechanism underlying a majority, if not all, of PAH cases. Although hemodynamic, stress-independent, occlusive neointimal lesions can be formed in cases, such as severe airway inflammation, the possibility that these cases develop PAH is unlikely. Whereas ~70% loss of pulmonary vascular volume is needed to induce mild PH, and much more loss (probably ~90%) is required for severe PH, morphological analyses of PAH lungs, obtained by biopsy at the time of diagnosis (or even by autopsy), do not appear to show such extended distribution of vessel occlusions (82, 107).
Hemodynamic Stress Is Not the Most Important Driver in the Pathogenesis of Occlusive Neointimal Lesions in PAH, by Marlene Rabinovitch
The key points of the argument are that structural remodeling precedes hemodynamic changes in many forms of PH, various animal models show signs of vascular remodeling and formation of neointimal and plexogenic lesions before onset of PH, PH is no longer reversible following pressure offloading in infants >2 yr, in animal models, pressure offloading reverses muscularization but not vessel loss, and furthermore, current vasodilator therapy does not reverse PH.
The initiation of structural remodeling by elevation in pulmonary arterial pressure is a feature of congenital heart disease and possibly heart failure with preserved or reduced ejection fraction but not the vast majority of cases of PH related to lung disease, schistosomiasis or HIV, drugs or toxins, autoimmunity, portal hypertension, sickle cell disease, or hereditary PAH or IPAH. The latter conditions are all associated with progressive remodeling and inflammation that ultimately lead to elevation in pressure when there are occlusive neointimal lesions and loss or obliteration of peripheral microvessels at the level of alveolar ducts and walls. In experimental studies in rats injected with the toxin monocrotaline (MCT), endothelial injury and structural remodeling precede the elevation in pulmonary arterial pressure. Only when there is baseline PH can we detect an increase in vasoreactivity in response to hypoxia or a vasoconstrictor, such as norepinephrine (93). Moreover, in experimentally induced schistosomiasis, extensive neointimal and plexogenic lesions occur without PH (34), and this is also seen in the transgenic mouse that overexpresses S100A4 following inoculation with murine gamma herpes virus (51, 104).
We have, however, investigated the extent to which reversibility of PH could occur following pressure offloading of the lung vasculature. This was preceded by studies in patients with a congenital heart defect in which it was shown that pulmonary vascular changes can regress following banding of the main pulmonary artery and could permit surgical correction without residual elevation in pulmonary vascular resistance (20). In our own studies, we found that reversibility of PH in a child with a congenital heart defect was dependent both on the age of the child and the severity of the vascular changes evident on a lung biopsy. When infants were repaired in the first 6 mo of life, even extensive, obliterative changes did not preclude return to normal pulmonary hemodynamics, as observed at a cardiac catheter study 1 yr later. This either reflects regression of the lesions, evident on the biopsy, or new growth of blood vessels, making those that are obliterated hemodynamically insignificant. In patients repaired at any age, reversibility of PH was possible if there was only medial hypertrophy. However, in ~50% of infants repaired beyond 9 mo of age and in all of those repaired beyond 2 yr of age, features of neointimal formation and occlusive lesions were associated with persistent or progressive increases in pulmonary artery pressure and resistance (83, 85). There have been anecdotal reports of single lung transplantation in patients with advanced pulmonary vascular disease in which the nontransplanted lung was examined after rejection of the donor lung, and the vascular disease had regressed. However, in these studies, it was uncertain whether the mechanism was related to pressure offloading or to the anti-inflammatory-immunosuppressive therapies.
We previously showed in experimental studies in the rat that banding of the left pulmonary artery prevented the vascular changes of increased muscularization following exposure to chronic hypoxia but not the loss of peripheral arteries (86). We also carried out studies where we transplanted a lung after established PH and vascular changes were induced by the toxin MCT. Here too, we found that we could reverse the intense increase in muscularity of the pulmonary circulation but not the loss of peripheral arteries (79). In the study by Abe and colleagues (1), rats were given the vascular endothelial growth factor inhibitor SU5416, followed by 3 wk of hypoxia and 2 wk in room air before they underwent left pulmonary artery banding to pressure offload the remodeled lung. After 5 wk of banding, the reversal of neointimal changes is impressive, as is the reduction of perivascular inflammation. However, the mortality is high, and this is a model that can spontaneously reverse the pulmonary hypertensive changes over time. This argues that although the vascular disease is impressive, it is still not reflective of the severity encountered in the clinical setting. Moreover, the feasibility of pressure offloading in the clinical setting must be considered. The creation of a pulmonary arterial band will further compromise an already vulnerable right ventricle that is under high pressure.
There is no evidence to date that current vasodilator therapy can reverse advanced pulmonary vascular changes that are associated with chronic perivascular inflammation (84). In contrast, use of agents that promote apoptosis of proliferating smooth muscle cells (SMCs) and restore BMPR2 signaling and endothelial health, such as the elastase inhibitor elafin (77), immunosuppressive FK506 (106), or BMPR2 ligand BMP9 (58), is effective in reversing experimental PH that was severe, as well as the abnormal phenotype observed in human pulmonary arterial ECs (PAECs) and SMCs.
PH Is a Disease of EC Apoptosis Not Proliferation, by Duncan Stewart
The key points of the argument are that PAH is a disease of microvascular rarefaction; EC injury and apoptosis are central triggers for the development of PAH; EC apoptosis leads to microvascular loss by either direct or indirect mechanisms, directly due to degeneration of fragile distal lung arterioles and indirectly, by leading to the emergence of growth-dysregulated, hyperproliferative cells; marked pruning of the arteriolar bed is an early finding in PAH models, occurring in parallel to increases in arterial pressures and before evidence of obliterative arterial remodeling; proliferative, occlusive arterial remodeling may be a consequence, not a cause, of hemodynamic abnormalities in experimental PAH; and the location of plexiform lesion development (the pulmonary vs. systemic vascular bed) might shed light on the pathomechanism.
PAH is a disease of microvascular rarefaction. The lung is unique among the body’s organs in that it must accommodate all of the cardiac output and does so at pressures that are a little more than venous. This remarkable feat is accomplished by virtue of the sheer extent of the lung microvasculature, such that only a fraction is needed under resting conditions, and low lung arterial pressure and impedance to blood flow are maintained even during peak exercise by the recruitment of this excess vascular capacity. Thus PH only begins to manifest after extensive loss of functional microvasculature. Lung vascular pruning has been observed in animal models of PH (52, 117, 127), as well as in PAH patients, with a close correlation of vascular volume with the disease severity (71, 72, 90). Together, the current literature strongly supports the idea that PAH is a disease of microvascular rarefaction.
EC apoptosis is a central trigger for the development of PAH. Commonly used experimental models of PAH, including the MCT and SU5416-hypoxia models, exhibit widespread lung EC apoptosis (49, 113). Moreover, we reported increases in lung EC apoptosis in a “hyper-responsive substrain” of Sprague-Dawley rats, which develops severe PAH with administration of a single dose of the VEGF receptor-2 inhibitor SU5416, even in the absence of hypoxia (48). Notably, pan-caspase inhibitors inhibited EC apoptosis and prevented development of PAH in the rat MCT and SU5416-hypoxia models (49, 113). As well, endothelial-targeted apoptosis has been shown to be sufficient to induce a PAH phenotype in a transgenic mouse model (33). With respect to human PAH, disease-causing mutations in BMPR2 result in increased susceptibility to EC apoptosis (17, 54, 114). Together, these findings suggest that apoptosis of pulmonary vascular EC is necessary and sufficient to cause PAH.
EC apoptosis leads to microvascular loss by either direct or indirect mechanisms. There are both direct and indirect mechanisms by which EC apoptosis could lead to the functional loss of microcirculation. First, apoptosis of ECs could lead directly to a degenerative loss of arteriolar-capillary continuity, particularly at the level of the fragile precapillary lung arteriolar bed (15). Second, persistent EC apoptosis may lead indirectly to the selection of apoptosis-resistant and growth-dysregulated ECs, which in turn, “pile up” in the lumen, leading to complex, occlusive arterial remodeling (i.e., plexiform arteriopathy) (119). Although by far, the greatest attention has been paid to the indirect, “proliferative” hypothesis, we posit that the data, both from experimental models and human studies, argue against this being a driving mechanism, particularly in the early stages of this disease. In contrast, we would suggest that the available evidence better supports the direct “degenerative” hypothesis as a mechanism by which EC injury and apoptosis lead to progressive increases in vascular resistance and loss of microvascular area in this disease (14, 15).
Marked pruning of the arteriolar bed is an early finding in PAH models, occurring in parallel to increases in arterial pressures and before evidence of obliterative arterial remodeling. Perhaps the strongest argument relates to the timing of the major events implicated in the development of PAH, particularly in the SU5416-hypoxia model, one of the only models that most faithfully reproduces the histopathological features of the human disease. In this model, loss of microvessels, as imaged by microcomputed tomography, precedes the development of PAH and parallels the levels of EC apoptosis. In fact, elevation of pulmonary arterial pressures is observed only after >75% of the arteriolar bed has been lost (21), after which, there is a steep linear relationship, with any additional loss of microvessels resulting in substantial increments in pressure. In contrast, evidence for occlusive proliferative arteriolar remodeling occurs later in this model, only after the development of severe PAH (21, 115).
Proliferative, occlusive arterial remodeling may be a consequence, not a cause, of hemodynamic abnormalities in experimental PAH. Protracted exposure of the pulmonary vascular bed to high pressure and flow, as occurs in Eisenmenger’s syndrome, is sufficient to result in the complex lesions (19). Furthermore, the banding of the left pulmonary artery prevented complex lesion formation in the ipsilateral lung in the rat SU5416-hypoxia model, and lesions were only seen in the contralateral, nonbanded lung (1). Moreover, unilateral pulmonary artery banding, 5 wk after administration of SU5416, reversed established complex arterial lesions in the affected lung (1). Likewise, in a PAH patient who had undergone successful unilateral lung transplantation, decades before succumbing to an unrelated cause, a postmortem study revealed regression of plexiform lesions, which were initially abundant in the explanted lung, in the nontransplanted lung (55).
Location, location, location: do plexiform lesions arise from the pulmonary or systemic vascular bed? In an intriguing recent report, Abman’s group (29) has suggested that the plexiform lesions may not even arise in the pulmonary circulation at all but in the bronchial circulation, as a result of the opening of intrapulmonary bronchial anastomoses in response to increased pulmonary arterial pressures overloading the bronchial venous bed. This concept is further supported by a report that patients harboring BMPR2 mutations exhibit more marked bronchial arterial remodeling and angiogenesis and are more prone to hemoptysis than patients without these mutations (118). Patients with BMPR2 mutations are known to exhibit a more severe PAH phenotype, present earlier, and have a worse prognosis than those with idiopathic disease (27), consistent with a possible relationship between severity of hemodynamic abnormalities and remodeling within the bronchial venous bed. If indeed plexiform lesions arise in the systemic bronchial circulation, then it follows that they cannot be the cause of pulmonary microvascular rarefaction in PAH.
Overall, the evidence suggests that EC apoptosis plays a central role in the pathogenesis of PAH by leading to lung microvascular loss by degeneration of precapillary arterioles and that complex proliferative changes may be a consequence, not necessarily a cause, of the severe pulmonary hemodynamic changes in this disease.
PH Is a Disease of Proliferation and Not EC Apoptosis or Endothelial Reprogramming in PAH: Apoptosis Is Not the Whole Story, by Stephen Y. Chan
The key points or the argument are that pulmonary arterial endothelial dysfunction in PAH extends far beyond the apoptotic phenotype, endothelial proliferation and resistance to apoptosis are primary pathophenotypes demonstrated in PAH, there is temporo-spatial balance: initial PAEC apoptosis precedes the development of hyperproliferative and pathogenic PAECs, and such spatio-temporal progression of endothelial dysfunction has significant clinical implications.
Endothelial dysfunction in PAH depends on multifaceted reprogramming events, only a portion of which involves endothelial apoptosis. In contrast to the perhaps more unilateral proproliferative phenotypes of diseased SMCs in PAH, the pathogenic reprogramming of PAECs in PAH is more complex and less well understood. PAEC apoptosis has been observed readily in multiple types of animal and human forms of PAH and plays a causative role, particularly early on in PAH, to trigger pathogenesis (23, 33, 58). Such apoptosis may trigger abnormalities in angiogenesis and contribute to the pathophenotype of pulmonary vascular rarefaction that is typical of PAH. Yet, PAEC dysfunction in PAH extends far beyond the apoptotic phenotype alone.
Endothelial proliferation and resistance to apoptosis are primary pathophenotypes demonstrated in PAH. In addition to apoptosis, increased PAEC proliferation has been noted by multiple groups and linked to PAH pathogenesis (124). Historically, this has included the study of plexiform lesions [previously described as “disorganized endothelial proliferation” (43)]. Moreover, recent studies (50) have demonstrated that cultured PAECs isolated from human PAH patients demonstrate increased proliferation compared with nondiseased control PAECs. Importantly, by modulating either microRNAs (50) or matrix stiffness (10), which consequently impact such endothelial proliferation, PAH manifestations improved in rodent models of PAH, indicating the importance of such proliferation to overall disease development. Other studies have isolated highly proliferative ECs from PAH patients as well (26). Studies in other animal models of PH, such as IL-6 transgenic mice (108), long-term potassium two pore domain channel subfamily K member 3 (or KCNK3) inhibition in rats (3), and MCT rats (50), have all verified increased PAEC proliferation in diseased arterioles. In the context of our work, increased PAEC proliferation and relative protection against apoptosis (10) would also appear to be a logical consequence of increased vascular stiffness (60, 81), as seen in other biological matrix contexts.
A spatio-temporal model of endothelial reprogramming in PAH may explain these apparent discrepancies in endothelial pathophenotypes. Overall, these data support the widely cited theory that initial PAEC apoptosis precedes the development of hyperproliferative and pathogenic PAECs that drive later stages of disease. This was originally described by Voelkel and colleagues (94) in 2005 and later described by Michelakis (69) as a spatio-temporal balance.
Such a spatio-temporal progression of endothelial pathophenotypes invokes one of several models of endothelial dysfunction in PAH. A spatio-temporal balance could predict one of multiple molecular models of pathogenic PAEC evolution in the course of PAH. First, separate populations of ECs may exist that are either anatomically or temporally distinct. Second, a single population of ECs may exist that cycles between apoptotic and proliferative signaling. Finally, a single or distinct population of proliferative ECs may exist that transforms cellular identity, such as in endothelial-to-mesenchymal transition, which has been observed in PAH (40, 87, 110). To interrogate these possibilities rigorously, the application of single-cell discovery platforms, coupled with pulse-chase labeling in vivo to endothelial biology, is likely required, as demonstrated previously in pulmonary artery SMC biology (35, 97, 98).
Such spatio-temporal progression of endothelial dysfunction has significant clinical implications. If a molecular mechanism can be established to explain the spatio-temporal progression of endothelial reprogramming in PAH, then there will be substantial implications for diagnosis and clinical management of PAH. First, the advent of new endothelial diagnostics of PAH could be envisioned that reflect the specific stage of disease. Second, based on the model’s prediction of a time dependency for endothelial apoptosis vs. proliferation, endothelial-specific therapies could be developed for more narrow windows of administration, thus optimizing efficacy and minimizing side effects. Prescreening for endothelial progression could allow for identification of “super-responders” to tailored drugs and could offer much-needed guidance of clinical trial design for precision therapies in the future.
BMPR2 Is the Central Therapeutic Target in PAH, by Nicholas W. Morrell
The key points of the argument are that there remains a major unmet clinical need to develop transformative, new treatments for PAH that target the underlying pathobiology; genetic evidence provides strong target validation for drug discovery; loss-of-function mutations in BMPR2 are the most common genetic cause of PAH; once PAH occurs, the presence of BMPR2 mutations predicts a poor clinical outcome; a reduction in BMPR2 expression or function is also central to nongenetic forms of PAH; restoration of BMPR2 expression or function reverses established PAH in preclinical genetic and nongenetic models; and BMPR2 represents a highly validated therapeutic target and should be a major focus for drug development in PAH.
PAH remains a rare but severe disease with a poor prognosis, despite the availability of licensed drugs that target key vasodilator pathways. These drugs show efficacy by improving symptoms and exercise capacity, often temporarily, but their impact on mortality is limited (44). There is no evidence that these drugs reverse the underlying, extreme pulmonary vascular remodeling that characterizes human forms of PAH (107). Thus there remains an urgent unmet need to develop therapies that directly target the key molecular and cellular pathways involved in PAH pathobiology.
Drug discovery is a lengthy and expensive process, with the vast majority of drugs failing to make it through to the clinic. There are many reasons for failure, but an important factor is the failure to provide compelling target validation for the pathway being targeted. In other words, can a particular pathway be causally implicated in the disease, and does manipulation of that pathway result in disease reversal in valid preclinical systems, e.g., cells and animal models? Strong validation of a molecular target can be provided by genetic evidence implicating the target in patients with the disease (76). Heterozygous germline mutations in the gene encoding BMPR2 are the most common genetic cause of PAH (27). In addition, mutations have been identified in several other components of the BMPR2 pathway in heritable forms of PAH (61). Therefore, the evidence that the BMPR2 pathway plays a central role in the initiation of PAH is overwhelming. A recent meta-analysis also demonstrated that patients with a BMPR2 mutation are younger at diagnosis, present with more severe disease, and have a worse survival than PAH patients without these mutations (27). This important study showed that BMPR2 dysfunction is not only important in disease initiation but also adversely impacts the clinical course of disease. Taken together, the genetic data available from these patient populations strongly suggest that the targeting of the BMPR2 pathway should be a high priority for drug development for PAH. Approximately 25–30% of patients, presenting with what appears to be IPAH, will possess an underlying mutation in BMPR2 (27). However, therapies targeting BMPR2 will also be relevant to patients without mutations, since reduced expression of BMPR2 has been shown to be a feature of many forms of PAH in animal models and patients (7, 16). Moreover, experimental approaches targeting BMPR2 are efficacious in both genetic and nongenetic preclinical models of PAH (58).
Several proof-of-concept studies have shown that BMPR2 can be targeted to prevent and reverse preclinical models of PH. The approaches include the following: 1) repurposed drugs as activators of BMP signaling, such as tacrolimus (106), 2) inhibition of BMPR2 lysosomal degradation by chloroquine (25, 59), 3) BMPR2 gene therapy directed at the pulmonary vascular endothelium (89), 4) reversal of BMPR2 cleavage and transcriptional suppression by inhibition of TNF-α (etanercept) (46), and 5) direct and selective agonism of the BMPR2 endothelial receptor complex by BMP9 (58). In addition, specific mutations that introduce premature termination codons in BMPR2 may be amenable to small molecules that promote translational readthrough (24), and mutations that cause protein misfolding and retention within the endoplasmic reticulum can be corrected with chemical chaperones (100).
Many of the above approaches are ready to be tested in the clinic. Indeed tacrolimus was recently tested in a small, placebo-controlled experimental medicine study of a mixed group of patients with PAH (105). Perhaps not surprisingly, given the case mix in this small, single-center study (only 17 patients received the drug), the results with tacrolimus were not impressive. Although this was predominantly a safety and tolerability study, the authors suggested that patients exhibiting the greatest increase in biomarkers of BMP signaling demonstrated some clinical benefit. One important message from this trial is that although the BMPR2 pathway is strongly validated as a therapeutic target, the best drug approach to activate the pathway remains to be determined. Ideally, such an approach would be selective for the endothelial BMPR2 receptor complex that is implicated from human genetic studies. The use of selective agonists, such as BMP9 and BMP10, provides one possible solution, but there may be others.
In summary, there is now overwhelming evidence supporting the prioritization of the BMPR2 pathway as a therapeutic target in PAH, with truly disease-modifying potential. Academic researchers have led the way in validating this key target, but the pharmaceutical industry needs to take up the challenge to help write the next chapter in the remarkable history of PAH therapies.
BMPR2 Signaling Is Not the Central Therapeutic Target in PAH, by Stephen L. Archer
The key points of the argument are that PAH usually affects patients who have no BMPR2 mutation; BMPR2 expression is reduced in PAH, regardless of the patient’s mutation status; BMPR2 mutations are not the only mutations that promote familial PAH; preclinical rat models of PAH lack BMPR2 mutations, and most murine models with a BMPR2 mutation do not develop PAH; and BMPR2 is an important risk factor for PAH and is highly relevant in familial PAH; however, there are insufficient data to consider BMPR2 signaling as the cause or the central therapeutic target in PAH.
I was assigned the role of refuting the hypothesis that the BMPR2 mutation is the central therapeutic target in PAH in both patients and preclinical models. This is an easy task, since the literature shows that most cases of PAH occur in patients who lack BMPR2 mutations. Moreover, most models of PAH are created in rodents that have no BMPR2 mutation. In murine models in which BMPR2 is deleted or mutated in vascular cells, PAH is of modest severity or occurs sporadically. By refuting the primacy of BMPR2 mutations in the etiology of PAH, I argued against the value of BMPR2 signaling as a “central therapeutic target.” I conceded to my worthy opponent the fact that BMPR2 is downregulated in most forms of PH, even those lacking mutations, and indeed, in secondary PH, as the basis for his case is that the pathway might have therapeutic significance. I gave BMPR2 mutations their due—as factors that are permissive of the development of PAH. In an attempt to find common ground, I noted that BMPR2 mutation and BMPR2 downregulation both stimulate abnormalities in the mitochondrial-metabolic pathway that are involved in the etiology of PAH. These mitochondrial-metabolic abnormalities, although downstream, are more therapeutically accessible as targets for anti-proliferative therapies.
PAH is a pulmonary vascular disease, characterized by excessive vasoconstriction, vascular obstruction, fibrosis, and inflammation. It results in premature death from right ventricular failure. Whereas there are ~10 approved therapies for PAH, most are primarily vasodilators. In contrast, there is growing recognition that vascular obliteration/narrowing and stiffening are of greater importance in PAH. Increased cell proliferation is a hallmark of all vascular cells in PAH, including SMCs (PASMC) (62, 63), PAEC (13), and fibroblasts (PAFib) (126). Histologically, the hyperproliferative phenotype of PAH vascular cells manifests as intimal hyperplasia and plexiform lesions (PAECs), medial hypertrophy (PASMC), and adventitial fibrosis (PAfib). This promotes vasoconstriction, obstruction, and stiffening, respectively. Thus the idea of the targeting of pathways that promote increased rates of cell proliferation has appeal. Mutations in the BMPR2 gene do occur in some PAH patients and can promote increased rates of cell proliferation, making the proposition worthy of debate. It is noteworthy that in PAH, cells have pathological adaptations in mitochondrial metabolism and dynamics that ensure that rapid proliferation is not thwarted by energy limitation, mitochondrial-mediated apoptosis, or inadequate rates of mitotic fission (4, 65). Although not the topic of this debate, PAH is also a syndrome in which inflammation, autoimmunity, endothelial dysfunction, and thrombosis play important roles. As will be discussed, there is an intersection between the BMPR2 pathway and the mitochondrial pathways in PAH.
The tenets of my case are the following: PAH usually affects patients who have no BMPR2 mutation. In familial or heritable PAH, BMPR2 mutations are common and pathologically important. However, familial PAH is rare, accounting for <10% of all PAH. Most cases of PAH are idiopathic or associated with other diseases, such as a CTD. BMPR2 mutations are rare in these cohorts, which account for over 90% of all PAH (67). Admittedly, BMPR2 mutations increase the risk of developing PAH, roughly 10,000-fold, from a population prevalence of 15–26 per million people (45) to a prevalence of 200,000 per million people in BMPR2 mutation carriers (53). In addition, it is clear that those patients with PAH, who have a BMPR2 mutation, present younger and have more severe disease (27). PAH patients who are BMPR2 mutation carriers have a modest, 1.27-fold increase in all-cause mortality compared with noncarriers (27). There is biological plausibility to the pathological effects of BMPR2 mutations in PAH. Specifically, BMPR2 mutations cause resistance to the anti-proliferative effects of transforming growth factor-β1 and BMP (74). However, even in families affected by PAH, a BMPR2 mutation has a disease penetrance of only 27% [albeit greater in females (42%) than in males (14%)] (53). The limited penetrance of a BMPR2 mutation in familial PAH families is evidence that the BMPR2 mutation is a risk factor for PAH, not a cause. Additional pathological stimuli, such as drugs, inflammation, or epigenetic changes, are usually required to precipitate disease, which is also true in rodent models of PAH. More importantly, the vast majority of patients with PAH lacks any mutation in the BMPR2 gene. Preliminary data from the National Heart, Lung, and Blood Institute PAH Biobank at Cincinnati Children’s Hospital Medical Center have been provided by the study’s Principle Investigator Dr. William Nichols (Cincinnati Children’s Hospital Medical Center/University of Cincinnati). The incidence of pathogenic/suspected pathogenic gene variants identified using panel sequencing of 12 genes in 2,251 PAH patients is 10.8%. Pathogenic/suspected pathogenic variants are less frequent in associated PAH (5.8%) and more common in IPAH (13.2%; personal communication, Dr. William Nichols). Thus BMPR2 mutations are a risk factor for PAH but are not found in most patients with PAH.
BMPR2 expression is reduced in PAH, regardless of the patient’s mutation status. My worthy opponent has published data showing that BMPR2 expression is downregulated in the peripheral lung tissue of patients with advanced PAH, regardless of their mutation status (7). Indeed, BMPR2 expression is downregulated even in secondary PH, a syndrome in which the BMPR2 mutation is not implicated. BMPR2 expression is also downregulated in the MCT rodent PAH model. We restored BMPR2 expression in this model by airway nebulization of an adenovirus carrying the normal BMPR2 gene (68). However, the restoration of BMPR2 expression in pulmonary arteries did not regress experimental PAH. Together, these data characterize the BMPR2 pathway as one that is dysregulated in PH (regardless of the cause), rather than a primary driver of PAH (68).
BMPR2 mutations are not the only mutations that promote familial PAH. BMPR2 is not the only mutated gene associated with familial PAH. Mutations that are pathologically relevant have also been reported in a number of genes associated with the bone morphogenic proteins and transforming growth factor-β pathways, including activin A receptor-like type 1 (or ACVRL1), endoglin, caveolin 1, and gene encoding for mothers against decapentaplegic homolog 9 (or SMAD9) (103). Mutations that promote PAH have also been reported involving the ion channel long-term potassium two pore domain channel subfamily K member 3 (or KCNK3) and the cell-cycle regulator eukaryotic translation initiation factor 2α kinase 4 (or EIF2AK4). Thus, even in familial PAH where BMPR2 mutations are important (accounting for ~75% of cases) (103), other mutations are involved.
Preclinical rat models of PAH lack BMPR2 mutations, and most murine models with a BMPR2 mutation do not develop PAH. The MCT and SU5416-hypoxia models of PAH are generated in rats that have no BMPR2 mutation. These models replicate human PAH reasonably well and are commonly used to test therapeutic agents in PAH drug development. Most murine models, in which a BMPR2 mutation is expressed, or haploinsufficiency of the gene is introduced, do not spontaneously develop PAH. Those that develop PAH do so only sporadically or when challenged with another pathological stimulus. An exception is the vascular SMC-specific, tetracycline-conditional, dominant-negative BMPR2 transgenic mice (121), which spontaneously develop PAH. Likewise, mice with one mutant BMPR2 allele (lacking exons 4 and 5) develop mild PAH and increased medial thickness of small pulmonary arteries; however, they lack the complex vascular lesions of PAH (9). Mice with a heterozygous or homozygous BMPR2 EC deletion mutation also develop PAH; however, for reasons that are unclear, this only occurs in a minority of mice (39%) (39). Mice with a BMPR2 heterozygous knockout do not develop PH unless serotonin, a vasoconstrictor and mitogen, is coadministered (57). In another study, BMPR2-haploinsufficient mice were also normal at baseline, with no difference from wild-type mice in lifespan, right ventricular systolic pressure, or lung histology (101). Only when these mice were challenged with a vasoconstrictor stressor (adenovirus expressing 5-lipoxygenase) did they develop PH (101). Mice with a U6-small hairpin RNA BMPR2 knockdown, which caused a 90% loss of BMPR2 expression, had no increase in pulmonary vascular resistance; rather, their phenotype included mucosal hemorrhage, incomplete mural cell coverage on vessel walls, and gastrointestinal hyperplasia (56). Thus BMPR2 haploinsufficiency is a risk factor that predisposes mice to PAH, rather than a sufficient stimulus to cause PAH on its own. This is consistent with the incomplete disease penetrance of BMPR2 mutations in humans.
Rat models of PAH are created in inbred animals that lack a BMPR2 mutation. These rats manifest a mitochondrial-metabolic abnormality that is also found in human PAH. This mitochondrial-metabolic phenotype can also result from BMPR2 mutations. I propose that these downstream mitochondrial-metabolic abnormalities, whether caused by BMPR2 mutations or diverse other mechanisms, such as epigenetically driven activation of hypoxia-inducible factor 1α (6), are more central to the pathogenesis of PAH and represent a key hub in the proliferation pathways, thus making it a more therapeutically appealing target.
The preclinical models induced by MCT or SU5416-hypoxia manifest the Warburg phenomenon (an increase in the rate of uncoupled glycolysis that occurs hand in hand with suppression of the mitochondrial oxidative metabolism) (88, 109). The Warburg metabolic phenotype preserves a cell’s energetic capacity (by upregulating glycolysis), while reducing mitochondrial oxidative metabolism, thereby decreasing mitochondrial-mediated apoptosis. This confers a survival advantage to PAH cells. The BMPR2 pathway intersects with the Warburg pathway in that downregulation of BMPR2 expression (or BMPR2 gene mutation) increases the expression of pyruvate kinase, the terminal enzyme in glycolysis, in PAEC (5, 13). Caruso et al. (13) recently showed that mutation of BMPR2 or downregulation of BMPR2 was sufficient to activate glycolysis and contribute to the Warburg phenomenon. They found that BMPR2 downregulation/BMPR2 mutation somehow decreased production of microRNA-124, which increased expression of polypyrimidine tract-binding protein 1, a factor that controls splice variant expression. Polypyrimidine tract-binding protein 1 upregulation increased the expression of a proglycolytic variant of pyruvate kinase PKM2. Increased expression of PKM2, the distal enzyme in glycolysis, promotes an increase in the Warburg metabolism (13). Thus abnormal BMPR2 signaling may simply be another route to achieve the Warburg pathway in PAH. A discussion of Caruso’s paper is included in a recent editorial (5).
Vascular cells from preclinical models of PAH that lack a BMPR2 mutation also manifest increased mitochondrial fission (63). The division of mitochondria occurs in coordination with mitosis, ensuring equitable distribution of mitochondria to the daughter cells. Recent studies from our laboratory show that in PAH and cancer, an increased rate of mitotic fission is required to sustain high rates of cell proliferation (4). Mitotic fission is mediated by the GTPase and dynamin-related protein 1 and its binding partners. The inhibition of mitotic fission (by targeting dynamin-related protein 1) slows cell proliferation, increases apoptosis, and can regress experimental PAH. This pathway interacts with the BMPR2 pathway. The reduction of PAEC expression of BMPR2 increases mitochondrial membrane potential, accelerates glycolysis, and induces mitochondrial fission, contributing to the proinflammatory state of PAH (23). Thus it appears that BMPR2 deficiency (or gene mutation) promotes a proliferative and inflammatory state in PAEC, PAFib, and PASMC, in part, by creating a mitochondrial-metabolic shift to Warburg metabolism and increased mitotic fission, as reviewed in Archer (5).
In conclusion, <10% of cases of PAH can be attributed to BMPR2 mutation, and even in familial PAH gene mutation, is better characterized as a risk factor than as a cause of the disease. This is also true in rodent models in which BMPR haploinsufficiency is rare enough to elicit PH in the absence of a concomitant vasoconstrictor stimulus. BMPR2 mutations can contribute to the cancer-like phenotype of PAH by creating the Warburg metabolism and mitochondrial fission in vascular cells within the pulmonary circulation. Recently, studies in PAH patients have established the safety and hinted at the efficacy of the targeting of the Warburg metabolic phenotype using dichloroacetate, an inhibitor of pyruvate dehydrogenase kinase that restores mitochondrial oxidative function (70); in contrast, there are no such data demonstrating the value of restoration of BMPR2 function in patients. Thus BMPR2 is an important risk factor for PAH and is highly relevant in familial PAH; however, there are insufficient data to support the proposition that BMPR2 signaling should “be considered the central therapeutic target in PAH.”
I concluded my debate with Dr. Morrell by quoting a comment made in 1958 by Nobel Prize in Physics Laureate Niels Bohr to Wolfgang Pauli regarding the latter’s presentation on Heisenberg’s field theory of elementary particles: “We are all agreed that your theory is crazy. The question that divides us is whether it is crazy enough to have a chance of being correct.” With the application of this test to the question of the centrality of BMPR2 to the pathogenesis of PAH, I believe the good-humored answer must be no!
SUMMARY
The present debate is aimed to illuminate current conceptual controversies on the pathogenesis of PAH with no intention to resolve obvious conflicts in opinion; in fact, as highlighted in the respective pro and con sections, there is, in all cases, good direct or indirect evidence both supporting and refuting a certain concept, necessitating the need for further in-depth exploration of the mechanistic basis of this heterogeneous disease.
MOVING FORWARD APPROACHING THE CONTROVERSIES
Resolution of these controversies is not an academic exercise but will have major implications for translational research and clinical practice: should we focus resources to target autoimmunity, hemodynamics, proliferation or apoptosis, BMPR2, or the glycolytic switch? Are there preferential time windows for one or another, and/or are there patient populations or subphenotypes that may particularly profit from a specific intervention? Or more general, can we infer PAH mechanisms, treatment targets, and therapeutic efficacy from our animal models of experimental PH, which often involve exposure to hypoxia or agents injurious to the endothelium?
More precise phenotyping of patients over the course of their disease with genomic, proteomic, as well as metabolomic approaches might unmask subgroups of patients, with or without pathogenetic features of autoimmune disease, who then can be tested for tailor-made therapies, such as IL-6 inhibition. Coculture assays with endothelial and SMCs incorporating flow, pressure, and shear stress might uncover pathways and mechanisms important for flow or pressure-associated vascular dysfunction, remodeling, and EC function and thus help to understand better the reciprocal interdependence between hemodynamic stress and occlusive neointimal lesions. Clinically, studies in severe PAH patients bridged with awake extracorporeal membrane oxygenation before lung transplantation (95) might shed light on the value of offloading the cardiopulmonary circulation to improve right/left ventricular function and potentially pulmonary vascular remodeling and lesion formation. The role of endothelial apoptosis, vessel regression, and/or reduced angiogenesis in the pulmonary vasculature, as well as clonal endothelial proliferation in PH, may be addressed by careful lineage-tracing experiments, single-cell analyses, as well as three-dimensional imaging of the pulmonary and bronchial circulation. Furthermore, the identification of surrogate biomarkers that reflect normal and deficient BMPR2 signaling in the pulmonary circulation in PAH patients would help validate target engagement of novel BMPR2 targeting therapies, as well as help to identify potential clinical responders.
Importantly, the conceptual gaps in understanding PAH pathogenesis are often paralleled by corresponding interventional gaps, whereas for some of these targets, novel interventions are presently tested, such as rituximab to block B cells in PAH or dichloroacetate to improve mitochondria function; others, such as endothelial apoptosis and vessel loss, still lack appropriate interventions.
We hope that the present pro-con debate can draw a new generation of enthusiastic researchers into this vibrant field of science and entice further discussions and research to bridge existing gaps for a better understanding and therapy of this fatal disease.
GRANTS
Support for this work was provided by the following: Heart and Stroke Foundation of Canada (Grant G-16-00013171), Deutsche Forschungsgesellschaft (Grant KU1218/9-1), Deutsche Zentrum für Herz-Kreislauf-Forschung e. V., Deutsches Zentrum fuer Lungenforschung, and Ontario Research Excellence Fund SCORR (Grant RE07-086; to W. M. Kuebler); National Heart, Lung, and Blood Institute (NHLBI; Grants HL122887, HL014985, HL125739, HL120001, and HL138473; to M. R. Nicolls); Oesterreichische Nationalbank (Grant 16682), Oesterreichischer Auslandsdienst fuer Wissenschaftlich-Technische Zusammenarbeit (Grant Hu07/2016), and International Ph.D. Program University of Graz (to A. Olschewski); Grants-in-Aid for Scientific Research from the Japan Society for the Promotion Science (26461146 and 17K09591; to K. Abe); NHLBI (Grants R01 HL074186, R01 HL087118, R01 HL122887, R01 HL138473, P01 HL108797, R24 HL123767, and K12 HL120001; to M. Rabinovitch); NHLBI (Grants R01 HL124021, HL122596, and HL138437), National Center for Advancing Translational Sciences (Grant UH2 TR002073), and American Heart Association Established Investigator Award (18EIA33900027; to S. Y. Chan); Canadian Institutes of Health Research (CIHR) Foundation Award (FDN_143291) and Ontario Research Excellence Fund (ORF RE07-086; to D. Stewart); British Heart Foundation Chair of Cardiopulmonary Medicine (to N. W. Morrell); CIHR Foundation, NHLBI (Grants RO1-HL071115 and 1RC1HL099462), Tier 1 Canada Research Chair in Mitochondrial Dynamics, William J. Henderson Foundation, CIHR Vascular Network, and Canadian Vascular Network (to S. L. Archer); and NHLBI (Grant R01 HL128734), Department of Defense (Grant PR161256), Wall Center for Pulmonary Vascular Disease Stanford, and Pulmonary Hypertension Association Career Development Grant (to E. Spiekerkoetter).
DISCLOSURES
W. M. Kuebler has served as a consultant for Boehringer Ingelheim Pharma GmbH & Co. KG (modest) and GlaxoSmithKline Research & Development (modest). S. Y. Chan has served as a consultant for Actelion (significant), Gilead, Pfizer, and Vivus (modest). N. W. Morrell is a founder and director of Morphogen-IX. E. Spiekerkoetter has served as scientific adviser for Selten Pharma Inc. and Vivus (modest). No other conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.S. conceived and designed research; E.S. analyzed data; E.S. interpreted results of experiments; E.S. prepared figures; W.M.K., M.R.N., A.O., K.A., M.R., D.S., S.Y.C., N.W.M., S.L.A., and E.S. drafted manuscript; W.M.K., M.R.N., A.O., K.A., M.R., D.S., S.Y.C., N.W.M., S.L.A., and E.S. edited and revised manuscript; W.M.K., M.R.N., A.O., K.A., M.R., D.S., S.Y.C., N.W.M., S.L.A., and E.S. approved final version of manuscript.
ACKNOWLEDGMENTS
Dr. S. L. Archer thanks Dr. William Nichols for providing preliminary data from the National Heart, Lung, and Blood Institute PAH Biobank at Cincinnati Children’s Hospital Medical Center as to the prevalence of gene mutations in PAH. Elements of illustration were used from Servier Medical Art and modified under a Creative Common Attribution 3.0 Generic License (https://smart.servier.com/).
REFERENCES
- 1.Abe K, Shinoda M, Tanaka M, Kuwabara Y, Yoshida K, Hirooka Y, McMurtry IF, Oka M, Sunagawa K. Haemodynamic unloading reverses occlusive vascular lesions in severe pulmonary hypertension. Cardiovasc Res 111: 16–25, 2016. doi: 10.1093/cvr/cvw070. [DOI] [PubMed] [Google Scholar]
- 2.Abe K, Toba M, Alzoubi A, Ito M, Fagan KA, Cool CD, Voelkel NF, McMurtry IF, Oka M. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation 121: 2747–2754, 2010. doi: 10.1161/CIRCULATIONAHA.109.927681. [DOI] [PubMed] [Google Scholar]
- 3.Antigny F, Hautefort A, Meloche J, Belacel-Ouari M, Manoury B, Rucker-Martin C, Péchoux C, Potus F, Nadeau V, Tremblay E, Ruffenach G, Bourgeois A, Dorfmüller P, Breuils-Bonnet S, Fadel E, Ranchoux B, Jourdon P, Girerd B, Montani D, Provencher S, Bonnet S, Simonneau G, Humbert M, Perros F. Potassium channel subfamily K member 3 (KCNK3) contributes to the development of pulmonary arterial hypertension. Circulation 133: 1371–1385, 2016. doi: 10.1161/CIRCULATIONAHA.115.020951. [DOI] [PubMed] [Google Scholar]
- 4.Archer SL. Mitochondrial dynamics—mitochondrial fission and fusion in human diseases. N Engl J Med 369: 2236–2251, 2013. doi: 10.1056/NEJMra1215233. [DOI] [PubMed] [Google Scholar]
- 5.Archer SL. Pyruvate kinase and Warburg metabolism in pulmonary arterial hypertension: uncoupled glycolysis and the cancer-like phenotype of pulmonary arterial hypertension. Circulation 136: 2486–2490, 2017. doi: 10.1161/CIRCULATIONAHA.117.031655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Archer SL, Marsboom G, Kim GH, Zhang HJ, Toth PT, Svensson EC, Dyck JR, Gomberg-Maitland M, Thébaud B, Husain AN, Cipriani N, Rehman J. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: a basis for excessive cell proliferation and a new therapeutic target. Circulation 121: 2661–2671, 2010. doi: 10.1161/CIRCULATIONAHA.109.916098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, Morrell NW. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation 105: 1672–1678, 2002. doi: 10.1161/01.CIR.0000012754.72951.3D. [DOI] [PubMed] [Google Scholar]
- 8.Becker MO, Kill A, Kutsche M, Guenther J, Rose A, Tabeling C, Witzenrath M, Kühl AA, Heidecke H, Ghofrani HA, Tiede H, Schermuly RT, Nickel N, Hoeper MM, Lukitsch I, Gollasch M, Kuebler WM, Bock S, Burmester GR, Dragun D, Riemekasten G. Vascular receptor autoantibodies in pulmonary arterial hypertension associated with systemic sclerosis. Am J Respir Crit Care Med 190: 808–817, 2014. doi: 10.1164/rccm.201403-0442OC. [DOI] [PubMed] [Google Scholar]
- 9.Beppu H, Ichinose F, Kawai N, Jones RC, Yu PB, Zapol WM, Miyazono K, Li E, Bloch KD. BMPR-II heterozygous mice have mild pulmonary hypertension and an impaired pulmonary vascular remodeling response to prolonged hypoxia. Am J Physiol Lung Cell Mol Physiol 287: L1241–L1247, 2004. doi: 10.1152/ajplung.00239.2004. [DOI] [PubMed] [Google Scholar]
- 10.Bertero T, Oldham WM, Cottrill KA, Pisano S, Vanderpool RR, Yu Q, Zhao J, Tai Y, Tang Y, Zhang YY, Rehman S, Sugahara M, Qi Z, Gorcsan J III, Vargas SO, Saggar R, Saggar R, Wallace WD, Ross DJ, Haley KJ, Waxman AB, Parikh VN, De Marco T, Hsue PY, Morris A, Simon MA, Norris KA, Gaggioli C, Loscalzo J, Fessel J, Chan SY. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J Clin Invest 126: 3313–3335, 2016. doi: 10.1172/JCI86387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breitling S, Hui Z, Zabini D, Hu Y, Hoffmann J, Goldenberg NM, Tabuchi A, Buelow R, Dos Santos C, Kuebler WM. The mast cell-B cell axis in lung vascular remodeling and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 312: L710–L721, 2017. doi: 10.1152/ajplung.00311.2016. [DOI] [PubMed] [Google Scholar]
- 12.Bull TM, Cool CD, Serls AE, Rai PR, Parr J, Neid JM, Geraci MW, Campbell TB, Voelkel NF, Badesch DB. Primary pulmonary hypertension, Castleman’s disease and human herpesvirus-8. Eur Respir J 22: 403–407, 2003. doi: 10.1183/09031936.03.00006903. [DOI] [PubMed] [Google Scholar]
- 13.Caruso P, Dunmore BJ, Schlosser K, Schoors S, Dos Santos C, Perez-Iratxeta C, Lavoie JR, Zhang H, Long L, Flockton AR, Frid MG, Upton PD, D’Alessandro A, Hadinnapola C, Kiskin FN, Taha M, Hurst LA, Ormiston ML, Hata A, Stenmark KR, Carmeliet P, Stewart DJ, Morrell NW. Identification of microRNA-124 as a major regulator of enhanced endothelial cell glycolysis in pulmonary arterial hypertension via PTBP1 (polypyrimidine tract binding protein) and pyruvate kinase M2. Circulation 136: 2451–2467, 2017. doi: 10.1161/CIRCULATIONAHA.117.028034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaudhary KR, Stewart DJ. Go with the (back) flow: what can retrograde perfusion teach us about arterial remodeling in pulmonary arterial hypertension? Am J Physiol Lung Cell Mol Physiol 314: L797–L798, 2018. doi: 10.1152/ajplung.00065.2018. [DOI] [PubMed] [Google Scholar]
- 15.Chaudhary KR, Taha M, Cadete VJ, Godoy RS, Stewart DJ. Proliferative versus degenerative paradigms in pulmonary arterial hypertension: have we put the cart before the horse? Circ Res 120: 1237–1239, 2017. doi: 10.1161/CIRCRESAHA.116.310097. [DOI] [PubMed] [Google Scholar]
- 16.Chen NY, D Collum S, Luo F, Weng T, Le TT, M Hernandez A, Philip K, Molina JG, Garcia-Morales LJ, Cao Y, Ko TC, Amione-Guerra J, Al-Jabbari O, Bunge RR, Youker K, Bruckner BA, Hamid R, Davies J, Sinha N, Karmouty-Quintana H. Macrophage bone morphogenic protein receptor 2 depletion in idiopathic pulmonary fibrosis and group III pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 311: L238–L254, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ciumas M, Eyries M, Poirier O, Maugenre S, Dierick F, Gambaryan N, Montagne K, Nadaud S, Soubrier F. Bone morphogenetic proteins protect pulmonary microvascular endothelial cells from apoptosis by upregulating α-B-crystallin. Arterioscler Thromb Vasc Biol 33: 2577–2584, 2013. doi: 10.1161/ATVBAHA.113.301976. [DOI] [PubMed] [Google Scholar]
- 18.Colvin KL, Cripe PJ, Ivy DD, Stenmark KR, Yeager ME. Bronchus-associated lymphoid tissue in pulmonary hypertension produces pathologic autoantibodies. Am J Respir Crit Care Med 188: 1126–1136, 2013. doi: 10.1164/rccm.201302-0403OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corno AF, Tozzi P, Genton CY, von Segesser LK. Surgically induced unilateral pulmonary hypertension: time-related analysis of a new experimental model. Eur J Cardiothorac Surg 23: 513–517, 2003. doi: 10.1016/S1010-7940(03)00025-3. [DOI] [PubMed] [Google Scholar]
- 20.Dammann JF Jr, Ferencz C. The significance of the pulmonary vascular bed in congenital heart disease. III. Defects between the ventricles or great vessels in which both increased pressure and blood flow may act upon the lungs and in which there is a common ejectile force. Am Heart J 52: 210–231, 1956. doi: 10.1016/0002-8703(56)90260-5. [DOI] [PubMed] [Google Scholar]
- 21.Deng Y, Chaudhary KR, Yang A, Rowe KJ, Stewart DJ. Protracted endothelial cell apoptosis leads to direct microvascular loss as a major mechanism of severe pulmonary arterial hypertension in the rat SU5416-hypoxia mode (Abstract) Am J Respir Crit Care Med 195: A7214, 2017. [Google Scholar]
- 22.Dickinson MG, Bartelds B, Borgdorff MA, Berger RM. The role of disturbed blood flow in the development of pulmonary arterial hypertension: lessons from preclinical animal models. Am J Physiol Lung Cell Mol Physiol 305: L1–L14, 2013. doi: 10.1152/ajplung.00031.2013. [DOI] [PubMed] [Google Scholar]
- 23.Diebold I, Hennigs JK, Miyagawa K, Li CG, Nickel NP, Kaschwich M, Cao A, Wang L, Reddy S, Chen PI, Nakahira K, Alcazar MA, Hopper RK, Ji L, Feldman BJ, Rabinovitch M. BMPR2 preserves mitochondrial function and DNA during reoxygenation to promote endothelial cell survival and reverse pulmonary hypertension. Cell Metab 21: 596–608, 2015. doi: 10.1016/j.cmet.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drake KM, Dunmore BJ, McNelly LN, Morrell NW, Aldred MA. Correction of nonsense BMPR2 and SMAD9 mutations by ataluren in pulmonary arterial hypertension. Am J Respir Cell Mol Biol 49: 403–409, 2013. doi: 10.1165/rcmb.2013-0100OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunmore BJ, Drake KM, Upton PD, Toshner MR, Aldred MA, Morrell NW. The lysosomal inhibitor, chloroquine, increases cell surface BMPR-II levels and restores BMP9 signalling in endothelial cells harbouring BMPR-II mutations. Hum Mol Genet 22: 3667–3679, 2013. doi: 10.1093/hmg/ddt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duong HT, Comhair SA, Aldred MA, Mavrakis L, Savasky BM, Erzurum SC, Asosingh K. Pulmonary artery endothelium resident endothelial colony-forming cells in pulmonary arterial hypertension. Pulm Circ 1: 475–486, 2011. doi: 10.4103/2045-8932.93547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans JD, Girerd B, Montani D, Wang XJ, Galiè N, Austin ED, Elliott G, Asano K, Grünig E, Yan Y, Jing ZC, Manes A, Palazzini M, Wheeler LA, Nakayama I, Satoh T, Eichstaedt C, Hinderhofer K, Wolf M, Rosenzweig EB, Chung WK, Soubrier F, Simonneau G, Sitbon O, Gräf S, Kaptoge S, Di Angelantonio E, Humbert M, Morrell NW. BMPR2 mutations and survival in pulmonary arterial hypertension: an individual participant data meta-analysis. Lancet Respir Med 4: 129–137, 2016. doi: 10.1016/S2213-2600(15)00544-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frost AE, Badesch DB, Barst RJ, Benza RL, Elliott CG, Farber HW, Krichman A, Liou TG, Raskob GE, Wason P, Feldkircher K, Turner M, McGoon MD. The changing picture of patients with pulmonary arterial hypertension in the United States: how REVEAL differs from historic and non-US contemporary registries. Chest 139: 128–137, 2011. doi: 10.1378/chest.10-0075. [DOI] [PubMed] [Google Scholar]
- 29.Galambos C, Sims-Lucas S, Abman SH, Cool CD. Intrapulmonary bronchopulmonary anastomoses and plexiform lesions in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 193: 574–576, 2016. doi: 10.1164/rccm.201507-1508LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galiè N, Corris PA, Frost A, Girgis RE, Granton J, Jing ZC, Klepetko W, McGoon MD, McLaughlin VV, Preston IR, Rubin LJ, Sandoval J, Seeger W, Keogh A. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol 62, Suppl: D60–D72, 2013. doi: 10.1016/j.jacc.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 31.Godfrey VL, Wilkinson JE, Rinchik EM, Russell LB. Fatal lymphoreticular disease in the scurfy (sf) mouse requires T cells that mature in a sf thymic environment: potential model for thymic education. Proc Natl Acad Sci USA 88: 5528–5532, 1991. doi: 10.1073/pnas.88.13.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Godfrey VL, Wilkinson JE, Russell LB. X-Linked lymphoreticular disease in the scurfy (sf) mutant mouse. Am J Pathol 138: 1379–1387, 1991. [PMC free article] [PubMed] [Google Scholar]
- 33.Goldthorpe H, Jiang JY, Taha M, Deng Y, Sinclair T, Ge CX, Jurasz P, Turksen K, Mei SH, Stewart DJ. Occlusive lung arterial lesions in endothelial-targeted, fas-induced apoptosis transgenic mice. Am J Respir Cell Mol Biol 53: 712–718, 2015. doi: 10.1165/rcmb.2014-0311OC. [DOI] [PubMed] [Google Scholar]
- 34.Graham BB, Mentink-Kane MM, El-Haddad H, Purnell S, Zhang L, Zaiman A, Redente EF, Riches DW, Hassoun PM, Bandeira A, Champion HC, Butrous G, Wynn TA, Tuder RM. Schistosomiasis-induced experimental pulmonary hypertension: role of interleukin-13 signaling. Am J Pathol 177: 1549–1561, 2010. doi: 10.2353/ajpath.2010.100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greif DM, Kumar M, Lighthouse JK, Hum J, An A, Ding L, Red-Horse K, Espinoza FH, Olson L, Offermanns S, Krasnow MA. Radial construction of an arterial wall. Dev Cell 23: 482–493, 2012. doi: 10.1016/j.devcel.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hensley MK, Levine A, Gladwin MT, Lai YC. Emerging therapeutics in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 314: L769–L781, 2018. doi: 10.1152/ajplung.00259.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hinchcliff M, Khanna S, Hsu VM, Lee J, Almagor O, Chang RW, Steen V, Chung L; PHAROS Investigators . Survival in systemic sclerosis-pulmonary arterial hypertension by serum autoantibody status in the Pulmonary Hypertension Assessment and Recognition of Outcomes in Scleroderma (PHAROS) registry. Semin Arthritis Rheum 45: 309–314, 2015. doi: 10.1016/j.semarthrit.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoeper MM, Huscher D, Ghofrani HA, Delcroix M, Distler O, Schweiger C, Grunig E, Staehler G, Rosenkranz S, Halank M, Held M, Grohé C, Lange TJ, Behr J, Klose H, Wilkens H, Filusch A, Germann M, Ewert R, Seyfarth HJ, Olsson KM, Opitz CF, Gaine SP, Vizza CD, Vonk-Noordegraaf A, Kaemmerer H, Gibbs JS, Pittrow D. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: results from the COMPERA registry. Int J Cardiol 168: 871–880, 2013. doi: 10.1016/j.ijcard.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 39.Hong KH, Lee YJ, Lee E, Park SO, Han C, Beppu H, Li E, Raizada MK, Bloch KD, Oh SP. Genetic ablation of the BMPR2 gene in pulmonary endothelium is sufficient to predispose to pulmonary arterial hypertension. Circulation 118: 722–730, 2008. doi: 10.1161/CIRCULATIONAHA.107.736801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hopper RK, Moonen JR, Diebold I, Cao A, Rhodes CJ, Tojais NF, Hennigs JK, Gu M, Wang L, Rabinovitch M. In pulmonary arterial hypertension, reduced BMPR2 promotes endothelial-to-mesenchymal transition via HMGA1 and its target slug. Circulation 133: 1783–1794, 2016. doi: 10.1161/CIRCULATIONAHA.115.020617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huertas A, Phan C, Bordenave J, Tu L, Thuillet R, Le Hiress M, Avouac J, Tamura Y, Allanore Y, Jovan R, Sitbon O, Guignabert C, Humbert M. Regulatory T cell dysfunction in idiopathic, heritable and connective tissue-associated pulmonary arterial hypertension. Chest 149: 1482–1493, 2016. doi: 10.1016/j.chest.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Huetsch JC, Suresh K, Bernier M, Shimoda LA. Update on novel targets and potential treatment avenues in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 311: L811–L831, 2016. doi: 10.1152/ajplung.00302.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 43, Suppl S: 13S–24S, 2004. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 44.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaïci A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Cottin V, Degano B, Jaïs X, Montani D, Souza R, Simonneau G. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 122: 156–163, 2010. doi: 10.1161/CIRCULATIONAHA.109.911818. [DOI] [PubMed] [Google Scholar]
- 45.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Simonneau G. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 173: 1023–1030, 2006. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 46.Hurst LA, Dunmore BJ, Long L, Crosby A, Al-Lamki R, Deighton J, Southwood M, Yang X, Nikolic MZ, Herrera B, Inman GJ, Bradley JR, Rana AA, Upton PD, Morrell NW. TNFα drives pulmonary arterial hypertension by suppressing the BMP type-II receptor and altering NOTCH signalling. Nat Commun 8: 14079, 2017. doi: 10.1038/ncomms14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jais X, Launay D, Yaici A, Le Pavec J, Tchérakian C, Sitbon O, Simonneau G, Humbert M. Immunosuppressive therapy in lupus- and mixed connective tissue disease-associated pulmonary arterial hypertension: a retrospective analysis of twenty-three cases. Arthritis Rheum 58: 521–531, 2008. doi: 10.1002/art.23303. [DOI] [PubMed] [Google Scholar]
- 48.Jiang B, Deng Y, Suen C, Taha M, Chaudhary KR, Courtman DW, Stewart DJ. Marked Strain-specific differences in the SU5416 rat model of severe pulmonary arterial hypertension. Am J Respir Cell Mol Biol 54: 461–468, 2016. doi: 10.1165/rcmb.2014-0488OC. [DOI] [PubMed] [Google Scholar]
- 49.Jurasz P, Courtman D, Babaie S, Stewart DJ. Role of apoptosis in pulmonary hypertension: from experimental models to clinical trials. Pharmacol Ther 126: 1–8, 2010. doi: 10.1016/j.pharmthera.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 50.Kim J, Kang Y, Kojima Y, Lighthouse JK, Hu X, Aldred MA, McLean DL, Park H, Comhair SA, Greif DM, Erzurum SC, Chun HJ. An endothelial apelin-FGF link mediated by miR-424 and miR-503 is disrupted in pulmonary arterial hypertension. Nat Med 19: 74–82, 2013. doi: 10.1038/nm.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim YM, Haghighat L, Spiekerkoetter E, Sawada H, Alvira CM, Wang L, Acharya S, Rodriguez-Colon G, Orton A, Zhao M, Rabinovitch M. Neutrophil elastase is produced by pulmonary artery smooth muscle cells and is linked to neointimal lesions. Am J Pathol 179: 1560–1572, 2011. doi: 10.1016/j.ajpath.2011.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kugathasan L, Ray JB, Deng Y, Rezaei E, Dumont DJ, Stewart DJ. The angiopietin-1-Tie2 pathway prevents rather than promotes pulmonary arterial hypertension in transgenic mice. J Exp Med 206: 2221–2234, 2009. [Correction in J Exp Med 23: 206, 2009. 10.1084/jem.20090389102109c.] doi: 10.1084/jem.20090389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Larkin EK, Newman JH, Austin ED, Hemnes AR, Wheeler L, Robbins IM, West JD, Phillips JA III, Hamid R, Loyd JE. Longitudinal analysis casts doubt on the presence of genetic anticipation in heritable pulmonary arterial hypertension. Am J Respir Crit Care Med 186: 892–896, 2012. doi: 10.1164/rccm.201205-0886OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lavoie JR, Ormiston ML, Perez-Iratxeta C, Courtman DW, Jiang B, Ferrer E, Caruso P, Southwood M, Foster WS, Morrell NW, Stewart DJ. Proteomic analysis implicates translationally controlled tumor protein as a novel mediator of occlusive vascular remodeling in pulmonary arterial hypertension. Circulation 129: 2125–2135, 2014. doi: 10.1161/CIRCULATIONAHA.114.008777. [DOI] [PubMed] [Google Scholar]
- 55.Levy NT, Liapis H, Eisenberg PR, Botney MD, Trulock EP. Pathologic regression of primary pulmonary hypertension in left native lung following right single-lung transplantation. J Heart Lung Transplant 20: 381–384, 2001. doi: 10.1016/S1053-2498(00)00153-4. [DOI] [PubMed] [Google Scholar]
- 56.Liu D, Wang J, Kinzel B, Müeller M, Mao X, Valdez R, Liu Y, Li E. Dosage-dependent requirement of BMP type II receptor for maintenance of vascular integrity. Blood 110: 1502–1510, 2007. doi: 10.1182/blood-2006-11-058594. [DOI] [PubMed] [Google Scholar]
- 57.Long L, MacLean MR, Jeffery TK, Morecroft I, Yang X, Rudarakanchana N, Southwood M, James V, Trembath RC, Morrell NW. Serotonin increases susceptibility to pulmonary hypertension in BMPR2-deficient mice. Circ Res 98: 818–827, 2006. doi: 10.1161/01.RES.0000215809.47923.fd. [DOI] [PubMed] [Google Scholar]
- 58.Long L, Ormiston ML, Yang X, Southwood M, Gräf S, Machado RD, Mueller M, Kinzel B, Yung LM, Wilkinson JM, Moore SD, Drake KM, Aldred MA, Yu PB, Upton PD, Morrell NW. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat Med 21: 777–785, 2015. doi: 10.1038/nm.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Long L, Yang X, Southwood M, Lu J, Marciniak SJ, Dunmore BJ, Morrell NW. Chloroquine prevents progression of experimental pulmonary hypertension via inhibition of autophagy and lysosomal bone morphogenetic protein type II receptor degradation. Circ Res 112: 1159–1170, 2013. doi: 10.1161/CIRCRESAHA.111.300483. [DOI] [PubMed] [Google Scholar]
- 60.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 196: 395–406, 2012. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Machado RD, Southgate L, Eichstaedt CA, Aldred MA, Austin ED, Best DH, Chung WK, Benjamin N, Elliott CG, Eyries M, Fischer C, Gräf S, Hinderhofer K, Humbert M, Keiles SB, Loyd JE, Morrell NW, Newman JH, Soubrier F, Trembath RC, Viales RR, Grünig E. Pulmonary arterial hypertension: a current perspective on established and emerging molecular genetic defects. Hum Mutat 36: 1113–1127, 2015. doi: 10.1002/humu.22904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marsboom G, Toth PT, Ryan JJ, Hong Z, Wu X, Fang YH, Thenappan T, Piao L, Zhang HJ, Pogoriler J, Chen Y, Morrow E, Weir EK, Rehman J, Archer SL. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ Res 110: 1484–1497, 2012. doi: 10.1161/CIRCRESAHA.111.263848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marsboom G, Wietholt C, Haney CR, Toth PT, Ryan JJ, Morrow E, Thenappan T, Bache-Wiig P, Piao L, Paul J, Chen CT, Archer SL. Lung 18F-fluorodeoxyglucose positron emission tomography for diagnosis and monitoring of pulmonary arterial hypertension. Am J Respir Crit Care Med 185: 670–679, 2012. doi: 10.1164/rccm.201108-1562OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marsh LM, Jandl K, Grünig G, Foris V, Bashir M, Ghanim B, Klepetko W, Olschewski H, Olschewski A, Kwapiszewska G. The inflammatory cell landscape in the lungs of patients with idiopathic pulmonary arterial hypertension. Eur Respir J 51: 1701214, 2018. doi: 10.1183/13993003.01214-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marshall JD, Bazan I, Zhang Y, Fares WH, Lee PJ. Mitochondrial dysfunction and pulmonary hypertension: cause, effect, or both. Am J Physiol Lung Cell Mol Physiol 314: L782–L796, 2018. doi: 10.1152/ajplung.00331.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maston LD, Jones DT, Giermakowska W, Howard TA, Cannon JL, Wang W, Wei Y, Xuan W, Resta TC, Gonzalez Bosc LV. Central role of T helper 17 cells in chronic hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 312: L609–L624, 2017. doi: 10.1152/ajplung.00531.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J, Harrington RA, Anderson JL, Bates ER, Bridges CR, Eisenberg MJ, Ferrari VA, Grines CL, Hlatky MA, Jacobs AK, Kaul S, Lichtenberg RC, Lindner JR, Moliterno DJ, Mukherjee D, Pohost GM, Rosenson RS, Schofield RS, Shubrooks SJ, Stein JH, Tracy CM, Weitz HH, Wesley DJ; ACCF/AHA . ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation 119: 2250–2294, 2009. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 68.McMurtry MS, Moudgil R, Hashimoto K, Bonnet S, Michelakis ED, Archer SL. Overexpression of human bone morphogenetic protein receptor 2 does not ameliorate monocrotaline pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 292: L872–L878, 2007. doi: 10.1152/ajplung.00309.2006. [DOI] [PubMed] [Google Scholar]
- 69.Michelakis ED. Spatio-temporal diversity of apoptosis within the vascular wall in pulmonary arterial hypertension: heterogeneous BMP signaling may have therapeutic implications. Circ Res 98: 172–175, 2006. doi: 10.1161/01.RES.0000204572.65400.a5. [DOI] [PubMed] [Google Scholar]
- 70.Michelakis ED, Gurtu V, Webster L, Barnes G, Watson G, Howard L, Cupitt J, Paterson I, Thompson RB, Chow K, O’Regan DP, Zhao L, Wharton J, Kiely DG, Kinnaird A, Boukouris AE, White C, Nagendran J, Freed DH, Wort SJ, Gibbs JS, Wilkins MR. Inhibition of pyruvate dehydrogenase kinase improves pulmonary arterial hypertension in genetically susceptible patients. Sci Transl Med 9: eaao4583, 2017. doi: 10.1126/scitranslmed.aao4583. [DOI] [PubMed] [Google Scholar]
- 71.Michelakis ED, Wilkins MR, Rabinovitch M. Emerging concepts and translational priorities in pulmonary arterial hypertension. Circulation 118: 1486–1495, 2008. doi: 10.1161/CIRCULATIONAHA.106.673988. [DOI] [PubMed] [Google Scholar]
- 72.Moledina S, de Bruyn A, Schievano S, Owens CM, Young C, Haworth SG, Taylor AM, Schulze-Neick I, Muthurangu V. Fractal branching quantifies vascular changes and predicts survival in pulmonary hypertension: a proof of principle study. Heart 97: 1245–1249, 2011. doi: 10.1136/hrt.2010.214130. [DOI] [PubMed] [Google Scholar]
- 73.Montani D, Achouh L, Marcelin AG, Viard JP, Hermine O, Canioni D, Sitbon O, Simonneau G, Humbert M. Reversibility of pulmonary arterial hypertension in HIV/HHV8-associated Castleman’s disease. Eur Respir J 26: 969–972, 2005. doi: 10.1183/09031936.05.00133904. [DOI] [PubMed] [Google Scholar]
- 74.Morrell NW, Yang X, Upton PD, Jourdan KB, Morgan N, Sheares KK, Trembath RC. Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-beta(1) and bone morphogenetic proteins. Circulation 104: 790–795, 2001. doi: 10.1161/hc3201.094152. [DOI] [PubMed] [Google Scholar]
- 75.Mouthon L, Guillevin L, Humbert M. Pulmonary arterial hypertension: an autoimmune disease? Eur Respir J 26: 986–988, 2005. doi: 10.1183/09031936.05.00112105. [DOI] [PubMed] [Google Scholar]
- 76.Nelson MR, Tipney H, Painter JL, Shen J, Nicoletti P, Shen Y, Floratos A, Sham PC, Li MJ, Wang J, Cardon LR, Whittaker JC, Sanseau P. The support of human genetic evidence for approved drug indications. Nat Genet 47: 856–860, 2015. doi: 10.1038/ng.3314. [DOI] [PubMed] [Google Scholar]
- 77.Nickel NP, Spiekerkoetter E, Gu M, Li CG, Li H, Kaschwich M, Diebold I, Hennigs JK, Kim KY, Miyagawa K, Wang L, Cao A, Sa S, Jiang X, Stockstill RW, Nicolls MR, Zamanian RT, Bland RD, Rabinovitch M. Elafin reverses pulmonary hypertension via caveolin-1-dependent bone morphogenetic protein signaling. Am J Respir Crit Care Med 191: 1273–1286, 2015. doi: 10.1164/rccm.201412-2291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nicolls MR, Taraseviciene-Stewart L, Rai PR, Badesch DB, Voelkel NF. Autoimmunity and pulmonary hypertension: a perspective. Eur Respir J 26: 1110–1118, 2005. doi: 10.1183/09031936.05.00045705. [DOI] [PubMed] [Google Scholar]
- 79.O’Blenes SB, Fischer S, McIntyre B, Keshavjee S, Rabinovitch M. Hemodynamic unloading leads to regression of pulmonary vascular disease in rats. J Thorac Cardiovasc Surg 121: 279–289, 2001. doi: 10.1067/mtc.2001.111657. [DOI] [PubMed] [Google Scholar]
- 80.Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurtry IF. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res 100: 923–929, 2007. doi: 10.1161/01.RES.0000261658.12024.18. [DOI] [PubMed] [Google Scholar]
- 81.Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep 15: 1243–1253, 2014. doi: 10.15252/embr.201439246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pietra GG, Edwards WD, Kay JM, Rich S, Kernis J, Schloo B, Ayres SM, Bergofsky EH, Brundage BH, Detre KM. Histopathology of primary pulmonary hypertension. A qualitative and quantitative study of pulmonary blood vessels from 58 patients in the National Heart, Lung, and Blood Institute, Primary Pulmonary Hypertension Registry. Circulation 80: 1198–1206, 1989. doi: 10.1161/01.CIR.80.5.1198. [DOI] [PubMed] [Google Scholar]
- 83.Rabinovitch M, Castaneda AR, Reid L. Lung biopsy with frozen section as a diagnostic aid in patients with congenital heart defects. Am J Cardiol 47: 77–84, 1981. doi: 10.1016/0002-9149(81)90293-9. [DOI] [PubMed] [Google Scholar]
- 84.Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res 115: 165–175, 2014. doi: 10.1161/CIRCRESAHA.113.301141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rabinovitch M, Haworth SG, Castaneda AR, Nadas AS, Reid LM. Lung biopsy in congenital heart disease: a morphometric approach to pulmonary vascular disease. Circulation 58: 1107–1122, 1978. doi: 10.1161/01.CIR.58.6.1107. [DOI] [PubMed] [Google Scholar]
- 86.Rabinovitch M, Konstam MA, Gamble WJ, Papanicolaou N, Aronovitz MJ, Treves S, Reid L. Changes in pulmonary blood flow affect vascular response to chronic hypoxia in rats. Circ Res 52: 432–441, 1983. doi: 10.1161/01.RES.52.4.432. [DOI] [PubMed] [Google Scholar]
- 87.Ranchoux B, Antigny F, Rucker-Martin C, Hautefort A, Péchoux C, Bogaard HJ, Dorfmüller P, Remy S, Lecerf F, Planté S, Chat S, Fadel E, Houssaini A, Anegon I, Adnot S, Simonneau G, Humbert M, Cohen-Kaminsky S, Perros F. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation 131: 1006–1018, 2015. doi: 10.1161/CIRCULATIONAHA.114.008750. [DOI] [PubMed] [Google Scholar]
- 88.Rehman J, Archer SL. A proposed mitochondrial-metabolic mechanism for initiation and maintenance of pulmonary arterial hypertension in fawn-hooded rats: the Warburg model of pulmonary arterial hypertension. Adv Exp Med Biol 661: 171–185, 2010. doi: 10.1007/978-1-60761-500-2_11. [DOI] [PubMed] [Google Scholar]
- 89.Reynolds AM, Holmes MD, Danilov SM, Reynolds PN. Targeted gene delivery of BMPR2 attenuates pulmonary hypertension. Eur Respir J 39: 329–343, 2012. doi: 10.1183/09031936.00187310. [DOI] [PubMed] [Google Scholar]
- 90.Rich JD, Rich S. Clinical diagnosis of pulmonary hypertension. Circulation 130: 1820–1830, 2014. doi: 10.1161/CIRCULATIONAHA.114.006971. [DOI] [PubMed] [Google Scholar]
- 91.Rich S, Kieras K, Hart K, Groves BM, Stobo JD, Brundage BH. Antinuclear antibodies in primary pulmonary hypertension. J Am Coll Cardiol 8: 1307–1311, 1986. doi: 10.1016/S0735-1097(86)80301-1. [DOI] [PubMed] [Google Scholar]
- 92.Rose NR, Bona C. Defining criteria for autoimmune diseases (Witebsky’s postulates revisited). Immunol Today 14: 426–430, 1993. doi: 10.1016/0167-5699(93)90244-F. [DOI] [PubMed] [Google Scholar]
- 93.Rosenberg HC, Rabinovitch M. Endothelial injury and vascular reactivity in monocrotaline pulmonary hypertension. Am J Physiol 255: H1484–H1491, 1988. doi: 10.1152/ajpheart.1988.255.6.H1484. [DOI] [PubMed] [Google Scholar]
- 94.Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, Voelkel NF. Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J 19: 1178–1180, 2005. doi: 10.1096/fj.04-3261fje. [DOI] [PubMed] [Google Scholar]
- 95.Salman J, Ius F, Sommer W, Siemeni T, Kuehn C, Avsar M, Boethig D, Molitoris U, Bara C, Gottlieb J, Welte T, Haverich A, Hoeper MM, Warnecke G, Tudorache I. Mid-term results of bilateral lung transplant with postoperatively extended intraoperative extracorporeal membrane oxygenation for severe pulmonary hypertension. Eur J Cardiothorac Surg 52: 163–170, 2017. doi: 10.1093/ejcts/ezx047. [DOI] [PubMed] [Google Scholar]
- 96.Schnader J, Schloo BL, Anderson W, Stephenson LW, Fishman AP. Chronic pulmonary hypertension in sheep: temporal progression of lesions. J Surg Res 62: 243–250, 1996. doi: 10.1006/jsre.1996.0202. [DOI] [PubMed] [Google Scholar]
- 97.Sheikh AQ, Lighthouse JK, Greif DM. Recapitulation of developing artery muscularization in pulmonary hypertension. Cell Reports 6: 809–817, 2014. doi: 10.1016/j.celrep.2014.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sheikh AQ, Misra A, Rosas IO, Adams RH, Greif DM. Smooth muscle cell progenitors are primed to muscularize in pulmonary hypertension. Sci Transl Med 7: 308ra159, 2015. doi: 10.1126/scitranslmed.aaa9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 62, Suppl: D34–D41, 2013. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 100.Sobolewski A, Rudarakanchana N, Upton PD, Yang J, Crilley TK, Trembath RC, Morrell NW. Failure of bone morphogenetic protein receptor trafficking in pulmonary arterial hypertension: potential for rescue. Hum Mol Genet 17: 3180–3190, 2008. doi: 10.1093/hmg/ddn214. [DOI] [PubMed] [Google Scholar]
- 101.Song Y, Jones JE, Beppu H, Keaney JF Jr, Loscalzo J, Zhang YY. Increased susceptibility to pulmonary hypertension in heterozygous BMPR2-mutant mice. Circulation 112: 553–562, 2005. doi: 10.1161/CIRCULATIONAHA.104.492488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Soon E, Holmes AM, Treacy CM, Doughty NJ, Southgate L, Machado RD, Trembath RC, Jennings S, Barker L, Nicklin P, Walker C, Budd DC, Pepke-Zaba J, Morrell NW. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 122: 920–927, 2010. doi: 10.1161/CIRCULATIONAHA.109.933762. [DOI] [PubMed] [Google Scholar]
- 103.Soubrier F, Chung WK, Machado R, Grünig E, Aldred M, Geraci M, Loyd JE, Elliott CG, Trembath RC, Newman JH, Humbert M. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol 62, Suppl: D13–D21, 2013. doi: 10.1016/j.jacc.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 104.Spiekerkoetter E, Alvira CM, Kim YM, Bruneau A, Pricola KL, Wang L, Ambartsumian N, Rabinovitch M. Reactivation of gammaHV68 induces neointimal lesions in pulmonary arteries of S100A4/Mts1-overexpressing mice in association with degradation of elastin. Am J Physiol Lung Cell Mol Physiol 294: L276–L289, 2008. doi: 10.1152/ajplung.00414.2007. [DOI] [PubMed] [Google Scholar]
- 105.Spiekerkoetter E, Sung YK, Sudheendra D, Scott V, Del Rosario P, Bill M, Haddad F, Long-Boyle J, Hedlin H, Zamanian RT. Randomised placebo-controlled safety and tolerability trial of FK506 (tacrolimus) for pulmonary arterial hypertension. Eur Respir J 50: 1602449, 2017. doi: 10.1183/13993003.02449-2016. [DOI] [PubMed] [Google Scholar]
- 106.Spiekerkoetter E, Tian X, Cai J, Hopper RK, Sudheendra D, Li CG, El-Bizri N, Sawada H, Haghighat R, Chan R, Haghighat L, de Jesus Perez V, Wang L, Reddy S, Zhao M, Bernstein D, Solow-Cordero DE, Beachy PA, Wandless TJ, Ten Dijke P, Rabinovitch M. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest 123: 3600–3613, 2013. doi: 10.1172/JCI65592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV, Jessup M, Grizzle WE, Aldred MA, Cool CD, Tuder RM. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med 186: 261–272, 2012. doi: 10.1164/rccm.201201-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, Waxman AB. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res 104: 236–244, 2009. doi: 10.1161/CIRCRESAHA.108.182014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sutendra G, Michelakis ED. The metabolic basis of pulmonary arterial hypertension. Cell Metab 19: 558–573, 2014. doi: 10.1016/j.cmet.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 110.Suzuki T, Carrier EJ, Talati MH, Rathinasabapathy A, Chen X, Nishimura R, Tada Y, Tatsumi K, West J. Isolation and characterization of endothelial-to-mesenchymal transition cells in pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 314: L118–L126, 2018. doi: 10.1152/ajplung.00296.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tamosiuniene R, Nicolls MR. Regulatory T cells and pulmonary hypertension. Trends Cardiovasc Med 21: 166–171, 2011. doi: 10.1016/j.tcm.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tamosiuniene R, Tian W, Dhillon G, Wang L, Sung YK, Gera L, Patterson AJ, Agrawal R, Rabinovitch M, Ambler K, Long CS, Voelkel NF, Nicolls MR. Regulatory T cells limit vascular endothelial injury and prevent pulmonary hypertension. Circ Res 109: 867–879, 2011. doi: 10.1161/CIRCRESAHA.110.236927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, Tuder RM. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J 15: 427–438, 2001. doi: 10.1096/fj.00-0343com. [DOI] [PubMed] [Google Scholar]
- 114.Teichert-Kuliszewska K, Kutryk MJ, Kuliszewski MA, Karoubi G, Courtman DW, Zucco L, Granton J, Stewart DJ. Bone morphogenetic protein receptor-2 signaling promotes pulmonary arterial endothelial cell survival: implications for loss-of-function mutations in the pathogenesis of pulmonary hypertension. Circ Res 98: 209–217, 2006. doi: 10.1161/01.RES.0000200180.01710.e6. [DOI] [PubMed] [Google Scholar]
- 115.Toba M, Alzoubi A, O’Neill KD, Gairhe S, Matsumoto Y, Oshima K, Abe K, Oka M, McMurtry IF. Temporal hemodynamic and histological progression in Sugen5416/hypoxia/normoxia-exposed pulmonary arterial hypertensive rats. Am J Physiol Heart Circ Physiol 306: H243–H250, 2014. doi: 10.1152/ajpheart.00728.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tuder RM, Cool CD, Yeager M, Taraseviciene-Stewart L, Bull TM, Voelkel NF. The pathobiology of pulmonary hypertension. Endothelium. Clin Chest Med 22: 405–418, 2001. doi: 10.1016/S0272-5231(05)70280-X. [DOI] [PubMed] [Google Scholar]
- 117.Vitali SH, Hansmann G, Rose C, Fernandez-Gonzalez A, Scheid A, Mitsialis SA, Kourembanas S. The Sugen 5416/hypoxia mouse model of pulmonary hypertension revisited: long-term follow-up. Pulm Circ 4: 619–629, 2014. doi: 10.1086/678508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Voelkel NF, Bogaard HJ. Adding complexity to plexogenic arteriopathy. Eur Respir J 48: 1553–1555, 2016. doi: 10.1183/13993003.01867-2016. [DOI] [PubMed] [Google Scholar]
- 119.Voelkel NF, Cool C, Lee SD, Wright L, Geraci MW, Tuder RM. Primary pulmonary hypertension between inflammation and cancer. Chest 114, Suppl: 225S–230S, 1998. doi: 10.1378/chest.114.3_Supplement.225S. [DOI] [PubMed] [Google Scholar]
- 120.Voskuhl R. Sex differences in autoimmune diseases. Biol Sex Differ 2: 1, 2011. doi: 10.1186/2042-6410-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.West J, Fagan K, Steudel W, Fouty B, Lane K, Harral J, Hoedt-Miller M, Tada Y, Ozimek J, Tuder R, Rodman DM. Pulmonary hypertension in transgenic mice expressing a dominant-negative BMPRII gene in smooth muscle. Circ Res 94: 1109–1114, 2004. doi: 10.1161/01.RES.0000126047.82846.20. [DOI] [PubMed] [Google Scholar]
- 122.Witebsky E, Rose NR, Terplan K, Paine JR, Egan RW. Chronic thyroiditis and autoimmunization. J Am Med Assoc 164: 1439–1447, 1957. doi: 10.1001/jama.1957.02980130015004. [DOI] [PubMed] [Google Scholar]
- 123.Wright AF, Ewart MA, Mair K, Nilsen M, Dempsie Y, Loughlin L, Maclean MR. Oestrogen receptor alpha in pulmonary hypertension. Cardiovasc Res 106: 206–216, 2015. doi: 10.1093/cvr/cvv106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xu W, Erzurum SC. Endothelial cell energy metabolism, proliferation, and apoptosis in pulmonary hypertension. Compr Physiol 1: 357–372, 2011. doi: 10.1002/cphy.c090005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zabini D, Heinemann A, Foris V, Nagaraj C, Nierlich P, Bálint Z, Kwapiszewska G, Lang IM, Klepetko W, Olschewski H, Olschewski A. Comprehensive analysis of inflammatory markers in chronic thromboembolic pulmonary hypertension patients. Eur Respir J 44: 951–962, 2014. doi: 10.1183/09031936.00145013. [DOI] [PubMed] [Google Scholar]
- 126.Zhang H, Wang D, Li M, Plecitá-Hlavatá L, D’Alessandro A, Tauber J, Riddle S, Kumar S, Flockton A, McKeon BA, Frid MG, Reisz JA, Caruso P, El Kasmi KC, Ježek P, Morrell NW, Hu CJ, Stenmark KR. Metabolic and proliferative state of vascular adventitial fibroblasts in pulmonary hypertension is regulated through a microRNA-124/PTBP1 (polypyrimidine tract binding protein 1)/pyruvate kinase muscle axis. Circulation 136: 2468–2485, 2017. doi: 10.1161/CIRCULATIONAHA.117.028069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhao YD, Courtman DW, Deng Y, Kugathasan L, Zhang Q, Stewart DJ. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res 96: 442–450, 2005. doi: 10.1161/01.RES.0000157672.70560.7b. [DOI] [PubMed] [Google Scholar]