Abstract
MicroRNAs (miRNAs) are noncoding RNAs that regulate gene expression in many diseases, although the contribution of miRNAs to the pathophysiology of lung injury remains obscure. We hypothesized that dysregulation of miRNA expression drives the changes in key genes implicated in the development of lung injury. To test our hypothesis, we utilized a model of lung injury induced early after administration of intratracheal bleomycin (0.1 U). Wild-type mice were treated with bleomycin or PBS, and lungs were collected at 4 or 7 days. A profile of lung miRNA was determined by miRNA array and confirmed by quantitative PCR and flow cytometry. Lung miR-26a was significantly decreased 7 days after bleomycin injury, and, on the basis of enrichment of predicted gene targets, it was identified as a putative regulator of cell adhesion, including the gene targets EphA2, KDR, and ROCK1, important in altered barrier function. Lung EphA2 mRNA, and protein increased in the bleomycin-injured lung. We further explored the miR-26a/EphA2 axis in vitro using human lung microvascular endothelial cells (HMVEC-L). Cells were transfected with miR-26a mimic and inhibitor, and expression of gene targets and permeability was measured. miR-26a regulated expression of EphA2 but not KDR or ROCK1. Additionally, miR-26a inhibition increased HMVEC-L permeability, and the disrupted barrier integrity due to miR-26a was blocked by EphA2 knockdown, shown by VE-cadherin staining. Our data suggest that miR-26a is an important epigenetic regulator of EphA2 expression in the pulmonary endothelium. As such, miR-26a may represent a novel therapeutic target in lung injury by mitigating EphA2-mediated changes in permeability.
Keywords: bleomycin, EphA2, lung injury, miRNA, miR-26a
INTRODUCTION
Acute respiratory distress syndrome (ARDS) is a severe form of lung injury that causes significant morbidity and mortality in adults and children (33, 47, 61, 67). ARDS is a clinical syndrome of severe hypoxemia, decreased lung compliance, and respiratory failure. These physiological changes are triggered by disruption of the alveolar-capillary barrier, which leads to development of pulmonary edema and intense tissue inflammation (55). Despite decades of intensive research, no effective therapies that target these fundamental mechanisms currently exist (35, 43, 48). Previous research identified numerous genes induced in response to lung injury that contribute to disruption of barrier function (38, 54). New advances implicate a potential role for epigenetic mechanisms in the regulation of these genes and represent the potential for novel therapeutic targets (2, 45, 58).
MicroRNAs (miRNAs) are small, noncoding RNAs that act as endogenous gene silencing factors. miRNAs serve a central role in epigenetic regulation by controlling levels of protein expression through translational repression and mRNA degradation (24). As such, miRNA expression patterns control biological and pathological processes, and several specific miRNAs are currently under development for the treatment of human diseases (50). Distinct patterns of miRNA dysregulation occur in a variety of animal models of lung injury (17, 26, 29, 51, 57, 66). Investigating the role of miRNAs in the injured lung will provide important insight into key pathophysiological mechanisms and reveal numerous potential therapeutic targets.
Changes in miRNA expression are relevant for development of disease when they impact important downstream gene targets. EphA2, a member of the ephrin receptor tyrosine kinase family, is integral to the development of increased endothelial permeability in several animal models of lung injury (3, 4, 9, 25). In silico analysis predicts that miR-26a targets EphA2—a relationship confirmed in endothelial progenitor cells (68). We utilized in vitro and in vivo systems to test our hypothesis that miR-26a regulates EphA2 expression in lung endothelial cells, leading to increased permeability seen in lung injury.
METHODS
Mouse model.
Animal studies were approved by the University of Colorado Institutional Animal Care and Use Committee. Mice received standard chow and water ad libitum and were cohoused with four or five mice per cage in the vivarium with a 14-h:10-h light-dark schedule. Male wild-type 7-wk-old C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME) and acclimated at altitude in Denver for 1 wk before initiation of the treatments. Mice were randomly assigned to receive 100 µl of PBS or 0.1 U of bleomycin (Hospira, Lake Forest, IL) in 100 µl PBS by intratracheal (IT) aspiration. Mice were euthanized by isoflurane with cervical dislocation at 4 or 7 days posttreatment, followed by thoracotomy and harvesting of lung tissue.
Tissue harvesting.
Mice were anesthetized with inhaled isoflurane (1.5–4%) and euthanized by exsanguination through right ventricle aspiration followed by cervical dislocation. The chest was opened, and lungs were flushed with 5–10 ml of cold PBS via the right ventricle to remove blood. Both left and right lungs were flash-frozen and pulverized.
Bronchoalveolar lavage fluid.
To collect bronchoalveolar lavage fluid (BALF), the trachea was cannulated with a blunt 20-gauge needle, and the lungs were lavaged 5 times using 1 ml of ice-cold PBS. BALF samples were spun down at 700 g for 7 min; then, the cell pellet was resuspended into a total of 100 μl PBS. A 50-μl aliquot was diluted with 950 μl of PBS for a total cell count performed by a Vi-Cell Machine (Beckman Coulter, Indianapolis, IN). For the albumin quantification, the BALF supernatant was diluted 1:50,000, and albumin content was determined by ELISA using the mouse albumin kit (Bethyl Laboratories, Montgomery, TX).
Quantitative PCR.
RNA was isolated from lungs using the miRNeasy kit (Qiagen, Hilden, Germany) and cells using TRIzol, and cDNA was synthesized by miScriptII (Qiagen) for miRNAs and iScript (Bio-Rad, Hercules, CA) for mRNA. quantitative PCR (qPCR) for miR-26a and EphA2 was performed on an ABI7300 real-time PCR machine (Applied Biosystems, Foster City, CA) using miScript SYBR Green Kit (Qiagen) designed for mouse miR-26a (forward miR-26a 5′-GGTTCAAGTAATCCAGGATAGGCT-3′) and housekeeping gene U6 (forward 5′-CGGCAGCACATATACTAAAATTGGAAC GA-3′); and power SYBR Green (Applied Biosystems) designed for mouse EphA2 (forward mouse EphA2 5′-TTAGGGAGAAGGATGGTGAGTT-3′, reverse mouse EphA2 5′-GTTGCTGTTGACGAGGATGTT-3′; forward human EphA2 5′-ATGGAGCTCCAGGCAGCCCGC-3′, reverse human EphA2 5′-GCCATACGGGTGTGTGAGCCAGC-3′); and housekeeping gene 18S (forward 18s 5′-GCCGCTAGAGGTGAAATTCTT G-3′, reverse 18s 5′-CTTTCGCTCTGGTCCGTCTT-3′). qPCR for KDR and ROCK1 was performed on a QuantStudio 6 Flex PCR machine (Applied Biosystems) with the following TaqMan gene expression assays (Applied Biosystems): Rn18S (Mm03928990_g1), ROCK1 (Mm00485745_m1), KDR (Mm01222421_m1), 18S (Hs99999901_s1), ROCK1 (Hs01127699_m1), and KDR (Hs00911700_m1). All samples were run in triplicate.
miRNA Array.
Four control lung cDNAs and four bleomycin-injured cDNAs were pooled into single samples. Using the qPCR-based TaqMan Mouse miRNA array (Life Technologies, Foster City, CA), we investigated relative miRNA expression of 55 different miRNAs.
Target gene prediction and gene enrichment.
TargetScan (http://www.targetscan.org) was used to predict the gene targets of miR-26a. Predicted genes for miR-26-5p irrespective of site conservation were entered into Enrichr (http://amp.pharm.mssm.edu/Enrichr/). Predicted gene targets of both miR-26a-5p and miR-26b-5p were included in gene enrichment since both miRNAs share a seed sequence and have the same gene targets. The P value was used to rank biological pathways (7).
Cell culture and transfection.
Human lung microvascular endothelial cells (HMVEC-L) were maintained in EGM-2MV media (Lonza, Walkersville, MD) at 37° at 5% CO2 in a humidified incubator and used between passages 4 and 7. Cells were seeded 24 h before transfection in six-well plates or 60-mm dishes (Costar, Corning, NY) coated with fibronectin (Calbiochem-Millipore, Billerica, MA) at 1 µg/cm2. Cell density was 1 × 105 cells/well for RNA isolation or 1.5–2 × 105 cell/dish for protein isolation. RNAiMAX (Life Technologies) was used to transfect mirVana miR-26a mimic (ID MC10249), inhibitor (ID MH10249), and respective negative controls at 25 nM. Cells transfected with mimic inhibitor and respective negative controls were harvested at 72 h.
Western blot analysis.
Tissue was homogenized in T-PER buffer (Thermo Scientific, Carlsbad, CA), and cells were homogenized in RIPA buffer (Thermo Scientific) with the addition of protease inhibitor (Sigma-Aldrich, St. Louis, MO) and phosphatase inhibitors 2 and 3 cocktails (Sigma-Aldrich). Protein concentration was determined using the Pierce 660-nm protein assay reagent (Thermo Scientific). Twenty micrograms of protein were separated by gel electrophoresis using Criterion XT 4–12% Bis-Tris Precast Gel (Bio-Rad) with MES SDS running buffer (Life Technologies). Proteins were transferred to polyvinylidene fluoride membranes (Bio-Rad) with NuPAGE transfer buffer using a Novex Semi-Dry Blotter (Life Technologies). Membranes were activated in methanol and blocked in 5% nonfat dry milk in Tris-buffered saline containing 0.05% Tween 20 (TBST) for 1 h. Membranes were incubated in the following primary antibodies: rabbit polyclonal to EphA2 (Santa Cruz Biotechnology, Santa Cruz, CA) at 1:100 and mouse monoclonal to β-actin (Sigma-Aldrich) at 1:10,000 in 5% milk in TBST at 4°C overnight. We previously confirmed the specificity of the EphA2 antibody for EphA2, showing the 132-kDa EphA2 band was absent in cells after siRNA knockdown of EphA2 (3). The appropriate horseradish peroxidase conjugated goat anti-rabbit or mouse secondary antibody (EMD Millipore, Billerica, MA) was applied at 1:10,000 in TBST for 1 h at room temperature. Detection was accomplished using SuperSignal Femto Chemiluminescent substrates (Thermo Scientific). Bands were quantified by densitometry using Image Laboratory Software (Bio-Rad) or FluorChem HD 9900 Software (ProteinSimple, San Jose, CA).
Permeability assay.
HMVEC-L were seeded on biotinylated gelatin-coated 96-well plates (Corning, Corning, NY) at 4 × 104 cells/well. Human vascular endothelial growth factor (GIBCO-Life Technologies, Frederick, MD) was added to selected wells at 100 ng/ml. The permeability assay was performed by adding FITC-avidin solution directly to the culture medium at 25 μg/ml for 2–3 min. Matrix-bound FITC-avidin was then measured on a POLARstart Omega plate reader (BMG Labtech, Cary, NC). Phenol red-free endothelial basal media (Lonza) was used for washes and a final volume for absorbance readings (10). Each experimental condition was performed in 3–8 wells per plate. The assay was performed on 3 days. Cells were transfected with mimic, inhibitor, and respective negative controls, as described above, 24 h after seeding and permeability experiments were performed 72 h after transfection.
Vascular endothelial-cadherin immunofluorescence.
Human umbilical vein endothelial cells (HUVEC) (Lonza, Walkersville, MD) were seeded at 5 × 105 cells per well on Laboratory Tek 4-well chamber slides. Cells were transfected with miR-26a inhibitor or negative control at 25 nM and siRNA for EphA2 (Thermo Scientific) or scramble siRNA-A (Santa Cruz Biotechnology) at 5 nM (3). Cells were cotransfected with the following combinations: miRNA negative control + scrambled siRNA, miRNA negative control + EphA2 siRNA, miR-26a inhibitor + scrambled siRNA, and miR-26a inhibitor + EphA2 siRNA. After 72 h, the cells were fixed with 4% PFA in PBS for 10 min, rinsed with PBS, and permeabilized by a 10-min incubation in 0.1% Triton X100 containing PBS. After 1 h of blocking with 10% goat serum in PBS and 0.1% Tween-20, the cells were incubated with anti-VE-cadherin conjugated to Alexa Fluor 488 (sc-9989 AF488; Santa Cruz Biotechnology) at 1:100 at room temperature. The cells were then mounted in mounting medium with DAPI (H1500; Vector Laboratory). The confocal fluorescence images were captured with an Olympus FV-1000 with a ×20 objective lens (Tokyo, Japan) (46).
In vivo delivery of miR-26a mimic.
Delivery of mirVana miR-26a mimic (ID MC10249; Life Technologies) and negative control (Cat. No. 4464061; Life Technologies) was performed with neutral lipid emulsion Max Suppressor In Vivo RNA-LANCErII (Bio Scientific, Austin, TX). The miRNA was prepared in the delivery reagent per the manufacturerʼs instructions and passed through an extruder membrane (Avanti Polar Lipids, Alabaster, AL) to form 100-nm particles. Mice were treated with IT bleomycin, as described above. Four days after bleomycin administration, mice were injected via tail vein with 40 μg (~2 mg/kg) of miR-26a mimic or negative control in 100 μl of lipid solution (53). Mice were harvested 7 days after bleomycin administration, and tissue and BALF were collected and processed as described above.
EphA2 immunofluorescence.
Lungs were inflation-fixed with 4% paraformaldehyde at 20 cmH2O pressure for 30 min followed by 4% paraformaldehyde for 48 h at room temperature and 70% ethanol until processed into 5-μm sections. Lung sections were deparaffinized with CitriSolv and rehydrated in graded ethanol. Tissues were processed for antigen retrieval in 10 mM sodium citrate, pH 6.0, for 5 min, washed in PBS for 5 min, blocked with 5% goat serum in PBS for 1 h, and then incubated with rabbit polyclonal α-EphA2 antibody at 1:25 dilution (Santa Cruz Biotechnology) at 4°C overnight followed by secondary goat anti-rabbit Alexa Fluor 594 (Invitrogen). Sections were next incubated with primary rabbit polyclonal α-von Willebrand Factor at 1:100 dilution (cat. no. F3520; Sigma) at 4°C overnight followed by secondary goat anti-rabbit Alexa Fluor 488 (Invitrogen). Lung sections were examined by confocal microscopy with a Zeiss laser-scanning microscope (LSM) 780 (Oberkochen, Germany).
In situ hybridization.
Lungs were inflation-fixed and sectioned as described above. In situ hybridization (ISH) on lung sections was performed using miRCURY LNA microRNA ISH optimization kit 5 (Exiqon, Vedbaek, Denmark), according to the manufacturer guidelines. Slides were deparaffinized with xylene and ethanol and treated with Proteinase-K. ISH was performed with DIG-labeled detection probes for miR-26a, U6 (positive control), and scrambled miRNA (negative control). Visualization performed with anti-DIG antibody conjugated with AP, which converts NBT and BCIP into dark blue precipitate. Fast Red was applied as a nuclear counterstain (Vector, Burlingame, CA).
Flow cytometry.
Whole lungs were mechanically digested in Liberase TM (0.4 mg/ml in HBSS) in a GentleMacs Dissociator (Miltenyi Biotec) using the program (m_lung_01_2) and then incubated in a shaking water bath at 37°C. Lungs were then mechanically digested using program (m_lung_02_1) for 10 s. Single-cell suspensions were then sequentially filtered through a 70- and 40-µm cell strainer. Pelleted cells were then stained for using fluorescently conjugated antibodies (BD Biosciences) against endothelial (CD31-BV421, clone MEC 13.3) and epithelial (CD326-BB515, clone G8.8) markers for 30 min at a 1/100 concentration. Dead cells were stained using LIVE/DEAD fixable near-IR dead cell kit (Molecular Probes) at a 1/100 concentration. After being washed, cells were then fixed and stained per PrimeFlow (Invitrogen) manufacturer instructions. In brief, target hybridization was performed using a miR26a-5p target probe (Type I, Affymetrix); then, the signal was amplified using preamplifier and amplifier DNA (Type I) and detected by the addition of fluorescent label probes (Alexa Fluor 647). UltraComp (eBiosciences) compensation beads were used to determine instrument voltages and adjust compensation as necessary. Unstained cells and fluorescence-minus one (FMO) controls were used to determine gate regions. Flow cytometry analysis was performed at the University of Colorado Cancer Center Flow Cytometry Core using a ZE5 cell analyzer (Yeti, Propel Laboratories, Fort Collins, CO), and data analysis was performed on Kaluza Analysis 1.5a (Beckman Coulter).
Statistical analysis.
Data were analyzed by unpaired t-test or two-way ANOVA followed by Tukey’s multiple-comparisons test to determine differences between treatment groups using Prism software (GraphPad Software, La Jolla, CA). Data are expressed as means ± SE. Significance was defined as P < 0.05. Outliers were removed using the Grubb test, where only one outlier was removed.
RESULTS
IT bleomycin increased lung permeability.
To evaluate the effect of IT bleomycin on lung permeability, we measured the alveolar cell count and protein content 7 days after bleomycin administration. We found a significant increase in both markers of lung permeability at the 7-day time point postbleomycin (Fig. 1, A and B).
Fig. 1.
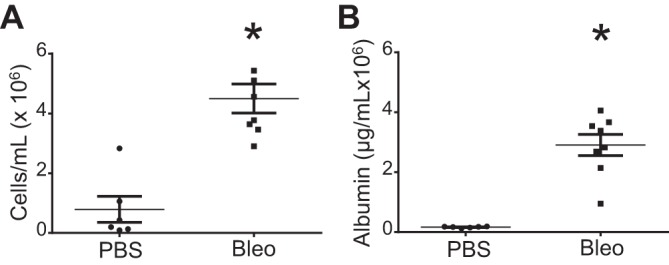
Increased permeability after intratracheal (IT) bleomycin. Mice were treated with 100 µl of PBS or 0.1 U of bleomycin in 100 µl of PBS. Bronchoalveolar lavage fluid collected 7 days after treatment. Alveolar cell count (A) and albumin (B) at 7 days. Data are displayed as means ± SE; n = 6 or 7. *P < 0.05 compared with PBS by unpaired t-test.
IT bleomycin decreased multiple lung miRNAs.
To evaluate miRNAs that changed in response to bleomycin, we measured the expression levels of 55 miRNAs in lungs pooled from four mice, 4 days posttreatment with IT bleomycin compared with PBS-treated control mice. We found 45 miRNAs decreased greater than twofold in the pooled lung samples, while no miRNAs were increased (Table 1). The greatest decrease occurred in miR-1 (8.4-fold), miR-26a (7.5-fold), miR-30b (7.7-fold), and miR-30c (8.8-fold).
Table 1.
Lung miRNA dysregulation after intratracheal bleomycin
| miRNA | Fold Change | miRNA | Fold Change |
|---|---|---|---|
| mRNA expression change: >6-fold | |||
| 1 | −8.42 | 30b | −7.77 |
| 26a | −7.46 | 30c | −8.84 |
| mRNA expression change: between 2- and 6-fold | |||
| 10a | −3.91 | 126-5p | −2.59 |
| 10b | −2.74 | 146a | −3.15 |
| 15b | −5.76 | 150 | −4.75 |
| 16 | −4.44 | 182 | −3.53 |
| 22 | −2.00 | 183 | −2.93 |
| 23a | −5.65 | 191 | −2.43 |
| 23b | −5.67 | 194 | −2.46 |
| 24 | −3.72 | 195 | −4.58 |
| 25 | −2.66 | 199b | −3.53 |
| 26b | −2.53 | 200a | −3.31 |
| 27b | −3.05 | 203 | −5.95 |
| 29a | −2.18 | 218 | −2.49 |
| 30a | −3.32 | 425 | −2.00 |
| 30d | −2.90 | 451 | −2.04 |
| 98 | −3.47 | let-7a | −2.36 |
| 99a | −2.59 | let-7b | −2.05 |
| 99b | −2.24 | let-7c | −2.11 |
| 125a-5p | −3.54 | let-7d | −3.12 |
| 125b-5p | −3.86 | let-7e | −2.10 |
| 126-3p | −3.62 | let-7g | −3.31 |
| mRNA expression change: <2-fold | |||
| 9 | −1.47 | 34c | −1.3 |
| 19b | −1.69 | 138 | −1.4 |
| 21 | −1.01 | 140 | −1.64 |
| 27a | −1.89 | let-7i | −1.51 |
| 30e | −1.85 | ||
Mice were treated with 100 µl of PBS or 0.1 U of bleomycin in 100 µl of PBS and harvested after 4 days. Four lung samples from each PBS and bleomycin group were pooled, and quantitative PCR-based miRNA array was performed. miRNA expression decreased by greater than 6-fold, between 2- and 6-fold, and less than twofold in bleomycin-treated lungs.
Predicted miR-26a gene targets regulate cell adhesion.
We analyzed predicted downstream gene targets for miR-26a due to its significant decrease in the bleomycin-injured lung and known relationship with EphA2, a receptor tyrosine kinase implicated in enhanced permeability in lung injury (9, 68). TargetScan was utilized to identify predicted target genes for miR-26a (including miR-26a-5p and miR-26b-5p), and 4,655 target genes were subsequently entered into Enrichr to identify the top biological pathways. Regulation of cell adhesion was highly ranked by P value and included 93 predicted target genes in several different categories, illustrated in Fig. 2. Among this group, we identified three miR26a target genes that would be expected to increase permeability when their expression increased: EphA2, KDR, and ROCK1. On the basis of these analyses, we proceeded to test the impact of bleomycin of these three miR26a target genes by qPCR in lung homogenates.
Fig. 2.
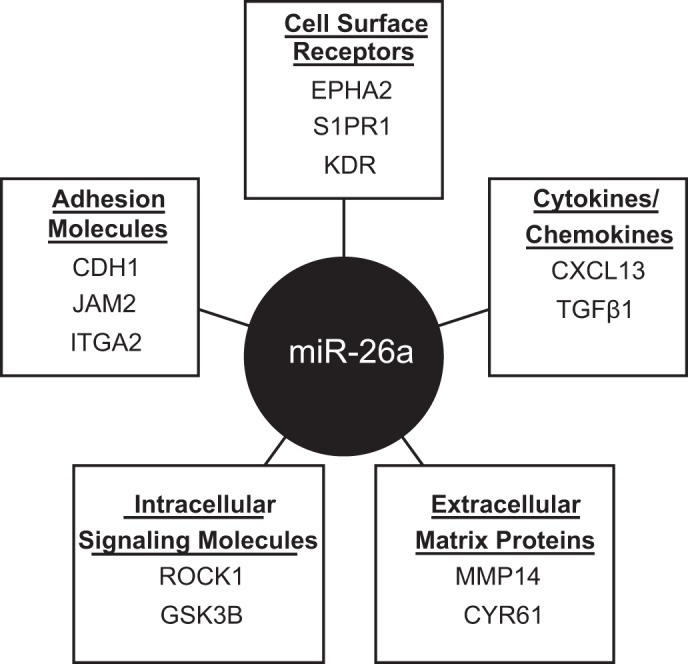
Predicted gene targets of miR-26a-involved cell adhesion. Predicted gene targets of miR-26a identified using TargetScan. Predicted gene targets entered into Enrichr to perform gene enrichment and biologic pathways ranked by combined score. Twelve of the 95 predicted gene targets of miR-26a involved in regulation of cell adhesion were selected and divided into categories: cell surface receptors, cytokines/chemokines, extracellular matrix proteins, intracellular signaling molecules, and adhesion molecules.
IT bleomycin decreased lung miR-26a and increased lung EphA2 expression.
To confirm whether miR-26a is decreased after IT bleomycin, we measured lung miR-26a levels by qPCR. Although the array indicated that miR-26a decreased at 4 days, when we validated these findings in a new cohort of mice by qPCR, miR-26a levels were unchanged 4 days after IT bleomycin but decreased significantly at 7 days (Fig. 3A). Therefore, we performed the remaining analyses at 7 days posttreatment. Concurrent with the decrease in miR26a, EphA2 increased 7 days posttreatment with bleomycin (Fig. 3B). In contrast to EphA2, KDR, and ROCK1 significantly decreased at 7 days, suggesting that their expression was not regulated by miR26a in this setting (Fig. 3, C and D). Consistent with the mRNA data, lung EphA2 protein significantly increased at 7 days (Fig. 3E). All comparisons between groups were performed with samples run on the same gel. Bands presented in the figures were run on the same gel with nonessential lanes removed. EphA2 was identified at 132 kDa in both lung and cells (Fig. 3, F and G); this was validated previously as the correct band size by the manufacturer and our previous publication, in which this band was absent in endothelial cells after siRNA knockdown of EphA2 (2). The two lower nonspecific bands were not included in the analysis.
Fig. 3.
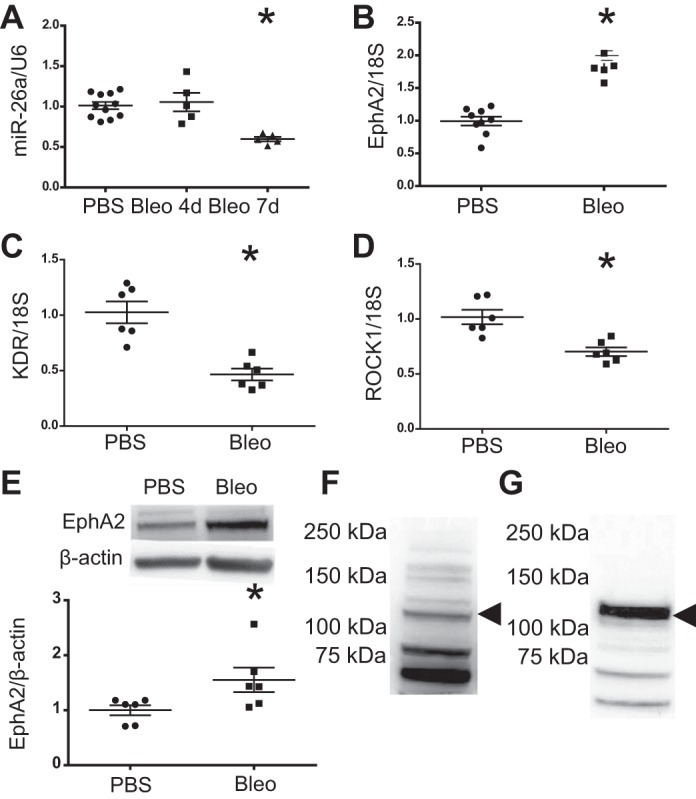
Lung miR-26a, EphA2, KDR, and ROCK1 after intratracheal (IT) bleomycin. Mice were treated with 100 µl of PBS or 0.1 U of bleomycin in 100 µl of PBS. Lungs were harvested 4 and 7 days after treatment. A: lung miR-26a at 4 and 7 days. Lung EphA2 (B), KDR (C), and ROCK1 (D) mRNA levels at 7 days. E: relative densitometry for Lung EphA2 protein at 7 days with a representative Western blot image. Data displayed as means ± SE; n = 5–11, *P < 0.05 compared with PBS by unpaired t-test. U6, small nuclear RNA U6; 18S, ribosomal protein 18S. F: Western blot for lung tissue with EphA2 band at 132 kDa and nonspecific lower MW bands (not included in densitometry). G: Western blot for cell protein with EphA2 band at 132 kDa and nonspecific lower MW bands. Black arrowhead denotes EphA2.
In HMVEC-L, miR-26a level regulated EphA2 expression.
To determine whether miR-26a regulated EphA2 in HMVEC-L, we performed in vitro experiments with HMVEC-L transfected with either the miR-26a inhibitor or mimic. We first confirmed the efficacy of the inhibitor or mimic transfection and demonstrated that the miR-26a inhibitor significantly decreased miR-26a expression while transfection with miR-26a mimic significantly increased miR-26a levels compared with respective negative controls (Fig. 4, A and B). We then measured EphA2 mRNA by qPCR and EphA2 protein by Western blot analysis. The miR-26a inhibitor increased EphA2 mRNA and protein by 30% compared with negative control. Conversely, the miR-26a mimic decreased EphA2 mRNA and protein by 40–50% compared with negative control (Fig. 4, C–F). To assess whether other miR-26a predicted target gene expression changed with miR-26a modulation and served as a negative control, we measured KDR and ROCK1 mRNA expression after transfection with miR-26a inhibitor or mimic. ROCK1 expression did not change with either miR-26a inhibitor or mimic, while KDR expression decreased with miR-26a mimic but did not significantly change with miR-26a inhibitor (Fig. 4, G–J).
Fig. 4.
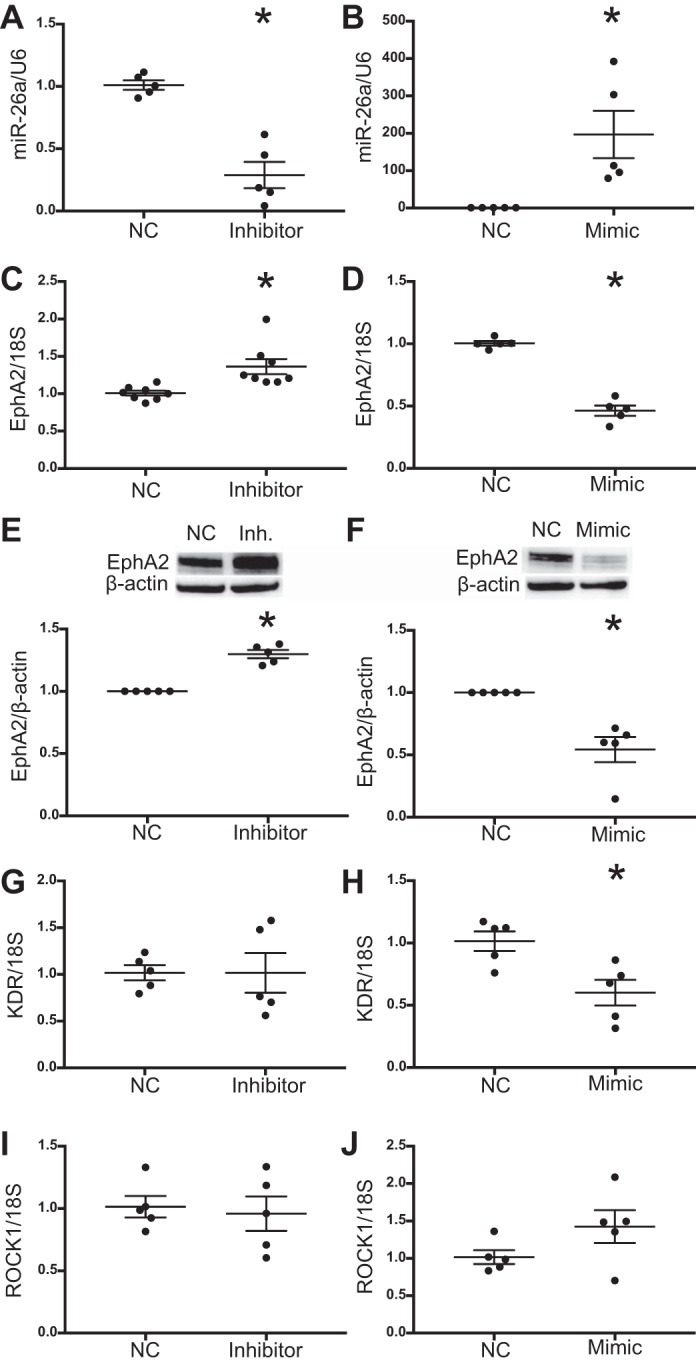
miR-26a regulates EphA2 expression in human lung microvascular endothelial cells (HMVEC-L). Cells were transfected with miR-26a mimic, inhibitor, or respective negative control (NC) and harvested at 72 h. miR-26a levels after transfection with miR-26a inhibitor (A) and mimic (B). EphA2 mRNA levels after transfection with miR-26a inhibitor (C) and mimic (D). Relative densitometry of EphA2 protein levels after transfection with miR-26a inhibitor (E) and mimic (F) with representative Western blots. KDR mRNA levels after transfection with miR-26a inhibitor (G) and mimic (H). ROCK1 mRNA levels after transfection with miR-26a inhibitor (I) and mimic (J). Data are displayed as means ± SE; n = 5–8, *P < 0.05 compared with NC determined by unpaired t-test. U6, small nuclear RNA U6; 18S, ribosomal protein 18S.
Inhibition of miR-26a increased HMVEC-L permeability.
We used a FITC-labeled avidin permeability assay to assess the role of miR-26a in regulation of HMVEC-L permeability. The data represent a set of wells seeded simultaneously and tested on a single day. The experiments were repeated on two subsequent days and showed a consistent pattern of response. As previously reported, VEGF stimulation increased permeability in control HMVEC-L (Fig. 5A). Transfection with miR-26a inhibitor increased permeability of HMVEC-L cells by 50% when compared with its negative control, while transfection with miR-26a mimic did not impact permeability of HMVEC-L (Fig. 5, B and C).
Fig. 5.
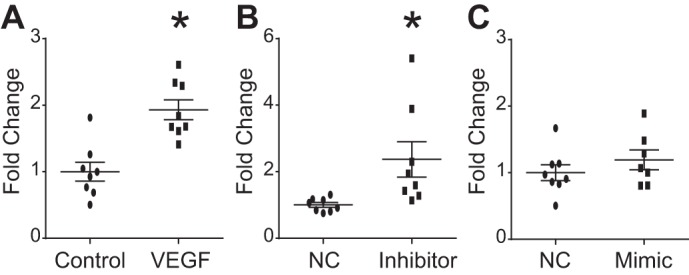
miR-26a inhibition increased human lung microvascular endothelial cell (HMVEC-L) permeability. HMVEC-L seeded on biotinylated gelatin-coated 96-well plates. Transfection with mimic, inhibitor, or negative control (NC) performed 24 h after seeding. Seventy-two hours after transfection, 25 μg/ml FITC-avidin solution was added for 2–3 min, and absorbance of matrix-bound FITC-avidin was measured to determine permeability. Results displayed as fold change in absorbance compared with NC. A: HMVEC-L permeability with and without VEGF (100 ng/ml) stimulation. HMVEC-L permeability after transfection with miR-26a inhibitor (B) and mimic (C) and respective NC. Data displayed as means ± SE; n = 7 or 8, *P < 0.05 compared with NC determined by unpaired t-test.
Increased permeability following inhibition of miR-26a was blocked by knockdown of EphA2.
To determine whether the increased endothelial cell permeability observed after miR-26a inhibition was mediated by increased EphA2, we evaluated cell monolayer integrity via VE-cadherin expression by immunofluorescence (IF) in HUVECs following dual transfection with the miR-26a inhibitor and EphA2 siRNA. HUVECs were used instead of HMVEC-L because HUVECs, but not HMVEC-L, tolerated the stress of the dual transfections sufficiently to assess endothelial cell barrier formation (1). The miR-26a inhibitor induced EphA2 in HUVECs, similar to HMVEC-L (data not shown). Dual transfection with the two negative controls (negative control miRNA + scrambled siRNA) or with the negative control miRNA + EphA2 siRNA showed normal HUVEC morphology with strong VE cadherin staining and no intercellular gaps (Fig. 6, A and B). In contrast, dual transfection with the miR-26a inhibitor + scrambled siRNA negative control showed gaps between cells and qualitatively decreased VE-cadherin staining, as an indicator of impaired cell-cell contact (Fig. 6C) (15, 25, 46). Dual transfection with the miR-26a inhibitor + siEphA2 preserved VE-cadherin expression and gap formation (Fig. 6D). The experiment was repeated three or four times, and a representative image is shown from cells treated and processed simultaneously. We confirmed that EphA2 siRNA reduced EphA2 levels to baseline in cells treated with miR-26a inhibitor + EphA2 siRNA (Fig. 6E).
Fig. 6.
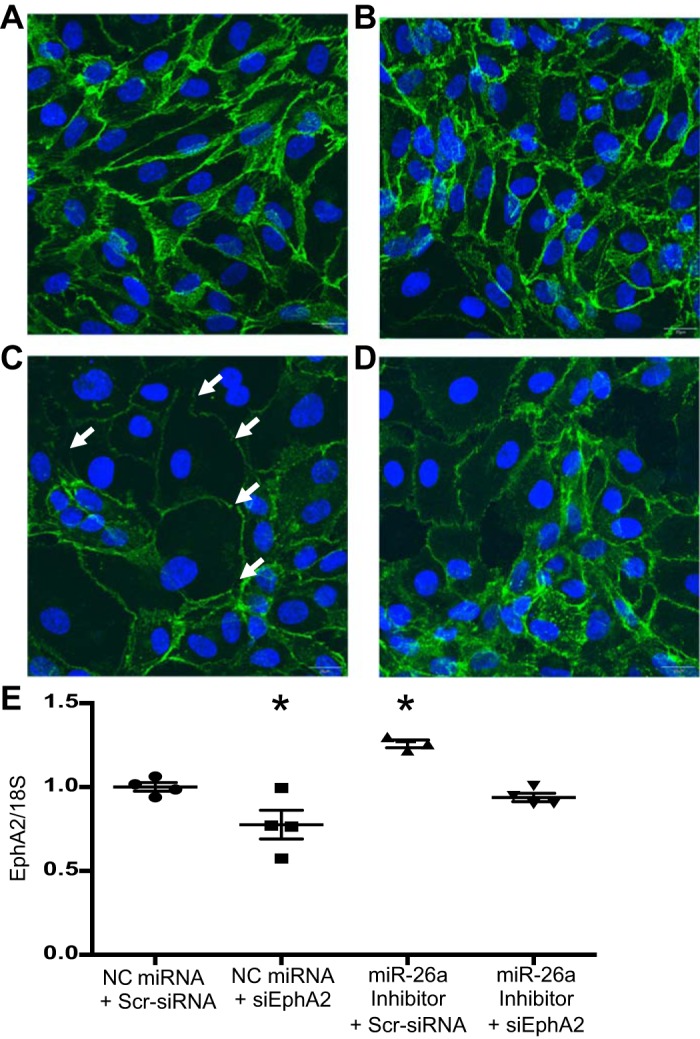
miR-26a inhibition increased human umbilical vein endothelial cells gap formation in an EphA2-dependent manner. Cells were transfected with miR-26a inhibitor, siEphA2, and/or respective negative control (NC) miRNA, or scrambled siRNA and immunofluorescence (IF) staining for VE-cadherin (green) and DAPI (blue) was performed at 72 h and visualized by confocal microscopy. Representative IF images after transfection with negative control miRNA + scrambled siRNA (A), negative control miRNA + EphA2 siRNA (B), miR-26a inhibitor + scrambled siRNA (C), miR-26a inhibitor + siEphA2 (D). Images shown at ×20 magnification. White arrows indicate gaps in paracellular adherens junctions. E: EphA2 mRNA levels after cotransfection. Data are displayed as means ± SE; n = 3 or 4, 18S, ribosomal protein 18S; Scr, scrambled. *P < 0.05 compared with dual negative controls (negative control miRNA + scrambled siRNA).
Delivery of miR-26a in vivo.
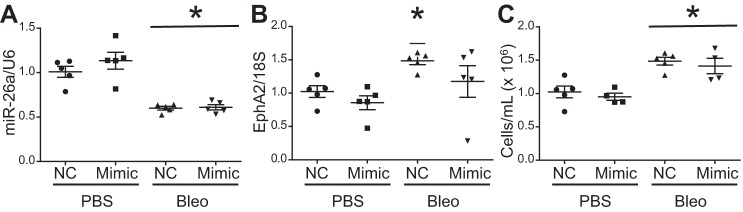
We used a commercially available neutral lipid carrier to deliver miR-26a mimic in vivo 4 days after bleomycin administration. With this delivery method, we did not restore lung miR-26a levels or demonstrate a decrease in EphA2 mRNA expression or alveolar cell count at the 7-day time point (Fig. 7, A–C).
Fig. 7.
Delivery of miR-26a in vivo did not alter miR-26a, EphA2, or permeability. Mice were treated with 100 µl of PBS or 0.1 U of bleomycin in 100 µl of PBS followed by 40 µg of miR-26a mimic 4 days after treatment. Lungs and bronchoalveolar lavage fluid collected 7 days after treatment. Lung miR-26a (A) and EphA2 (B) at 7 days. C: alveolar cell count at 7 days. Data displayed as means ± SE; n = 5, *P < 0.05 compared with PBS treated groups by two-way ANOVA followed by Tukey’s post hoc analysis. U6, small nuclear RNA U6; 18S, ribosomal protein 18S.
EphA2 and miR-26a expressed in multiple cell types.
To localize the source of miR-26a and EphA2, we performed ISH and IF. We found that miR-26a was expressed in multiple cell types (Fig. 8, A–D) and that EphA2 expression increased qualitatively in both endothelial and nonendothelial cells 7 days after bleomycin injury (Fig. 8, E–H). ISH and IF were performed on three samples from each group (PBS and bleomycin) to ensure reproducibility. To determine the changes in miR-26a expression in both endothelial and epithelial cells, we performed flow cytometry to identify miR-26a within lung endothelial and epithelial cells. While miR-26a was expressed in both cell types, in response to bleomycin, miR-26a decreased only in endothelial cells (Fig. 8, I–J).
Fig. 8.
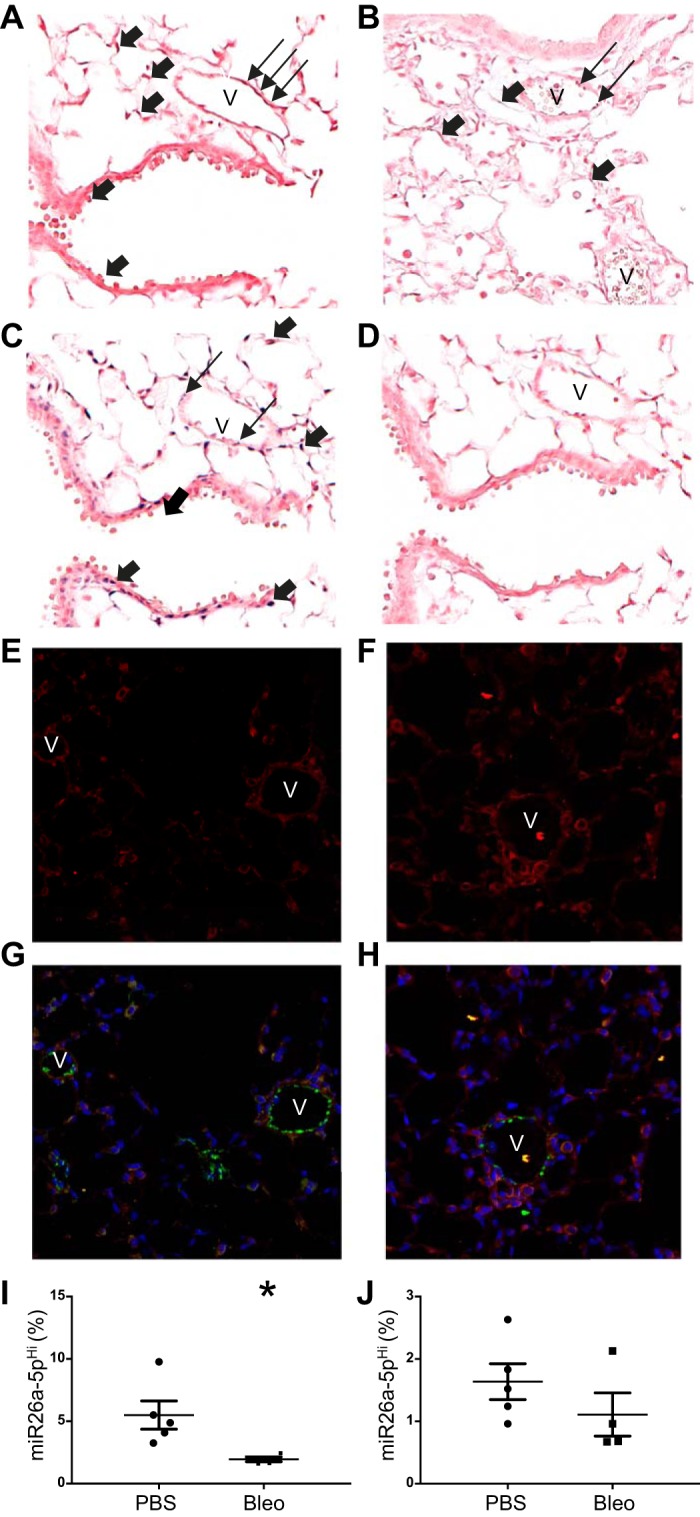
Localization of lung miR-26a and EphA2. Mice were treated with 100 µl of PBS or 0.1 U of bleomycin in 100 µl of PBS, and lungs were harvested 7 days after treatment. In situ hybridization (ISH) with dark blue staining for miR-26a after PBS (A) and bleomycin (B) treatment. ISH with dark blue staining for U6 (C) and negative control miRNA (D) after PBS treatment. Immunofluorescence staining for EphA2 (red), VWF (green), and DAPI (blue) after PBS (E and G) and bleomycin (F and H) treatment. Percentage of lung endothelial (I) and epithelial (J) cells expressing miR-26a. Images shown at ×20 magnification. V, vessel. Thick arrows denote positive staining in nonendothelial cells, whereas the thin arrows denote positive staining in endothelial cells. Data displayed as means ± SE; n = 4 or 5. *P < 0.05 compared with PBS as determined by unpaired t-test. U6, small nuclear RNA U6; 18S, ribosomal protein 18S.
DISCUSSION
Noncoding RNA, including miRNAs, regulate diverse normal and pathologic biologic processes, and miRNA modulation has shown therapeutic benefit in human disease (50). Disruption of the alveolar-capillary barrier is central to the development of lung injury, a devastating condition with no disease-modifying therapies, and thus, it is important to identify miRNAs altered in lung injury that control expression of genes important in the regulation of endothelial permeability (6). One miRNA of interest is miR-26a, given its demonstrated regulation of EphA2—a key receptor tyrosine kinase that our group and others demonstrated is increased in multiple experimental lung injury models and is vital to maintenance of endothelial barrier function (3, 4, 16, 25, 68). Our laboratory previously reported that EphA2 knockout blunts bleomycin-induced lung injury and that EphA2 stimulation increases lung endothelial cell permeability (3, 4). In this study, we demonstrated that miR-26a is decreased in a mouse model of bleomycin-induced lung injury, coinciding with increased EphA2 expression and alveolar edema, and that loss of miR-26a drives an increase in EphA2 expression and enhances permeability in HMVEC-L. As such, miR-26a may represent a novel therapeutic target in lung injury by mitigating EphA2-mediated changes in permeability.
Our first observation was that multiple miRNAs decreased in the lung 4 days following bleomycin treatment. We evaluated the lung miRNA profile at 4 days posttreatment with bleomycin as an unbiased approach to evaluate an early time point associated with enhanced alveolar-capillary permeability observed in the bleomycin-induced lung injury model (13, 34, 36, 39). The miRNA array provided insight into the complex regulatory network of miRNAs within the injured lung. Among the miRNA analyzed in the array, we focused on miR-26a, which significantly decreased in the bleomycin-injured lung and is predicted to regulate EphA2 and other gene targets involved in maintenance of the alveolar-capillary barrier. Other investigators have evaluated the time course of changes in the lung miRNA profile after bleomycin, including at 3- and 7-day time points; subsequent analyses of their findings focused on miRNA gene targets important in inflammation and apoptosis and late time points associated with lung fibrosis (59). Similar to our findings, multiple miRNAs were significantly altered after bleomycin, but the miRNAs included in the arrays differed, making direct comparison to our results difficult.
The array data served to identify miR-26a as a key downregulated miRNA in the lung, and qPCR validation studies demonstrated that miR-26a was consistently decreased at 7 days posttreatment with bleomycin. We speculate that the discrepancy in the time course of miR-26a between the microarray and qPCR were likely related to the limited number of samples in the microarray. Subsequent results were, thus, built upon the reproducible and significant loss of miR-26a by qPCR at 7 days. We also confirmed an increase in lung permeability by alveolar protein and cell count at 7 days posttreatment with bleomycin, consistent with our previous report, in which we tested alveolar edema and cellular influx across a time course and reported a peak at 7 days posttreatment with bleomycin (34, 39). Consistent with our data, miR-26a was also reported to decrease 4 wk after bleomycin administration (30, 31, 59). Lung miRNA profiles have also been reported in other experimental models of lung injury, and miR-26a decreases in numerous models, including saline lavage, mechanical ventilation, influenza infection, allergic inflammation, and lung cancer (12, 17, 21, 26, 32, 41, 51, 65). While miRNAs comprise a sophisticated and dynamic regulatory network, the conservation of miR-26a downregulation in multiple injury models and over time after bleomycin injury, combined with its predicted role in the regulation of EphA2 and other genes important in regulation cell adhesion, supports an important role for miR-26a in disruption of endothelial barrier function.
We focused our subsequent investigations on the relationship between miR-26a and EphA2 in both the lung and pulmonary endothelial cells. First, we demonstrated the decrease in miR-26a correlated with an increase in EphA2 in our model at 7 days. Targeting of EphA2 by miR-26a is both predicted by in silico analysis and confirmed by functional validation of miR-26a regulation of EphA2 expression in endothelial progenitor cells by luciferase-EphA2 3′UTR activity assay (68). Given the well-established role of EphA2 in the development of enhanced lung permeability and evidence for miR-26a regulation of EphA2 expression, our findings suggest that the miR-26a/EphA2 axis is important in the development of increased permeability after bleomycin injury (3, 4). However, miRNA binding is promiscuous, and miR-26a has thousands of additional gene targets predicted by in silico analysis. Some of these gene targets, including ROCK1 and KDR, also have important roles in the development of enhanced permeability in lung injury but were not increased in our model of bleomycin-induced lung injury (14, 52).
We next demonstrated that manipulation of miR-26a levels in lung endothelial cells leads to consistent and significant reciprocal changes in EphA2 levels, further supporting the role of the miR-26a/EphA2 axis in the development of lung injury. Control of EphA2 expression by miR-26a and miR-26b has been demonstrated in hepatocellular carcinoma and pituitary gland cells but not in the pulmonary endothelium (23, 28, 60). These previous findings are consistent with our results, but because miRNA effects on gene expression are cell specific, determination of the epigenetic regulation of EphA2 expression in the pulmonary endothelium is vital to understanding the pathogenesis of lung injury. Both ROCK1 and KDR are gene targets that are potentially regulated by miR-26a and implicated in the development of increased permeability in lung injury (14, 52). Despite the predicted relationship between miR-26a, ROCK1, and KDR, neither of these gene targets exhibited the same tonic relationship as miR-26a and EphA2. While exogenous miR-26a is capable of inhibiting KDR in vitro, the loss of endogenous miR-26a conversely did not increase KDR expression in lung endothelial cells and, therefore, would not account for the observed increased permeability.
Our final major observation was that miR-26a inhibition increased endothelial cell permeability via upregulation of EphA2. The increase in EphA2 expression due to loss of miR-26a could account for enhanced HMVEC-L permeability in the absence of exogenous ephrin, as increased EphA2 expression can lead to ligand-independent transactivation of the EphA2 receptor (9, 27, 37, 42). To confirm the contribution of EphA2 in the increased permeability observed with the miR-26a inhibitor, we assessed the integrity of VE-cadherin adherens junctions in endothelial cells transfected with both the miR-26a inhibitor and siRNA against EphA2. Although we and other investigators have been able to assess HMVEC-L barrier function in cells after transfection with either miRNA mimics/inhibitors or siRNA molecules, we were unable to repeat experiments in HMVEC-L after dual transfections due to the consequent impaired cell barrier formation (1). Thus, we repeated the experiments in an alternative cell type, HUVECs, which better tolerate stressful conditions, and were able to use VE-cadherin staining to assess endothelial cell barrier formation through adherens junctions, as previously reported (15, 25, 46). These studies confirmed that the loss of barrier integrity seen after miR-26 inhibition was blocked when EphA2 was knocked down. Additional miR-26a gene targets may also contribute to the increased permeability demonstrated with miR-26a inhibition. Although we did not detect an increase in KDR and ROCK1 mRNA levels with the miR26a inhibitor, we did not evaluate protein content. It is also possible that expression of additional miR-26a gene targets that regulate cell adhesion was altered. Consistent changes in HMVEC-L permeability were not induced by transfection with the miR-26a mimic. The mimic reduced EphA2 expression by half, although the residual EphA2 expression could have been sufficient to preserve permeability. Additional miR-26a gene targets involved in the redundant mechanisms to maintain endothelial cell barrier integrity may also contribute to the lack of effect seen with the mimic.
Although our findings are the first to implicate miR-26a in the regulation of endothelial permeability, it already has a well-established role in the regulation of angiogenesis and pulmonary fibrosis (5, 8, 19, 20, 22, 31, 44, 62). This previous work, combined with the direct relationship between angiogenesis and permeability, further supports the role of miR-26a in maintenance of barrier function (40). Investigators have discovered other miRNA-gene target pairs important in the regulation of endothelial permeability (6). Our findings likewise support the importance of miRNA regulation of the alveolar-capillary barrier and add miR-26a to the growing list of miRNAs that are potential therapeutic targets for lung injury. IT delivery of exogenous miR-26a to the lung has been shown to blunt the fibrotic response after IT bleomycin by targeting CTGF (31). Although this finding supports the potential for supplementation of miR-26a as a treatment modality, it also highlights the importance of accounting for other miRNA gene targets when developing therapeutic strategies that utilize miRNAs. As such, miRNA delivery vehicles that target the tissue and cells affected by injury have the potential to direct miRNA treatment to the desired target genes in a given disease state.
To determine whether we could modulate miR-26a and, thus, alter EphA2 expression and permeability in vivo, we administered intravenous miR-26a mimic to bleomycin-treated mice. We selected an established delivery method using a commercially available lipid emulsion, which has been successfully used to deliver miRNA in other models of lung disease, including pulmonary hypertension and lung cancer (49, 53, 56). This approach was ineffective in altering whole lung miR-26a levels in our model, and consequently, we did not observe a change in EphA2 or markers of permeability. It is possible that the commercially available delivery system we used did not sufficiently protect the miRNA molecule from degradation or deliver sufficient levels to the lung endothelial cells. Effective delivery of exogenous miRNA is a common technical challenge, and a wide variety of solutions have been proposed (11, 18, 50, 63, 64). Intratracheal administration of miR-26a has previously been described and reported to protect from bleomycin-induced pulmonary fibrosis, but investigators did not evaluate outcomes until day 21 (30). Our ISH and flow cytometry studies confirmed that while miR-26a is expressed in multiple lung cell types, a significant decrease was observed only in the endothelial cells early after bleomycin treatment, indicating that an intravenous administration should provide the most direct delivery mechanism for abrogating the increased vascular permeability seen in acute lung injury. Future studies will test delivery of miR-26a via functionalized nanoparticles to improve delivery to the injured vascular endothelium.
In summary, we characterized miRNA dysregulation in bleomycin-induced lung injury, including a significant decrease in miR-26a. We further demonstrated the relationship of miR-26a and EphA2 expression in HMVEC-L. Most importantly, we established that miR-26a inhibition enhanced permeability in these cells. The role of the miR-26a/EphA2 axis in the development of lung injury and as a possible therapeutic warrants further study.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grant 5-R01-HL-119533-03 (to T. Carpenter, E. Nozik-Grayck, U. Kompella, and C. Sucharov). Imaging experiments were performed in the University of Colorado Anschutz Medical Campus Advance Light Microscopy Core supported in part by National Center for Advancing Translational Science Colorado Clinical and Translational Science Award Grant UL1-TR001082.
DISCLAIMERS
Contents are the authors' sole responsibility and do not necessarily represent official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.J.G., C.U.V., A.K.U., T.C.C., C.C.S., and E.N.-G. conceived and designed research; R.J.G., L.H.-L., A.A., J.K.M., C.U.V., U.K., T.C.C., C.C.S., and E.N.-G. performed experiments; R.J.G., L.H.-L., A.A., J.K.M., C.U.V., A.K.U., U.K., K.G.B., T.C.C., C.C.S., and E.N.-G. analyzed data; R.J.G., L.H.-L., J.K.M., C.U.V., A.K.U., U.K., K.G.B., T.C.C., C.C.S., and E.N.-G. interpreted results of experiments; R.J.G., L.H.-L., C.C.S., and E.N.-G. prepared figures; R.J.G., C.C.S., and E.N.-G. drafted manuscript; R.J.G., L.H.-L., A.A., C.U.V., A.K.U., U.K., K.G.B., T.C.C., C.C.S., and E.N.-G. edited and revised manuscript; R.J.G., L.H.-L., A.A., J.K.M., C.U.V., A.K.U., U.K., K.G.B., T.C.C., C.C.S., and E.N.-G. approved final version of manuscript.
REFERENCES
- 1.Aman J, Weijers EM, van Nieuw Amerongen GP, Malik AB, van Hinsbergh VWM. Using cultured endothelial cells to study endothelial barrier dysfunction: challenges and opportunities. Am J Physiol Lung Cell Mol Physiol 311: L453–L466, 2016. doi: 10.1152/ajplung.00393.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao Y, Lyu YI, Tang J, Li Y. MicroRNAs: novel regulatory molecules in acute lung injury/acute respiratory distress syndrome. Biomed Rep 4: 523–527, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carpenter TC, Schroeder W, Stenmark KR, Schmidt EP. Eph-A2 promotes permeability and inflammatory responses to bleomycin-induced lung injury. Am J Respir Cell Mol Biol 46: 40–47, 2012. doi: 10.1165/rcmb.2011-0044OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cercone MA, Schroeder W, Schomberg S, Carpenter TC. EphA2 receptor mediates increased vascular permeability in lung injury due to viral infection and hypoxia. Am J Physiol Lung Cell Mol Physiol 297: L856–L863, 2009. doi: 10.1152/ajplung.00118.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chai ZT, Kong J, Zhu XD, Zhang YY, Lu L, Zhou JM, Wang LR, Zhang KZ, Zhang QB, Ao JY, Wang M, Wu WZ, Wang L, Tang ZY, Sun HC. MicroRNA-26a inhibits angiogenesis by down-regulating VEGFA through the PIK3C2α/Akt/HIF-1α pathway in hepatocellular carcinoma. PLoS One 8: e77957, 2013. doi: 10.1371/journal.pone.0077957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chamorro-Jorganes A, Araldi E, Suárez Y. MicroRNAs as pharmacological targets in endothelial cell function and dysfunction. Pharmacol Res 75: 15–27, 2013. doi: 10.1016/j.phrs.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, Clark NR, Ma’ayan A. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14: 128, 2013. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Zhang K, Xu Y, Gao Y, Li C, Wang R, Chen L. The role of microRNA-26a in human cancer progression and clinical application. Tumour Biol 37: 7095–7108, 2016. doi: 10.1007/s13277-016-5017-y. [DOI] [PubMed] [Google Scholar]
- 9.Coulthard MG, Morgan M, Woodruff TM, Arumugam TV, Taylor SM, Carpenter TC, Lackmann M, Boyd AW. Eph/Ephrin signaling in injury and inflammation. Am J Pathol 181: 1493–1503, 2012. doi: 10.1016/j.ajpath.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 10.Dubrovskyi O, Birukova AA, Birukov KG. Measurement of local permeability at subcellular level in cell models of agonist- and ventilator-induced lung injury. Lab Invest 93: 254–263, 2013. doi: 10.1038/labinvest.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujita Y, Takeshita F, Kuwano K, Ochiya T. RNAi therapeutic platforms for lung diseases. Pharmaceuticals (Basel) 6: 223–250, 2013. doi: 10.3390/ph6020223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao S, Li J, Song L, Wu J, Huang W. Influenza A virus-induced downregulation of miR-26a contributes to reduced IFNα/β production. Virol Sin 32: 261–270, 2017. doi: 10.1007/s12250-017-4004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gasse P, Mary C, Guenon I, Noulin N, Charron S, Schnyder-Candrian S, Schnyder B, Akira S, Quesniaux VFJ, Lagente V, Ryffel B, Couillin I. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J Clin Invest 117: 3786–3799, 2007. doi: 10.1172/JCI32285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han J, Ding R, Zhao D, Zhang Z, Ma X. Unfractionated heparin attenuates lung vascular leak in a mouse model of sepsis: role of RhoA/Rho kinase pathway. Thromb Res 132: e42–e47, 2013. doi: 10.1016/j.thromres.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Hirano M, Hirano K. Myosin di-phosphorylation and peripheral actin bundle formation as initial events during endothelial barrier disruption. Sci Rep 6: 20989, 2016. doi: 10.1038/srep20989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong JY, Shin MH, Douglas IS, Chung KS, Kim EY, Jung JY, Kang YA, Kim SK, Chang J, Kim YS, Park MS. Inhibition of EphA2/EphrinA1 signal attenuates lipopolysaccharide-induced lung injury. Clin Sci (Lond) 130: 1993–2003, 2016. doi: 10.1042/CS20160360. [DOI] [PubMed] [Google Scholar]
- 17.Huang C, Xiao X, Chintagari NR, Breshears M, Wang Y, Liu L. MicroRNA and mRNA expression profiling in rat acute respiratory distress syndrome. BMC Med Genomics 7: 46, 2014. doi: 10.1186/1755-8794-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huleihel L, Sellares J, Cardenes N, Álvarez D, Faner R, Sakamoto K, Yu G, Kapetanaki MG, Kaminski N, Rojas M. Modified mesenchymal stem cells using miRNA transduction alter lung injury in a bleomycin model. Am J Physiol Lung Cell Mol Physiol 313: L92–L103, 2017. doi: 10.1152/ajplung.00323.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Icli B, Dorbala P, Feinberg MW. An emerging role for the miR-26 family in cardiovascular disease. Trends Cardiovasc Med 24: 241–248, 2014. doi: 10.1016/j.tcm.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Icli B, Wara AK, Moslehi J, Sun X, Plovie E, Cahill M, Marchini JF, Schissler A, Padera RF, Shi J, Cheng HW, Raghuram S, Arany Z, Liao R, Croce K, MacRae C, Feinberg MW. MicroRNA-26a regulates pathological and physiological angiogenesis by targeting BMP/SMAD1 signaling. Circ Res 113: 1231–1241, 2013. doi: 10.1161/CIRCRESAHA.113.301780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang C, Yu H, Sun Q, Zhu W, Xu J, Gao N, Zhang R, Liu L, Wu X, Yang X, Meng L, Lu S. Extracellular microRNA-21 and microRNA-26a increase in body fluids from rats with antigen induced pulmonary inflammation and children with recurrent wheezing. BMC Pulm Med 16: 50, 2016. doi: 10.1186/s12890-016-0216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang H, Chen Y, Yu T, Zhao X, Shan H, Sun J, Zhang L, Li X, Shan H, Liang H. Inhibition of lncRNA PFRL prevents pulmonary fibrosis by disrupting the miR-26a/Smad2 loop. Am J Physiol Lung Cell Mol Physiol ajplung.00434.2017, 2018. doi: 10.1152/ajplung.00434.2017. [DOI] [PubMed] [Google Scholar]
- 23.Jin Q, Li XJ, Cao PG. MicroRNA-26b enhances the radiosensitivity of hepatocellular carcinoma cells by targeting EphA2. Tohoku J Exp Med 238: 143–151, 2016. doi: 10.1620/tjem.238.143. [DOI] [PubMed] [Google Scholar]
- 24.Jonas S, Izaurralde E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat Rev Genet 16: 421–433, 2015. doi: 10.1038/nrg3965. [DOI] [PubMed] [Google Scholar]
- 25.Larson J, Schomberg S, Schroeder W, Carpenter TC. Endothelial EphA receptor stimulation increases lung vascular permeability. Am J Physiol Lung Cell Mol Physiol 295: L431–L439, 2008. doi: 10.1152/ajplung.90256.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SM, Choi H, Yang G, Park KC, Jeong S, Hong S. microRNAs mediate oleic acid-induced acute lung injury in rats using an alternative injury mechanism. Mol Med Rep 10: 292–300, 2014. doi: 10.3892/mmr.2014.2155. [DOI] [PubMed] [Google Scholar]
- 27.Lennon FE, Mirzapoiazova T, Mambetsariev N, Mambetsariev B, Salgia R, Singleton PA. Transactivation of the receptor-tyrosine kinase ephrin receptor A2 is required for the low molecular weight hyaluronan-mediated angiogenesis that is implicated in tumor progression. J Biol Chem 289: 24043–24058, 2014. doi: 10.1074/jbc.M114.554766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H, Sun Q, Han B, Yu X, Hu B, Hu S. MiR-26b inhibits hepatocellular carcinoma cell proliferation, migration, and invasion by targeting EphA2. Int J Clin Exp Pathol 8: 4782–4790, 2015. [PMC free article] [PubMed] [Google Scholar]
- 29.Li W, Ma K, Zhang S, Zhang H, Liu J, Wang X, Li S. Pulmonary microRNA expression profiling in an immature piglet model of cardiopulmonary bypass-induced acute lung injury. Artif Organs 39: 327–335, 2015. doi: 10.1111/aor.12387. [DOI] [PubMed] [Google Scholar]
- 30.Liang H, Gu Y, Li T, Zhang Y, Huangfu L, Hu M, Zhao D, Chen Y, Liu S, Dong Y, Li X, Lu Y, Yang B, Shan H. Integrated analyses identify the involvement of microRNA-26a in epithelial-mesenchymal transition during idiopathic pulmonary fibrosis. Cell Death Dis 5: e1238, 2014. doi: 10.1038/cddis.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang H, Xu C, Pan Z, Zhang Y, Xu Z, Chen Y, Li T, Li X, Liu Y, Huangfu L, Lu Y, Zhang Z, Yang B, Samuel G, Lu Y, Shan H, Du Z. The anti-fibrotic effects and mechanisms of MicroRNA-26a in idiopathic pulmonary fibrosis. Mol Ther 10.1038/mt.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long H, Xiang T, Luo J, Li F, Lin R, Liu S, Jiang S, Hu C, Chen G, Wong E, Wan Y, Li QJ, Zhu B. The tumor microenvironment disarms CD8+ T lymphocyte function via a miR-26a-EZH2 axis. OncoImmunology 5: e1245267, 2016. doi: 10.1080/2162402X.2016.1245267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.López-Fernández Y, Azagra AM, de la Oliva P, Modesto V, Sánchez JI, Parrilla J, Arroyo MJ, Reyes SB, Pons-Ódena M, López-Herce J, Fernández RL, Kacmarek RM, Villar J; Pediatric Acute Lung Injury Epidemiology and Natural History (PED-ALIEN) Network . Pediatric acute lung injury epidemiology and natural history study: incidence and outcome of the acute respiratory distress syndrome in children. Crit Care Med 40: 3238–3245, 2012. doi: 10.1097/CCM.0b013e318260caa3. [DOI] [PubMed] [Google Scholar]
- 34.Ma L, Shaver CM, Grove BS, Mitchell DB, Wickersham NE, Carnahan RH, Cooper TL, Brake BE, Ware LB, Bastarache JA. Kinetics of lung tissue factor expression and procoagulant activity in bleomycin induced acute lung injury. Clin Transl Med 4: 63, 2015. doi: 10.1186/s40169-015-0063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol 6: 147–163, 2011. doi: 10.1146/annurev-pathol-011110-130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295: L379–L399, 2008. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miao H, Li DQ, Mukherjee A, Guo H, Petty A, Cutter J, Basilion JP, Sedor J, Wu J, Danielpour D, Sloan AE, Cohen ML, Wang B. EphA2 mediates ligand-dependent inhibition and ligand-independent promotion of cell migration and invasion via a reciprocal regulatory loop with Akt. Cancer Cell 16: 9–20, 2009. doi: 10.1016/j.ccr.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Millar FR, Summers C, Griffiths MJ, Toshner MR, Proudfoot AG. The pulmonary endothelium in acute respiratory distress syndrome: insights and therapeutic opportunities. Thorax 71: 462–473, 2016. doi: 10.1136/thoraxjnl-2015-207461. [DOI] [PubMed] [Google Scholar]
- 39.Mouradian GC, Gaurav R, Pugliese S, Kasmi El K, Hartman B, Hernandez-Lagunas L, Stenmark K, Bowler RP, Nozik-Grayck E. SOD3 R213G SNP blocks murine bleomycin-induced fibrosis and promotes resolution of inflammation. Am J Respir Cell Mol Biol 56: 362–371, 2017. doi: 10.1165/rcmb.2016-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagy JA, Benjamin L, Zeng H, Dvorak AM, Dvorak HF. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis 11: 109–119, 2008. doi: 10.1007/s10456-008-9099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Otsuki T, Ishikawa M, Hori Y, Goto G, Sakamoto A. Volatile anesthetic sevoflurane ameliorates endotoxin-induced acute lung injury via microRNA modulation in rats. Biomed Rep 3: 408–412, 2015. doi: 10.3892/br.2015.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paraiso KH, Das Thakur M, Fang B, Koomen JM, Fedorenko IV, John JK, Tsao H, Flaherty KT, Sondak VK, Messina JL, Pasquale EB, Villagra A, Rao UN, Kirkwood JM, Meier F, Sloot S, Gibney GT, Stuart D, Tawbi H, Smalley KS. Ligand-independent EPHA2 signaling drives the adoption of a targeted therapy-mediated metastatic melanoma phenotype. Cancer Discov 5: 264–273, 2015. doi: 10.1158/2159-8290.CD-14-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pierrakos C, Karanikolas M, Scolletta S, Karamouzos V, Velissaris D. Acute respiratory distress syndrome: pathophysiology and therapeutic options. J Clin Med Res 4: 7–16, 2012. doi: 10.4021/jocmr761w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qian X, Zhao P, Li W, Shi ZM, Wang L, Xu Q, Wang M, Liu N, Liu LZ, Jiang BH. MicroRNA-26a promotes tumor growth and angiogenesis in glioma by directly targeting prohibitin. CNS Neurosci Ther 19: 804–812, 2013. doi: 10.1111/cns.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rajasekaran S, Pattarayan D, Rajaguru P, Sudhakar Gandhi PS, Thimmulappa RK. MicroRNA regulation of acute lung injury and acute respiratory distress syndrome. J Cell Physiol 231: 2097–2106, 2016. doi: 10.1002/jcp.25316. [DOI] [PubMed] [Google Scholar]
- 46.Rajput C, Tauseef M, Farazuddin M, Yazbeck P, Amin MR, Avin Br V, Sharma T, Mehta D. MicroRNA-150 suppression of angiopoetin-2 generation and signaling is crucial for resolving vascular injury. Arterioscler Thromb Vasc Biol 36: 380–388, 2016. doi: 10.1161/ATVBAHA.115.306997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med 353: 1685–1693, 2005. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 48.Shafeeq H, Lat I. Pharmacotherapy for acute respiratory distress syndrome. Pharmacotherapy 32: 943–957, 2012. doi: 10.1002/j.1875-9114.2012.01115. [DOI] [PubMed] [Google Scholar]
- 49.Trang P, Wiggins JF, Daige CL, Cho C, Omotola M, Brown D, Weidhaas JB, Bader AG, Slack FJ. Systemic delivery of tumor suppressor microRNA mimics using a neutral lipid emulsion inhibits lung tumors in mice. Mol Ther 19: 1116–1122, 2011. doi: 10.1038/mt.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Rooij E, Kauppinen S. Development of microRNA therapeutics is coming of age. EMBO Mol Med 6: 851–864, 2014. doi: 10.15252/emmm.201100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vaporidi K, Vergadi E, Kaniaris E, Hatziapostolou M, Lagoudaki E, Georgopoulos D, Zapol WM, Bloch KD, Iliopoulos D. Pulmonary microRNA profiling in a mouse model of ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 303: L199–L207, 2012. doi: 10.1152/ajplung.00370.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vohra PK, Hoeppner LH, Sagar G, Dutta SK, Misra S, Hubmayr RD, Mukhopadhyay D. Dopamine inhibits pulmonary edema through the VEGF-VEGFR2 axis in a murine model of acute lung injury. Am J Physiol Lung Cell Mol Physiol 302: L185–L192, 2012. doi: 10.1152/ajplung.00274.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallace E, Morrell NW, Yang XD, Long L, Stevens H, Nilsen M, Loughlin L, Mair KM, Baker AH, MacLean MR. A sex-specific microRNA-96/5-hydroxytryptamine 1B axis influences development of pulmonary hypertension. Am J Respir Crit Care Med 191: 1432–1442, 2015. doi: 10.1164/rccm.201412-2148OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang T, Gross C, Desai AA, Zemskov E, Wu X, Garcia AN, Jacobson JR, Yuan JX, Garcia JG, Black SM. Endothelial cell signaling and ventilator-induced lung injury: molecular mechanisms, genomic analyses, and therapeutic targets. Am J Physiol Lung Cell Mol Physiol 312: L452–L476, 2017. doi: 10.1152/ajplung.00231.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 56.Wiggins JF, Ruffino L, Kelnar K, Omotola M, Patrawala L, Brown D, Bader AG. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Res 70: 5923–5930, 2010. doi: 10.1158/0008-5472.CAN-10-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie L, Zhou J, Zhang S, Chen Q, Lai R, Ding W, Song C, Meng X, Wu J. Integrating microRNA and mRNA expression profiles in response to radiation-induced injury in rat lung. Radiat Oncol 9: 111, 2014. doi: 10.1186/1748-717X-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xie N, Liu G. ncRNA-regulated immune response and its role in inflammatory lung diseases. Am J Physiol Lung Cell Mol Physiol 309: L1076–L1087, 2015. doi: 10.1152/ajplung.00286.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xie T, Liang J, Guo R, Liu N, Noble PW, Jiang D. Comprehensive microRNA analysis in bleomycin-induced pulmonary fibrosis identifies multiple sites of molecular regulation. Physiol Genomics 43: 479–487, 2011. doi: 10.1152/physiolgenomics.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yuan B, Yu WY, Dai LS, Gao Y, Ding Y, Yu XF, Chen J, Zhang JB. Expression of microRNA-26b and identification of its target gene EphA2 in pituitary tissues in Yanbian cattle. Mol Med Rep 12: 5753–5761, 2015. doi: 10.3892/mmr.2015.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest 133: 1120–1127, 2008. doi: 10.1378/chest.07-2134. [DOI] [PubMed] [Google Scholar]
- 62.Zgheib C, Liechty KW. Shedding light on miR-26a: Another key regulator of angiogenesis in diabetic wound healing. J Mol Cell Cardiol 92: 203–205, 2016. doi: 10.1016/j.yjmcc.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 63.Zhang D, Lee H, Zhu Z, Minhas JK, Jin Y. Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol 312: L110–L121, 2017. doi: 10.1152/ajplung.00423.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y, Wang Z, Gemeinhart RA. Progress in microRNA delivery. J Control Release 172: 962–974, 2013. doi: 10.1016/j.jconrel.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang YH, Wu LZ, Liang HL, Yang Y, Qiu J, Kan Q, Zhu W, Ma CL, Zhou XY. Pulmonary surfactant synthesis in miRNA-26a-1/miRNA-26a-2 double knockout mice generated using the CRISPR/Cas9 system. Am J Transl Res 9: 355–365, 2017. [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou T, Garcia JG, Zhang W. Integrating microRNAs into a system biology approach to acute lung injury. Transl Res 157: 180–190, 2011. doi: 10.1016/j.trsl.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zimmerman JJ, Akhtar SR, Caldwell E, Rubenfeld GD. Incidence and outcomes of pediatric acute lung injury. Pediatrics 124: 87–95, 2009. doi: 10.1542/peds.2007-2462. [DOI] [PubMed] [Google Scholar]
- 68.Zuo K, Zhi K, Zhang X, Lu C, Wang S, Li M, He B. A dysregulated microRNA-26a/EphA2 axis impairs endothelial progenitor cell function via the p38 MAPK/VEGF pathway. Cell Physiol Biochem 35: 477–488, 2015. doi: 10.1159/000369713. [DOI] [PubMed] [Google Scholar]